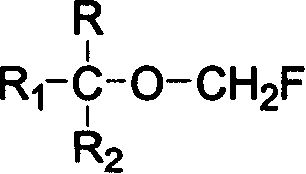
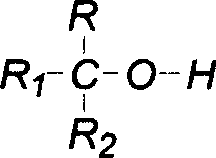
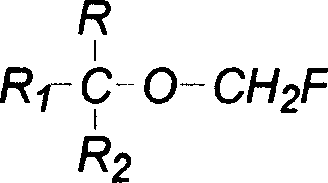
Single fluorine substituted methyl ether preparation method
A monofluoromethyl ether, fluorinated technology, applied in the field of preparation of monofluoromethyl ether, can solve the problem of low reaction conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 168g of hexafluoroisopropanol, 11.3g of boron trifluoride etherate complex and 90g of paraformaldehyde into a 250mL single-necked bottle equipped with a magnetic stirring and reflux device, heat and reflux for 5 hours, and after the reaction is completed, 186g is obtained by distillation. According to GC analysis, it contains 90.5% of sevoflurane, and the yield is 84%.
Embodiment 2
[0027] Add 168g of hexafluoroisopropanol, 6.7g of anhydrous aluminum trifluoride, 120mL of chloroform and 90g of paraformaldehyde into a 500mL single-necked bottle with a magnetic stirring and reflux device, heat and reflux for 5 hours, and after the reaction is complete, distill to obtain 164g , containing sevoflurane 88.2% by GC analysis, yield: 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com