Disulfiram implant and preparation method thereof
A technology of disulfiram and implants, which is applied in the field of medicine, can solve the problems of poor patient compliance and low bioavailability, and achieve the effects of improving compliance, avoiding the first-pass effect, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
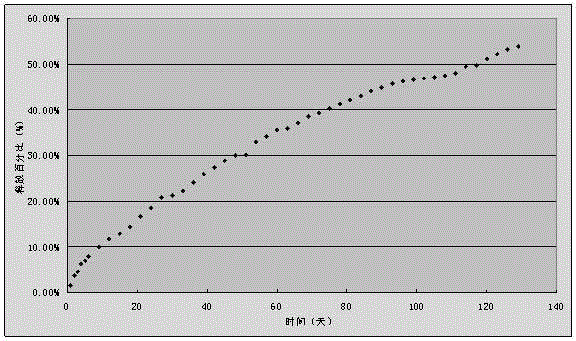
Embodiment 1
[0036] A disulfiram implant, 80g of disulfiram, 20g of racemic polylactic acid, the selective viscosity coefficient of racemic polylactic acid is 0.9dl / g (chloroform, 25°C), and the average molecular weight is about 80,000;
[0037] The preparation method is,
[0038] 1) Prepare 0.5%~3% PVA aqueous solution as the continuous item, polylactic acid and disulfiram are dissolved in dichloromethane as the dispersed phase,
[0039] 2) Stir the dispersed phase at a stirring speed of 800 rpm;
[0040] 3) Slowly add the dispersed phase to the continuous phase while stirring;
[0041] 4) Continue stirring to volatilize the organic solvent to prepare microspheres, and the size of the obtained microspheres is 30-50 microns;
[0042] 5) Compress plain tablets with 5kN pressure, and coat with 6% polylactic acid dichloromethane solution. The diameter of the tablet is 8mm, the hardness test size is 6kg, and the thickness of the coating is 0.1mm.
[0043] Using the in vitro release test met...
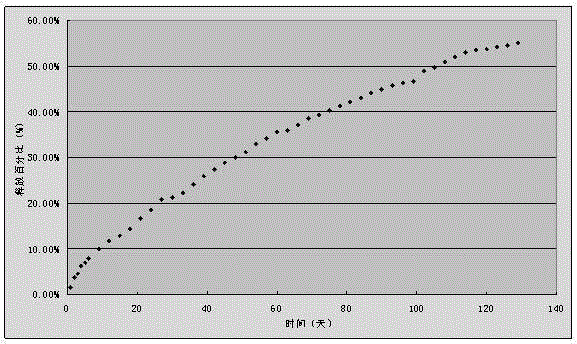
Embodiment 2
[0045] A disulfiram implant, 60g of disulfiram, 40g of racemic polylactic acid, the selective viscosity coefficient of racemic polylactic acid is 0.7dl / g (chloroform, 25°C), and the average molecular weight is about 70,000;
[0046] The preparation method is,
[0047] 1) Prepare 0.5%~3% PVA aqueous solution as the continuous item, and dissolve polylactic acid and disulfiram in dichloromethane as the dispersed phase;
[0048] 2) Stir the dispersed phase at a stirring speed of 800 rpm;
[0049] 3) Slowly add the dispersed phase to the continuous phase while stirring;
[0050] 4) Continue stirring to volatilize the organic solvent to prepare microspheres, and the size of the obtained microspheres is 30-50 microns;
[0051] 5) Press 10kN pressure into plain tablets, and coat with 3% polylactic acid dichloromethane solution. The tablet diameter is 8mm, the hardness test size is 7kg, and the coating thickness is 0.2mm.
[0052] Using the in vitro release test method, continuous mea...
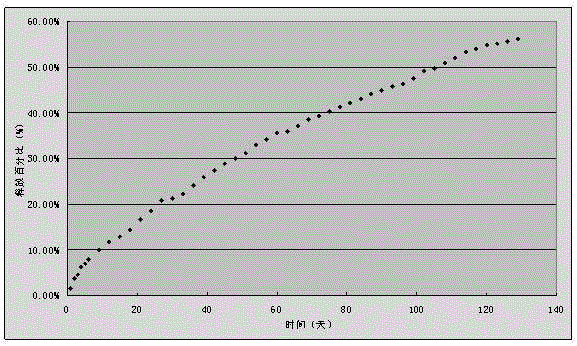
Embodiment 3
[0054] A disulfiram implant, 60g of disulfiram, 40g of racemic polylactic acid, the selective viscosity coefficient of racemic polylactic acid is 0.7dl / g (chloroform, 25°C), and the average molecular weight is about 70,000;
[0055] The preparation method is,
[0056] 1) Prepare 0.5%~3% PVA aqueous solution as the continuous item, and dissolve polylactic acid and disulfiram in dichloromethane as the dispersed phase;
[0057] 2) Stir the dispersed phase at a stirring speed of 800 rpm;
[0058] 3) Slowly add the dispersed phase to the continuous phase while stirring;
[0059] 4) Continue stirring to volatilize the organic solvent to prepare microspheres, and the size of the obtained microspheres is 30-50 microns;
[0060] 5) Press 10kN pressure into plain tablets, and coat with 5% polylactic acid dichloromethane solution. The tablet diameter is 8mm, the hardness test size is 7kg, and the coating thickness is 0.2mm.
[0061] Using the in vitro release test method, continuous m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com