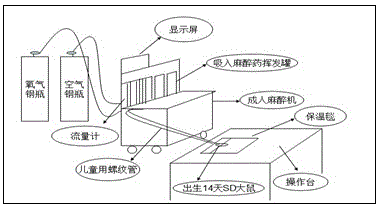
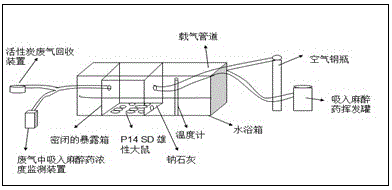
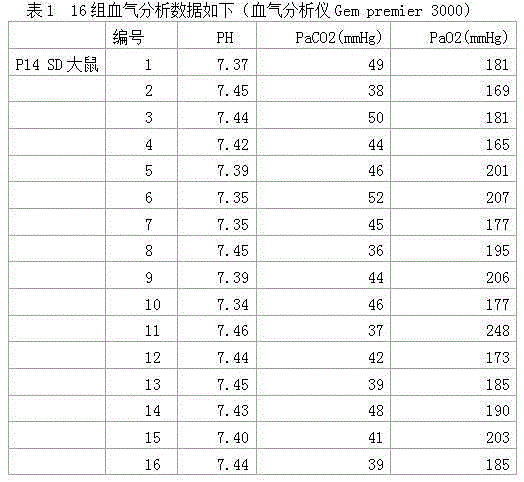
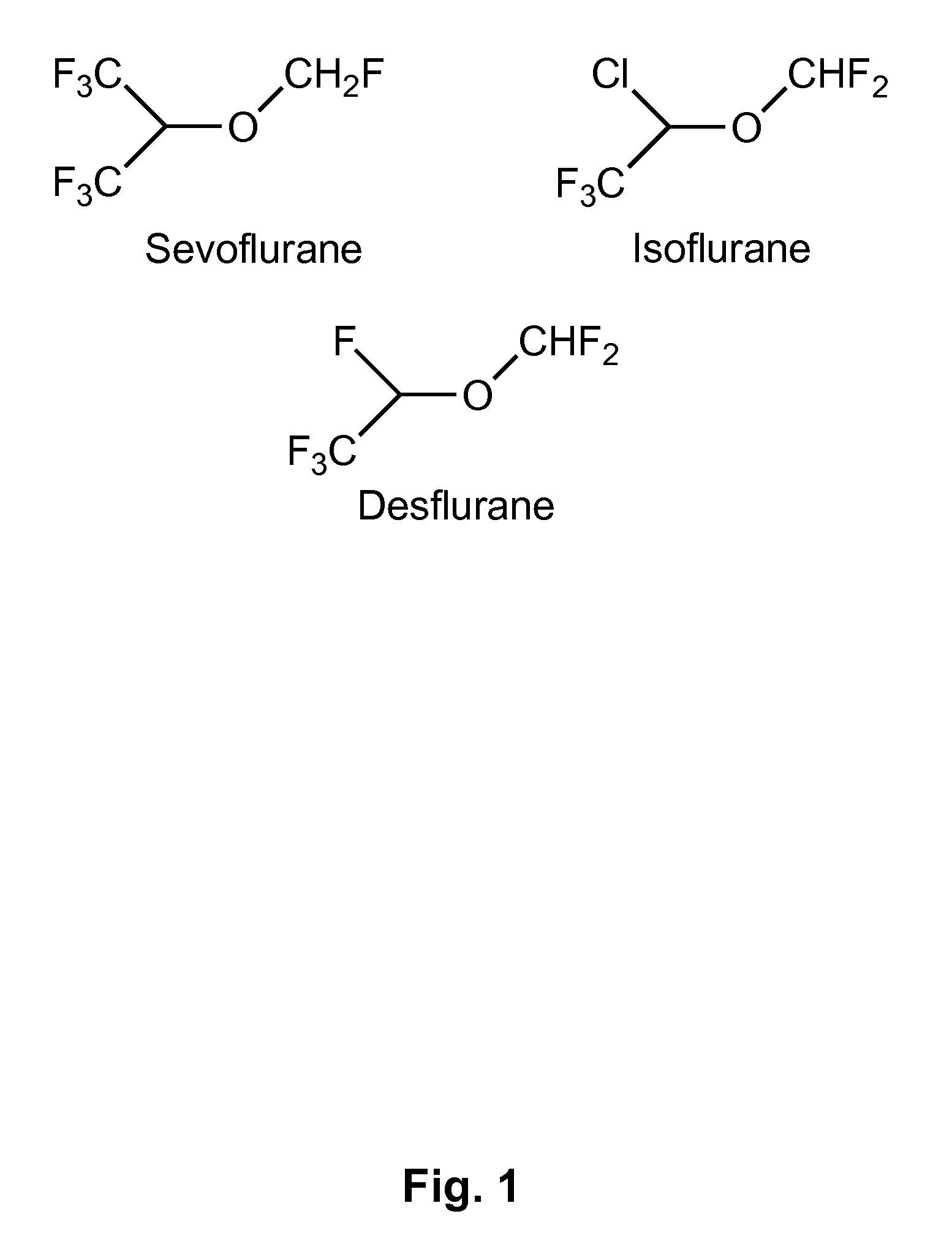
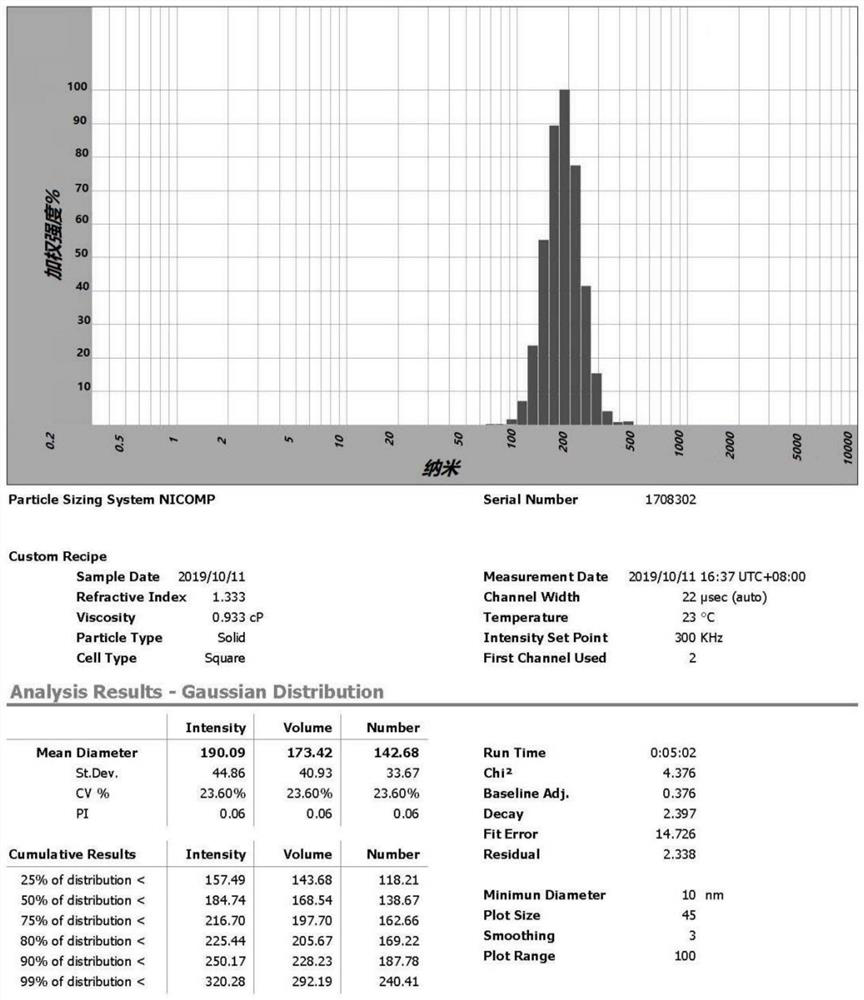
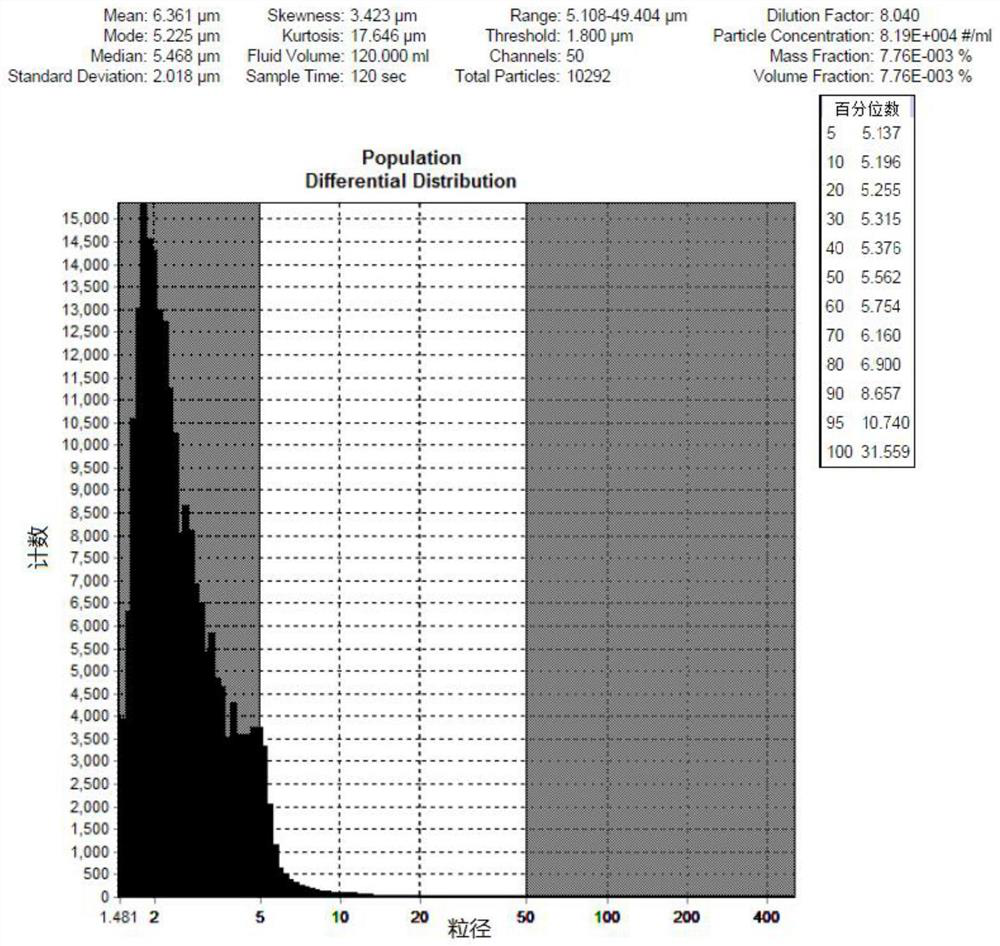
Patents
Literature
46 results about "Volatile anesthetic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
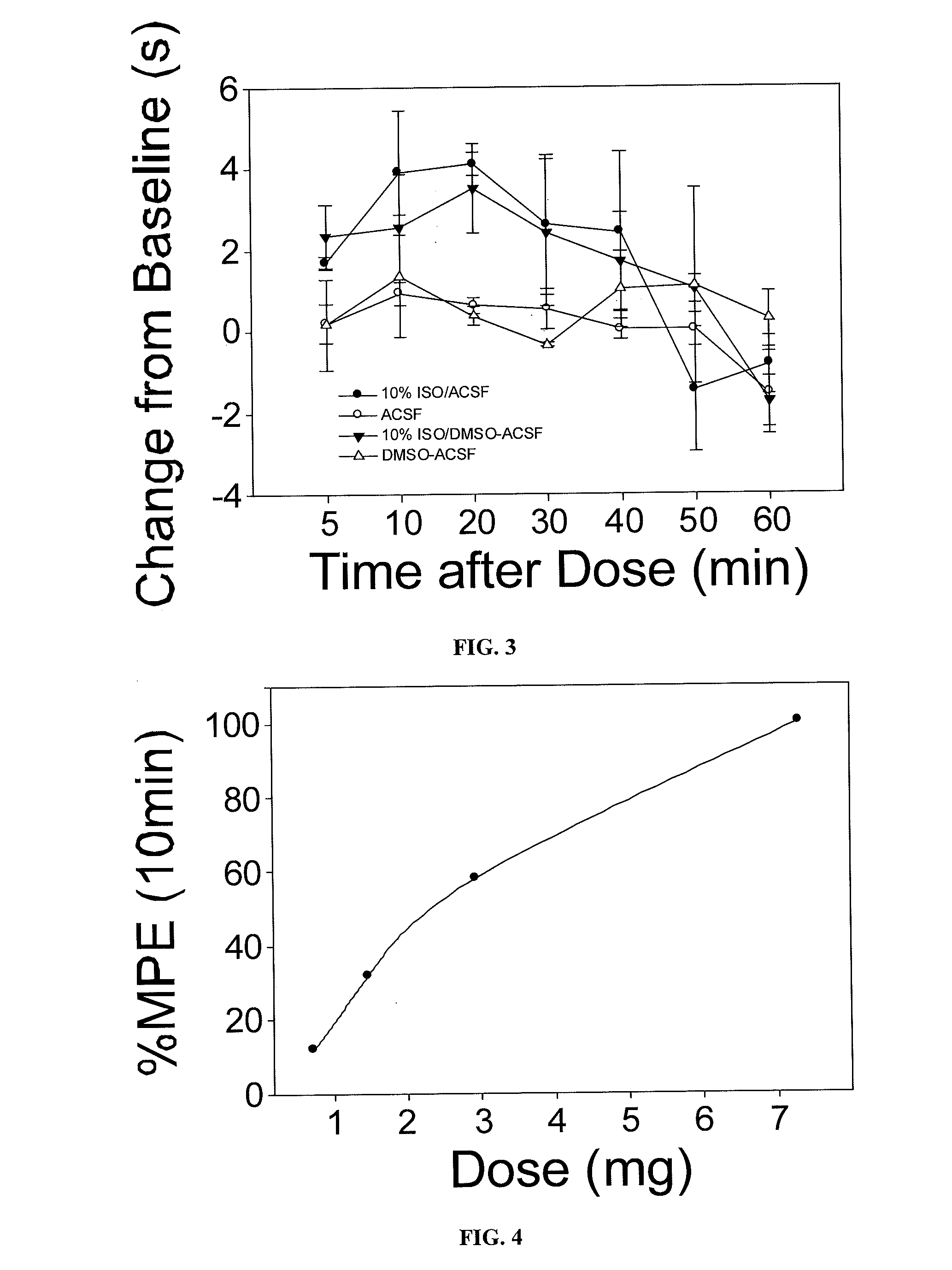
Volatile anesthetic agents, such as halothane, isoflurane, and sevoflurane, are the drugs most commonly used to maintain the state of general anesthesia. They have long been known to provide some protection against the effects of cardiac ischemia and reperfusion.
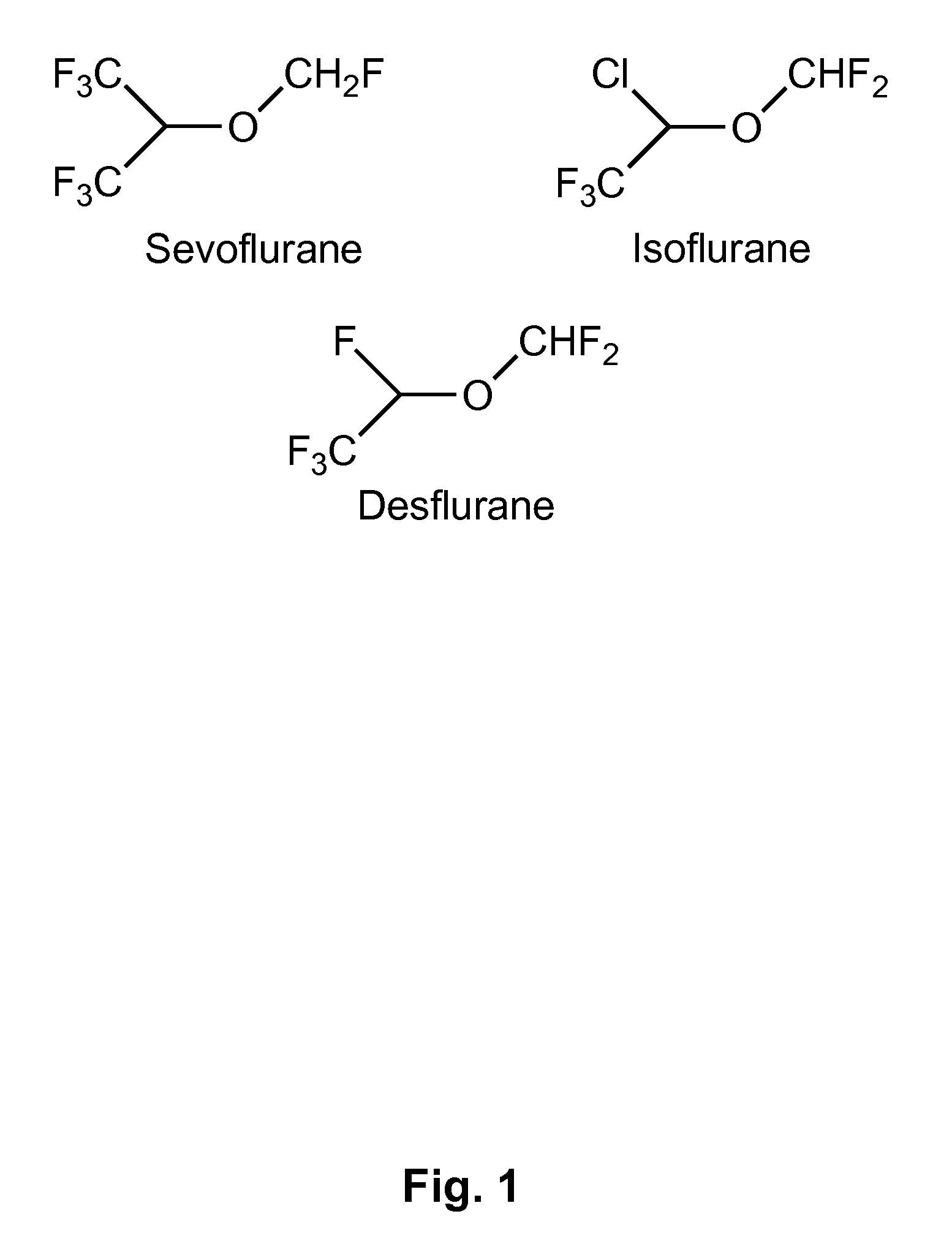
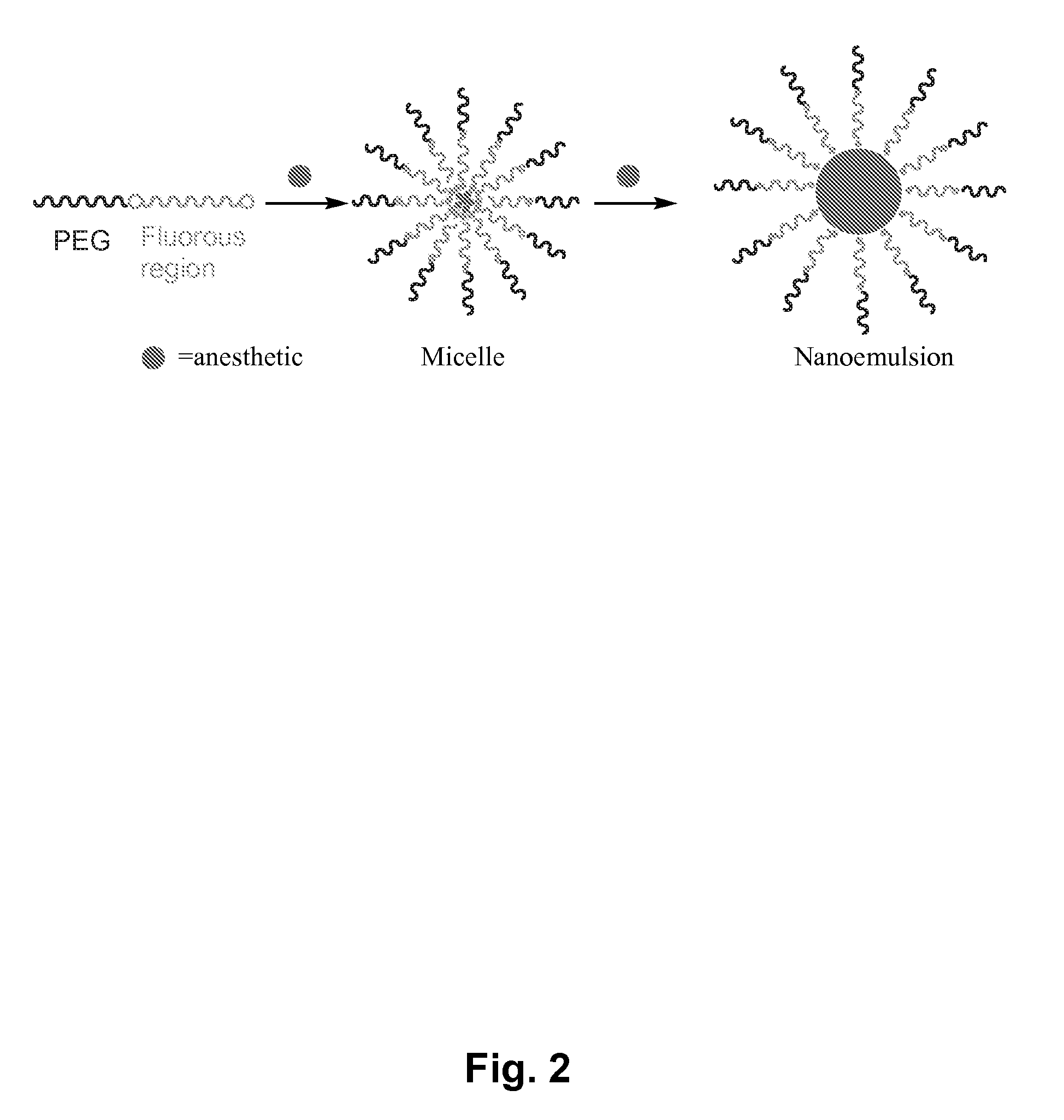
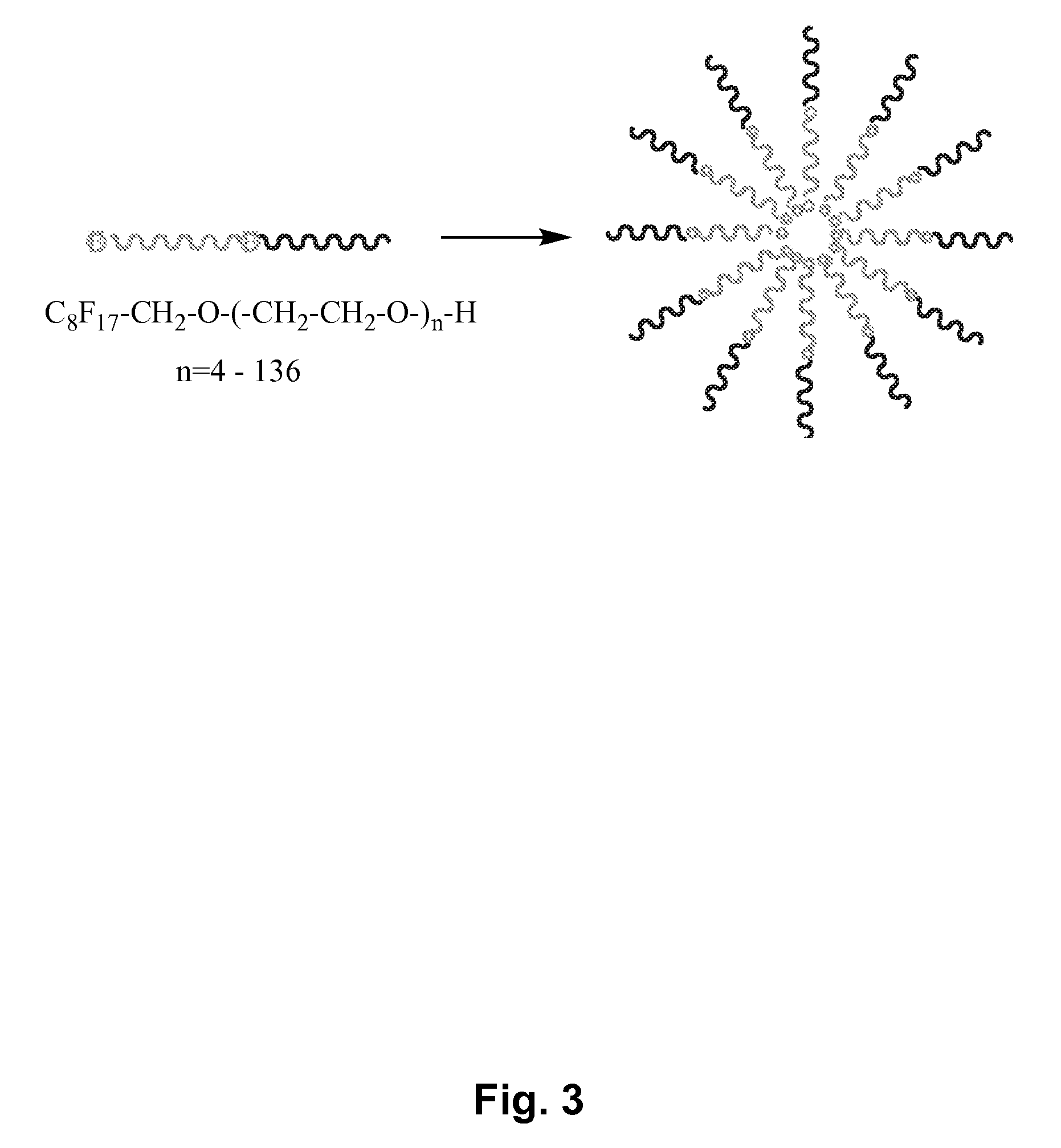
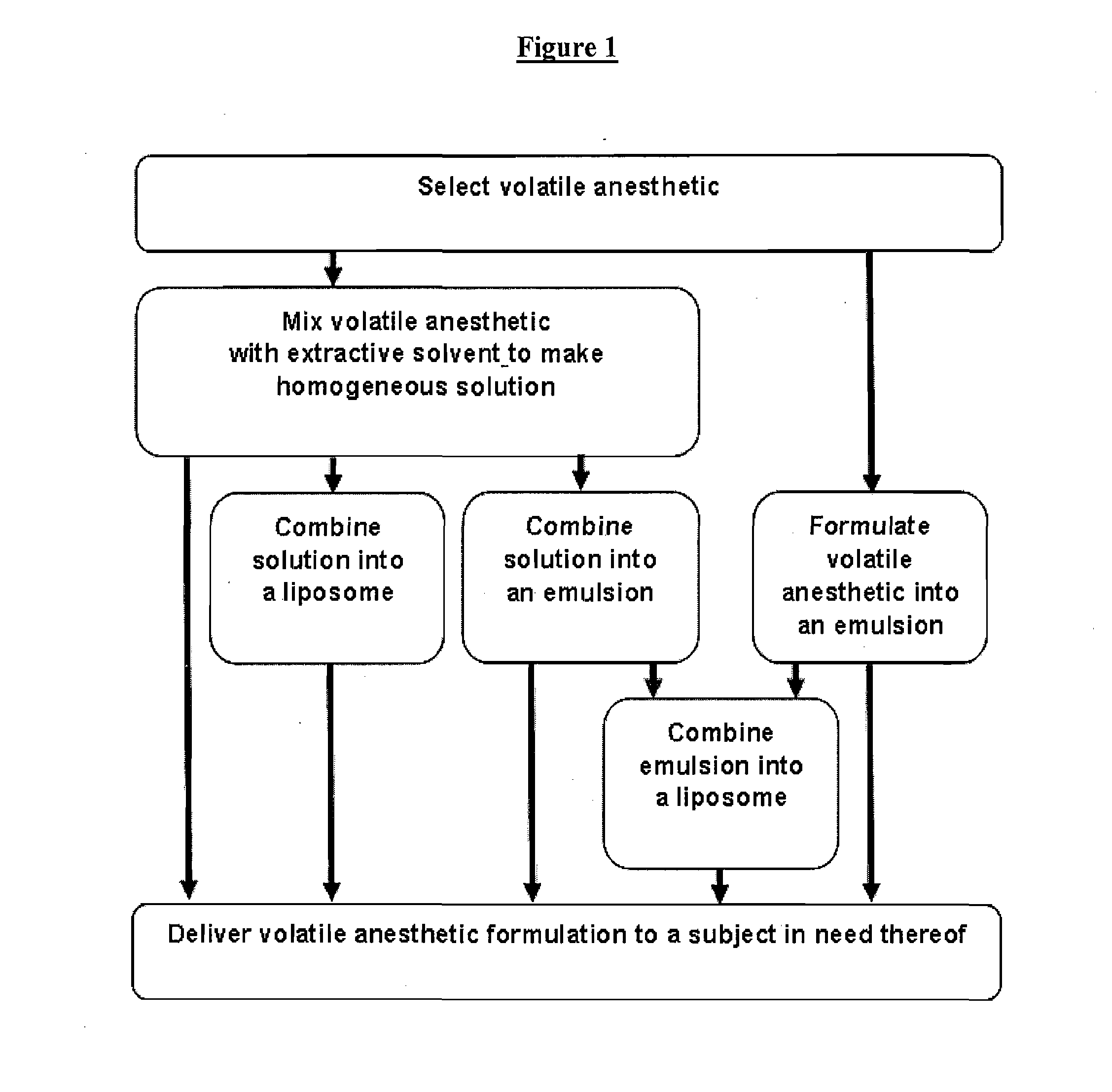
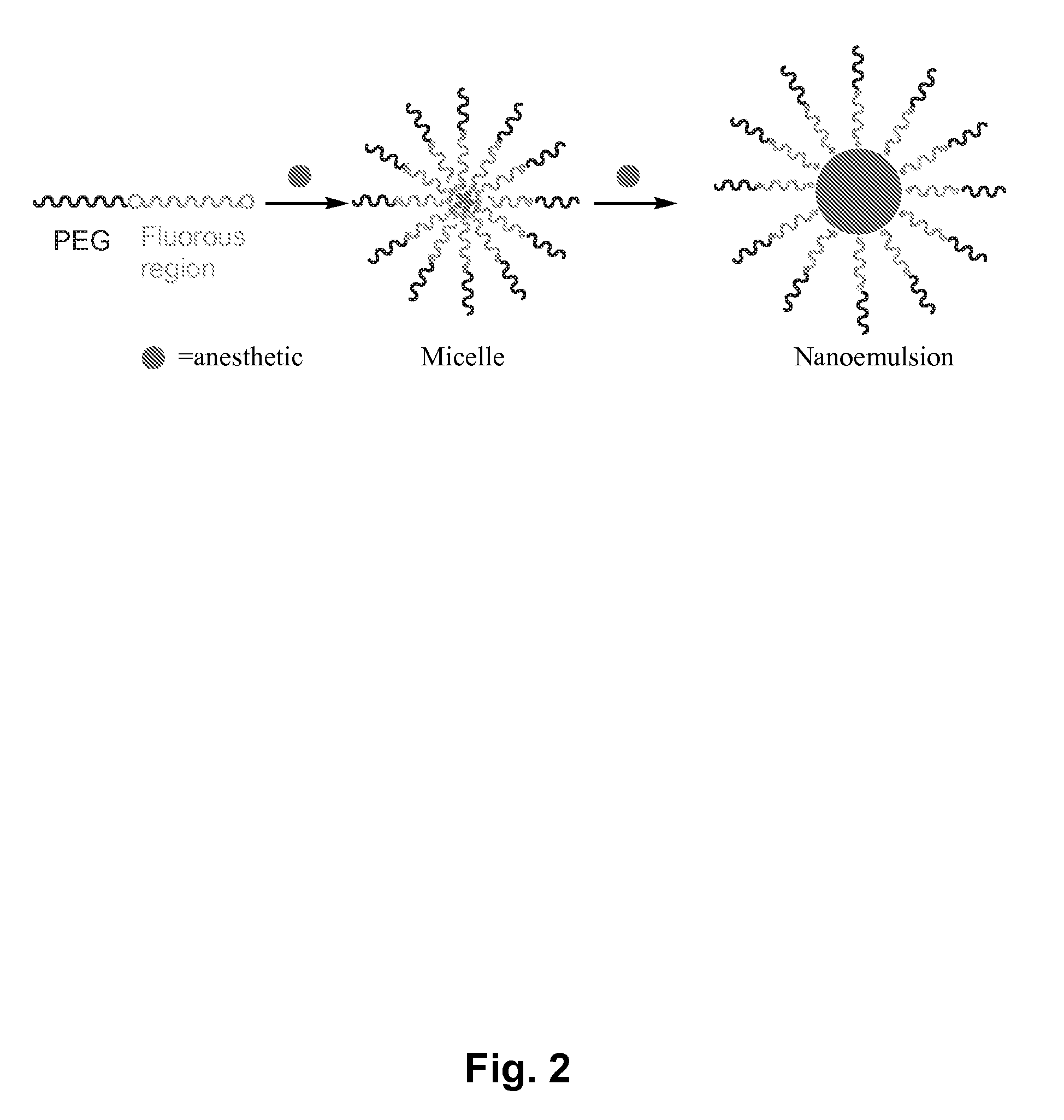
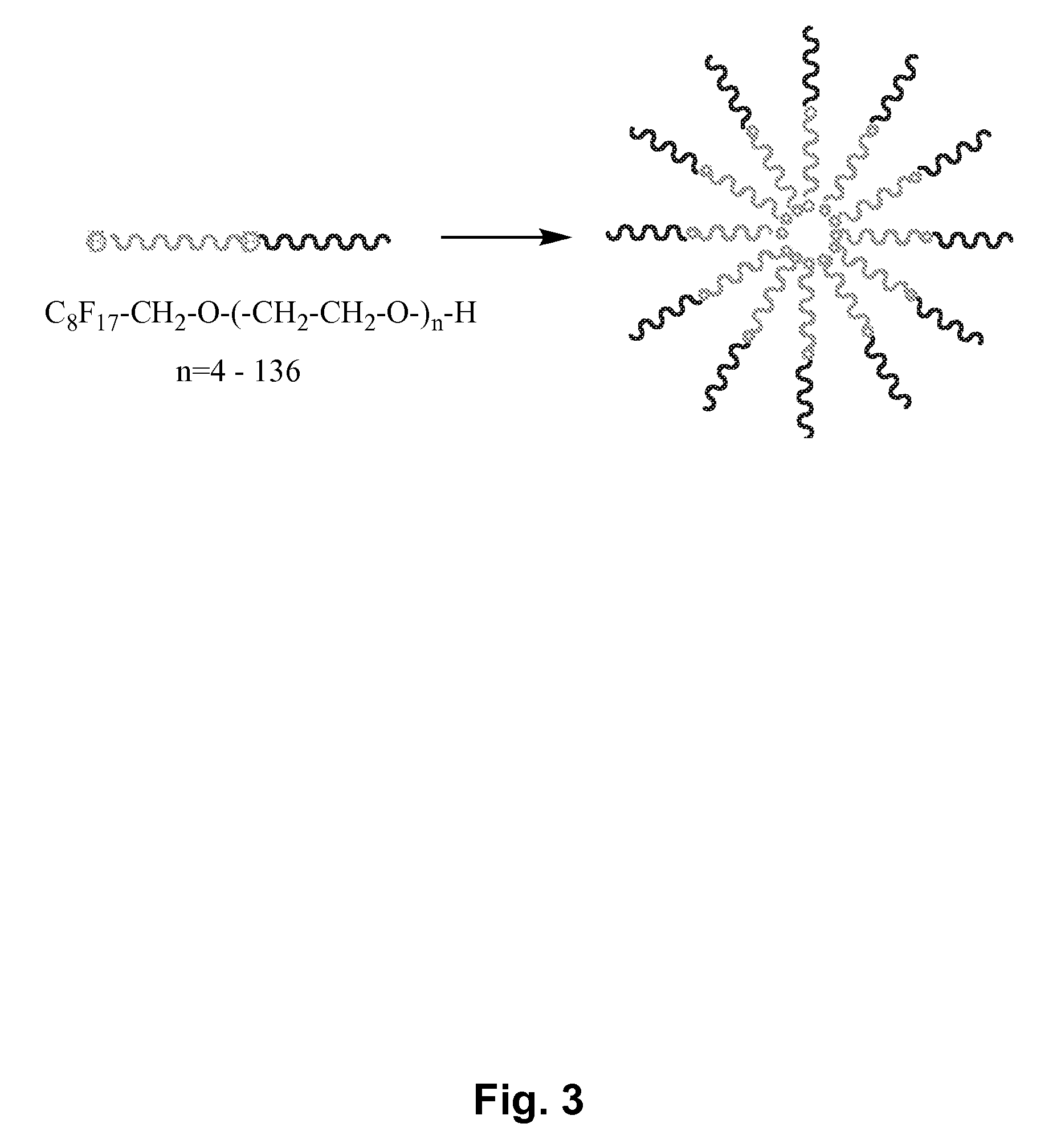
Fluoropolymer-based emulsions for the intravenous delivery of fluorinated volatile anesthetics
InactiveUS20080234389A1Maintenance can be inducedReduce interfacial tensionBiocideNervous disorderAnesthetic AgentEmulsion
The present invention provides therapeutic formulations, including therapeutic emulsions and nanoemulsions, and related methods for the delivery of fluorinated therapeutic compounds, including an important class of low boiling point perfluorinated and / or perhalogenated volatile anesthetics. Emulsion-based fluorinated volatile anesthetic formulations compatible with intravenous administration are provided that are capable of delivering and releasing amounts of fluorinated volatile anesthetic compounds effective for inducing and maintaining anesthesia in patients. Intravenous delivery of the present emulsion-based fluorinated volatile anesthetic formulations permits anesthetic levels in a patient to be selectively adjusted very rapidly and accurately without the need to hyperventilate patients and without the use of irritating agents.
Owner:WISCONSIN ALUMNI RES FOUND
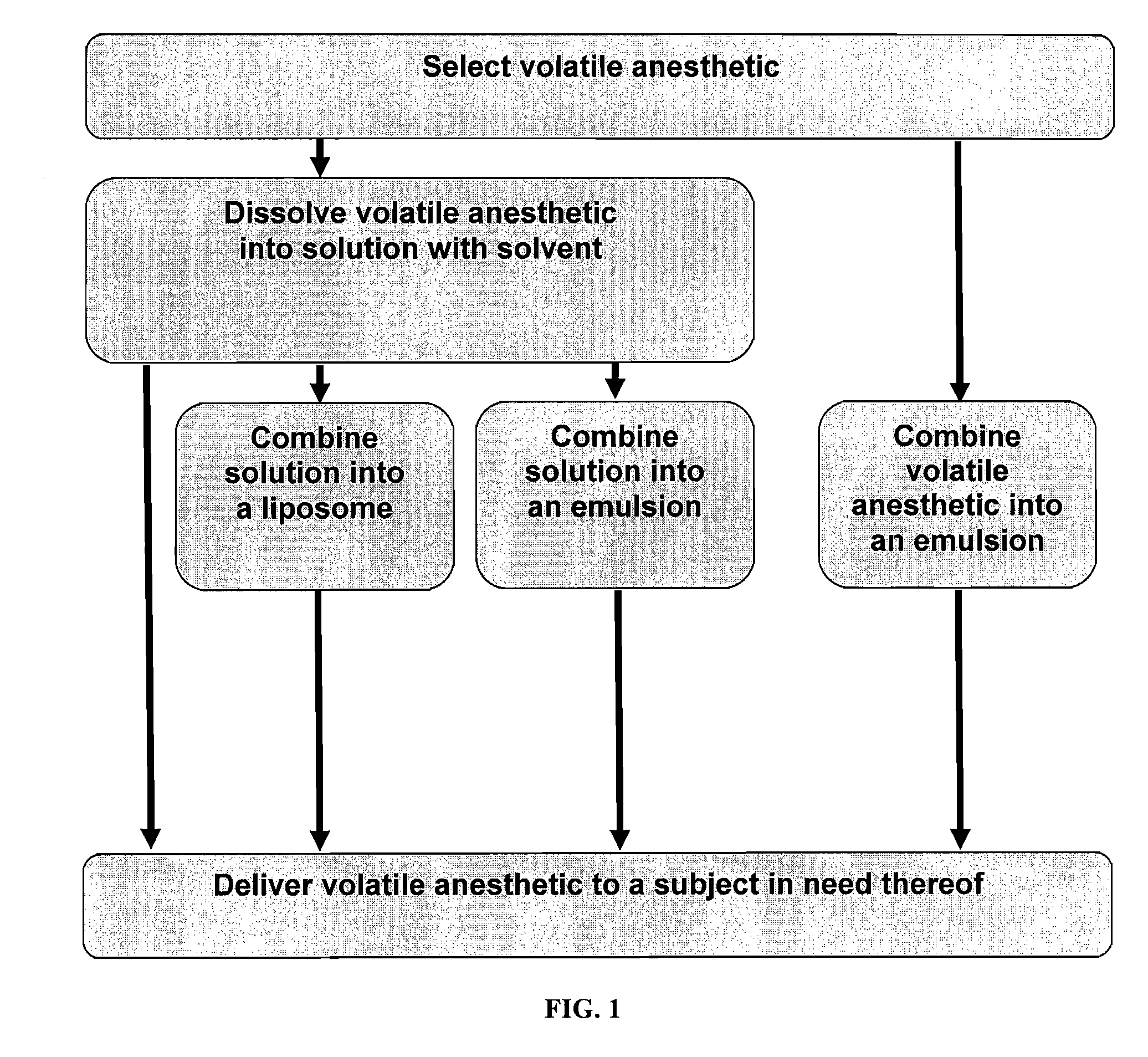
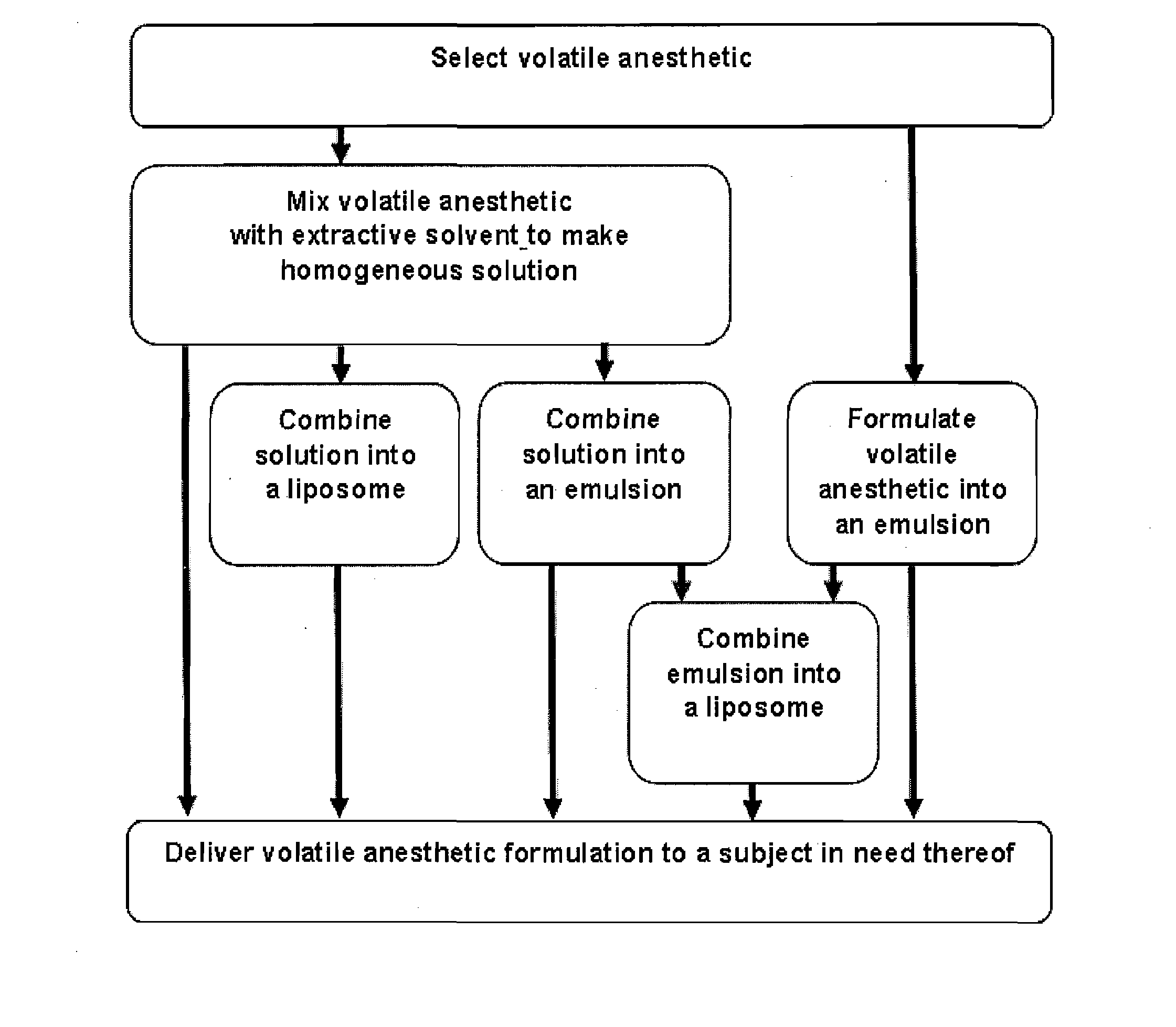
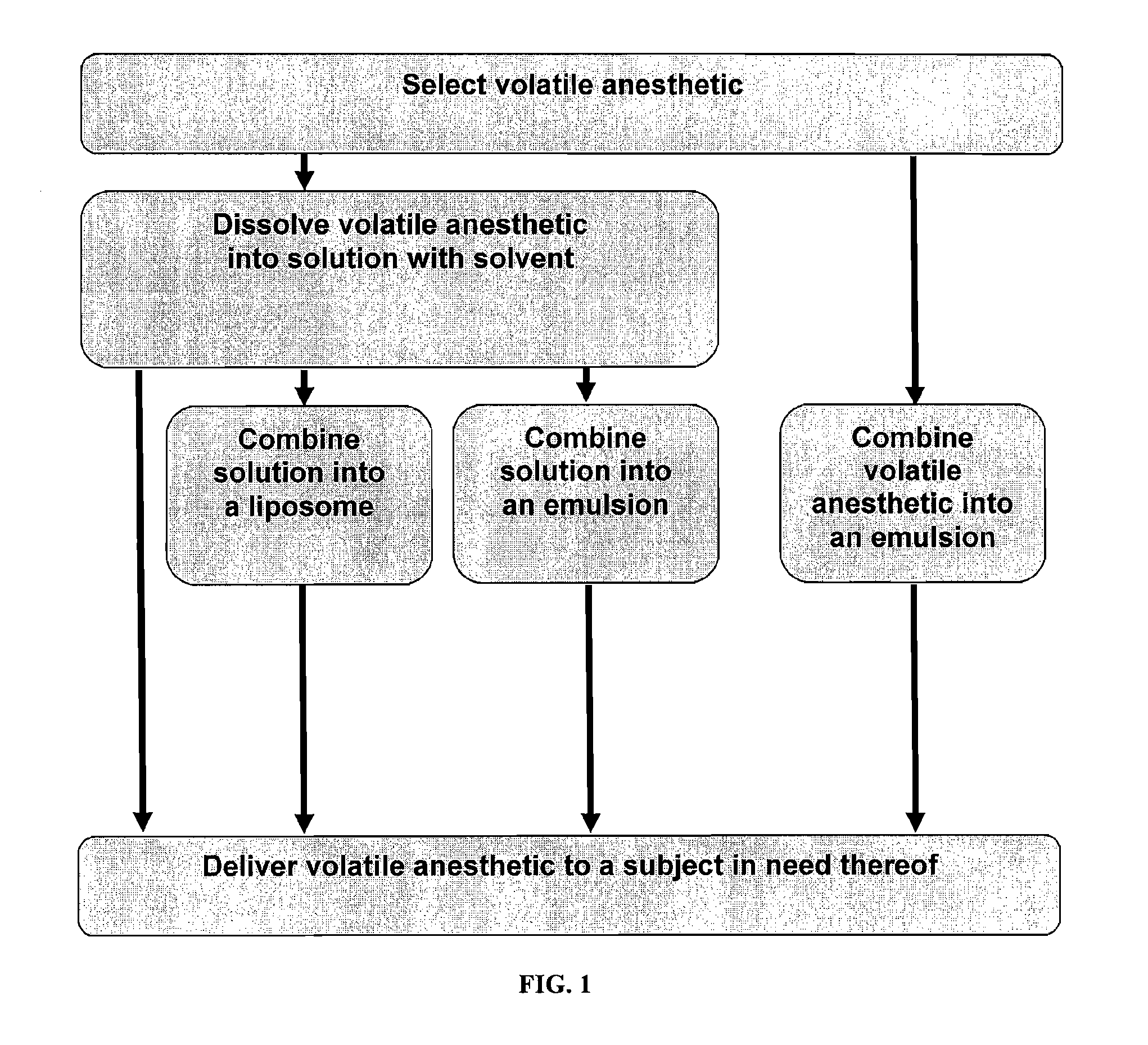
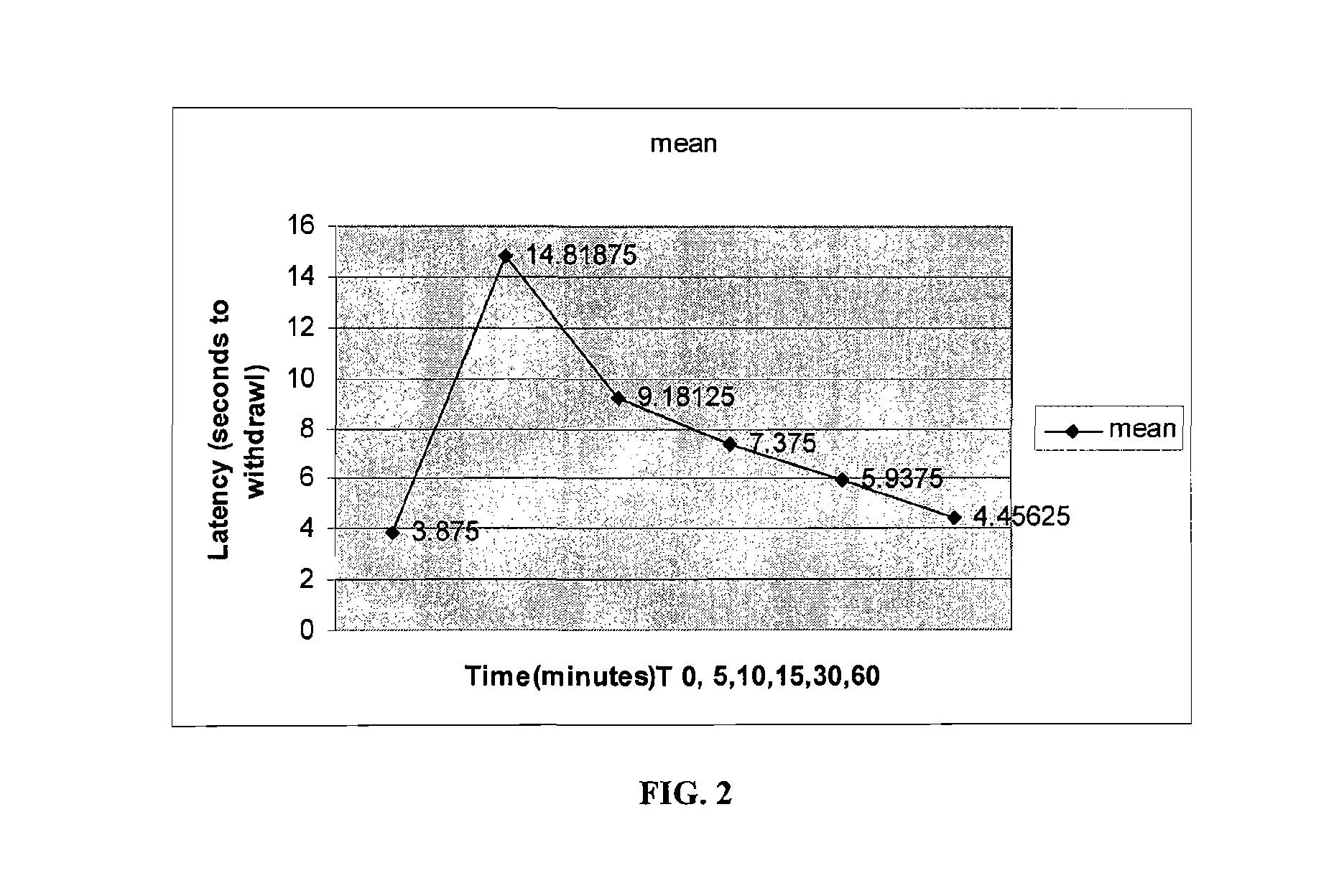
Volatile Anesthetic Compositions and Methods of Use
InactiveUS20110159078A1Excellent characteristicsImprove propertiesBiocideNervous disorderEmulsionMotor function
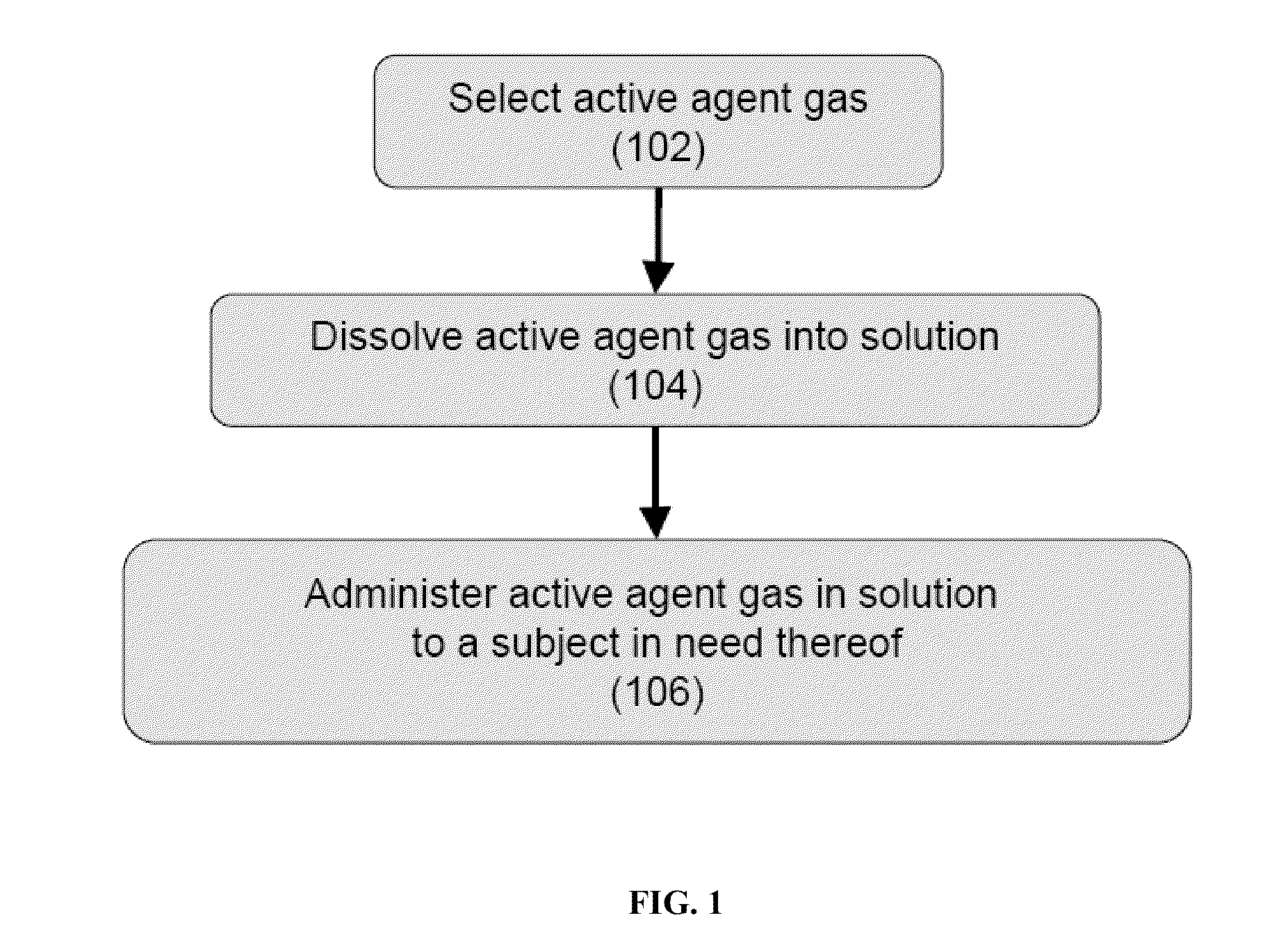
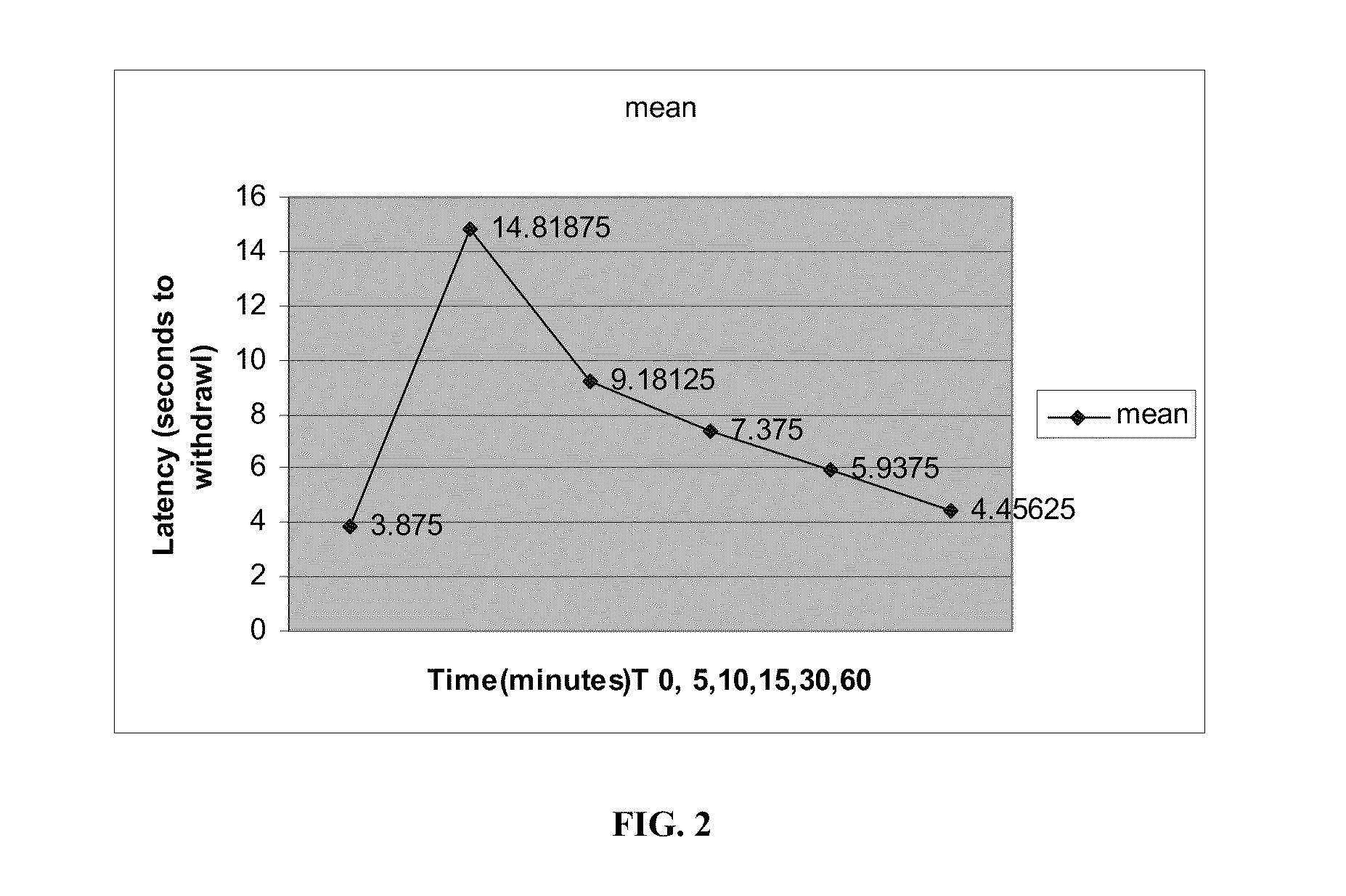
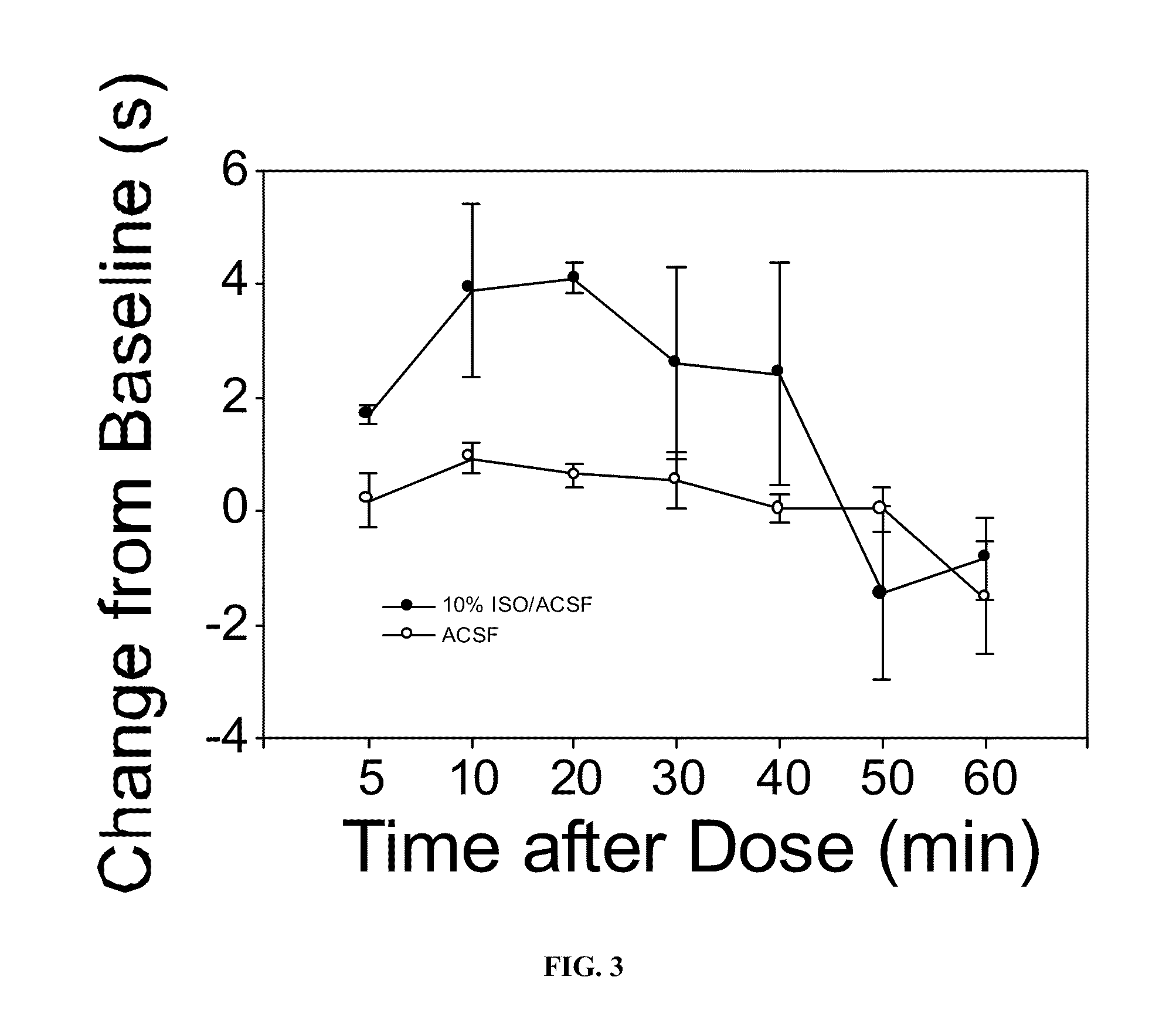
The present invention provides methods for reducing pain in a subject in need thereof by delivering a volatile anesthetic in a solution or an emulsion that can additionally include an extractive solvent in an amount effective to reduce pain without substantially interfering with motor function. Chronic or acute pain may be treated, or the volatile anesthetic may be delivered as a regional anesthetic to a subject to anesthetize a portion of the subject prior to surgery. Dosing regimes including a one-time administration, continuous and / or periodic administration are contemplated.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
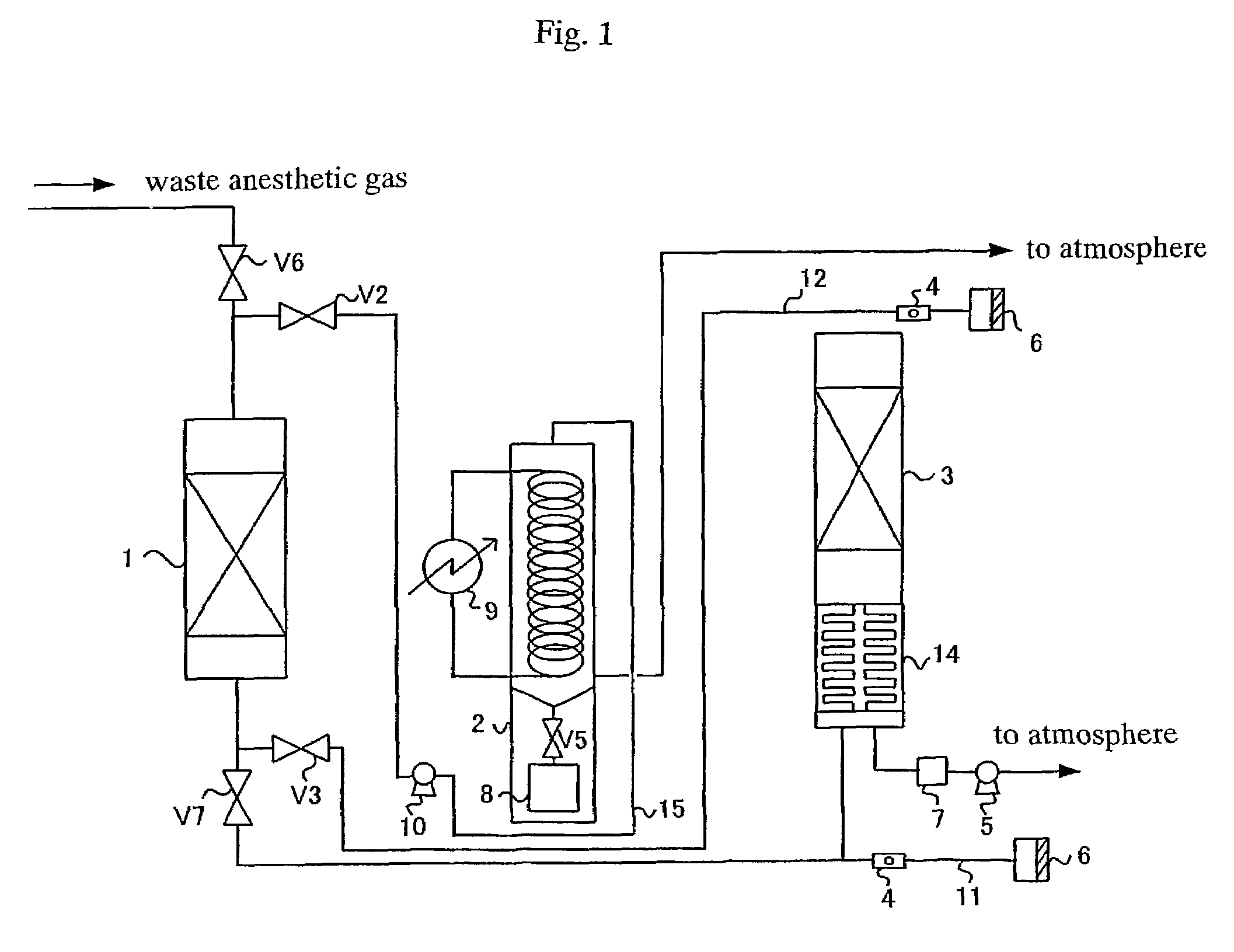
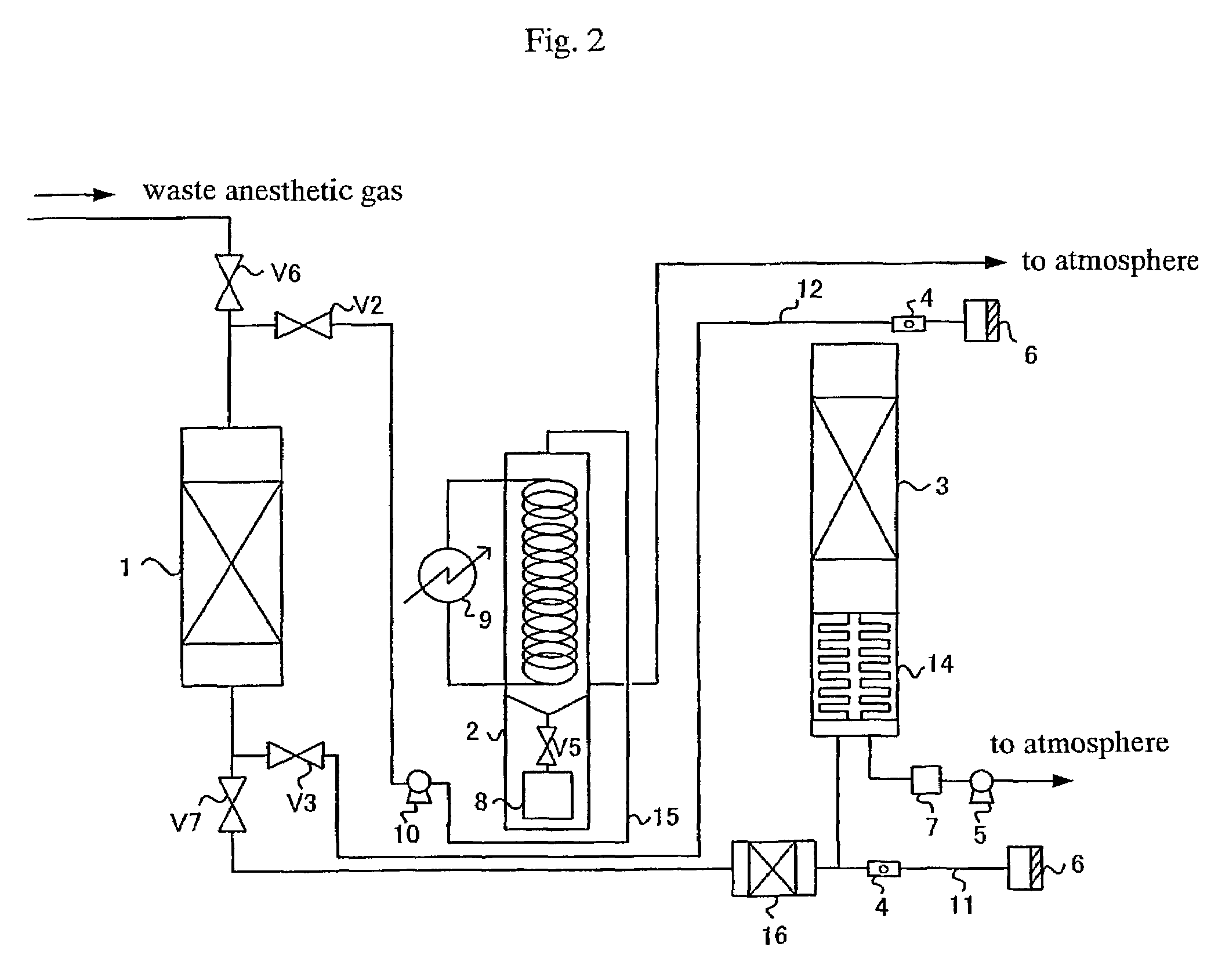
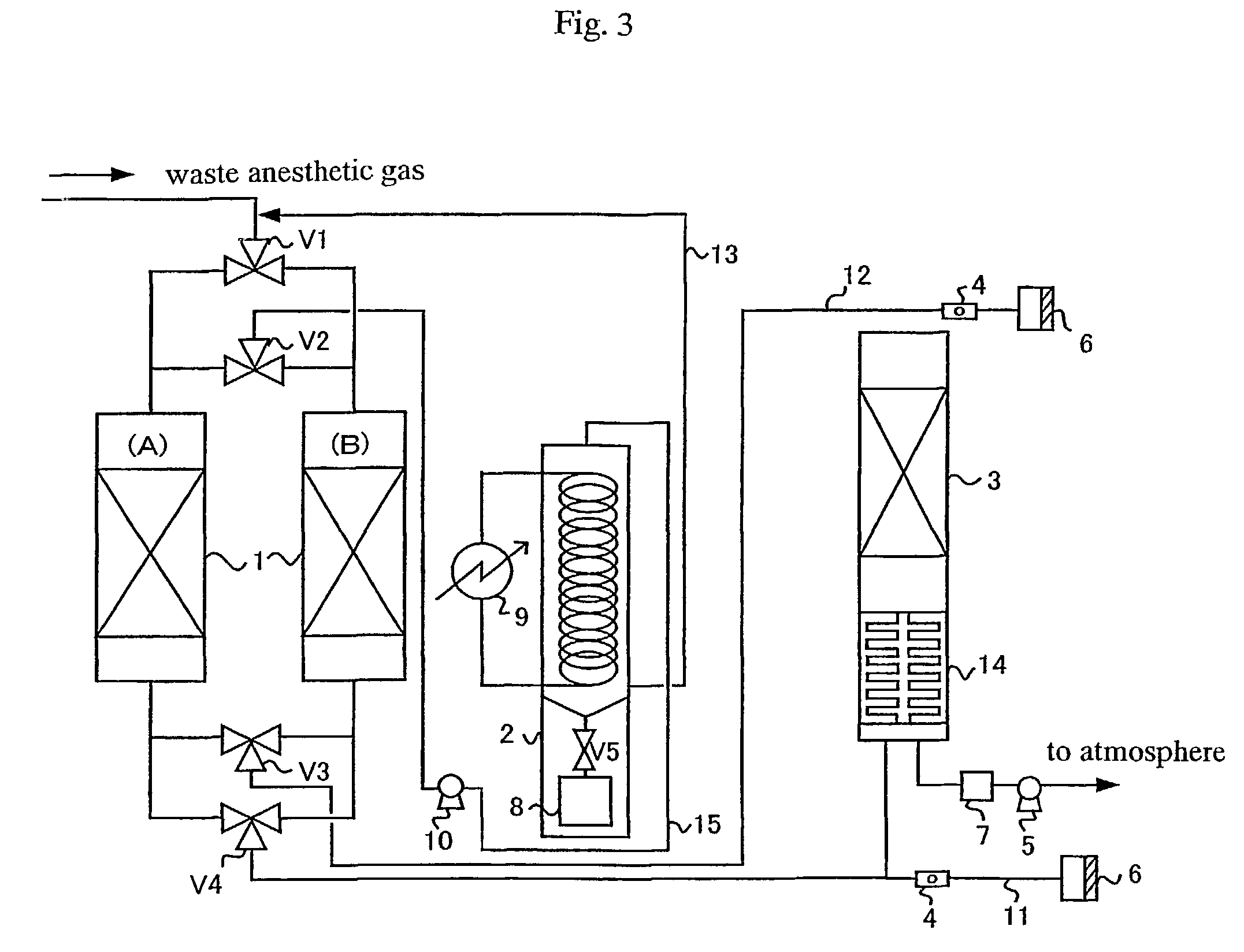
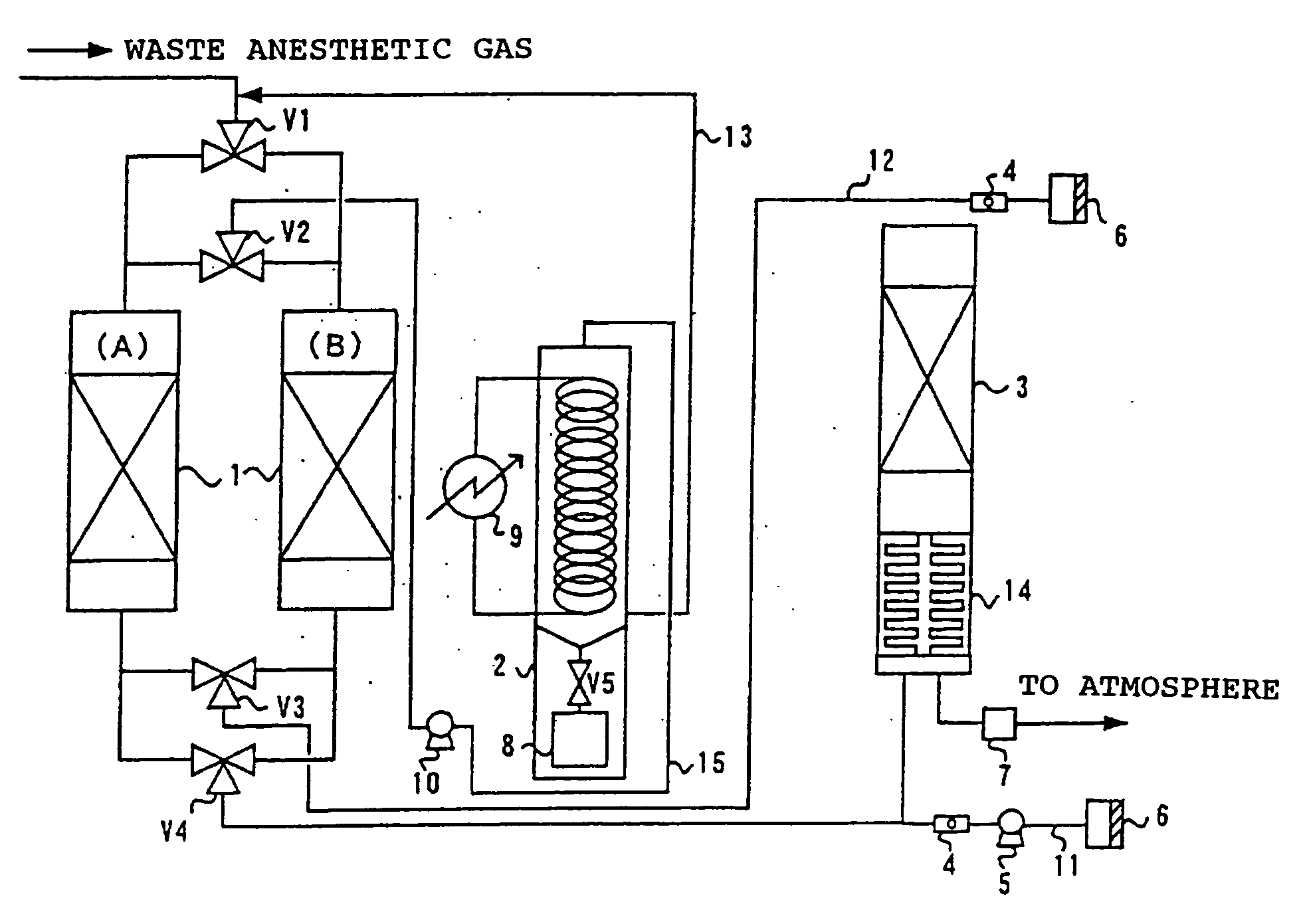
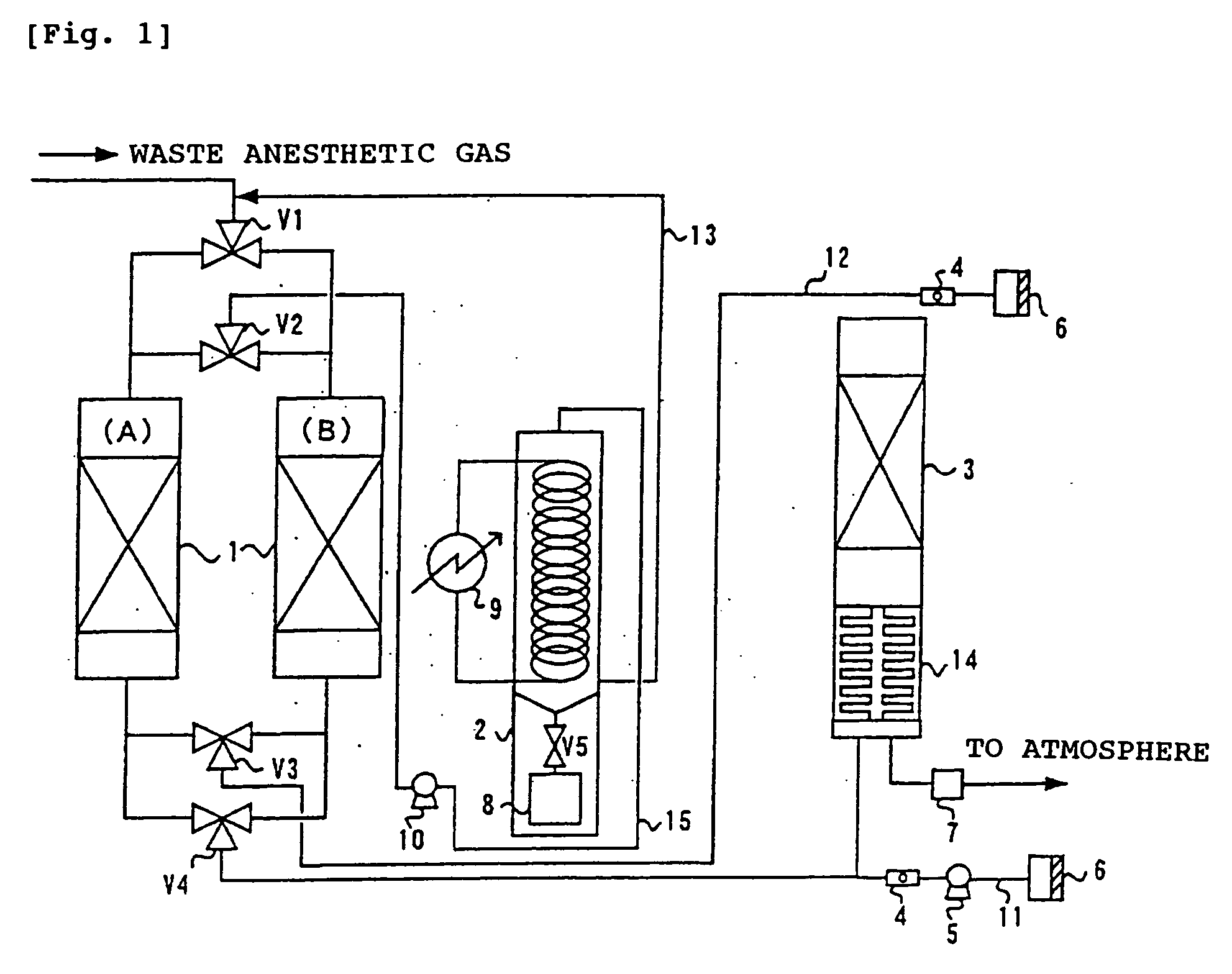
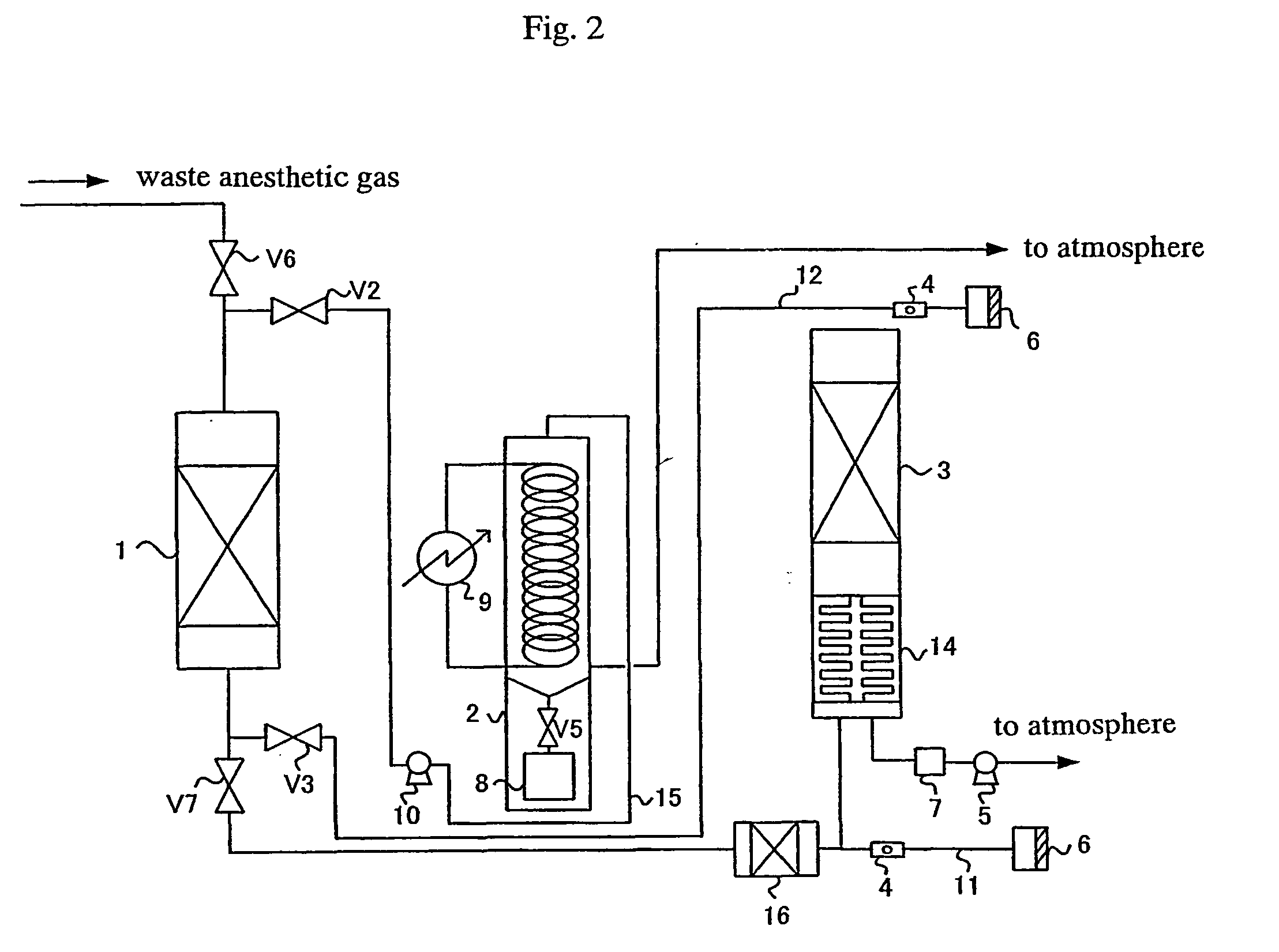
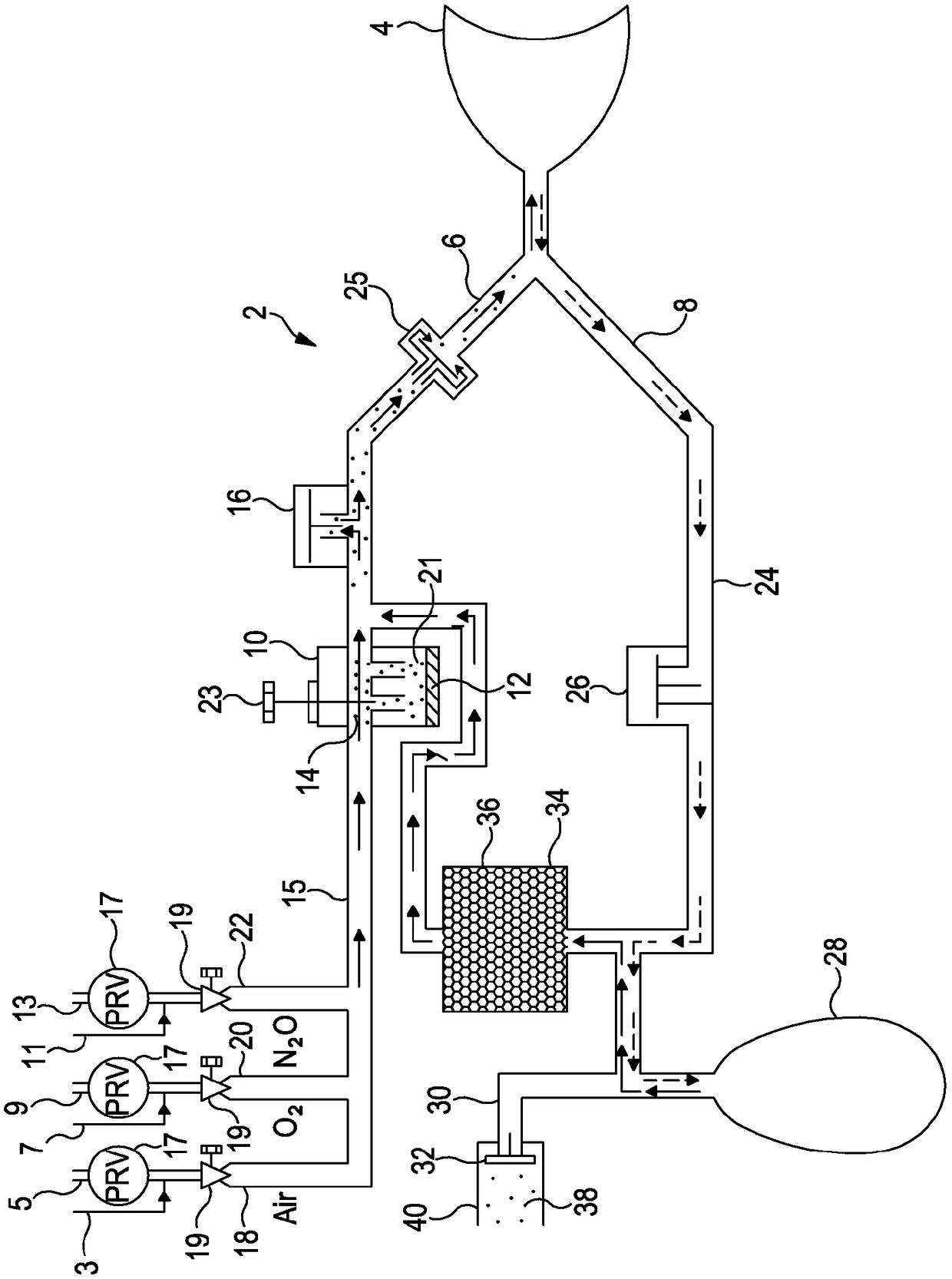
Process for treating waste anesthetic gas
To provide a process and an apparatus for treating a waste anesthetic gas containing a volatile anesthetic and nitrous oxide discharged from an operating room by introducing the gas into an adsorbing cylinder filled with an adsorbent, where the volatile anesthetic contained in the waste anesthetic gas is adsorbed and thereby removed, and successively introducing the gas into a catalyst layer filled with a nitrous oxide decomposition catalyst, where nitrous oxide is decomposed into nitrogen and oxygen. By using the process and the apparatus for treating a waste anesthetic gas of the present invention, a volatile anesthetic having a possibility of destroying the ozone layer or nitrous oxide as a global warming gas can be made harmless while preventing the release into atmosphere.
Owner:RESONAC HOLDINGS CORPORATION
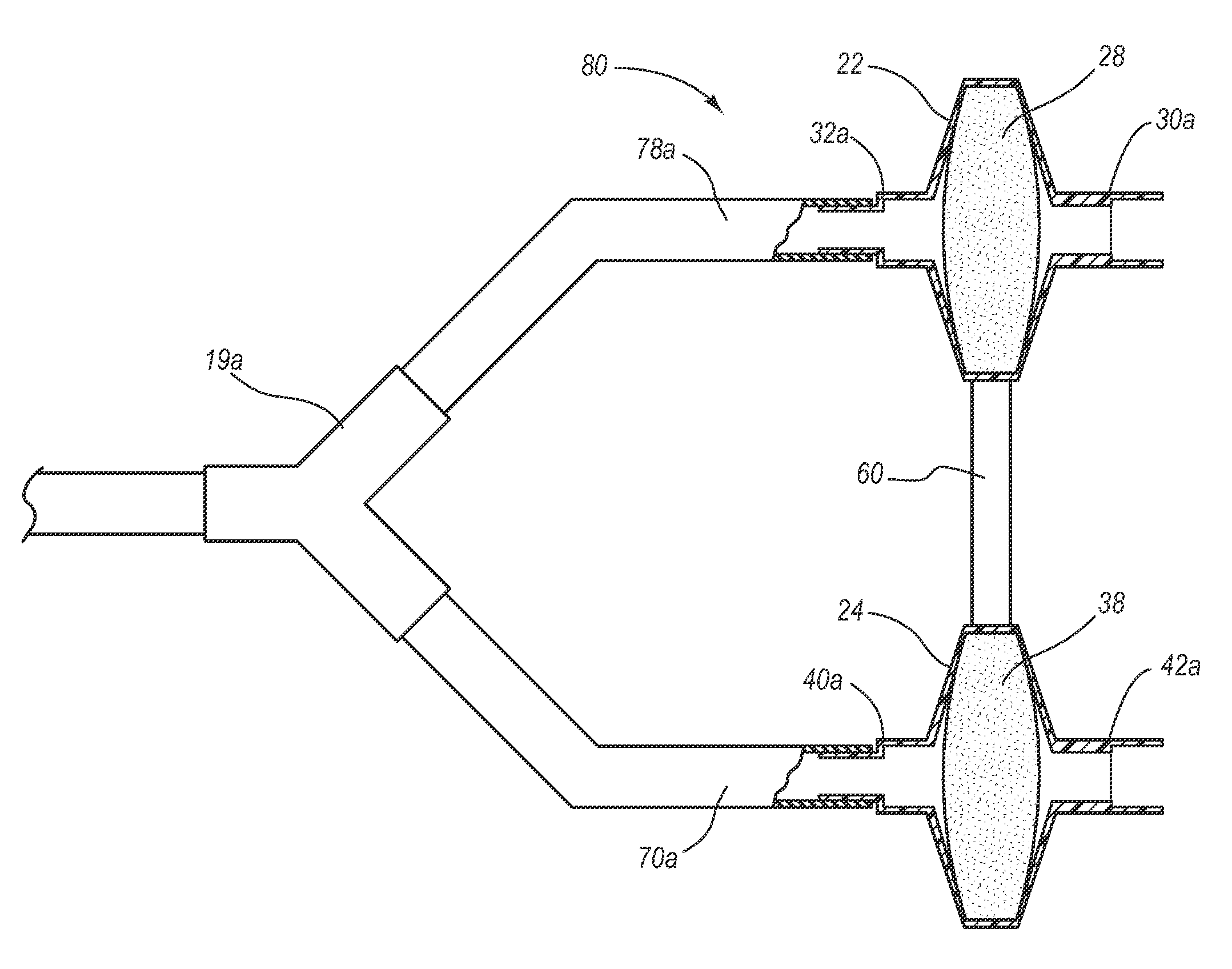
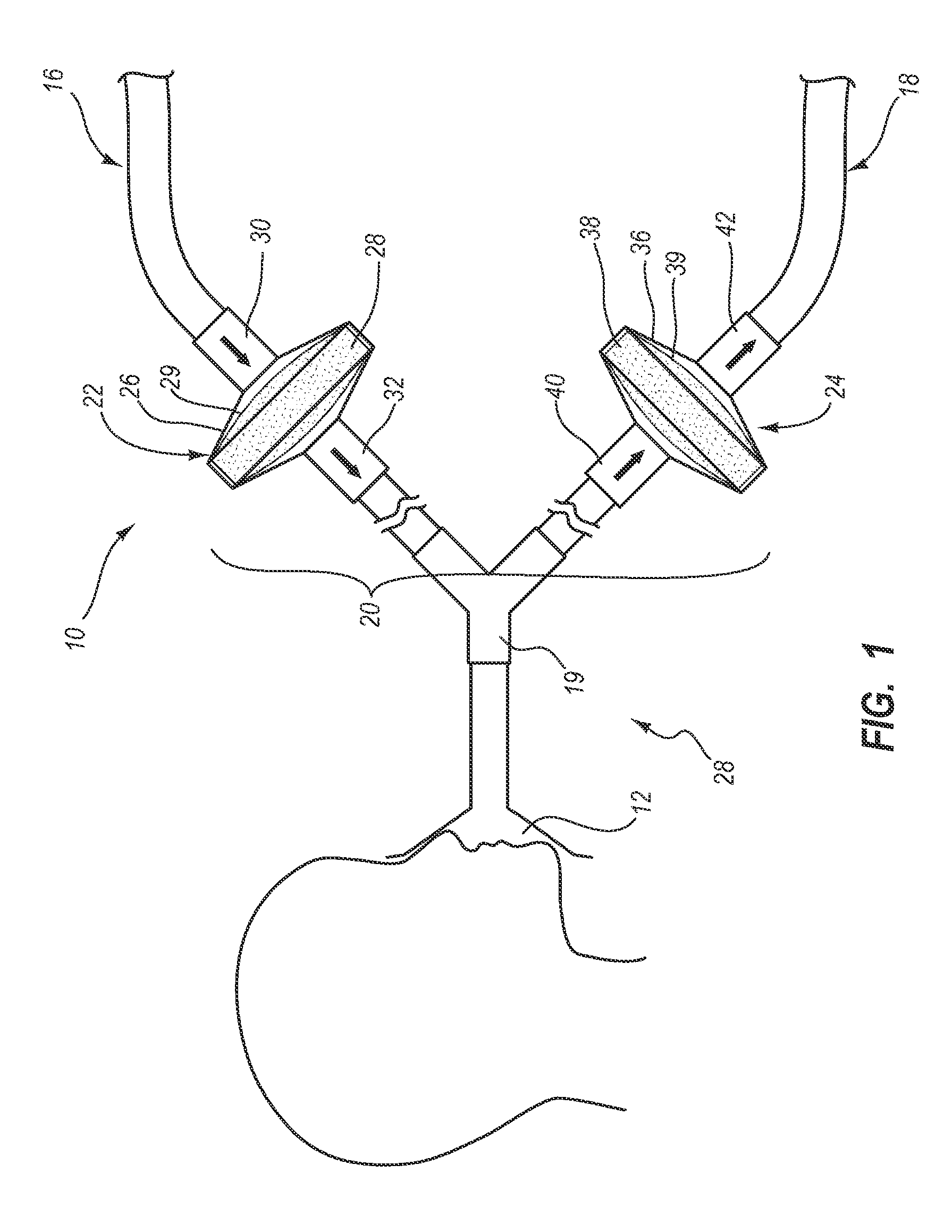
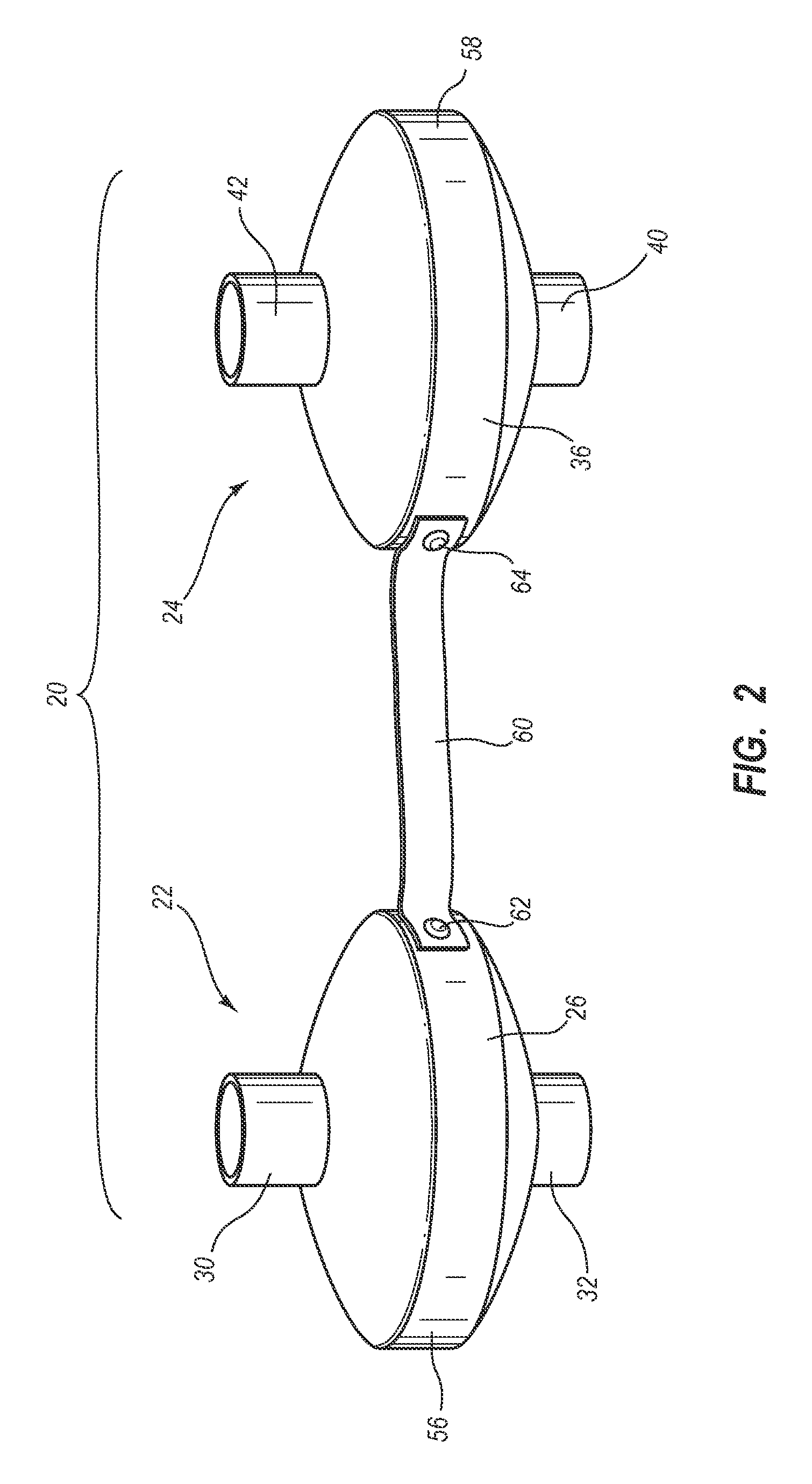
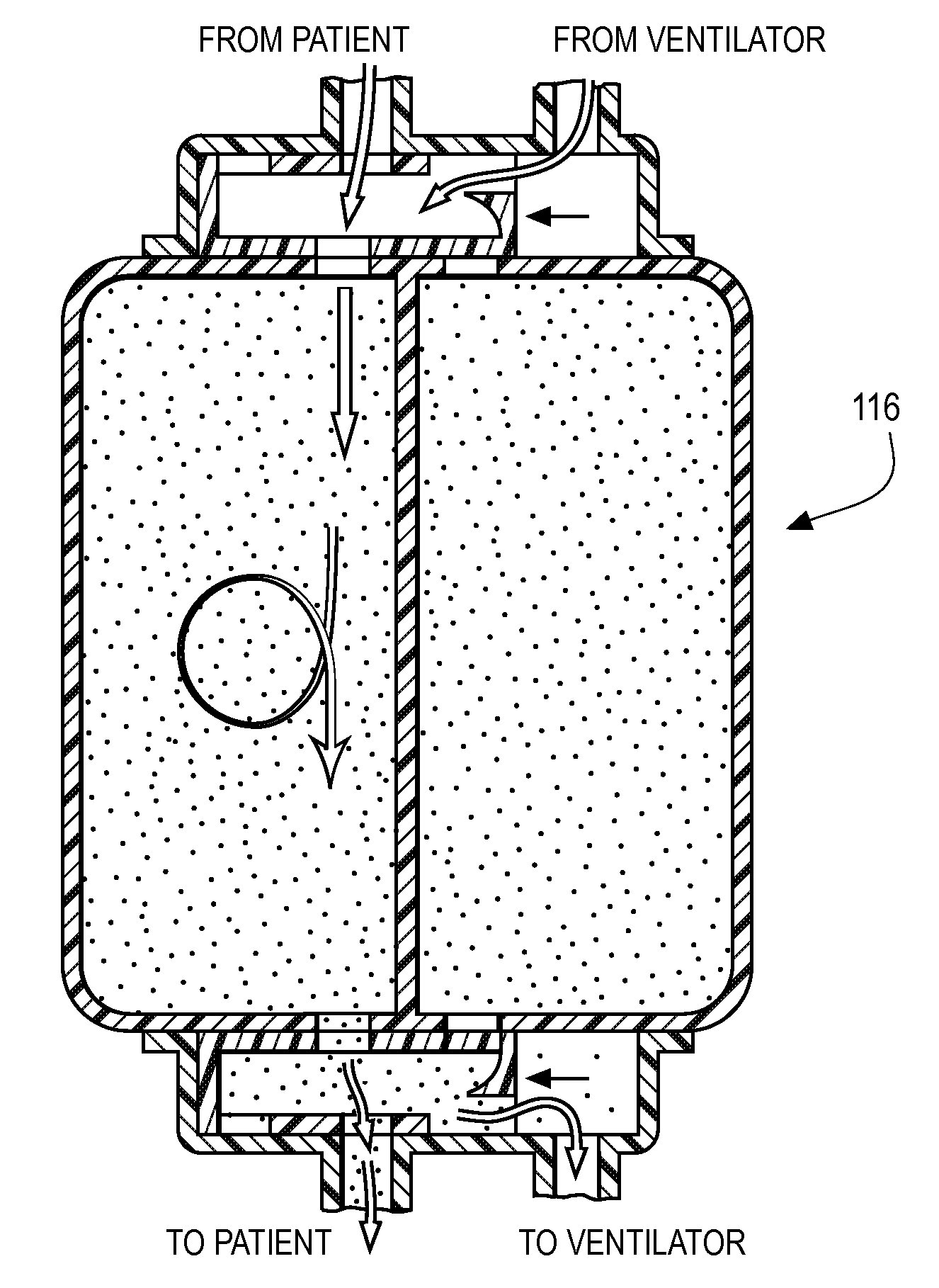
System, method and apparatus for removal of volatile anesthetics for malignant hyperthermia
ActiveUS20100269828A1Minimize impactEfficient removalRespiratorsBreathing filtersMalignant hyperthermiaExpiratory limb
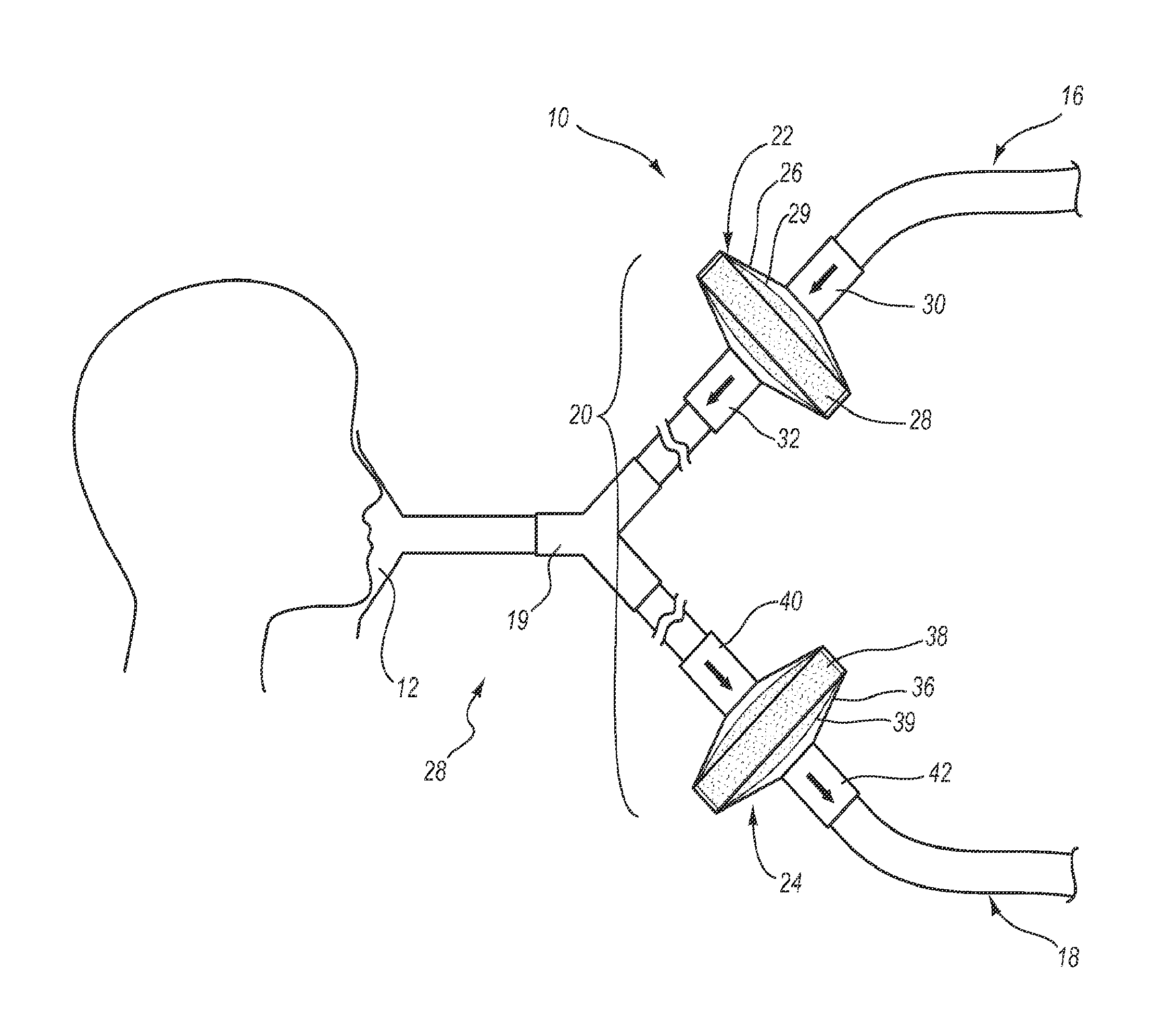
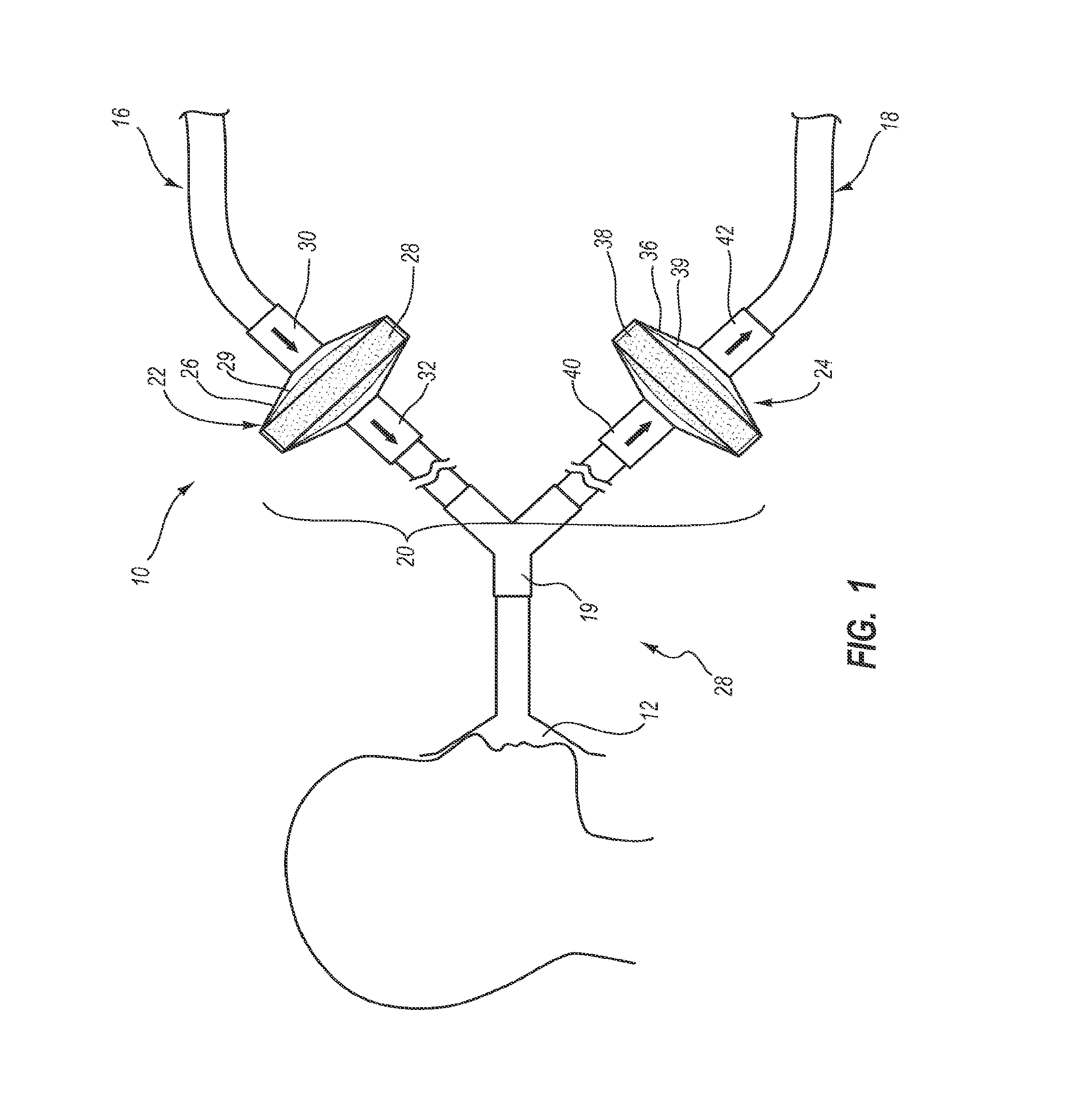
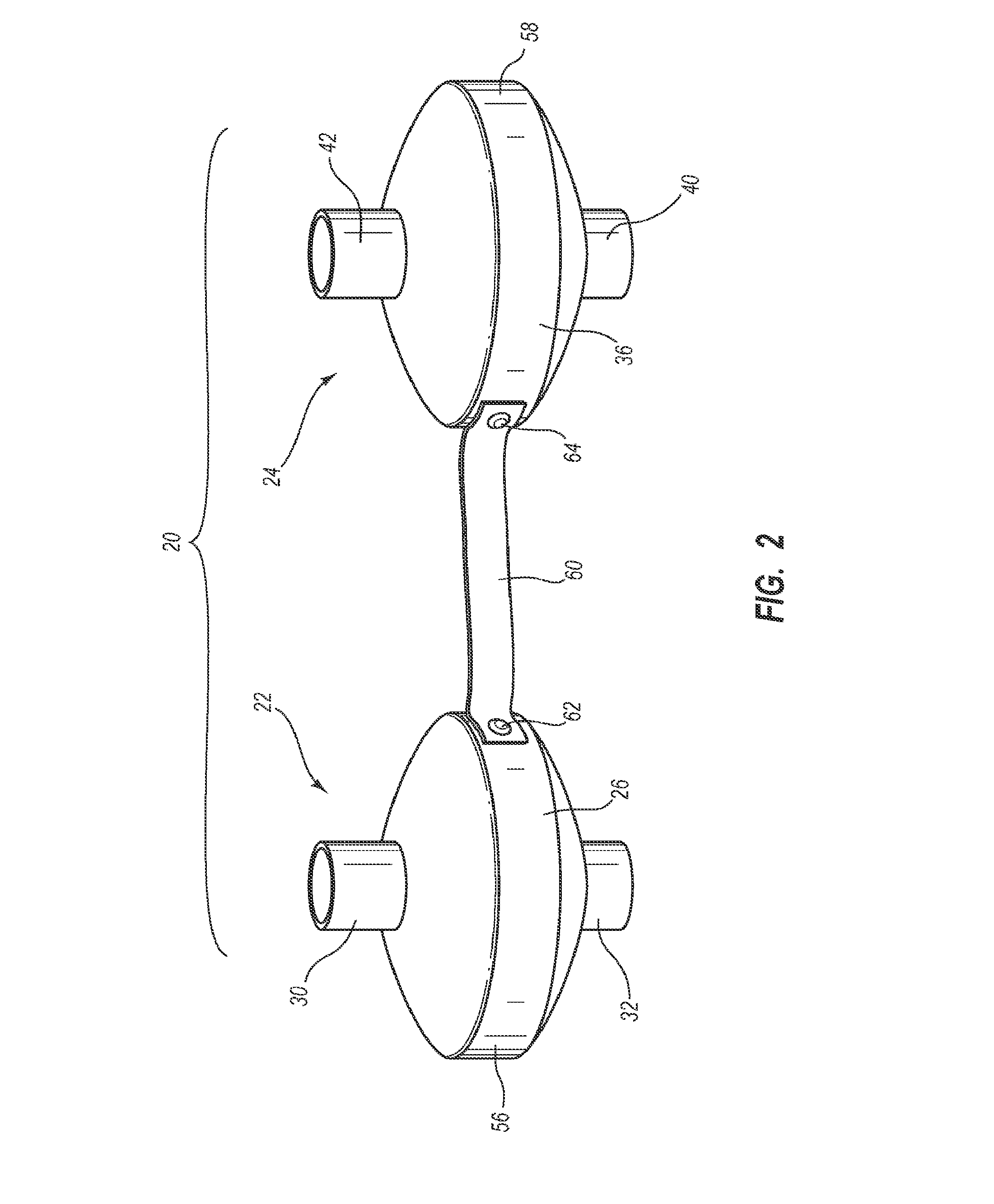
Systems, methods, and apparatus for removing volatile anesthetics from an anesthesia or ventilation system to minimize the effects of malignant hyperthermia in susceptible patients. According to one aspect of the present invention, a system for removing volatile anesthetics is provided. A first filter component placed in fluid communication with an inspiratory limb of an anesthesia or ventilation system such that volatile anesthetics will pass through the first filter component during operation of the anesthesia or ventilation system. A second filter component is operably coupled to the expiration port of the anesthesia or ventilation system such that gases passing through the expiratory limb of the anesthesia or ventilation system pass through the second filter component. The first filter component and second filter component are adapted to effectively remove volatile anesthetics passing through the respective filters.
Owner:DYNASTHETICS
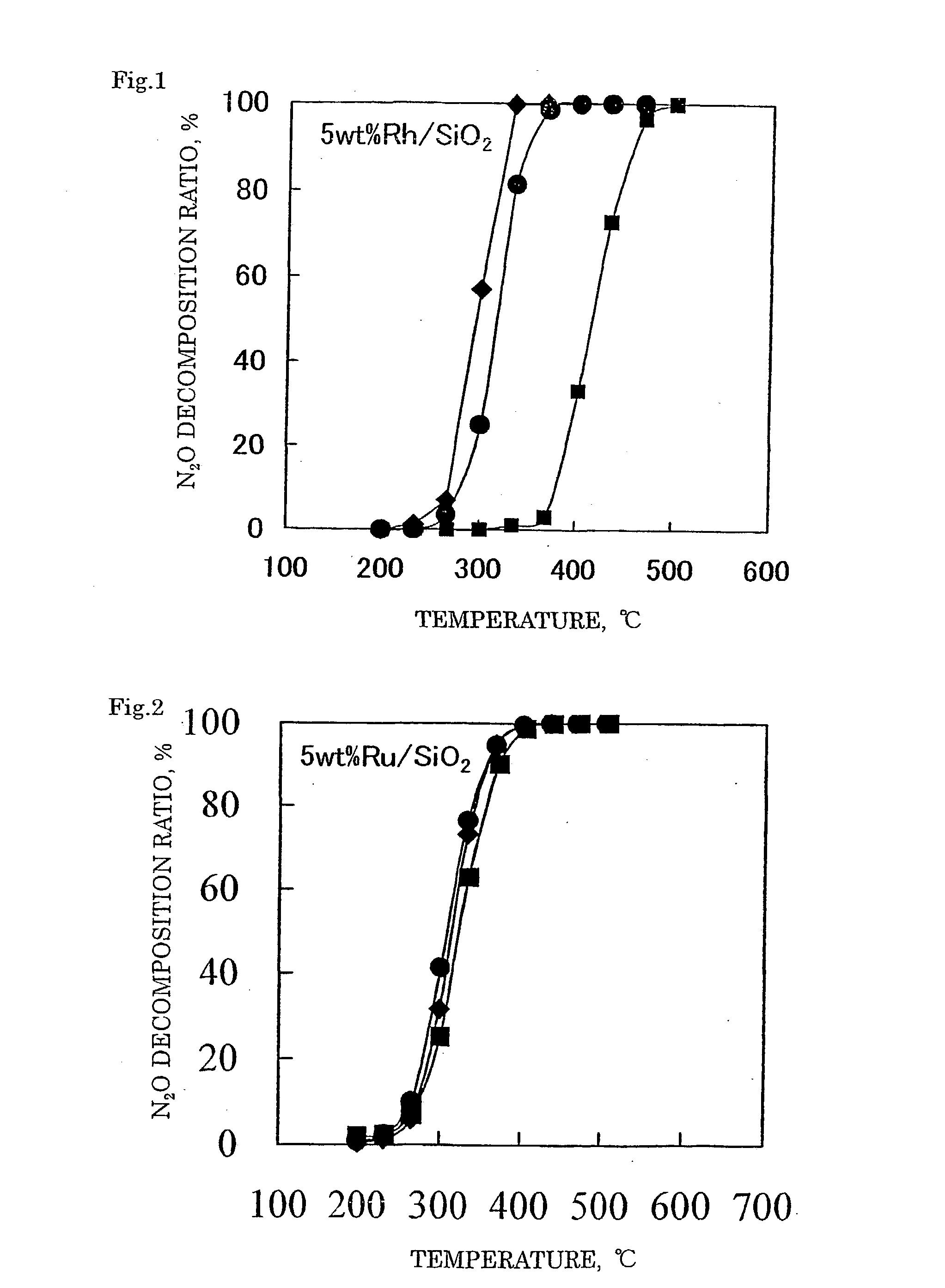
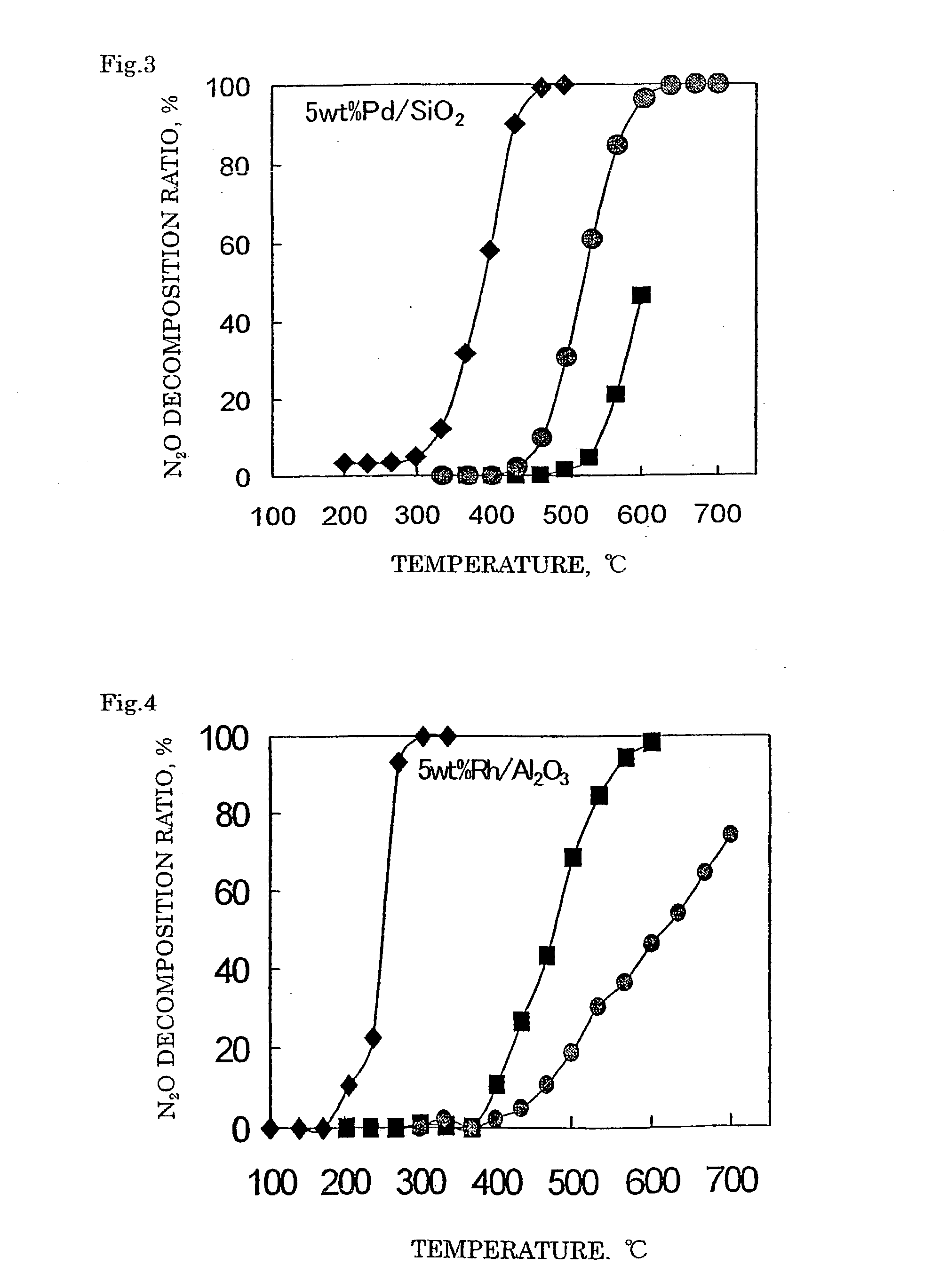
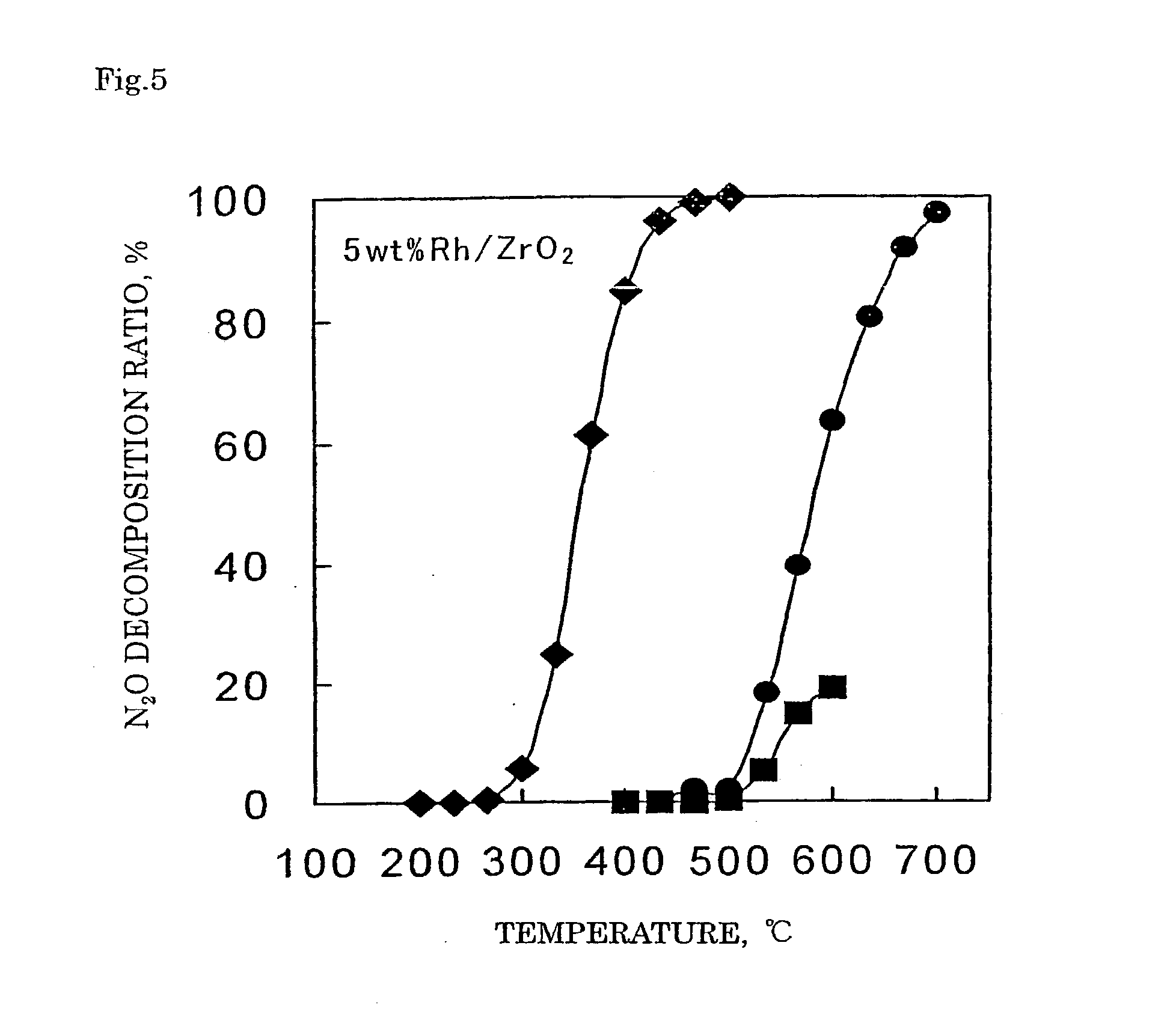
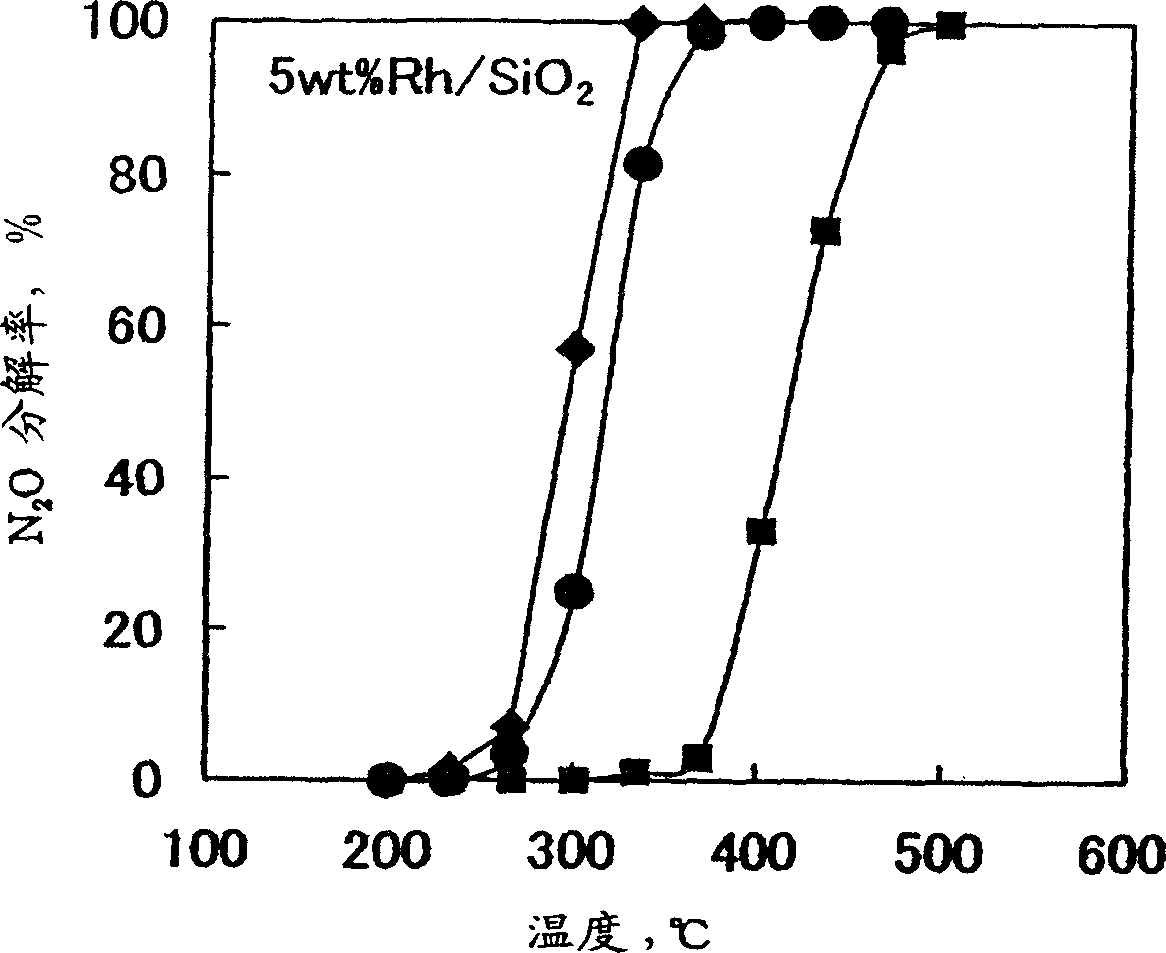
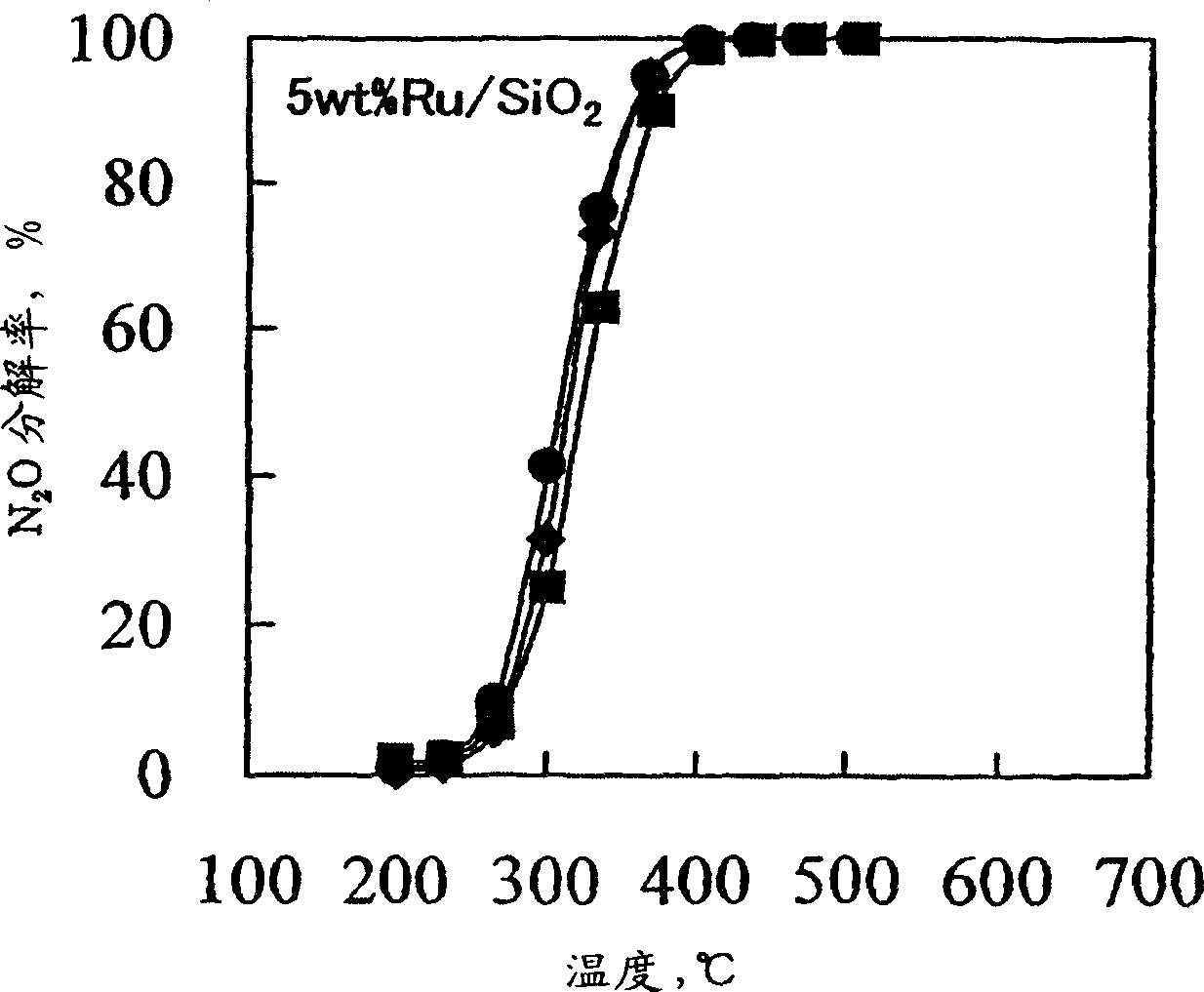
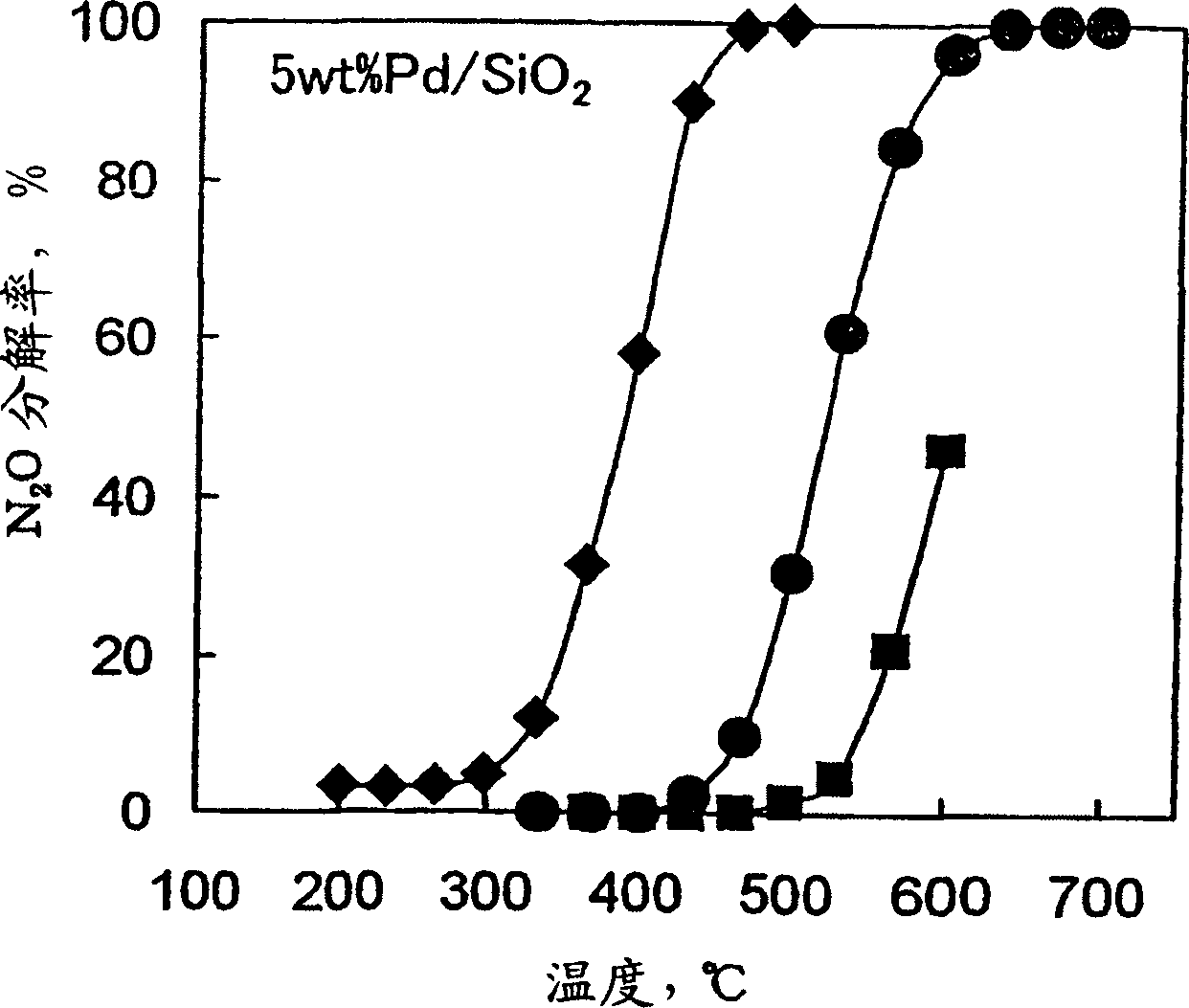
Decomposition catalyst for nitrous oxide, prcocess for producing the same and process for decomposing nitrous oxide
InactiveUS20030181324A1Return to normal activitiesReduce amount of NOx generatedNitrous oxide captureGas treatmentDecompositionAnesthetic gases
To provide a catalyst obtained by loading at least one noble metal selected from the group consisting of rhodium, ruthenium and palladium on a support selected from silica and silica alumina, and a method for decomposing nitrous oxide using the catalyst thereof. The catalyst for decomposing nitrous oxide of the present invention cannot be easily affected by a volatile anesthetic contained in a waste anesthetic gas, can recover the activity by activation and regeneration even when deteriorated, and can reduce the amount of NOx generated to less than the allowable concentration.
Owner:SHOWA DENKO KK
Novel Formulations of Volatile Anesthetics and Methods of Use for Reducing Inflammation
ActiveUS20120171281A1Reduce vaporizationReduce evaporationBiocideHalogenated hydrocarbon active ingredientsAnesthesiaInflammation
The present invention provides methods for treating inflammation or a wound in a subject in need of such wound treatment or inflammation treatment by delivering a volatile anesthetic to the wound or the inflammation site.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
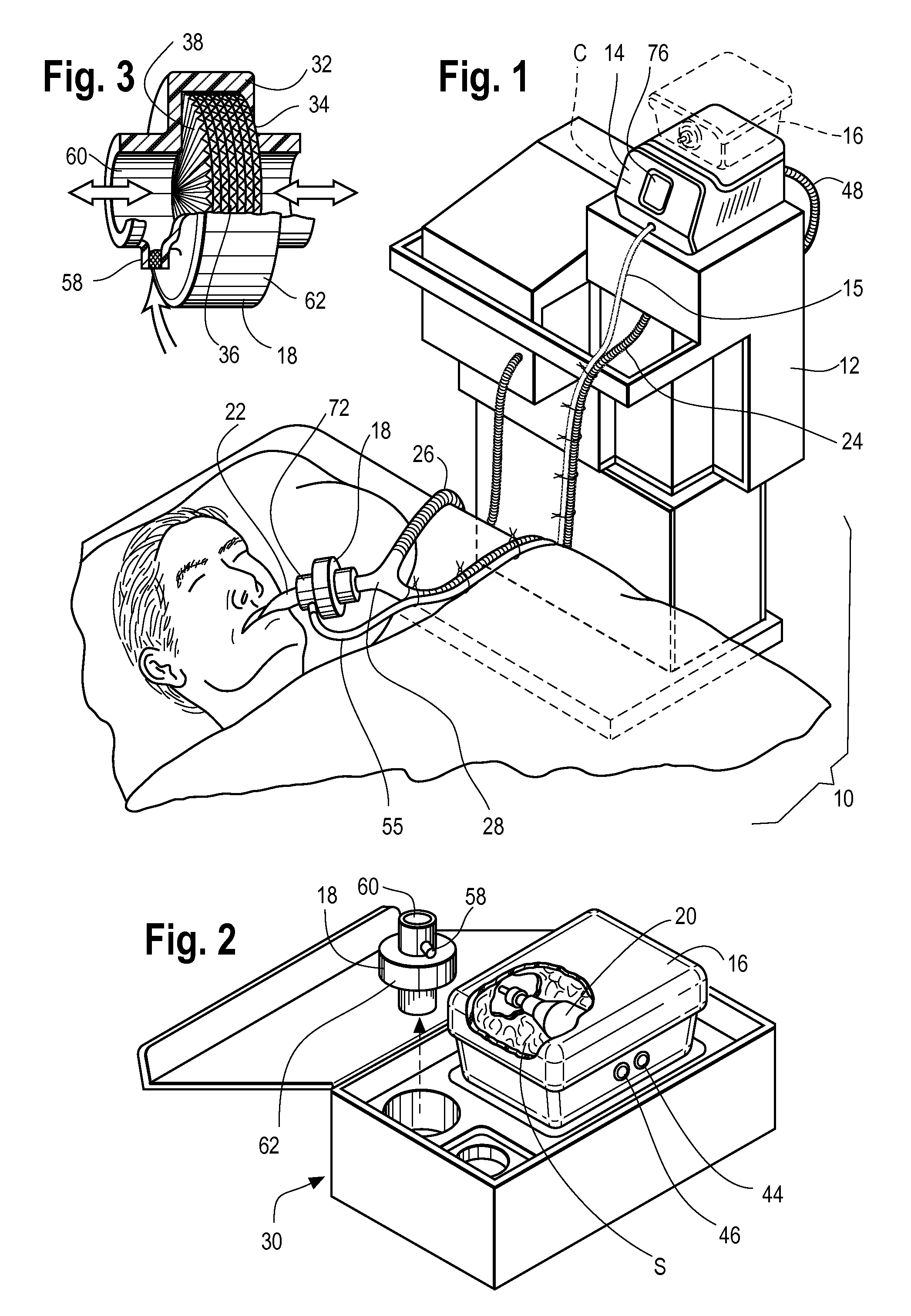
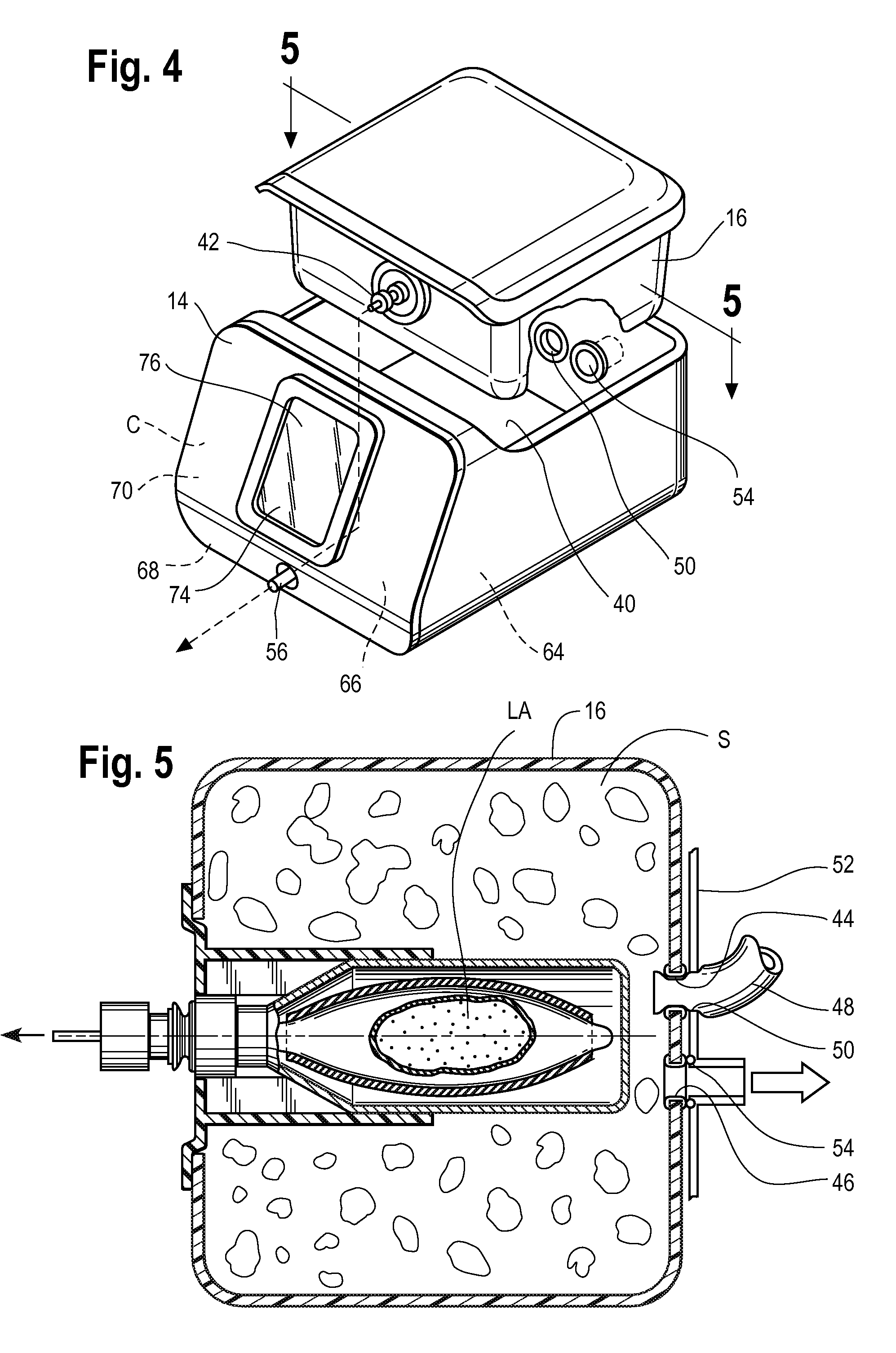
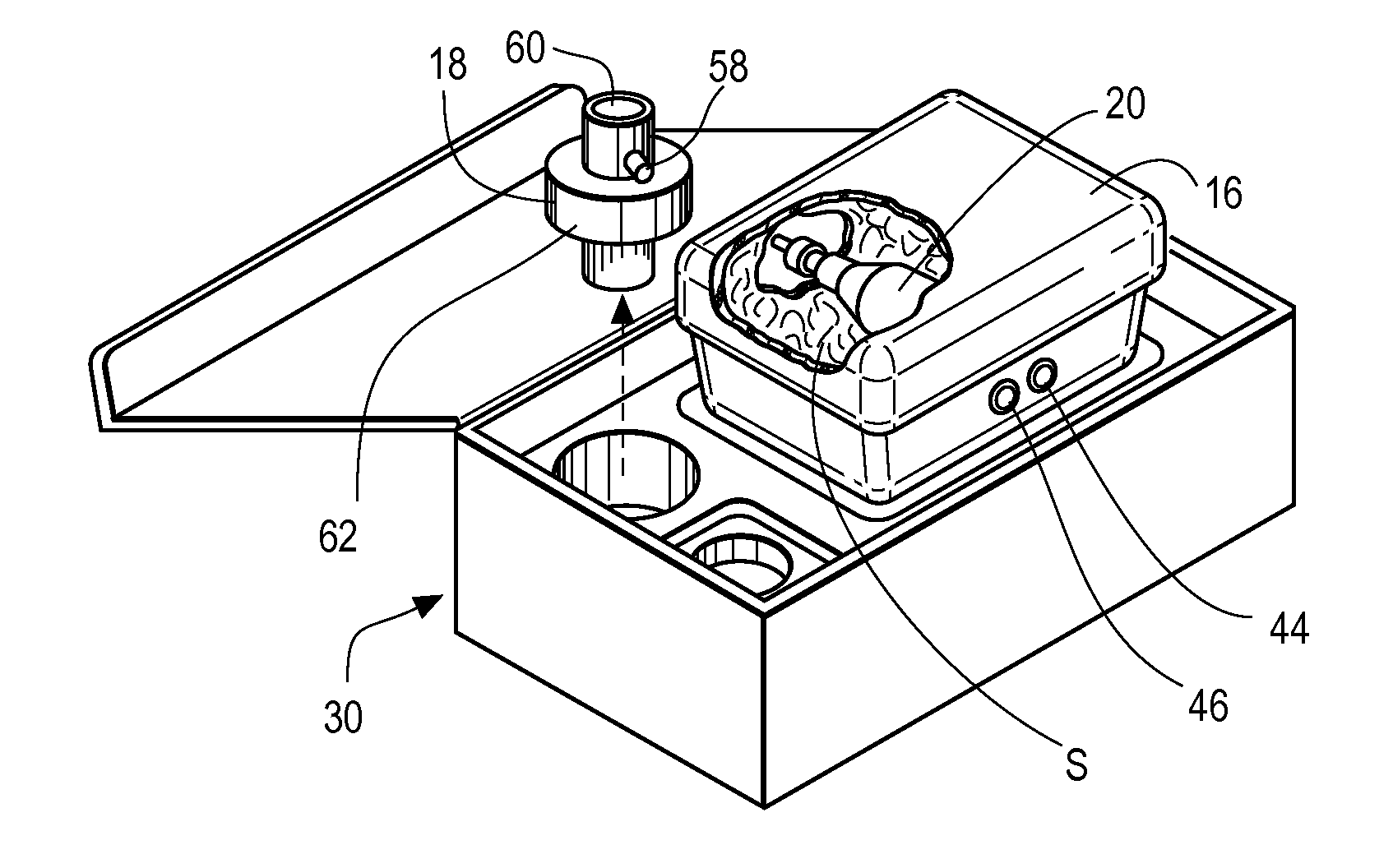
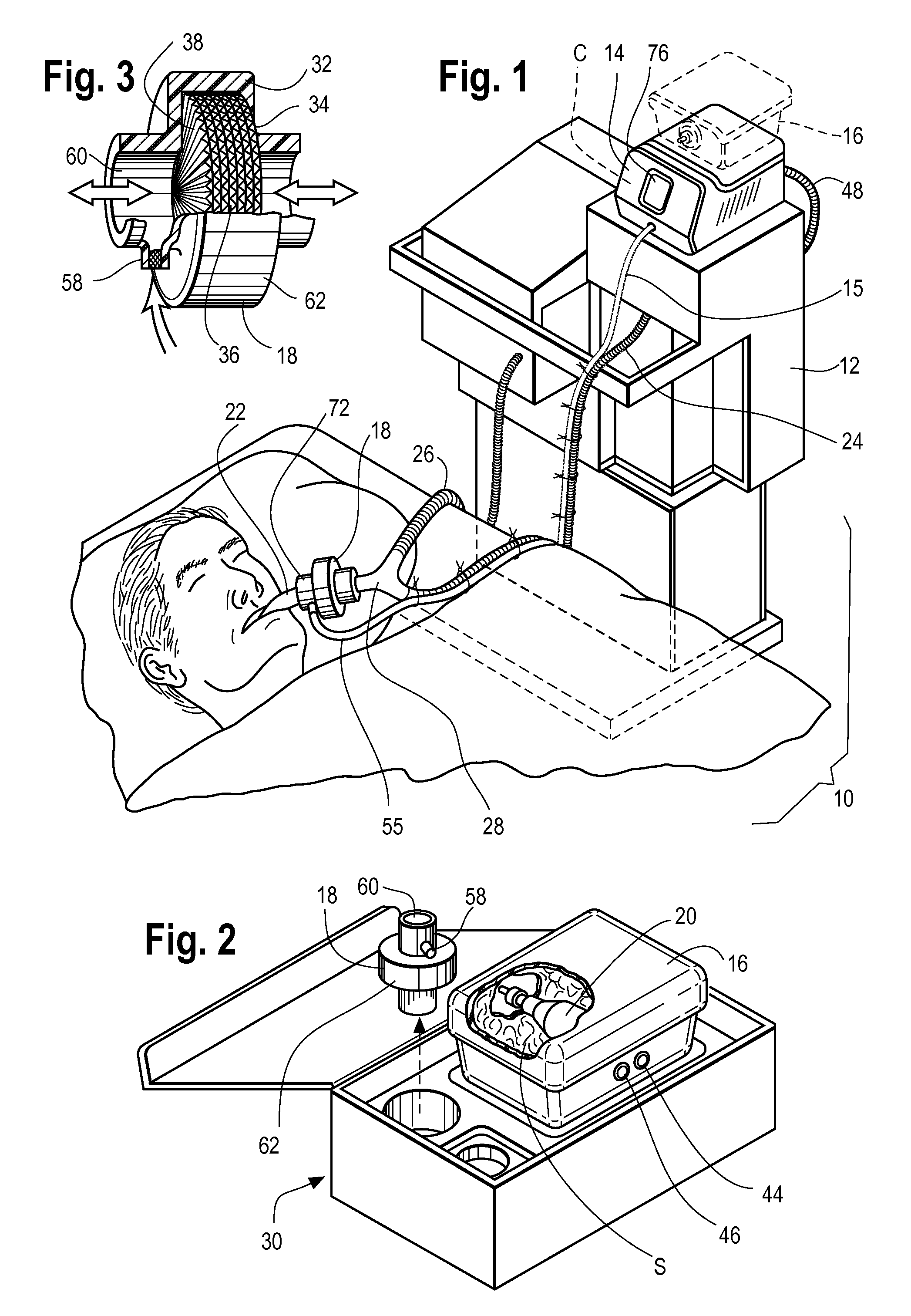
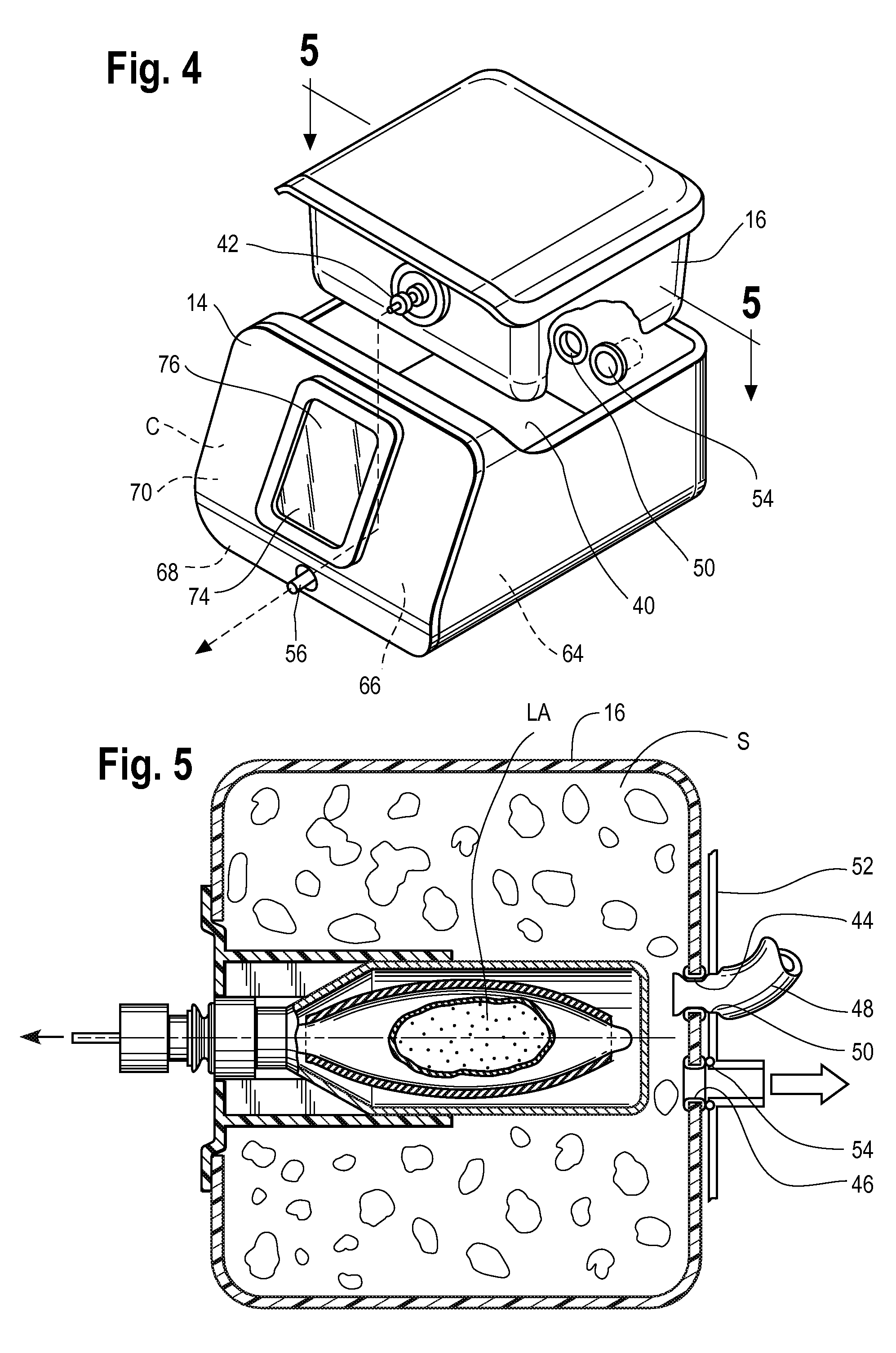
Inhaled anesthetic agent therapy and delivery system
InactiveUS20100212668A1Decrease and replace useShorten the lengthTracheal tubesBreathing filtersMedical intensive care unitSurgical intensive care unit
A therapy utilizing inhaled anesthetic agents (such as desflurane, sevoflurane, isoflurane, or xenon) for the sedation of patients outside of the immediate perioperative space such as in the medical intensive care unit (MICU) and the surgical intensive care unit (SICU). The therapy includes controlled delivery of volatile anesthetic agents to patients undergoing ventilatory support on an ICU ventilator over extended periods of time. A system which provides for the delivery of anesthetic agents includes an anesthetic agent vaporizer element, an anesthetic agent reflector, and a plug-in cassette which contains both a cartridge housing liquid phase volatile anesthetic agent and an anesthetic vapor scrubbing medium.
Owner:BAXTER INT INC +1
Inhaled anesthetic agent therapy and delivery system
InactiveUS8267081B2Decrease and replace useShorten the lengthTracheal tubesBreathing filtersMedical intensive care unitSurgical intensive care unit
A therapy utilizing inhaled anesthetic agents (such as desflurane, sevoflurane, isoflurane, or xenon) for the sedation of patients outside of the immediate perioperative space such as in the medical intensive care unit (MICU) and the surgical intensive care unit (SICU). The therapy includes controlled delivery of volatile anesthetic agents to patients undergoing ventilatory support on an ICU ventilator over extended periods of time. A system which provides for the delivery of anesthetic agents includes an anesthetic agent vaporizer element, an anesthetic agent reflector, and a plug-in cassette which contains both a cartridge housing liquid phase volatile anesthetic agent and an anesthetic vapor scrubbing medium.
Owner:BAXTER INT INC +1
Anesthesia device for animal experiment
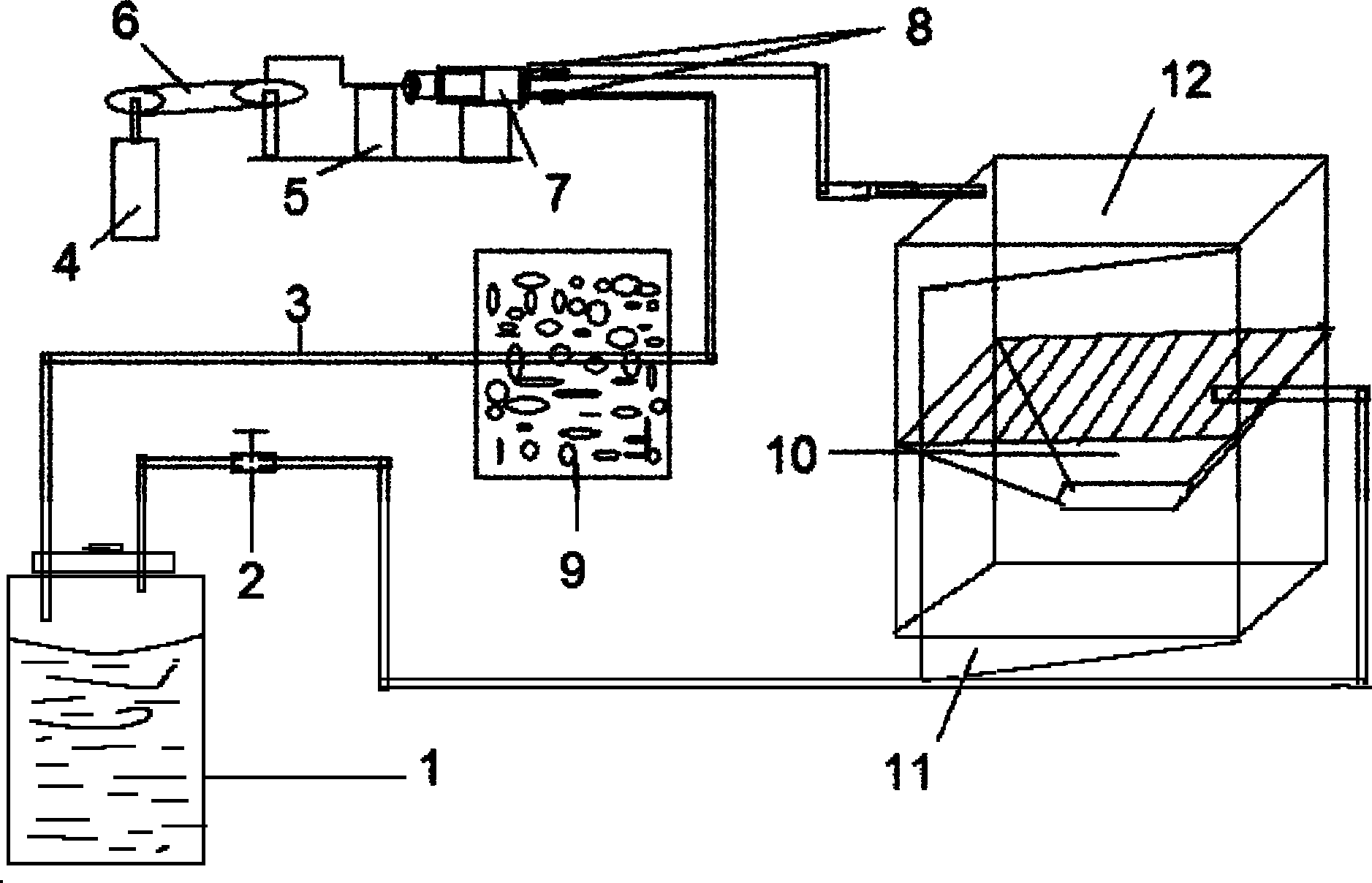
InactiveCN102138834AReduce self-injuryReduce pollutionVeterinary instrumentsAnesthetic AgentEngineering
The invention discloses an anesthesia device for an animal experiment. The device is characterized by comprising an anesthesia case, a closed vessel filled with a volatile anesthetic; a seal door which can be opened is formed on the front side of the anesthesia case; communicating pipes which are communicated with the inside and the outside of the anesthesia case are arranged on two sides of the anesthesia case respectively; the communicating pipe on one side is arranged on the middle lower part of the case wall, and the communicating pipe on the other side is arranged on the upper part of the case wall; a sealing cover is arranged on the top of the closed vessel; and two straight pipes communicating the inside and the outside of the volatile anesthetic vessel are embedded on the sealing cover, one straight pipe is connected with the communicating pipe on the middle lower part of the case wall on one side of the anesthesia case through an anesthetic recycle device, and the other straight pipe is connected with the communicating pipe on the upper part on the case wall on the other side of the anesthesia case through a hose with a valve.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
Process and apparatus for treating waste anesthetic gas
[Problem to be Solved]To provide a process and an apparatus for treating a waste anesthetic gas containing a volatile anesthetic and nitrous oxide, discharged from an operating room. [Means to Solve the Problem]A waste anesthetic gas containing a volatile anesthetic and nitrous oxide is introduced into an adsorbing cylinder filled with an adsorbent, where the volatile anesthetic contained in the waste anesthetic gas is adsorbed and thereby removed, and successively this gas is introduced into a catalyst layer filled with a nitrous oxide decomposition catalyst, where nitrous oxide is decomposed into nitrogen and oxygen.
Owner:SHOWA DENKO KK
Decomposition catalyst for nitrous oxide, process for producing the same and process for decomposing nitrous oxide
The invention provides a catalyst obtained by loading at least one noble metal selected from the group consisting of rhodium, ruthenium and palladium on a support selected from silica and silica alumina, and a method for decomposing nitrous oxide using the catalyst thereof. The catalyst for decomposing nitrous oxide of the present invention cannot be easily affected by a volatile anesthetic contained in a waste anesthetic gas, can recover the activity by activation and regeneration even when deteriorated, and can reduce the amount of NOx generated to less than the allowable concentration.
Owner:RESONAC HOLDINGS CORPORATION
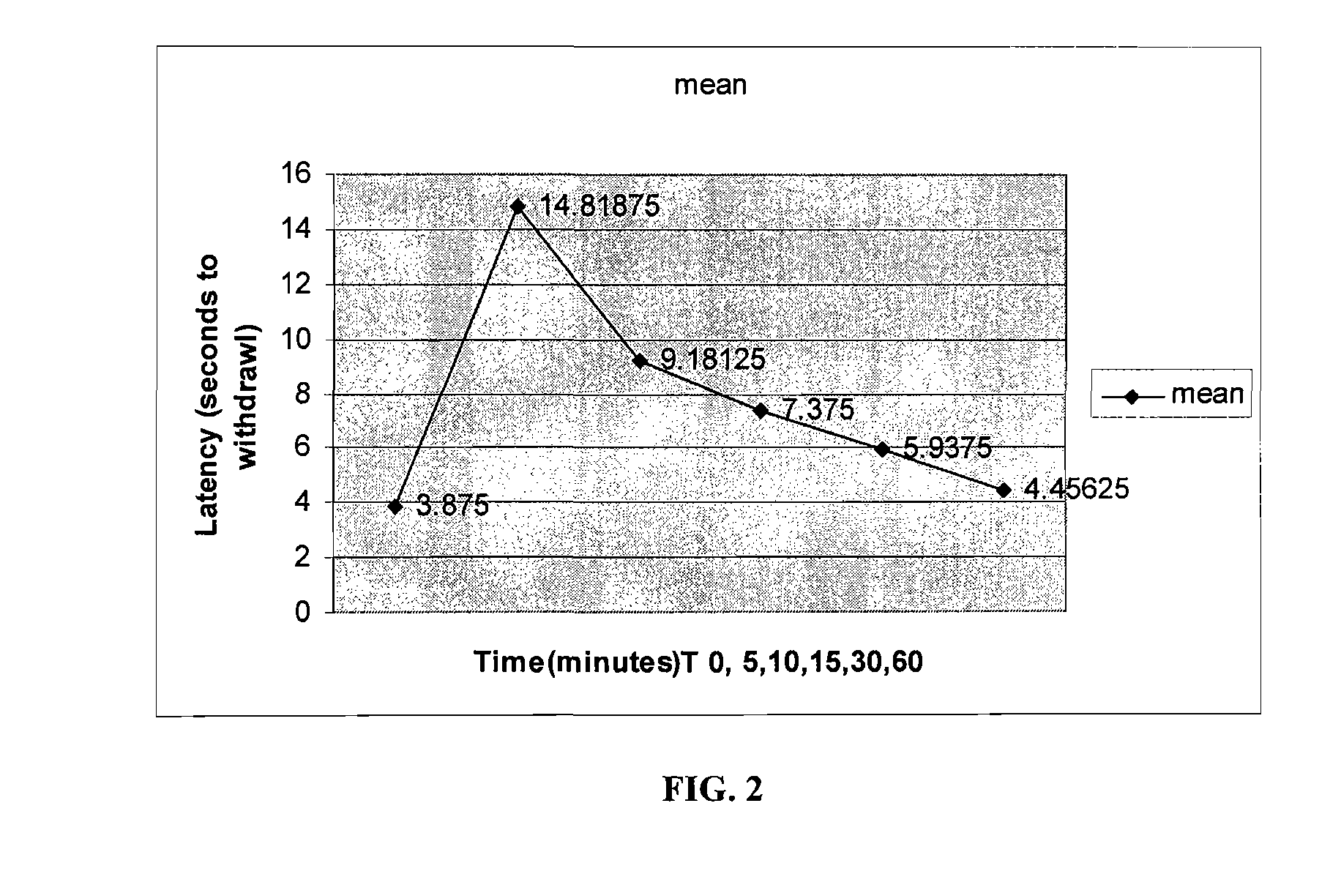
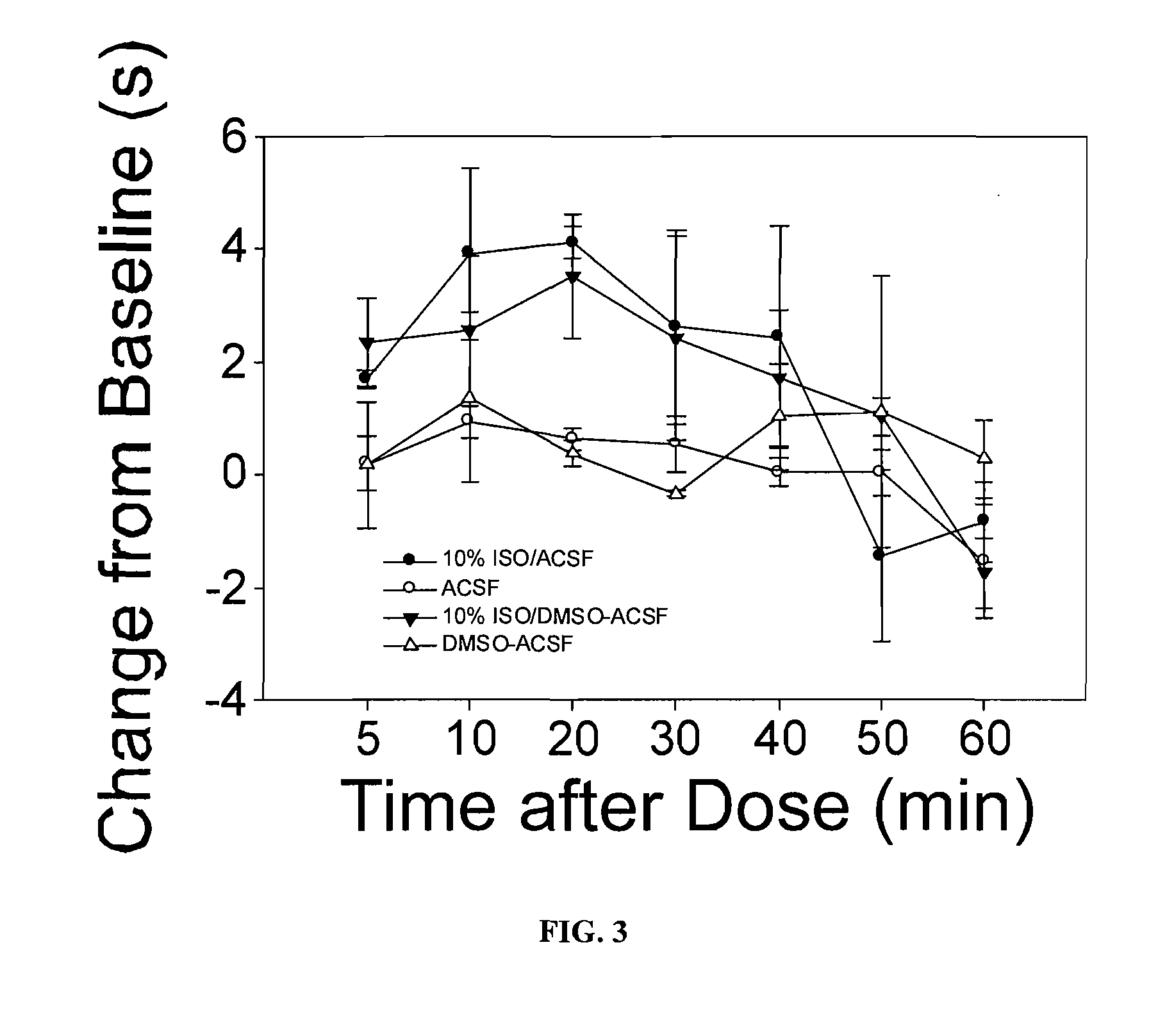
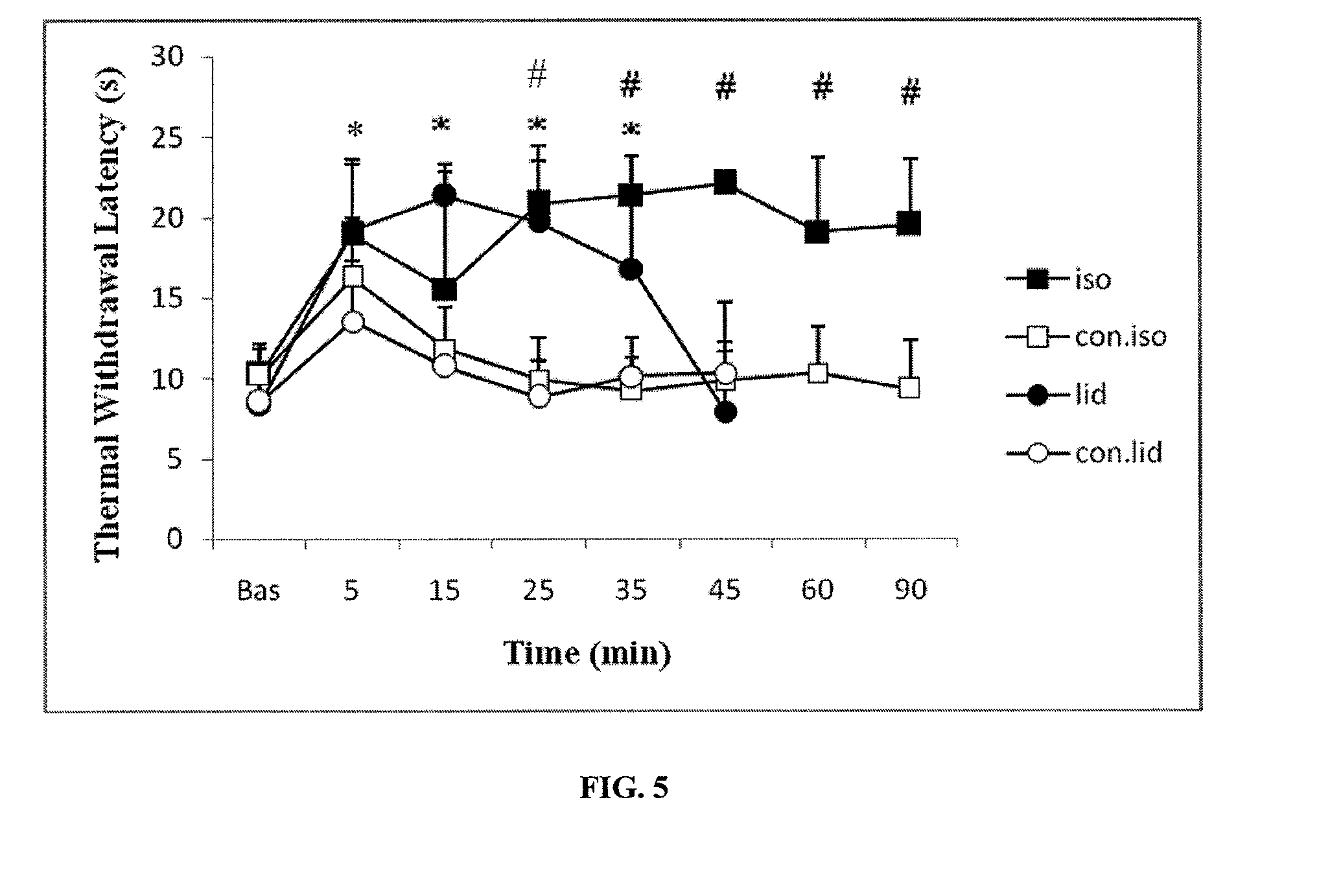
Volatile anesthetic compositions comprising extractive solvents for regional anesthesia and/or pain relief
ActiveUS20110039944A1Rapidly titratableRapid onsetHalogenated hydrocarbon active ingredientsBiocideRegimenSolvent
The present invention provides methods for reducing pain in a subject in need of such pain reduction by delivering, e.g., intrathecally or epidurally, a volatile anesthetic dissolved in a solution comprising an extractive solvent, e.g., DMSO or NMP, in an amount effective to reduce pain. Chronic or acute pain may be treated, or the anesthetic may be delivered as a regional anesthesia to a subject to anesthetize a portion the subject prior to a surgery, hi certain embodiments, isoflurane, halothane, enflurane, sevoflurane, desflurane, methoxyflurane, or mixtures thereof may be used. Dosing regimens including a one-time administration, continuous and / or periodic administration are contemplated.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
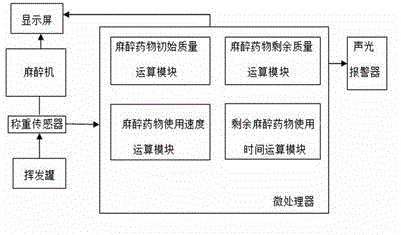
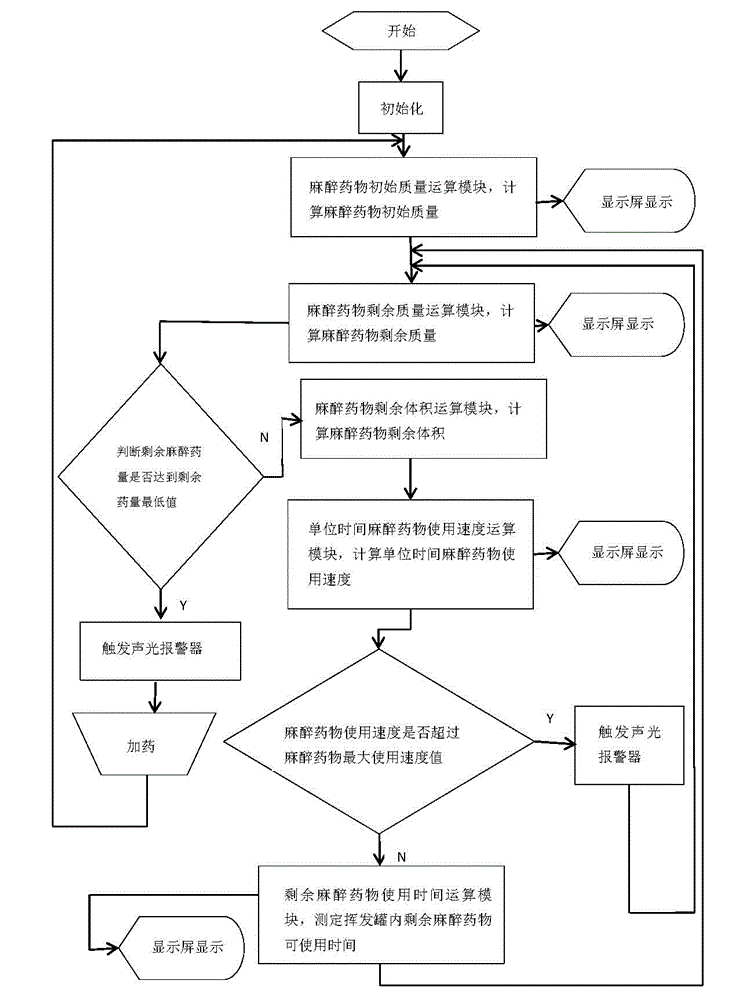
Volatile anesthetic dosage monitoring system and method for realizing same
InactiveCN103330984AKnowing the qualityUse the speed to master in real timeRespiratorsElectricityMonitoring system
The invention discloses a volatile anesthetic dosage monitoring system, belongs to the technical field of medical monitoring and solves the problems that in the prior art, the initially added anesthetic mass cannot be detected, the residual anesthetic mass cannot be detected in real time, the using speed of anesthetic cannot be detected, and the residual using time of residual anesthetic cannot be detected. The system comprises an anaesthesia machine which is provided with a display screen and a volatilization pot, wherein a weighing sensor is arranged between the anaesthesia machine and the volatilization pot. One end of the weighing sensor is electrically connected with a microprocessor which comprises an anesthetic initial mass operational module, an anesthetic residual mass operational module, an anesthetic residual volume operational module, a unit time anesthetic using speed operational module and a residual anesthetic using time operational module. The microprocessor is electrically connected with the display screen on the anesthesia machine. The invention further discloses a method for realizing the volatile anesthetic dosage monitoring system.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Method for managing experimental animal respiration
InactiveCN102743238AAddress respiratory management issuesReduced traumatic stress responseTracheal tubesEvaluation of blood vesselsIschemic hypoxiaPharmacology
The invention provides a method for managing experimental animal respiration, which utilizes a clinic anesthesia machine for the anesthetization of the animal experiment, performs tracheal cannula on an animal, adopts an animal temperate maintenance instrument to keep warm, and keeps the body temperature at the physical level (37 plus or minus 0.5 DEG C) all the time during the anesthesia process. The method is used for solving the animal respiration management problem during the traditional anesthesia process, the same inhalation anesthesia concentration and the stable body environment are also maintained during the whole anesthesia process. By the method, not only is the stress response of the experimental animal reduced, but also the vital sign stability of the experimental animal can be maintained during the inhalation anesthesia process of a long time (such as 4 hours), the mortality is low and the reviving can be stable, and the influences of trauma stress, hypoxia-ischemia, hypercapnia, acidosis and other interference factors on the experimental result can be eliminated. The method effectively controls the respiratory tract of the experimental animal, and also maintains the inhalation anesthesia of the volatile anesthetics with constant concentration, which is output to the animal.
Owner:温州医学院附属第二医院
Fluoropolymer-based emulsions for the intravenous delivery of fluorinated volatile anesthetics
InactiveUS9000048B2Maintenance can be inducedReduce interfacial tensionBiocideNervous disorderAnesthetic AgentEmulsion
The present invention provides therapeutic formulations, including therapeutic emulsions and nanoemulsions, and related methods for the delivery of fluorinated therapeutic compounds, including an important class of low boiling point perfluorinated and / or perhalogenated volatile anesthetics. Emulsion-based fluorinated volatile anesthetic formulations compatible with intravenous administration are provided that are capable of delivering and releasing amounts of fluorinated volatile anesthetic compounds effective for inducing and maintaining anesthesia in patients. Intravenous delivery of the present emulsion-based fluorinated volatile anesthetic formulations permits anesthetic levels in a patient to be selectively adjusted very rapidly and accurately without the need to hyperventilate patients and without the use of irritating agents.
Owner:WISCONSIN ALUMNI RES FOUND
Volatile anesthetic compositions and methods of use
ActiveUS20130273141A1Excellent characteristicsImprove propertiesBiocideNervous disorderEmulsionSolvent
The present invention provides methods for reducing pain in a subject in need thereof by delivering a volatile anesthetic in a solution or an emulsion that can additionally include an extractive solvent in an amount effective to reduce pain without substantially interfering with motor function. Chronic or acute pain may be treated, or the volatile anesthetic may be delivered as a regional anesthetic to a subject to anesthetize a portion of the subject prior to surgery. Dosing regimes including a one-time administration, continuous and / or periodic administration are contemplated.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
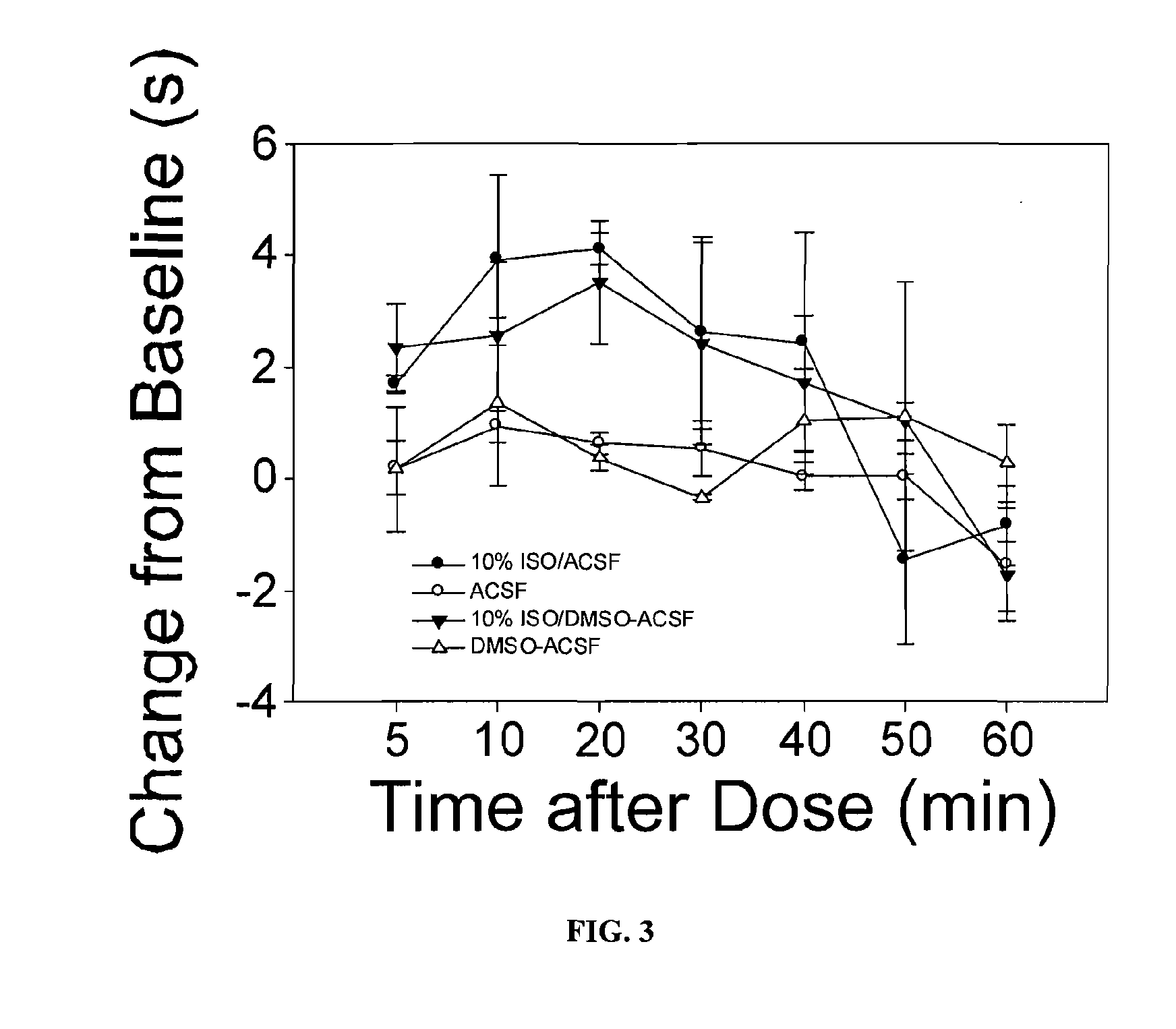
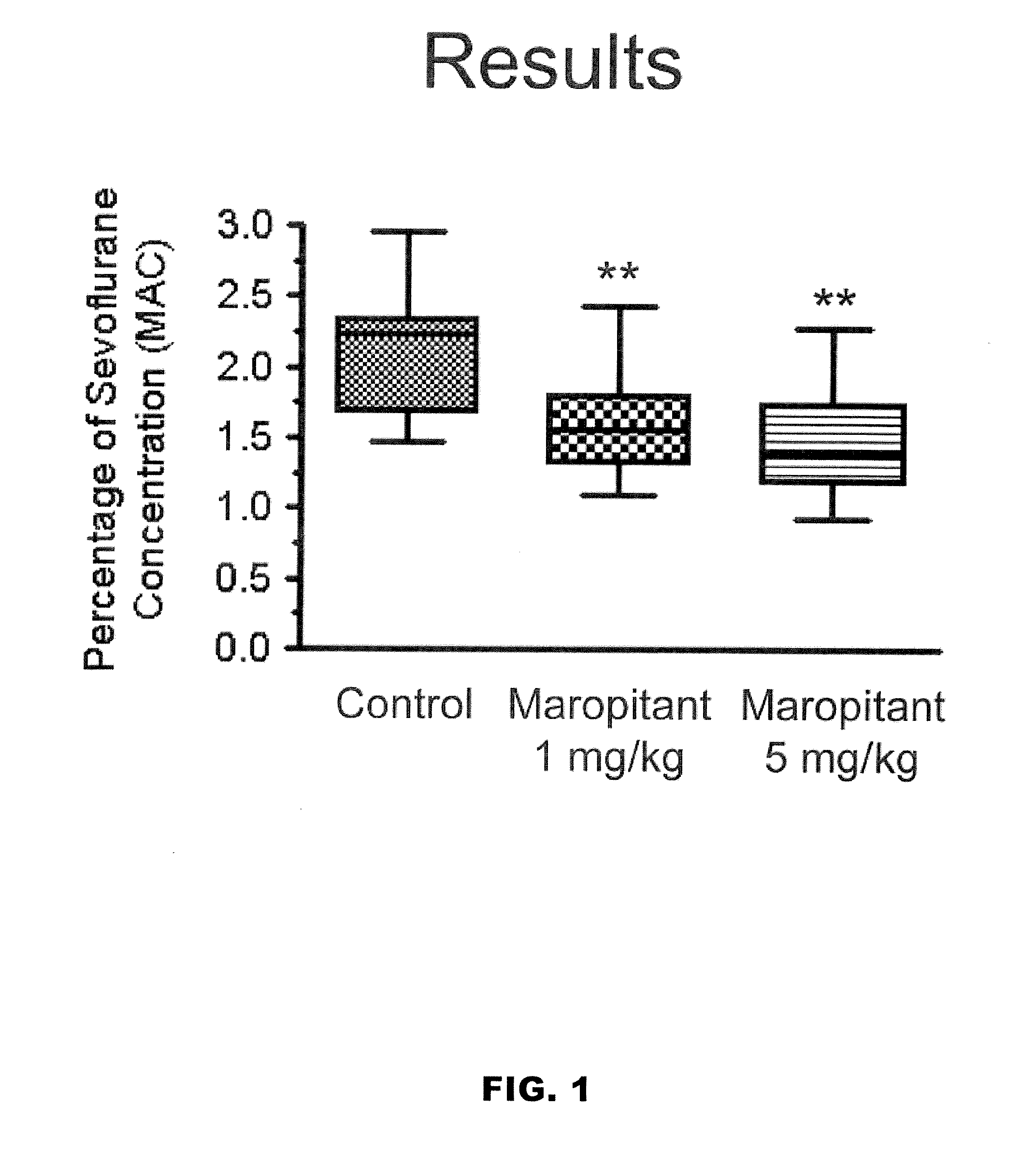
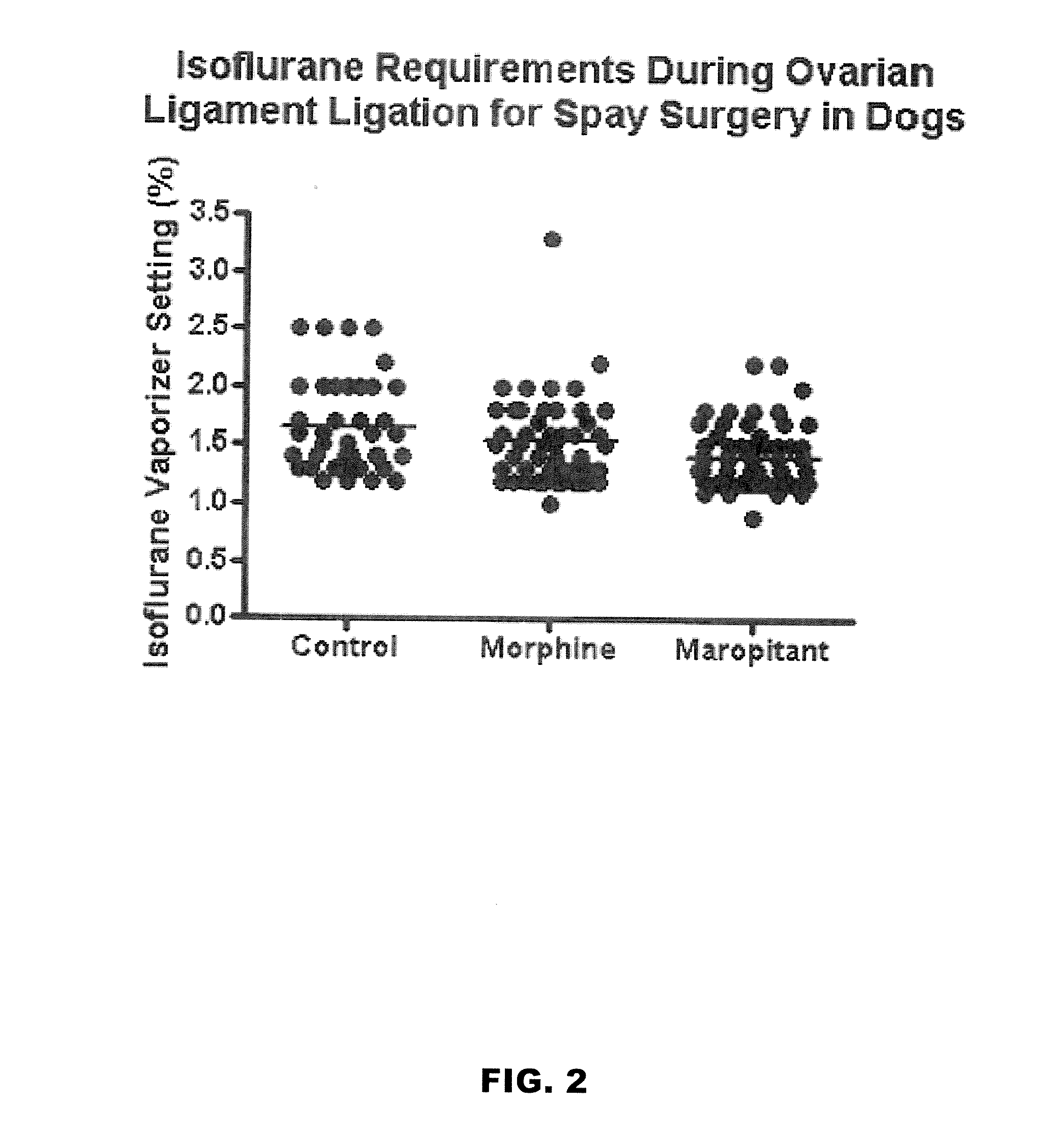
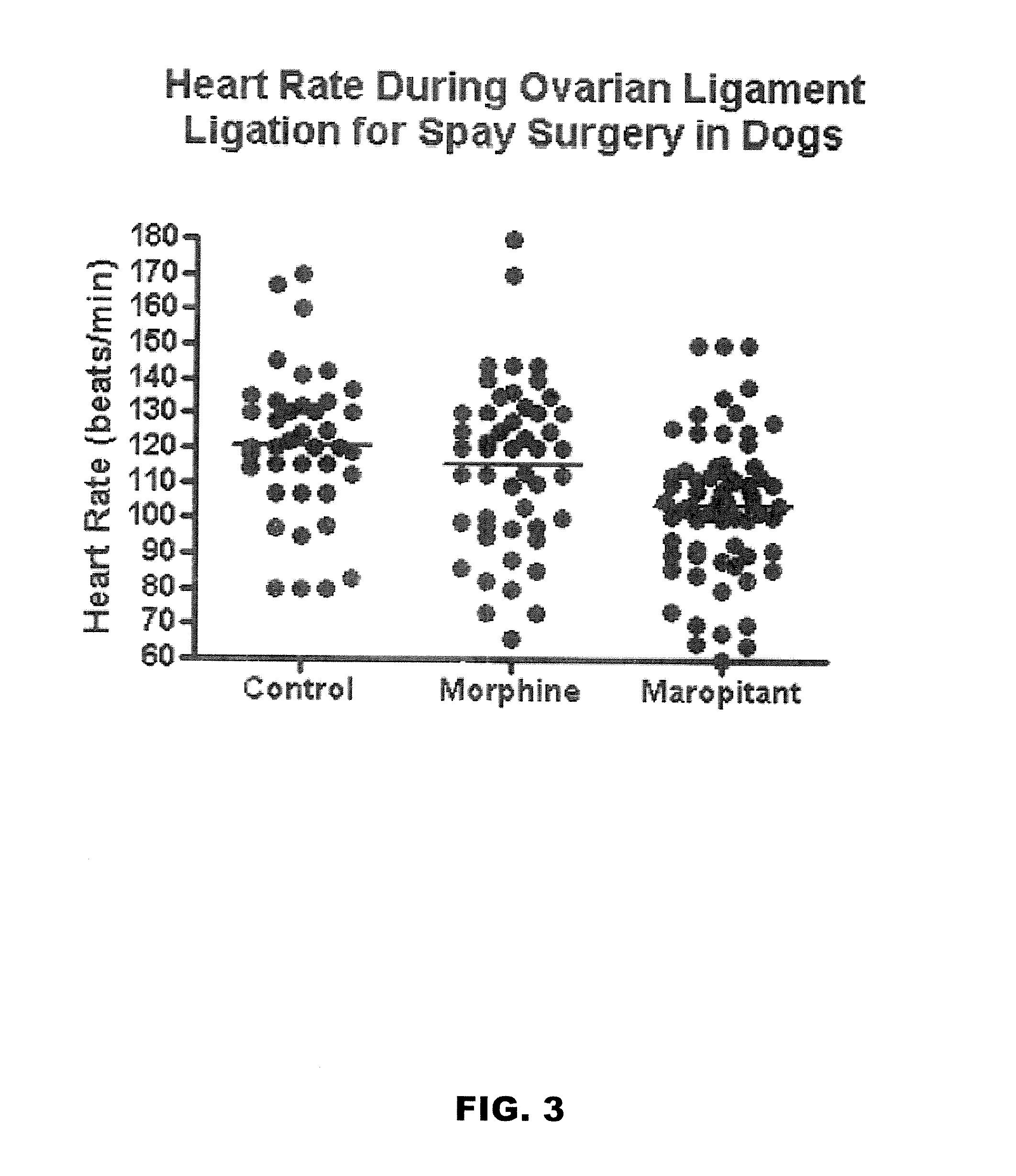
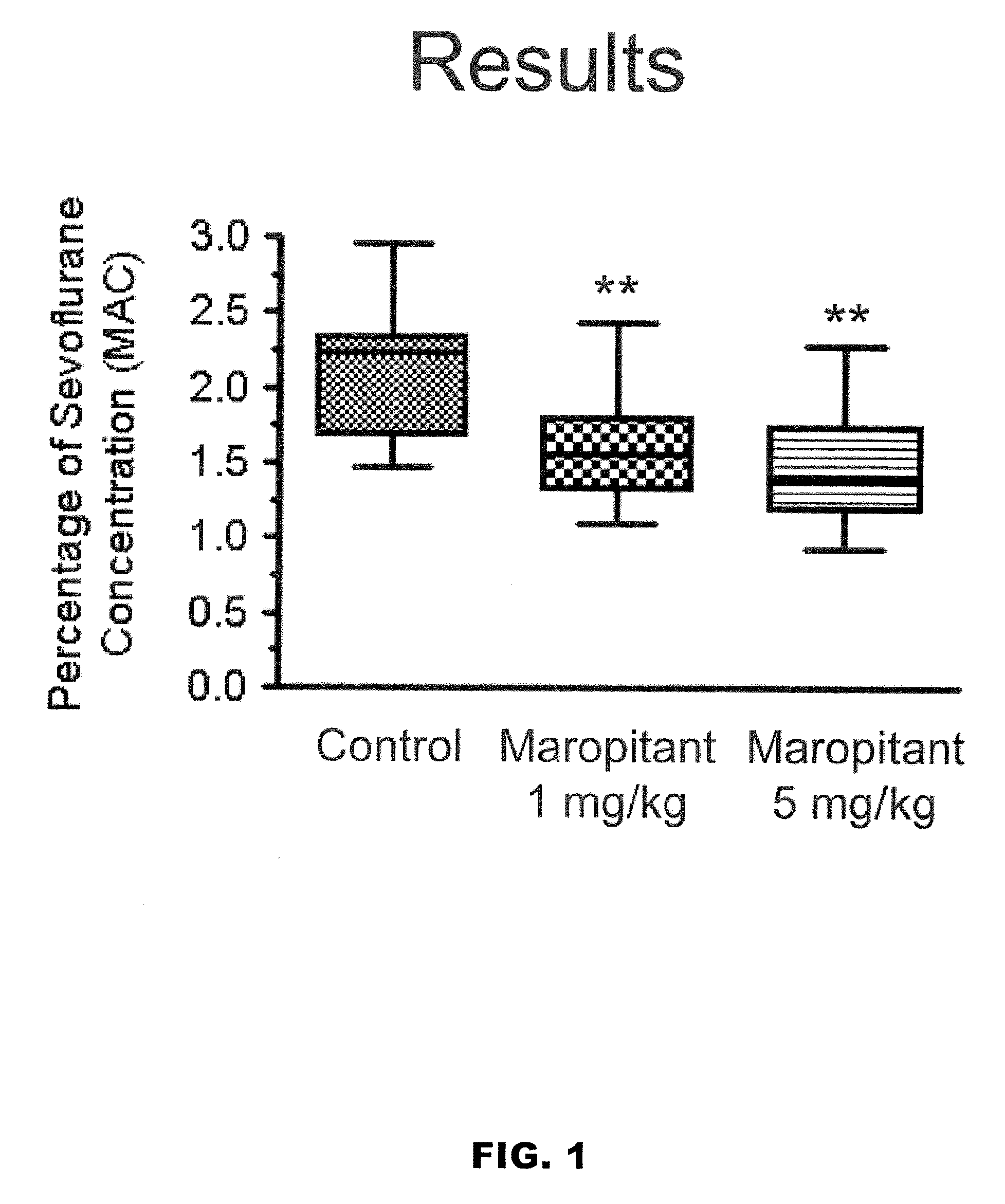
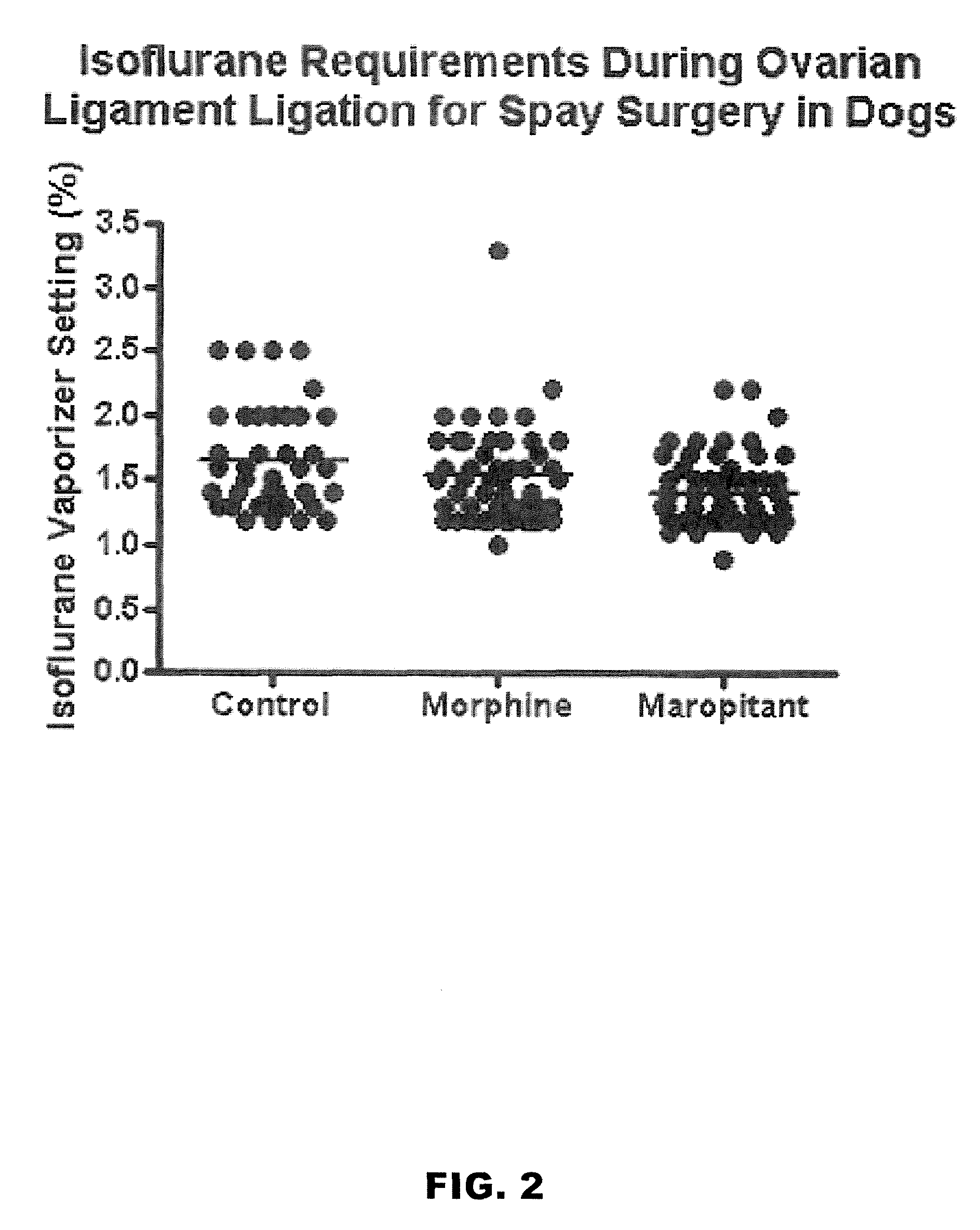
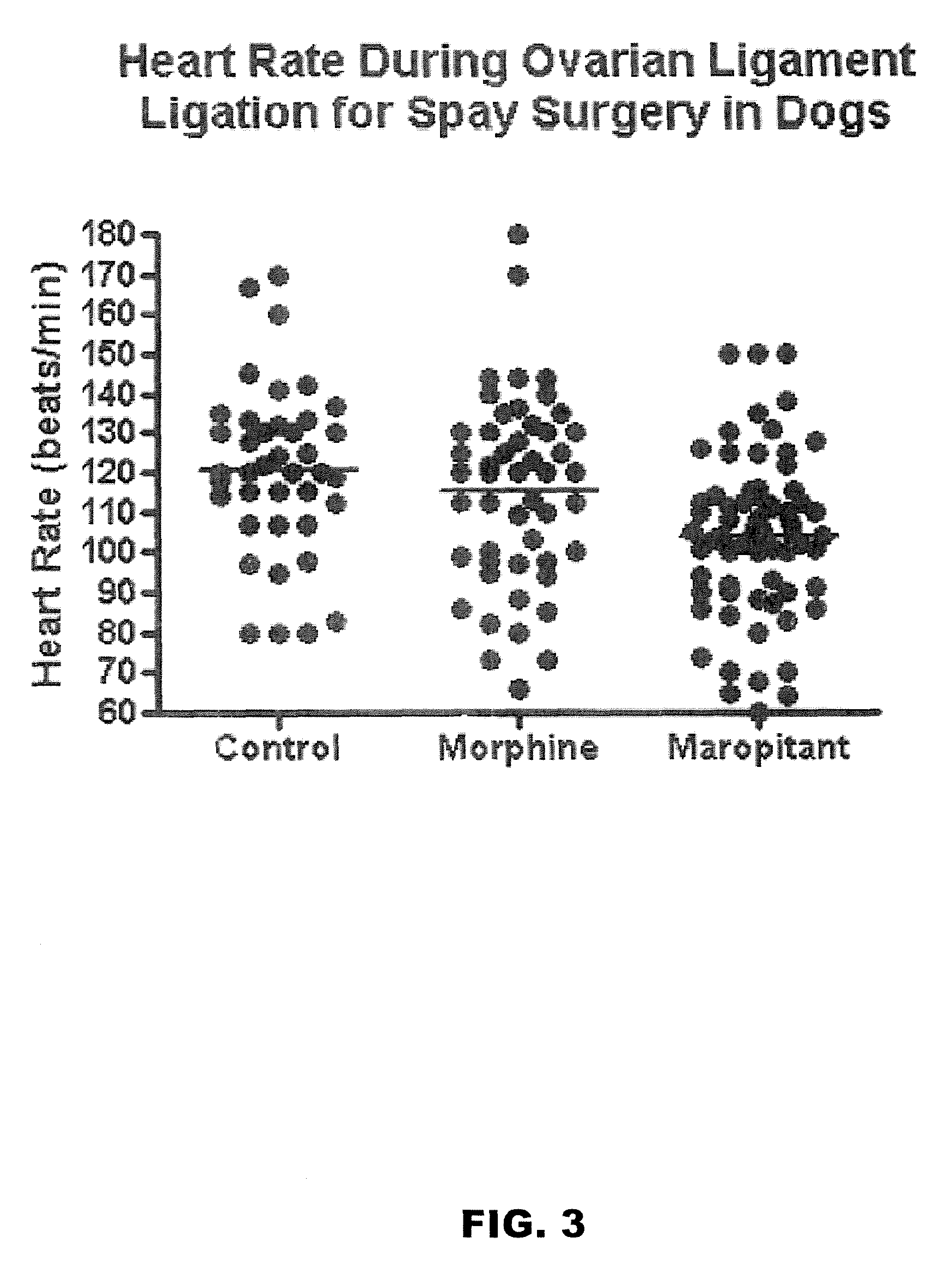
Use of nk-1 receptor antagonists in management of visceral pain
Methods for managing visceral pain in mammalian subjects are described, in which a NK-1 receptor antagonist is administered to the subject before, during or after administration of general anesthesia. The methods and uses of NK-1 receptor antagonists described herein provide improved visceral pain management and MAC reduction when used with volatile anesthetics for general anesthesia.
Owner:COLORADO STATE UNIVERSITY
Methods for delivering volatile anesthetics for regional anesthesia and/or pain relief
ActiveUS20110269843A1Rapidly titratableRapid onsetHalogenated hydrocarbon active ingredientsBiocideRegimenMethoxyflurane
The present invention provides methods for reducing pain in a subject in need of such pain reduction by delivering, e.g., intrathecally or epidurally, a volatile anesthetic such as a halogenated ether compound in an amount effective to reduce pain. Chronic or acute pain may be treated, or the anesthetic may be delivered to the subject to anesthetize the subject prior to a surgery. In certain embodiments, isoflurane, halothane, enflurane, sevoflurane, desflurane, methoxyflurane, xenon, and mixtures thereof may be used. Dosing regimens including a one-time administration, continuous and / or periodic administration are contemplated.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
System, Method and Apparatus for Removal of Volatile Anesthetics for Malignant Hyperthermia
ActiveUS20120325213A1Minimize impactEfficient removalRespiratorsBreathing filtersMalignant hyperthermiaExpiratory limb
Systems, methods, and apparatus for removing volatile anesthetics from an anesthesia or ventilation system to minimize the effects of malignant hyperthermia in susceptible patients. According to one aspect of the present invention, a system for removing volatile anesthetics is provided. A first filter component placed in fluid communication with an inspiratory limb of an anesthesia or ventilation system such that volatile anesthetics will pass through the first filter component during operation of the anesthesia or ventilation system. A second filter component is operably coupled to the expiration port of the anesthesia or ventilation system such that gases passing through the expiratory limb of the anesthesia or ventilation system pass through the second filter component. The first filter component and second filter component are adapted to effectively remove volatile anesthetics passing through the respective filters.
Owner:ORR JOSEPH +2
Passive portable anesthesia machine
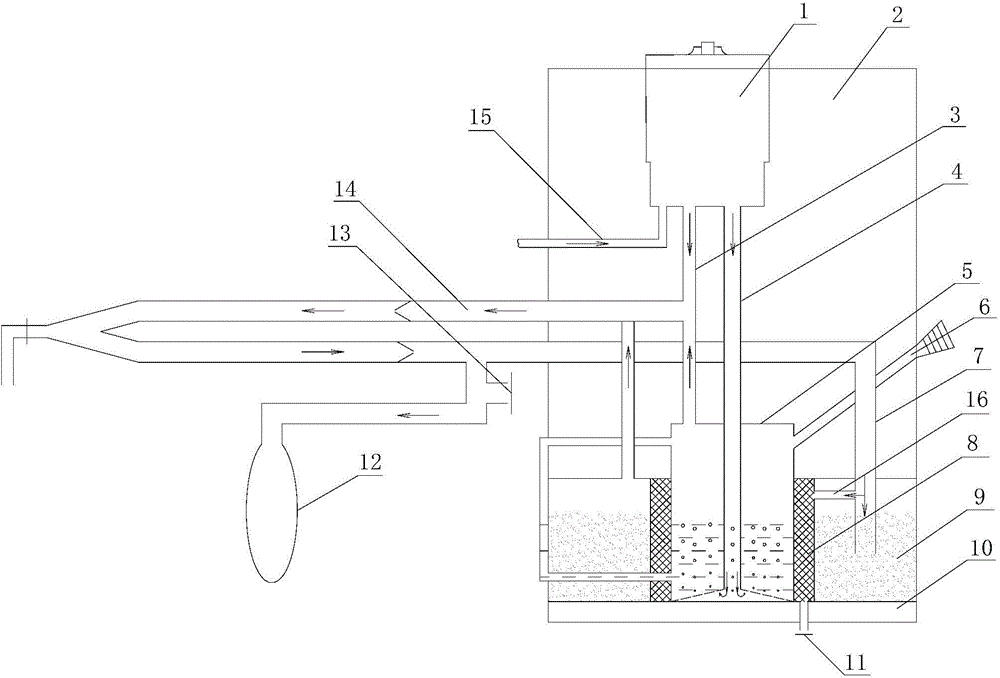
The invention relates to a passive portable anesthesia machine. The passive portable anesthesia machine comprises a box, a breathing pipeline, an exhaling loop and an oxygen pipe, and is characterized in that an anesthetic gas concentration regulating and controlling device is arranged on the upper part inside the box; an anesthetic waste gas absorbing device is mounted at the bottom inside the box; the anesthetic waste gas absorbing device comprises an anesthetic storage tank, a waste gas absorbing particle storage tank A and a waste gas absorbing particle storage tank B; a waste gas pressure reducing valve A is connected with the bottom of the waste gas absorbing particle storage tank A; a primary anesthetic exhausting pipe and a secondary anesthetic exhausting pipe are connected with the bottom of the anesthetic gas concentration regulating and controlling device; one end of the exhaling loop extends out of the box, and the other end of the exhaling loop is communicated with the waste gas absorbing particle storage tank B; the oxygen pipe is communicated with the anesthetic gas concentration regulating and controlling device; the breathing pipeline is communicated with the secondary anesthetic exhausting pipe. The passive portable anesthesia machine has the advantages of being light in weight, wide in application range, free of an external power supply, capable of maintaining the concentration of a volatile anesthetic constant, and capable of maintaining the temperature of the anesthetic constant by heat generated during reaction of water and carbon dioxide in gas exhaled by a patient with the waste gas absorbing device.
Owner:TIANJIN PLASTICS RES INST CO LTD
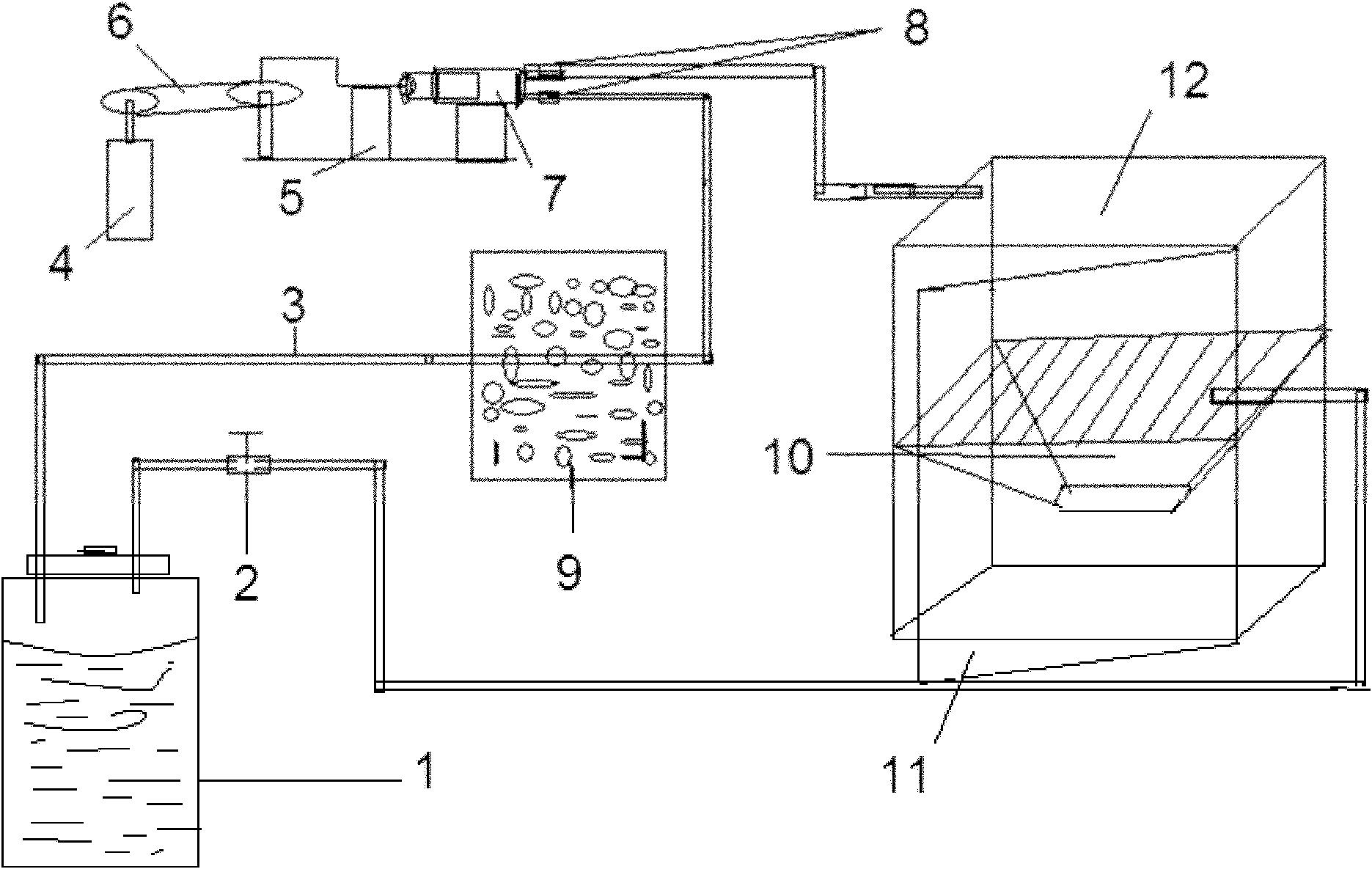
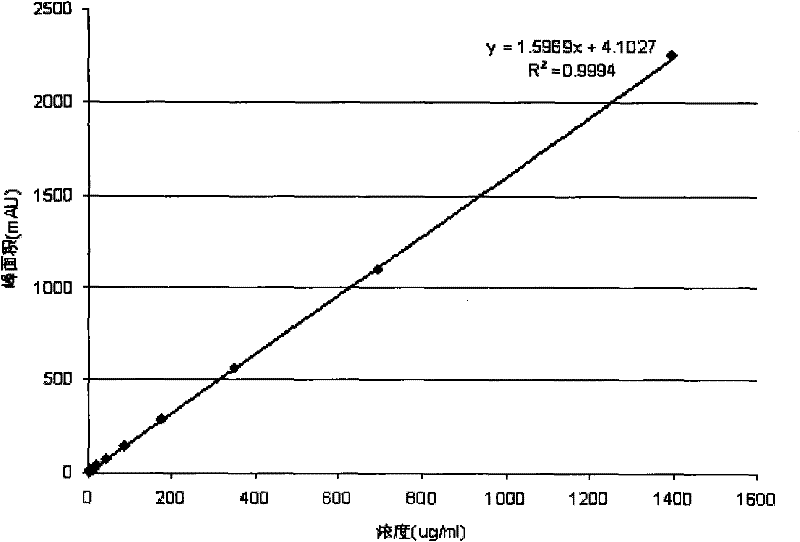
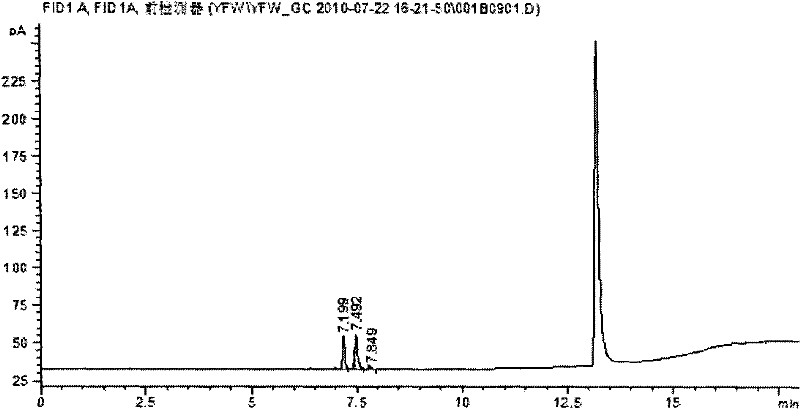
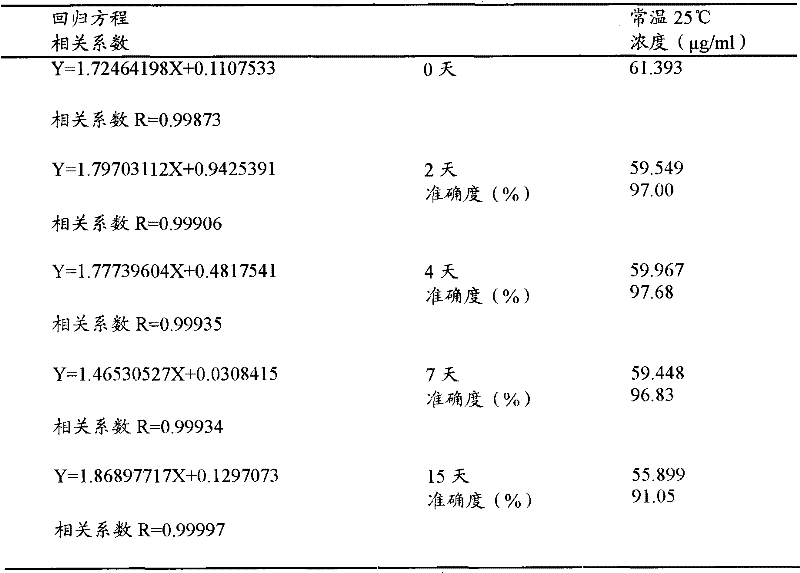
Method for measuring concentration of volatile anesthetics in samples by chromatography
InactiveCN102175780AConcentration determination is simpleRapid concentration determinationComponent separationTissue fluidBulk samples
The invention discloses a method for measuring concentration of volatile anesthetics in samples by automatic headspace gas chromatography. The method is suitable for measuring the concentrations of volatile anesthetics like methoxyflurane, enflurane, isoflurane, sevoflurane and desflurane in samples. The samples can be solutions containing the substances to be measured, as well as can be in vitro biological samples containing the substances to be measured, such as blood, urine, tissue fluid. The method is simple, fast and exact, suitable for measuring and analyzing laboratory samples, and suitable for result measurement of a plurality of samples in clinical monitoring.
Owner:YICHANG HUMANWELL PHARMA
Improvements to the manufacture and remanufacture of volatile anaesthetic agents using supercritical fluids
PendingCN108697968AReduce manufacturing costSimple reaction conditionsRespiratorsGas treatmentHalocarbonBiomedical engineering
An anaesthetic halocarbon capture system is provided. The system comprises a pressure-intolerant sleeve containing filter material for capturing one or more types of anaesthetic halocarbon prior to supercritical fluid extraction, and a pressure-tolerant housing into which the sleeve can be inserted so as to permit exposure of the sleeve contents to pressures required for supercritical fluid extraction.
Owner:SAGETECH MEDICAL EQUIP
Electric continuous flow device of circulation loop
ActiveCN102500027BSolve the problem of low head pressureIncrease kinetic energyRespiratorsControl signalCo2 absorption
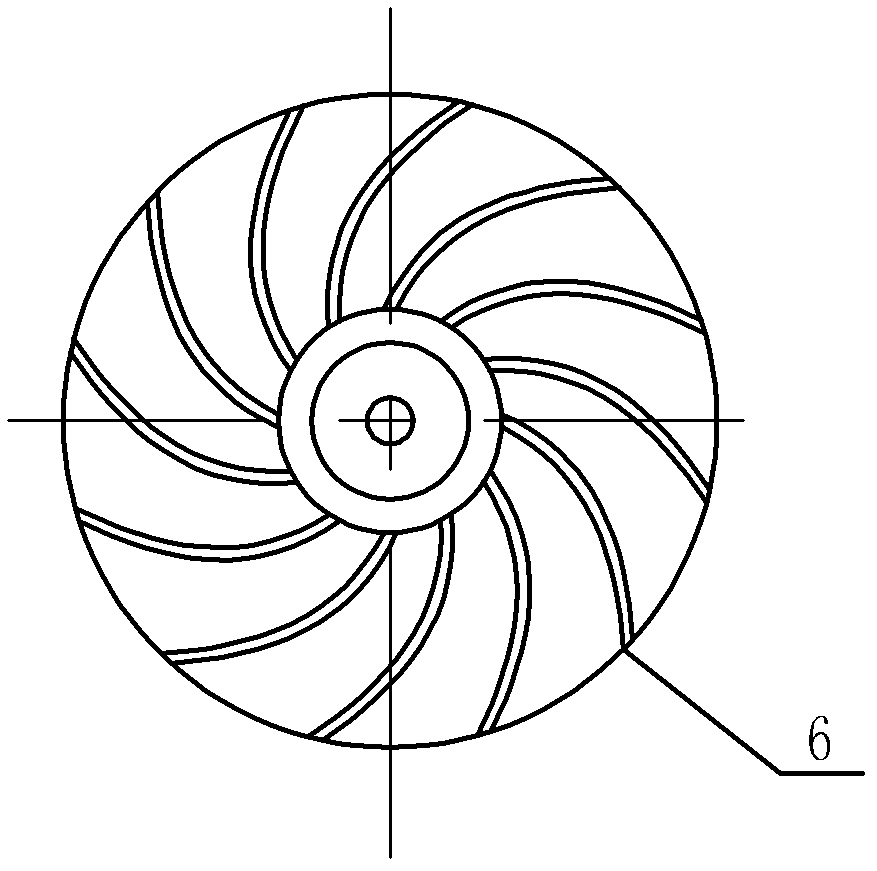
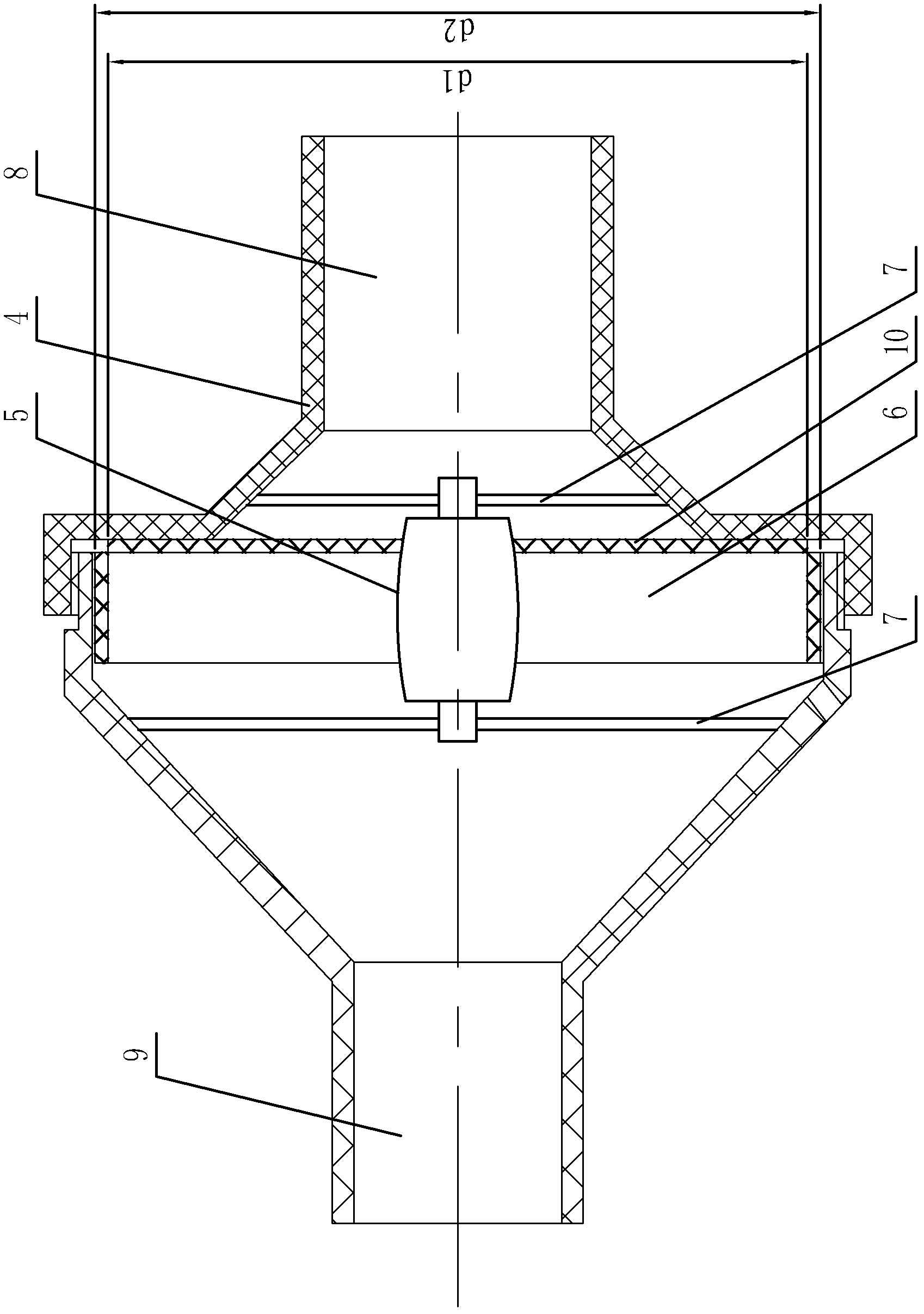
An electric continuous flow device of a circulation circuit relates to a continuous flow device of a circulation loop, and solves the problems that a semi-open anesthetic loop for pediatric anesthesia causes low respiratory efficiency, the unutilized oxygen and volatile anesthetics are discharged into the atmosphere, medical resources are wasted, the environment is polluted, and a pediatric anesthetic close circulation loop has large resistance and high respiratory power consumption. A disc flow deflector is fixedly installed on a fan blade and is positioned at one side of an air inlet side, the external diameter of the disc flow deflector is d1, the external diameter of the fan blade is d2, and di equals to d2 minus 4mm; and the control signal output end of a touch screen is connected with the control signal input end of an external rotor electric machine, the signal output end of the external rotor electric machine is connected with the signal input end of the touch screen, the loop inspiration port of a respiration loop is communicated with the inspiration end of a disposable anesthetic loop through the external rotor electric machine, and the loop expiration port of the disposable anesthetic loop is communicated with a respiration loop through a dioxide carbon absorption unit. The electric continuous flow device is used in the anesthetic loop systems for children and adults.
Owner:李恩有 +3
Use of formulations containing halogenated volatile anesthetics for the preparation of formulations for the treatment of patients whose tissues have ischemic events by parenteral administration
InactiveCN102283821AHalogenated hydrocarbon active ingredientsSenses disorderNeurophysinsCardiac muscle
Provided is a method of treating a patient having a tissue that is subject to an ischemic event. The method is conducted by parenterally administering a formulation containing a halogenated volatile anesthetic in an amount effective to improve the tissue's resistance to or tolerance of the ischemic event. In preferred embodiment of the invention, the amount of the formulation administered to the patient is sub-anesthetic. The formulation can be administered prior to, concurrently with, or after the ischemic event. The method can be used, for example, for treatment of patients having myocardial or neuronal tissue that is subject to an ischemic event.
Owner:BAXTER INT INC
Novel method for transdermal measurement of volatile anesthetics
ActiveUS20180325429A1High-precision detectionHighly accurateCell electrodesSurgeryHuman skinPerspiration
Devices and methods of making and using the device for the non-invasive detection of volatile anesthetics are provided. The devices are capable of measuring the concentration of volatile anesthetics transdermally and in a non-invasive manner. The devices and methods can be applied in detection of volatile anesthetics in samples collected from human skin perspiration.
Owner:FLORIDA INTERNATIONAL UNIVERSITY +1
Method for the preparation of volatile Anesthetics
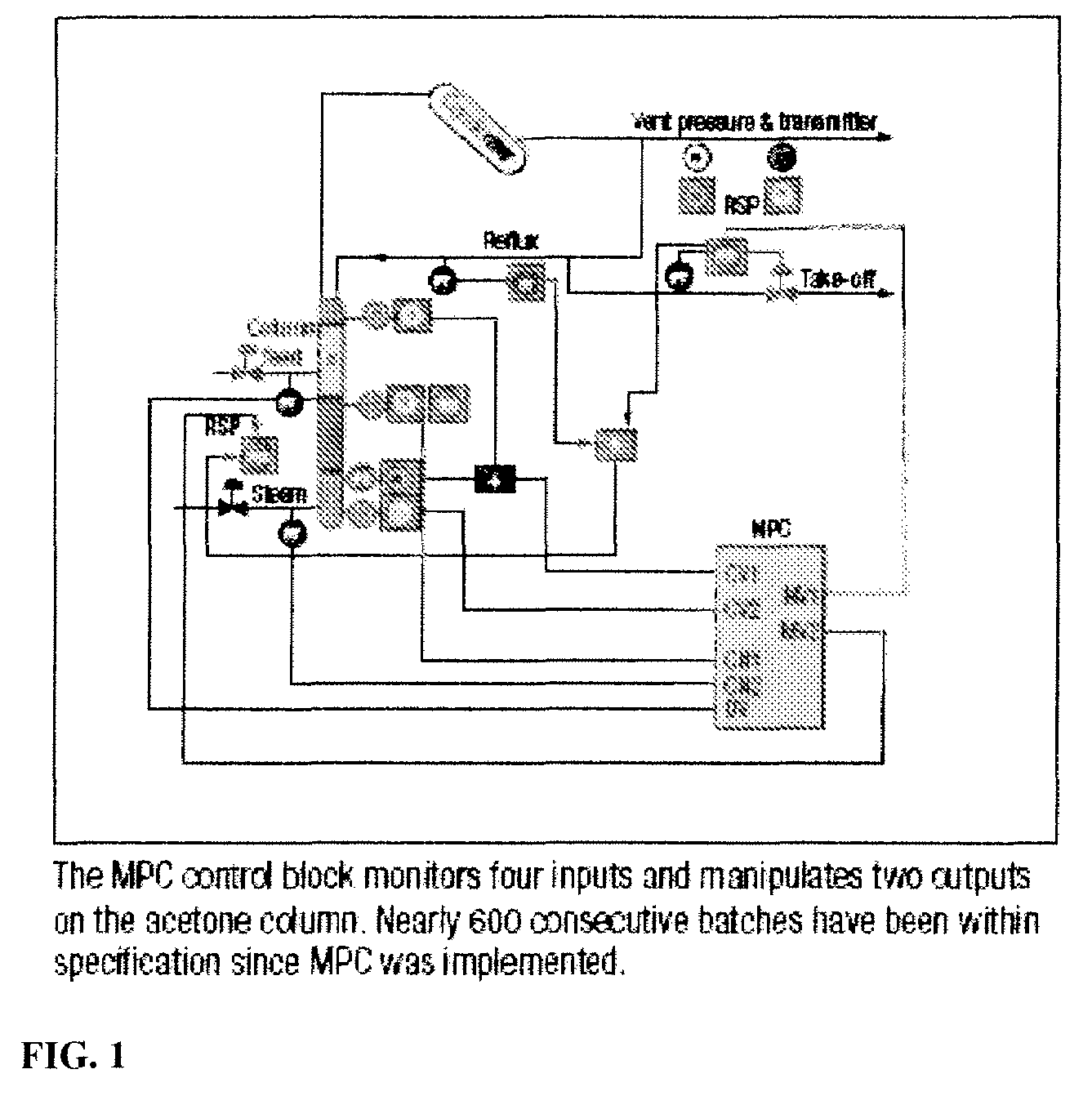
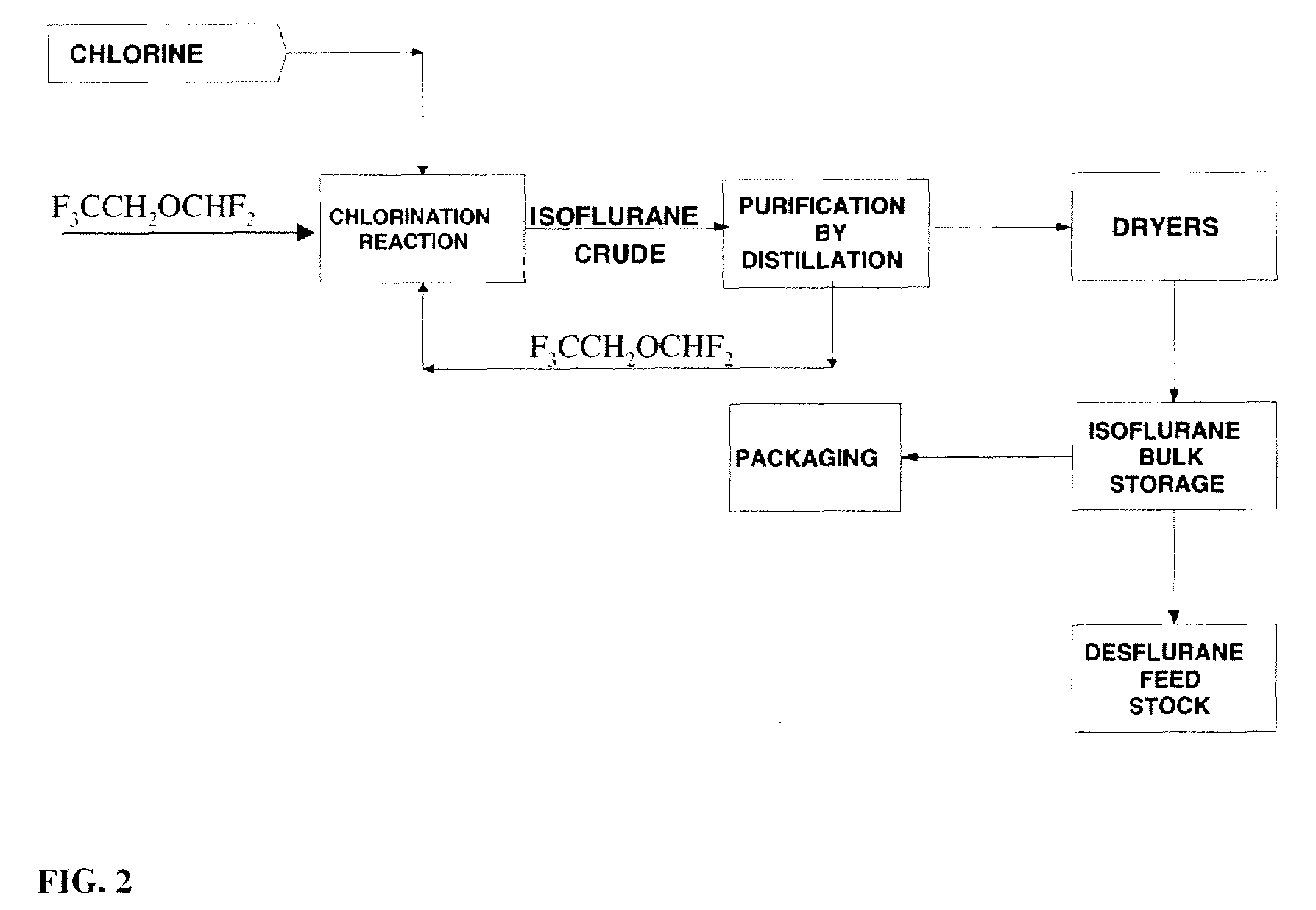
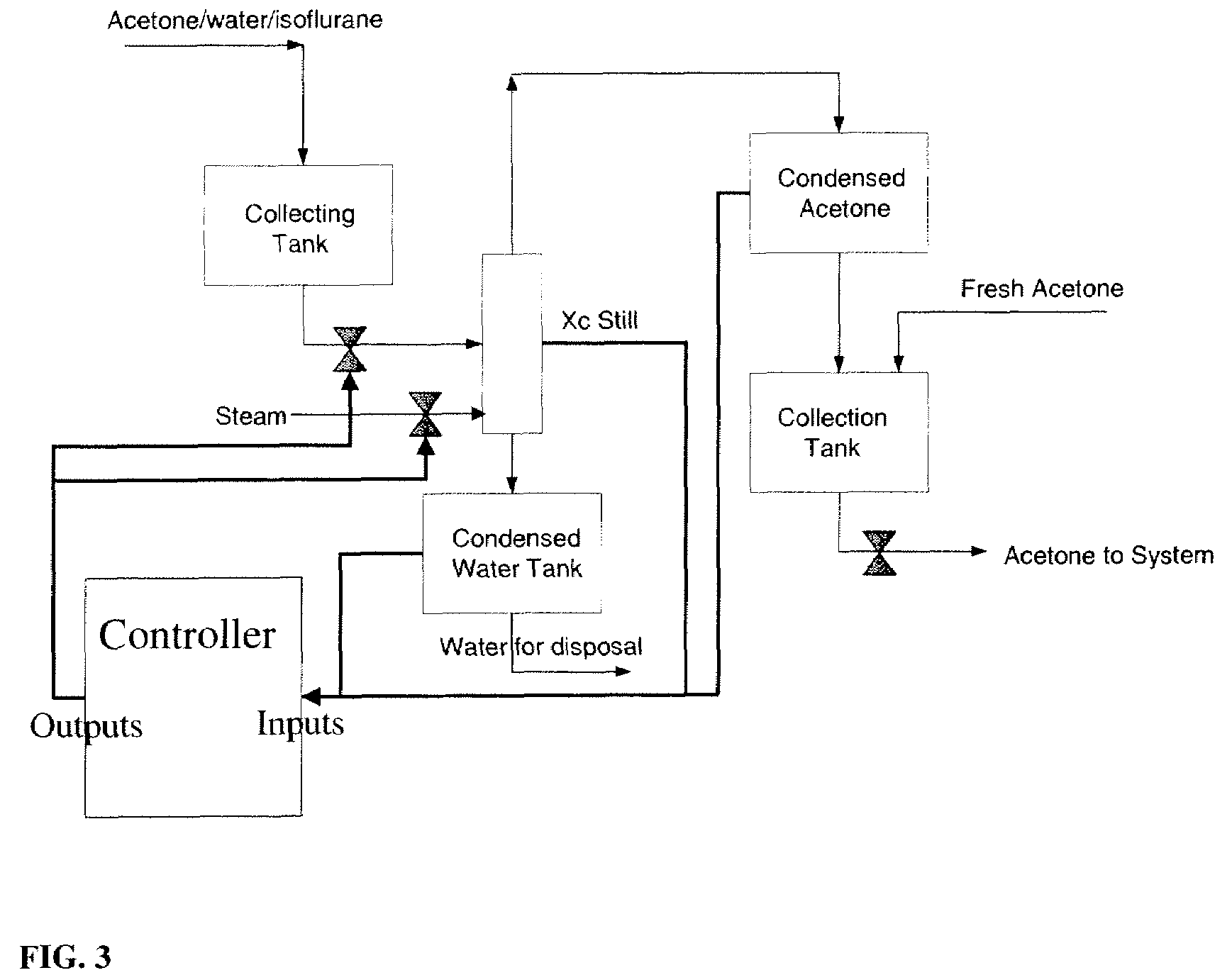
ActiveUS7605291B2Improve distillation efficiencyOrganic compound preparationCarbonyl compound separation/purificationAutomatic controlVolatile anesthetic
The present invention is concerned with the automated control of the synthesis and purification of components useful in the synthesis and purification of volatile anesthetics. The invention is particularly useful in its application to the purification of acetone used in the purification of isoflurane.
Owner:BAXTER INT INC +1
Electric continuous flow device of circulation loop
ActiveCN102500027ASolve the problem of low head pressureIncrease kinetic energyRespiratorsSemi openControl signal
An electric continuous flow device of a circulation circuit relates to a continuous flow device of a circulation loop, and solves the problems that a semi-open anesthetic loop for pediatric anesthesia causes low respiratory efficiency, the unutilized oxygen and volatile anesthetics are discharged into the atmosphere, medical resources are wasted, the environment is polluted, and a pediatric anesthetic close circulation loop has large resistance and high respiratory power consumption. A disc flow deflector is fixedly installed on a fan blade and is positioned at one side of an air inlet side, the external diameter of the disc flow deflector is d1, the external diameter of the fan blade is d2, and di equals to d2 minus 4mm; and the control signal output end of a touch screen is connected with the control signal input end of an external rotor electric machine, the signal output end of the external rotor electric machine is connected with the signal input end of the touch screen, the loop inspiration port of a respiration loop is communicated with the inspiration end of a disposable anesthetic loop through the external rotor electric machine, and the loop expiration port of the disposable anesthetic loop is communicated with a respiration loop through a dioxide carbon absorption unit. The electric continuous flow device is used in the anesthetic loop systems for children and adults.
Owner:李恩有 +3
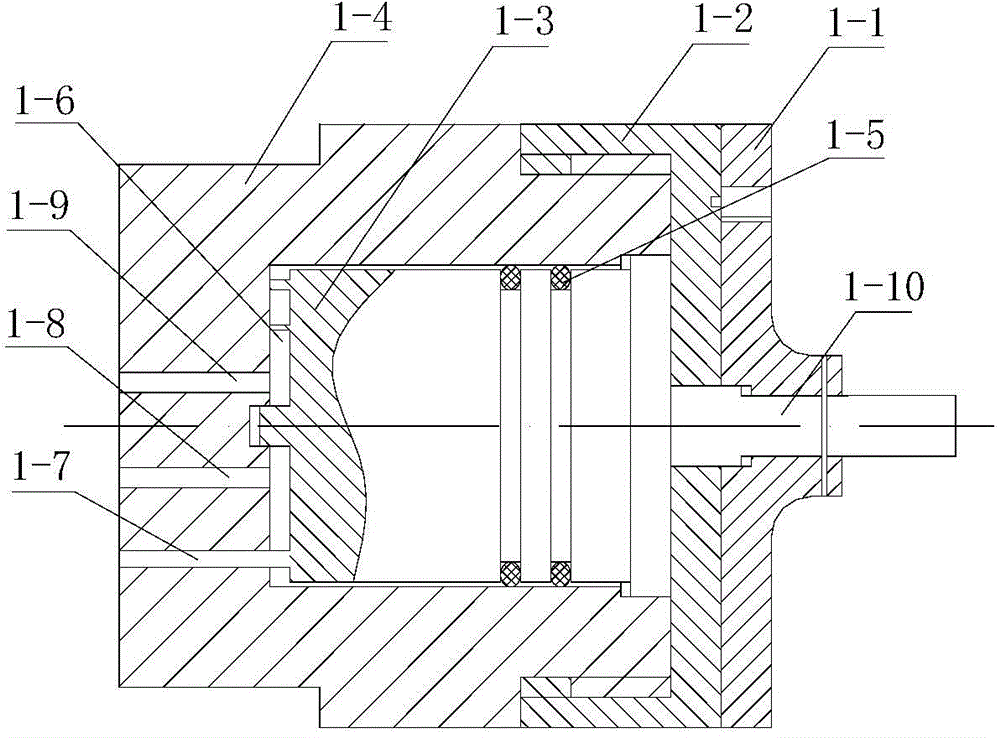
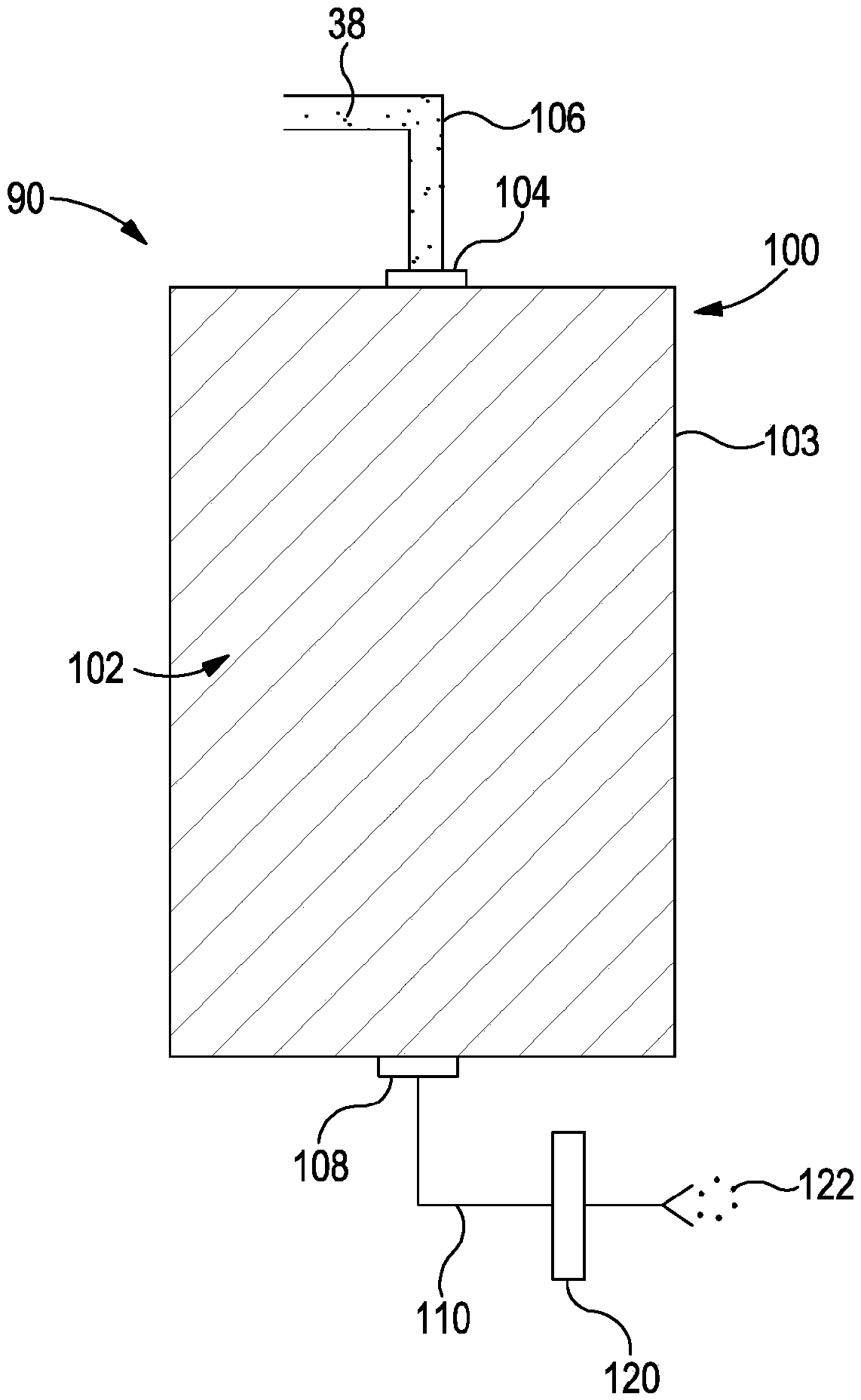
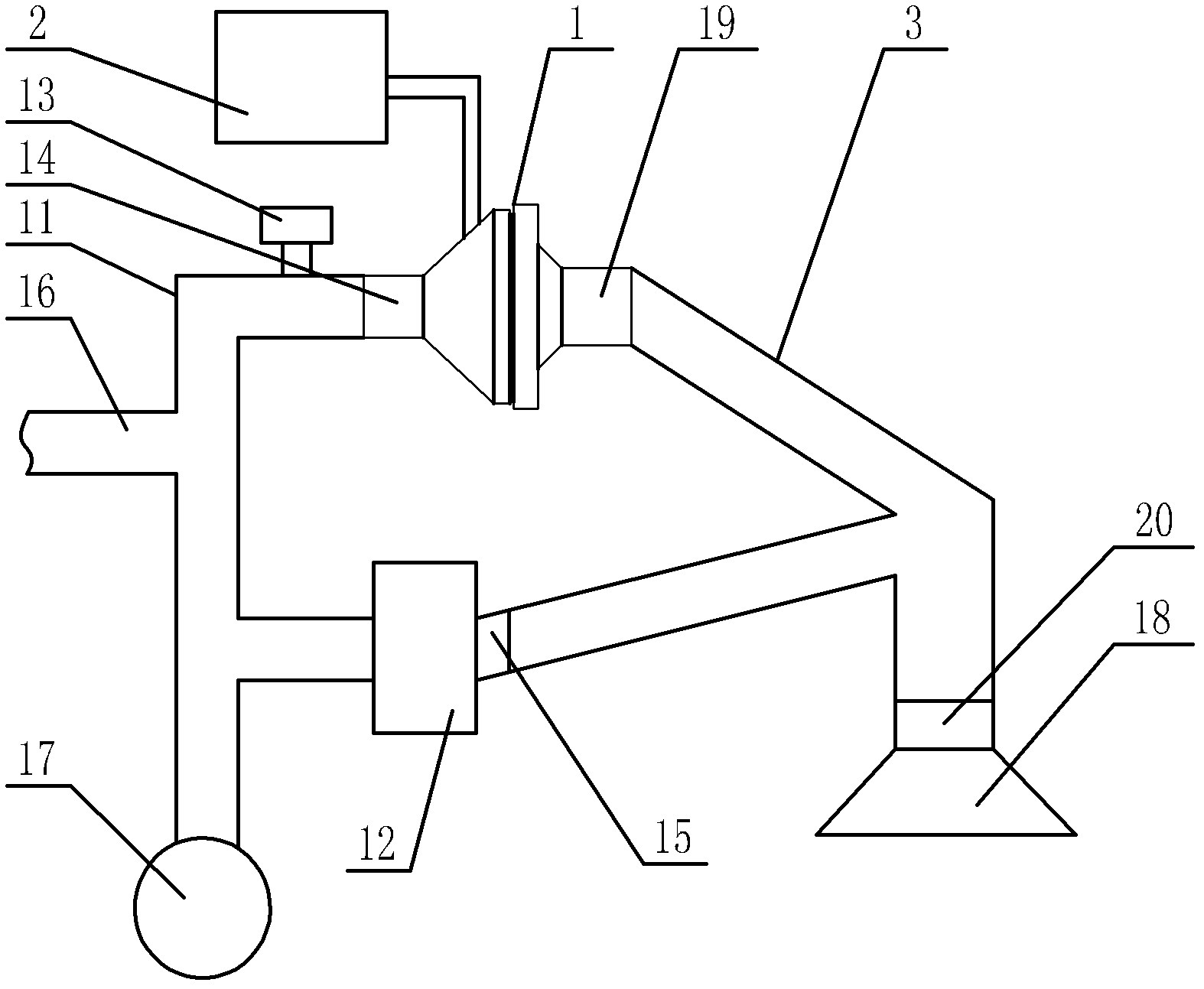
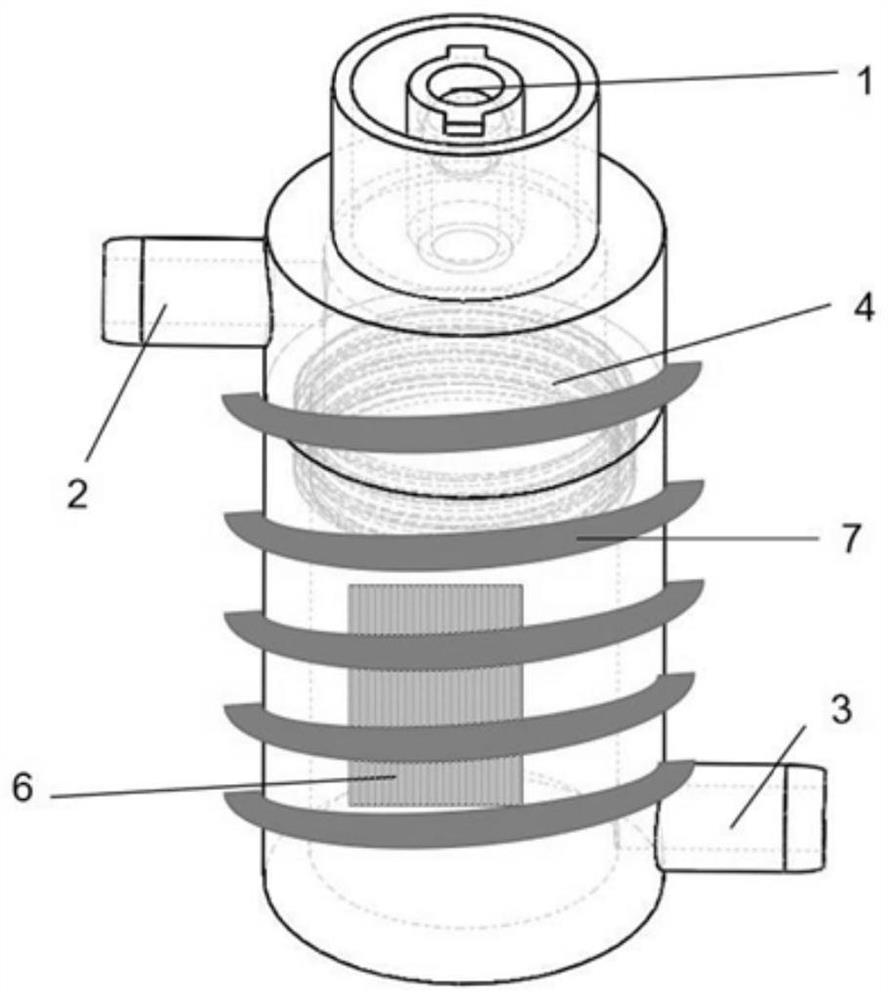
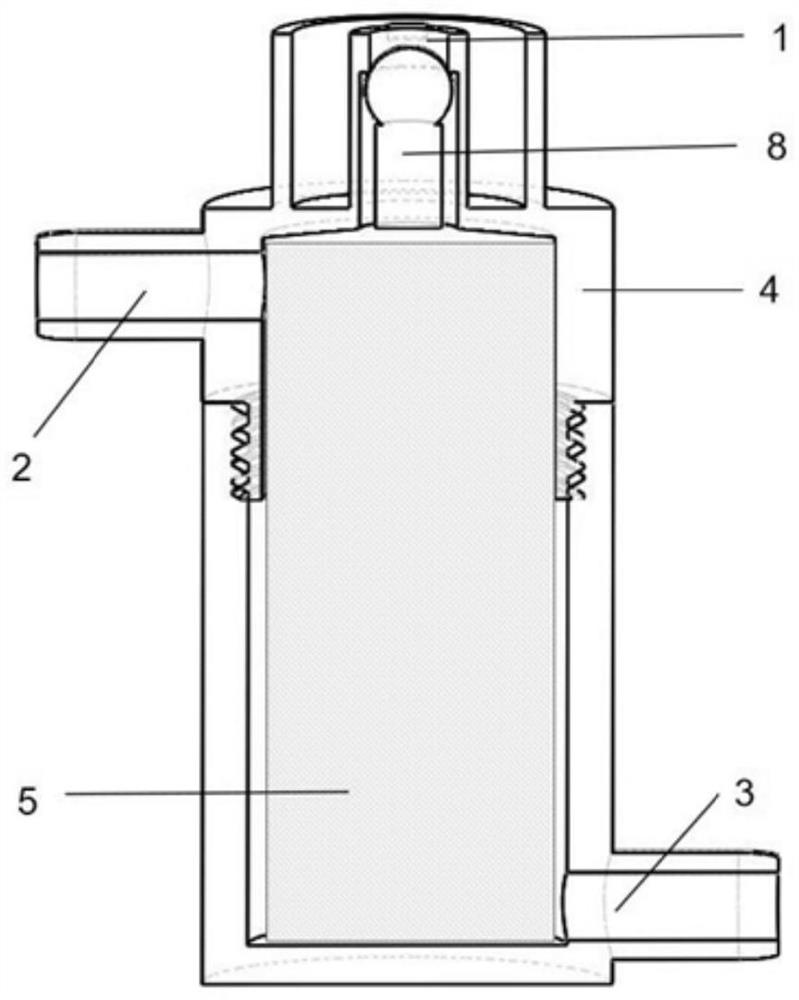
Medicine volatilization tank for anaesthesia machine
The invention belongs to the technical field of medical devices, and particularly relates to a medicine volatilization tank for an anaesthesia machine, the medicine volatilization tank comprises a volatilization cavity and absorbent cotton arranged in the volatilization cavity, the volatilization cavity is provided with an anesthetic inlet and two air vents, and anesthetic is injected into the absorbent cotton through the anesthetic inlet to be volatilized; the two air vents are respectively used as a gas inlet and an anesthetic mixed gas outlet, and gas introduced from one air vent is mixed with a volatile anesthetic and is discharged from the other air vent; the anesthetic is injected into the absorbent cotton for volatilization, so that waste of the anesthetic can be avoided, and stable anesthetic concentration can be guaranteed.
Owner:SHANDONG UNIV
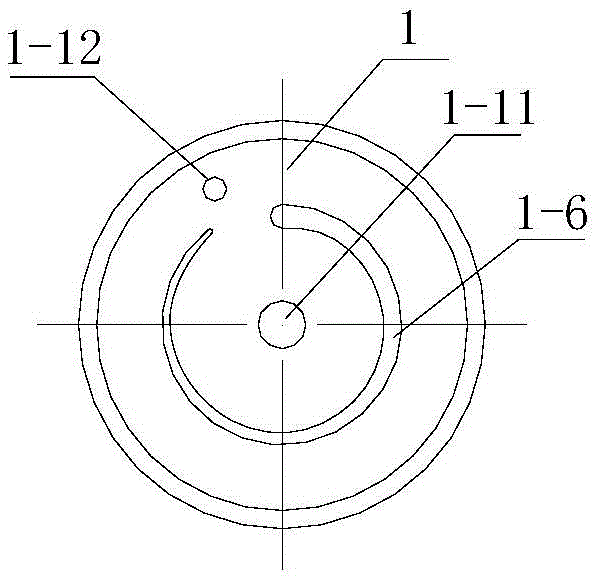
Preparation method and composition of fat emulsion for volatile anesthetic injection
PendingCN114099433AStable traitsRealize initial separate emulsificationInorganic non-active ingredientsAnaestheticsAnesthesiaOrganic chemistry
The invention discloses a preparation method of a fat emulsion composition for volatile anesthetic injection, which comprises the following steps: distributing emulsifiers into a first emulsifier and a second emulsifier according to a weight ratio of 1: 10-10: 1, and forming blank emulsion by the first emulsifier and oil for injection, the blank emulsion and the second part of emulsifier participate in forming a pre-emulsion, and the pre-emulsion and the volatile anesthetic participate in forming the fat emulsion composition for volatile anesthetic injection. In addition, the invention also provides an improved fat emulsion composition for volatile anesthetic injection, and the fat emulsion composition can not generate a bottle flushing phenomenon during high-temperature sterilization, and can be stored at room temperature for a long time.
Owner:YICHANG HUMANWELL PHARMA
Use of NK-1 receptor antagonists in management of visceral pain
Methods for managing visceral pain in mammalian subjects are described, in which a NK-1 receptor antagonist is administered to the subject before, during or after administration of general anesthesia. The methods and uses of NK-1 receptor antagonists described herein provide improved visceral pain management and MAC reduction when used with volatile anesthetics for general anesthesia.
Owner:COLORADO STATE UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com