Ramelteon sublingual tablets and preparation method thereof
A technology for ramelteon and sublingual tablet, applied in the field of ramelteon sublingual tablet and preparation thereof, can solve the problems of poor patient compliance, increased preparation cost, cumbersome and time-consuming, etc., and achieves absorption promotion and bioavailability improvement. The effect of improving the degree of compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
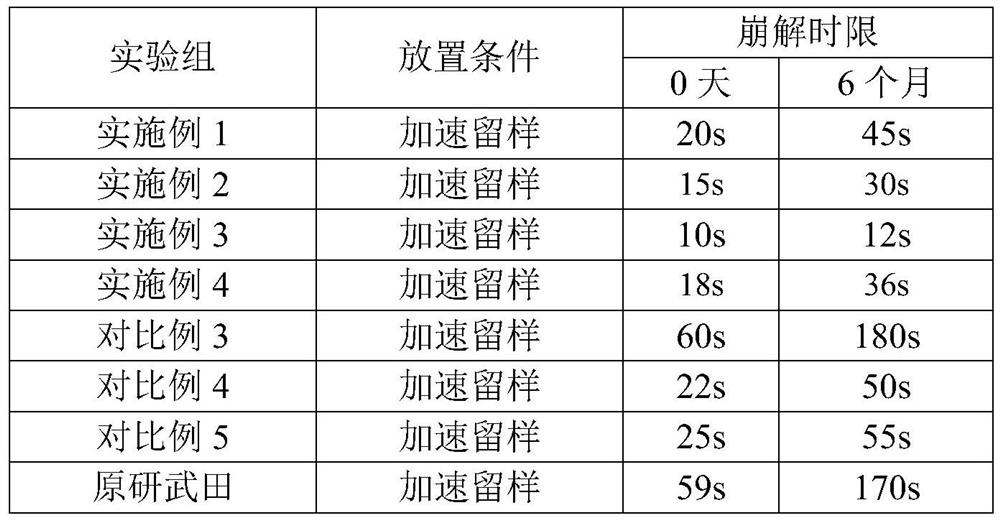
Embodiment 1
[0032] The prescription and technology of embodiment 1 ramelteon sublingual tablet
[0033] See below for the prescription. Assuming that the specification of ramelteon is 0.25 mg / tablet and the prescription quantity is 10,000 tablets, the dosage (unit: g) and weight ratio of each component are as follows:
[0034] Ramelteon 2.5g(0.25%) Mannitol 956g(95.6%) Crospovidone 40g(4%) Magnesium stearate 1g(0.1%) mint flavor 0.5g(0.05%) total 1000g(100%)
[0035] The preparation method is as follows:
[0036] (1) Get the ramelteon of prescription quantity and pass through 80 mesh sieves, then take the mannitol of prescription quantity, crospovidone and peppermint essence and pass through 60 mesh sieves respectively; Take each material of prescription quantity, for subsequent use;
[0037] (2) Place the ramelteon obtained in step (1) and the mannitol of 1 / 4 prescription amount in a ball mill and grind and mix for 2 hours to obtain a ramelteo...
Embodiment 2
[0040] The prescription and technology of embodiment 2 ramelteon sublingual tablet
[0041] See below for the prescription. Assuming that the specification of ramelteon is 0.25 mg / tablet and the prescription quantity is 10,000 tablets, the dosage (unit: g) and weight ratio of each component are as follows:
[0042] Ramelteon 2.5g(0.25%) Mannitol 956g(95.6%) Crospovidone 20g(2%) Sodium carboxymethyl starch 20g(2%) Magnesium stearate 1g(0.1%) mint flavor 0.5g(0.05%) total 1000g(100%)
[0043] The preparation method is the same as in Example 1, only the disintegrant in steps (1) and (3) is replaced by the Crospovidone of Example 1 and the Crospovidone and sodium carboxymethyl starch of the cost embodiment .
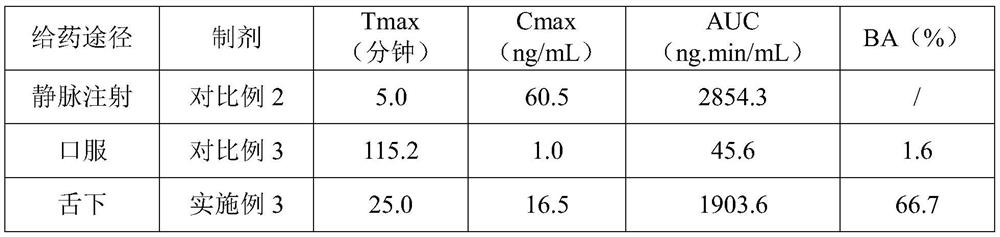
Embodiment 3
[0044] The prescription and technology of embodiment 3 ramelteon sublingual tablet
[0045] See below for the prescription. Assuming that the specification of ramelteon is 0.25 mg / tablet and the prescription quantity is 10,000 tablets, the dosage (unit: g) and weight ratio of each component are as follows:
[0046] Ramelteon 2.5g(0.25%) Mannitol 956g(95.6%) Crospovidone 13.3g(1.33%) Sodium carboxymethyl starch 26.7g(2.67%) Magnesium stearate 1g(0.1%) mint flavor 0.5g(0.05%) total 1000g(100%)
[0047] The preparation method is the same as Example 1, only the disintegrant in steps (1) and (3) is replaced by the Crospovidone of Example 1 and the Crospovidone and sodium carboxymethyl starch of the cost embodiment .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com