Aerosol inhalation device
a technology of aerosol particles and inhalation chambers, which is applied in the direction of inhalators, medical devices, other medical devices, etc., can solve the problems of reduced patient compliance, oral candidiasis and dysphonia and systemic side effects, and aerosol particles that are not easily absorbed and deposited in the mouth, throat and pharynx walls,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
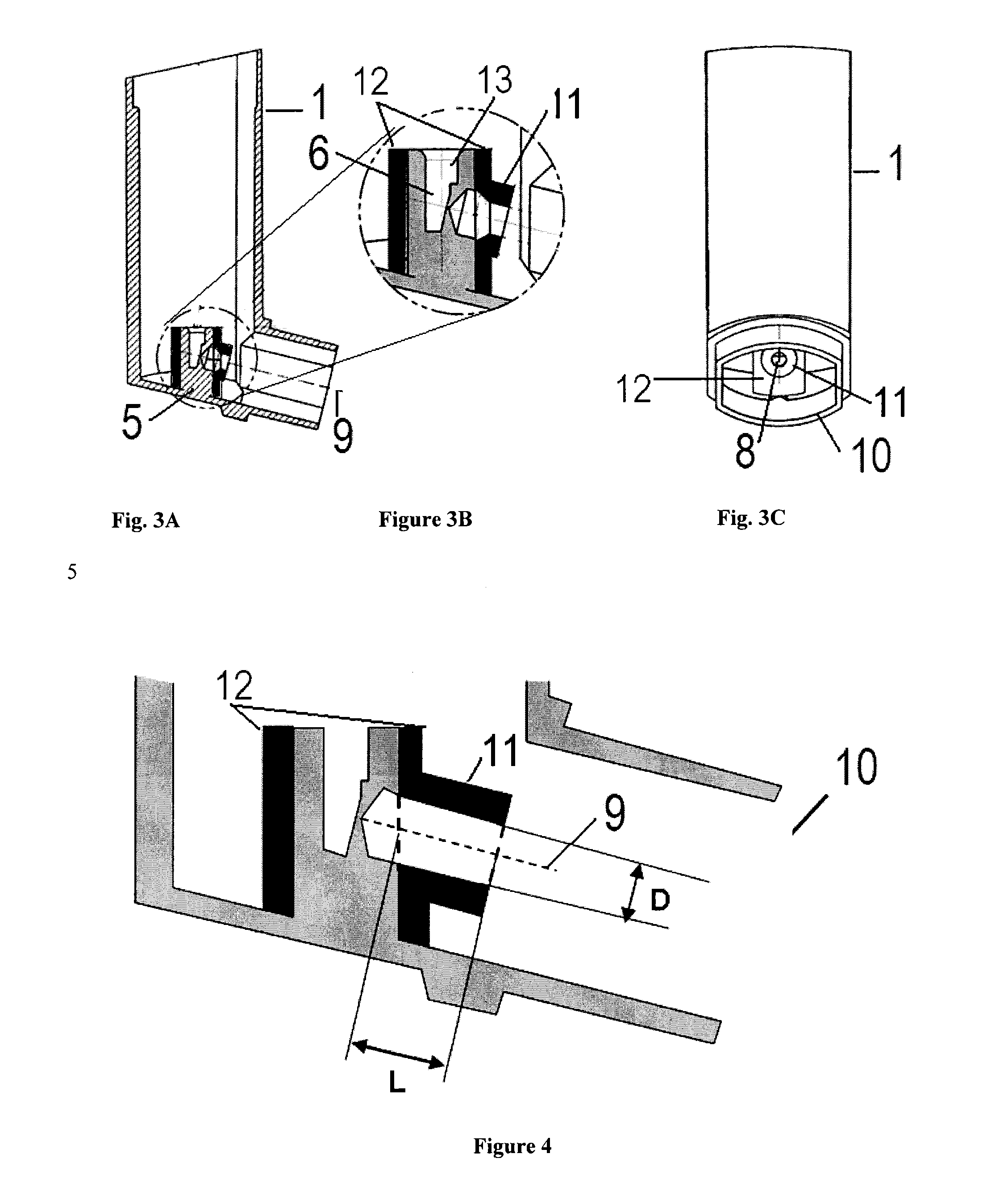
[0132]A range of tubular elements of predetermined dimensions were designed and manufactured. These were designed to allow them to be easily fitted to a conventional actuator block, thus converting the actuator into an actuator according to the present invention with a recessed orifice aperture design. FIGS. 3b and 4 show the basic premise of the design and the respective diameter D and length L of the geometries tested. The smallest internal diameter of 3 mm is less than the diameter of the orifice cone therefore was chamfered to prevent an impact surface and potential dead space from being created. The largest tube diameters required a step detail to enable the extra width to be catered for, as the internal diameter was wider than the nozzle block of the actuator (FIG. 5).
[0133]The tubular elements were manufactured in UV Curable Acrylic Plastic. All prototype tubes were fitted on actuators with 0.30 mm orifice diameter.
[0134]The actuator prototypes based on FIGS. 2 to 5 were prod...
example 1b
[0145]A range of single molded actuators plus tubular elements of 10 mm length and 6 mm diameter with smoothed or stepped nozzle tube features as shown in FIGS. 16A-B and in FIGS. 17A-C were manufactured and tested in their performances. The seven variants produced, depending also from the orifice diameters and the material types: Polypropylene (PP: Ineos 100-GA3), polymethylmetacrylate (PMMA: Aultglas V825T), Polycarbonate (PC: Makrolon 2405, are shown in Table 5
TABLE 5Single moulded actuators plus tubular elementsmanufactured according to Example 1B.SteppedOrifice DiameterPrototype N.FeatureMaterial(mm)269YesPolypropylene0.32270YesPolypropylene0.30271NoPolycarbonate0.30272NoPolycarbonate0.32273YesPolycarbonate0.30274YesPolycarbonate0.32275NoPolymethylmetacrylate0.32
example 2
[0146]An actuator fitted with one of the preferred tube element (i.e. Prototype 204 of Example 1A) with a 10 mm length (L) and 6 mm internal diameter (D) was tested to challenge the robustness of the concept. Through-can-life testing was carried out to assess the drug delivery performance of the prototype, in particular when no patient washing is performed, i.e. a “worst case” scenario. The plume temperature profile was also measured, to determine whether the presence of the nozzle tube could potentially reduce the “cold-freon” effect.
Through-Life-Testing.
[0147]Prototype 204 was coupled with a new, un-primed can containing the solution formulation of beclometasone dipropionate 250 μg / dose (BDP 250 of Table 1). Four doses were delivered to a waste Dose Unit Sampling Apparatus (DUSA; Copley Instruments, UK) every five minutes and all shot weights recorded. The five minute period allowed the ethanol to evaporate and the area around the orifice and nozzle tube to dry; this could be expe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com