Preparation method of S-3-aminoquinuclidine dihydrochloride
A technology of aminoquinine dihydrochloride and aminoquinine, applied in the field of preparation of S-3-aminoquinine dihydrochloride, can solve the problem of affecting yield and cost, affecting yield and cost, inconvenient operation, etc. problem, to achieve the effect of high yield, low cost and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
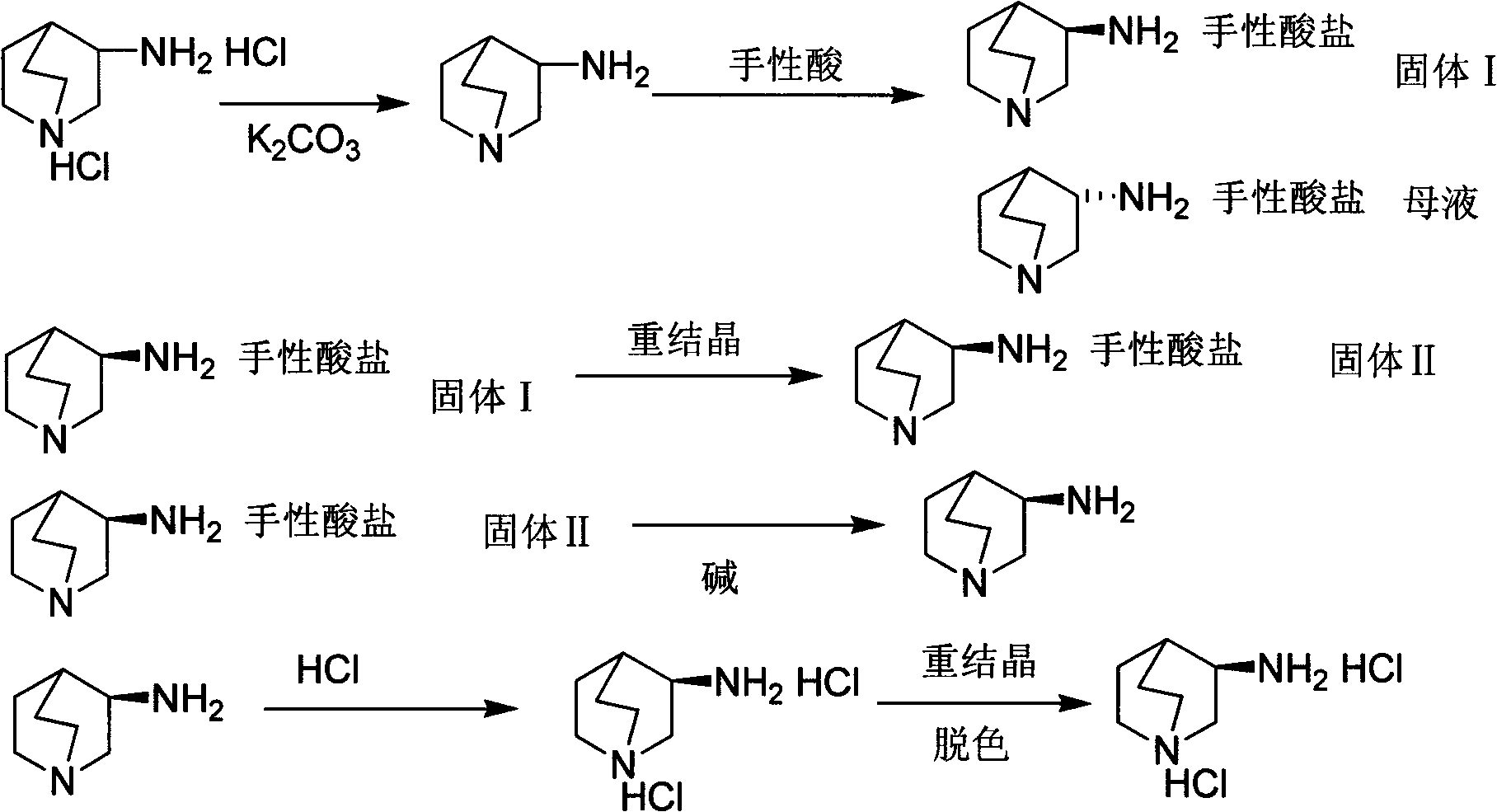
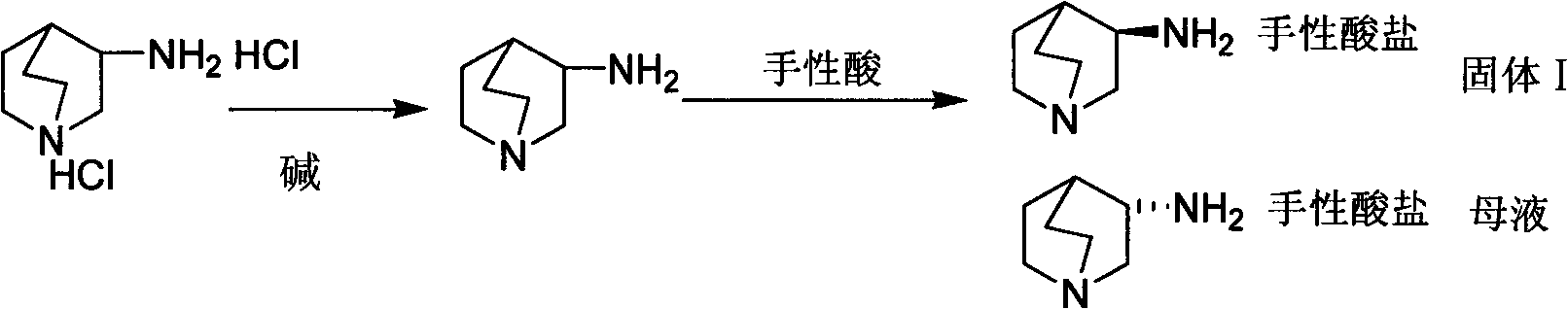
[0029] The preparation method of S-3-aminoquinine dihydrochloride is achieved through the following steps:
[0030] 1) Preparation of S-3-aminoquinine-D-tartrate
[0031] Add 10g of 3-aminoquinine dihydrochloride and 150ml of methanol to a 250ml single-neck flask, cool to 0-5°C in an ice-water bath, add 6.9g of potassium carbonate, stir at room temperature for 1h after the addition, filter to remove the inorganic salt of potassium chloride, Under stirring at 50°C, 7.3 g of D-tartaric acid was added to the mother liquor and stirred to form a salt for 1 hour. A large amount of solid was precipitated. The solid was filtered, washed with a small amount of solvent, and dried to obtain 7.2 g.
[0032] Mp: 213-215℃, [α] D 20 = -35.0 0 (C=1, H 2 O).
[0033] 2) Recrystallization of S-3-aminoquinine-D-tartrate,
[0034] Measure 50ml of methanol and place it in a 250ml single-necked flask, add the salt obtained in step 1), raise the temperature to 50°C, stop heating after the salt is compl...
Embodiment 2
[0044] The preparation method of S-3-aminoquinine dihydrochloride is achieved through the following steps:
[0045] 1) Preparation of S-3-aminoquinine-D-camphorsulfonate
[0046] Add 10g of 3-aminoquinine dihydrochloride, 150ml of absolute ethanol to a 250ml single-necked flask, cool to 0-5°C in an ice-water bath, slowly add 8.5g of sodium bicarbonate, the entire adding process lasts for 1h, and stir at room temperature after the addition is complete 1.5h, filter to remove the inorganic salt sodium chloride, add 12.8g of D-camphorsulfonic acid to the mother liquor under stirring at 30℃, stir to form a salt for 2h, solids will precipitate out, filter the solids, wash the solids with a small amount of solvent, and dry to get 9.9 g
[0047] Mp: >300℃, [α] D20 = -49.5 0 (C=1, H 2 O).
[0048] 2) Recrystallization of S-3-aminoquinine-D-camphorsulfonate,
[0049] Measure 60ml of absolute ethanol and place it in a 250ml single-necked bottle, add the salt obtained in step 1), raise the te...
Embodiment 3
[0059] The preparation method of S-3-aminoquinine dihydrochloride is achieved through the following steps:
[0060] 1) Preparation of S-3-aminoquinine-L-dibenzoyl tartrate
[0061] Add 10g of 3-aminoquinine dihydrochloride, 150ml of isopropanol to a 250ml single-necked flask, cool to 0-5°C in an ice-water bath, and slowly add 8.3g of sodium acetate. The entire adding process lasts for 1h. After the addition, stir at room temperature for 2h. , Remove the inorganic salt sodium chloride by filtration, add 18.9 g of L-dibenzoyl tartaric acid to the mother liquor under stirring at 20°C, stir to form a salt for 3 hours, a large amount of solids will precipitate out, filter the solids, wash the solids with a small amount of solvent, and dry to obtain 14.0g
[0062] Mp: 179-181℃, [α] D 25 = +135 0 (C=1, H 2 O).
[0063] 2) Recrystallization of S-3-aminoquinine-D-dibenzoyl tartrate,
[0064] Measure 70ml isopropanol into a 250ml single-neck bottle, add the salt obtained in step 1), raise ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com