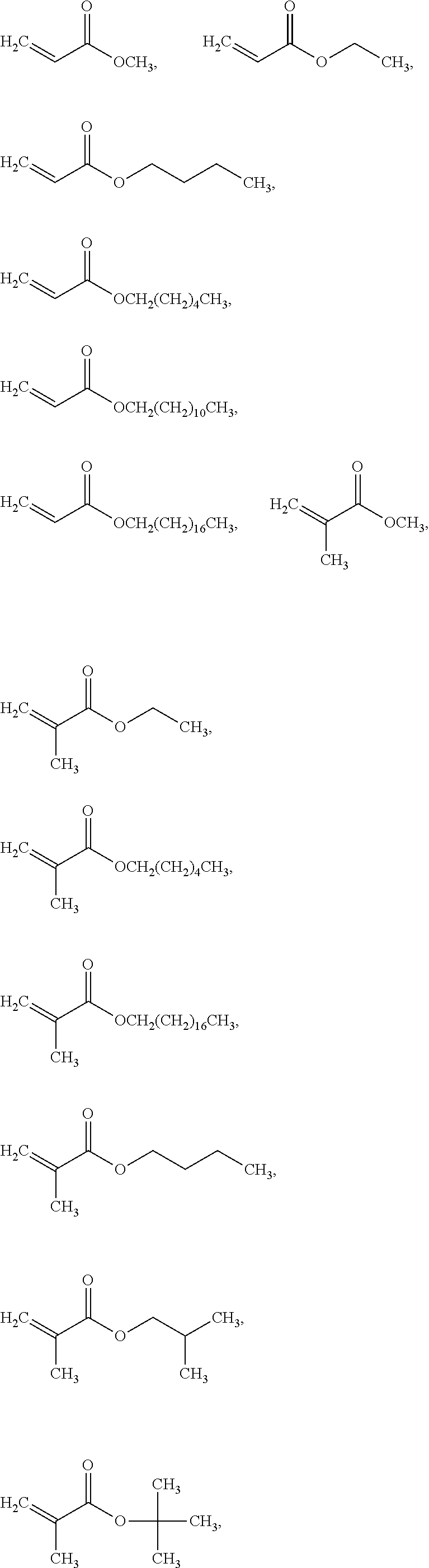
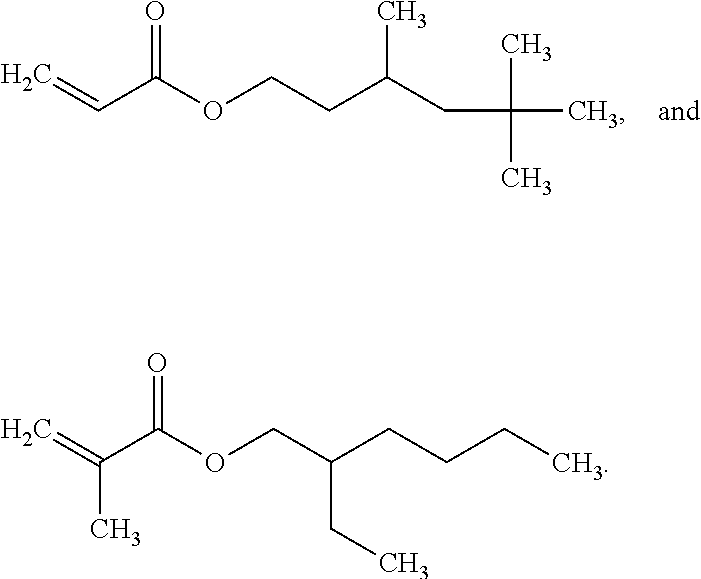
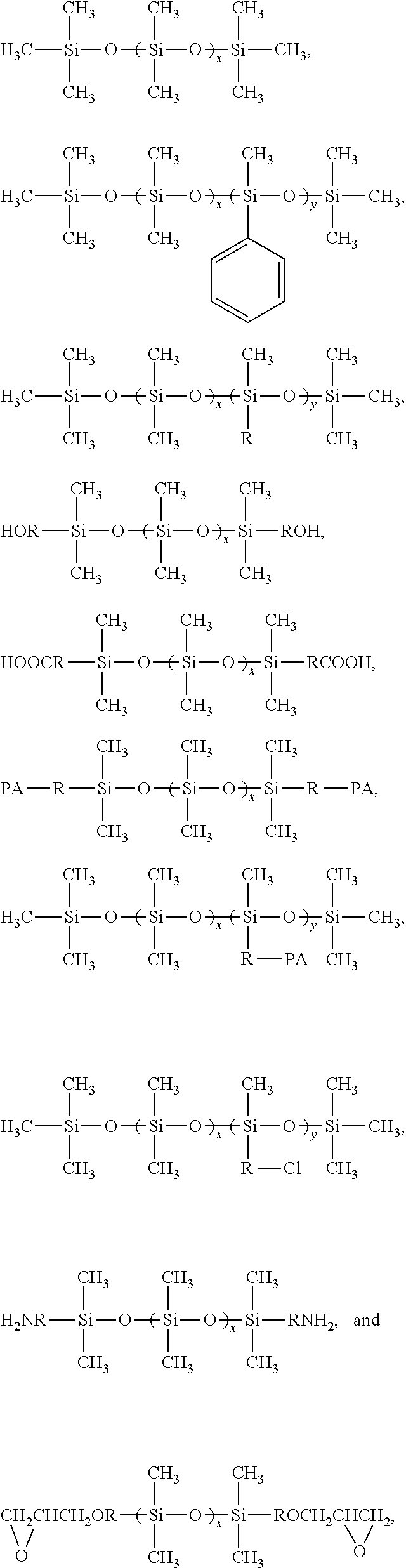
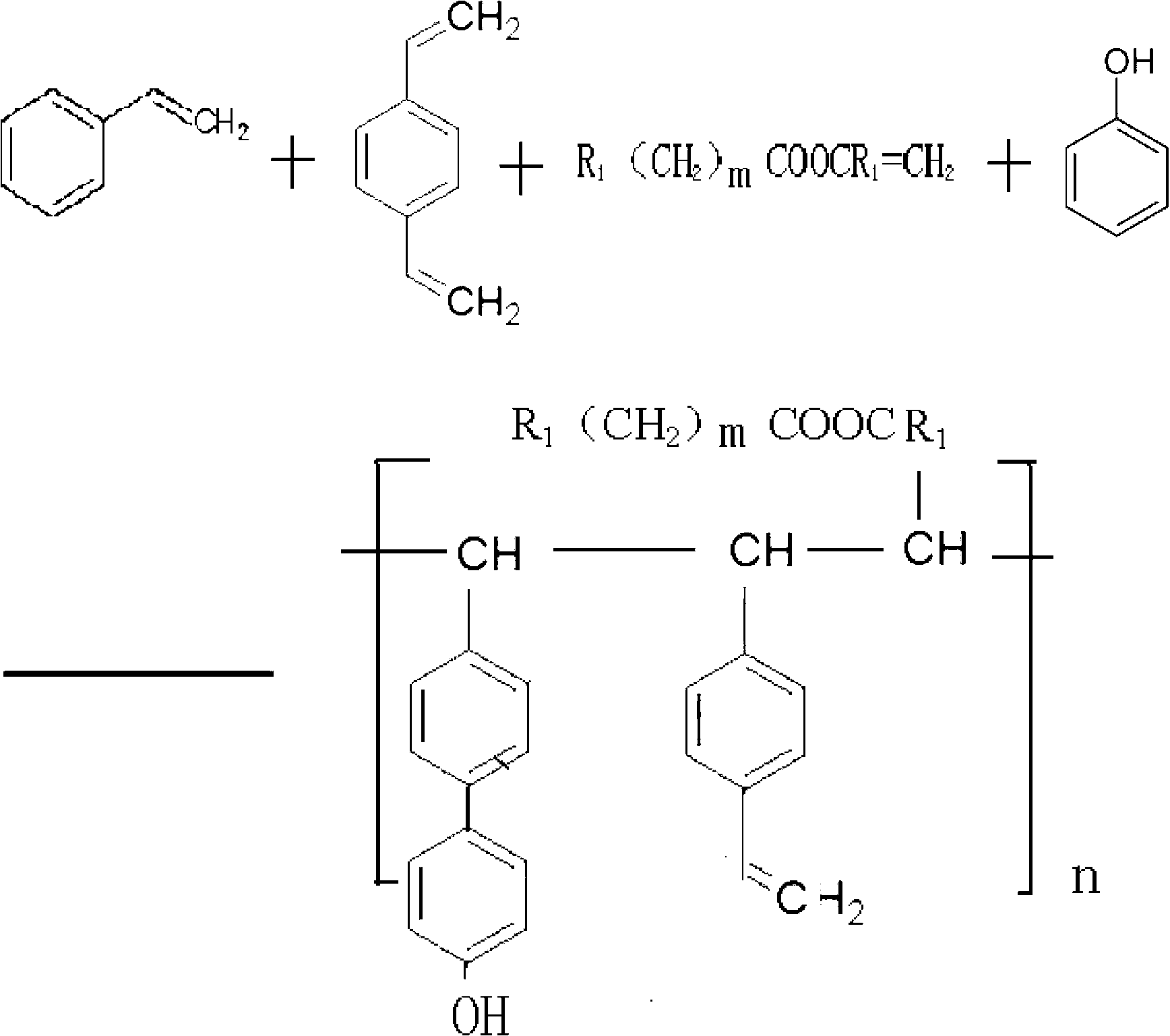
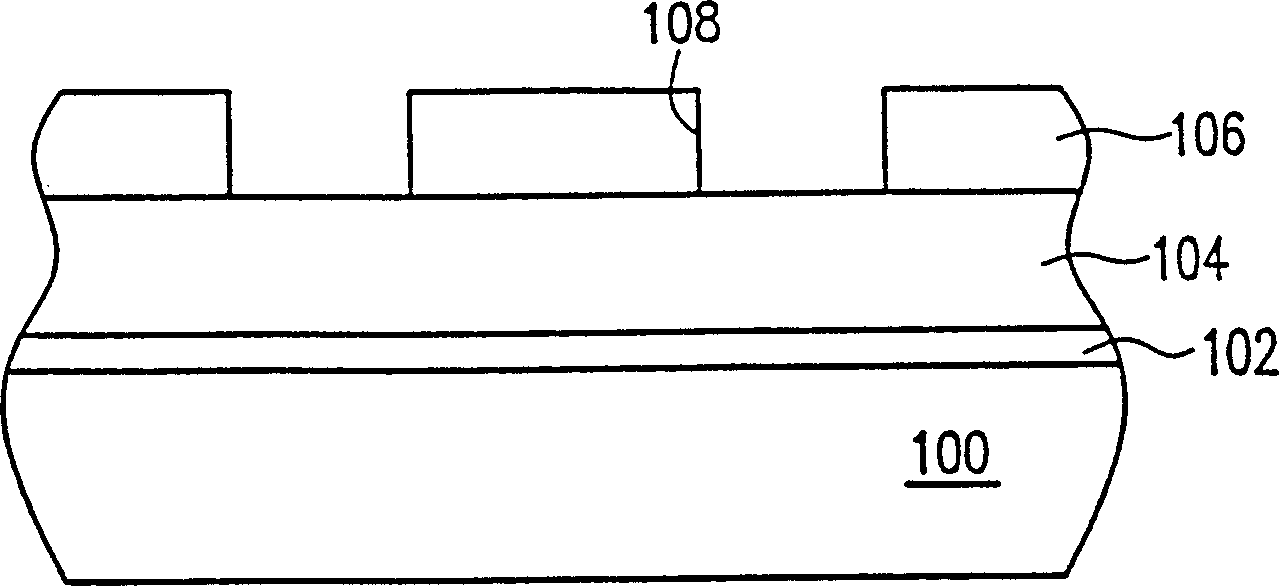
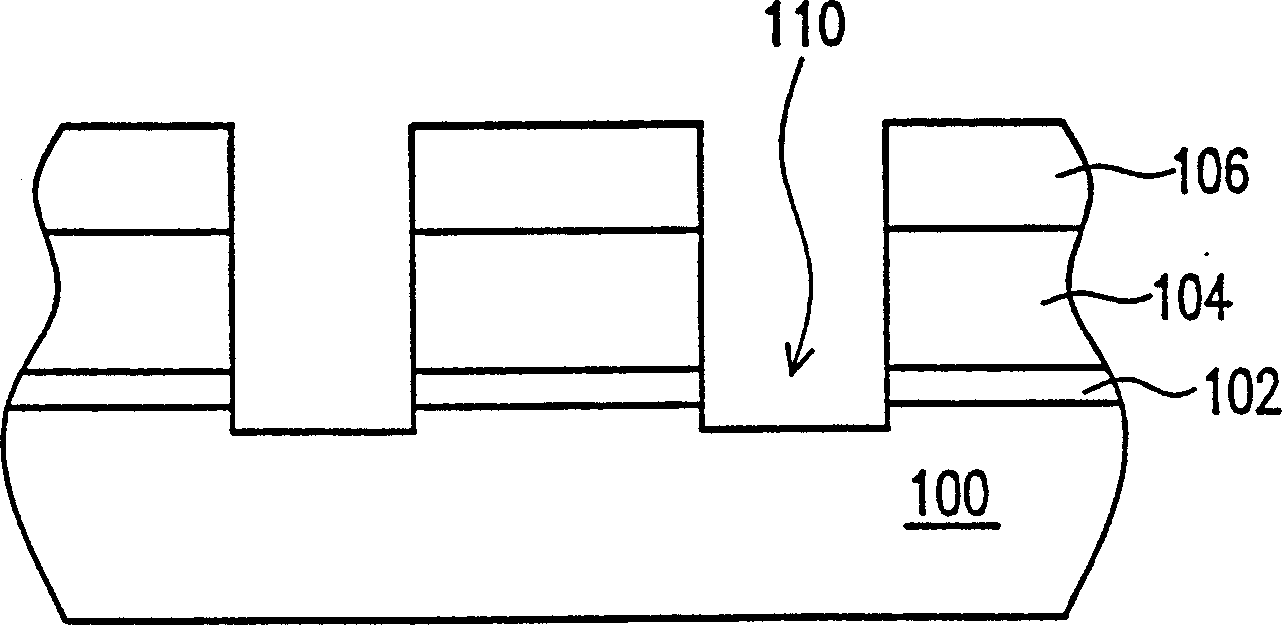
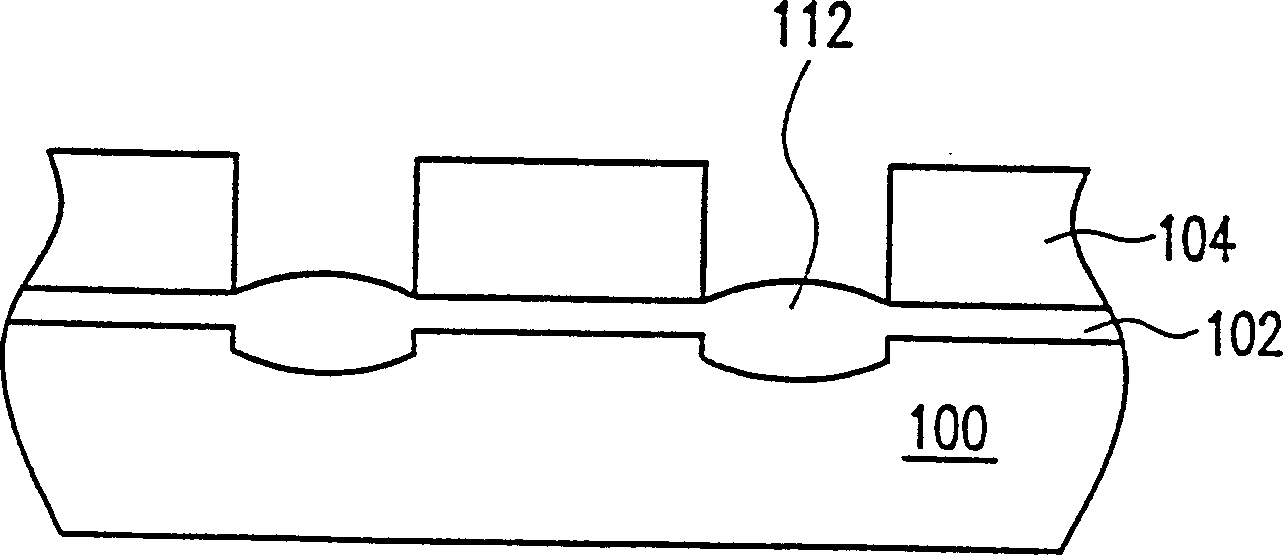
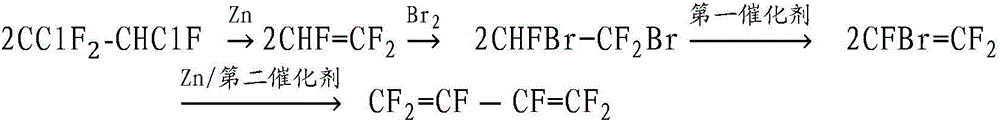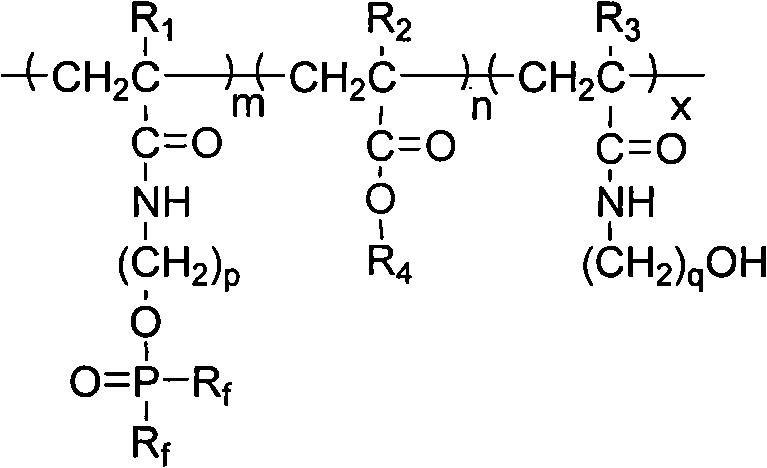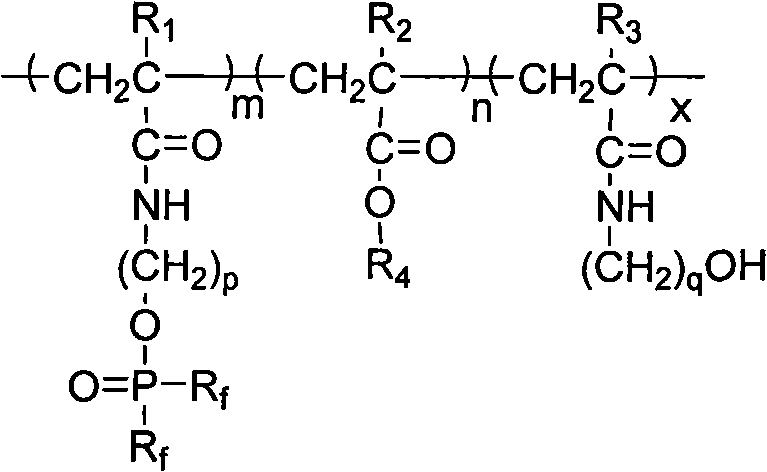Patents
Literature
88 results about "Halothane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Halothane sold under the brand name Fluothane among others, is a general anesthetic. It can be used to start or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful in those who are difficult to intubate. It is given by inhalation.
Method for continuous measurement of flux of gases in the lungs during breathing
ActiveUS20050217671A1RespiratorsOperating means/releasing devices for valvesAutonomous breathingEngineering
A method of calculating the flux of any gas (x) in a CBC circuit for a ventilated or a spontaneous breathing subject, for example said gas(x) being; a) an anesthetic such as but limited to; i)N2O; ii) sevoflurane; iii) isoflurane; iv) halothane; v) desflurame; or the like b) Oxygen; c) Carbon dioxide; or the like utilizing the following relationships; Flux of gas(x)=SGF (FSX−FEX) wherein SGF=Source of gas flow into the breathing circuit (CBC circuit) in liters / minute as read from the gas flow meter as set by the anesthesiologist; FSX=Fractional concentration of gas X in the source gas (which is set by the anesthesiologist); FEX=Fractional concentration of gas X in the end expired gas as determined by a portable gas analyzer, or the like.
Owner:THORNHILL SCI INC
Method for continuous measurement of flux of gases in the lungs during breathing
ActiveUS7913690B2RespiratorsOperating means/releasing devices for valvesContinuous measurementOxygen
A method of calculating the flux of any gas (x) in a CBC circuit for a ventilated or a spontaneous breathing subject, for example said gas(x) being; a) an anesthetic such as but limited to; i)N2O; ii) sevoflurane; iii) isoflurane; iv) halothane; v) desflurame; or the like b) Oxygen; c) Carbon dioxide; or the like utilizing the following relationships; Flux of gas(x)=SGF (FSX−FEX) wherein SGF=Source of gas flow into the breathing circuit (CBC circuit) in liters / minute as read from the gas flow meter as set by the anesthesiologist; FSX=Fractional concentration of gas X in the source gas (which is set by the anesthesiologist); FEX=Fractional concentration of gas X in the end expired gas as determined by a portable gas analyzer, or the like.
Owner:THORNHILL SCI INC
Preparation method of surface-modified self-healing type microcapsule
InactiveCN103316617AImprove the impact of mechanical propertiesCoatingsMicroballoon preparationEpoxyPolymer science
The invention discloses a preparation method of a surface-modified self-healing type microcapsule. The preparation method comprises the steps of synthesizing the self-healing type microcapsule of Poly-urea formaldehyde coated epoxy resin firstly, and then performing surface modification treatment on the microcapsule by using fluothane coupling agent. By adopting the surface-modified self-healing type microcapsule, the affect on the mechanical property of a composite material caused by the self-healing type microcapsule is significantly improved, and therefore, the method provided by the invention is more suitable for the surface modification of the self-healing type microcapsule; furthermore, as the fluorine groups are existent on the surface of the microcapsule, the surface energy of a film can be reduced, and the hydrophobic property and the antifouling property are improved as well.
Owner:GUANGDONG PHARMA UNIV
Fluorine-containing hyperbranched polyester acrylic ester and method of preparing the same
The invention discloses fluorinated hyperbranched polyester acrylate and a preparation method thereof. Fluoride-alcohol and hydroxy acrylate are chosen to react with diisocyanate respectively to achieve two semi-additive products, and the two semi-additive products in proper proportions are used to react with hydroxyl-terminated hyperbranched polymer, to achieve hyperbranched polymer fluorine terminated bases with acrylic acid ester base groups and halothane base groups linked to the hyperbranched molecular framework through carbamate base groups as well as acrylic acid modified hyperbranched polymer. The iso-cyan acid radical reaction of the method has high activity, the Fluoro-alcohol conversion rate is high, the raw materials are cheap and easy to obtain, the operation is easy, and capable of facilitating industrial production. The fluoric hyperbranched polyester acrylate achieved by the method can be applied to ultraviolet-light solidification coating, the achieved coating has sound hydrophobic and oleophobic performances, and the industrial application is very broad.
Owner:UNIV OF SCI & TECH OF CHINA
Working fluid of a blend of 1,1,1,3,3-pentafluoropane, 1,1,1,2,3,3-hexafluoropropane, and 1,1,1,2-tetrafluoroethane and method and apparatus for using
InactiveUS20090049856A1Organic chemistryCompression machines with non-reversible cycleChemistryWorking fluid
There is a working fluid for a heating and cooling. The fluid is a blend of about 1% to about 98% by mass 1,1,1,3,3-pentafluoropane, about 1% to about 98% by mass 1,1,1,2,3,3-hexafluoropropane, and about 1% to about 98% by mass 1,1,1,2-tetrafluoroethane. The 1,1,1,3,3-pentafluoropane, 1,1,1,2,3,3-hexafluoropropane, and 1,1,1,2-tetrafluoroethane are about 90% or more by mass of the blend. There are also an apparatus that uses the blend and methods for heating and cooling using the blend.
Owner:HONEYWELL INT INC
Method for preparing hexafluoro-1,3-butadiene
ActiveCN106495982AIncrease added valueRaw materials are cheap and easy to getPreparation by dehalogenationPreparation by hydrogen halide split-offGas phaseTwo step
The invention relates to a method for preparing hexafluoro-1,3-butadiene. The method comprises the following steps: performing reduction dechloridation on 1,2-dichloro-1,1,-2-halothane with zinc powder to obtain a CF2=CHF gas phase intermediate product, introducing into liquid bromine to carry out an addition reaction, and obtaining CBrF2CHBrF; evaporating CBrF2CHBrF in an evaporator to generate a gas, and using a reaction tube filled with a first catalyst to remove one molecule HBr and generate trifluorovinyl bromide; performing two steps of reactions on the trifluorovinyl bromide under the action of the zinc powder, the solvent and a second catalyst, thereby obtaining the hexafluoro-1,3-butadiene. The method has the advantages of being simple in process, high in yield, easy in raw material obtaining, easy in industrialization, green and environment-friendly.
Owner:湖北广钢气体电子材料有限公司
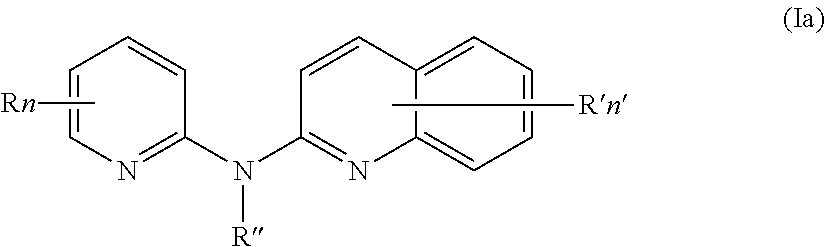
Compounds for preventing, inhibiting, or treating cancer, aids and/or premature aging
The manufacture and use of compounds of formula (Ia) or a pharmaceutically acceptable salt thereof for preventing, inhibiting or treating cancer, AIDS and / or premature aging. The compounds of formula (Ia) being:where:R independently represents a hydrogen atom, a halogen atom, a (C1-C3)alkyl group, a —CN group, a hydroxyl group, a —COOR1 group, a (C1-C3)fluoroalkyl group, a —NO2 group, a —NR1R2 group, or a (C1-C3)alkoxy group;R′ is a hydrogen atom, a halogen atom, a (C1-C3)alkyl group, a —NO2 group, a (C1-C3)alkoxy group, or a —NR1R2 group; andR1 and R2 are a hydrogen atom or a (C1-C3) alkyl group.
Owner:ABIVAX +3
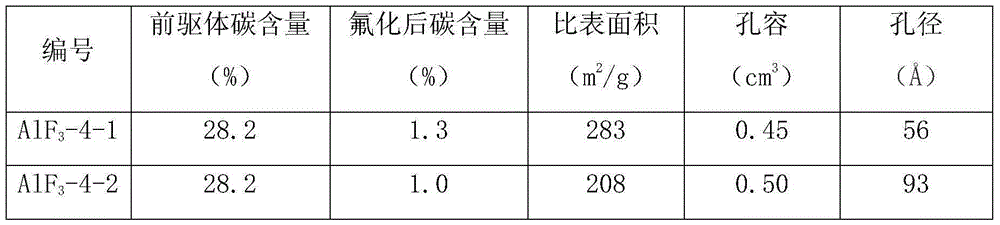
Aluminum fluoride catalyst with high specific surface area and application thereof
ActiveCN106256429AShorten the fluoridation timeWon't clogPreparation by hydrogen halide split-offPhysical/chemical process catalystsAluminum fluorideAlcohol
The invention discloses an aluminum fluoride catalyst with a high specific surface area. Ethyl ether of HF and / or an alcoholic solution are / is firstly added into an organic aluminum solution for reacting to obtain a catalyst precursor, and then the aluminum fluoride catalyst is obtained through fluoridation. The specific surface area of the aluminum fluoride catalyst can reach 200 m<2> / g to 320 m<2> / g, and the aluminum fluoride catalyst is suitable for synthesis of 1-chloro-2,2,2-halothane or 3,3,3-trifluoropropene or 1,1,1,2,3-pentachloropropane or 2,3,3,3-tetrafluoropropene.
Owner:陕西中化蓝天化工新材料有限公司 +2
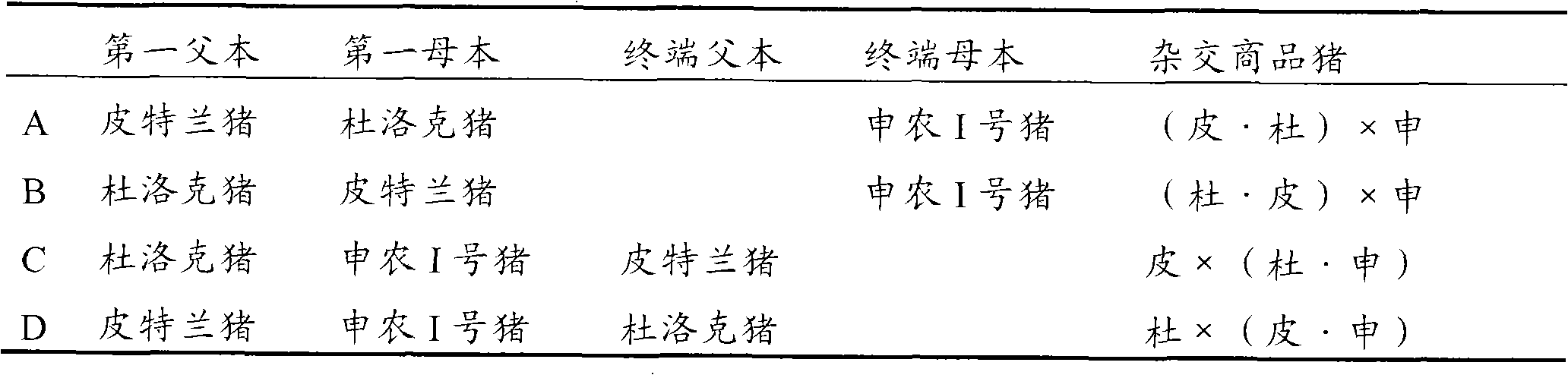
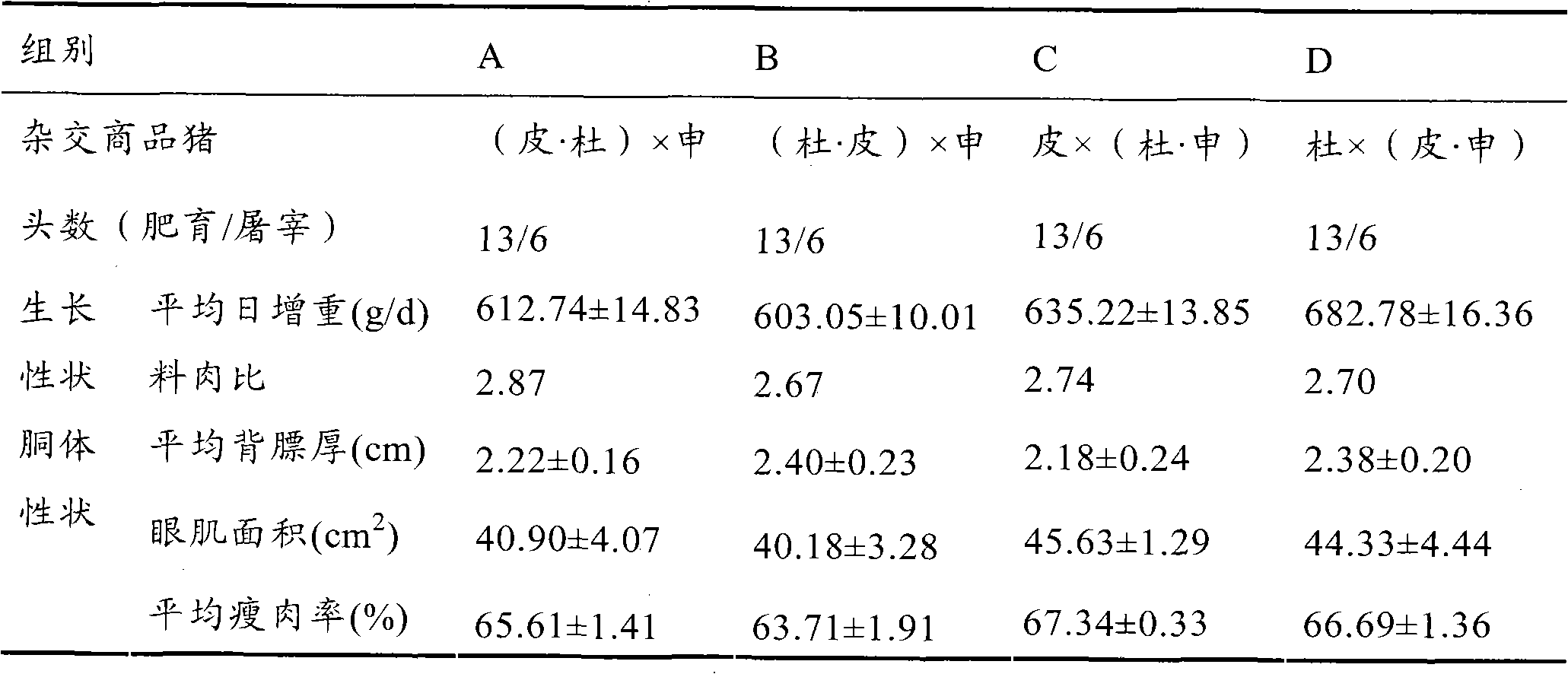
Mating system selective breeding method for producing high-quality commercial pig
The invention discloses a mating system selective breeding method for producing a high-quality commercial pig, comprising the following steps: A) taking halothane-free recessive gene Pietrain as a first male parent of a mating system, taking Duroc as a first female parent of the mating system, and hybridizing to obtain Pierre cross-bred pig; and B) selecting the Pierre cross-bred pig as a second male parent of the mating system, taking Shannon No. I pig as a terminal female parent, and hybridizing to obtain the high-quality commercial pig. The average litter size of the pluriparous sows of the mating system terminal female parent of the invention is not less than 12, the growth speed of the high-quality commercial pig is up to above 700 g / d, lean meat percentage is above 62%, feed conversion ratio is not higher than 3.2, intramuscular fat content is not lower than 2.5%, and no PSE meat exists.
Owner:SHANGHAI ELITE AGRI SCI TECH GROUP +2
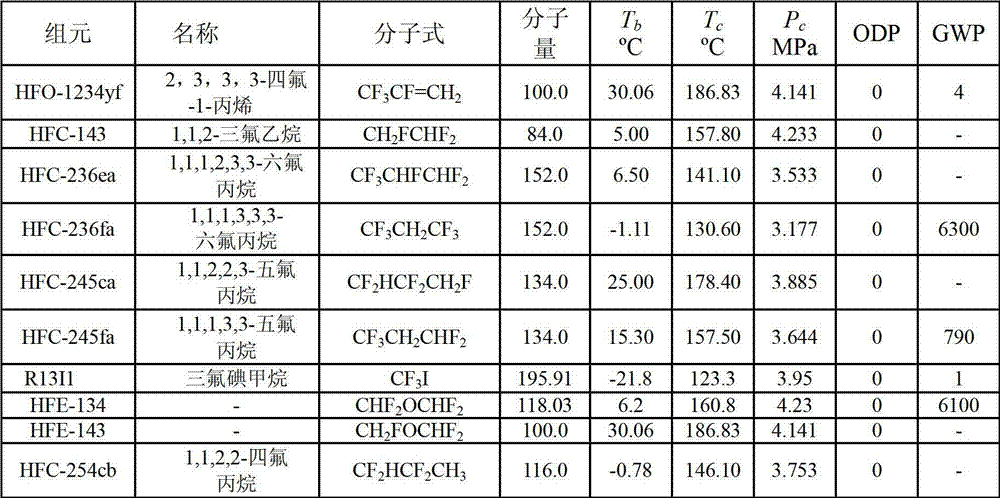
Organic Rankine cycle mixed refrigerant containing HFC-143(1,1,2-halothane)
The invention discloses an organic Rankine cycle mixed refrigerant containing HFC-143(1,1,2-halothane). The organic Rankine cycle mixed refrigerant containing HFC-143 is suitable to be used as refrigerating fluid of a low temperature Rankine cycle system of which the evaporation temperature is 60 DEG C to 100 DEG C. The mixed refrigerant comprises HFO-1234yf (CF3CF=CF2, 2, 3, 3, 3-teflon-1-propylene), HFC-143 (1,1,2-halothane) and HFC-245fa (1,1,1,3,3,3-hexafluoropropane) according to different mass ratio. The preparation method comprises the step of physically mixing the three components according to an appointed ratio at the normal temperature to obtain the corresponding mixed refrigerant. The mixed refrigerant does not damage ozone layers, is lower in greenhouse effect, and accords with environment protection requirements; and thermal parameters are proper and cycling performance is excellent. Under a designed working condition, the efficiency of the Rankine cycle is about 9.5%, and unit mass output net work is basically over 18KJ / kg.
Owner:TIANJIN UNIV
Method for producing trichlorotrifluoroethane by high-temperature gas phase chlorination method
The invention belongs to the technical field of chemical engineering and particularly relates to a method for producing trichlorotrifluoroethane by a high-temperature gas phase chlorination method. According to the method for producing trichlorotrifluoroethane by the high-temperature gas phase chlorination method, a one-step segmented different-temperature serially-connected high-temperature gas phase is adopted for synthesizing 1.1.1 trichlorotrifluoroethane through chlorination, chlorine is added into halothane gas for further reaction to complete the production, and the steps are few. Meanwhile, the cost of raw materials is low, and the halothane is 27000Yuan / ton. The conversion rate is as high as 27 to 35 percent. The reaction time only needs 20 to 40 seconds, the yield is high, and 30 percent of the cost can be saved through being compared with that of other methods for synthesizing identical products.
Owner:AZUREWAVE TEHNOLOGIES INC
Preparation method of thioglycoside compound
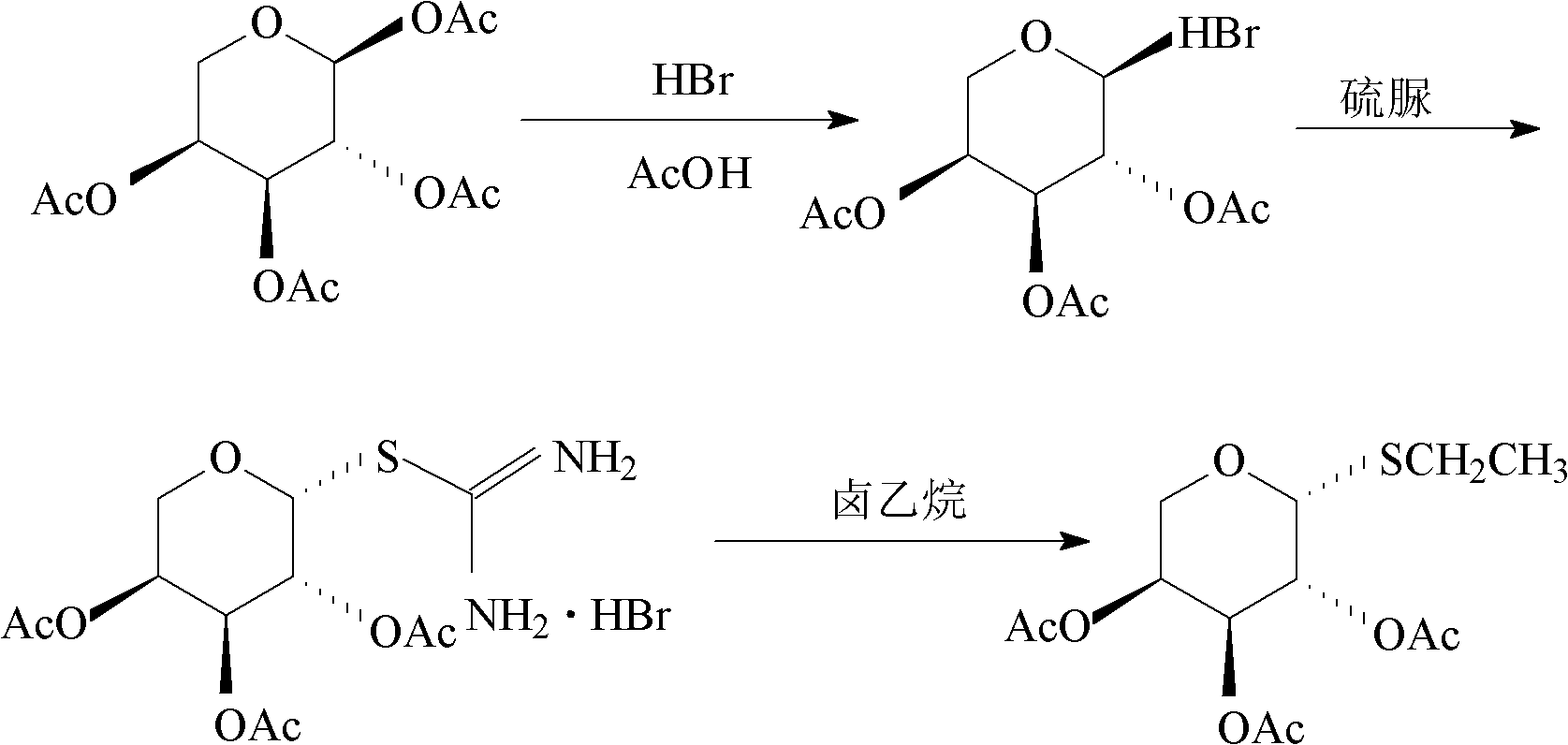
ActiveCN102627674AAvoid harmEasy post-processingSugar derivativesSugar derivatives preparationThioureaHuman health
The invention provides a preparation method of a thioglycoside compound. The preparation method comprises the following steps of: (a) dissolving halogenated acetylated sugar and thiourea into a first organic solvent and reacting under a heating condition to obtain S-glycosyl isothiourea halide; and (b) reacting the S-glycosyl isothiourea halide and halothane in mixed solution of a second organic solvent and organic amine to obtain the thioglycoside compound. In the entire reaction process, the thiourea and the halothane are mainly selected and used as reactants, ethanethiol with offensive odor and toxicity is not required to be selected and used, and the hazard caused to human health and environment is effectively avoided. In addition, according to the method provided by the invention, an obtained product is extremely easy to separate crystals, the pH is not required to be adjusted, and the post-treatment operation is simple.
Owner:济南尚博生物科技有限公司
Commercial pork pig multiple crossbreeding method
The invention discloses a commercial pork pig multiple crossbreeding method. The method comprises the following steps of A) taking Pietrain pig with non-halothane allogene as the first male parent of multiple crossbreeding and Meishan pig as the first female parent of multiple crossbreeding for hybridization to obtain Pietrain-Meishan-crossed hybrid pig; B) taking Duroc pig as the second male parent of multiple crossbreeding and the Pietrain-Meishan-crossed hybrid pig as the terminal female parent of multiple crossbreeding for hybridization to obtain commercial pork pig. According to the commercial pork pig multiple crossbreeding method, the average litter size of multiparity sow of the first female parent of multiple crossbreeding is not less than 14.5; the hybrid high-quality commercial pork pig obtains an average daily gain up to more than 680 g / d (gramme per day) and a lean meat rate up to more than 64% and a feed conversion rate as little as 2.75, avoids PSE (pale-soft-exudative) meat and obtains an intramuscular fat percentage up to 2.5%.
Owner:SHANGHAI XIANGXIN ANIMALS & FOWLS +1
Mold releasing agent
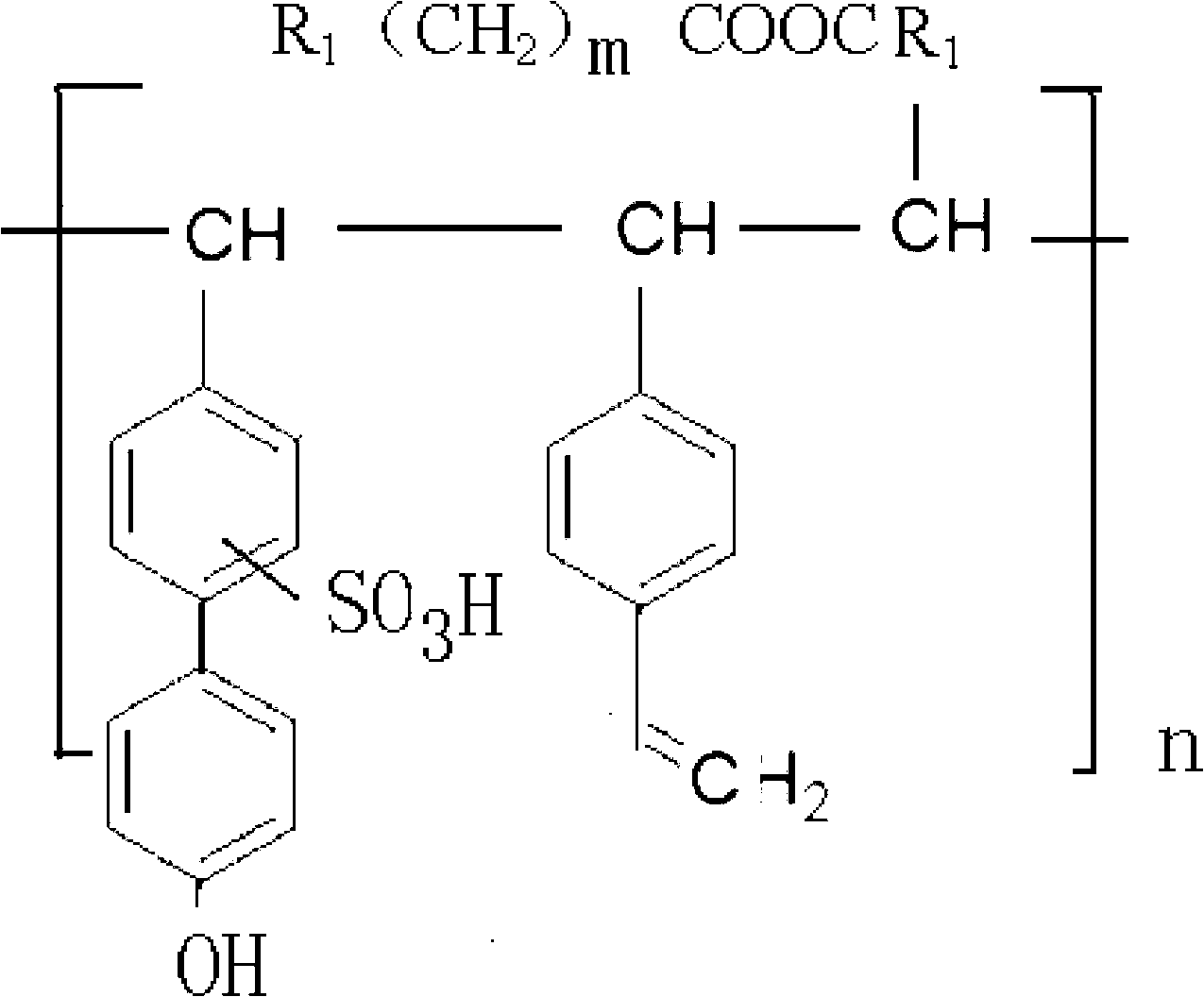
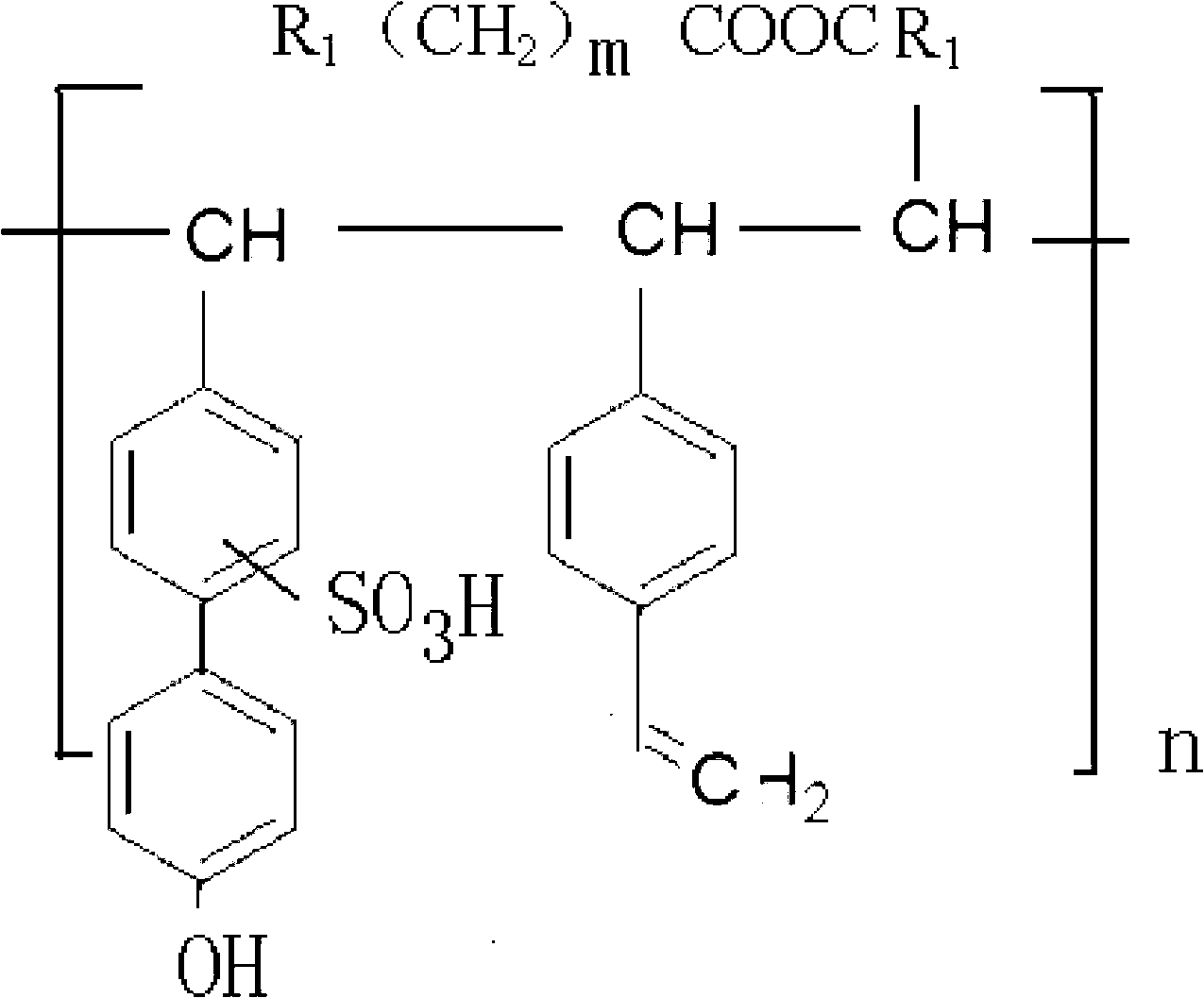
InactiveUS20160002377A1Comparable releasing performanceComparable longevityCoatingsFluorine containingHalothane
Owner:TAIWAN FLUORO TECH
Resin catalyst applied to preparation of phenol through decomposition of cumene hydroperoxide (CHP) and preparation method for resin catalyst
ActiveCN102698801AImprove oxidation resistanceExtended service lifeOrganic chemistryOrganic compound preparationOxidation resistantIon-exchange resin
The invention discloses a resin catalyst applied to preparation of phenol through decomposition of cumene hydroperoxide (CHP) and a preparation method for the resin catalyst. The preparation method comprises the following steps of: by a macroporous styrene ion exchange resin aggregation production process, adding a (methyl) crylic acid halothane-based ester monomer and the phenol, aggregating, sulfonating, and thus obtaining the resin catalyst. The prepared catalyst is high in oxidation resistance and mechanical strength, has long service life, can be widely applied in production, and has an extremely good application prospect in the field of preparation of the phenol through a CHP decomposition reaction.
Owner:KAIRUI ENVIRONMENTAL PROTECTION TECH
Silicon release agent and preparation method thereof
The invention discloses a silicon release agent and a preparation method of the silicon release agent. The main ingredients of the silicon release agent are expressed through the following chemical general formula: A-L-B or A-L-Rf, the L is a bifunctional crosslinking agent (Linker); the A comprises an alkoxy silane conjugate having a hydrolysed siloxane functional group structure or an active oxygen-containing functional group conjugate; the B comprises a siloxane conjugate comprising an active functional group, or a carbon-containing oxane conjugate, or a Vinyl / Allyl / (methyl) acrylate conjugate, or an amido-containing carbon oxide conjugate; the Rf comprises a fluorine-containing carbon alkane conjugate, or a fluothane ether molecule, or a fluorinated conjugate ingredient. The silicon release agent can be applied to molding a plastic rubber resin, the polyurethane resin demolds quite easily when the silicon release agent is evenly coated on a polyurethane resin forming mold, and an even thermoset polyurethane resin molding material having no crack, gap or unfilled corner is obtained.
Owner:漳州丰笙新材料有限公司
Method for preparing trifluorochloroethylene by halothane
The invention discloses a method for preparing trifluorochloroethylene by halothane, which includes the step: subjecting the halothane to a reaction with chlorine and hydrogen in a fixed bed reactor respectively, wherein the chlorine is subjected to a reaction at a position of an inlet of the a reactor, the hydrogen is subjected to the reaction at the position 3m away from the inlet of the reactor, the temperature of the catalytic reaction is between 200 DEG C and 300 DEG C, the molar ratio of halothane and the chlorine is 1: (3-5), and the molar ratio of the halothane and the hydrogen is 1: (1-2). According to the method for preparing the trifluorochloroethylene by the halothane, traditional process is abandoned by the whole technological process, the trifluorochloroethylene is prepared by directly subjecting the halothane to respectively reacting with the chlorine and the hydrogen at different positions of the fixed bed reactor, and thereby the method is low in cost and high in yield.
Owner:CHANGSHU 3F FLUOROCHEM IND CO LTD
Method for preparing triethyl silicane
The invention belongs to the field of the preparation of medicinal intermediates, and particularly relates to a method for preparing triethyl silicane. The method comprises the following steps of: performing a condensation reaction of trichlorosilane, halothane and magnesium powder in an alkane or arene organic solvent containing an ether catalyst, and purifying a reaction production to obtain the triethyl silicane. By the method for preparing the triethyl silicane, the obtained product has fewer impurities, and the product content is over 99 percent; and the preparation method has low cost, less pollution and mild reaction condition, is easy and convenient to operate, and is convenient for industrial production.
Owner:河南豫辰药业股份有限公司
Temperature-sensitive tri-block polymer, reduction and ultrasonic-sensitive core-shell structural microgel with same and application of reduction and ultrasonic-sensitive core-shell structural microgel
InactiveCN107254053ASensitive to temperatureWith temperatureAerosol deliveryOintment deliveryCross-linkHalothane
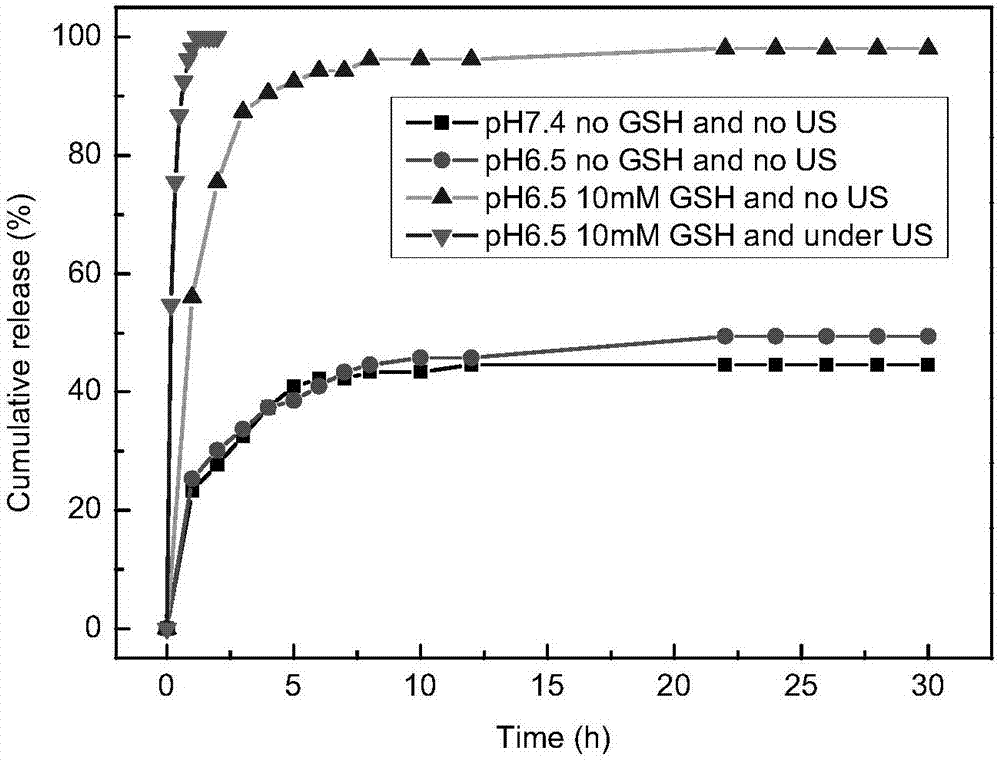
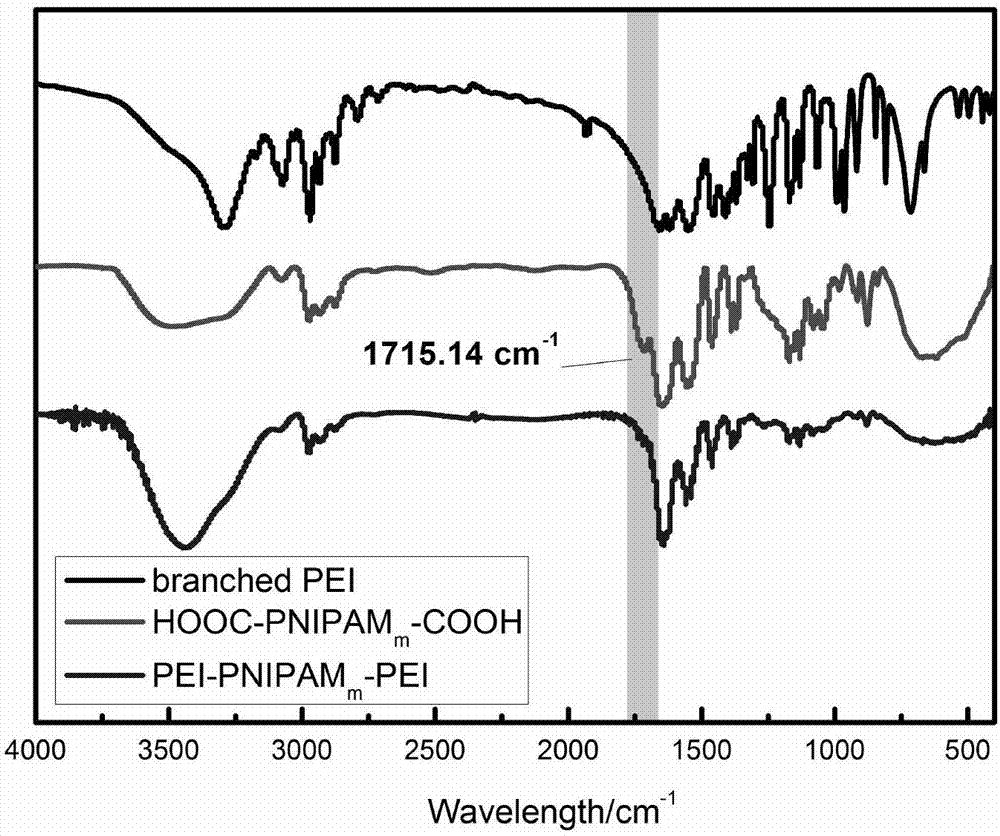
The invention belongs to the field of physical chemistry, and provides a temperature-sensitive tri-block polymer, reduction and ultrasonic-sensitive core-shell structural microgel with the same and application of the reduction and ultrasonic-sensitive core-shell structural microgel. The structure of the temperature-sensitive tri-block polymer is PEI<m>-b-PNIPAM<n>-b-PEI<m>, the temperature-sensitive tri-block polymer is amphiphilic at the lower critical solution temperatures (LCST) and can be subjected to self-assembly in aqueous solution to obtain micelle solution with uniform particle sizes in stable states, PNIPAM<n> is used as a core of the micelle solution, and PEI is used as a shell of the micelle solution. Addition can be carried out on micelle and cross-linking agents BAC<y> to obtain reduction-sensitive gel shells, and disulfide bonds on the shells can be selectively cracked by reducing reagents such as GSH, TCEP and DTT. The temperature-sensitive tri-block polymer, the reduction and ultrasonic-sensitive core-shell structural microgel and the application have the advantages that halothane molecules with gas-liquid phase change properties can be loaded, accordingly the core-shell structural microgel with ultrasonic response can be obtained, and the purpose of target medicine administration can be achieved.
Owner:EAST CHINA UNIV OF SCI & TECH
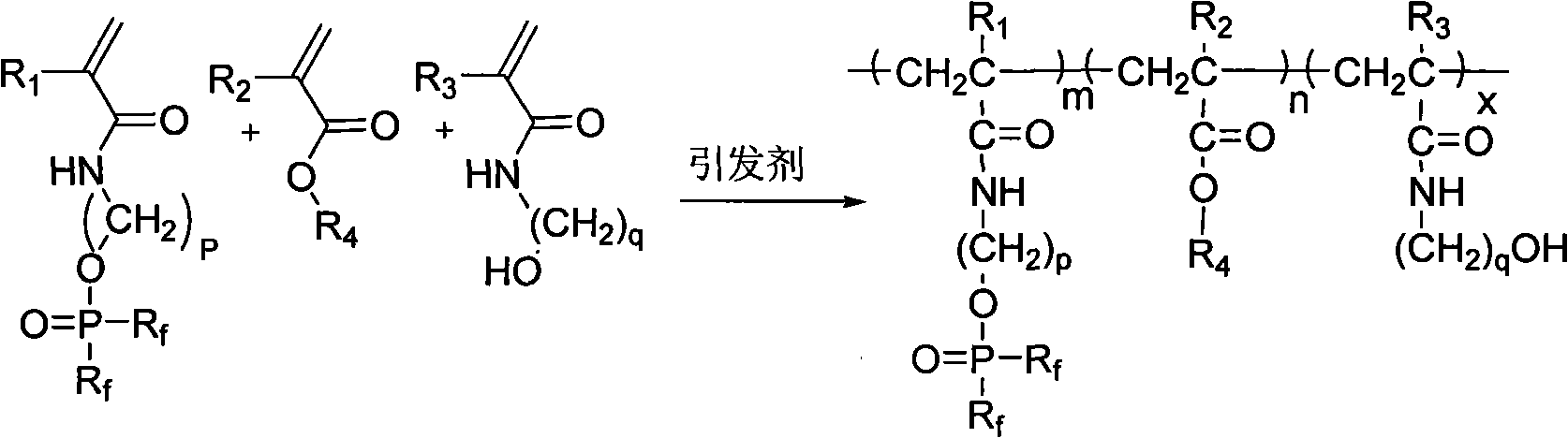
Acrylamide copolymer containing halothane and phosphoric acid ester as well as preparation method and application thereof
InactiveCN101831031ANo emissionsNo pollution in the processVegetal fibresPhosphoric Acid EstersFluorescence
The invention relates to an acrylamide copolymer containing halothane and phosphoric acid ester as well as a preparation method and the application thereof. The preparation method comprises the steps of: (1) dissolving monomer, crosslinking monomer and non-fluoro long-chain monomer of the acrylamide copolymer containing halothane and phosphoric acid ester and emulsifier in deionized water and solvent for ultrasonically pre-emulsifying for 1-5h to obtain milk-white pre-emulsion; (2) under the protection of N2, heating 1 / 3 of the pre-emulsion to 60-90 DEG C, slowly dropping an initiator aqueous solution for initiating reaction, stopping dropping after the emulsion has blue fluorescence, combining the residual initiator aqueous solution with the pre-emulsion, slowing dropping the combined residual initiator aqueous solution and pre-emulsion into reaction liquid, stopping reaction after continuously reacting for 1-5h at 60-90 DEG C, and cooling down to the room temperature to obtain the required emulsion PFPA (Per Fluoro Propionic Anhydride). In the invention, cotton fabric processed by the copolymer emulsion has excellent performances of resisting water, oil and flame, the processing process is simple, and greater development potency is provided.
Owner:DONGHUA UNIV
Difluoroethanol synthesis process
InactiveCN108069821AFew stepsSimple processOrganic compound preparationHydroxy compound preparationReaction temperaturePotassium
The invention relates to a difluoroethanol synthesis process. The difluoroethanol synthesis process comprises the following steps: firstly adding gamma-butyrolactone and an alkali metal hydroxide intoa reactor, introducing 2,2-difluoro-1-halothane, rapidly heating to the reaction temperature, processing a feed liquid through a centrifugal machine after the reaction is finished, rectifying an obtained clear liquid in a separation tower to obtain difluoroethanol; centrifuging, enabling a solid to enter a washing kettle, washing with methyl alcohol or ethanol, processing a washing liquid throughthe centrifugal machine, drying an obtained solid through a drier to obtain potassium chloride, conducting vacuum distillation on the centrifuged clear liquid in a distilling kettle, recycling methylalcohol or ethanol, obtaining a solid of potassium hydroxybutyrate after distilling, drying through the drier, and returning to a reaction system to continue to react. By adopting the process, the operation is simple, the economic efficiency is relatively high, and the process is suitable for large-scale production.
Owner:XIAN MODERN CHEM RES INST
Plasma etching gas
InactiveCN1405855ADoes not affect etch critical dimensionsImprove etch uniformitySemiconductor/solid-state device manufacturingHalothaneNitrogen gas
The gas is suitable for the etching machine of etching silicon oxide to etch silicon layer. The gas at least includes the partial substitutional halothane gas, the entire substitutional halothane gas, argon gas and nitrogen gas. The ratio between the partial substitutional halothane gas and the entire substitutional halothane gas is about 3:1 to 15:1.
Owner:MACRONIX INT CO LTD
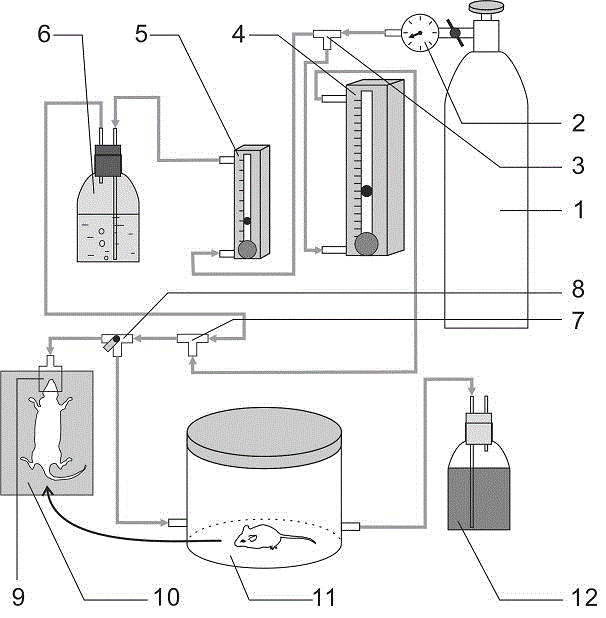
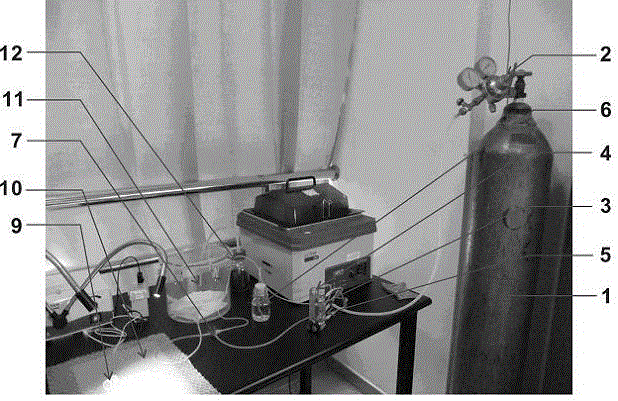
Simple small animal inhalation anesthesia machine
The invention belongs to the technical field of small animal anesthesia and relates to a simple economical small animal inhalation anesthesia machine which comprises a gas steel bottle, a gas adjusting valve, a three-way pipe I, a gas flow meter I, a gas flow meter II, an isoflurane gas volatilization bottle, a three-way pipe II, an adjustable three-way pipe, an anaesthetic mask, an animal body constant temperature system, an anesthesia induction box and a gas recycling bottle. The gas steel bottle is connected with the gas adjusting valve, the three-way pipe I, the gas flow meter I, the gas flow meter II, the isoflurane gas volatilization bottle, the three-way pipe II, the adjustable three-way pipe, the anaesthetic mask, the animal body constant temperature system, the anesthesia induction box and the gas recycling bottle in sequence. Using results show that the simple economical small animal inhalation anesthesia machine is simple and reasonable in structure, various parts are fixed and combined in a coordinating mode, small animals can be subjected to anesthesia control in short time or long time, accurate anesthesia depth is guaranteed, safety is good, after operation, the morbidity and the mortality of the small animals are low, and the operation success rate is high.
Owner:FUDAN UNIV
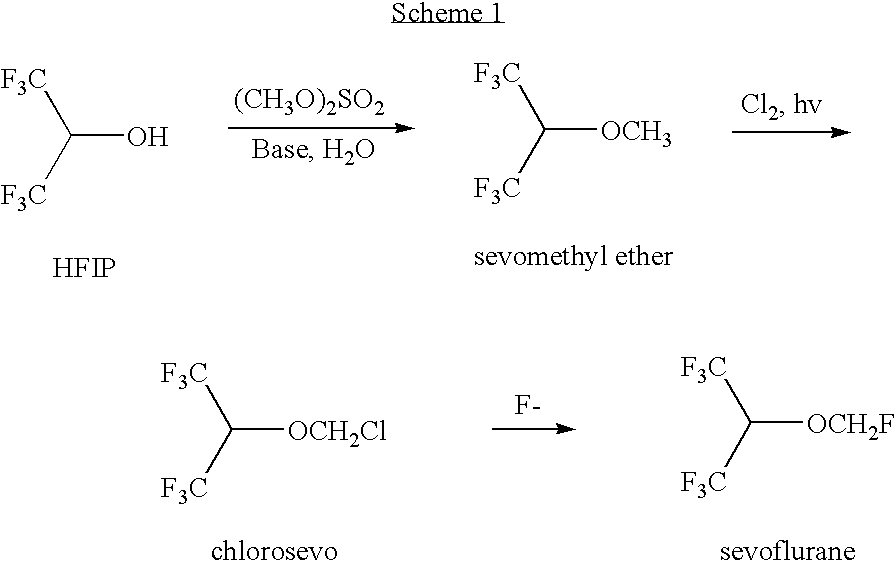
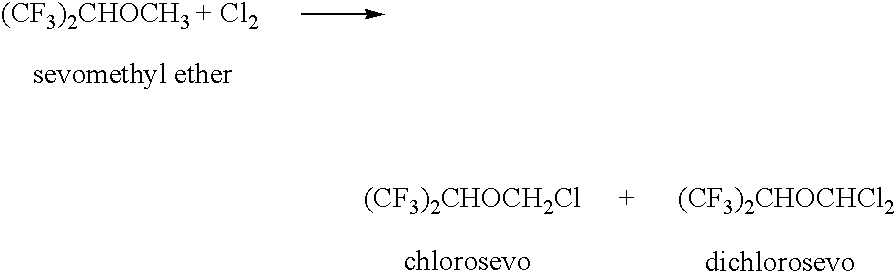
Process for recovery of 1,1,1,3,3,3-hexafluoroisopropanol from the waste stream of sevoflurane synthesis
ActiveUS6987204B2Useful anesthetic propertyRapid onset of anesthesiaEther separation/purificationOrganic compound preparationPropanolAcid hydrolysis
Owner:BAXTER INT INC
Inhalational compound anaesthetic agent
InactiveCN104623084ARapid induction of anesthesiaShort duration of anesthesiaPharmaceutical delivery mechanismAnaestheticsRadix AconitiInduction anesthesia
The invention provides an inhalational compound anaesthetic agent. The inhalational compound anaesthetic agent is characterized by comprising an A agent and a B agent, wherein the A agent serves as an inducer and comprises nitrous oxide, sodium chloride, perfume and a compound emulgator; the B agent serves as an anesthesia retaining agent and comprises flos daturae, radix aconiti agrestis, sevoflurane, sodium chloride, perfume and a compound emulgator; according to the A agent, the content of nitrous oxide is 1-5%, the content of the compound emulgator is 15-25%, and the content of the perfume is 0.1-0.3%; according to the B agent, the content of flos daturae is 0.5-0.9g / 100mL, the content of radix aconiti agrestis is 0.1-0.5g / 100mL, and the content of the sevoflurane is 1.5-2.5%, the content of the compound emulgator is 10-20%, and the content of the perfume is 0.1-0.3%. The inhalational compound anaesthetic agent is simple to operate during use; the A agent can be used for quickly inducing anesthesia and can be directly inhaled by an inhaling device; the B agent is used for retaining anesthesia, can be inhaled by a matched anesthesia machine and can also be infused into the body by other manners; the infusing time and dosage can be flexibly arranged according to the needs of the operation; and the inhalational compound anaesthetic agent can be applied to complex or long-time operation.
Owner:黄玉华
Etching method for anti-reflecting coating layer on organic substrate
InactiveCN1437073AAffect critical dimensionsAvoid etchingSemiconductor/solid-state device manufacturingPhotosensitive material processingPlasma GasesHalothane
The etching method of the present invention is that after material layer, anti-reflecting base organic coating layer and photoresist pattern are formed successively on the substrate, etching plasma gas including at least partially substituted halothane gas is used to etch the anti-reflecting base organic coating layer. During the etching, halothane polymer is formed on the side wall of the photoresist pattern to prevent the side wall from being etched.
Owner:MACRONIX INT CO LTD
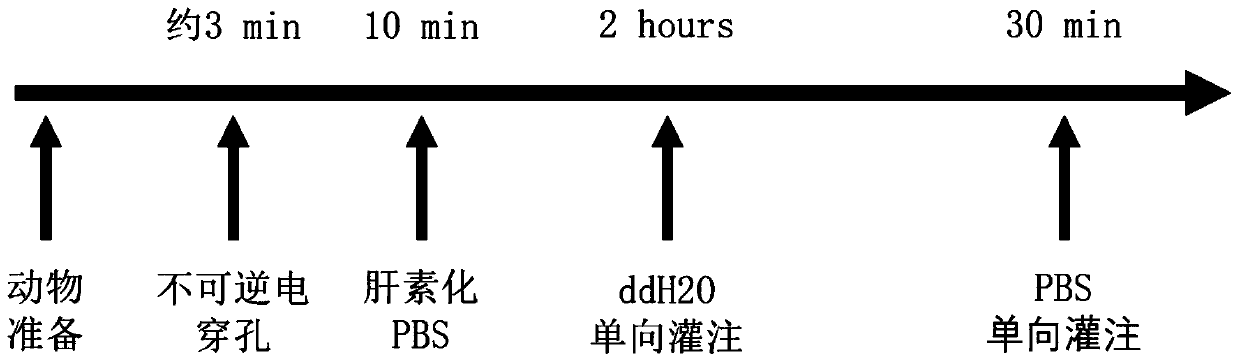
Liver acellular stent construction method based on irreversible electroporation technology
PendingCN111518744AAvoid damageShort timeCell dissociation methodsVertebrate cellsVena portaAcellular scaffold
The invention relates to a liver acellular stent construction method based on an irreversible electroporation technology. The method comprises the following steps of 1.1) weighing a rat, carrying outgas inhalation anesthesia by using isoflurane, maintaining the gas inhalation anesthesia, after the anesthesia is effective, disinfecting abdominal skin of the SD rat in a supine position by using 75%alcohol, and opening the abdomen layer by layer to expose the liver; 1.2) clamping a left lateral lobe area of the rat liver by using a caliper type electrode, and performing high-voltage pulsed electric field treatment; 1.3) after the irreversible electroporation treatment is finished, performing transhepatic portal vein catheterization, perfusing with heparinized PBS (Phosphate Buffer Solution), cutting off the subhepatic inferior vena cava until the liver is full and the color is gradually lightened, gradually dissociating tissues and ligaments around the liver, taking down the liver, andimmersing the liver into the heparinized PBS; 1.4) putting the taken liver into a clean container, and performing one-way perfusion with deionized water, and 1.5) after the deionized water perfusion is finished, performing one-way perfusion with PBS for 30 minutes to complete the preparation of the stent. By the method disclosed by the invention, the construction efficiency of the in-vitro acellular stent can be effectively improved under the condition of not applying harmful chemical preparations.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
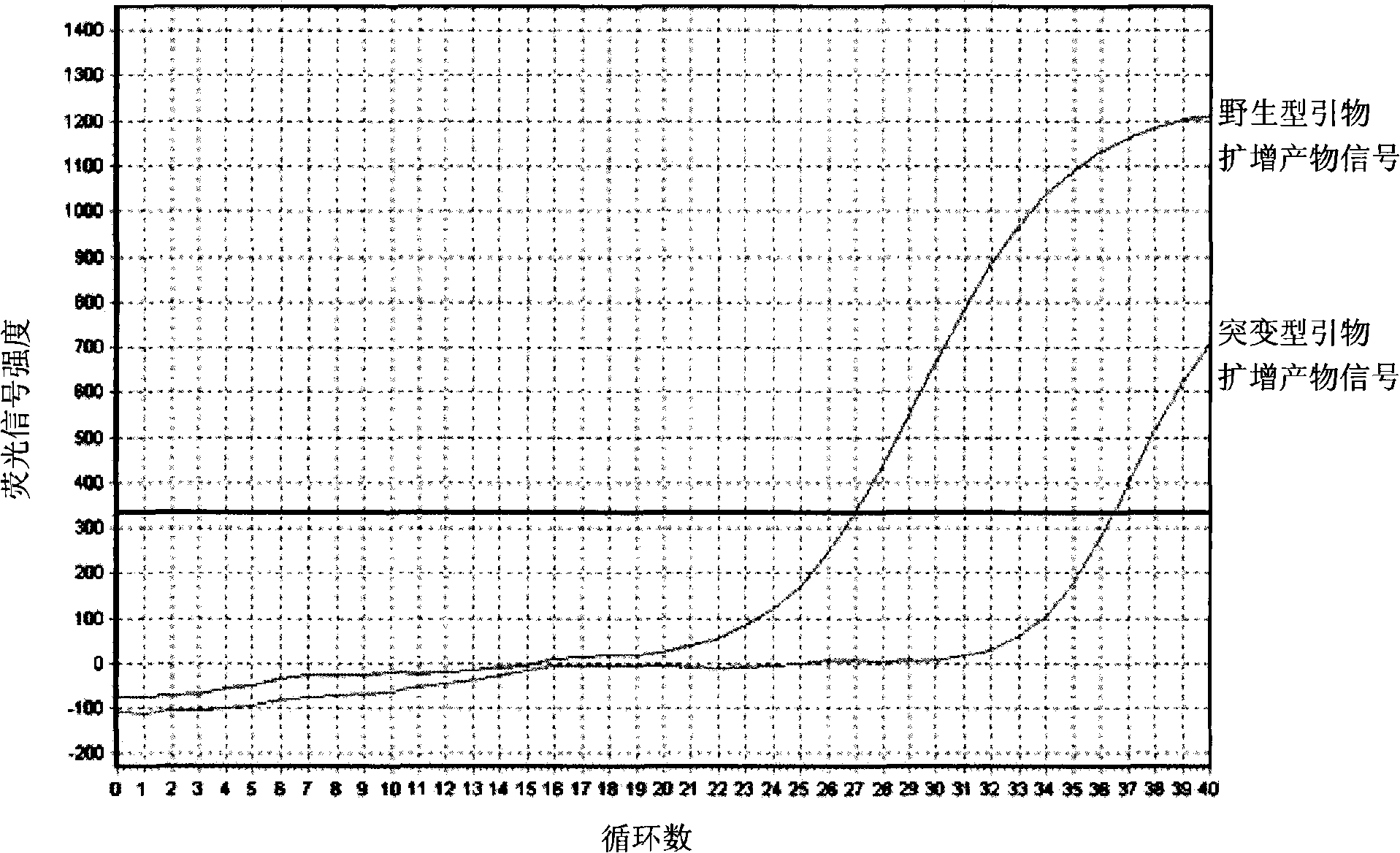
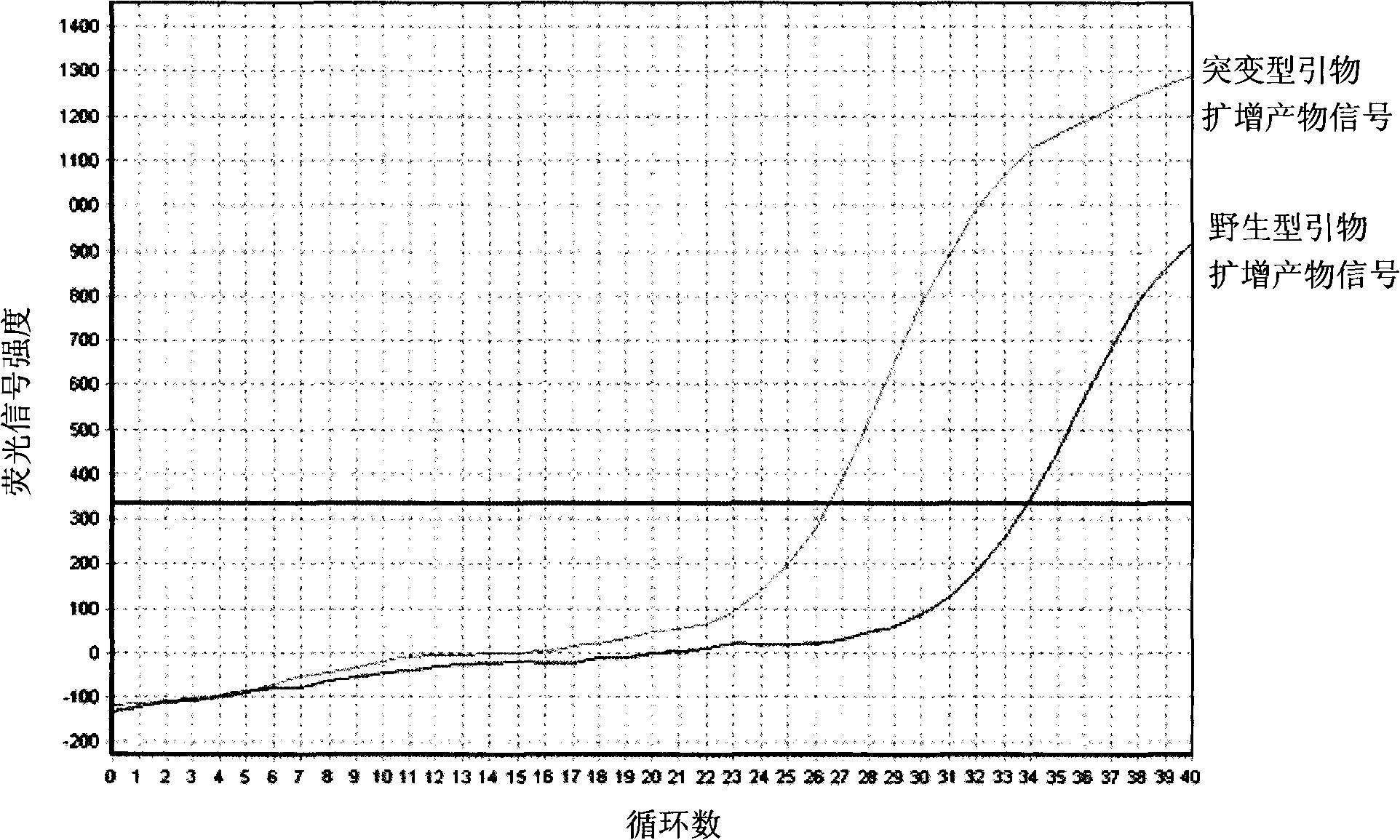
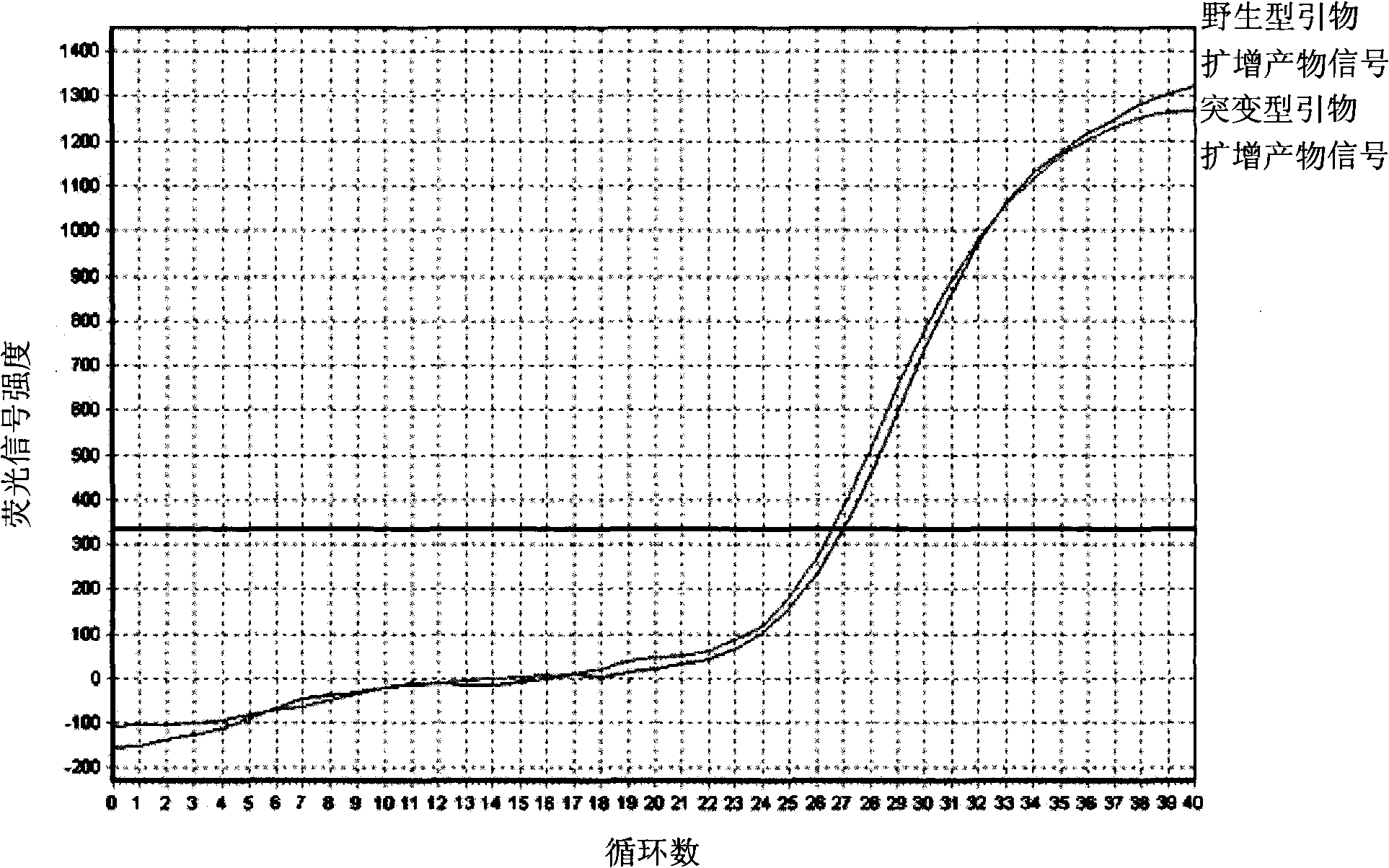
Detection method of halothane genotype in swine
InactiveCN101358245AFew stepsHigh precisionMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceGenotype
The invention provides a genotype test method for a pig halothane gene. Two groups of different primers are schemed out according to the base sequences of two ends of the mutant site of the pig halothane gene, sample DNA respectively receives the PCR amplification with simultaneously, the change of the product amount is monitored through the fluorescent signal intensity change to judge the genotype of the sample DNA. The invention solves the technical problems of low accuracy, many operation steps, complicate process, long time and toxic test reagents of the methods for testing the genotype of the pig halothane gene in the prior art, improves the test accuracy, simplifies the operation procedures, and avoids the use of the toxic and harmful reagents.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
Preparation of sevoflurane with negligible water content
InactiveCN101180250AReduce moisture contentEther separation/purificationOrganic compound preparationMolecular sieveMedicine
Provided is a sevoflurane anesthetic product which can remain substantially undegraded after long periods of storage, as well as a method for preparing the product. The product comprises sevoflurane in a glass container having a water content of less than 130 ppm. The method comprises drying sevoflurane having a water content of greater than 130 ppm to a water content les than 130 ppm. A preferred method of drying comprises contacting a sevoflurane composition having a water content of greater than 130 ppm with alumina-containing molecular sieves such that the water content is reduced to lessthan 130 ppm.
Owner:PIRAMAL CRITICAL CARE
Preparation method of pentafluoroethane
ActiveCN100999439AReduce wasteSolve the problem of unstable ratioPreparation by halogen replacementTetrachloroethyleneHydrogen fluoride
This invention relates to a preparation method of pentafluoroethane (HFC-125). It takes hydrogen fluoride (HF) and tetrachloroethene (PCE) as raw material, used two reaction vessel through two-step gaseous phase fluoridation to prepare HFC-125. The first reactor mainly fluorate PCE to synthesize 2,2 - dichloro-1 ,1,1 - halothane (HCFC-123) and 2-chloro-1 ,1,1,2 - tetrafluoroethane (HCFC-124); the second reaction mainly fluorate at least one of HCFC-123, HCFC-124 to synthesize HFC-125. Using distillation column to take PCE from the products of first reactor, and then circulate to first reactor. Take phase separator to separate HF, HCFC-123 and HCFC-124 from products of first and second reactor, HF can circulate to first and second reactor for continues reaction, HCFC-123 and HCFC-124 can be recycled to the second reactor continued reaction, but also can through distillation and separation to obtain HCFC-123 and HCFC-124 products. The invention mainly for prepare pentafluoroethane.
Owner:XIAN MODERN CHEM RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com