Aerosol Drug Inhibition of Lung Cancer
a technology of lung cancer and aerosol, which is applied in the field of aerosol drug inhibition of lung cancer, can solve the problems of cancer-related deaths, limited use of traditional systemic routes of drug delivery, and limited drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Chemicals
[0042] Paclitaxel was obtained from SuperGen (San Ramon, Calif.). Cyclosporin A was purchased from Chemwerth (Woodridge, Conn.). Dilauroylphosphatidylcholine (DLPC) was purchased from Avanti Polar Lipids (Alabaster, Ala.). Organic solvents (HPLC grade) were obtained from Fisher Scientific. Sterile water for irrigation was purchased from Baxter Healthcare Corporation (Deerfield, Ill.).
example 2
Animals
[0043] Female BALB / c mice (7-8 weeks old) were obtained from Harlan-Sprague Dawley (Indianapolis, Ind.) and housed in standard cages with food and water provided ad libitum. Experiments were performed with the approval of the Institutional Animal Care and Use Committee.
example 3
Cell Culture and Animal Model
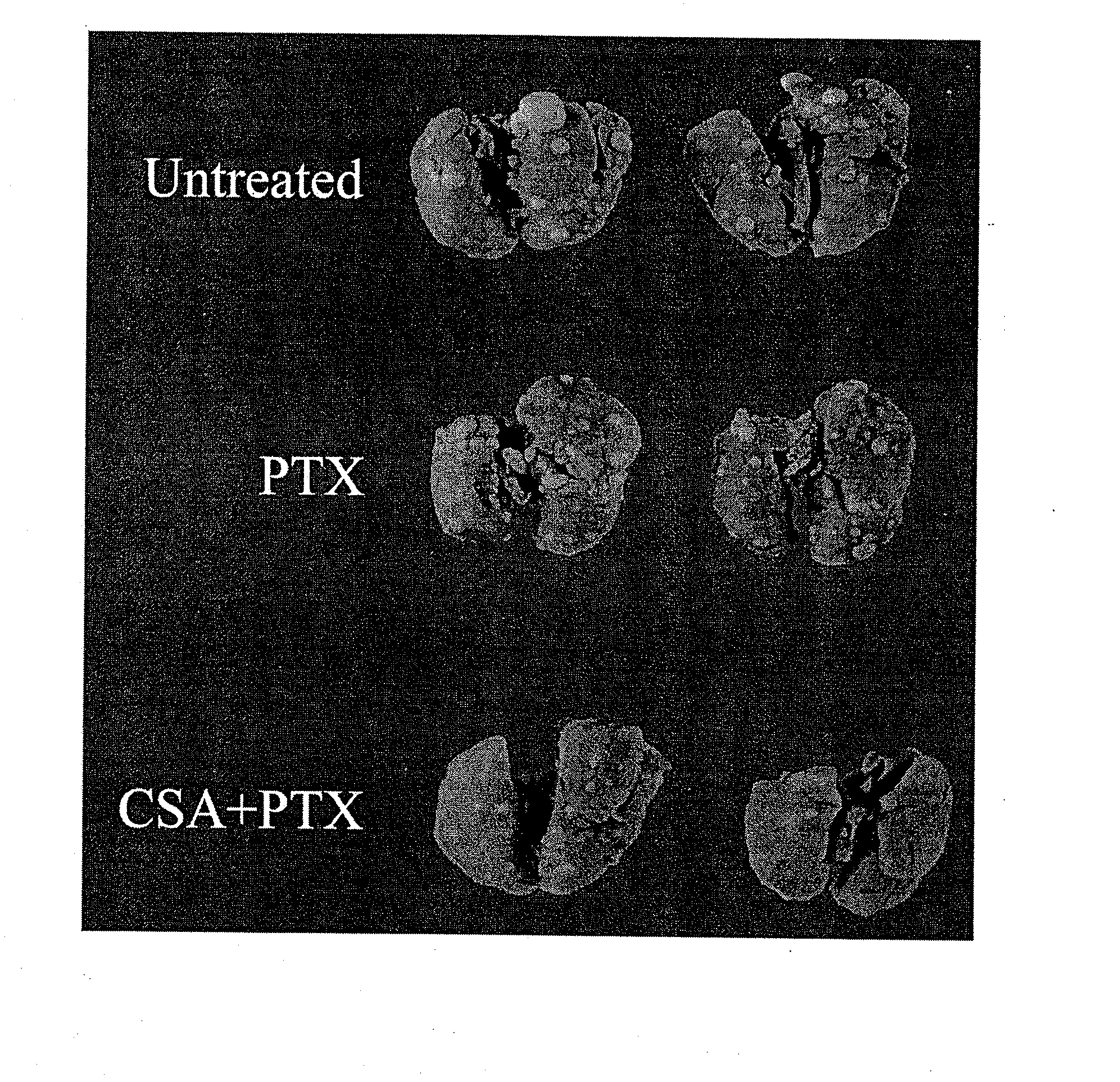
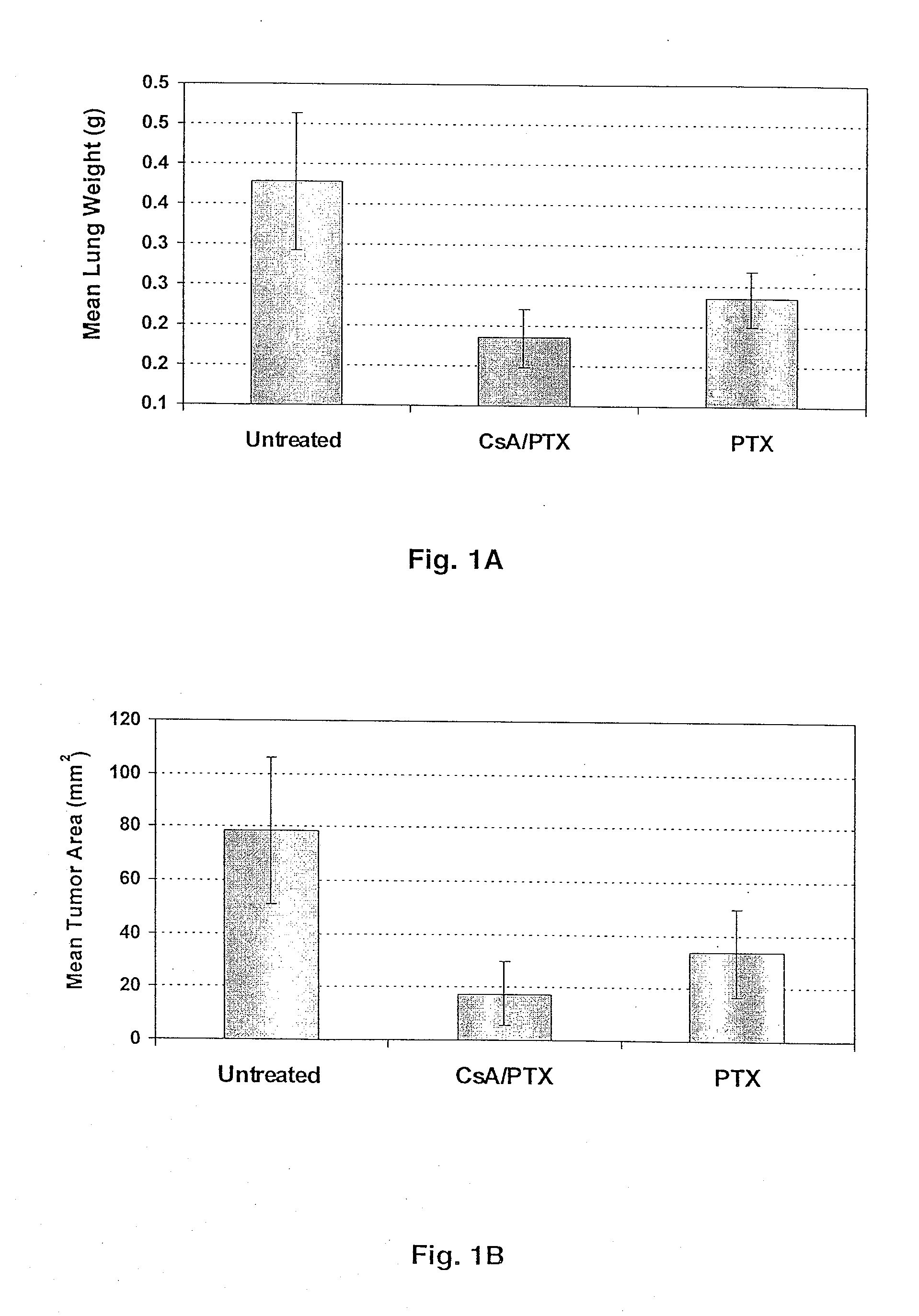
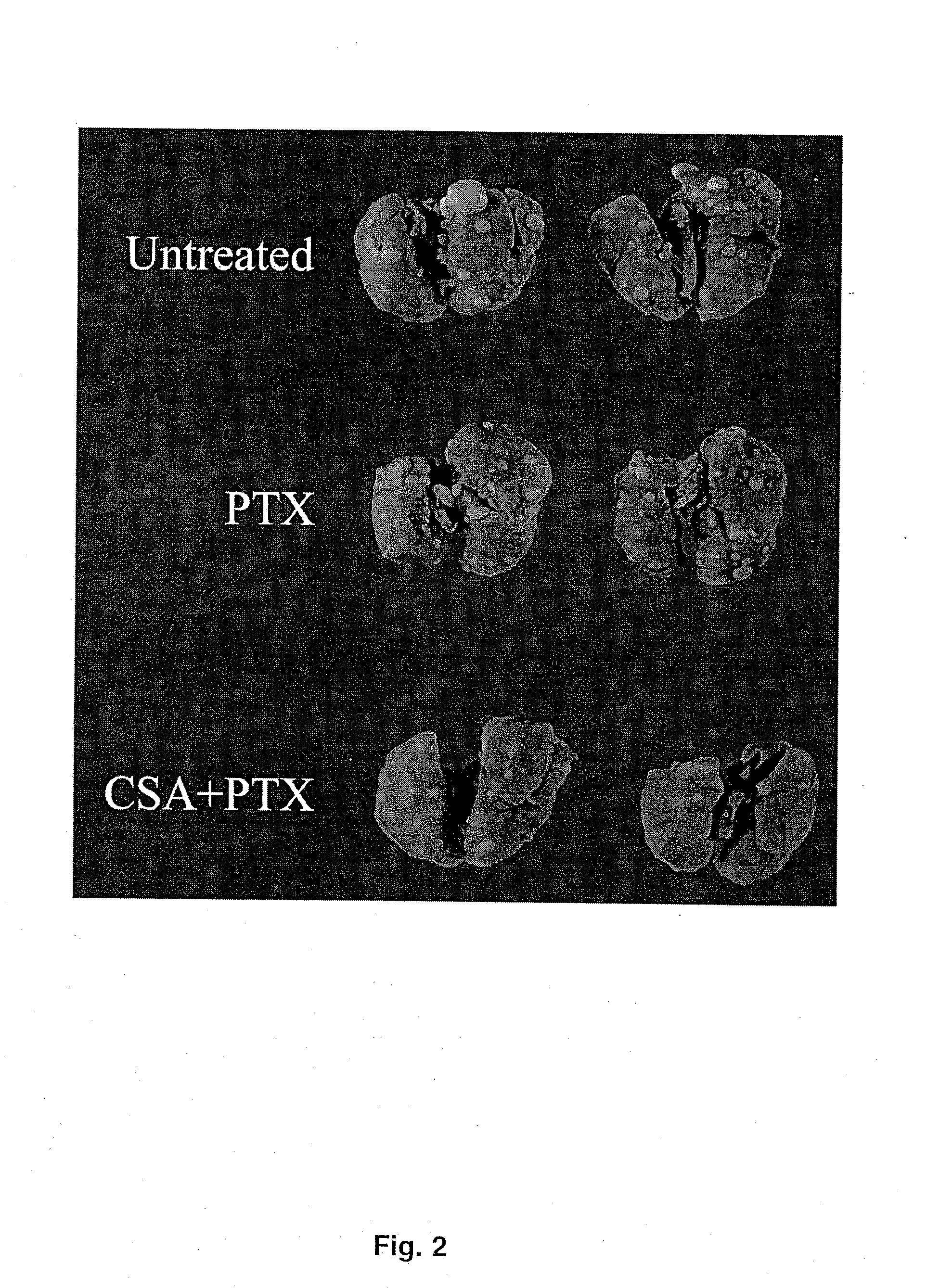
[0044] The mouse renal carcinoma cell line (Renca) was provided by and maintained by serial passages as described by Dr. Robert Wiltrout, National Cancer Institute (Frederick, Md.). Prior to in vivo implantation, Renca cells were cultured in vitro for 2 passages as described previously (14). To induce pulmonary metastases, 100,000 cells were injected intravenously in 0.2 ml saline via tail vein in syngeneic BALB / c mice.
PUM
| Property | Measurement | Unit |
|---|---|---|
| transition temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com