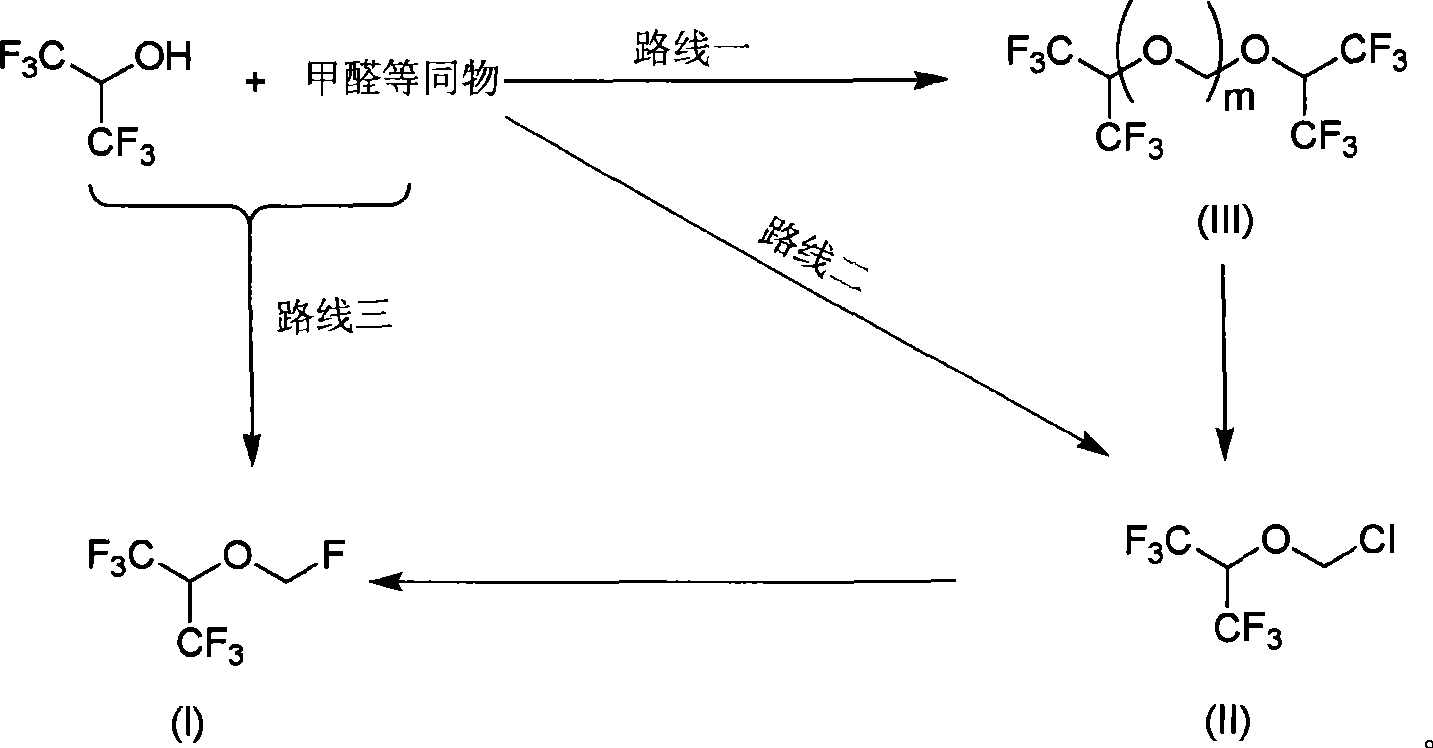
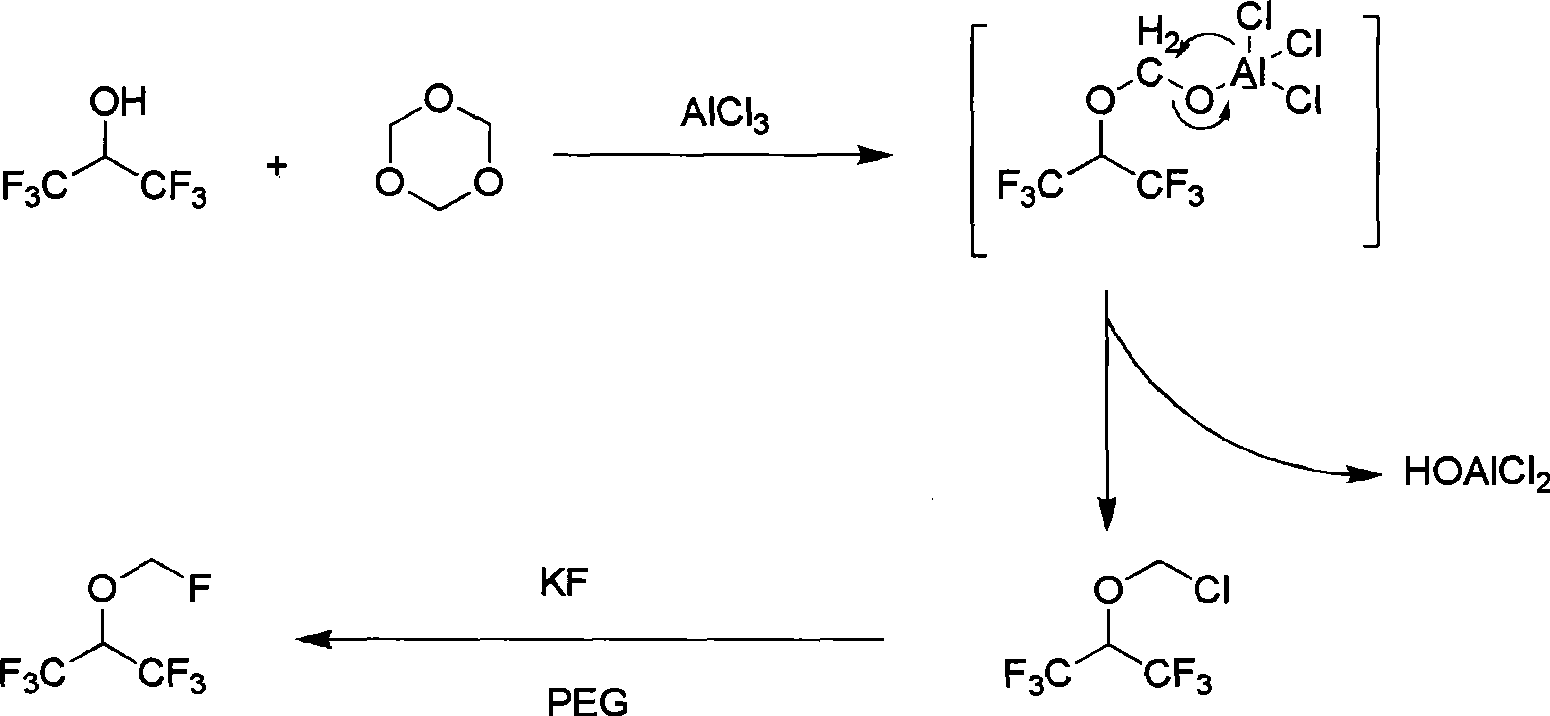
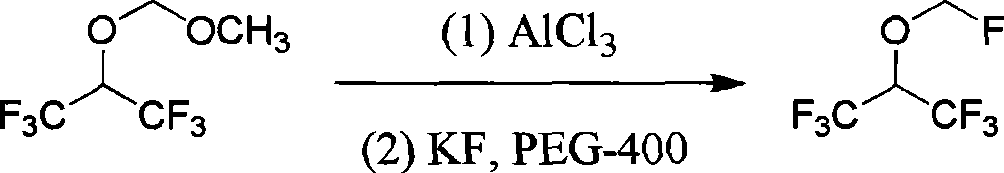Sevoflurane synthesizing method
A sevoflurane, a new synthesis technology, applied in ether preparation, organic chemistry and other directions, can solve the problems of decreased yield and purity, unsuitable for industrial production, raw materials need to be recovered, etc., and achieves easy operation, easy control of reaction conditions, and three wastes discharge. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Add 400 g of hexafluoroisopropanol, 84 g of paraformaldehyde and 30 ml of concentrated sulfuric acid into a 500 ml flask, stir and heat to 45-50° C., react for 2 hours, stop stirring, cool down to room temperature, and set aside for later use.
[0066] 300.0 g of sevoflurane crude product and 340 g of anhydrous aluminum trichloride were added to a 3000ml three-necked flask, cooled to 0 °C, and the reaction solution for use in Example 1 was added dropwise with stirring, and the temperature of the reaction solution was controlled not to exceed 10 °C. After completion of the reaction at 0 °C for 0.5 hours, heating to 25-30 °C, after 10 hours of reaction, 1000 ml of polyethylene glycol was added, 232 g of anhydrous potassium fluoride was added with stirring, and the reaction was heated and refluxed under normal pressure for 4 hours; All fractions were collected to obtain 661.5 g of crude sevoflurane (GC 97.36%, yield 70.1%). The crude product was rectified to obtain 584.7 g...
Embodiment 2
[0068] Add 400 g of hexafluoroisopropanol, 84 g of paraformaldehyde, and 40 ml of concentrated sulfuric acid into a 500 ml flask, stir and heat to 45-50 ° C, react for 2 hours, stop stirring, cool down to room temperature, and separate the lower layer of concentrated sulfuric acid for later use.
[0069] 300.0 g of sevoflurane crude product and 340 g of anhydrous aluminum trichloride were added to a 3000ml three-necked flask, cooled to 0 °C, and the reaction solution for use in Example 1 was added dropwise with stirring, and the temperature of the reaction solution was controlled not to exceed 10 °C. After completion of the reaction at 0 °C for 0.5 hours, heating to 25-30 °C, after 10 hours of reaction, 1000 ml of polyethylene glycol was added, 232 g of anhydrous potassium fluoride was added with stirring, and the reaction was heated and refluxed under normal pressure for 4 hours; All fractions were collected to obtain 676.5 g of crude sevoflurane (GC 97.36%, yield 73.2%). The...
Embodiment 3
[0072] Add 300.0 g of sevoflurane crude product, 340 g of anhydrous aluminum trichloride, 400 g of hexafluoroisopropanol, and 84 g of paraformaldehyde into a 3000-ml three-necked flask, cool down to 0 °C, and add 40 ml of concentrated sulfuric acid dropwise with stirring to control the reaction solution. The temperature does not exceed 10 °C, after the addition is completed, the reaction is carried out at 0 °C for 0.5 hours, and the temperature is heated to 25-30 °C. After 10 hours of reaction, 1000 ml of polyethylene glycol is added, and 232 g of anhydrous potassium fluoride is added with stirring, and the reaction is heated and refluxed under normal pressure. 4 hours; atmospheric distillation, collecting all fractions to obtain a total of 636.5 g of crude sevoflurane (GC 97.36%, yield 65.3%). The crude product was rectified to obtain 542.3 g of sevoflurane (GC 99.998%, yield 85.2%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com