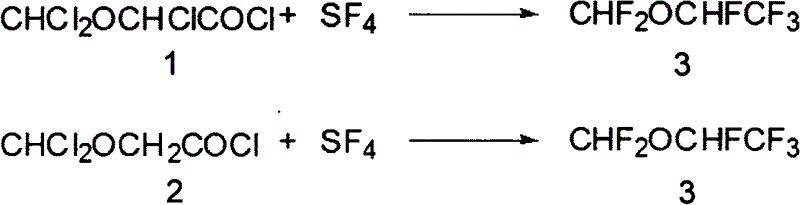
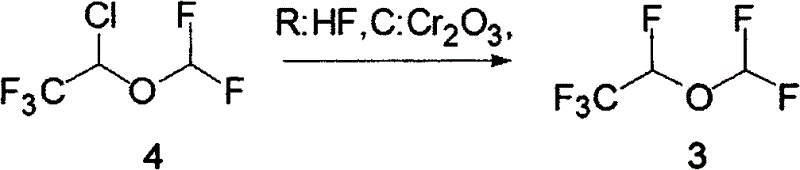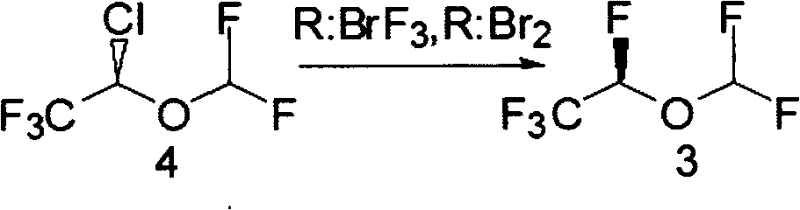Synthesis method of desflurane
A synthetic method, the technology of desflurane, applied in the field of medicine and chemical industry, can solve the problems of slow reaction rate, low reaction temperature, easy explosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Fluorination of 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether
[0030] Add 144ml of diethylene glycol, 39.79g of dry potassium fluoride into the autoclave, add 66.46g of isoflurane, seal and stir, heat up to 220-230°C, and react for 10-12 hours. After the reaction, put the autoclave in Cool at room temperature overnight, and then circulate the refrigerated liquid into the autoclave to cool down. After about 4 hours, until the pressure no longer drops, open the autoclave, pour the reaction solution and residue into a round bottom flask, heat and distill, and condense with the refrigerated liquid The condenser was refluxed, and fractions were collected to obtain 29.9 g of a crude product of desflurane, with a GC content of 88.63% and a yield of 43.8%.
Embodiment 2
[0031] Example 2: Purification of 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether
[0032] Use a rectification column to rectify 61.7g of crude desflurane product with a GC content of 85%. ℃, the receiving bottle was kept warm in an ice-salt bath in a self-made vacuum flask. At first, it was controlled to be in a total reflux state, and the fractions were collected after a continuous reflux was formed. The reflux ratio was controlled to be 1:1. The collected desflurane distillation product was 42g, with a GC content of 99.89% and a distillation yield of about 80%. The distillation residual liquid can be recycled in the next batch.
[0033] 1HNMR (400MHz, CDC13) δ: 6.42(t, 1H, -CH), 5.90(d, 1H, -CH).
[0034] 19FNMR (367.5MHz, CDC13) δ: -85.10 (s, 3F, -CF3), -85.76 (d, 1F, -CF, J=157.65), -87.20 (d, 1F, -CF, J=157.65), -146.91(s, 1F, -CF).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com