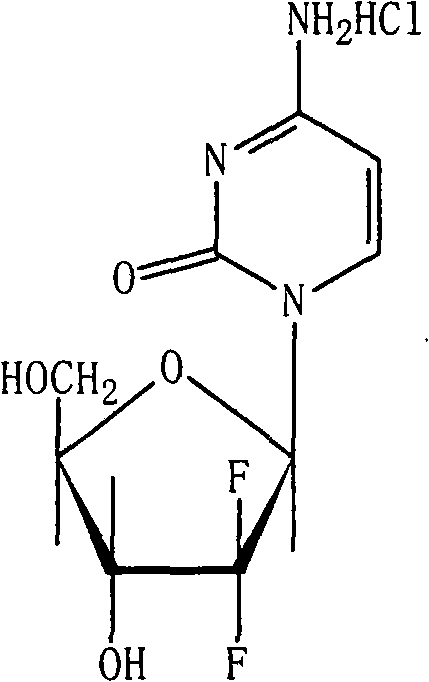
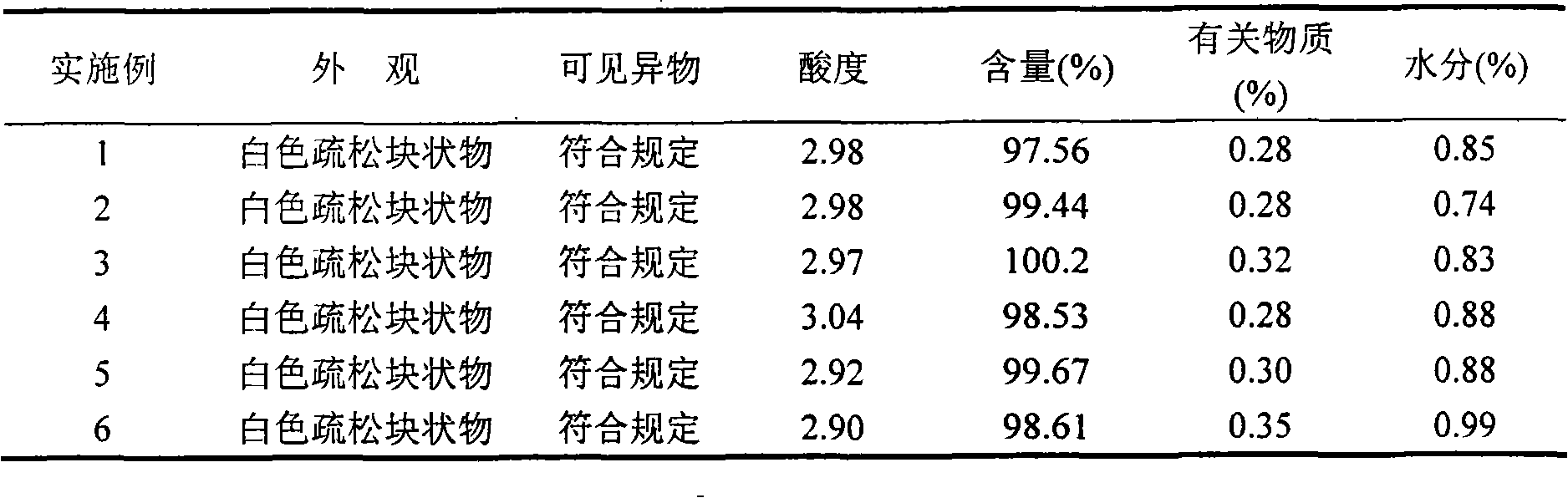
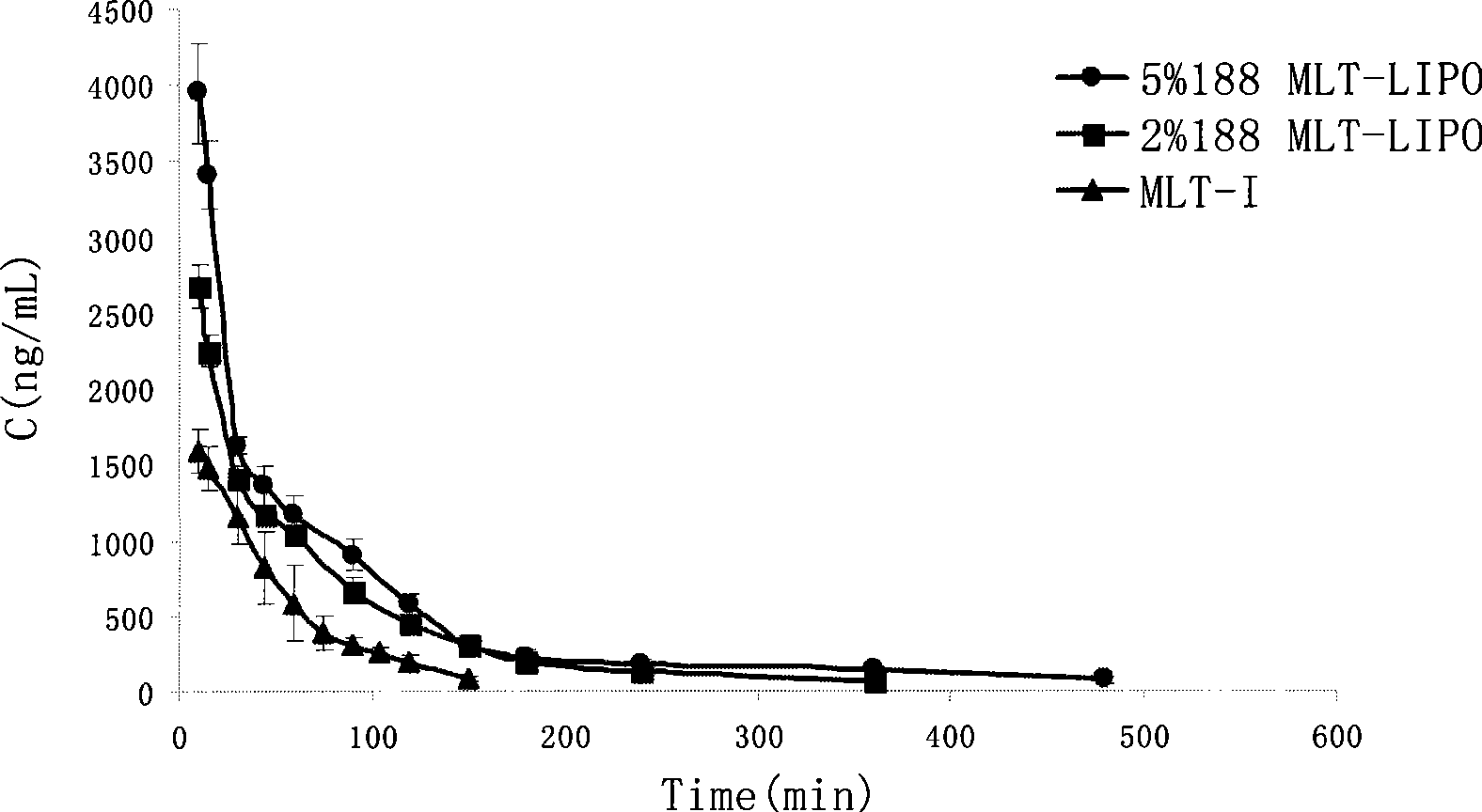
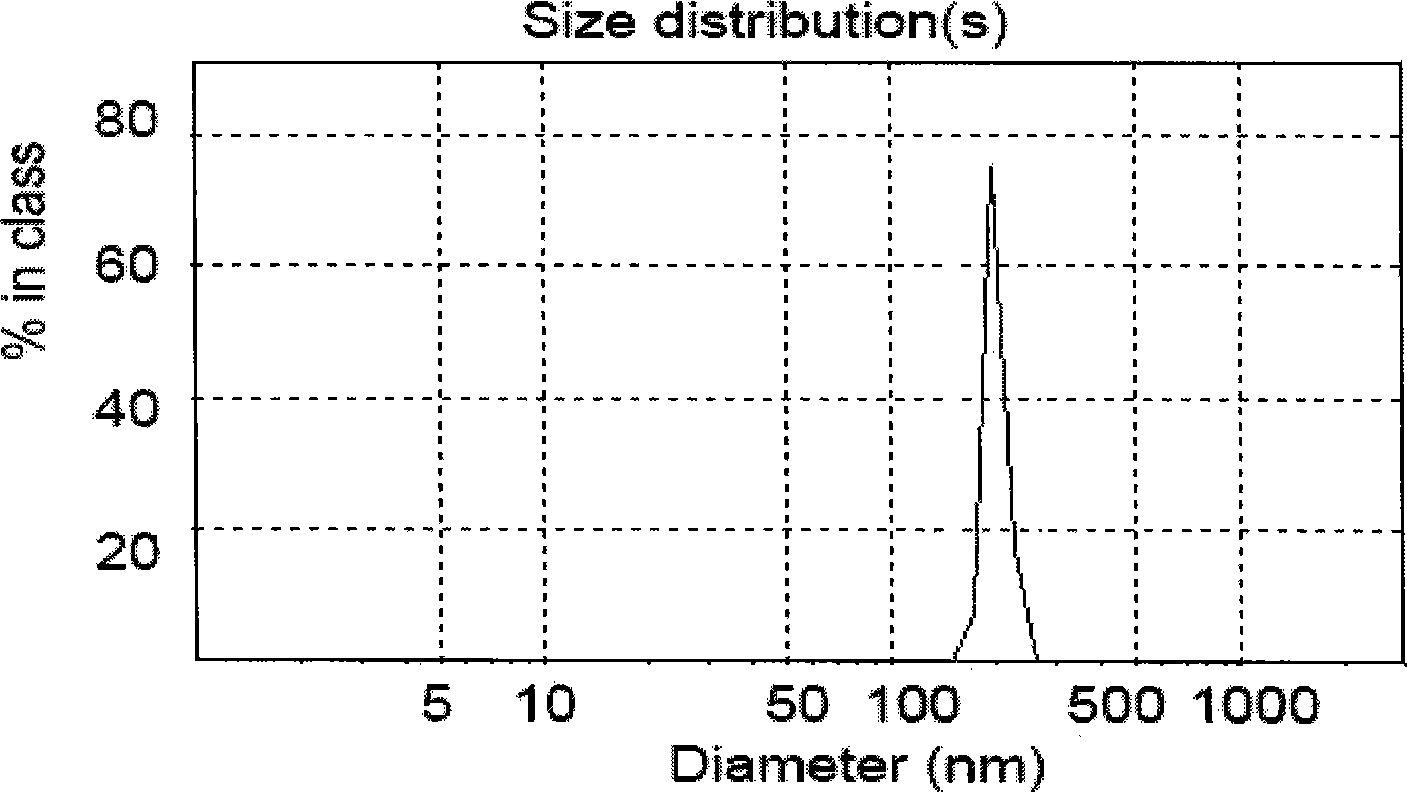
Patents
Literature
402results about How to "Easy to industrialize mass production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gemcitabine hydrochloride lyophilized powder injection
ActiveCN101564381AImprove stabilityLow content of related substancesPowder deliveryOrganic active ingredientsPancreas CarcinomaDrug
The invention relates to a gemcitabine hydrochloride lyophilized powder injection and a preparation method thereof. The gemcitabine hydrochloride lyophilized powder injection prepared by the method can be used as a therapeutic medicament for treating middle and late non-small cell lung cancer, pancreatic cancer and the like. The gemcitabine hydrochloride lyophilized powder injection is characterized by consisting of gemcitabine hydrochloride, mannitol and sodium acetate, wherein the weight ratio of the gemcitabine hydrochloride to the mannitol is 1:0.5-5, and the weight ratio of the gemcitabine hydrochloride to the sodium acetate is 1:0.01-0.1. The preparation method comprises the following steps: taking the mannitol and the sodium acetate; dissolving the mannitol and the sodium acetate by adding injection water; adding the gemcitabine hydrochloride to the mixture, stirring and dissolving the mixture, and adjusting the pH to between 2.7 and 3.3; fixing the volume; filtering the product by a 0.22 mu m microporous membrane; filling, dishing up, lyophilizing, and compressing; taking the product out of a box, and tying the product with an aluminum-plastic composite cover; and inspecting the quality, and packaging the product after passing the quality inspection to obtain the gemcitabine hydrochloride lyophilized powder injection.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Natural dye mordanting dyeing or mordanting printing product and preparation method thereof
ActiveCN105113290AHigh color fastnessUniform base colorFibre treatmentDyeing processMordantNatural dye
The invention provides a method for preparing a natural dye mordanting dyeing or mordanting printing product. The preparation method comprises the steps of performing pretreatment on a fabric, using cation modified working solution to perform cation modification on the fabric, and obtaining cation-modified fabric; using natural dye to dye the cation-modified fabric and obtaining a natural dye dyeing fabric; using a mordant working solution or mordant working solution foam to perform mordanting dyeing or mordanting printing on the natural dye dyeing fabric at a predetermined area, performing steaming or baking at 80-180 DEG C for 10 s to 25 min, performing washing, softening and shaping, and obtaining the natural dye mordanting dyeing or mordanting printing product. The invention further provides the natural dye mordanting dyeing or mordanting printing product obtained according to the preparation method. The natural dye mordanting dyeing or mordanting printing product obtained according to the preparation method is good in color fastness and uniform in base color, the printing part is dark in color, the material loss is less, and a fabric is softer.
Owner:GUANGDONG ESQUEL TEXTILES CO LTD
Apitoxin liposome preparation and preparation method thereof
InactiveCN101391098AReduce releaseImprove bioavailabilityPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectPhospholipid
The invention relates to the field of medicine preparation, in particular to a melittin lipidosome preparation. The lipidosome preparation is composed of one part of melittin, 5-40 parts of phospholipid, 1.3-10 parts of cholesterin and 10-140 parts of poloxamer. The invention also discloses the preparing method of the melittin lipidosome preparation. The melittin lipidosome preparation not only can delay the medicine release in the lipidosome, prong the circulating time in the blood and improve the bioavailability of the medicine, but also can obviously reduce the side effect of the medicine and improve the adaptability of a patient.
Owner:安徽省百春制药有限公司 +1
Normal-temperature high-effective formaldehyde catalytic-oxidizing catalyst, preparation method, and application thereof
InactiveCN107096527ASimple preparation stepsShort cycleGas treatmentDispersed particle separationCatalytic oxidationManganese oxide
A high-effective catalyst used for removing indoor formaldehyde at normal temperature includes a carrier, a noble active component, and an additive. The carrier is a mesoporous oxide including at least one of Al2O3, TiO2, SiO2, cerium oxide, cobalt oxide and manganese oxide; the active component includes a less amount of at least one of noble metals Pd, Pt, Au and Ag; the additive is at least one of Na, K, Fe, Co, Ce and Ni. The catalyst can completely convert the formaldehyde into CO2 and H2O at normal temperature under relative humidity of 50-90%. The catalyst has high catalytic-oxidizing activity and strong anti-moisture capability and is suitable for removal of the formaldehyde in a sealed or a semi-sealed space.
Owner:XI'AN PETROLEUM UNIVERSITY
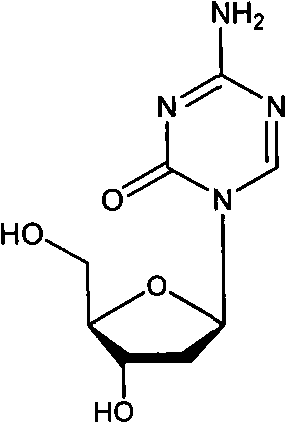
Decitabine freeze-dried powder injection
ActiveCN101584670AImprove stabilityLow content of related substancesOrganic active ingredientsPowder deliveryPhosphateFreeze-drying
The invention relates to a decitabine freeze-dried powder injection and a preparing method thereof. The prepared decitabine freeze-dried powder injection is used for treating myelodysplastic syndrome (MDS). The decitabine freeze-dried powder injection contains decitabine, utilizes the mixed solvent composed of the tert-butyl alcohol and the injection water in the preparation process, wherein the concentration of the decitabine in the mixed solvents is 2.5-5 mg / ml; and the volume ratio of the solvents is: 5-50% of tert-butyl alcohol and the balance of injection water. The potassium dihydrogen phosphate and the sodium hydroxide may be added for the pH regulator. The preparation process comprises the following steps: measuring tert-butyl alcohol, adding injection water, potassium dihydrogen phosphate and sodium hydroxide, stirring and mixing evenly, cooling to 2-15 DEG C, heat preserving, adding decitabine, stirring to dissolve, filtering, filling, plugging, disking, freeze-drying, pressing plug, out box, tying and packing after quality test qualification.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Pantoprazole sodium freeze-dried powder injection and preparation method thereof
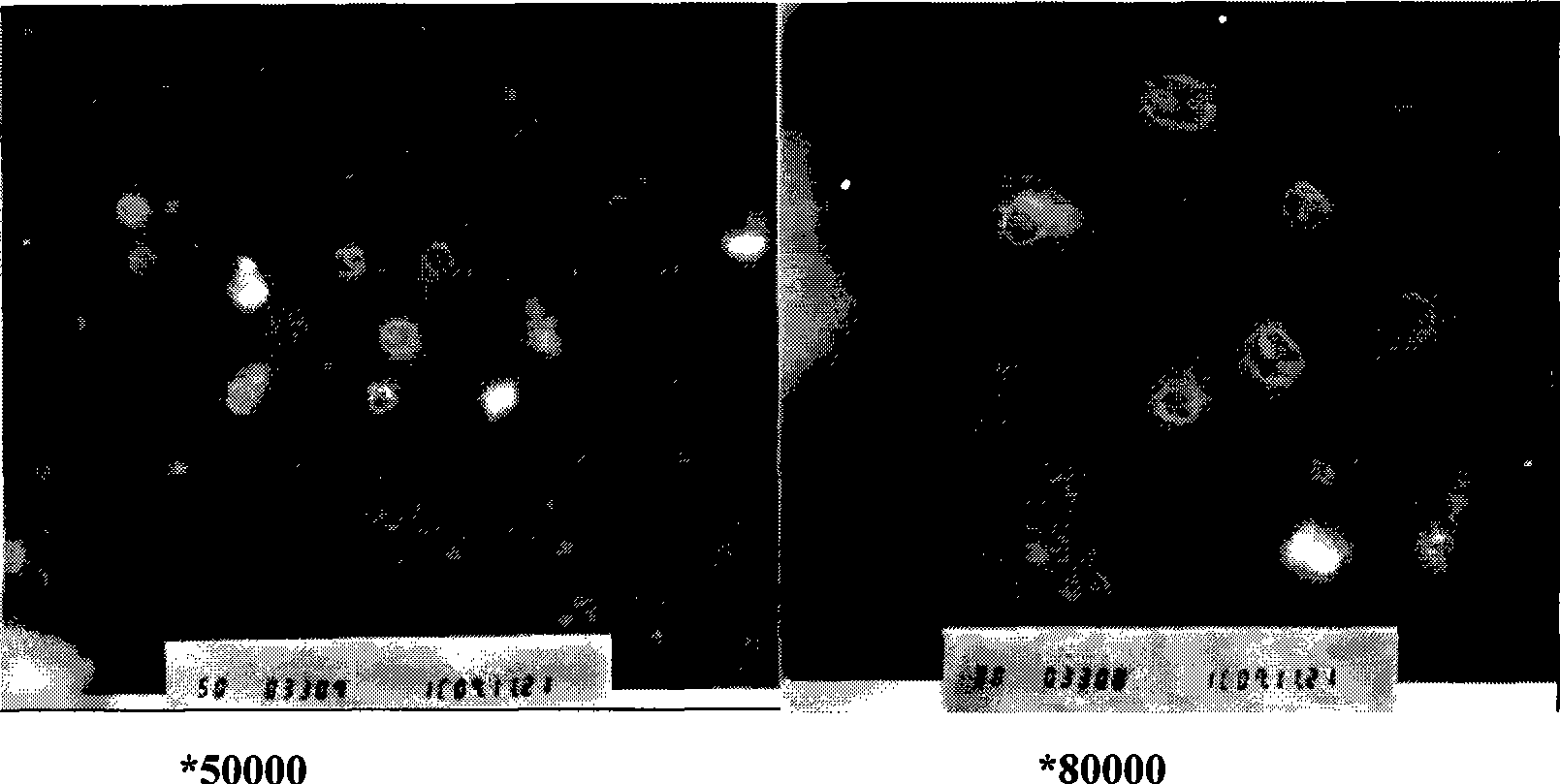
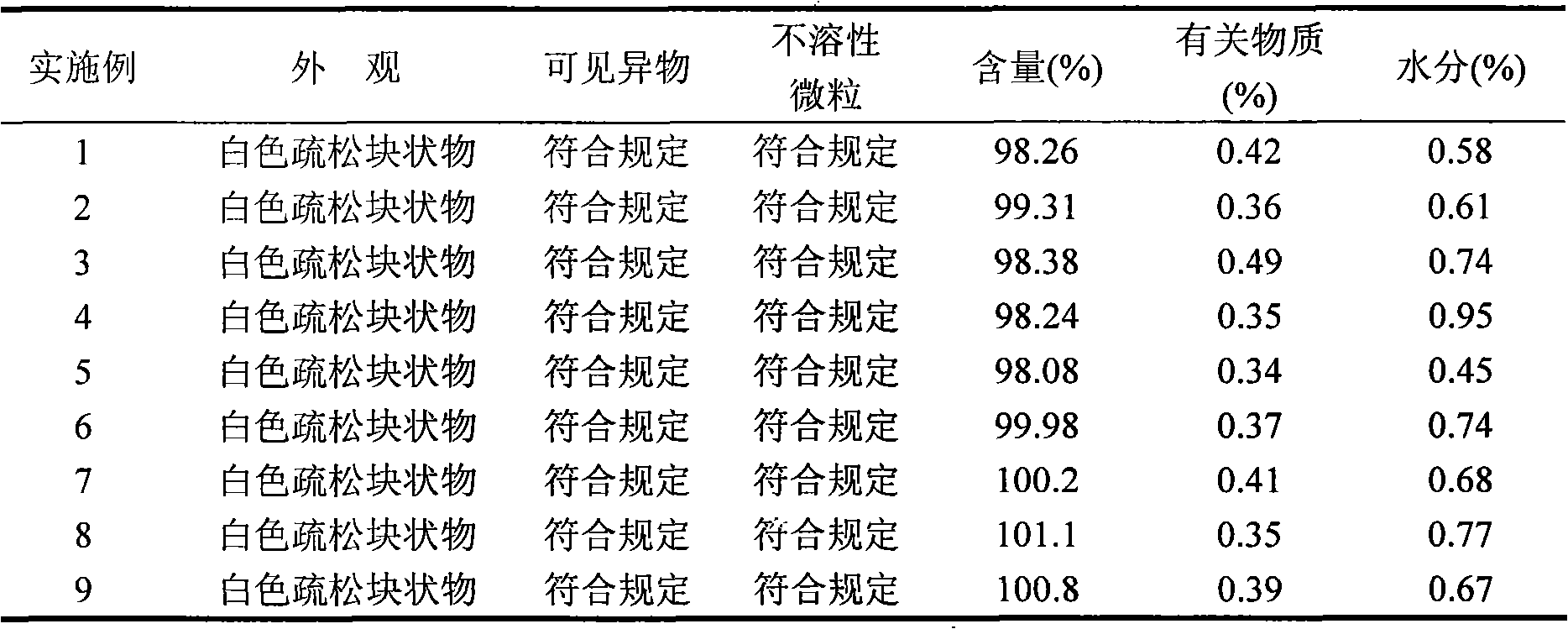
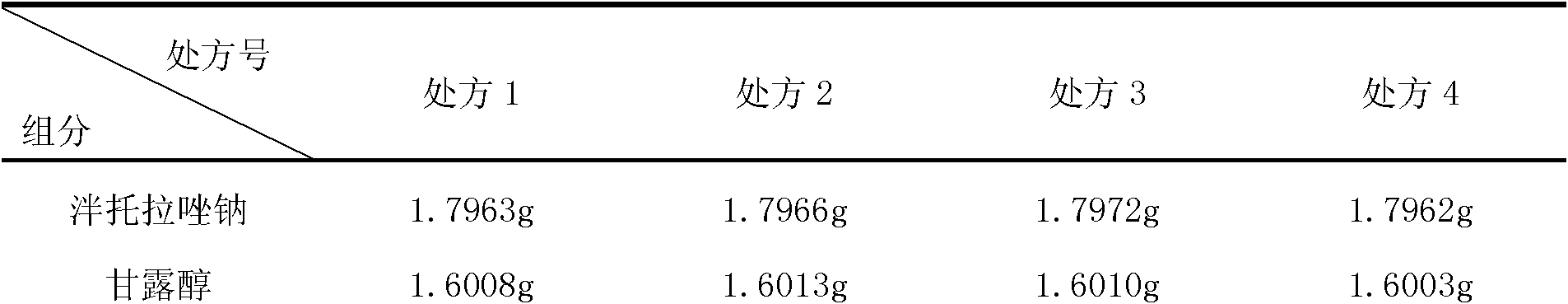
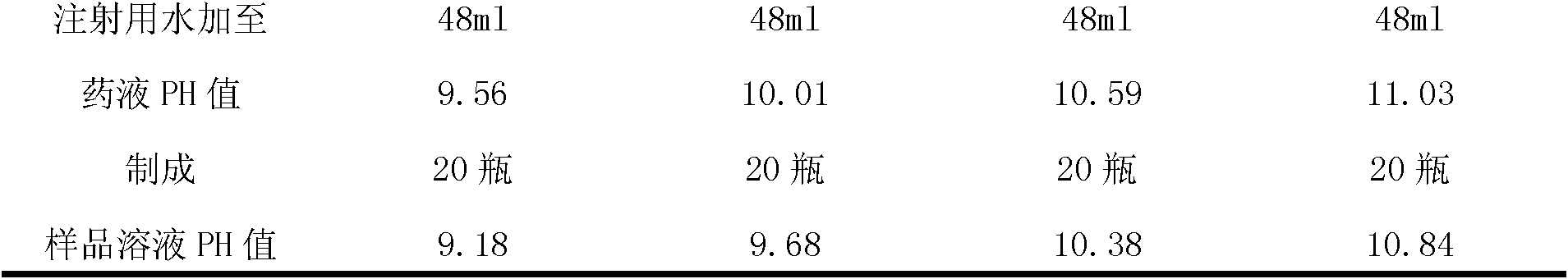
ActiveCN102085190AShorten the secondary drying timeGood lookingOrganic active ingredientsPowder deliveryCLARITYFreeze-drying
The invention relates to a pantoprazole sodium freeze-dried powder injection and a preparation method thereof. The powder injection is prepared from pantoprazole sodium and mannitol, wherein the consumption ratio of the pantoprazole sodium to the mannitol is (1:0.8)-(1:1.6), and the PH value is 10.5-11.0. In the invention, by lowering the pre-freezing temperature, properly lowering the freezing temperature, maintaining the lowered freezing temperature for a proper time, properly shortening two-stage drying time and carrying out other adjustment processes, good appearance and quality of the product can be kept under the condition that the content of the mannitol is low, the processes are reliable and feasible, and the effect is obvious. The prepared product has low content of related substances and has controllable quality, and the freeze-dried product has good clarity and formability after being redissolved.
Owner:HAINAN JINRUI PHARMA CO LTD
Method for removing ginkgolic acid from ginkgo leaf extract by extraction method
InactiveCN1470487ASave raw materialsSimple and fast operationOrganic compounds purification/separation/stabilisationSolid solvent extractionAlcoholOrganic solvent
The present invention discloses a method for removing ginkgolic acid from ginkgo leaf extract by orgnaic solvent extraction process, and said process mainly includes: extracting and drying. In which the refining procedure includes: adding methyl alcohol or ethyl alcohol to pulverized ginkgo leaf extract, stirring, dissolving, standing still, filtering, reduced pressure concentrating and drying; and its extracting procedure includes: stirring the pulverzed ginkgo leaf extract with ethyl alcohol or acetone, dissolving and placing said material into extract, adding paraffins material, uniformly mixing them, standing still and demixing, removing upper layer, repeatedly operating lower layer for several times, drying.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Antibacterial functional fibers
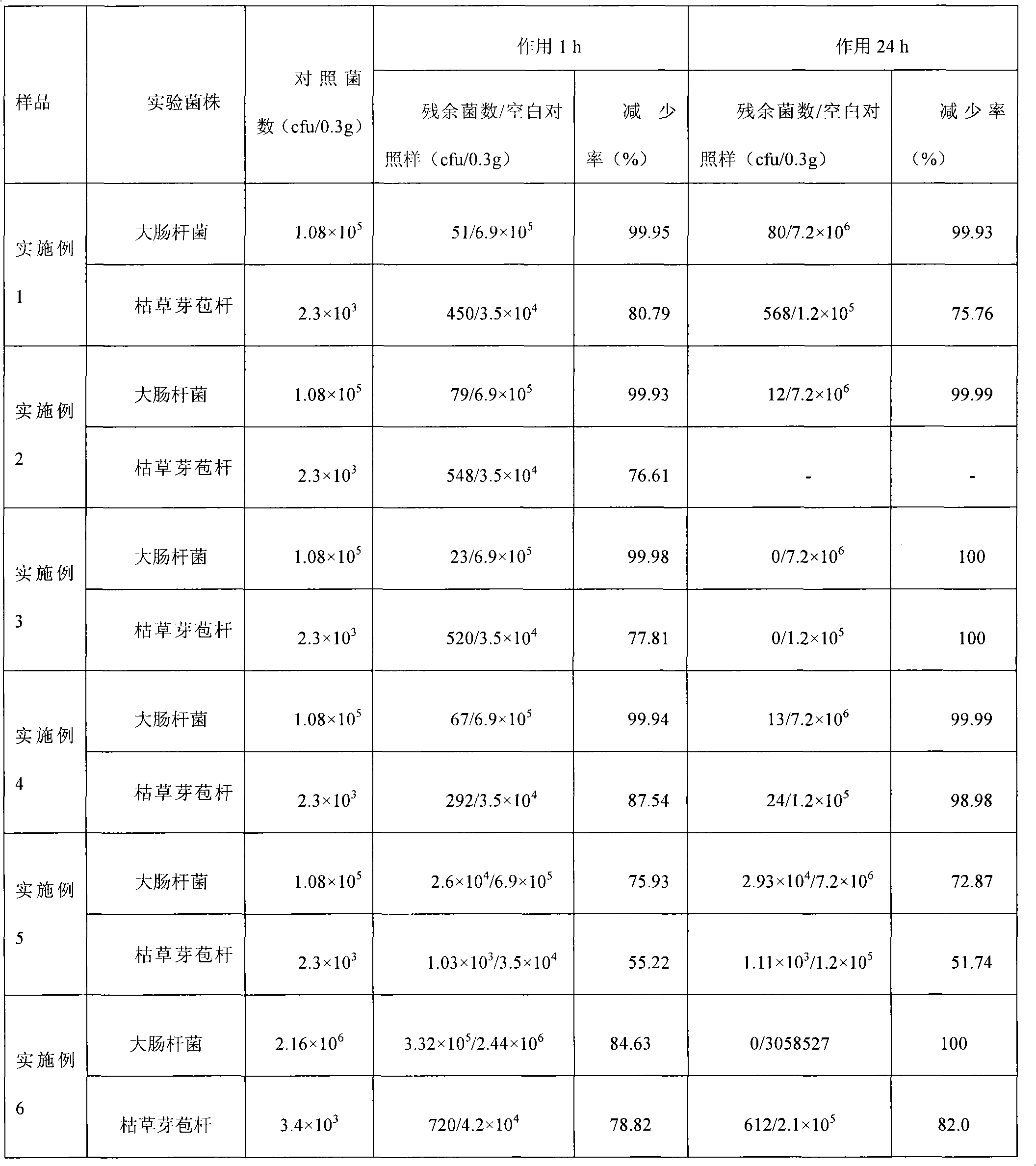
ActiveCN101812801ARich sourcesThe synthesis process is simpleFibre treatmentPolymer scienceNonwoven fabric
The invention relates to antibacterial functional fibers and belongs to the field of polymer material. In the invention, carrier fibers and aqueous solution of an inorganic metal antibacterial agent are mixed and stirred at a constant temperature of 0 to 100 DEG C for 10 minutes to 24 hours first, then the fibers are taken out and washed by deionized water till the pH value of the fibers is 6.5 to 7.5, and finally the fibers are dried at 40 to 60 DEG C to give the target product. In the invention, the raw materials are widely available, the synthesis process is simple, the synthesis process is easy to control, and industrial production is easy to realize. The fibers have a broad antibacterial spectrum and long-lasting antibacterial performance, is safe and reliable, and can be made into health-care underwear, insoles, operation clothes, nurse clothes, medical gauzes and the like. The fibers can also be made into nonwoven fabrics used for making filter elements of air purifiers for killing bacteria and germs in the air, and therefore have a promising application prospect.
Owner:718TH RES INST OF CHINA SHIPBUILDING INDAL CORP
Method for preparing herbicide
ActiveCN101602770AHigh yieldReduce yieldBiocideOrganic chemistryDistillationVapor phase chromatography
The invention relates to a method for preparing an herbicide, and provides a method for preparing a natural herbicide, which has the advantages of mild reaction conditions, simple process, low cost and easy industrialized mass production. The method comprises the following steps of: dissolving alpha-terpineol insolvent, and adding alkali, metallic oxides and peroxides; stopping the reaction when the gas chromatographic analysis shows that the content of the alpha-terpineol is less than 0.5 percent; filtering and recovering the metallic oxides; distilling filtrate distillation to recover solvent, obtaining 1,2-epoxide; dissolving the 1,2-epoxide in the solvent, and adding acid; stopping the reaction when the gas chromatographic analysis shows that the content of the 1,2-epoxide is less than 2 percent; distilling reaction solution to recover solvent, obtaining an isomerized product of the 1,2-epoxide; dissolving the isomerized product of the 1,2-epoxide in the reaction solvent, adding alkali for stirring, and adding 2-methyl benzylchloride; stopping the reaction when the gas chromatographic analysis shows that the content of the isomerized product is less than 2 percent; pouring the reaction solution in water, adjusting the pH value of the mixed solution to neutral, and separating out organic phases; and obtaining the finished product by the water phase extraction, the combination of the organic phases, and distillation and recovery of the solvent.
Owner:云南森美达生物科技股份有限公司
Puerarin nanocrystalline medical composition and preparation method thereof
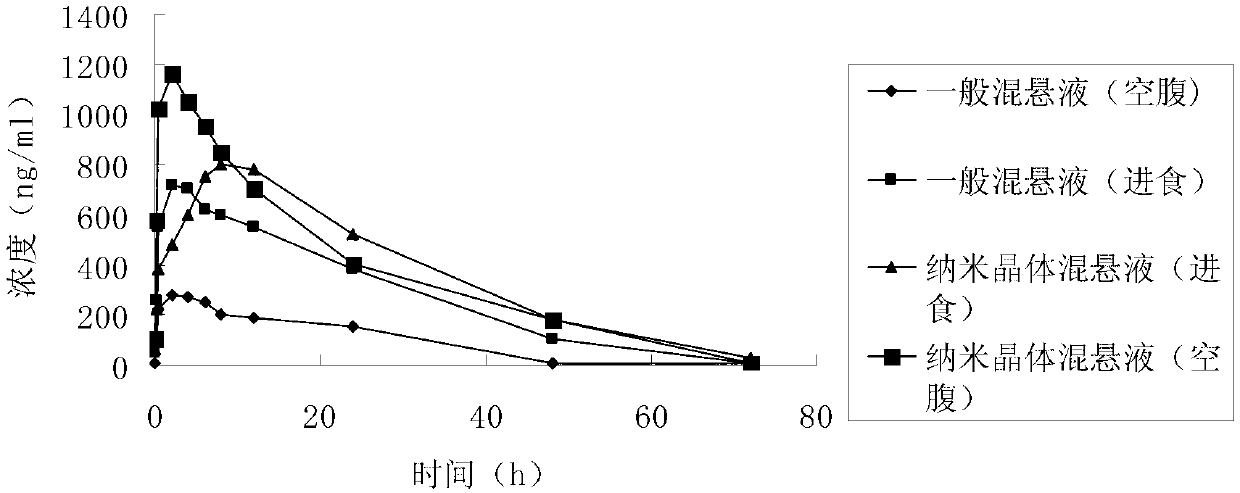
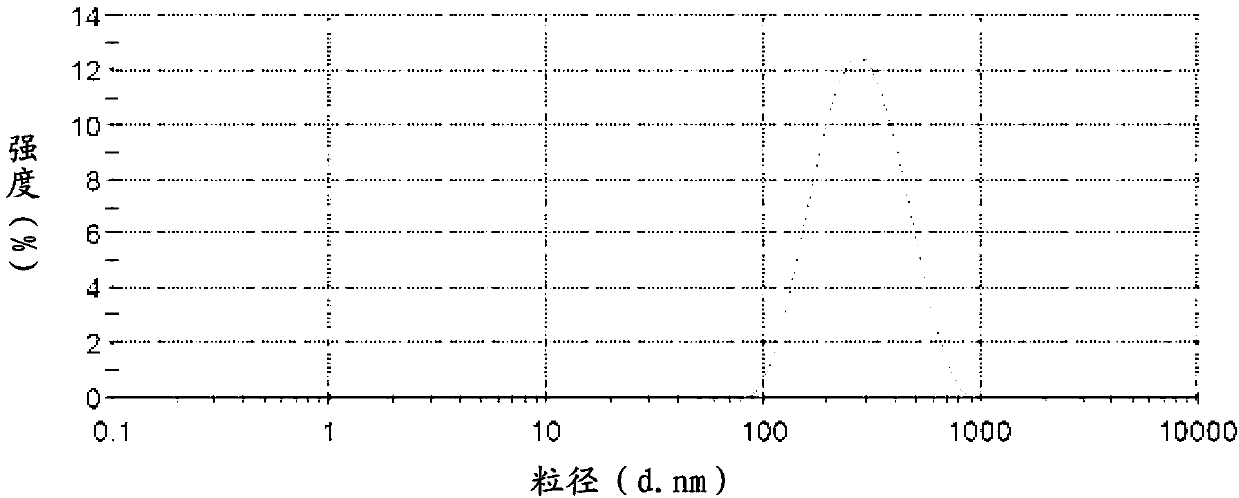
InactiveCN103211759AImprove stabilityImprove solubilityOrganic active ingredientsMetabolism disorderSolubilityFOOD EFFECT
The invention belongs to the technical field of medicines and relates to a puerarin nanocrystalline medical composition and a preparation method and application thereof. Specifically, the puerarin composition is a puerarin nanocrystalline composition. The puerarin composition provided by the invention remarkably improves the solubility and bioavailabilityof puerarin and reduces or eliminates the food effect of the medicine, and is good in stability and can be prepared into various common preparation forms easily, the stability and effect of the medicine are improved, so that the composition has good prospect.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
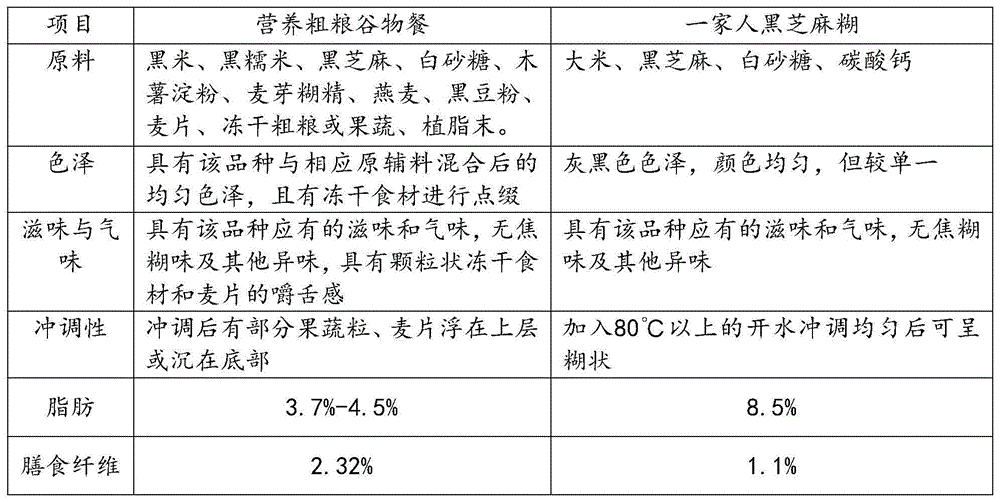
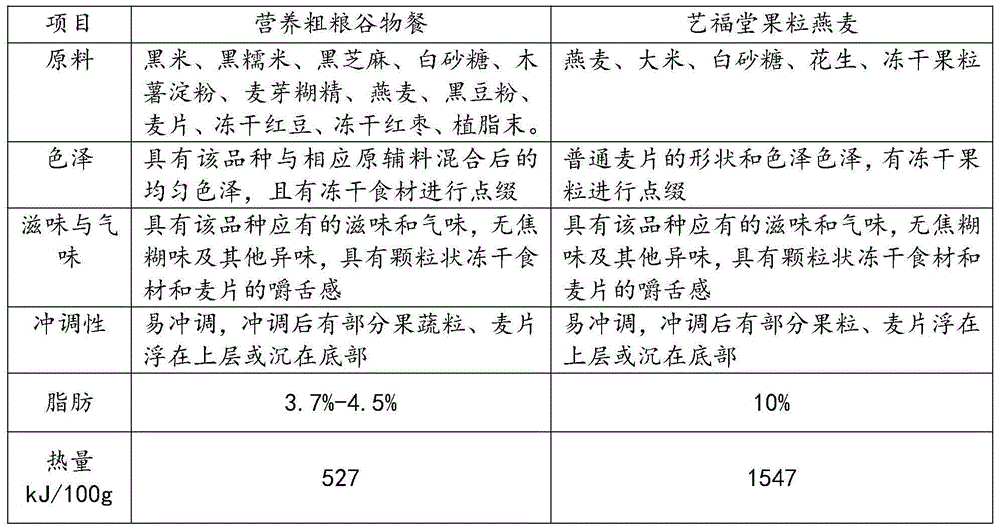
Nutritional whole-grain cereal meal and preparation method thereof
InactiveCN104642933AIn line with the concept of healthy eatingIncrease heatFood preparationFreeze-dryingSemen
The invention discloses a nutritional whole-grain cereal meal and a preparation method thereof, and belongs to the field of food processing. The nutritional whole-grain cereal meal is prepared from the following raw materials in parts by weight: 15-25 parts of black rice, 5-10 parts of semen sesami nigrum, 5-10 parts of oat, 1-5 parts of black glutinous rice, 1-5 parts of black bean flour, 1-5 parts of oatmeal, 1-2 parts of purple sweet potatoes and purple Chinese yam or 1-2 parts of freeze-drying raw materials. The preparation method comprises the following working procedures of frying the semen sesami nigrum, puffing the black rice and the black glutinous rice, crushing raw materials, mixing and the like. The nutritional whole-grain cereal meal has the characteristics of low content of fat, high content of proteins, dietary fibers and vitamins, relatively reasonable nutrition match, relatively convenience in preparation, and rich mouthfeel, can effectively remedy the problems of poor nutrition replenishment, low proteins, inconvenience in eating, poor mouthfeel and the like in existing foods (especially the food for breakfast), so that the dietary structure for the breakfast becomes reasonable.
Owner:GUANGXI HEIWULEI FOOD GRP
Ternary positive electrode material, preparation method thereof and lithium ion battery
ActiveCN111200120AReduce the amount of residual alkaliImprove cycle performanceCell electrodesSecondary cellsPhysical chemistryLithium-ion battery
The invention provides a ternary positive electrode material, a preparation method thereof and a lithium ion battery. The ternary positive electrode material is mainly composed of a high-nickel ternary material core and a cobalt borate coating layer. The preparation method comprises the steps of 1) mixing a boron source and a cobalt source, and sintering in a protective atmosphere to obtain cobaltborate; and 2) mixing cobalt borate with the high-nickel ternary material, and heating in an oxidizing atmosphere to obtain the ternary positive electrode material. According to the ternary positiveelectrode material provided by the invention, the high-nickel ternary material core, the cobalt borate coating layer and the lithium cobalt borate positioned between the high-nickel ternary material core and the cobalt borate coating layer are matched with one another, so that the ternary positive electrode material is low in residual alkali content and good in rate discharge capacity and cycle performance. The preparation method provided by the invention is short in process, simple to operate and easy for industrial large-scale production.
Owner:SHENZHEN CITY BATTERY NANOMETER TECH
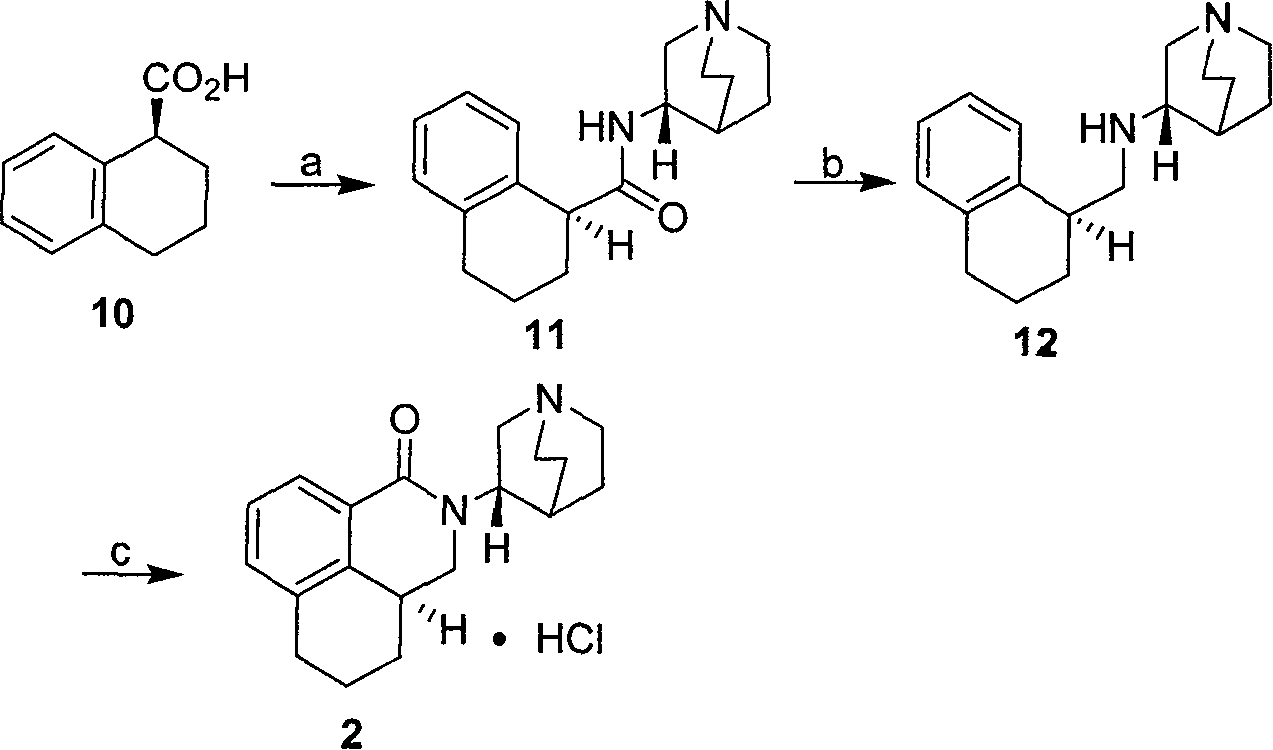
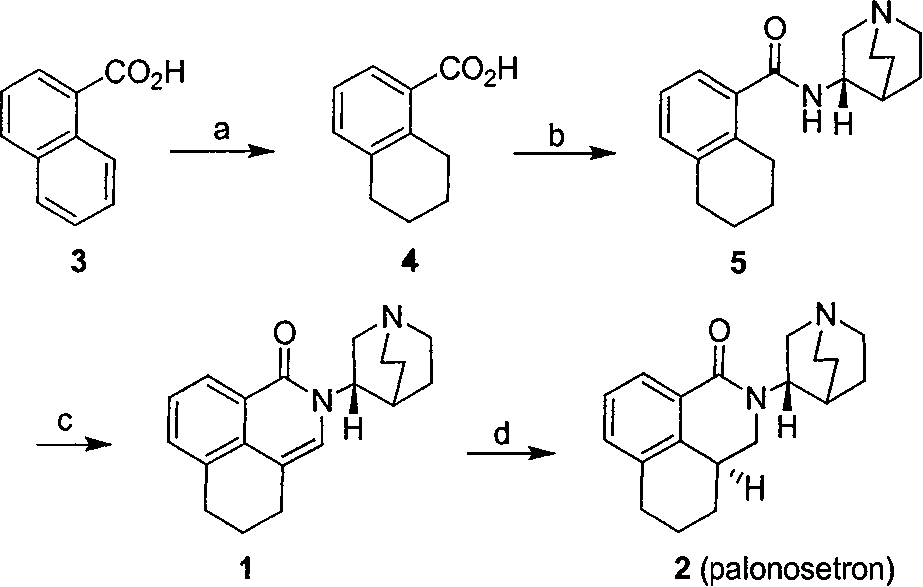
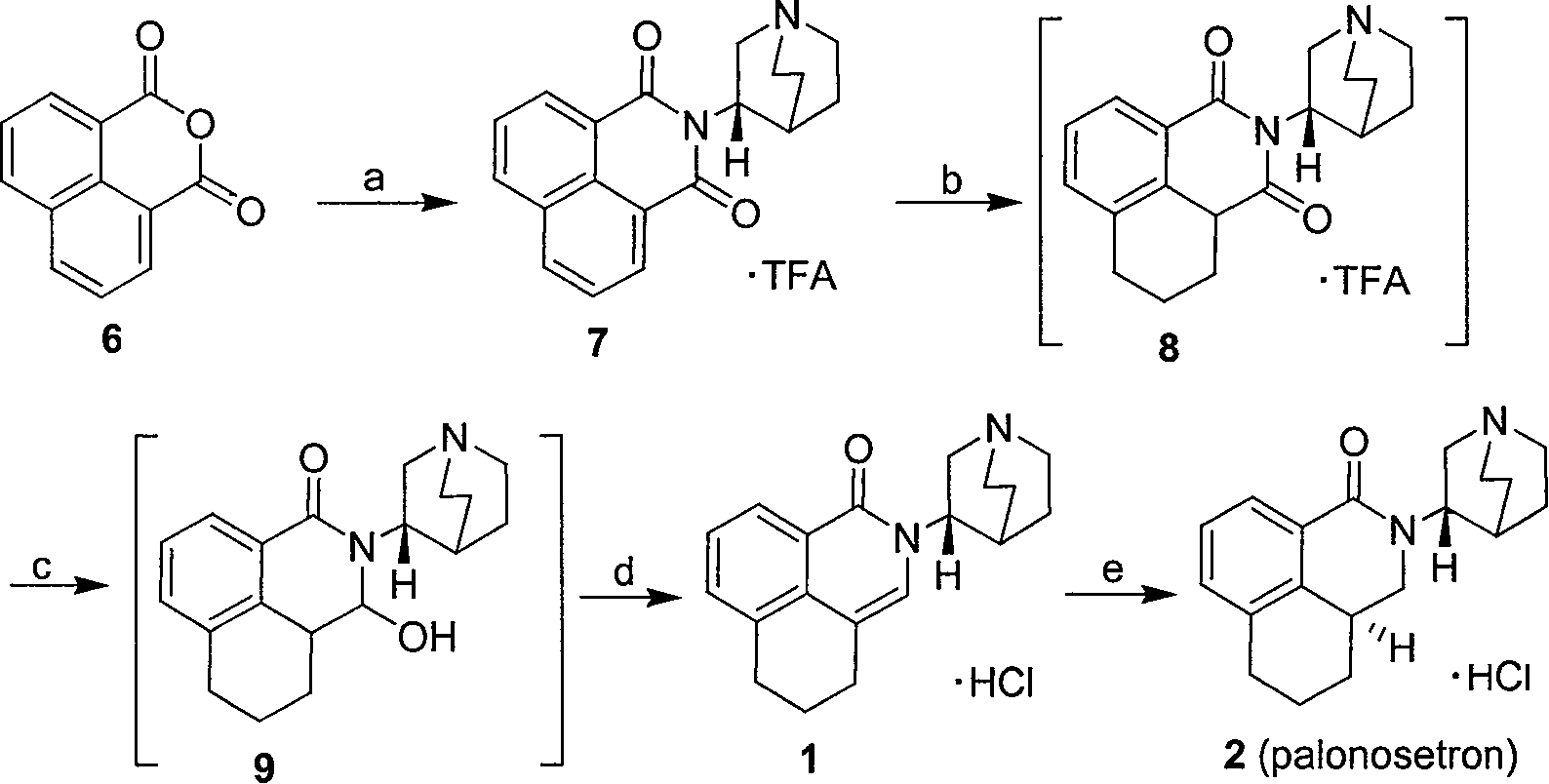
Method for synthesizing palonosetron hydrochloride
The invention discloses a novel synthesis method of palonosetron hydrochloride, which comprises that (1) (S)-tetralin formic acid is reacted with thionyl chloride and (S)-3-amido-quinine cyclic amine, to obtain (S, S)-quinuclidine tetralin formamide, (2), (S, S)-quinuclidine tetralin formamide is reacted with reductant and boron trifluoride diethyl etherate, to obtain (S, S)-tetralin methyl quinine cyclic amine, (3), (S, S)-tetralin methyl quinine cyclic amine is reacted with diphosgene to be added and reacted in boron trifluoride diethyl etherate solution, while the product is added and reacted with alcaine and water, to obtain palonosetron hydrochloride. And the synthesis route is represented as above: a: SOCI2, (S)-3-aminoquinuclidine, b: NaBH4, BF3OEt2, c: BF3OEt2, CICO2CCI3.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Dry garment leather base and preparation process thereof
InactiveCN106118034ASensualImprove tensile propertiesGrip property fibresProcess engineeringMaterials science
The invention provides a dry garment leather base and a preparation process thereof. The preparation process includes the steps: 1) base cloth treatment: completely soaking base cloth in mixed solution of soluble calcium salt and leather softeners, mangling the base cloth and then ironing and drying the base cloth by an ironing roller; 2) coating the base cloth with 200-500g / m<2> of garment leather base foaming slurry by a scraping method; 3) drying the base cloth in a drying oven; 4) base rolling: cooling and rolling the base cloth by a cooling roller to prepare the dry garment leather base. The prepared dry garment leather base has good moisture and air permeability, is quite soft in hand feeling and good in rebound resilience and wear resistance, and can meet higher performance requirements and environmental protection standards.
Owner:HEFEI KETIAN WATERBORNE TECH CO LTD
Preparation method of lithium manganese iron phosphate positive electrode material
PendingCN113929073ARealize NanoizationAchieve primary particle sizeSecondary cellsPositive electrodesLithium iron phosphatePhysical chemistry
The invention discloses a method for preparing lithium manganese iron phosphate by a solid phase method. The preparation method comprises the following steps: weighing a certain amount of a manganese source and an iron source according to a molar ratio of 7:3, weighing a lithium source, a phosphorus source, a carbon source and a dopant according to a certain stoichiometric ratio, adding pure water, carrying out ball milling and sanding, controlling the sanding particle size D50 to be less than or equal to 300 nm, and carrying out spray drying to obtain brown precursor powder; and sintering the precursor under the protection of a nitrogen atmosphere, controlling the sintering temperature to be 600-700 DEG C, then performing crushing and screening, and removing iron to obtain the lithium manganese iron phosphate positive electrode material. The lithium manganese iron phosphate prepared by the method is simple in process and easy to control in process, compared with existing lithium iron phosphate and ternary materials, the lithium manganese iron phosphate is lower in cost and higher in voltage platform, and meanwhile, the obtained lithium manganese iron phosphate has good electrical performance and cycle performance.
Owner:HUBEI WANRUN NEW ENERGY TECH DEV
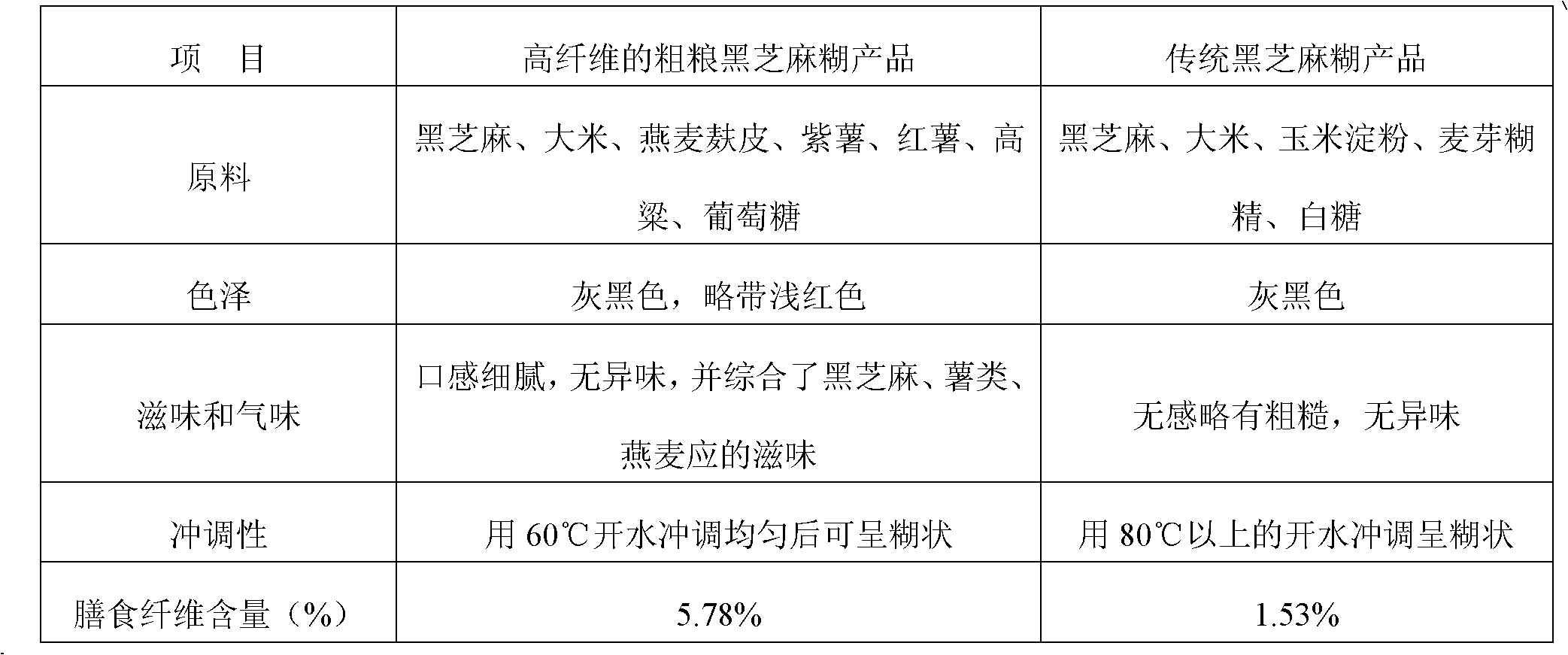
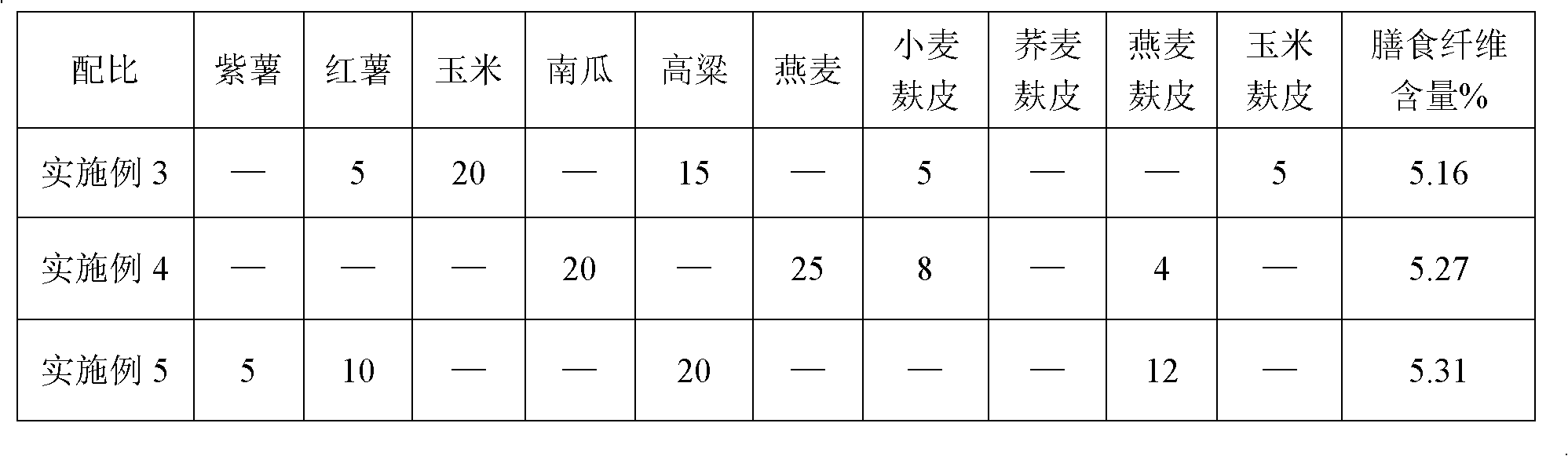
High-fiber coarse food grain black sesame paste and preparation method thereof
ActiveCN102210466AIn line with the concept of healthy eatingHigh nutritional valueFood preparationFiberGranularity
The invention provides high-fiber coarse food grain black sesame paste and a preparation method thereof. The black sesame paste consists of the following components in percentage by weight: 10 to 15 percent of black sesame, 25 to 35 percent of rice, 10 to 15 percent of bran superfine powder, and 35 to 45 percent of coarse food grains. The invention also provides a method for manufacturing high-fiber coarse food grain black sesame paste, which comprises the following steps: frying and finely grinding black sesame; crushing and puffing the rice and the coarse food grains after frying and finelygrinding to prepare flour; performing ultra-fine pulverization on puffed bran to obtain bran ultra-fine flour (the granularity is required to be less than or equal to 50 micron meters); and mixing the raw materials according to a weight ratio, wherein the dietary fiber content of the product is improved to over 5 percent. In the method, the black sesame paste is scientifically combined with high-fiber coarse food grains, the defects of overhigh heat and less dietary fiber content can be overcome, and the black sesame conforms to the healthy dietary concept of modern consumers; and associationof the raw materials ensures that the nutrition can be more complete, and the higher nutrition value is, and the functions of regulating intestines and stomach and preventing Three High (high blood pressure, high cholesterol, high blood sugar) can be realized after long term administration.
Owner:HUNAN RENRENJIA FOOD
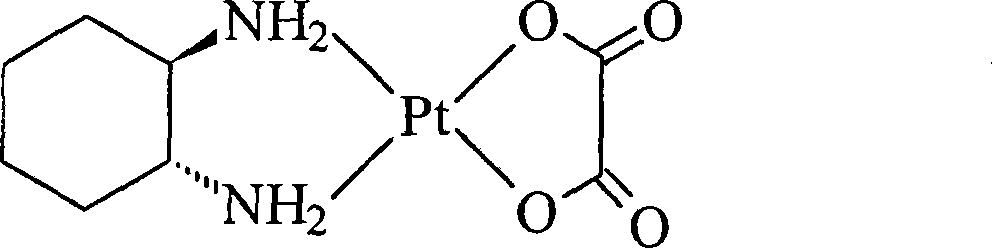
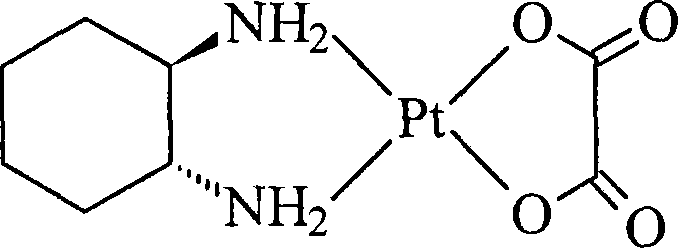
Oxaliplatin lyophilized powder injection and preparing method thereof
ActiveCN101199506AGood reproducibilityLess impuritiesPowder deliveryPharmaceutical non-active ingredientsCITRATE ESTERMANNITOL/SORBITOL
The invention relates to oxaliplatin freeze-dried injection, which is characterized in that the invention is prepared by the method that aqueous solution is freeze-dried. The aqueous solution contains oxaliplatin, mannitol and citrate, wherein, the concentration of the oxaliplatin in the aqueous solution is 2.5 to 6.25 mg / ml; the concentration of the mannitol in the aqueous solution is 25 to 200 mg / ml; and the concentration of the citrate in the aqueous solution is 2 to 20 mg / ml. And sodium citrate can also be added into the aqueous solution so as to adjust the pH of the aqueous solution. The preparation processes are as following: the oxaliplatin is placed inside the container, 80 percent amount of water for injection is added, and then the water for injection is mixed so that the oxaliplatin can be dissolved and mixed evenly in the water for injection; after that, the mannitol and the citrate are added into the water for injection, and then the water for injection is mixed so that the mannitol and the citrate can be dissolved and mixed evenly in the water for injection; the content of the intermediate is measured; if the content of the intermediate is qualified, the volume of the water for injection is fixed to full amount; in the aseptic condition, the water for injection is filtered until to be clear by a microporous membrane of 0.22 microns; the filtered solution is filled into an aseptic silin bottle; part of the aseptic silin bottle is plugged with a butyl rubber closure; and then the aseptic silin bottle is filled in the tray to be sent into the freeze dryer to be freeze-dried; the mouth of the aseptic silin bottle is rolled; the quality of the filtered solution is inspected; and the aseptic silin bottle is packaged.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Roxburgh rose vinegar beverage and preparation method thereof
InactiveCN102919938ARich types of processed productsEfficient use ofVinegar preparationFood scienceFlavorAlcohol
The invention discloses a roxburgh rose vinegar beverage and a preparation method thereof. The roxburgh rose vinegar beverage is a liquid beverage which is prepared by fermenting the following components in parts by weight: 10 to 50 parts of roxburgh rose dregs, 1 to 5 parts of roxburgh rose fruits and / or 4 to 8 parts of roxburgh rose juice. According to the preparation method, the roxburgh rose dregs are used as the main raw material, and a small amount of roxburgh rose fruits and / or roxburgh rose juice is used to prepare the roxburgh rose vinegar beverage; the preparation process mainly comprises the steps of alcohol fermentation, acetic fermentation, filtration, dilution and seasoning; and compared with a product of the prior art, the prepared roxburgh rose vinegar beverage is rich in nutrient components and genuine in flavor, keeps the unique flavor of roxburgh roses, is convenient and easy to produce, and is lower in cost and easier for industrial mass production. Moreover, the roxburgh rose vinegar beverage is prepared from the commonly discarded roxburgh rose dregs as the main raw material, thus having great significance for comprehensive development and full utilization of the roxburgh roses; and the utilization ratio of the roxburgh roses and the generated economic benefit are far higher than those of the prior art.
Owner:季晓燕 +1
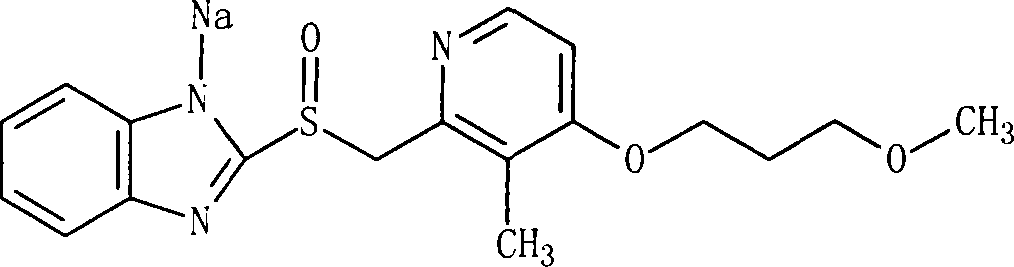
Sodium rebeprazole freeze-dried injection
InactiveCN101249078ASmall irritation to human bodyImprove securityPowder deliveryOrganic active ingredientsVialAdjuvant
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for producing low-temperature peanut oil
InactiveCN1470627AAvoid damageGuaranteed nutritional valueFatty-oils/fats productionOrganic solventChemistry
The production method of low temp. peanut oil includes the procedures of breaking, extracting crude oil and refinement, in concrete, said method adopts the following steps: adding broken peanut vernel into peanut oil, grinding to make pulp; grinding to 60-180 meshes and press-filtering at 30-70 deg.C to obtain oil pump, and making the oil content of peanut cake be reduced to 2-4% of obtain peanutcrude oil ,heating the crude oil to 50-80 deg.C and adding water whose adding quantity is 10-30% of oil weight, stirring, precipitating, taking supernatant oil, heating to 60-80 deg.C, dehydrating under the condition of the vacuum for 10-30 min. and filtering at 0.1-0.3 MPa so as to obtain the low-temp. peanut oil.
Owner:冀中能源邢台矿业集团有限责任公司
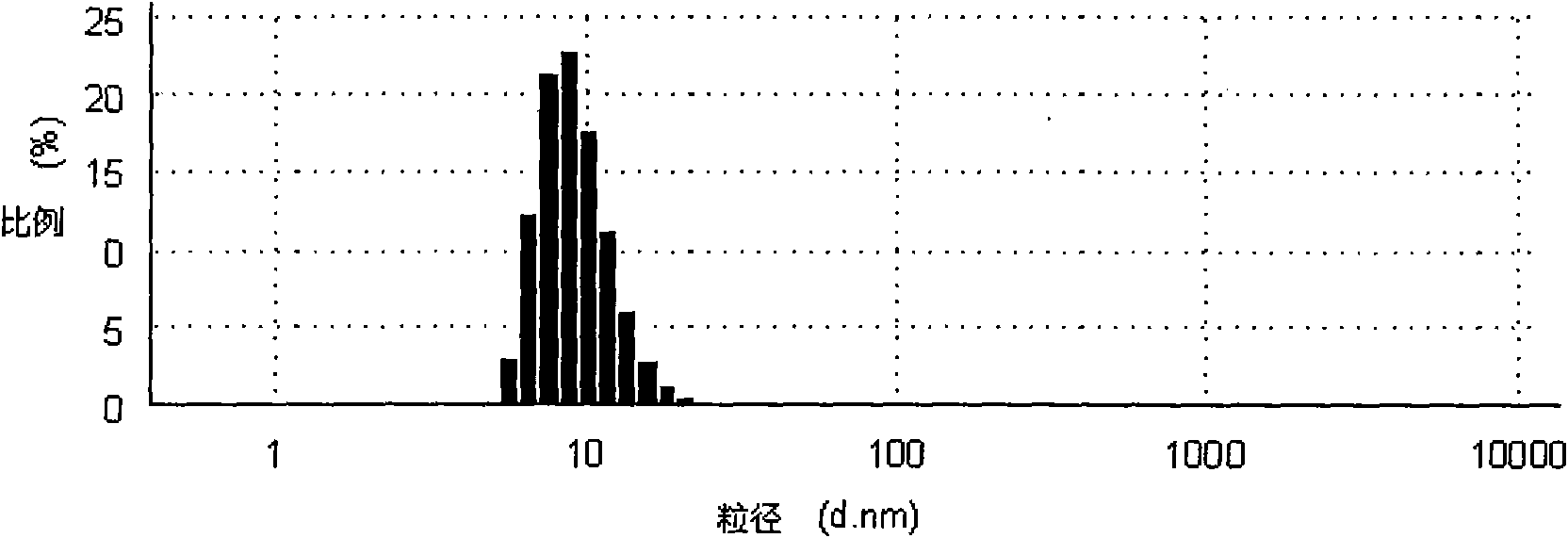
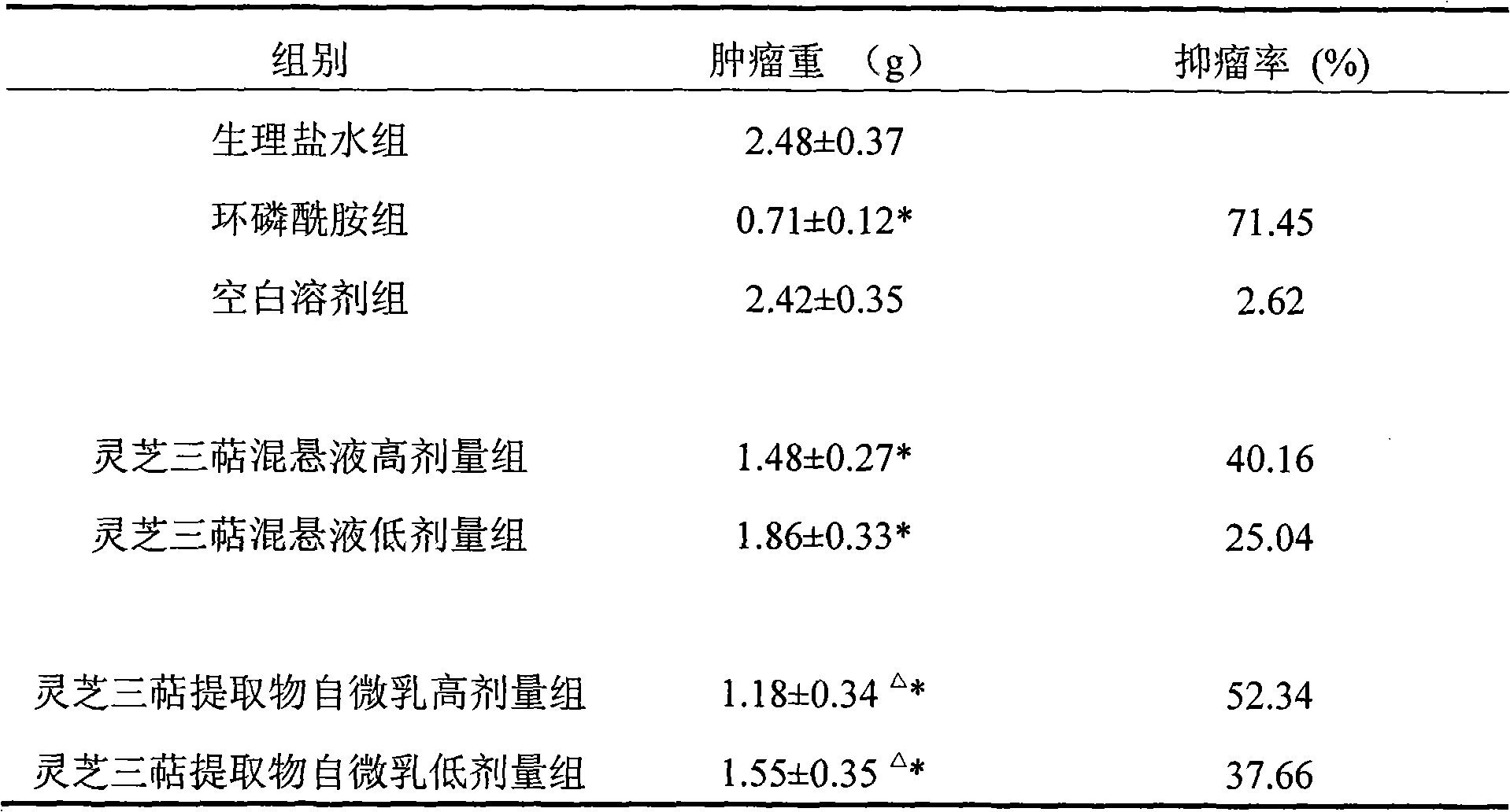
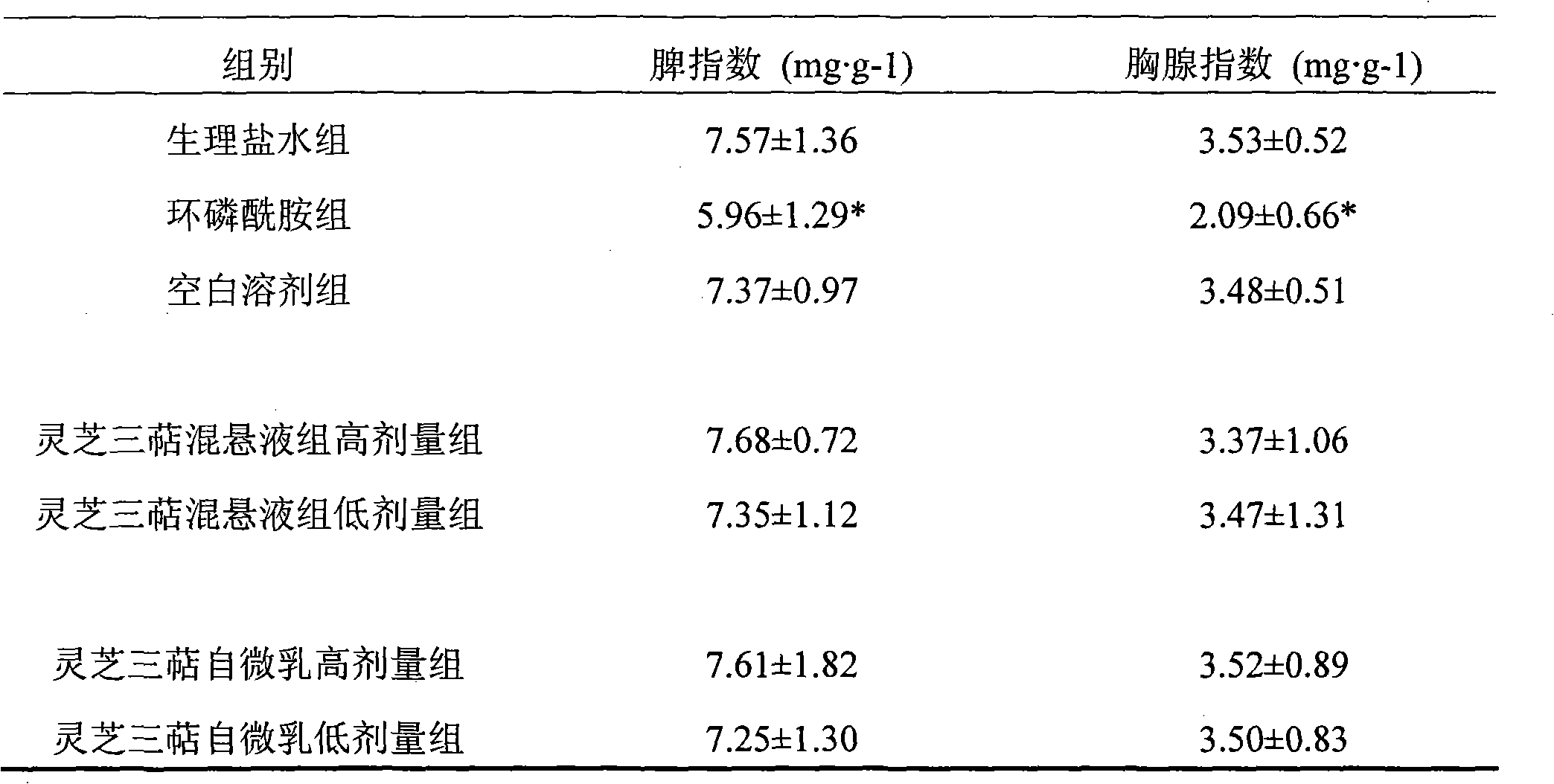
Self-microemulsion nanometer composition of ganodenic acid extract and preparation method thereof
ActiveCN101601695AEasy to prepareGood emulsifying performanceEmulsion deliveryAntineoplastic agentsDrug adsorptionSurface-active agents
The invention relates to a preparation method of a self-microemulsion nanometer composition of ganodenic acid extract; the self-microemulsion nanometer composition takes ganodenic acid extract as crude drug, and the mixture of surface active agent, cosurfactant, and oil phase is adopted as drug carrier; the preparation method is characterized in that: the components are as follows by weight part: 10 parts of ganodenic acid extracts, 50-400 parts of surface active agents, 100-800 parts of cosurfactants, and 15 to 600 parts of oil phase; the ganodenic acid extract is total triterpenoids in ganoderma lucidum which is obtained by extracting lucid ganoderma fruiting body with a supercritical CO2 extracting method. In the method, the total triterpenoids in ganoderma lucidum, which is an insolubility drug, is prepared into self-microemulsion formulation, so as to improve the dissolution rate of the drug, promote the drug adsorption and be beneficial to improving the bio-availability; in addition, the drug can keep a dissolution state in intestine so as to better to permeate through cell membranes dispersedly, thereby improving the drug effect of the drug and enhancing the in-vivo anti-tumor effect.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Method for preparing supported type nano Pt(Pt-M)/carrier catalyst
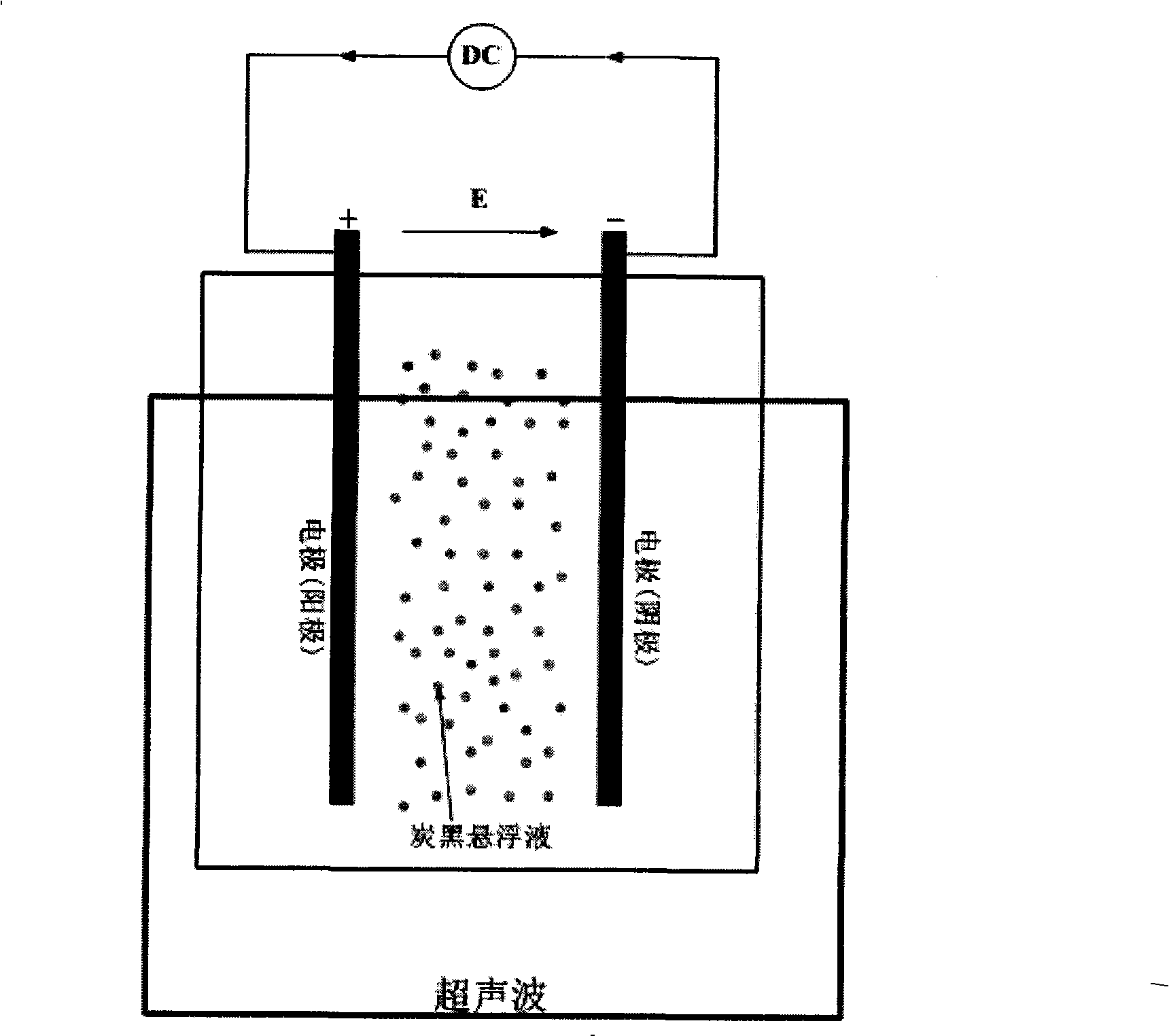
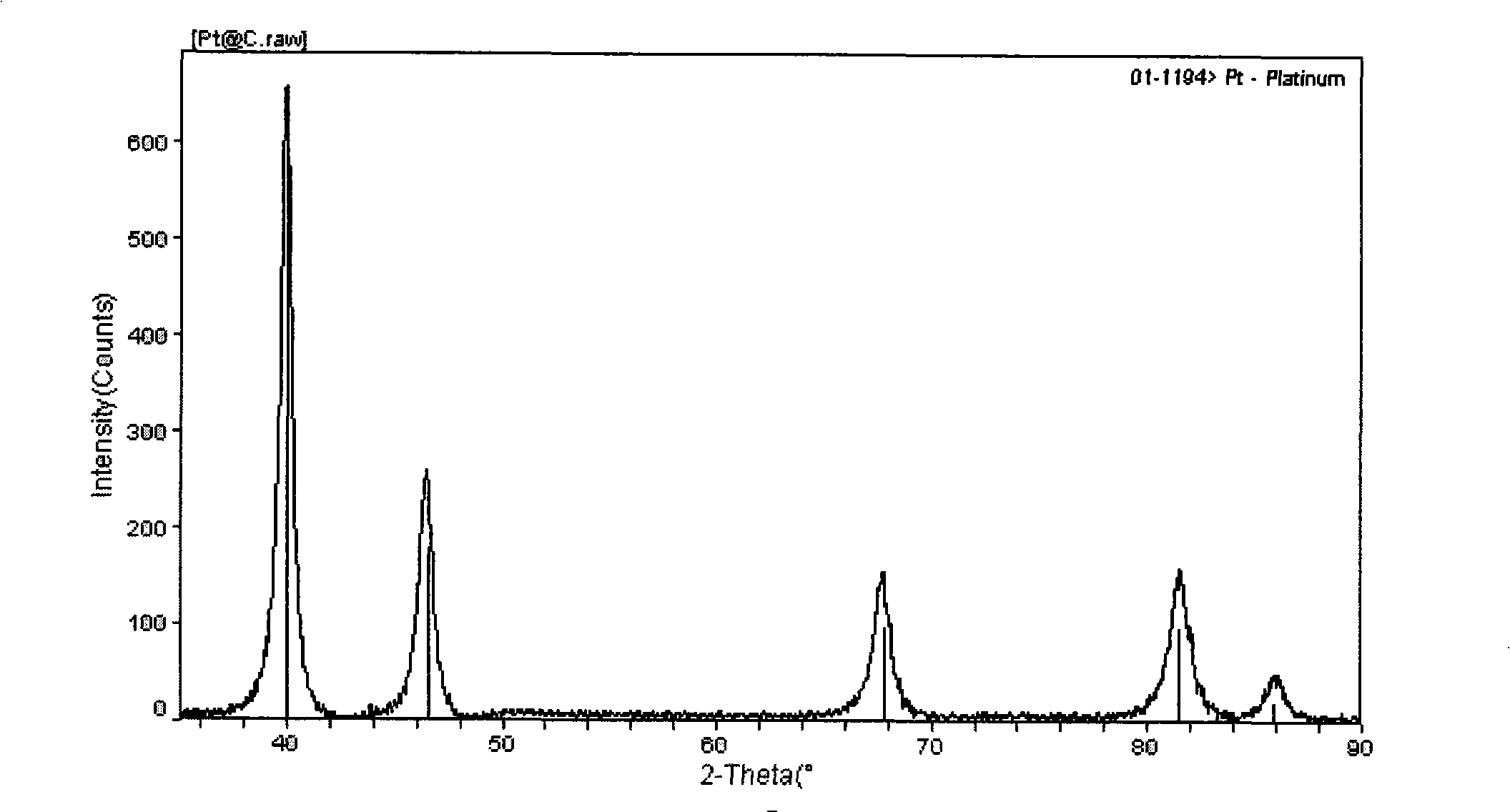
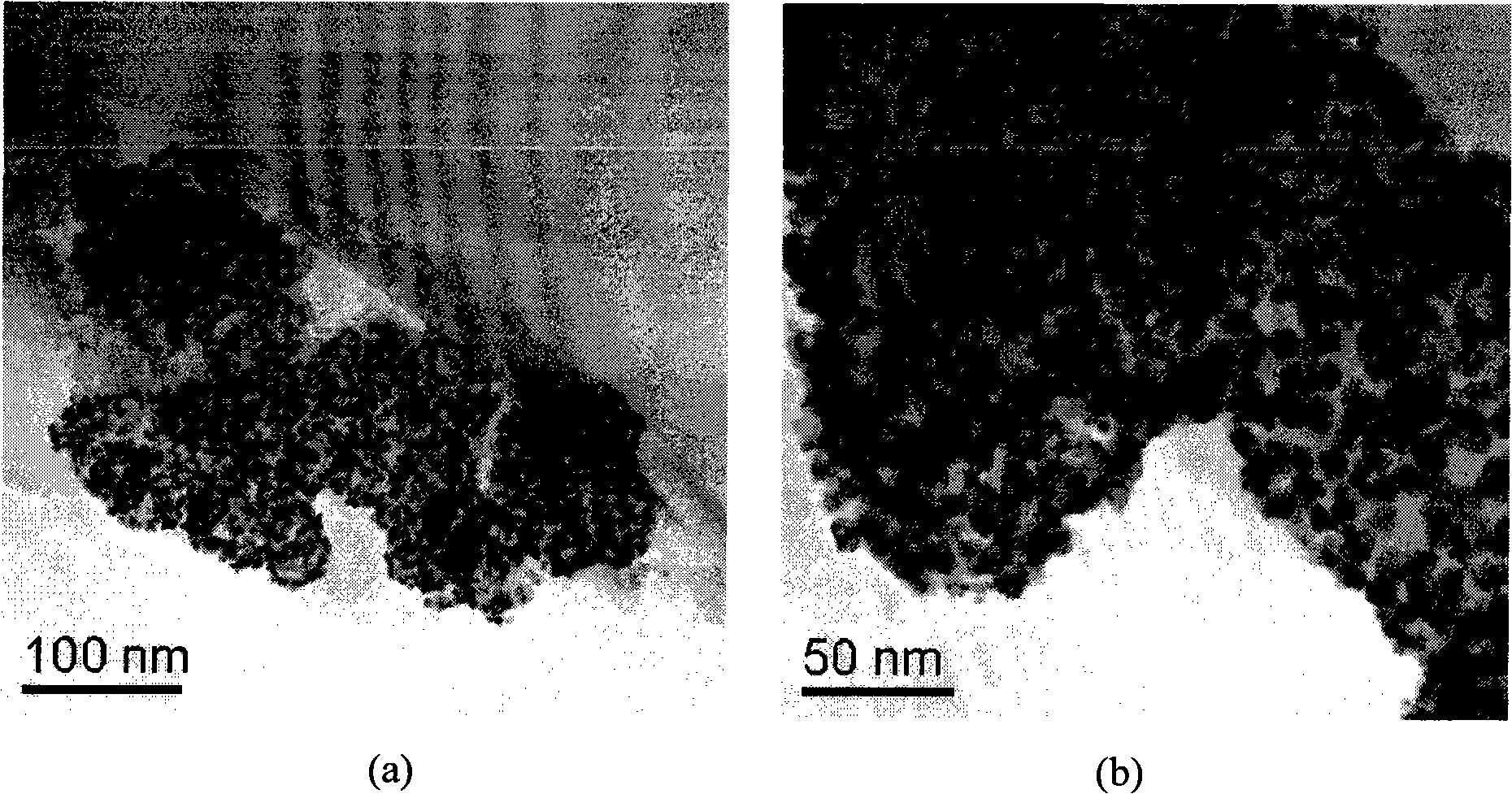
InactiveCN101352683AReduce manufacturing costLong electrode lifeCell electrodesMetal/metal-oxides/metal-hydroxide catalystsChemistryVolt-ampere
The invention relates to a preparation method of a load-typed nanometer Pt (Pt-M) / carrier catalyst, which comprises that a carrier is uniformly dispersed in a chloroplatinic acid solution to obtain an electrolyte which is then infused to a ultrasound-imposed double-platinum-electrode electrolysis bath for electrodeposition, so as to obtain a catalyst suspension of a nanometer Pt (Pt-M) catalyst which is uniformly loaded on the carrier and then separated to obtain the load-typed nanometer Pt (Pt-M) / carrier catalyst. The preparation method loads the nanometer Pt (Pt-M) on the carrier directly, thus avoiding the defects of high-temperature reduction or use of reducers for preparation of other methods; the preparation method does not require to conduct separate activation or modification treatment to the carrier and has the advantages of simple process and catalyst with adjustable size, low cost and no environmental pollution, thus being a universal method easy for industrialized massive production. By determining with cyclic volt-ampere curves, in terms of performance, 40 percent load-typed nanometer Pt / C catalyst prepared by the invention is superior to similar products produced by the worldwide advanced Johnson Matthey Company.
Owner:NANJING UNIV
Preparation method of high-purity hyodeoxycholic acid
The invention discloses a preparation method of high-purity hyodeoxycholic acid. The preparation method comprises the steps: weighing raw materials, adding strong alkaline and water, saponifying at the temperature of 95-100 DEG C for 16-24h, cooling to room temperature, standing, siphoning to remove a supernatant liquid, adding water into paste in the lower layer, stirring for dissolving the paste, adding strong acid to acidize until a congo red test paper turns to be blue, adding ethyl acetate, stirring for extracting for 20-50min, standing for layering, removing a water phase, adding water into the ethyl acetate to wash until the pH value of the water phase is equal to 6-7, adding the ethyl acetate into anhydrous sodium sulfate to dehydrate, carrying out activated carbon decoloration, filtering, concentrating until precipitates are separated out, cooling, filtering or squeezing and drying in vacuum; adding an alcohol solvent, stirring for dissolving the above product, slowly adding an alkaline organic solvent, stirring, cooling, separating out a great number of precipitates, filtering and drying; and adding water and sodium hydroxide, stirring for dissolving, dropwise adding hydrochloric acid with a volume concentration of 1:1, stirring, filtering and drying to obtain the hyodeoxycholic acid. The invention aims to provide the preparation method of the hyodeoxycholic acid with simple process, high yield and high purity.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
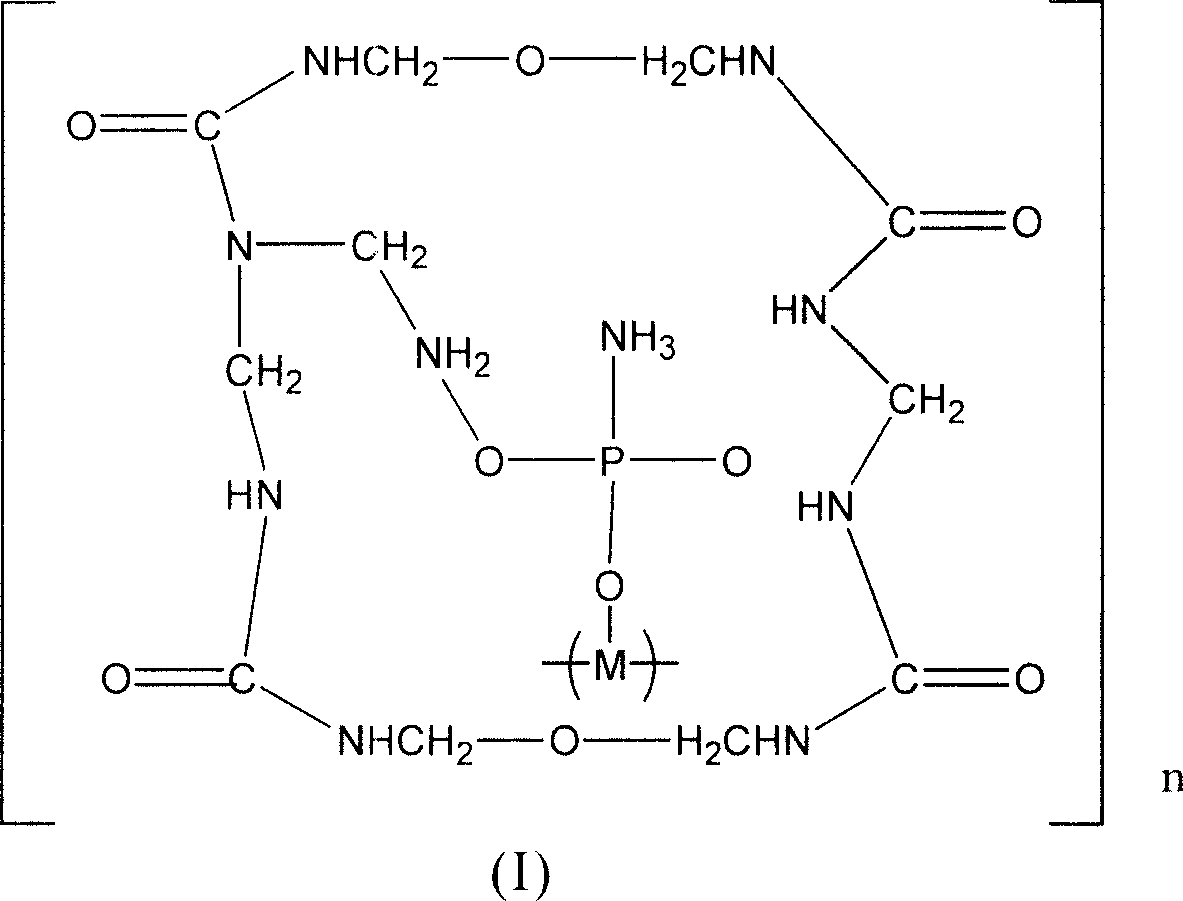
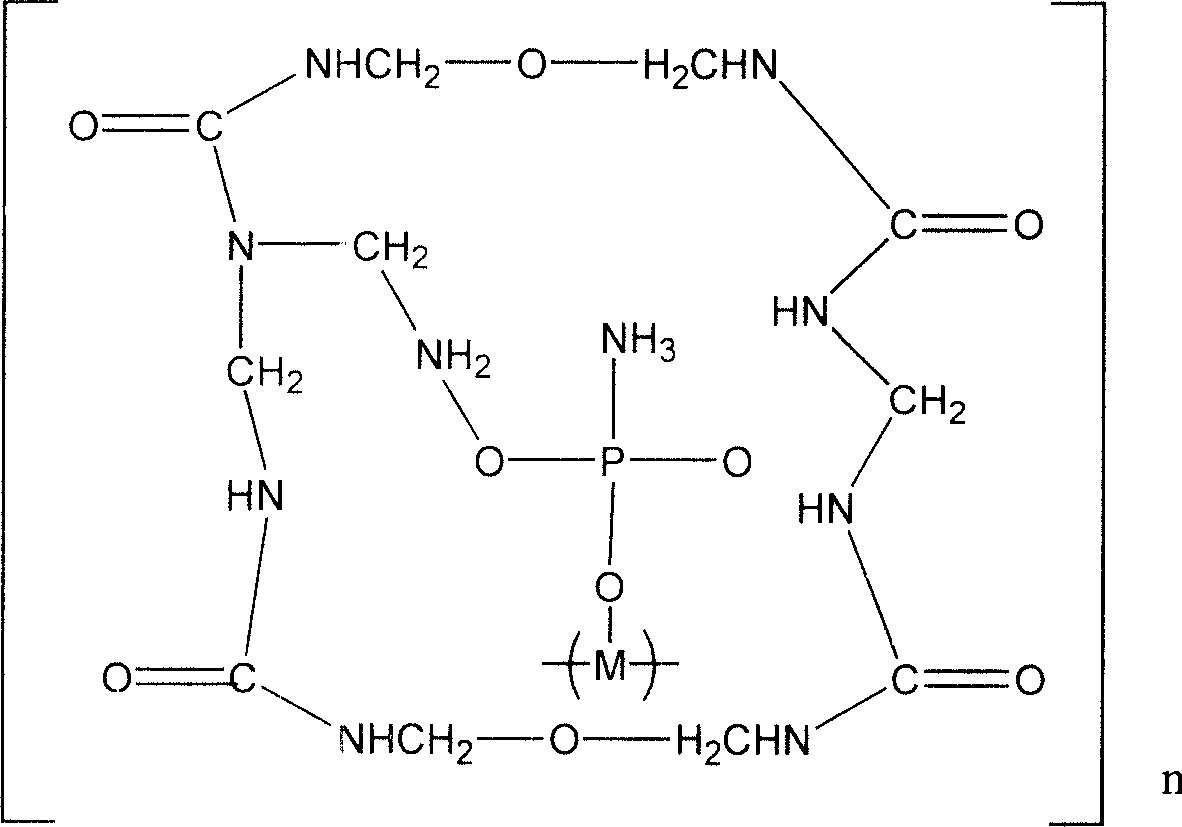
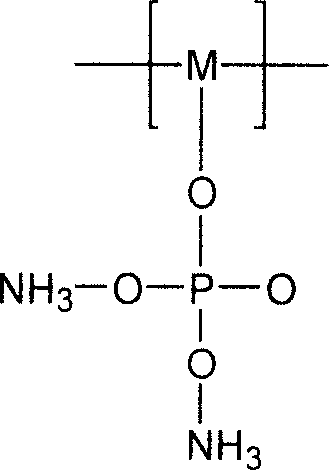
Expansion type flame-retardant and its preparation method
InactiveCN101121892AEasy to prepareEasy to industrialize mass productionFireproof paintsPhosphatePhosphoric acid
The invention discloses a novel inflatable fire-retardant of general formula (I). In terms of preparation method, a reaction of polyhydroxy compound and phosphoric acid or ammonium phosphate salt is conducted to achieve phosphate ester; a reaction of urea and formaldehyde is conducted to achieve urea-formaldehyde resin; and then the achieved phosphate ester and urea-formaldehyde resin are solidified by crosslinking to achieve the final product. The fire-retardant is applied to various polymers for fire-retarding, particularly applicable to fire-refractory coatings for inflatable fire-retarding.
Owner:SHANDONG JUANCHENG TAIDOU HIGH TECH MATERIAL
Pnenolic aldehyde type adsorption resin and preparation method thereof
The invention discloses a preparing method of pnenolic aldehyde type adsorbing resin and preparing, which comprises the following steps: comprising large hole spherical (or granular) with fenol unit pnenolic aldehyde type absorbing resin, large hole spherical (or granular) with catechol unit pnenolic aldehyde type absorbing resin and large hole granular pnenolic aldehyde type absorbing resin with phloroglucine unit; choosing cavaform (or formaldehyde water solution), fenol or catechol or phloroglucine raw material to prepare large hole spherical (or granular) pnenolic aldehyde type adsorbing resin; adopting anti-phase floating polycondensation method or solution polycondensation method to synthesize under existence of special hole agent ethandiol (or glycerin); finishing synthesized reaction under existence of acid catalyst. This preparing method is simple and can finish with one step, which can simplify process and decrease cost.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
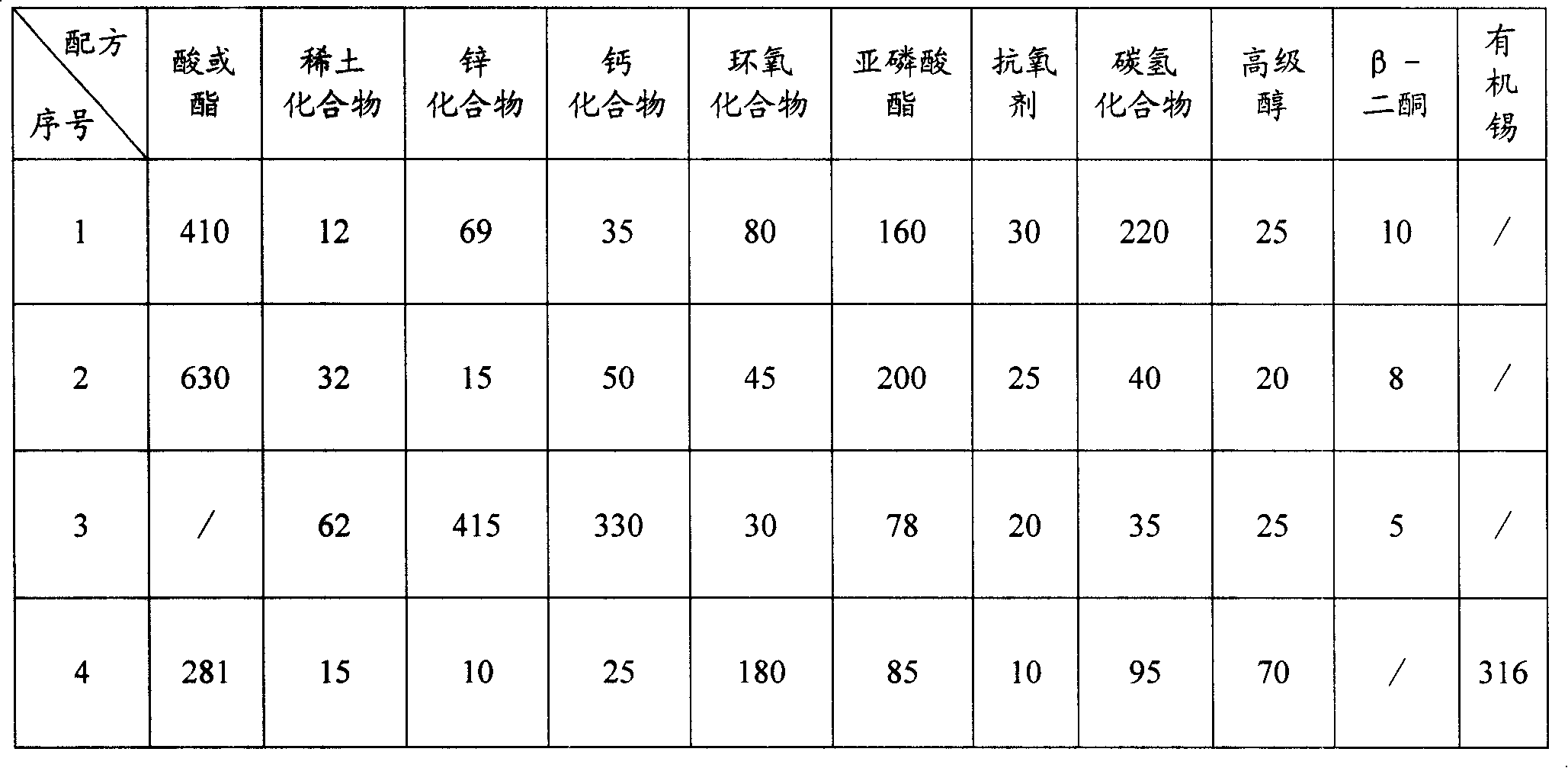
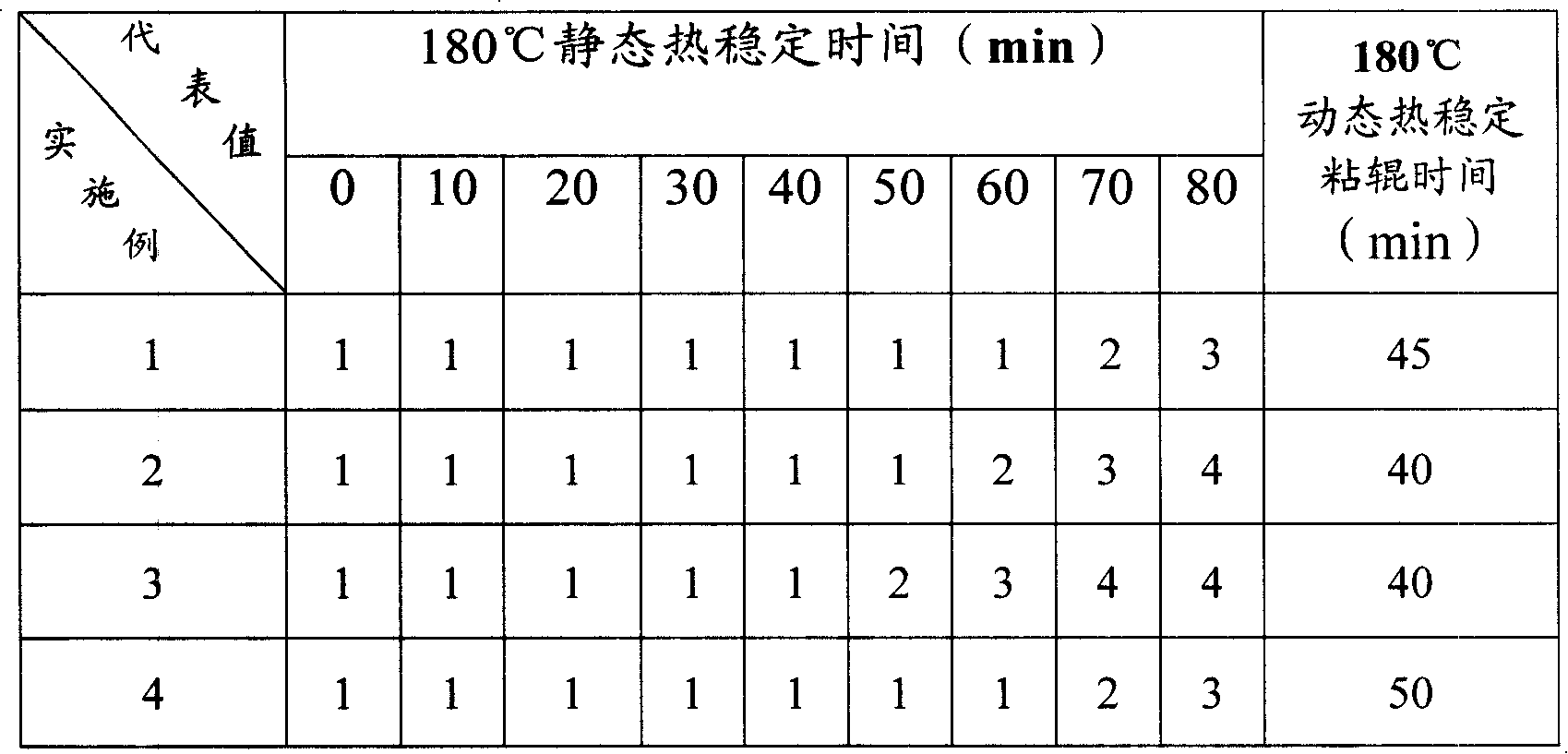
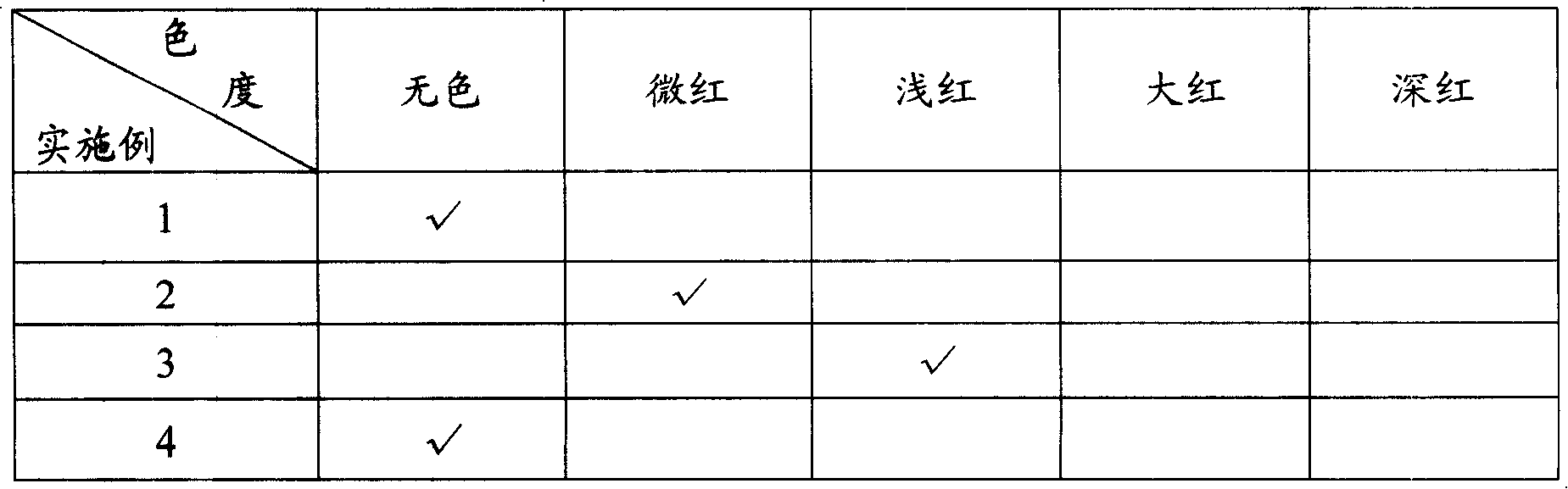
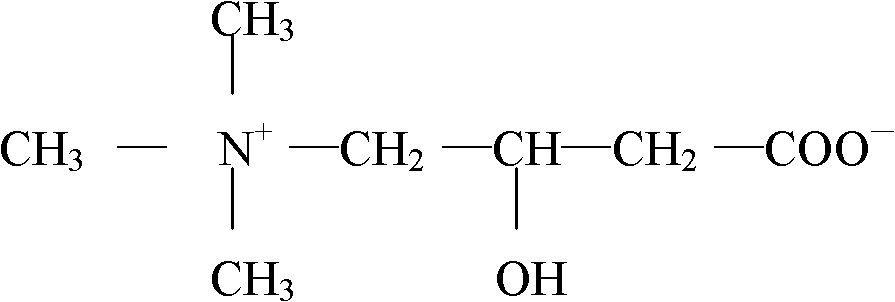
Liquid rare earth calcium zinc stabilizer and preparation method thereof
The present invention provides liquid rare-earth calcium-zinc stabilizer and the preparation method. The liquid rare-earth calcium-zinc stabilizer is compound made of rare-earth calcium-zinc compound and effectively cooperative material. The beneficial effects of the liquid rare-earth calcium-zinc stabilizer and the preparation method provided by the present invention is mainly shown in that (1) the liquid rare-earth calcium-zinc stabilizer has the advantages of high product quality, no toxin, environmental protection, no toxic heavy metal component in recipe, strong practicability and wide application range, which is particularly applicable to the transparent packaging material of food, candy and medicine, as well as the advantages of good processing performance, no precipitation, excellent lubricating performance, superior transparency, thermal stability and oxidation resistance; (2) the process condition is stable and easy to control, thus being applicable to industrialized mass production; the process flow has the advantages of simplicity, environment friendliness, easy access to raw materials and less energy consumption, thus greatly decreasing the production cost; in addition, the whole production process has anhydrous operation, safety and convenience, no discharge of three wastes and less environmental pollution.
Owner:高春福
Levocarnitine composition for injection and preparation method of levocarnitine composition
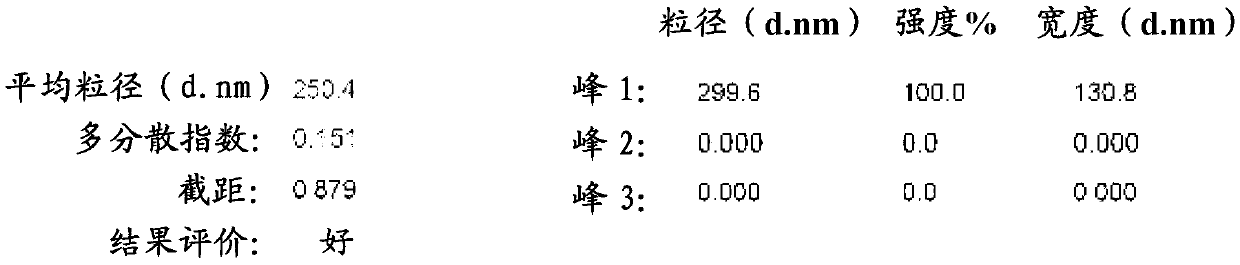
ActiveCN102327238AReduce dosageReduce skeletonOrganic active ingredientsPowder deliveryPorosityFreeze-drying
The invention relates to a levocarnitine composition for injection and a preparation method of the levocarnitine composition; the levocarnitine composition is lyophilized powder containing levocarnitine and mannitol, wherein the weight ratio of the levocarnitine to the mannitol is 1:(0.75-1.25); the average particle diameter of the lyophilized powder is 90-130 nm, and the porosity is 94-98%. The preparation method comprises the steps of: 1) preparing: weighing levocarnitine and mannitol, putting the levocarnitine and the mannitol in a preparing tank, adding injection water, agitating to enable the levocarnitine and the mannitol to be completely dissolved and uniformly mixing, regulating the pH to 5.7-6.3 through a 0.1mol / L hydrochloric acid solution; 2) decarburizing and sterile filtering; 3) sterile packaging; 4) vacuum freeze drying to obtain the levocarnitine composition . The levocarnitine composition has the advantages of simple preparation, advanced technique, uniform quality, excellent stability, better redissolution capability and clinic medicine application safety.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
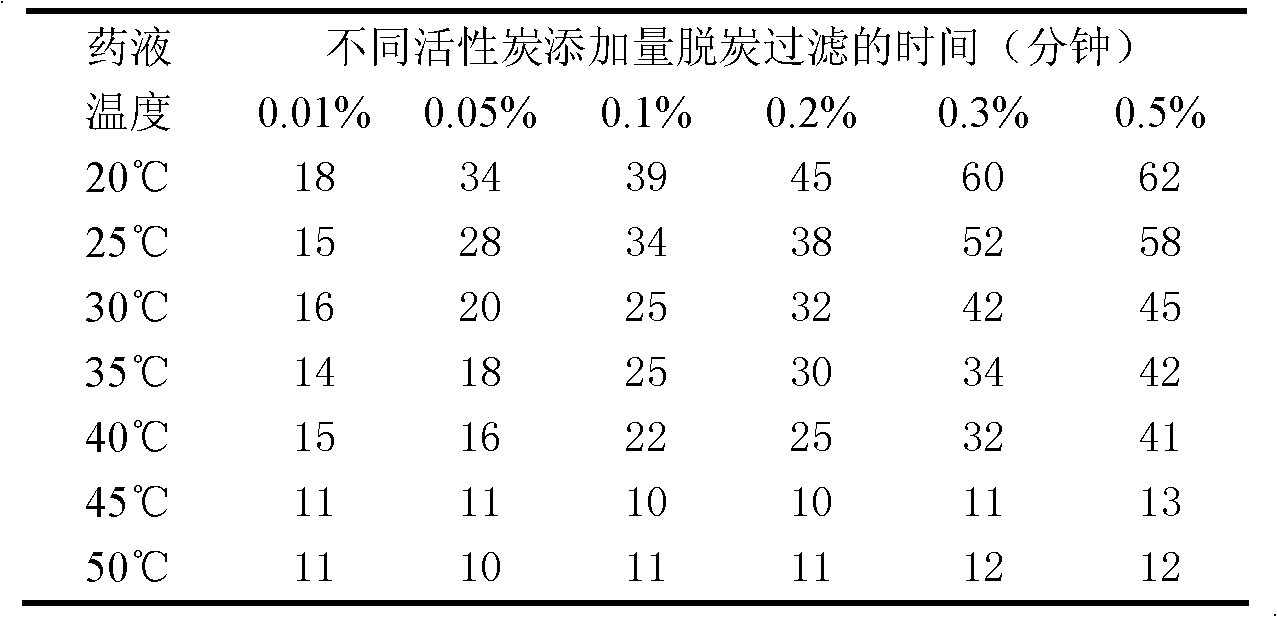
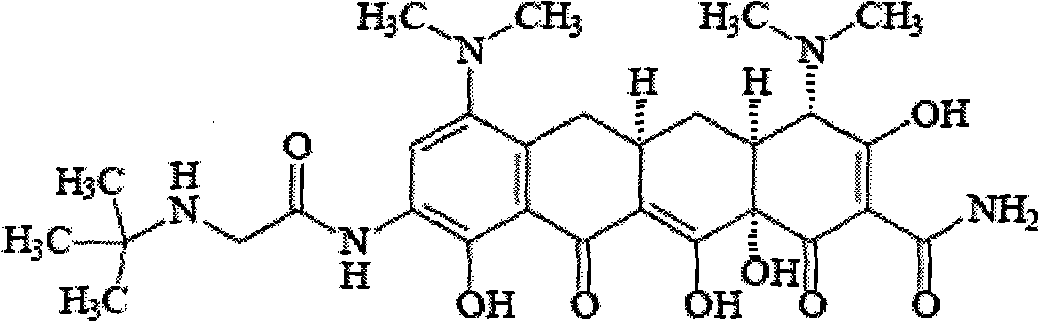
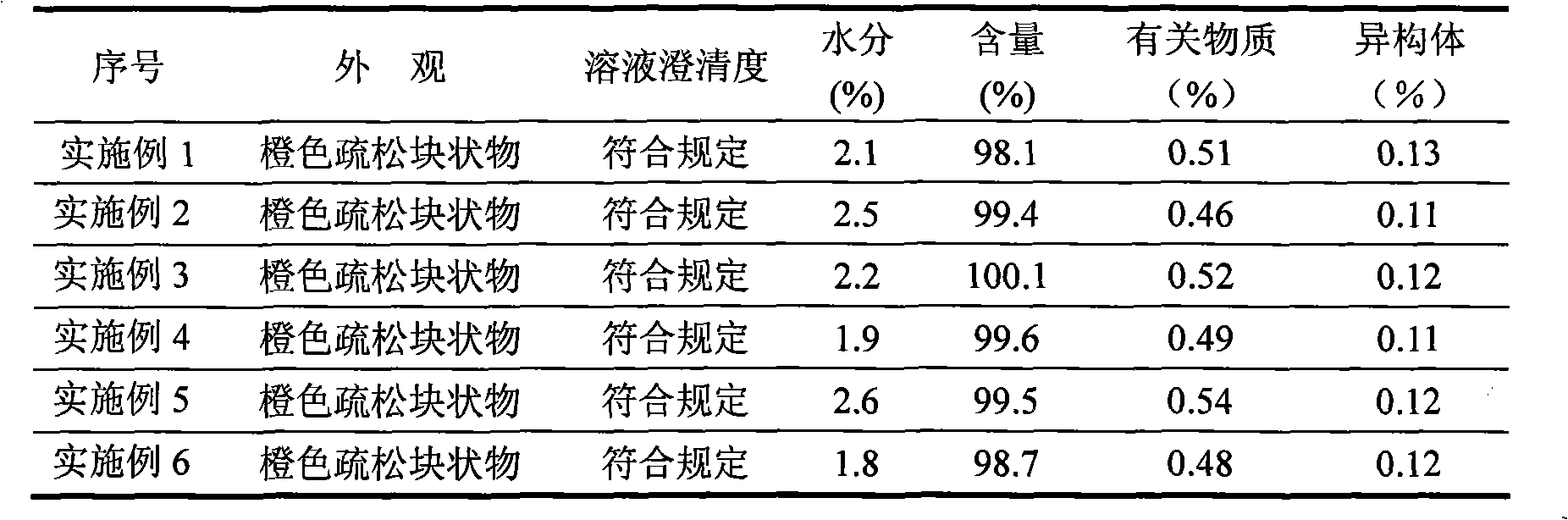
Tigecycline freeze-dried injection
ActiveCN101401812AImprove stabilityLow content of related substancesAntibacterial agentsPowder deliverySulfite saltFreeze-drying
The invention relates to a tigecycline freeze-dried powder injection, which consists of tigecycline, dextran, sodium sulfite and sodium citrate according to the following weight proportion: 10 portions of the tigecycline, 10 to 90 portions of the dextran, 0.1 to 3 portions of anhydrous sodium sulfite, and 0.1 to 3 portions of the sodium citrate. The pH value of the tigecycline freeze-dried powder injection is between 7.0 and 9.0. The preparing process comprises the following steps: firstly, dissolving the dextran by 80 percent of water for injection; secondly, adding the sodium citrate and the anhydrous sodium sulfate into the mixture after cooling; thirdly, adding the tigecycline into the mixture after dissolving and evenly stirring the sodium citrate and the anhydrous sodium sulfate, and mixing the mixture evenly; and fourthly, regulating the pH to between 7.0 and 9.0, adding a needle activated carbon into the mixture, stirring and adsorbing the mixture, removing the activated carbon from the mixture, and fixing the capacity. The solution is filtered through two microporous membranes and then are filled in a cillin bottle, and the tigecycline freeze-dried powder injection is obtained through semi-stoppering, spanning, freeze-drying, introducing nitrogen, performing tamponade, taking out the injection from a box, rolling a mouth, passing the quality inspection, and packaging.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
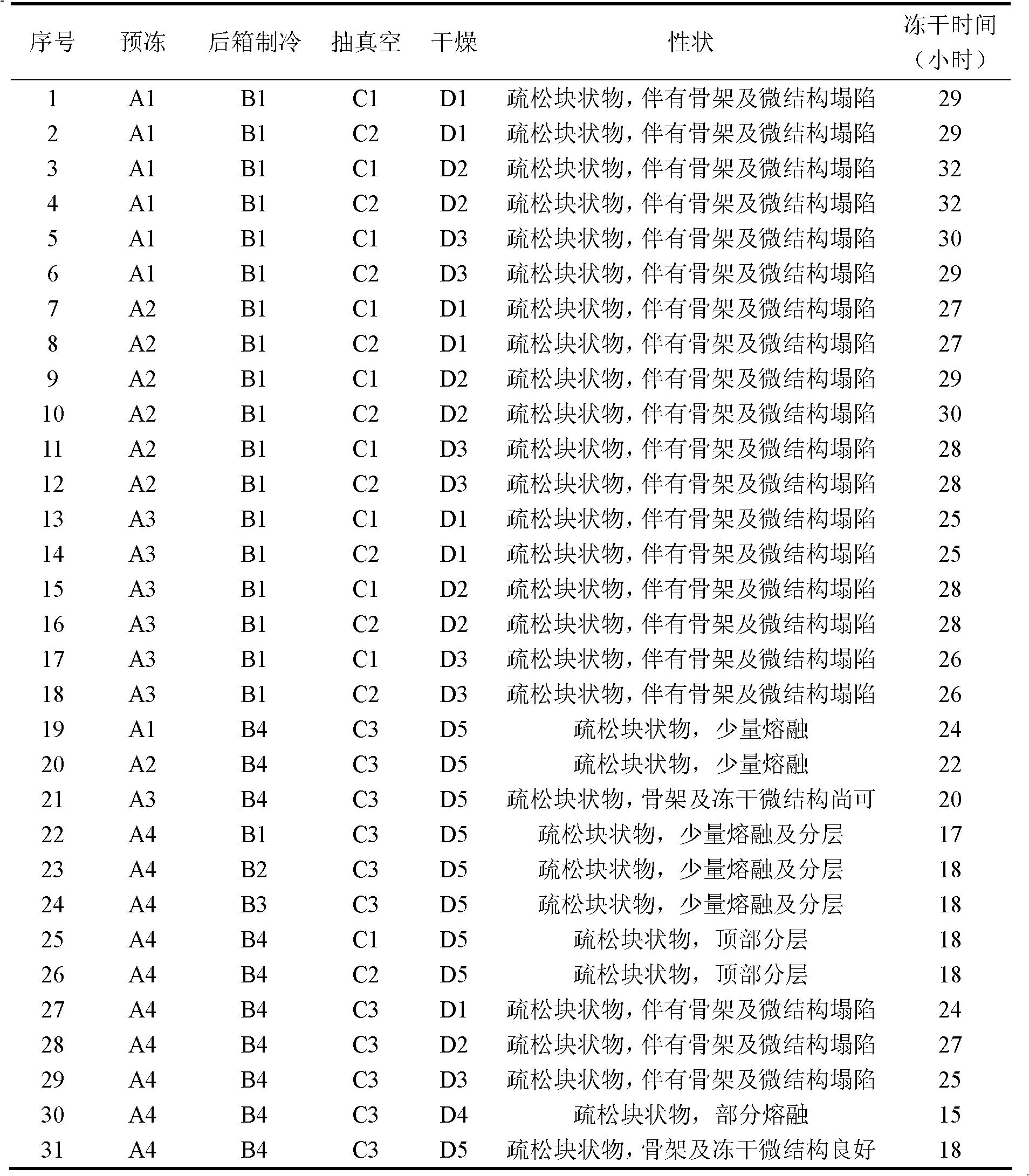
Preparation method of terlipressin acetate lyophilized powder for injection
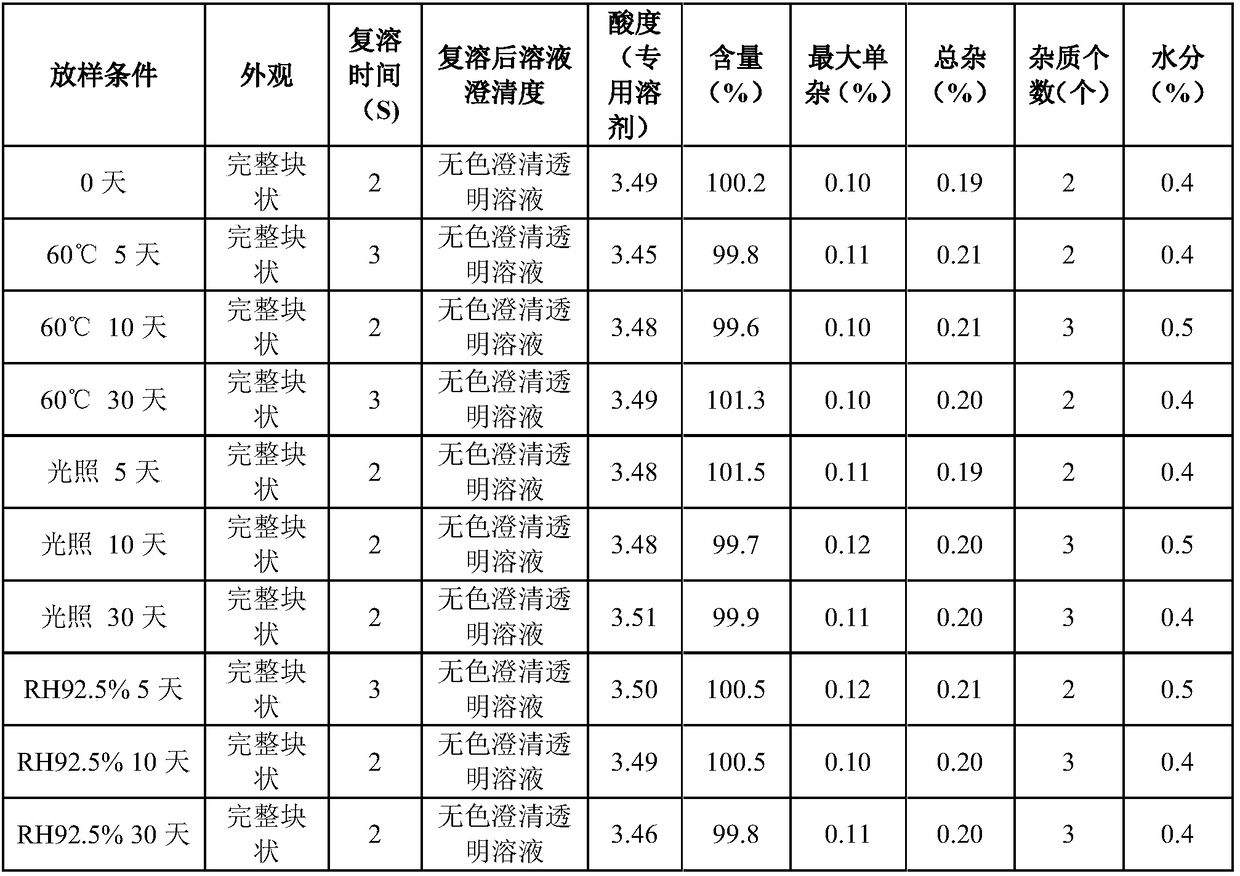
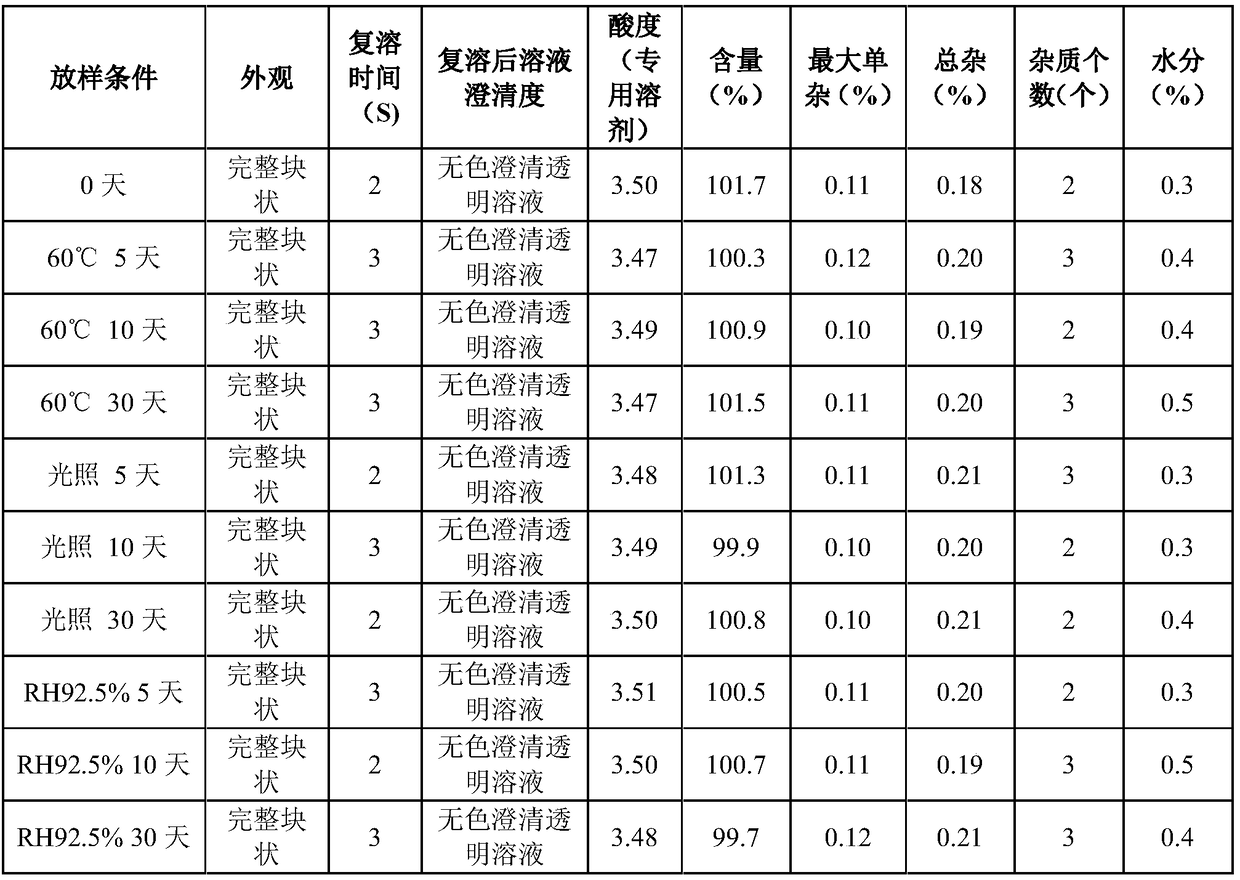
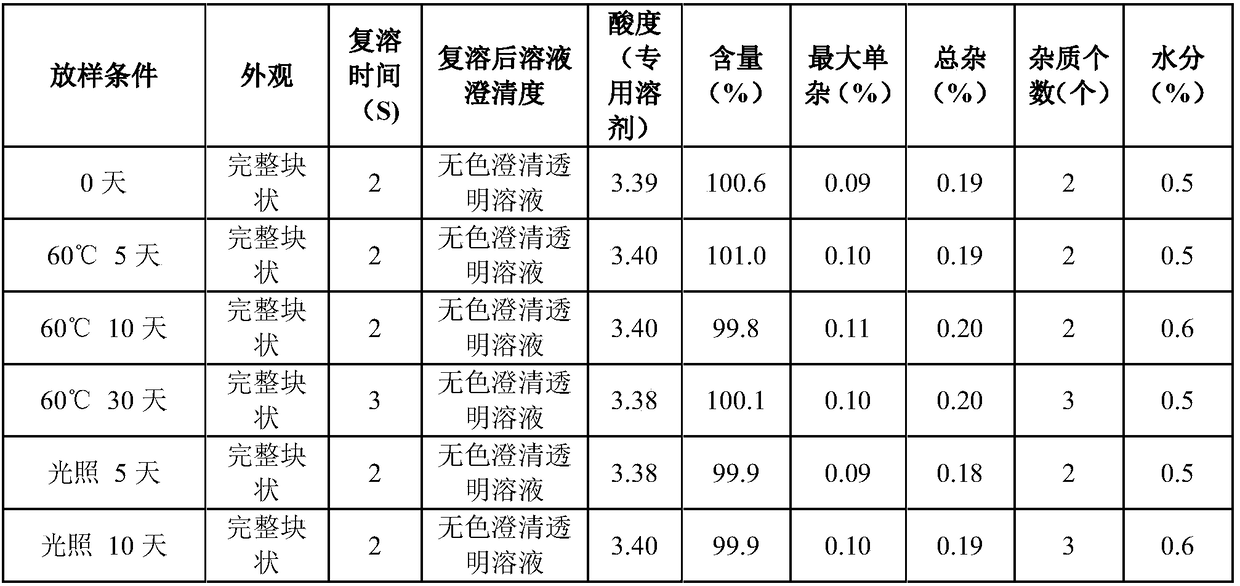
ActiveCN109223720AReduce moisture contentImprove stabilityPowder deliveryPeptide/protein ingredientsDrugs solutionMedicine
The invention relates to the pharmaceutical field, in particular to a preparation method of terlipressin acetate freeze-dried powder injection for injection. A method for prepare a terlipressin acetate lyophilized powder for injection comprises that following step of: pre-freezing the drug solution in stages, and then sequentially drying the drug solution in stages for the first time and drying the drug solution in stages for the second time. The invention can effectively improve the phenomenon of spraying bottle, and ensure that the prepared terlipressin acetate freeze-dried powder injectionhas good stability, complete sample appearance, rapid resolution and low moisture content.
Owner:南京康舟医药科技有限公司
Prepn of polygelatine peptide injection
InactiveCN1387912AImprove production pass rateControl product qualityPeptide/protein ingredientsBlood disorderPeptideChemistry
The polygelatin peptide injection is prepared with gelatin of ox bone, pig bone, etc. and through dissolving, hot degradation in controlled condition, cross-linking with non-toxic cross-linking agent, mixing with electrolyte, adsorption, clarification and stepped filtering to separate cross-linked spherical molecule. During the preparation, the molecular weight of the cross-linked matter is well controlled.
Owner:武汉华龙生物制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com