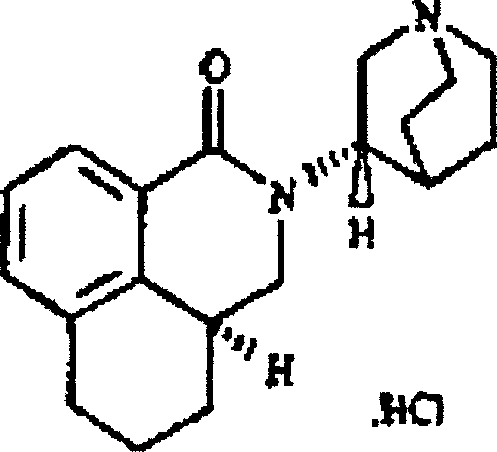
Stabilized Parinaudsijones large-capacity injection
A technology of palonosetron and injection, which is applied in the field of large-capacity injection, and can solve the problems of poor compliance, large irritation, and high concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Prescription Composition Specifications (30ml: 0.25mg)
[0020] Palonosetron Hydrochloride 0.25mg
[0023] pH 6.0-8.0
[0024] Water for injection 30ml
[0025] According to the prescription ratio, weigh the prescribed amount of palonosetron hydrochloride and the required pharmaceutical excipients, add water and stir to dissolve, adjust the pH to 6.0-8.0 with hydrochloric acid and sodium hydroxide, apply activated carbon to remove pyrogens, and divide Pack, fill the bottle with nitrogen gas, stopper and press the cap, and steam sterilize at 100°C for 30 minutes.
Embodiment 2
[0027] Prescription Composition Specifications (50ml: 0.25mg)
[0028] Palonosetron Hydrochloride 0.25mg
[0030] Ascorbic acid 1.5mg
[0031] pH 6.5-7.5
[0032] Water for injection 50ml
[0033] According to the prescription ratio, weigh the prescribed amount of palonosetron hydrochloride and the required pharmaceutical excipients respectively, add water and stir to dissolve, adjust the pH to 6.5-7.5 with hydrochloric acid and sodium hydroxide, apply activated carbon to remove pyrogens, and divide Pack, fill the bottle with nitrogen gas, stopper and press the cap, and steam sterilize at 100°C for 30 minutes.
Embodiment 3
[0035] Prescription Composition Specifications (100ml: 0.25mg)
[0036] Palonosetron Hydrochloride 0.25mg
[0037] Sodium chloride 850mg
[0038] Sodium bisulfite 3mg
[0039] pH 6.5-7.5
[0040] Water for injection 100ml
[0041] According to the prescription ratio, weigh the prescribed amount of palonosetron hydrochloride and the required pharmaceutical excipients respectively, add water and stir to dissolve, adjust the pH to 6.5-7.5 with hydrochloric acid and sodium hydroxide, apply activated carbon to remove pyrogens, and divide Pack, fill the bottle with nitrogen gas, stopper and press the cap, and steam sterilize at 100°C for 30 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com