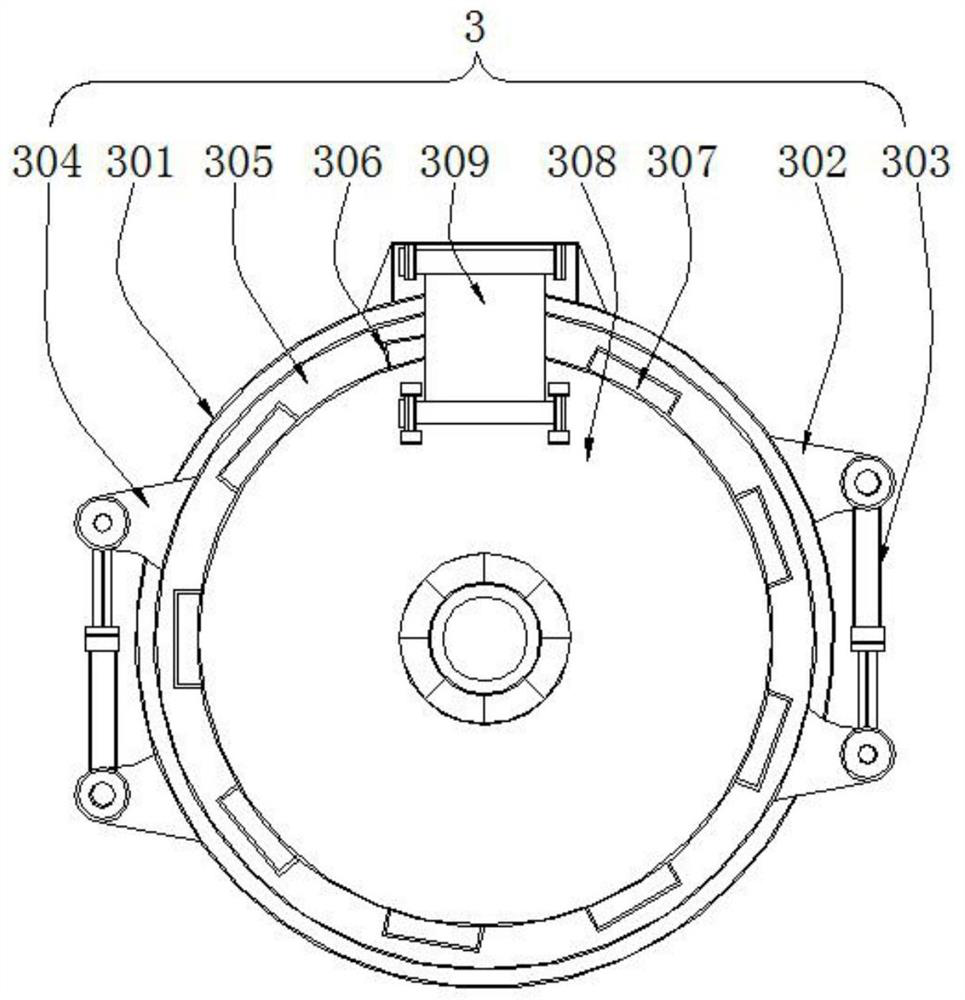
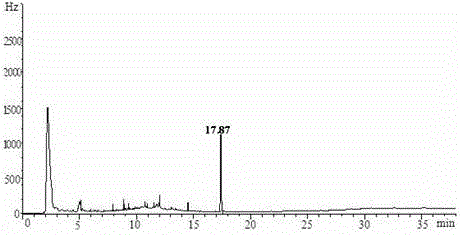
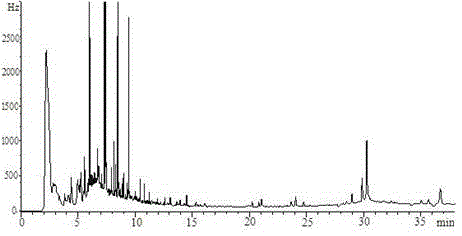
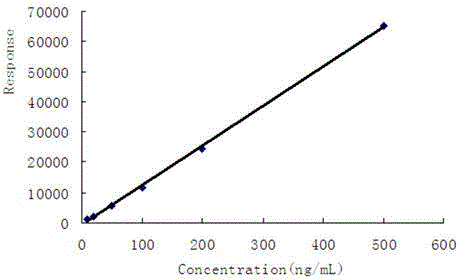
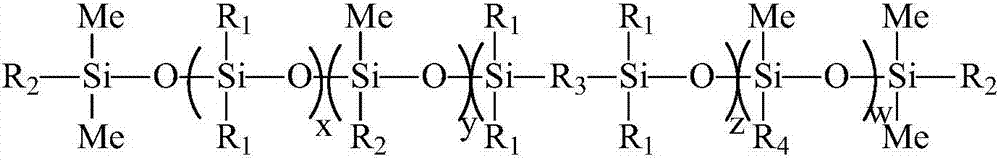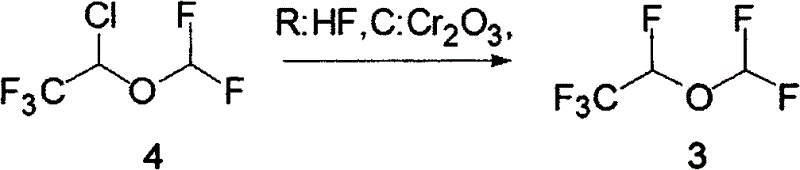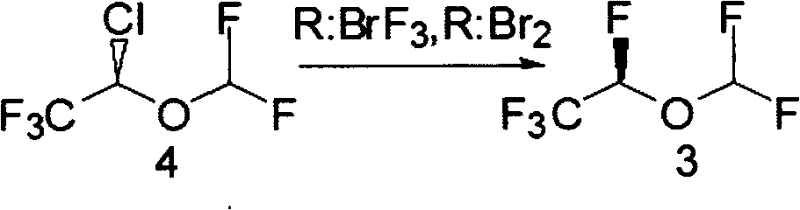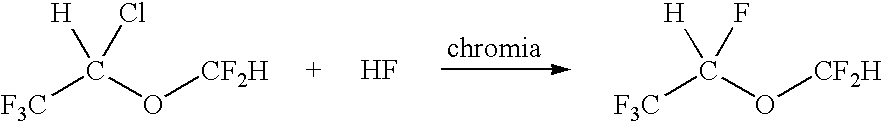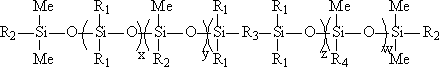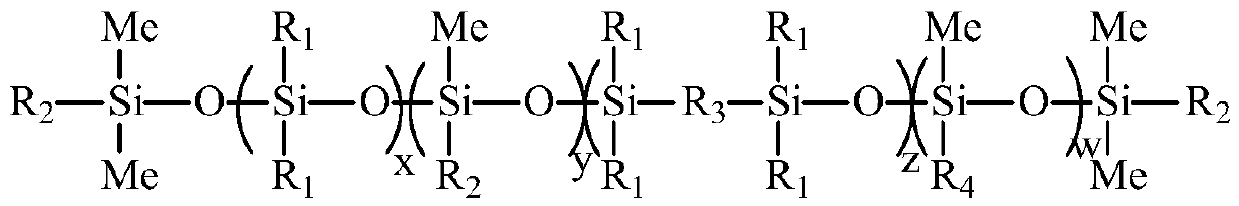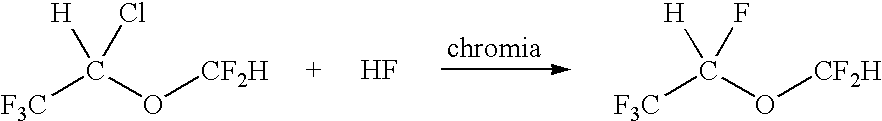Patents
Literature
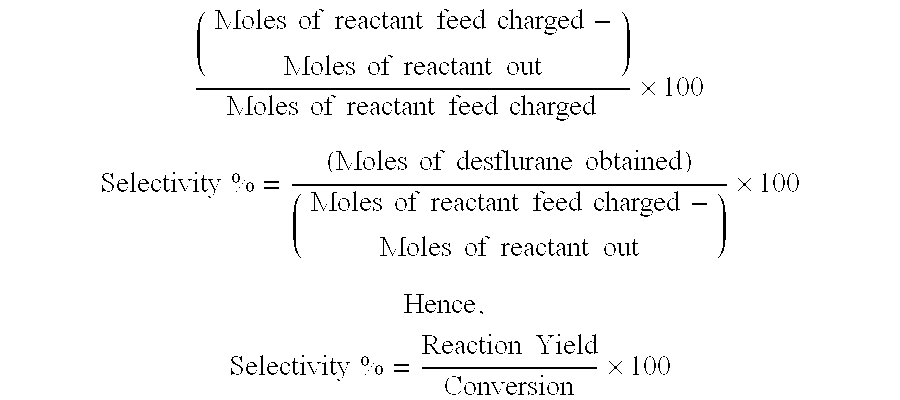
33 results about "Desflurane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Desflurane (1,2,2,2-tetrafluoroethyl difluoromethyl ether) is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane, and isoflurane, it is a racemic mixture of (R) and (S) optical isomers (enantiomers). Together with sevoflurane, it is gradually replacing isoflurane for human use, except in economically undeveloped areas, where its high cost precludes its use. It has the most rapid onset and offset of the volatile anesthetic drugs used for general anesthesia due to its low solubility in blood.
Anesthetic agent recovery
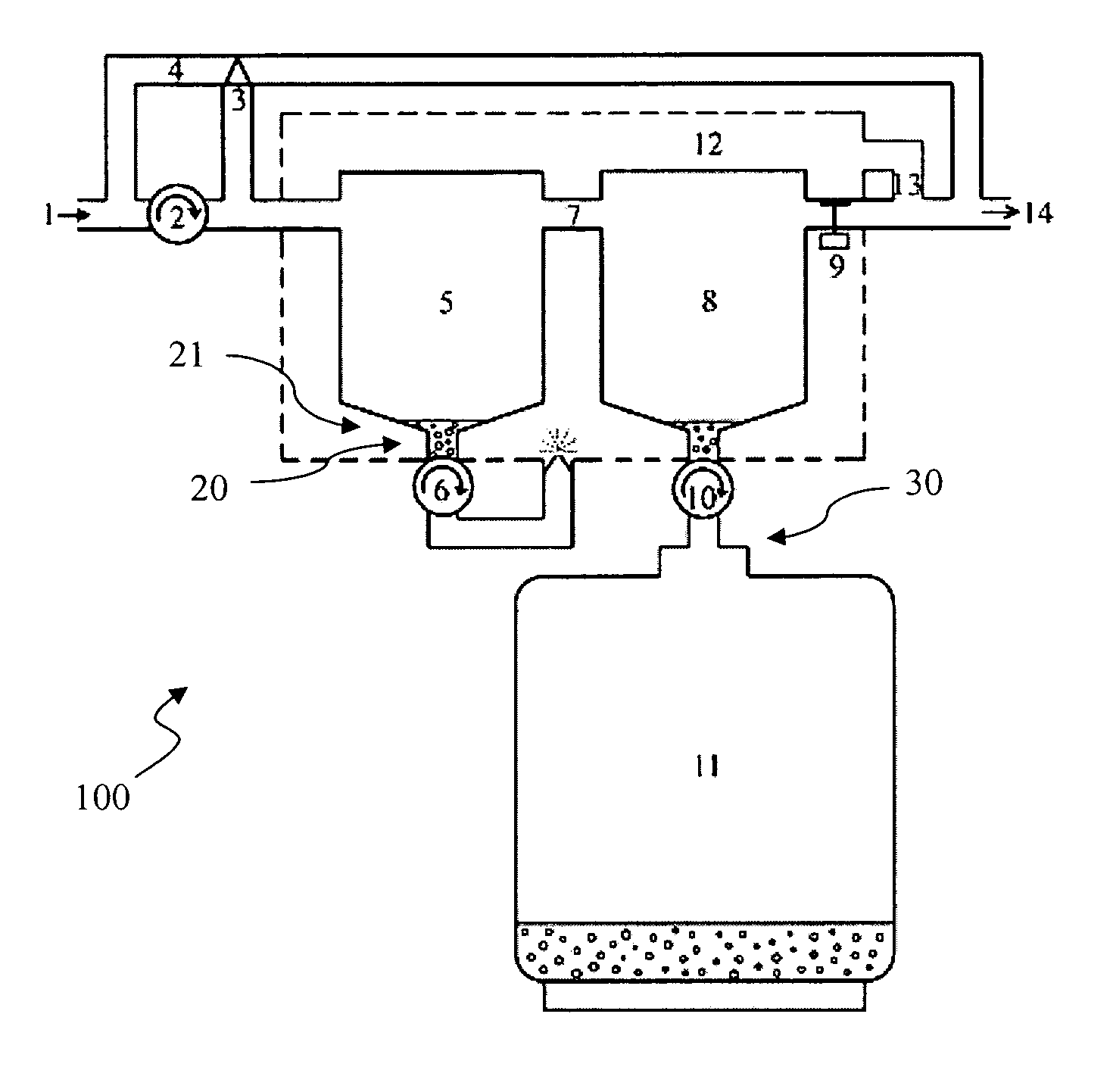
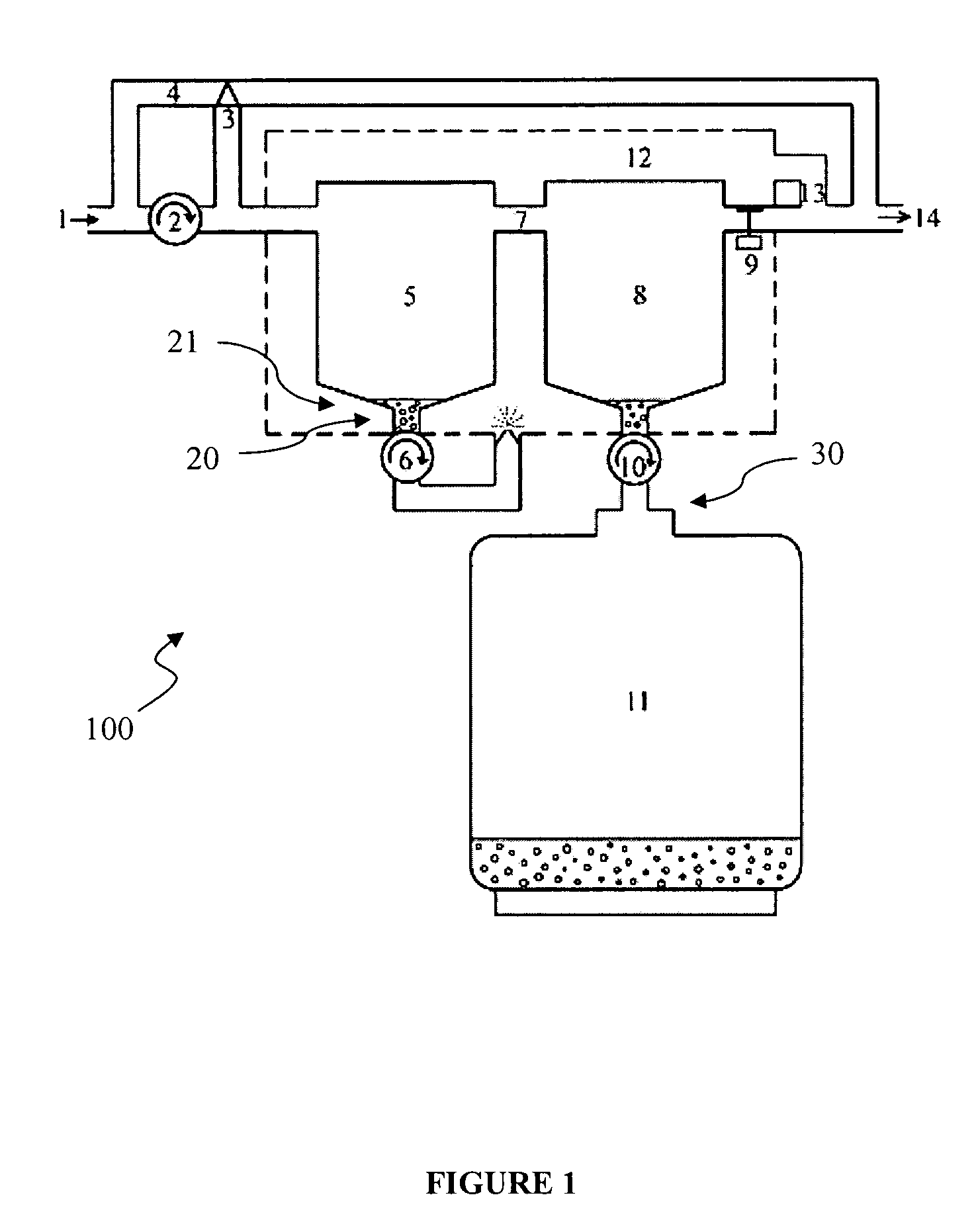
Disclosed and claimed herein are devices and method for the recovery of one or more anesthetic agents after they have been exhaled from a patient undergoing surgery and before they have been vented to the atmosphere. Typical anesthetic agents include, but are not limited to, isoflurane, desflurane, sevoflurane, and the like. Recovery of the anesthetic agents should result in numerous benefits including, but not limited to, reduction of their production costs, protection of the environment, and the like.
Owner:ROCK MICHAEL
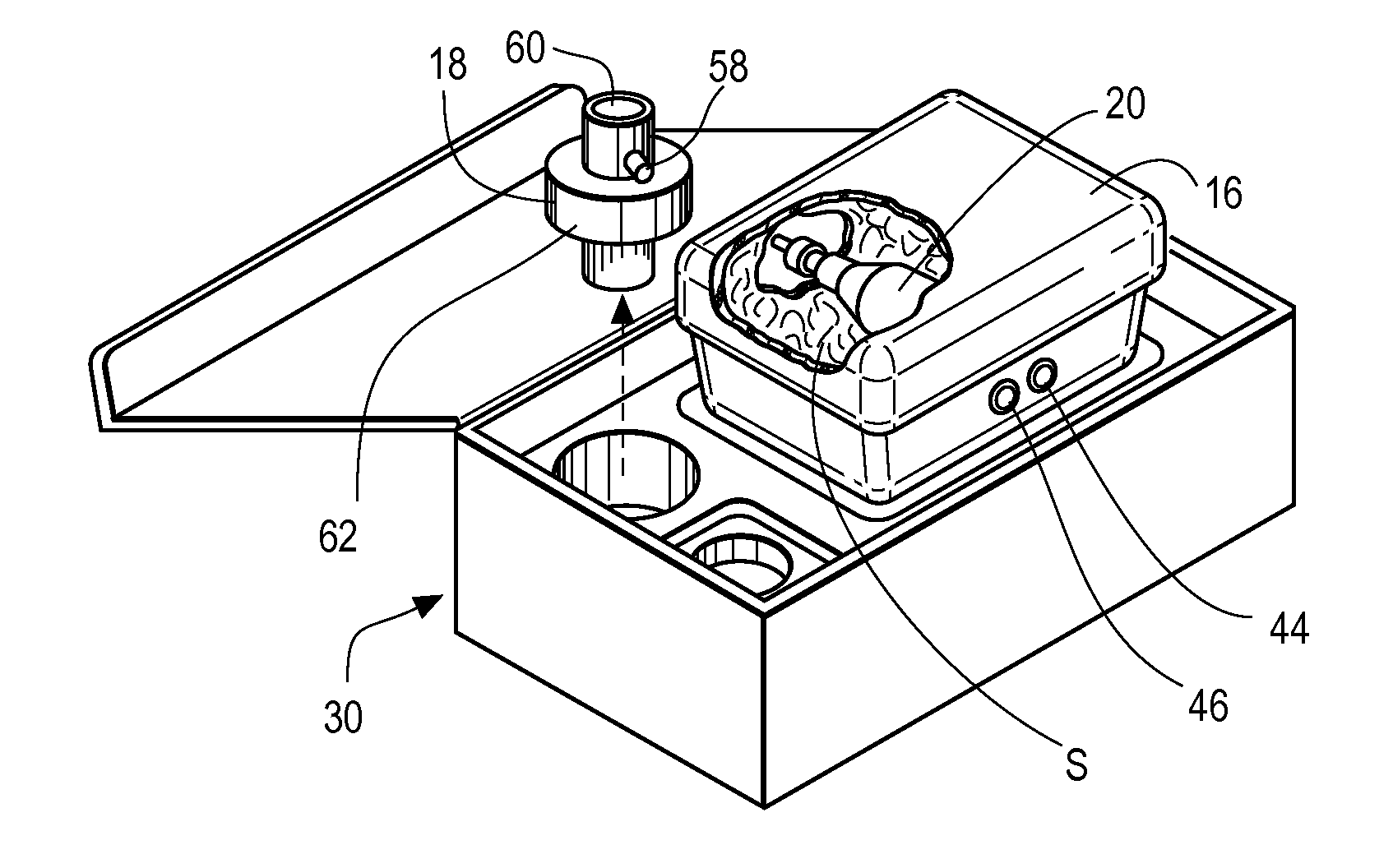
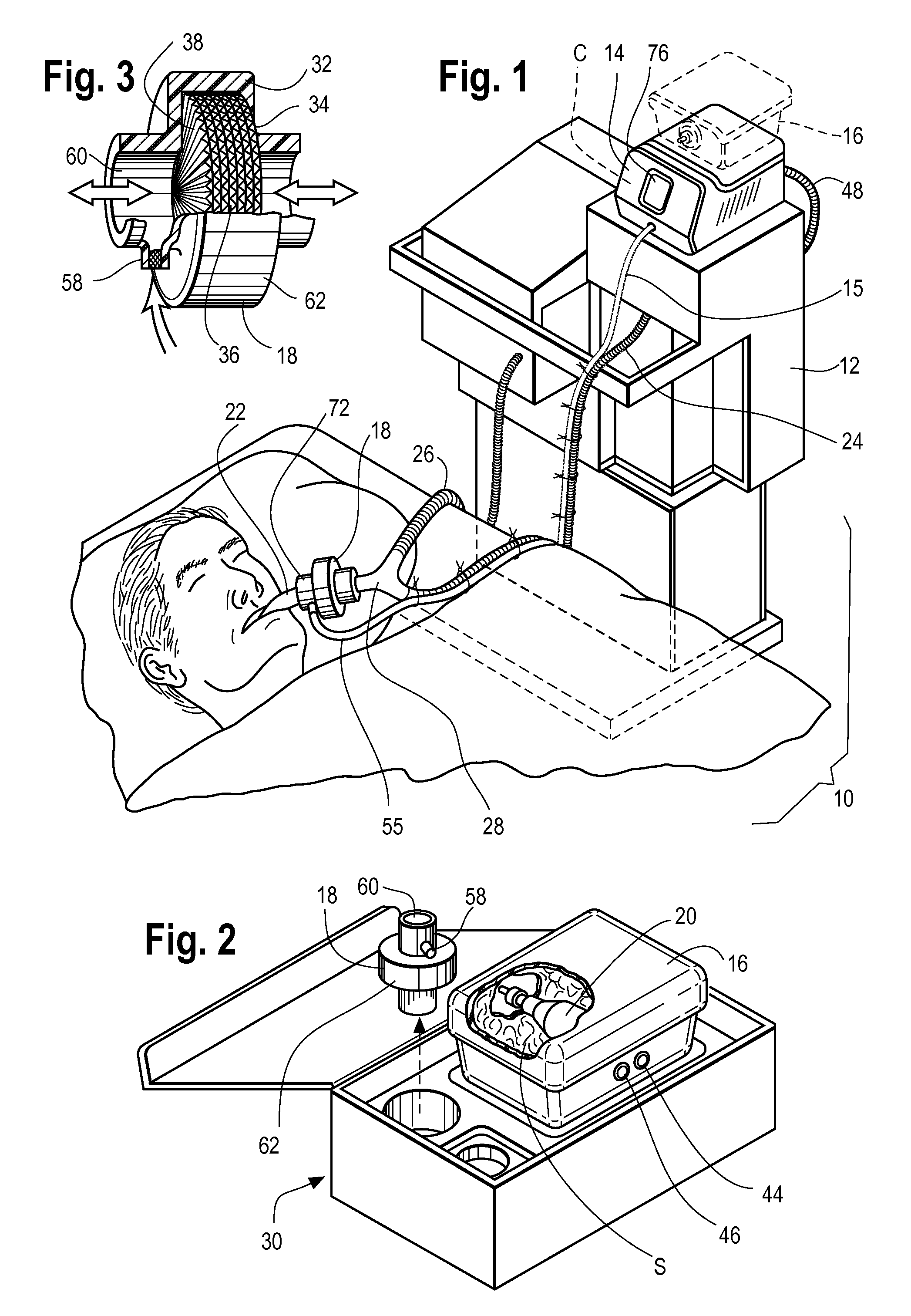
Inhaled anesthetic agent therapy and delivery system
InactiveUS20100212668A1Decrease and replace useShorten the lengthTracheal tubesBreathing filtersMedical intensive care unitSurgical intensive care unit
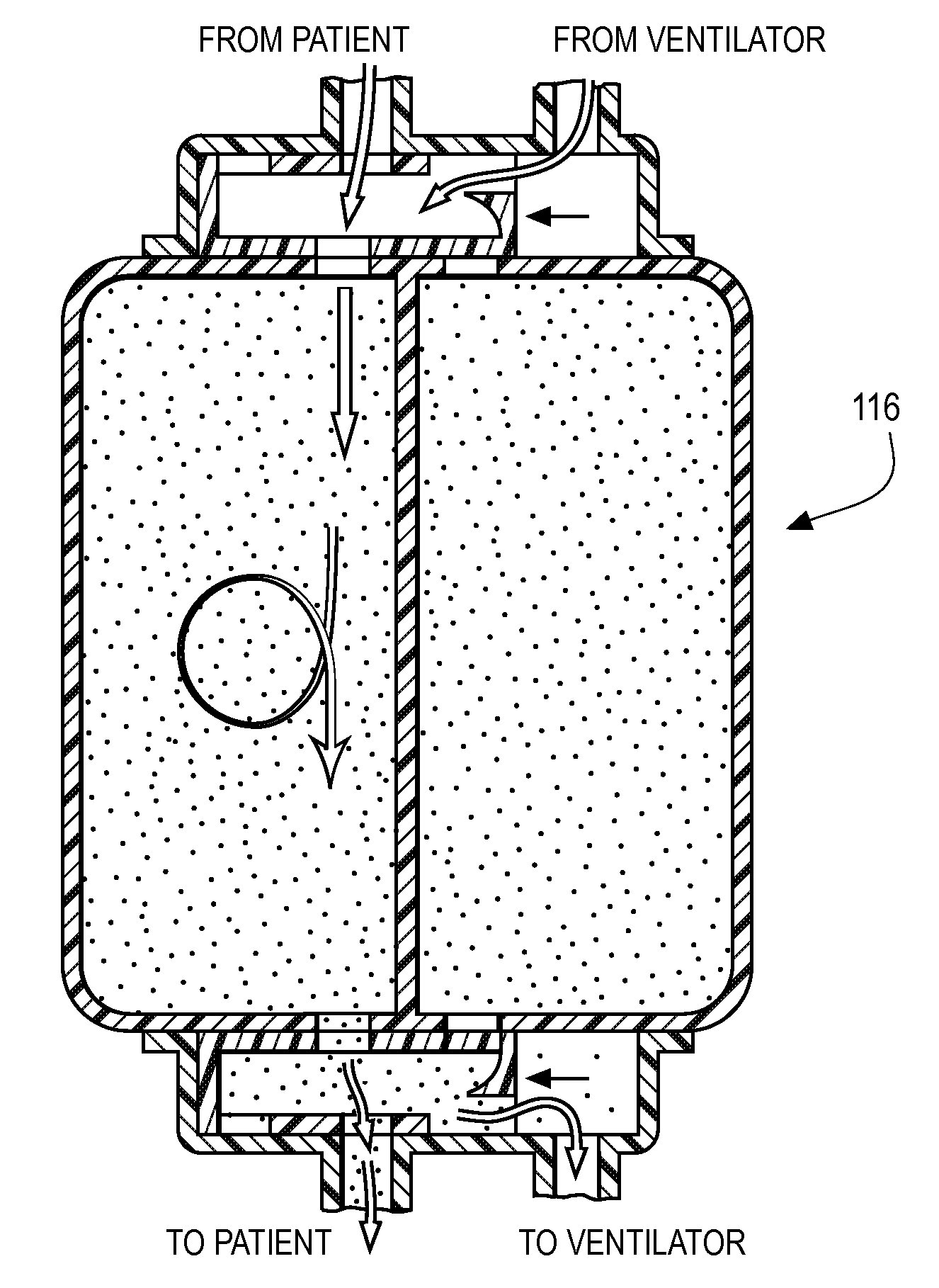
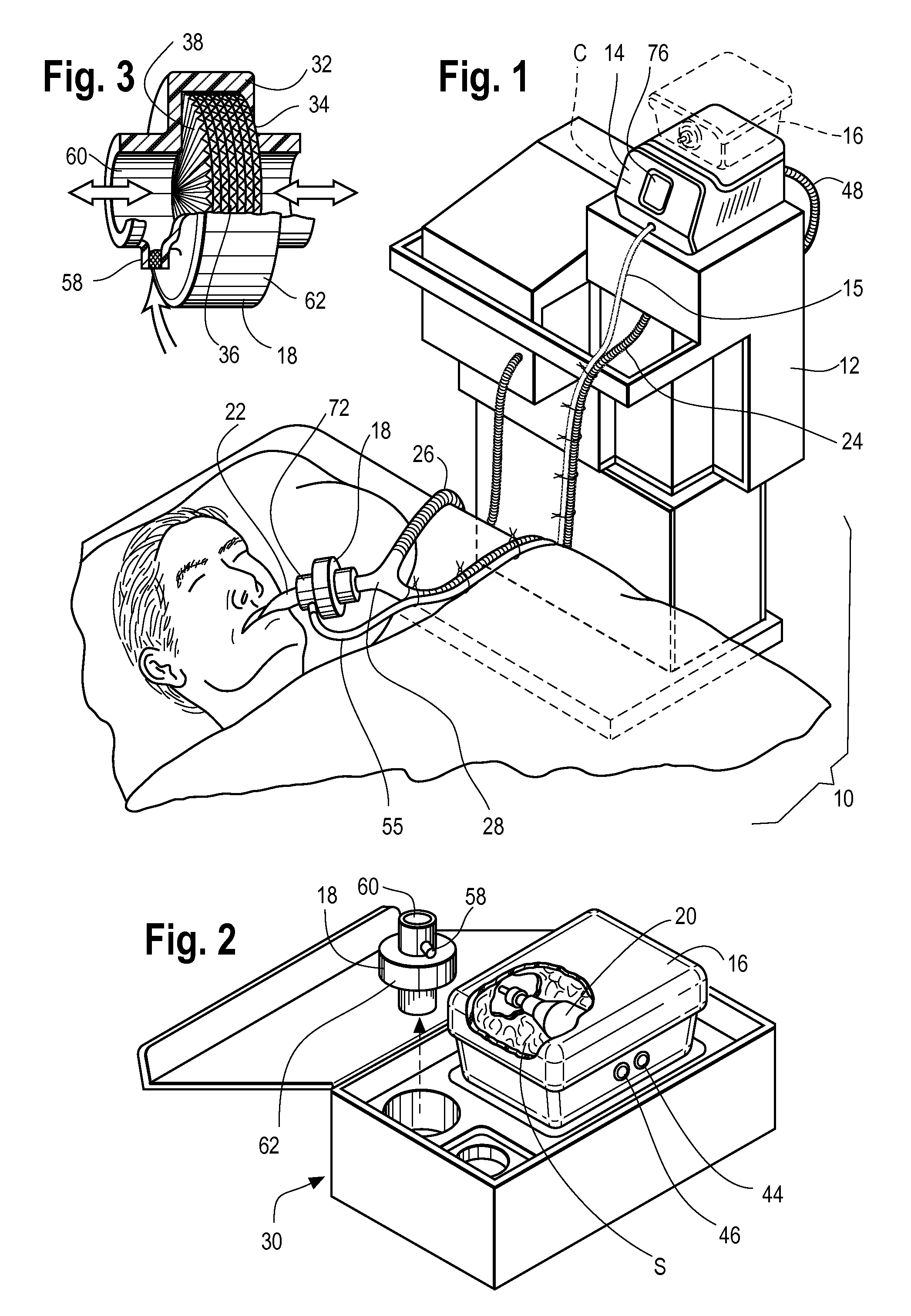
A therapy utilizing inhaled anesthetic agents (such as desflurane, sevoflurane, isoflurane, or xenon) for the sedation of patients outside of the immediate perioperative space such as in the medical intensive care unit (MICU) and the surgical intensive care unit (SICU). The therapy includes controlled delivery of volatile anesthetic agents to patients undergoing ventilatory support on an ICU ventilator over extended periods of time. A system which provides for the delivery of anesthetic agents includes an anesthetic agent vaporizer element, an anesthetic agent reflector, and a plug-in cassette which contains both a cartridge housing liquid phase volatile anesthetic agent and an anesthetic vapor scrubbing medium.
Owner:BAXTER INT INC +1
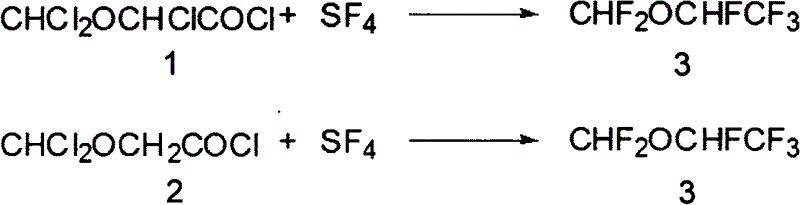
Process for production of 1,2,2,2-tetrafluoro ethyl difluoro methyl ether
InactiveUS20060205983A1High yieldPromote conversionOrganic chemistryOrganic compound preparationHydrogen fluorideEther
A method for the preparation of 1,2,2,2-tetrafluoroethyl difluoromethyl ether (CF3CHFOCHF2, Desflurane) is provided, which comprises reacting CF3CHClOCHF2 (isoflurane) and hydrogen fluoride in the presence of antimony pentafluoride such that desflurane is formed.
Owner:FIRST NIAGARA BANK
Inhaled anesthetic agent therapy and delivery system
InactiveUS8267081B2Decrease and replace useShorten the lengthTracheal tubesBreathing filtersMedical intensive care unitSurgical intensive care unit
A therapy utilizing inhaled anesthetic agents (such as desflurane, sevoflurane, isoflurane, or xenon) for the sedation of patients outside of the immediate perioperative space such as in the medical intensive care unit (MICU) and the surgical intensive care unit (SICU). The therapy includes controlled delivery of volatile anesthetic agents to patients undergoing ventilatory support on an ICU ventilator over extended periods of time. A system which provides for the delivery of anesthetic agents includes an anesthetic agent vaporizer element, an anesthetic agent reflector, and a plug-in cassette which contains both a cartridge housing liquid phase volatile anesthetic agent and an anesthetic vapor scrubbing medium.
Owner:BAXTER INT INC +1
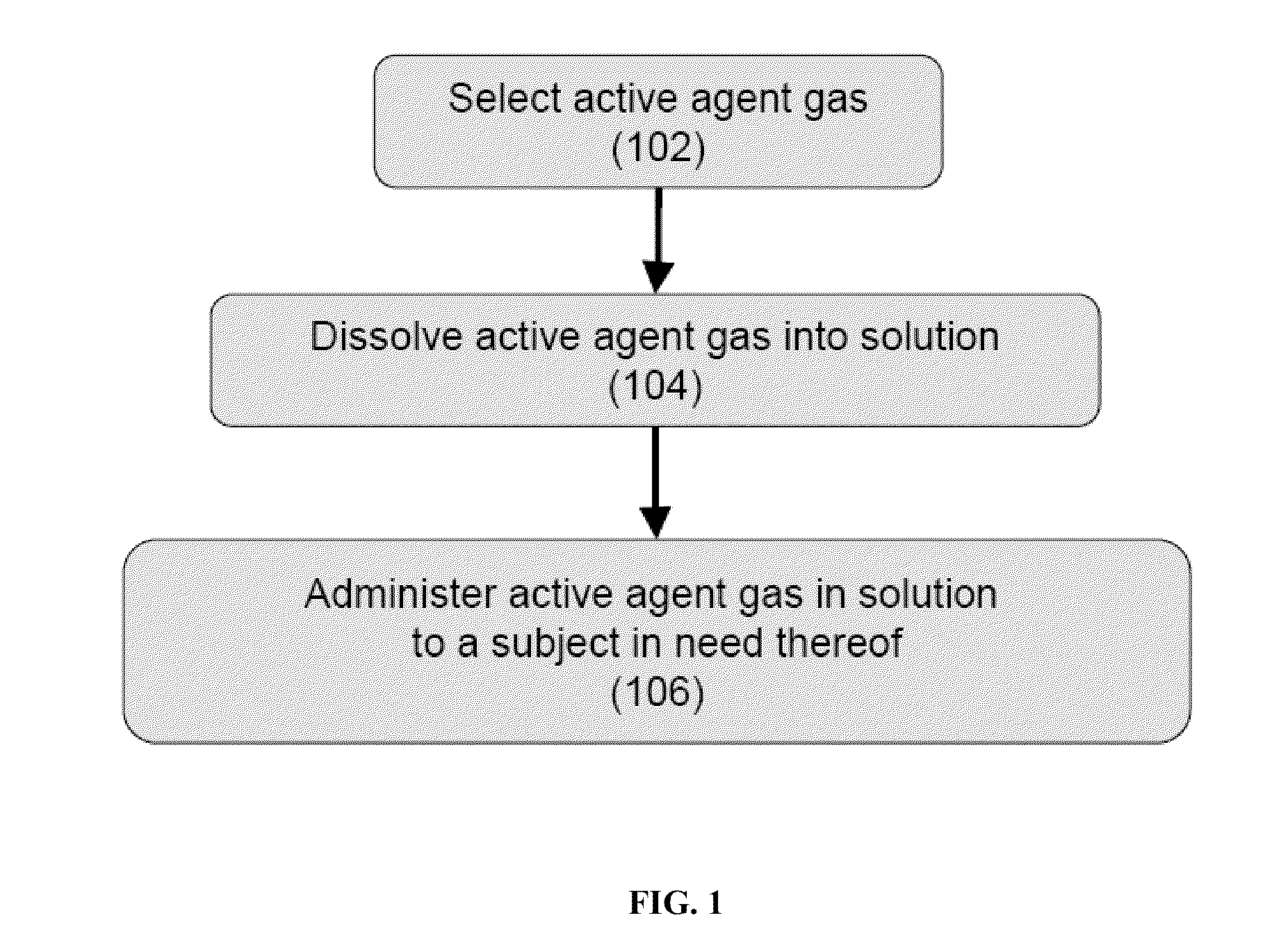
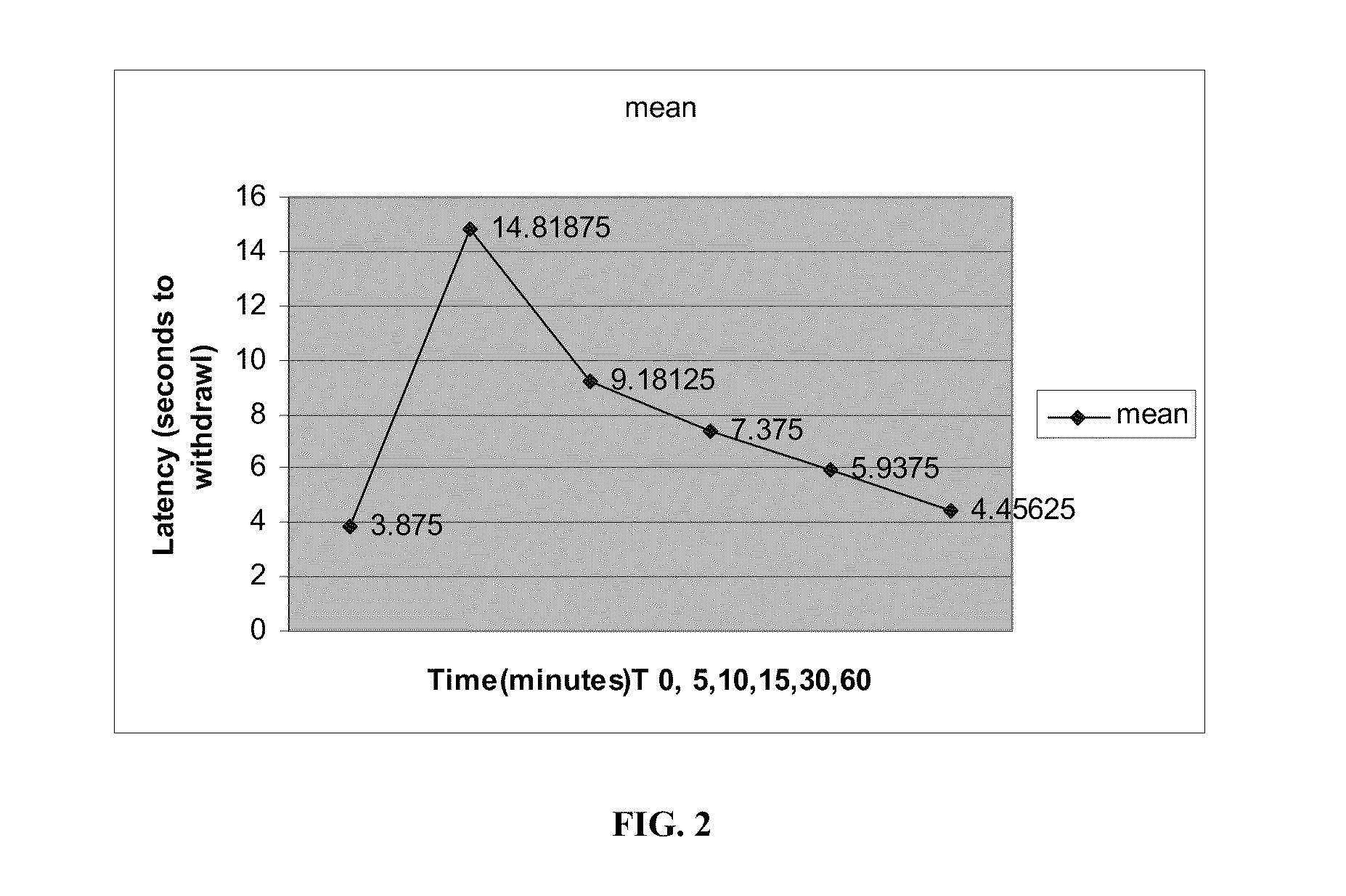
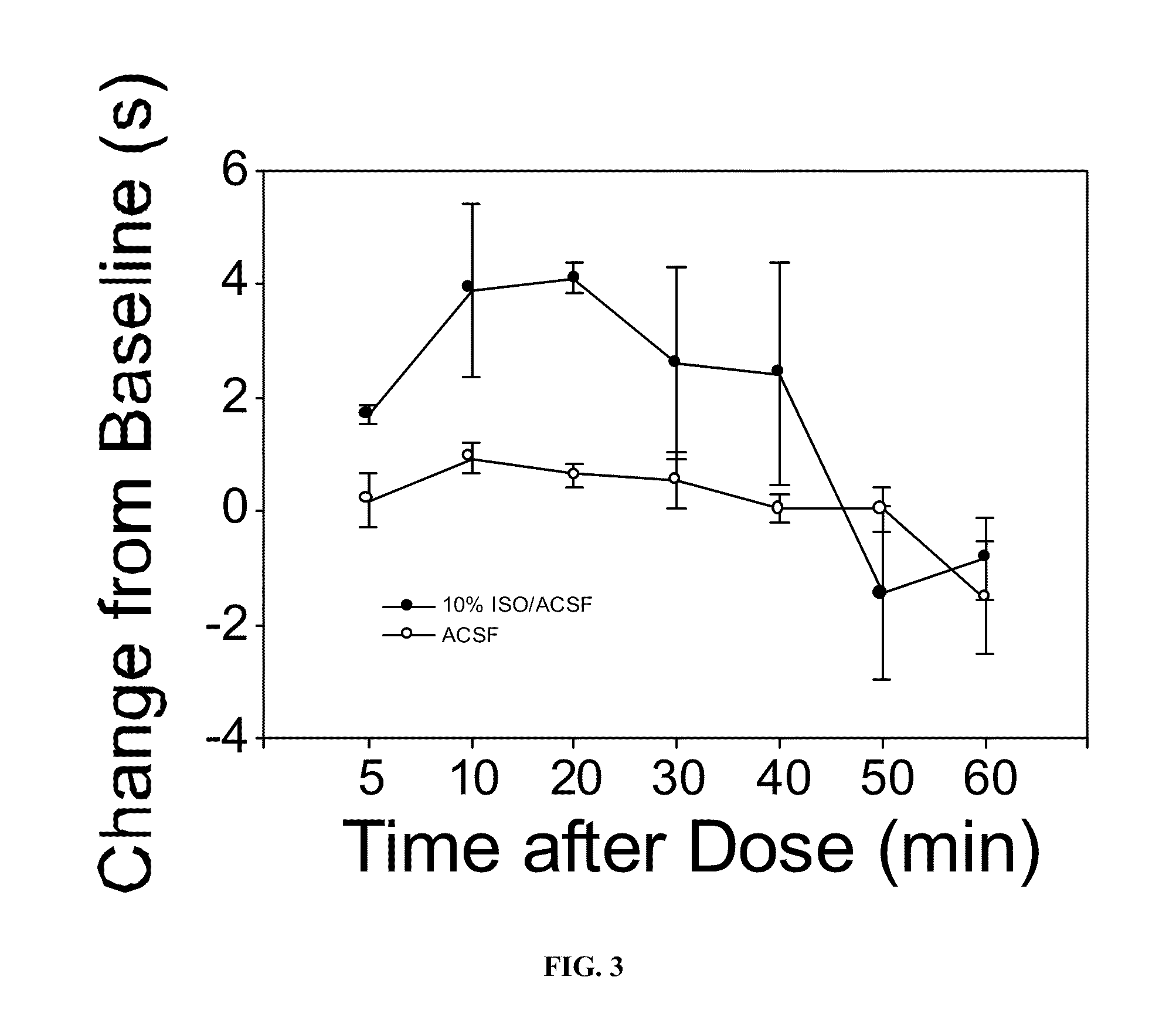
Volatile anesthetic compositions comprising extractive solvents for regional anesthesia and/or pain relief
ActiveUS20110039944A1Rapidly titratableRapid onsetHalogenated hydrocarbon active ingredientsBiocideRegimenSolvent
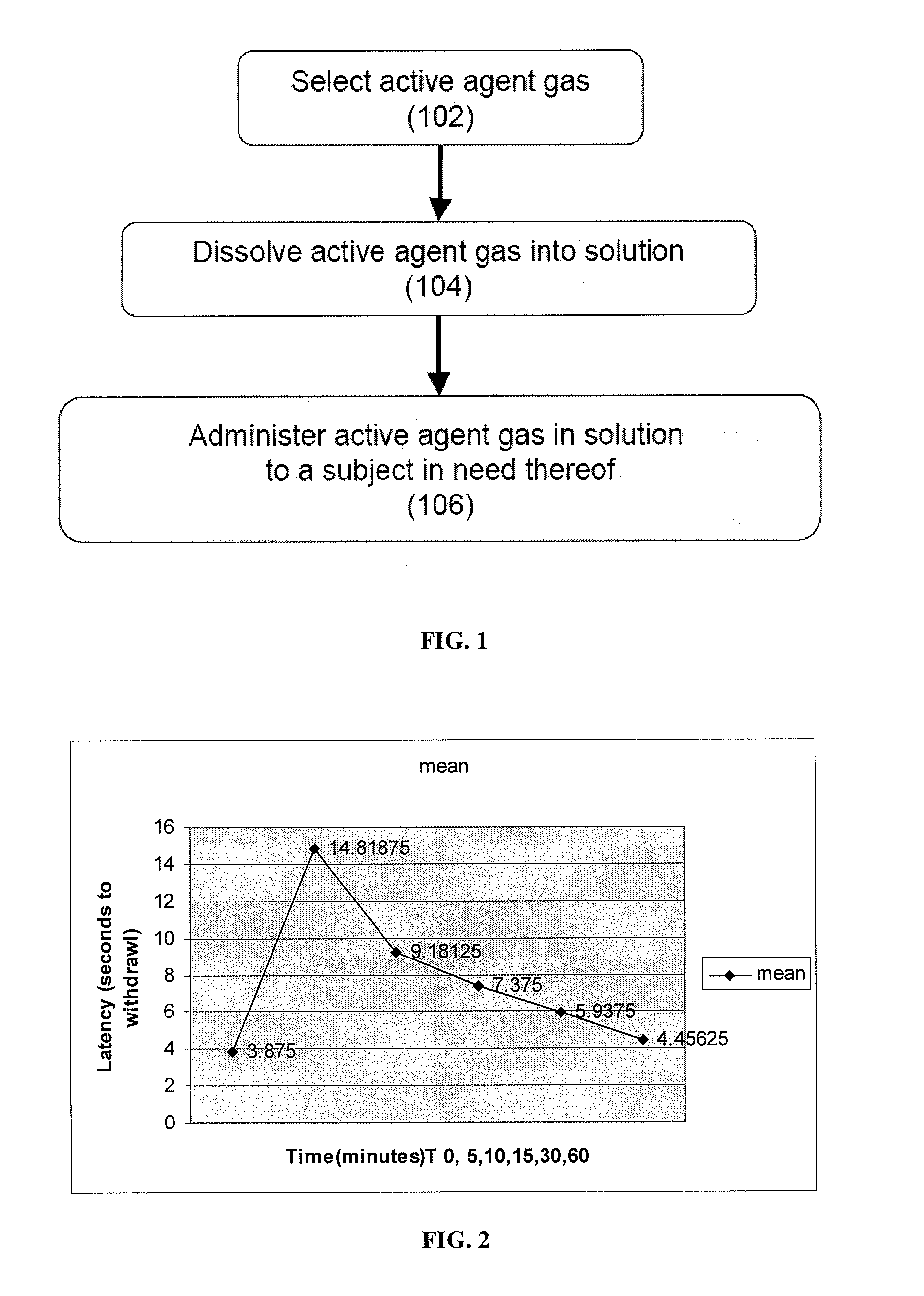
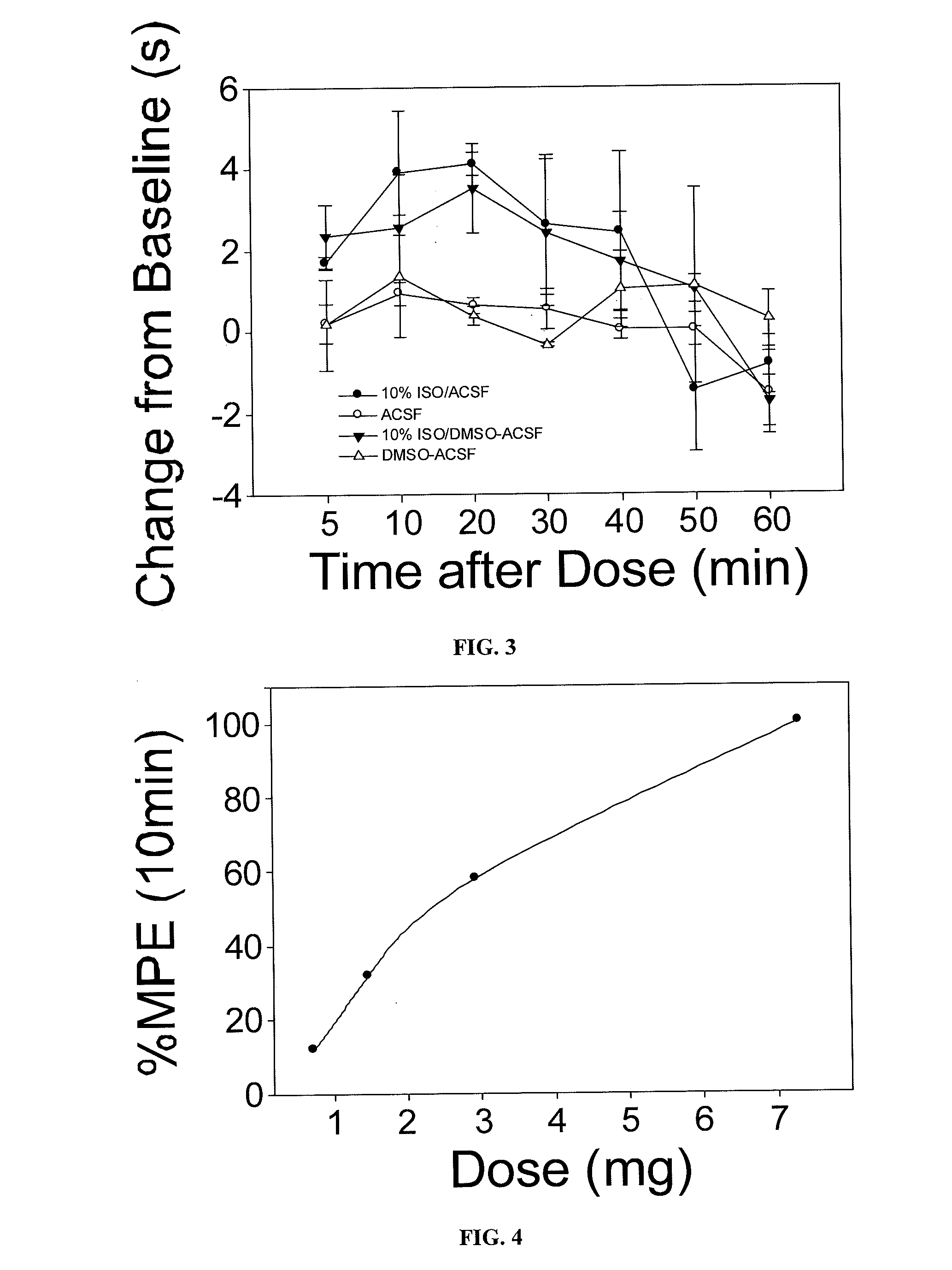
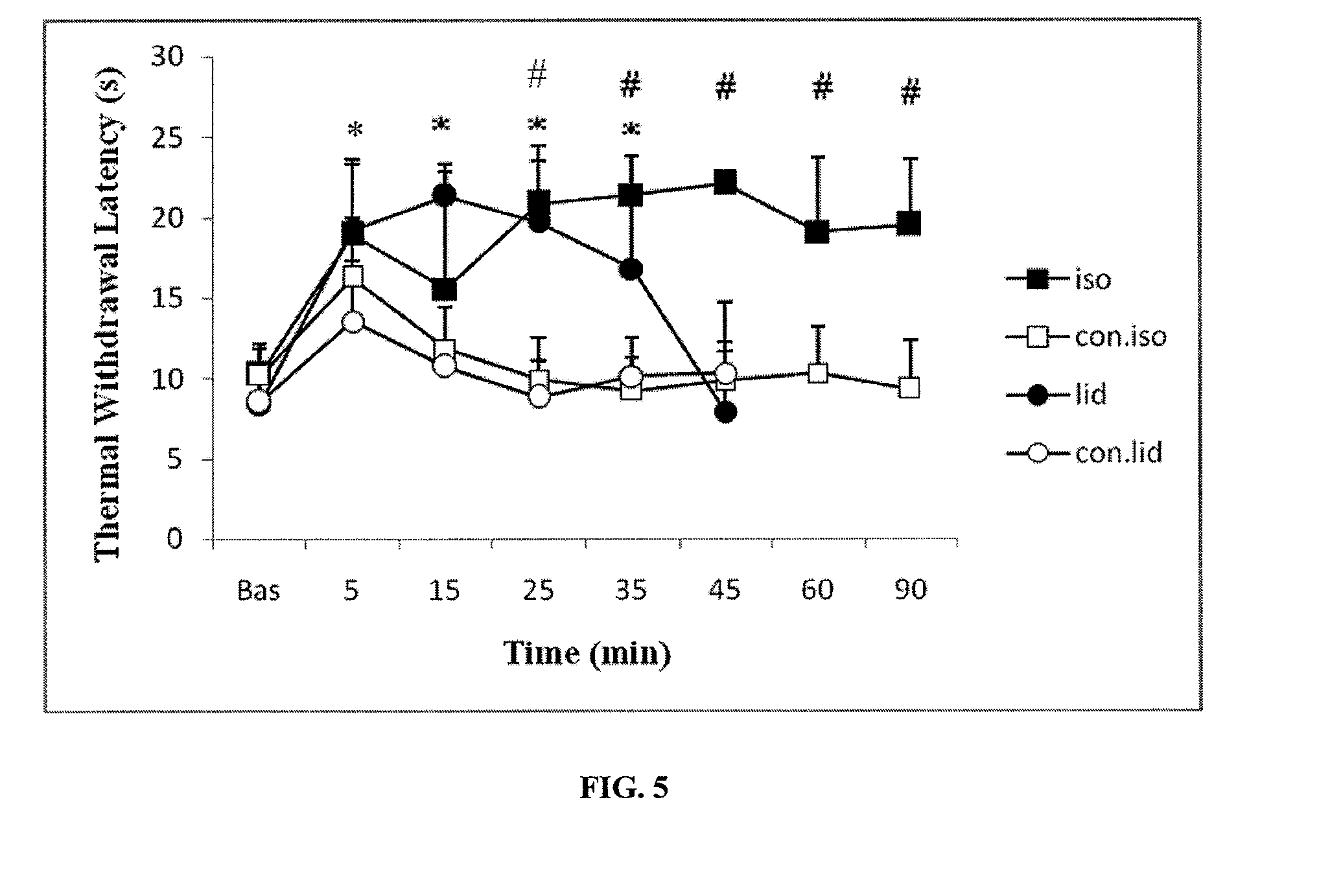
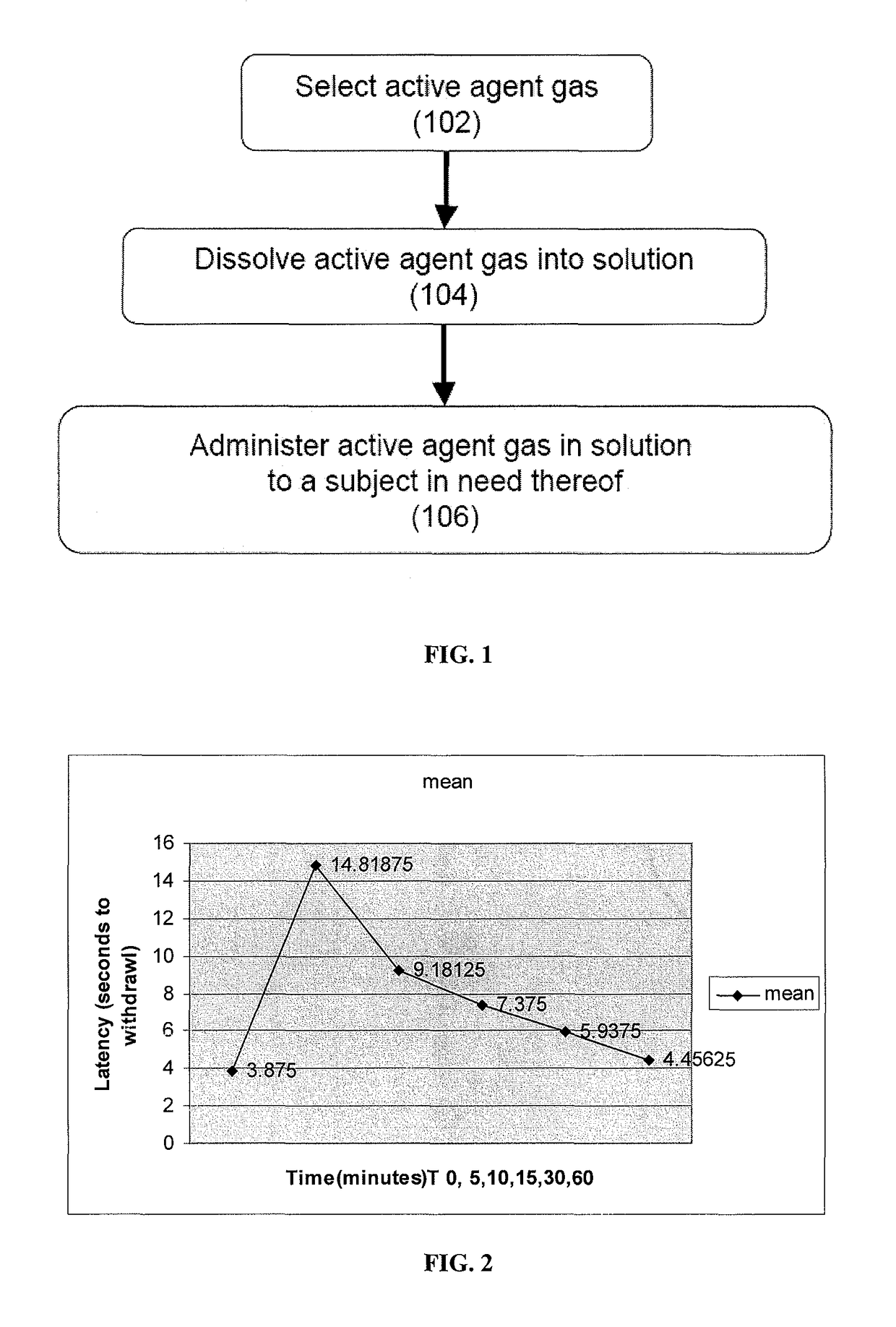
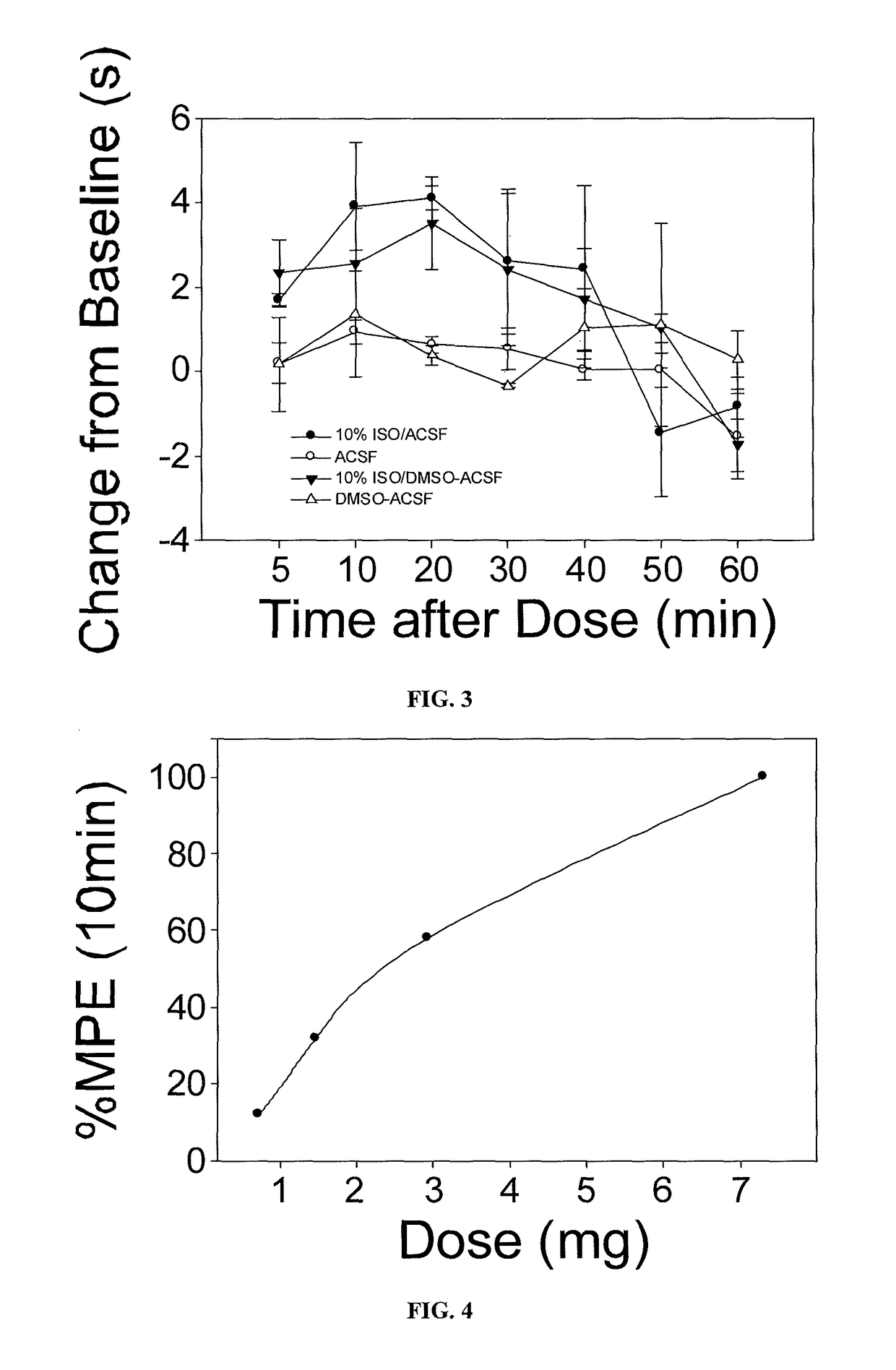
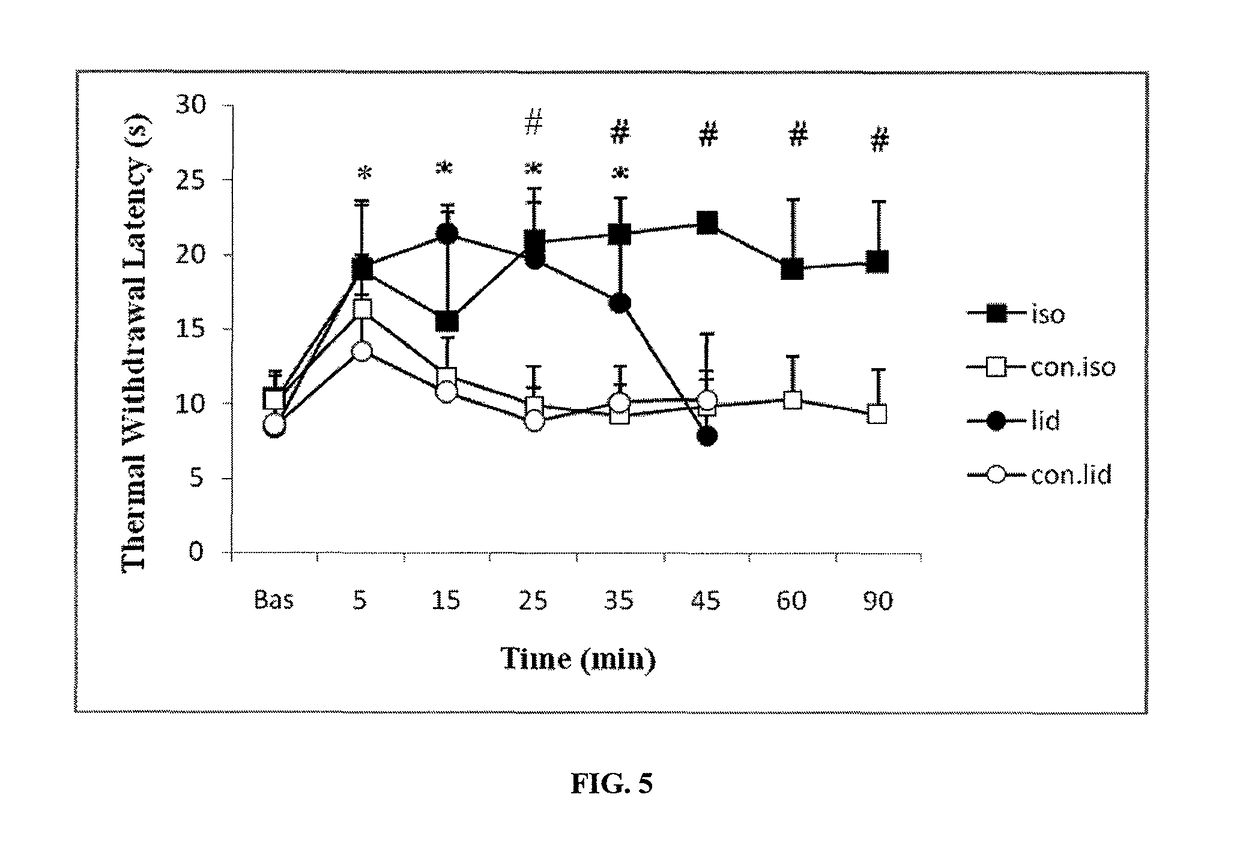
The present invention provides methods for reducing pain in a subject in need of such pain reduction by delivering, e.g., intrathecally or epidurally, a volatile anesthetic dissolved in a solution comprising an extractive solvent, e.g., DMSO or NMP, in an amount effective to reduce pain. Chronic or acute pain may be treated, or the anesthetic may be delivered as a regional anesthesia to a subject to anesthetize a portion the subject prior to a surgery, hi certain embodiments, isoflurane, halothane, enflurane, sevoflurane, desflurane, methoxyflurane, or mixtures thereof may be used. Dosing regimens including a one-time administration, continuous and / or periodic administration are contemplated.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
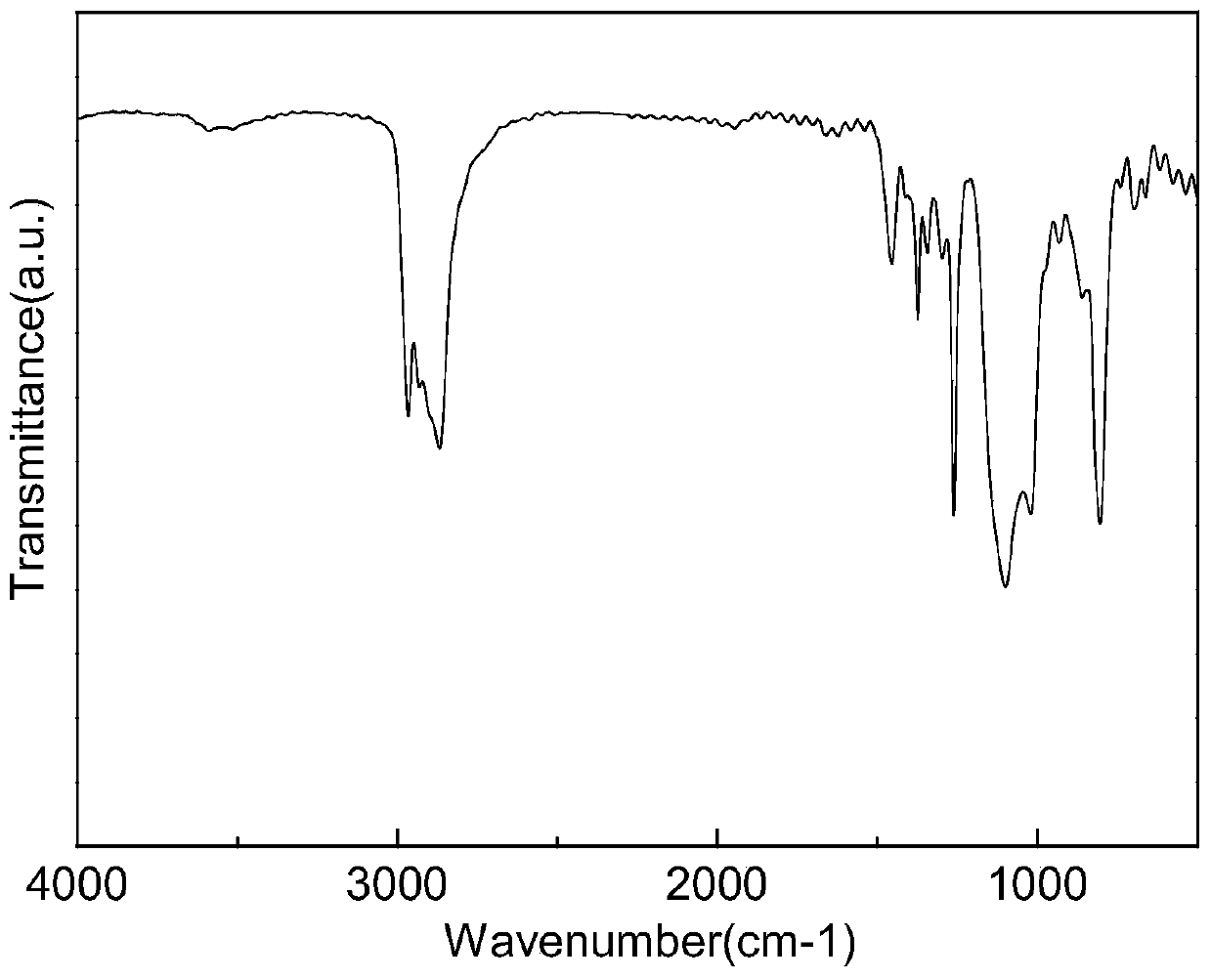
UV (Ultraviolet) curing fluorine-silicon release agent and preparation method thereof
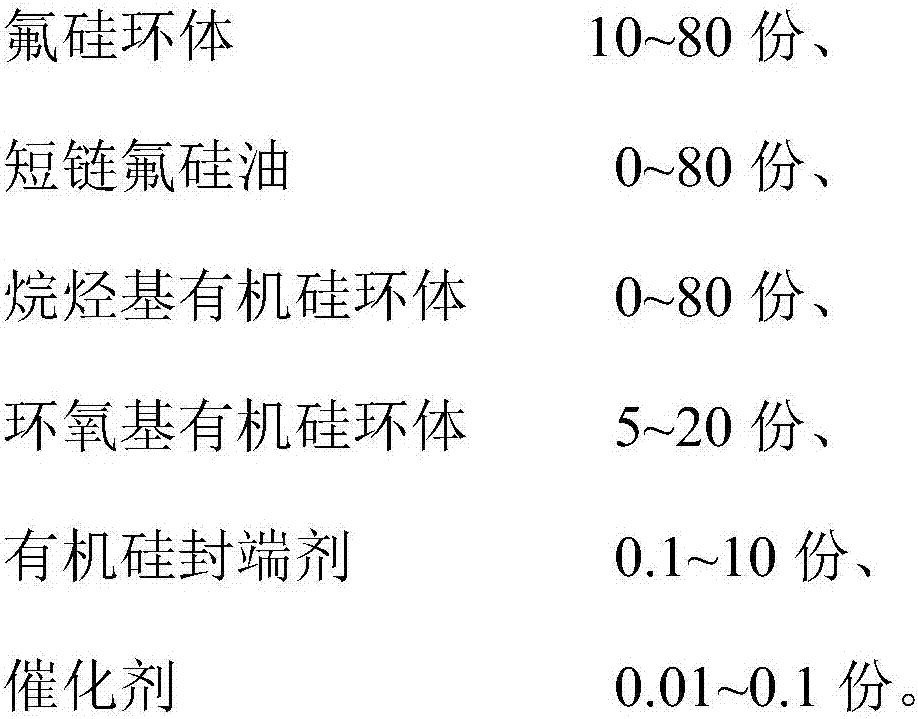
The invention discloses a UV (Ultraviolet) curing fluorine-silicon release agent and a preparation method thereof. The structure of the UV curing fluorine-silicon release agent disclosed by the invention is shown as follows, wherein R1 is CnH2n+1, and n is equal to 1-9; R2 is one of the structural formulas as shown in the specification; R3 is C2H4CnF2nC2h4, and n is equal to 1-9; R4 is C2H4Rf, and Rf is desflurane group or perfluoroalkyl; and x is equal to 1-5000, y is equal to 1-5000, z is equal to 0-5000, and w is equal to 1-5000. According to the UV curing fluorine-silicon release agent disclosed by the invention, the preparation method is simple, and the problems such as high-temperature curing and cheap catalysts can be solved. The structural formula is as shown in the specification.
Owner:安庆龙驰氟硅新材料有限公司
Anesthetic Agent Recovery
Owner:ROCK MICHAEL
Methods for delivering volatile anesthetics for regional anesthesia and/or pain relief
ActiveUS20110269843A1Rapidly titratableRapid onsetHalogenated hydrocarbon active ingredientsBiocideRegimenMethoxyflurane
The present invention provides methods for reducing pain in a subject in need of such pain reduction by delivering, e.g., intrathecally or epidurally, a volatile anesthetic such as a halogenated ether compound in an amount effective to reduce pain. Chronic or acute pain may be treated, or the anesthetic may be delivered to the subject to anesthetize the subject prior to a surgery. In certain embodiments, isoflurane, halothane, enflurane, sevoflurane, desflurane, methoxyflurane, xenon, and mixtures thereof may be used. Dosing regimens including a one-time administration, continuous and / or periodic administration are contemplated.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Synthesis method of desflurane
InactiveCN102617298AHigh yieldHigh purityOrganic chemistryOrganic compound preparationSynthesis methodsEther
The invention provides a synthesis method of desflurane. According to the synthesis method, 1-chlorine-2,2,2-trifluoride ethyl difluoro methyl ether (isoflurane) is used as raw materials, 2-difluoro methyl-1,1,1,2-tetrafluoroethane (desflurane) is obtained through fluorination reaction, and the yield reaches 43.8 percent. The yield is further improved on the basis of the prior art, the cost is reduced, the purity of products is improved, the operability is enhanced, and the synthesis method is suitable for industrialized production.
Owner:EAST CHINA UNIV OF SCI & TECH
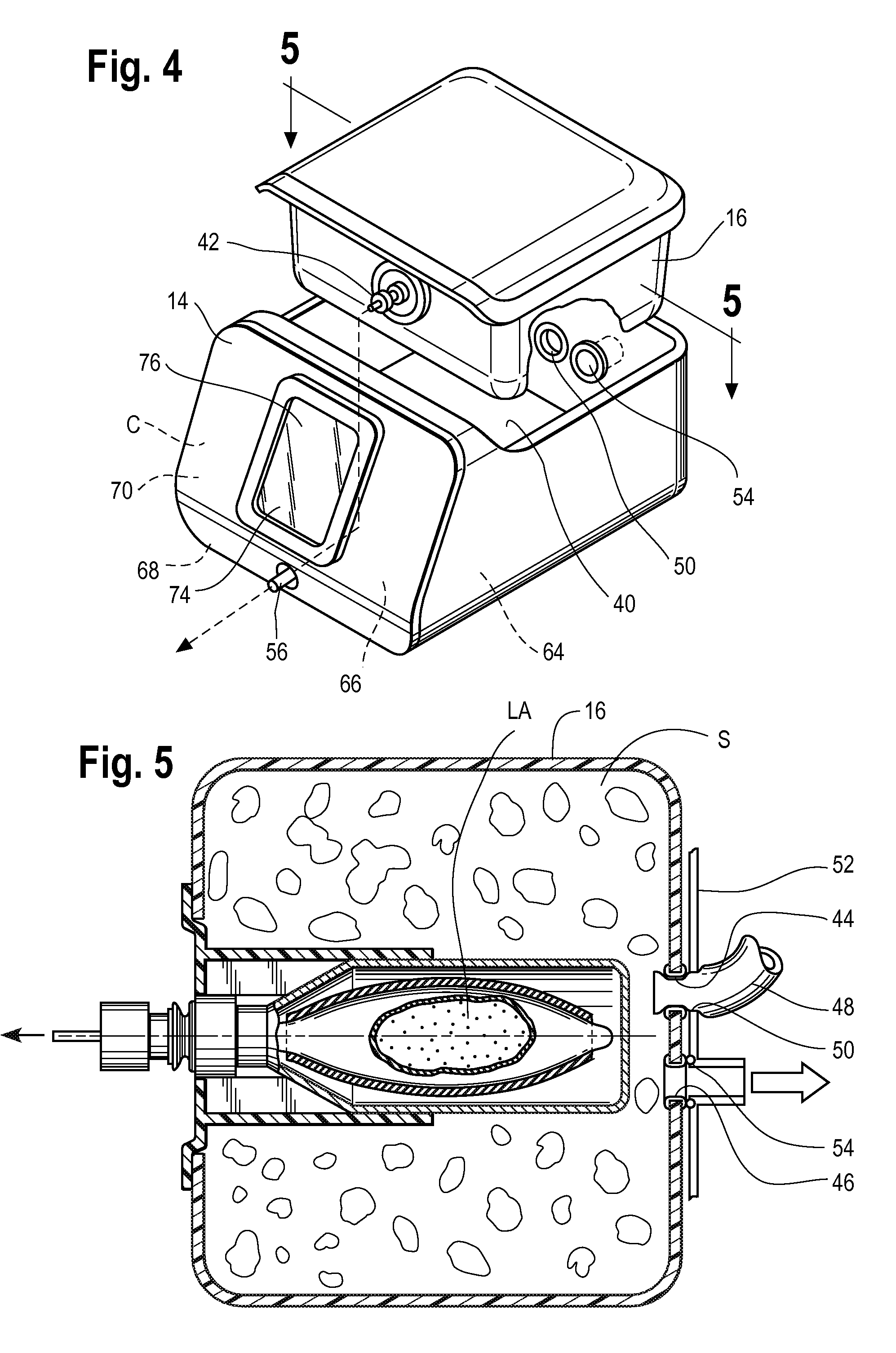
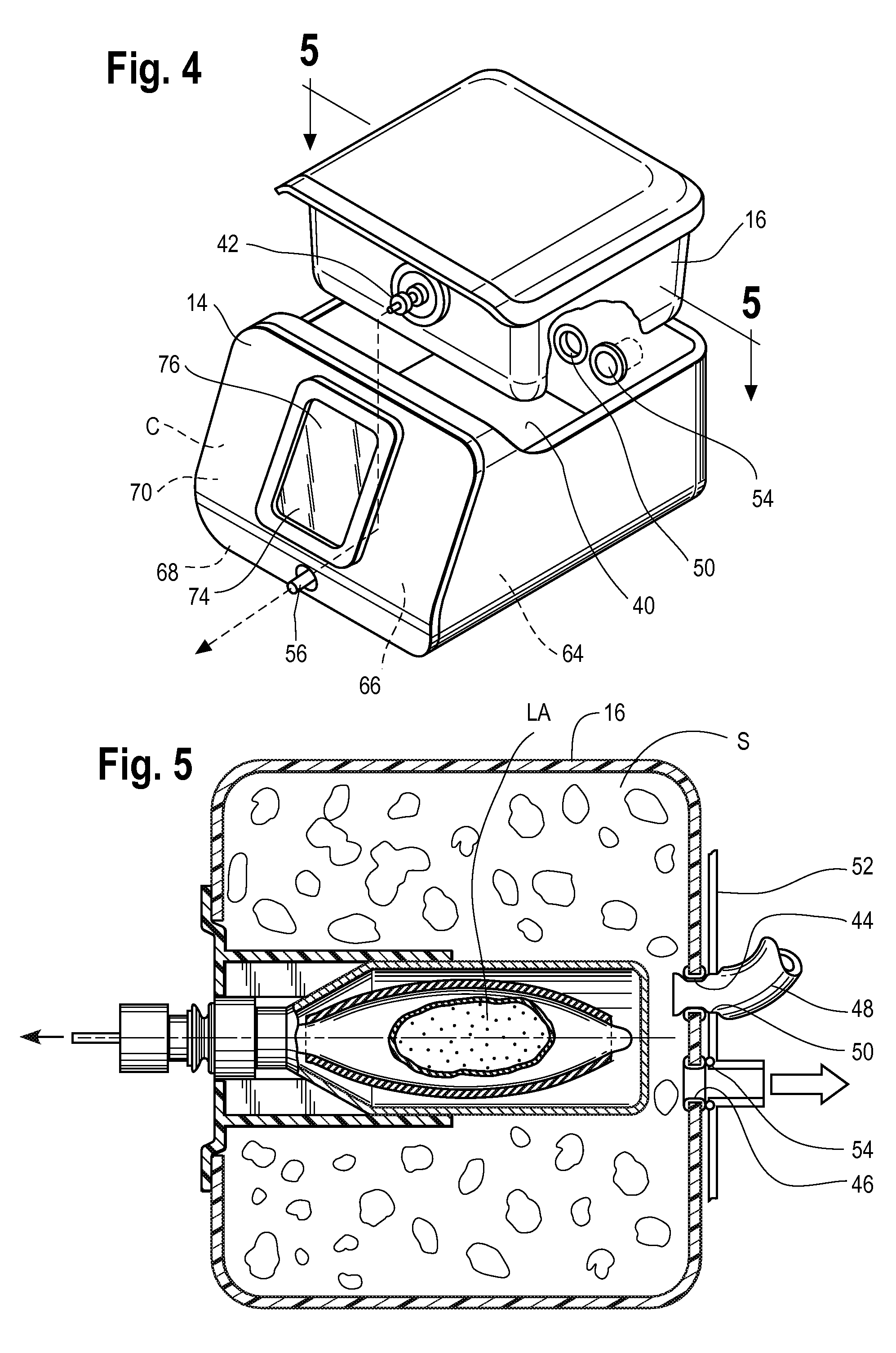
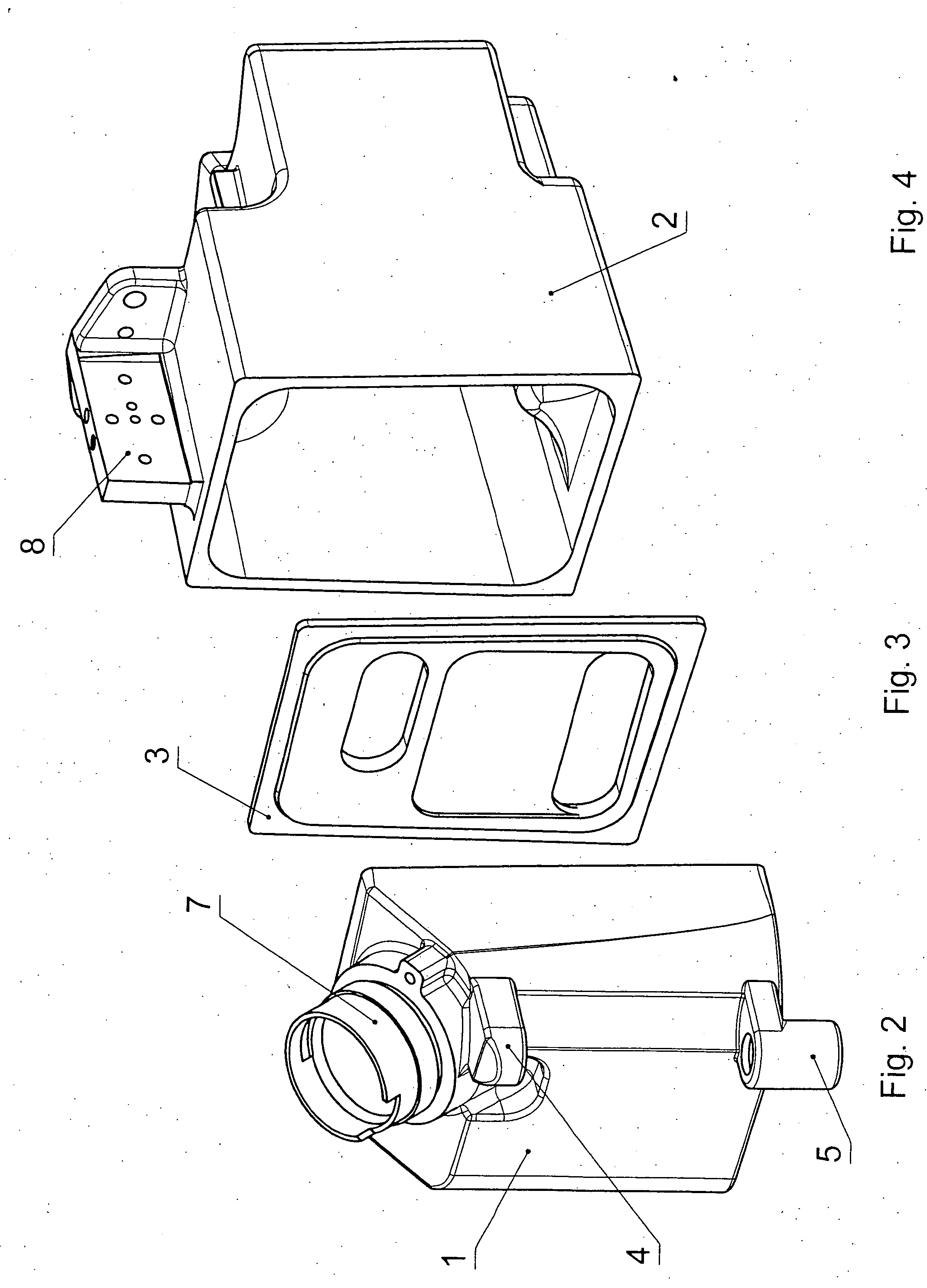
Pressure-resistant tank for liquids
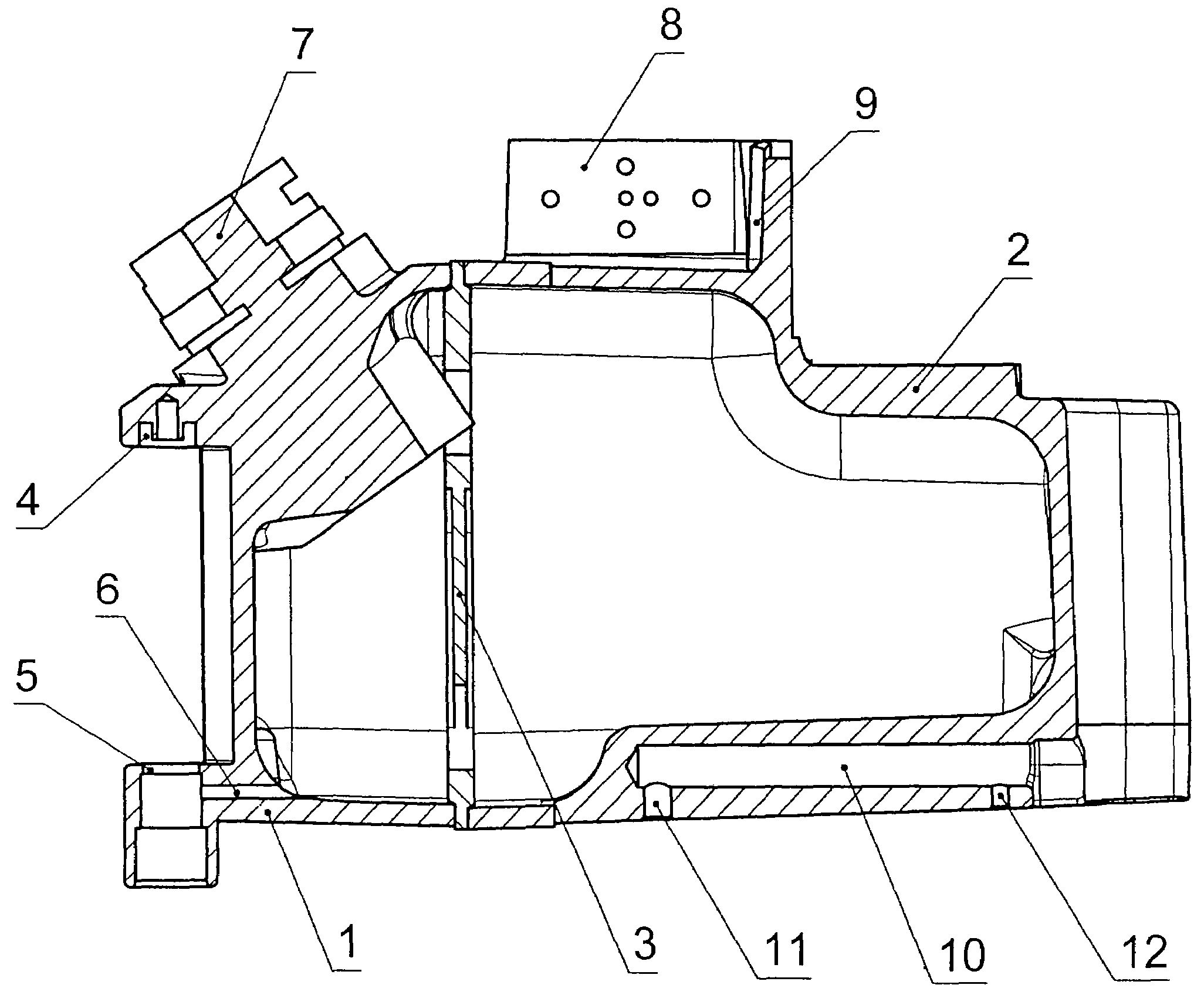
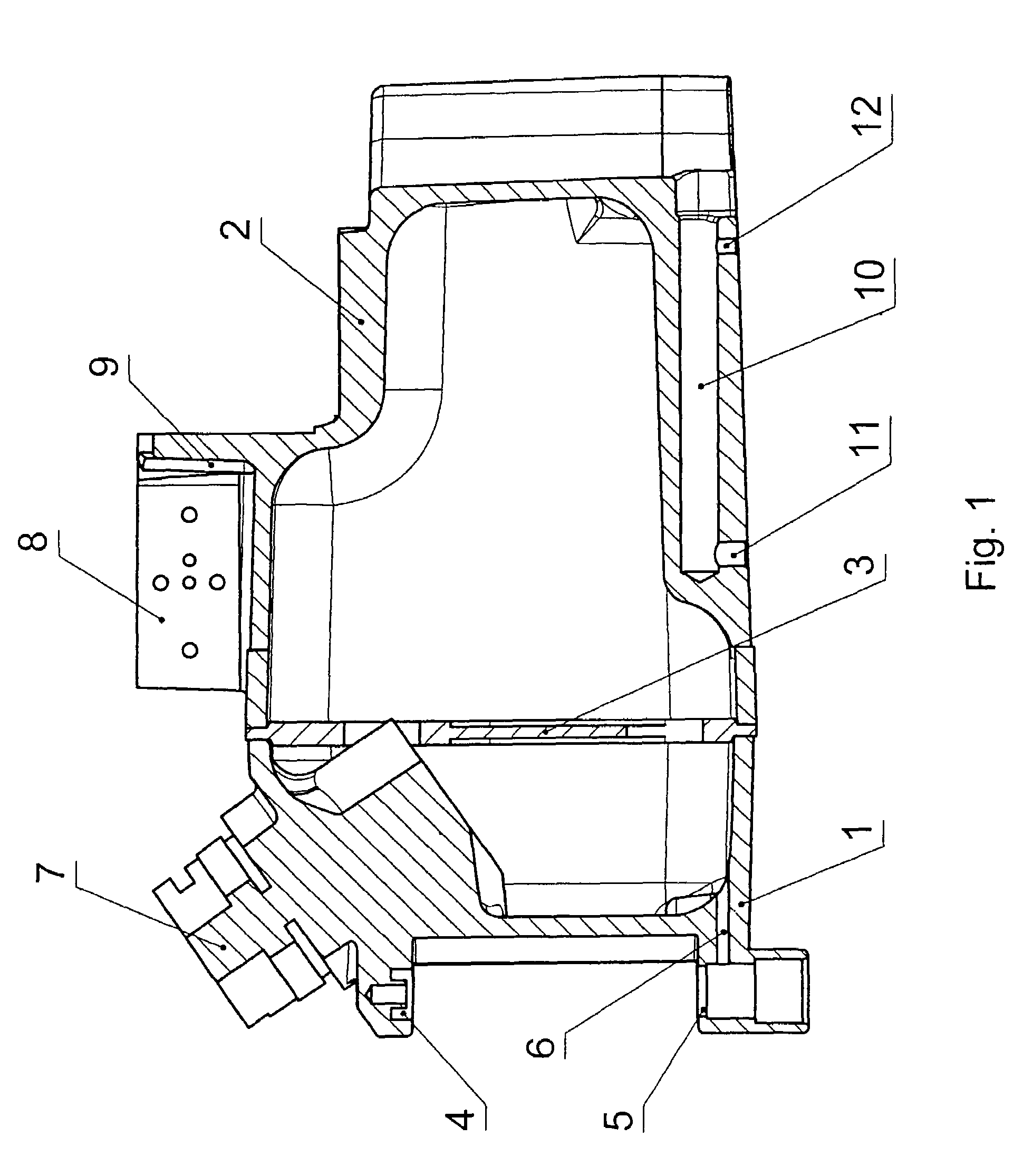
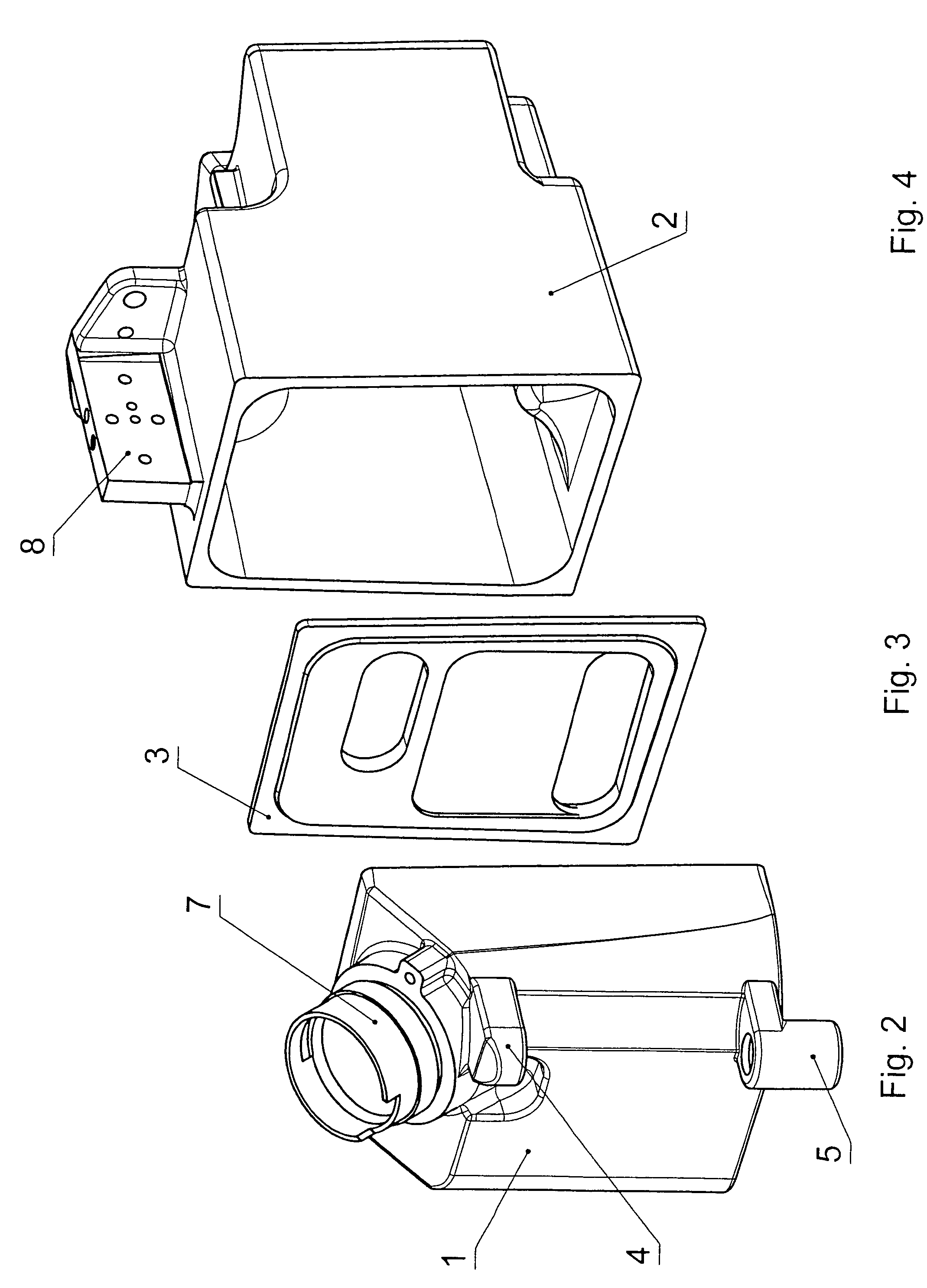
ActiveUS20060070622A1Save spaceSimple designRespiratorsOther heat production devicesAnesthetic AgentHydrogen content
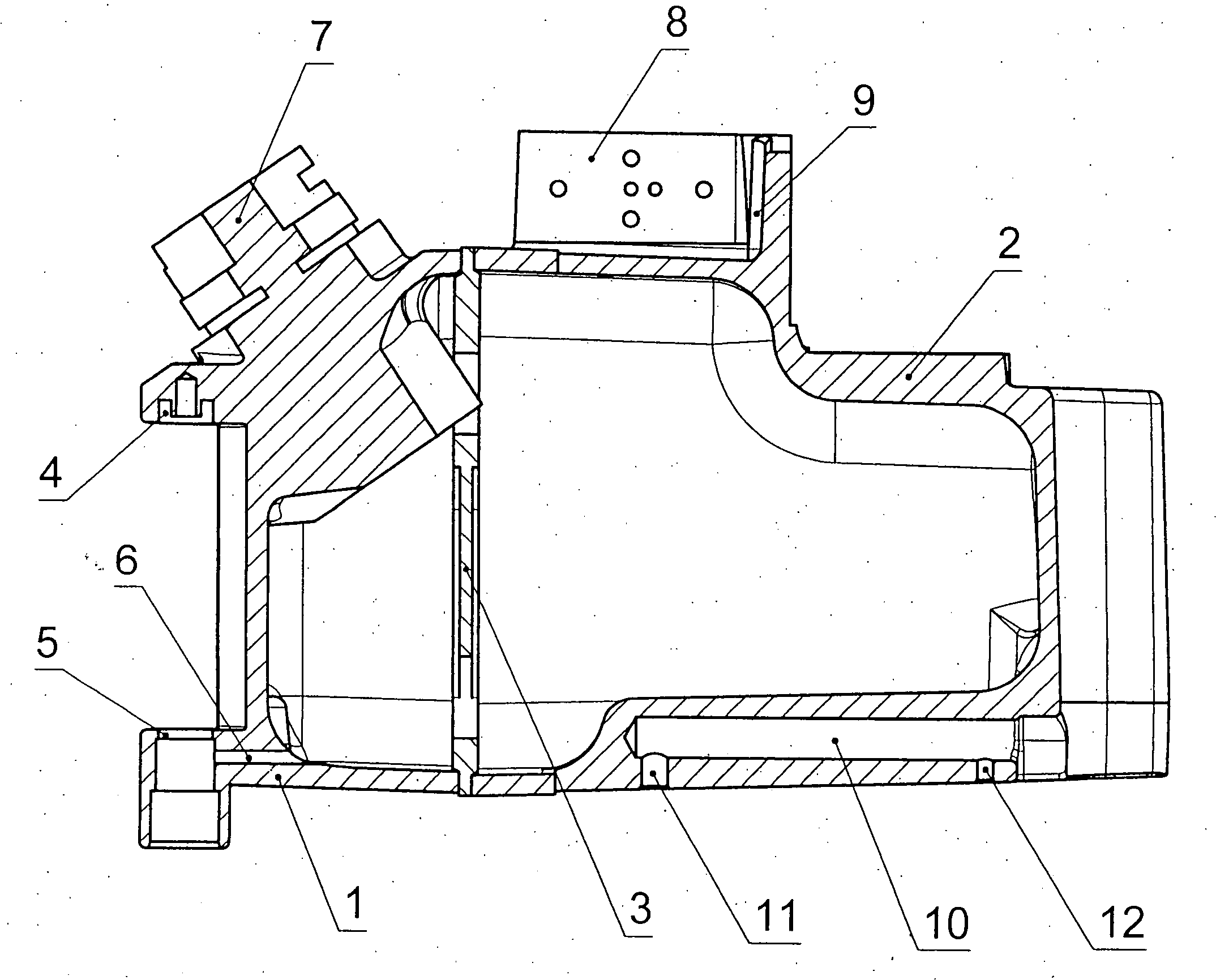
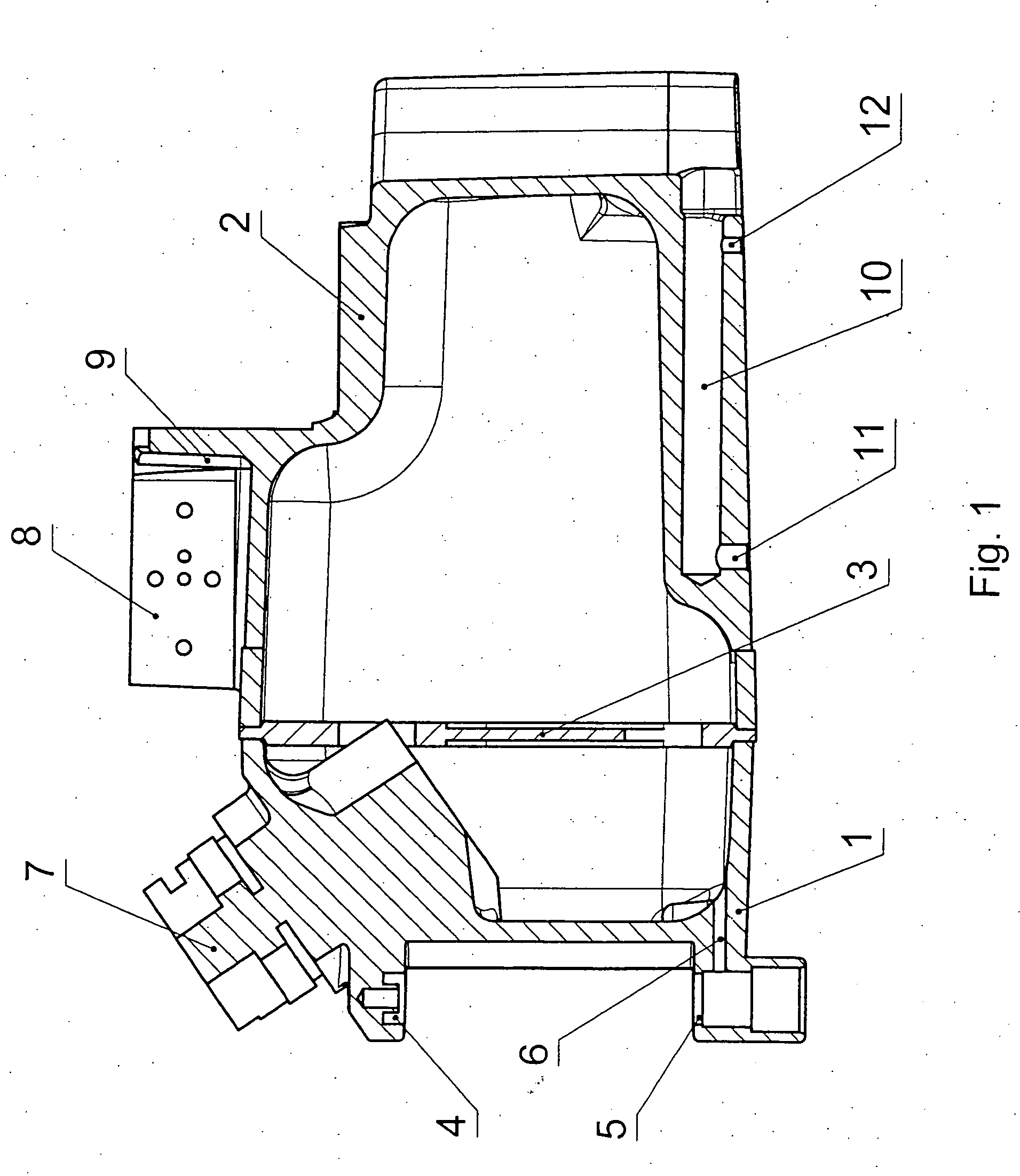
A pressure-resistant tank for liquids includes a plurality of differently shaped diecast parts (1, 2) made of a first aluminum alloy (cast alloy). The diecast parts (1, 2) are connected by means of a closed weld seam welded with an electron beam with the use of a second aluminum alloy (wrought alloy). The second aluminum alloy has especially a hydrogen content of less than 0.2 mL per 100 g of wrought alloy. The tank for liquids is especially an anesthetic tank and the anesthetic is desflurane.
Owner:DRAGERWERK AG
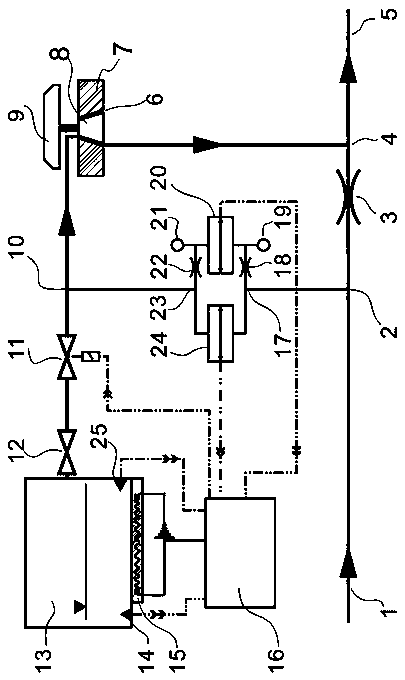
Inhalational anesthetic evaporation device
PendingCN110339443ALow boiling pointThe heat supplement needed to solve the evaporation is notRespiratorsInhalatorsAnesthetic AgentAnesthetic gases
The invention relates to the technical field of medical devices, in particular to an inhalational anesthetic evaporation device. The device includes a first gas path, one end of the first gas path isprovided with an inlet, and the other end of the first gas path is provided with a mixed gas outlet; the first gas path is connected with a second gas path and a third gas path communicated with eachother; the connection portion of the second gas path and the first gas path is provided with a pressure monitoring diversion opening, and the other end of the second gas path is connected with a pressure monitoring unit; the connection portion of the third gas path and the first gas path is provided with a gas mixing hole; the pressure monitoring unit is connected with a seventh gas path through asixth gas path, one end of the seven gas path is communicated with an evaporation chamber unit, and the other end of the seven gas path is connected with an anesthetic gas control unit. The problemsare solved that the boiling point of desflurane is low, heat required by evaporation cannot be supplemented in time and added medicines are leaked, and meanwhile the evaporation control problem of clinical application of desflurane is solved.
Owner:BEIJING YIANFENG TECH CO LTD
Synthesis of Fluorinated Ethers
ActiveUS20080132731A1Organic compound preparationEther preparation by ester reactionsHydrogen fluorideGas phase
A process for preparing fluorinated ethers, such as desflurane, comprises reacting the corresponding chlorinated ether, such as isoflurane, with anhydrous hydrogen fluoride in the vapor phase in the presence of chromia catalyst. It is emphasized that this abstract is provided to comply with the rules requiring an abstract which will allow a searcher or other reader quickly to ascertain the subject matter of the technical disclosure. It is submitted with the understanding that it will not be used to interpret or limit the scope or meaning of the appended issued claims.
Owner:HALOCARBON PROD CORP
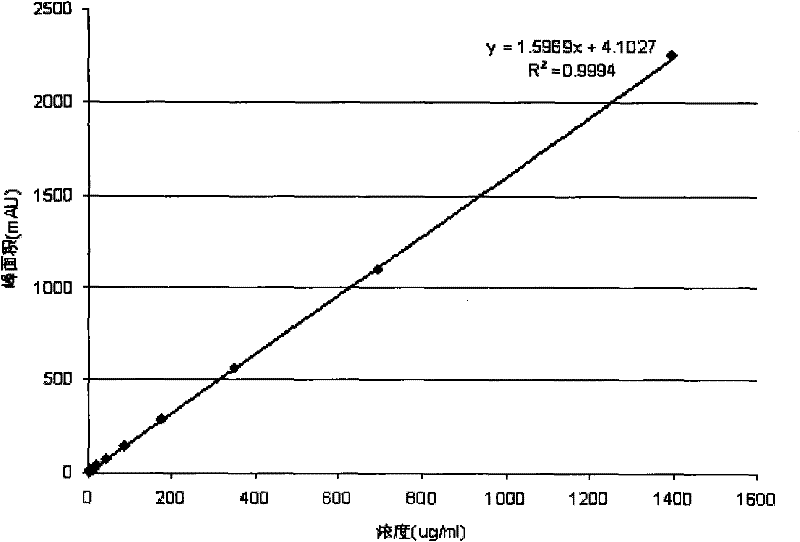
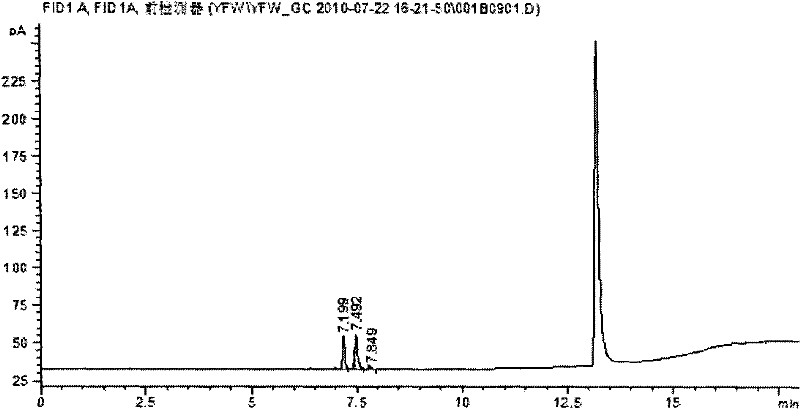
Method for measuring concentration of volatile anesthetics in samples by chromatography
InactiveCN102175780AConcentration determination is simpleRapid concentration determinationComponent separationTissue fluidBulk samples
The invention discloses a method for measuring concentration of volatile anesthetics in samples by automatic headspace gas chromatography. The method is suitable for measuring the concentrations of volatile anesthetics like methoxyflurane, enflurane, isoflurane, sevoflurane and desflurane in samples. The samples can be solutions containing the substances to be measured, as well as can be in vitro biological samples containing the substances to be measured, such as blood, urine, tissue fluid. The method is simple, fast and exact, suitable for measuring and analyzing laboratory samples, and suitable for result measurement of a plurality of samples in clinical monitoring.
Owner:YICHANG HUMANWELL PHARMA
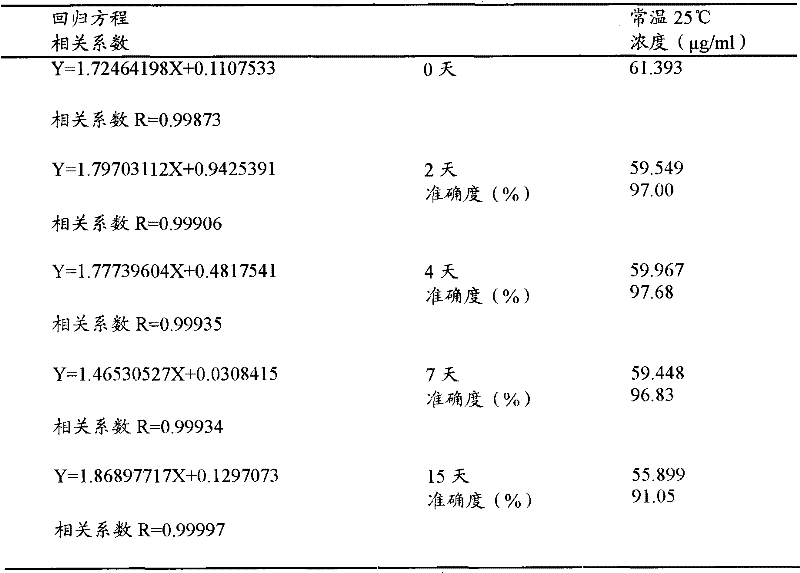
Deflurane evaporation tank capable of automatically detecting liquid level
InactiveCN113350811AGuaranteed purityEasy to useDispersed particle filtrationEvaporator accessoriesGas leakEngineering
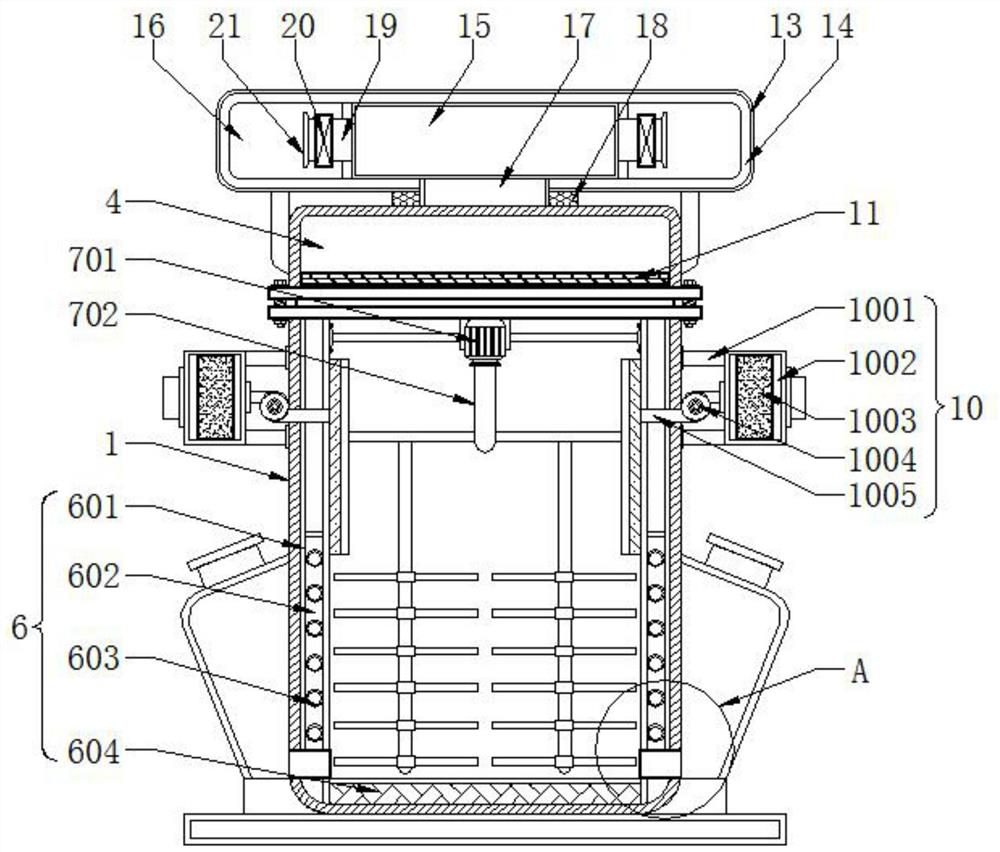
The invention discloses a desflurane evaporation tank capable of automatically detecting the liquid level, and relates to the technical field of desflurane evaporation tanks. The desflurane evaporation tank comprises a bearing assembly and a sealing assembly, and the left side of the upper end of the bearing assembly is provided with an evaporation assembly; the evaporation assembly comprises an evaporation tank, a visible window, an automatic liquid level monitor, a heating pipe, a heat preservation sleeve, a condensate water guide pipe and a condensate water collecting tank, and the visible window is arranged in the middle of the front end of the evaporation tank. Through a limiting block arranged in the outer side of the top cover and a limiting groove formed in the inner side of a tight ring, the tight ring can rotate clockwise at the upper end of a sealing ring through pushing of an electric push rod, when the top cover is closed, the limiting block is just embedded into the limiting groove, and then through rotation of the tight ring, the limiting block is rotated to be clamped into the limiting groove, so that the effect of closing and fixing the top cover and the evaporation tank body is achieved, the tightness of the closing mode is better, and the situation of gas leakage of the evaporation tank body is effectively avoided.
Owner:深圳市普博医疗科技股份有限公司
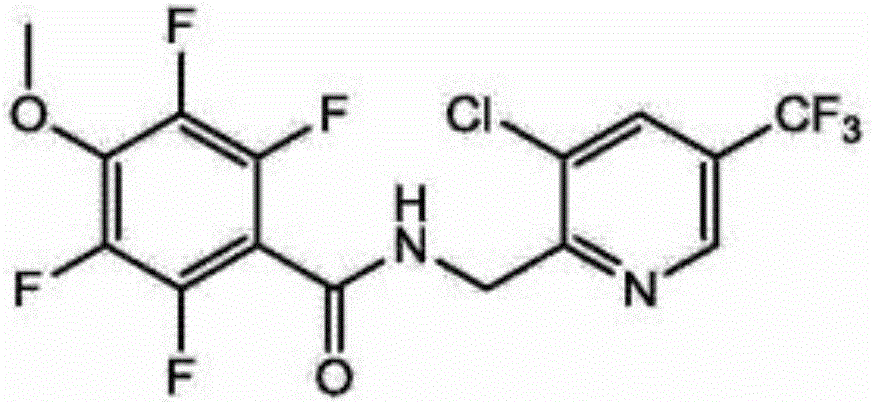
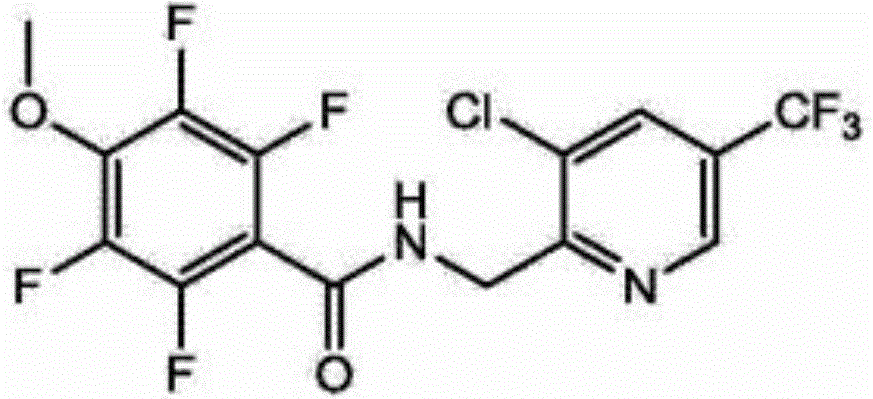
ECD determination method for residual quantity of N-(3-chloro-5-(trichloromethyl)pyridyl-2-methyl)-2,3,5,6-tetrafluoro-4-methoxybenzamide in vegetables and fruits
InactiveCN104678024AAvoid interferenceSimple and fast operationComponent separationAcetic acidElectron capture detector
The invention discloses an ECD determination method for residual quantity of N-(3-chloro-5-(trichloromethyl)pyridyl-2-methyl)-2,3,5,6-tetrafluoro-4-methoxybenzamide in vegetables and fruits. The ECD determination method comprises the following steps: homogenizing by using acetonitrile or an acetonitrile solution containing 1% acetic acid to extract residual desflurane bacteria amide in a sample; after dispersing and purifying an extract by using ethidene diamine-N-propylsilane (PSA) and octadecylsilane bonded phase (C18) matrix, determining by using a gas chromatography-electron capture detector (GC-ECD); quantifying by an external standard method. By the ECD determination method, the average recycling rate is 93.3-98.3%, the average relative standard deviation (RSD) is 4.1-6.5%, and the determination limit is lower than 3.16 [mu]g / kg; the ECD determination method has the advantages of simple, convenient and rapid operation, high sensitivity, good repeatability and accurate quantification; by the ECD determination method, a technical requirement on a uniform residue limit of 0.01mg / kg can be met; a powerful technical support is provided for guaranteeing food safety of people in China and healthy development of export trade.
Owner:河北德诺商品检测技术服务有限公司
Separation/Purification of Desflurane from Hydrogen Fluoride
ActiveUS20080306309A1Ether separation/purificationOrganic compound preparationHydrogen fluorideSubject matter
An azeotrope comprising desflurane (CF3CFHOCF2H) and hydrogen fluoride (HF). The azeotrope can be prepared by fractionally distilling a crude mixture of desflurane and HF. Purer desflurane can be readily and easily separated from the azeotrope. It is emphasized that this abstract is provided to comply with the rules requiring an abstract which will allow a searcher or other reader quickly to ascertain the subject matter of the technical disclosure. It is submitted with the understanding that it will not be used to interpret or limit the scope or meaning of the appended issued claims.
Owner:HALOCARBON PROD CORP
Pressure-resistant tank for liquids
A pressure-resistant tank for liquids includes a plurality of differently shaped diecast parts (1, 2) made of a first aluminum alloy (cast alloy). The diecast parts (1, 2) are connected by means of a closed weld seam welded with an electron beam with the use of a second aluminum alloy (wrought alloy). The second aluminum alloy has especially a hydrogen content of less than 0.2 mL per 100 g of wrought alloy. The tank for liquids is especially an anesthetic tank and the anesthetic is desflurane.
Owner:DRAGERWERK AG
Seizure control compositions and methods of using same
An anti-seizure composition includes a therapeutically effective combination of a halogenated ether, a benzodiazepine, and a barbiturate. The halogenated ether may selected from one or more of isoflurane, desflurane and sevoflurane. The benzodiazepine may be diazepam and the barbiturate may be phenobarbital. Also, a method for treating a seizure event may include administering a therapeutically effective amount of the combination.
Owner:MARSH WANG MEDICAL SYST LLC
Desflurane bacteria amide and metconazole-containing sterilization composition and application thereof
InactiveCN105831127AThe mechanism of action is differentReduce dosageBiocideDead animal preservationDiseaseBiotechnology
The invention discloses a desflurane bacteria amide and metconazole-containing sterilization composition and application thereof. The sterilization composition contains desflurane bacteria amide and metconazole which serve as main effective components, wherein the mass ratio of desflurane bacteria amide to metconazole is (1-70):(1-50). The sterilization composition can be applied to prevention and treatment of cereal, fruit tree and vegetable diseases, and is better in synergetic effect; the drug resistance of bacteria can be overcome and delayed; the sterilization speed is high, the lasting period is long, and the application cost is reduced; the prevention and treatment effect is obviously better than those of other single dosages. The desflurane bacteria amide and epoxiconazole-containing sterilization composition can be used for preventing and treating fungoid on crops, particularly for preventing and treating fungal diseases such as downy mildew, potato late blight, rice sheath blight, cotton seedling damping-off, wheat powdery mildew, wheat glume blight and wheat rust, and such prevention and treatment effect is obviously better than that of the single dosage.
Owner:NANJING HUAZHOU PHARMA
Methods for treating or preventing organophosphate poisoning
ActiveUS11291637B2Halogenated hydrocarbon active ingredientsHydroxy compound active ingredientsPhosphateOrganophosphorus Poisoning
The invention relates to methods of treating or preventing organophosphate poisoning in a subject in need thereof, comprising administering to the subject isoflurane, enflurane, halothane, sevoflurane, desflurane, xenon or argon in a therapeutically effective amount.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
A kind of UV light curing fluorosilicone release agent and preparation method thereof
The invention discloses a UV (Ultraviolet) curing fluorine-silicon release agent and a preparation method thereof. The structure of the UV curing fluorine-silicon release agent disclosed by the invention is shown as follows, wherein R1 is CnH2n+1, and n is equal to 1-9; R2 is one of the structural formulas as shown in the specification; R3 is C2H4CnF2nC2h4, and n is equal to 1-9; R4 is C2H4Rf, and Rf is desflurane group or perfluoroalkyl; and x is equal to 1-5000, y is equal to 1-5000, z is equal to 0-5000, and w is equal to 1-5000. According to the UV curing fluorine-silicon release agent disclosed by the invention, the preparation method is simple, and the problems such as high-temperature curing and cheap catalysts can be solved. The structural formula is as shown in the specification.
Owner:安庆龙驰氟硅新材料有限公司
Separation/purification of desflurane from hydrogen fluoride
ActiveUS8283500B2Ether separation/purificationOrganic compound preparationHydrogen fluorideSubject matter
An azeotrope comprising desflurane (CF3CFHOCF2H) and hydrogen fluoride (HF). The azeotrope can be prepared by fractionally distilling a crude mixture of desflurane and HF. Purer desflurane can be readily and easily separated from the azeotrope. It is emphasized that this abstract is provided to comply with the rules requiring an abstract which will allow a searcher or other reader quickly to ascertain the subject matter of the technical disclosure. It is submitted with the understanding that it will not be used to interpret or limit the scope or meaning of the appended issued claims.
Owner:HALOCARBON PROD CORP
Purification of desflurane containing R-123
ActiveUSH2234H1RespiratorsEther separation/purification1,1,1,2-TetrafluoroethaneIntermediate product
A process for preparing 2-difluoromethoxy-1,1,1,2-tetrafluoroethane (desflurane) comprising the following steps:(a) providing a first mixture comprising desflurane and 2,2-dichloro-1,1,1-trifluoroethane (R-123);(b) adding water to the first mixture to form a second mixture; and(c) fractionally distilling the second mixture to yield a desflurane product purer in R-123 than said the mixture.Also described is a purified desflurane product prepared by the process, and intermediate products comprising water added to a mixture of desflurane and R-123.
Owner:ULTRA MEK
Desflurane bacteria amide and epoxiconazole-containing sterilization composition and application thereof
InactiveCN105831128AThe mechanism of action is differentReduce dosageBiocideDead animal preservationDiseaseTreatment effect
The invention discloses a desflurane bacteria amide and epoxiconazole-containing sterilization composition and application thereof. The sterilization composition contains desflurane bacteria amide and epoxiconazole which serve as main effective components, wherein the mass ratio of desflurane bacteria amide to epoxiconazole is (1-70):(1-50). The sterilization composition can be applied to prevention and treatment of cereal, fruit tree and vegetable diseases, and is better in synergetic effect; the drug resistance of bacteria can be overcome and delayed; the sterilization speed is high, the lasting period is long, and the application cost is reduced; the prevention and treatment effect is obviously better than those of other single dosages. The desflurane bacteria amide and epoxiconazole-containing sterilization composition can be used for preventing and treating fungoid on crops, particularly for preventing and treating fungal diseases such as downy mildew, late blight, banded sclerotial blight, damping off, powdery mildew and pseudocercosporella herpotrichoides, and such prevention and treatment effect is obviously better than that of the single dosage.
Owner:NANJING HUAZHOU PHARMA
A kind of ecd determination method of fluoxamide residues in vegetables and fruits
InactiveCN104678024BAvoid interferenceSimple and fast operationComponent separationRelative standard deviationChemistry
The invention discloses an ECD determination method for residual quantity of N-(3-chloro-5-(trichloromethyl)pyridyl-2-methyl)-2,3,5,6-tetrafluoro-4-methoxybenzamide in vegetables and fruits. The ECD determination method comprises the following steps: homogenizing by using acetonitrile or an acetonitrile solution containing 1% acetic acid to extract residual desflurane bacteria amide in a sample; after dispersing and purifying an extract by using ethidene diamine-N-propylsilane (PSA) and octadecylsilane bonded phase (C18) matrix, determining by using a gas chromatography-electron capture detector (GC-ECD); quantifying by an external standard method. By the ECD determination method, the average recycling rate is 93.3-98.3%, the average relative standard deviation (RSD) is 4.1-6.5%, and the determination limit is lower than 3.16 [mu]g / kg; the ECD determination method has the advantages of simple, convenient and rapid operation, high sensitivity, good repeatability and accurate quantification; by the ECD determination method, a technical requirement on a uniform residue limit of 0.01mg / kg can be met; a powerful technical support is provided for guaranteeing food safety of people in China and healthy development of export trade.
Owner:河北德诺商品检测技术服务有限公司
Synthesis of fluorinated ethers
InactiveUS9150480B2Organic compound preparationEther preparation by ester reactionsHydrogen fluorideGas phase
A process for preparing fluorinated ethers, such as desflurane, comprises reacting the corresponding chlorinated ether, such as isoflurane, with anhydrous hydrogen fluoride in the vapor phase in the presence of chromia catalyst. It is emphasized that this abstract is provided to comply with the rules requiring an abstract which will allow a searcher or other reader quickly to ascertain the subject matter of the technical disclosure. It is submitted with the understanding that it will not be used to interpret or limit the scope or meaning of the appended issued claims.
Owner:HALOCARBON PROD CORP
Process for production of 1,2,2,2-tetrafluoroethyl difluoromethyl ether (desflurane)
InactiveUS8378149B2Improve conversion efficiencyHigh yieldOrganic chemistryOrganic compound preparationHydrogen fluorideEther
Owner:PIRAMAL ENTERPRISES LTD
Deflurane evaporation tank with automatic crystal removal structure
PendingCN113476868AImprove functionalityKnow the water levelHollow article cleaningEvaporation apparatusEngineeringAnesthetic gases
The invention discloses a desflurane evaporation tank with an automatic crystal removal structure, and relates to the technical field of desflurane, the desflurane evaporation tank comprises a tank body, an evaporation heating assembly and a crystal removal assembly, an upper cover is mounted above the tank body through a connecting flange, and locking bolts are uniformly distributed on the outer side of the connecting flange; the liquid adding assemblies are arranged on the two sides of the lower portion of the outer portion of the tank body, the evaporation heating assembly is arranged on the inner side of the tank body and comprises a vacuum layer, a heating layer, a heating coil and a heating bottom plate, the heating layer is arranged on the inner side of the vacuum layer, the heating coil is arranged in the heating layer, a heating bottom plate is fixed to the bottom side of the interior of the tank body, and a driving assembly is installed on the upper portion of the interior of the tank body. According to the desflurane evaporation tank with the automatic crystal removal structure, crystals or blocky crystals on the inner wall of the tank body are removed through the crystal removal assembly, anesthetic gas can be collected in a centralized mode, the concentration of anesthetic gas can be adjusted, and the functionality is good.
Owner:深圳市普博医疗科技股份有限公司
Volatile anesthetic compositions comprising extractive solvents for regional anesthesia and/or pain relief
ActiveUS9675544B2Excellent characteristicsImprove propertiesHalogenated hydrocarbon active ingredientsBiocideRegimenSolvent
The present invention provides methods for reducing pain in a subject in need of such pain reduction by delivering, e.g., intrathecally or epidurally, a volatile anesthetic dissolved in a solution comprising an extractive solvent, e.g., DMSO or NMP, in an amount effective to reduce pain. Chronic or acute pain may be treated, or the anesthetic may be delivered as a regional anesthesia to a subject to anesthetize a portion the subject prior to a surgery, hi certain embodiments, isoflurane, halothane, enflurane, sevoflurane, desflurane, methoxyflurane, or mixtures thereof may be used. Dosing regimens including a one-time administration, continuous and / or periodic administration are contemplated.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Electron evaporator for inhalant anesthetic
The invention relates to the technical field of medical apparatuses, in particular to an electron evaporator for inhalant anesthetic. The electron evaporation device comprises an evaporator body, wherein the evaporator body comprises a bracket assembly, a medicine storing chamber assembly, a valve body assembly and a dial assembly; the medicine storing chamber assembly is fixed to the front end ofthe valve body assembly through two screws; the bracket assembly is fixed to the side of the valve body assembly through two screws II; and the dial assembly is fixed to the upper end of the valve body assembly through three screws III, and the housing is fixed to the valve body assembly and the bracket assembly to form the entire machine. The electron evaporator solves the problems that desflurane is low in boiling point, caloric required by evaporation cannot be supplemented in time, and can solve the problem of evaporation control for clinical application of the desflurane.
Owner:北京毅安峰技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com