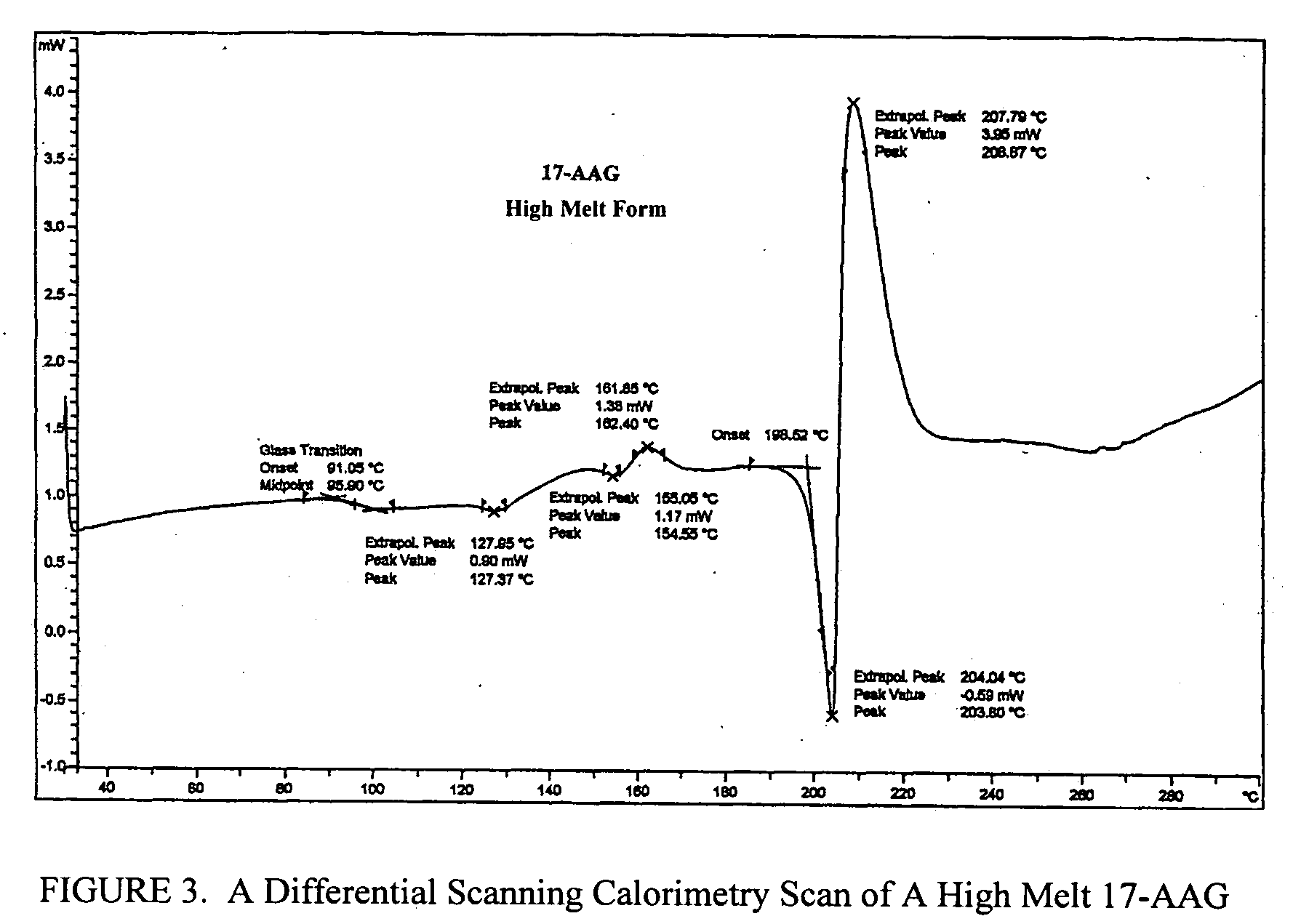
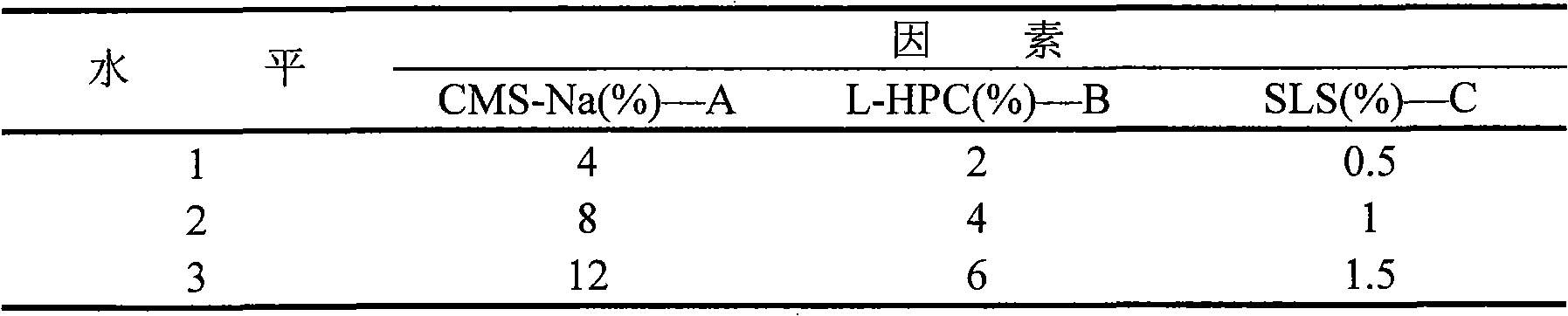
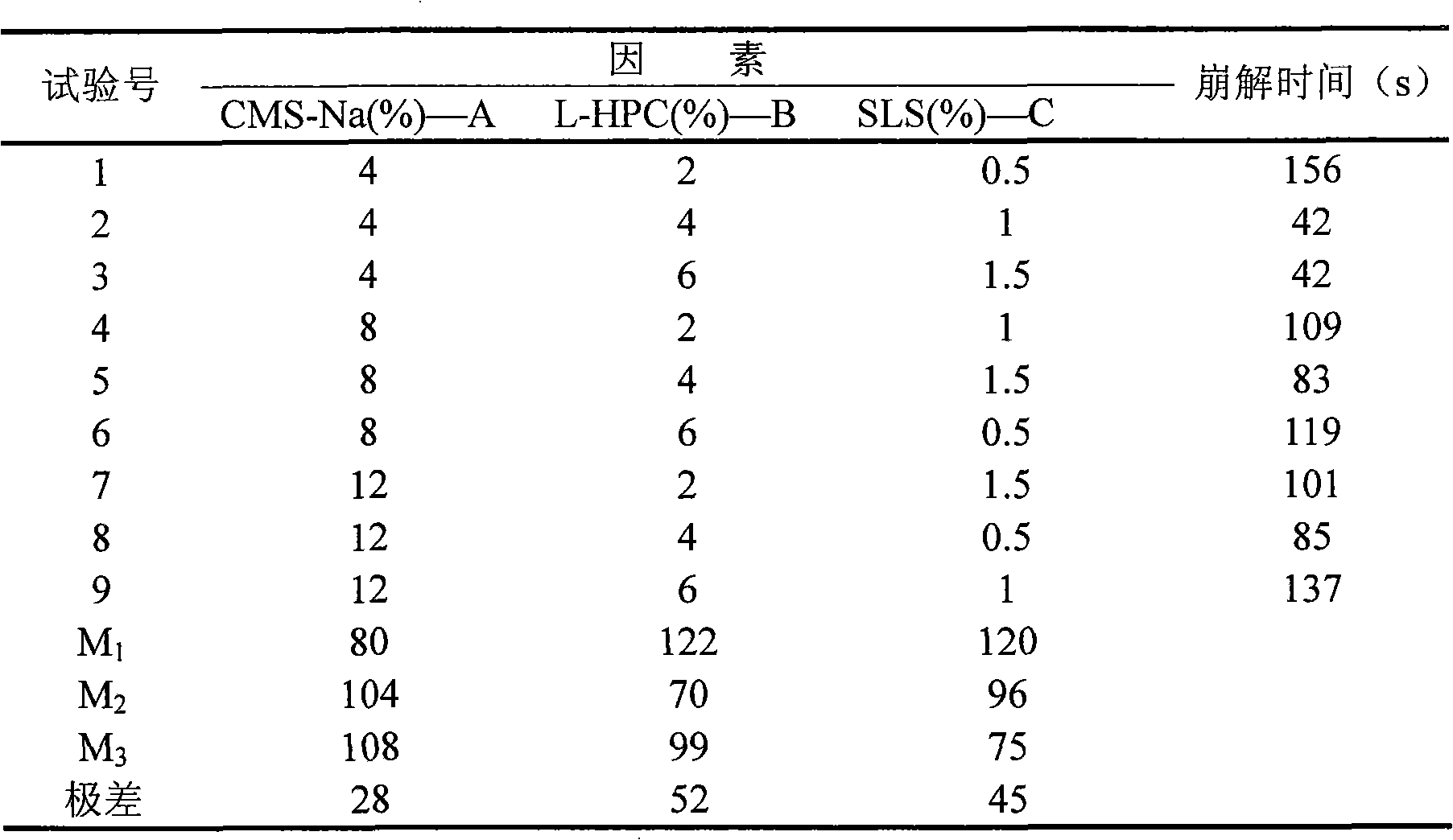
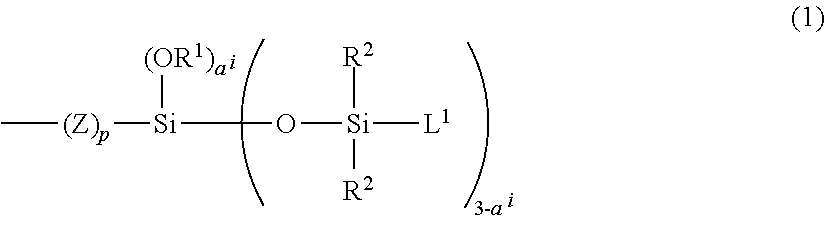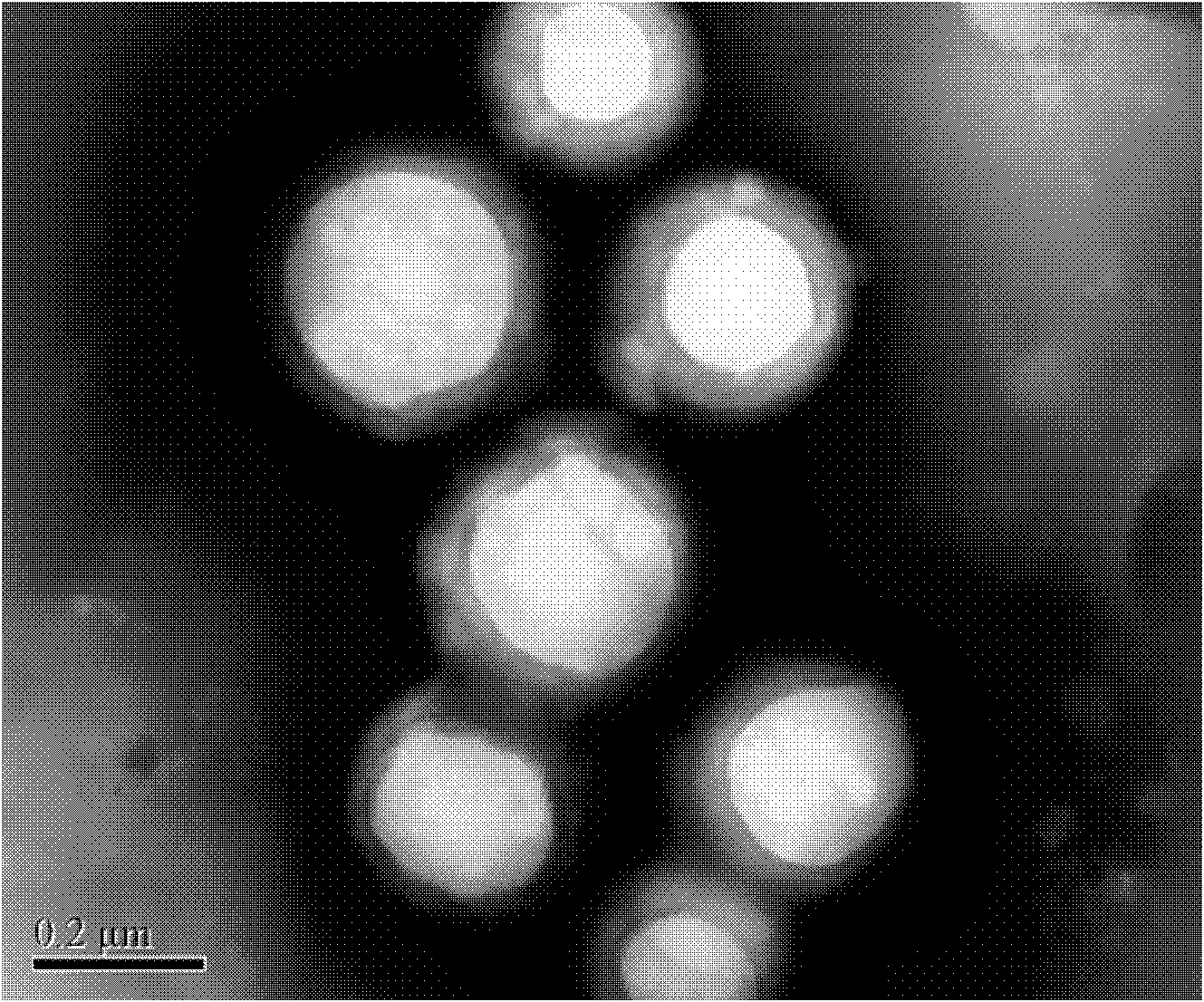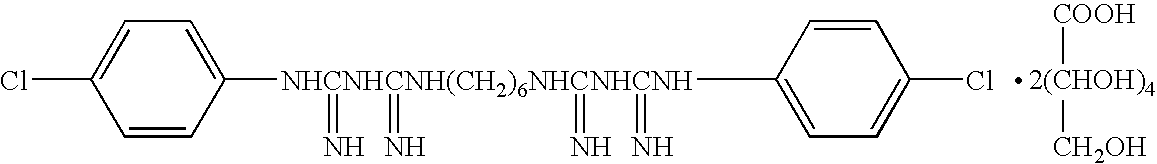Patents
Literature
212results about How to "Good formulation stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
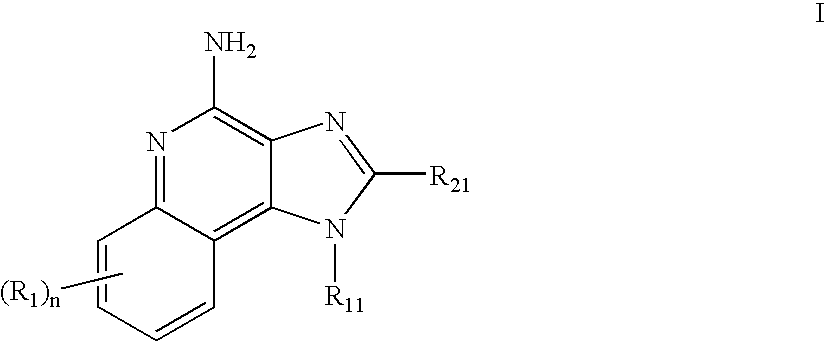
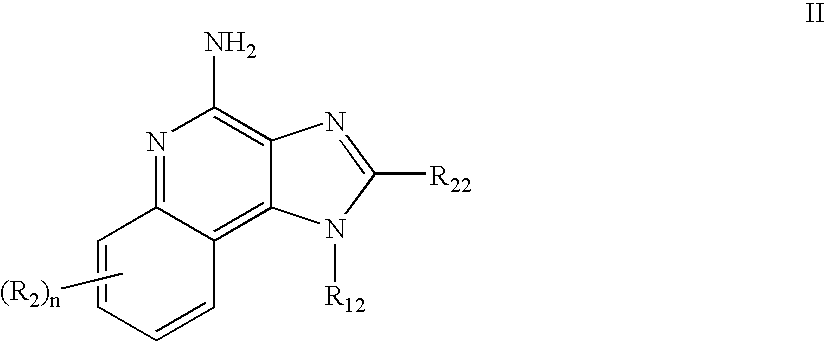
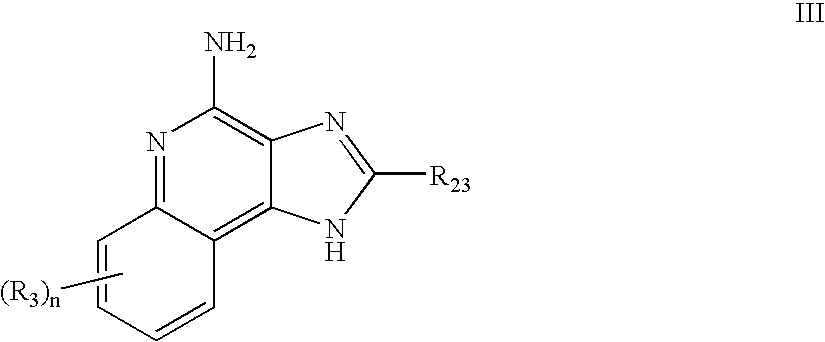
Immune response modifier formulations and methods
Pharmaceutical formulations including an immune response modifier (IRM) compound having a 2-aminopyridine moiety fused to a five-membered nitrogen-containing heterocyclic ring; a preservative system including a sorbic acid preservative selected from the group consisting of sorbic acid, esters thereof, salts thereof, and combinations thereof; an antioxidant; and an optional chelating agent.
Owner:MEDICIS PHARMA CORP
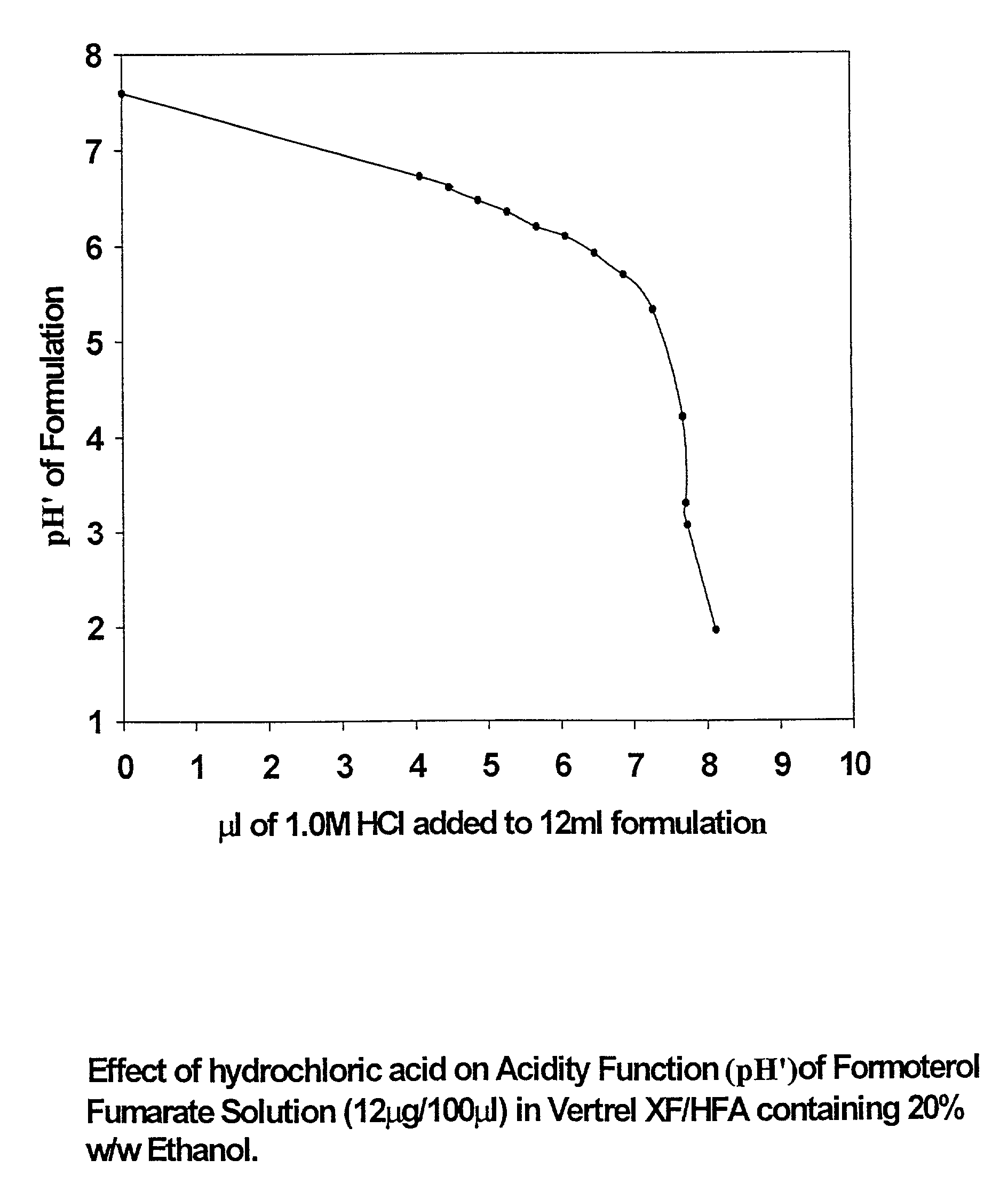
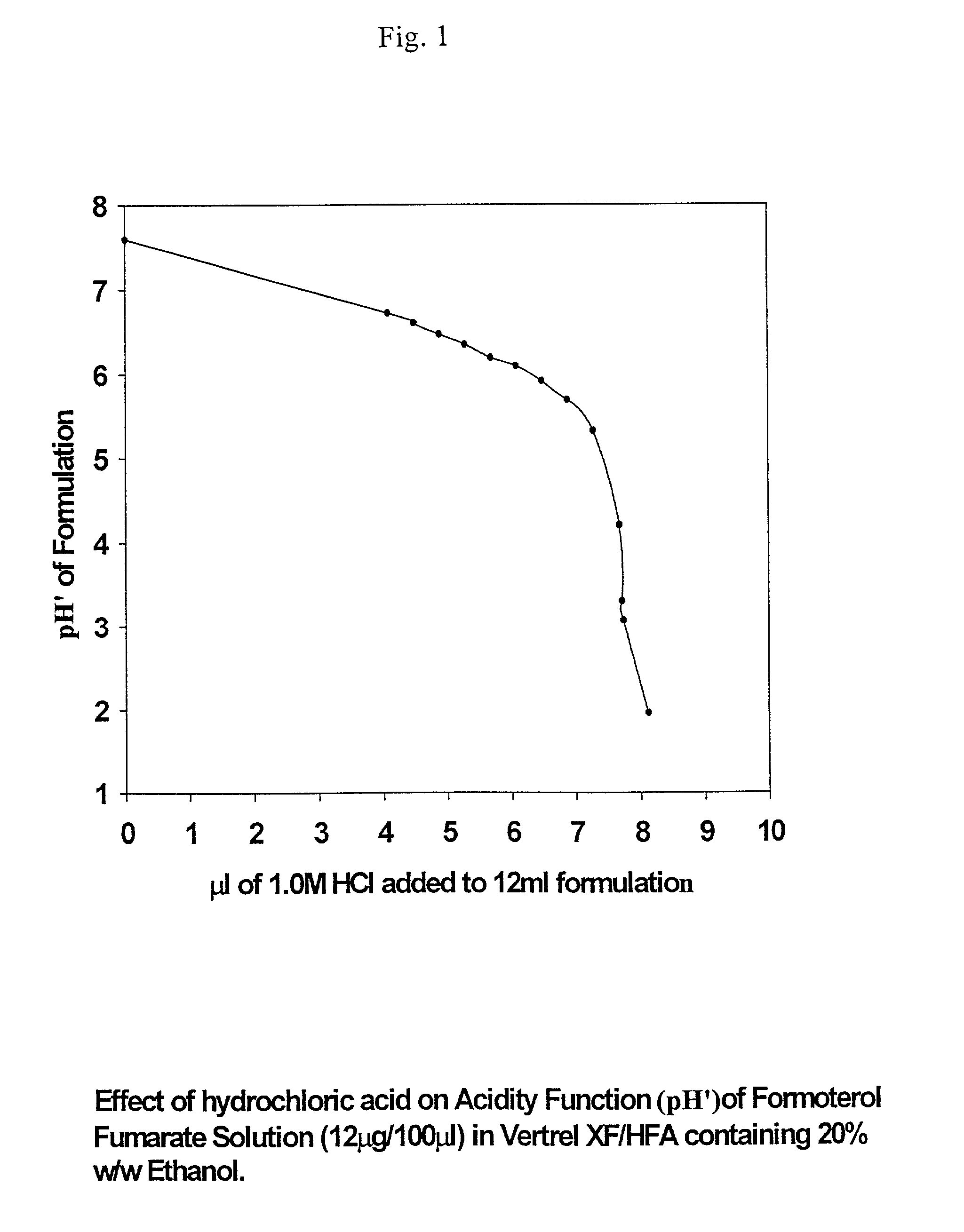
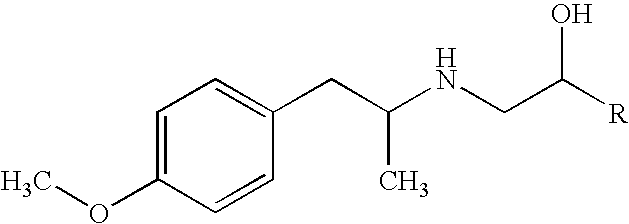
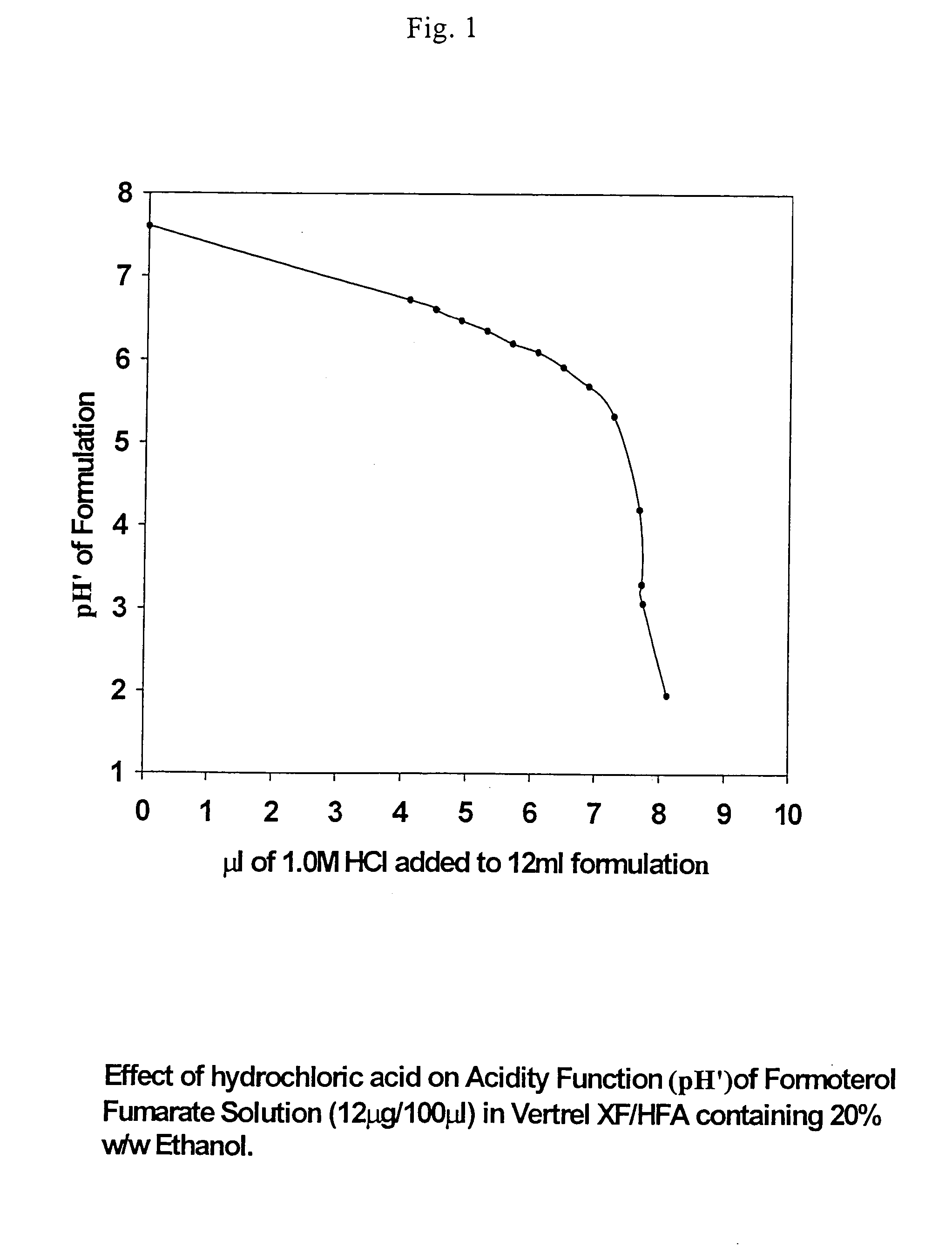
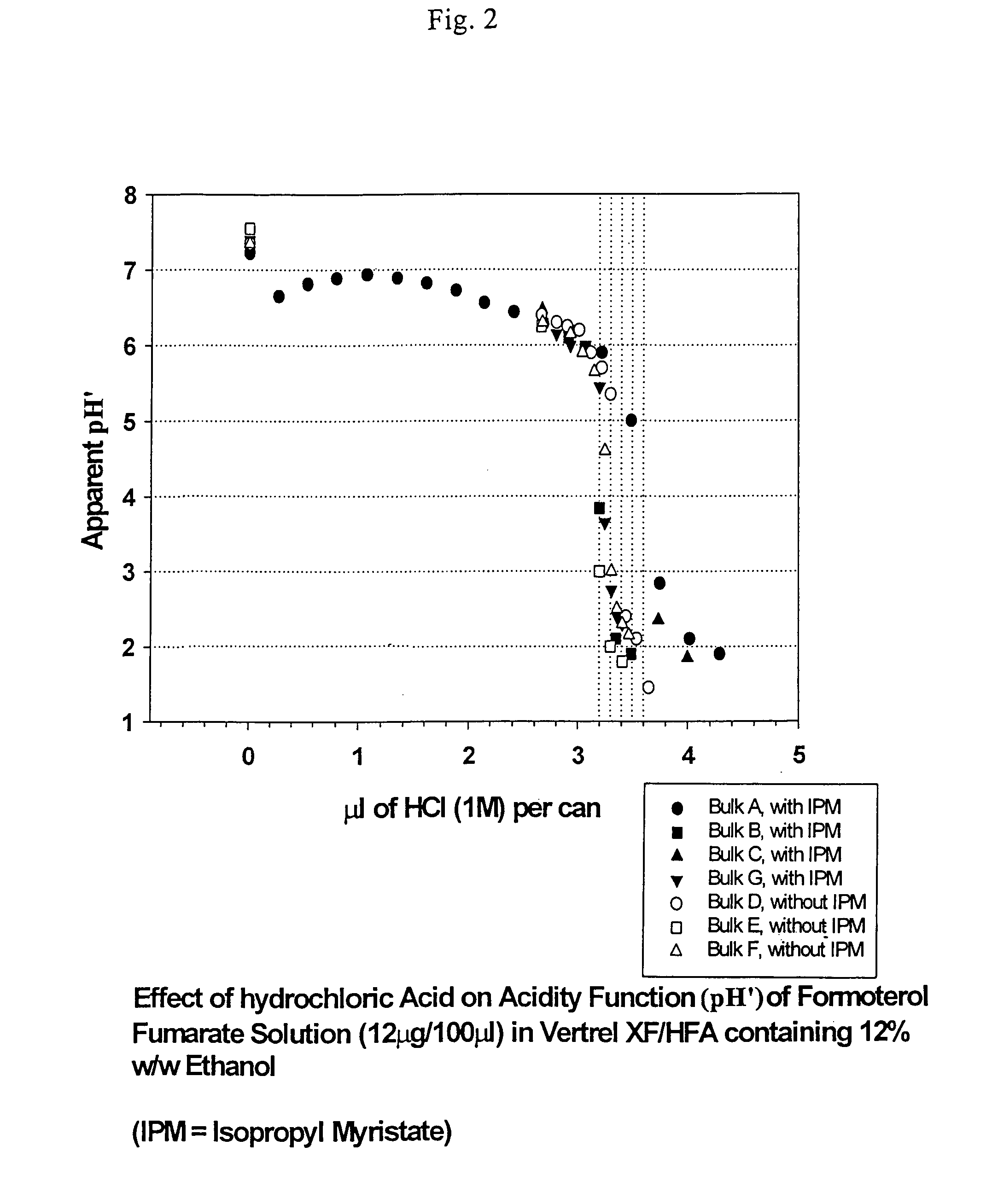
Stable pharmaceutical solution formulations for pressurised metered dose inhalers
An aerosol solution composition for use in an aerosol inhaler comprises an active material, a propellant containing a hydrofluoroalkane, a cosolvent and optionally a low volatility component to increase the mass median aerodynamic diameter (MMAD) of the aerosol particles on actuation of the inhaler. The composition is stabilized by using a small amount of mineral acid and a suitable can having part or all of its internal metallic surfaces made of stainless steel, anodized aluminium or lined with an inert organic coating.
Owner:CHIESI FARM SPA
Liquid Compositions of Insoluble Drugs and Preparation Methods Thereof
ActiveUS20130156853A1Good biocompatibilityEliminate hidden dangersBiocideCarbohydrate active ingredientsPhospholipidSolvent
A liquid composition of an insoluble medicament and a preparation method thereof are disclosed. The composition includes insoluble medicament, oil for injection, phospholipid, and solvent; the percentage by weight of each component is as follows: insoluble medicament 0.01-10%, oil for injection 0%-20%, phospholipid 10-80%, solvent 20-89%. The preparation method for the composition includes the following steps: dissolving an insoluble medicament into solvent or oil for injection or a mixture thereof firstly, and then adding other components, and mixing uniformly; or dissolving an insoluble medicament into a mixture of other components, and mixing uniformly; or dissolving an insoluble medicament into part of solvent firstly, and then adding into a mixed solvent of other components and the remaining solvent, and mixing uniformly.
Owner:PEKING UNIV
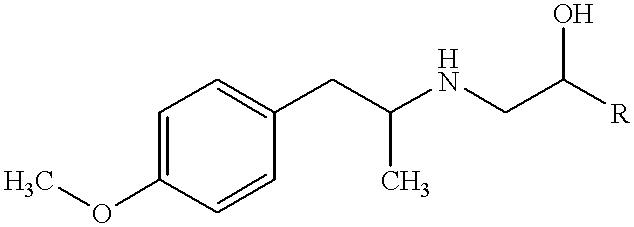
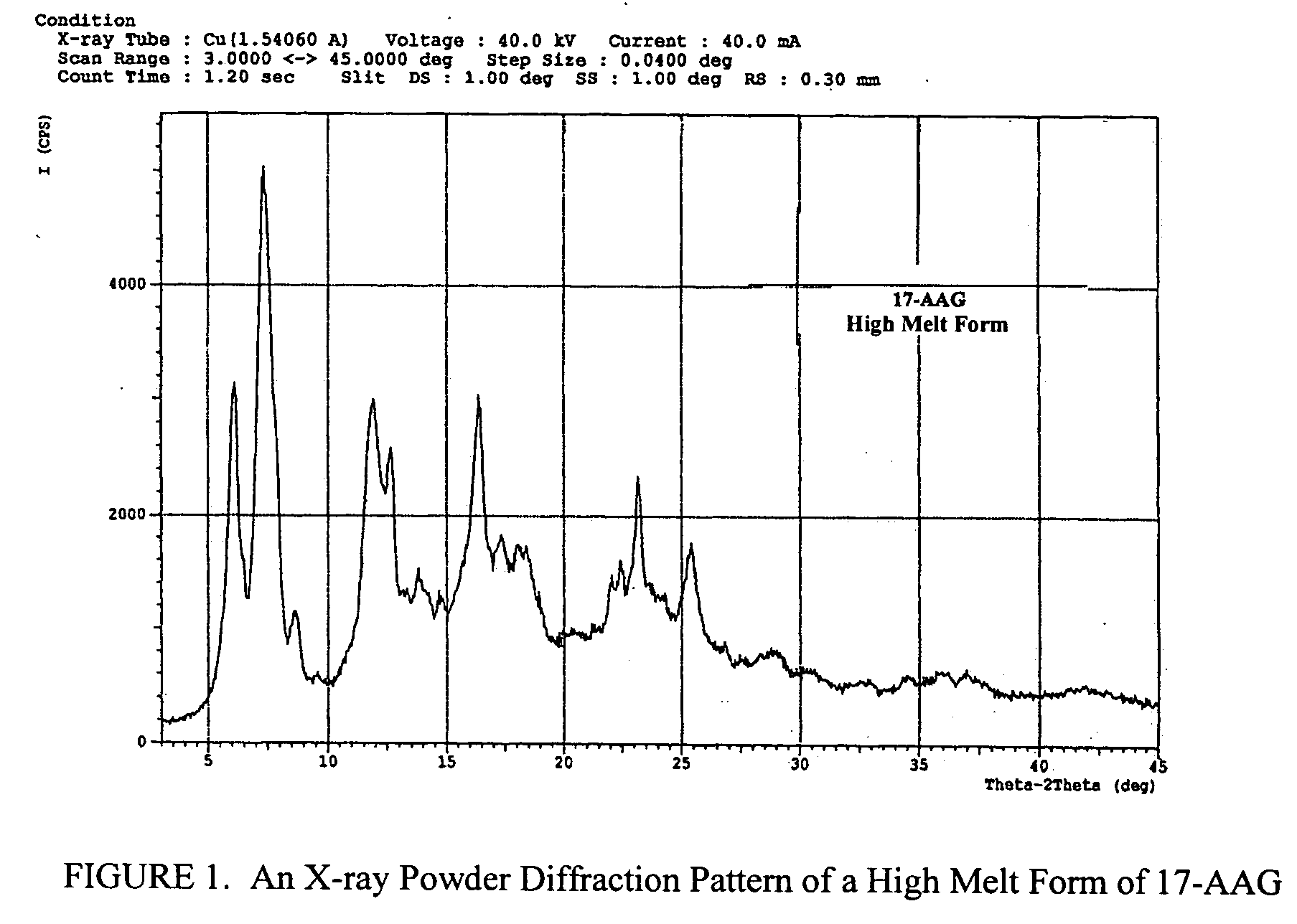
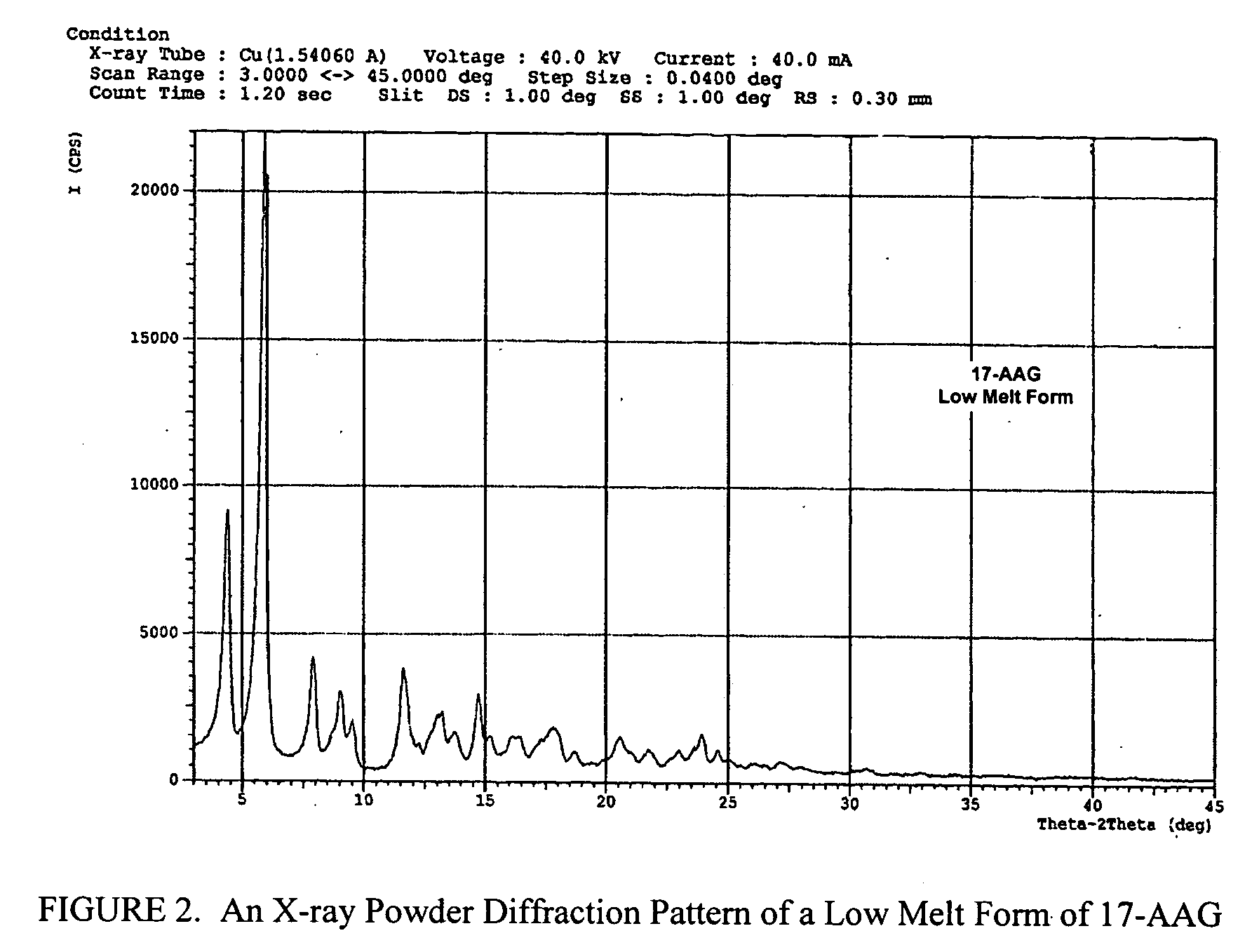
Method of stabilizing imiquimod
InactiveUS7902242B2Short lifeImpaired stabilityBiocideOrganic chemistryPharmaceutical formulationOleic Acid Triglyceride
Pharmaceutical formulations and methods including an immune response modifier (IRM) compound and an oleic acid component are provided where stability is improved by using oleic acid have low polar impurities such as peroxides.
Owner:MEDICIS PHARMA CORP
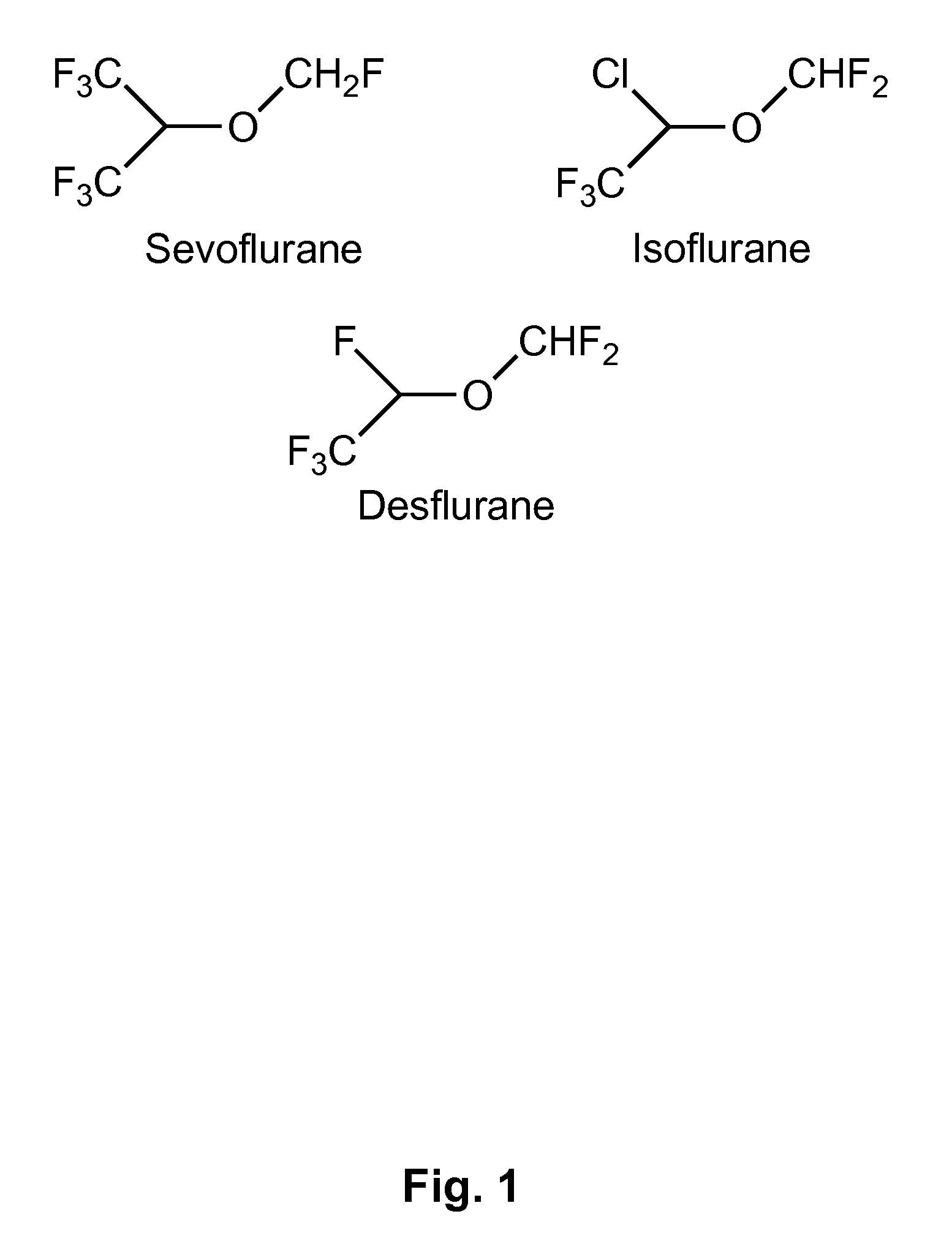
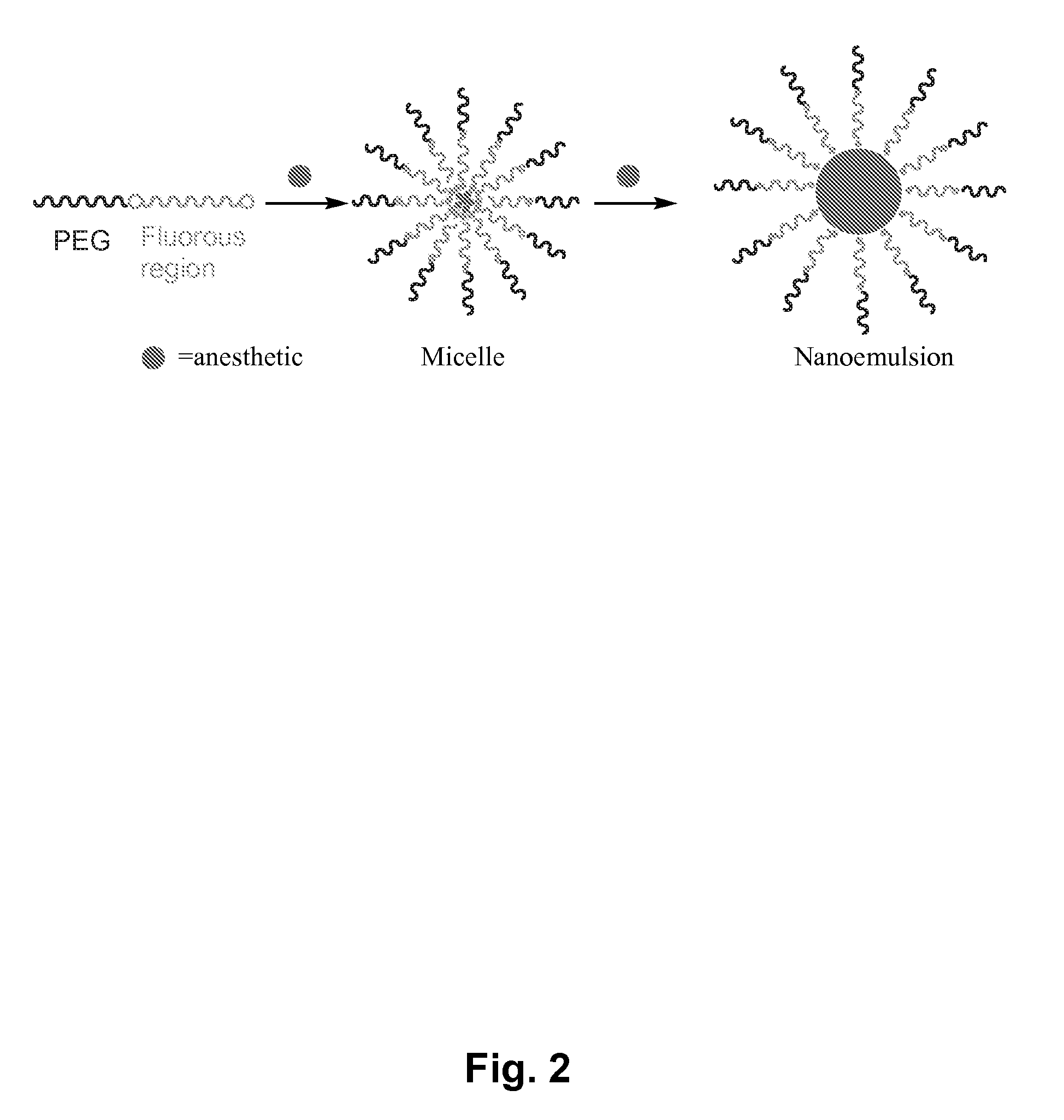
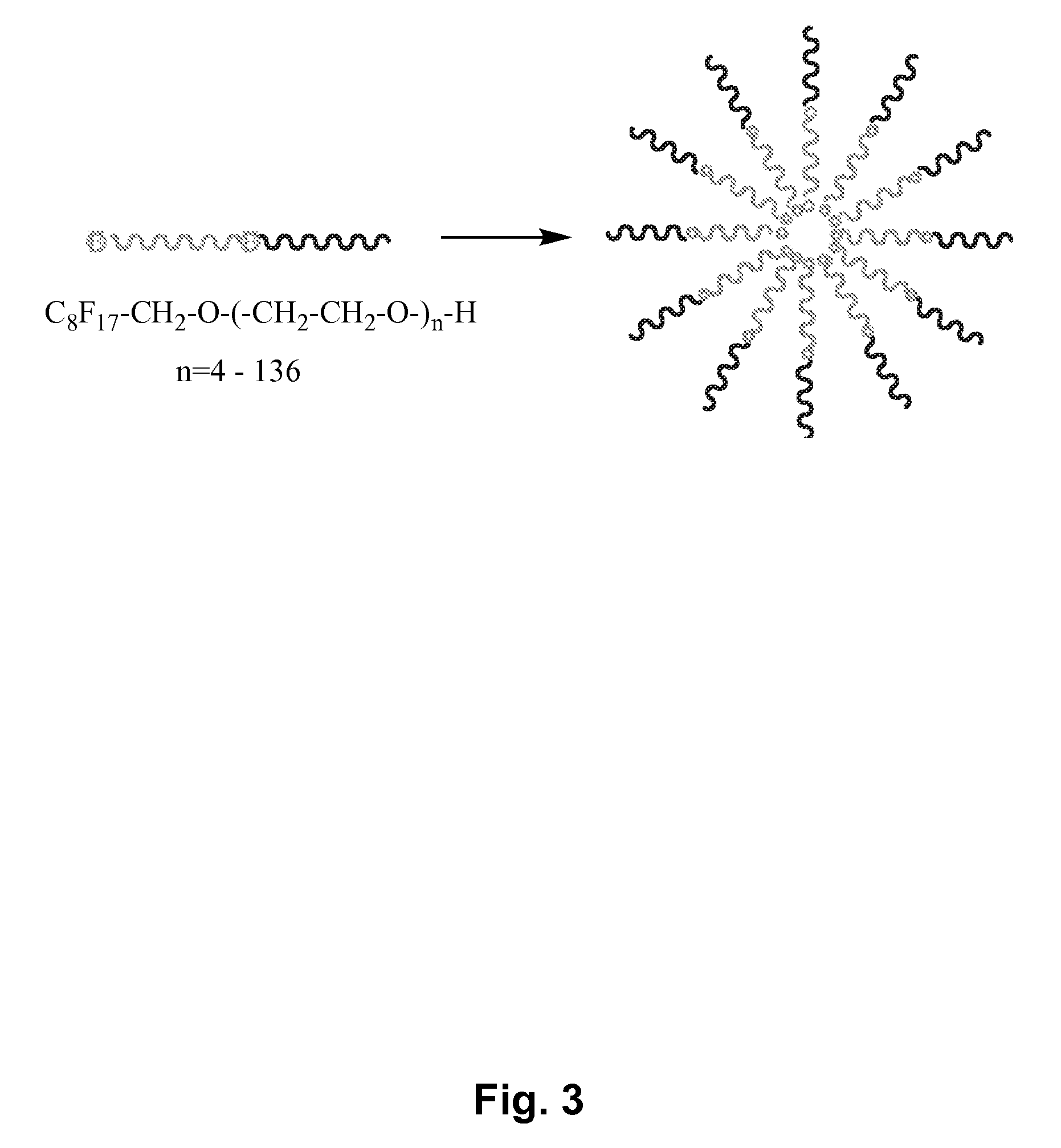
Fluoropolymer-based emulsions for the intravenous delivery of fluorinated volatile anesthetics
InactiveUS20080234389A1Maintenance can be inducedReduce interfacial tensionBiocideNervous disorderAnesthetic AgentEmulsion
The present invention provides therapeutic formulations, including therapeutic emulsions and nanoemulsions, and related methods for the delivery of fluorinated therapeutic compounds, including an important class of low boiling point perfluorinated and / or perhalogenated volatile anesthetics. Emulsion-based fluorinated volatile anesthetic formulations compatible with intravenous administration are provided that are capable of delivering and releasing amounts of fluorinated volatile anesthetic compounds effective for inducing and maintaining anesthesia in patients. Intravenous delivery of the present emulsion-based fluorinated volatile anesthetic formulations permits anesthetic levels in a patient to be selectively adjusted very rapidly and accurately without the need to hyperventilate patients and without the use of irritating agents.
Owner:WISCONSIN ALUMNI RES FOUND
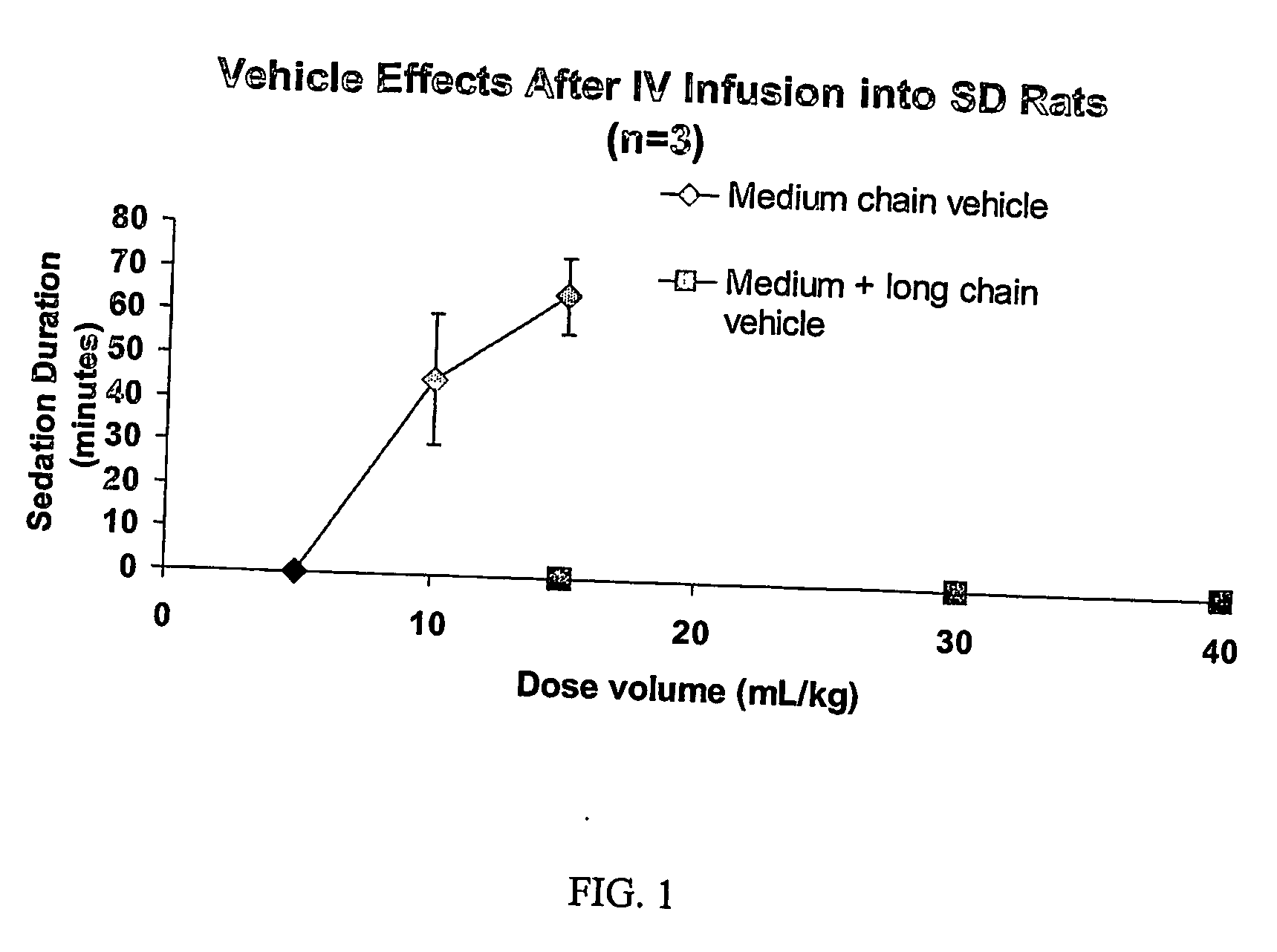
Drug formulations having long and medium chain triglycerides
InactiveUS20060148776A1Good formulation stabilitySimple pharmacyBiocideLyophilised deliveryPharmaceutical formulationCentral nervous system
Drug formulations having emulsifying agents and both medium and long chain triglycerides are described. In preferred embodiments, the long chain triglycerides negate or lessen deleterious central nervous system effects that are caused by medium chain triglycerides.
Owner:CONFORMAL THERAPEUTICS CORP (US)
Oral pharmaceutical formulations and methods for producing and using same
InactiveUS20060067953A1Reduce the environmentLow toxicityBiocideSolution deliveryCompound (substance)Phospholipid
Owner:CONFORMAL THERAPEUTICS CORP (US)
Engine coolant
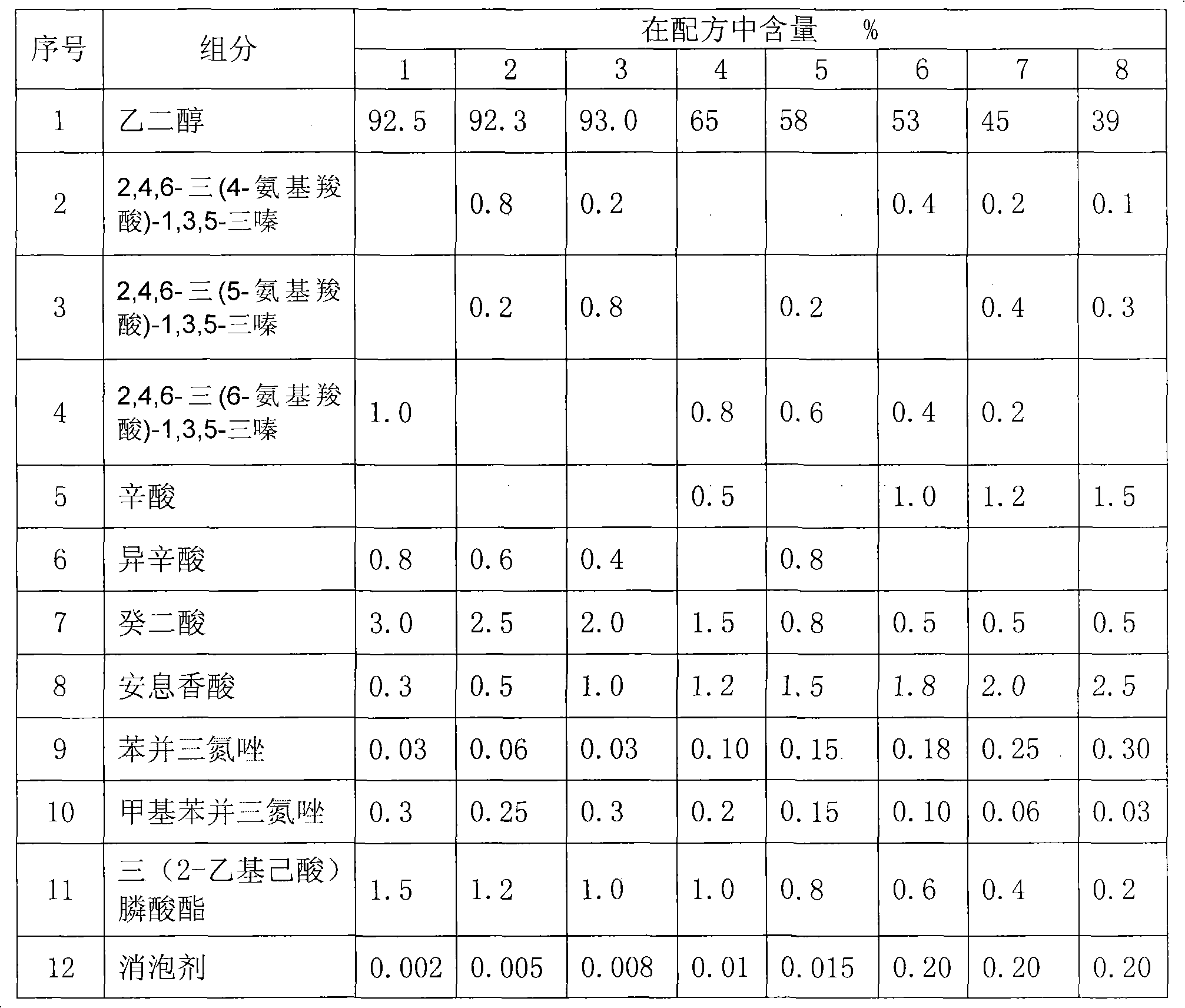
ActiveCN101768428AAvoid corrosionGood corrosion inhibitionHeat-exchange elementsIonAutomotive engineering
The invention provides an all-organic type engine coolant which comprises glycol, fat carboxylic acid, tricarboxylic acid, benzoic acid, tri (2-ethylhexoic acid) phosphonate, imidazoles compound, defoamer and deionized water. The engine coolant of the invention contains no inorganic salts, such as amines, borate, nitrate, nitrite, silicate, and the like, so the engine coolant is environment-friendly, can prevent all the contacted metal and nonmetal materials in the engine cooling system from being corroded, has an obvious engine coolant effect on various metals, particularly solves the problem that the cast aluminum using a traditional organic acid engine coolant becomes black generally, can be stabled in a relative low density and bigger PH value range, and has the advantages of long-time storage and use.
Owner:北京蓝星清洗有限公司
Stable pharmaceutical solution formulations for pressurised metered dose inhalers
InactiveUS20040047809A1Avoid leachingGood formulation stabilityBiocideDispersion deliveryDrugs solutionAlkane
An aerosol solution composition for use in an aerosol inhaler comprises an active material, a propellant containing a hydrofluoroalkane, a cosolvent and optionally a low volatility component to increase the mass median aerodynamic diameter (MMAD) of the aerosol particles on actuation of the inhaler. The composition is stabilized by using a small amount of mineral acid and a suitable can having part or all of its internal metallic surfaces made of stainless steel, anodized aluminium or lined with an inert organic coating.
Owner:CHIESI FARM SPA
Copolymer Having Carbosiloxane Dendrimer Structure, And Composition And Cosmetic Containing The Same
ActiveUS20120263662A1Improve stabilityGood compatibilityCosmetic preparationsBody powdersDendrimerPolymer science
The present invention relates to a copolymer having a specified carbosiloxane dendrimer structure and a long-chain alkyl group with 14 to 22 carbon atoms in a specified ratio, and a cosmetic comprising the copolymer as a cosmetic raw material. The cosmetic raw material has good miscibility with other cosmetic raw materials, and therefore, can improve stability of the formulation in a cosmetic, as well as can provide good water resistance, sebum resistance, glossiness, tactile sensation and / or adhesive properties to the hair and / or skin to a cosmetic, and also provide a cosmetic formed by blending the aforementioned cosmetic raw material, which exhibits superior surface protective properties, outer appearance and / or sensation during use.
Owner:DOW TORAY CO LTD
Surgical adhesive formulations and methods of preparation
InactiveUS20050215748A1Enhanced hydrogen bondingOptimized adhesiveOrganic active ingredientsSurgical adhesivesSurgical adhesiveAdditive ingredient
This application describes specific ratios of raw ingredients and methods of combining and reacting those ingredients to obtain polyurethane prepolymers optimized for the special purpose of forming bonds to living tissue, or of bulking or sealing it. Preferred prepolymers are based on polyalkylene oxides, particular copolymers of ethylene oxide and propylene oxide. Important method steps are rigorous drying and deionization, and rigorous control of temperature during synthesis and use.
Owner:PROMETHEAN SURGICAL DEVICES
Oral care compositions comprising zinc amino acid halides
ActiveUS9913784B2Lower pHGood formulation stabilityCosmetic preparationsToilet preparationsMouth carePyrrolidinones
Owner:COLGATE PALMOLIVE CO
Skin preparations for external use
InactiveUS20060099161A1Superb effectAvoid decompositionBiocideCosmetic preparationsStainingSkin treatments
A skin treatment composition comprising anti-bacterial zeolite and trisalt ethylenediaminehydroxyethyl triacetate. A skin treatment composition comprising anti-bacterial zeolite and alum and / or dried alum. A skin treatment composition comprising anti-bacterial zeolite and polyoxyethylene polyoxypropylene 2-decyltetradecyl ether. The object is to provide a skin treatment composition containing anti-bacterial zeolite that exhibits the effect of preventing discoloration of the skin treatment compositions and / or reducing the degree of discoloration. Another object is to provide a skin treatment composition that is superior in terms of formulation stability and tactile sensation during use. Yet another object is to provide a skin treatment composition having the effect of preventing the skin treatment composition from staining clothing and / or reducing the degree of such staining.
Owner:SHISEIDO CO LTD
Chemical copper plating solution and technique thereof
InactiveCN101078111AAvoid pollutionLow costLiquid/solution decomposition chemical coatingChemical solutionCopper plating
A kind of chemical solution used to copperize, the following are the weight percent of the major components of it: blue vitriod0.8-3, Fe vitriol 0-0.5, complex agent 3-6, sodium hypophosphorous acid 4-6, ammonia sulfate 0.5-1, sulfourea 0-0.01. The following is the procedure of the copper coating method with the above copper coating solution: to widen and activate the plastic board->to clean->to make it electric->to clean->to coat nickel-copper alloy on it by chemical method. The following are the content of the chemical method: the plastic pretreated according the above description will be immersed in the above chemical solution used to copperize, the pH of which has been adjusted to 10-13 by vitriol and stranger ammonia water, the working temperature of it is 20-70deg.C, the time to copperize is 5-20 minutes. The chemical solution used to copperize in this patent does not contain formaldehyde, it improve the working circumstance, and is easy to control, the cost of it is lower, and it own high economical benefit and society benefit.
Owner:MITAC PRECISION TECH CO LTD SHUNDE DISTRICT FOSHAN CITY
External preparation for skin
InactiveUS20100021405A1Good formulation stabilityEffective penetrationCosmetic preparationsBiocideHigh concentrationPreparing skin
The present invention provides an external preparation for skin comprising a phospholipid having an iodine value of 80 to 110, ethanol in an amount of 55 to 83 wt % and water in an amount of 15 to 43 wt %, which is improved in it's preparation stability by suppressing an increase in an acid value of phospholipid. The present invention also provides an external preparation for skin which is improved in percutaneous absorption of medically effective ingredient(s). The present invention provides further a method for suppressing an increase in an acid value of phospholipid by comprising phospholipid having high iodine value and high concentration of ethanol and water, as well as a method for improving percutaneous absorption of medically effective ingredient(s) in external preparation for skin.
Owner:ROHTO PHARM CO LTD
Liquid compositions of insoluble drugs and preparation methods thereof
ActiveUS9339553B2Good biocompatibilityEliminate hidden dangersBiocideCarbohydrate active ingredientsPhospholipidSolvent
A liquid composition of an insoluble medicament and a preparation method thereof are disclosed. The composition includes insoluble medicament, oil for injection, phospholipid, and solvent; the percentage by weight of each component is as follows: insoluble medicament 0.01-10%, oil for injection 0%-20%, phospholipid 10-80%, solvent 20-89%. The preparation method for the composition includes the following steps: dissolving an insoluble medicament into solvent or oil for injection or a mixture thereof firstly, and then adding other components, and mixing uniformly; or dissolving an insoluble medicament into a mixture of other components, and mixing uniformly; or dissolving an insoluble medicament into part of solvent firstly, and then adding into a mixed solvent of other components and the remaining solvent, and mixing uniformly.
Owner:PEKING UNIV
Inverse emulsions comprising avermectins and cosmetic/dermatological applications thereof
ActiveUS20090035338A1Good chemical stabilityImprove physical stabilityCosmetic preparationsBiocideEmulsionAvermectin
Physically and chemically stable, oxidation-resistant, cosmetic / dermatological inverse emulsions contain a therapeutically effective amount of at least one avermectin compound, notably ivermectin, a glycolic or aqueous / glycolic dispersed hydrophilic phase, a continuous lipophilic phase and an emulsifier having an HLB ranging from 2 and 7, and are useful for the treatment of a variety of dermatological conditions / afflictions, e.g., rosacea.
Owner:GALDERMA HLDG SA
Pharmaceutical composition for parenteral administration of idebenone
InactiveUS20100130619A1Formula stableHigh shear mixingBiocideNervous disorderParenteral nutritionIdebenone
Owner:ALPHARX
Pemetrexed disodium liposome injection
InactiveCN103040748AInhibit aggregationLarge particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityMedicine
The invention discloses pemetrexed disodium liposome injection which is mainly made of pemetrexed disodium and a preparation method thereof, distearoyl phosphatidyl glycerol, dipalmitoyl phosphatidyl choline, PEG600, cholesterol and mannitol. The liposome injection has the advantages that the particle size of liposome is small, the liposome is uniformly distributed, the encapsulation efficiency is high, the leakage rate is low, the stability is good, the solubility of the pemetrexed disodium and the quality of injection products are improved, the toxic and side effect is reduced, and the curative effect is improved.
Owner:海南路易丹尼生物科技有限公司
Parecoxib sodium pharmaceutical composition for injection
InactiveCN102512383ASolve the problem of unstable pH value when exposed to lightHigh yieldPowder deliveryAntipyreticDrug utilisationParecoxib sodium
The invention relates to a stable parecoxib sodium pharmaceutical composition for injection. The pharmaceutical composition specifically comprises parecoxib sodium and additive for injection, wherein the additive at least contains a phosphatic buffer with a pH of 7.8-8.0, and other additive is selected from mannitol and sodium hydroxide. The parecoxib sodium pharmaceutical composition for injection provided by the invention is filled in a colorless neutral borosilicate glass tube injection bottle, has good stability, stable pH and little stimulation on skin, and is convenient for transportation and storage. The parecoxib sodium pharmaceutical composition for injection provides reasonable preparation prescription and preparation technology for clinical medicament usage and is easy for realization of industrialization.
Owner:TIANJIN SONGRUI MEDICAL TECH
Irinotecan preparation
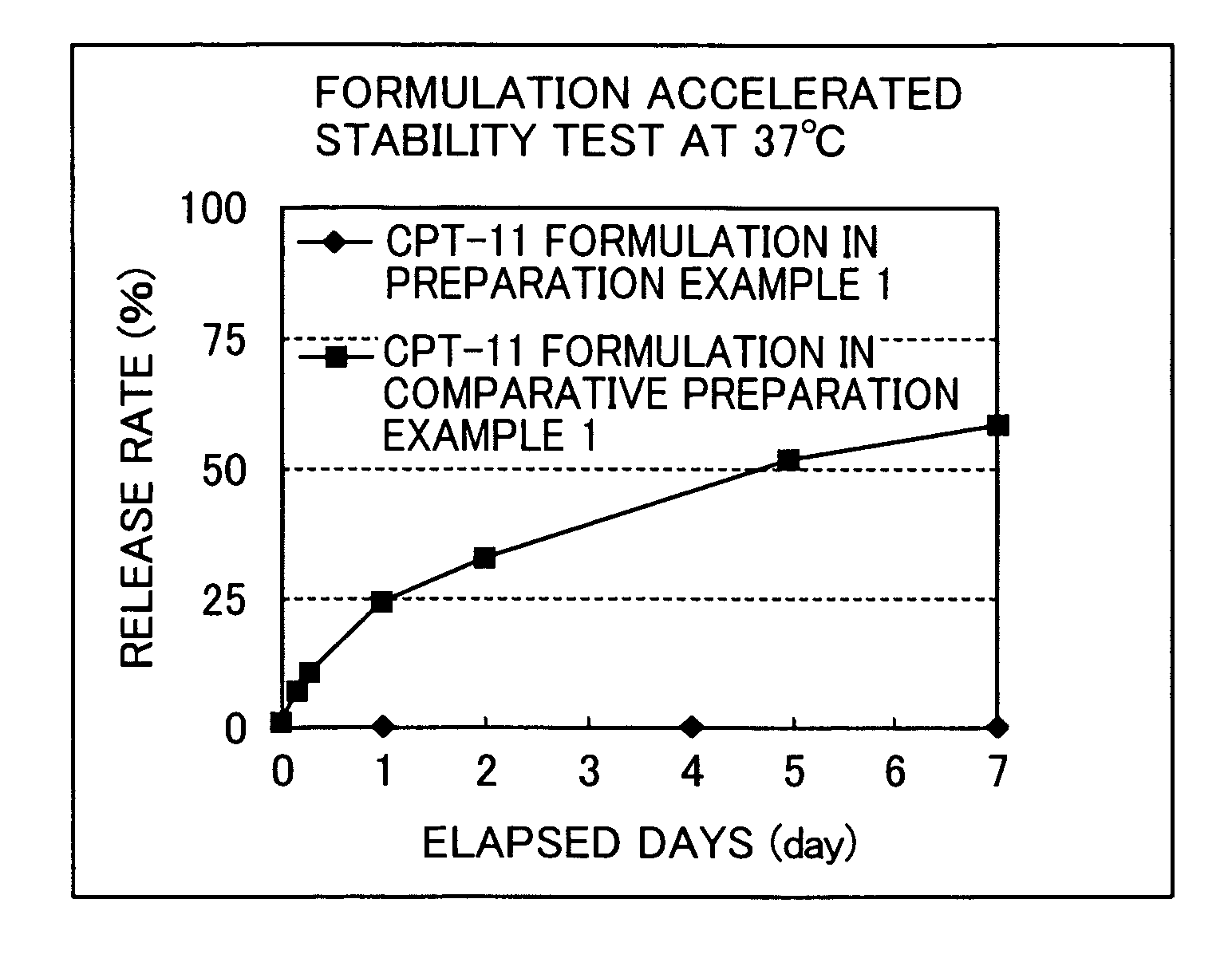
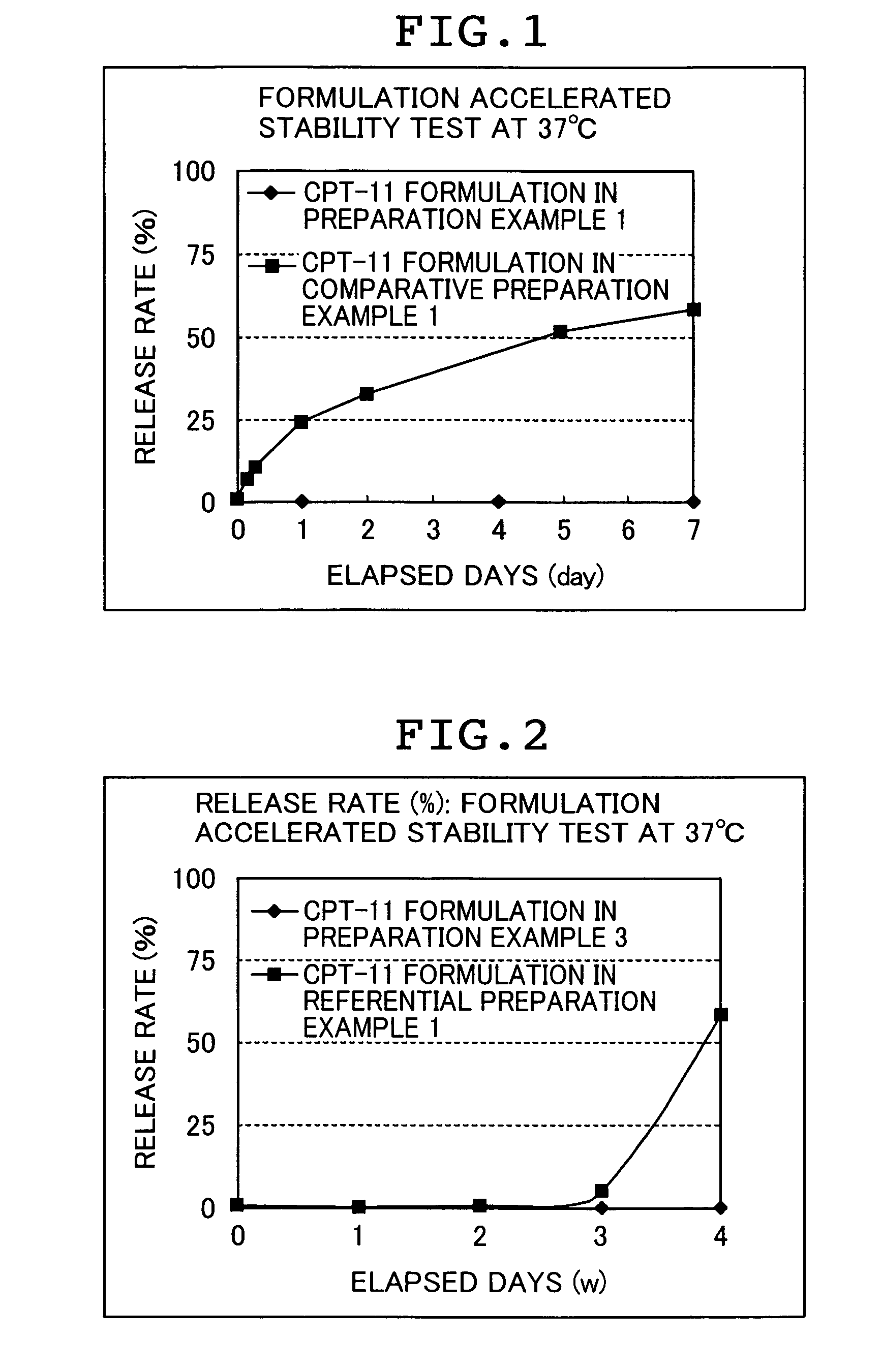
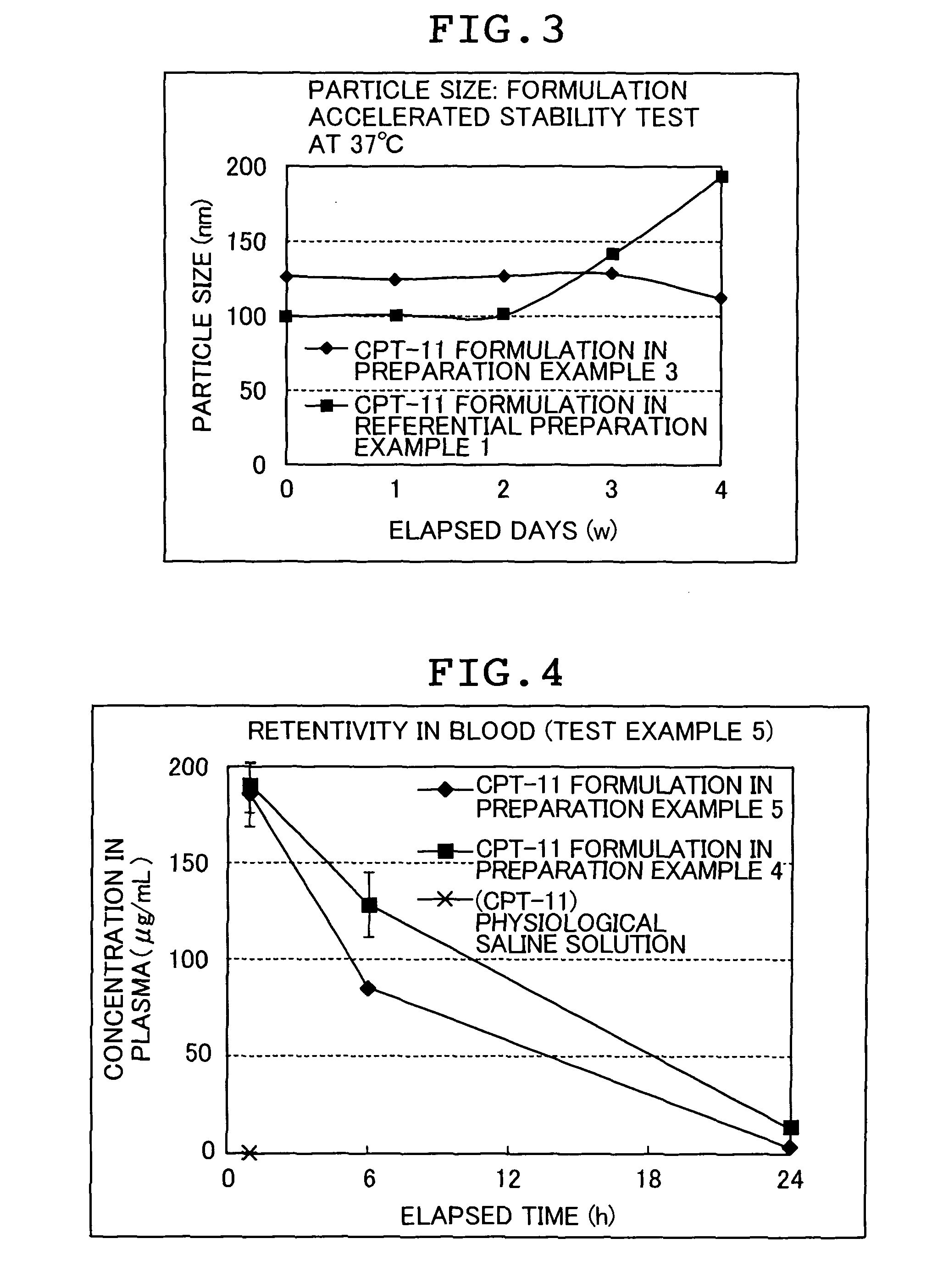
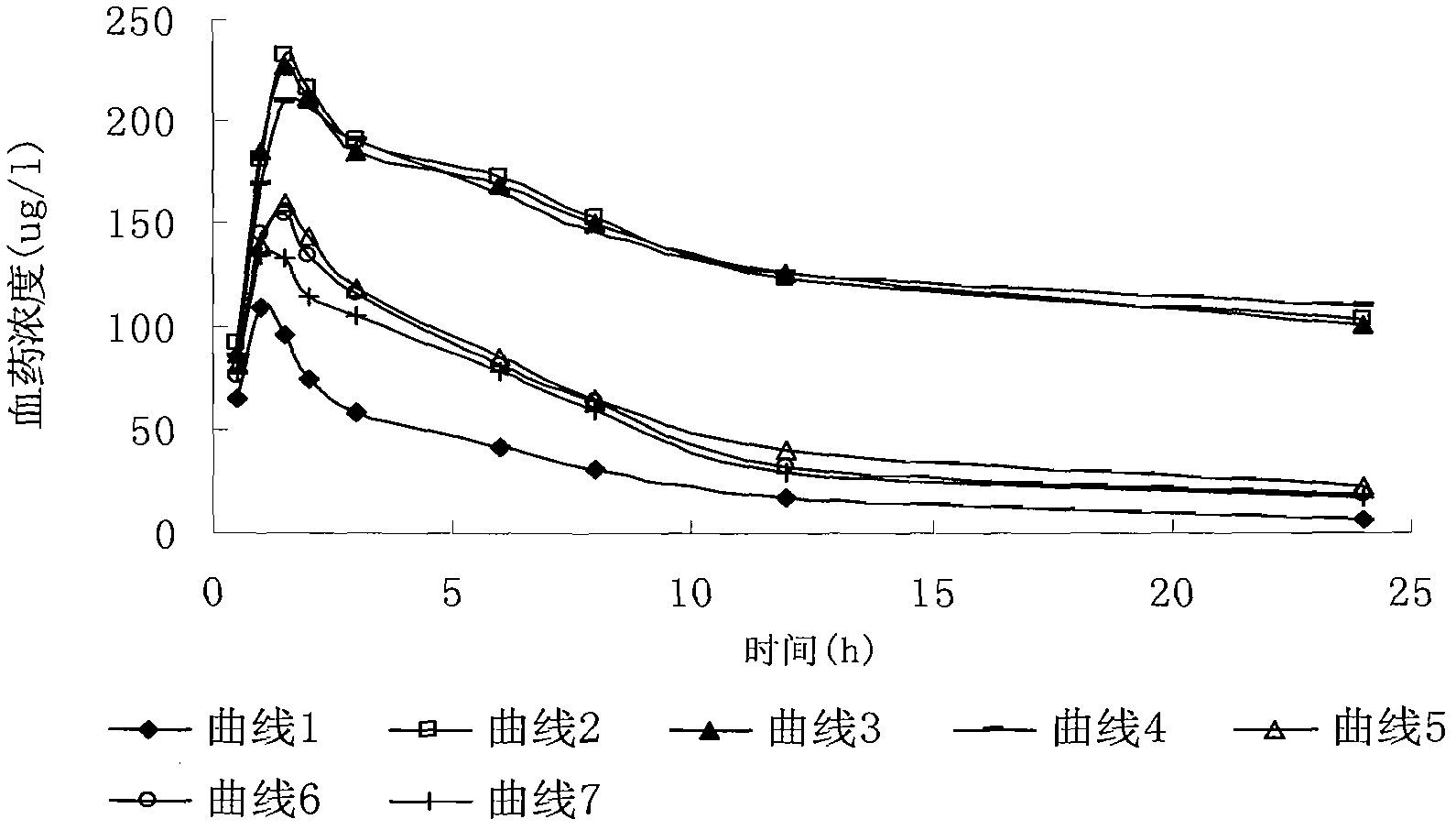
ActiveUS7846473B2Enhance memoryGood formulation stabilityBiocidePharmaceutical non-active ingredientsLipid formationHigh concentration
Provided is an irinotecan formulation capable of supporting irinotecan and / or a salt thereof in a closed vesicle carrier at a high concentration and existing in blood for a long period of time by dramatically improved retentivity in blood compared to a conventionally known irinotecan liposome formulation. That is, an irinotecan formulation including a closed vesicle formed by a lipid membrane, in which irinotecan and / or a salt thereof is encapsulated at a concentration of at least 0.07 mol / mol (drug mol / membrane total lipid mol). There is an ion gradient between an inner aqueous phase and an outer aqueous phase in the irinotecan formulation. The closed vesicle is preferably liposome, in which only the outer surface of the liposome is preferably modified with a surface-modifying agent containing a hydrophilic polymer.
Owner:TERUMO KK +1
W/O diaper cream for babies and children as well as preparation method thereof
ActiveCN103690451AImprove permeabilityPromote recoveryCosmetic preparationsToilet preparationsButtocksSODIUM PYRROLIDONE CARBOXYLATE
The invention relates to W / O diaper cream for babies and children as well as a preparation method thereof. The W / O diaper cream comprises a component A, a component B and a component C, wherein the component A consists of 12-20% of grease, 3-5% of emulsifier and 0.05-0.1% of antioxidant according to the total weight of the diaper cream; the component B consists of 65-80% of de-ionized water, 0.05-0.15% of allantoin, 3-5% of humectant and 0.5-0.8% of stabilizer according to the total weight of the diaper cream; the component C consists of 0.3-0.8% of cortex moutan extracting solution, 0.05-0.2% of rutin extract and 0.1-0.3% of pyrrolidone carboxylic acid sodium salt according to the total weight of the diaper cream. The diaper cream has an obvious treating effect on the red buttocks phenomenon caused by the irritation of diapers, and can effectively restrain the bacteria growth so as to take the preventing and curing effects on the irritation of sensitive skin on the buttocks of the babies and children.
Owner:FROG PRINCE (FUJIAN) BABY & CHILD CARE PROD CO LTD
Composite drug carried microsphere, minocycline hydrochloride nano controlled-release composite drug carried microsphere system and preparation method thereof
InactiveCN101836961ALow toxicityGood slow releaseAntibacterial agentsTetracycline active ingredientsMicrosphereCholesterol
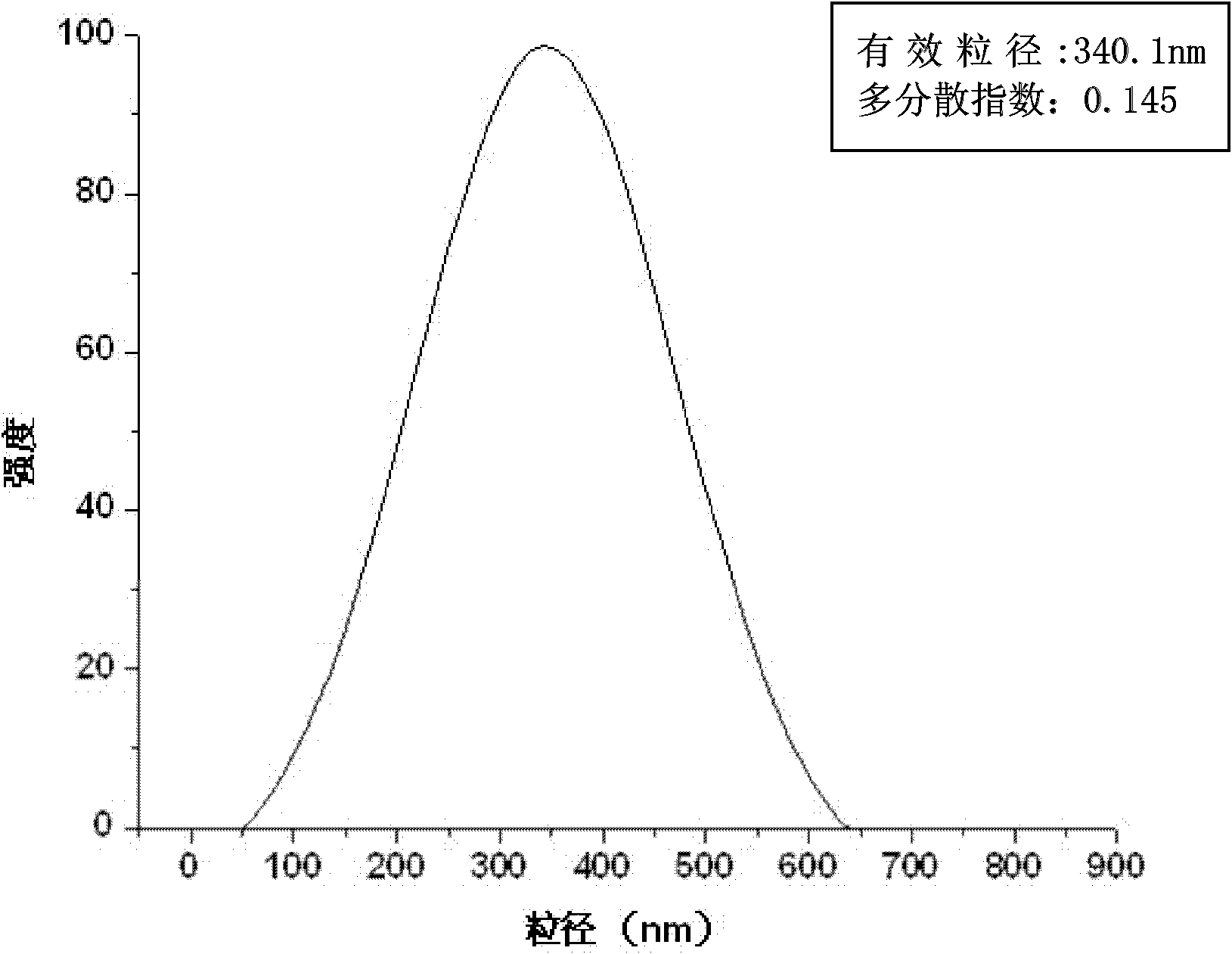
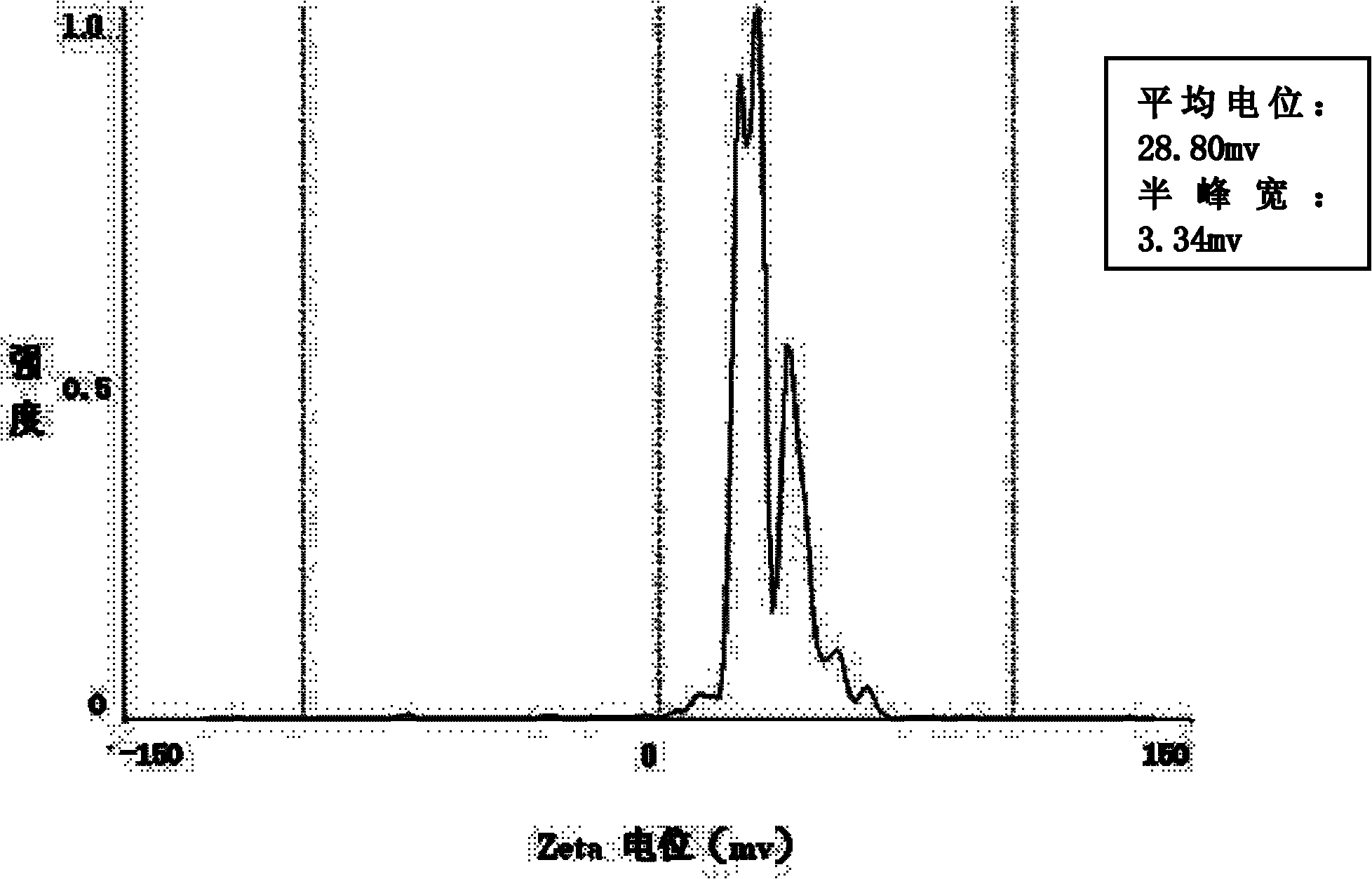
The invention relates to a composite drug carried microsphere, a minocycline hydrochloride nano controlled-release composite drug carried microsphere system and a preparation method thereof. A drug carried system with a nuclear shell structure is formed by embedding minocycline hydrochloride inside a poly D,L-lactide-co-glycolic acid polymer microsphere and covering a cationic polymeric liposome prepared from O-QACMC modified by polyethylene glycol, O-QACMC and cholesterol outside the poly D, L-lactide-co-glycolic acid polymer microsphere; and the composite drug carried microsphere system covered and carried with the minocycline hydrochloride has the grain diameter ranging from 340 nm to 400 nm and positive surface Zeta electric potential. The composite drug carried microsphere system can be remained in a water solution for at least 2 months, has high entrapment rate reaching larger than 90 percent on drugs and strong drug carrying capacity reaching 9 percent. The minocycline hydrochloride nano controlled-release composite drug carried microsphere system has the characteristics of uniform and controllable grain diameter, good preparation stability, simple preparation process, high drug carrying rate, favorable controlled release function, and the like, and is suitable for batch production.
Owner:TIANJIN UNIV
Dilution-type sterilizer composition
InactiveUS20070025948A1Prevent precipitationGood formulation stabilityAntibacterial agentsBiocideAdditive ingredientEther
The present invention provides a germicidal antiseptic composition for dilution, which is superior in preparation stability, and free of formation of insoluble precipitation even when diluted with water containing an inorganic ion (e.g., tap water etc) when in use, thereby retaining superior germicidal disinfection ability. The present invention relates to a germicidal antiseptic composition for dilution, which is an aqueous liquid comprising chlorhexidine gluconate as a main ingredient, (1) 1-10 w / v % of chlorhexidine gluconate; (2) 1-10 w / v % of one or more selected from the group consisting of a polyoxyethylene alkyl ether and a polyoxyethylene alkenyl ether, each having an HLB of 10-15 and a congeal point of not more than 35° C.; (3) 0.001-0.5 w / v % of a water-soluble organic monocarboxylic acid having 2 to 6 carbon atoms; and (4) water.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Oral composition
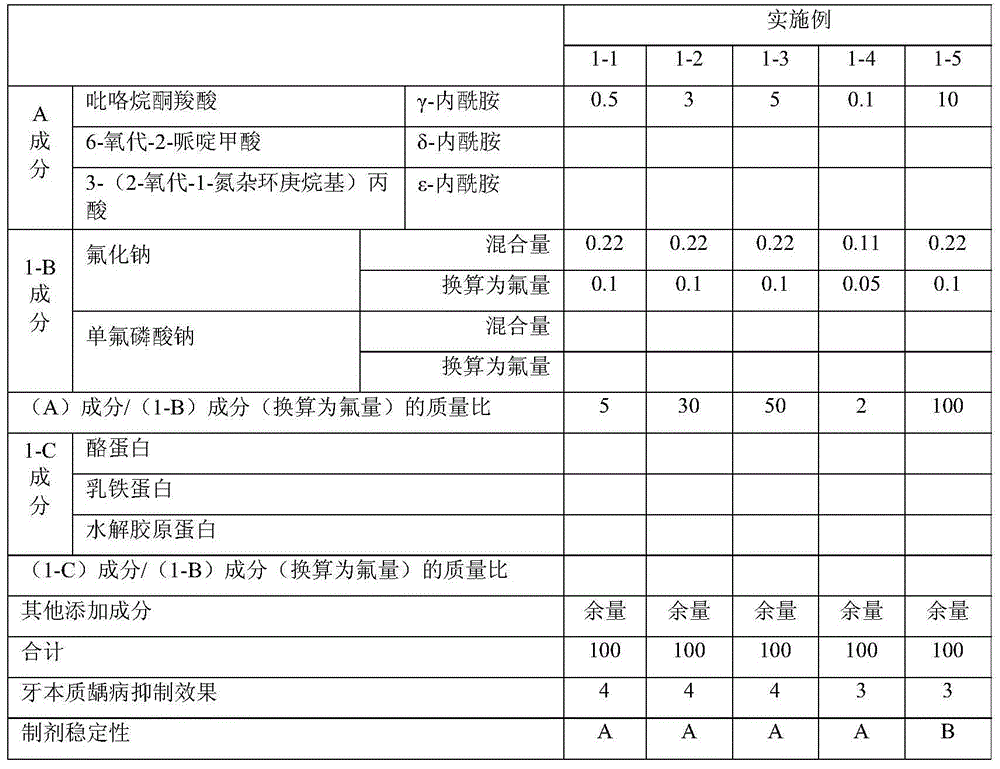
ActiveCN103826605AExcellent dental caries prevention effectGood formulation stabilityCosmetic preparationsToilet preparationsMedicineDentin hypersensitivity
The purpose of the present invention is to provide a sole component that can exhibit a function as an oral composition, such as the prevention of dentinal caries, the prevention or amelioration of dentin hypersensitivity, the inhibition of the formation of stains, the removal of stains or the like, or a combination of two or more of such components. The present invention provides an oral composition containing a component (A) which is a compound having at least one lactam skeleton selected from the group consisting of a gamma-lactam skeleton, a delta-lactam skeleton and an epsilon-lactam skeleton and also having an acidic group and / or a salt of the compound.
Owner:LION CORP
General flavanone capsule of desmodium styracifolium and preparation method and application thereof
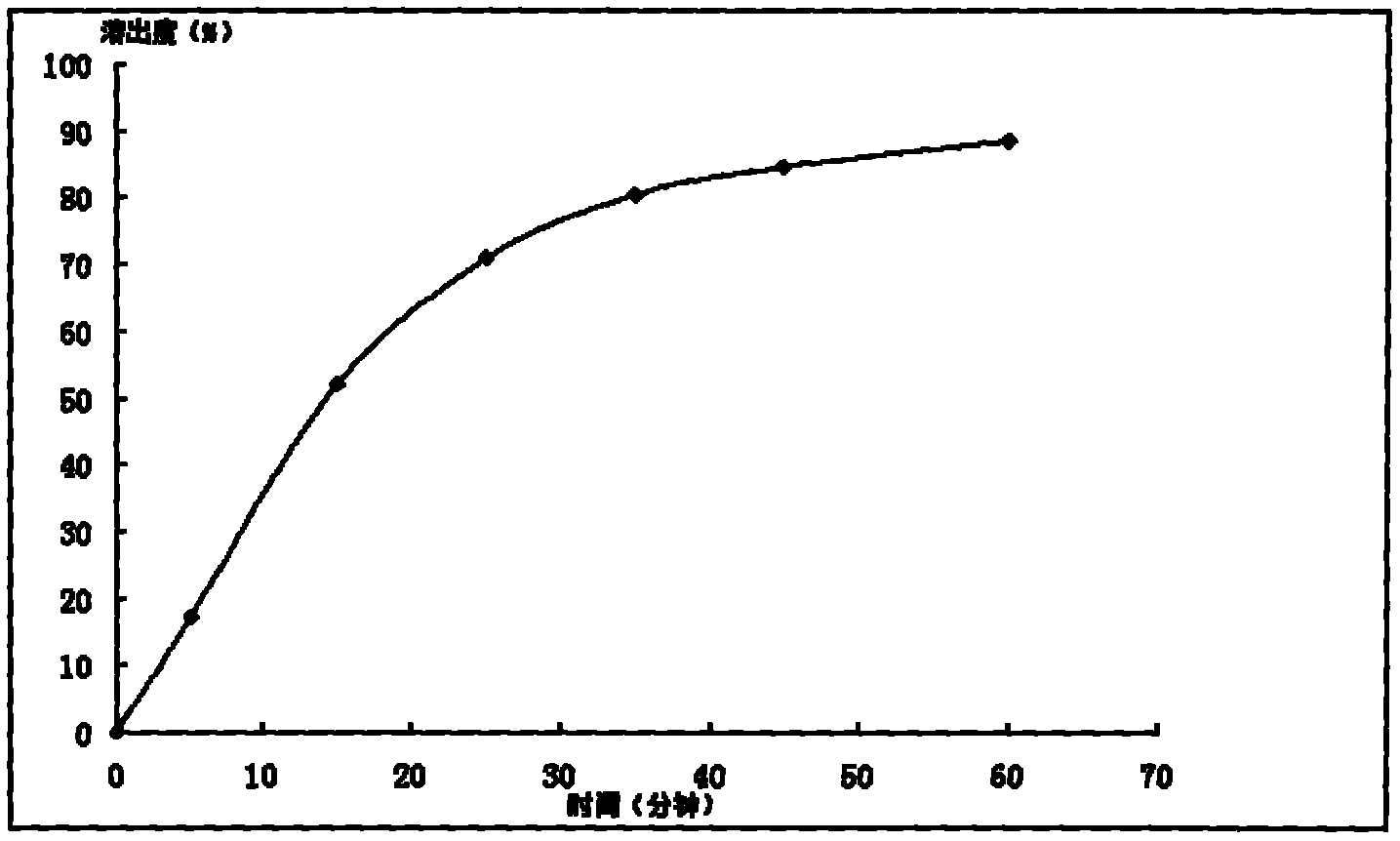
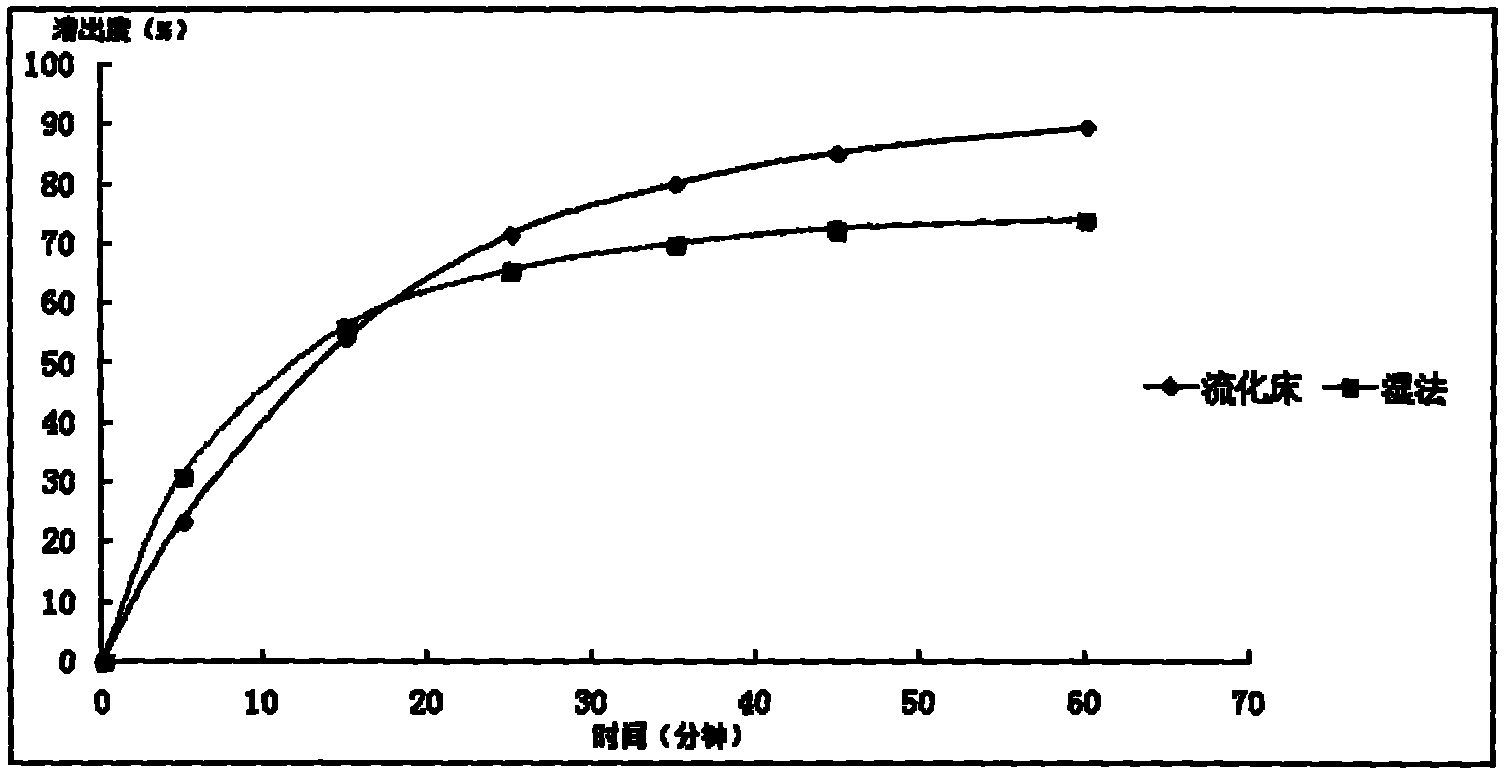
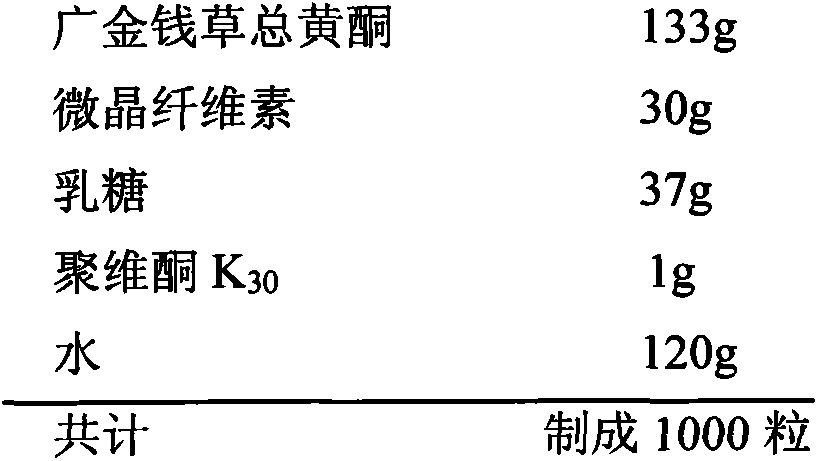
InactiveCN103893246AHigh dissolution rateGood quality and stabilityUrinary disorderGranular deliveryAlcoholMedicine
The invention provides a general flavanone capsule of desmodium styracifolium and a preparation method and application thereof, wherein the general flavanone capsule of the desmodium styracifolium comprises total flavanone of the desmodium styracifolium, which is an alcohol extract of the desmodium styracifolium, and pharmaceutically acceptable medicinal excipients. The general flavanone capsule of the desmodium styracifolium disclosed by the invention has the characteristics of being explicit in effective material basis, controllable in quality standard, good in drug dissolution degree, good in quality stability, significant in pharmacology and drug efficacy, less in dosage, safe and convenient to take, and completely applicable to industrial massive production.
Owner:HUMANWELL HEALTHCARE GRP +1
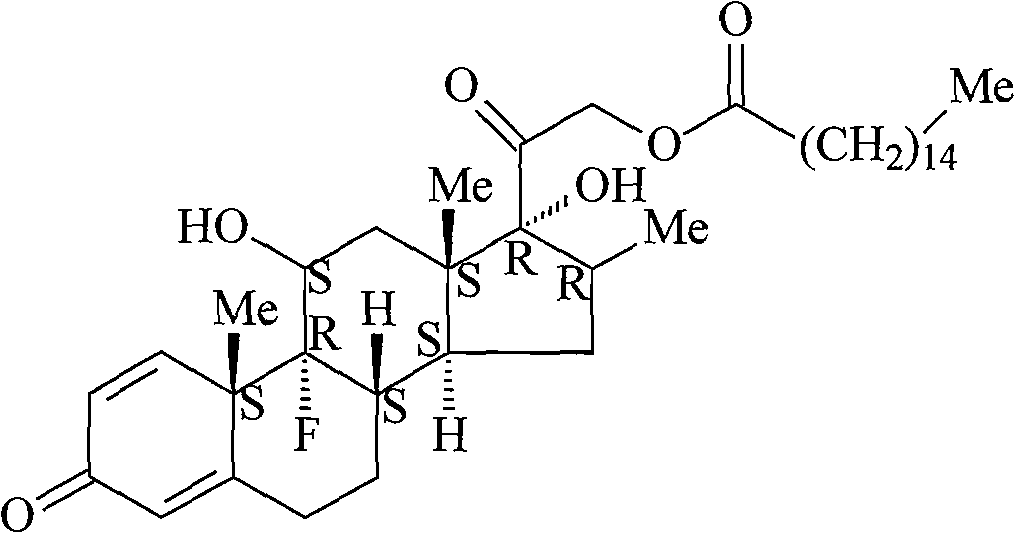
Dexamethasone palmitate acid liposome injection
InactiveCN102366411AWon't breakHigh encapsulation efficiencyOrganic active ingredientsPowder deliverySide effectCholesterol
The invention discloses a dexamethasone palmitate acid liposome injection and a preparation method thereof. The liposome injection is prepared from dexamethasone palmitate, dispermaceti phospholipids, cholesterol, tween80, glucose and dextran according to a certain weight ratio. The liposome injection provided by the invention has good preparation stability. During a freezing process, rupture of liposome due to fusion and ice crystals are prevented. After long-term storage, good entrapment rate is maintained in the liposome. With the injection and the method provided by the invention, dissolubility of dexamethasone palmitate is improved, quality of the preparation product is improved, toxic and side-effects are reduced, retention time of the medicine in systemic circulation is prolonged, bioavailability of the medicine is improved, and the curative effect is improved. The preparation method is simple, and the method is suitable for industrial productions.
Owner:HAINAN YONGTIAN PHARMA INST
Cosmetic or dermatological preparation for application on wet skin
ActiveUS20130108572A1Improve sensory propertyIncrease stabilityCosmetic preparationsHair removalMicrocrystalline waxHydrocarbon
An aqueous cosmetic or dermatological preparation for application on wet or moist skin which is substantially emulsifier-free and comprises at least two different polyacrylic acid polymers, at least two different C14-22 fatty alcohols, and at least about 13% by weight of microcrystalline wax, preferably in combination with one or more hydrocarbon oils.
Owner:BEIERSDORF AG
Copolymer having carbosiloxane dendrimer structure, and composition and cosmetic containing the same
ActiveUS10047199B2Good compatibilityGood formulation stabilityOrganic active ingredientsCosmetic preparationsDendrimerTactile sensation
The present invention relates to a copolymer having a specified carbosiloxane dendrimer structure and a long-chain alkyl group with 14 to 22 carbon atoms in a specified ratio, and a cosmetic comprising the copolymer as a cosmetic raw material. The cosmetic raw material has good miscibility with other cosmetic raw materials, and therefore, can improve stability of the formulation in a cosmetic, as well as can provide good water resistance, sebum resistance, glossiness, tactile sensation and / or adhesive properties to the hair and / or skin to a cosmetic, and also provide a cosmetic formed by blending the aforementioned cosmetic raw material, which exhibits superior surface protective properties, outer appearance and / or sensation during use.
Owner:DOW TORAY CO LTD
Preparation of Glabridin dispersible tablets and use of the tablets in reducing blood sugar as medicament active composition
ActiveCN101347495AWide variety of sourcesSimple preparation processMetabolism disorderPill deliverySide effectDiabetes model
The present invention discloses a preparation process of glycyrrhiza flavonoid dispersible tablet and the application of the tablet as the active component in decreasing blood sugar. The activity test proves three dosage groups of the glycyrrhiza flavonoid dispersible tablet all play a significant role in decreasing blood sugar of hyperglycemic model mice caused by alloxan, adrenalin and dextrose; the role in decreasing blood sugar is equal to or more significant than similar sugar-reducing medicines in clinical use. At the same time, the 'three more and one less' symptom of the diabetic model mouse is obviously relieved and especially the drinking amount is obviously reduced. The mouse acute toxicity test proves that under the maximum dosage, the mouse has no toxic side effect and the weight growth is normal. The source of raw materials is wide, the main sugar-reducing component is taken from natural medicines and the sugar-reducing effect is obvious; therefore, the present invention is suitable for long-term use. The present invention with simple preparation technology and low production cost is suitable for large-scale industrial production.
Owner:JIANGSU KANION PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com