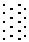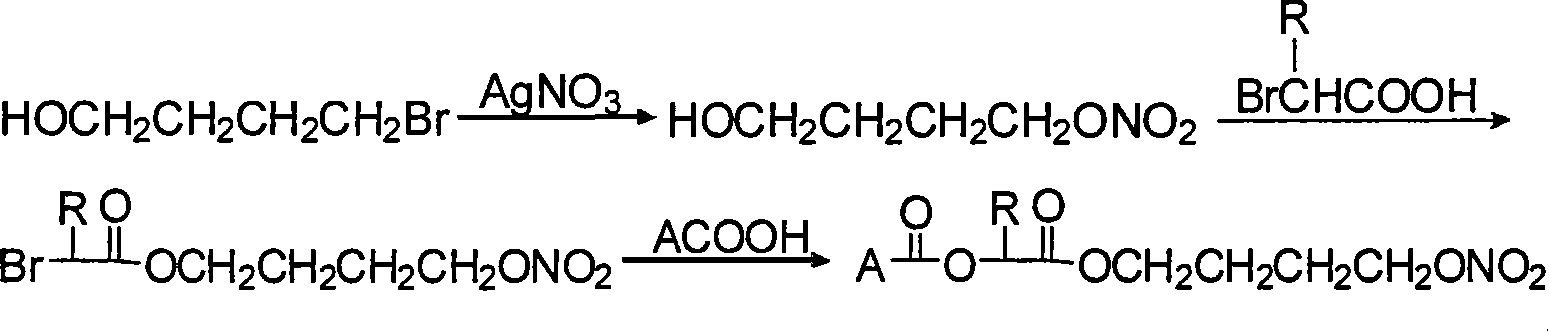Patents
Literature
70 results about "Piroxicam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Piroxicam is used to reduce pain, swelling, and joint stiffness from arthritis.
Analgesic composition for topical use
An analgesic composition, is disclosed which comprises a mixture of piroxicam, dexamethasone, ketamine, lidocaine injection, dimethyl sulfoxide, gabapentin and Vanicream™, preferably in the form of a cream or ointment. The composition is applied topically for the relief of pain of arthritis, neuropathy, post-herpetic (shingles) conditions, sore muscles, tendons and ligaments, and local reactions to insect bites or stings.
Owner:CATHCART CELEVATORON H
Herbal formulation useful as a therapeutic and cosmetic applications for the treatment of general skin disorders
InactiveUS6200570B1Useful applicationDrawback can be obviatedCosmetic preparationsBiocideHerbal preparationsSalicylic acid
The invention provides a herbal formulation useful as a therapeutic and cosmetic applications for the treatment of general skin disorders, the composition comprising at least two or more plant extracts in the form of oil or powder or mixtures thereof, the plant extracts being selected from the group consisting of Gymnena sylvestrae water extract 3 to 6 wt. %; Tridax procumbens water extract 3 to 6 wt. %; its methanolic extract 4 to 6 wt. %, Allium sativum oil hexane extract 1 to 3 wt. %; dried juice of Aloe vera 2 to 6 wt. %; Gum Olibanum powder in the natural form 4 to 7 wt. %; Gum Olibanum resinoid organic solvent extract 3 to 8 wt. %; and resinoid free Gum Olibanum meal 5 to 10 wt. %., optionally, including any drug having anti-inflammatory and wound healing property or mixture thereof, the drug being selected from the group consisting of Disclofenac sodium 1-3 wt. %, Salicyclic acid 1 to 4 wt. %, Piroxicam 1 to 2 wt. %, Turmeric powder 0.1 to 1 wt. %, a base containing aqueous cream or a gel containing carbopol ranging between 1 to 4 wt. %, emulsifying ointment ranging between 20 to 40 wt. %, preservatives ranging between 0.05-0.3% and a humecant ranging between 1-4 wt. %, and remaining water to make 100 wt. %.
Owner:COUNCIL OF SCI & IND RES
Method for measuring 12 types of remaining medicine in water environment through separation and enrichment
ActiveCN105424825ASimplified processing stepsImprove extraction efficiencyComponent separationWater dischargePretreatment method
The invention relates to a method for measuring 12 types of remaining medicine in a water environment through separation and enrichment at the same time, and belongs to the field of safety detection of a trace of organic pollutant residue in the water environment. The content of 12 types of frequently-used medicine in the water environment (drinking water, faucet water, river water and water discharged into and out of sewage treatment plants) is directly measured with an ultra performance liquid-chromatography-mass spectrometer (UPLC-MS / MS) as a detection tool after a water sample is subjected to solid phase extraction combined with ultrasonic-assisted dispersion liquid-liquid micro-extraction (UA-DLLME) separation and enrichment. The 12 types of antibiotic include ketoprofen, ciprofloxacin, tinidazole, tolfenamic acid, sulfadiazine, sulindac, naproxen, sulfamethoxazole, chloramphenicol, cefuroxime axetil, piroxicam and mefenamic acid. Inspection and optimization are conducted on a sample pretreatment method and instrument detection conditions of the water sample, and the optimal UA-DLLME method is established and is successfully applied to practical sample detection. Compared with a traditional method, the method has the advantages of being high in sensitivity, high in extraction and recycle rate, wide in suitable object, friendly to the environment, and the like.
Owner:SHENYANG PHARMA UNIVERSITY +1
Topical formulation having effects on alleviating pain/inflammation caused by herpes virus infection
InactiveUS7132452B2Fasten skin recoveryPromote recoveryPowder deliveryBiocideComplete remissionTolmetin
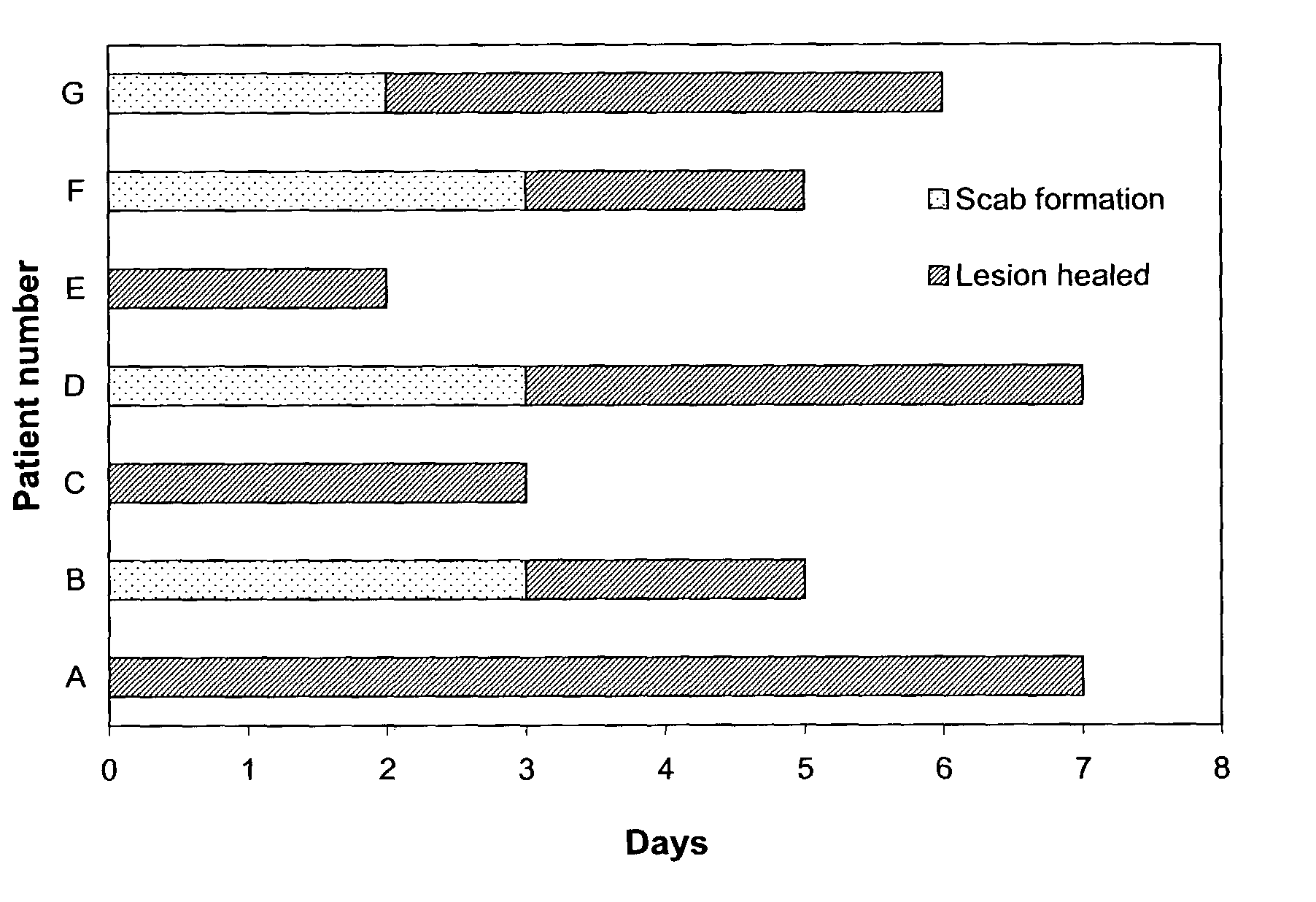
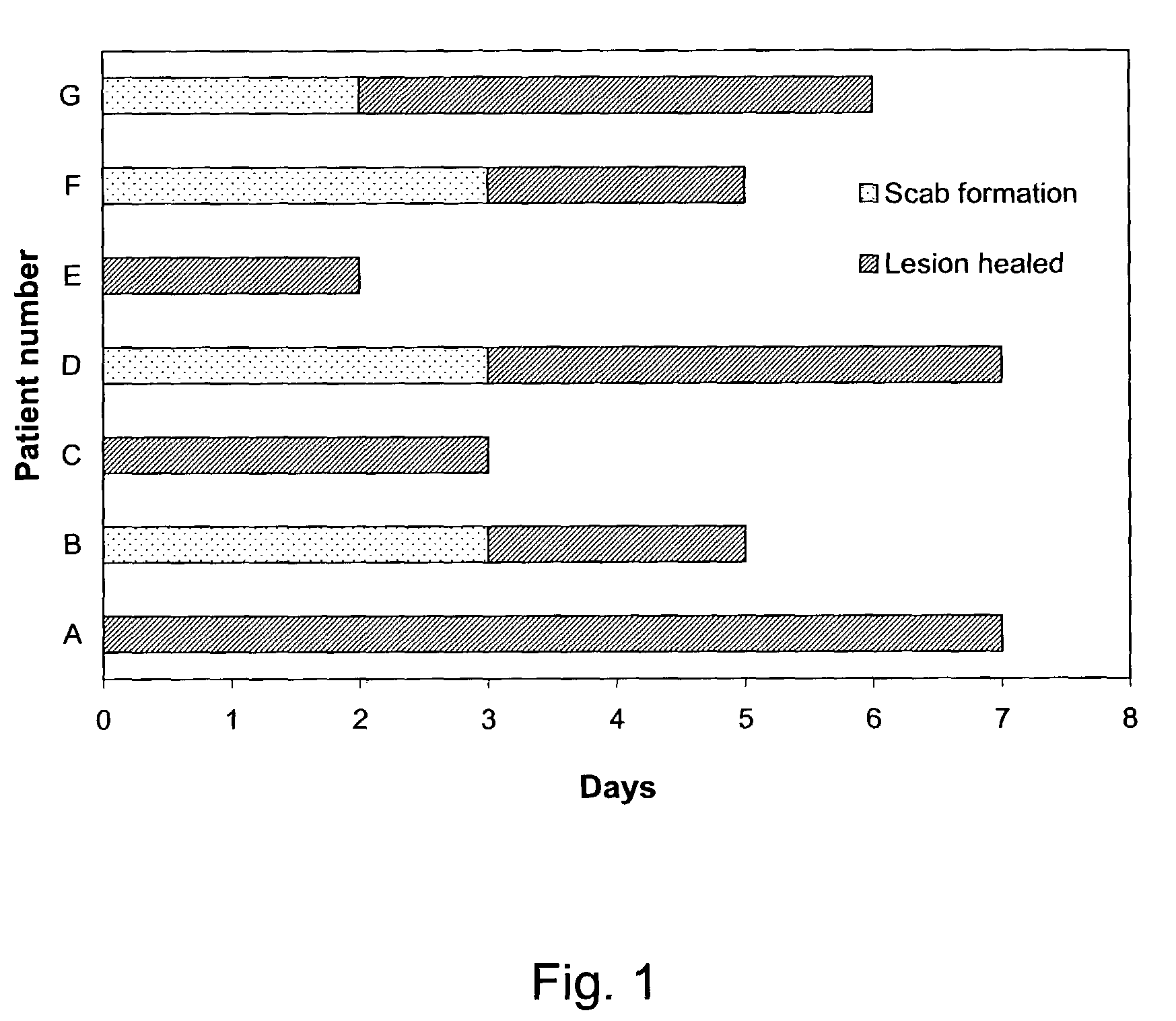
The present invention provides a topical formulation containing NSAID, particularly diclofenac. The topical formulation is particularly useful for alleviating pain / inflammation associated with infection caused by herpes virus, especially herpes simplex virus (HSV) and varicella-zoster virus (VZV). Similar relief can be achieved where diclofenac is replaced with another non-steroidal anti-inflammatory drug (NSAID), which includes, without limitation, etodolac, ketorolac, bromfenac, diflunisal, ibuprofen, fenoprofen, ketoprofen, naproxen, suprofen, meclofenamate, mefenamic acid, piroxicam, meloxicam, indomethacin, sulindac, phenylbutazone, oxyphenbutazone, and tolmetin. The topical formulation is further characterized by its fast relief on pain and / or inflammation associated with infection caused by herpes virus, i.e., a complete relief in no more than seven (7) days after the application of the topical formulation on skins of patients.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
Nonsteroidal antiinflammatories with nitric oxide donors and its preparation method
InactiveCN101053662ALittle side effectsMild reaction conditionsAntipyreticAnalgesicsIndometacinSide effect
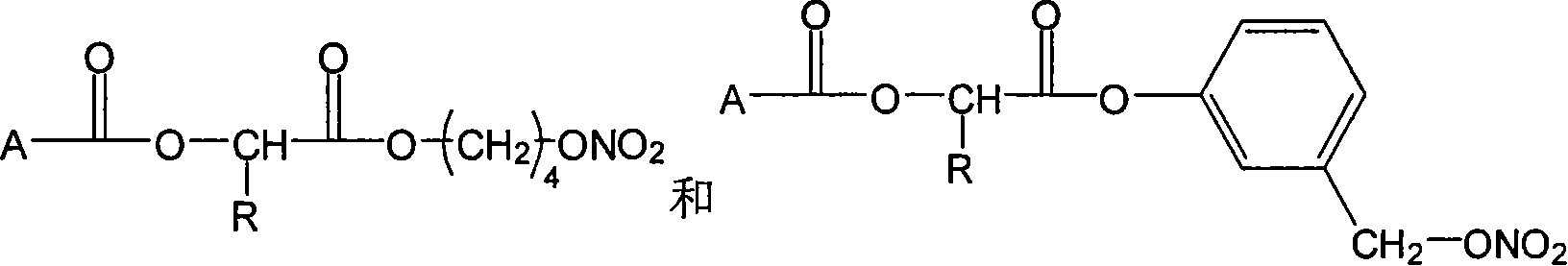
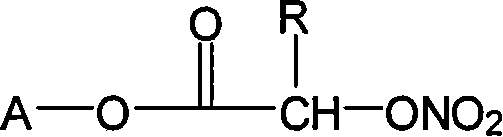
The invention relates to a non-steroidal anti-inflammatory drug with nitrogen oxide donor and a method for preparing same, which can be used to eliminate inflammation, relieve fever and stop pain, and can decrease the frequently seen side effect thereof on the gastrointestinal tract. The structure of which is A-O(X)-CO-O(y)-B-ONO2,wherein, A is the non-steroidal anti-inflammatory group; B is the connection group, when x=0, y=1; when x=1 then y=0. The the non-steroidal anti-inflammatory groups includes aspirin, diclofenac, indometacin, lumiracoxib, brufen, ketoprofen, naproxen, piroxicam, and meloxicam. The preparation process are that the bromhydrin (or hydroxybenzene) reacts with silver nitrate into hydroxy nitrate, then reacts with bromo acid into the connection group of nitrogen oxide donor, then connects with the non-steroidal anti-inflammatory drug; or the non-steroidal anti-inflammatory drug condensates with bromo acid into a bromide intermediate, then reacts with silver nitrate into the non-steroidal anti-inflammatory drug with nitrogen oxide donor.
Owner:江苏吴中苏药医药开发有限责任公司
Nucleoside derivative for preventing and treating inflammatory reaction as well as application thereof
ActiveCN109912598ALittle side effectsSimple structure and processOrganic chemistryAntipyreticIndometacinDisease
The invention provides a nucleoside derivative for preventing and treating inflammatory reaction as well as application of the derivative to preparation of medicines for preventing and treating inflammatory diseases. The nucleoside derivative for preventing and treating inflammatory reaction, which is provided by the invention, can obviously improve the disease conditions of pancreatitis, hepatitis, arthritis and the like, can improve the damaged and inflammatory indexes of organs and has the effect better than that of a positive control medicine indometacin. Compared with the traditional anti-inflammatory medicines aspirin, ibuprofen, indometacin, butazodine, diclofenac, piroxicam and glucocorticoid, the nucleoside derivative for preventing and treating inflammatory reaction, which is provided by the invention, has the advantage that the side effect is obviously smaller.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
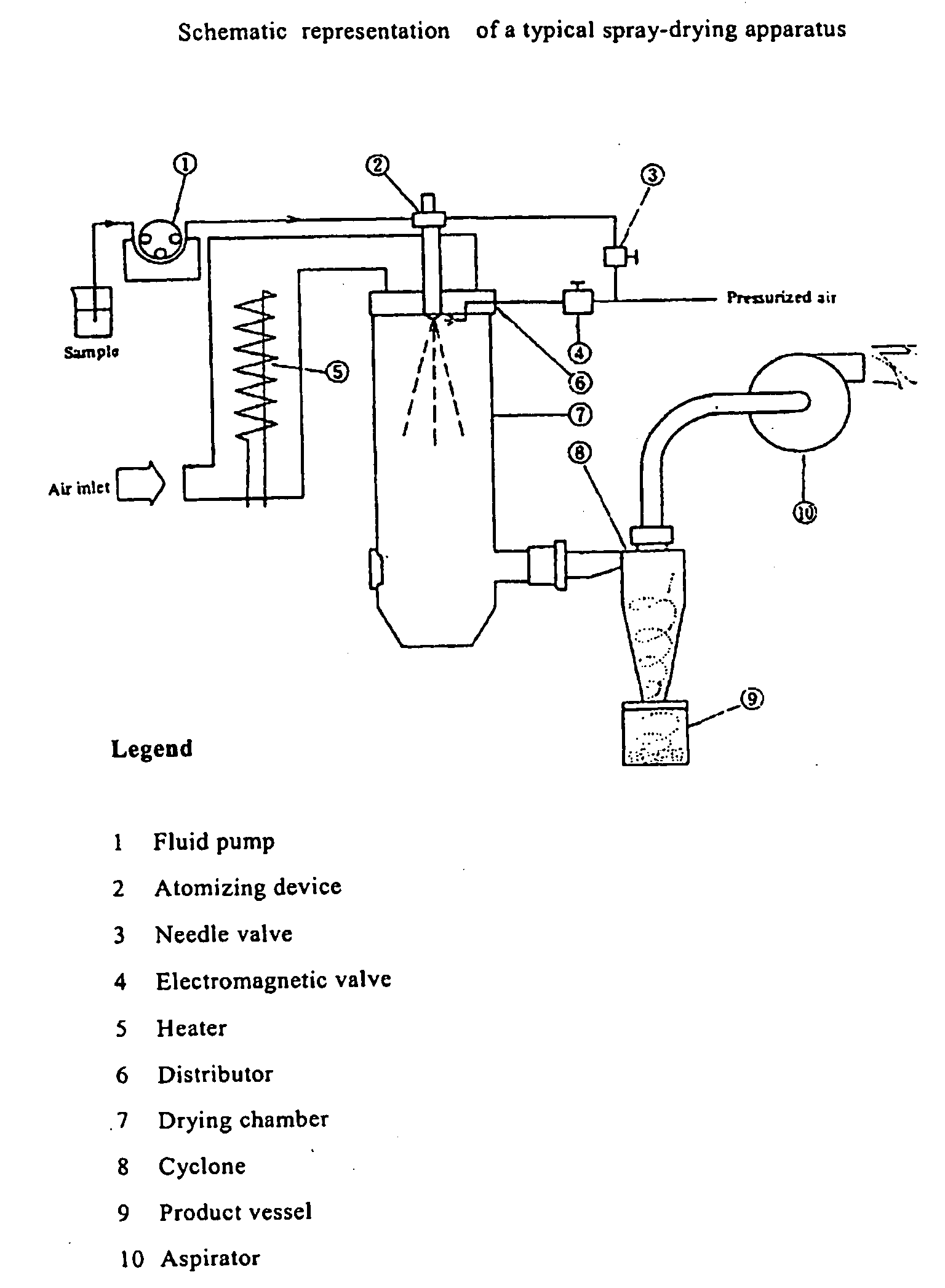
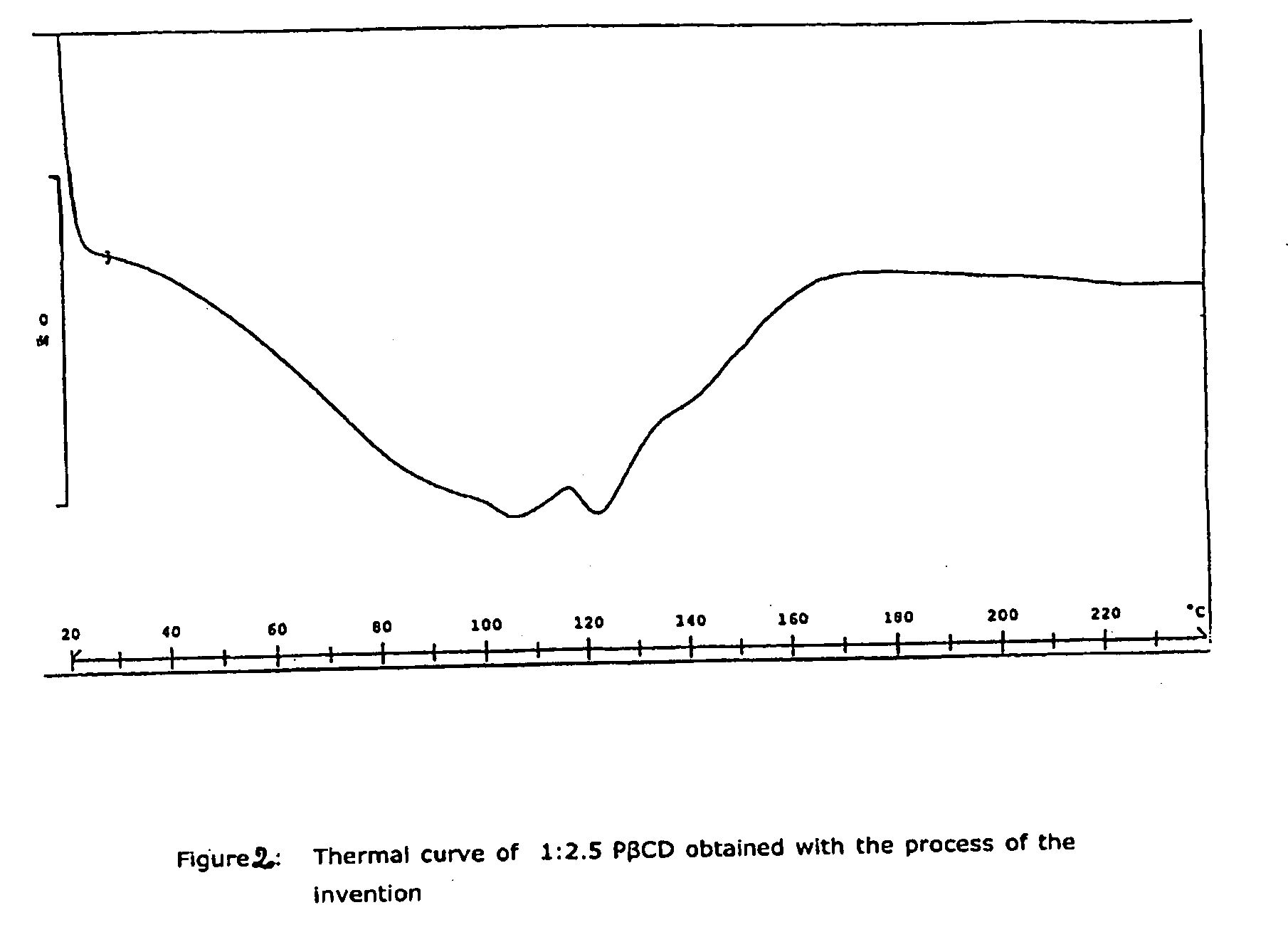
Process for the Preparation of a Piroxicam: Betacyclodextrin Inclusion Compound
The present invention relates to a process for the preparation of an inclusion compound of piroxicam with β-cyclodextrin by spray-drying, applicable on a pilot or industrial scale. The obtained product have optimal physico-chemical characteristics as well as technological and biopharmaceutical properties and it is suitable for preparing solid pharmaceutical compositions for the oral administration.
Owner:CHIESI FARM SPA
Detection card and method for rapidly detecting content of piroxicam (PIR) in traditional Chinese medicines capable of dispelling wind and eliminating dampness
ActiveCN109444054ARapid Qualitative DetectionSolve prone to false detectionMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsChemical compositionRheumatism
The invention belongs to the technical field of detection of traditional Chinese medicine preparations, particularly relates to a detection card for rapidly detecting content of illegally added piroxicam (PIR) in traditional Chinese medicine preparations capable of dispelling wind and eliminating dampness and further discloses a method for rapidly detecting content of illegally added piroxicam intraditional Chinese medicine preparations capable of dispelling wind and eliminating dampness. Through the detection card for rapidly detecting content of the illegally added piroxicam in traditionalChinese medicine preparations capable of dispelling wind and eliminating dampness, reaction processes of extracting and developing the piroxicam in traditional Chinese medicine preparations are capable of rapidly carrying out qualitative detection of piroxicam added in the traditional Chinese medicine preparations; a handheld machine capable of carrying out qualitative analysis in the prior art isused for carrying out qualitative detection of the content of piroxicam; the detection card can be used for carrying out rapid on-site detection on the content of the chemical component which is piroxicam in Chinese patent medicines capable of dispelling wind and eliminating dampness; the defects of high cost and high time consumption of the existing instruments are overcome; the problems of highpossibility of occurrence wrong detection and detection omission due to diversity of interference factors in the samples of the existing kit are solved.
Owner:北京倍肯恒业科技发展股份有限公司
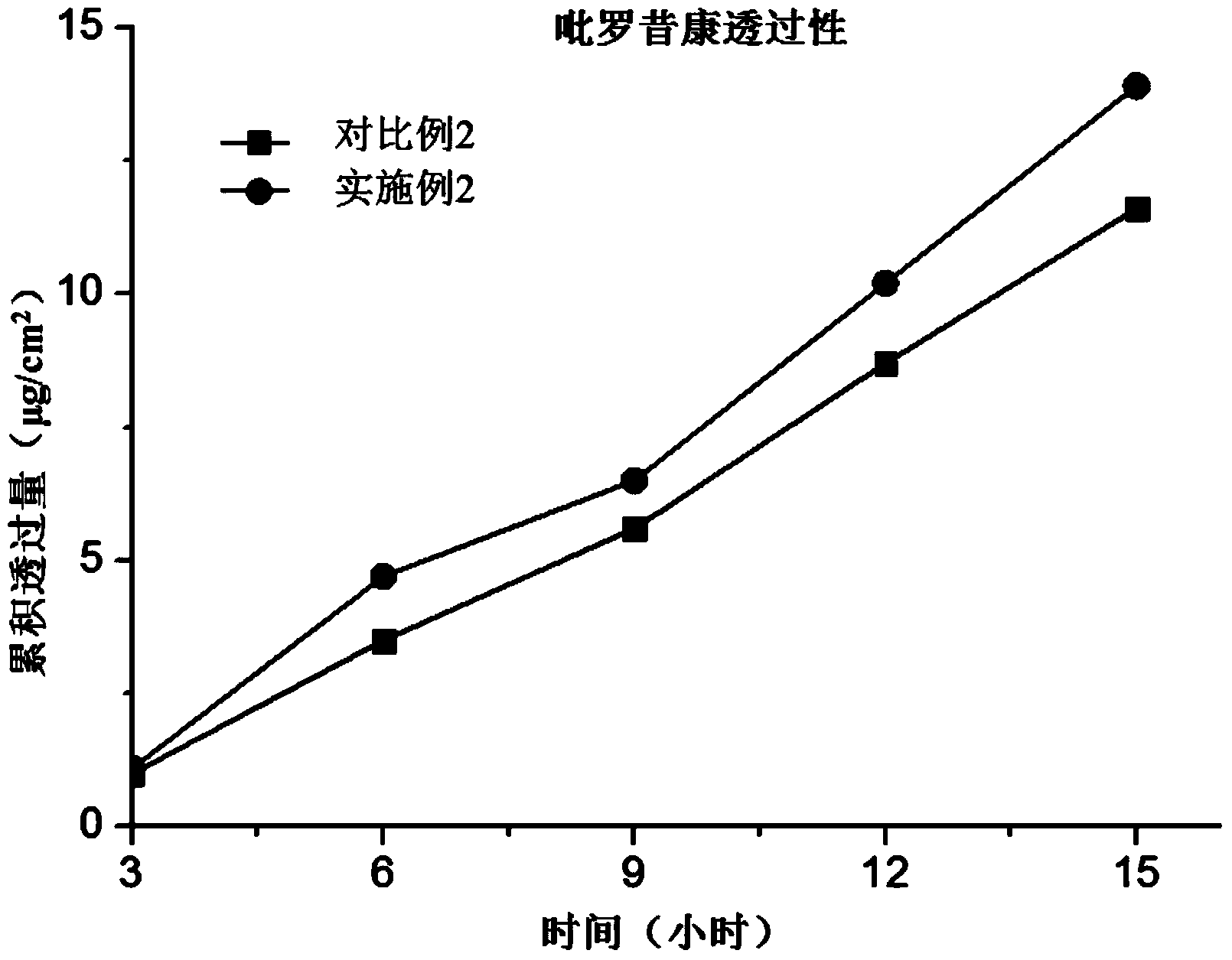
Piroxicam Transdermal Composition to Treat Plantar Fasciitis
ActiveUS20140371211A1Improve treatment outcomesRelieve painOintment deliveryPharmaceutical non-active ingredientsSkin permeabilityEffective treatment
A transdermal composition and method to be used as a treatment for plantar fasciitis is provided. Transdermal composition may include a combination of about 2% w / w to about 5% w / w of piroxicam and about 95% w / w to about 98% w / w of a natural permeation enhancement (NPE) composition. The NPE composition may increase the skin permeability, enhancing the transdermal delivery flux of piroxicam via a single transdermal application, thus, reducing the time of treatment. Transdermal composition may be applied upon an area of treatment, which may include myofascial trigger points linked to pain caused by plantar fasciitis, thus treating this condition more effectively. Moreover, employing a long acting NSAID such as piroxicam, in combination with the NPE composition, may act as a faster and effective treatment for an inflammatory process compared to typical treatments.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Piroxicam-containing transdermal-absorption preparation and preparation method thereof
ActiveCN104173322AExcellent percutaneous absorptionDoes not inhibit releaseOrganic active ingredientsAntipyreticBULK ACTIVE INGREDIENTActive ingredient
The invention relates to a piroxicam-containing transdermal-absorption preparation and a preparation method thereof. The transdermal-absorption preparation comprises piroxicam serving as an active ingredient, and levobupivacaine or pharmaceutically acceptable salt thereof serving as an absorption enhancer. According to the transdermal absorption preparation, the piroxicam has extremely excellent transdermal absorbability due to the absorption promoting effect of the levobupivacaine, and the effect is a specific effect generated by the combination of levobupivacaine and piroxicam, wherein a relatively good effect can be achieved as long as a small amount of levobupivacaine is provided. The piroxicam-containing transdermal absorption preparation, namely a patch, is extremely useful in clinical application and has a great medical effect.
Owner:苏州盛泽科技创业园发展有限公司
Piroxicam beta-cyclodextrin inclusion compounds and preparation method of tablets thereof
InactiveCN101766822AEasy to operateEasy to industrialize mass productionOrganic active ingredientsAntipyreticOral medicationAqueous solution
The invention relates to piroxicam beta-cyclodextrin inclusion compounds and a preparation method of tablets thereof. The preparation method comprises the following steps of: after keeping an alkaline aqueous solution (the pH value is 8.0-11.0) of a component at the temperature of 70-80 DEG C for 1-3 hours, spraying and drying the alkaline aqueous solution at the temperature of 70-80 DEG C and drying in vacuum. Compared with the product obtained by using the method in the prior art, the appearance of the obtained product and the properties of the technology using the product for producing preparations are obviously improved, and the product provides superior conditions for preparing oral-administration preparations.
Owner:GUANGZHOU OUHUA PHARMA
Piroxicam medicinal preparation and preparing method thereof
InactiveCN1919198AEasy to useGood antipyretic and analgesic effectOrganic active ingredientsAntipyreticAdjuvantMedicine
The invention relates to a pharmaceutical preparation of piroxicam and process for preparation, wherein the pharmaceutical preparation comprises 0.5-10 weight parts of piroxicam as active composition, 5-95 weight parts of medicinal adjuvant, and 5-90 weight parts of casting agent. The preparation has the advantages of easiness in use, higher safety and reliability, and better analgesic and analgesic effects.
Owner:湖北南洋药业有限公司
Two-component pharmaceutical composition for the treatment of pain
ActiveUS20100093712A1Safe and effective painReduce the amount requiredBiocidePowder deliveryDiseaseRegimen
The present invention is directed to a pharmaceutical composition that includes a combination of about 2-5 milligrams of a non-steroidal anti-inflammatory drug and from about 2-30 milligrams of an opioid analgesic in a single pharmaceutical dosage unit that can provide effective chronic pain management with the added benefit of reduced side effects such as withdrawal and gastrointestinal disorders. The non-steroidal anti-inflammatory drug may be piroxicam and the opioid analgesic may be buprenorphine. The present invention also provides for a method of managing pain in a patient that includes administering the pharmaceutical composition previously described. The pharmaceutical composition previously described may be administered in a single or multiple dosage regimen.
Owner:BRIDGE THERAPEUTICS INC
Method for purifying piroxicam
The invention relates to a method for purifying piroxicam. The method comprises the following steps that (1) xylene and dioxane are mixed at the volume ratio of (1 to 5):1 to obtain a mixed solvent; (2) crude piroxicam is added to the mixed solvent and heated until completely dissolved; (3) the mixture is filtered at a high temperature, and filtrate is cooled to precipitate out a solid; and (4) filtering is carried out, and a filter cake is dried to obtain pure piroxicam. According to the method, piroxicam is recrystallized through the solvent prepared by mixing xylene and dioxane, the solubility of piroxicam is good, the solvent to solute ratio is decreased, the purification cost of piroxicam is lowered, the recovery rate of piroxicam is high, the purity of piroxicam is higher, and the purification quality of piroxicam is effectively improved.
Owner:CHONGQING SANSHENG IND CO LTD
Fengtongning composite preparation and preparation method thereof
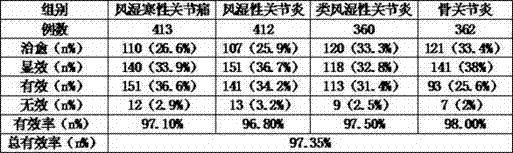
The present invention discloses a Fengtongning composite preparation and a preparation method thereof. The composite preparation is prepared from the following raw medicines, by weight, 6-12 parts of thunder god vine, 8-20 parts of sabia japonica maxim, 7-15 parts of incised notopterygium rhizome and root, 6-12 parts of wild celery, 3-10 parts of rheum, 6-12 parts of common monkshood mother-root, 5-10 parts of angelica archang lica, 7-15 parts of kusnezoff monkshood mother-root, 7-15 parts of angelica, 8-20 parts of Chinese clematis root and rhizome, 8-15 parts of piroxicam, 10-20 parts of diclofenac sodium, 7-15 parts of oryzanol, 8-18 parts of vitamin B1, and 6-12 parts of vitamin B6. The composite preparation has effects of rheumatism removing, blood circulation activating, blood stasis dissipating, meridian passage dredging, swelling subsiding and analgesia, and provides significant treatment effects for bone and muscle pain, gouty arthritis, rheumatism, rheumatoid arthritis, osteoarthritis and the like, wherein the total efficiency is 97.35%.
Owner:丁海望
Piroxicam gel preparation and preparation method thereof
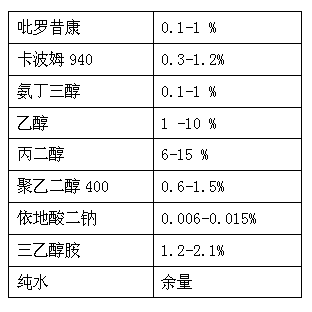
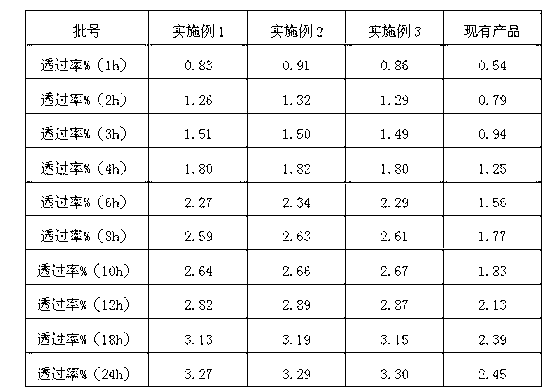
ActiveCN102697703ASolve insolubleImprove absorption rateOrganic active ingredientsAntipyreticGel preparationDisodium Edetate
The invention provides a piroxicam gel preparation and a preparation method of the piroxicam gel preparation. The gel preparation comprises the following components in percentages by weight: 0.1-1% of piroxicam, 0.3-1.2% of Carbomer 940, 0.1-1% of tromethamine, 1-10% of ethanol, 6-15% of propylene glycol, 0.6-1.5% polyethylene glycol 400, 0.006-0.015%Edetate Disodium, and 1.2-2.1% of triethanolamine. Piroxicam is dissolved by using tromethamine to solve the problem that tromethamine is difficult to dissolve in water, the penetrability of piroxicam to the skin is improved, the absorption rate of piroxicam through the skin is increased, the curative effect is obviously improved by local topical administration for curing the local pain, and a novel administration method for the patients is also provided. The gel preparation is semi-solid gel having good transdermal absorption property; the piroxicam is completely dissolved and dispersed in the gel preparation, thereby facilitating the pain relief for the patient. The preparation process is simple, and the preparation is stable and reliable in quality.
Owner:江苏小林制药有限公司
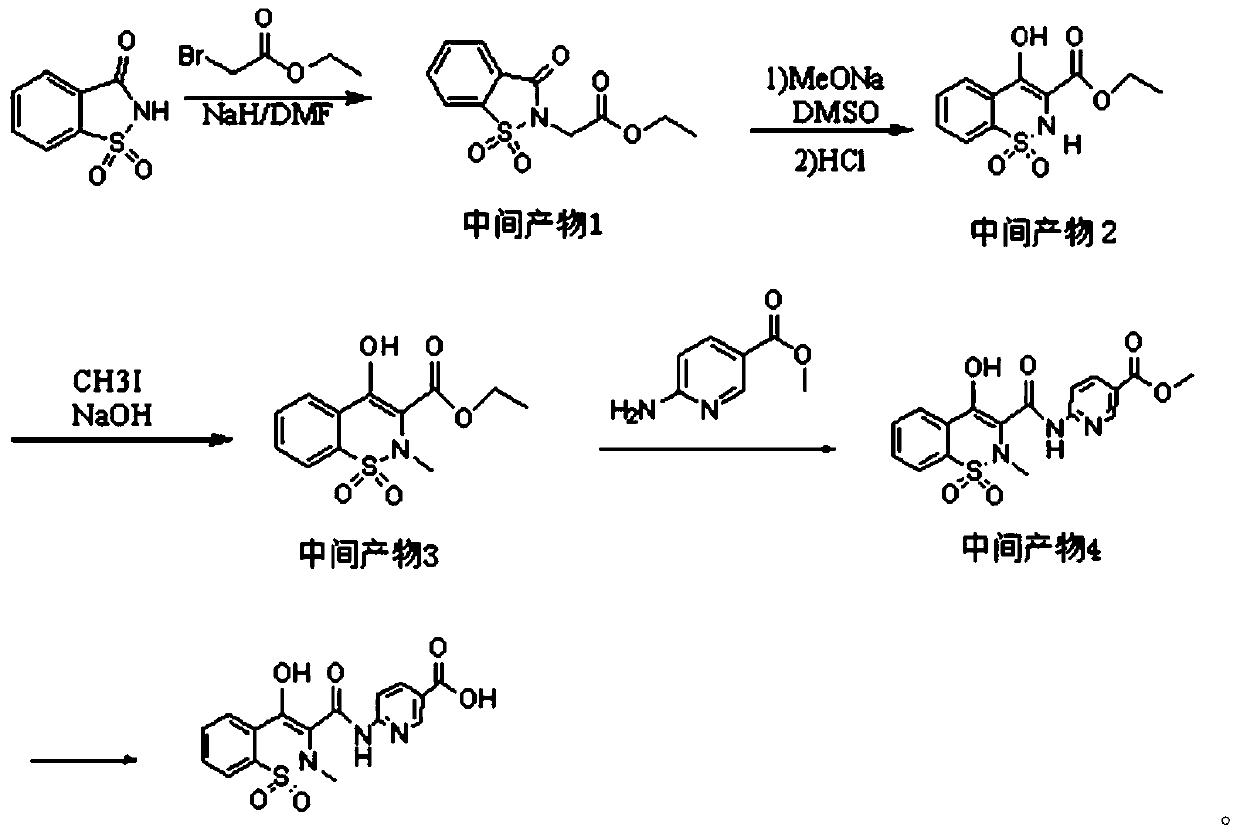
Piroxicam hapten and preparation method and application thereof
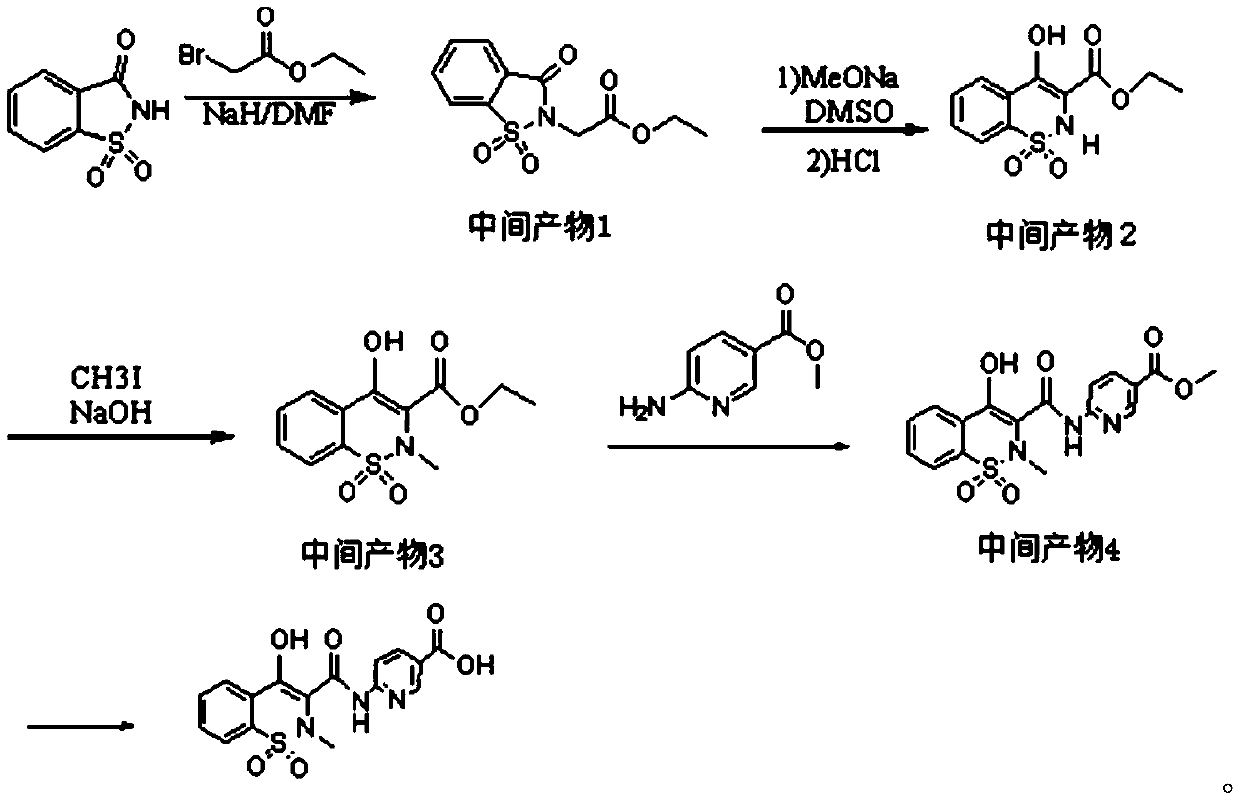
InactiveCN110330488APreserve the characteristic structureImprove immunityOrganic chemistryBiological testing6-aminonicotinic acidSynthesis methods
The invention aims to provide a piroxicam hapten and a synthesis method and application thereof, belonging to the field of drug safety detection. The preparation method comprises the following steps:1) mixing saccharin and ethyl bromoacetate for reacting to prepare an intermediate product 1; 2) mixing and extracting the intermediate product 1 with DMSO and MeONa to obtain an intermediate product2; 3) mixing the intermediate product 2 and CH3I for reacting to obtain an intermediate product 3; 4) mixing the intermediate product 3 and 6-aminonicotinic acid methyl ester for reacting to obtain anintermediate product 4; 5) after adding of the intermediate product 4 into a reagent for miscibility reaction, purifying to obtain the final product, namely the piroxicam hapten. Immunological detection method has the advantages of high sensitivity, strong specificity, simple sample pretreatment, short detection time, strong stability, no need of special equipment and reagents, intuitive judgmentof results and the like, thus being particularly suitable for rapid detection of illegal addition of piroxicam chemical drugs in vast basic inspection personnel and large quantities of anti-rheumatism Chinese patent medicines.
Owner:SHENZHEN BIOEASY BIOTECHNOLOGY CO LTD
Novel combination and use
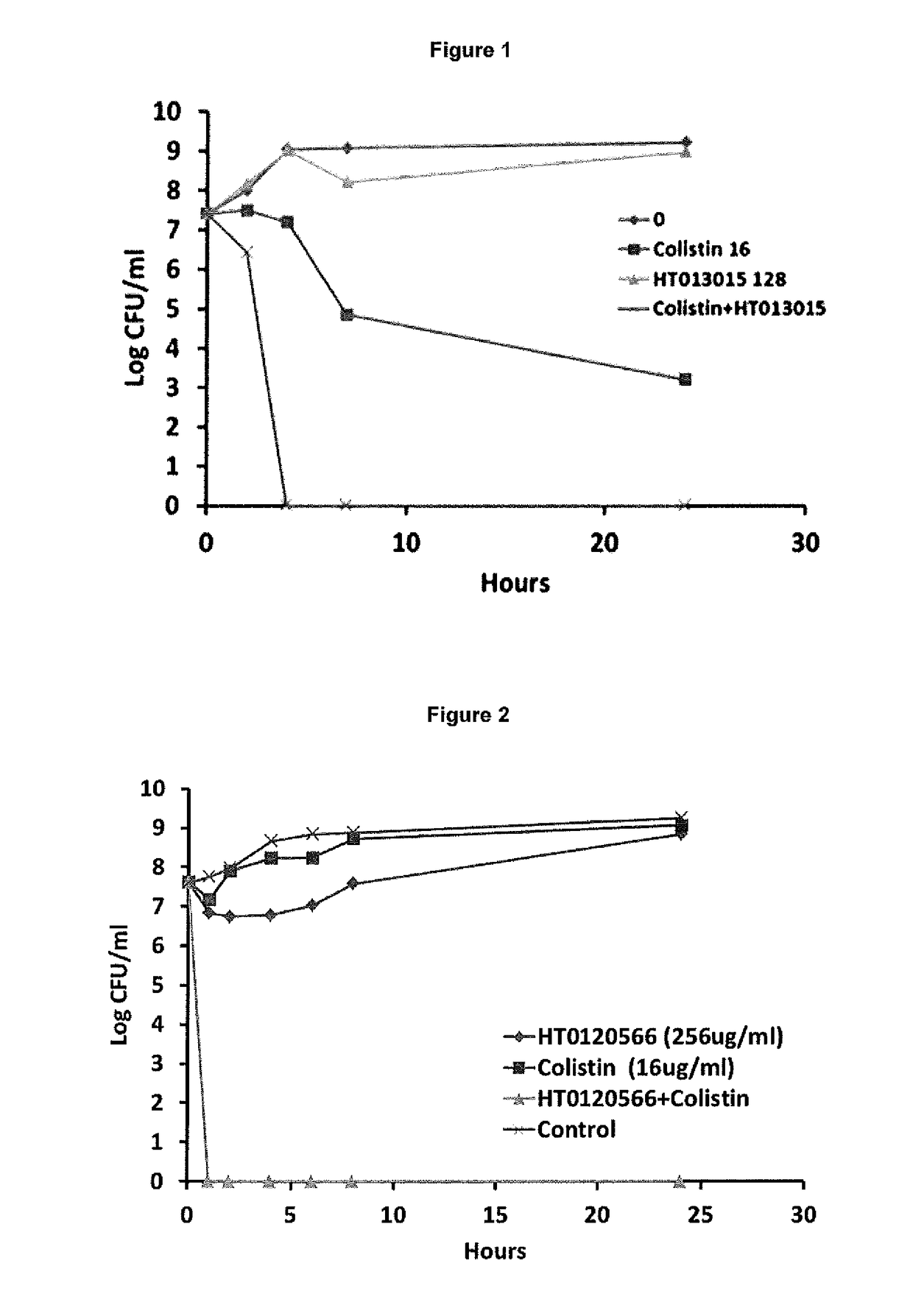
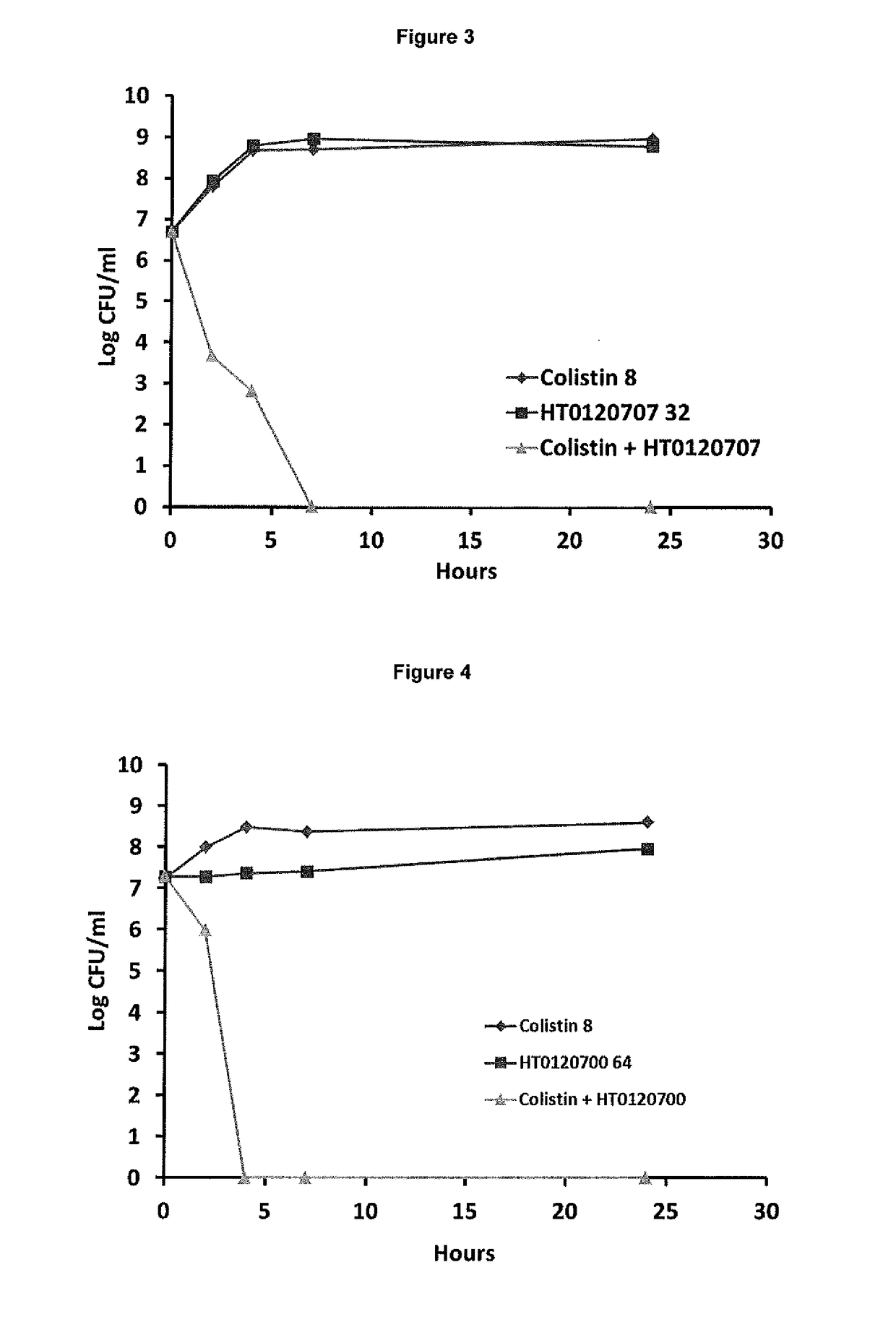
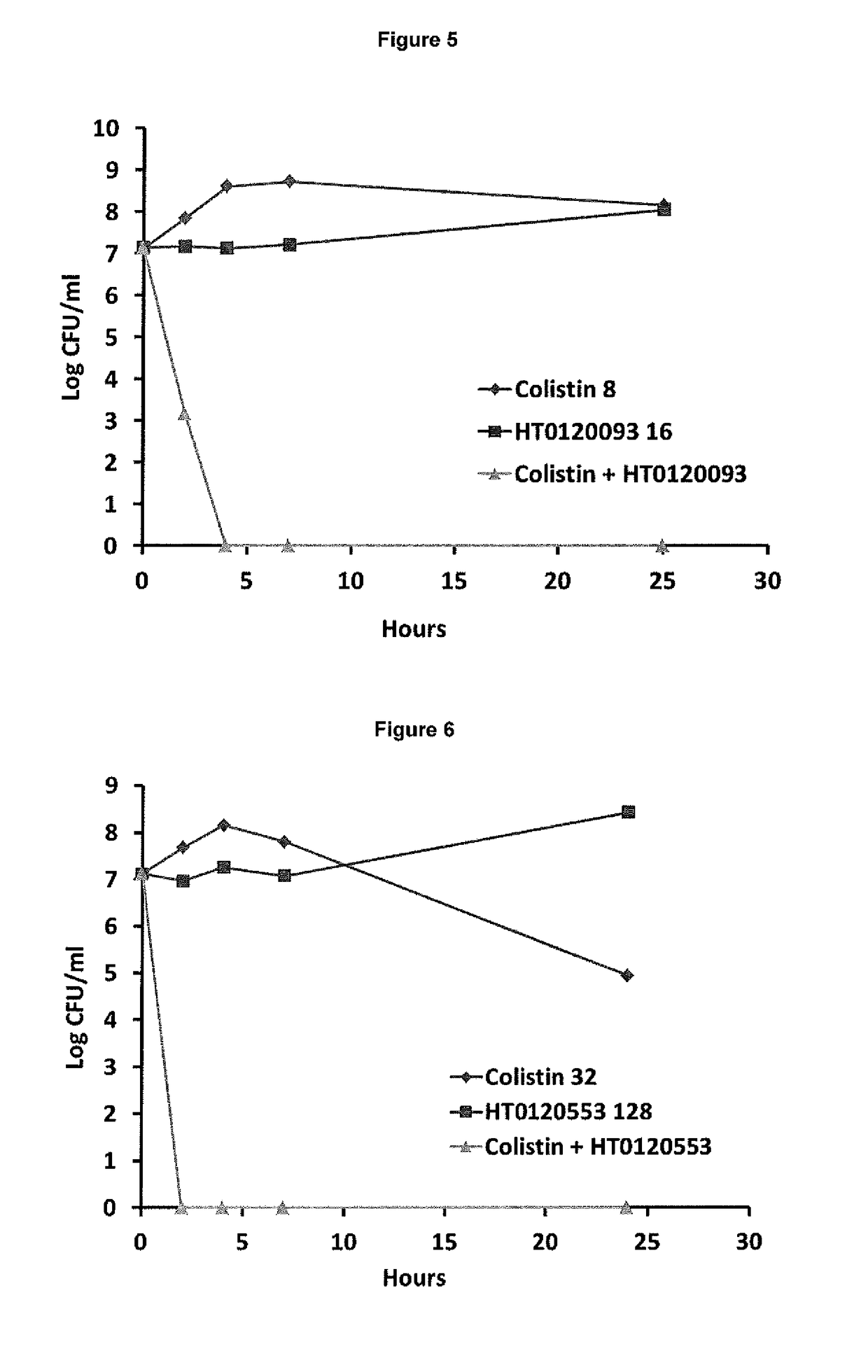
The present invention relates to the use of one or more compounds selected from the following: caffeic acid, thymol, aspirin, benzydamine hydrochloride, diclofenac sodium, flurbiprofen, ibuprofen, indomethacin, trifluoperazine hydrochloride, chlorprothixene hydrochloride, triflupromazine hydrochloride, suloctidil, thioridazine hydrochloride, dichlorophen, saccharin and piroxicam, in combination with a polymyxin selected from colistin or polymyxin B or a pharmaceutically acceptable derivative thereof, for use in the treatment of a microbial infection, and in particular for killing clinically latent microorganisms associated with microbial infections. The invention also provides a combination comprising suloctidil or a pharmaceutically acceptable derivative or prodrug thereof, and a polymyxin selected from polymyxin E and polymyxin B or a pharmaceutically acceptable derivative thereof. This combination is particularly useful for the treatment and / or prevention of microbial infections.
Owner:HELPERBY THERAPEUTICS LTD
Piroxicam cataplasm
InactiveCN107028921AFast transdermal absorptionLow drug releaseOrganic active ingredientsAntipyreticDihydroxyaluminum aminoacetateGlycerol
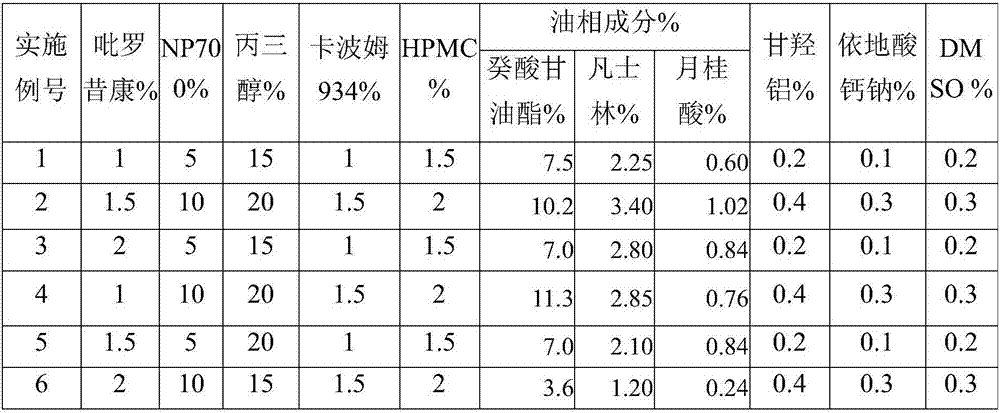
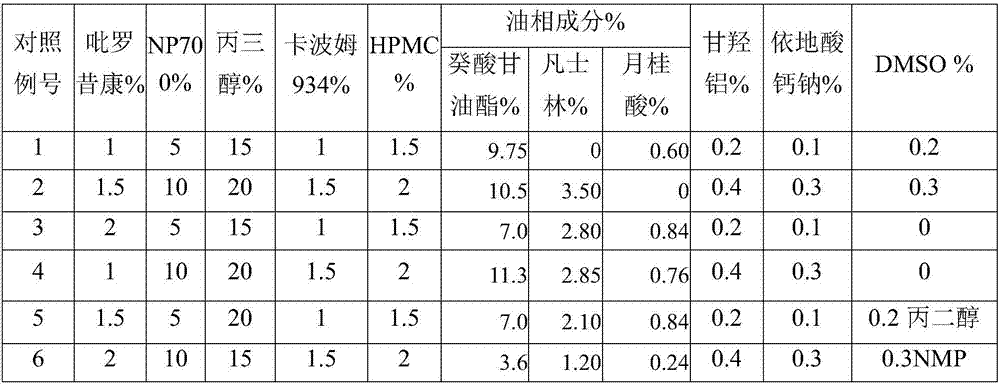
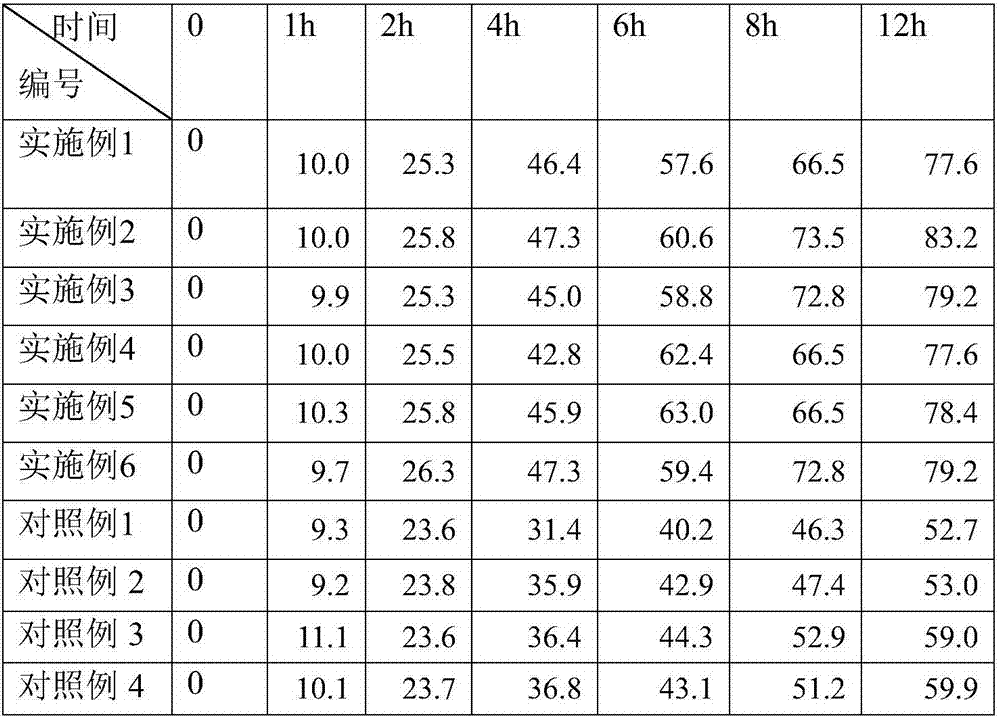
The invention discloses piroxicam cataplasm. The piroxicam cataplasm comprises back lining layers, medicine storages and protective layers, and is characterized in that the medicine storages comprise, by weight, 1%-2% of piroxicam, 10%-15% of oil-phase components, 5%-10% of partially neutralized sodium polyacrylate, 15%-20% of glycerol, 1%-1.5% of carbomer 934, 1.5%-2% of hydroxyl propyl methyl celluloses (HPMC), pH (potential of hydrogen) regulators, 0.2%-0.4% of dihydroxyaluminum aminoacetate, 0.1%-0.3% of calcium disodium edetate, 0.2%-0.3% of dimethyl sulfoxide (DMSO), 1%-3% of fillers and the balance water, and the piroxicam is used as an active component; the oil-phase components comprise caprin, Vaseline and lauric acid, a weight ratio of the caprin to the Vaseline to the lauric acid is 1-1.2:0.3-0.4:0.08-0.12, and the piroxicam is dispersed in oil phases; the partially neutralized sodium polyacrylate is used as a water-phase component.
Owner:北京茗泽中和药物研究有限公司
Macaca fascicularis P450 2C18 medical metabolic enzyme and co-expression recombinant carrier with macaca fascicularis P450 oxidoreductase
The invention discloses cynomolgus monkey P450 2C18 drug metabolizing enzyme and a co-expression recombinant vector of the cynomolgus monkey P450 2C18 drug metabolizing enzyme and cynomolgus monkey P450 oxidoreductase. The cynomolgus monkey P450 2C18 drug metabolizing enzyme can catalyze hydroxylation of piroxicam and a gene sequence of the cynomolgus monkey P450 2C18 drug metabolizing enzyme or a complementary sequence of the cynomolgus monkey P450 2C18 drug metabolizing enzyme is a sequence in SEQ ID NO:1 with the mutation rate of 0-1%. The co-expression recombinant vector of the cynomolgus monkey P450 2C18 drug metabolizing enzyme and the cynomolgus monkey P450 oxidoreductase comprises an open reading frame of the sequence of the cynomolgus monkey P450 2C18 drug metabolizing enzyme and the open reading frame of the sequence of the cynomolgus monkey P450 oxidoreductase. The sequence or the complementary sequence of the cynomolgus monkey P450 oxidoreductase is shown in SEQ ID NO:2. Protein which is expressed by a heterogeneous source of the invention only represents the cynomolgus monkey P450 2C18 hypotype and the system is closer to the real situation of metabolism in vivo of cynomolgus monkeys.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Process for preparation of inclusion compounds between a non-steroidal anti-inflammatory drug and betacyclodextrin by microwave treatment
InactiveCN1638806ANanomedicinePharmaceutical non-active ingredientsMicrowave ovenAntiinflammatory drug
A process for the preparation of inclusion complexes of a drug (piroxicam or ibuprofen) and a cyclodextrin, characterised in that: a) the drug and cyclodextrin, in the form of finely divided powders, are mixed in the presence of aqueous or hydroalcoholic solutions, ammonia solutions or acid solutions; b) the resulting mixture is treated in a microwave oven; c) the resulting product is dried under vacuum at room temperature or with heating.
Owner:CHIESI FARM SPA
External medicine composition preparation with anti-inflammation detumescence pain-easing function
ActiveCN101416976BSignificant relief from treatmentGood curative effectOrganic active ingredientsNervous disorderDiseaseBULK ACTIVE INGREDIENT
The invention relates to an externally-used medical compound preparation having the functions of being anti-inflammatory, antipyretic and analgesic. The preparation is characterized in that the preparation is prepared by the following raw materials of active ingredients with the following weight percentages: 0.01 percent to 20 percent of aescin or salts thereof acceptable in pharmacy, 0.01 percent to 10 percent of one or more of ketoprofen, tiaprofenic acid, tiaprofenic salts acceptable in the pharmacy, meloxicam, piroxicam and naproxen, and the rest amount of accessories. The externally-usedmedical composition can be used for preparing any externally-used preparation acceptable in the pharmacy. The preparation of the invention can be used for treating arthrodynia, hyperosteogeny, gout, neuropathic pains, traumatic injuries, chronic eparsalgia, various closed injuries and other diseases, and has strong practicability.
Owner:JIANGSU KANION PHARMA CO LTD
Chinese and western medicine compound medicine for treating osteoarthritis and preparation method thereof
InactiveCN112402584AQuick cureAdjust compatibilityHeavy metal active ingredientsOrganic active ingredientsCyathula officinalisRadix Astragali seu Hedysari
The invention provides a Chinese and western medicine compound medicine for treating osteoarthritis. The Chinese and western medicine compound medicine for treating osteoarthritis is composed of traditional Chinese medicine components and western medicine components, the traditional Chinese medicine components comprise Chinese starjasmine stem, radix salviae miltiorrhizae, radix astragali, liquorice, native copper, herba epimedii and radix cyathulae, and the western medicine components comprise glucosamine, yak bone collagen peptide, piroxicam and aluminum hydroxide. The natural Chinese herbalmedicines and western medicines are organically combined, compatibility and applicability of the Chinese and western medicines are adjusted, and the Chinese and western medicine compound medicine fortreating osteoarthritis is prepared. The Chinese and western medicine compound medicine for treating osteoarthritis improves a microcirculation, promotes blood flow, reduces swelling and pain, increases bone density while quickly treating osteoarthritis, protects gastric mucosa, avoids side effects, has a good applicability to patients, and fundamentally improves healing effects of patients withmild, moderate and severe osteoarthritis.
Owner:魏县魏州冀南医院
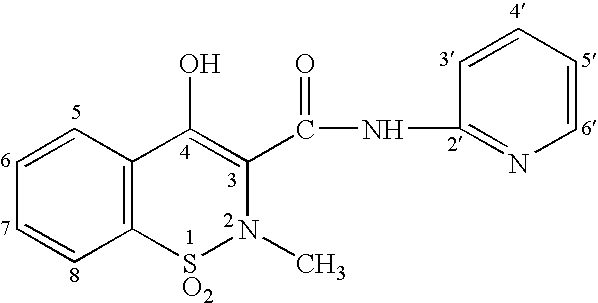
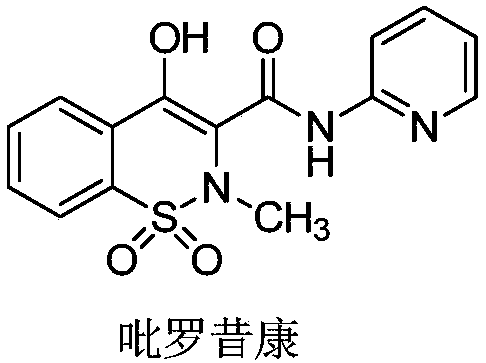
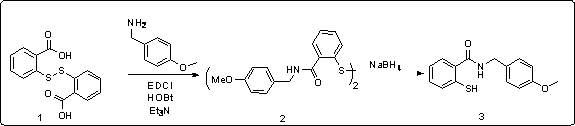
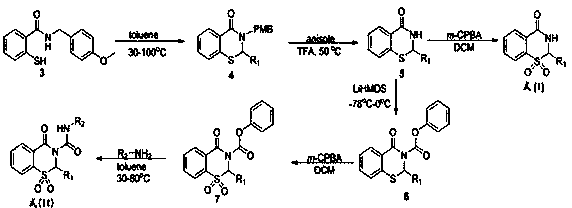
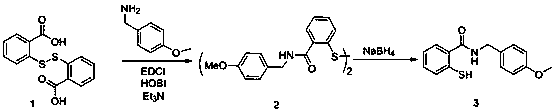
Synthetic method of piroxicam
ActiveCN108623579AHigh reaction conversion rateHigh yieldOrganic chemistryChemical synthesisCarboxylic acid
The invention belongs to the field of chemical synthesis, and more specifically relates to a synthetic method of piroxicam. The synthetic method of piroxicam comprises following steps: 1, sodium saccharin and ethyl chloroacetate are taken as initial raw materials, and condensation reaction is carried out so as to obtain 3-oxo-1,2-benzoisothiazoline-2-methyl acetate-1,1-dioxide; 2, sodium methylateis added into the product in step 1, reaction is carried out under catalytic effect of potassium iodide, and 2-methyl-3,4- dioxo-4-oxo-2H-1,-2- benzothiazine-3-carboxylic acid methyl ester-1, 1-dioxide is obtained under the effect of DMSO; 3, the above product is reacted with 2-aminopyridine at 130 DEG C for 10h so as to obtain a high purity finished product. According to the synthetic method, potassium iodide is taken as a catalyst to increase the reaction conversion rate of step 2 obviously, impurity content is controlled effectively; and the total yield is increased to 69%.
Owner:ZHENGZHOU MINGZE MEDICAL TECH
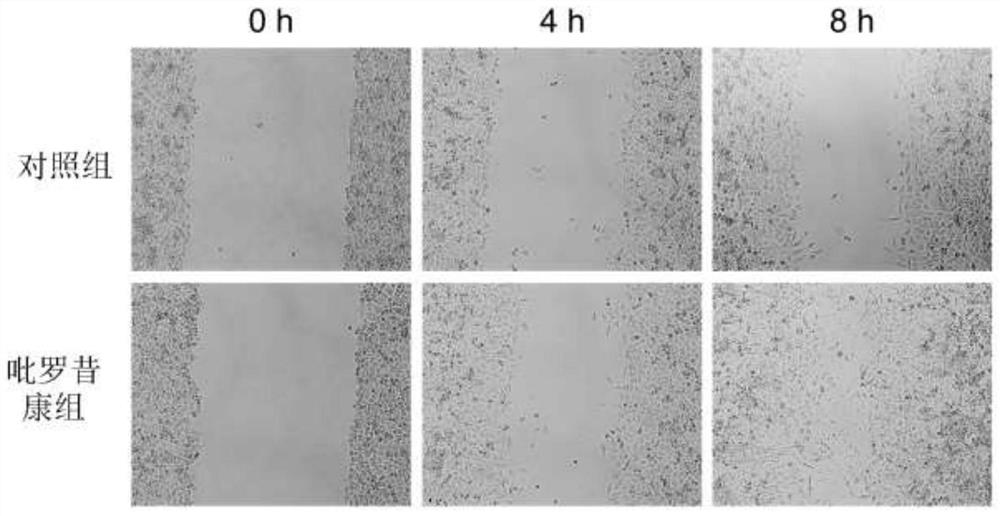
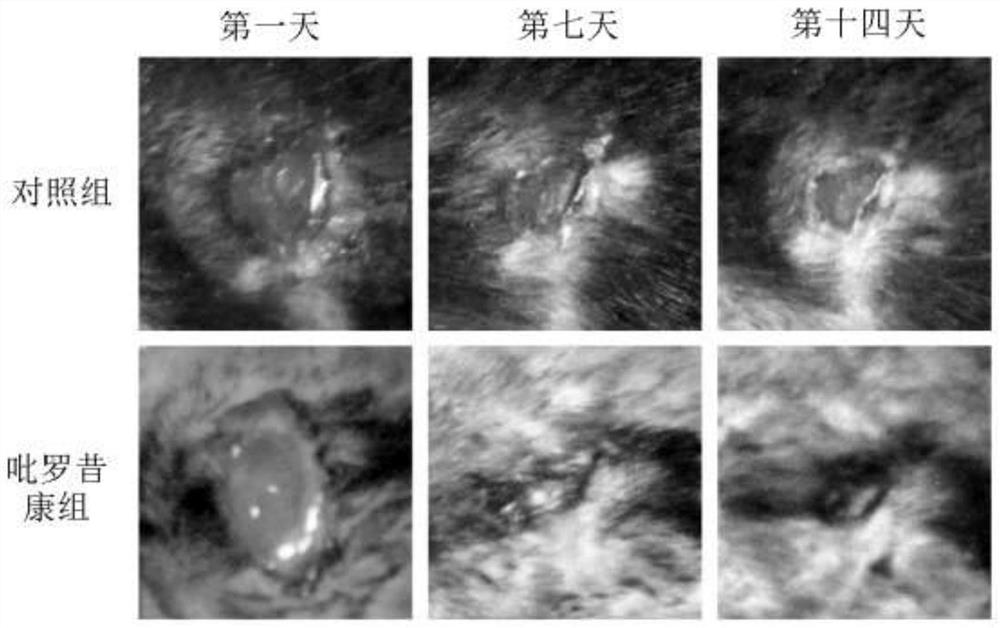
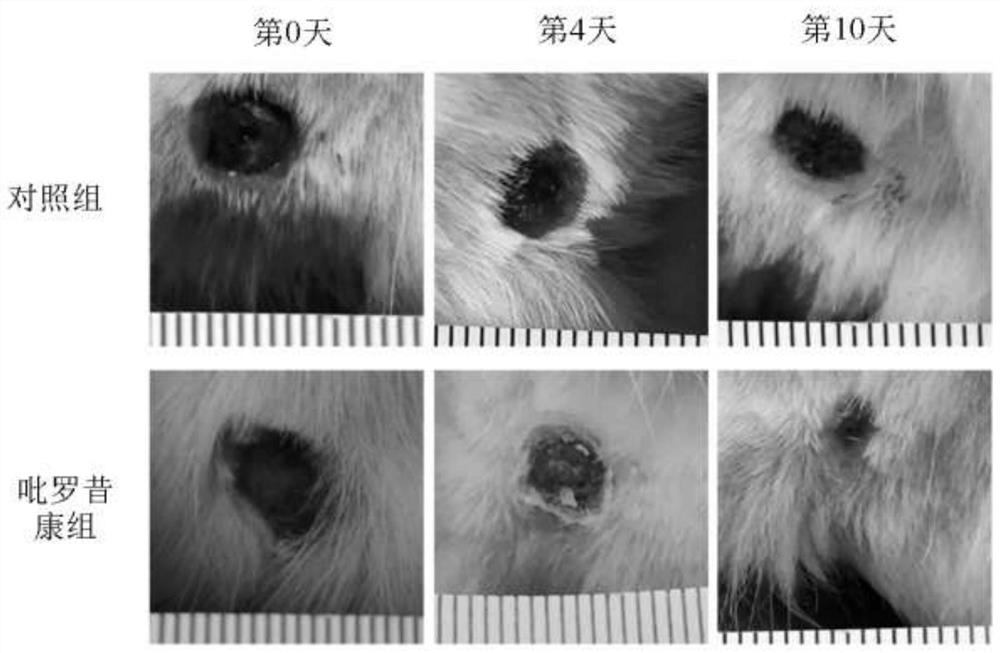
Application of piroxicam in preparation of medicine for treating skin ulcer and preparation method of medicine
PendingCN112402426AEffective treatmentPromote migrationOrganic active ingredientsMetabolism disorderPharmaceutical AidsDiabetic ulcers
The invention discloses application of piroxicam in preparation of a medicine for treating skin ulcer and a preparation method of the medicine. The medicine is prepared from piroxicam and pharmaceutically acceptable auxiliary materials. The piroxicam can promote migration of human umbilical vein endothelial cells (HUVEC cells) under high glucose stimulation, so that healing of diabetic foot ulcerwounds is promoted; and the piroxicam has a remarkable curative effect on skin ulcer, especially diabetic foot ulcer.
Owner:SICHUAN UNIV
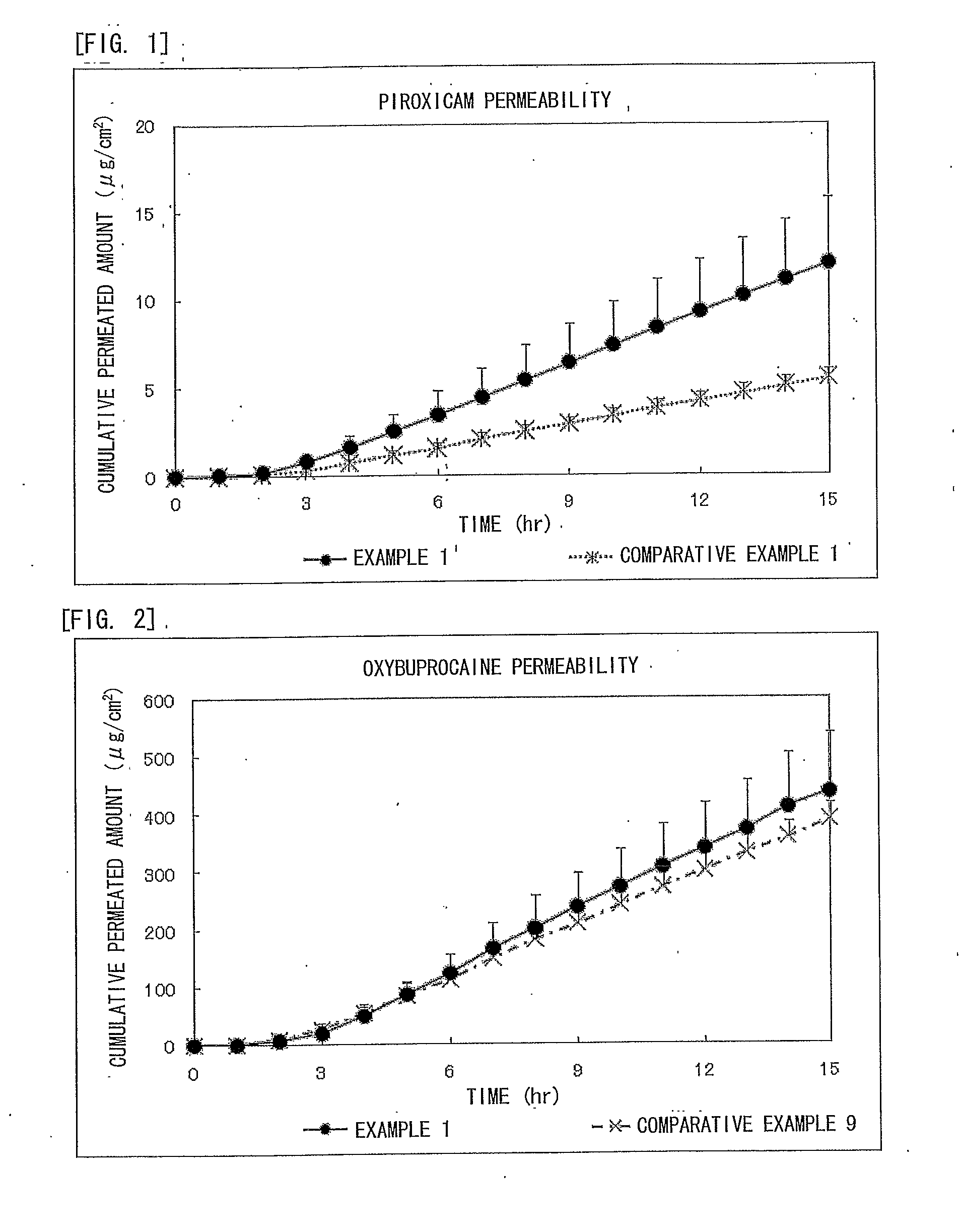
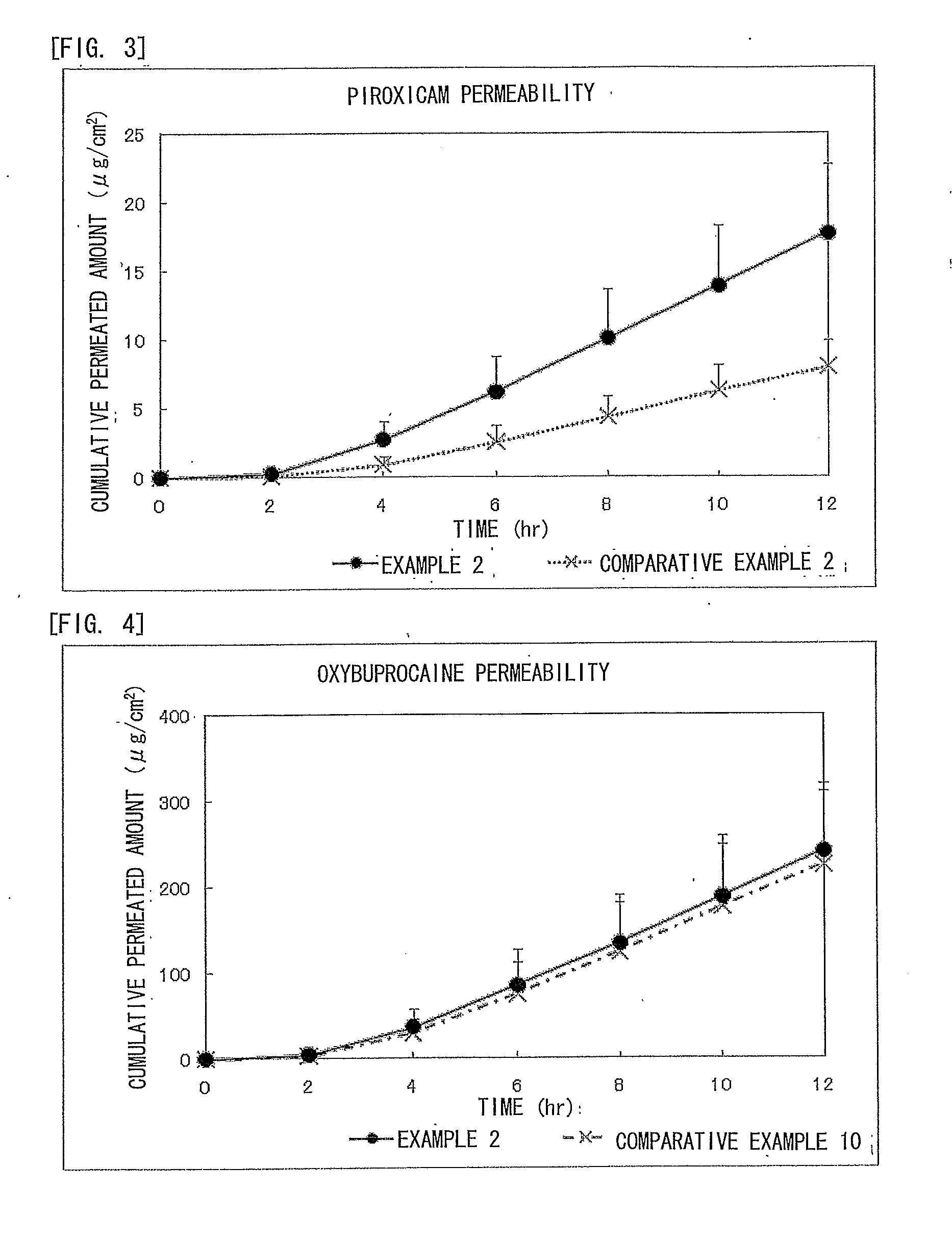
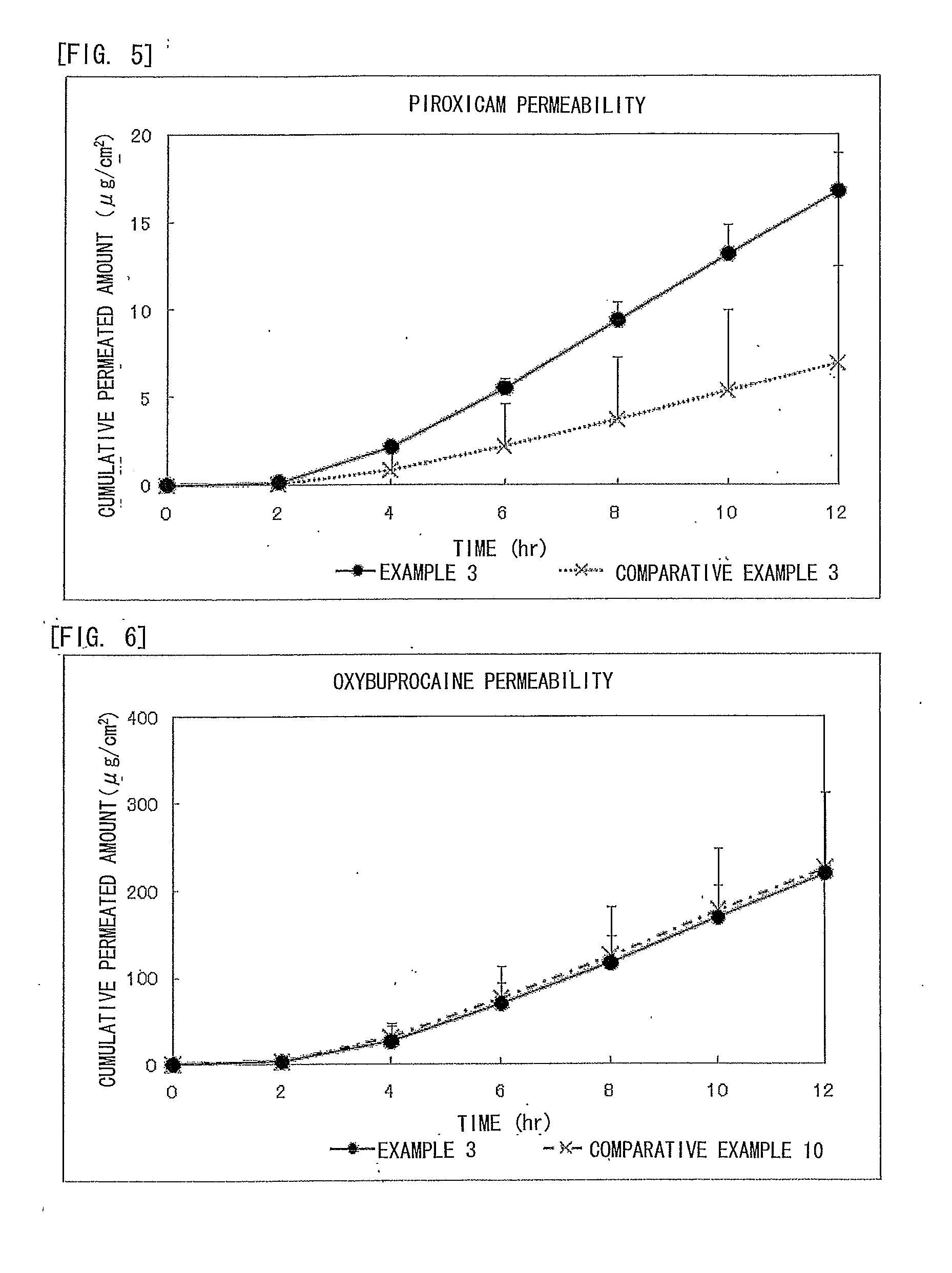
Piroxicam-Containing Transdermally Absorbable Preparation
ActiveUS20120309749A1Facilitated releaseGood analgesic effectAntipyreticAnalgesicsAnalgesics effectsNon steroidal anti inflammatory
An adhesive patch is provided in which piroxicam is formulated as a non-steroidal anti-inflammatory analgesic. In particular, provided is a piroxicam-containing transdermally absorbable adhesive patch in which an absorption promoter to piroxicam is formulated to achieve high anti-inflammatory and analgesic effects without inhibiting releasing of these drugs. The piroxicam-containing transdermally absorbable adhesive patch contains piroxicam as a medicinal component and oxybuprocaine or a pharmaceutically acceptable salt thereof as an absorption promoter. In the piroxicam-containing transdermally absorbable adhesive patch, the content of piroxicam is from 0.1% to 5% by weight to the total weight of a drug-containing plaster and the content of oxybuprocaine or the pharmaceutically acceptable salt thereof is from 1% to 30% by weight to the total weight of the drug-containing plaster.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Piroxicam tablets and preparation process thereof
InactiveCN103860513AQuality improvementRapid dissolutionOrganic active ingredientsAntipyreticMagnesium stearateArthritis
The invention discloses piroxicam tablets and a preparation process and purposes thereof. The product consists of piroxicam, lactose, pregelatinized starch, hydroxypropyl methyl cellulose, carboxymethyl starch sodium, polysorbate-80, 15% pregelatinized starch for slushing, superfine silica powder, magnesium stearate and a gastric soluble film coating premix. The production method comprises the steps: preparing tablet cores, coating and the like. The piroxicam tablets are used for treating 30 cases of rheumatoid arthritis, have good curative effects of diminishing inflammation and relieving pain and have slight influence on erythrocyte sedimentation rate, the total effective rate is 88 percent, and the piroxicam tablets have the characteristics of low administration frequency, stable blood concentration, long duration and the like.
Owner:崔书豪
Eye nursing gel and preparation method thereof
InactiveCN105963677AEliminate edemaRelieve eye fatigueSenses disorderHydroxy compound active ingredientsNedocromil SodiumVitamin B12
The invention discloses an eye nursing gel and a preparation method of the eye nursing gel. The eye nursing gel is mainly prepared from the following components in parts by weight: 3-10 parts of hydroxypropyl methyl cellulose, 1-4 parts of guar gum, 0.1-0.3 part of nedocromil sodium, 0.1-0.5 part of betaxolol, 0.2-0.6 part of piroxicam, 0.1-0.3 part of sulphate, 0.1-0.2 part of dipotassium glycyrrhizinate, 0.05-0.15 part of bacitracin, 0.8-1.2 parts of epinephrine bitartrate, 1.5-3 parts of taurine, 0.3-1 part of procyanidine, 0.4-1 part of vitamin A, 0.4-1 part of vitamin B12, 0.01-0.03 part of a preservative, 2-5 parts of a buffer agent, 2-8 parts of an iso-osmotic agent, and 70-120 parts of a gel substrate. According to the eye nursing gel, the components are mixed to accelerate the recovery of the injured eye tissue, prevent the infectious complications, and alleviate the eye discomfort, and therefore, a very good nursing role is played on the eyes; the eye nursing gel can effectively alleviate eyestrain, eliminate black eyes and edemas on the peripheries of the eyes, resist wrinkles as well as activate and relax the skin, and has certain effects for preventing shortsightedness, recovering the vision health, controlling and preventing eye diseases, and the like.
Owner:宋钦
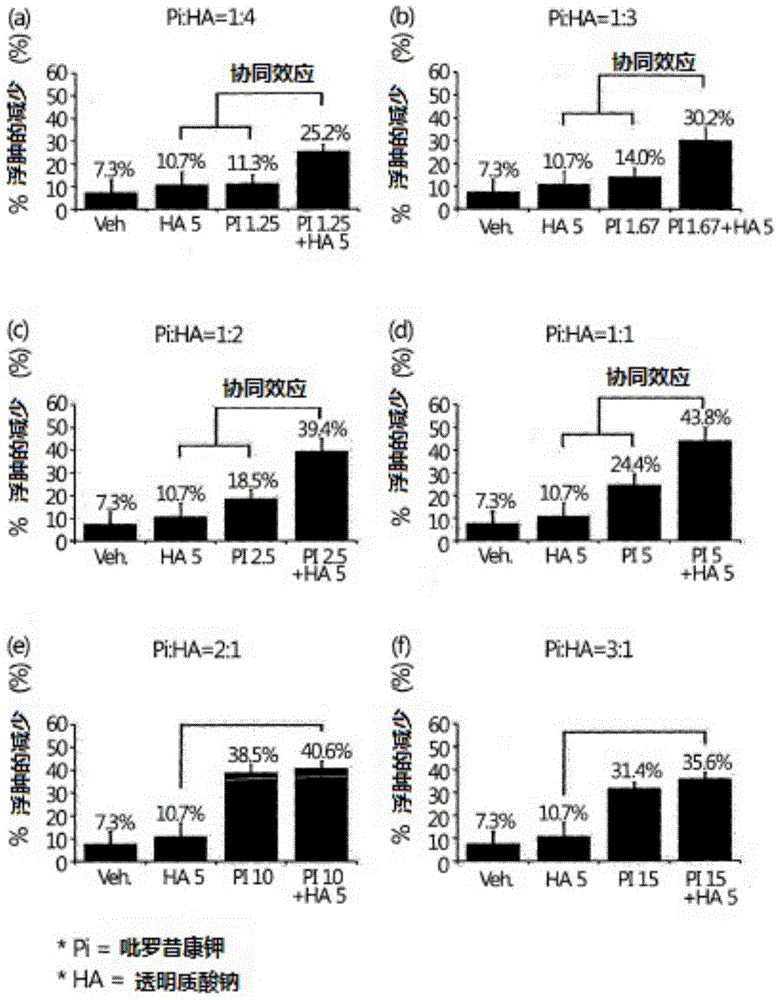
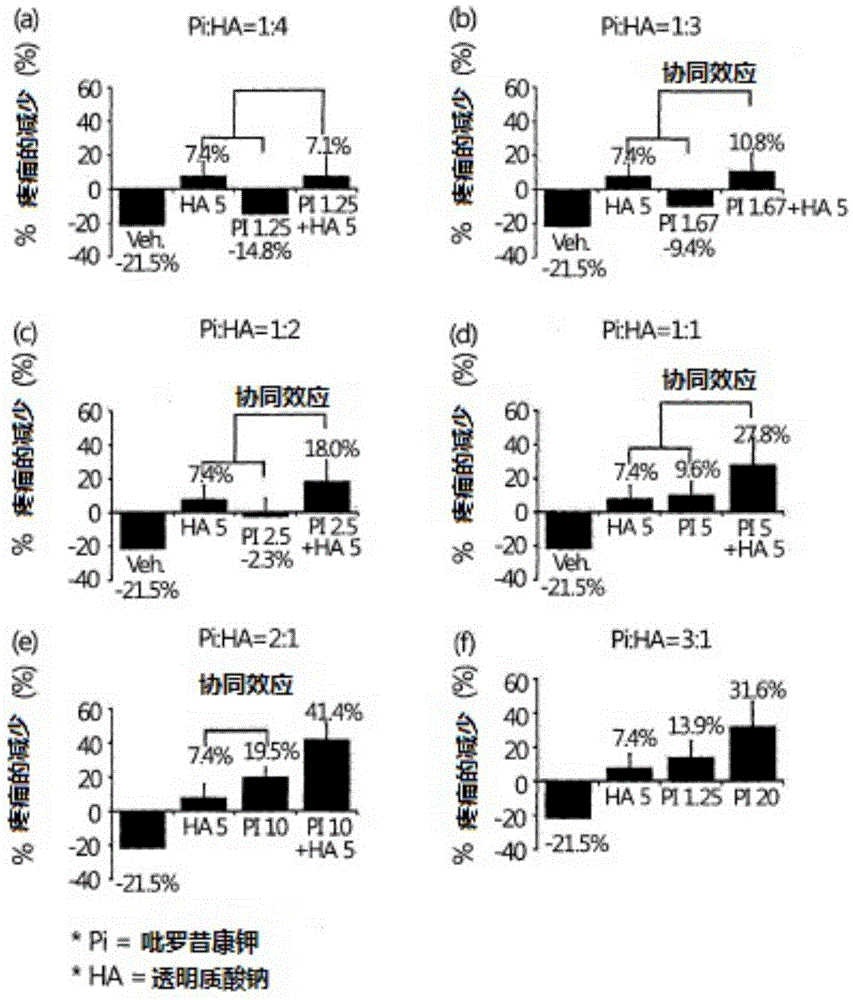
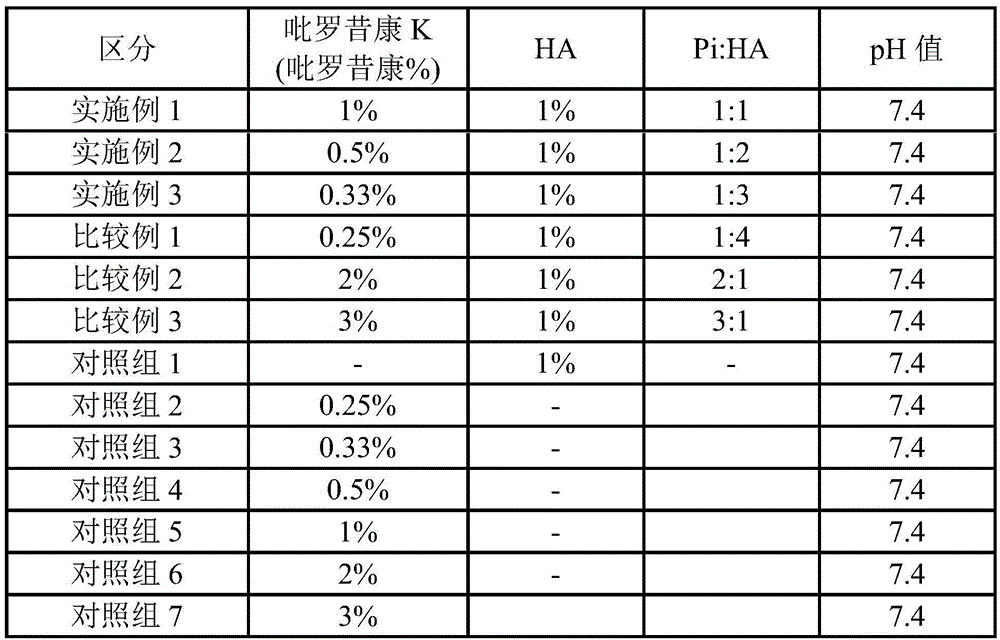
Liquid pharmaceutical composition containing piroxicam and hyaluronic acid for the treatment of osteoarthritis
InactiveCN105473146AInorganic non-active ingredientsSkeletal disorderTherapeutic effectAnalgesics effects
The present invention relates to the pharmaceutical composition for the treatment of osteoarthritis comprising piroxicam or pharmaceutically acceptable salt thereof and hyaluronic acid or pharmaceutically acceptable salt thereof at a specific ratio that generates synergistic effect on both anti-inflammatory and analgesic effects simultaneously. The present invention provides a pharmaceutical composition for the treatment of osteoarthritis comprising 0.25-10.0 wt% of piroxicam or its pharmaceutically acceptable salt and 0.5-5.0 wt% of hyaluronic acid or its pharmaceutically acceptable salt, where the weight ratio between piroxicam or its pharmaceutically acceptable salt and hyaluronic acid or its pharmaceutically acceptable salt is between 1:1 and 1:3. The liquid pharmaceutical composition according to the present invention has an outstanding therapeutic effect simultaneously on both inflammation and pain, thus providing an injectable formulation for intra-articular injection with synergistic effect on the treatment of osteoarthritis.
Owner:DONG A ST CO LTD
1,3-benzothiazinone compound as well as synthesis method and application thereof
InactiveCN109384740AHigh activityLow toxicityAntibacterial agentsOrganic active ingredientsMeloxicamSynthesis methods
The invention discloses a 1,3-benzothiazinone compound which has a chemical structural formula as shown in the description, in the formula, R1 is C1-C4 straight chain alkyl or methyl carbethoxy respectively; and R2 is a heterocyclic compound or substituted phenyl. The compound disclosed by the invention has activity equivalent to or higher than those of known medicines such as meloxicam and piroxicam at a cell level, has anti-inflammation, anti-cancer and antibacterial activity, has toxicity lower than those of xicam compounds in the market, and is safe and effective.
Owner:LANZHOU UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com