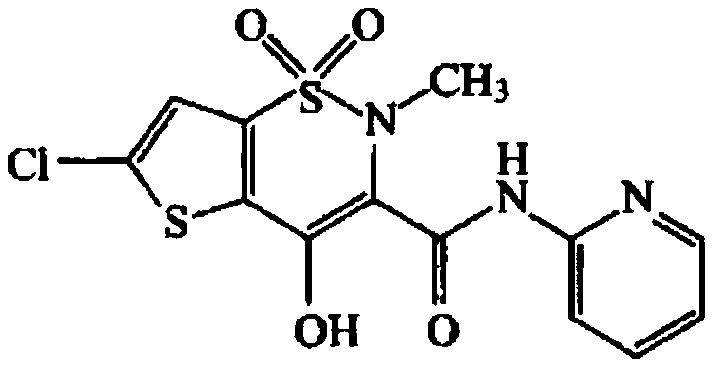
Lornoxicam freeze-dried orally disintegrating tablet and preparation method thereof
A technology of lornoxicam and orally disintegrating tablets, which can be applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as gritty feeling and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
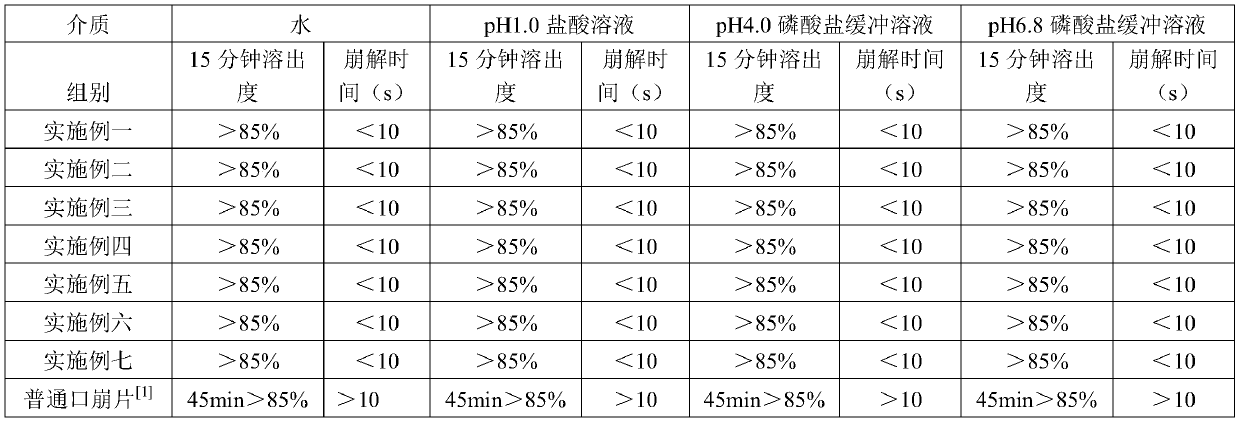
Image
Examples
Embodiment 1
[0030] ingredient Amount of each component (g) Lornoxicam0.8 gelatin0.8 Mannitol8.0 aspartame1.0 Strawberry flavor0.4
[0031] The preparation steps of freeze-dried orally disintegrating tablets are as follows:
[0032] Dissolve: add the adhesive to the water, mix and heat to 50℃, continue to mix until completely dissolved; add the evenly mixed matrix proppant and other excipients and the mixture of lornoxicam, stir until uniform, continue to add flavors and Essence, stir and mix evenly, dilute the mixture with water to 80ml;
[0033] Injection mould: absorb 0.8ml / piece of liquid medicine and inject the liquid medicine into the mould;
[0034] Quick freezing: make the liquid medicine quickly freeze into a solid at -70℃;
[0035] Freeze-drying: The temperature of the freeze-drying machine is reduced to -45°C, and the mold containing the liquid medicine is placed in the freeze-drying machine. After half an hour, vacuum is applied. When the vacuum degree reaches below 10 Pa, keep...
Embodiment 2
[0041] ingredient Amount of each component (g) Lornoxicam1.0 gelatin0.7 Mannitol7.0 sucrose1.0 Orange flavor0.4
[0042] The preparation steps of freeze-dried orally disintegrating tablets are as follows:
[0043] Dissolve: add the adhesive to the water, mix and heat to 50°C, continue to mix until completely dissolved; add the well-mixed matrix proppant and the mixture of other excipients and lornoxicam, stir until uniform, continue to add flavors and Essence, stir and mix well, dilute the mixture with water to 70ml;
[0044] Injection mould: absorb 0.7ml / piece of liquid medicine and inject liquid medicine into the mould;
[0045] Quick freezing: make the liquid medicine quickly freeze into a solid at -80℃;
[0046] Freeze-drying: The temperature of the freeze-drying machine is cooled to -45°C, the mold containing the liquid medicine is placed in the freeze-drying machine, and the vacuum is drawn after half an hour.
[0047] When the vacuum is below 30Pa, keep it warm for 2 hou...
Embodiment 3
[0053] ingredient Amount of each component (g) Lornoxicam0.4 gelatin1.0 Mannitol8.0 sucrose1.0 Orange flavor0.4
[0054] The preparation steps of freeze-dried orally disintegrating tablets are as follows:
[0055] Dissolve: add the adhesive to the water, mix and heat to 50°C, continue to mix until completely dissolved; add the well-mixed matrix proppant and the mixture of other excipients and lornoxicam, stir until uniform, continue to add flavors and Essence, stir and mix evenly, dilute the mixture with water to 100ml;
[0056] Injection mould: absorb 1ml / piece of liquid medicine and inject the liquid medicine into the mould;
[0057] Quick freezing: make the liquid medicine quickly freeze into a solid at -90℃;
[0058] Freeze-drying: The temperature of the freeze-drying machine is cooled to -45°C, the mold containing the liquid medicine is put into the freeze-drying machine, and the vacuum is drawn after half an hour
[0059] When the vacuum is below 15 Pa, keep it warm for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com