Patents
Literature
66 results about "Aerosol drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
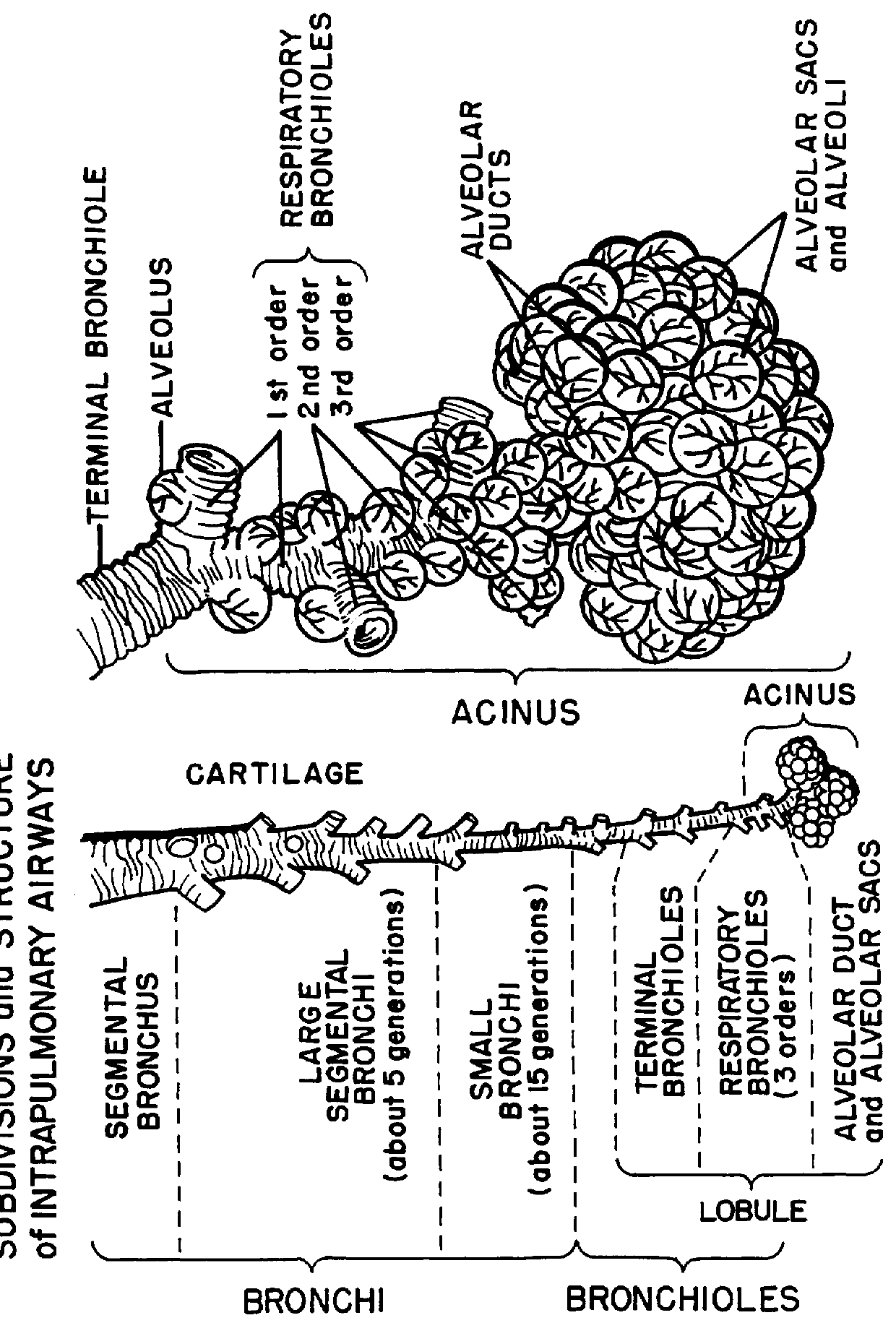
Physical and chemical substances used as medical aerosols include drugs that act as bronchodilators and decongestants, such as epinephrine, ephedrine, isoproterenol, atropine, and the steroids. Wetting agents administered as aerosols to render the bronchial secretions more liquid include tyloxapol and acetylcysteine.
Method and a tobramycin aerosol formulation for treatment prevention and containment of tuberculosis
A method for treatment, prevention and containment of acute and chronic tuberculosis using a preservative-free concentrated tobramycin aerosol formulation delivering tobramycin to the lung endobronchial space including alveoli in an aerosol having mass medium average diameter predominantly between 1 to 5 mu . The method comprises administration of tobramycin in concentration one to ten thousand times higher than the minimal inhibitory concentration of Mycobacterium tuberculosis. A method for containment of and decreasing infectivity periods of tuberculosis patients to shorter periods of time.
Owner:CHIRON CORP
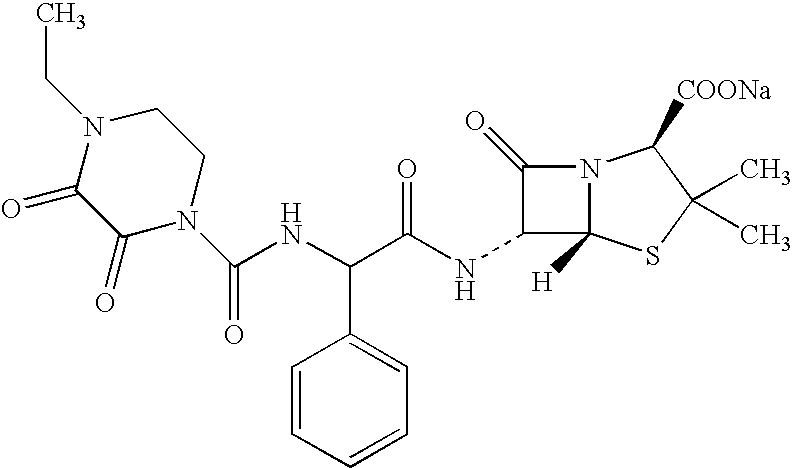
Aerosol drug formulations containing hydrofluoroalkanes and alkyl saccharides
InactiveUS6932962B1Function increaseGood dispersionPowder deliveryDispersion deliveryAerosol drugsActive agent
Aerosol formulations suitable for use in pressurised metered dose inhalers comprise a hydrofluoroalkane propellant, an medicament for inhalation and a surfactant which is a a C8–C16 fatty acid or salt thereof, a bile salt, a phospholipid, or an alkyl saccharide.
Owner:ASTRAZENECA AB
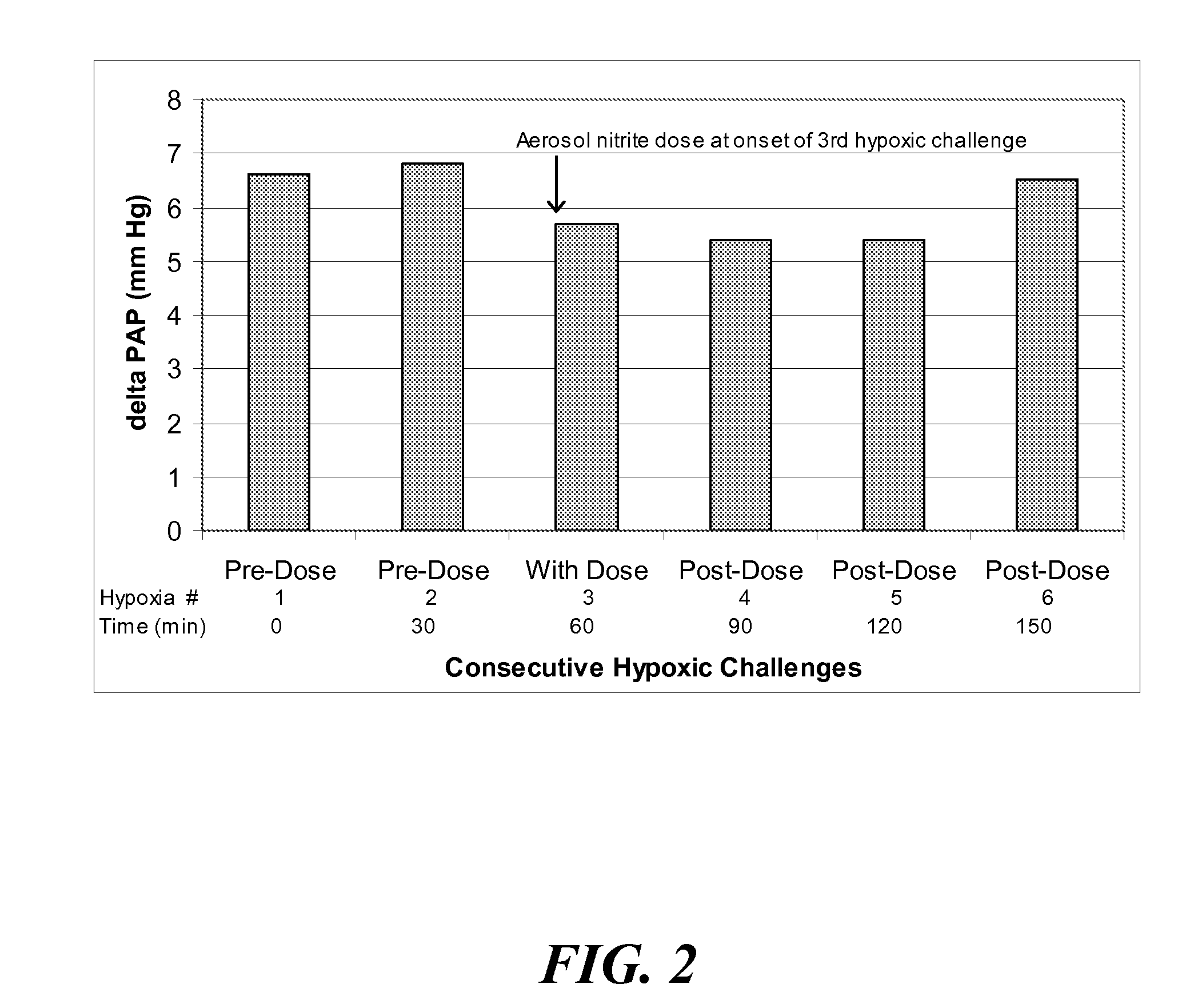
Aerosolized nitrite and nitric oxide -donating compounds and uses thereof
Disclosed herein are formulations of nitrite, nitrite salt, or nitrite- or nitric oxide-producing compounds suitable for aerosolization and use of such formulations for aerosol administration of nitrite, nitrite salt, or nitrite- or nitric oxide-donating compounds for the treatment of pulmonary arterial hypertension, intra-nasal or pulmonary bacterial infections, or to treat or prevent ischemic reperfusion injury of the heart, brain and organs involved in transplantation. In particular, inhaled nitrite, nitrite salt, or nitrite- or nitric oxide-donating compound specifically formulated and delivered to the respiratory tract for the indications is described. Compositions include all formulations, kits, and device combinations described herein. Methods include inhalation procedures and manufacturing processes for production and use of the compositions described.
Owner:AIRES PHARMA
Device and method for delivering an aerosol drug
Owner:PATHFINDER INNOVATIONS LLC +1
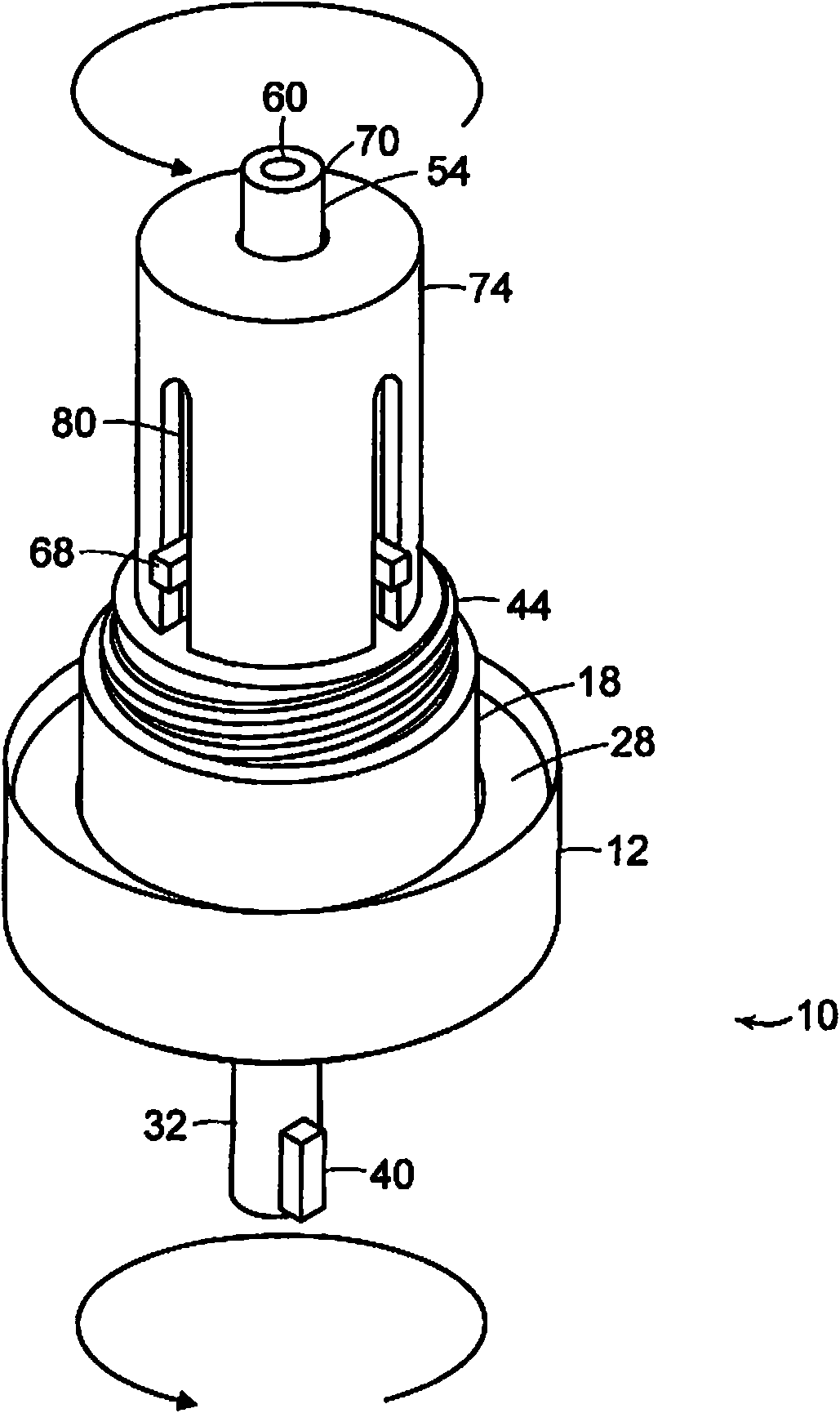
Variable dose aerosol drug canister
A variable volume medicament valve, inhaler; and method of treatment including a housing, and a plug insertable into the housing wherein a volume of medicament to be released by the valve is defined by a distance between a lower surface of the plug and a top surface of the housing and wherein the volume is variable.
Owner:ABBOTT LAB INC
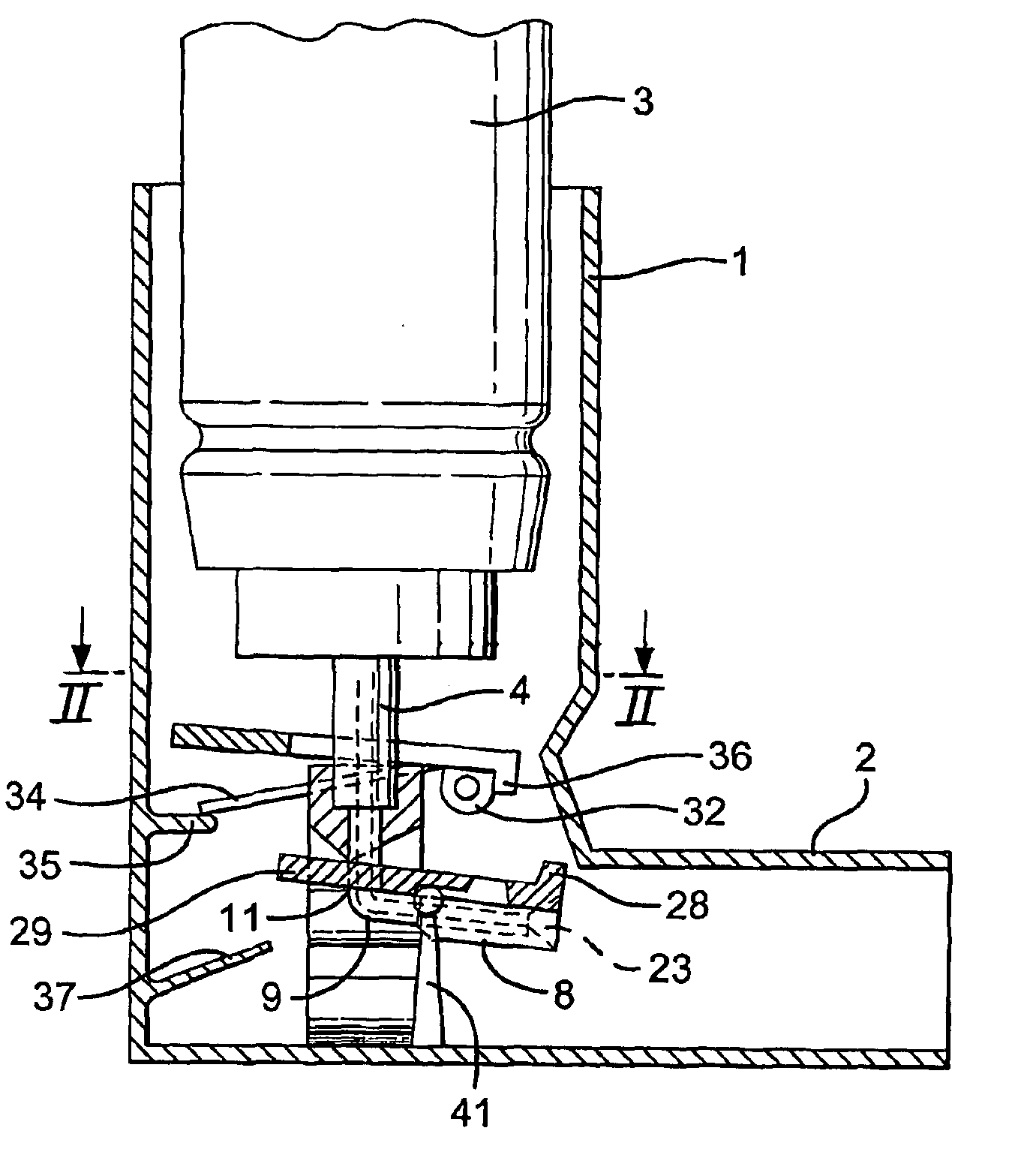
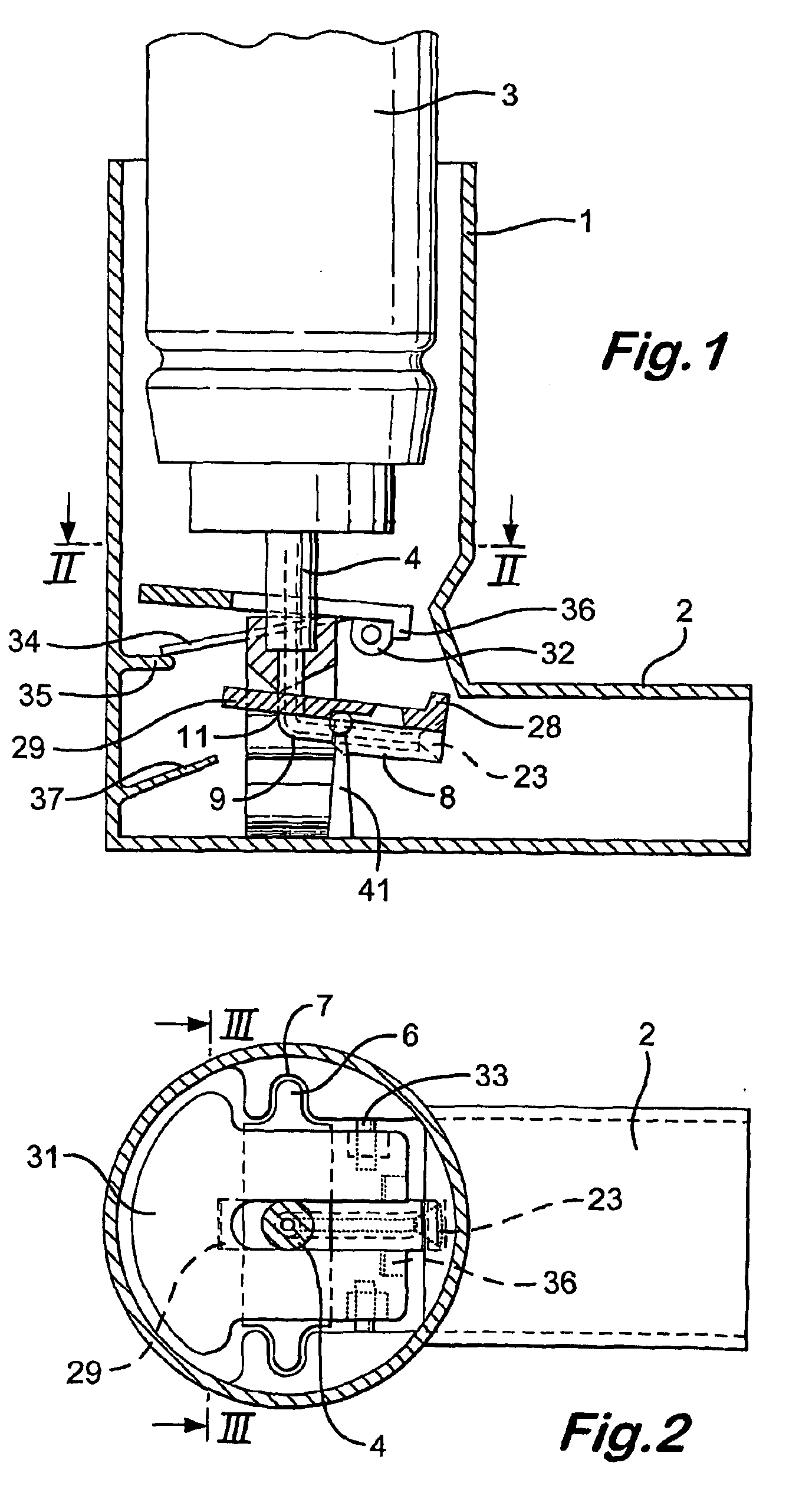
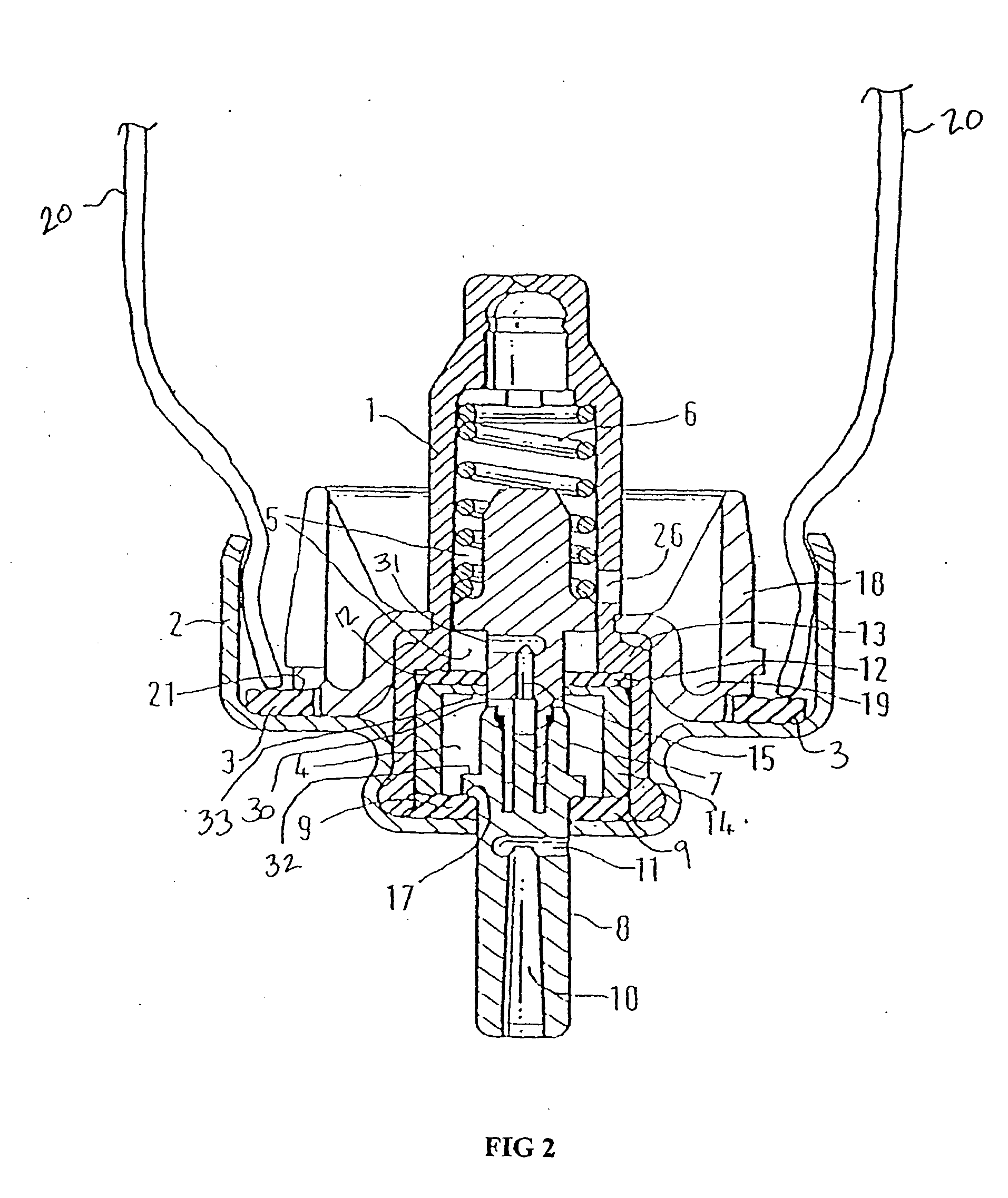
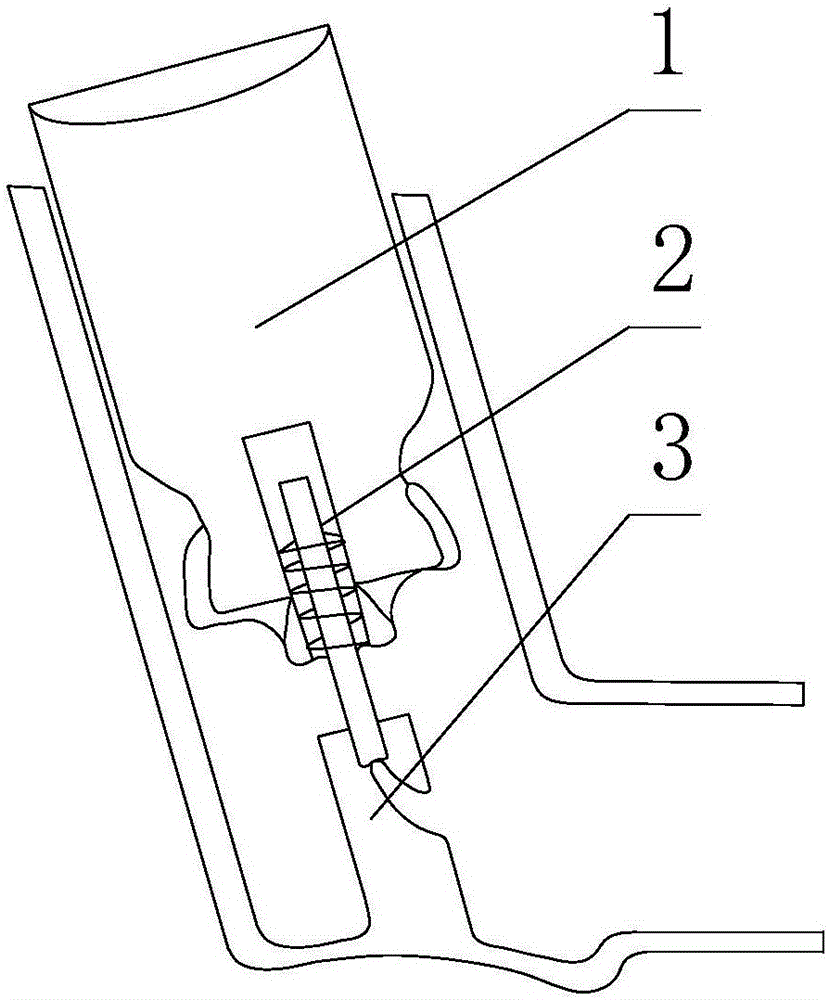
Firing flap dispenser
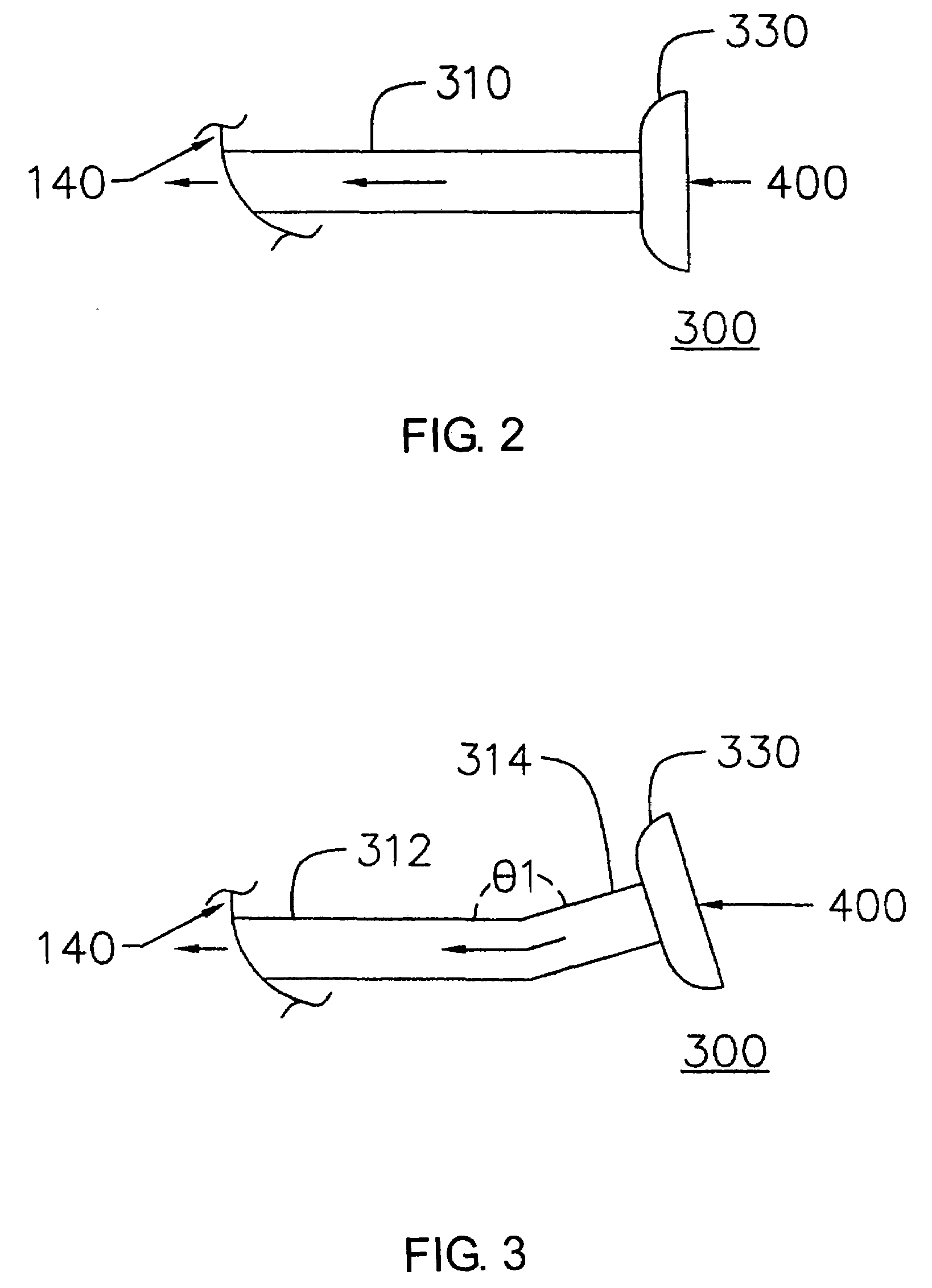
A breath actuated dispenser there-shown has a generally L-shaped hollow body 1 with a mouthpiece 2. An aerosol drug can 3 is mounted in the body. The can has a dispensing spout 4, which engages a receptor moulding 5 incorporating a living hinge 11, a movable outlet member 8 and a kink valve 9. A flap member 31 is pivotally mounted between the can and the receptor moulding. Breathing in through the dispenser by the patient will cause the flap to be drawn down against its spring 34. The outlet member is then tipped down by the spring 37 to point out of the mouthpiece 2, whence the dose is dispensed by opening of the kink valve.
Owner:CLINICAL DESIGNS
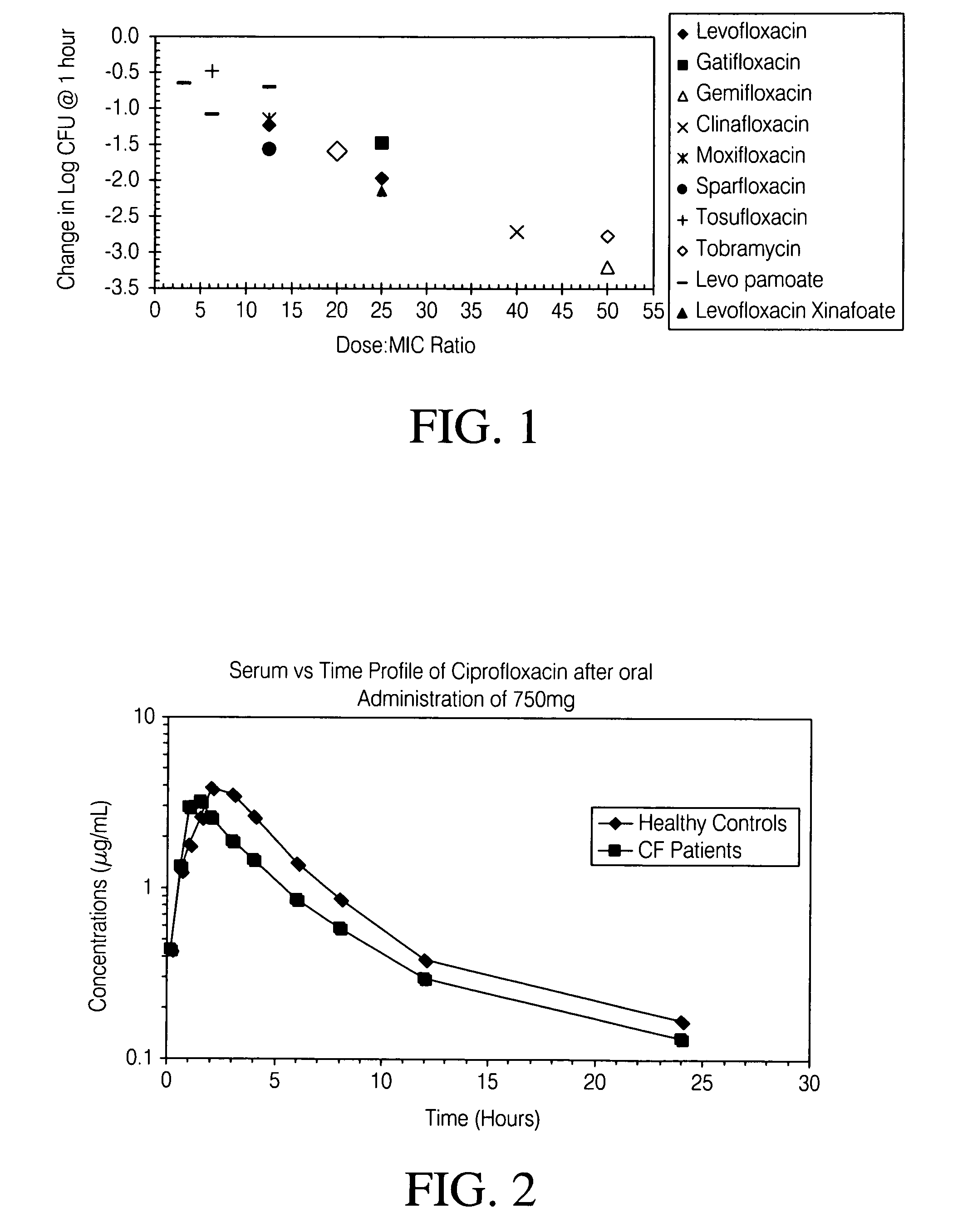
Aerosolized fluoroquinolones and uses thereof
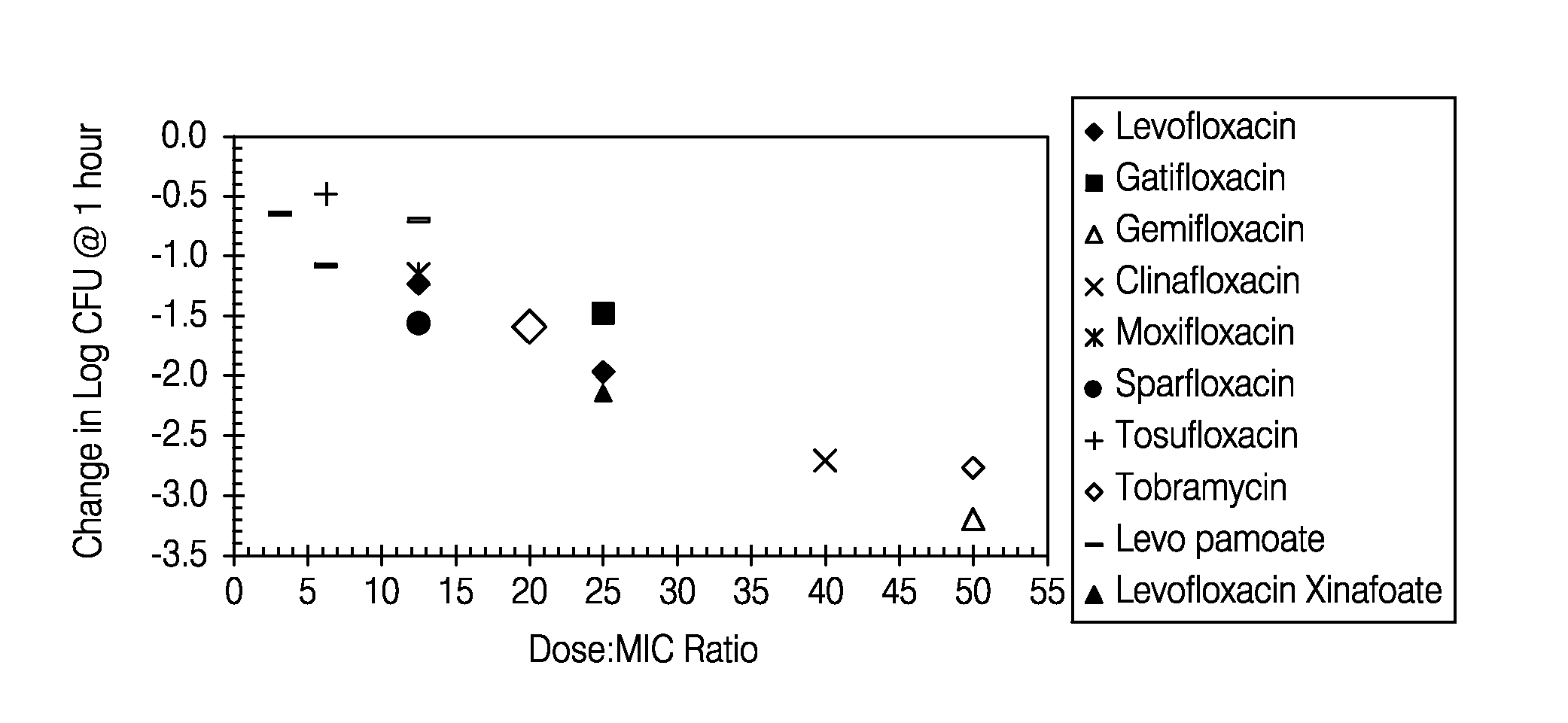
ActiveUS7838532B2Reduce riskHigh levelPowder deliveryHeavy metal active ingredientsAerosol drugsLevofloxacin
Owner:HORIZON ORPHAN LLC
Aerosol Drug Inhibition of Lung Cancer
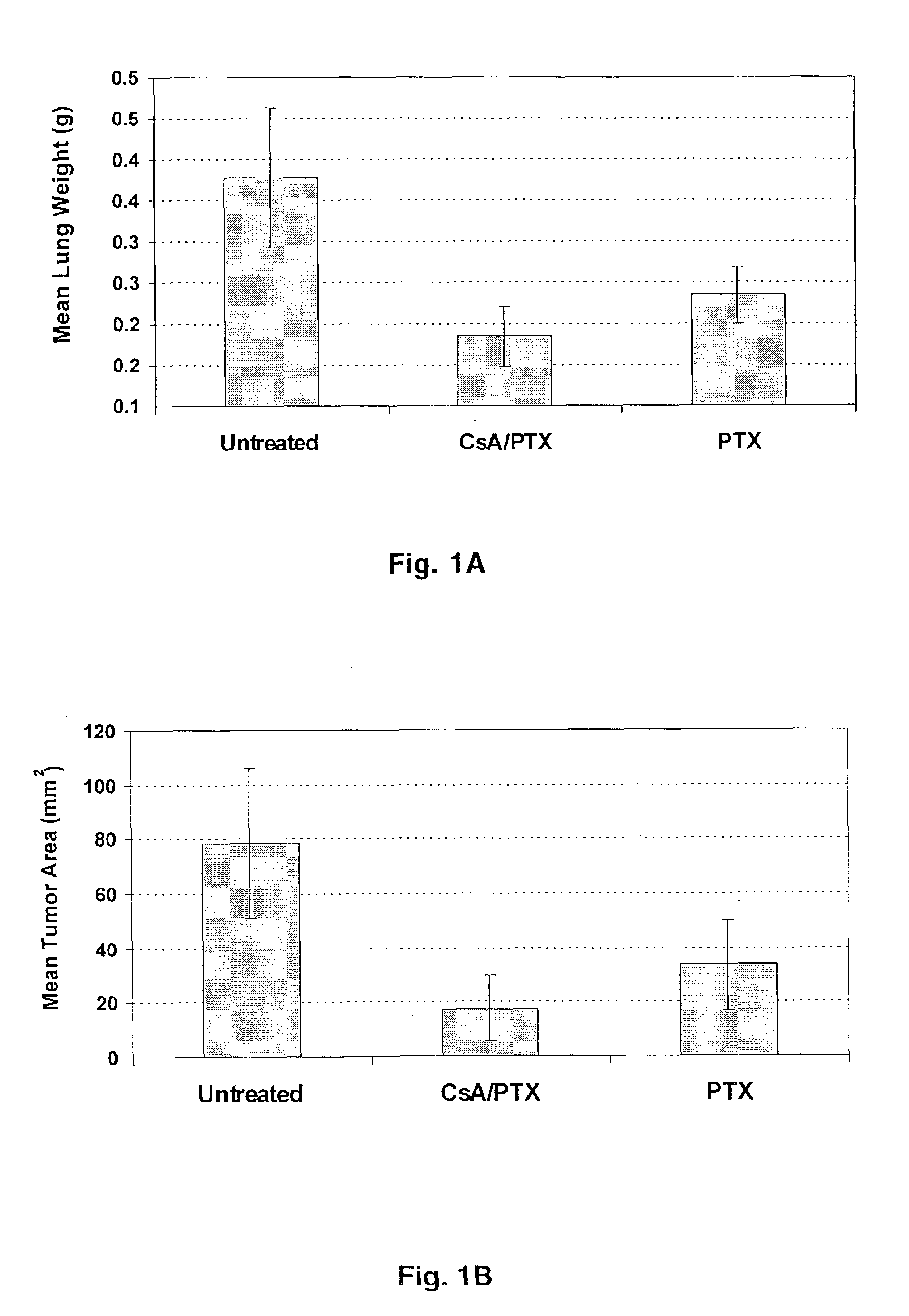
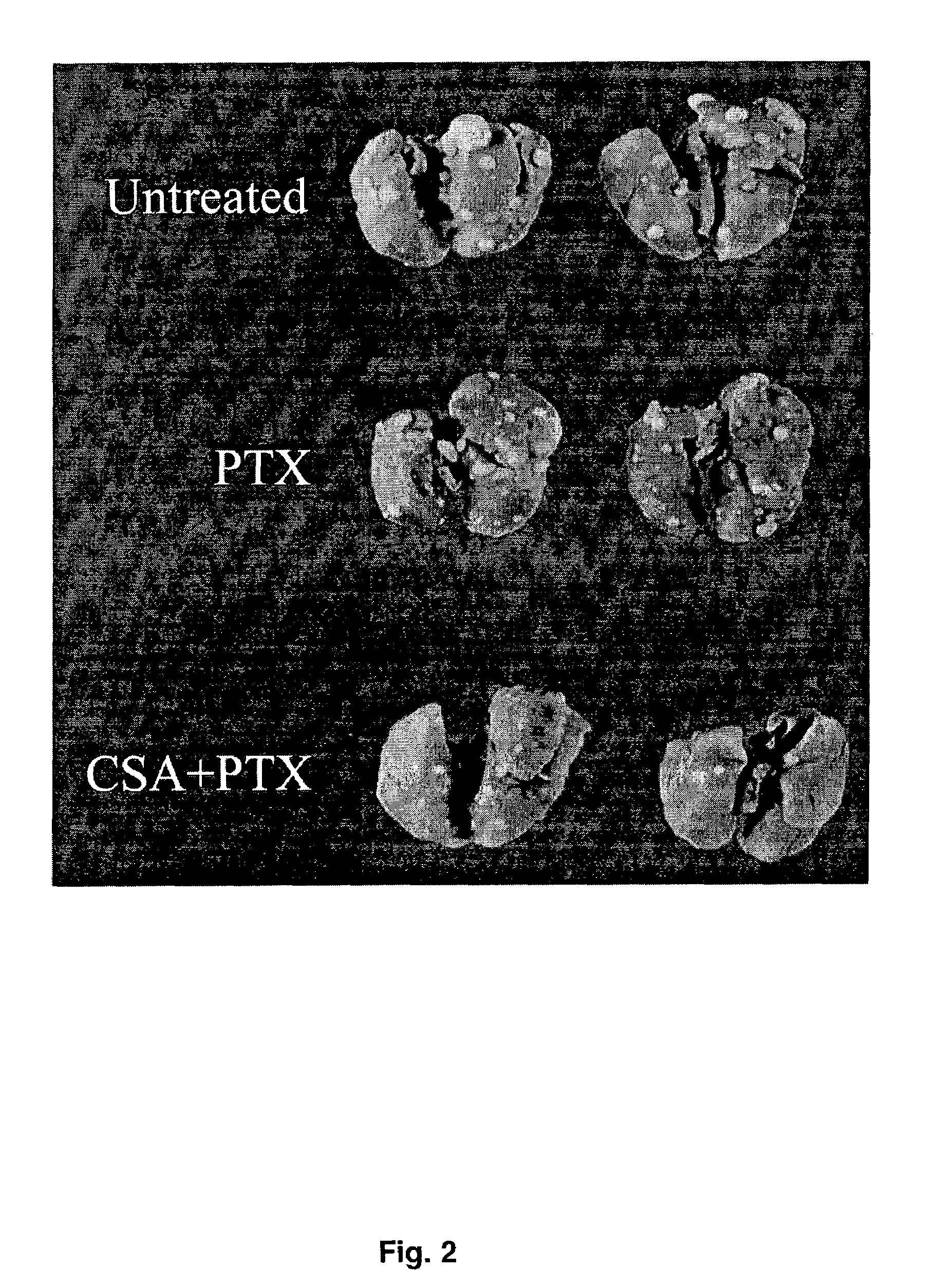
The present invention provides a method of inhibiting growth of lung metastases in an individual comprising the steps of administering a dose of a lipid-drug enhancer liposomal complex and, in sequence, administering a dose of a lipid-anticancer drug liposomal complex. Furthermore, the lipid-drug enhancer liposomal complex may be administered in a continuing dose with the lipid-anticancer drug liposomal complex whereby both liposomal complexes are mixed in the nebulizer. Methods of inhibiting growth of lung metastases in an individual by the sequential administration via aerosolization of a dilauroylphosphatidylcholine-cyclosporin A liposomal complex and a dilauroylphosphatidylcholine-paclitaxel liposomal complex are also provided.
Owner:KNIGHT J VERNON +2
Aerosol generator for drug formulation
InactiveUS20030230303A1Constant flow rate of the fluidBlock deliveryRespiratorsLiquid surface applicatorsAerosol drugsAerosol spray
An aerosol generator such as a hand-held inhaler and method of delivering aerosol to a user inhaling on an outlet of a mouthpiece when a pressure drop is detected within the mouthpiece. A medicated fluid passing through a capillary passage is heated sufficiently to vaporize the fluid and form the aerosol by condensation of the vaporized fluid as it admixes with air. Air is supplied to the mouthpiece through an air passage which is initially closed during detection of the pressure drop. A metering chamber allows consistent delivery of precise doses of fluid to the capillary passage. The pressure drop is detected before air is supplied to the mouthpiece with the result that the aerosol can be quickly delivered to the user as the user begins to inhale on the mouthpiece. The quick delivery of aerosol provides more efficient use of the user's lung capacity.
Owner:PHILIP MORRIS USA INC
Aerosol drug inhibition of lung metastases
The present invention provides a method of inhibiting growth of lung metastases in an individual comprising the steps of administering a dose of a lipid-drug enhancer liposomal complex and, in sequence, administering a dose of a lipid-anticancer drug liposomal complex. Furthermore, the lipid-drug enhancer liposomal complex may be administered in a continuing dose with the lipid-anticancer drug liposomal complex whereby both liposomal complexes are mixed in the nebulizer. Methods of inhibiting growth of lung metastases in an individual by the sequential administration via aerosolization of a dilauroylphosphatidylcholine-cyclosporin A liposomal complex and a dilauroylphosphatidylcholine-paclitaxel liposomal complex are also provided.
Owner:RES DEVMENT FOUND
Device and method for delivering an aerosol drug
A device and method for delivering a therapeutic drug to a patient in the form of an aerosol includes a heat generating chamber and an aerosol forming chamber separated by a heat conductor. A heating element is disposed within the heat generating chamber in heat conducting relation with the heat conductor. A substrate having a therapeutic drug and an aerosol forming agent deposited thereon is disposed within the aerosol forming chamber in heat conducting relation with the heat conductor. The heating element ignites a fuel source consisting essentially of a combustible liquefied gas to generate heat that is conducted to the heat conductor and then to the substrate via the heat conductor. The heat generated by the heating element activates the aerosol forming agent to volatilize the therapeutic drug into an aerosol drug contained within the aerosol forming chamber and made available to be inhaled by the patient.
Owner:PATHFINDER INNOVATIONS LLC +1
Respiratory equipment spacer assembly
A spacer assembly for a ventilator or other respiratory equipment for dispensing aerosol drugs from metered dose inhaler (MDI) canisters or nebulized drugs from a nebulizer into a respiratory gas stream delivered from a ventilator or other respiratory equipment connected to a patient. The improvements involve optimizing the shape of the spacer assembly body member and providing an efficient MDI nozzle assembly to allow maximal evaporation of the propellant before the propellant droplets impact the walls of the body member while providing a compact volume for directing the output of an MDI canister or a nebulizer into the gas stream.
Owner:SALTER LABS
Triggering circuit for an aerosol drug-dispensing device
InactiveUS7357133B2Preventing erroneous triggeringLess sensitiveRespiratorsMedical devicesElectrical resistance and conductanceAerosol drugs
An electronic gas flow triggering circuit for use in an aerosol drug dispensing device has a voltage source and a self-nulling circuit to which voltage is supplied from the voltage source. A hot wire anemometer filament forms a component of the bridge circuit which is adapted to maintain a constant resistance of the anemometer filament, the bridge drive voltage being dependent upon the gas flow across the anemometer filament. A comparator compares the bridge output voltage with a reference voltage and provides a triggering signal to operate the device if the bridge drive voltage is greater than the reference voltage.
Owner:THE TECHNOLOGY PARTNERSHIP PLC
Face mask
A face mask for use in gas delivery applications, such as breathable gas or aerosol drug systems. The face mask includes a body having a peripheral edge for placement against the face of a wearer. The peripheral edge defines a single chamber over the nose and the mouth of the wearer. An inlet opening is formed on a surface of the body for supplying inhalation gas to the nose through the chamber. A vent assembly inwardly extends from the surface of the body to the chamber and seals around the mouth for passing exhalation gas to the exterior of the body.
Owner:MESURE TECH
Aerosols for sinunasal drug delivery
InactiveUS8852557B2Convenient treatmentReduce gapOrganic active ingredientsBiocideDiseaseNasal cavity
Owner:PARI PHARMA GMBH
Pharmaceutical Metered Dose Inhaler and Methods Relating Thereto
InactiveUS20080190418A1Improve stabilityDecreased drop in FPMRespiratorsOrganic active ingredientsEngineeringPharmaceutical formulation
Metering valves for use in a metered dose inhaler that include a valve body, a first stem seal including a first elastomeric material, a second stem seal including a second elastomeric material different from the first elastomeric material, and a valve stem slidably engaged with at least one of the first stem seal and the second stem seal as well as sealed containers configured to contain an aerosol pharmaceutical formulation that include a container having an opening therein, a cap covering the opening in the container, a metering valve adjacent the cap, and a cap seal positioned between the cap and the container to provide a sealed container where the metering valve include at least one stem seal that includes a first elastomeric material, and the cap seal includes a second elastomeric material different from the first elastomeric material are described.
Owner:GLAXO GROUP LTD
Face mask
A face mask for use in gas delivery applications, such as breathable gas or aerosol drug systems. The face mask includes a body having a peripheral edge for placement against the face of a wearer. The peripheral edge defines a single chamber over the nose and the mouth of the wearer. An inlet opening is formed on a surface of the body for supplying inhalation gas to the nose through the chamber. A vent assembly inwardly extends from the surface of the body to the chamber and seals around the mouth for passing exhalation gas to the exterior of the body.
Owner:MESURE TECH
Treating cystic fibrosis with antibiotics via an aerosol drug
A method of treating respiratory disorders by delivering antibiotic to the lung alveoli using an aerosol drug delivery system.
Owner:WYETH LLC
Aerosolized fluoroquinolones and uses thereof
ActiveUS20100158957A1Reduce riskHigh levelAntibacterial agentsPowder deliveryAerosol drugsLevofloxacin
Disclosed herein are formulations of fluoroquinolones suitable for aerosolization and use of such formulations for aerosol administration of fluoroquinolone antimicrobials for the treatment of pulmonary bacterial infections. In particular, inhaled levofloxacin specifically formulated and delivered for bacterial infections of the lungs is described. Methods include inhalation protocols and manufacturing procedures for production and use of the compositions described.
Owner:HORIZON ORPHAN LLC
Hand-thrown aerosol fire extinguisher
InactiveCN101850164AMake up for the lack of mobile useFlexible useFire rescueElectricityCombustion chamber
The invention discloses a hand-thrown aerosol fire extinguisher, comprising a casing, an igniter, an aerosol generator containing an aerosol drug column, a combustion chamber, a cooling chamber and an aerosol nozzle, wherein, the igniter in the aerosol generator is a delay element and an ignition element; the ignition element is a Lanyard-actuated ignition element, a push type ignition element or a thermosensitive ignition element; the ignition element is connected with the delay element; the tail end of the delay element is arranged in the aerosol drug column; and a grip or a handle is arranged on the casing. The hand-thrown aerosol fire extinguisher of the invention adopts the Lanyard-actuated ignition or push type ignition to replace electric ignition adopted in the existing aerosol extinguisher, does not has the problems of false tripping or no starting occurring in electric ignition in an extinguishing process, does not has safety potential for hurting people due to explosion, and has lower manufacturing cost, longer storage period and convenience for flexible use. The hand-thrown aerosol fire extinguisher of the invention belongs to an unfixed extinguisher which has the characteristics of environmentally friendliness, safety, reliability and no damage on protectors, and is suitable for fire-fighting of various relative sealing spaces.
Owner:HUBEI WEIDONG HLDG GROUP
Medical aerosol formulation
A medicinal formulation is disclosed. The formulation comprises: a therapeutic amount of a protein or peptide medicament, a fluid for containing said medicament having a molecular size ranging from 1 K Dalton to about 150 K Daltons, a fluid carrier for containing the medicament, and a stabilizer selected from an amino acid, a derivative thereof or a mixture of the foregoing.
Owner:AEROPHARM TECH
Variable dose aerosol drug canister
Owner:ABBOTT RESPIRATORY LLC
Aerosol precursor containing effective components of oral drug for treating rhinitis and method for dispersing effective components into nanoscale droplets
InactiveCN104688688APromote absorptionLarge specific surface areaAerosol deliveryPharmaceutical non-active ingredientsFlavorAerosol drugs
The invention relates to an aerosol precursor containing effective components of an oral drug for treating rhinitis. The aerosol precursor comprises glycerine, propylene glycol, 1,3 butanediol, flavor components and oral dug extract for treating rhinitis, wherein the mass ratio of the glycerine to the propylene glycol to the 1,3 butanediol to the flavor components to the oral dug extract for treating rhinitis is (40-45):(20-25):(0-10):(0-10):(1-10). The invention further relates to a method for dispersing the effective components of the oral drug for treating rhinitis into nanoscale droplets, and an aerosol containing nanoscale droplets. The aerosol precursor contains the effective components of the oral drug for treating rhinitis, is placed in an electronic cigarette utensil containing an electric heating device to be heated to produce nanoscale droplets, and the human body can better absorb the effective components.
Owner:CHINA TOBACCO YUNNAN IND
Inhalation aerosol of plant extract for treating asthma and preparation method
InactiveCN101569684AGood curative effectLow toxicityAerosol deliveryRespiratory disorderAerosol drugsIrritation
The invention provides an inhalation aerosol of plant extract for treating asthma and a preparation method. The plant extract for treating asthma is prepared from the following materials by the weight portion: 5 to 20 of bunge pricklyash seed, 12 to 85 of argy wormwood leaf, 5 to 35 of asarum and 5 to 35 of Chinese ephedra. The invention is characterized in that lipophilic plant extract is initiatively mixed with hydro-fluoro alkane with relatively larger polarity for preparing a quantitative inhalation aerosol of plant extract. When sprayed out, the medicament changes into small milky particles after touching water, and the milky particles are adsorbed quickly by human body to take effect. The prepared inhalation aerosol reduces medicament irritation and toxicity, enhances medicament absorption rate and transmucosal property, further enhances medicament bioavailability, strengthens medicament curative effect, and improves the medicament taking adaptability of patients.
Owner:浙江省中药研究所有限公司 +1
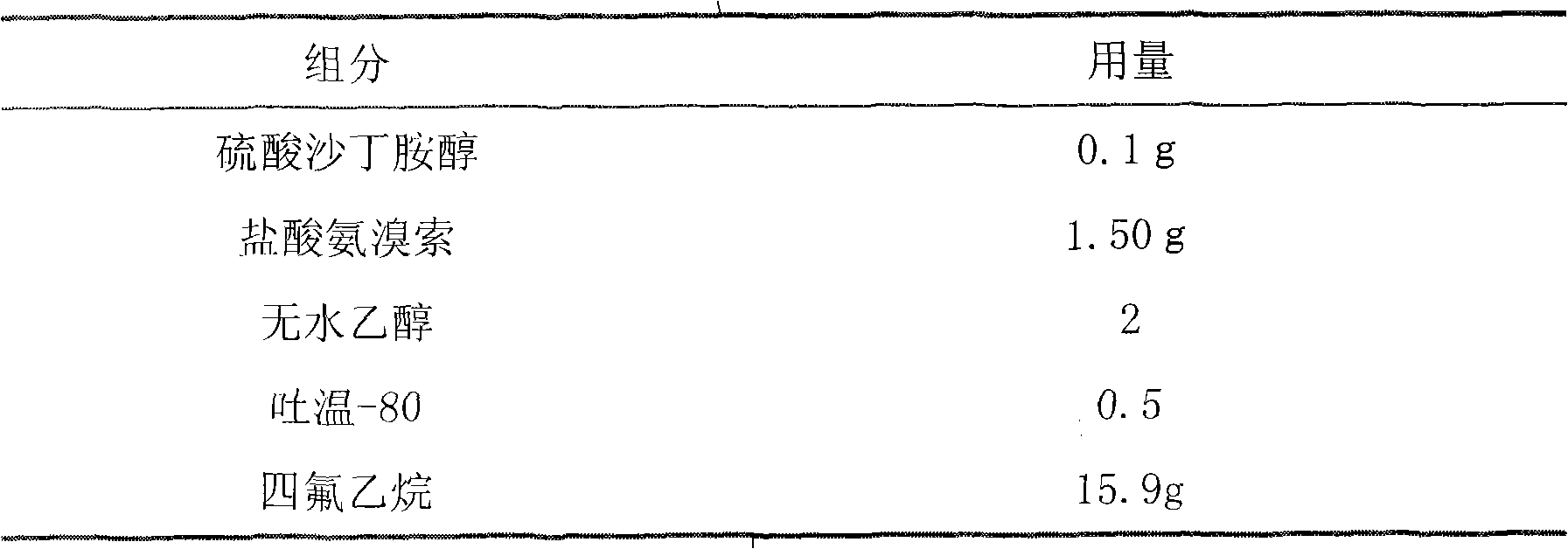
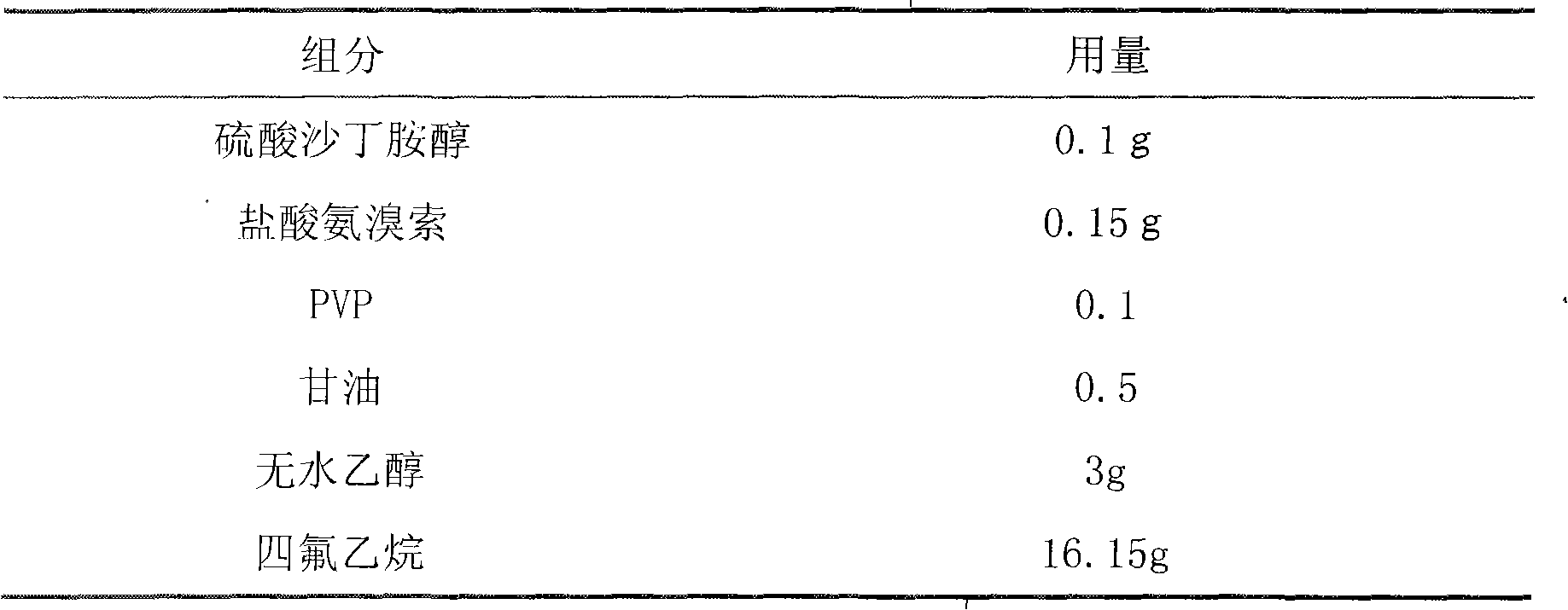
Aerosol taking salbutamol and ambroxol as active ingredients
Owner:北京利乐生制药科技有限公司
Aerosol preparation and quantitative inhalation aerosol
ActiveCN106581010AImprove protectionAvoid damageOrganic active ingredientsDispersion deliveryAerosol drugsAerosol spray
The invention discloses an aerosol preparation and quantitative inhalation aerosol. The preparation comprises active components and a liquefaction propellant used as an active component solvent, wherein the active components comprise budesonide and formoterol fumarate; the liquefaction propellant is hydrofluorocarbon; the preparation further comprises PVPk25 (Polyvinyl Pyrrolidone k25) and PEG1000 (Polyethylene Glycol 1000), which are added into the liquefaction propellant; the content of PVPk25 in the aerosol preparation is 0.00005wt%-0.003wt%; the content of PEG1000 in the aerosol preparation is 0.001wt%-0.01wt%; and the aerosol comprises the preparation. According to the aerosol preparation and the aerosol, provided by the invention, when the aerosol is sprayed out, the gathering of drug particles can be effectively avoided or alleviated and the dosage of fine particles is improved.
Owner:SICHUAN PURITY PHARM CO LTD
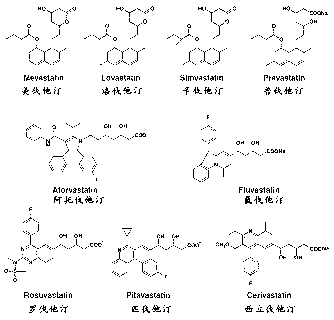
Application of preparing statins aerosol inhalant in airway inflammation disease
The invention discloses an application of manufacturing statins gas spray in airway inflammation disease. The aerosol refers to a non-chlorofluorocarbon (CFC) solution formula. The fine subfraction (FPF) of the formula is high. The result display of a gas spray inhalant thermodynamic experience is quick and the effect is high so as to be used for treating bronchial asthma and a chronic obstructive pulmonary disease (COPD). The aerosol inhalant has the advantages of being good in anti-inflammation of airway and reducing adverse reaction caused by oral large doses.
Owner:谢诒诚
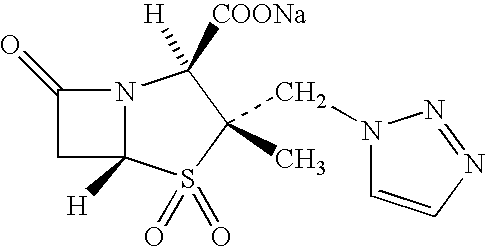
Solution-type metered dose inhalation aerosol for treating respiratory diseases and preparation method thereof
InactiveCN103784401AEasy to expandGood against airway inflammationAerosol deliveryRespiratory disorderDiseaseAerosol drugs
The invention discloses a solution type metered dose inhalation aerosol of glycopyrronium bromide or derivatives thereof and a preparation method thereof. The aerosol comprises a main drug, a cosolvent, a surfactant and a propellant. The aerosol is high in fine particle fraction (FPF) and good in placement stability under long-term reserving and accelerating conditions, and the FPF does not have remarkable variation. Pharmacodynamic test results indicate that the aerosol is quick in response, can be used for treating bronchial asthma and chronic obstructive pulmonary disease, and has excellent effects of resisting airway inflammation, airway fibrosis and airway remodeling.
Owner:BEIJING FSWELCOME TECH DEV +1
Medicinal aerosol formulations
The use of a microparticle loosening agent in a pharmaceutical aerosol formulation comprising a suspension of drug particles in a propellant, the microparticle loosening agent having a very small mass median diameter, which is less than 1 micron, preferably less than 300nm. Particulate loosening agents include, for example, vitamin C, sugars, polysaccharides, amino acids, organic and inorganic salts, urea, and propiododon.
Owner:3M INNOVATIVE PROPERTIES CO
Nebulised antibiotics for inhalation therapy
Owner:帕锐制药两和公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com