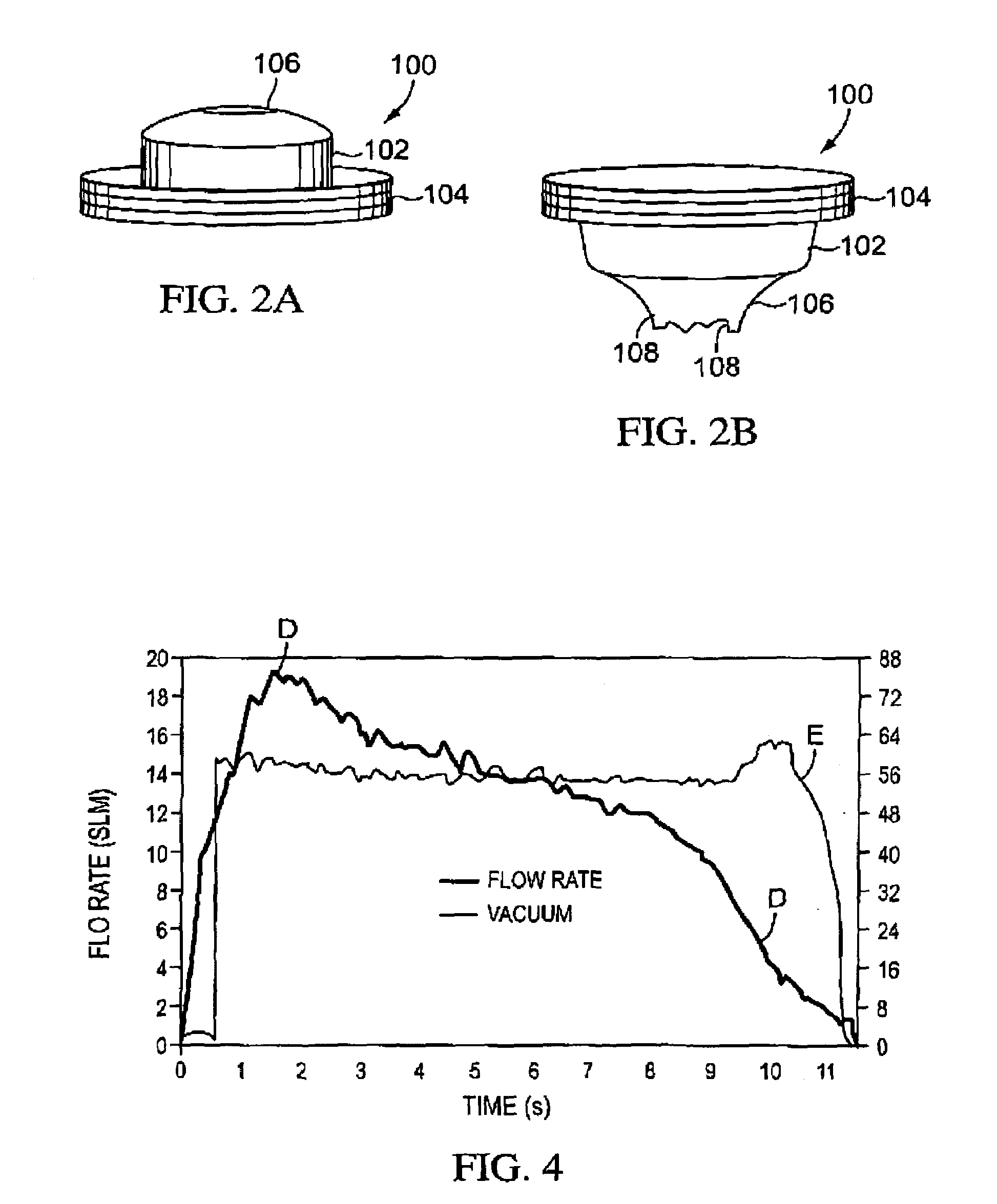
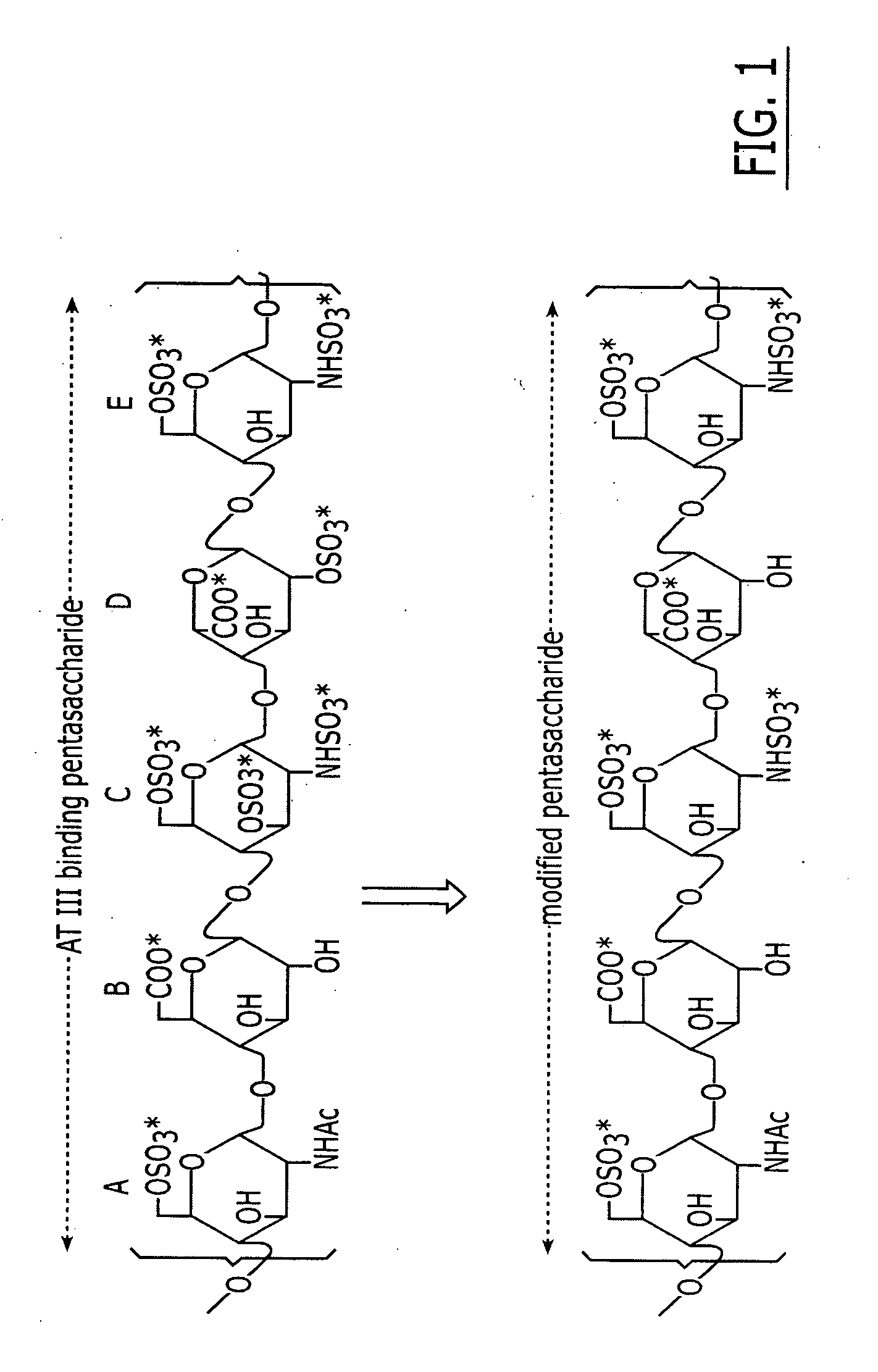
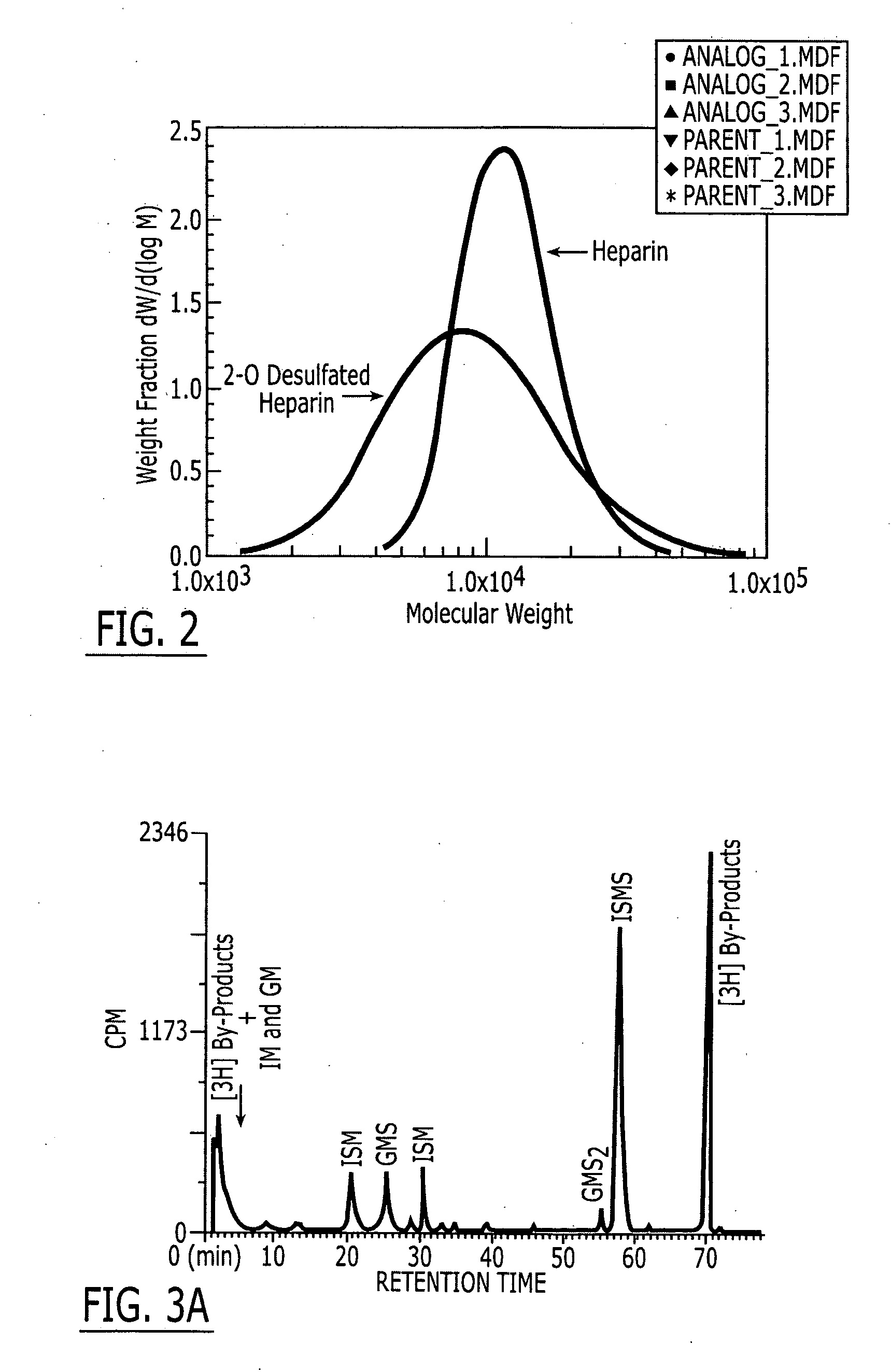
Patents
Literature
581 results about "Aerosolization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aerosolization is the process or act of converting some physical substance into the form of particles small and light enough to be carried on the air i.e. into an aerosol. Aerosolization refers to a process of intentionally oxidatively converting and suspending particles or a composition in a moving stream of air for the purpose of delivering the oxidized particles or composition to a particular location.
Electronic smoking article comprising one or more microheaters
The present disclosure relates to an electronic smoking article that provides for improved aerosol delivery. Particularly, the article comprises one or more microheaters. In various embodiments, the microheaters provide for improved control of vaporization of an aerosol precursor composition and provide for reduced power requirements to achieve consistent aerosolization. The present disclosure further relates to methods of forming an aerosol in a smoking article.
Owner:RAI STRATEGIC HLDG INC
Electronic smoking article comprising one or more microheaters
The present disclosure relates to an electronic smoking article that provides for improved aerosol delivery. Particularly, the article comprises one or more microheaters. In various embodiments, the microheaters provide for improved control of vaporization of an aerosol precursor composition and provide for reduced power requirements to achieve consistent aerosolization. The present disclosure further relates to methods of forming an aerosol in a smoking article.
Owner:RAI STRATEGIC HLDG INC
Powder formulation disintegrating system and method for dry powder inhalers
A disperser for dry powders which can be used with different dose systems, dose weights ranging from 2 to 25 mg and different types of powder formulation. In one embodiment, the disperser acts both as a de-agglomeration (disintegration; aerosolization) means and as an air classifier for especially adhesive mixtures. Only fine drug particles are emitted whereas the larger agglomerates and carrier crystals are retained by the disperser. Another embodiment enables time controlled release of carrier crystals in these mixtures. Yet another embodiment has optimized performance with spherical pellets, containing no carrier crystals. Other possible embodiments of the invention make it possible to control the total inhaler resistance and the powder deposition in the upper respiratory tract by means of the addition of a so-called sheath flow of clean air. Modifications also enable carrier retainment in the mouthpiece and elimination of the tangential flow component of the discharge cloud.
Owner:ASTRAZENECA AB
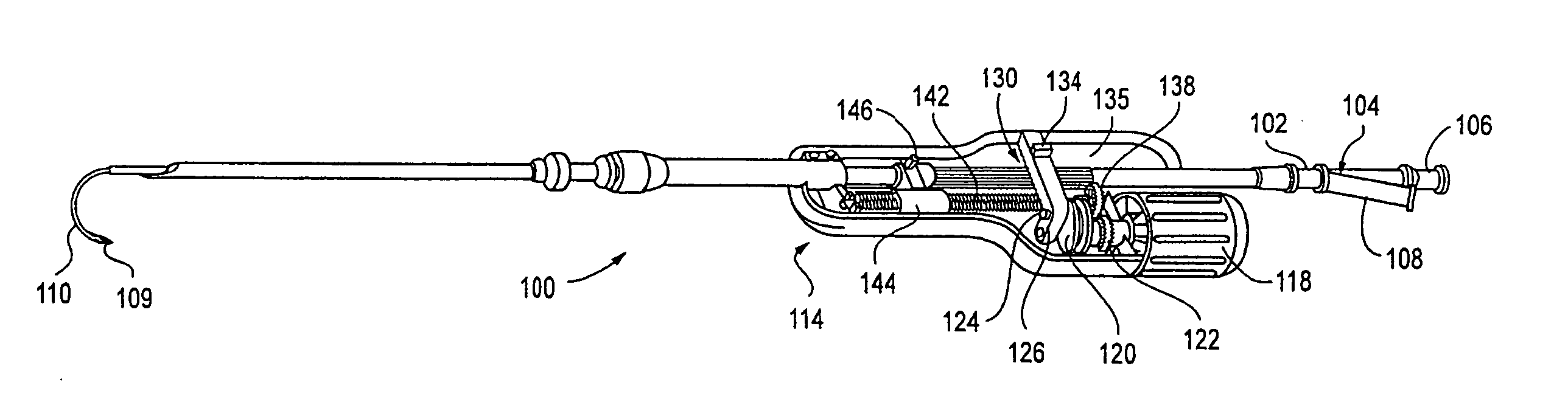
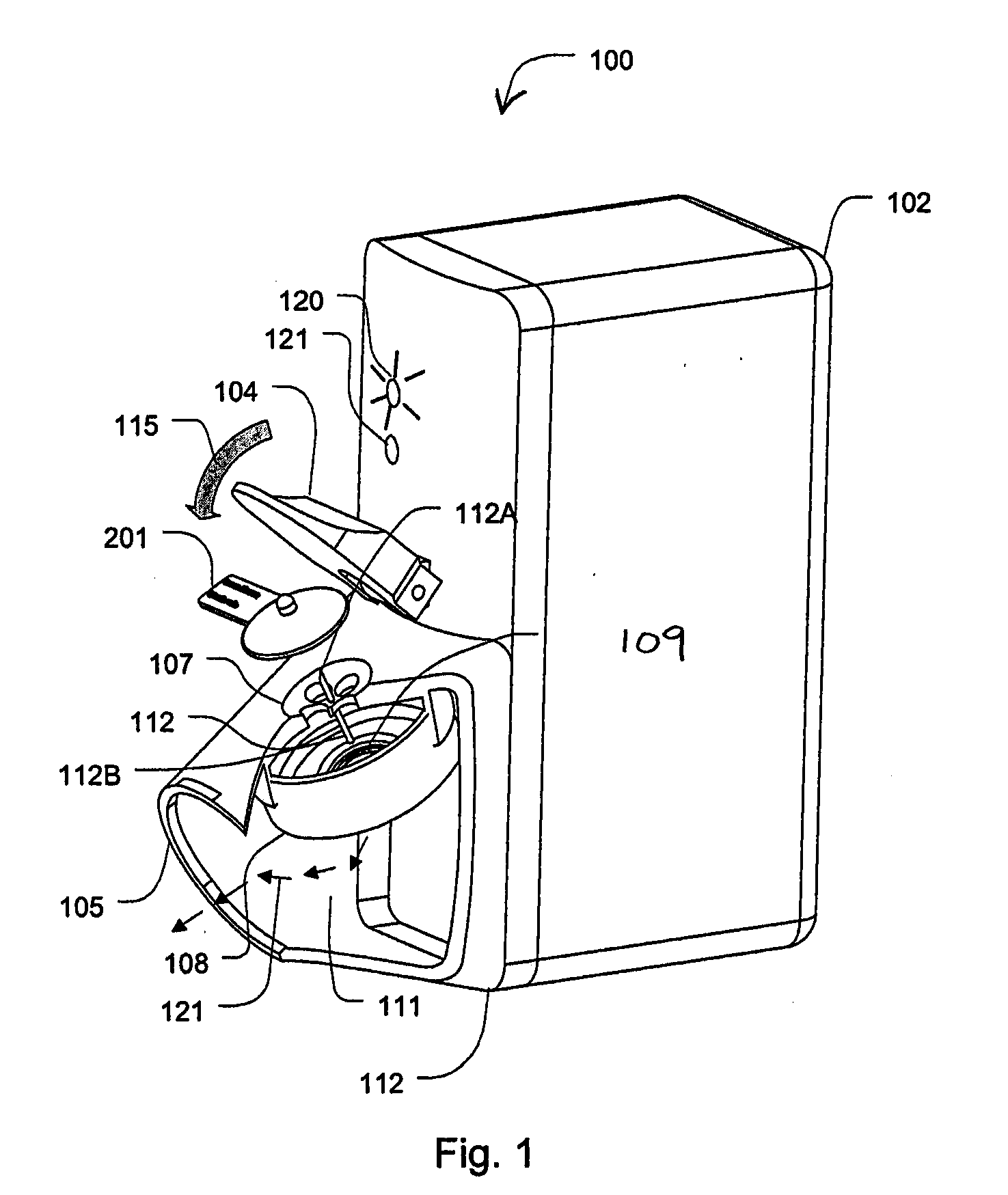
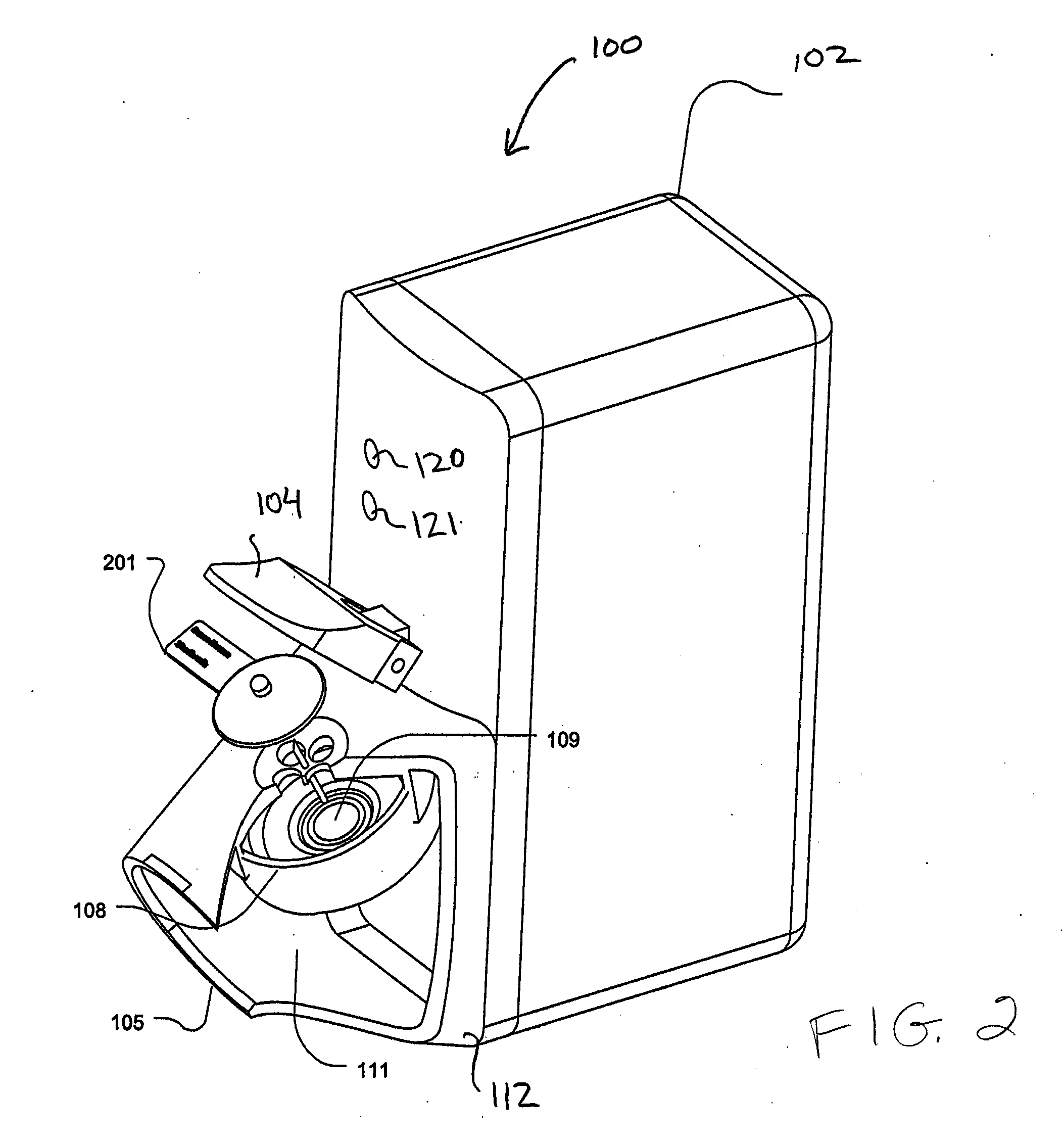
System and method for manipulating a catheter for delivering a substance to a body cavity
ActiveUS20050125002A1Make up for deficienciesGuide needlesEar treatmentINTRODUCTION deviceAerosolization
A system and method for adjusting a catheter to create a medicated atmosphere in an organ, or body cavity is disclosed. The system comprises a catheter, such as an aerosolization catheter, that can be manipulated during use and an introduction device for the introduction and manipulation, by rotational and / or axial positioning, of the aerosolization catheter. The method includes inserting the catheter into a body cavity via an introducer apparatus and adjusting an angle or orientation of the exit end of the catheter so that a substance provided to the catheter will be controllably applied to the body cavity at desired locations.
Owner:TRUDELL MEDICAL INT INC
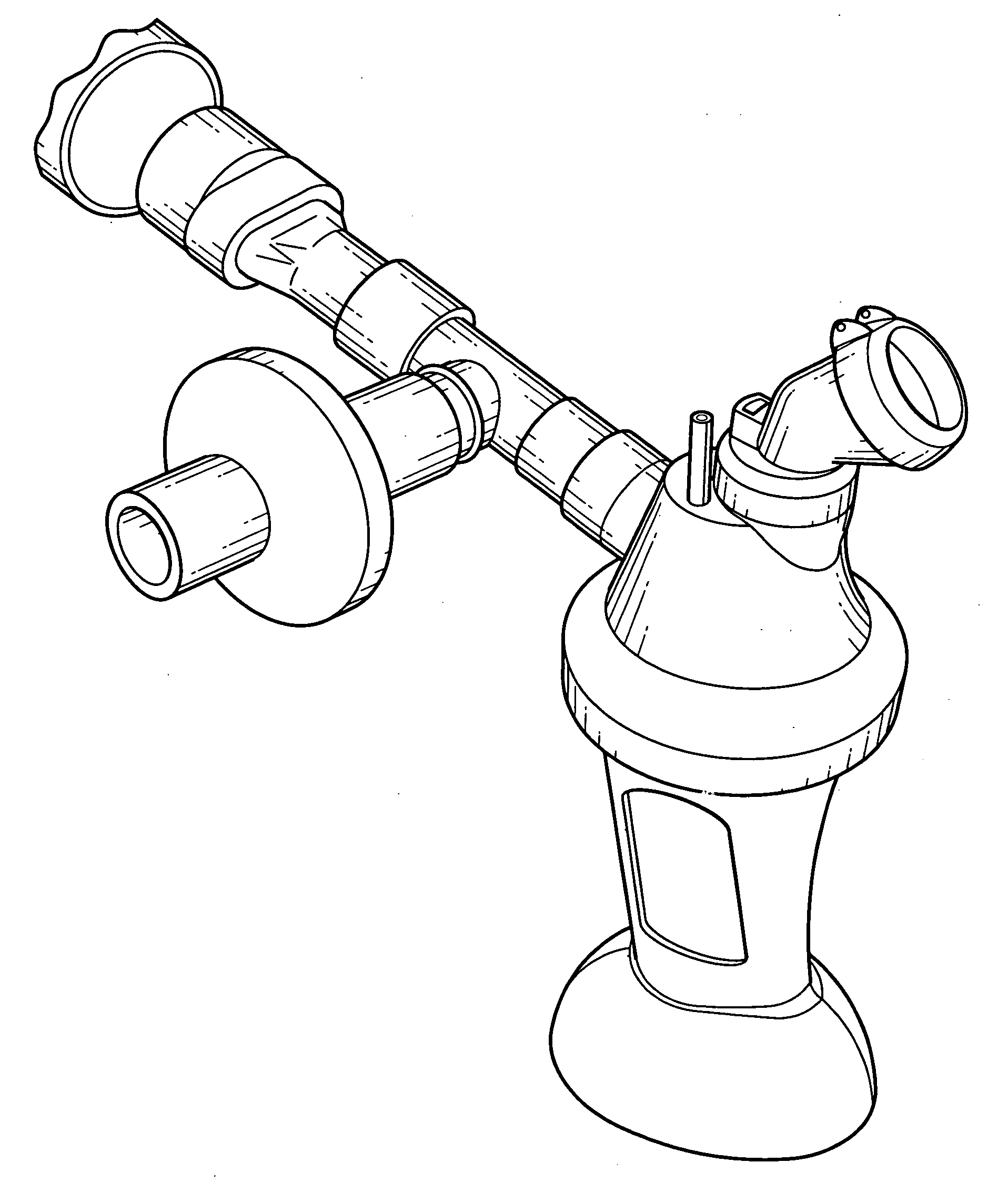
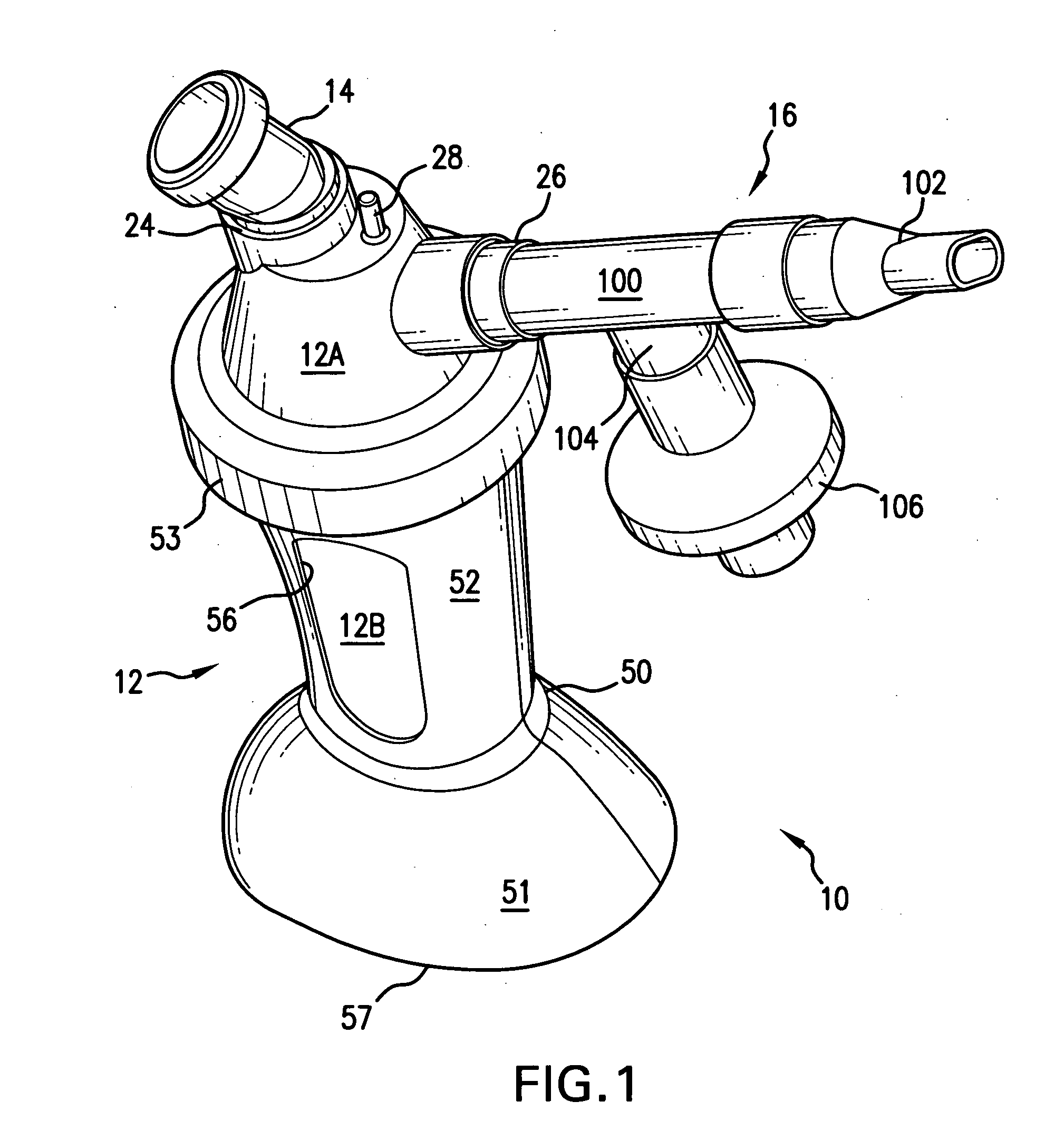
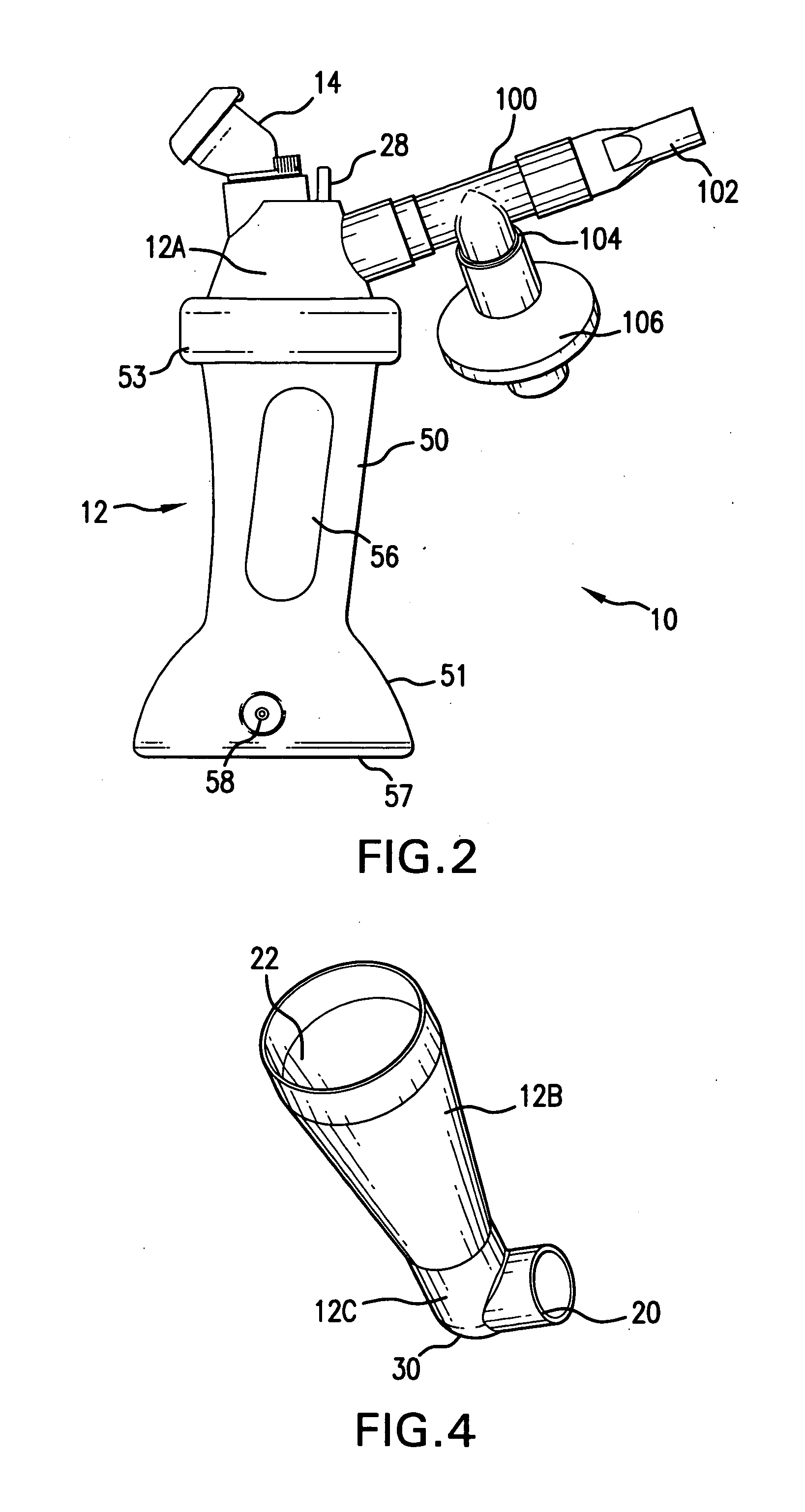
Aerosolization device
InactiveUS20110108025A1Reduce needImprove security levelTracheal tubesRespiratory masksNebulizerAerosol deposition
An aerosol transfer device (10) for medical aerosol generators comprises a body (12), fluidically coupled to a nebulizer (14) and to a patient interface (16). An ambient air intake (20) is formed into a lower body (12C). The body is shaped and configured to optimize mixing of ambient air from the ambient air intake and the aerosol generated by the nebulizer, resulting in the formation of an aerosol plume having optimum characteristics for delivery of the aerosol to the patient's pulmonary system, such as the central or deep lung regions. The shape and dimensions of the body are further designed to minimize aerosol deposition, thus improving delivery efficiency.
Owner:NEKTAR THERAPEUTICS INC
Systems and methods for clearing aerosols from the effective anatomic dead space
InactiveUS6845770B2Reduce amountImprove delivery efficiencyRespiratorsMovable spraying apparatusContinuationEngineering
An aerosolization device comprises a housing having a mouthpiece, an aerosol generator, a flow sensor and a controller. The controller is configured to begin operation of the aerosol generator upon receipt of a signal from the flow sensor indicating that a threshold flow rate has been achieved by a user when inhaling a tidal breath through the mouthpiece, and to stop operation after the passage of an operation time period that is selected such that continuation of the tidal breath delivers substantially all of the aerosol to the lungs.
Owner:NOVARTIS FARMA
Systems and methods for aerosol delivery of agents
InactiveUS20040134494A1Effective treatmentAvoid pollutionControlling ratio of multiple fluid flowsRespiratory masksUltrasonic nebulizersAerosol delivery
Aerosol delivery systems and methods for delivering an agent to a patient are described herein. The present invention includes embodiments comprising an insulated receptacle connected to a body to hold a vial of an agent to be delivered to a patient. The vial is located in an inverted position within the receptacle and connected to the housing. One or more reusable thermal packs can be located on the inner sides of the receptacle, to maintain a selected temperature surrounding the vial. The agent is administered to a patient by placing a prong into one of the patient's orifices and then activating an aerosol delivery system. Such systems comprise jet aerosolization and pneumatic and ultrasonic nebulizers and preferably are portable.
Owner:CREARE INC +1
Powder formulation disintegrating system and method for dry powder inhalers
A disperser for dry powders which can be used with different dose systems, dose weights ranging from 2 to 25 mg and different types of powder formulation. In one embodiment, the disperser acts both as a de-agglomeration (disintegration; aerosolization) means and as an air classifier for especially adhesive mixtures. Only fine drug particles are emitted whereas the larger agglomerates and carrier crystals are retained by the disperser. Another embodiment enables time controlled release of carrier crystals in these mixtures. Yet another embodiment has optimized performance with spherical pellets, containing no carrier crystals. Other possible embodiments of the invention make it possible to control the total inhaler resistance and the powder deposition in the upper respiratory tract by means of the addition of a so-called sheath flow of clean air. Modifications also enable carrier retainment in the mouthpiece and elimination of the tangential flow component of the discharge cloud.
Owner:ASTRAZENECA AB
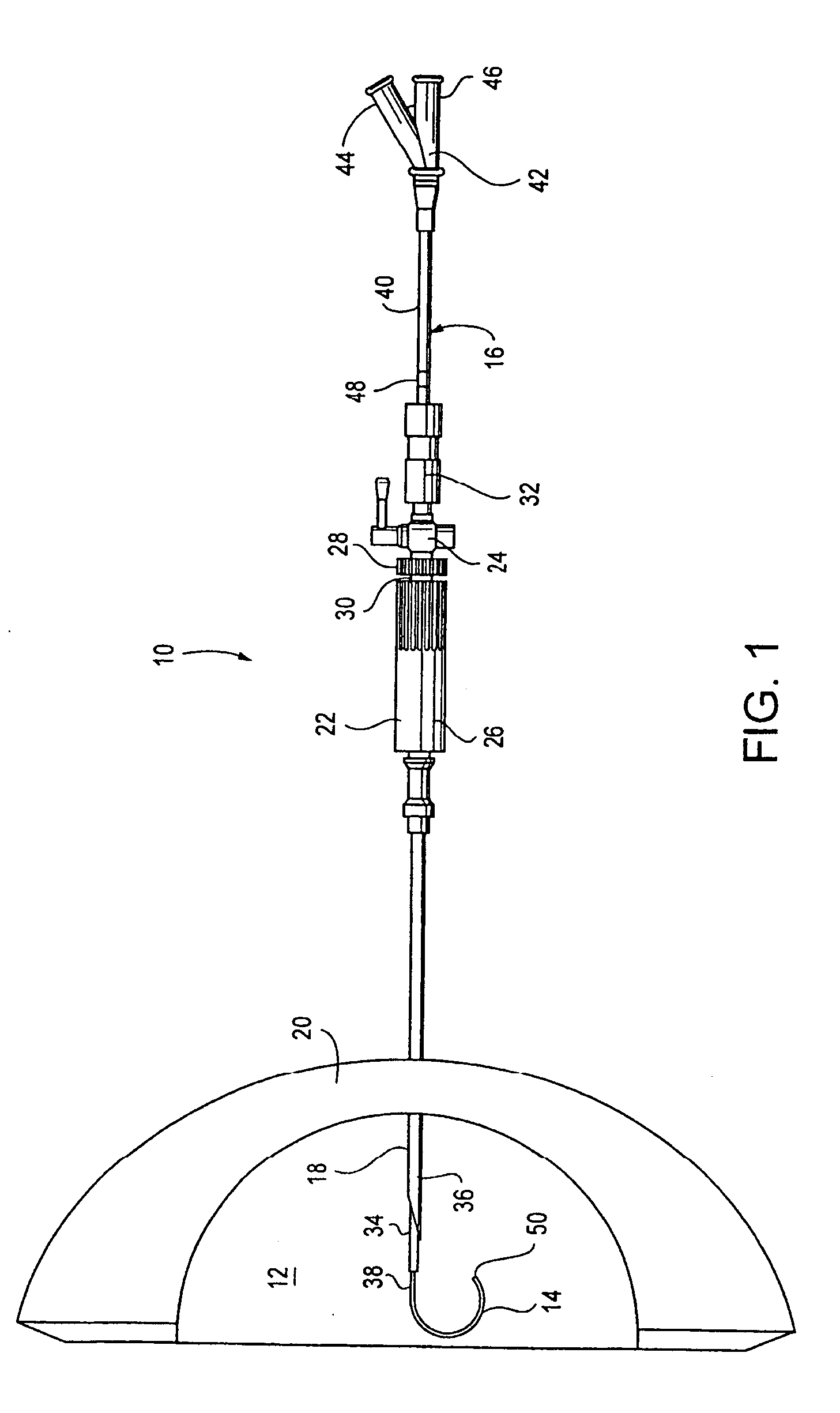
System and method for delivering a substance to a body cavity
ActiveUS20050137529A1Efficient and safe and effective applicationSurgical needlesMedical devicesDrug aerosolPost operative
A system and method for creating a medicated atmosphere in an organ, or body cavity is disclosed. The system includes a flexible aerosolization catheter that can be manipulated during use, a device for the introduction of the aerosolization catheter, a medication delivery apparatus configured to control delivery of a medication to the catheter, a gas delivery apparatus in communication with the catheter, a gas pressure relief apparatus configured to relieve pressure in the organ or body cavity, and a central controller in communication with the medication delivery apparatus, gas delivery apparatus, and gas pressure relief apparatus control of the various means. The method includes providing insufflation gas and an aerosol of medication to an organ or body cavity while controlling overall pressure in the organ or cavity. The method may also include re-entering a patient through at least one port to apply gas and an aerosolized medicament, in either a post-operative procedure or in a chemotherapy context.
Owner:NORTHGATE TECH
Aerosolized nitrite and nitric oxide -donating compounds and uses thereof
Disclosed herein are formulations of nitrite, nitrite salt, or nitrite- or nitric oxide-producing compounds suitable for aerosolization and use of such formulations for aerosol administration of nitrite, nitrite salt, or nitrite- or nitric oxide-donating compounds for the treatment of pulmonary arterial hypertension, intra-nasal or pulmonary bacterial infections, or to treat or prevent ischemic reperfusion injury of the heart, brain and organs involved in transplantation. In particular, inhaled nitrite, nitrite salt, or nitrite- or nitric oxide-donating compound specifically formulated and delivered to the respiratory tract for the indications is described. Compositions include all formulations, kits, and device combinations described herein. Methods include inhalation procedures and manufacturing processes for production and use of the compositions described.
Owner:AIRES PHARMA
Ultrasonic Aerosol Generator
InactiveUS20060249144A1Low costFormula stableLiquid spraying apparatusMedical atomisersElectricityHand held
An ultrasonic aerosol generator for delivering a liquid formulation in an aerosolized form at a high output rate of greater than 0.5 mL / minute, preferably greater than 1.0 mL / minute, and with diameters in a respirable size range, methods of using this device and kits including the device are described herein. The ultrasonic aerosol generator (10) contains at least (a) a liquid reservoir / aerosolization chamber (11), (b) a piezoelectric engine (12), (c) a relief aperture (13), and (d) an aerosol delivery element (20). Preferably the aerosolized particles that are delivered to the user through the aerosol delivery element have an average aerodynamic diameter of between 1 and 20 μm, more preferably between 1 and 10 μm, and most preferably between 1 and 5 μm. Optionally, the ultrasonic aerosol generator is designed to deliver more than one formulation simultaneously, preferably a low cost and / or stable formulation is administered simultaneously with a more expensive and / or labile formulation. In the preferred embodiment, the ultrasonic aerosol generator is a hand-held device designed for a single user.
Owner:PULMATRIX
Aerosolization device
InactiveUS20090114737A1Stop operationSelf-acting watering devicesMovable spraying apparatusElectricityAerosolization
In an aerosolization device, an aerosol generator is installed a position proximate to an opening at the bottom of a liquid container for aerosolizing a supplied liquid in the container and a sensor is provided to detect a variation of electric characteristic according to a condition whether or not the liquid in the container is in contact with the aerosol generator and the variation of electric characteristic is sent to a controller for computation, so as to control whether to continue operating or stop operating the aerosol generator. If the aerosol generator is controlled to stop its operation, the controller will drive an alarm device to send out an alarm signal at the same time.
Owner:HEALTH & LIFE CO LTD
Needle sheath device
InactiveUS6328713B1Avoid vibrationPrevent movementInfusion syringesInfusion needlesLocking mechanismAerosolization
The safety device of the instant invention is equipped with a fluid absorbable material at the portion of the sheath that meets the tip portion of the needle so that, as the sheath is pivoted to a position in substantial alignment with the needle, whatever fluid that has been collected at the needle is absorbed before the needle is fixedly retained by a locking mechanism, either integrated within the needle sheath or to the base and lower portion of the needle sheath, to thereby prevent splattering or aerosolization of contaminated fluid into the environment.
Owner:SMITHS MEDICAL ASD INC
Preservative-free single dose inhaler systems
An aerosolization system includes a squeezable container having a resilient container body. The container is configured to deliver a unit dosage of a liquid when squeezed a single time. The system also includes an aerosolizer that is constructed of a housing defining a mouthpiece, and an aerosol generator disposed in the housing. The aerosol generator comprises a vibratable membrane having a front face and a rear face, and a vibratable element used to vibrate the membrane. Further, the housing includes an opening that is adapted to receive a unit dosage of the liquid from the container. The opening provides a liquid path to the rear face of the vibratable membrane.
Owner:AERAMI THERAPEUTICS INC
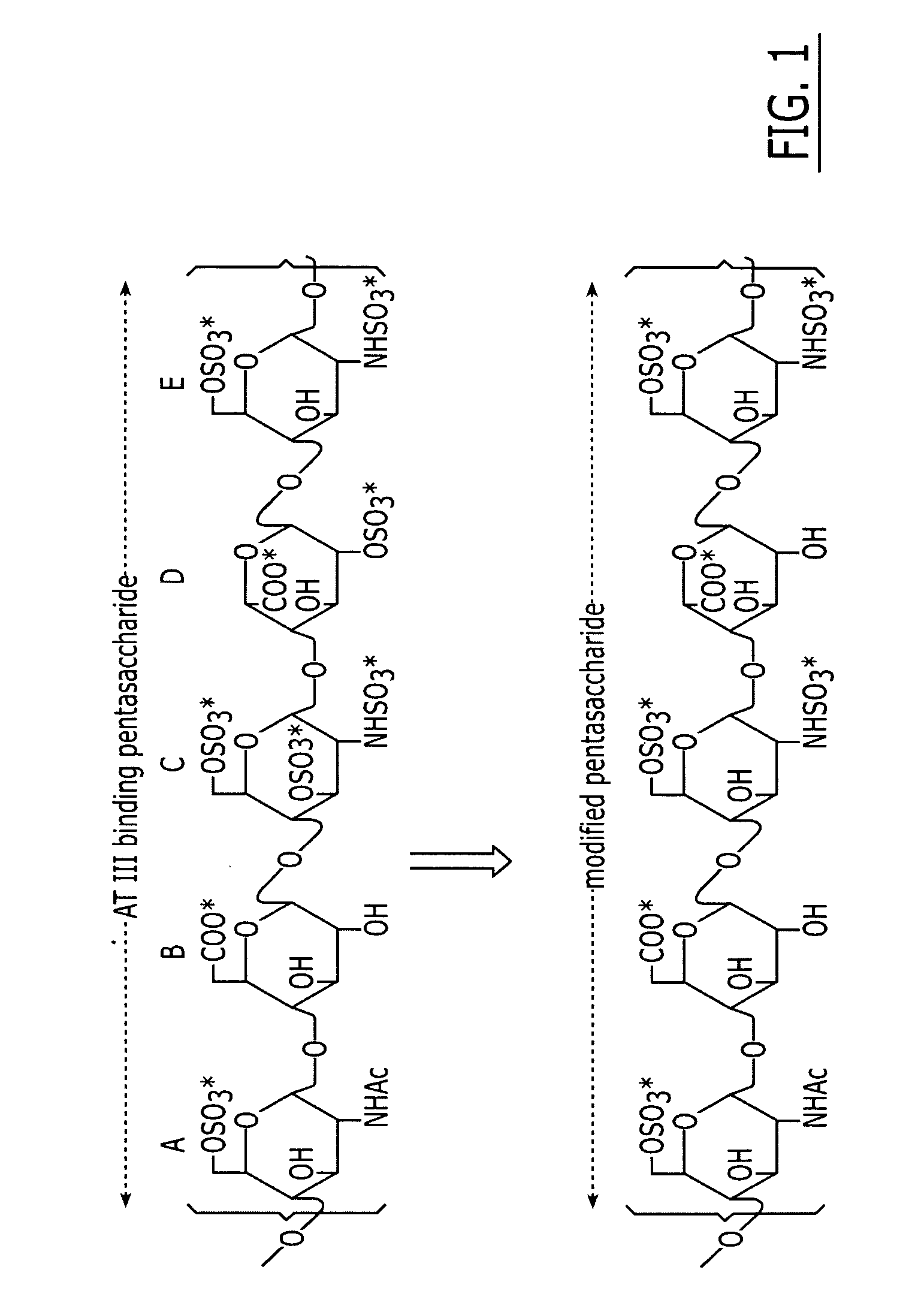
Method and medicament for anticoagulation using a sulfated polysaccharide with enhanced anti-inflammatory activity
InactiveUS20060040896A1Sufficient degreeGood anti-inflammatory activityOrganic active ingredientsBlood disorderSulfated polysaccharidesMedicine
A method and medicament for anticoagulating a patient with a sulfated polysaccharide mixture that demonstrates enhanced anti-inflammatory activity compared to anticoagulation with unfractionated heparin comprising various combinations of fully anticoagulant unfractionated heparin with 2-O desulfated heparin demonstrating reduced anticoagulant activity but enhanced anti-inflammatory actions. The medicament preferably is administered intravenously, by aerosolization or orally. Preferably, the 2-O desulfated heparin medicament includes a physiologically acceptable carrier which may be selected from the group consisting of physiologically buffered saline, normal saline and distilled water. Additionally provided is a method of synthesizing 2-O desulfated heparin in commercially practical quantities for the formulation of an anticoagulant 2-O desulfated heparin and heparin mixture.
Owner:CANTEX PHARMA
Systems and methods for aerosol delivery of agents
InactiveUS7225807B2Reduce and maintain ambient temperatureAvoid pollutionControlling ratio of multiple fluid flowsRespiratory masksUltrasonic nebulizersAerosol delivery
Aerosol delivery systems and methods for delivering an agent to a patient are described herein. The present invention includes embodiments comprising an insulated receptacle connected to a body to hold a vial of an agent to be delivered to a patient. The vial is located in an inverted position within the receptacle and connected to the housing. One or more reusable thermal packs can be located on the inner sides of the receptacle, to maintain a selected temperature surrounding the vial. The agent is administered to a patient by placing a prong into one of the patient's orifices and then activating an aerosol delivery system. Such systems comprise jet aerosolization and pneumatic and ultrasonic nebulizers and preferably are portable.
Owner:CREARE INC +1
Officinal health-care solid electronic aerosolization liquid and preparation method thereof
The invention provides an officinal health-care solid electronic aerosolization liquid, mainly comprising the following components by weight percent: 35-45% of propylene glycol, 30-35% of medicament, 10-15.0% of deionized water, 3-3.5% of tobacco leaf extract solution, 3-3.5% of tobacco flavour, 2-3.0% of excipient, 0.2-1.0% of calcium pectate and 0.3-0.8% of curing agent. The invention also provides a preparation method of the officinal health-care solid electronic aerosolizatioon liquid. The invention contains multiple officinal components having treating function on lungs, thus having treating function on symptoms such as dryness and itch of the throat and irritable cough which are caused by smoking, chronic pharyngitis and dryness of mouth and nose, thirst and dry cough, less sputum or distressed cough caused by the fact that sputum is glued; meanwhile the invention is solid, thus the stability of storing and use is improved, production and transportation are more convenient and flexible, and fragrance can be remained to be more complete.
Owner:FEELLIFE BIOSCI INT
Powder delivery device
ActiveUS20050205087A1Easily and advantageously manufacturedEasily and advantageously and distributedPowder deliverySurgeryHand heldEngineering
A pistol-shaped powder delivery device consolidates propellant pulse creation, powder storage, powder aerosolization and aerolsolized powdered delivery in a single device while providing dose, pattern and coverage control of the output spray. The single-hand-held, single-hand-operated device includes a housing having a handle portion that is selectively collapsed to generate a propellant pulse that is conveyed into and through powder stored in a reservoir mounted at an upward angle to the horizontal in a receptacle provided in the housing. Powder in the reservoir is caused to be aerosolized by the propellant pulse whereupon aerosolized powder exits the reservoir through an open end of, and elongate slots provided in the sidewall of, an elongate hollow member extending from an outlet in the receptacle through the stored powdered material into a head space in the reservoir. The outlet is connected a barrel portion for output of the aerosolized powder spray.
Owner:GENZYME CORP
Paper softening compositions containing low levels of high molecular weight polymers and soft tissue paper products comprising said compositions
Disclosed is a composition suitable for atomizing without excessive aerosolization in the form of an oil-in-water emulsion comprising: a) a continuous aqueous phase, and b) a discontinuous oil phase wherein the rheology of the aqueous phase is modified by the addition of a water-in-oil emulsion comprising: i) a high molecular weight polymer in a discontinuous aqueous phase, and ii) a continuous organic solvent phase. Preferred embodiments of the present invention relate to compositions for softening an absorbent paper tissue comprising a) a quaternary ammonium softening active ingredient; b) an electrolyte; c) a high molecular weight polymer emulsion comprising: i) from about 20% to about 40% by weight of the premix of a high molecular weight polymer; ii) from about 40% to about 60% of water; and iii) from about 20% to about 40% of an organic solvent; and d) a vehicle in which said softening active ingredient is dispersed.
Owner:THE PROCTER & GAMBLE COMPANY
Powder delivery device
ActiveUS7455248B2Efficiently and satisfactorily operablePowder deliverySurgeryHand heldCoverage control
A pistol-shaped powder delivery device consolidates propellant pulse creation, powder storage, powder aerosolization and aerolsolized powdered delivery in a single device while providing dose, pattern and coverage control of the output spray. The single-hand-held, single-hand-operated device includes a housing having a handle portion that is selectively collapsed to generate a propellant pulse that is conveyed into and through powder stored in a reservoir mounted at an upward angle to the horizontal in a receptacle provided in the housing. Powder in the reservoir is caused to be aerosolized by the propellant pulse whereupon aerosolized powder exits the reservoir through an open end of, and elongate slots provided in the sidewall of, an elongate hollow member extending from an outlet in the receptacle through the stored powdered material into a head space in the reservoir. The outlet is connected a barrel portion for output of the aerosolized powder spray.
Owner:GENZYME CORP
Aerosol generating and delivery device
ActiveUS7905229B2Efficient deliveryHigh viscositySpray nozzlesIsotope separationParticle flowNasal Cavity Epithelium
Particular aspects provide novel atomizers for generating particles over a broad range of MMAD size distributions, the eliminating the requirement for an impaction baffle in generating the desired particle sizes. In particular aspects, the atomization means communicates with a remote particle filter member configured and positioned to provide for particle size filtering. In additional aspects, the atomization means communicates with a particle dispersion chamber suitable to impart a desired particle flow pattern to particles within and exiting the dispersion chamber. In further aspects, the atomization means communicates with a nasal, ocular, oral or ‘vicinity’ adapter. The novel devices provide for targeted (e.g., nasal, ocular, oral, local vicinity), systemic, and / or topical delivery of an atomized liquid (e.g., via the nasal cavity, olfactory region, and mouth). Further exemplary aspects relate to aerosolization and delivery of perfume, fragrance, essential oil or cosmeceutical agents and the like.
Owner:KURVE THERAPEUTICS INC
Flow regulator for aerosol drug delivery and methods
InactiveUS7185651B2Sufficient flow rateEasy to useRespiratorsLiquid surface applicatorsVacuum levelEngineering
An aerosolization device comprises a housing having a mouthpiece, and a flow path arrangement in fluid communication with the mouthpiece. The flow path arrangement has a flow regulating valve and a threshold valve, where the threshold valve is configured to open at a first vacuum level and to close at a second vacuum level that is less than the first vacuum level. The housing includes a region that is adapted to hold a powder in fluid communication with the flow path arrangement so that air drawn through the mouthpiece opens the threshold valve once the first vacuum level is exceeded and remains open until the vacuum falls below the second vacuum level. The flow rate of the air drawn through the mouthpiece is regulated by the flow regulating valve to remain within a certain range while the threshold valve remains open.
Owner:NOVARTIS FARMA
Method and medicament for sulfated polysaccharide treatment of inflammation without inducing platelet activation and heparin-induced thrombocytopenia syndrome
InactiveUS20050282775A1Sufficient degreeNot induce anti-coagulant activityOrganic active ingredientsAntipyreticHeparin antibodySulfated polysaccharides
A method and medicament for treating inflammation in a patient with a sulfated polysaccharide without inducing platelet activation or thrombosis in the presence of heparin- and platelet factor 4-complex reactive antibodies using a 2-O desulfated heparin with an average degree of sulfation of 0.6 sulfate groups per monosaccharide or greater and an average molecular weight or 2.4 kD or greater. The medicament preferably is administered intravenously, by aerosolization or orally. Preferably, the 2-O desulfated heparin medicament includes a physiologically acceptable carrier which may be selected from the group consisting of physiologically buffered saline, normal saline, and distilled water. Additionally provided is a method of synthesizing 2-O desulfated heparin.
Owner:CANTEX PHARMA
Apparatus for providing aerosol for medical treatment and methods
Embodiments of the present invention include apparatus and methods for aerosolizing liquid. One embodiment of the invention provides an apparatus for generating an aerosol. The apparatus includes an actuator having a first face and a second face and defining an opening therethrough, as well as a vibratory element in mechanical communication with the actuator, and a sealing member configured to isolate the vibratory element from a surrounding environment. In accordance with certain embodiments, the apparatus further comprises an aerosolization element mounted on the actuator and disposed substantially over the opening, wherein the aerosolization element defines at least one aperture therethrough. Hence, the vibratory element may be operated to vibrate to cause movement of the aerosolization element in such a manner that a liquid at the first face of the aerosolization element can be dispensed as an aerosol through the at least one aperture. Some embodiments feature an electrode coupled to the vibratory element.
Owner:NOVARTIS FARMA
Pore structures for reduced pressure aerosolization
InactiveUS6855909B2Reduce weightIncreased patient mobilityDispensing apparatusAerosol deliveryDecrease weightSpray nozzle
A nozzle comprising a thin, flexible substantially planar polymeric film having a plurality of pores with structures allowing for generation of an aerosol at reduced extrusion pressure is disclosed. The pores can comprise at least two sections, or steps, in which the thickness of the membrane is reduced in stepwise fashion, or the pores can be tapered. Nozzles formed comprising pores having such structures permit aerosol generation at lower extrusion pressures, thereby allowing for decreased weight of aerosolization devices, increased efficiency, increased portability and increased battery life. The pore structures also allow for the use of thicker, more easily processed polymeric films in manufacturing while having a thinner, more efficient aerosolization area. The use of decreased extrusion pressures also results in increased uniformity in aerosol generation and improved reliability of other components.
Owner:ARADIGM
Liquid nebulization systems and methods
ActiveUS20160001019A1Increase the differential pressureRespiratorsMedical devicesInspiratory flowCatheter
Embodiments provide aerosolization device for providing aerosolized medicament to user. The aerosolization device includes conduit, aerosol generator, fluid receiving chamber, restrictor within the conduit, and indicator mechanism. Conduit has an inner wall and a mouthpiece end for causing an inspiratory flow. Aerosol generator includes a vibratable mesh laterally offset from the inner wall. Fluid receiving chamber receives liquid medicament. At least a portion of chamber is tapered such that liquid medicament is directed onto vibratable mesh for aerosolization. Restrictor defines a plurality of apertures that provide increases in pressure differential that vary with inspiratory flow rate within conduit and provide relatively laminar flow downstream of restrictor. Indicator mechanism indicates a state of flow parameters relative to a predefined range. Aerosol generator is configured to aerosolize at least a portion of liquid medicament only when flow parameters of the inspiratory flow are within range.
Owner:AERAMI THERAPEUTICS INC
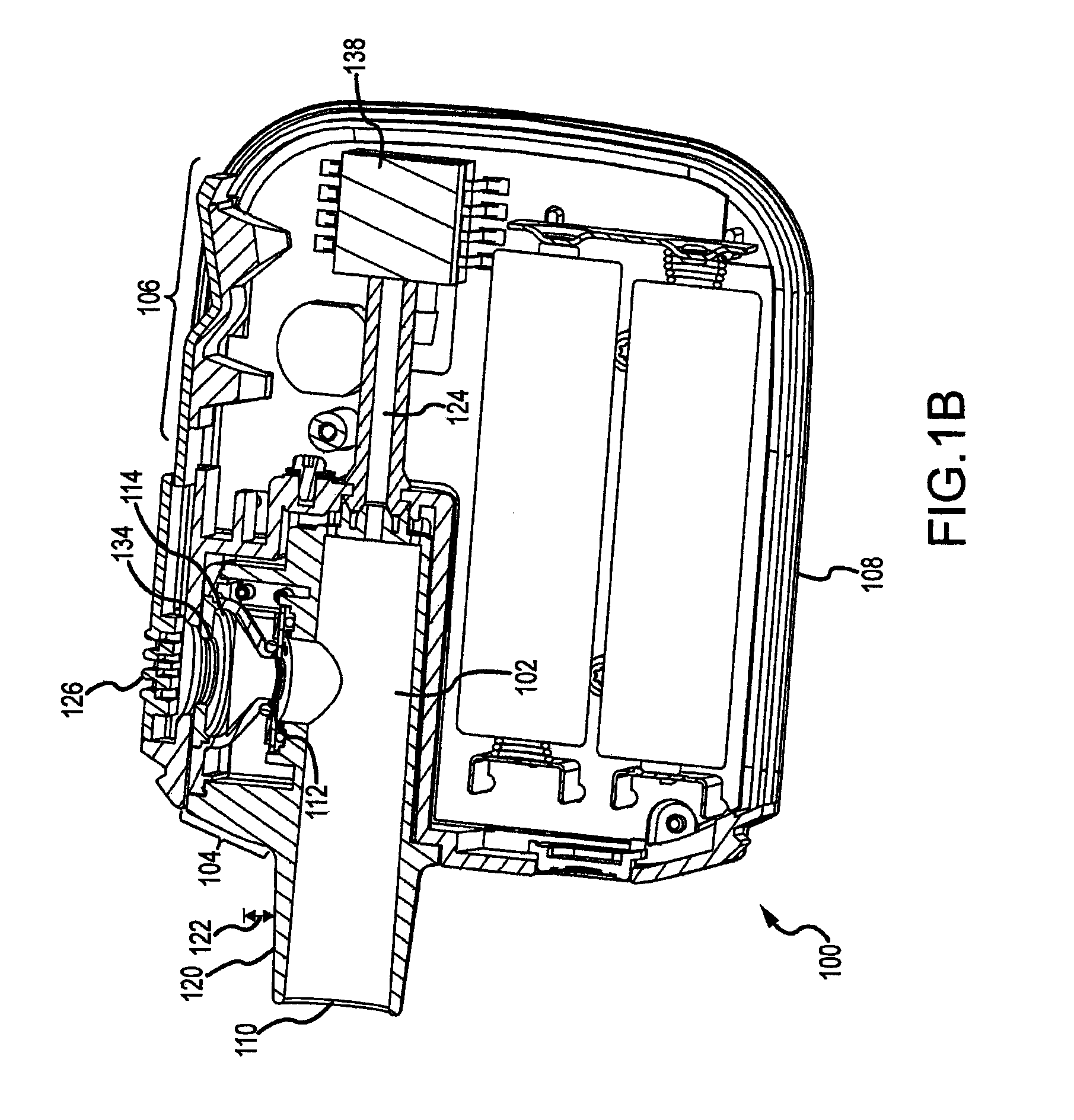
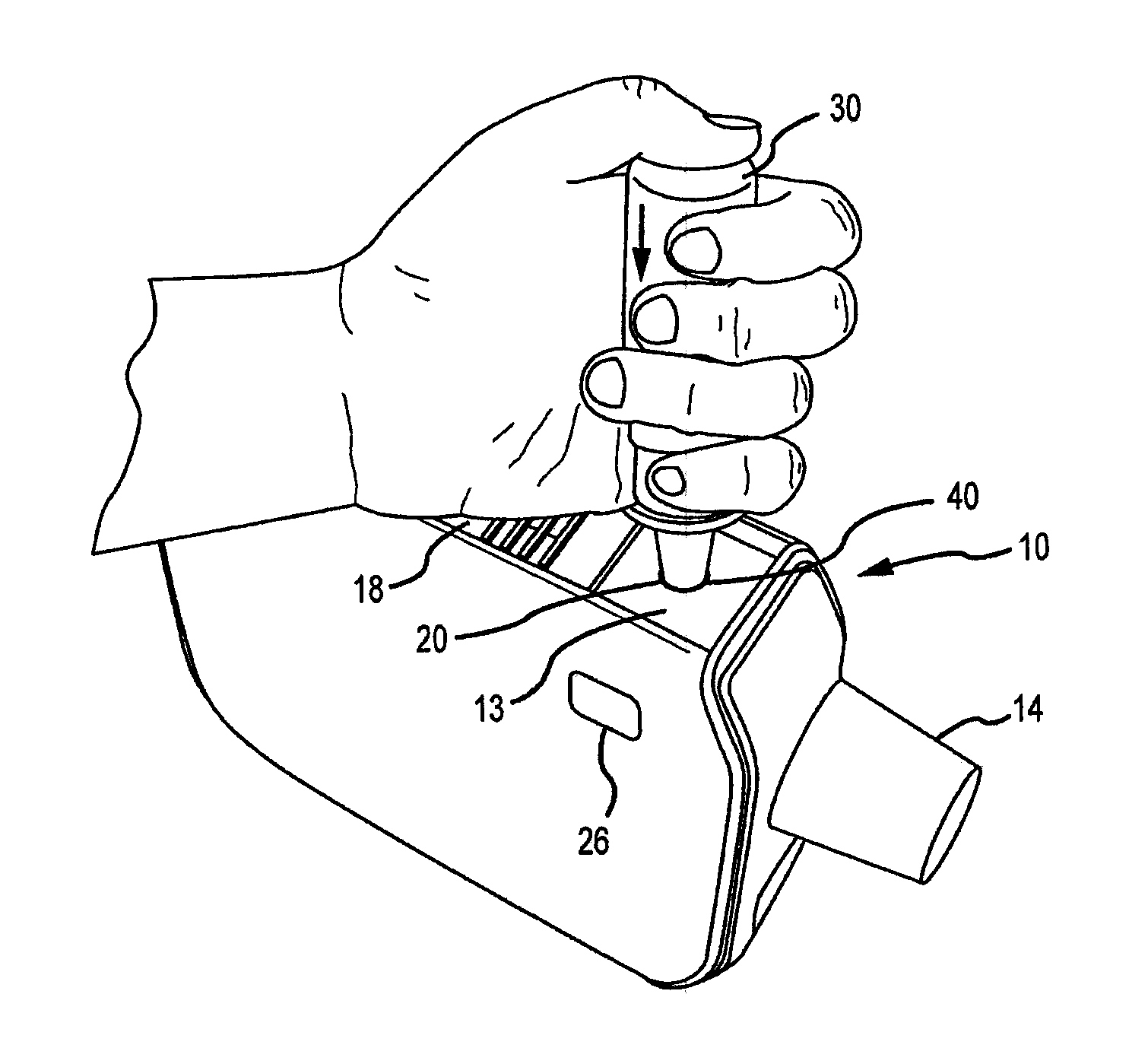
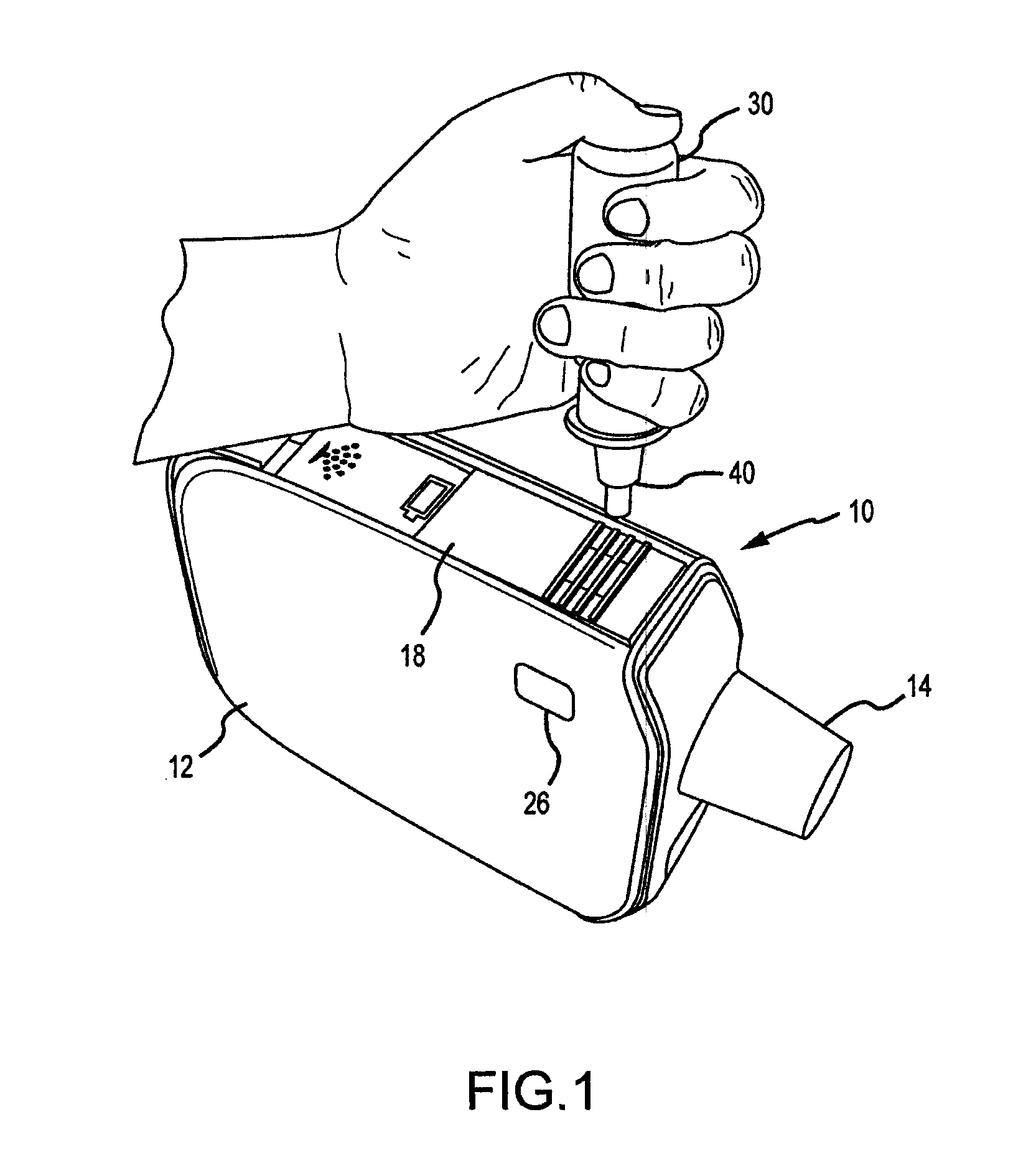
Methods and systems for supplying aerosolization devices with liquid medicaments
A method is described for supplying a metered amount of a liquid medicament to an aerosolizing device. The method utilizes a dispenser comprising an elongate dispenser body having a proximal end and a tip at a distal end through which a liquid medicament is dispensed. The dispenser further comprises a dispensing mechanism that operates to dispense a metered quantity of the liquid medicament from the tip each time the dispenser body is compressed. The dispenser is grasped with one hand such that the fingers wrap around the dispenser body, with the thumb closest to the proximal end and the last finger closest to the tip. The tip is inserted into an opening of an inhaler and the elongate body is forced toward the tip to cause the dispenser body to compress, thereby operating the dispensing mechanism and causing a metered quantity of the liquid medicament to eject into the inhaler.
Owner:DANCE BIOPHARM
Aerosolization system with flow restrictor and feedback device
ActiveUS20160001018A1Precise deliveryIncrease the differential pressureRespiratorsMedical devicesInspiratory flowCatheter
Owner:AERAMI THERAPEUTICS INC
Inhalable pharmaceutical compositions
ActiveUS20120321698A1Prevent foggingReduce deliveryAntibacterial agentsPowder deliveryChemical compositionAerosolization
Inhalable pharmaceutical compositions can include an aqueous dispersion of particles including a hydrophobic bioactive agent (e.g., CoQ10) suitable for continuous aerosolization. Due to their chemical composition and methods of manufacture, the pharmaceutical compositions exhibit distinctive physicochemical properties that provide advantageous aerosol transmission and output.
Owner:BPGBIO INC
Treatment and prevention of systemic bacterial infections in plants using antimicrobial metal compositions
An embodiment of the invention is a treatment for the mitigation and prevention of systemic infection of plants using materials derived from bactericidal metals wherein one metal is silver. The candidate bactericidal metal is preferably introduced in metallic, nanocrystalline, salt form, chelated form, or otherwise coupled form. Metal atoms, ions, molecules, clusters, or particles in concentrations between 0.001 to 100,000 parts per million (ppm) may be employed, wherein the preferred concentration of silver is sufficient to suppress bacterial viability. The bactericidal principle is preferably introduced to the plant by injection, ballistic insertion, pneumatic insertion, mechanical insertion, manual insertion, root application, aerosolization or spray in order to effect the treatment and prevention of systemic plant infections by bacterial agents of disease or reduced productivity.
Owner:NEWMAN KAREL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com