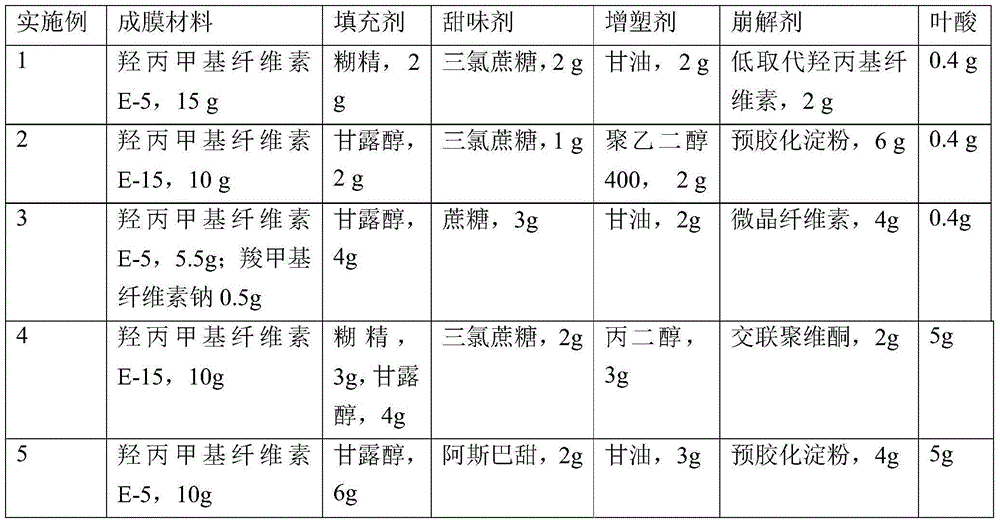
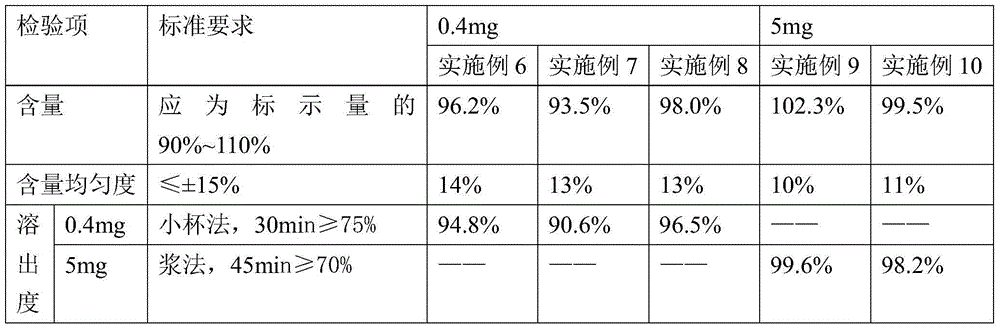
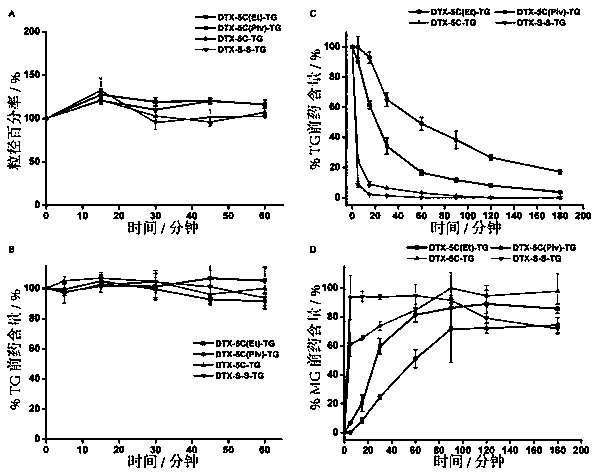
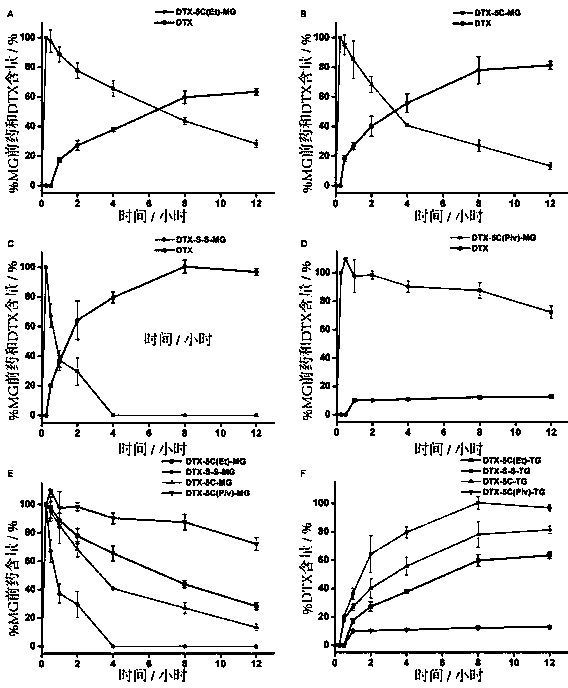
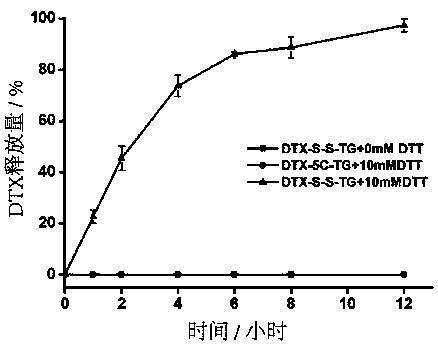
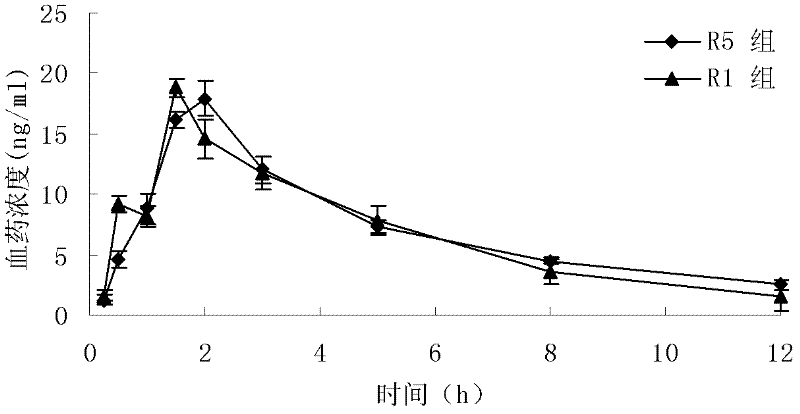
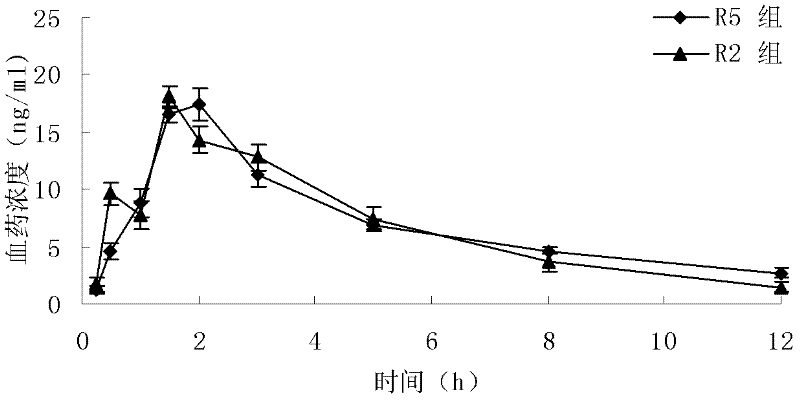
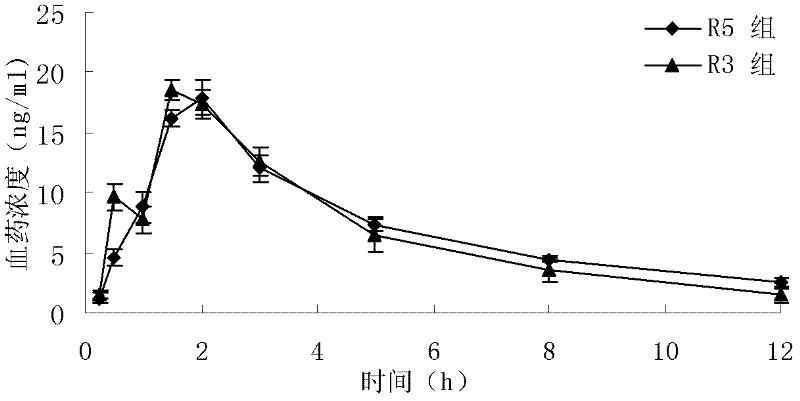
Patents
Literature
165 results about "First pass effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The first pass effect (also known as first-pass metabolism or presystemic metabolism) is a phenomenon of drug metabolism whereby the concentration of a drug is greatly reduced before it reaches the systemic circulation. It is the fraction of drug lost during the process of absorption which is generally related to the liver and gut wall. Notable drugs that experience a significant first-pass effect are imipramine, morphine, propranolol, buprenorphine, diazepam, midazolam, pethidine, cannabis, cimetidine, lidocaine, and nitroglycerin. In contrast some drugs are enhanced in potency: for example, the effect of the most commonly considered active ingredient in cannabis, THC, is enhanced by transformation of a significant portion into 11-hydroxy-THC that more readily crosses the blood brain barrier and thus achieves greater potency than the original THC. [Cannabis edible]
Compound liquorice lozenge and preparation process thereof
InactiveCN1433810ASatisfy the tasteSignificant effectUnknown materialsPill deliveryClinical efficacyGLYCYRRHIZA EXTRACT
The present invention discloses a compound licorice lozenge and its preparation method. Its prescription includes licorice extract powder and powdered opium, etc. and it is characterized by that it also includes off-odor removing agent, filling agent, mucilage and correctives, in which the off-odor removing agent can invest and adsorb the putrid odor produced by camphor and anise star oil, and said lozenge has good taste, has no special putrid odor, and its effective component can be directly acted on the pharyngeal portion, laryox portion and trachea region of oral cavity, so that it can obtain obvious effect for removing the phlegm, relieving cough, relieving inflammation and killing bacteria.
Owner:江西制药有限责任公司
Vagina administration mifepristone prepn and its composition and prepn process
InactiveCN1846703AHigh affinityImprove adhesionOrganic active ingredientsSuppositories deliverySide effectWhole body
The vagina administrated mifepristone preparation for birth control and health reproduction consists of mifepristone or nanometer mifepristone liposome particle as main component, excipient, osmosis promoter, pH regulator, preservative, surfactant, solvent, water and other pharmaceutically acceptable components, and may be prepared into gel, film or other preparation forms. The vagina administrated mifepristone preparation is absorbed through vagina mucous membrane into blood circulation, and has high bioavailability, lowered dosage, less toxic side effect and less stimulation.
Owner:程定超
Nimodipine gel for nasal cavity
InactiveCN1679561AFine and even textureModerate viscosityOrganic active ingredientsNervous disorderNasal cavityNose
A gel of nimodipine for nasal cavity is prepared from nimodipine, hydrophilic gel and pharmacologically acceptable additive (pH regulator, humectant, anticeptic, etc). Its advantages are high biological utilization and no toxin to vibrissae.
Owner:FUDAN UNIV
A dabigatran etexilate solid dispersion enteric-coated preparation and a preparing method thereof
InactiveCN106880845AInhibition of re-coalescenceHigh dissolution rateOrganic active ingredientsPharmaceutical delivery mechanismSolubilityPartition coefficient
A dabigatran etexilate enteric-coated preparation and a preparing method thereof are provided. Dabigatran etexilate that is a medicine active component is prepared into a solid dispersion through a hot melting extrusion technique, and further prepared into the enteric-coated preparation. The preparation overcomes dependence of solubility and oil-water partition coefficients of the dabigatran etexilate and salts thereof on pH values, improves membrane permeability of a medicine in intestinal juice, improves medicine absorption, avoids or reduces hepatic first-pass effects, increases medicine bioavailability, and avoids hydrolysis of water-insoluble dabigatran etexilate and adverse irritation of the medicine on the gastrointestinal tract. A process is simple. Medicine properties are stable. The preparation is convenient to take.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Chinese forest frog bactericidal peptide spray agent and its preparation
InactiveCN1583166APromote wound healingGood treatment effectPeptide/protein ingredientsAerosol deliveryPEG 400Tree frog
A spray of tree frog's antibacterial peptide for treating burn, scald, and skin infection is prepared from antibacterial peptide of tree frog, nipagin ethylester, PEG 400, sodium chloride and water through preparing auxiliary solution, adding the antibacterial peptide of tree frog and water, stirring and loading in sprayer.
Owner:JILIN UNIV
Permeability nose absorption type medicine for treating and preventing senile dementia, improving learning memory faculty and regulating blood brain barrier
InactiveCN1861060AEasy to controlPromote recoveryOrganic active ingredientsNervous disorderNoseEugenol
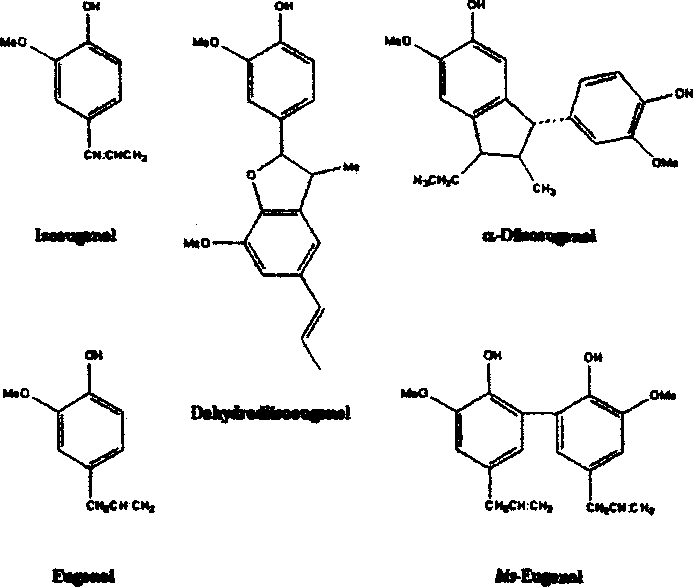
A snuff for preventing and treating senile dementia, improving memory and regulating the permeability of blood brain barrier is prepared from the active component(s) chosen from eugenol, its salt, its isomer and their mixture, and selective natural spice.
Owner:李光武
Freeze dried powder injection of coenzyme Q10 and its preparation process
ActiveCN1593392AImprove bioavailabilityOrganic active ingredientsPowder deliverySolubilityFreeze-drying
The invention discloses a freeze dried injection containing coenzyme Q#-[10], which comprises coenzyme Q#[10] and surface active agent, wherein the surface-active agent is the mixture of Twain and polyoxyl stearate, the medicinal active component being coenzyme Q#-[10], the weight ratio of coenzyme Q#[10] and Twain is 1:1-1:50, the weight ratio of coenzyme Q#[10] and pollyoxyl stearate is 1:1-1:50.
Owner:HAINAN PULIN PHARMA +2
Edaravone and (+)-2-camphol sublingual medicine composition
InactiveCN107773545AHydroxy compound active ingredientsPill deliveryPharmaceutical drugSublingual administration
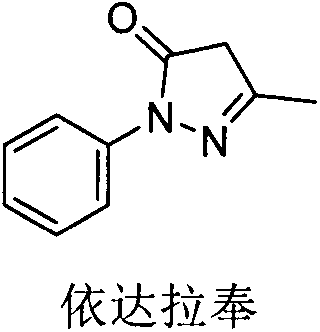
The invention discloses an edaravone and (+)-2-camphol composition sublingual medication preparation and a preparation method thereof, wherein the sublingual medication preparation is prepared from edaravone, (+)-2-camphol, excipients, filling agents, bonding agents, disintegrating agents, lubricating agents and the like. The sublingual medication preparation has the advantages that the first passeffect of the liver can be avoided; the sample stability is high; the transportation is convenient; the use is convenient, and the like.
Owner:YANTAI YENEPHARMA BIOMEDICAL
Ribavirin inhalation powder atomizing agent and its preparing process and anti-virus infection use
The Ribavirin inhalation is a kind of respiratory tract mucous membrane absorbed preparation comprising superfine Ribavirin powder and fine medicinal carrier powder and is administrated with special administrating device. It has high and fast target effect, safe antiviral function, no stimulation to mucous membrane and other advantages. The present invention further expands the clinical application range of Ribavirin.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Silibinin meglumine salt oral disintegration tablet preparation and its preparing method
InactiveCN1586471AQuality improvementEasy to takeOrganic active ingredientsMetabolism disorderOrally disintegrating tabletFibrillation
The present invention relates to one kind of orally disintegrated silibinin-meglumine salt tablet for protecting liver cell, stimulating the biological synthesis of protein inside liver cell, promoting recovery of damaged liver cell, resisting fibrillation, capturing free oxygen radical and inhibiting the deposition and infiltration of fat in liver, and its preparation. The orally disintegrated silibinin-meglumine salt tablet is prepared with silibinin-meglumine salt as main material, and through adding stuffing, disintegrating agent, corrective, flow assistant and other supplementary material, pressing into tablet and other steps. The present invention has the features of low production cost, fast disintegration, good taste, etc.
Owner:COSCI MED TECH CO LTD
Active substance-contained gel composite based on multilayer liquid crystal framework and method for producing same
ActiveCN102614109AIncrease concentrationImprove stabilityNervous disorderPharmaceutical delivery mechanismChemistryLiquid crystal
The invention discloses an active substance-contained gel composite based on a multilayer liquid crystal framework and a method for producing the same, wherein the gel composite comprises the following components in percentage by weight: 0.1-35% of liquid crystal gel surfactant, 0.2-30% of permeable skin penetration enhancer, 0.5-30% of liquid paraffin, 0.1-20% of gel host material, 3-90% of water, 0.1-15% of active substance and 3-90% of ethanol solution. The active substance-contained gel composite based on the multilayer liquid crystal framework not only has better efficiency, but also can reduce the medicine taking frequency, so the compliance of a user is increased; and meanwhile, a permeable skin way avoids the first-pass effect after the medicine is orally taken and then passes by gastrointestinal tract and liver, so higher bioavailability is obtained.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
Application of composition to the preparation of antiepileptic drug
ActiveCN103599115AAddress serious deficienciesImprove antiepileptic effectNervous disorderHydroxy compound active ingredientsFructose phosphateOral mucous membrane
The invention provides the application of a 1,6-fructose diphosphate sodium salt, fructose and / or borneol composition to the preparation of an antiepileptic drug. The composition consists of 1,6-fructose diphosphate sodium salt and fructose, or 1,6-fructose diphosphate sodium salt and borneol combination, or 1,6-fructose diphosphate sodium salt, fructose and borneol. The composition provided by the invention can improve in vivo stability of 1, 6 1,6-fructose diphosphate, in order to maintain high level of 1,6-fructose diphosphate in the brain for a long time and make the 1,6-fructose diphosphate sodium salt exert antiepileptic effect for a long time. The composition provided by the invention particularly employs chewable tablets, orally disintegrating tablets and dispersible tablets as dosage forms for the antiepileptic drug, and the dosage forms can be directly absorbed in oral mucosa, avoid hepatic first-pass effect and further increase the level of 1,6-fructose diphosphate in the brain, thereby further improving the antiepileptic efficacy of the drug.
Owner:ZHEJIANG UNIV
Oral loratadine disintegrating tablet and its prepn
ActiveCN1739513AReduce stimulationQuick effectPill deliveryImmunological disordersDiseaseOrally disintegrating tablet
The present invention belongs to the field of medicine technology, and is especially oral loratadine disintegrating tablet for treating allergic diseases and its preparation process. The oral loratadine disintegrating tablet includes loratadine as effective medicine component, and excipient mixture comprising disintegrating agent, stuffing, soluble polyol and penetrant. The oral loratadine disintegrating tablet consists of loratadine 20-50 wt%, disintegrating agent 5-15 wt%, stuffing 10-30 wt%, soluble polyol 30-60 wt%, and penetrant 1-5 wt%. The oral loratadine disintegrating tablet, after being disintegrated fast, can cover gastrointestinal mucous membrane widely, and has fast acting, no first pass effect, high bioavailability, and convenient taking.
Owner:HAINAN PULIN PHARMA +1
Oral folic acid instantly-dissolved membrane and preparation method thereof
InactiveCN104127370AGreat tasteImprove Medication AdherenceOrganic active ingredientsNervous disorderPlasticizerHepatic first pass effect
The invention discloses a preparation method of an oral folic acid instantly-dissolved membrane, and the method comprises the following steps: (1) weighing raw materials and auxiliary materials comprising a film-forming material, a filler, a sweetening agent, a plasticizer, a disintegrating agent and folic acid; (2) evenly mixing the folic acid, the sweetening agent, the disintegrating agent, the filler and the film-forming material, adding purified water, stirring evenly; after full swelling, adding the plasticizer, placing for completely swelling, degassing, and coating; and (3) drying, demoulding, cutting to obtain the oral folic acid instantly-dissolved membrane. The oral folic acid instantly-dissolved membrane tastes good, the oral folic acid instantly-dissolved membrane can be taken without drinking water, and can be instantly dissolved in the oral cavity without first pass effect, medication compliance of patients is high, and the need of the use of special flavoring agent is eliminated. The preparation method is simple, short in time and low in cost.
Owner:天津市聚星康华医药科技有限公司
Diversine hydrochloride spray and its preparation process
InactiveCN1478475AGood effectPromote absorptionOrganic active ingredientsAntipyreticCurative effectSolvent
A spray of diversine hydrochloride is prepared from the tuduranine hydrochloride, percutaneous promoter and solvent. Its advantages are high percutaneous effect and curative effect, quick absorption, and low toxic by-effect.
Owner:西安利君制药股份有限公司
Triglyceride prodrug based on lymphatic mediated transport and preparation method thereof
ActiveCN109851593APromote lymphatic transportAvoid first pass effectOrganic active ingredientsOrganic chemistryTG - TriglycerideGlycerol
The invention belongs to the technical field of medicines, and relates to a triglyceride prodrug based on lymphatic mediated transport, in particular to a triglyceride prodrug with different linkagesand based on lymphatic mediated transport and preparation and application of the triglyceride prodrug in drug delivery. The present invention provides the prodrug with different connecting keys and asynthesis method thereof. The structure of the prodrug is as follows, and X, R1, R2, n and m are described in the claims and specifications. According to the prodrug provided by the invention, the digestion and absorption mechanism of glycerol in the gastrointestinal tract is simulated by using the structure of triglycerides, the lymphatic transport of drugs is promoted, the first-pass effect is avoided, and the prodrug has targeting property and can obviously enhance or improve the bioavailability of drugs.
Owner:SHENYANG PHARMA UNIVERSITY +1
Loratadine lyophilized tablets and preparation method thereof
ActiveCN102451169AReduce dosageFast and fully dispersedOrganic active ingredientsPill deliveryOlder peopleMedicine
The invention relates to loratadine lyophilized tablets, and a lyophilization prescription and a technology thereof. The loratadine lyophilized tablets provided by the invention are prepared from a main medicine loratadine and medicinal auxiliary materials. When the tablets are taken, no water is used, and the tablets disintegrate rapidly in mouths. Therefore, the tablets are suitable for patients with swallowing difficulties such as old people and children. Also, the tablets are suitable to be used in trips with water source shortages. The tablets are advantaged in convenient administration, fast absorption, small first-pass effect, and small irritation to gastrointestinal mucous membranes. The market prospect of the tablets is wide. With the lyophilized tablets provided by the invention, side effects of loratadine can be substantially reduced. Also, the invention relates to a preparation method of the loratadine lyophilized tablets.
Owner:BEIJING QUANTUM HI TECH PHARMA TECHCO
Rotundine orally disintegrating tablets and preparation method thereof
InactiveCN102451165AGreat tasteSimple materialOrganic active ingredientsNervous disorderHigh absorptionSide effect
The invention relates to rotundine orally disintegrating tablets and a prescription and process for preparing the rotundine orally disintegrating tablets by adopting a freeze drying method. The rotundine orally disintegrating tablets are prepared from rotundine as a main medicine and pharmaceutic adjuvants, can be taken without water, are capable of rapidly disintegrating after entering the mouth, are suitable for taking by patients suffered from dysphagia, such as the old, children and the like, are simultaneously suitable for medications in the travel journey under the condition of being not easy to obtain a water source, has the advantages of convenience for taking, high absorption speed, small fast pass effect, little irritation to digestive tract mucosa, wide market application prospect and the like, and are capable of remarkably reducing side effects of rotundine. In addition, the invention also relates to a preparation method of the rotundine orally disintegrating tablets.
Owner:QUANTUM HI TECH BEIJING RES INST
Ginseng oral disintegration tablet and its preparing process
InactiveCN1586597AQuality improvementEasy to takeNervous disorderDigestive systemAdhesiveOrally disintegrating tablet
The present invention relates to oral disintegrated tablet of ginseng for nourishing primordial Qi, invigorating spleen, benefiting lung, promoting the secretion of saliva and tranquilizing. Ginseng as main material as well as stuffing, disintegrating agent, corrective, flow assistant, lubricant and other supplementary material are prepared into the oral disintegrated tablet. Into the supplementary material, adhesive, coating material and / or effervescent agent may be added, if necessary. The oral disintegrated tablet of the present invention has excellent crushing degree, fast disintegration, good taste and low cost, and may be taken with no water and high curative effect.
Owner:COSCI MED TECH CO LTD
Intravenous injection liquid of coenzyme Q10, and its prepn. method
ActiveCN1270702CImprove bioavailabilityImprove thermal stabilityOrganic active ingredientsDigestive systemMedicineSolvent
Owner:刘红 +1
Compound orally disintegrating tablet containing loratadine and ambroxol and preparation process thereof
InactiveCN1994304AImprove bioavailabilityIncrease blood concentrationOrganic active ingredientsPill deliveryOrally disintegrating tabletLoratadine
The invention relates to a luleitadingmione oral calving tablet which can treat allergic rhinitis, etc. wherein, it has quick adsorption, high biological utilization, and simple intake method without water. It uses Loratadine and bromamine acid as materials, with some findings.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
American ginseng and its extract oral disintegration tablet and its preparing process
InactiveCN1586596AQuality improvementEasy to takeAntibacterial agentsNervous disorderAdhesiveOrally disintegrating tablet
The present invention relates to oral disintegrated tablet of American ginseng and its extract for treating vital energy deficiency, Yin deficiency, dyspneic cough, blood trace found in phlegm, etc. American ginseng and its extract as main material as well as stuffing, disintegrating agent, corrective, flow assistant, lubricant and other supplementary material are prepared into the oral disintegrated tablet. Into the supplementary material, adhesive, coating material and / or effervescent agent may be added, if necessary. The oral disintegrated tablet of the present invention has excellent crushing degree, fast disintegration, good taste and low cost, and may be taken with no water and high curative effect.
Owner:COSCI MED TECH CO LTD
Hydrophilic plaster for treating dysmenorrhea
InactiveCN1973836ANo stimulationAvoid first pass effectOrganic active ingredientsAntipyreticCross-linkOrganic solvent
The hydrophilic plaster for treating dysmenorrheal consists of indometacin 0.15-6 wt%, salbutamol 0.01-3 wt%, transdermal promoter 0.5- 10 wt%, humectant 5-40 wt%, hydrophilic polymer material 4-20 wt%, cross-linking agent 0.1-3 wt%, and cross-linking regulator 0.01-2 wt%, and is prepared through common plaster preparing process. Compared with traditional preparation, the present invention contains no organic solvent, and has high safety, reduced toxic side effect and other advantages.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hydrophillia Babu agent for treating depression
InactiveCN100998579AComfortable to useOrganic active ingredientsNervous disorderMolecular materialsDistilled water
A hydrophilic percutaneous medicine for treating depression is proportionally prepared from fluoxetine hydrochloride, hydrophilic high-molecular material, cross-linking agent, cross-linking regulator, humectant, percutaneous promoter, and distilled water.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Disulfiram implant and preparation method thereof
ActiveCN104825416AImprove complianceMeet clinical needsOrganic active ingredientsNervous disorderHuman bodyPharmaceutical drug
The invention relates to a disulfiram implant and a preparation method thereof. The disulfiram medicinal composition can be slowly and stably released in a human body, and the disulfiram implant comprises 60-80 parts of disulfiram and 20-40 parts of a biodegradable high polymer material. The disulfiram implant can be injected and implanted into the human body, and patients suffering from alcohol dependence can be treated so as to avoid repeated drinking. The therapeutic concentration can be maintained for a long time after administration, a first-pass effect is avoided, the bioavailability is improved, the compliance of the patients is improved, and the clinical requirements of the patients can be met.
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
Jiawei Yupingfeng gel, preparation method and purpose thereof
ActiveCN104491003AAddressing chronic UV damageSignificant effectAerosol deliveryOintment deliveryOfficinalBaical Skullcap Root
The invention discloses Jiawei Yupingfeng gel, a preparation method and a purpose thereof and belongs to pharmaceutical products with traditional Chinese herbal medicine. The Jiawei Yupingfeng gel is prepared through weighing milkvetch root, bighead atractylodes rhizome, Chinese parsnip root and baical skullcap root according to weight parts, and extracting and condensing to obtain extract through ethanol; adding pharmaceutic accessories (water-soluble polymer matrix-Carbopol, wetting agent-glycerol, propylene glycol, penetration enhancer-azone, pH regulating agent-triethanolamine, preservative-ethylparaben and pure water) to the extract according to the weight-to-volume ratio, and stirring uniformly. The Jiawei Yupingfeng gel is based on treating an internal illness by external treatment, and effective parts or effective ingredients in the traditional Chinese medicine raw materials are extracted to prepare into the gel according to the external transdermal absorption feature; the gel is directly used for the skin damaged by ultraviolet, and the gel does not need the first pass effect of the liver and reduces the damage of gastrointestinal tract enzyme to the gel; the gel can obviously improve the light aging skin damage; the gel has outstanding curative effect in the light aging treatment aspect.
Owner:TIANJIN NANKAI HOSPITAL
Preparation method and application of capsaicin-collagen sponge
ActiveCN104490760AImproves transdermal penetrationAvoid first pass effectOrganic active ingredientsPharmaceutical delivery mechanismFreeze-dryingEthylic acid
The invention relates to the technical field of external medicines for hypertrophic scars, and particularly relates to a preparation method and an application of capsaicin-collagen sponge. The preparation method comprises the following steps: 1 preparing collagen powder from tendo calcaneus; 2 extracting the collagen powder with pepsin, so as to obtain an extract liquid; 3 centrifugally collecting supernate; 4 separating out collagen by adding sodium hydroxide, and washing, so as to prepare high-purity collagen; 5 redissolving the collagen with ethylic acid; and 6 evenly mixing the collagen liquid, glycerinum and capsaicin, and then carrying out freeze-drying, so as to obtain the capsaicin-collagen sponge. The capsaicin-collagen sponge prepared by the method is applied to tissue trauma repair and inhibition of the hypertrophic scars in the tissue trauma repair process, and has the advantage of good percutaneous permeability; the drug liver first-pass effect can be avoided; and the thrill to the skin can be reduced.
Owner:GUANGDONG MEDICAL UNIV
Lornoxicam freeze-dried orally disintegrating tablet and preparation method thereof
InactiveCN110652500AEasy to takeDisintegrates quicklyOrganic active ingredientsAntipyreticOrally disintegrating tabletLornoxicam
The invention provides a lornoxicam freeze-dried orally disintegrating tablet and a preparation method thereof. The lornoxicam freeze-dried orally disintegrating tablet consists of lornoxicam and a matrix, wherein the single dosage effective component lornoxicam is 4-10mg; and the matrix comprises 0.8-10mg of an adhesive, 10-200mg of a framework support agent, 0-10mg of a flavoring agent and 0-10mg of an essence. The preparation method of the lornoxicam freeze-dried orally disintegrating tablet provided by the invention comprises steps of dissolution, mold injection, quick freezing, freeze-drying and product packaging. The lornoxicam freeze-dried orally disintegrating tablet provided by the invention is simple and convenient to take and rapid to disintegrate, can be dissolved immediately after being orally taken, can be directly taken without water and is rapid to absorb, and a first pass effect can be avoided.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Voriconazole suppository and preparation method and application thereof
ActiveCN102525883AAvoid first pass effectLittle side effectsOrganic active ingredientsAntimycoticsSide effectWhole body
The invention provides a voriconazole suppository and a preparation method and application thereof. The suppository comprises an effective dose of voriconazole, a dispersion carrier and a matrix, wherein the matrix comprises a forming agent, an emulsifying agent and a suspension aid agent. The preparation method comprises the following steps of: dissolving or dispersing voriconazole in the dispersion carrier, and uniformly mixing with the molten matrix to obtain the suppository; or adding the dispersion carrier into the molten matrix, and adding the voriconazole powder and the suspension aid agent, and uniformly mixing to obtain the suppository. The voriconazole suppository is applied to rectal or gynecological cavity administration and to the therapy for local or whole-body deep fungus infection. The invention provides a safe and effective cavity or rectal administration drug form which avoids the first-pass effect of liver, directly reaches the focus, reduces the medicine dosage, isparticularly suitable for the therapy for local deep fungus infection in gynecological cavity, pelvic cavity, accessory and the like, and ensures little side effect and good curative effect.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY +1
Non-steroidal anti-inflammatory drug orally disintegrating tablet and producing method thereof
ActiveCN102579376AGreat tasteSimple materialOrganic active ingredientsAntipyreticSide effectOrally disintegrating tablet
The invention discloses a non-steroidal anti-inflammatory drug orally disintegrating tablet and a producing method thereof, and relates to the non-steroidal anti-inflammatory drug orally disintegrating tablet and a prescription and a process utilizing the freeze-drying method to produce the non-steroidal anti-inflammatory drug orally disintegrating tablet. The non-steroidal anti-inflammatory drug orally disintegrating tablet is produced by main drug and pharmaceutic adjuvant, no water is needed while users take the tablet, and the tablet disintegrates rapidly after being delivered to the mouth, thereby the tablet is suitable for dysphagia patients such as the old, children and the like; the tablet is suitable for traveling under the condition that water is not easy to get; the non-steroidal anti-inflammatory drug orally disintegrating tablet has the advantages of convenience in taking, rapid absorption, small first pass effect, small irritation to digestive tract mucosa and the like, the tablet has a broad application prospect in the market, and the non-steroidal anti-inflammatory drug orally disintegrating tablet is capable of effectively reducing side effects of non-steroidal anti-inflammatory drugs. In addition, the invention further relates to the non-steroidal anti-inflammatory drug orally disintegrating tablet producing method.
Owner:BEIJING QUANTUM HI TECH PHARMA TECHCO
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com