Palonosetron transdermal patch and preparation method thereof
A patch and agar base technology, applied in the field of medicine, can solve problems such as poor compliance, unfavorable use by patients, inconvenient administration of injections, etc., and achieve the effect of simple production process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
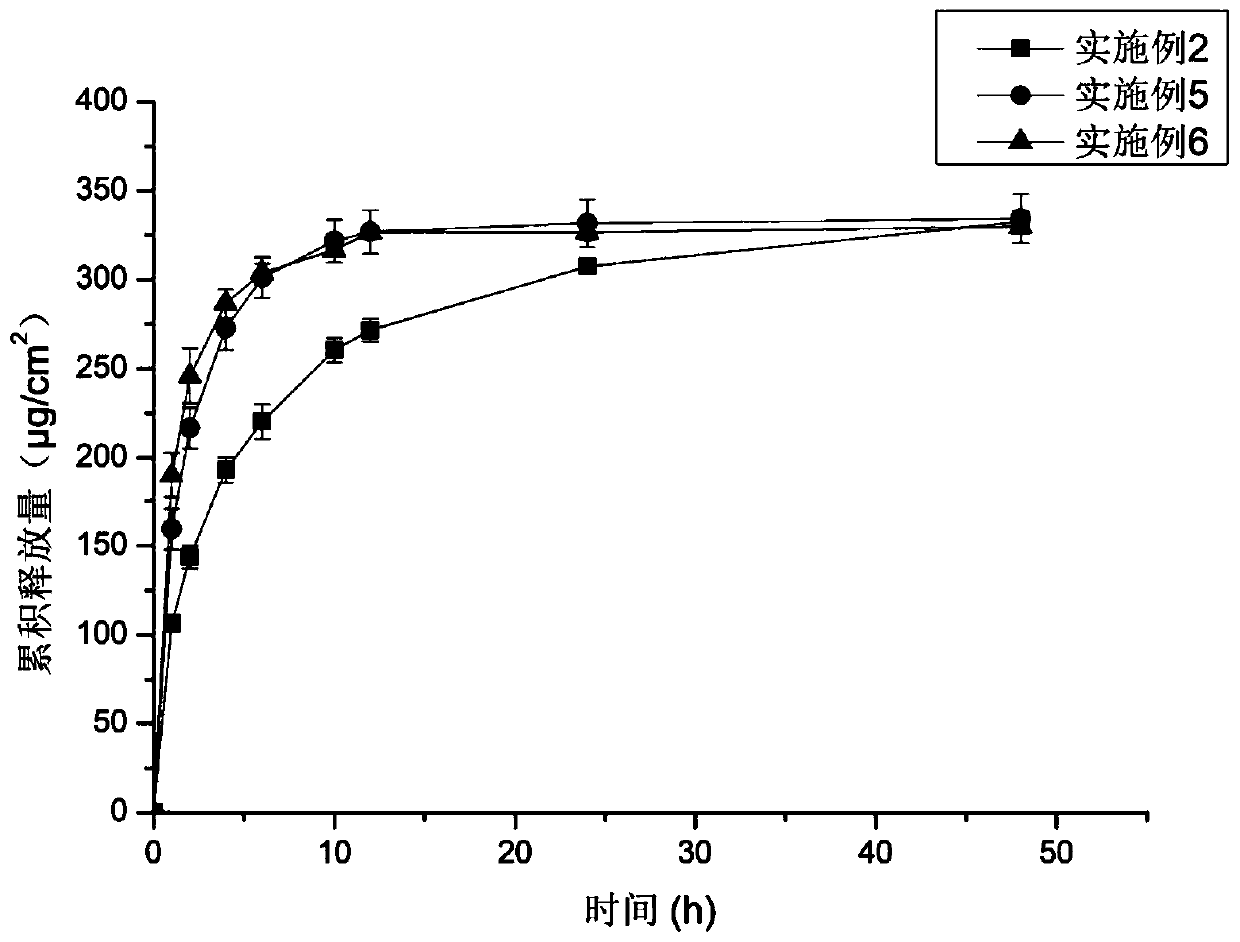
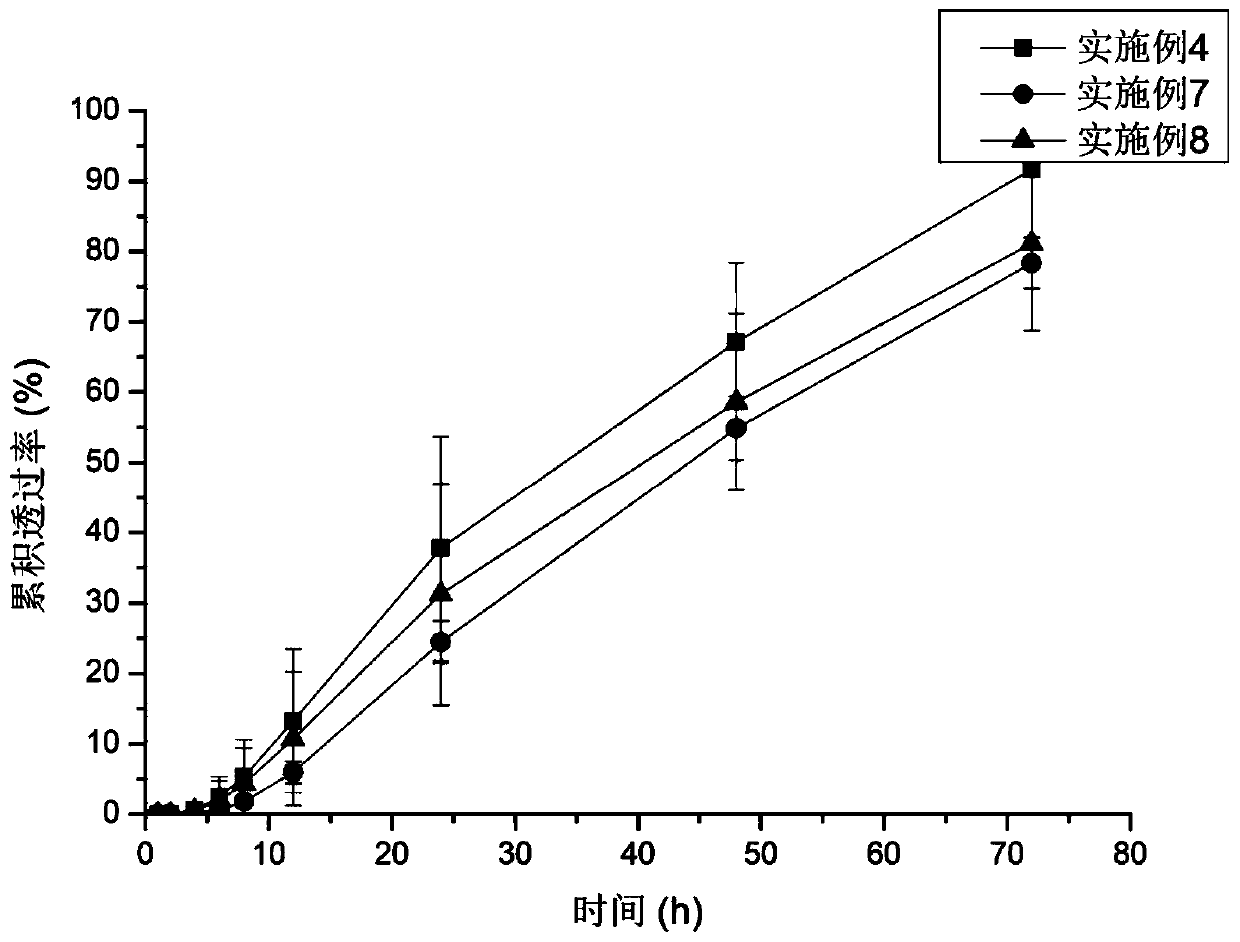
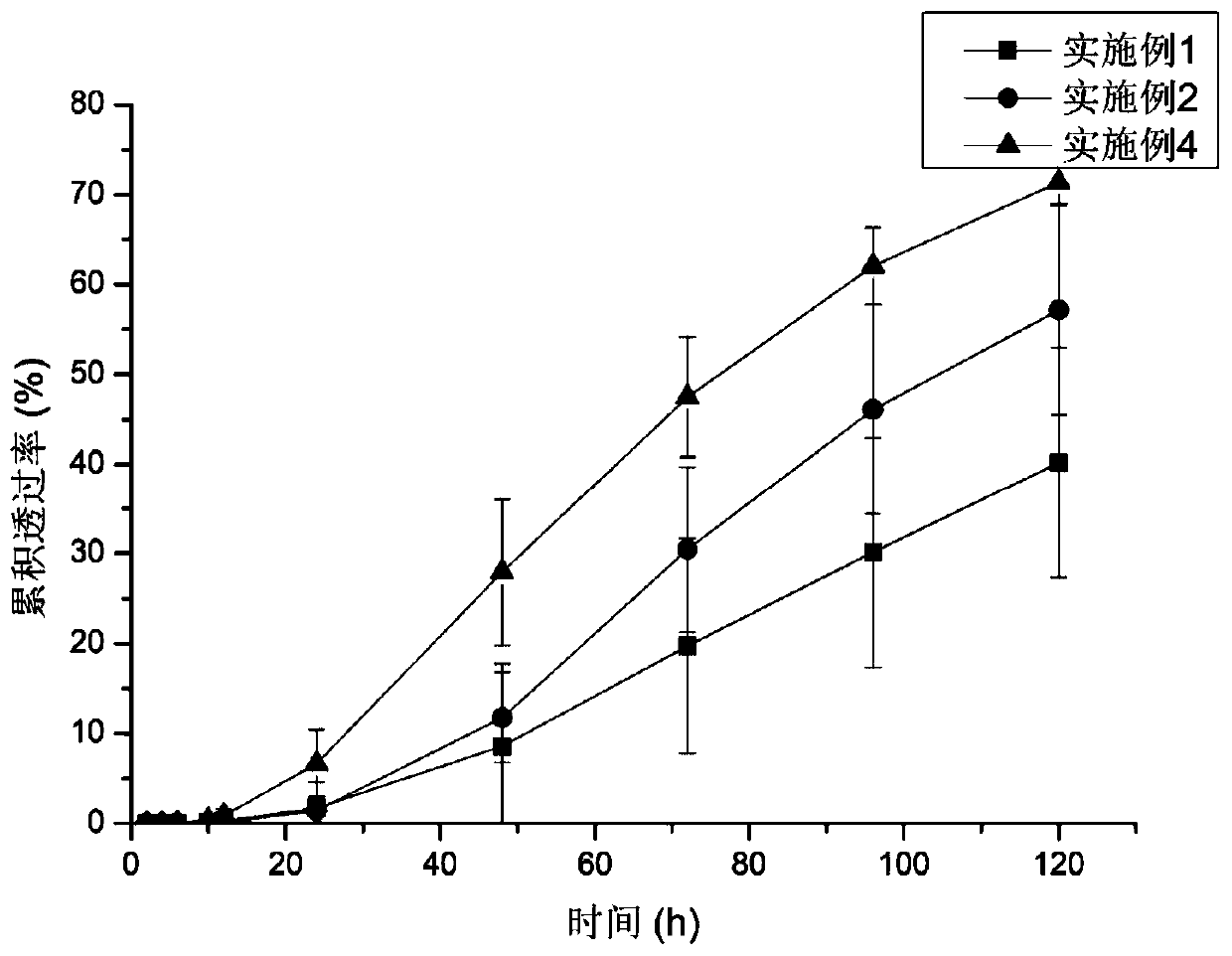
Examples
Embodiment 1
[0047] Dissolve 0.1g (5% w / w) palonosetron base in 5.278g polyacrylate pressure-sensitive adhesive, add 0.002g vitamin E, add 2ml ethyl acetate and mix well, let stand at room temperature for 1h to degas, transfer Coating on the anti-adhesive layer, drying the solvent, and compounding the upper backing layer to form a palonosetron transdermal patch.
Embodiment 2
[0049]Dissolve 0.1g of palonosetron base in 4.722g of polyacrylate pressure-sensitive adhesive, add 0.2g of IPM and 0.002g of vitamin E, add 2ml of ethyl acetate and mix well, let it stand at room temperature for 1h to degas, transfer coating on On the anti-adhesive layer, the solvent is dried in the drying process, and the upper backing layer is compounded to form a palonosetron transdermal patch.
Embodiment 3
[0051] Swell 0.2g E100 with ethyl acetate, add 4.167g polyacrylic acid pressure-sensitive adhesive to form a uniform pressure-sensitive adhesive matrix, dissolve 0.1g palonosetron base in the matrix, add 0.2g IPM and 0.002g VE , add 2ml of ethyl acetate and mix well, let it stand at room temperature for 1h to degas, transfer and coat on the anti-adhesive layer, dry the solvent during the process, and compound the upper backing layer to form a palonosetron transdermal patch.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com