Palonosetron percutaneous absorption patch and preparation method thereof
A technology of palonosetron and absorption accelerator, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
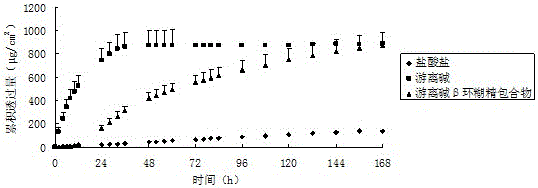
[0027] 0.5 g of palonosetron hydrochloride was dissolved in 20 ml of distilled water, and an aqueous solution of sodium hydroxide was added dropwise until no white turbidity occurred. Extract with 120ml of ethyl acetate for 5 times, combine the extracts, add an appropriate amount of anhydrous sodium sulfate for dehydration, and then dry by rotary evaporation to obtain palonosetron free base.
Embodiment 2
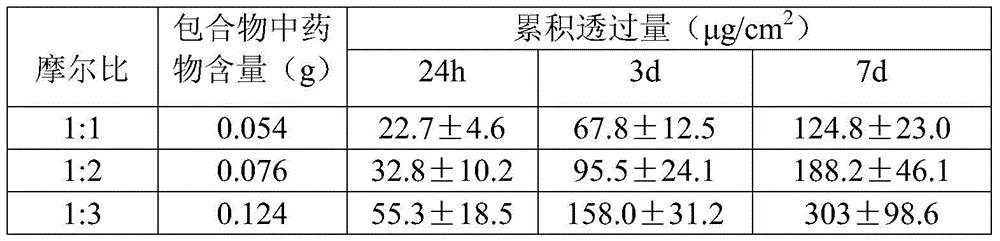
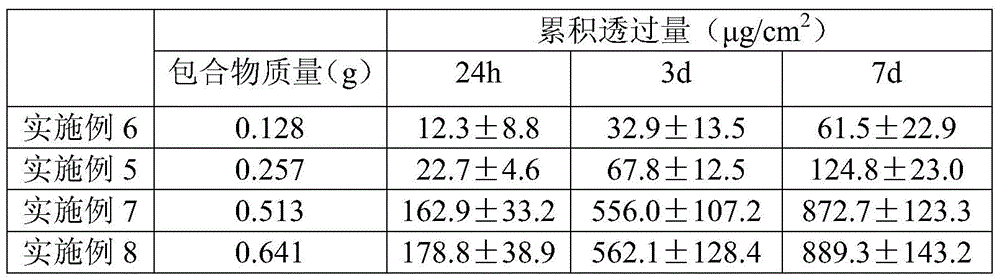
[0029]Disperse 0.121g of palonosetron hydrochloride (equivalent to 0.108g free base) into 2.55g of the polyacrylate pressure-sensitive adhesive DT-2287 of American National Starch Co. Dry at 60°C for 15 minutes, then cover with a backing material including PVC or non-woven fabric, and die-cut to make a patch with a thickness of 30 μm.
Embodiment 3
[0031] Palonosetron free base 0.108g obtained by ethyl acetate extraction was dispersed in 2.55g polyacrylate pressure-sensitive adhesive DT-2287 of National Starch Company of the United States, fully mixed, evenly coated on the release layer, and after 60 After drying at ℃ for 15 min, it was covered with a backing material including PVC or non-woven fabric, and punched to make a patch with a thickness of 30 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com