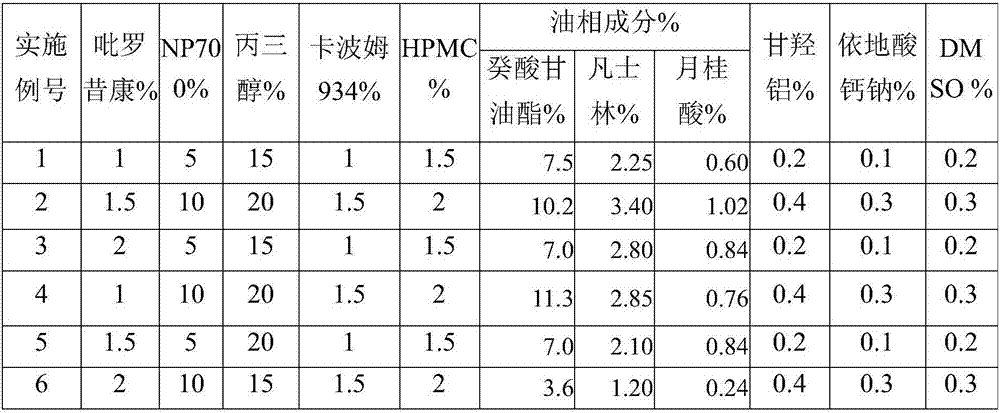
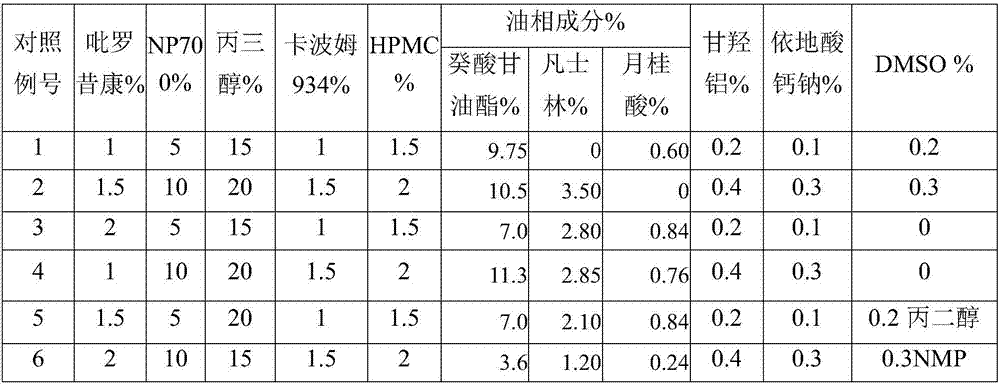
Piroxicam cataplasm
A technology of piroxicam and piroxicam, applied in anti-inflammatory agents, non-central analgesics, medical preparations of non-active ingredients, etc., can solve the problem of large drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
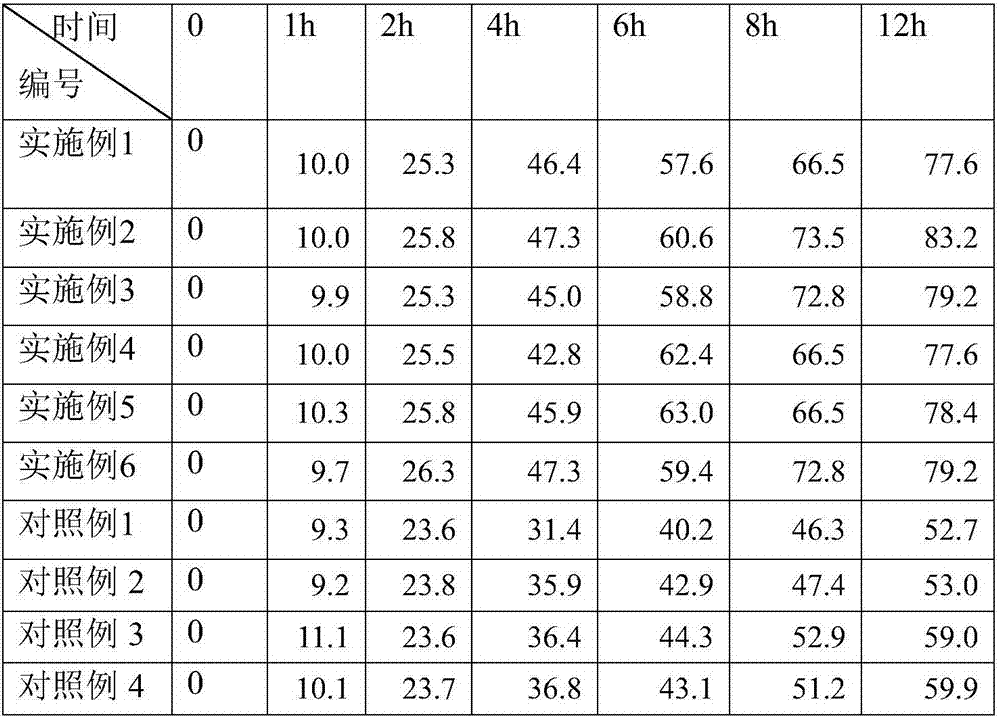
[0019] Pharmacological Example 1 In Vitro Release Experiment
[0020] According to the method provided in the third method (paddle and disk method, used for transdermal patch) in the second appendix XD of the Chinese Pharmacopoeia 2010 edition, the emergency release of the patch obtained in Examples 1 to 6 is measured . The specific method is as follows
[0021] In the test, physiological saline was used as the release medium, and the release medium was added into the dissolution cup, pre-warmed to (32±0.5°C) to remove the protective layer of the cataplasm, cut into a size of 2.5cmx7.5cm, and put it flat into a dialysis bag (molecular weight cut-off 14,000 ), with the release side facing up, placed between two layers of discs, so that the edges of the disc clamp the two ends of the dialysis bag, and then wrap and fix with rubber bands to fix the disc. Take 6mL samples from the dissolution vessel at 10min, 20min, 30min, 45min, 60min, 90min, 2h, 2.5h, 3h and 4h respectively, a...
Embodiment 2
[0022] Pharmacological embodiment 2, in vitro transdermal experiment
[0023] The modified Franz diffusion cell method was used to conduct an in vitro transdermal test with the cataplasm patch prepared in Examples 1-6 and Comparative Examples 1-6, using the abdominal skin of isolated 3-month-old rats as a barrier. The specific experimental method is:
[0024] After 3-month-old healthy rats were anesthetized and killed, the abdominal hair was removed with scissors, the undamaged skin was removed, and the subcutaneous tissue was removed. After washing, they were respectively fixed at the release port of the Franz diffusion cell, and pH 7.4 phosphoric acid was added to the receiving chamber. The buffer is used as a release medium to keep the endothelial layer in close contact with the solution. Put the cataplasm with the protective layer removed on the skin, adjust the water bath so that the temperature of the outer layer is constant at (32±0.5)°C, and the stirring speed is 100r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com