Patents
Literature
47 results about "Palonosetron" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to prevent nausea and vomiting caused by cancer drug treatment (chemotherapy). It is also used to prevent nausea and vomiting after surgery.
Method of treating post operative nausea and vomiting
InactiveUS20060074101A1Improve effectivenessLess incidenceBiocideNervous disorderNausea sicknessGynecology
Owner:HELSINN HEALTHCARE SA
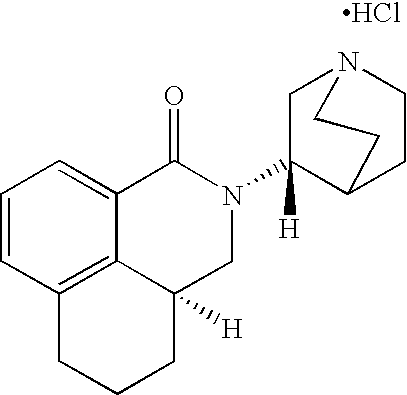
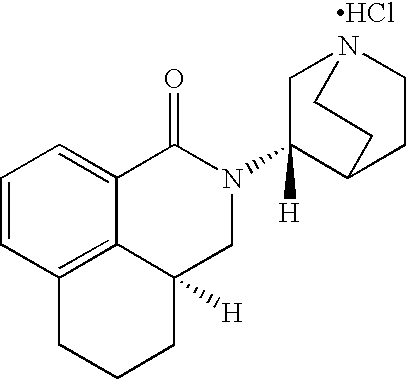
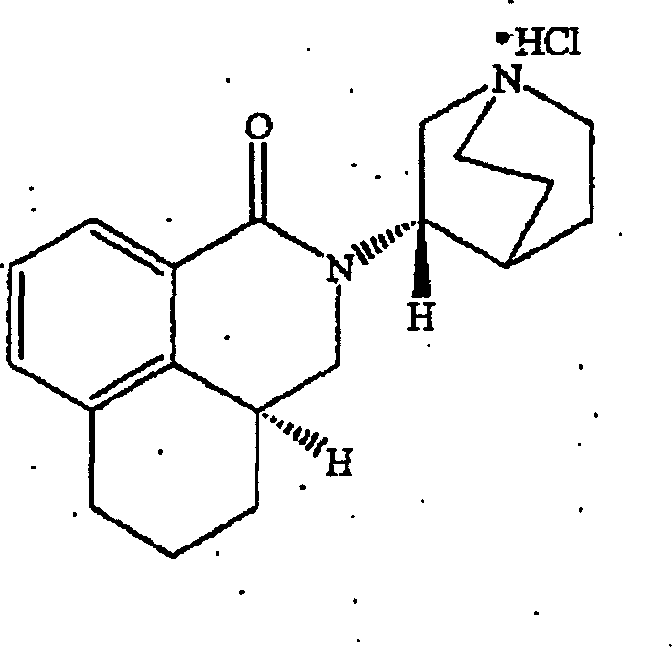
Liquid pharmaceutical formulations of palonosetron
ActiveUS7947725B2Improve stabilityPreventing and reducing emesisBiocideOrganic active ingredientsPalonosetronPharmaceutical formulation
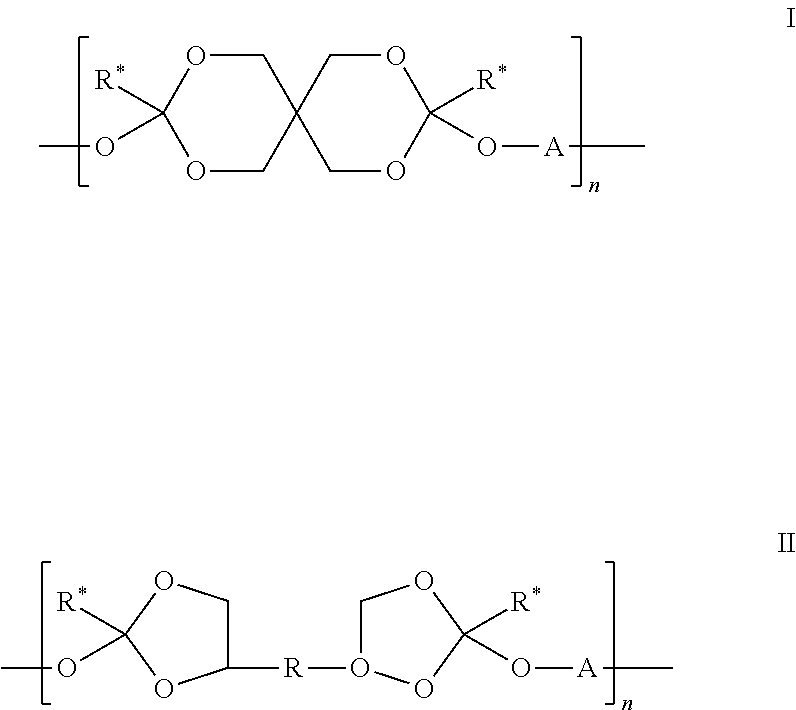
The present invention relates to shelf-stable liquid formulations of palonosetron for reducing chemotherapy and radiotherapy induced emesis with palonosetron. The formulations are particularly useful in the preparation of intravenous and oral liquid medicaments.
Owner:HELSINN BIREX PHARMA +2
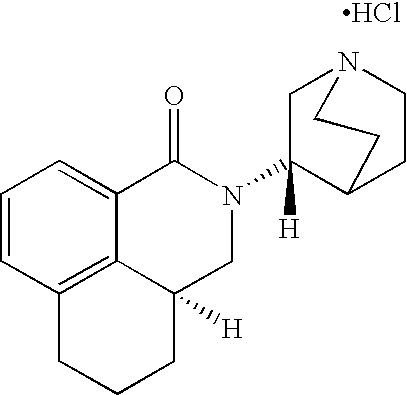
Liquid pharmaceutical formulations of palonosetron
InactiveUS20060167072A1Improve stabilityPreventing and reducing emesisBiocideOrganic active ingredientsPalonosetronPharmaceutical formulation
The present invention relates to shelf-stable liquid formulations of palonosetron for reducing chemotherapy and radiotherapy induced emesis with palonosetron. The formulations are particularly useful in the preparation of intravenous and oral liquid medicaments.
Owner:HELSINN HEALTHCARE SA
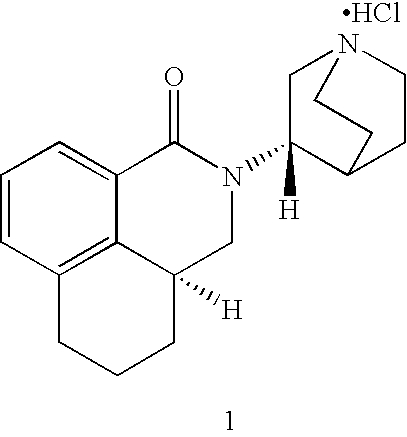
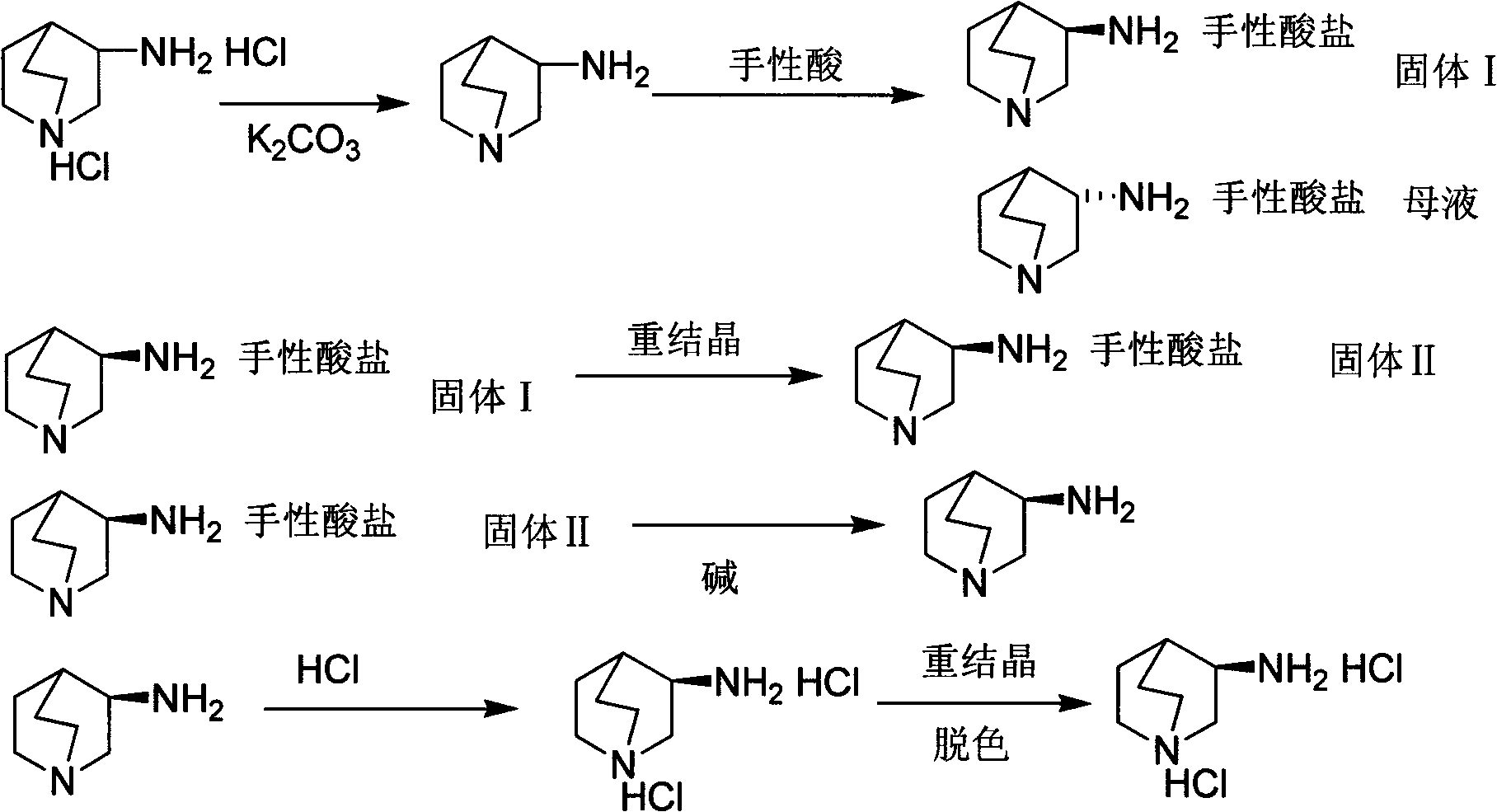
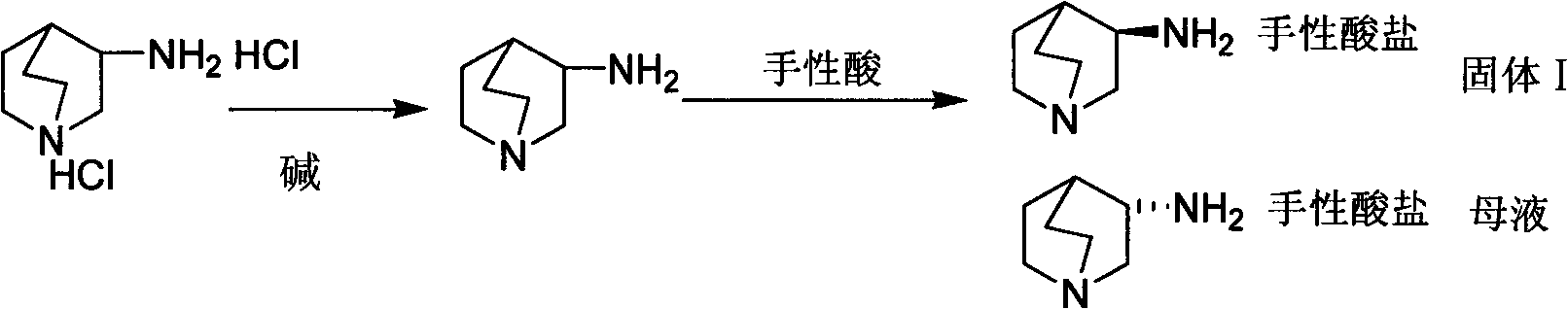
Preparation method of S-3-aminoquinuclidine dihydrochloride
InactiveCN101613349ASimple methodHigh yieldOrganic chemistry3-aminoquinuclidine dihydrochloridePalonosetron
The invention relates to a preparation method of S-3-aminoquinuclidine dihydrochloride which is obtained by taking 3-aminoquinuclidine dihydrochloride as a raw material and carrying out resolving, salifying by resolving, recrystallizing, resolving, salifying, decoloring and crystallizing by concentration. The S-3-aminoquinuclidine dihydrochloride serves as an important raw material for preparing palonosetron. In the method, the 3-aminoquinuclidine dihydrochloride is taken as the raw material and is directly subjected to chiral acid resolution in proper solvent without derivation, with resolution ratio over 40% and optical purity over 98%. The method has simple synthetic method and low production cost, has total product yield over 35% and can be used for industrial production.
Owner:WUHAN UNIV OF TECH
Palonosetron oral transmucosal film or patch
ActiveUS9937122B2Easy to receiveQuick releaseOrganic active ingredientsDigestive systemMentholPlasticizer
The present invention provides a pharmaceutical composition for delivering palonosetron through the buccal mucosa or sublingual mucosa. The pharmaceutical composition comprises 0.05-35% (w / w) of palonosetron, 40-90% of a film forming agent, 1-10% (w / w) of a plasticizer, 5-25% (w / v) of an adhesive agent, and 0.1-5% of a penetration enhancing agent. A preferred plasticizer is a polysorbate. A preferred adhesive agent is polyvinylpyrrolidone or carboxymethylcellulose. A preferred penetration enhancing agent is peppermint oil or menthol.
Owner:LP PHARM (XIAMEN) CO LTD
Liquid pharmaceutical formulations of palonosetron
ActiveUS20130289065A1Improve stabilityPreventing and reducing emesisBiocideDispersion deliveryPalonosetronPharmaceutical formulation
The present invention relates to shelf-stable liquid formulations of palonosetron for reducing chemotherapy and radiotherapy induced emesis with palonosetron. The formulations are particularly useful in the preparation of intravenous and oral liquid medicaments.
Owner:HELSINN BIREX PHARMA +2
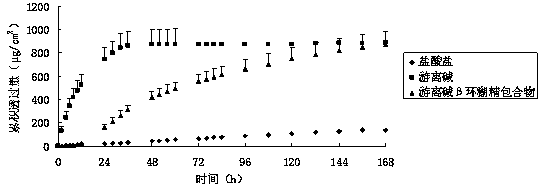
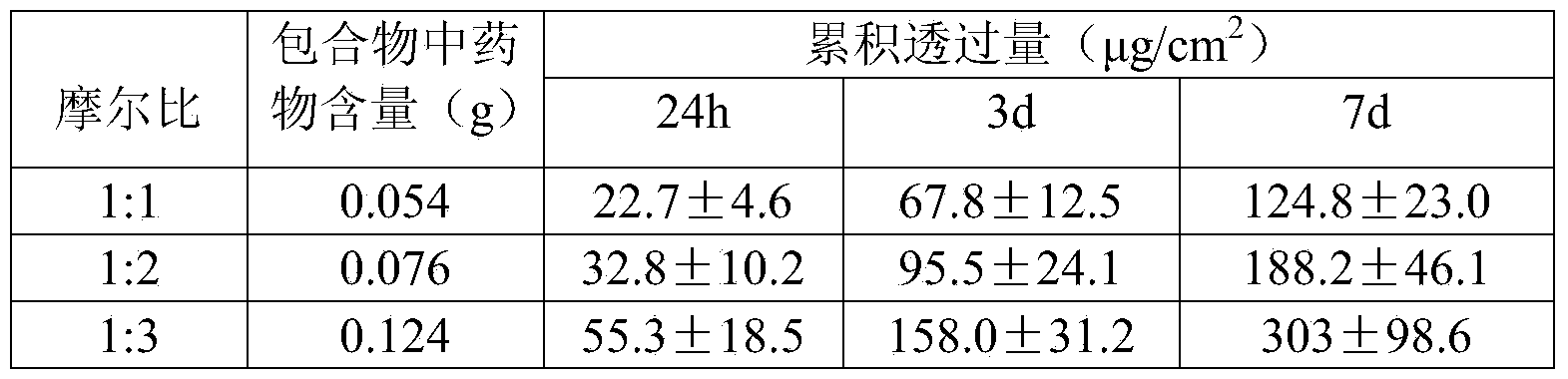
Percutaneous-absorption palonosetron patch and preparation method thereof
ActiveCN104069505AReduce complianceOrganic active ingredientsDigestive systemPalonosetronPercutaneous absorption
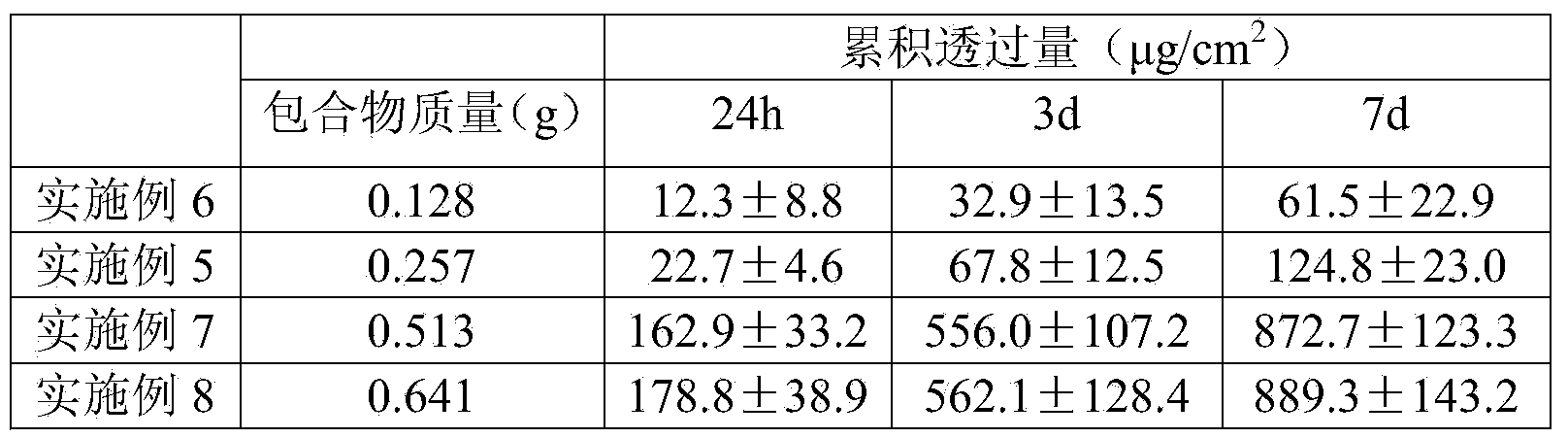
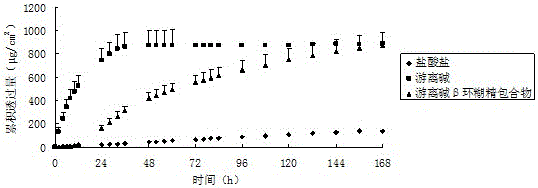
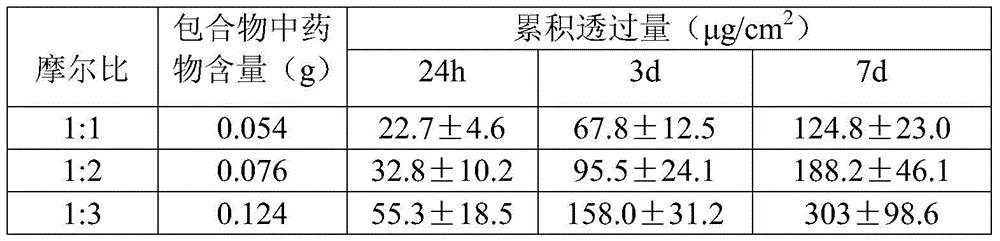
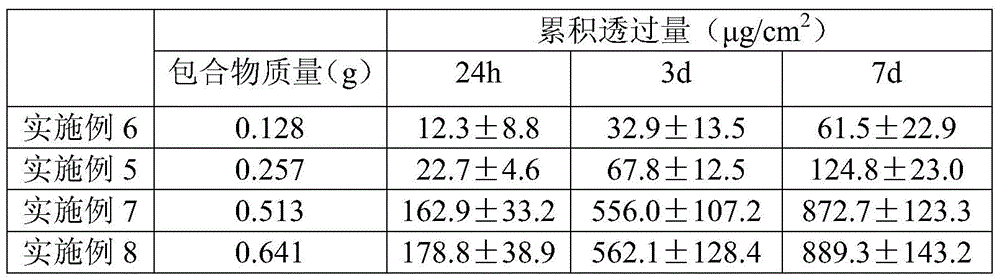
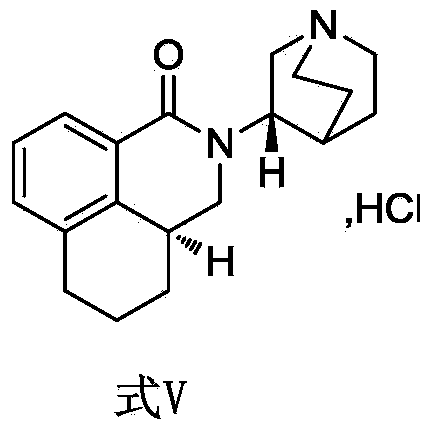
The invention belongs to the technical field of medicines and relates to a percutaneous-absorption palonosetron patch and a preparation method thereof. The patch is composed of a backing layer, a medicine-carrying pressure-sensitive adhesive layer and an anti-sticking layer; the medicine-carrying pressure-sensitive adhesive layer comprises a beta-cyclodextrin inclusion compound of palonosetron free alkali, a pressure-sensitive adhesive and a percutaneous absorption enhancer, wherein the beta-cyclodextrin inclusion compound of the palonosetron free alkali accounts for 4.78%-20.09% of the total weight, preferably 9%-19%, the pressure-sensitive adhesive accounts for 68.80%-95.22% of the total weight and the percutaneous absorption enhancer accounts for 0-11.11wt% of the total weight. In the beta-cyclodextrin inclusion compound of the palonosetron free alkali, the ratio of the palonosetron free alkali to the beta-cyclodextrin inclusion compound is 1: (1-3). The percutaneous-absorption palonosetron patch is provided with the beta-cyclodextrin inclusion compound of the palonosetron free alkali, and therefore, compared with a patch with non-inclusion medicine, the slow release of the medicine within 7 days is realized.
Owner:SHENYANG PHARMA UNIVERSITY
Liquid pharmaceutical formulations of palonosetron
ActiveUS20130261150A1Improve stabilityPreventing and reducing emesisBiocideDispersion deliveryPalonosetronPharmaceutical formulation
The present invention relates to shelf-stable liquid formulations of palonosetron for reducing chemotherapy and radiotherapy induced emesis with palonosetron. The formulations are particularly useful in the preparation of intravenous and oral liquid medicaments.
Owner:HELSINN ADVANCED SYNTHESIS +2
Liquid pharmaceutical formulations of palonosetron
ActiveUS20130261149A1Improve stabilityPreventing and reducing emesisBiocideDispersion deliveryPalonosetronPharmaceutical formulation
The present invention relates to shelf-stable liquid formulations of palonosetron for reducing chemotherapy and radiotherapy induced emesis with palonosetron. The formulations are particularly useful in the preparation of intravenous and oral liquid medicaments.
Owner:HELSINN BIREX PHARMA +2
Palonosetron formulation
Owner:TEVA PHARM USA INC
Intranasal pharmaceutical preparation for treating vomit caused by cancer chemo-treatment, radiation-treatment or operation
InactiveCN101040857AVomiting worksEasy to manufactureOrganic active ingredientsAerosol deliveryNasal cavityNose
The invention provides an agent fed via nasal to treat the emesis caused by cancer chemotherapy, radiotheraphy or surgery. The inventive agent comprises palosnon drug, or medical accept salt, and medical effective findings. The inventive agent fed via nasal can effectively be absorbed by schneiderian membrane to restrain emesis, without simulation on schneiderian membrane and little cilium toxicity.
Owner:吴祥根
Palonosetron oral cavity membrane preparation and preparation method thereof
InactiveCN102652739AAvoid bitternessDissolved and absorbed completelyOrganic active ingredientsDigestive systemQuick FreezePalonosetron
The invention relates to the technical field of biological medicine, in particular to a palonosetron oral cavity membrane preparation and a preparation method thereof. The palonosetron oral cavity membrane preparation and the preparation method thereof have the following advantages: (1) the oral cavity membrane preparation overcomes the bitter taste of palonosetron, has a good mouthfeel, and is completely dissolved and absorbed so that a patient can easily accept the palonosetron, and the oral cavity membrane preparation can be taken in any occasion without water, and is particularly suitable for patients who undergo chemotherapy and possibly vomit if drinking water; (2) the palonosetron oral cavity membrane preparation can quickly release a medicine and has the characteristics of quick effect acting and quick release; and (3) the palonosetron oral cavity membrane preparation is simple in production process and low in cost and is convenient to carry.
Owner:FOSHAN DAYI TECH LTD
Palonosetron for the treatment of chemotherapy induced emeses
InactiveUS20060079545A1Improve effectivenessLess incidenceBiocideDigestive systemPalonosetronRadical radiotherapy
Methods and compositions for reducing chemotherapy and radiotherapy induced emises with 5-HT3 receptor antagonists are disclosed, especially with palonosetron.
Owner:HELSINN HEALTHCARE SA
Method of treating post operative nausea and vomiting
Owner:HELSINN HEALTHCARE SA
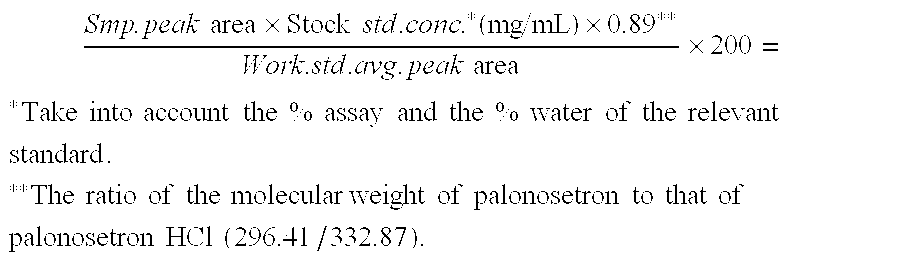
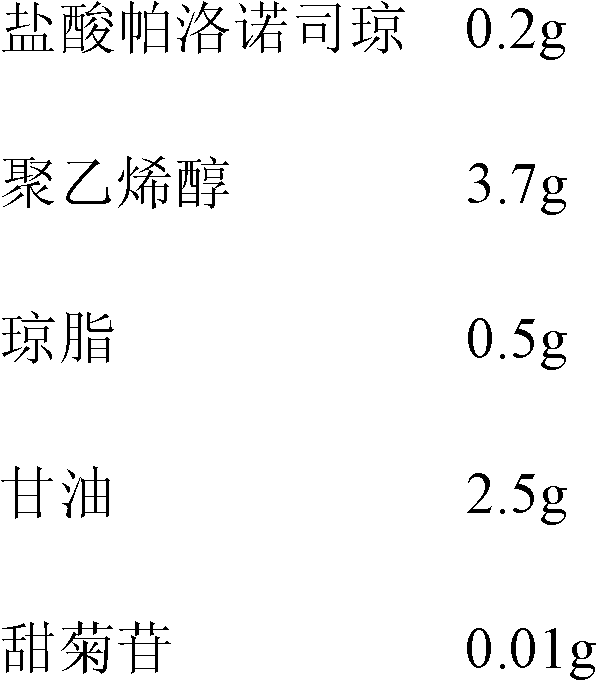
Oral spray or aerosol containing Palonosetron
The invention relates to an oral spray or aerosol containing Palonosetron, which comprises Palonosetron or other medical salt thereof and auxiliary materials which are acceptable on pharmacy and are selected from buffer, flavoring agent, antiseptic, chelating agent, polar solvent or non-polar solvent. The active components in the spray or the aerosol can be rapidly absorbed through mucous menbrane of mouth to take effect rapidly, and has high bioavailability.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Palonosetron percutaneous absorption patch and preparation method thereof
ActiveCN104069505BReduce complianceOrganic active ingredientsDigestive systemPercutaneous absorptionPalonosetron
The invention belongs to the technical field of medicine, and relates to a palonosetron percutaneous absorption patch and a preparation method thereof. The patch of the invention is composed of a backing layer, a drug-loaded pressure-sensitive adhesive layer and an anti-adhesive layer; Absorption enhancer. Wherein the β-cyclodextrin inclusion complex of palonosetron free base accounts for 4.78%-20.09% of the total weight, preferably 9%-19%, the pressure-sensitive adhesive accounts for 68.80%-95.22% by weight, and the transdermal absorption accelerator Accounting for 0~11.11wt% of the total weight. In the β-cyclodextrin inclusion compound of palonosetron free base, the ratio of the palonosetron free base to the β-cyclodextrin inclusion compound is 1:1-3. The palonosetron percutaneous absorption patch provided by the present invention is selected from the β-cyclodextrin inclusion complex of palonosetron free base, which successfully achieves a drug-free absorption within 7 days compared with the drug-unincluded patch. Release slowly.
Owner:SHENYANG PHARMA UNIVERSITY
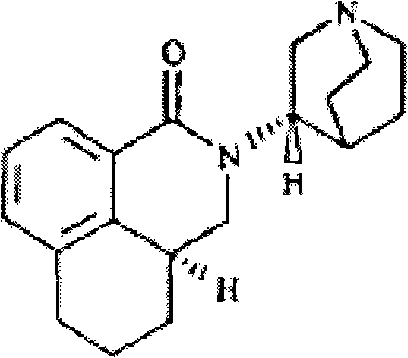
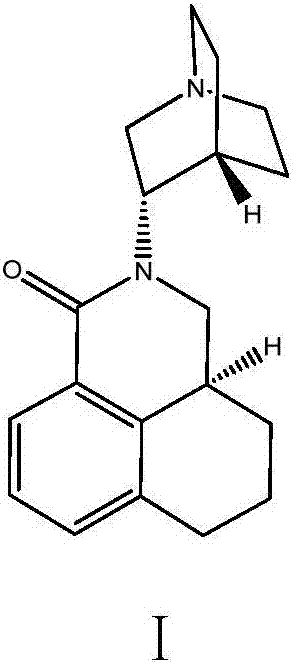
Method for synthesizing palonosetron metabolite
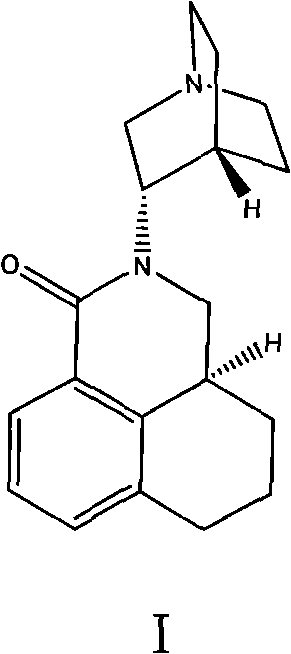
The invention belongs to the technical field of medicines, and in particular relates to a novel method for synthesizing palonosetron metabolite (3aS, 3'S)-2-[-1-aza-bicyclo-[2.2.2]cyn-3-yl]-2,3,3a,4,5,6-hexahydro-1-oxo-1H-benzo[de] isoquinoline nitric oxide. According to the method, the compound as shown in the formula I and / or a derivative of the compound is adopted as a raw material, and the compound or the derivative is reacted in an inert organic solvent in the presence of an oxidant as shown in the formula IV, thereby obtaining the palonosetron metabolite as shown in the formula III. Compared with the prior art, the method has the advantages that the used raw materials and oxidant are easily available, the reaction condition is gentle, the operation is simple, the yield is high, the nitric oxide can be massively prepared, and thus great pharmaceutical research significance and pharmaceutical industrial values are achieved.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Liquid pharmaceutical formulations of palonosetron
ActiveUS20060167073A1Improve stabilityPreventing and reducing emesisBiocideOrganic active ingredientsMedicinePalonosetron
The present invention relates to shelf-stable liquid formulations of palonosetron for reducing chemotherapy and radiotherapy induced emesis with palonosetron. The formulations are particularly useful in the preparation of intravenous and oral liquid medicaments.
Owner:HELSINN ADVANCED SYNTHESIS +2
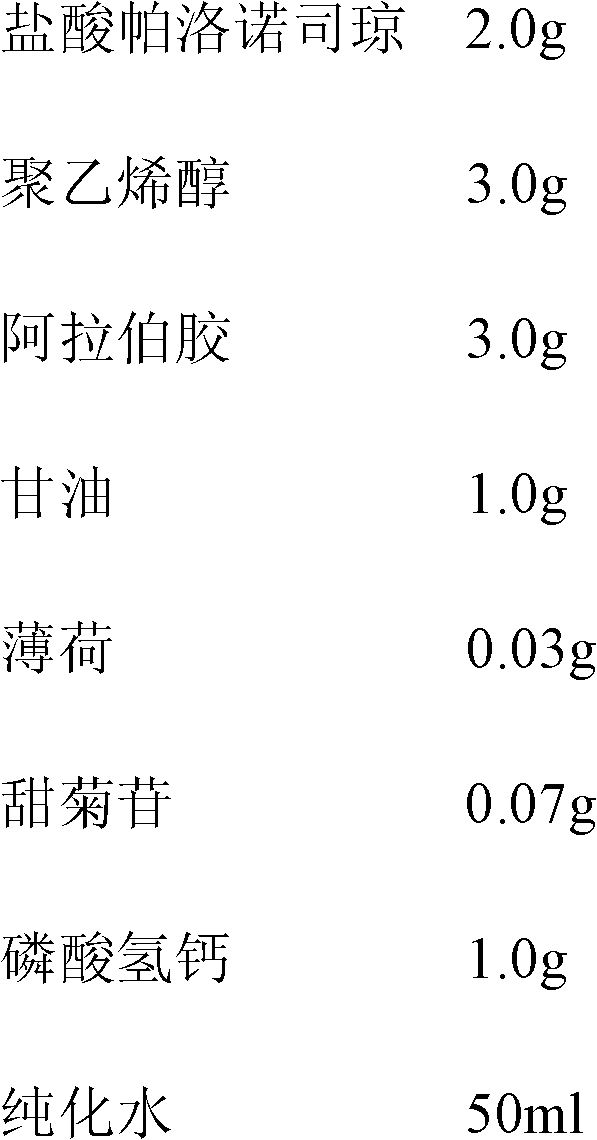
Stabilized Parinaudsijones large-capacity injection
A stable high-capacity injection of Paluonuosiqiong or its medicinal salt for treating the vomiting reaction caused by chemcotherapy or radiotherapy of cancer with low irritation and high compliance is disclosed.
Owner:深圳万乐药业有限公司
Liquid pharmaceutical formulations of palonosetron
ActiveUS20130261592A1Improve stabilityPreventing and reducing emesisBiocideDispersion deliveryPalonosetronPharmaceutical formulation
The present invention relates to shelf-stable liquid formulations of palonosetron for reducing chemotherapy and radiotherapy induced emesis with palonosetron. The formulations are particularly useful in the preparation of intravenous and oral liquid medicaments.
Owner:HELSINN ADVANCED SYNTHESIS +2
Palonosetron transdermal patch and preparation method thereof
ActiveCN109875982ASimple production processReduce manufacturing costOrganic active ingredientsDigestive systemTransdermal patchMedicine
The invention discloses a palonosetron transdermal patch and a preparation method thereof. The palonosetron transdermal patch is composed of a backing layer, a medicine carrying pressure-sensitive adhesive layer and an anti-bonding layer. The medicine carrying pressure-sensitive adhesive layer comprises palonosetron, polyacrylate resin, a polyacrylate pressure-sensitive adhesive, a chemical penetration enhancer and an antioxidant, the palonosetron accounts for 1% to 5% of the total weight of the medicine carrying pressure-sensitive adhesive layer, the medicine content of the patch ranges from0.5 mg to 20.0 mg, preferably 0.5 mg to 2.0 mg, and the area of the patch is smaller than or equal to 10 cm<2>. According to the palonosetron transdermal patch, the polyacrylate resin is added, the medicine percutaneous penetration rate is increased, the content of unknown impurities is reduced, and the patch meets the dosing requirement of 5-7 days after being dosed for 3 days.
Owner:GUANGDONG HONGSHANHU PHARM CO LTD
Liquid pharmaceutical formulations of palonosetron
ActiveUS20060069114A1Improve stabilityPreventing and reducing emesisBiocideDigestive systemPharmaceutical drugPalonosetron
The present invention relates to shelf-stable liquid formulations of palonosetron for reducing chemotherapy and radiotherapy induced emesis with palonosetron. The formulations are particularly useful in the preparation of intravenous and oral liquid medicaments.
Owner:HELSINN BIREX PHARMA +2
Liquid pharmaceutical formulations of palonosetron
ActiveUS20060167071A1Improve stabilityPreventing and reducing emesisBiocideOrganic active ingredientsPalonosetronPharmaceutical formulation
The present invention relates to shelf-stable liquid formulations of palonosetron for reducing chemotherapy and radiotherapy induced emesis with palonosetron. The formulations are particularly useful in the preparation of intravenous and oral liquid medicaments.
Owner:HELSINN BIREX PHARMA +2
Palonosetron for the treatment or prevention of nausea and vomiting
ActiveUS20200345632A1Rapid long-term ameliorationFast systemic absorptionOrganic active ingredientsDigestive systemPalonosetronPharmaceutical medicine
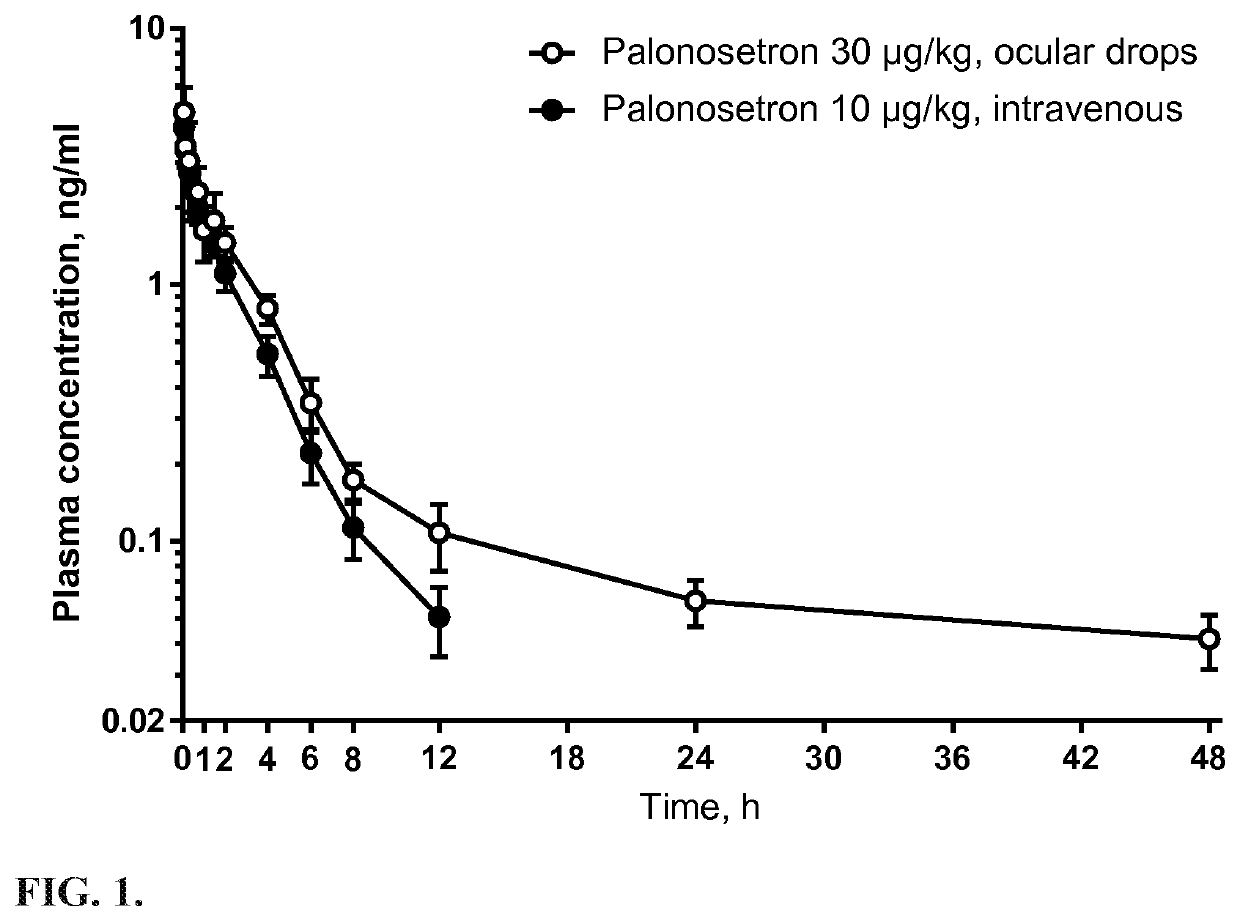
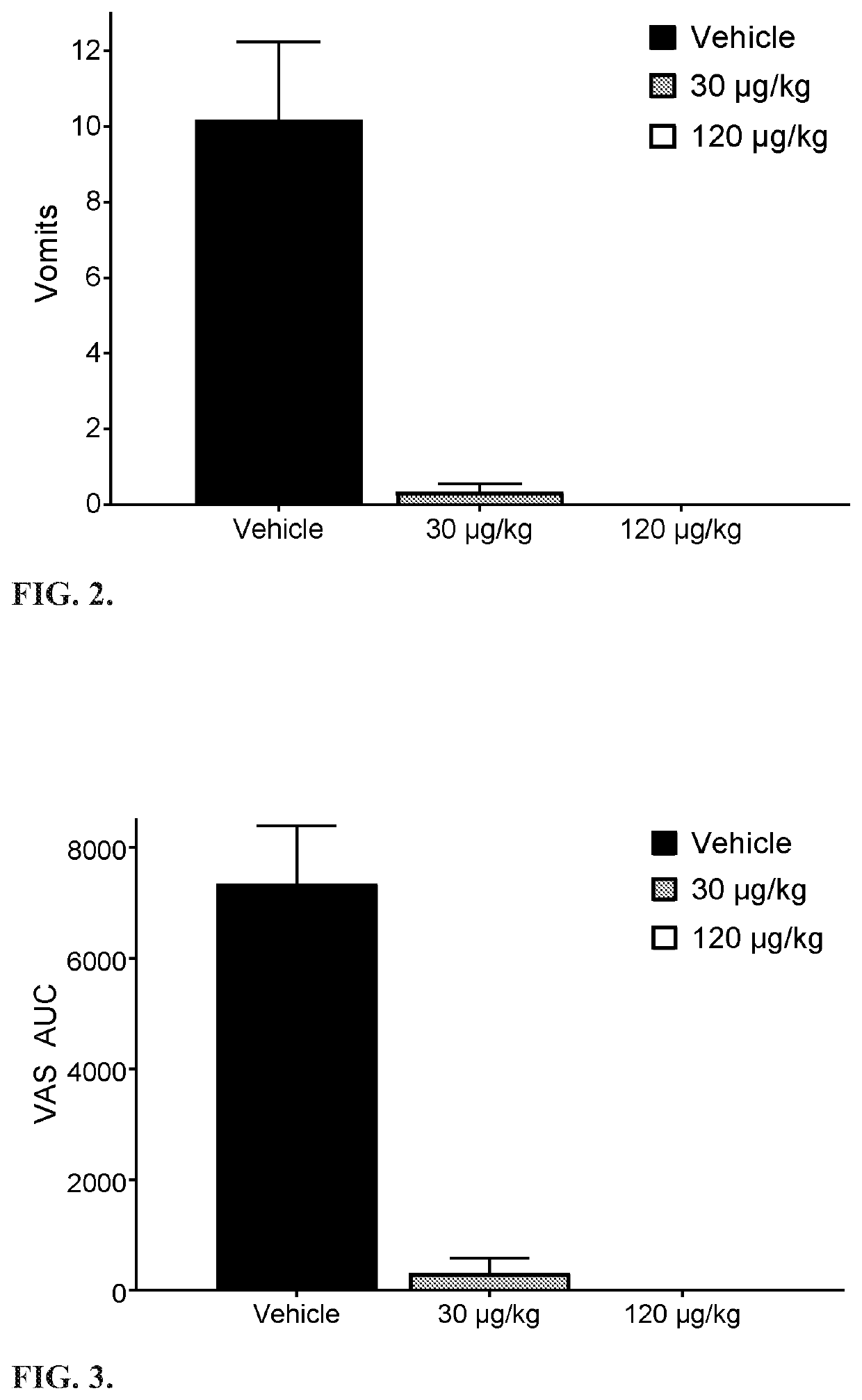
The invention relates to a method for treating or preventing nausea and vomiting. The method comprises administering an eye drop composition comprising palonosetron or a pharmaceutically acceptable salt thereof to the eye of the subject. The ocular administration results in fast systemic absorption, improved bioavailability compared to oral route and extended elimination time.
Owner:ORION CORP
Solid medicine composition comprising palonosetron
ActiveCN107137374AThe prescription process is simpleImprove stabilityOrganic active ingredientsDigestive systemAdhesivePalonosetron
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Palonosetron powder for injection and its preparing method
The present invention relates to a powder injection containing panorsqiong and its preparation method. It is made up by using panorsqiong or its salt (panorsqiong hydrochloride) and medicinal auxiliary material through a certain preparation process. Its product quality is stable.
Owner:孟繁浩
Pharmaceutical composition containing palonosetron
Owner:SAMYANG BIOPHARMLS CORP
Method of treating patients non-responsive to palonosetron
InactiveUS20170281779A1Measurable prevention reductionOrganic active ingredientsPharmaceutical delivery mechanism5-HT3 antagonistMedicine
A method for treating chemotherapy-induced nausea and vomiting in individuals undergoing chemotherapy and previously treated with, and whom failed to respond to, a 5-HT3 antagonist other than granisetron is described. Individuals who fail to respond to, for example, palonosetron, as evidenced by an inadequate prevention or attenuation of acute or delayed chemotherapy-induced nausea and vomiting, are treated with a semi-solid drug delivery vehicle that provides a sustained release of granisetron.
Owner:HERON THERAPEUTICS
Palonosetron solid medicine composition
InactiveCN103446072AThe prescription process is simpleImprove stabilityOrganic active ingredientsDigestive systemMedicinePalonosetron
The invention relates to a palonosetron solid medicine composition. The palonosetron solid medicine composition comprises palonosetron or a pharmaceutically acceptable salt and a pharmaceutically acceptable excipient of the palonosetron, and the solid medicine composition does not contain an adhesion agent.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Palonosetron oral transmucosal film or patch
ActiveUS20170367969A1Easy to receiveQuick releaseOrganic active ingredientsDigestive systemMentholPlasticizer
The present invention provides a pharmaceutical composition for delivering palonosetron through the buccal mucosa or sublingual mucosa. The pharmaceutical composition comprises 0.05-35% (w / w) of palonosetron, 40-90% of a film forming agent, 1-10% (w / w) of a plasticizer, 5-25% (w / v) of an adhesive agent, and 0.1-5% of a penetration enhancing agent. A preferred plasticizer is a polysorbate. A preferred adhesive agent is polyvinylpyrrolidone or carboxymethylcellulose. A preferred penetration enhancing agent is peppermint oil or menthol.
Owner:LP PHARM (XIAMEN) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com