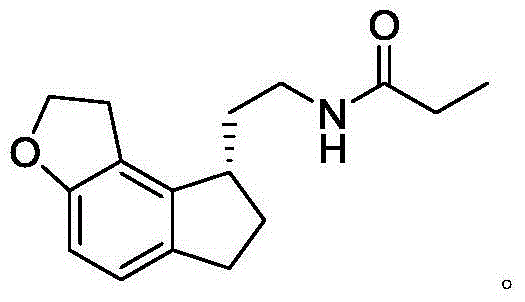
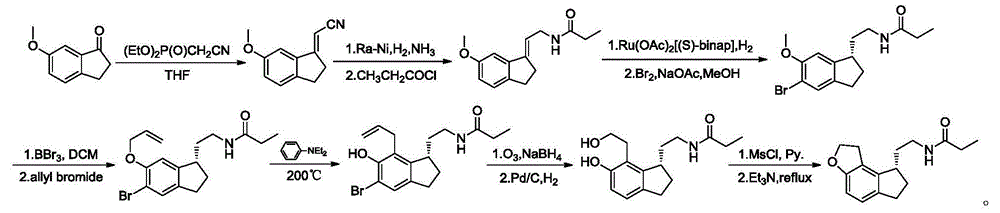
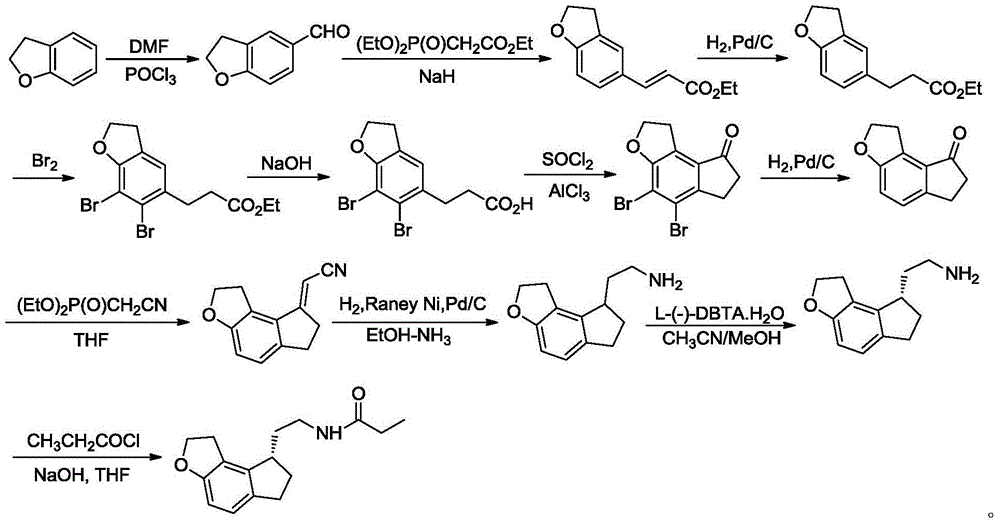
Synthetic preparation method for key intermediate of ramelteon
A technology for ramelteon and its intermediates, applied in the field of drug synthesis, can solve the problems of unavailable starting materials, heavy environmental pollution, and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Synthesis and preparation of 1-(2,3-dihydrobenzofuran-4-yl)-2-propen-1-ol (Ⅲ)
[0064] The reaction formula is as follows:
[0065]
[0066] Add 1050mL (1.05mol) of 1.0M tetrahydrofuran solution of vinylmagnesium bromide into the reaction flask, start stirring, cool the reaction solution to -10~-5°C, add dropwise 2,3-dihydrobenzofuran-4 -formaldehyde (148.2g, 1.00mol) in tetrahydrofuran (500mL) solution, and control the temperature of the reaction solution not to exceed -5°C. After the dropwise addition, keep the temperature at -10~-5°C and react for half an hour, then slowly raise the temperature to room temperature to continue the reaction. After 6 hours, 300 mL of saturated aqueous ammonium chloride solution was added, stirred for 30 minutes, and extracted three times with ethyl acetate (800 mL×3). The organic phase was washed once each with saturated sodium chloride solution (300 mL) and water (300 mL), dried over anhydrous sodium sulfate, filtered, ...
Embodiment 2
[0067] Example 2: Synthesis and preparation of 1-(2,3-dihydrobenzofuran-4-yl)-2-propen-1-one (IV)
[0068] The reaction formula is as follows:
[0069]
[0070]At room temperature, add 750 mL of dimethyl sulfoxide solvent into the reaction flask, and then add 150.0 g of 1-(2,3-dihydrobenzofuran-4-yl)-2-propen-1-ol described in formula III (0.85mol), stirring and dissolving, adding 360g (1.29mol) of 2-iodobenzoic acid (IBX) in batches at room temperature, and the addition was completed in about 10 minutes. After continuing to react at room temperature for 2 hours, TLC detected that the raw material point disappeared. Add 2.5L of ethyl acetate, stir for 15 minutes, filter, wash the filtrate twice with water (850mL×2), dry over anhydrous sodium sulfate, filter, and distill the filtrate to remove the solvent under reduced pressure to obtain 139.2g of a yellow oil. The rate is 94.1%.
Embodiment 3
[0071] Example 3: Synthesis and preparation of 1-(2,3-dihydrobenzofuran-4-yl)-2-propen-1-one (IV)
[0072] The reaction formula is as follows:
[0073]
[0074] Add 1500 mL of dichloromethane and 130.0 g (0.74 mol) of 1-(2,3-dihydrobenzofuran-4-yl)-2-propen-1-ol described in formula III to the reaction flask, stir to dissolve 410 g (0.97 mol) of (1,1,1-triacetoxy)-1,1-dihydro-1,2-phenyliodide-3(1H)-one (DMP) was added at room temperature. After continuing to react at room temperature for 2 hours, TLC detected that the raw material point disappeared. Add ethyl acetate 1000mL, 10% Na 2 S 2 o 3 Solution 150mL and saturated NaHCO 3 200 mL of the solution, stirred vigorously for 1 hour, separated the organic phase, washed once with saturated brine (800 mL) and water (800 mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was distilled off the solvent under reduced pressure to obtain a yellow oil The product was 124.8g, and the yield was 97.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com