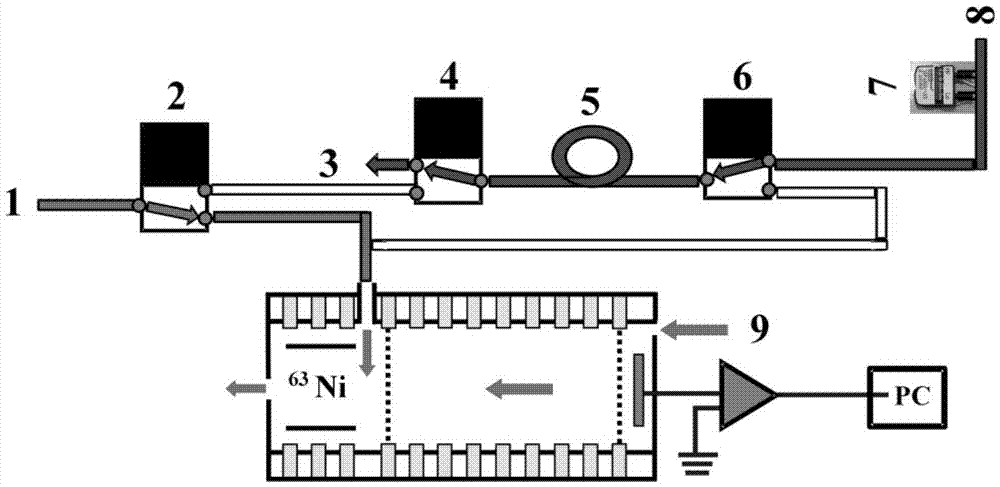
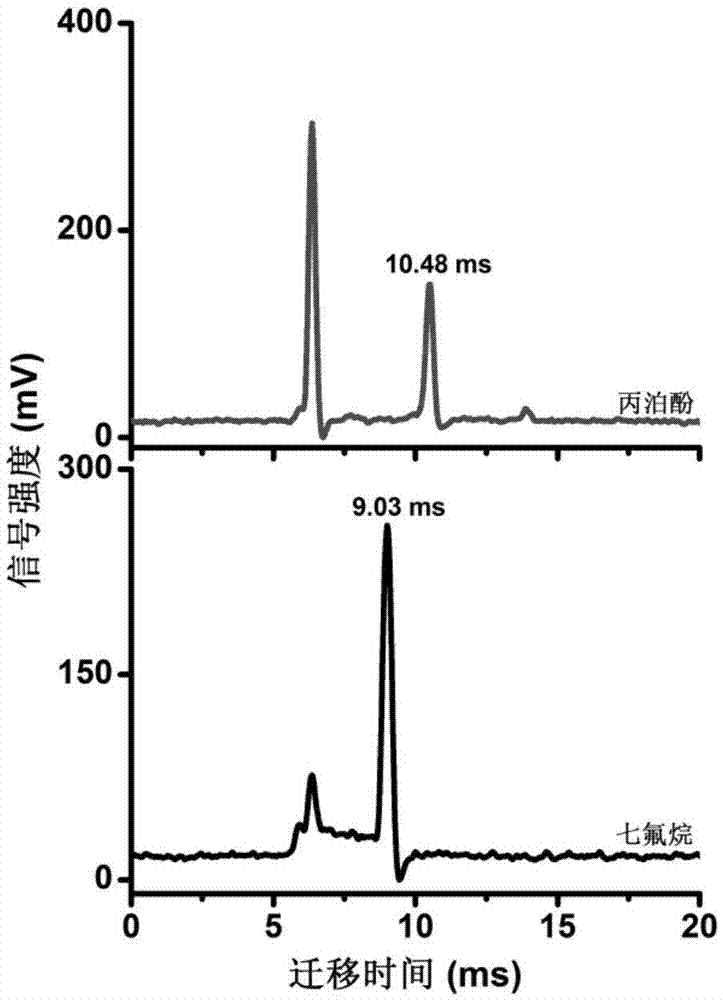
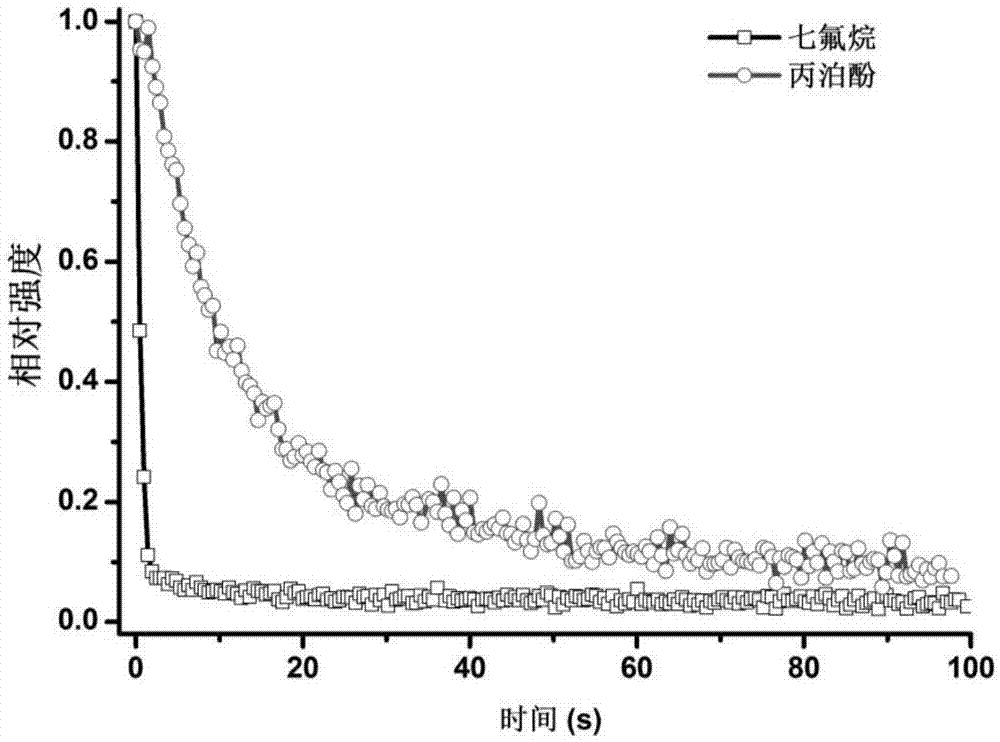
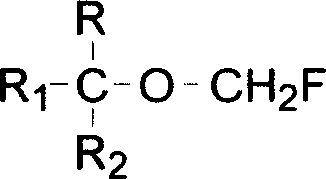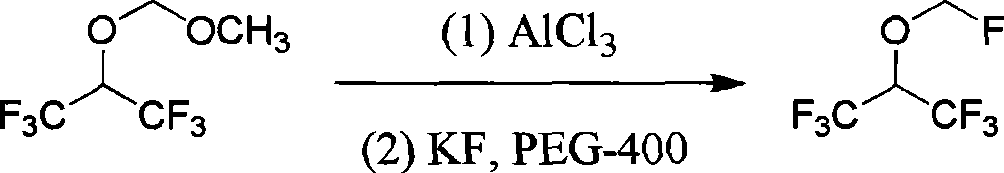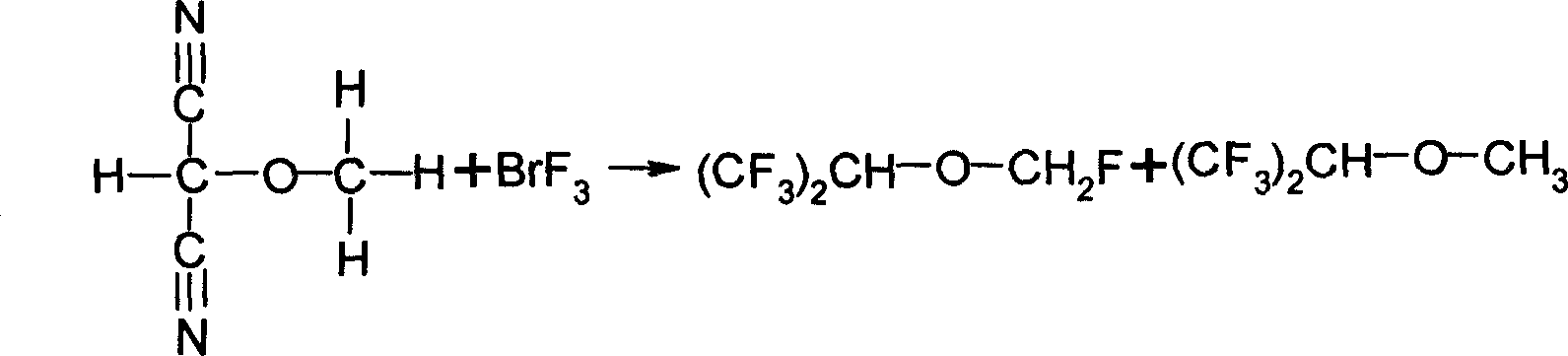Patents
Literature
96 results about "Sevoflurane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sevoflurane is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used as an inhalational anaesthetic for induction and maintenance of general anesthesia. After desflurane, it is the volatile anesthetic with the fastest onset and offset.
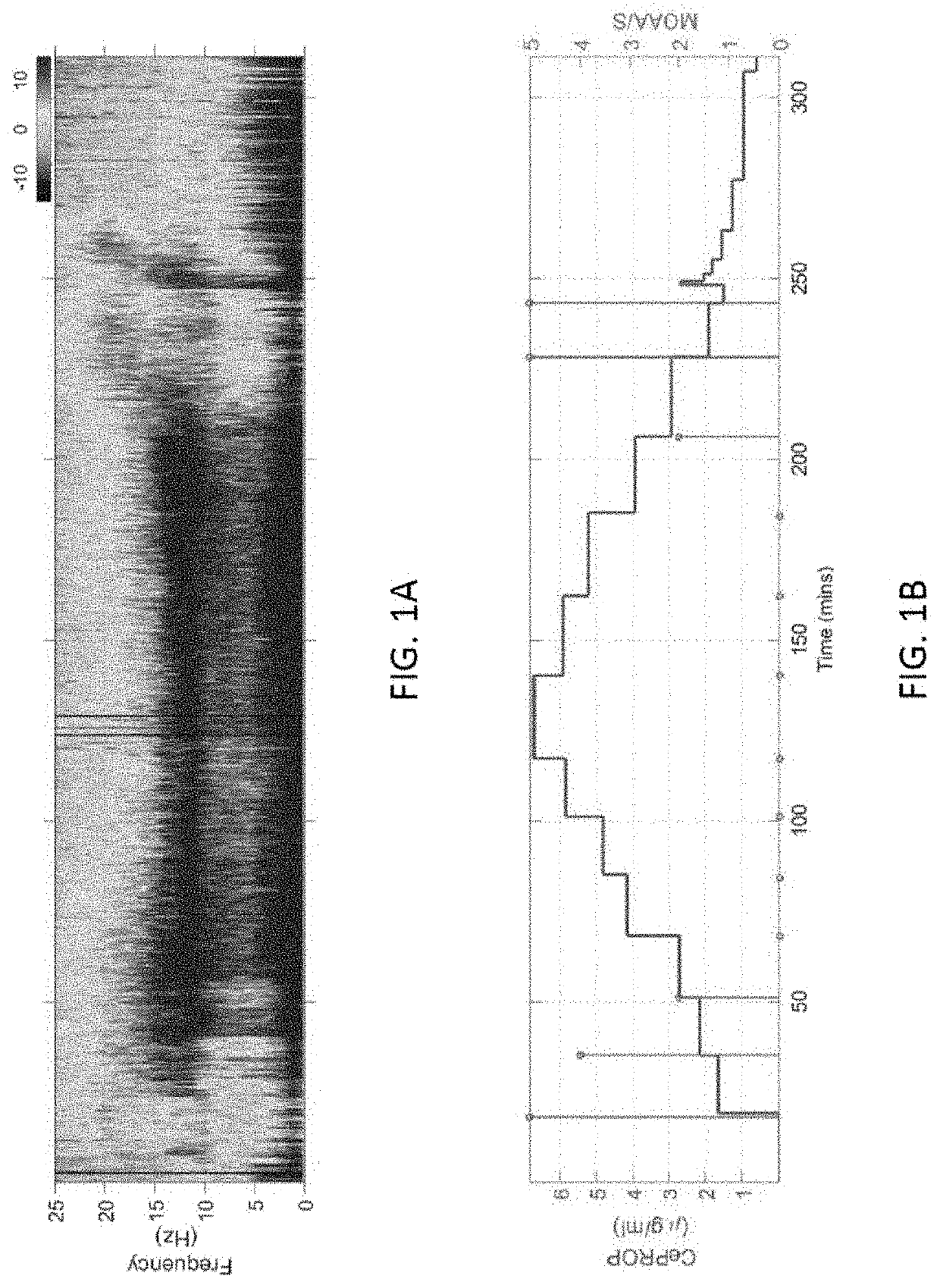
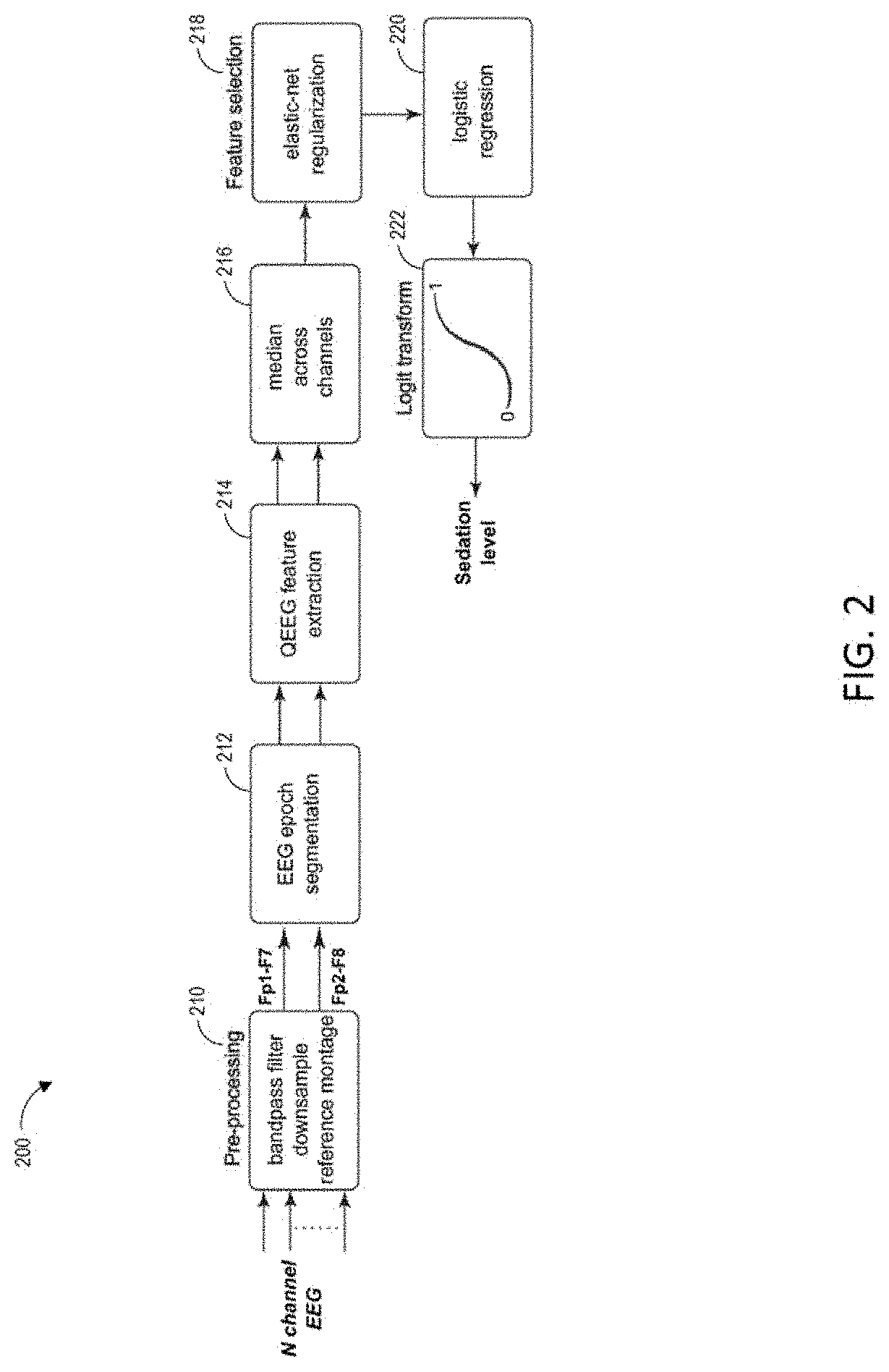
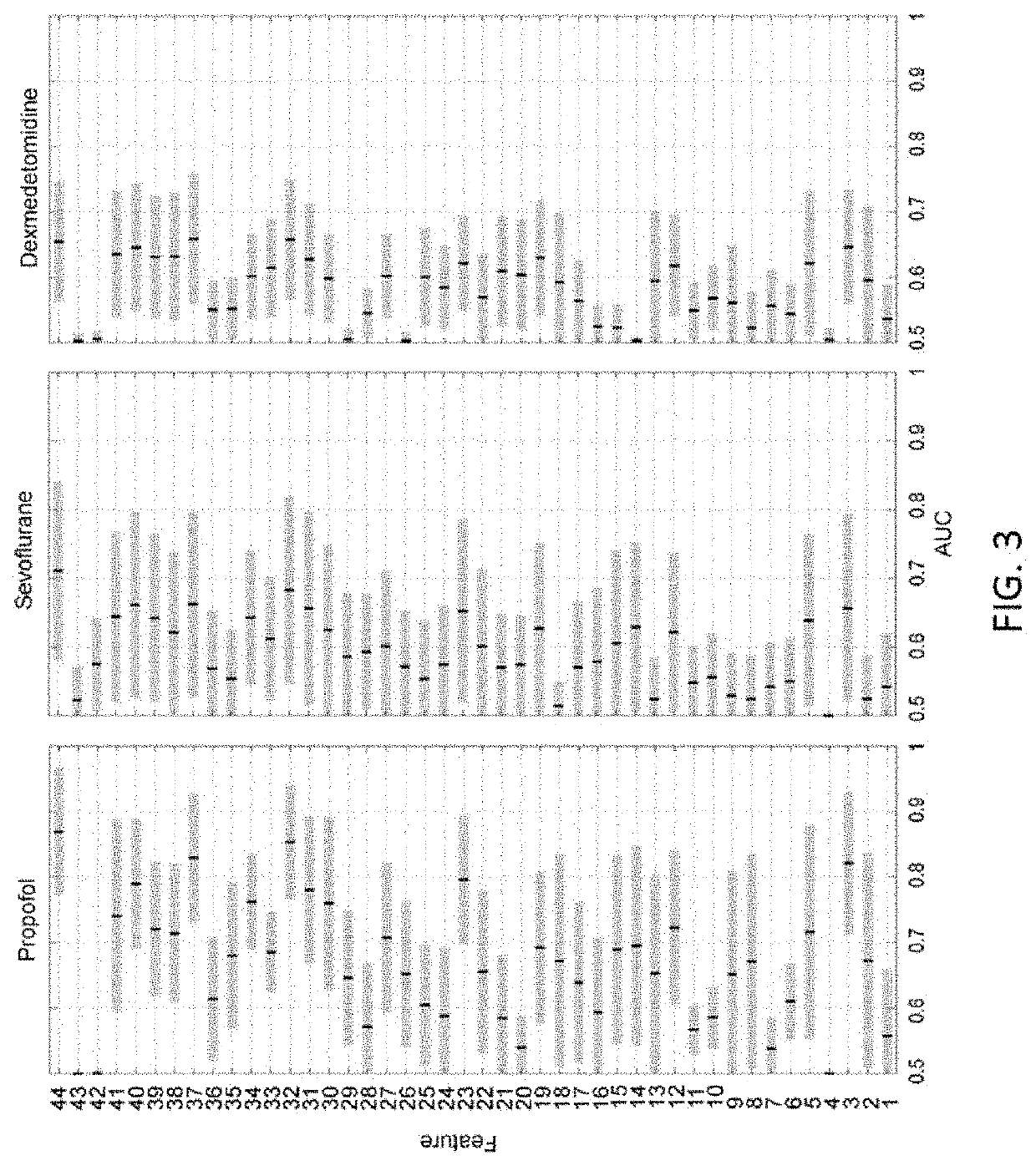
Combining multiple qeeg features to estimate drug-independent sedation level using machine learning
PendingUS20200253544A1Improve predictabilityImprove performanceElectroencephalographyMechanical/radiation/invasive therapiesSedative drugPhysical medicine and rehabilitation
The present disclosure describes systems and methods of estimating sedation level of a patient using machine learning. For example, the integration of multiple QEEG features into a single sedation level estimation system using machine learning could result in a significant improvement in the predictability of the levels of sedation, independent of the sedative drug used. The present disclosure advantageously allows for the incorporation of large numbers of QEEG features and machine learning into the next-generation monitors of sedation level. Different QEEG features may be selected for different sedation drugs, such as propofol, sevoflurane and dexmedetomidine groups. The sedation level estimation system can maintain a high performance for detecting MOAA / S, independent of the drug used.
Owner:MASIMO CORP
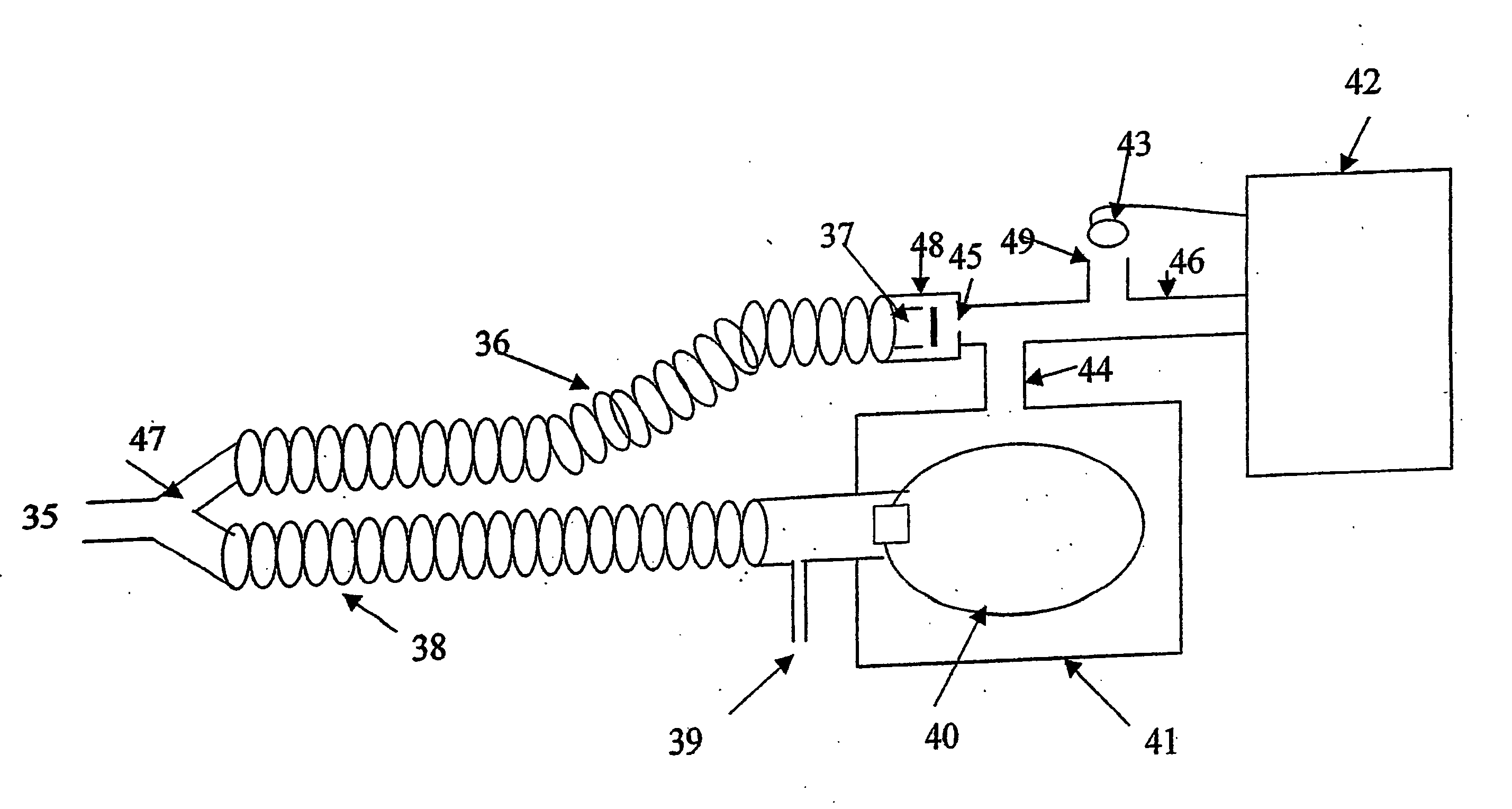
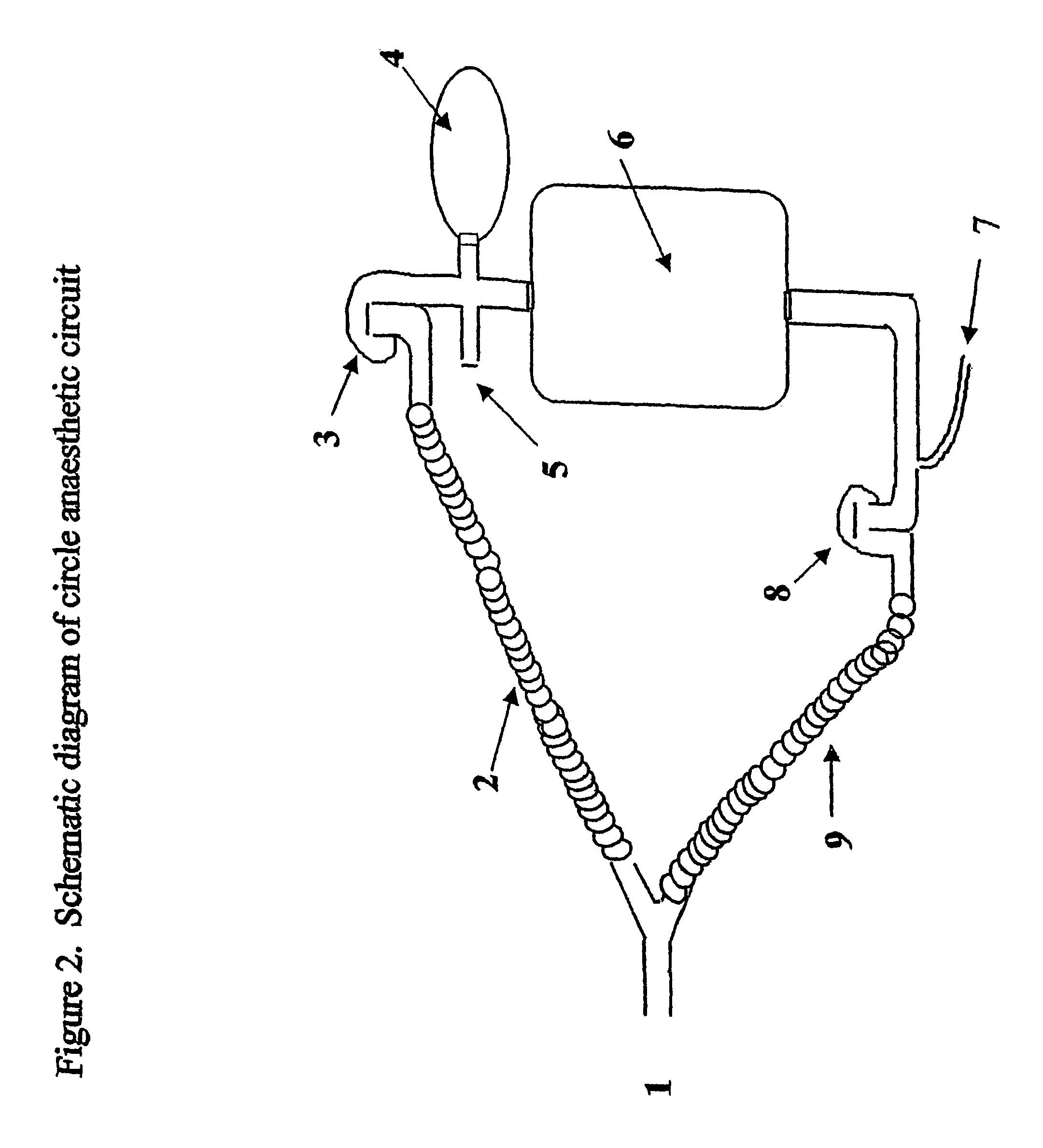
Method for continuous measurement of flux of gases in the lungs during breathing
ActiveUS20050217671A1RespiratorsOperating means/releasing devices for valvesAutonomous breathingEngineering
A method of calculating the flux of any gas (x) in a CBC circuit for a ventilated or a spontaneous breathing subject, for example said gas(x) being; a) an anesthetic such as but limited to; i)N2O; ii) sevoflurane; iii) isoflurane; iv) halothane; v) desflurame; or the like b) Oxygen; c) Carbon dioxide; or the like utilizing the following relationships; Flux of gas(x)=SGF (FSX−FEX) wherein SGF=Source of gas flow into the breathing circuit (CBC circuit) in liters / minute as read from the gas flow meter as set by the anesthesiologist; FSX=Fractional concentration of gas X in the source gas (which is set by the anesthesiologist); FEX=Fractional concentration of gas X in the end expired gas as determined by a portable gas analyzer, or the like.
Owner:THORNHILL SCI INC
Method for continuous measurement of flux of gases in the lungs during breathing
ActiveUS7913690B2RespiratorsOperating means/releasing devices for valvesContinuous measurementOxygen
A method of calculating the flux of any gas (x) in a CBC circuit for a ventilated or a spontaneous breathing subject, for example said gas(x) being; a) an anesthetic such as but limited to; i)N2O; ii) sevoflurane; iii) isoflurane; iv) halothane; v) desflurame; or the like b) Oxygen; c) Carbon dioxide; or the like utilizing the following relationships; Flux of gas(x)=SGF (FSX−FEX) wherein SGF=Source of gas flow into the breathing circuit (CBC circuit) in liters / minute as read from the gas flow meter as set by the anesthesiologist; FSX=Fractional concentration of gas X in the source gas (which is set by the anesthesiologist); FEX=Fractional concentration of gas X in the end expired gas as determined by a portable gas analyzer, or the like.
Owner:THORNHILL SCI INC
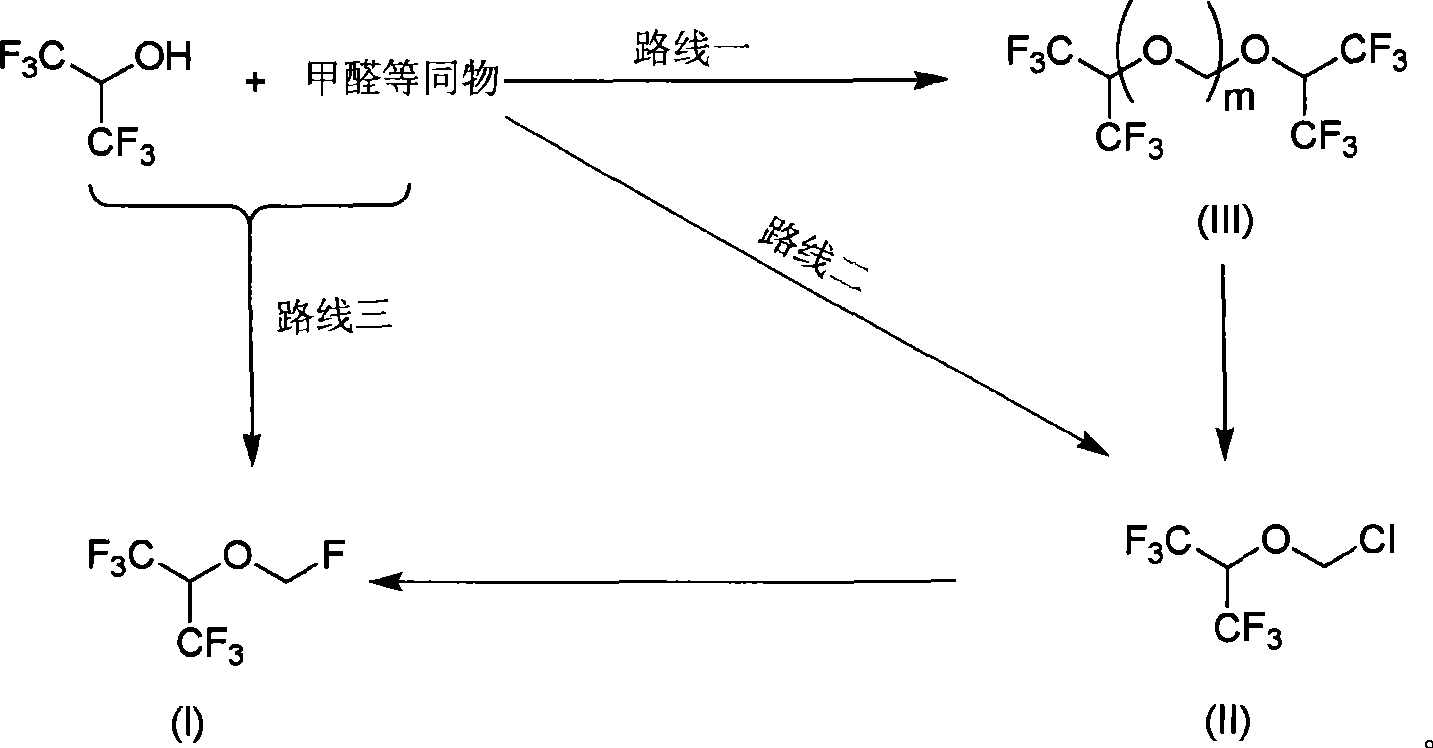
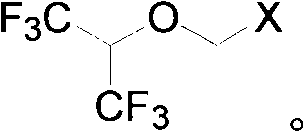
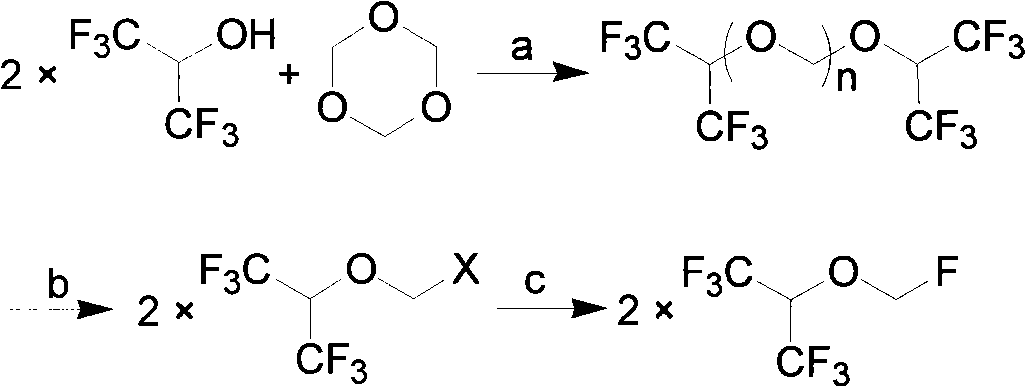
Process for preparing fluoro methyl ether
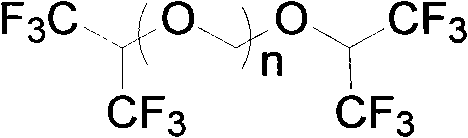
The invention relates to a process for preparing propylene glycol ester, more specifically fluoromethyl-2,2,2-trifluo-1-(trifluoromethyl) ethyl ether (sevoflurane), wherein the precursor reacts with fluorides at the presence of activating agents.
Owner:SHANDONG NEWTIME PHARMA
Distillation Method For The Purification Of Sevoflurane And The Maintenance Of Certain Equipment That May Be Used In The Distillation Process
InactiveUS20090275785A1Efficient separationEliminate needEther separation/purificationOrganic compound preparationDistillation methodImpurity
Processes for preparing commercial quantities of a stable, pharmaceutically acceptable sevoflurane substantially tree of impurities are claimed. In another embodiment, a process for removing reactive metal, salts from the surface of metallic equipment used in the distillation of sevoflurane and rendering a non-inert metallic surface of the metallic equipment inert.
Owner:HALOCARBON PROD CORP
Sevoflurane synthesizing method
The invention discloses a method for applying methylating halogen, which is a new method to use hexafluoro isopropanol, formaldehyde equivalent, strong acid, alchlor and metal fluoride as raw materials to react so as to synthesize high-purity and high-yield sevoflurane. The method is characterized in that the reaction condition is easy to control without unnecessary intermediate processes of separation and purification, the post-treatment is simple, mostly raw materials and auxiliary materials can be recovered and used repeatedly so that the yield of the 'three wastes' is quite little, the product yield is high, the production time of single batch is shortened greatly, and the production efficiency is improved, thus the method is applicable to large-scale industrial production.
Owner:福安药业集团重庆博圣制药有限公司 +1
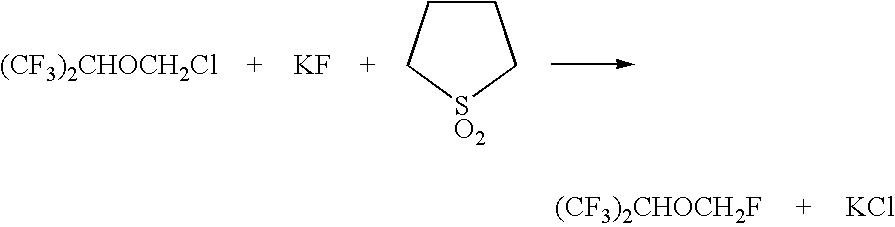
Method for the preparation of sevoflurane
ActiveUS20060205825A1Promote conversionHigh yieldBiocideOrganic chemistryPotassium fluoridePhase-transfer catalyst
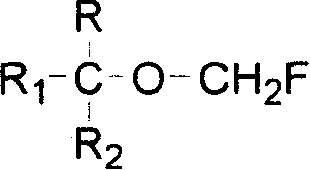
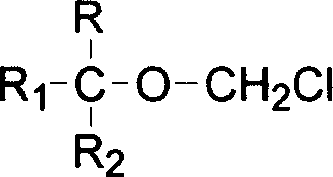
A method for the preparation of (CF3)2CHOCH2F (Sevoflurane) is presented, which comprises providing a mixture of (CF3)2CHOCH2Cl, potassium fluoride, water, and a phase transfer catalyst and reacting the mixture to form (CF3)2CHOCH2F.
Owner:FIRST NIAGARA BANK
Method for detecting propofol with elimination of sevoflurane interference
InactiveCN106872553AEliminate distractionsRealize continuous online monitoringPreparing sample for investigationMaterial analysis by electric/magnetic meansIon-mobility spectrometryComputer science
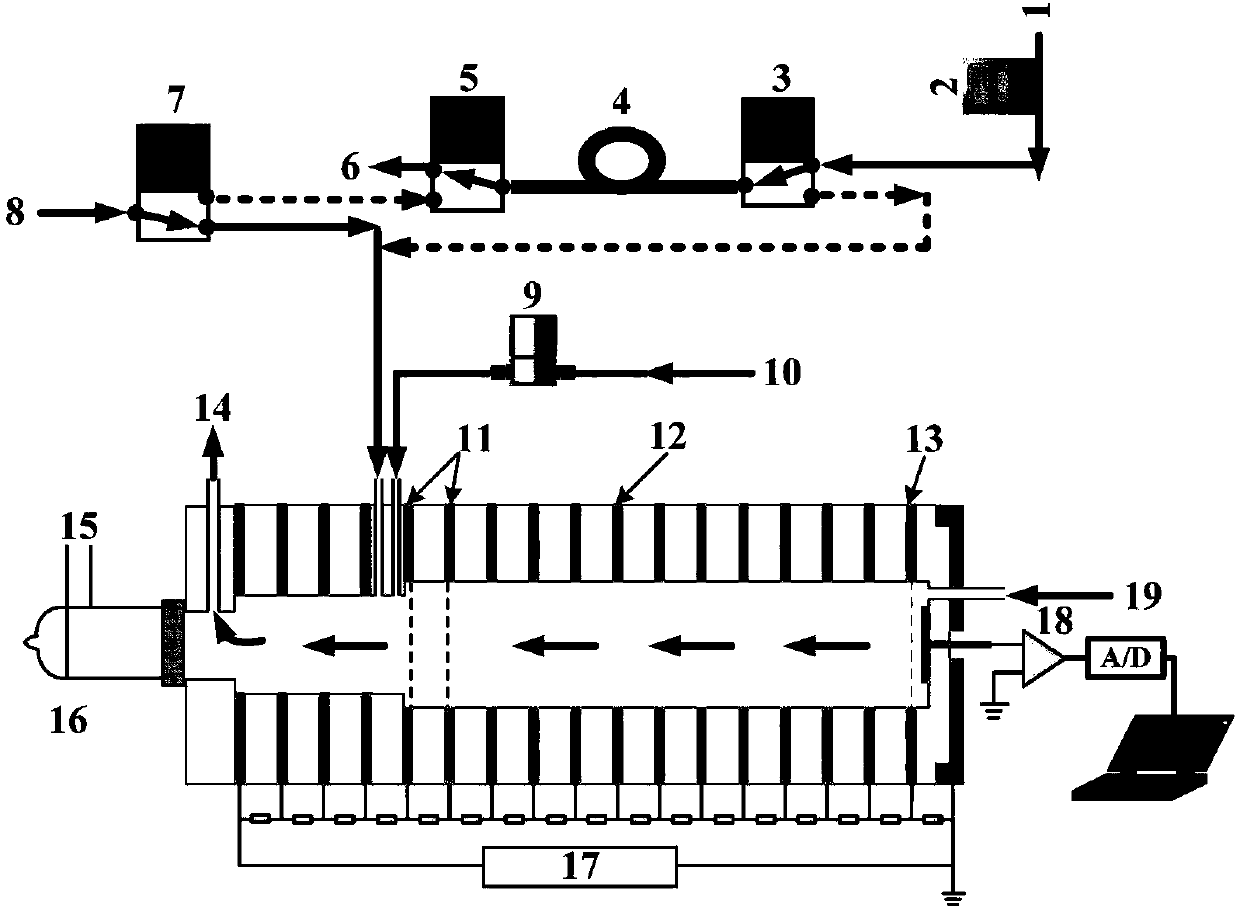
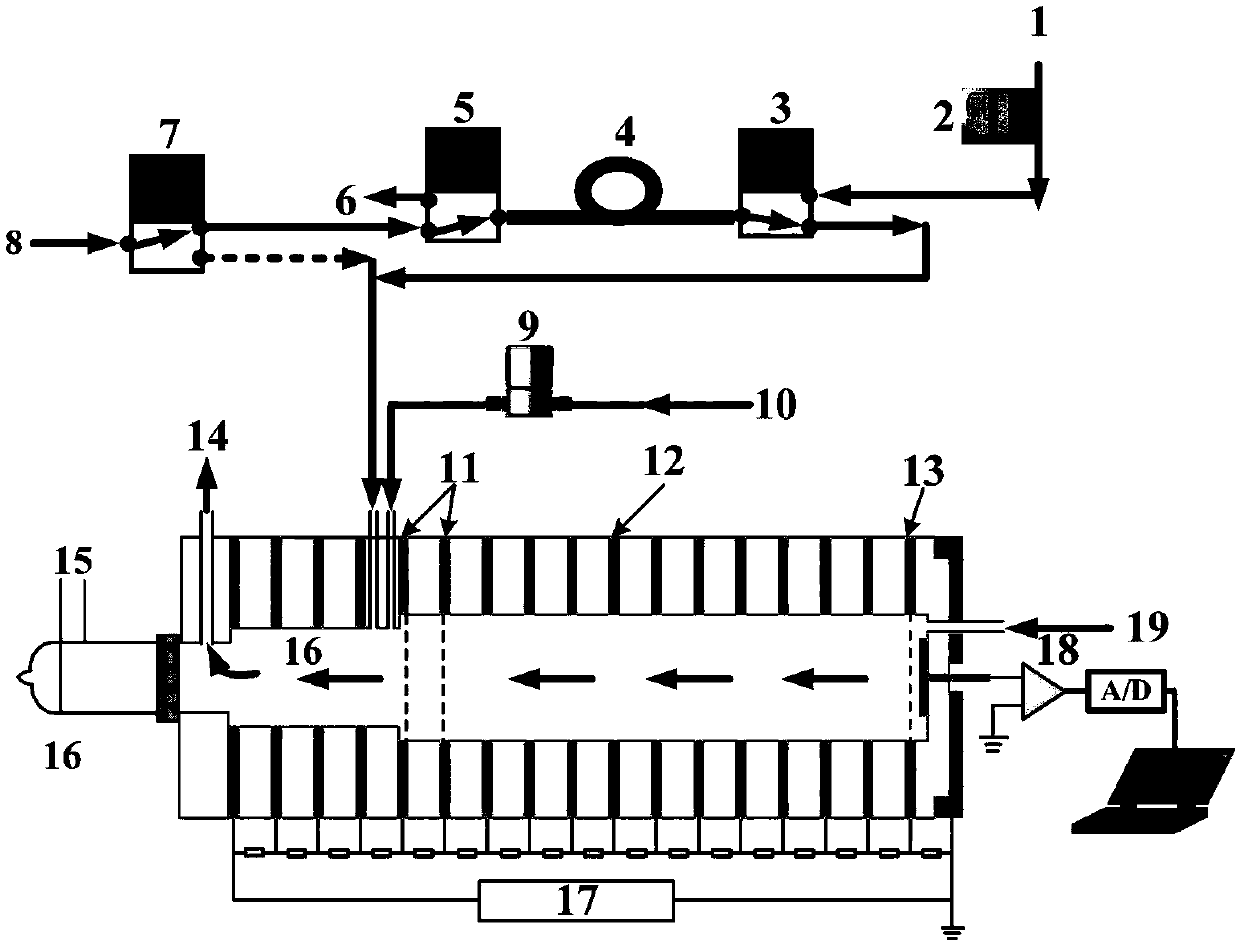
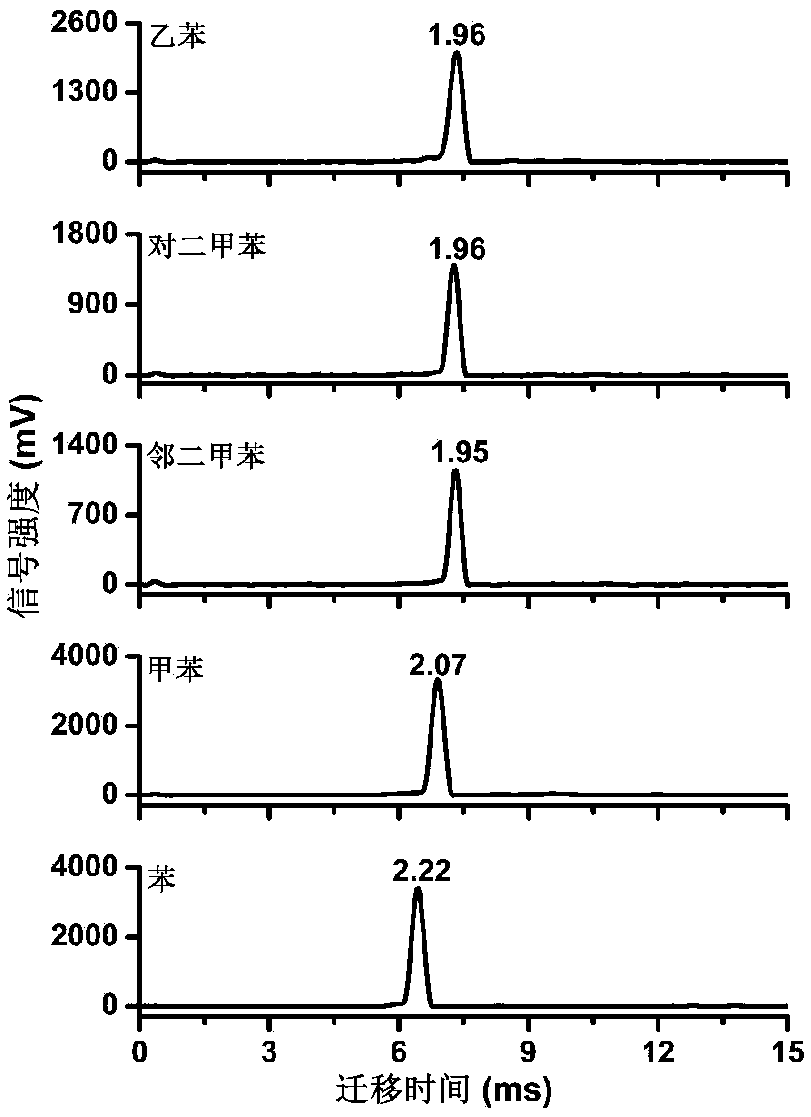
The invention discloses a method for detecting propofol with elimination of sevoflurane interference. Based on an ion mobility spectrometry technique and a time-resolved dynamic dilution sample introduction technique, the method realizes dynamic dilution of propofol and sevoflurane, thereby eliminating the interference of residual sevoflurane in anesthetics, and realizing continuous on-line monitoring of propofol in intra-operative expiratory air.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
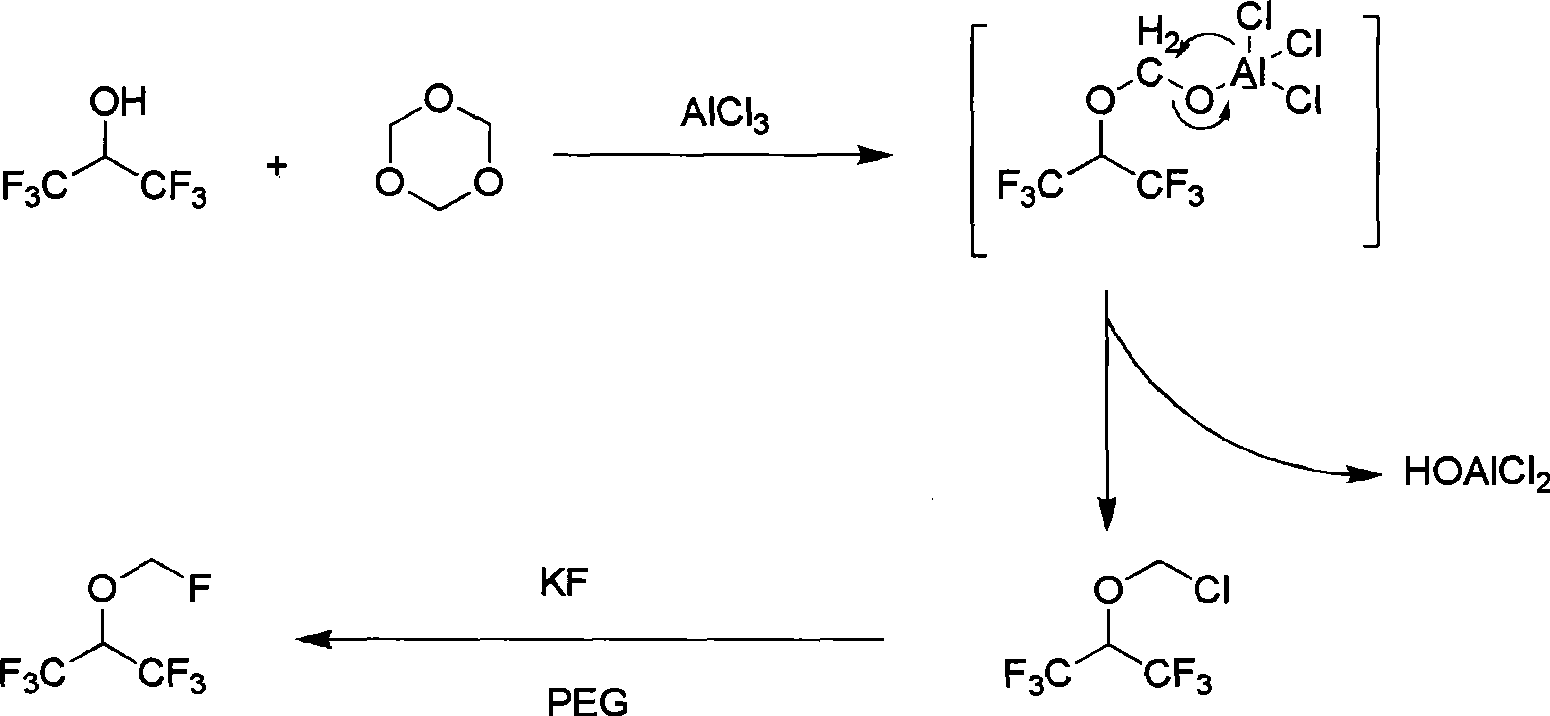
Method of synthesizing fluoromethyl-1,1,1,3,3,3-hexafluoroisopropyl ether
InactiveCN101058533ALow priceEasy to removeOrganic compound preparationEther preparationPropanolEther
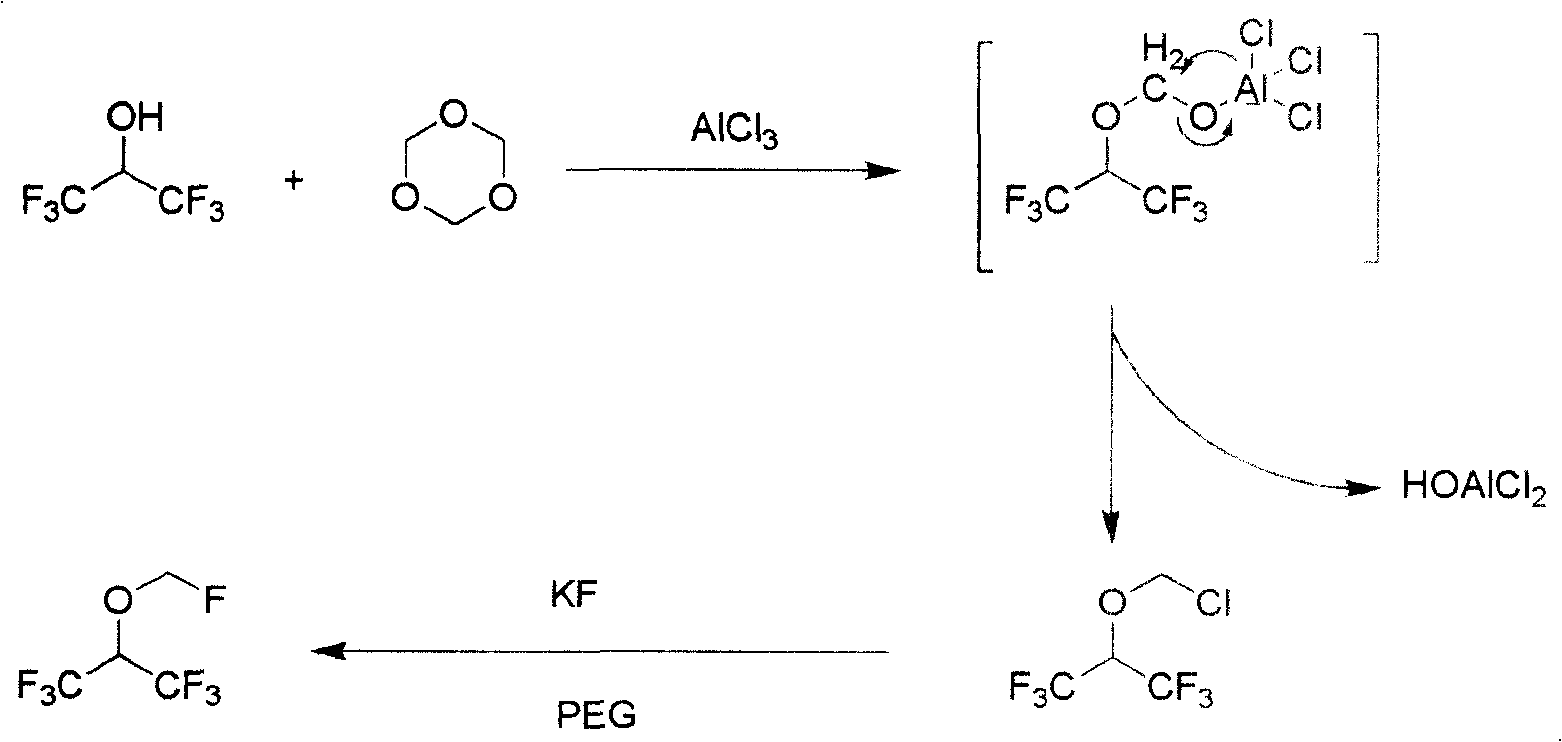
The invention discloses a synthesizing method of fluomethyl-1,1,1,3,3,3-hexafluoroisopropyl ether, which comprises the following steps: 1) reacting 1,1,1,3,3,3-hexafluorine-2-propanol and ,3,5-trioxymethylene or oligoformaldehyde and Lewis acid chloride to generate chloromethyl-1,1,1,3,3,3-hexafluoroisopropyl ether as intermediate and HOAlCl2 as by-product; adding 6N HCl to disintegrate to remove by-product HOAlCl2; 2) reacting chloromethyl-1,1,1,3,3,3-hexafluoroisopropyl ether and fluorination agent and solvent to produce the product; improving the selectivity of intermediate; removing by-product easily.
Owner:SHANGHAI HUMEI CHEM IND TECH DEV
Use of xenon as neuroprotectant in a neonatal subject
InactiveUS20090311340A1Reduce injuryEnhances isoflurane-induced apoptosisBiocideNervous disorderIntensive care medicineXenon
The present invention relates to the use of xenon in the preparation of a medicament for preventing and / or alleviating one or more anesthetic-induced neurological deficits in a neonatal subject. The present invention further relates to combinations of xenon and sevoflurane, and use thereof as preconditioning agents for administration prior to hypoxic-ischaemic injury.
Owner:IP2IPO INNOVATIONS LTD
Device and method for rapidly detecting sevoflurane online
ActiveCN105891191AQuick analysisGood choiceChemiluminescene/bioluminescenceElectricityTemperature control
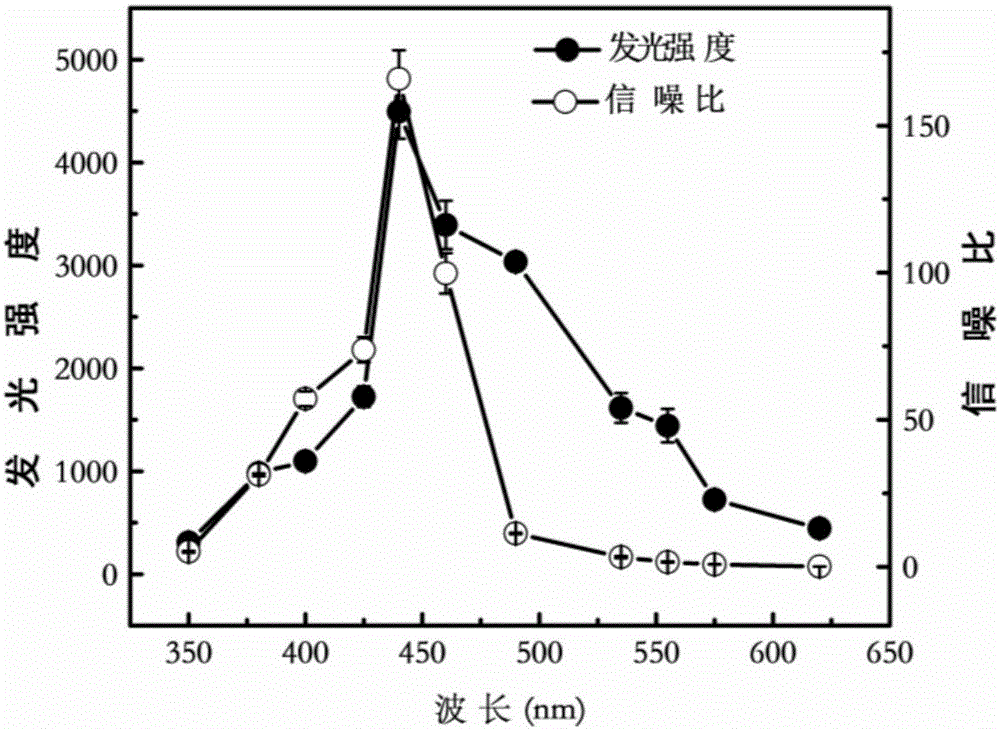
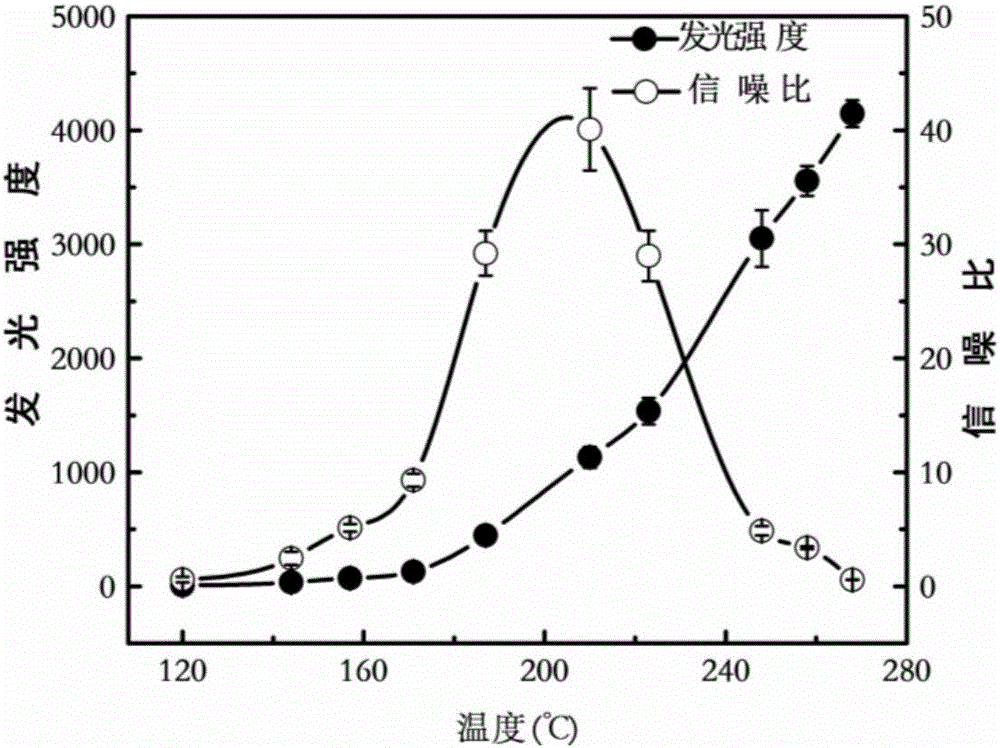
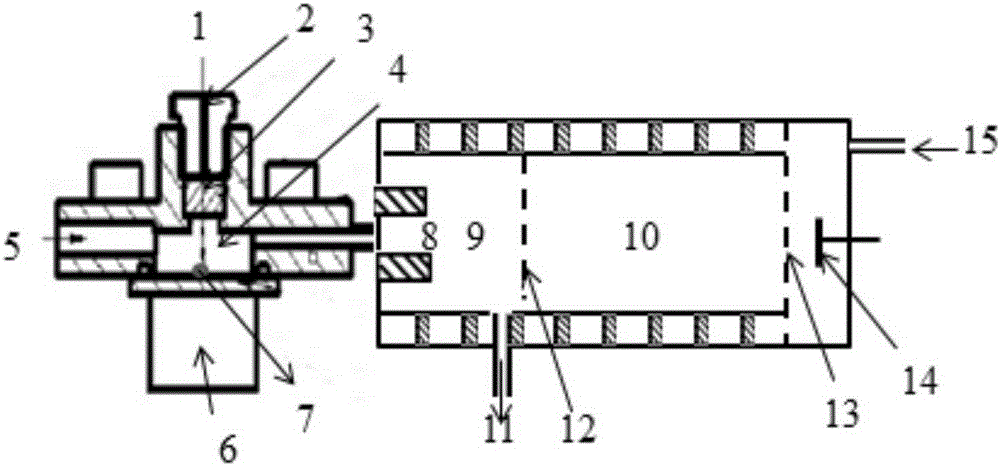
The invention discloses a device and a method for rapidly detecting sevoflurane online. The device comprises a sampling unit and a cataluminescence sensing unit, wherein the sampling unit comprises a sampling head and a double-channel air pump, the sampling head is connected with an air inlet of a first channel of the double-channel air pump, and an air inlet of a second channel of the double-channel air pump is communicated with air; the cataluminescence sensing unit comprises a chemiluminescence reaction chamber, a temperature controller, an optical splitter and a detector; a heating element is arranged in the chemiluminescence reaction chamber, and nanometer strontium oxide is sintered on the surface of the heating element; the temperature controller is electrically connected with the heating element, and the optical splitter is arranged between the chemiluminescence reaction chamber and the detector, so that light emitted from the chemiluminescence reaction chamber passes through the optical splitter and then is received and detected by the detector. According to the device and the method, nanometer strontium oxide is adopted as a sensor element, has good selectivity for sevoflurane and can be directly used for rapidly analyzing sevoflurane in complex samples.
Owner:SUN YAT SEN UNIV
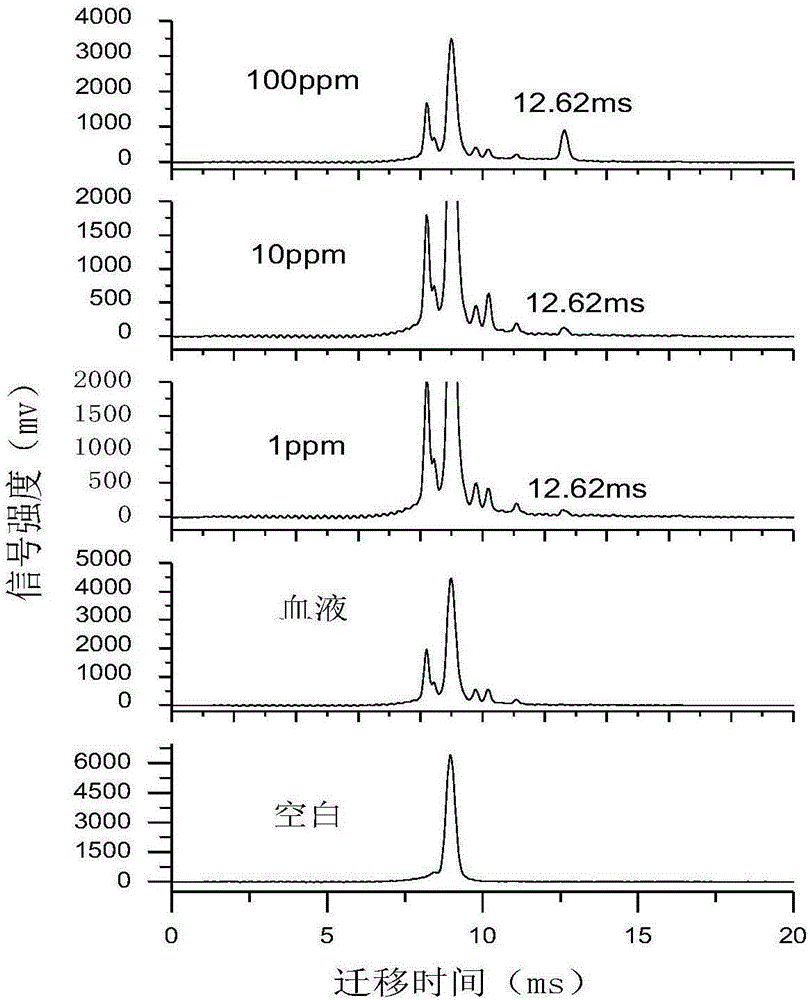
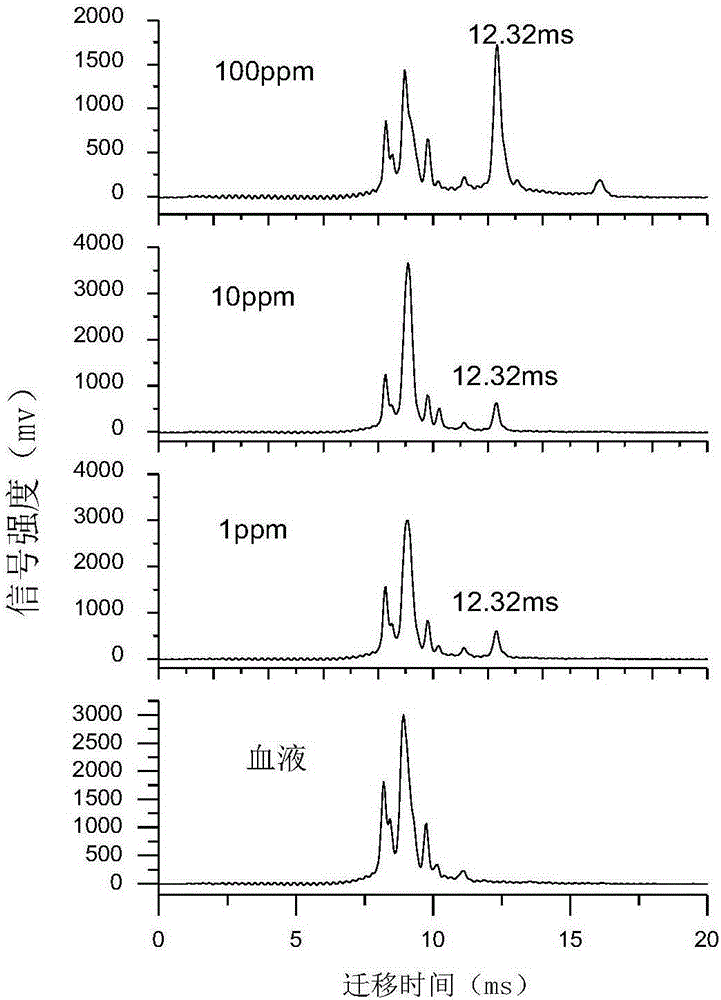
Online detector for sevoflurane in blood, and applications thereof
InactiveCN106645370ARealize sample injectionThe detection process is fastMaterial analysis by electric/magnetic meansSpectroscopyIon-mobility spectrometry
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Single fluorine substituted methyl ether preparation method
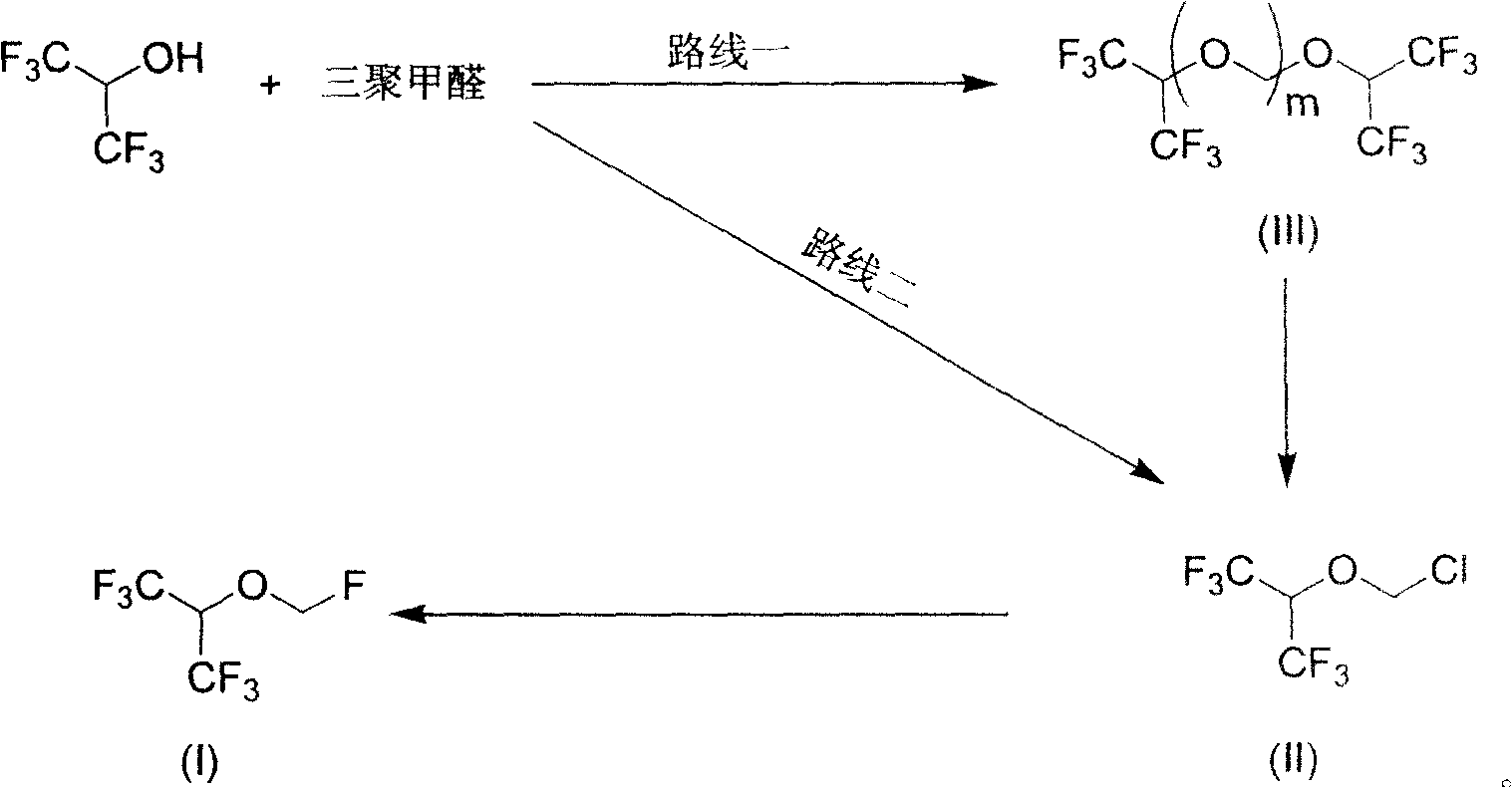
The invention provides a process for preparing monofluoromethyl ether, especially fluoromethyl-2,2,2-trifluoro-1-(trifluoromethyl) ethyl ether (sevoflurane), wherein its precursor hexafluoroisopropanol is reacted with trioxymethylene at the presence of fluoro-containing catalyst.
Owner:SHANDONG NEWTIME PHARMA
Process for synthesizing Sevoflurane
ActiveCN101314560AReduce consumptionEasy to storeOrganic compound preparationEther preparation by organic exchangeFluorideParaformaldehyde
Owner:LUNAN PHARMA GROUP CORPORATION
Intravenous injection/inhalation dual-purpose general anesthetic and preparation process thereof
InactiveCN1965815AHighlight substantiveReduce dosageHalogenated hydrocarbon active ingredientsPharmaceutical containersExtracorporeal circulationSide effect
The invention relates to a vein injection / oral general anesthetic, and relative production, wherein it comprises fluorocarbon and volatile general anesthetic, while the general anesthetic is alorbatalotane, enflurane, furane, sevoflurane, etc. And the invention can be injected in vein or orally taken, with low cost. It has simple control without side effect.
Owner:王晟
Preparation method of sevoflurane
InactiveCN103804151AAchieve reuseHigh yieldOrganic compound preparationEther preparationHindered amine light stabilizersPhase-transfer catalyst
The invention discloses a preparation method of sevoflurane. The sevoflurane is prepared from hexafluoroisopropanol, formaldehyde equivalent, aluminum trichloride, a phase transfer catalyst, hindered amine and a metal fluorinating agent as raw materials. The preparation method has the advantages of high yield, high purity, few byproducts and the like, can realize recycling of some raw materials, and is low in cost, easy to control industrially, environment-friendly and suitable for large-scale industrial production.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
Insecticidal and fungicidal composition containing hymexazol
ActiveCN104145965AAchieve the effect of bothExpand the effect of insecticidal and bactericidal spectrumBiocideFungicidesInsect pestBULK ACTIVE INGREDIENT
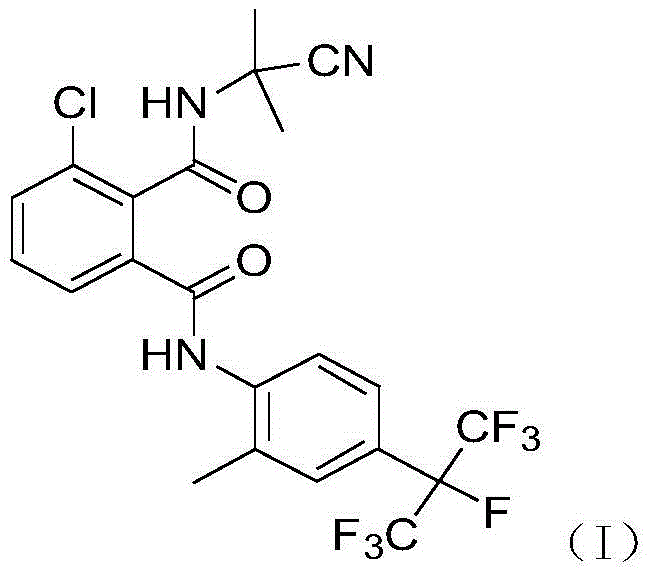
The invention discloses an insecticidal and fungicidal composition containing two active ingredients, wherein the first active ingredient is hymexazol and the second active ingredient is 3-chlorine-N1-(2-methyl-4-Sevoflurane isopropyl phenyl)-N2-(1-methyl-1-cyano ethyl) phthalic diamide. The insecticidal and fungicidal composition provided by the invention is suitable for prevention and control of diseases and insect pests of seedling-stage rice.
Owner:ZHEJIANG RES INST OF CHEM IND CO LTD +1
Process for the purification of fluromethyl 1,1,1,3,3,3,-hexafluoroisopropyl ether (sevoflurane)
A process for purifying crude fluoromethyl 1,1,1,3,3,3-hexafluoroisopropyl ether (sevoflurane). The crude sevoflurane is repeatedly washed with water under conditions and for a time sufficient to reduce the level of 1,1, 1,3,3, 3-hexafluoroisopropanol (HFIP) to no more than 200 ppm or no more than 100 ppm.
Owner:HALOCARBON PROD CORP
Efficient medical soda lime and preparation method thereof
PendingCN107243249AHigh hardnessAvoid the problem of blocking a person's breathing circuitDispersed particle separationAir quality improvementCalcium hydroxideHydrogen
The invention discloses efficient medical soda lime and a preparation method thereof. The efficient medical soda lime is prepared from the following raw materials in parts by weight: 80 to 85 parts of calcium hydroxide, 0.5 to 5 parts of calcium sulfate hemihydrate, 0.1 to 1 part of a moisture absorbent, 0.1 to 5 parts of a rigidity regulator, 1 to 5 parts of a de-foaming agent, 0.05 to 1 part of a pH (Potential of Hydrogen) indication dyeing agent and 12 to 19 parts of water. The efficient medical soda lime disclosed by the invention has the following advantages that the rigidity regulator and the foaming agent are combined to use and the defect of an existing product that the rigidity and CO2 absorption rate cannot be both considered is overcome; the moisture absorbent is added and is used for absorbing moisture and strong alkali in the prior art is replaced so that a condition that the strong alkali and anesthetics including sevoflurane and the like react to generate harmful olefin or CO is avoided; meanwhile, the moisture of the calcium hydroxide is kept and absorption of carbon dioxide of the next step is facilitated; the calcium hydroxide is used as a main acting component and reacts with the acidic gas CO2 to absorb the CO2; in a preparation process, a lot of heat is not generated; the preparation process is easy to control, the cost is low and a technology is simple, safe and controllable.
Owner:党银喜
Detection method of bacterial endotoxins in pharmaceutical raw materials
InactiveCN102565293AEnsure safetyEasy to detectTesting medicinal preparationsBiotechnologyBiochemistry
The invention relates to a detection method of bacterial endotoxins in pharmaceutical raw materials. The detection method comprises the following steps: selecting the pharmaceutical raw materials: selecting the pharmaceutical raw materials which are easy to volatilize and difficult to dissolve in water; preparing a test liquid: weighing and metering the selected pharmaceutical raw materials, heating to volatilize the pharmaceutical raw materials, and supplementing the equal quantity of water for testing the bacterial endotoxins till the original pharmaceutical mass to get the test liquid; and detecting the bacterial endotoxins in the pharmaceutical raw materials in the test liquid through a conventional method. According to the detection method, the bacterial endotoxins that are difficult to dissolve in the water and easy to volatilize, and can not be directly determined through a known method in the pharmaceutical raw materials, such as isoflurane, enflurane, sevoflurane and the like, can be determined, and the quality of sterile products can be controlled from a source, so that the detection method is highly significant in ensuring the safety of preparation productions. The addition of other reagents is not required during the detection process, and the existence of the bacterial endotoxins in samples can be simply, conveniently and reliably detected.
Owner:YICHANG HUMANWELL PHARMA
Chromatographic method for the analysis of both in process and finished sevoflurane
InactiveUS20050234268A1Ether separation/purificationOrganic compound preparationHydrogen fluorideCapillary gas chromatography
A method in which organic matter found in a crude sevoflurane may be separated, identified, and quantified using a CARBOWAX™ (polyethylene glycol) capillary gas chromatographic column or an alkyl polysiloxane capillary gas chromatographic column. Also provided is a process control method for the production of sevoflurane, wherein the content of a particular component in one of the following steps is determined, and in that, assuming this as a variable, the treatment condition of the step is adjusted: 1) a step of extracting, or cooling to form two layers, and / or distilling a mixture of crude sevoflurane and hydrogen fluoride (HF) in order to isolate the majority of the sevoflurane and 2) an optional step of purifying the crude sevoflurane and 3) a step of distilling crude sevoflurane. Also provided is a method for determining the impurity level of a purified sevoflurane that is acceptable for use in human / animal anesthesia.
Owner:HALOCARBON PROD CORP
Positive ion mobility spectrometry detection method for propofol in exhaled air
InactiveCN109781827ARealize continuous online monitoringEliminate the effects ofMaterial analysis by electric/magnetic meansIon-mobility spectrometryExhaled air
The invention discloses a positive ion mobility spectrometry detection method for propofol in exhaled air. The method is based on a time resolution dynamic dilution sampling device and a reagent molecule-assisted photoionization positive ion mobility spectrometry technology. Time-resolved dynamic dilution sampling of propofol molecules and water molecules in exhaled air is achieved, influences ofhumidity in the exhaled air and sevoflurane in composite anesthesia are eliminated, the propofol is quantified through signal intensity of the 2s propofol of time-resolved dynamic dilution sampling, and clinical continuous online monitoring of the propofol in the exhaled air is achieved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing sevoflurane
ActiveCN102199076AImprove conversion rateEasy to recycleOrganic chemistryOrganic compound preparationSolventFluoride
The invention relates to a method for preparing sevoflurane (i.e. methyl fluoride-2,2,2-trifluoro-1- (trifluoromethyl) ethylether), which comprises the steps of: reacting chloromethyl-2,2,2-trifluoro-1- (trifluoromethyl) ethylether) with fluoride. The method has the advantages of simple operation, low cost, high yield and easiness in recycling the solvent, and is suitable for industrialized production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Method for preparing sevoflurane
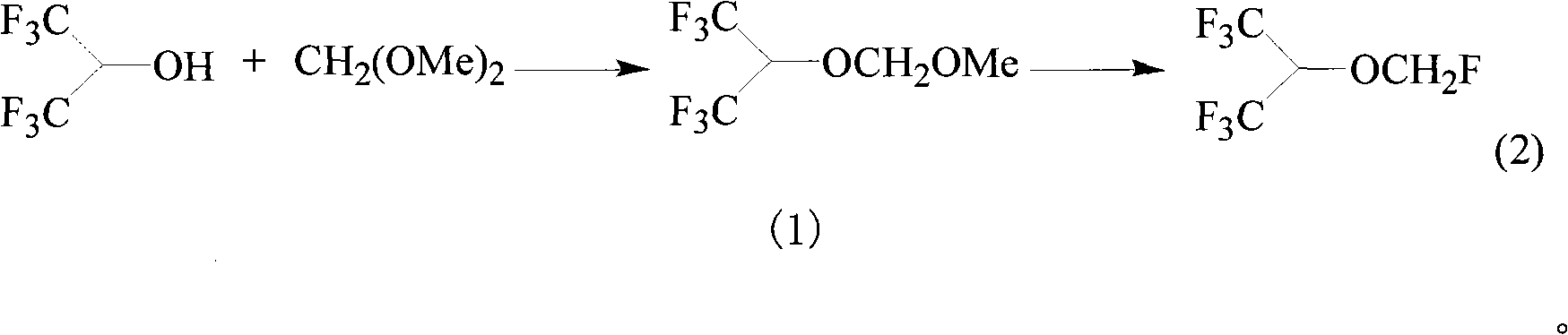
ActiveCN101337863AIncrease profitHigh yieldEther preparation by organic exchangeStrong acidsDimethoxymethane
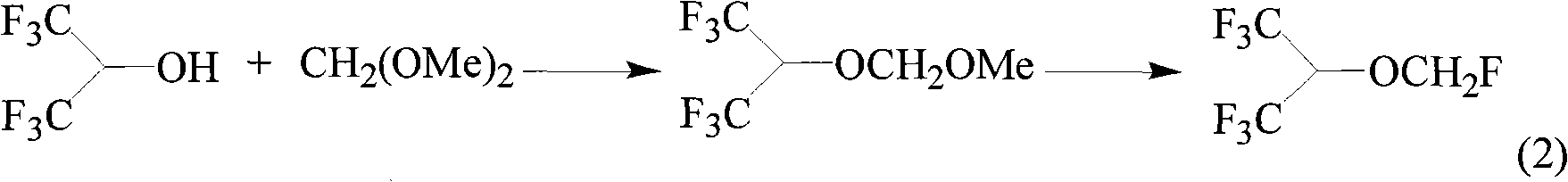
The invention relates to a method for preparing sevoflurane [fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl) ethyl ether], which comprises the following steps: reacting hexafluoroisopropanol with dimethoxymethane in the presence of a catalyst to obtain methoxymethylene hexafluoroisopropyl ether, and reacting methoxymethylene hexafluoroisopropyl ether with a fluoride in the presence of a strong acid to obtain the sevoflurane.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +2
New synthetic method of sevoflurane
ActiveCN100590111CReduce the discharge of three wastesLow costEther preparationHalogenBiochemical engineering
Owner:福安药业集团重庆博圣制药有限公司 +1
Inhalational compound anaesthetic agent
InactiveCN104623084ARapid induction of anesthesiaShort duration of anesthesiaPharmaceutical delivery mechanismAnaestheticsRadix AconitiInduction anesthesia
The invention provides an inhalational compound anaesthetic agent. The inhalational compound anaesthetic agent is characterized by comprising an A agent and a B agent, wherein the A agent serves as an inducer and comprises nitrous oxide, sodium chloride, perfume and a compound emulgator; the B agent serves as an anesthesia retaining agent and comprises flos daturae, radix aconiti agrestis, sevoflurane, sodium chloride, perfume and a compound emulgator; according to the A agent, the content of nitrous oxide is 1-5%, the content of the compound emulgator is 15-25%, and the content of the perfume is 0.1-0.3%; according to the B agent, the content of flos daturae is 0.5-0.9g / 100mL, the content of radix aconiti agrestis is 0.1-0.5g / 100mL, and the content of the sevoflurane is 1.5-2.5%, the content of the compound emulgator is 10-20%, and the content of the perfume is 0.1-0.3%. The inhalational compound anaesthetic agent is simple to operate during use; the A agent can be used for quickly inducing anesthesia and can be directly inhaled by an inhaling device; the B agent is used for retaining anesthesia, can be inhaled by a matched anesthesia machine and can also be infused into the body by other manners; the infusing time and dosage can be flexibly arranged according to the needs of the operation; and the inhalational compound anaesthetic agent can be applied to complex or long-time operation.
Owner:黄玉华
Preparation of sevoflurane with negligible water content
InactiveCN101180250AReduce moisture contentEther separation/purificationOrganic compound preparationMolecular sieveMedicine
Provided is a sevoflurane anesthetic product which can remain substantially undegraded after long periods of storage, as well as a method for preparing the product. The product comprises sevoflurane in a glass container having a water content of less than 130 ppm. The method comprises drying sevoflurane having a water content of greater than 130 ppm to a water content les than 130 ppm. A preferred method of drying comprises contacting a sevoflurane composition having a water content of greater than 130 ppm with alumina-containing molecular sieves such that the water content is reduced to lessthan 130 ppm.
Owner:PIRAMAL CRITICAL CARE
Composition of analgesic bioadhesive healing microspheres
ActiveUS20170035696A1Halogenated hydrocarbon active ingredientsEther/acetal active ingredientsMicrosphereNon ionic
A new composition of analgesic bioadhesive healing microspheres in which each microsphere comprises at least:a.—a layer of a polyanion, where the polyanion may be an alginate.b.—a core coated with a polyanion consisting of a triblock copolymer non-ionic surfactant; polexamer 188 or a mixture of them; and a volatile halogenated by-product anaesthetic ether agent of methyl-isopropyl-ether in contact with the internal part of the polyanion layer, which may be sevoflurane.c.—a coating of a polycation in contact with the external part of the polyanion layer, which may be chitosan.
Owner:FERNANDEZ GINES DAMASO
Novel carboxylic acid compound, use thereof, and process for producing the same
ActiveUS20090156861A1Efficient productionLow costOrganic compound preparationCarboxylic acid esters preparationMethylating AgentCarboxylic acid
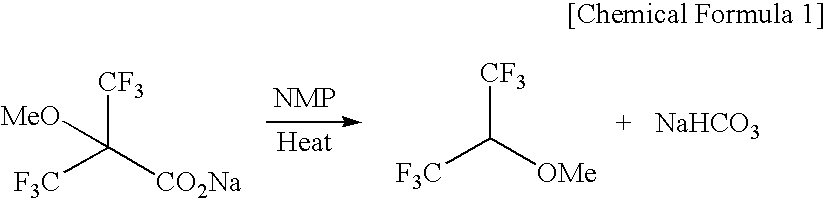
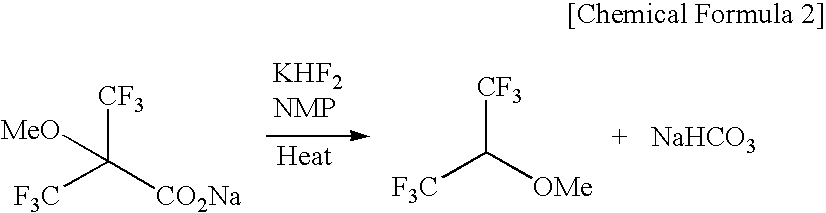
The present invention provides a process for producing 1,1,1,3,3,3-hexafluoro-2-methoxypropane, wherein a novel compound, 2-methoxy-2-trifluoromethyl-3,3,3-trifluoropropionic acid or a salt thereof is decarboxylated, or wherein an olefin compound represented by chemical formula (4): CF2═C(CF3)(OCH3) is reacted with a fluorinating agent; and a process for producing 2-methoxy-2-trifluoromethyl-3,3,3-trifluoropropionic acid or a salt thereof by reacting a hydroxycarboxylic ester with a methylating agent and then hydrolyzing the reaction product, or hydrolyzing the hydroxycarboxylic ester and then reacting the resulting product with a methylating agent.In accordance with the invention, 1,1,1,3,3,3-hexafluoro-2-methoxypropane, which is useful as a raw material for, for example, the anesthetic Sevoflurane, can be produced efficiently and at low cost.
Owner:DAIKIN IND LTD
Carboxylic acid ester, use of the same, and method for producing the same
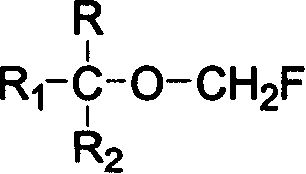
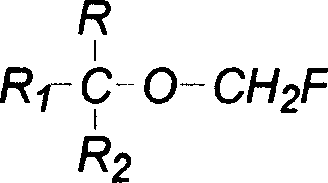
ActiveUS8013182B2High yieldValid conversionOrganic compound preparationCarboxylic acid esters preparationHalogenSulfur
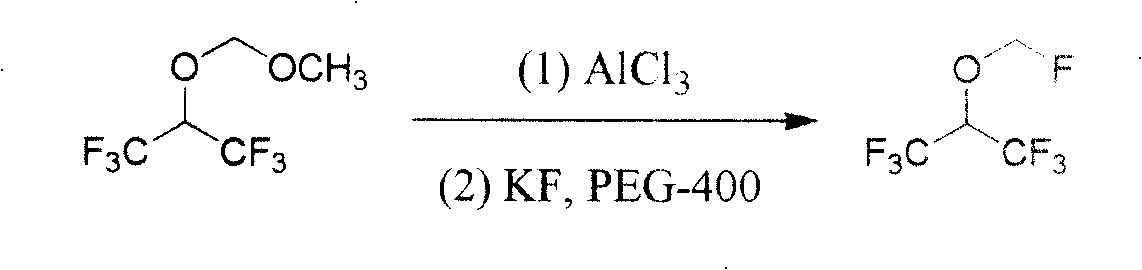
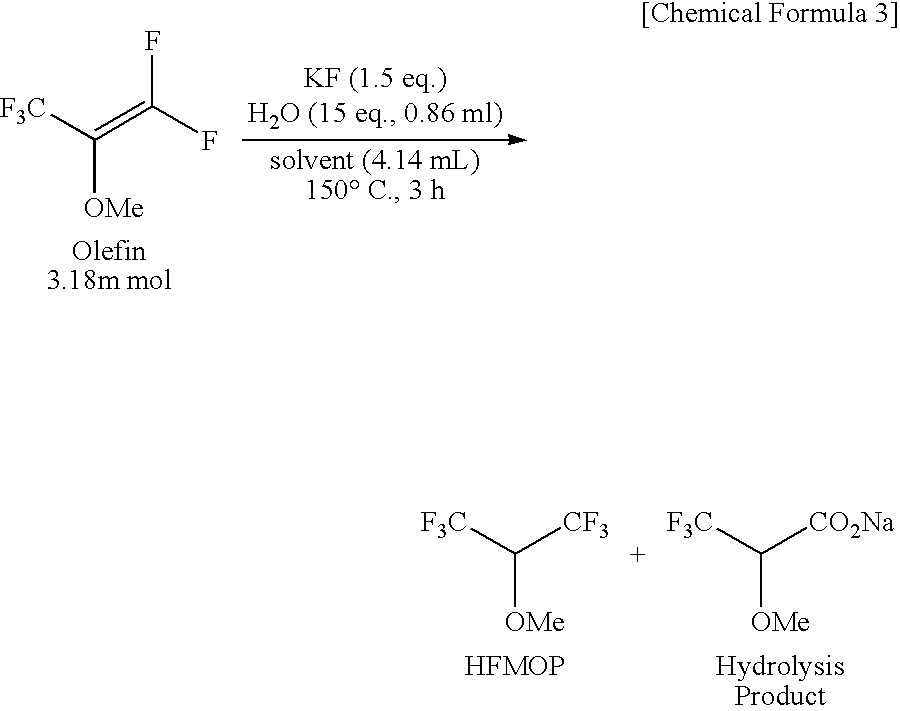
The present invention provides a process for producing a novel compound, i.e., α-chloromethoxycarboxylic acid ester represented by General Formula (1): (CF3)2C(OCH2Cl)COOR, wherein R is a hydrocarbon group which may be substituted with at least one atom selected from the group consisting of halogen, oxygen, nitrogen, and sulfur atoms, comprising reacting an α-methoxycarboxylic acid ester represented by General Formula (2): (CF3)2C(OCH3)COOR, wherein R is as defined above, with molecular chlorine; and a process for producing 1,1,1,3,3,3-hexafluoroisopropyl fluoromethyl ether represented by a chemical formula (CF3)2CH(OCH2F), comprising fluorinating and decarboxylating the α-chloromethoxycarboxylic acid ester.According to the present invention, 1,1,1,3,3,3-hexafluoroisopropyl fluoromethyl ether (sevoflurane), which is known as a compound having an anesthetic property, can be produced efficiently and at a low cost.
Owner:DAIKIN IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com