Oral dosage form comprising a therapeutic agent and an adverse-effect agent
a technology of adverse effects and therapeutic agents, which is applied in the direction of capsule delivery, microcapsules, drug compositions, etc., can solve the problems of drug abusers, defeat the controlled release design, and attract drug abusers, so as to reduce or eliminate the pharmacological effects of the therapeutic agent, suppress withdrawal symptoms, and reduce or eliminate the effect of the therapeutic agent's pharmacological activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
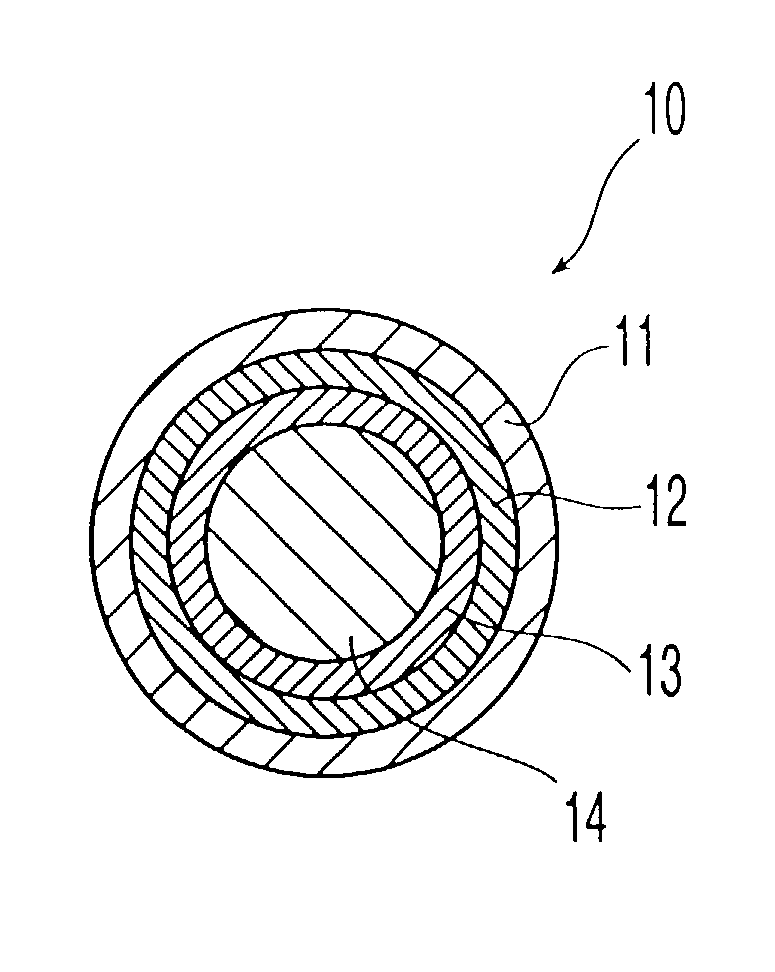
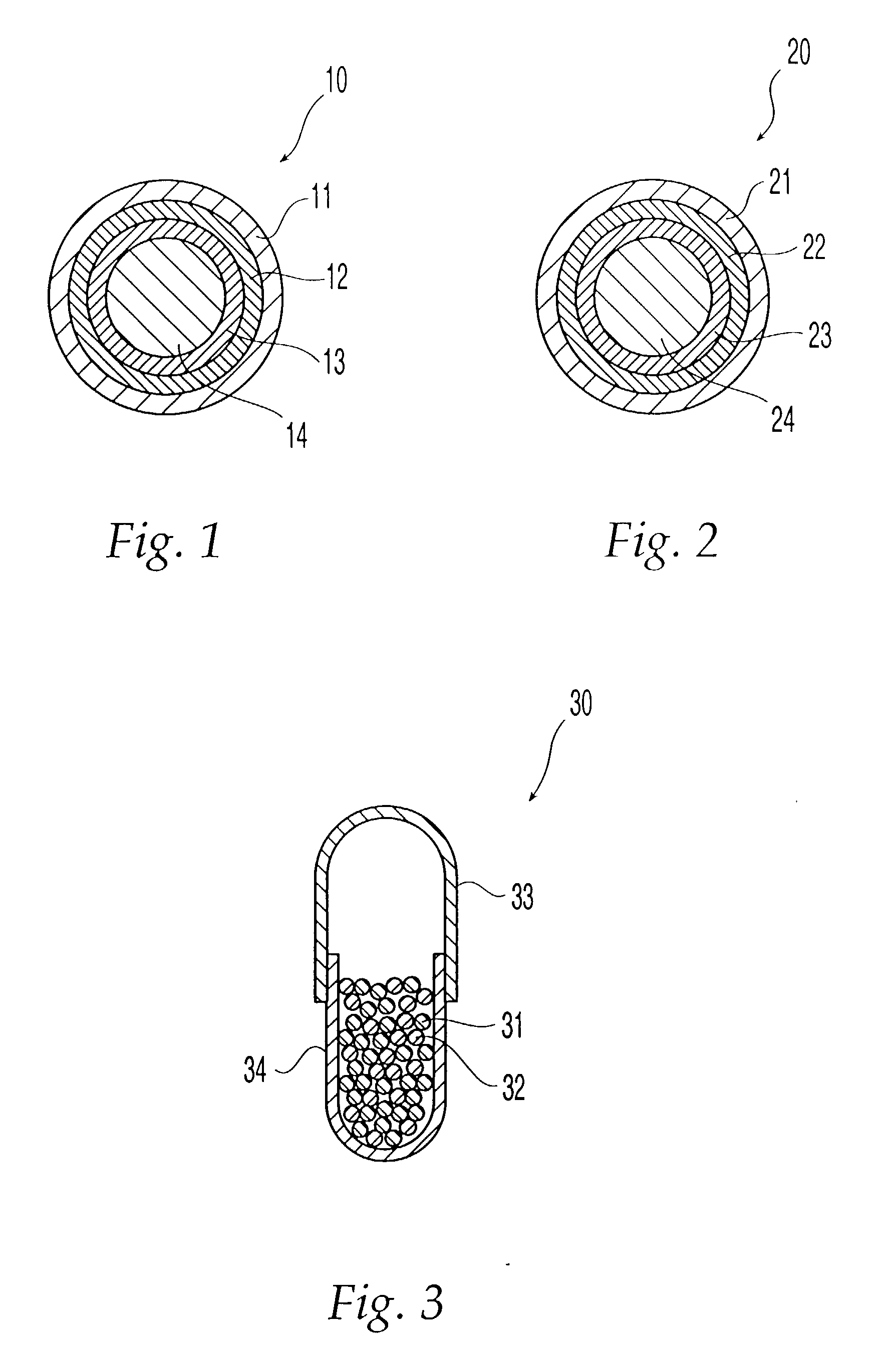
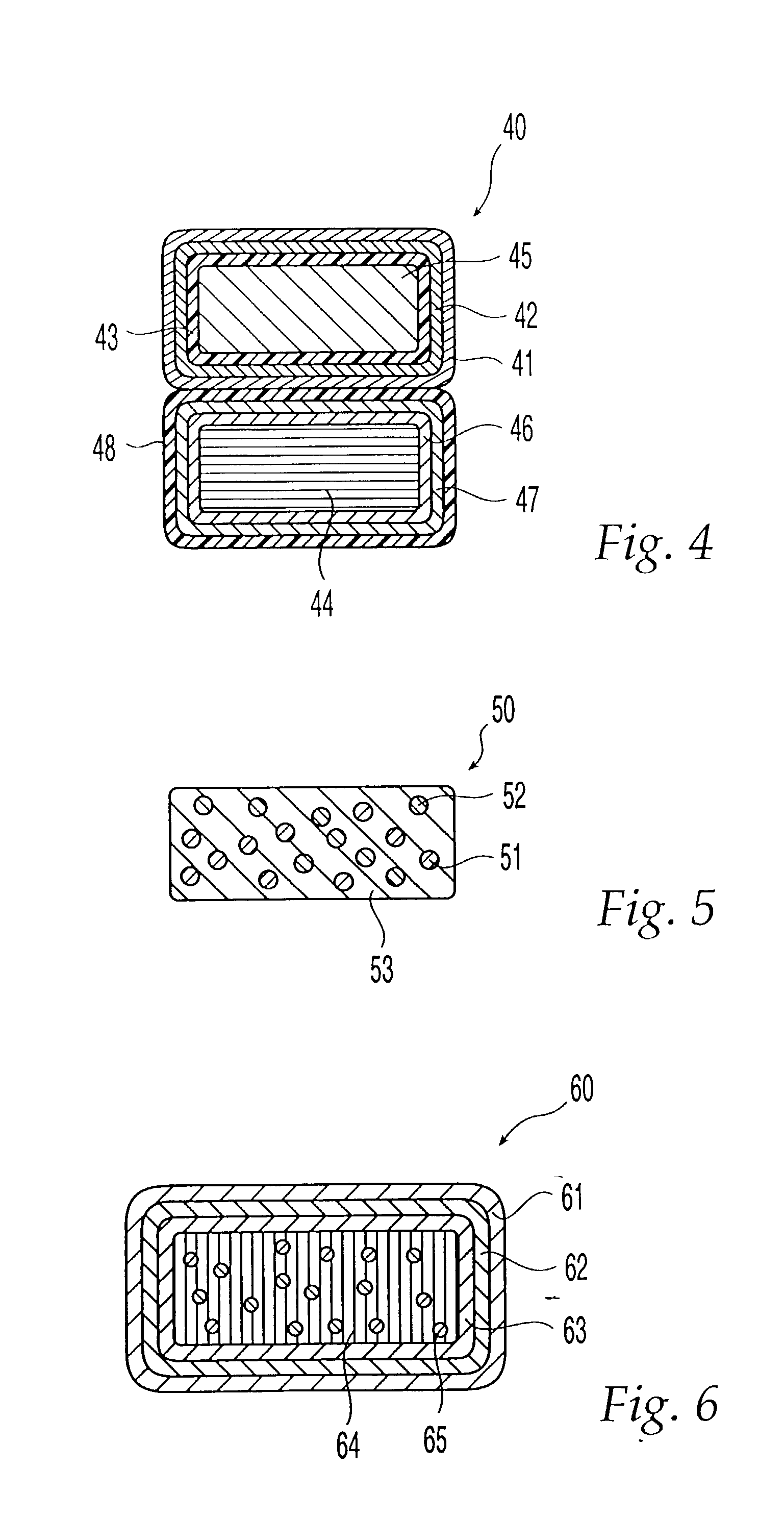
[0025] The term "adverse-effect agent," as used herein, means an agent that (A) reduces or eliminates one or more pharmacological effects of the therapeutic agent, such as a euphoric or toxic effect or (B) causes an undesired physiological reaction, such as emesis. In the oral dosage form of the invention, the second composition is coated with a layer that is substantially insoluble in the gastrointestinal tract. Thus, when the oral dosage form of the present invention is orally administered to a patient as intended, only the therapeutic agent is released in the gastrointestinal tract of the patient, and the adverse-effect agent is not released. If the oral dosage form is tampered with so that the coating on the second composition is damaged, however, then not only the therapeutic agent but also the adverse-effect agent are released upon administration.
second embodiment
[0026] In a second embodiment the second composition is coated with an outer base-soluble layer and an inner acid-soluble layer, which is not dissolved when orally administered to a patient.
third embodiment
[0027] In the oral dosage form of the invention, both the first composition and second composition have a coating comprising at least two layers, an acid-soluble layer and a base-soluble layer, but the order of the layers in the coating on the first composition is different from that of the layers in the coating on the second composition. The coating covering the first composition comprises an outer acid-soluble layer and an inner base-soluble layer, which are dissolved when orally administered to a patient. On the other hand, the coating covering the second composition comprises an outer base-soluble layer, which gets dissolved when orally administered, and an inner acid-soluble layer, which does not get dissolved when orally administered to a patient.
[0028] When orally administered to a patient, the oral dosage form passes through the stomach first, where its acidic environment dissolves the first composition's outer acid-soluble layer, and then passes into the small intestine, wh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com