Liquid pharmaceutical composition containing piroxicam and hyaluronic acid for the treatment of osteoarthritis
A technology of hyaluronic acid and piroxicam, which is applied in the direction of drug combination, medical preparations containing active ingredients, drug delivery, etc., and can solve problems such as different and unsuitable osteoarthritis treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
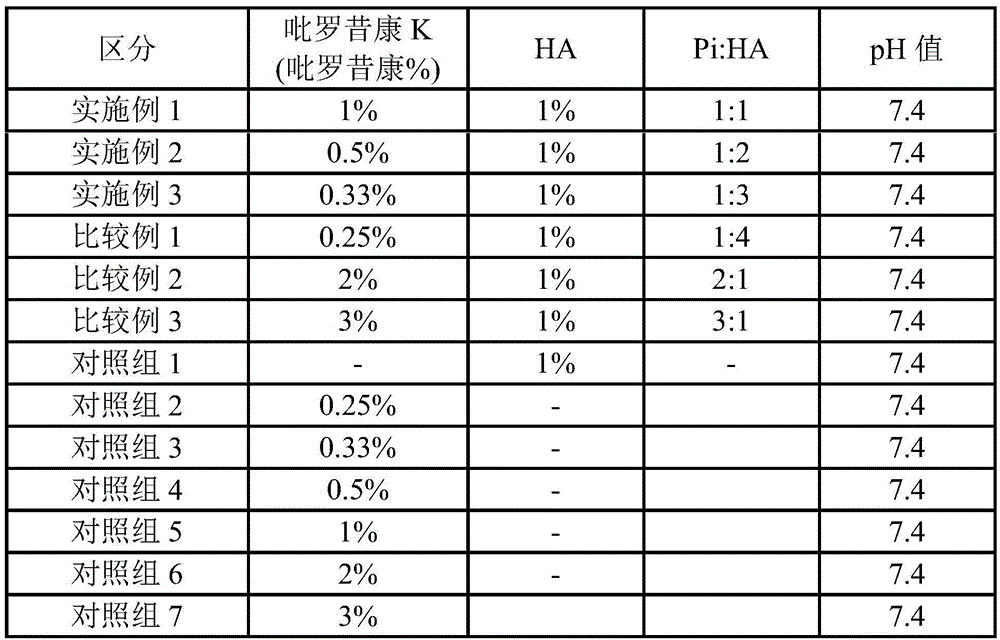
[0029] The present invention contains the preparation of the liquid composition of piroxicam and hyaluronic acid
[0030] Add 5.58 g of piroxicam potassium and an appropriate amount of solubilizer to about 350 ml of phosphate buffer (pH 7.4), then stir at 30° C. for 1 hour to dissolve the mixture, and then add phosphate buffer to a final volume of 500 ml. After sterilization with a syringe filter, 5.0 g of sodium hyaluronate was then added. The composition was then prepared as a final step with stirring at a temperature of 30-40° C. for 12 hours using an overhead mixer.
Embodiment 2 and 3
[0031] preparation of the liquid composition containing piroxicam and hyaluronic acid of the present invention
[0032] A liquid composition containing piroxicam and hyaluronic acid was prepared using the same method as in Example 1 above, but the contents were as shown in Table 1 below, and they were referred to as Example 2 and Example 3, respectively.
experiment example 1
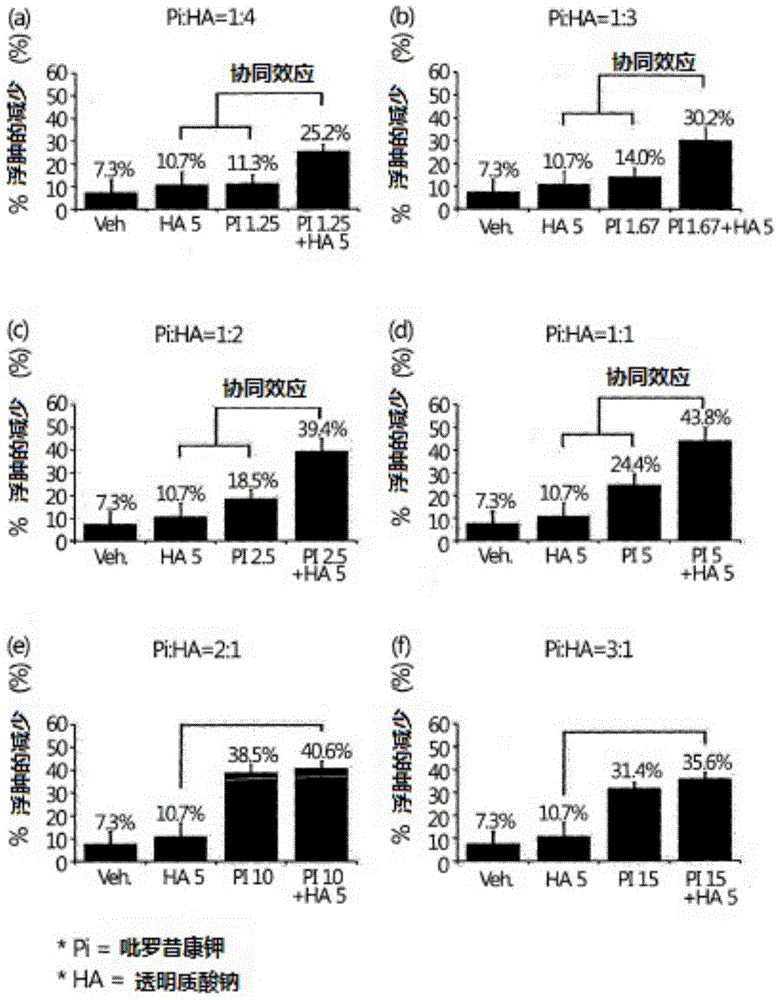
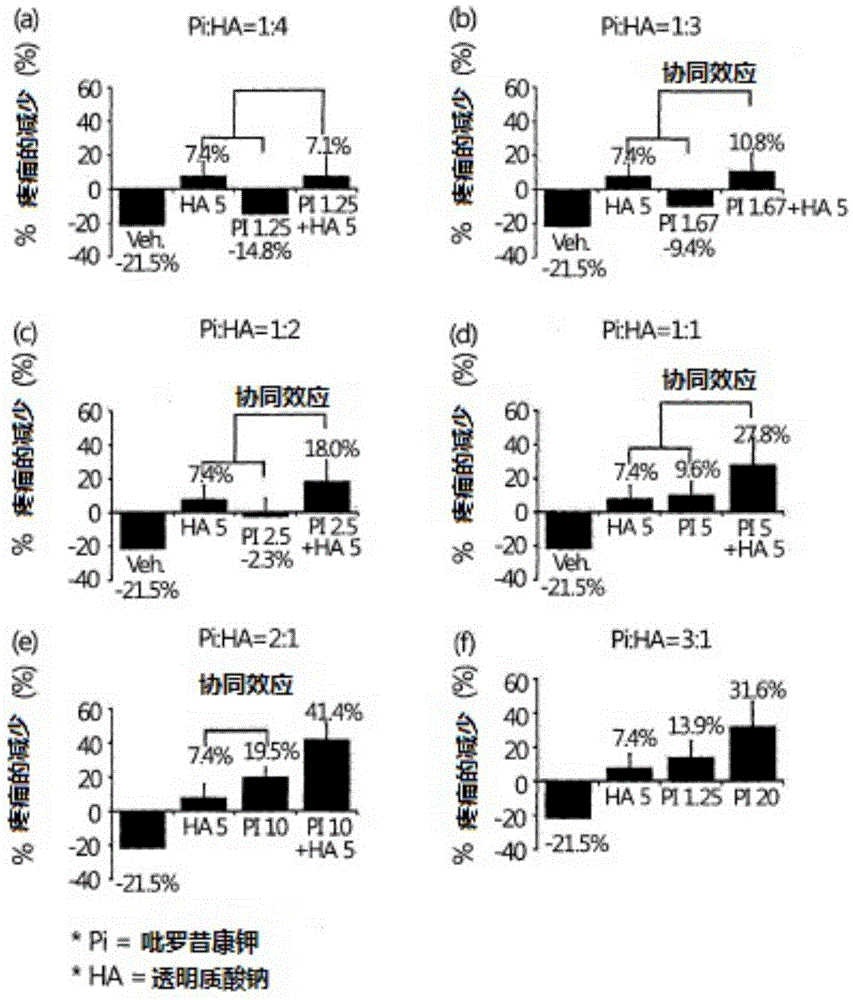
[0042] Anti-inflammatory efficacy evaluation in Mono-iodoacetate-induced osteoarthritis model
[0043] For this evaluation, 8-week-old male SD rats purchased from Daehan Biolink Co., Ltd. were stabilized for four weeks in a clean area (animal breeding room on the second basement floor of the new building of the research institute), wherein the temperature in the clean area was maintained at 23 ±2°C, relative humidity maintained at 40-60%, ventilation frequency maintained at more than 10 times / hour, ammonia concentration maintained at or below 20ppm, average illuminance at 150-300lux, and noise level maintained at or below 60 loudness units (phon), and the light and dark cycle was maintained at 12 hours. At the beginning of the experiment, Mono-iodoacetate (hereinafter referred to as MIA) was administered into the right joint cavity at 3mg / 30ul / only. One day after the administration, the joint diameters of the rats were measured, and they were divided into groups so that each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com