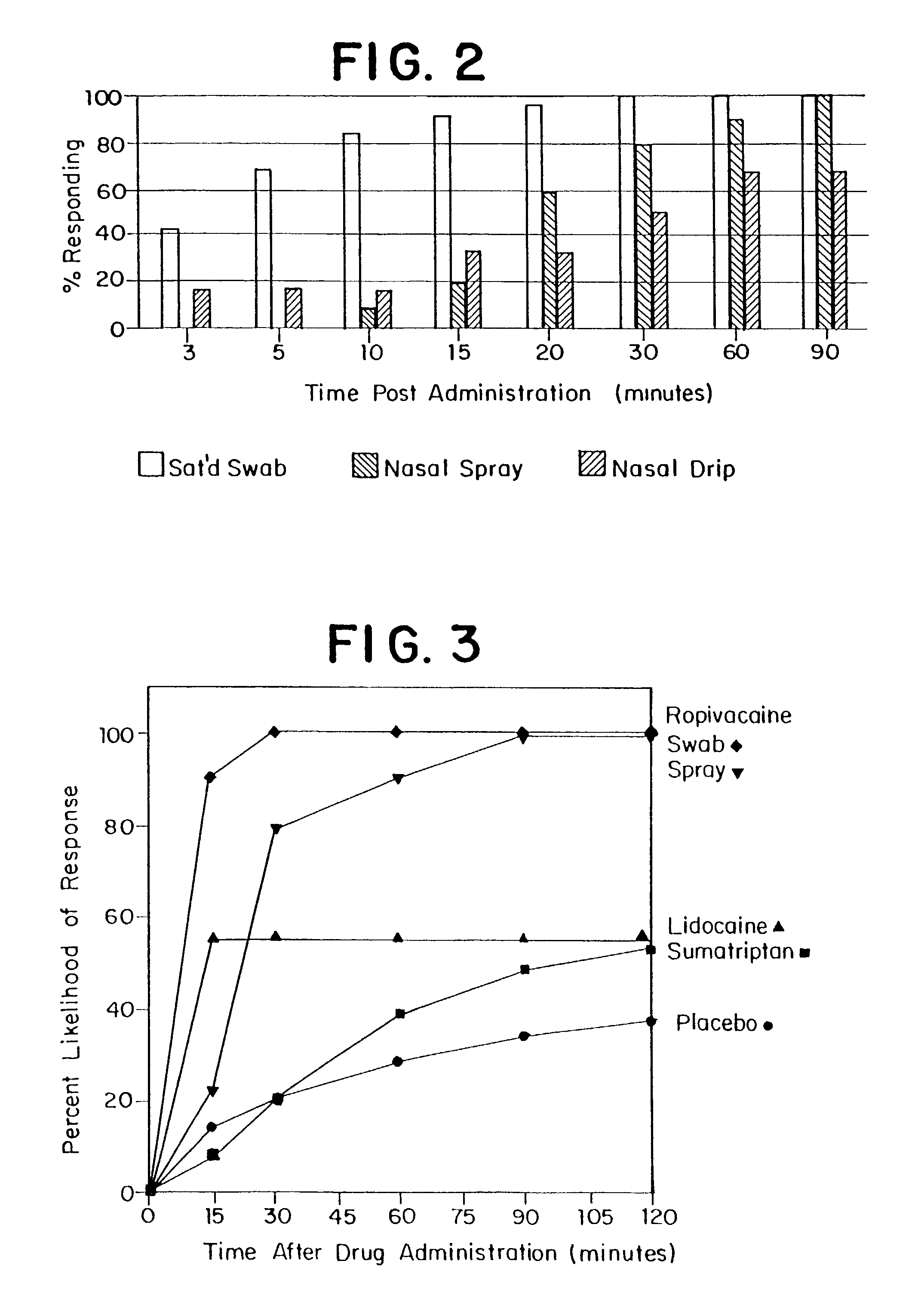
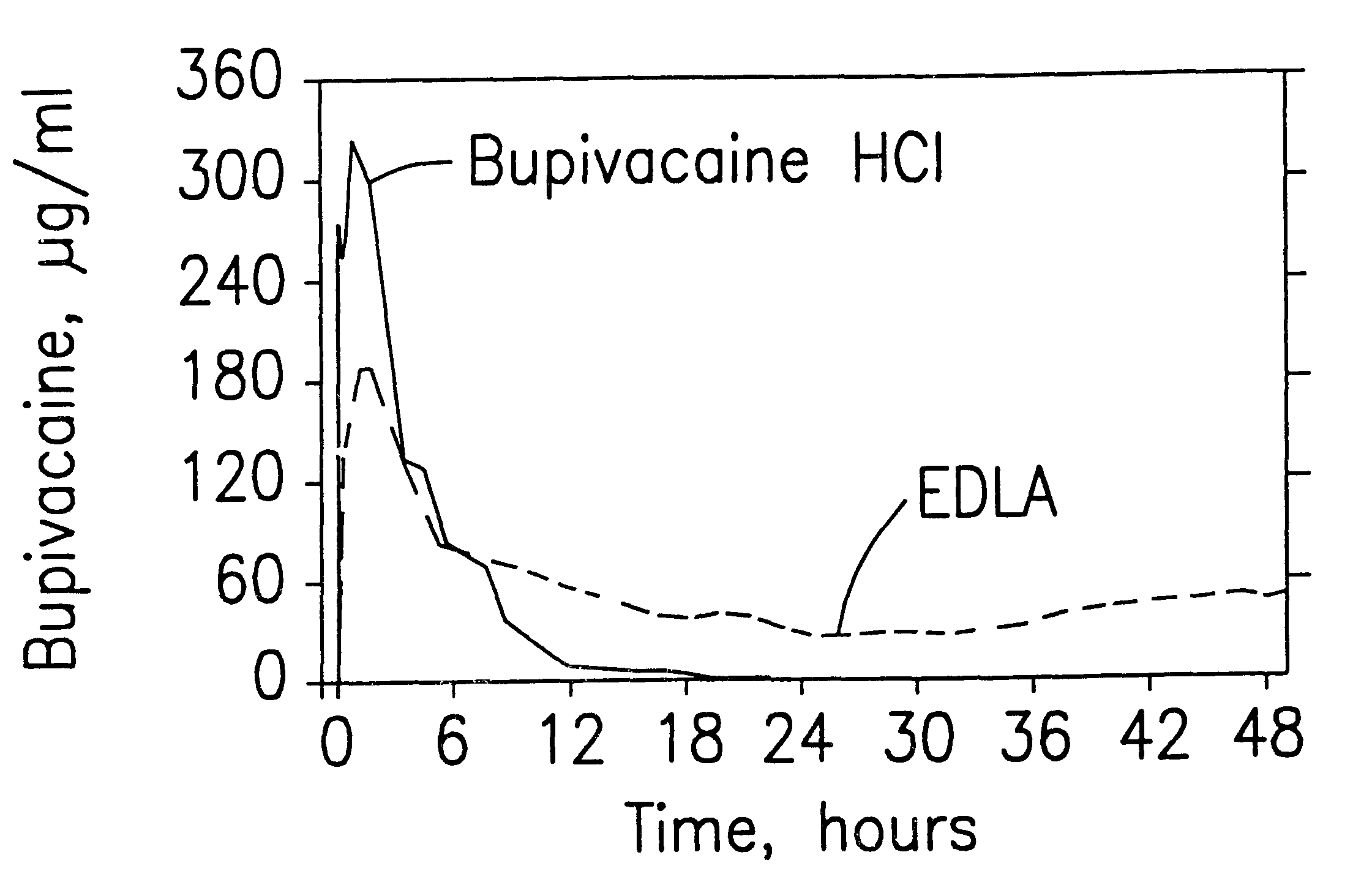
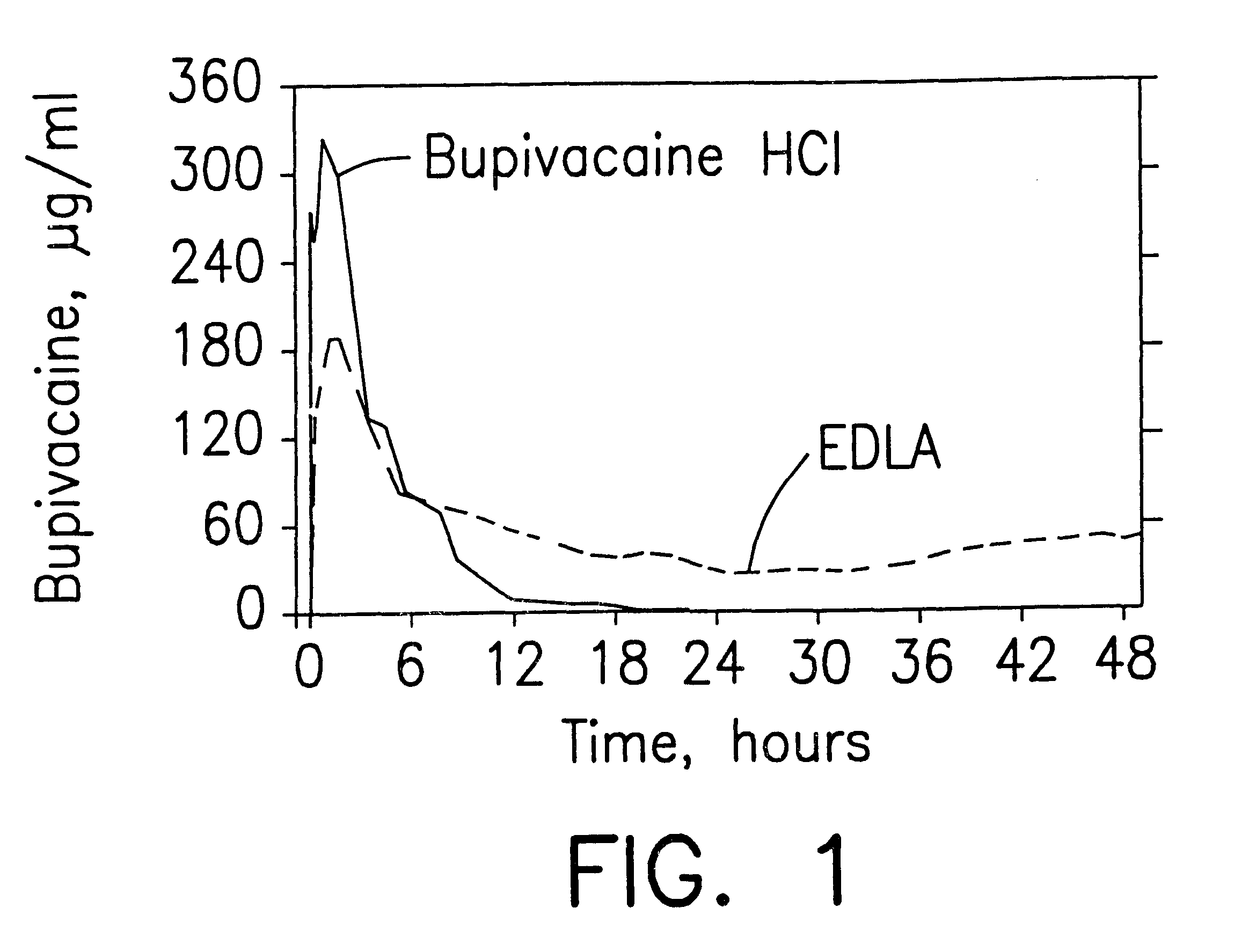
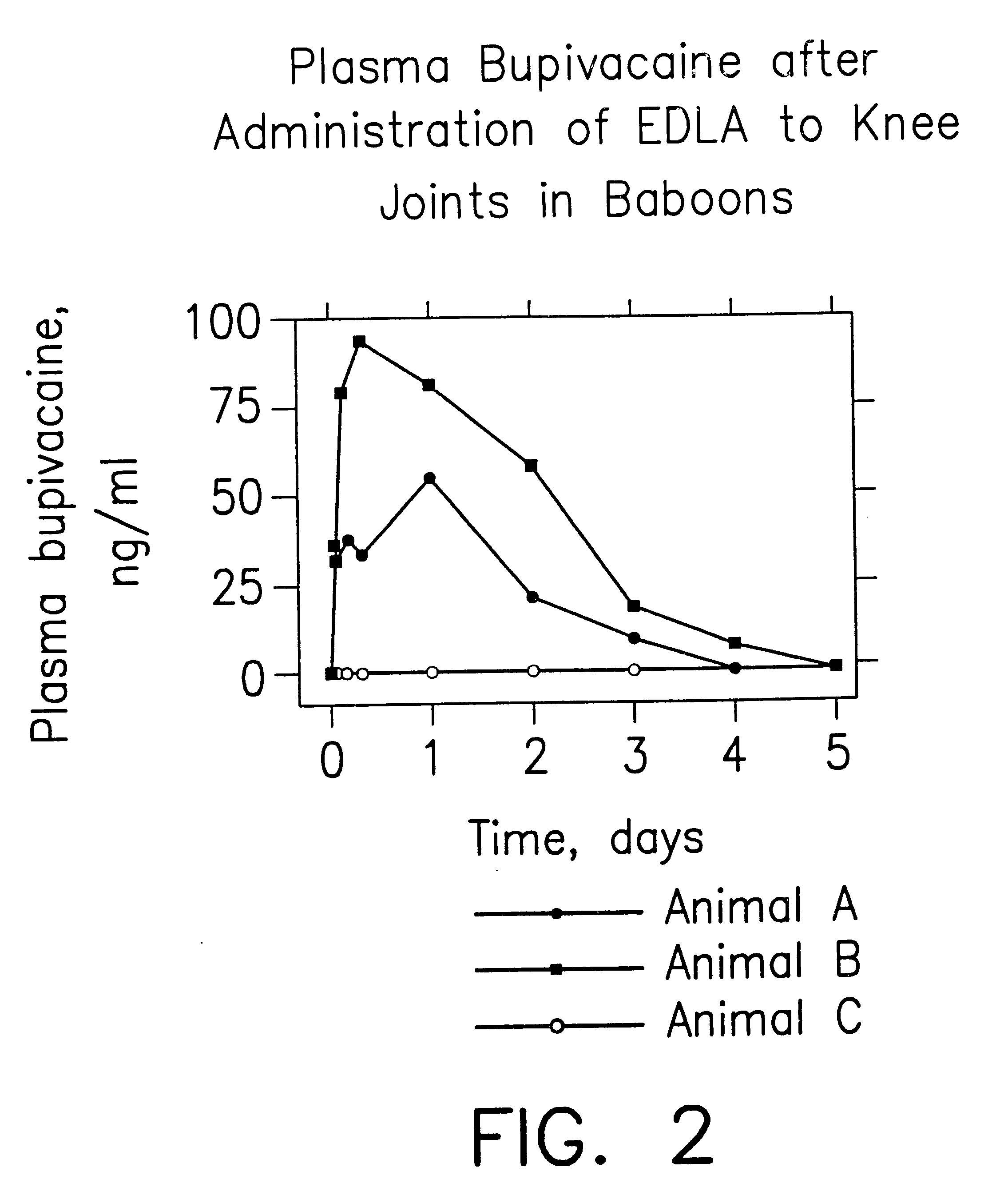
Patents
Literature
459 results about "Anaesthetic Agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anesthetic agent - a drug that causes temporary loss of bodily sensations. anaesthetic, anaesthetic agent, anesthetic. drug - a substance that is used as a medicine or narcotic. general anaesthetic, general anesthetic - an anesthetic that anesthetizes the entire body and causes loss of consciousness.
Transdermal drug delivery compositions and topical compositions for application on the skin
InactiveUS20090053290A1Improve biological activityEliminate side effectsPowder deliveryCosmetic preparationsRadio frequencyOtic Agents
Transdermal delivery compositions and topical compositions for application to the skin are provided. The transdermal delivery composition includes at least two penetrants working synergistically but by disparate biochemical pathways. In one embodiment, the transdermal delivery system includes benzyl alcohol and lecithin organogel. The transdermal delivery compositions are used in a variety of topical compositions as a means of transdermally delivering and topically administering different drugs and agents, including compositions promoting collagen biosynthesis, retinoids and skin lighteners, chemical denervation agents such as BOTOX®, anti-fungal agents, anesthetics and non-steroidal anti-inflammatory drugs (NSAIDs). In addition, these topical compositions may be used in combination with non-ablative treatment modalities, such as microdermabrasion, laser-based skin remodeling and radio-frequency-based skin remodeling.
Owner:NUVIANCE
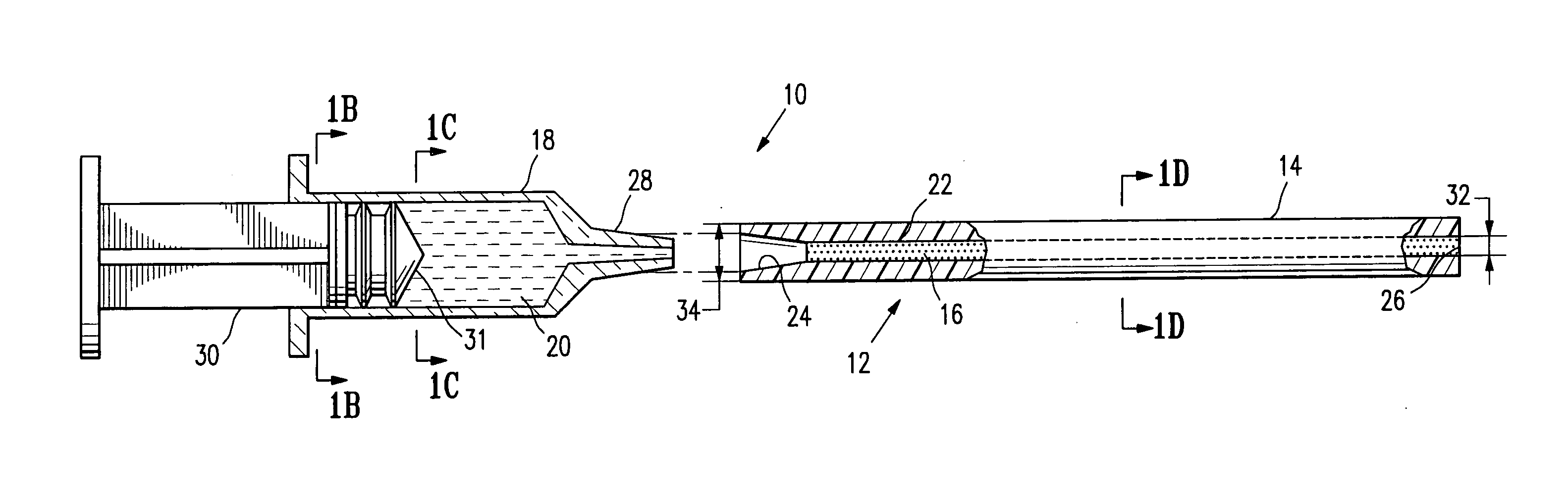
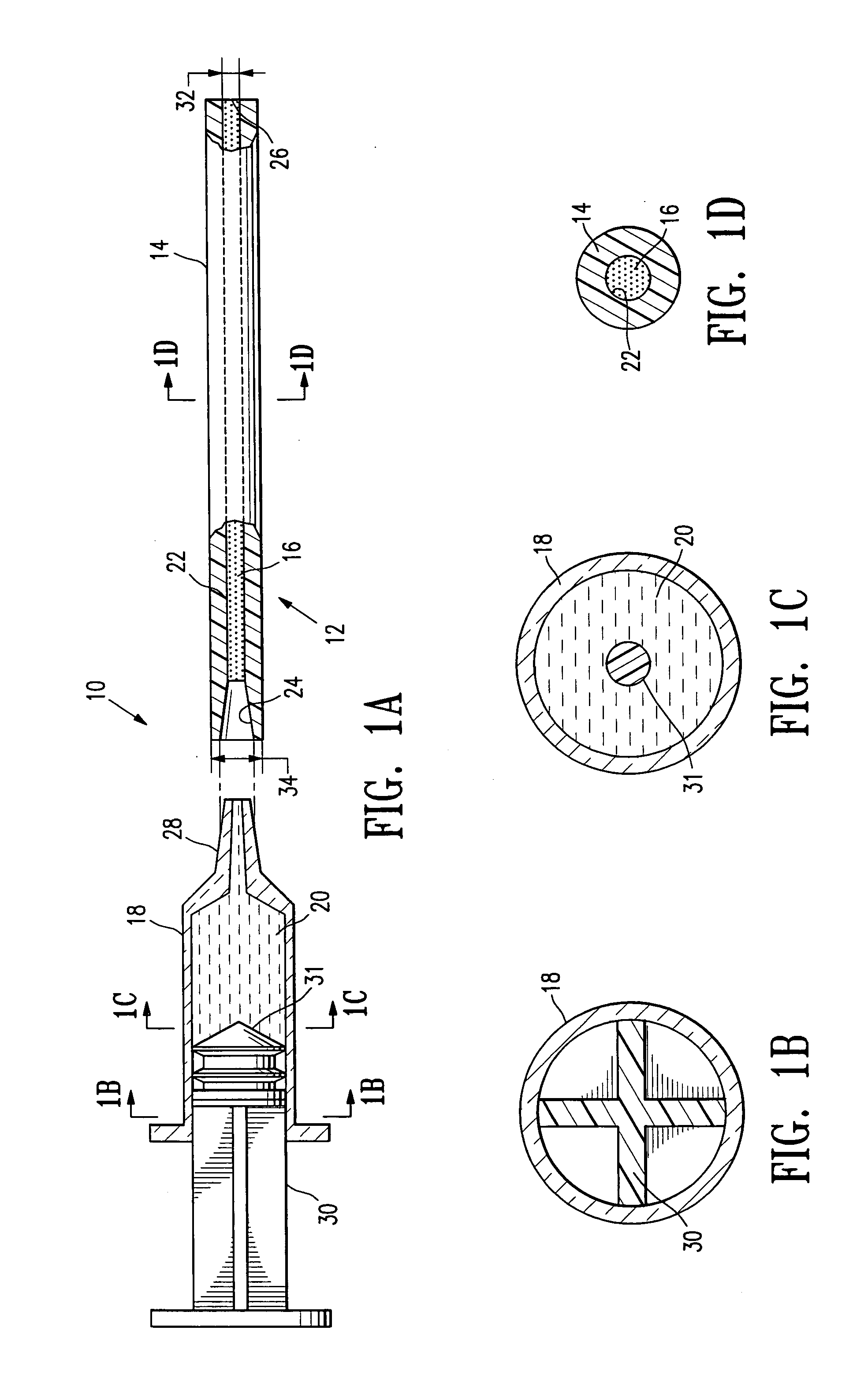
Method and device for painless injection of medication
InactiveUS7637891B2Simple and inexpensive to performSimple and inexpensive to and operateAmpoule syringesAutomatic syringesMedication injectionMedical treatment
A device for painlessly injecting medications, and a method for providing a substantially painless injection of medication into a patient that does not require the use of an anesthetic, that does not require the medical personnel to spend a substantial amount of time performing the injection procedure, that is relatively simple and inexpensive to perform and operate, and that provides a relatively high degree of safety for both the medical personnel and for the patient. The injection needle can have an outside diameter greater than 0.20 mm and less than about 0.38 mm. The medicament can be injected painlessly through the needle and into the patient at a substantially constant volumetric flow rate of about 0.05 μL / s to about 50 μL / s.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
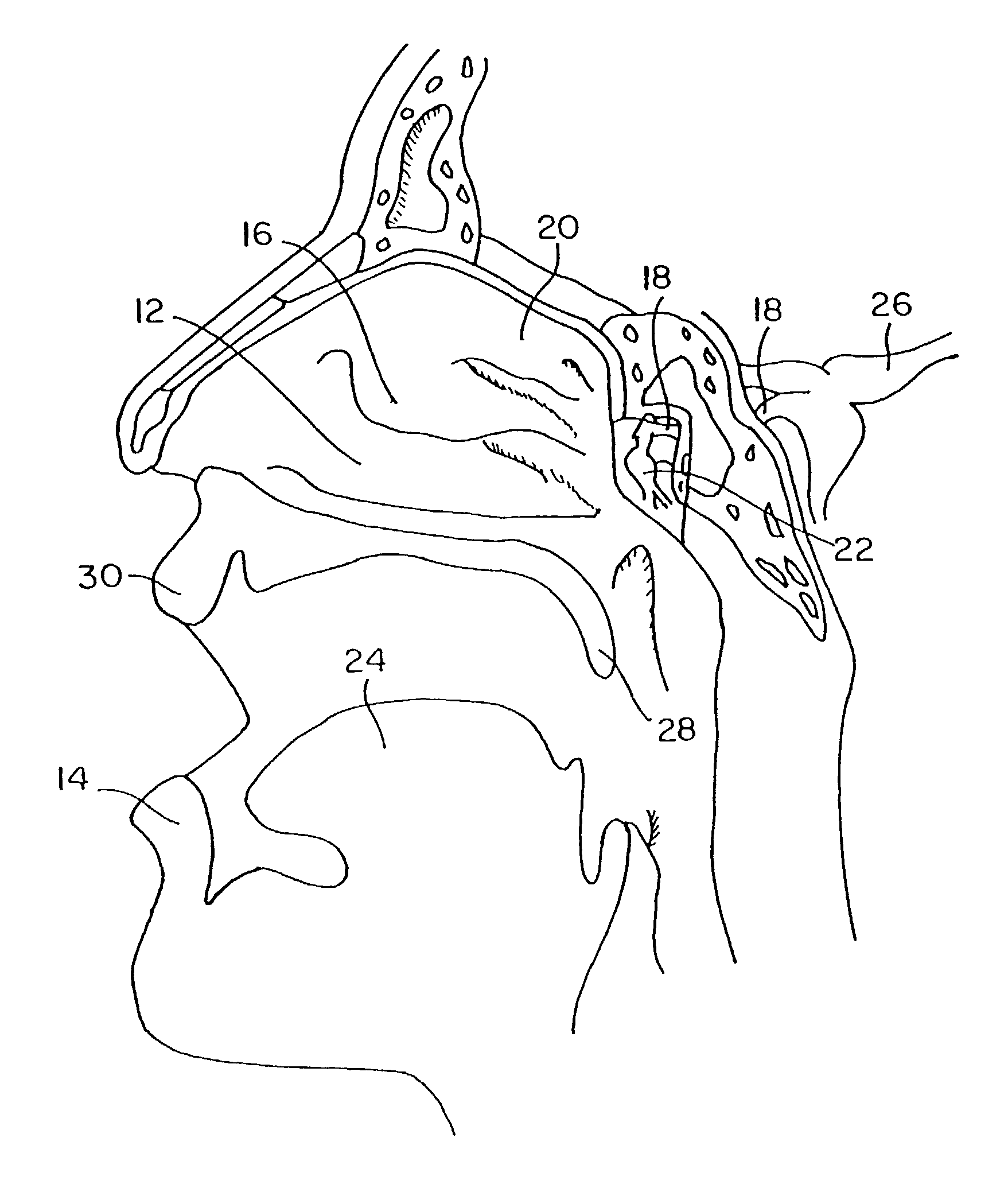
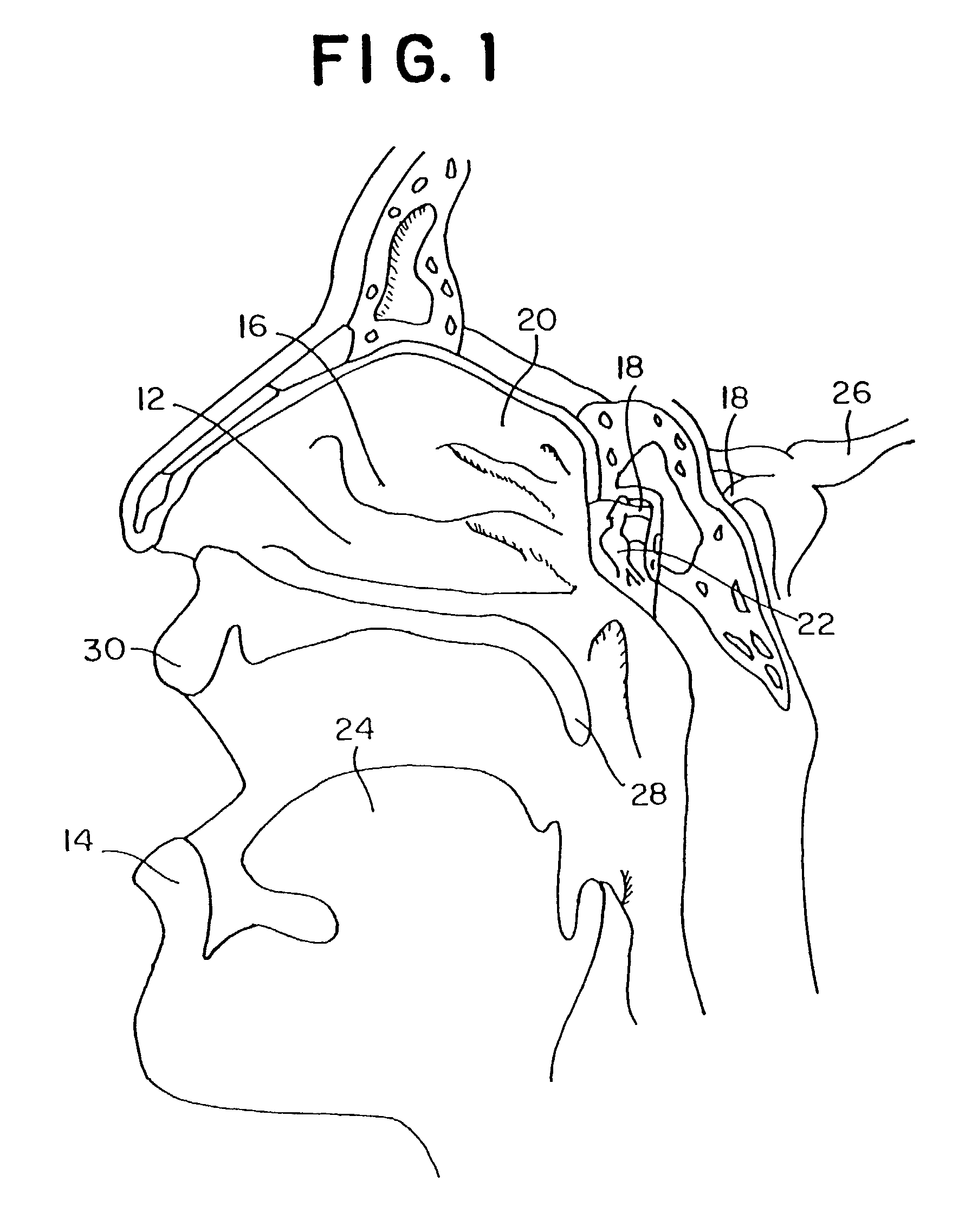
Method for directed intranasal administration of a composition
Methods, kits, apparatus, and compositions for inhibiting a cerebral neurovascular disorder, a muscular headache, or cerebral inflammation in a human patient are provided. The methods comprise intranasally administering to the patient a pharmaceutical composition comprising a local anesthetic, and preferably a long-acting local anesthetic ingredient. A composition useful for practicing the methods of the invention is described which comprises at least one local anesthetic in a pharmaceutically acceptable carrier, wherein the composition is formulated for intranasal delivery. Cerebral neurovascular disorders include migraine and cluster headache. Muscular headaches include tension headaches and muscle contraction headaches. A kit comprising the composition and an intranasal applicator and a method of systemically delivering a pharmaceutically active agent to an animal are also included in the invention. Apparatus for directed intranasal administration of the compositions of the invention and for performing the methods of the invention are also described.
Owner:BHL PATENT HLDG
Formulations and methods for providing prolonged local anesthesia
InactiveUS6451335B1Slow in-vitro releaseRelease the local anestheticAnaesthesiaGranular deliveryControlled releaseAnesthetic Agent
A formulation for inducing sustained regional local anesthesia in a patient comprising a substrate comprising a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material prolonging the release of the local anesthetic from the substrate to obtain a reversible local anesthesia when implanted or injected in a patient, and a non-toxic augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the augmenting agent. In preferred embodiments, the controlled release material is a low molecular weight, acid-terminated polymer. A further aspect of the invention is directed to such formulations which release the local anesthetic in two phases, the first a rapid "bolus" to initiate anesthesia and a second, slower release to maintain anesthesia.
Owner:EURO-CELTIQUE SA
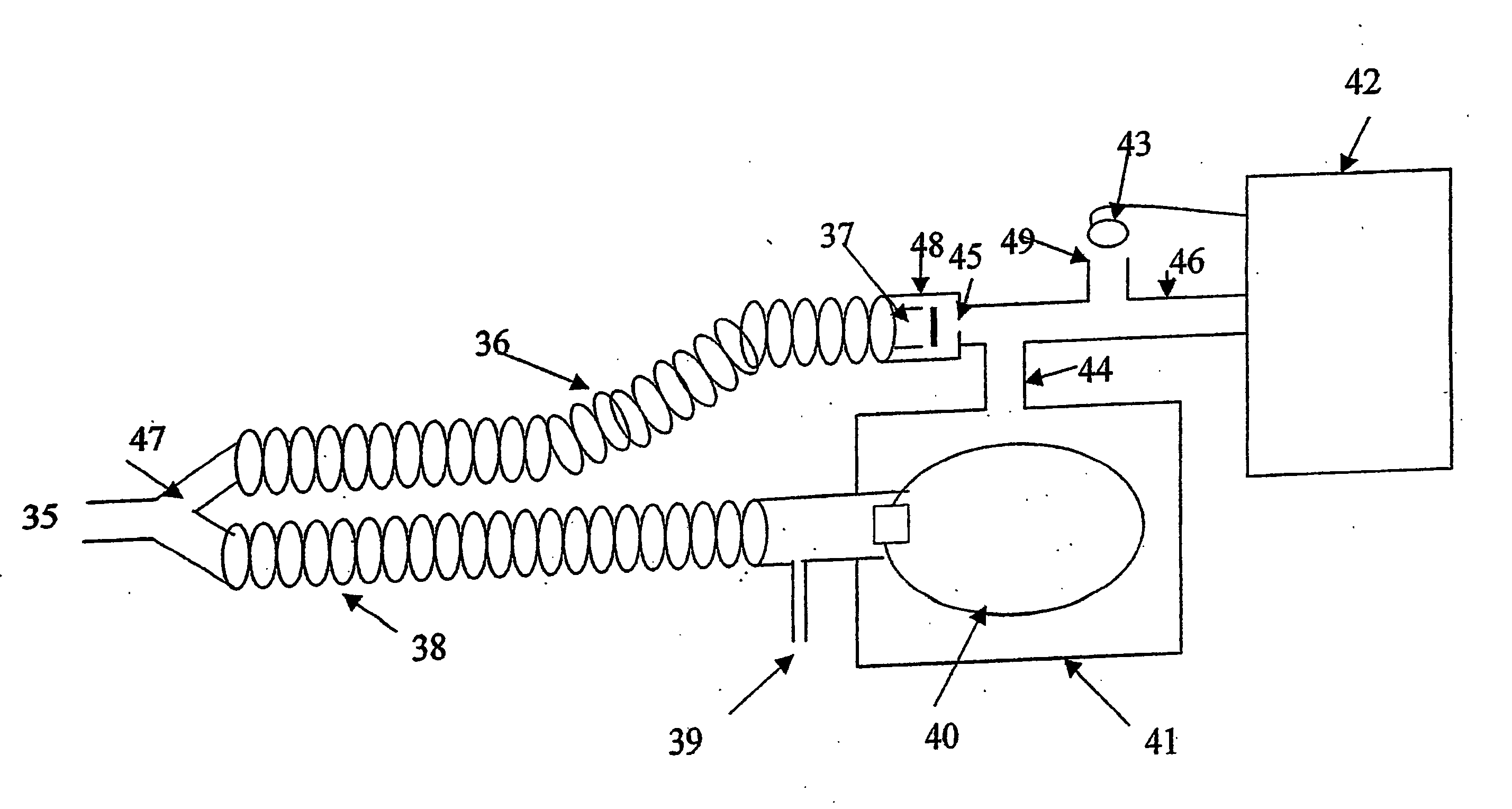
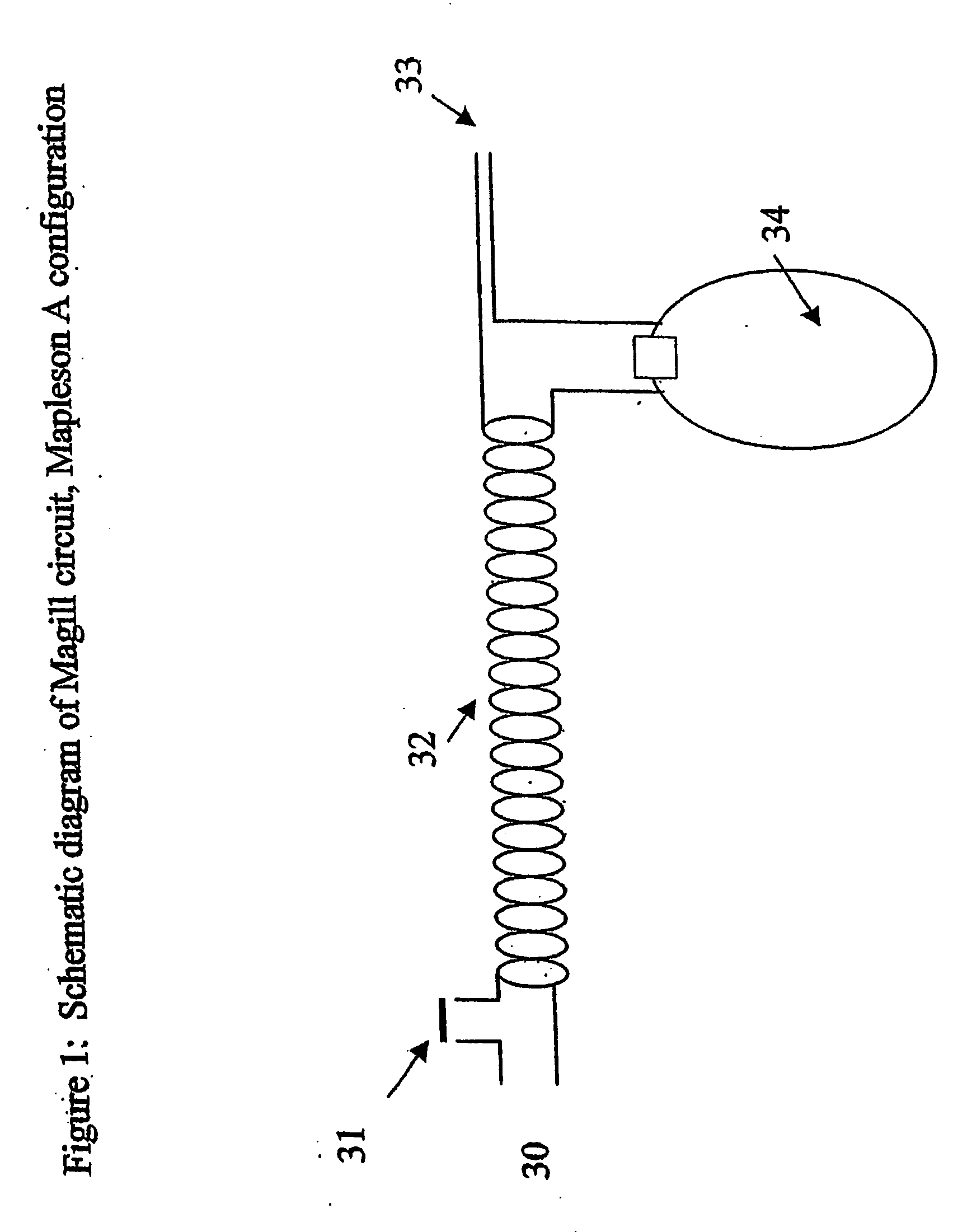
Method for continuous measurement of flux of gases in the lungs during breathing
ActiveUS20050217671A1RespiratorsOperating means/releasing devices for valvesAutonomous breathingEngineering
A method of calculating the flux of any gas (x) in a CBC circuit for a ventilated or a spontaneous breathing subject, for example said gas(x) being; a) an anesthetic such as but limited to; i)N2O; ii) sevoflurane; iii) isoflurane; iv) halothane; v) desflurame; or the like b) Oxygen; c) Carbon dioxide; or the like utilizing the following relationships; Flux of gas(x)=SGF (FSX−FEX) wherein SGF=Source of gas flow into the breathing circuit (CBC circuit) in liters / minute as read from the gas flow meter as set by the anesthesiologist; FSX=Fractional concentration of gas X in the source gas (which is set by the anesthesiologist); FEX=Fractional concentration of gas X in the end expired gas as determined by a portable gas analyzer, or the like.
Owner:THORNHILL SCI INC
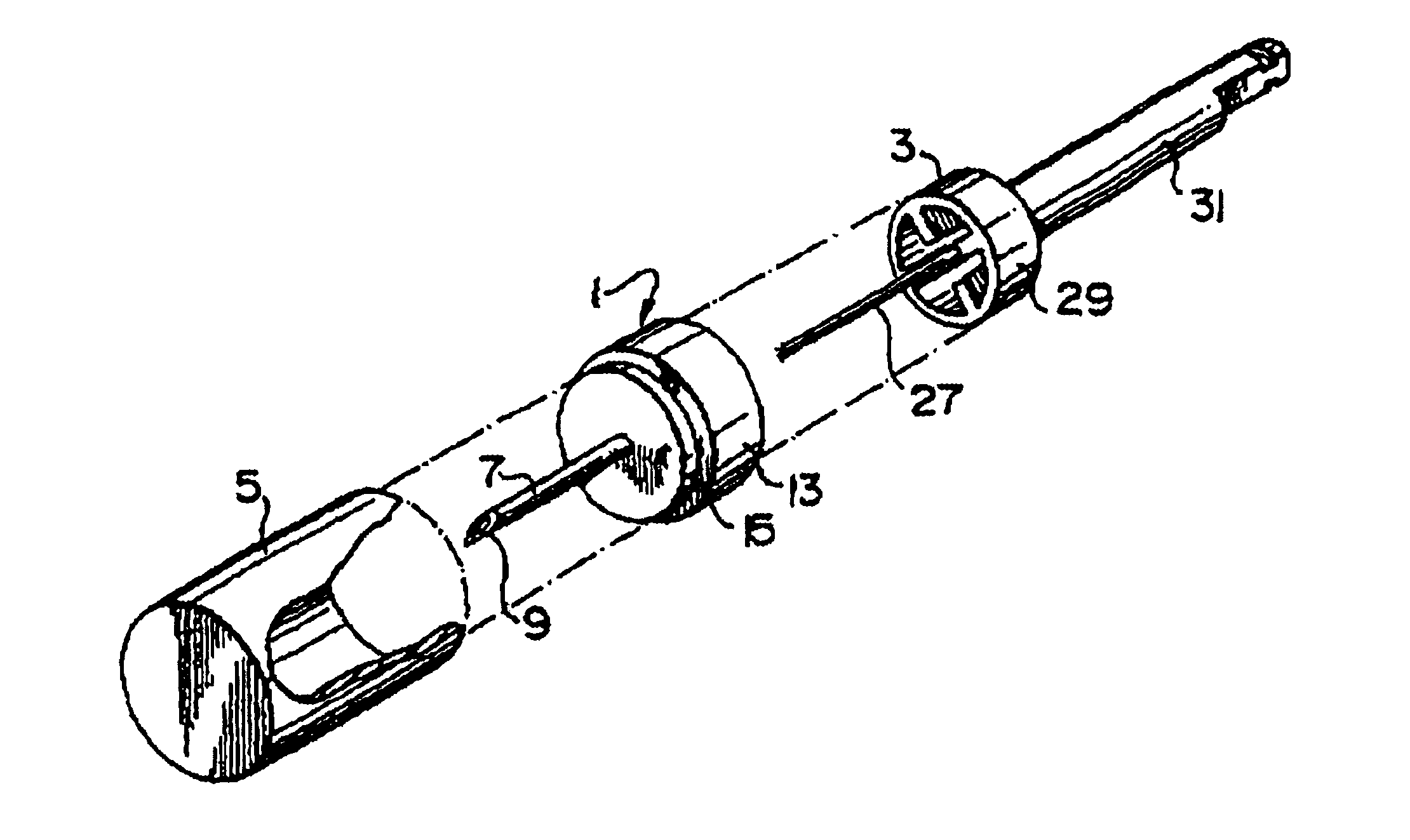
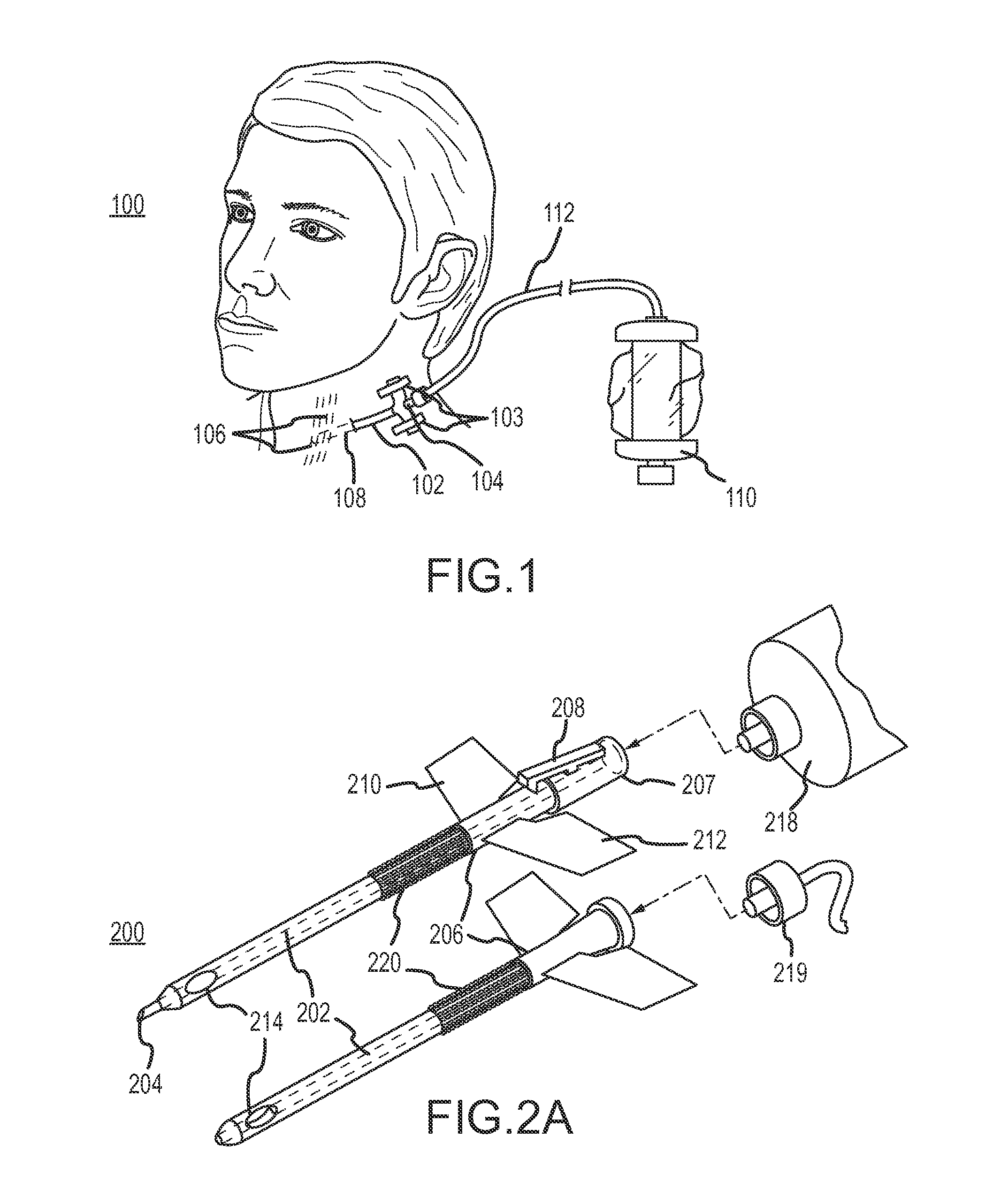
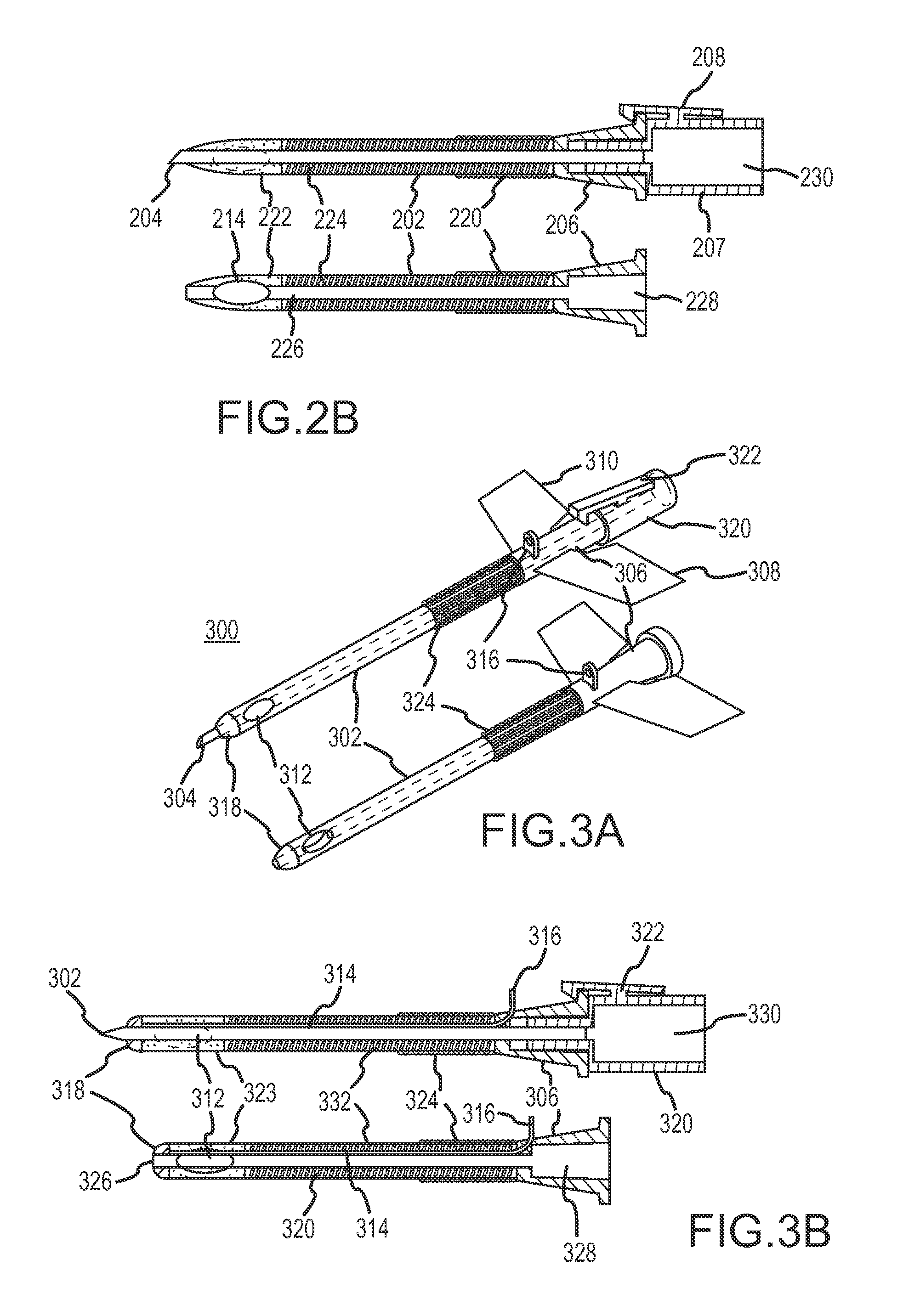
Device for targeted, catheterized delivery of medications
InactiveUS6905486B2Reduce disadvantagesReduce problemsSurgical needlesMedical devicesHypodermoclysisHypodermic needle
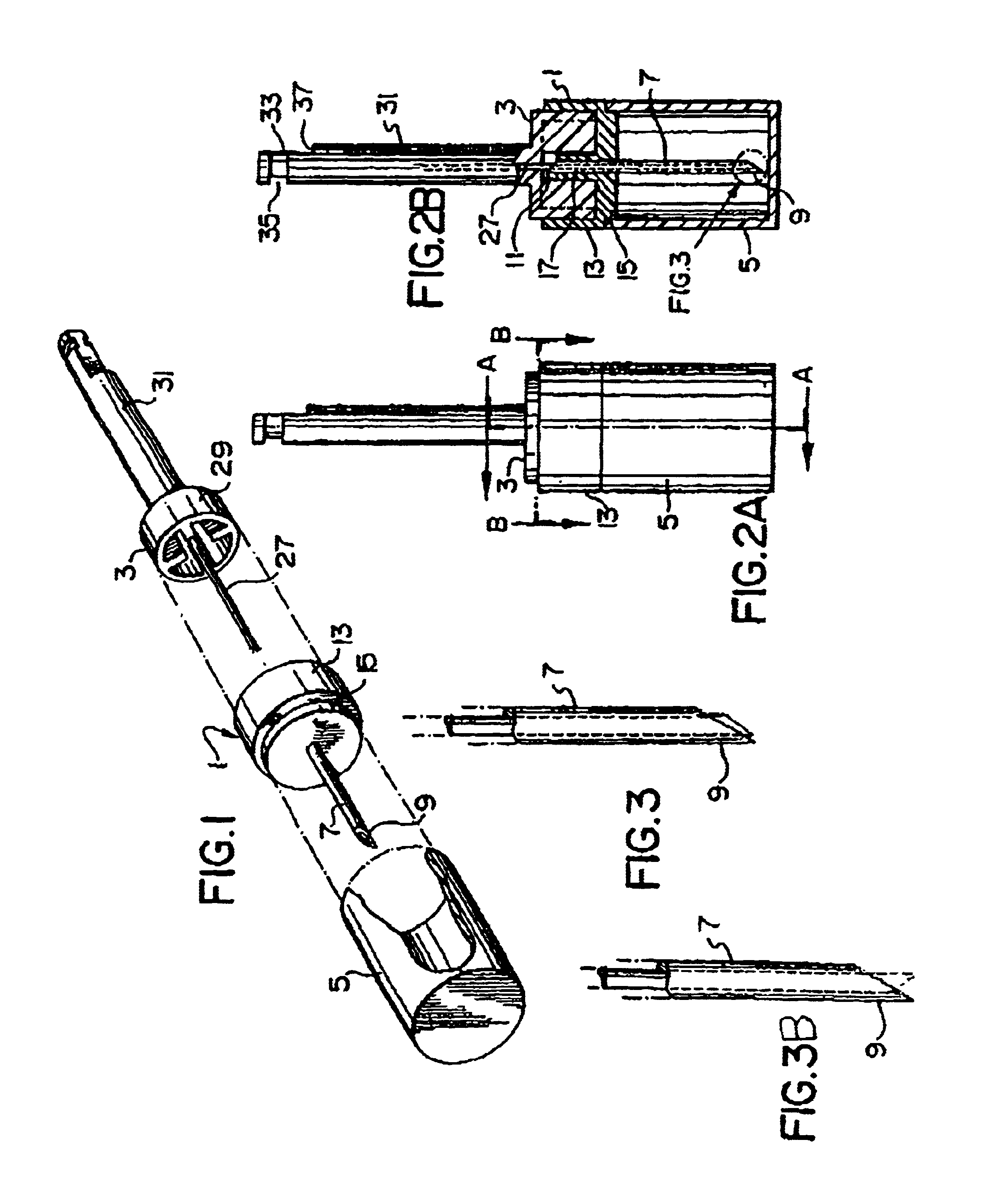
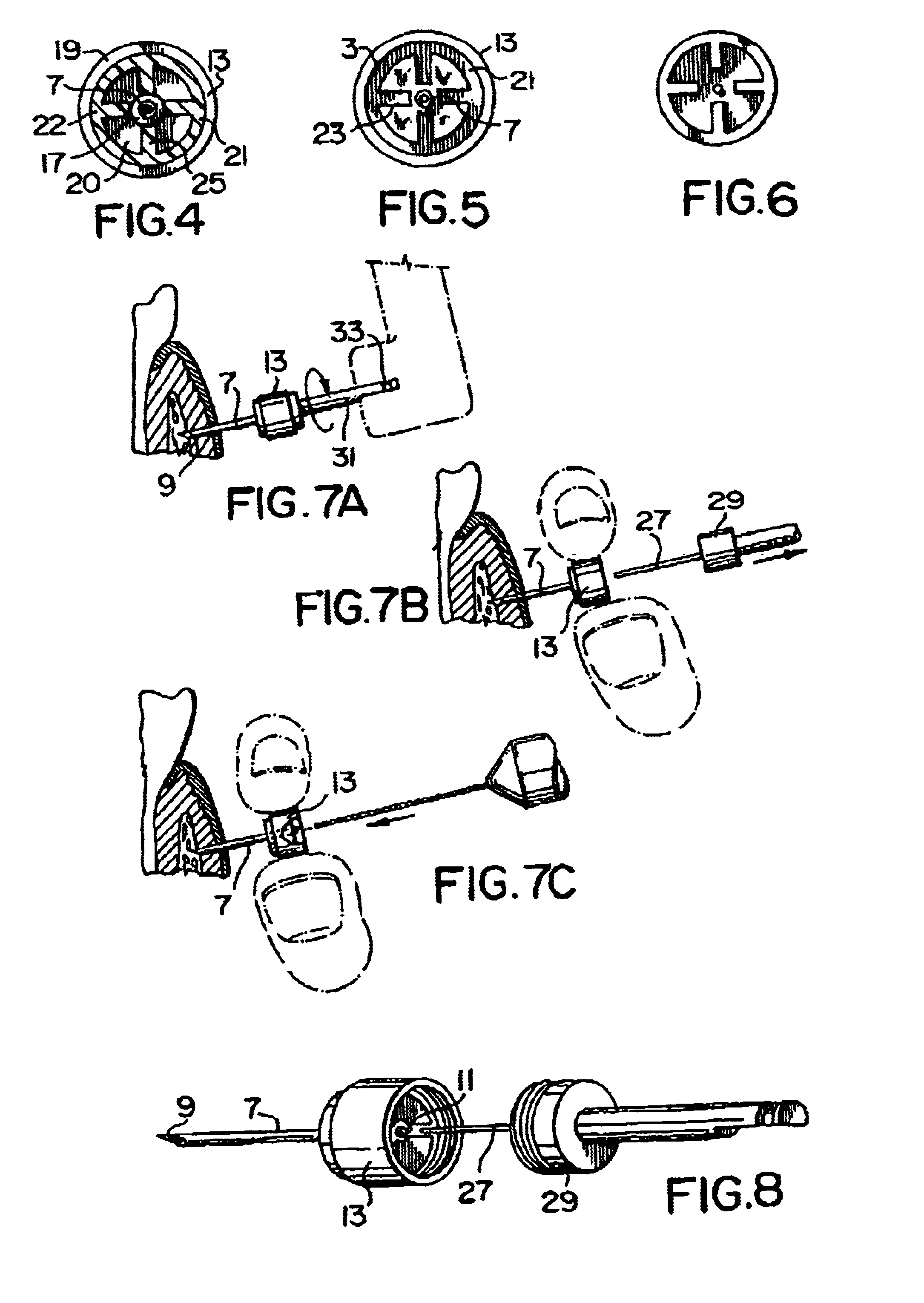
Apparatus and method for catheterized delivery or infusion of medication and anaesthesia are disclosed. The perforating catheter is first used to perforate the periodontal ligament and / or the cortical plate of bone tissue, and is then left in place and used as a catheter for insertion of a hypodermic needle of smaller gauge to deliver medication or anaesthesia to a target area. The perforator is a bevelled needle for drilling into the ligament or bone tissue. For drilling, the device comprises an adaptor which transmits the rotational movement from a dental hand piece or the like to the bevelled needle. A cap is also included for protecting the bevelled needle during storage of the device. The adaptor may have a rod which extends axially into the bevelled needle when the device is assembled for drilling. The rod is used to prevent the debris resulting from drilling from blocking the passage in the bevelled needle. As well, the rod reinforces the needle and maintains the alignment between the perforator and the adaptor for improved drilling efficiency. An adapter is disclosed which couples with the catheter once in place easing access to supply medication to difficult to reach areas.
Owner:INTROSAN DENTAL PROD
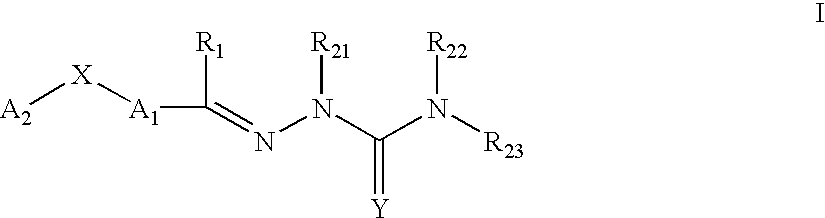
Carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones and the use thereof
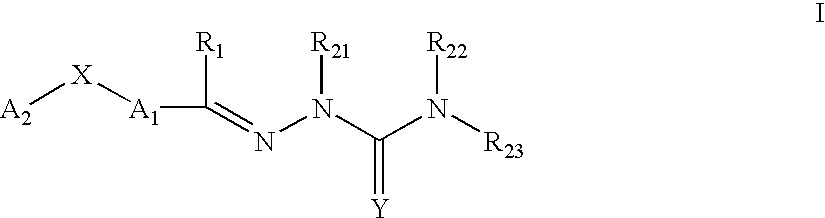
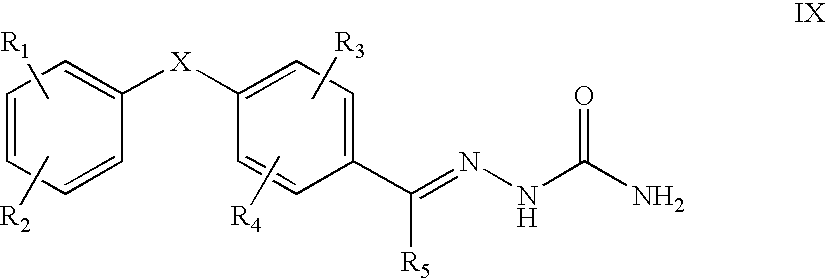
This invention is related to carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones represented by Formula I: ##STR1## or a pharmaceutically acceptable salt or prodrug thereof, wherein: Y is oxygen or sulfur; R.sub.1, R.sub.21, R.sub.22 and R.sub.23 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl; or R.sub.22 and R.sub.23, together with the N, form a heterocycle; A.sub.1 and A.sub.2 are independently aryl, heteroaryl, saturated or partially unsaturated carbocycle or saturated or partially unsaturated heterocycle, any of which is optionally substituted; X is one or O, S, NR.sub.24, CR.sub.25 R.sub.26, C(O), NR.sub.24 C(O), C(O)NR.sub.24, SO, SO.sub.2 or a covalent bond; where R.sub.24, R.sub.25 and R.sub.26 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl. The invention also is directed to the use of carbocycle and heterocycle substituted semicarbazones and thiosemicarbazones for the treatment of neuronal damage following global and focal ischemia, for the treatment or prevention of neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS), for the treatment and prevention of otoneurotoxicity and eye diseases involving glutamate toxicity and for the treatment, prevention or amelioration of pain, as anticonvulsants, and as antimanic depressants, as local anesthetics, as antiarrhythmics and for the treatment or prevention of diabetic neuropathy and urinary incontinence.
Owner:COCENSYS
Formulations and methods for providing prolonged local anesthesia
InactiveUS6521259B1Good effectPain controlPowder deliveryNervous disorderControlled releaseGlucocorticoid
A formulation and methods for inducing sustained regional local anesthesia in a patient comprising a substrate comprising a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material prolonging the release of the local anesthetic from the substrate to obtain a reversible local anesthesia when inplanted or injected in a patient, and a pharmaceutically acceptable, i.e., non-toxic, non-glucocorticoid augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the augmenting agent.
Owner:PURDUE PHARMA LP
Formulations and methods for providing prolonged local anesthesia
A formulation and methods for inducing sustained regional local anesthesia in a patient comprising a substrate comprising a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material prolonging the release of the local anesthetic from the substrate to obtain a reversible local anesthesia when implanted or injected in a patient, and a pharmaceutically acceptable, i.e., non-toxic, non-glucocorticoid augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the augmenting agent.
Owner:PURDUE PHARMA LP
Cavity-filling biopsy site markers
InactiveUS20050143656A1Easy to detectLuminescence/biological staining preparationSurgical needlesAnesthetic AgentMaximum dimension
The invention provides materials, devices and methods for marking biopsy sites for a limited time. The biopsy-marking materials are ultrasound-detectable bio-resorbable powders, with powder particles typically between about 20 microns and about 800 microns in maximum dimension, more preferably between about 300 microns and about 500 microns. The powders may be formed of polymeric materials containing cavities sized between about 10 microns and about 500 microns, and may also contain binding agents, anesthetic agents, hemostatic agents, and radiopaque markers. Devices for delivering the powders include tubes configured to contain the powders and to fit within a biopsy cannula, the powders being ejected by action of a syringe. Systems may include a tube containing powder, and a syringe containing sterile saline. The tube may be configured to fit within a biopsy cannula such as a Mammotome® or SenoCor 360™ cannula.
Owner:SENORX
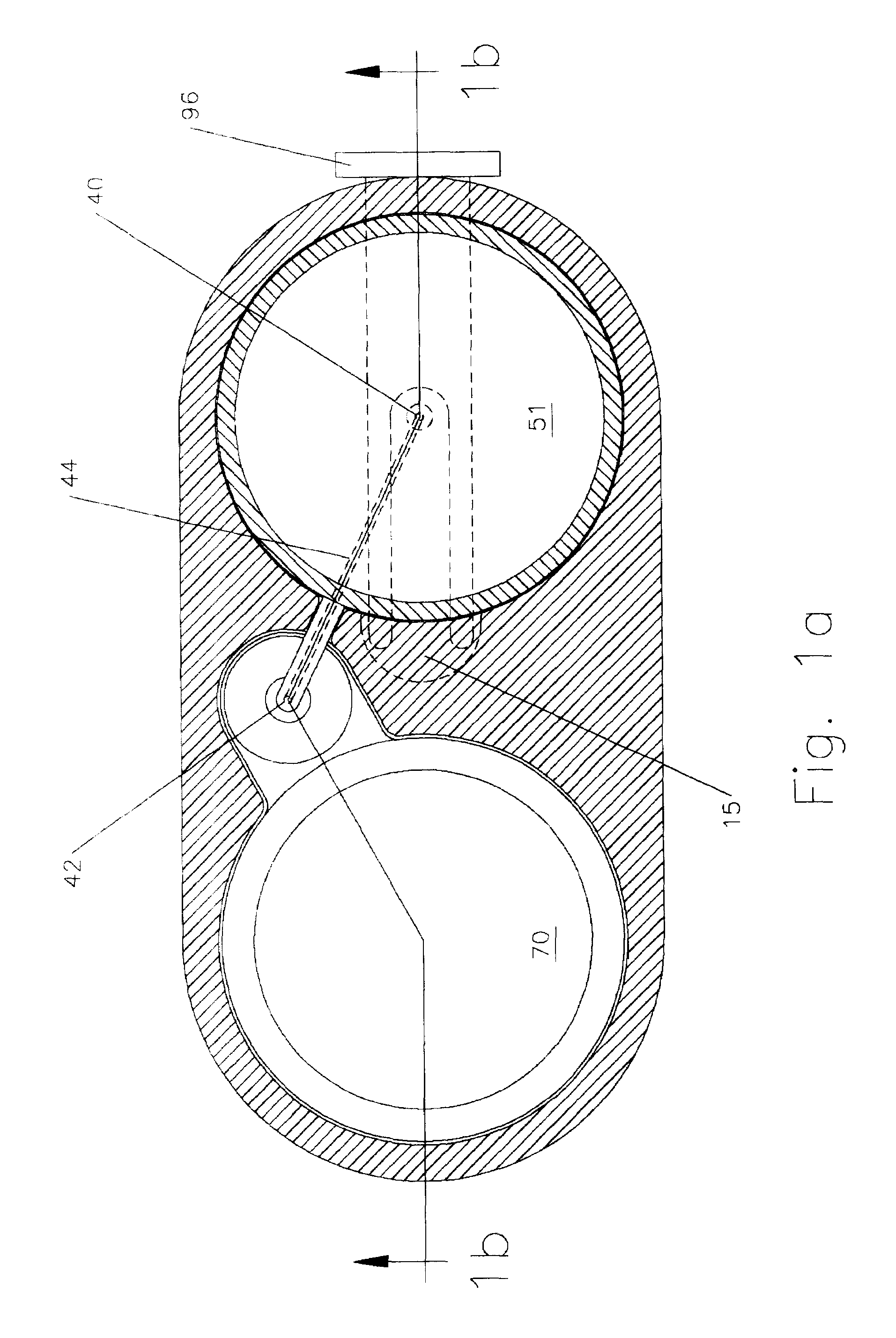
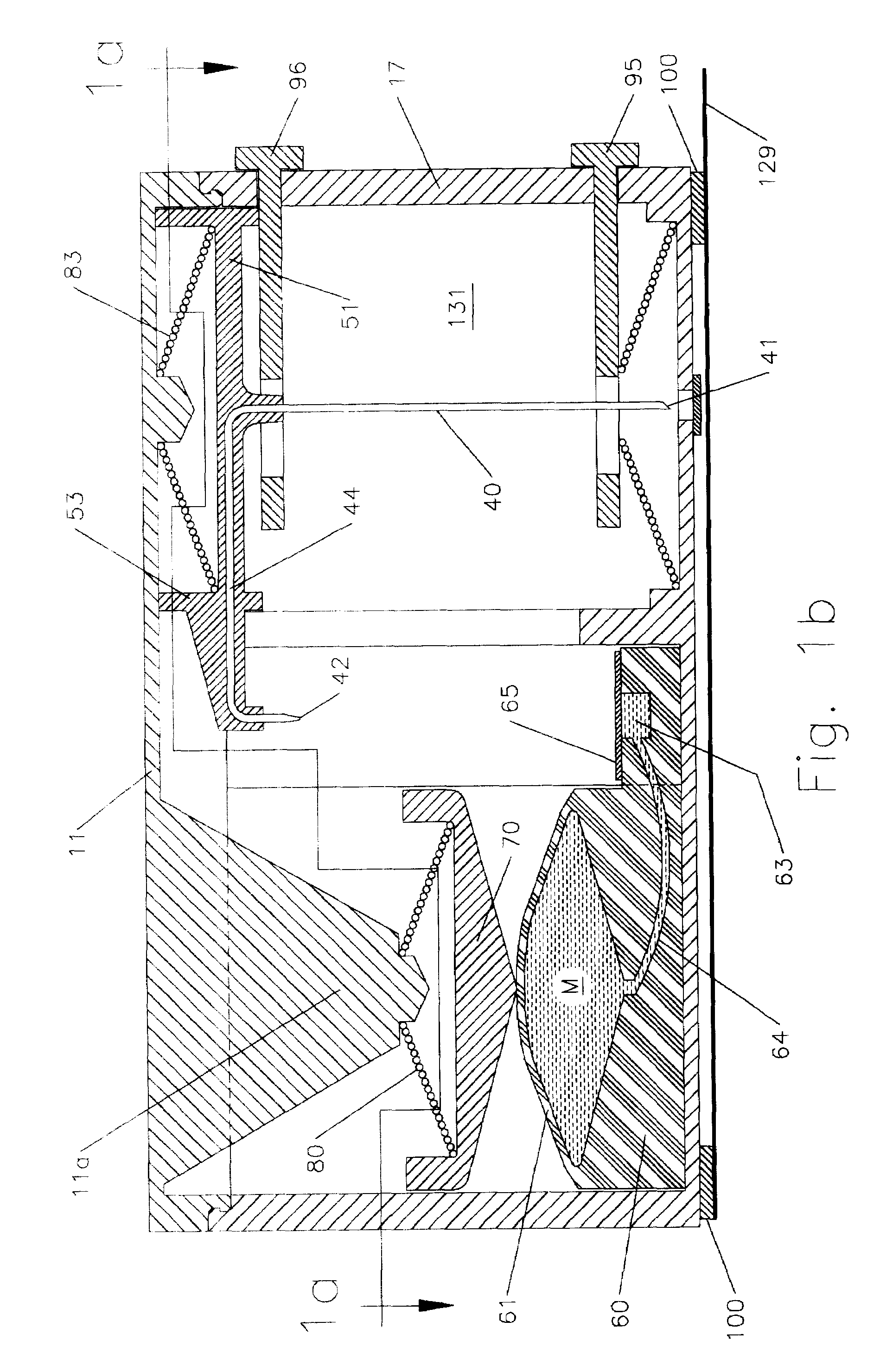
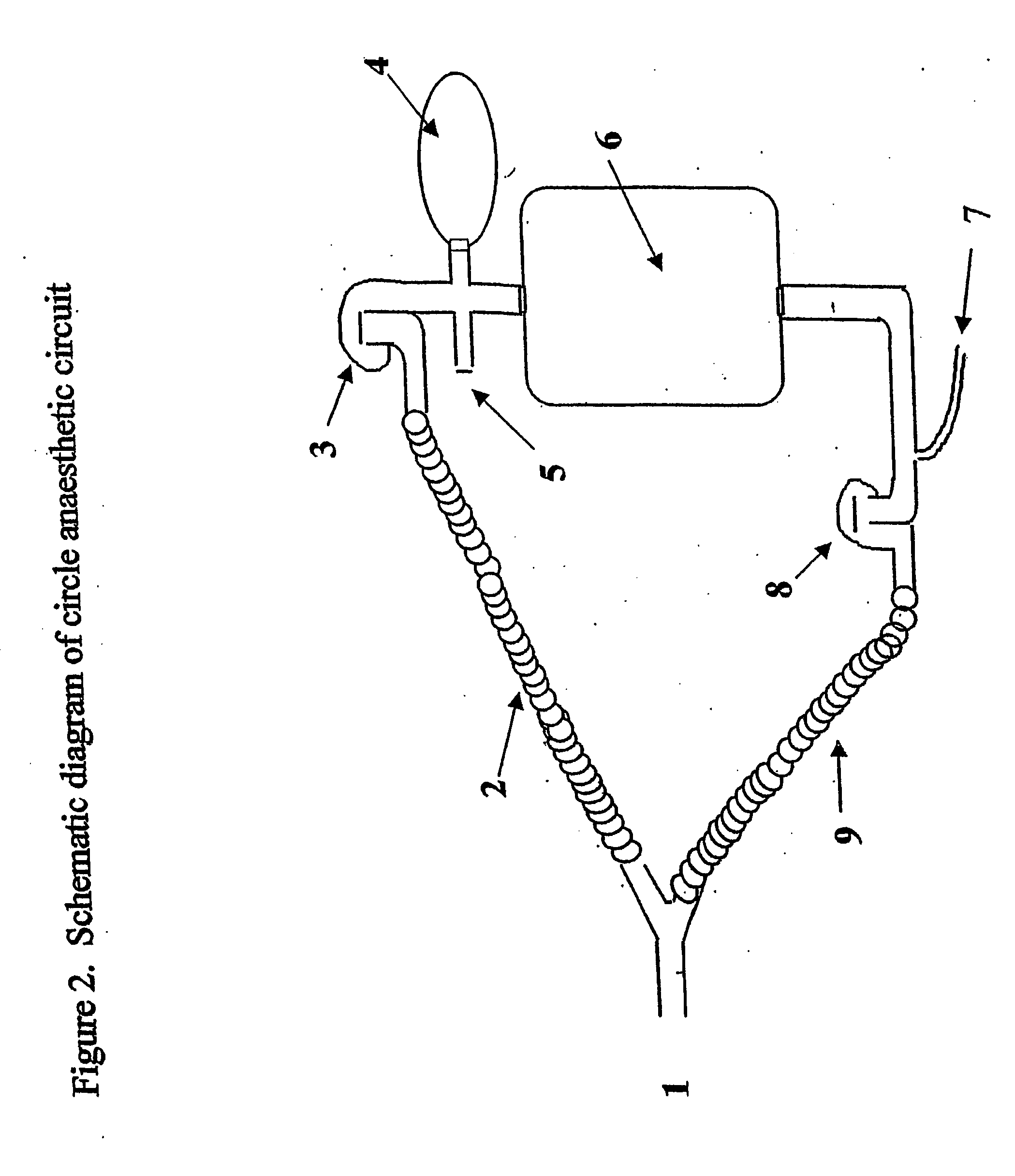
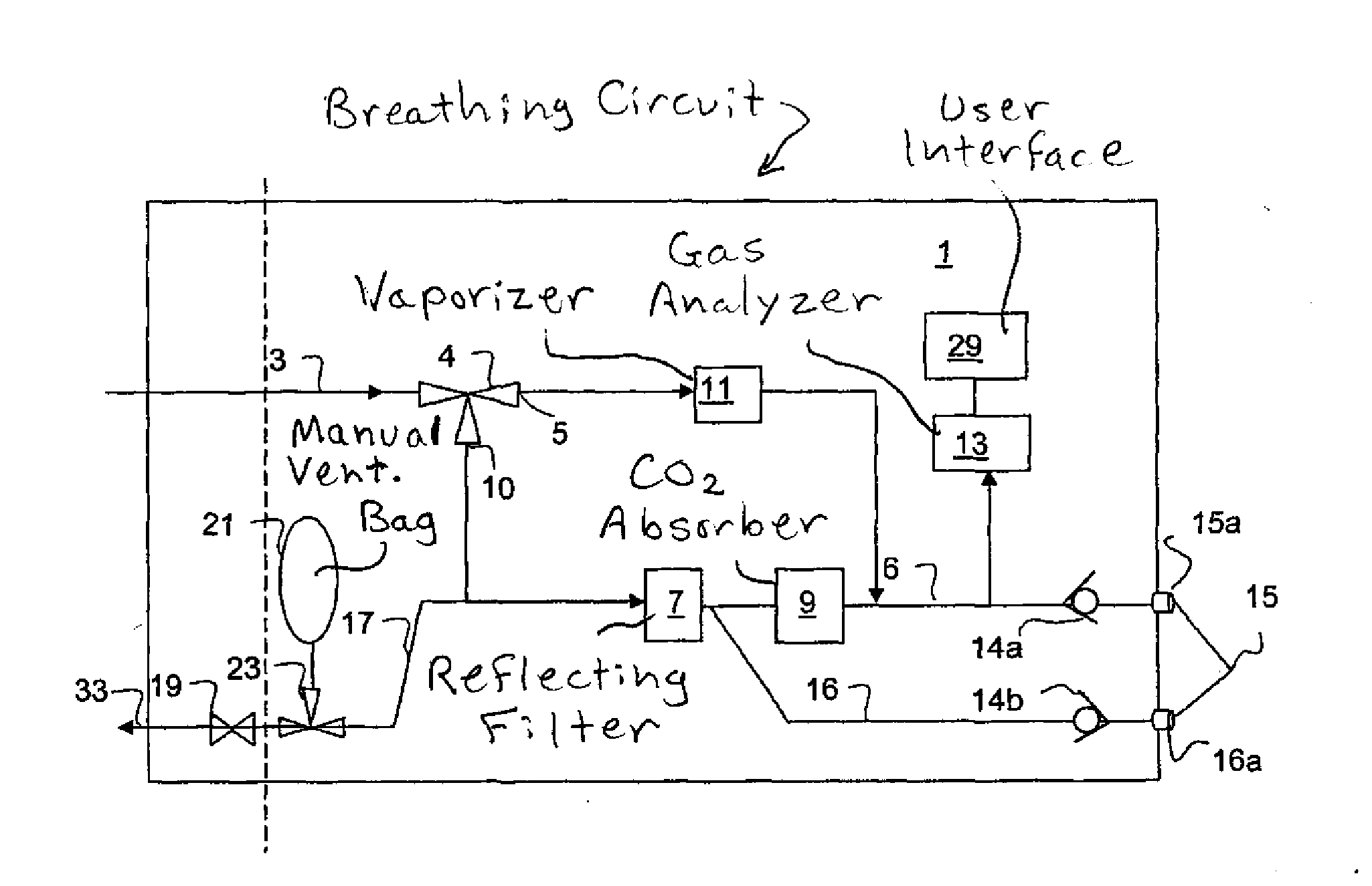
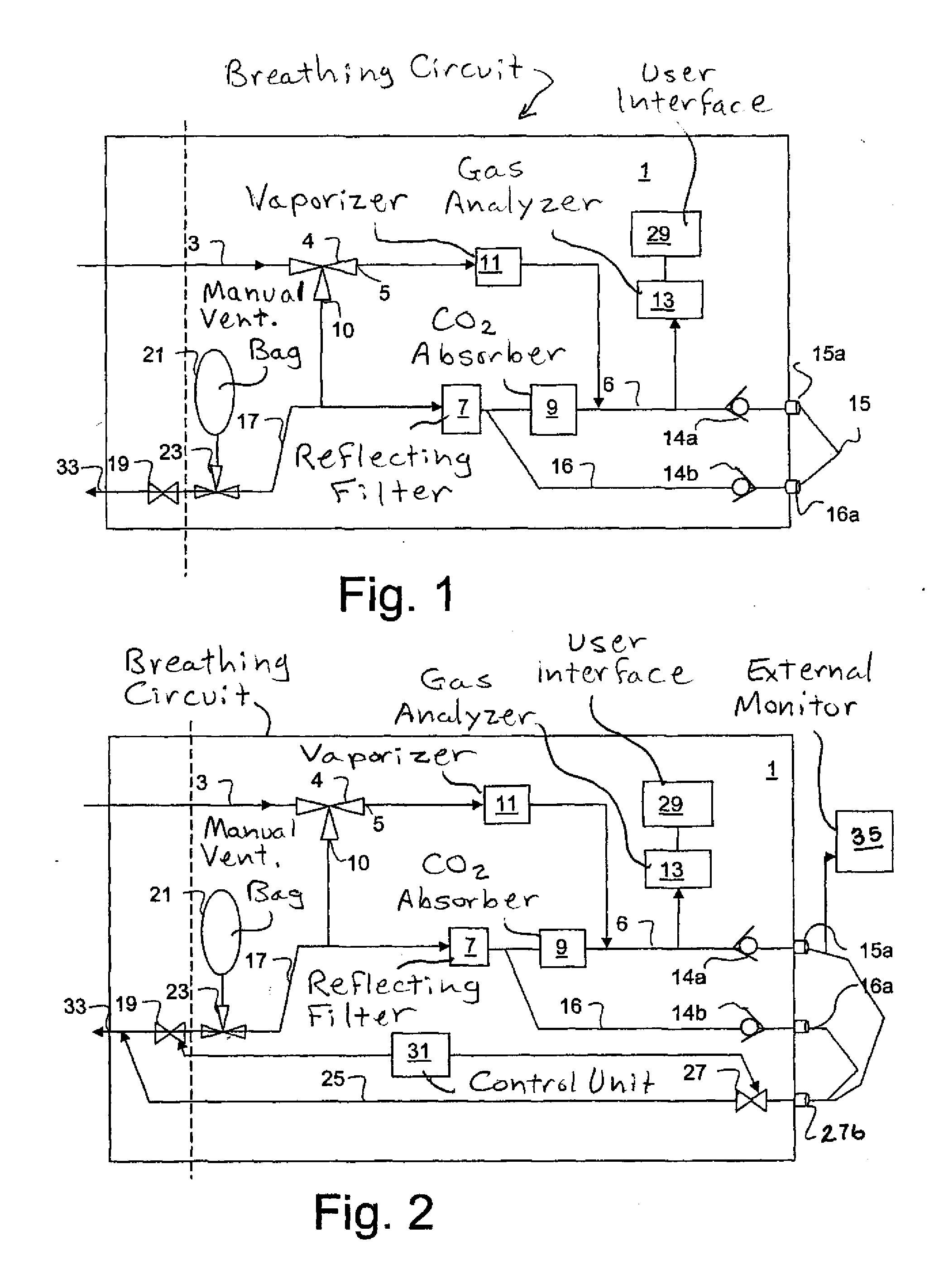
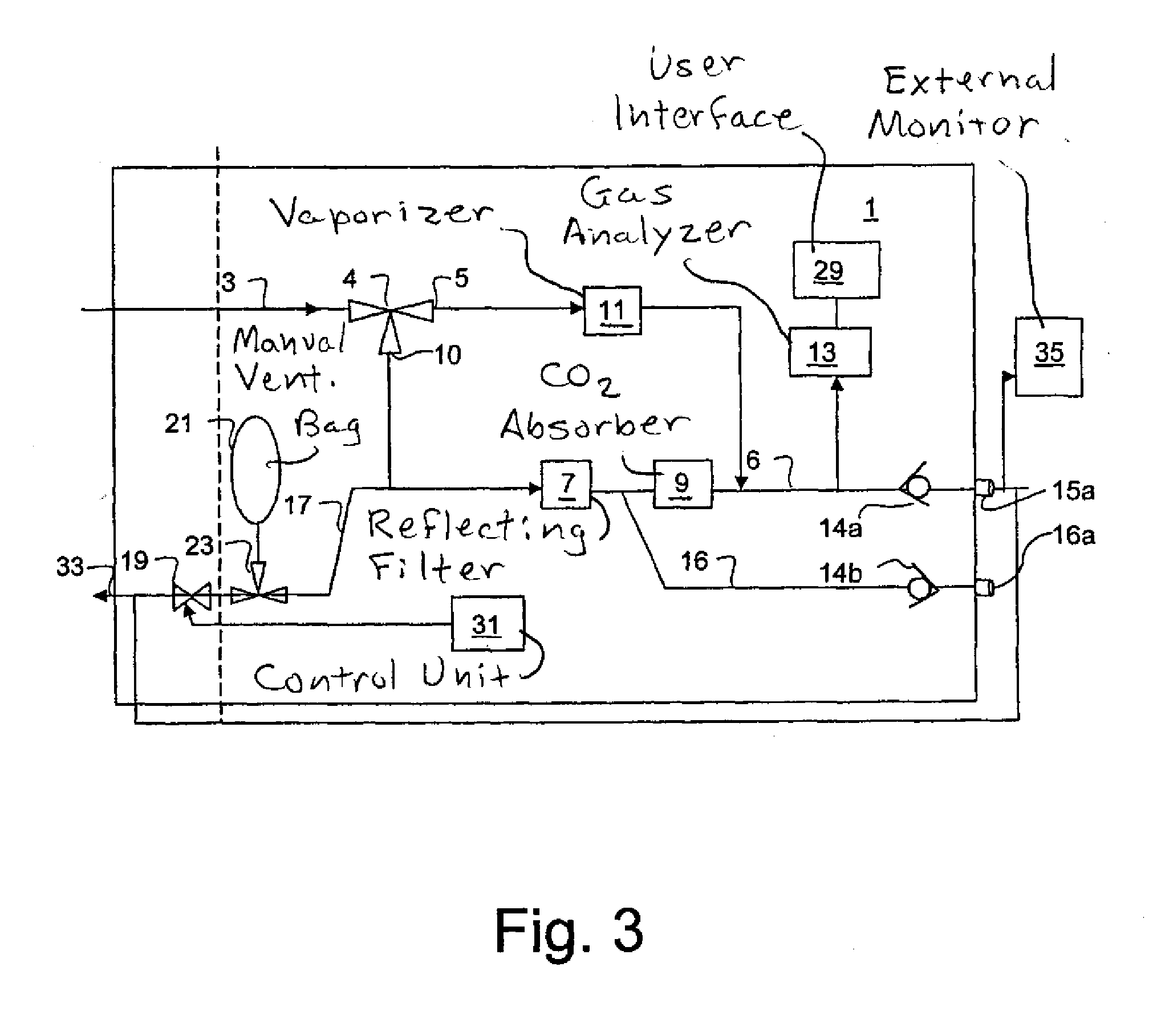
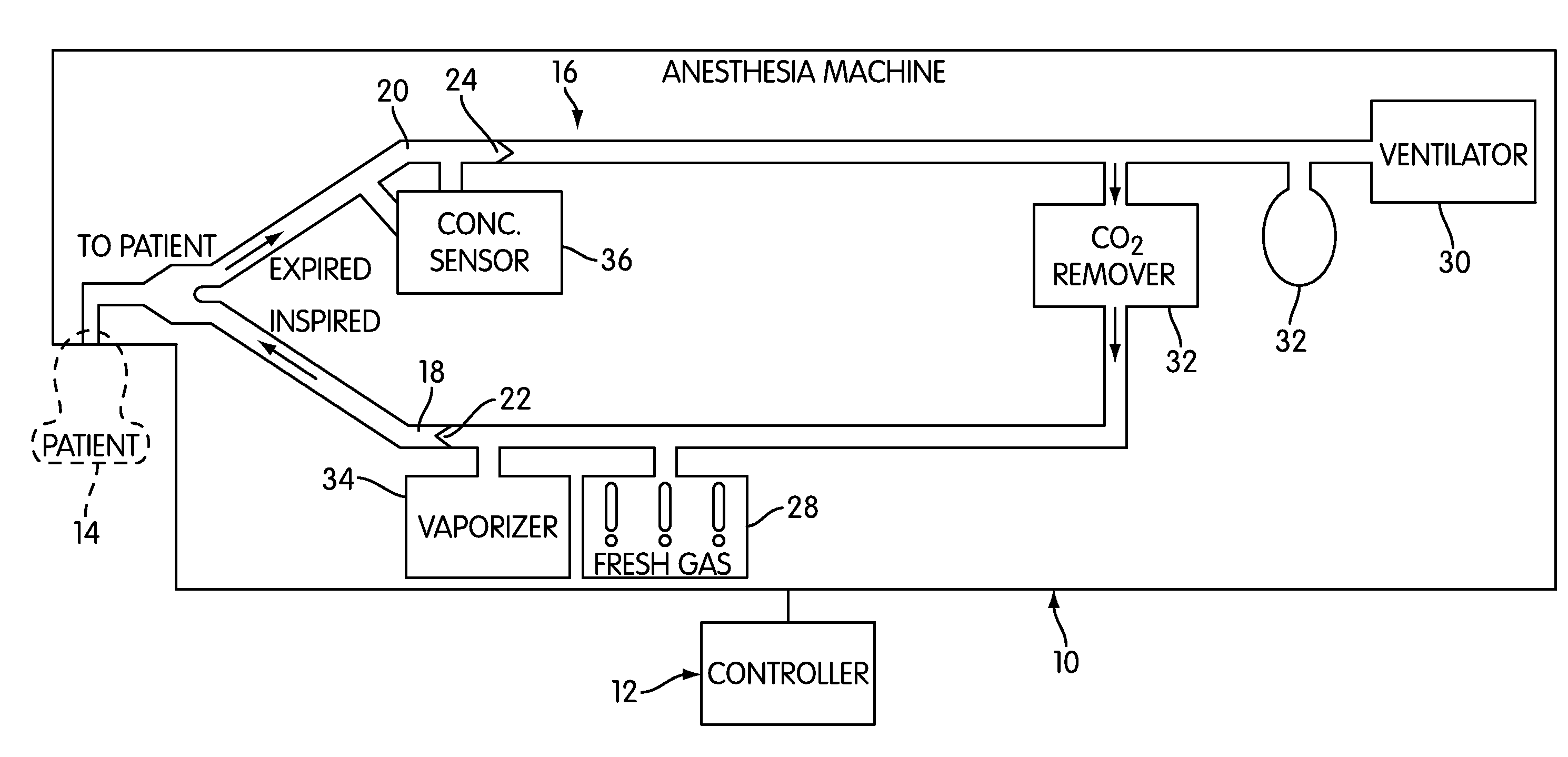
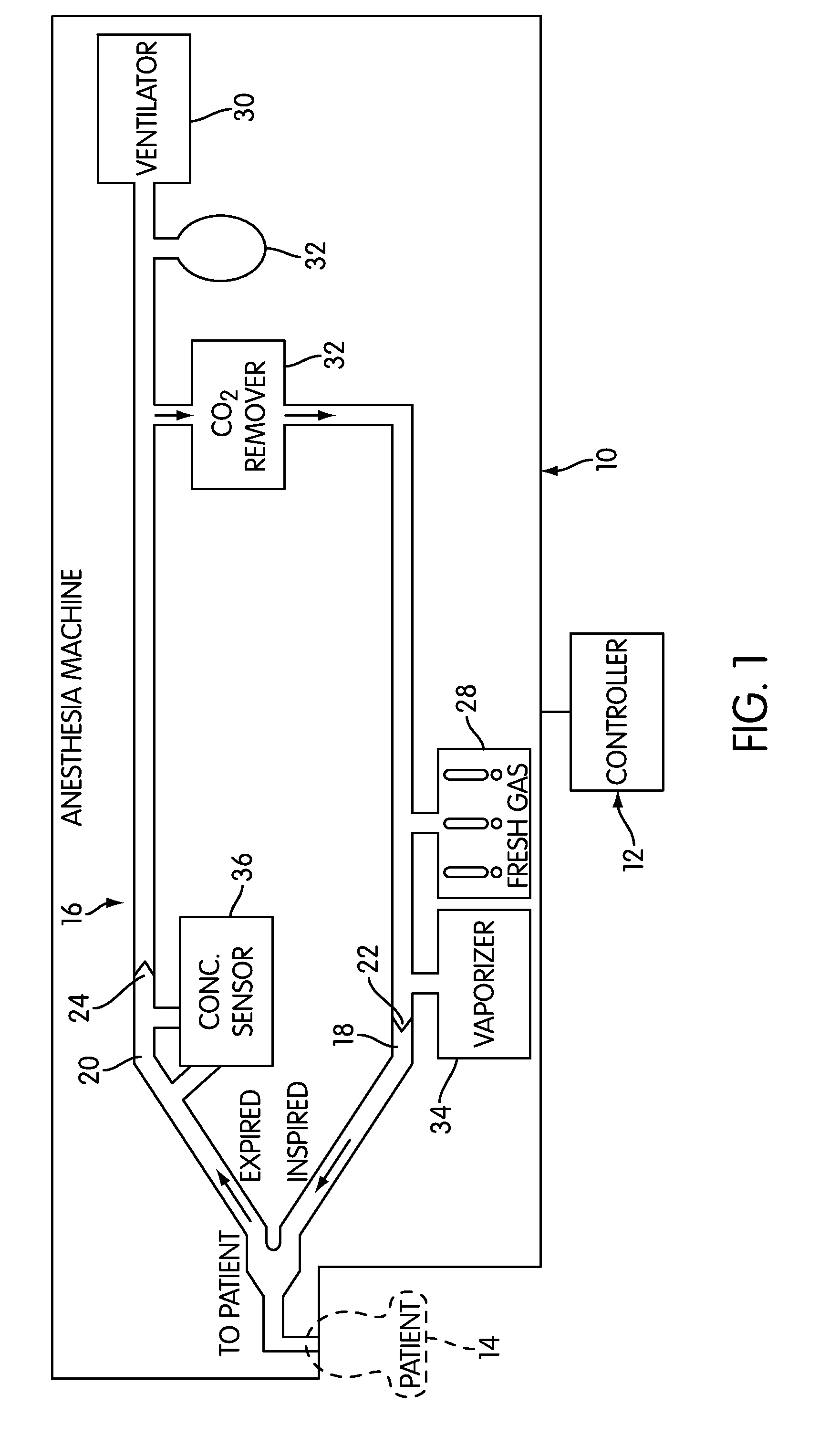
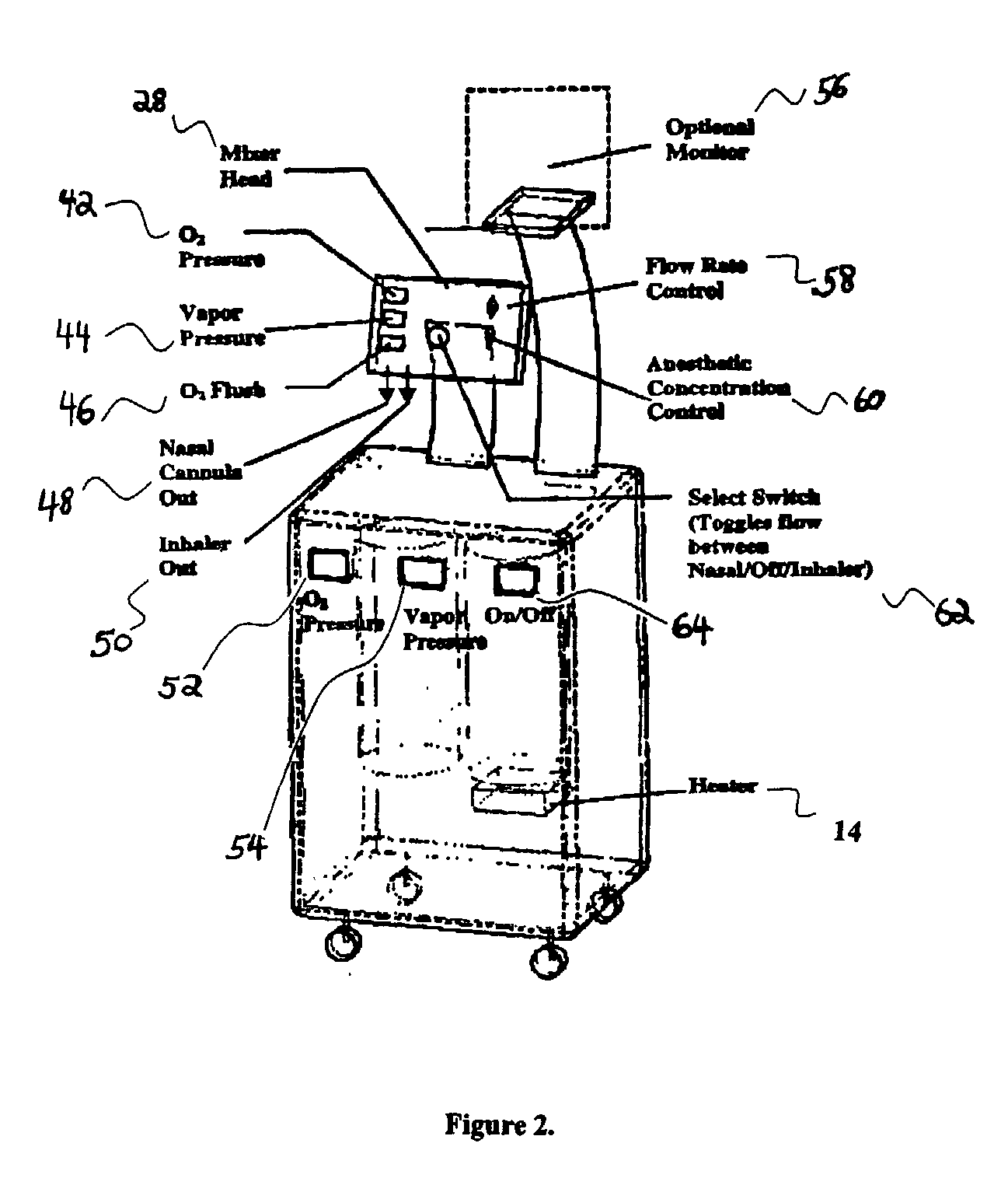
Anaesthesia apparatus and method for operating an anaesthesia apparatus
An anaesthesia apparatus has a control unit that controls a gas flow of a flushing gas through at least part of the anaesthesia apparatus so as to flush anaesthetic agent from the apparatus when the apparatus is not connected to a patient. In this way the remaining anaesthetic agent can be removed from the anaesthesia apparatus after use before connecting another patient. Pre-use check of the apparatus can be performed automatically.
Owner:MAQUET CRITICAL CARE
Continuous anesthesia nerve conduction apparatus, system and method thereof
ActiveUS20140025039A1Shorten operation timeEasy to handleSpinal electrodesGuide needlesElectricityAnesthetic Agent
The invention generally relates to a continuous anesthesia nerve conduction apparatus and method thereof, and more particularly to a method and system for use in administering a continuous flow or intermittent bolus of anesthetic agent to facilitate a continuous or prolonged nerve block. In one embodiment, the apparatus includes a sheath having a proximal end, a distal end and at least one lumen extending from the proximal end to the distal end. The sheath also includes an embedded conductive element for transmitting an electrical signal from a proximal portion of the sheath to a distal portion of the sheath. A cannula is arranged in the at least one lumen of the sheath and has a distal end protruding from a distal portion of the sheath. The cannula is electrically coupled to at least a portion of the embedded conductive element and is configured to provide nerve stimulation.
Owner:SOLODEX LLC
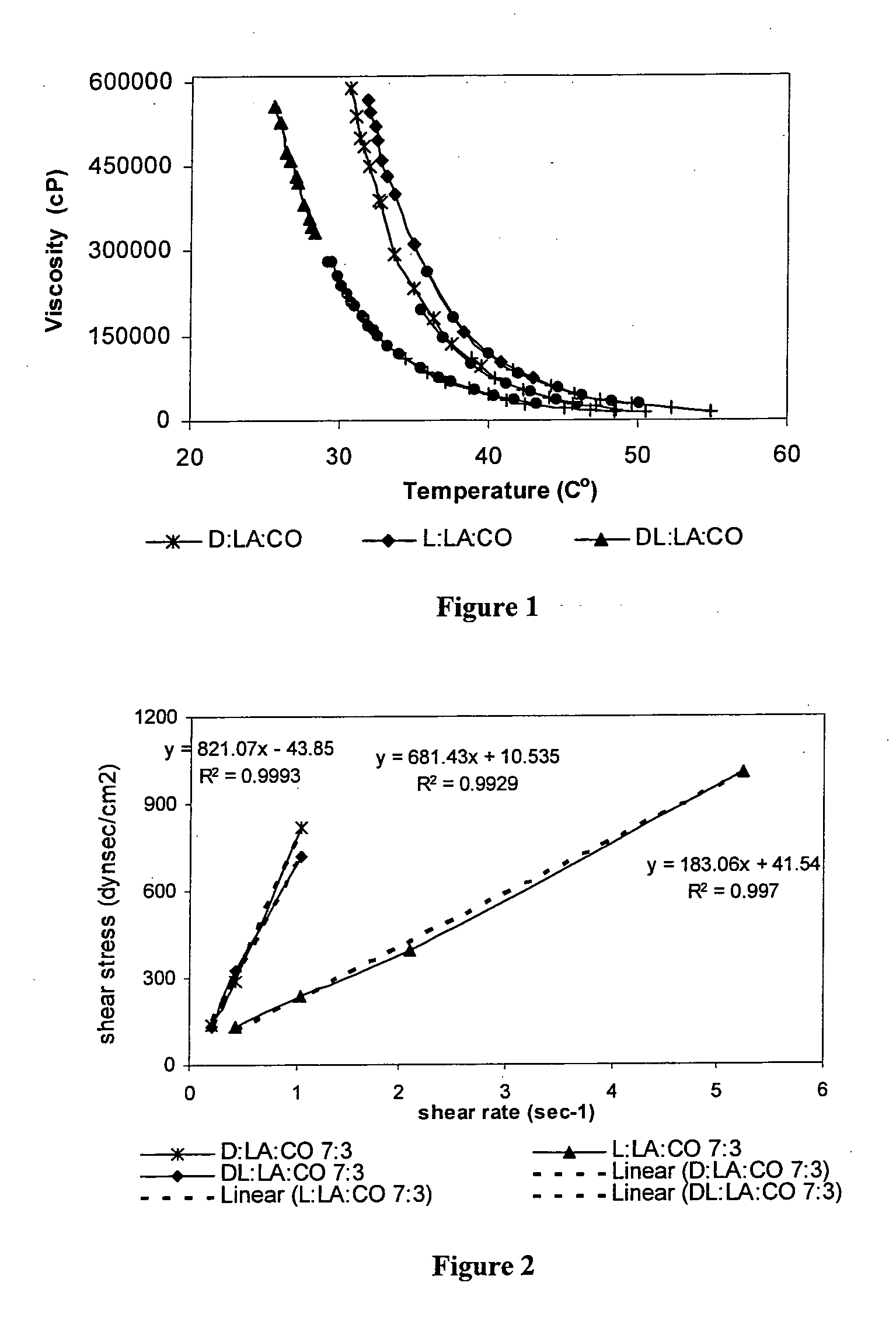
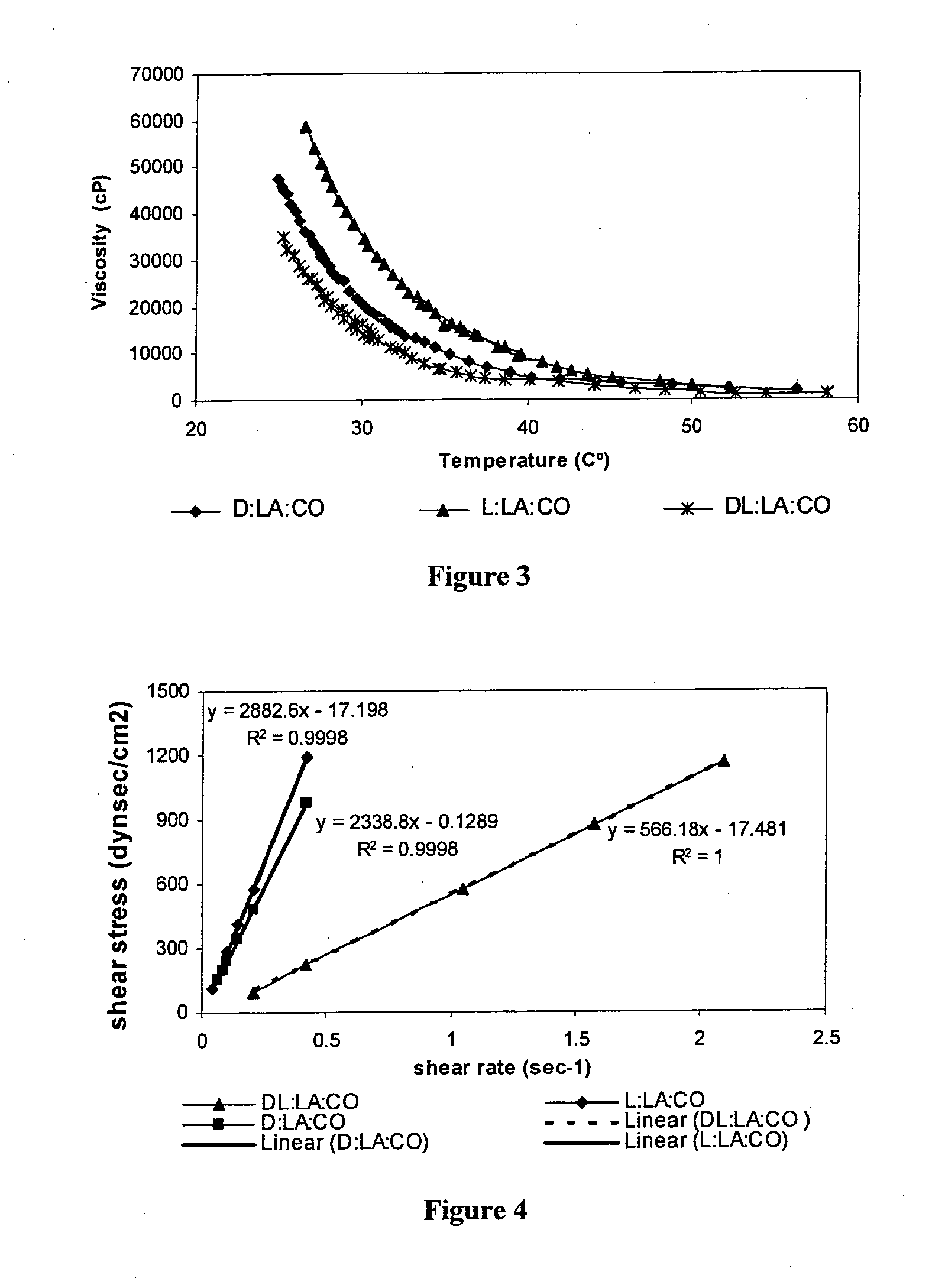
Injectable biodegradable polymer compositions for soft tissue repair and augmentation
InactiveUS20100260703A1Pharmaceutical delivery mechanismUrinary disorderInjectable polymersActive agent
Methods for soft tissue repair and / or augmentation using injectable, biodegradable polymers are described herein. In one embodiment, the polymer compositions are liquid or pastes at room temperature. In a preferred embodiment, the polymer composition contains liquid or pasty hydroxy fatty acid-based copolyesters, polyester-anhydrides, or combinations thereof. The viscosity of the polymers increases upon contact with bodily fluid to form a solid or semisolid implant suitable for soft tissue repair and / or augmentation. In another embodiment, the polymer composition contains particles of a polymer stereocomplex. One or more active agents may be incorporated into the polymer compositions. Suitable classes of active agents include local anesthetics, anti-inflammatory agents, antibiotics, analgesics, growth factors and agents that induce and / or enhance growth of tissue within the filled cavity or control the growth of a certain type of tissue, and combinations thereof. The polymer compositions may also contain one or more additives or excipients that modify the physical and / or mechanical properties of the polymer. The polymer compositions are typically administered by injection. The injectable polymers can be used for a variety of soft tissue repair and augmentation procedures.
Owner:POLYGENE LTD
Method and apparatus for monitoring intravenous (IV) drug concentration using exhaled breath
InactiveUS7104963B2Good correlationCost-effective and frequentRespiratorsRespiratory organ evaluationAnesthetic AgentMetabolite
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Tamper-proof narcotic delivery system
A composition for oral, transdermal or subdermal administration to a subject is described. The composition contains: (a) an agonist component; (b) an antagonist component containing at least one antagonist and having a delayed time of release; and (c) an immediate release antagonist removal component, where the subject includes a gastrointestinal tract and the antagonist removal component is present in an amount sufficient to substantially remove the antagonist component from the gastrointestinal tract of the subject before the time of release of the antagonist component. The composition may be delivered to the subject by a method which includes the step of administering the composition orally, transdermally or subdermally to the subject. When the composition of the present invention is administered orally, the method of the present invention may include the step of administering a potassium compound to the subject.
Owner:FARRELL JOHN J
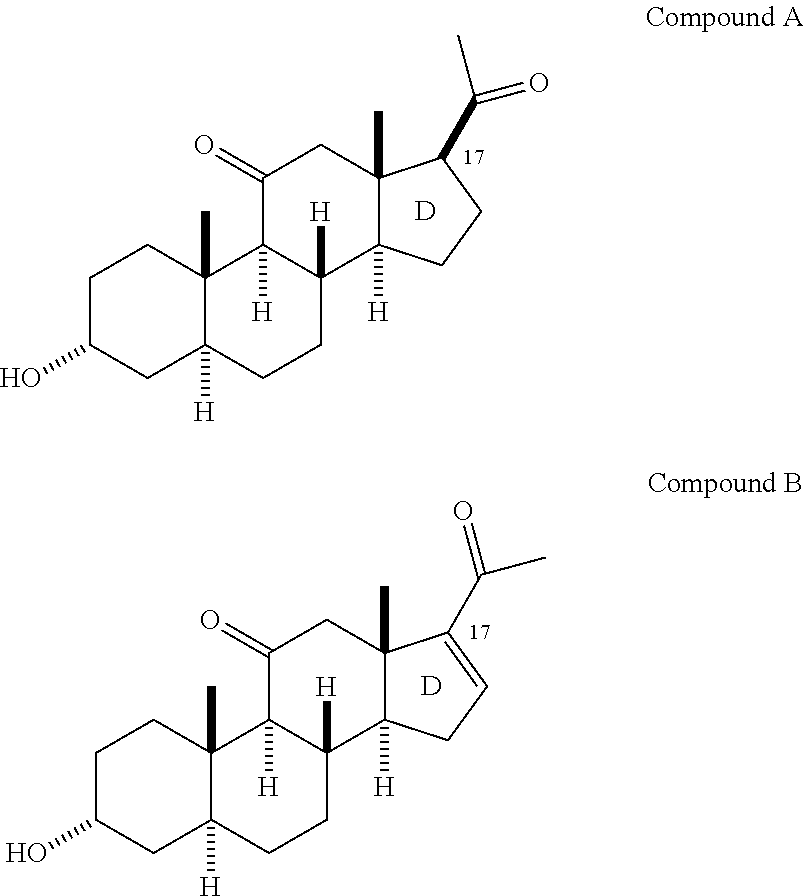
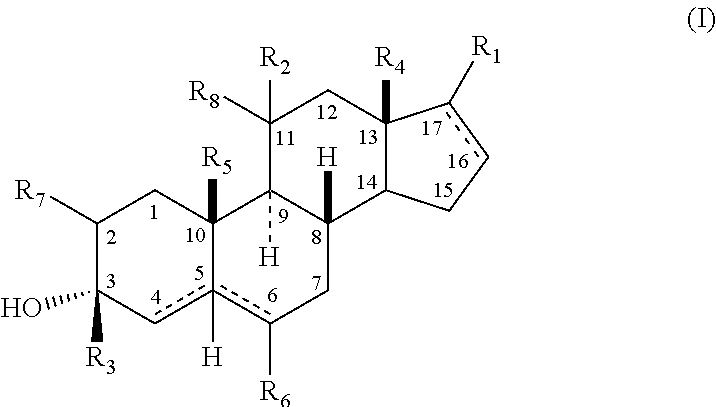
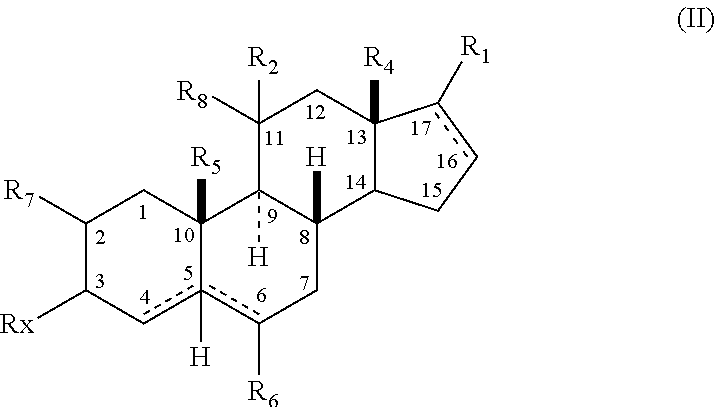
Neuroactive 19-alkoxy-17-substituted steroids, prodrugs thereof, and methods of treatment using same
The present disclosure is generally directed to neuroactive 19-alkoxy-17-substituted steroids as referenced herein, and pharmaceutically acceptable salts thereof, for use as, for example, an anesthetic, and / or in the treatment of disorders relating to GABA function and activity. The present disclosure is further directed to pharmaceutical compositions comprising such compounds.
Owner:SAGE THERAPEUTICS +1
Automated compulsory blood extraction system
InactiveUS6340354B1Cost-effectively mass producedPromote circulationIncision instrumentsWound drainsVeinHead size
A method and apparatus for the treatment of thrombosis, venous insufficiency, and the like, and in particular to an Automated Compulsory Blood Extraction System (ACBES) configured to provide an efficient and safe means for the measured extraction of blood utilizing a device providing, in effect, an artificial leech, but without the infection, control, care, and other limitations associated with the medicinal leech. The preferred embodiment of the present invention utilizes recent micro technological advances to provide a micro mechanical device which mimics and improves upon the bloodletting properties of the medicinal leech utilizing a micro mechanical valve, micropump, and micro sensor arraignment cooperating with a tertiary jaw array having teeth situated thereon. The preferred embodiment of the present invention contemplates an extraction device which may have a head size of one centimeter or less, and which may be utilized in number about the affected area of the patient to provide controlled, precision, pulsed blood extraction via vacuum induction, supplying a controlled dosage of anticoagulant, histamine anesthetic, or the like. Alternative embodiments of the present invention include an independent, single needle, stationary design configured primarily for emergency use, a multi-needle piston design, a large extraction area array design including concentric needles of adjustable depth, and a deep extraction needle design.
Owner:RAMBIN CHRISTOPHER L
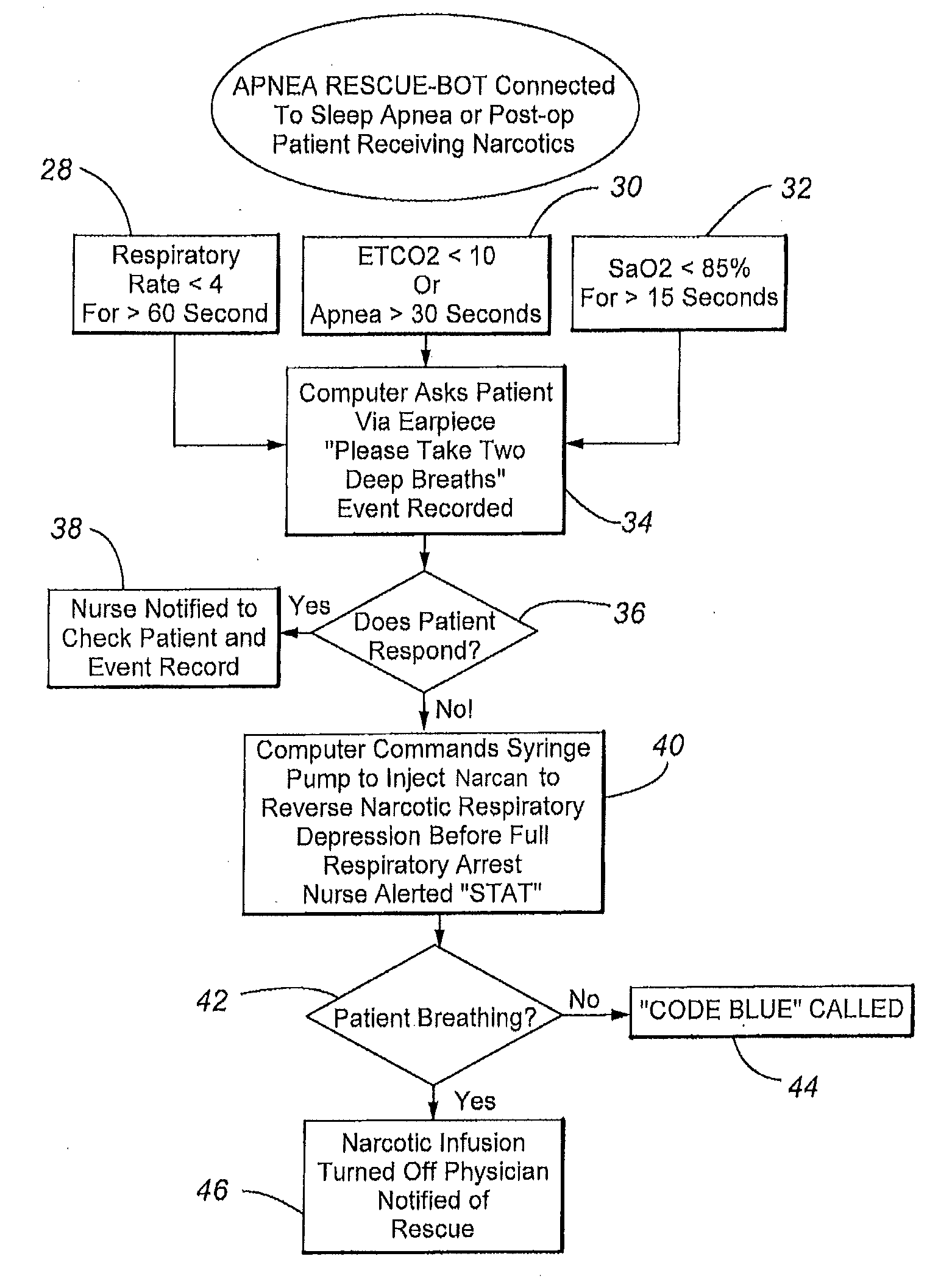
Systems And Methods For Using Photoplethysmography In The Administration Of Narcotic Reversal Agents
Provided according to embodiments of the present invention are methods of monitoring and treating respiratory depression that include securing a photoplethysmography (PPG) sensor to a central source site of an individual; administering a central nervous system (CNS) depressant to the individual; processing PPG signals front the PPG sensor with a computer in communication with the PPG sensor; and administering a narcotic reversal agent to the individual if the PPG signals or a physiological parameter derived therefrom are outside a preset value range. Related systems are also described.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
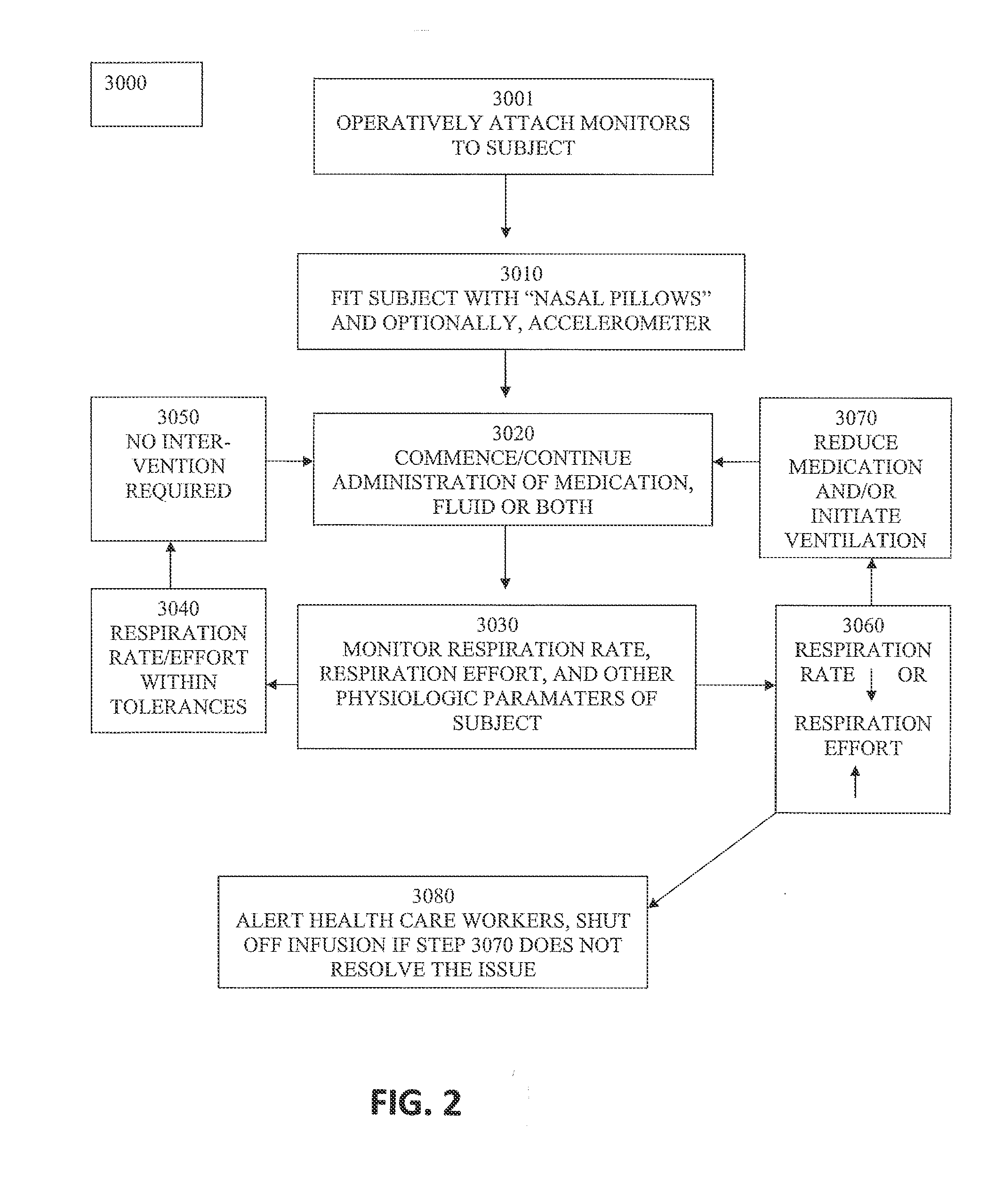
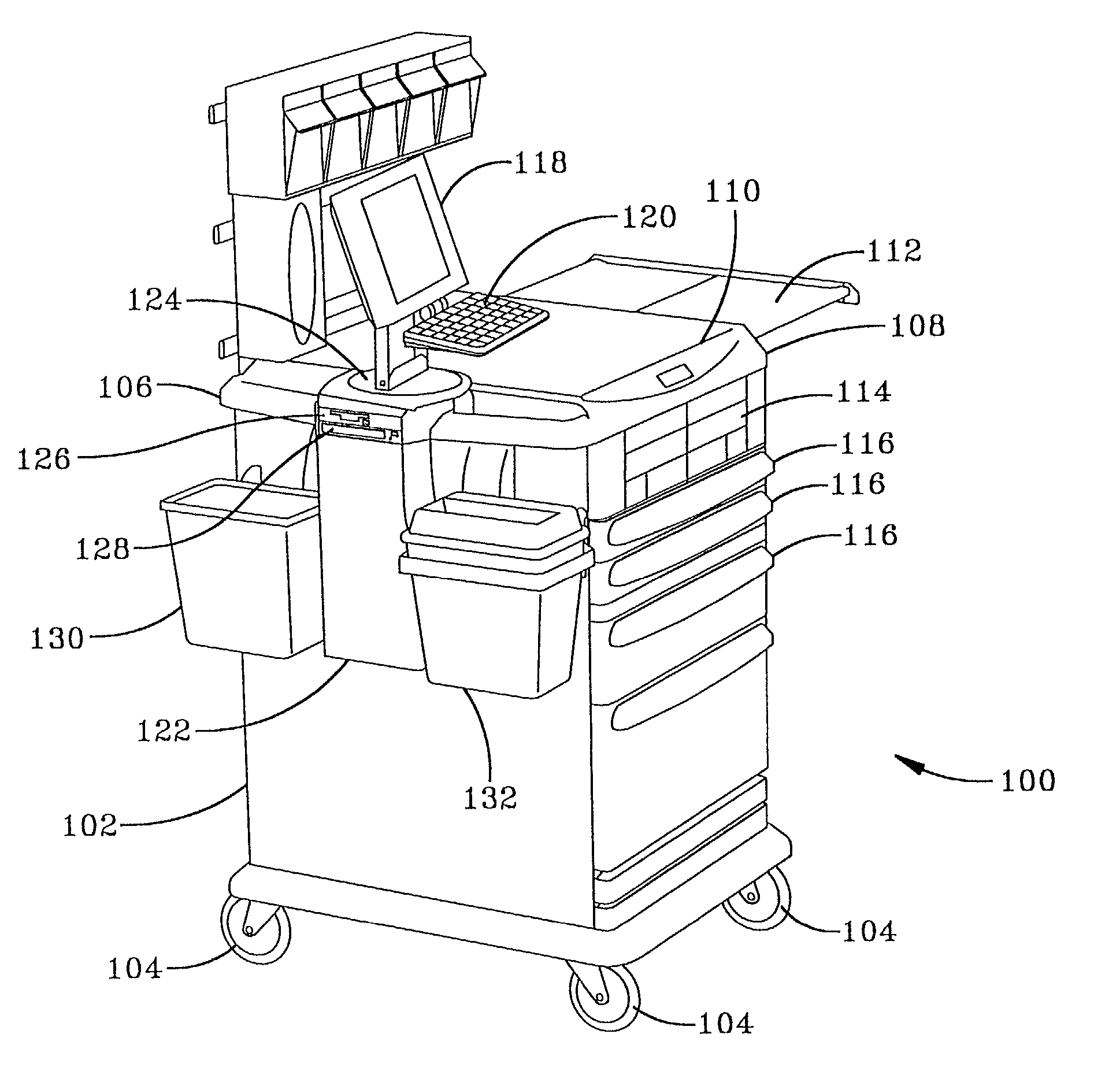
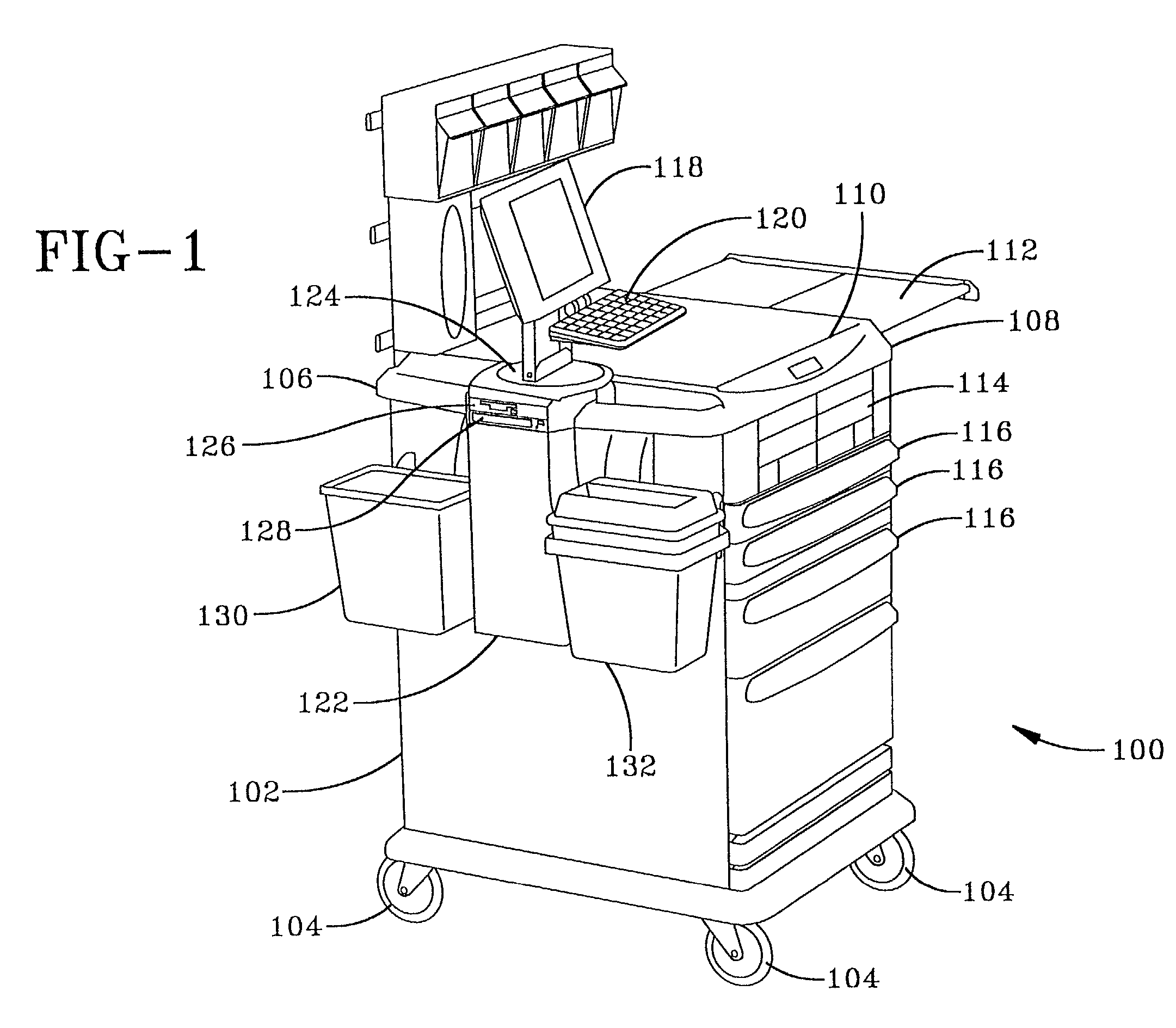
Anesthesia cart
InactiveUS20020013640A1Easy to processRespiratorsData processing applicationsAnesthesia cartDispensary
A computerized medication dispensing station that addresses anesthesia medication management and tracking problems is disclosed. Medications, including narcotic and non-narcotic, and supplies for use in anesthesia, are stored in secured, semi-secured, and unsecured containers of a mobile station. A computer housed in the station is used to track the anesthesiology items that have been removed from the station. For each item removed, the time of removal, who removed it, and to whom it was administered is tracked. Items that are not administered to a patient are returned to the pharmacy or wasted (i.e., disposed in accordance with regulations). Each type of event (administration to a patient, return, or waste) is documented so that a health care institution can track usage of items, including narcotic medications, for use in anesthesia.
Owner:PHOON FLORENCE H +5
Transdermal anesthetic and vasodilator composition and methods for topical administration
A composition for topical application comprising a therapeutically effective amount of a topical anesthetic, a safe and effective amount of a pharmaceutically acceptable topical vasodilator and a pharmaceutically acceptable carrier and a method of administering the composition to a mammal are disclosed.
Owner:SAMUELS PAUL J +1
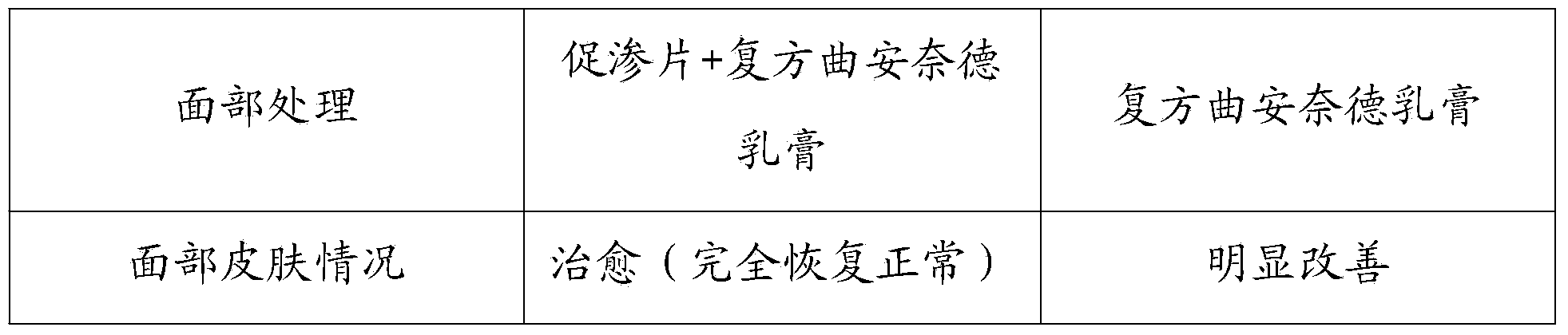
Photosensitizer composition for treating skin disorders
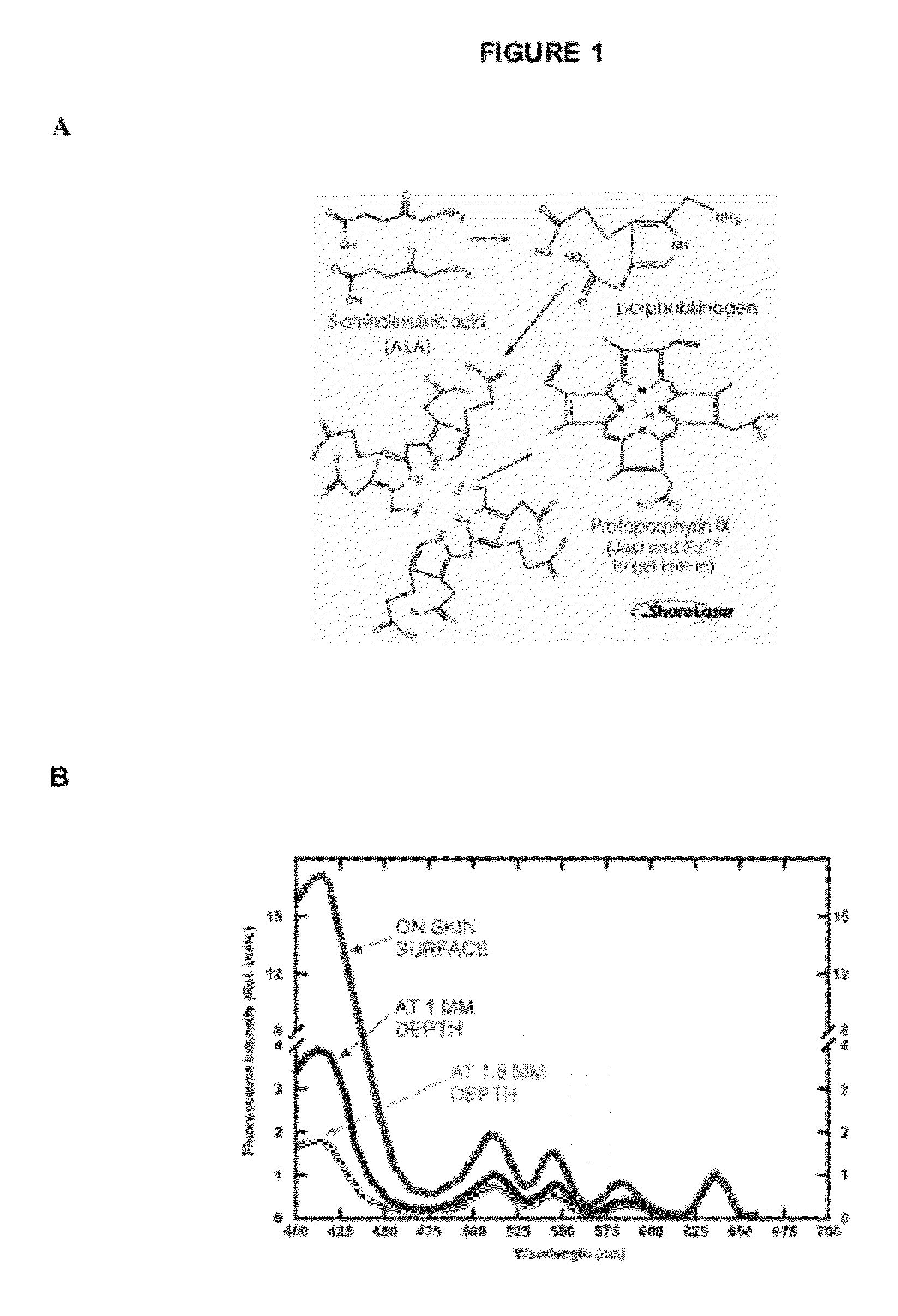
A composition comprising an active ingredient selected from the group of 5-aminolevulinic acid or a pharmaceutically acceptable salt or derivative thereof; and an aqueous carrier comprising at least one absorption enhancer, an anesthetic, hyaluronic acid and at least one acid selected from the group consisting of glycolic acid and lactic acid. The composition is useful in a photodynamic method of treating skin disorders.
Owner:MODI PANKAJ
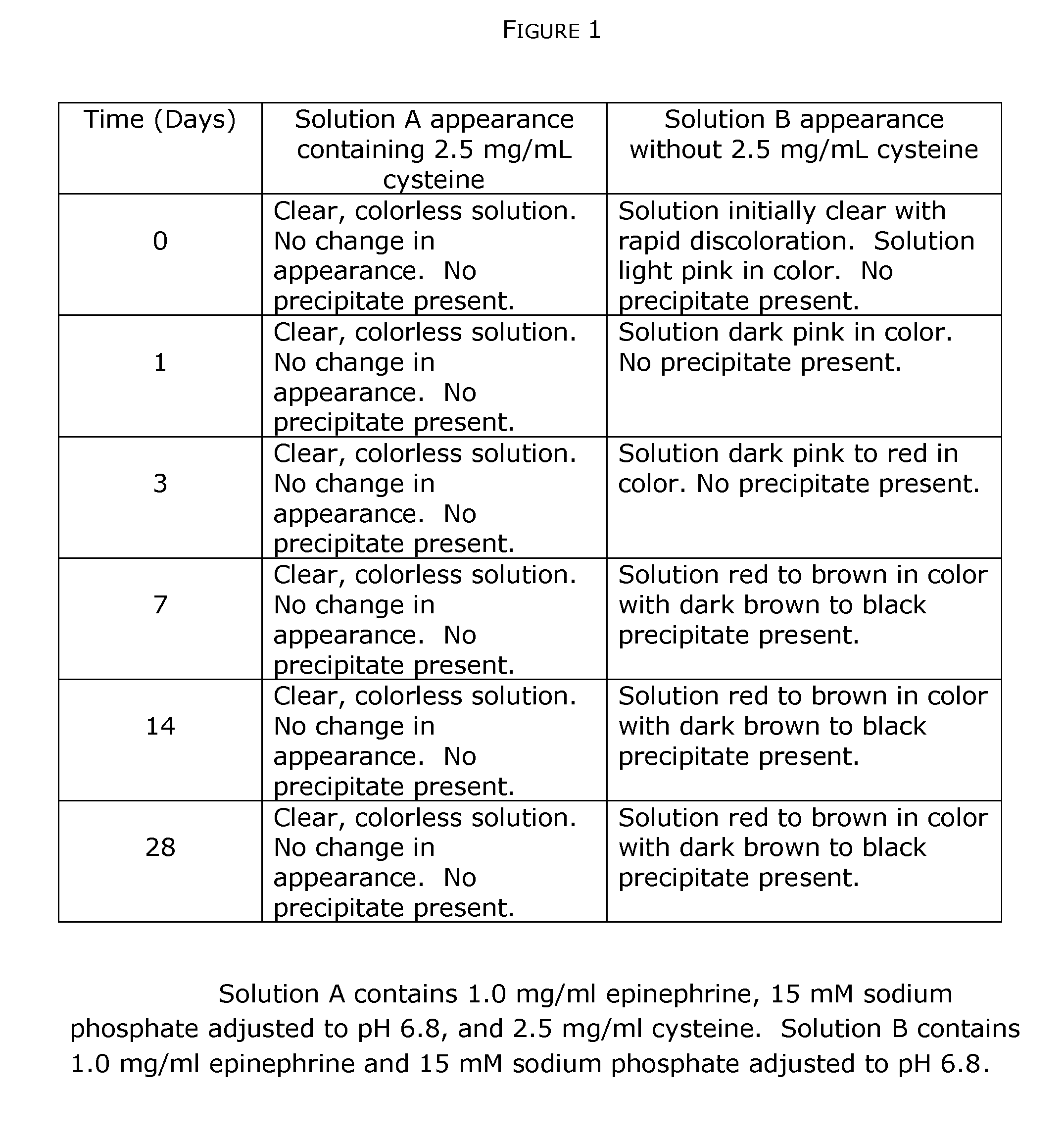
Stabilization of quinol composition such as catecholamine drugs
InactiveUS20120029085A1Stabilizing compoundReduced form requirementsBiocidePharmaceutical delivery mechanismCatecholaminePh buffering
Compositions and methods are provided for obtaining stabilized quinol compositions, such as catecholamine drugs (e.g., epinephrine solutions), and also for obtaining stable pharmaceutical formulations that comprise a stabilized quinol composition and a second pharmacologically active component such as a local anesthetic or other active drug ingredient having a reversibly protonated amine group. Stability is achieved through the inclusion of an appropriately selected pH buffer and a thiol agent, based on redox and pH buffering principles including pKa of the buffer and of the reversibly protonated amine group.
Owner:MACKAY JON
Neuroactive 13, 24-cyclo-18, 21-dinorcholanes and structurally related pentacyclic steriods
Owner:WASHINGTON UNIV IN SAINT LOUIS
Metabolic measure system including a multiple function airway adapter
InactiveUS20100036272A1Easy to reusePromote generationRadiation pyrometryMaterial analysis by observing effect on chemical indicatorAirway adaptorQuenching
A system (300) for measuring a metabolic parameter. The system includes an integrated airway adapter (20, 100, 200) capable of monitoring any combination of respiratory flow, O2 concentration, and concentrations of one or more of CO2, N2O, and an anesthetic agent in real time, breath by breath. Respiratory flow may be monitored with differential pressure flow meters under diverse inlet conditions through improved sensor configurations which minimize phase lag and dead space within the airway. Molecular oxygen concentration may be monitored by way of luminescence quenching techniques. Infrared absorption techniques may be used to monitor one or more of CO2, N2O, and anesthetic agents.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Sublingual and buccal film compositions
The present invention relates to products and methods for treatment of narcotic dependence in a user. The invention more particularly relates to self-supporting dosage forms which provide an active agent for treating narcotic dependence while providing sufficient buccal adhesion of the dosage form.
Owner:INDIVIOR UK
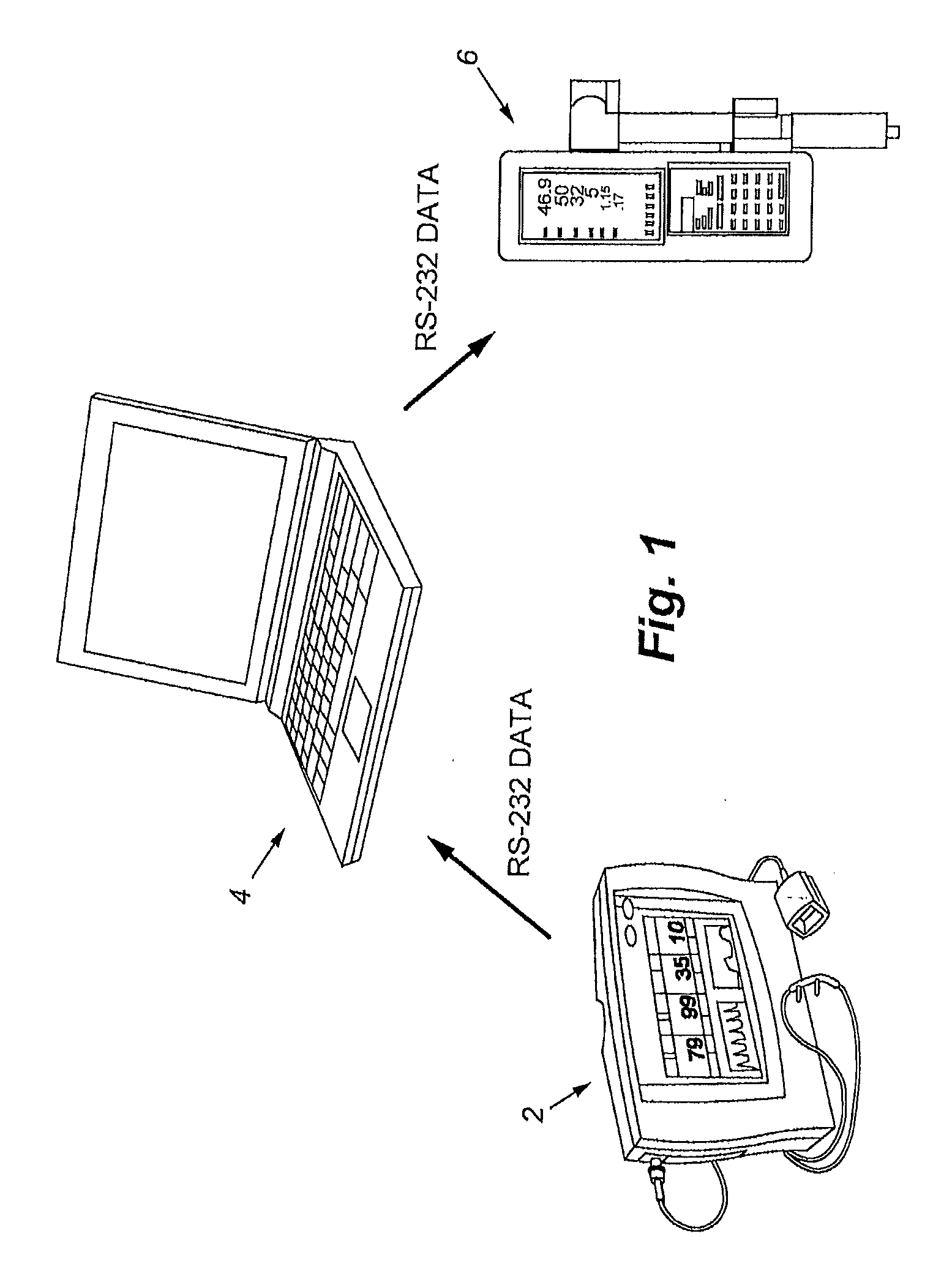
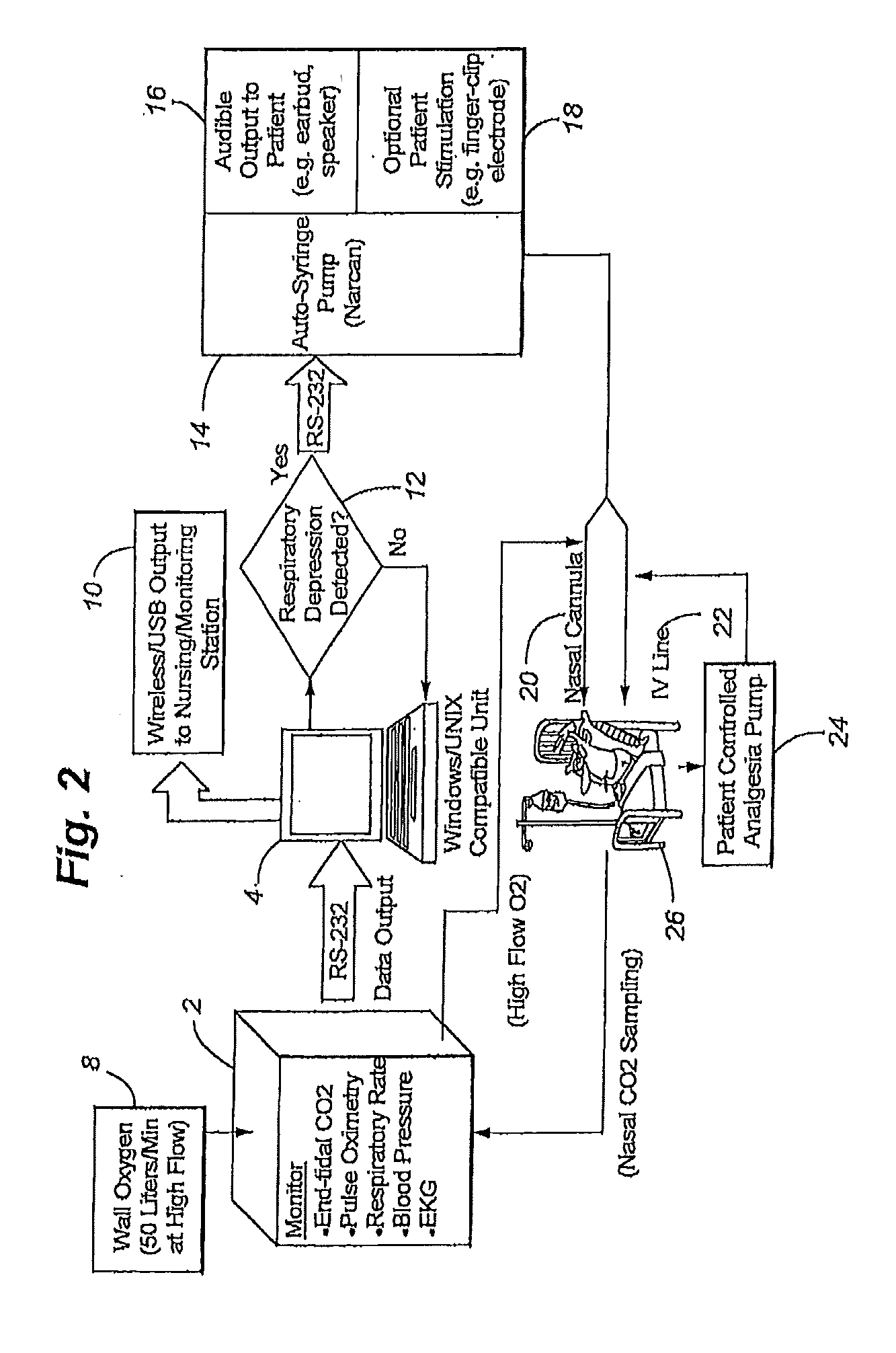
Apparatus and method of monitoring and responding to respiratory depression
ActiveUS20110290250A1Low component requirementsLower device costRespiratorsElectrotherapyNarcoticPost operative
The present disclosure involves a system for monitoring patients, and more specifically post-operative patients receiving narcotics, and a novel apparatus for automatically delivering a narcotic-reversing agent, including but not limited to the agent commonly known as naloxone, in response to dangerous respiratory conditions such as respiratory depression or other undesired consequences caused by reaction to narcotic dosage.
Owner:OLSON LLOYD VERNER +1
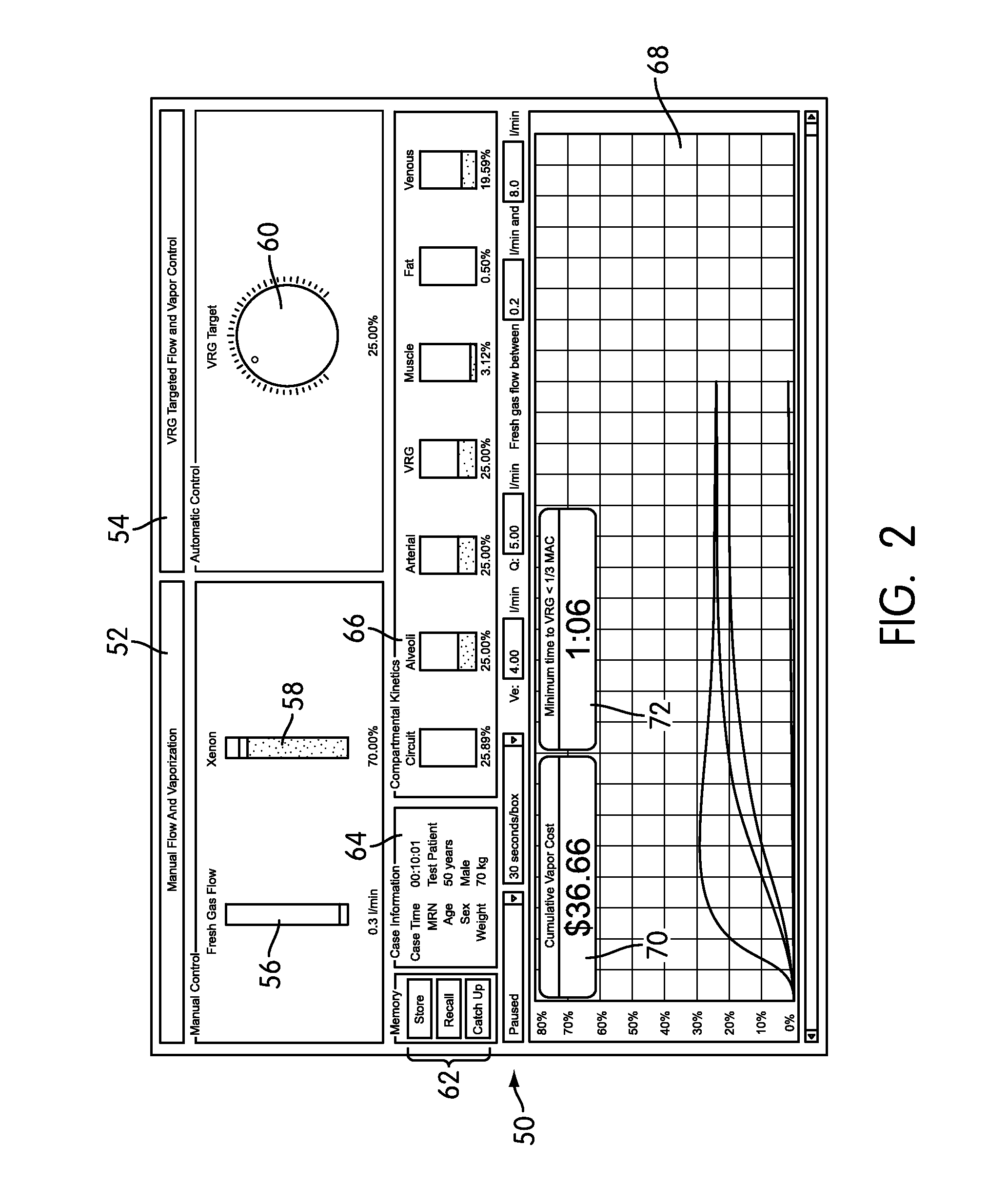
Anesthesia Simulator and Controller for Closed-Loop Anesthesia
Methods, systems, and software are disclosed for simulating and controlling anesthesia delivery and for implementing automatic closed-loop anesthesia with an inhaled anesthetic agent. The methods involve using a physiological simulation to forward calculate the effects of an inhaled anesthetic agent, and searching for gas administration characteristics, such as fresh gas flow rates and anesthetic concentrations or partial pressures, that will achieve a desired physiological anesthetic concentration or effect with minimal use of anesthetic agent and minimal fresh gas flow rate.
Owner:THE BRIGHAM & WOMENS HOSPITAL INC
Drug delivery system for conscious sedation
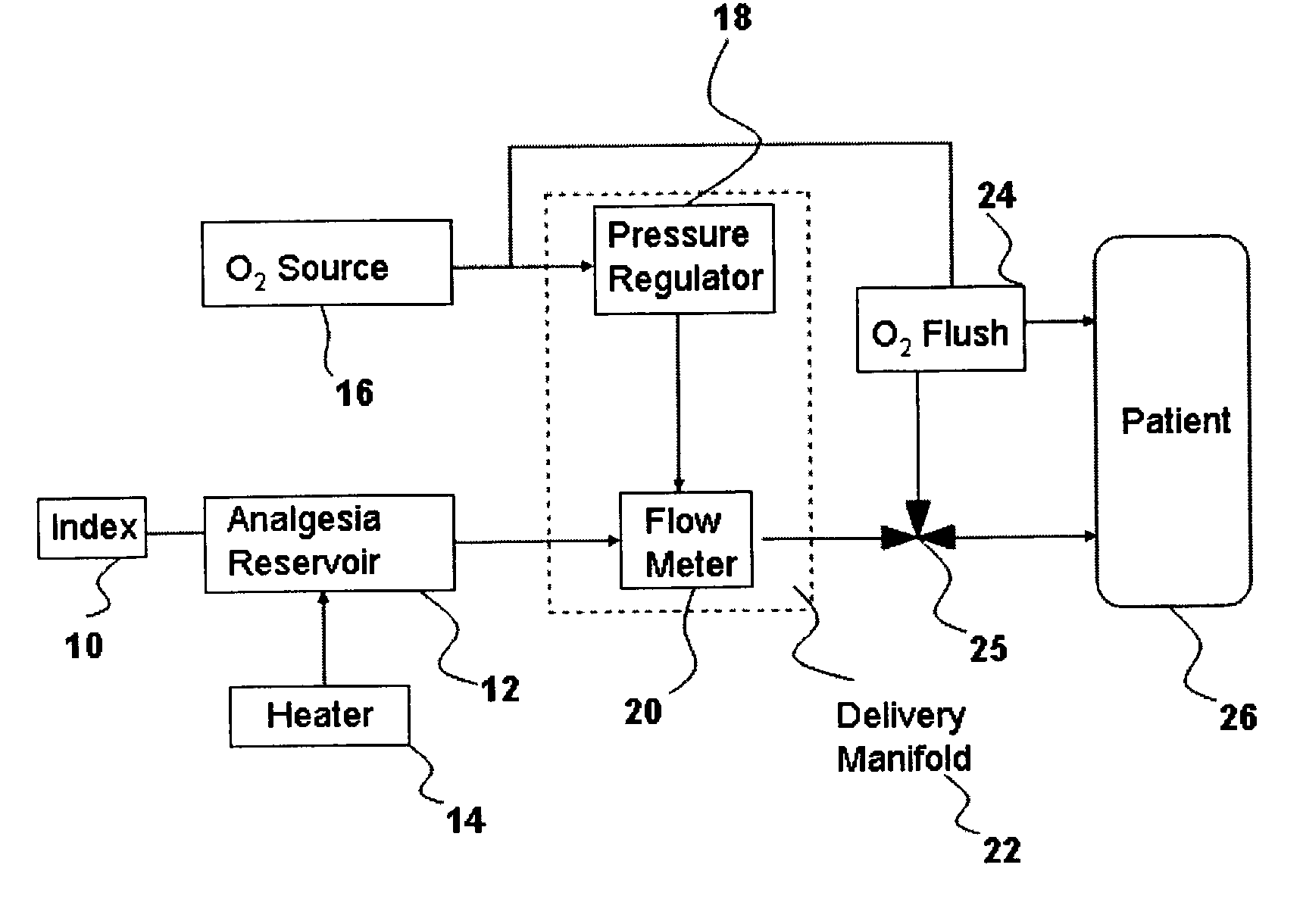
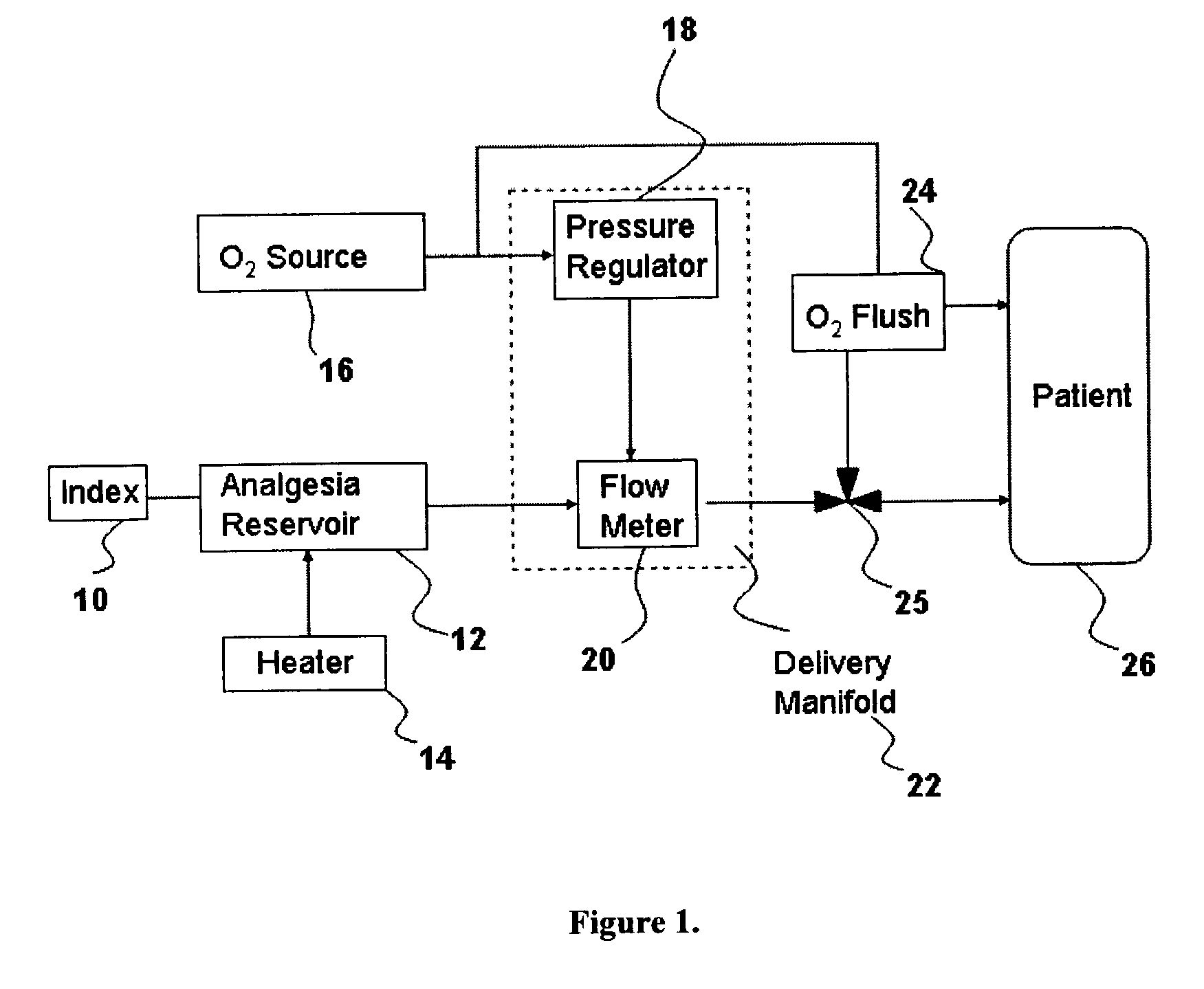
InactiveUS20030233086A1Increase oxygenationRelief the painHalogenated hydrocarbon active ingredientsNervous disorderAmnesiaSedation
Inhalant anesthetics are developed with a number of properties including rapid onset and recovery, controllability, and, ideally, a broad safety profile. The efficacy of these agents is measured by their ability to create anesthesia within the framework of the other desirable properties. The instant invention focuses on the dosage level where analgesia occurs but amnesia or lack of consciousness does not. In addition to identifying the dosage level where pain is sharply reduced or eliminated but awareness remains, a delivery system for safe and effective delivery of the agent is described.
Owner:FIRST NIAGARA BANK
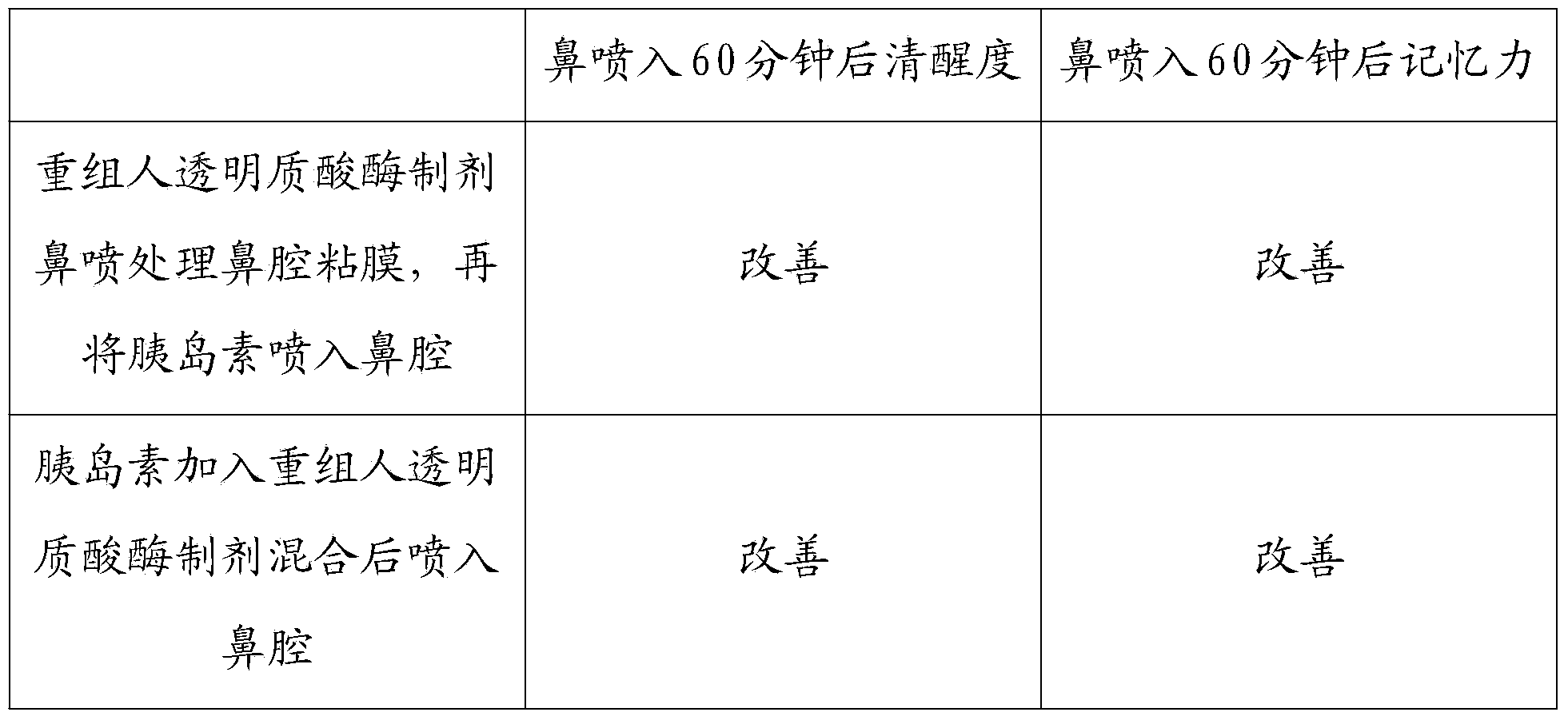
Recombined human hyaluronidase, production and purification method and preparations thereof, use method and application
InactiveCN103468662AImprove permeabilityPharmaceutical non-active ingredientsEnzymesNasal cavityDisease
The invention discloses a recombined human hyaluronidase, a production and purification method and preparations of the recombined human hyaluronidase, a use method and application. Recombined human hyaluronidase PH20 or human hyaluronidase human albumin fusion protein PH20-HSA or human hyaluronidase human immunoglobulin IgG2Fc fragment fusion protein PH20-IgFc is adopted by the recombined human hyaluronidase and used in the mucosa or the surface of the skin. The preparations of the recombined human hyaluronidase can be made into different types such as membrane preparations, spray preparations, lotion and freeze-dried powder spray and used for skin infiltration promotion of beauty nutrient substances, skin mucosa infiltration promotion of surface anesthetic, infiltration promotion of skin disease therapeutic medicine, mucosa infiltration promotion of biological tranquillizer, mucosa skin infiltration promotion of growth factors, mucosa infiltration promotion of hypoglycemic drug, mucosa nasal cavity infiltration promotion of nervous centralis nutrient substances and the like.
Owner:惠觅宙
Systems and methods for providing gastrointestinal pain management
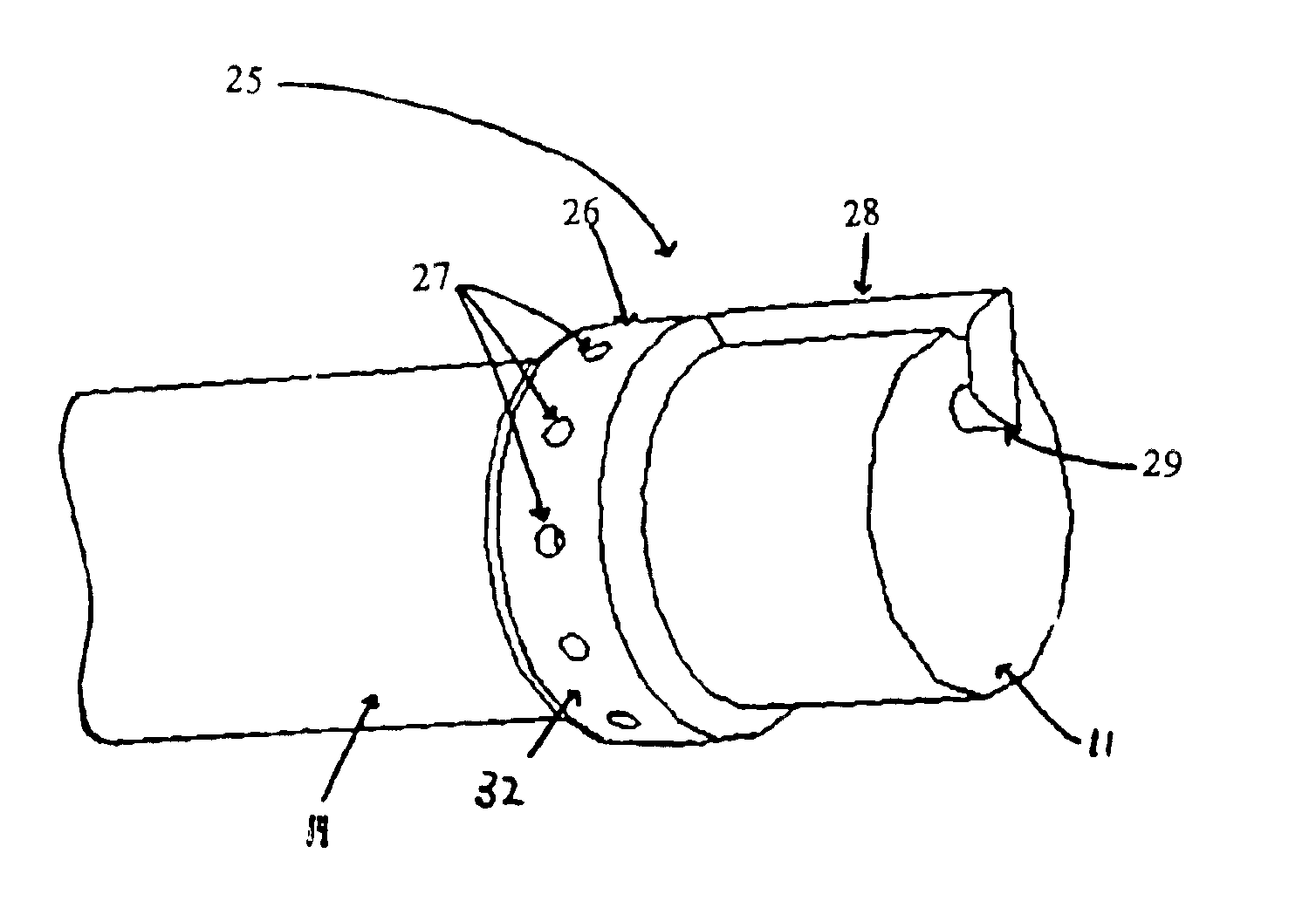
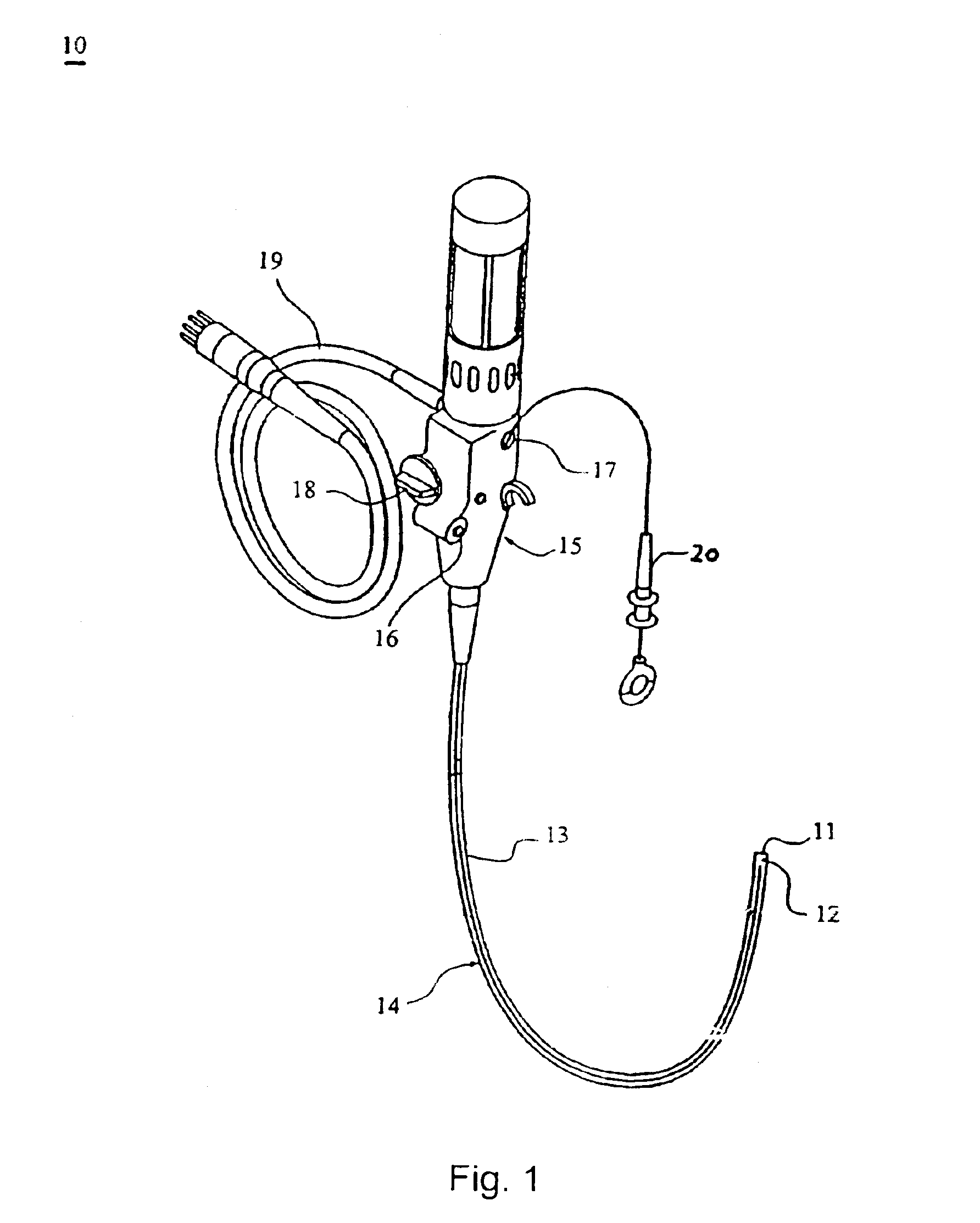
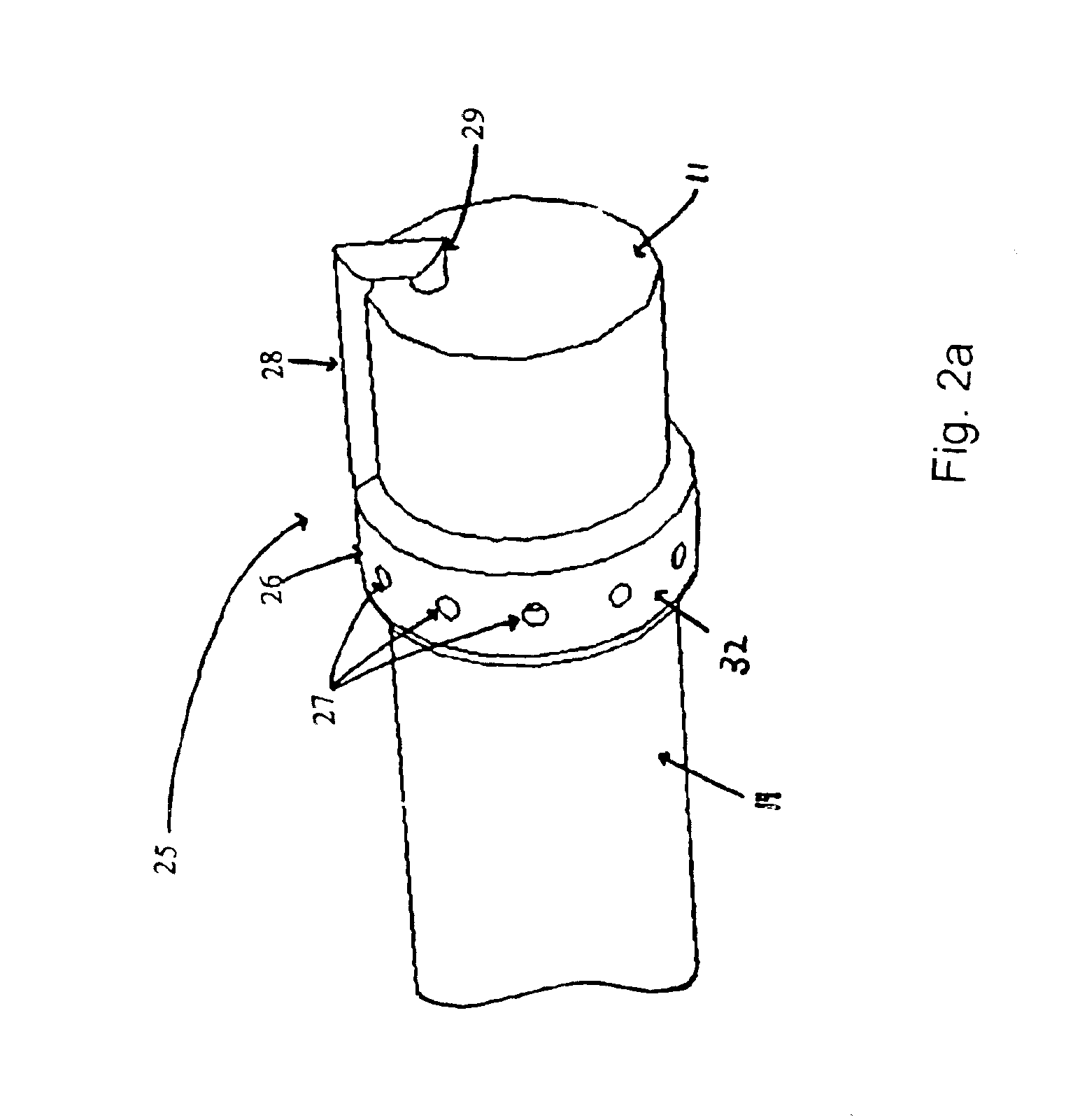
The present invention includes systems and methods for decreasing the pain and discomfort commonly associated with endoscopic procedures, where such procedures may be performed with lower dosage levels of sedative and analgesic drugs. The invention includes use of an anesthetic collar coupled to an endoscope with a flexible shaft. The anesthetic collar allows lubricants, local anesthetics, dyes, and / or other desirable fluids to be passed through the existing lumen of the flexible shaft into an annulus, where the fluid may be distributed through expulsion pores into the gastrointestinal tract. Utilizing the existing lumens found in endoscopes, the present invention allows those fluids that may reduce the pain and discomfort associated with endoscopies such as, for example, local anesthetics and lubricants, to be distributed in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, where such fluids may reduce the drug level requirements for sedative and analgesic agents. Alternatively, the endoscope may be redesigned for streamlined integration with the anesthetic collar or to accomplish the same function of distributing local anesthetics and lubricants, in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, The invention can also be used with endoscopes without existing lumens.
Owner:SCOTT LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com