Patents
Literature
95 results about "Aerodynamic diameter" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aerodynamic diameter. Expression of aerodynamic behavior of an irregularly shaped particle in terms of the diameter of an idealized particle; that is, aerodynamic diameter is the diameter of a sphere of unit density that has aerodynamic behavior identical to that of the particle in question.
Methods of spray-drying a drug and a hydrophobic amino acid
InactiveUS6372258B1High level of stabilityEasy to manufacturePowder deliveryOrganic active ingredientsMoistureParticle-size distribution
According to the subject invention, dispersible dry powder pharmaceutical-based compositions are provided, including methods for their manufacture and dry powder dispersion devices. A dispersible dry powder pharmaceutical-based composition is one having a moisture content of less than about 10% by weight (% w) water, usually below about 5% w and preferably less than about 3% w; a particle size of about 1.0-5.0 mum mass median diameter (MMD), usually 1.0-4.0 mum MMD, and preferably 1.0-3.0 mum MMD; a delivered dose of about >30%, usually >40%, preferably >50%, and most preferred >60%; and an aerosol particle size distribution of about 1.0-5.0 mum mass median aerodynamic diameter (MMAD), usually 1.5-4.5 mum MMAD, and preferably 1.5-4.0 MMAD. Such composition are of pharmaceutical grade purity.
Owner:NOVARTIS FARMA
Pharmaceutical aerosol composition
InactiveUS7601336B2Avoid and mitigate problemIncrease the diameterPowder deliveryCosmetic preparationsMedicineInhalation
A composition for use in an aerosol inhaler, the composition comprising an active material, a propellant containing a hydrofluoroalkane (HFA), a cosolvent and further comprising a low volatility component to increase the mass median aerodynamic diameter (MMAD) of the aerosol particles on actuation of the inhaler, filling of an aerosol container and use of the composition for the administration of active materials by inhalation.
Owner:CHIESI FARM SPA
Stable pharmaceutical solution formulations for pressurised metered dose inhalers
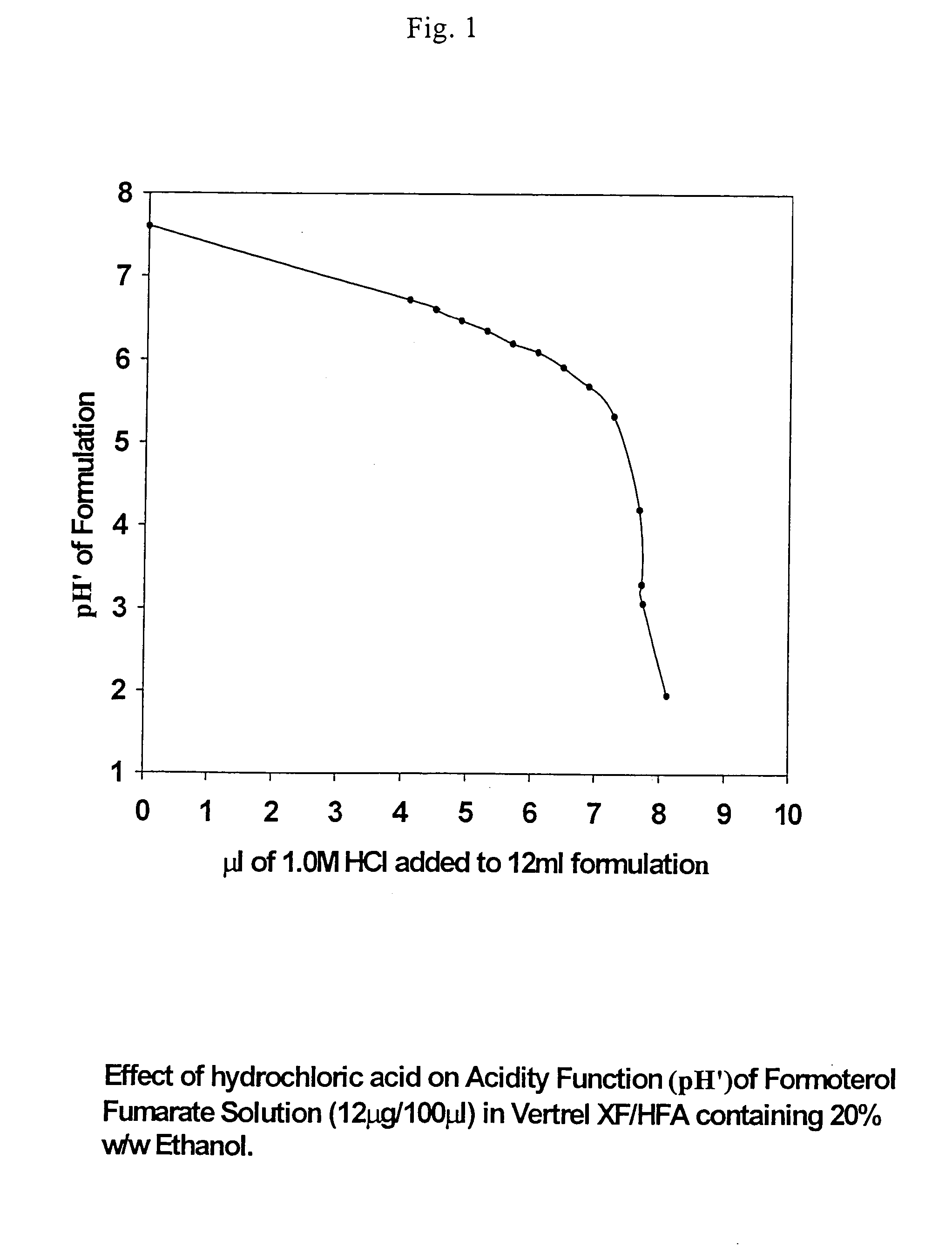
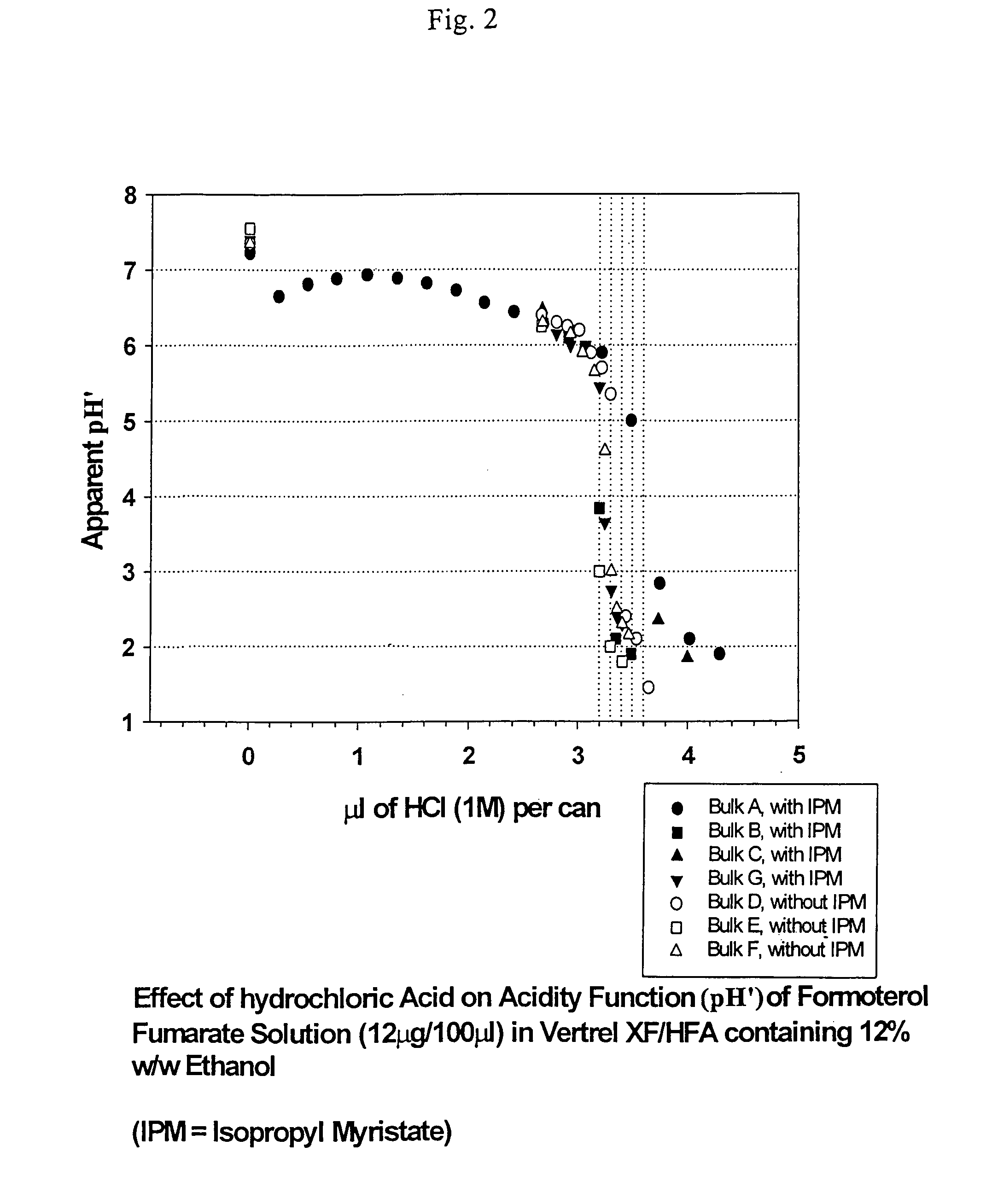
An aerosol solution composition for use in an aerosol inhaler comprises an active material, a propellant containing a hydrofluoroalkane, a cosolvent and optionally a low volatility component to increase the mass median aerodynamic diameter (MMAD) of the aerosol particles on actuation of the inhaler. The composition is stabilized by using a small amount of mineral acid and a suitable can having part or all of its internal metallic surfaces made of stainless steel, anodized aluminium or lined with an inert organic coating.
Owner:CHIESI FARM SPA
Ultrasonic Aerosol Generator
InactiveUS20060249144A1Low costFormula stableLiquid spraying apparatusMedical atomisersElectricityHand held
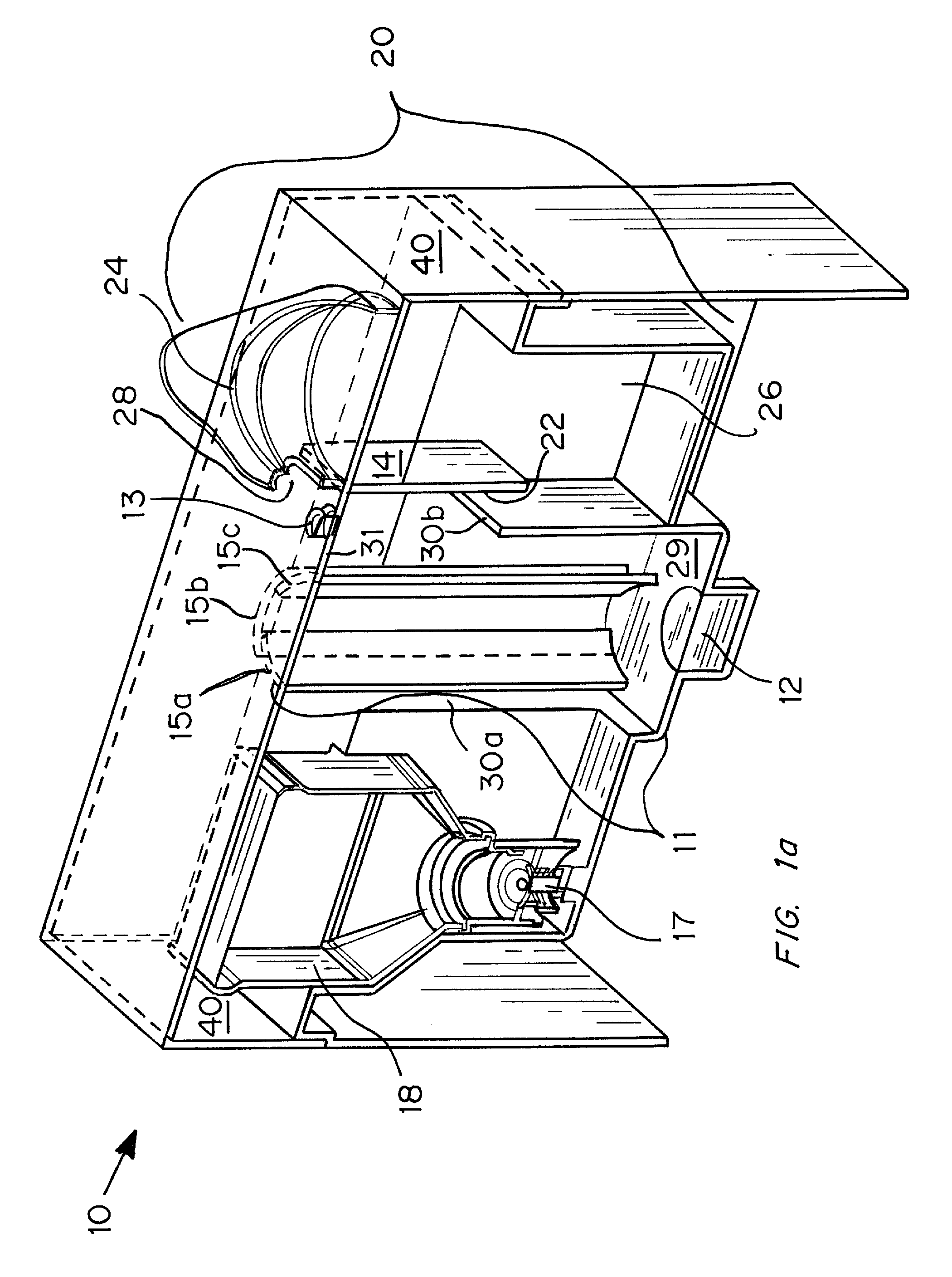
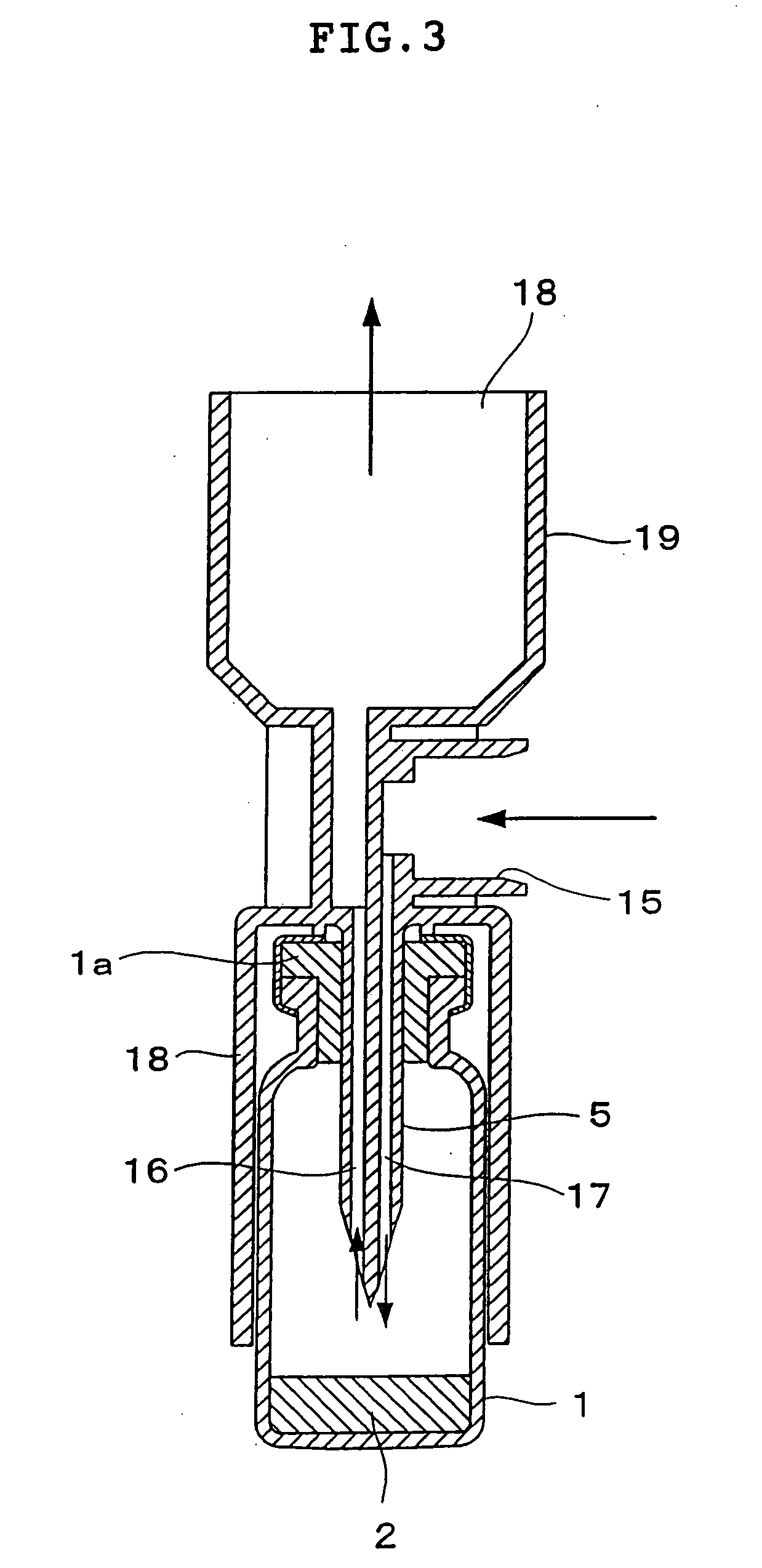
An ultrasonic aerosol generator for delivering a liquid formulation in an aerosolized form at a high output rate of greater than 0.5 mL / minute, preferably greater than 1.0 mL / minute, and with diameters in a respirable size range, methods of using this device and kits including the device are described herein. The ultrasonic aerosol generator (10) contains at least (a) a liquid reservoir / aerosolization chamber (11), (b) a piezoelectric engine (12), (c) a relief aperture (13), and (d) an aerosol delivery element (20). Preferably the aerosolized particles that are delivered to the user through the aerosol delivery element have an average aerodynamic diameter of between 1 and 20 μm, more preferably between 1 and 10 μm, and most preferably between 1 and 5 μm. Optionally, the ultrasonic aerosol generator is designed to deliver more than one formulation simultaneously, preferably a low cost and / or stable formulation is administered simultaneously with a more expensive and / or labile formulation. In the preferred embodiment, the ultrasonic aerosol generator is a hand-held device designed for a single user.
Owner:PULMATRIX
Insulin highly respirable microparticles
ActiveUS7625865B2High percentage of pharmaceutical activityHigh pHPowder deliveryPeptide/protein ingredientsMicroparticleVolumetric Mass Density
The invention describes novel dried powders of peptide therapeutic agent useful for producing highly respirable aerosols and the methods for their manufacture. Insulin is the peptide therapeutic agent in the preferred embodiment. The powders of insulin prepared for pulmonary administration are characterized by the peculiar structure and shape of the microparticles that allow the powder to flow and to be easy aerosolized. Typical dry powder of insulin described in this patent show corrugated, nonagglomerated microparticles with a low tapped density. The mean geometric diameter (particle size) ranges between 1.0 and 10.0 micron and the mass median aerodynamic diameter (MMAD) ranges between 1.0 and 4.0 micron. These insulin pulmonary powders exhibit in vitro a very high respirable fraction (>75%).
Owner:UNIV DEGLI STUDI DI PARMA
Aerated solids particle laser analyzer
ActiveCN101398367ADistinctive featuresThe test result is accurateBiological testingFluorescence/phosphorescenceFluorescenceUltraviolet
The invention provides an aerosol particle laser analyzer which online and continuously detects the aerodynamic diameter and particle quantity of the aerosol particles in the air one by one in real-time and identifies whether the particles are biological particles; the aerosol particle laser analyzer comprises a particle beam queuing acceleration sampling system wrapped by shell flows, a dual-peak laser aerodynamic diameter measurement system, a biological particle fluorescent detection system induced by ultraviolet laser, an ineffective and superposed particle identification circuit, data processing, displaying and memorizing software, and a communication module. The aerosol particle laser analyzer can not only detect the physical parameters such as aerodynamic diameter, particle quantity and the like of the aerosol particles, but also can judge whether the particles are active biological particles or not according to the natural characteristic that the active biological particle emits bioluminescence when being induced and can measure the parameters of the active biological particles such as the quantity, the concentration and the like; the aerosol particle laser analyzer has exact detection results and can be used conveniently and fast for detection; and the parts have long service life and the volume of the aerosol particle laser analyzer is small, thus being convenient for movable usage.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI +1
Effervescent powders for inhalation
ActiveUS20070031490A1Enhancing loosening, thinning, cleansing, and removing of mucus and extrinsic surface materialsBiocidePowder deliveryInhalable particlesActive agent
Effervescent powders comprising inhalable particles are disclosed, as are methods for preparing these powders. The inhalable carrier particles comprise an inorganic or organic carbonate, and an acid, and exhibit effervescence when exposed to water or humid air. The particles have a mass median aerodynamic diameter suitable for nasal, bronchial, or pulmonary administration. The inhalable particles may be used as carriers for active agents. The inhalable particles may also be used to enhance permeability of mucosal and surface barriers on an inner surface of the nose, mouth, airway, and / or lungs of a patient, as well as to loosen, thin, cleanse, and remove mucus and extrinsic surface materials from an inner surface of the nose, mouth, airway, and / or lungs of a patient in need thereof.
Owner:LOEBENBERG RAIMAR +3
System and method for converting optical diameters of aerosol particles to mobility and aerodynamic diameters
ActiveUS20140247450A1Low costProvide quicklyMaterial analysis by optical meansParticle size analysisOptical propertyRefractive index
A system and a method of measuring a particle's size in a select aerosol using the optical diameter of the particle to perform a mobility and / or aerodynamic diameter conversion without any knowledge about the particle's shape and its optical properties in the aerosol being characterized. In one example embodiment of the invention, the method includes generating a set of calibration data and finding the optimal refractive index and shape that best fits the calibration data. In addition, the method includes creating a new calibration curve that provides a mobility-equivalent or aerodynamic-equivalent diameter.
Owner:TSI INC
Stable pharmaceutical solution formulations for pressurised metered dose inhalers
InactiveUS20040047809A1Avoid leachingGood formulation stabilityBiocideDispersion deliveryDrugs solutionAlkane
An aerosol solution composition for use in an aerosol inhaler comprises an active material, a propellant containing a hydrofluoroalkane, a cosolvent and optionally a low volatility component to increase the mass median aerodynamic diameter (MMAD) of the aerosol particles on actuation of the inhaler. The composition is stabilized by using a small amount of mineral acid and a suitable can having part or all of its internal metallic surfaces made of stainless steel, anodized aluminium or lined with an inert organic coating.
Owner:CHIESI FARM SPA
Hemophilia treatment by inhalation of coagulation factors
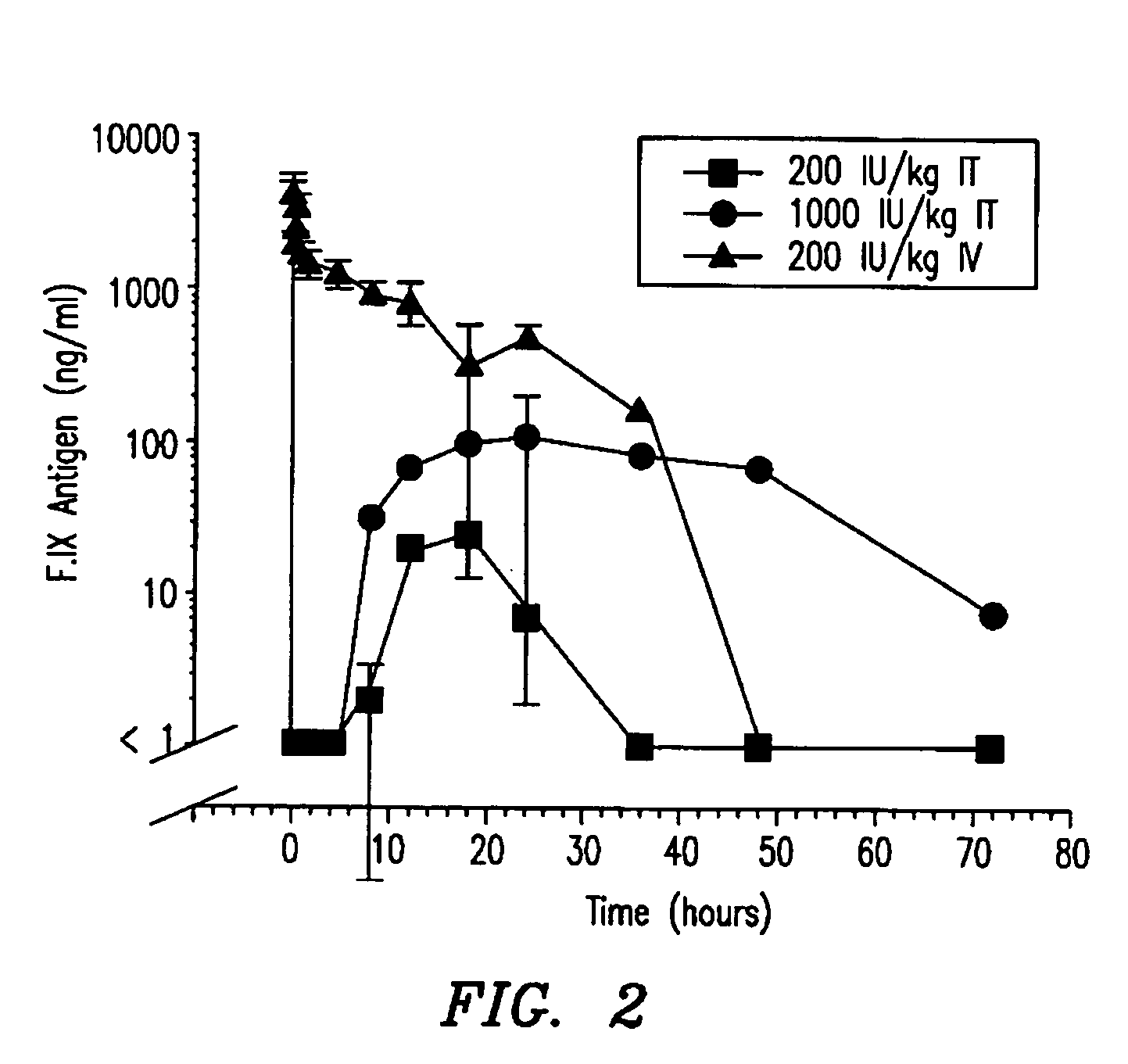
Hemophilia treatment by the inhalation of coagulation factors. Dry powder Factor IX is aerosolized to a mass median aerodynamic diameter of 4 μm or less, with at least 90% monomer content, at least 80% activity level, and 10% water or less. The aerosol is slowly, and deeply inhaled into the lung, and followed by a maximal exhale.
Owner:WYETH LLC +1
Composition for pulmonary administration comprising a drug and a hydrophobic amino acid
According to the subject invention. dispersible dry powder pharmaceutical-based compositions are provided. including methods for their manufacture and dry powder dispersion devices. A dispersible dry powder pharmaceutical-based composition is one having a moisture content of less than about 10% by weight (% w) water, usually below about 5% w and preferably less than about 3% w; a particle size of about 1.0-5.0 .mu.m mass median diameter (MMD), usually 1.0-4.0 .mu.m MMD, and preferably 1.0-3.0 .mu.m MMD; a delivered dose of about >30%, usually >40%, preferably >50%, and most preferred >60%: and an aerosol particle size distribution of about 1.0-5.0 .mu.m mass median aerodynamic diameter (MMAD), usually 1.5-4.5 .mu.m MMAD, and preferably 1.5-4.0 MMAD. Such composition are of pharmaceutical grade purity.
Owner:NOVARTIS FARMA
Interleukin-13 antagonist powders, spray-dried particles, and methods
A powder includes IL-13 antagonist, wherein the powder has a mass median aerodynamic diameter (MMAD) of less than about 10 μm. A composition includes a spray-dried particle including IL-13 antagonist. A method of administering IL-13 antagonist to the lungs of a subject includes: dispersing a dry powder composition involving IL-13 antagonist to form an aerosol; and delivering the aerosol to the lungs of the subject by inhalation of the aerosol by the subject, thereby ensuring delivery of the IL-13 antagonist to the lungs of the subject. A method of treating an IL-13-related condition includes: pulmonarily administering a therapeutically effective amount of a dry powder including IL-13 antagonist. A method of preparing IL-13 antagonist-containing powder involves: combining IL-13 antagonist, optional excipient, and solvent to form a mixture or solution; and spray drying the mixture or solution to obtain the powder.
Owner:NOVARTIS FARMA
Method for treatment of patients with cystic fibrosis
InactiveUS20130034534A1High trafficRespiratorsPowder deliveryControlled breathingCystic fibrosis lungs
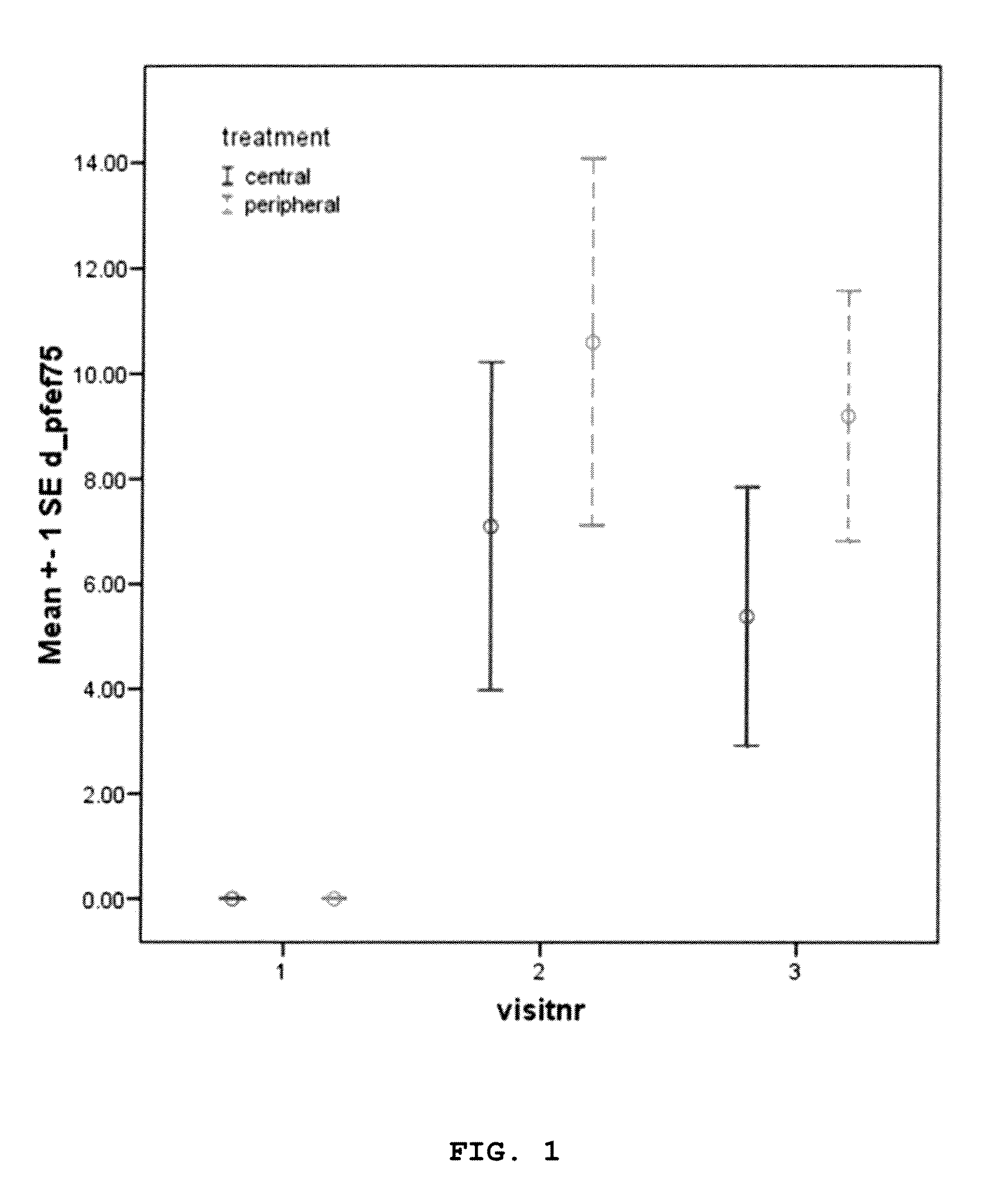
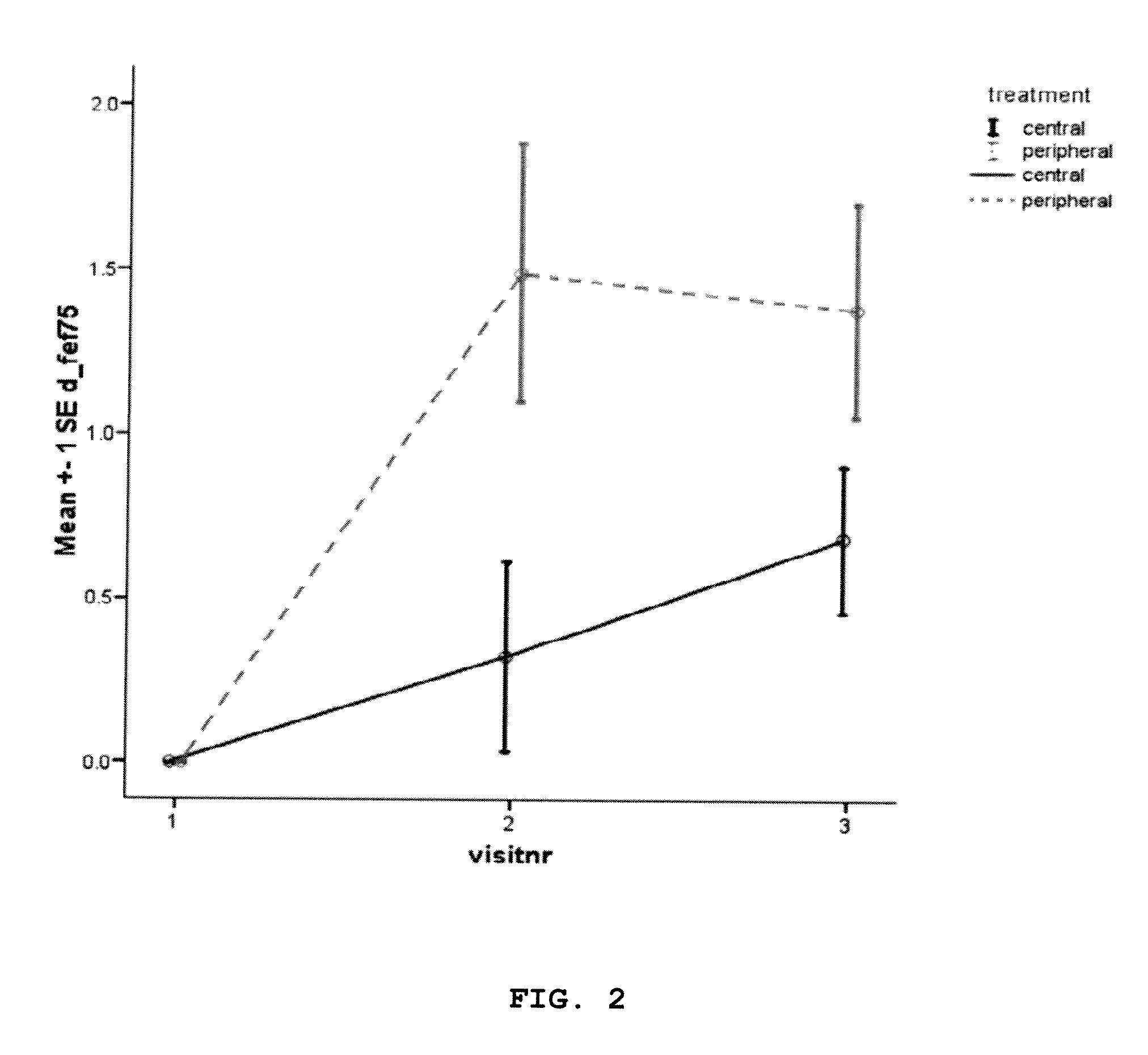
A method for treatment of patients with cystic fibrosis by providing an inhalable aerosol comprising a mucolytic compound (dornase alpha) administered into a patient's lungs according to a specific treatment protocol setting comprising a mucolytic drug containing aerosol having particles with a predetermined mass medial aerodynamic diameter (MMAD) delivered predominantly to a peripheral lungs using a nebulizing system able to administer said aerosol with overpressure and under controlled breathing conditions.
Owner:ERASMUS MC SOPHIA CHILDRENS HOSPITAL
Novel dry powder inhalation system for transpulmonary administration
InactiveUS20060073105A1Reduce manufacturing costHigh fine particle fractionPowder deliverySpray deliveryFreeze-dryingMedicine
The present invention provides a novel dry powder inhalation system suitable for transpulmonary administration. The dry powder inhalation system of the present invention characterized by using a combination of: (1) a vessel housing a freeze-dried composition prepared by freeze-drying a composition liquid containing ingredients in a non-dissolved form, and has: (i) a non-powder cake-like form, (ii) a disintegration index of 0.05 or more, and (iii) a property of becoming fine particles having a mean particle diameter (mass median aerodynamic diameter) of 10 microns or less or a fine particle fraction of 10% or more upon receipt of an air impact having an air speed of at least 1 m / sec and an air flow rate of at least 17 ml / sec; and (2) a device comprising a member capable of applying said air impact to the freeze-dried composition in said vessel, and a member for discharging the powder-form freeze-dried composition that has been made into fine particles.
Owner:OTSUKA PHARM CO LTD
Efficient low-resistance nano-fiber microscopic gradient structure filtering material and preparation method thereof
ActiveCN108796823ASimple preparation processUniform tapered tapered stacked structureMembrane filtersFiltration separationElectricitySurface layer
The invention discloses an efficient low-resistance nano-fiber microscopic gradient structure filtering material and a preparation method thereof. The filtering material comprises a nanometer fine filtering layer, a micrometer support primary filtering layer and protective surface layers, wherein the micrometer support primary filtering layer and the nanometer fine filtering layer are interactively overlaid, and arranged between the two protective surface layers. The nanometer fine filtering layer has a grid structure, and the nanometer fine filtering layer is composed of a plane matrix fiberlayer and cone structures, wherein fibers between the tips of the cone structures and a grid matrix fiber layer have an oriented structure along the tips and the plane matrix fiber layer. By means ofthe filtering material without electricity, the efficiency of filtering NaCl aerosol with the mass median aerodynamic diameter of 0.26 micrometer is 99.9-99.999%, and the pressure is reduced to 130-300 Pa; by means of the filtering material which is treated by electricity, the efficiency of filtering the NaCl aerosol with the mass median aerodynamic diameter of 0.26 micrometer is 99.9-99.999%, andthe pressure is reduced to 30-250 Pa.
Owner:SOUTH CHINA UNIV OF TECH +1
Use of simple amino acids to form porous particles
InactiveUS20070104658A1Lowering overall particle manufacturing costImproved formulabilityPowder deliveryOrganic active ingredientsDiagnostic agentMedicine
Particles having a tap density of less than 0.4 g / cm3 include a hydrophobic amino acid or salt thereof and a therapeutic, prophylactic or diagnostic agent or any combination thereof. Preferred particles include a phospholipid, have a median geometric diameter between about 5 and about 30 microns and an aerodynamic diameter between about 1 and about 5 microns. The particles can be formed by spray-drying and are useful for delivery to the pulmonary system.
Owner:CIVITAS THERAPEUTICS
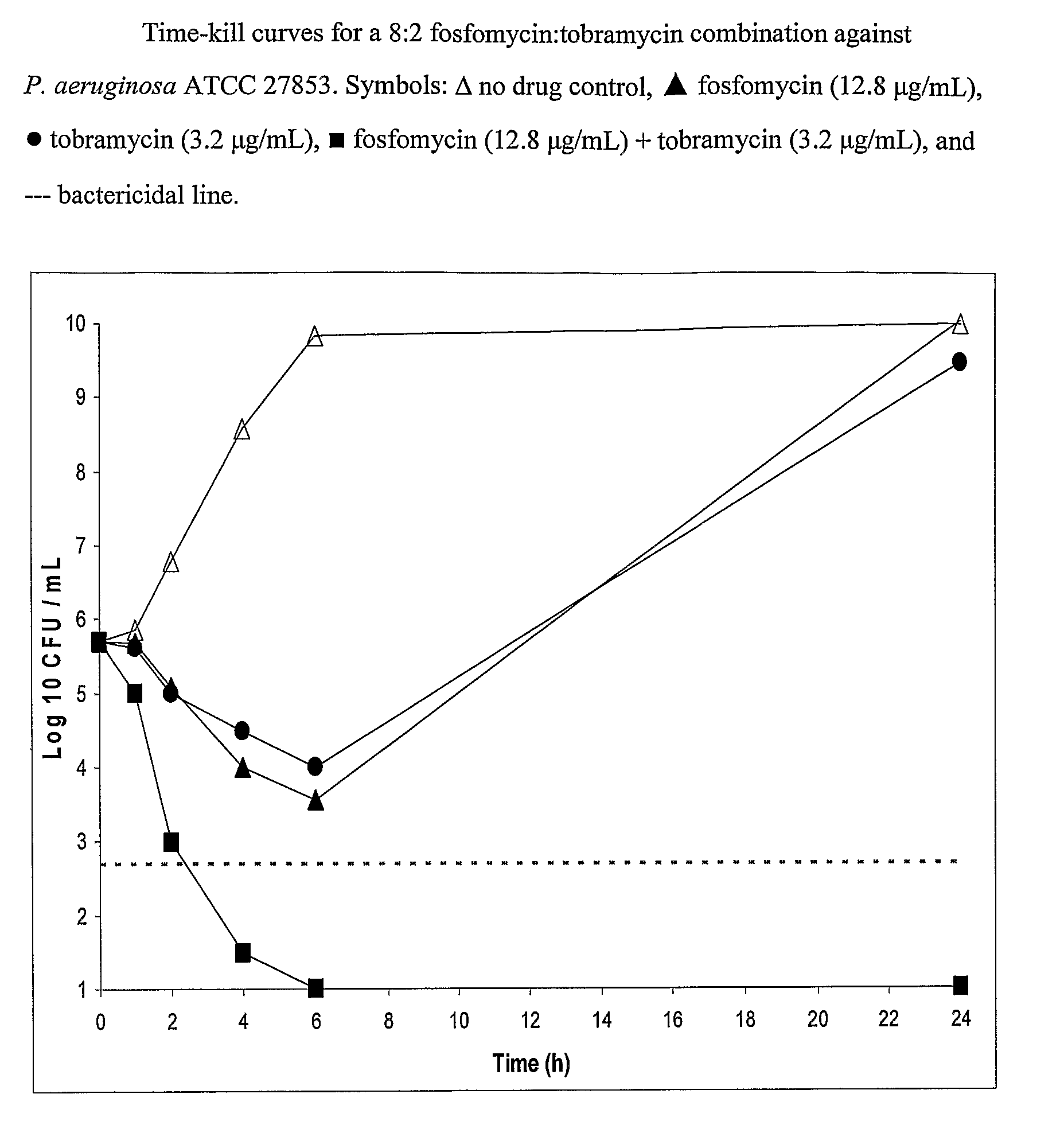
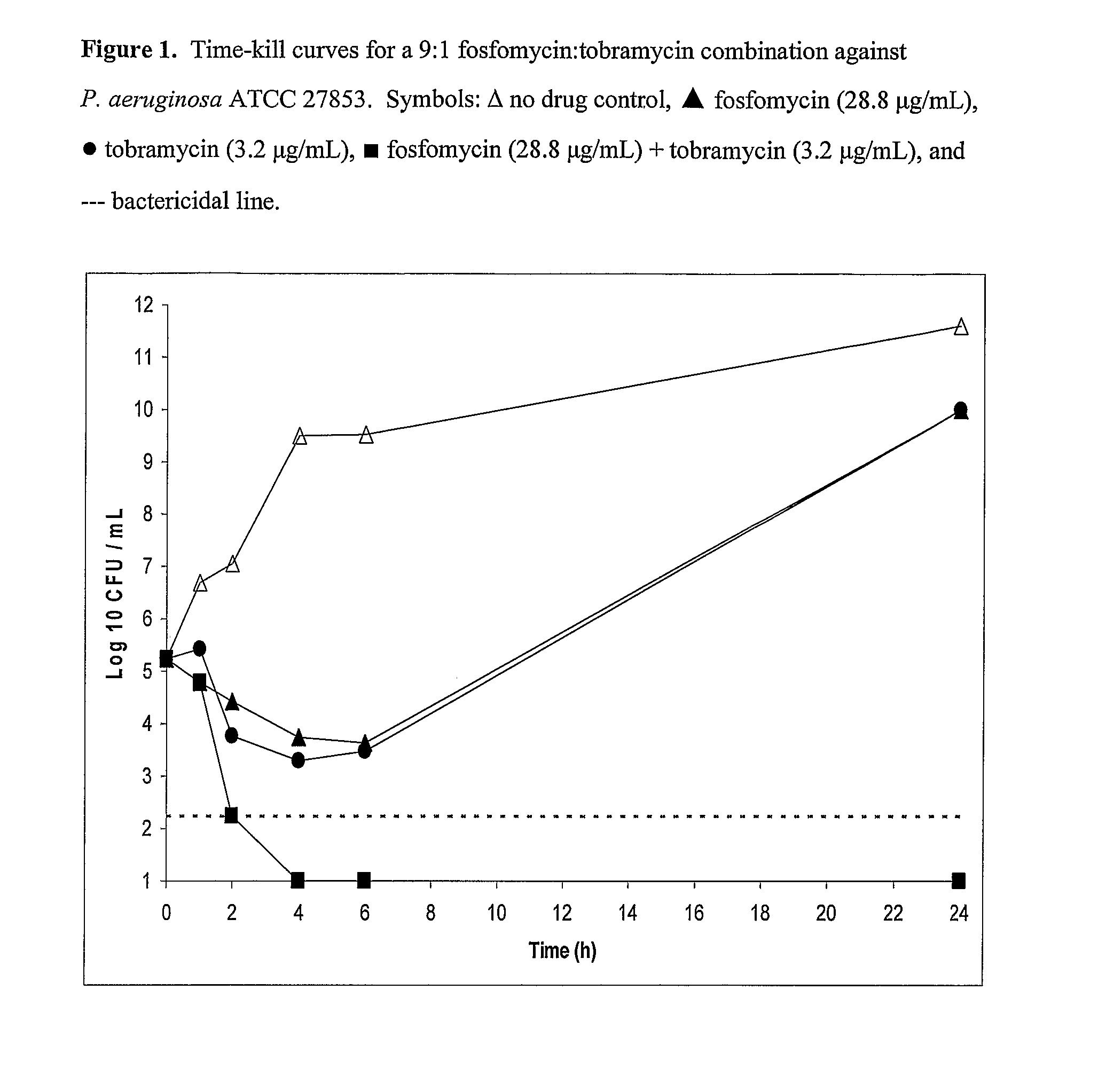
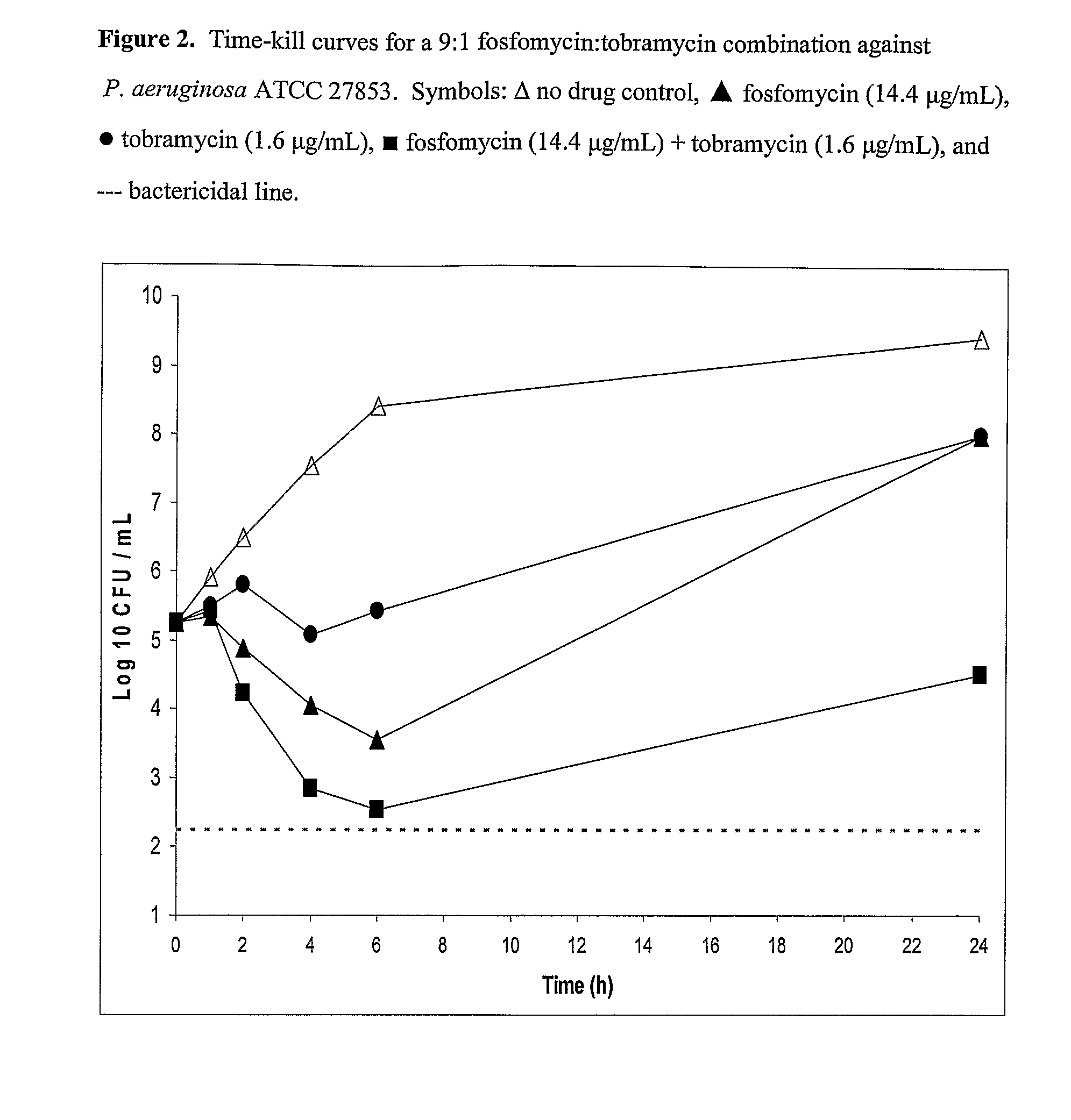
Aerosolized fosfomycin/aminoglycoside combination for the treatment of bacterial respiratory infections
InactiveUS7943118B2Reduce developmentIncreases the post antibiotic affect (PAE)Antibacterial agentsBiocideBacterial respiratory infectionTobramycin
Owner:GILEAD SCI INC
Aerodynamic size spectrometer
InactiveCN102608004AAvoid lossHigh sensitivityParticle size analysisParticle suspension analysisPhotovoltaic detectorsParticle beam
The invention discloses an aerodynamic size spectrometer, which comprises a sample flow injection device, a particle beam collimation and acceleration nozzle, a shell flow device, a light beam focusing and reshaping device, a photoelectric detector, a signal detecting and processing module, and a micro-control and display module. The aerodynamic size spectrometer adopts flight time to measure aerodynamic diameter, and preceding stage particle size cutting and sampling is not needed, so that the particle loss is avoided, the sensitivity is high, and the accuracy of detection is high.
Owner:北京汇丰隆生物科技发展有限公司
Method of preparing porous polymer microspheres by high voltage electrostatic anti-solvent process
ActiveCN102941043AInorganic non-active ingredientsMicroballoon preparationPolymer scienceDecomposition
The present invention discloses a method of preparing porous polymer microspheres by high voltage electrostatic anti-solvent process. Under the effect of high voltage electrostatic force, water-in-oil emulsion is discharged from an injection needle and is added dropwise to an anti-solvent. The organic solvent in the water-in-oil emulsion and the anti-solvent are miscible with each other, so that the polymer droplets takes the water emulsion droplets as nucleus and are cured and precipitated on surfaces of the nucleus, and the polymer microsphere structure containing pore-foaming agent water emulsion droplets is formed. The gas generated by ammonium hydrogen carbonate decomposition inside the microspheres because of emulsification opens holes on the surfaces of the polylactic acid microspheres to generate the porous structural microspheres. The present invention can be used for preparing drug carriers for inhaled pulmonary drug delivery. The experimental results show that: porous microspheres having a large geometric particle size can be prepared under ideal process parameters; the degree of sphericity is good, the surface is rough, the surface has a large number of pores of different sizes, and the interior is filled with through-hole structure; and increasing the input amount of ammonium hydrogen carbonate, the geometric particle size of the product can be significantly improved and the aerodynamic diameter can be reduced.
Owner:HUAQIAO UNIVERSITY
Three-stage dust sampler
InactiveUS20070269349A1Smooth collection efficiencyEasy to operateWithdrawing sample devicesLaboratory apparatusInhalable particlesThree stage
A three-stage dust sampler makes use of a porous foam as the impactor substrates. The sampler includes an annular inlet which conforms to ISO / CEN / ACGIH inhalable sampling criteria, two impactor stages to classify thoracic and respirable particles and a final filter to collect respirable particles after the impactors. For 100 ppi polymer foam substrates, and a flow rate of 3.2 lpm, the collection efficiency curves of the thoracic and respirable dust matches ISO / CEN / ACGIH criteria when the jet-to-plate distance is 1.0. The cut-off aerodynamic diameter is 9.6 and 4.0 μm for the first and second impactor stages, respectively, and √{square root over (stk50)} is 0.39 for both stages. The collection efficiencies for solid particles is equal to that for liquid particles, indicating that there is no solid particle bouncing from the foam substrates.
Owner:INST OCCUPATIONAL SAFETY & HEALTH COUNCIL LABOR AFFAIRS EXECUTIVE YUAN
Aerosolizing nozzle and method of operating such aerosolizing nozzle
ActiveUS9573147B1High viscosityReduce surface tensionSpray nozzlesMedical devicesAcute angleEngineering
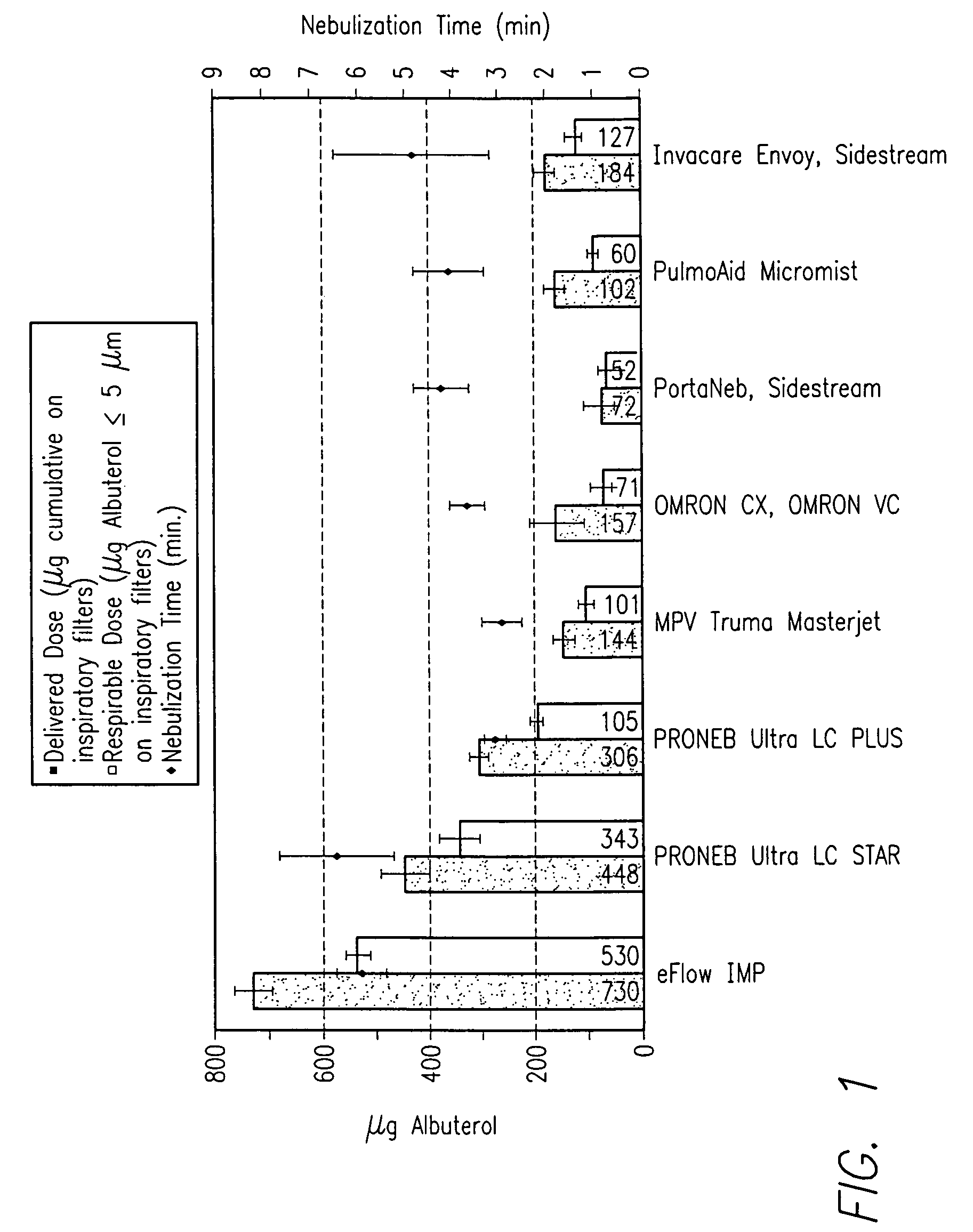
A nozzle and a method of generating an aerosol from a fluid and a gas by operating the nozzle. The nozzle has an aerosol exit orifice of a larger diameter and a fluid exit orifice of a smaller diameter aligned on a common central axis. A pressurized gas from a pressurized gas exit in close proximity to the fluid exit orifice intersects a fluid jet exiting from the fluid exit orifice at that acute angle and in a distance from the aerosol exit orifice. The method includes aerosolizing a fluid with a viscosity exceeding 4 cSt delivering an inhalable medication at a rate of more than 1 ml / minute, thereby delivering a medication at a mass flow rate of at least 30 mg / minute in form of the fluid particles having a mass median aerodynamic diameter (MMAD) of 6 μm or less.
Owner:KAER BIOTHERAPEUTICS CORP
Targeted delivery of lidocaine and other local anesthetics and a method for treatment of cough and tussive attacks
An anti-tussive nebulized solution for targeted delivery of lidocaine into conducting and central airways. A method for treatment of cough and tussive attacks or episodes using said lidocaine solution. A nebulized lidocaine solution administered in daily dose from about 10 mg to 80 mg of lidocaine dissolved in a saline and nebulized into an aerosol having a mass median aerodynamic diameter 3 μm to 10 μm and a geometric standard deviation less than 1.7 using an electronic nebulizer.
Owner:GILEAD SCI INC
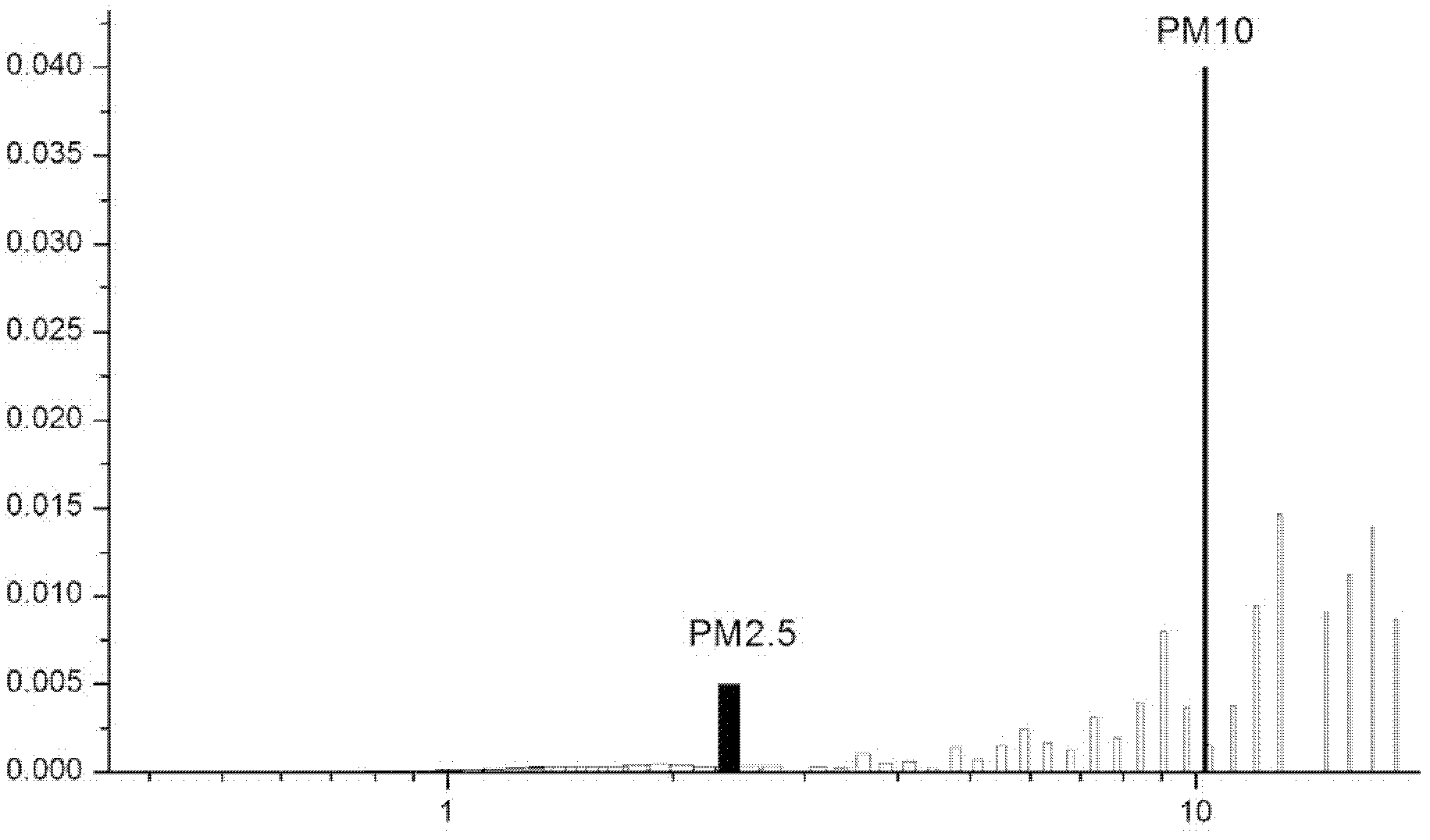
Nanometer screen window for preventing PM2.5 and method for preparing screen window material
ActiveCN105178835AFixed tightlyWaterproofSemi-permeable membranesDispersed particle filtrationFiberEngineering
The invention discloses a nanometer screen window for preventing PM2.5 and a method for preparing a screen window material. The nanometer screen window (NSW-2.5) is composed of a window frame, carbon non-woven fabrics and a nanometer filtering membrane, wherein the nanometer filtering membrane is made of dispersed nanometer acetate fibers, a plurality of nanopores with the pore diameter being 0.5-1 mum are formed, and the porosity of the nanometer filtering membrane is 85-98 percent. The NSW-2.5 can isolate particles with the aerodynamic diameter larger than 2.5 mum in the air and meanwhile keeps good daylighting and ventilation effects. The NSW-2.5 screen window is detachable and is convenient to wash. Moreover, as the nanometer filtering membrane is made of fiber materials, the NSW-2.5 screen window can be rolled and is convenient to store. The NSW-2.5 screen window belongs to the field of household environmental-protection equipment, has the advantages of being low in cost and easy to dismount and wash, realizes integrated mechanized production, and is more easily accepted by the market.
Owner:中地泓通工程技术有限公司
Single-particle aerosol online ionization source and realization method thereof
ActiveCN1838370AImprove quality resolutionImprove ionization hit rateTime-of-flight spectrometersIon sources/gunsLow voltageImage resolution
This invention provides a article aerosol on-line ionization power, which is arranged outside the speeding area of the flying time quality analysis device, which includes ionization laser, low pressure radio frequency four poles, and the laser beam sent from the ionization laser is eradiated into the incenter of the low pressure radio frequency four poles vertically. The ionization power applying method has the following steps: measuring diameter of the single grain aerosol by aerodynamics, then before the flight time quality analyzer enters, iorizating the single grain into plasma, and the positive-negtive ions produced is focalized into ion beam which has very little spcace and then is sent into the flight time quality analyzer. And this invenktion also combining the ionization zone with the aerodynamics diameter measuring zone together, when diameter measuring is done, aerosol inoizates, which reduces the excursion distance of the aerosol, an improves transmission efficient greatly;and low-voltage radio-frequency quadrupole pole's concentrating effect improves the differentiate rate of the flight time quality analyzer.
Owner:KUSN HEXIN MASS PECTRUM TECH +1
Targeted Lung Delivery of Citrulline and/or Another Nitric Oxide Precursor and a Method for Treatment of Pulmonary Deficiency of Nitric Oxide in Cystic Fibrosis and Other Pulmonary Diseases
A method of treatment of cystic fibrosis and other pulmonary diseases identified by nitric oxide deficiency, comprising administration of a nebulized solution of citrulline and / or another nitric oxide precursor as an inhalable aerosol or an inhalable dry powder for targeted delivery into conducting and central airways. Citrulline or another nitric oxide precursor is formulated as a composition having predetermined limited volume, salinity, pH and osmolality. The composition is nebulized into an aerosol having a mass median aerodynamic diameter (MMAD) between 2 μm and 6 μm.
Owner:ACTIVAERO
Medicinal aerosol compositions with a functionalized polyethyleneglycol excipient
The invention comprises a medicinal aerosol composition comprising a propellant, an excipient comprising a functionalized polyethyleneglycol, and a drug. The invention also comprises particulate medicinal compositions comprising particles with a mean mass aerodynamic diameter of less than about 10 microns that incorporate an excipient comprising a functionalized polyethyleneglycol.
Owner:KINDEVA DRUG DELIVERY LP
Particulate materials
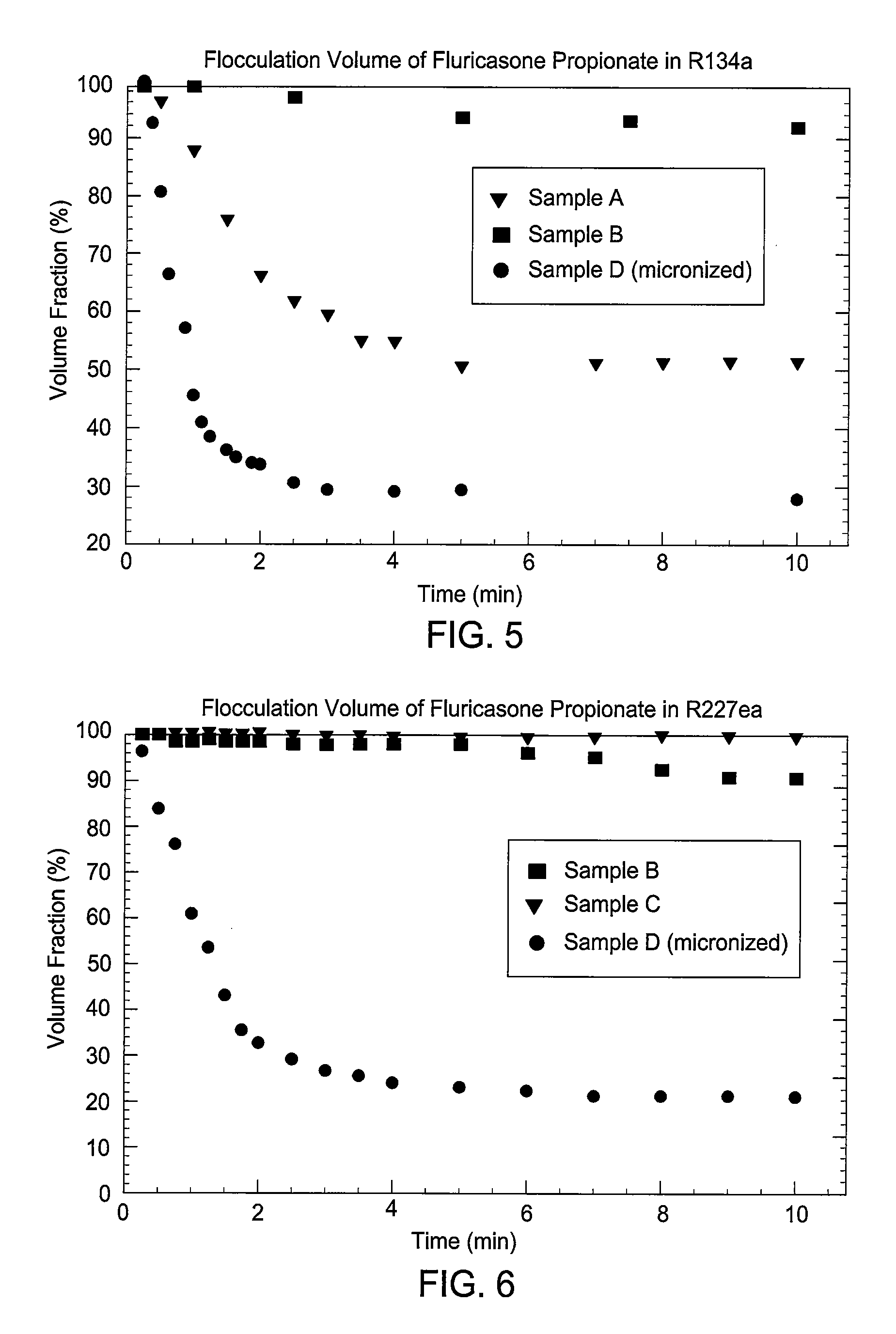
Embodiments of the invention relate to particles of active substances, methods for preparing the particles, formulations containing the particles, and metered dose inhalers containing the particles or formulations. In one embodiment, an inhaler contains an aerosol formulation containing a particulate active substance of non-micronized, solid particles having a mass median aerodynamic diameter of less than 10 μm. The particles may be suspended in a nonsolvent hydrofluorocarbon fluid vehicle (e.g., HFA 134a or 227ea) at a concentration within a range from about 0.2% w / v to about 5% w / v. The formulation exhibits a flocculation volume of about 85% or greater about 1 minute after mixing the particulate active substance and the vehicle. The particulate active substance may contain salmeterol xinafoate, budesonide, salbutamol sulfate, dihydroergotamine mesylate, risperidone-(9-hydroxy)-palmitate, bromocriptine mesylate, or derivatives thereof. In some examples, the active substance is dihydroergotamine mesylate.
Owner:NEKTAR THERAPEUTICS INC
Targeted delivery of lidocaine and other local anesthetics and a method for treatment of cough and tussive attacks
An anti-tussive nebulized solution for targeted delivery of lidocaine into conducting and central airways. A method for treatment of cough and tussive attacks or episodes using said lidocaine solution. A nebulized lidocaine solution administered in daily dose from about 10 mg to 80 mg of lidocaine dissolved in a saline and nebulized into an aerosol having a mass median aerodynamic diameter 3 μm to 10 μm and a geometric standard deviation less than 1.7 using an electronic nebulizer.
Owner:GILEAD SCI INC
Microparticle formulations for sustained-release of bioactive compounds
Owner:POWDER PHARM INC
Aerosol inhalation device
InactiveUS20140060531A1Eliminate requirementsReduce carryRespiratorsMedical devicesSide effectMedicine
Actuators for an aerosol inhalation device containing a housing adapted to receive an aerosol canister containing a pressurized medicament formulation, a mouthpiece portion through which the user inhales, a nozzle block and an orifice and a tubular element extending in the mouthpiece portion from the orifice aperture in a longitudinal axis substantially aligned with a longitudinal axis of the mouthpiece portion provide a significant reduction in the non-respirable, coarse fraction of the emitted aerosol medicament via inertial impaction and retention in the actuator than in the oro-pharynx, with consequent less associated side effects and oral candidiasis in the patient. In addition the presence of the tubular element has minimal, negligible impact on the fine particle dose and on the particle size distribution (PSD) of the delivered particles having aerodynamic diameter lower than 9 μm.
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com