Aluminum fluoride catalyst with high specific surface area and application thereof
A technology with high specific surface area and catalyst, which is applied in the direction of aluminum fluoride, physical/chemical process catalyst, aluminum halide, etc., can solve the problems of expensive raw material aluminum isopropoxide, complicated preparation process, and organic impurities, and achieve shortening of fluorine The effect of time-saving and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
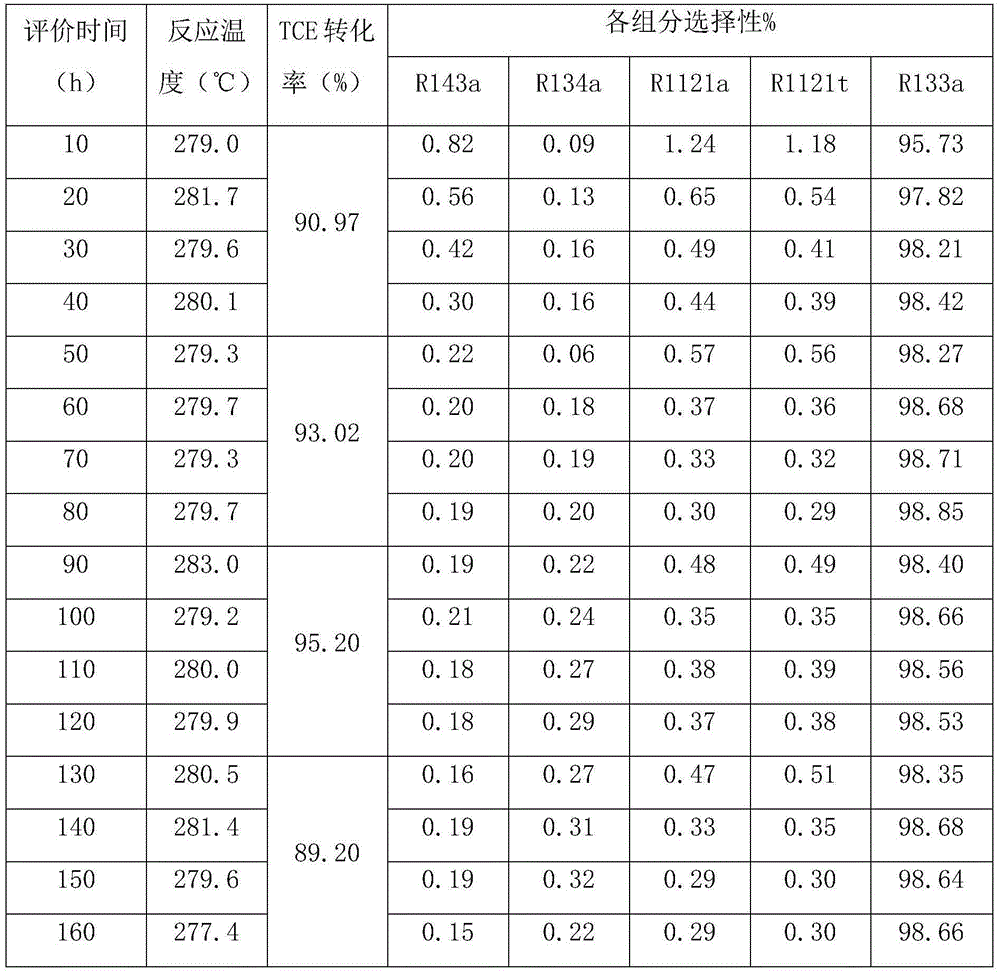
Examples
Embodiment 1
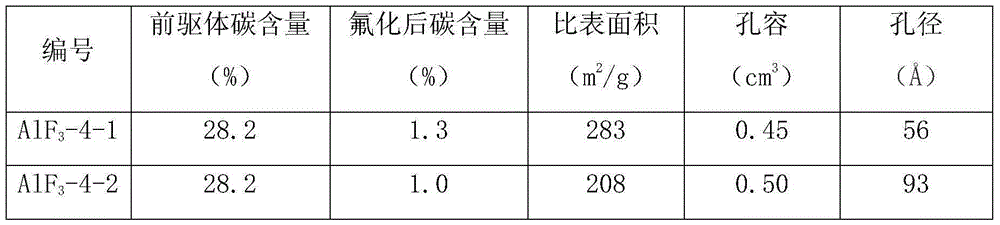
[0038] Take 61.58g Al(t-BuO) 3 Dissolve in 500ml of isopropanol and stir to form an aluminum solution. At room temperature, dissolve 15.0 g of anhydrous HF in HF-Et 2 O solution (12.5mol / L) was added to the above aluminum solution, stirred vigorously, stopped stirring after 4 hours of reaction, and left to age for 4 hours. Use a rotary evaporator or a centrifuge to remove the volatile components in the product, and dry them in a vacuum oven to obtain a high specific surface area aluminum fluoride precursor, denoted as AlF 3 -1.
Embodiment 2
[0040] Take 61.58g Al(t-BuO) 3 Dissolve in 500ml of isopropanol and stir to form an aluminum solution. At room temperature, dissolve 30.0 g of anhydrous HF in HF-Et 2 O solution (12.5mol / L) was added to the above aluminum solution, stirred vigorously, stopped stirring after 4 hours of reaction, and left to age for 4 hours. Use a rotary evaporator or a centrifuge to remove the volatile components in the product, and dry them in a vacuum oven to obtain a high specific surface area aluminum fluoride precursor, denoted as AlF 3 -2.
Embodiment 3
[0042] Take 61.58g Al(t-BuO) 3 Dissolve in 500ml of isopropanol and stir to form an aluminum solution. At room temperature, dissolve 10.0 g of anhydrous HF in HF-Et 2 O solution (12.5mol / L) was added to the above aluminum solution, stirred vigorously, stopped stirring after 4 hours of reaction, and left to age for 4 hours. Use a rotary evaporator or a centrifuge to remove the volatile components in the product, and dry them in a vacuum oven to obtain a high specific surface area aluminum fluoride precursor, denoted as AlF 3 -3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com