Fluorine-containing hyperbranched polyester acrylic ester and method of preparing the same
A polyester acrylate and acrylate-based technology, applied in the field of UV-curable hydrophobic and oleophobic materials, can solve problems such as pollution, isocyanate toxicity, and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
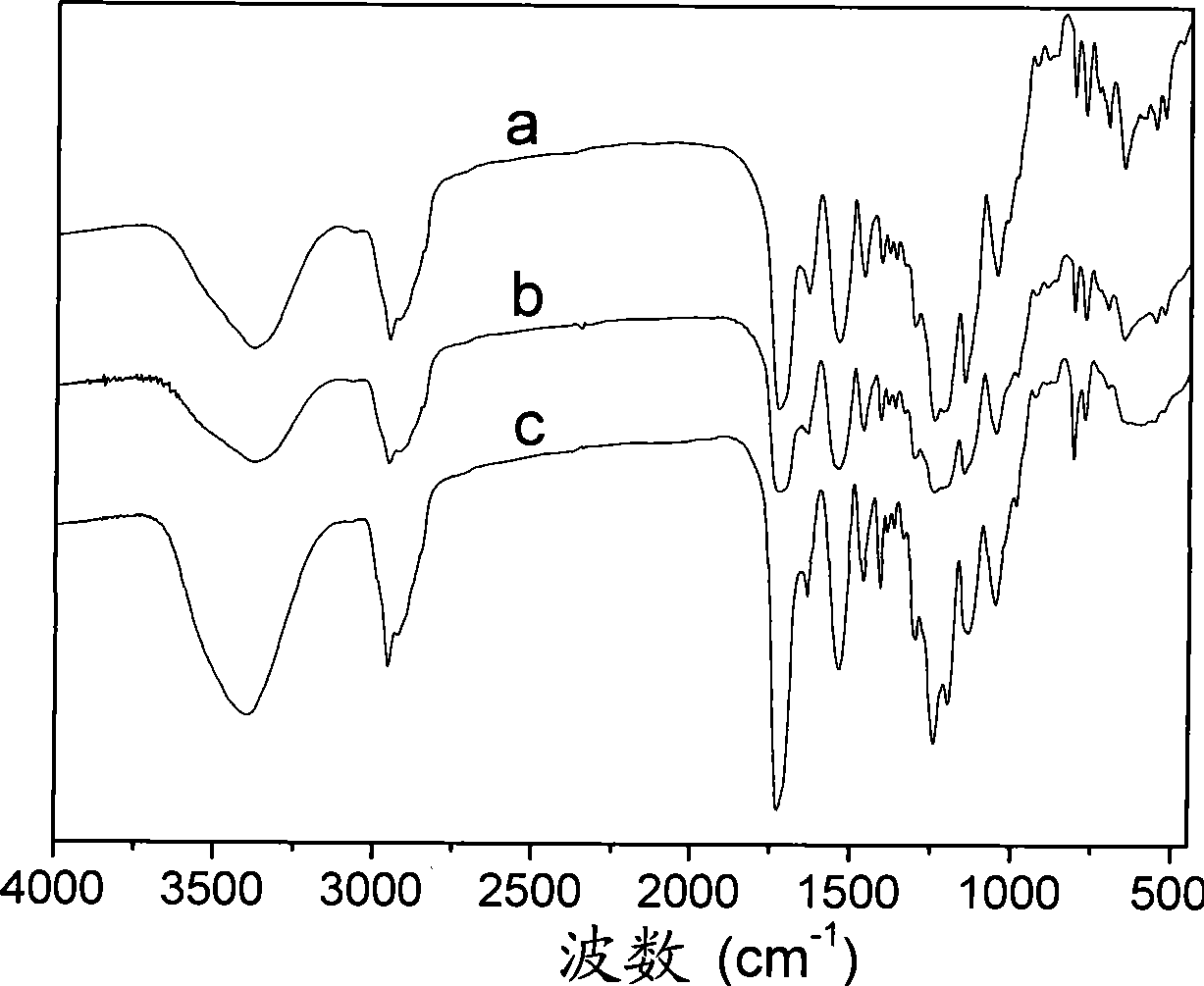
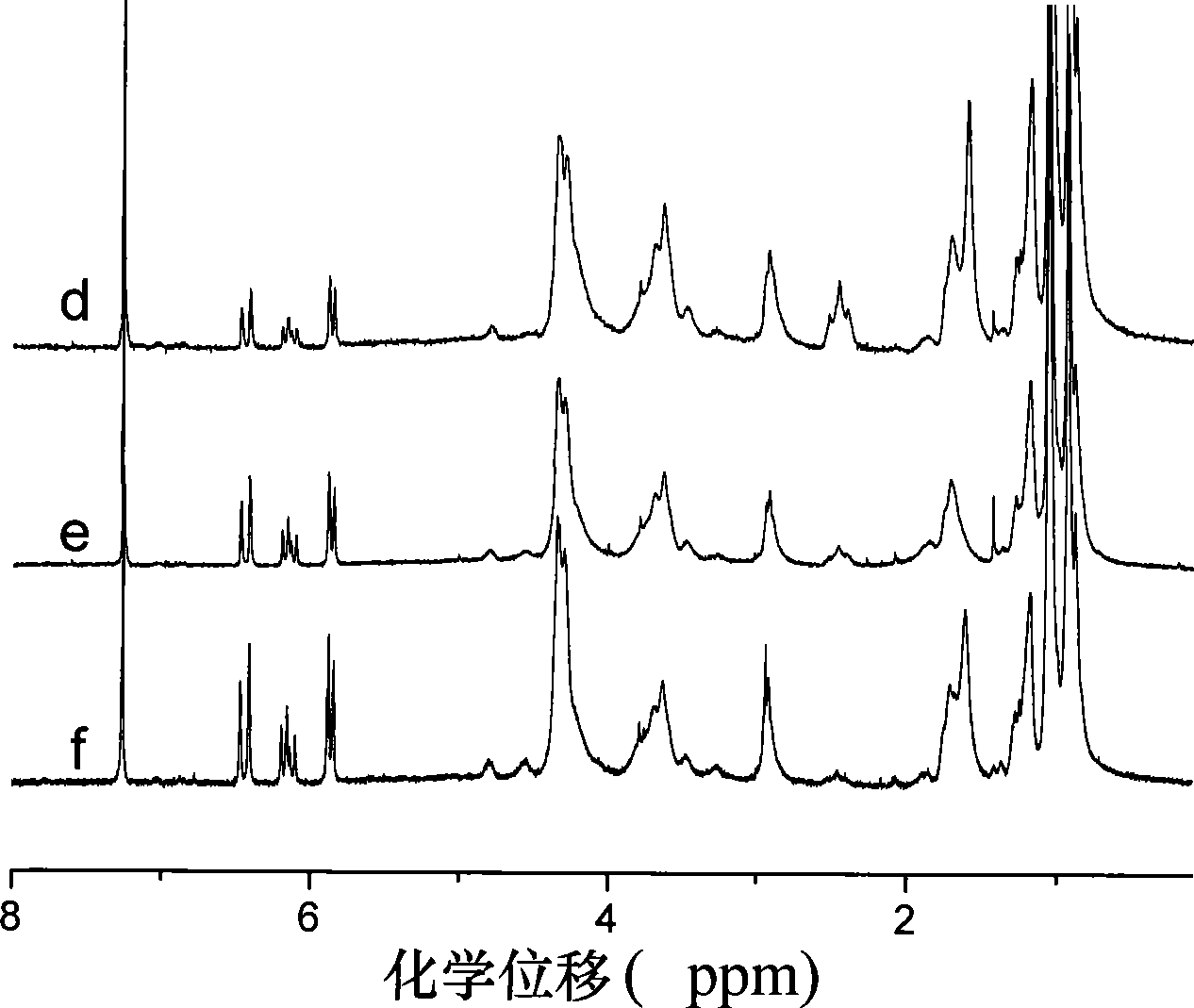
Image
Examples
Embodiment 1
[0033] Under nitrogen protection, 4.45 g of isophorone diisocyanate (IPDI) was added to a three-necked flask reactor equipped with a condensation and stirring device, diluted with 20 mL of N, N-dimethylacetamide (DMAc), and added 0.1wt% of the catalyst dibutyl tin laurate (DBTDL), then slowly drip the DMAc solution containing 9.74g of perfluorooctyl alcohol in this solution with a dropping funnel, after the dropwise addition, the temperature is raised to 50°C, React for 12 hours to obtain the first semi-addition product A1; at the same time, add IPDI 4.45g in another three-necked flask reactor equipped with condensation and stirring devices, dilute with 20mLDMAc, and add 0.1wt% catalyst DBTDL , and then slowly dropwise add the DMAc solution containing 2.43g of hydroxyethyl acrylate (HEA) to the solution with a dropping funnel, after the dropwise addition, the temperature is raised to 50°C, and the reaction is carried out for 12 hours to obtain the second semi-addition product B...
Embodiment 2
[0046] Under the protection of nitrogen, add IPDI 4.45g in the three-necked flask reactor equipped with condensation and stirring device, dilute with 20mL dioxane, and add 0.5wt% catalyst DBTDL, then use the dropping funnel to slowly add to the solution Add dropwise a dioxane solution containing 9.74 g of perfluorooctyl ethanol. After the dropwise addition, heat up to 50° C. and react for 12 hours to obtain the first semi-addition product A2; Add IPDI 18.80g in the three-necked flask reactor of the condensing and stirring device, dilute with 20mL dioxane, and add the catalyst DBTDL of 0.5wt%, then use the dropping funnel to slowly drop the dioxane containing HEA 9.72g in the solution. Hexane solution, after the dropwise addition, was heated up to 50°C, and reacted for 12 hours to obtain the second semi-addition product B2; BoltornH30 11.25g was added to a three-necked flask reactor equipped with a condensation and stirring device, and 50mL dihydrogen After the oxyhexane was di...
Embodiment 3
[0058] Under the protection of nitrogen, add IPDI 4.45g into the three-necked flask reactor equipped with condensation and stirring device, dilute with 20mL dimethyl sulfoxide, and add 0.3wt% catalyst DBTDL, then use the dropping funnel to slowly add to the solution Add dropwise a dimethyl sulfoxide solution containing 9.74 g of perfluorooctyl ethanol. After the dropwise addition, heat up to 50° C. and react for 12 hours to obtain the first semi-addition product A3; Add IPDI 9.90g to the three-necked flask reactor of the condensation and stirring device, dilute with 20mL dimethyl sulfoxide, and add 0.3wt% catalyst DBTDL, then slowly add distillate containing HEA 9.72g in the solution with a dropping funnel. After the addition of methyl sulfoxide solution, the temperature was raised to 50°C, and the reaction was carried out for 12 hours to obtain the second semi-addition product B3; 22.84 g of BoltornH40 was added to a three-necked flask reactor equipped with a condensation and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com