Patents
Literature
603results about How to "Reduce pain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
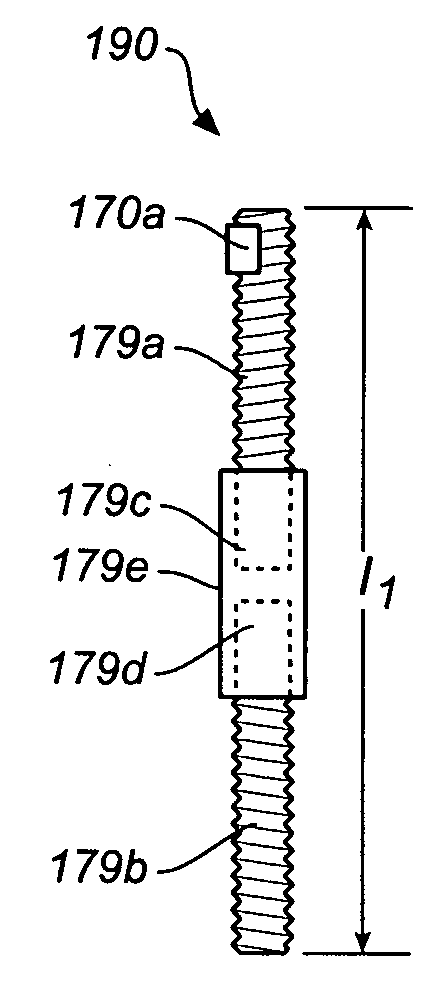
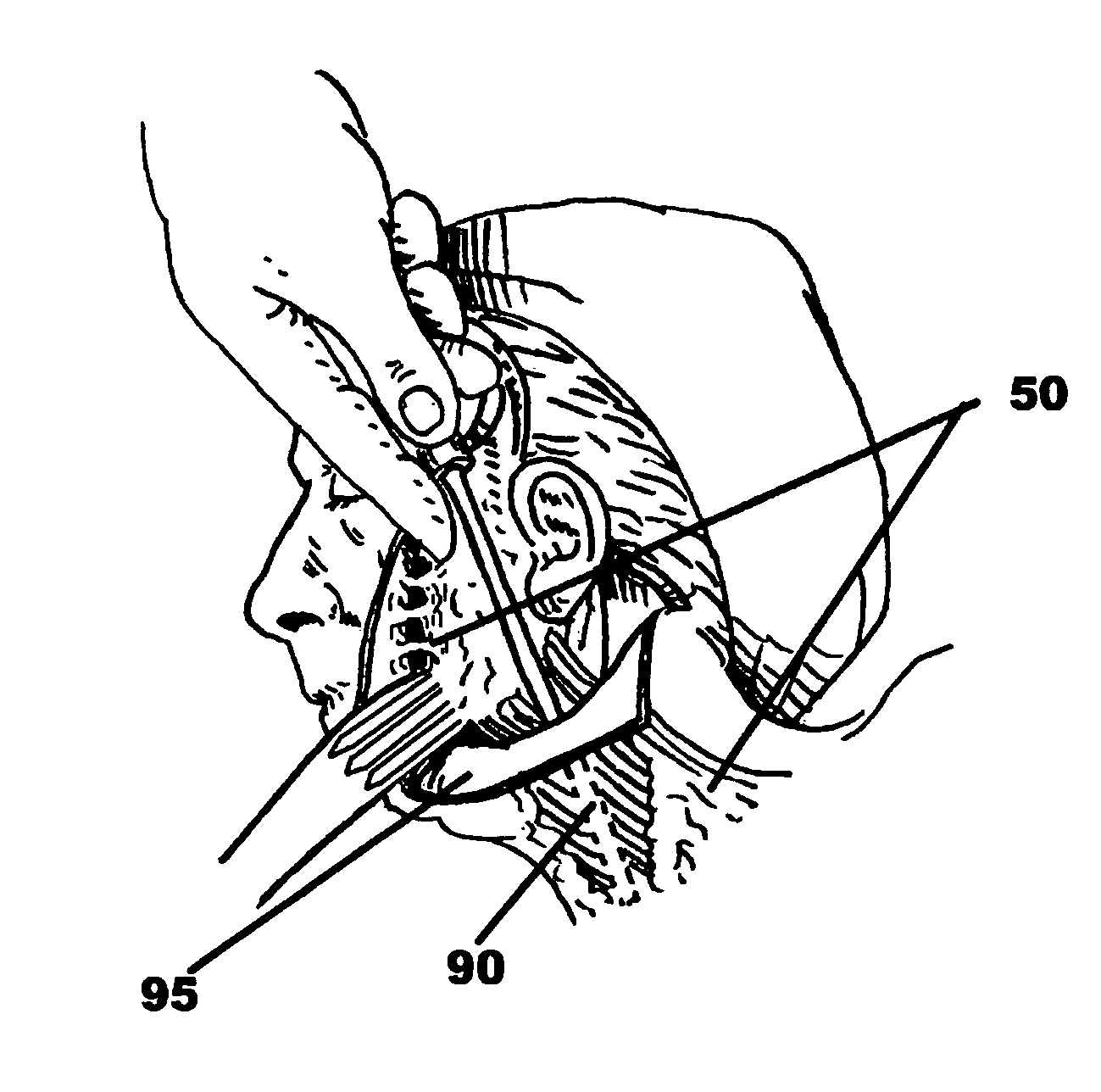
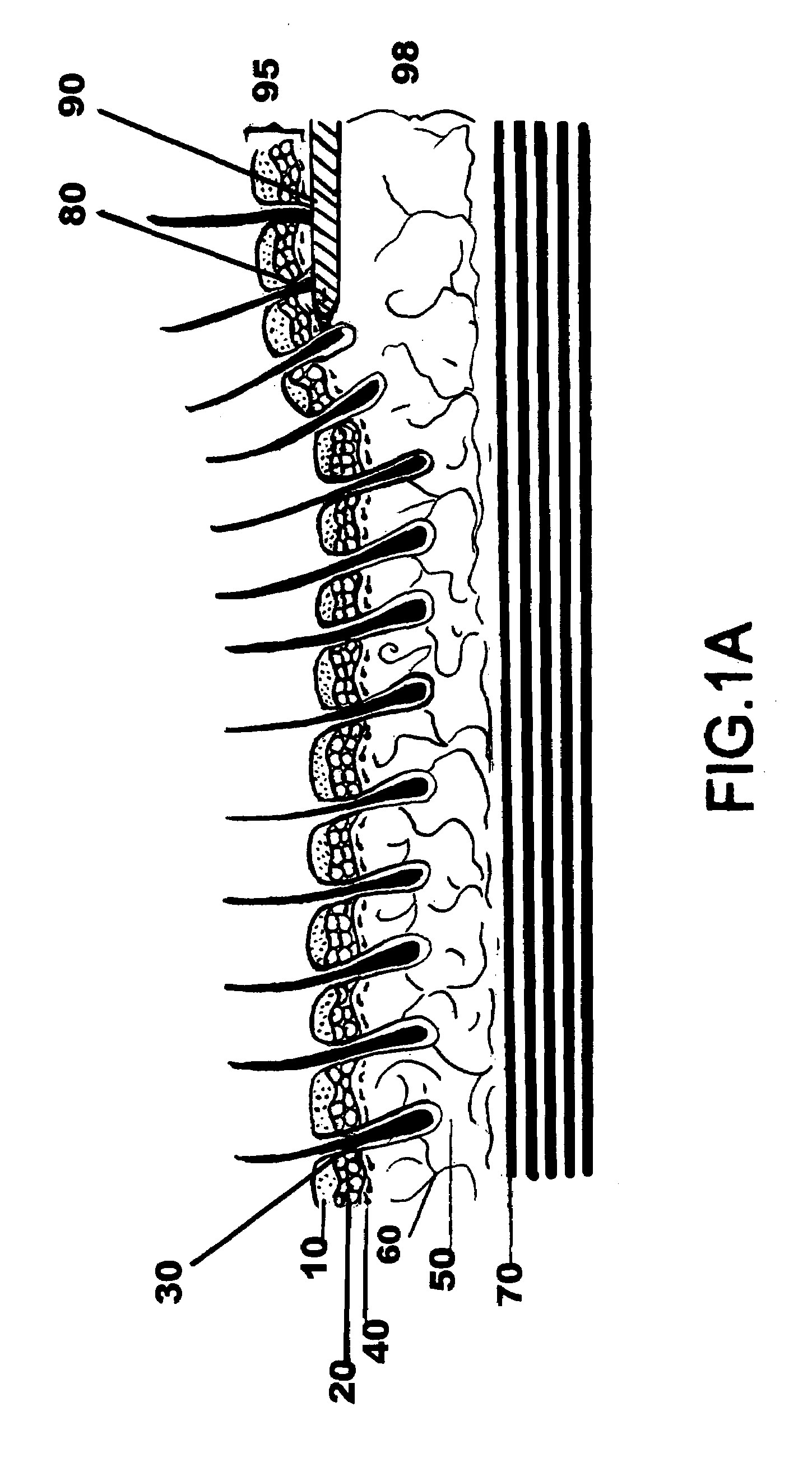
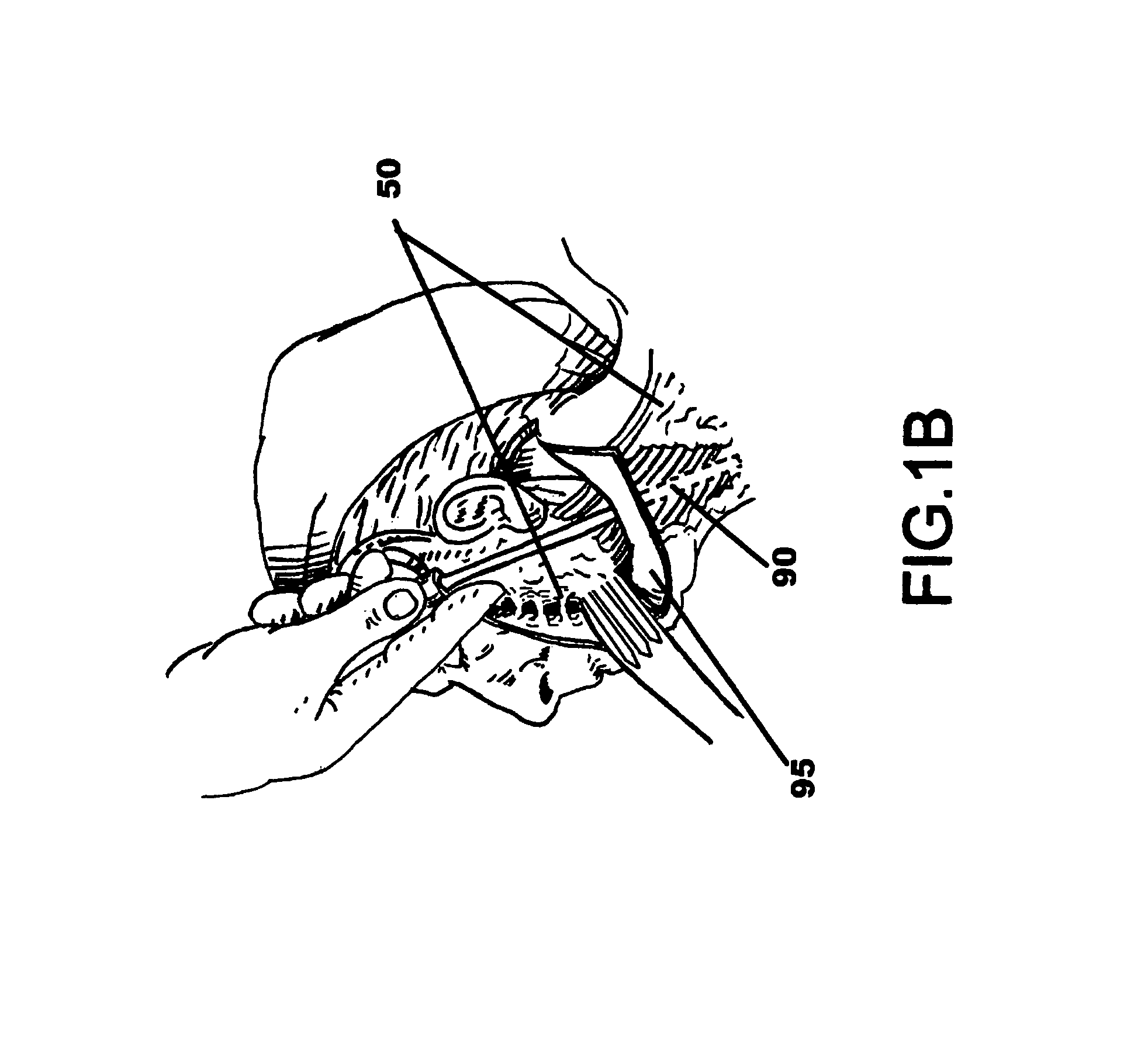
Drug depot implant designs
ActiveUS7727954B2Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injuryChronic pain
Owner:WARSAW ORTHOPEDIC INC
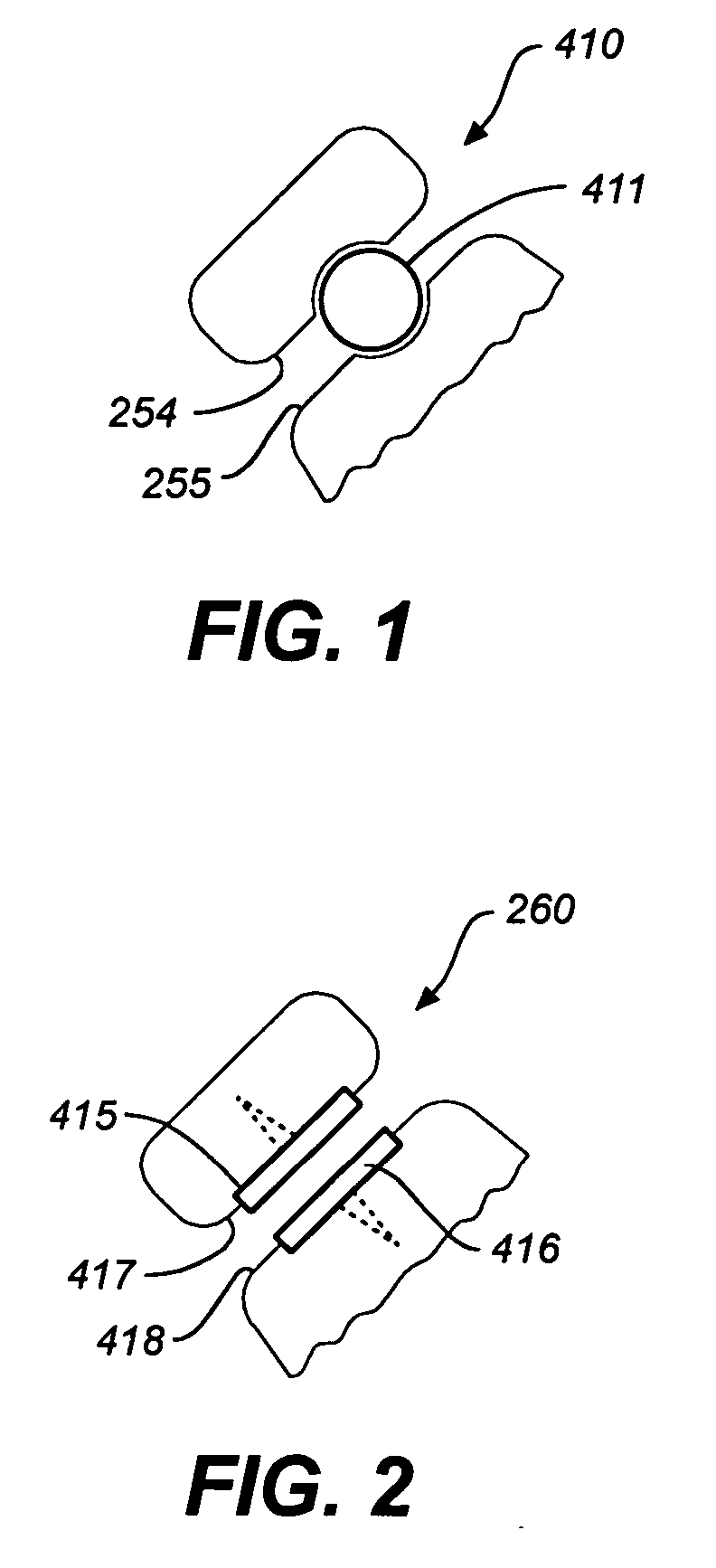
Facet device and method
InactiveUS20060036323A1Alleviating discomfort and or deformityLess discomfortInternal osteosythesisDiagnosticsProsthesisSacroiliac joint
Owner:K2M +1
Spine treatment devices and methods
InactiveUS20060036259A1Reduce deformityAvoid damageInternal osteosythesisDiagnosticsBiomedical engineering
Owner:ALBANY MEDICAL COLLEGE +1
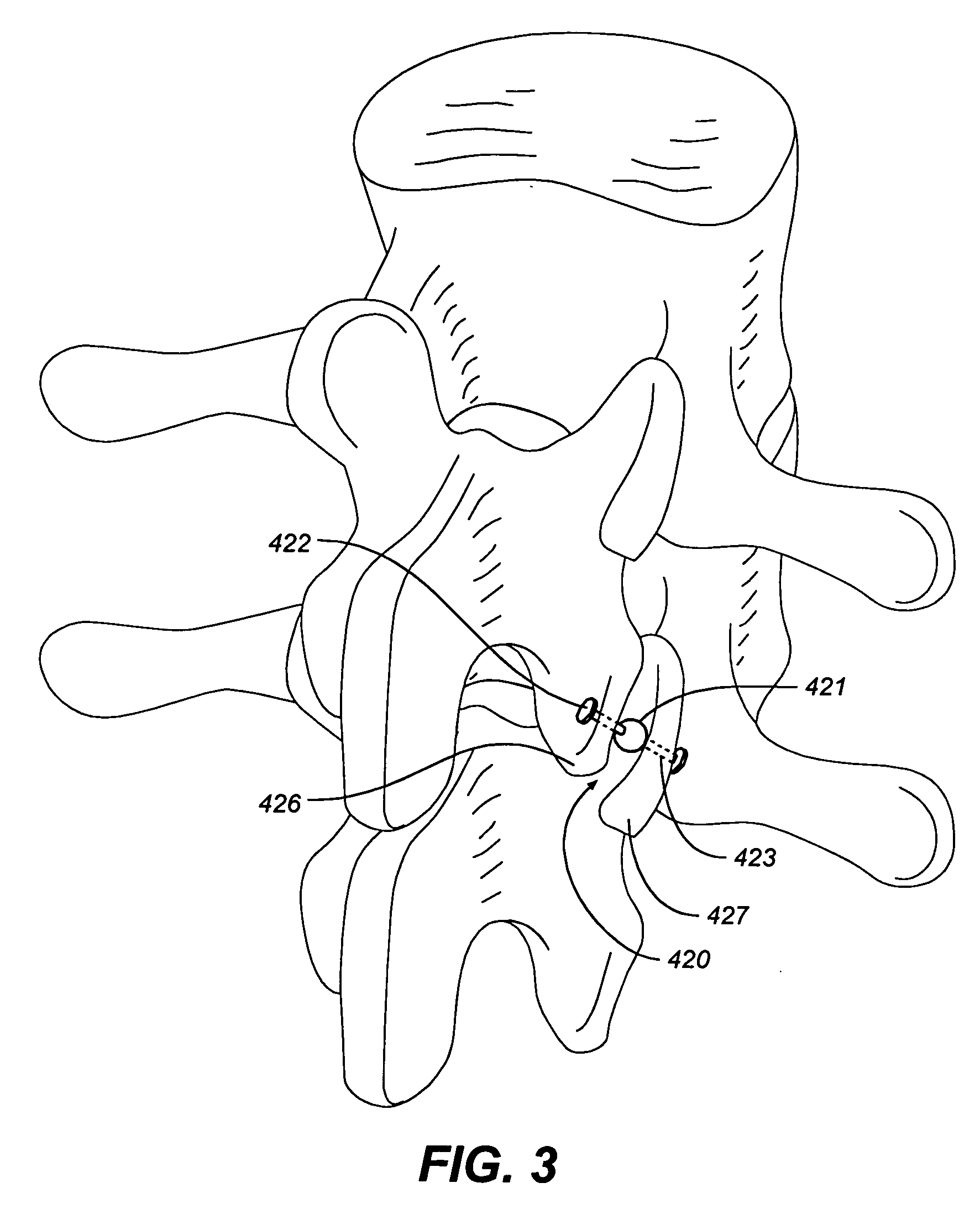
Systems and methods for stabilization of bone structures
InactiveUS20070100341A1Reduce painLong-term complicationInternal osteosythesisJoint implantsSmall incisionSpinal locomotion
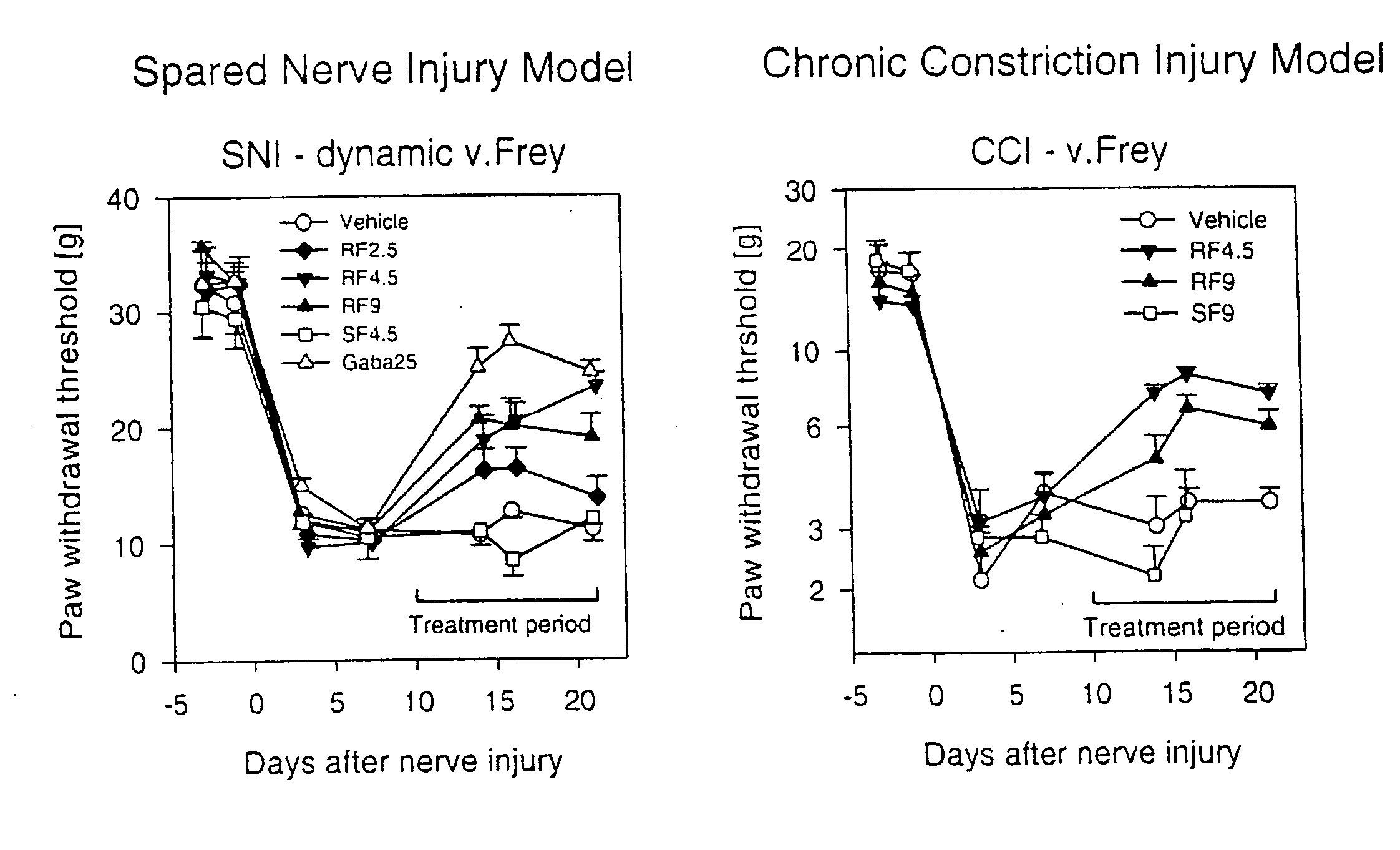
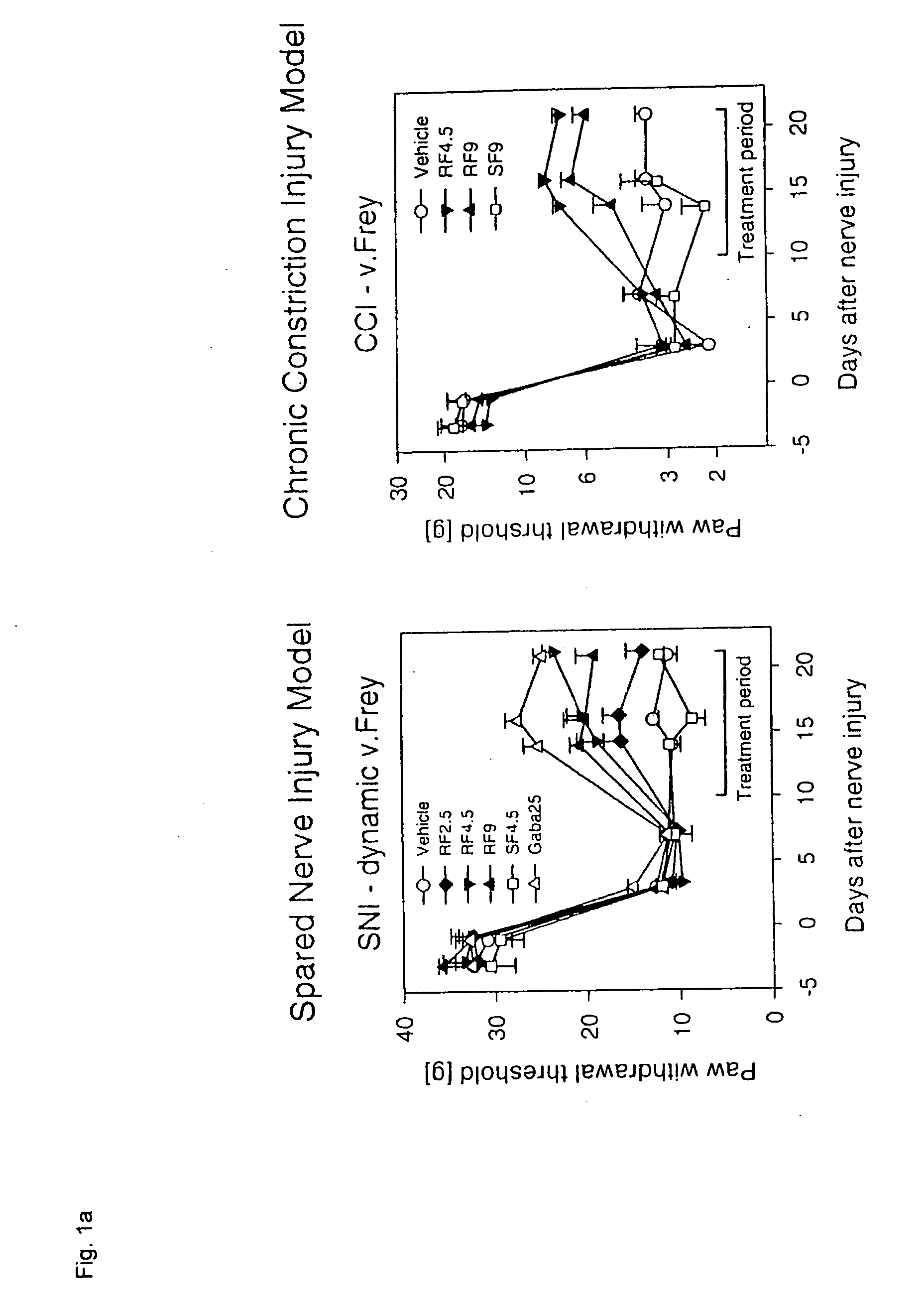
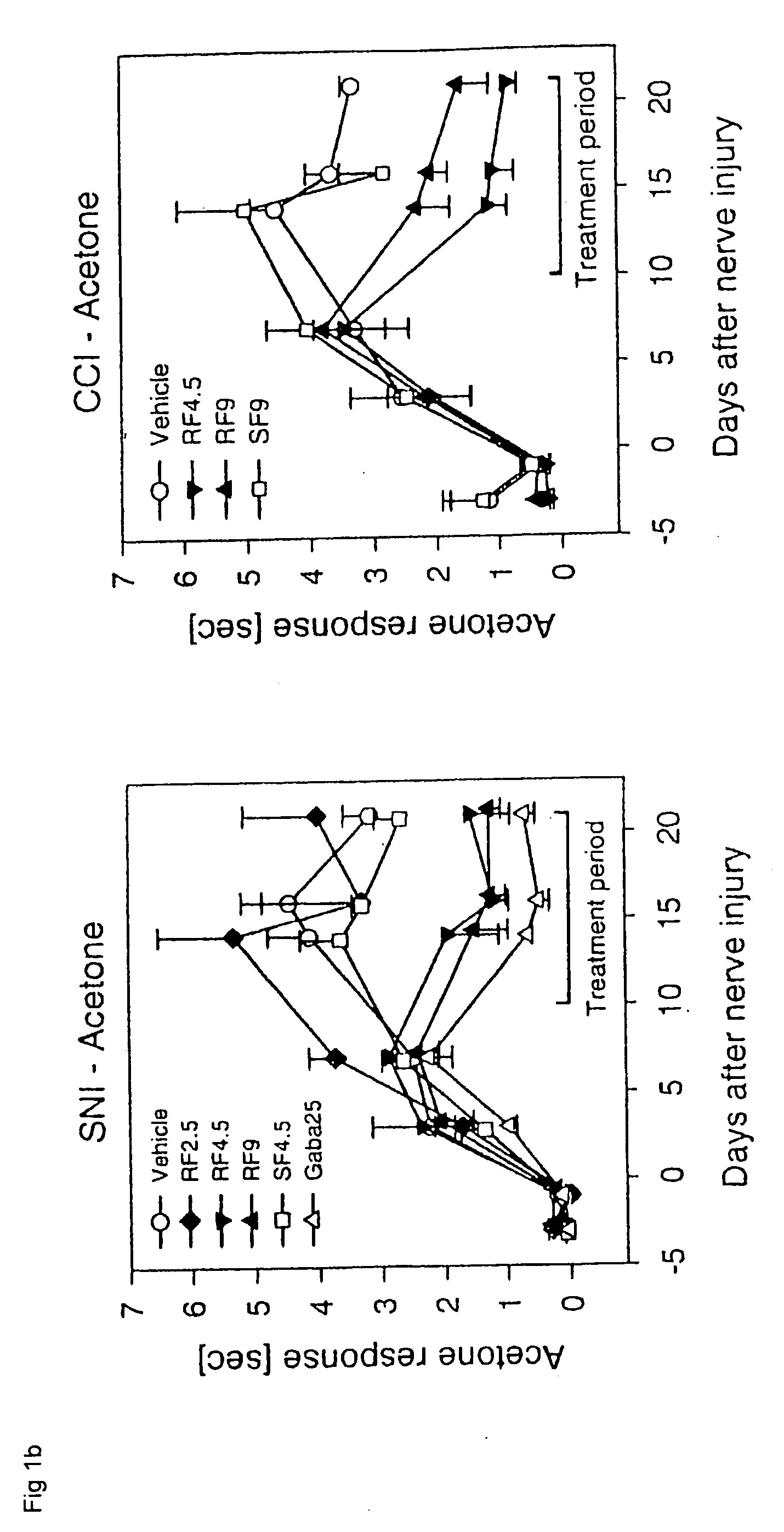
A dynamic bone stabilization system is provided. The system may be placed through small incisions and tubes. The system provides systems and methods of treating the spine, which eliminate pain and enable spinal motion, which effectively mimics that of a normally functioning spine. Methods are also provided for stabilizing the spine and for implanting the subject systems.
Owner:EXACTECH INC
Facial tissue strengthening and tightening device and methods
InactiveUS7494488B2Improve efficacyImprove securityUltrasound therapyElectrotherapyForms of energyFacial tissue
A device is described that can be used quickly and accurately by surgeons to provide uniform facial tissue planes that are tunnel-free and wall-free thus optimizing face lifting, tightening, and implant delivery. The device is comprised of a shaft with a substantially planar tip further comprised of relative protrusions and energized relative recession lysing segments. Forward motion of the device precisely divides and energizes various tissue planes causing contraction, especially via the fibrous tissues. Other forms of energy and matter can be delivered down the shaft to further enhance desirable tissue modification and contraction.
Owner:WEBER PAUL J
Pelvic disorder treatment device
InactiveUS6862480B2Reduce decreaseRelieving pelvic painUltrasonic/sonic/infrasonic diagnosticsElectrotherapyInterstitial cystitisFecal incontinence
A device for treating a medical condition is provided, and a surgical procedure for implanting the device is disclosed. The device includes a sensor, which is adapted to generate a signal responsive to a state of a patient, and at least one electrode, which is adapted to be coupled to a pelvic site of the patient. A control unit is adapted to receive the signal, to analyze the signal so as to distinguish between an imminent stress incontinence event and an imminent urge event, and, responsive to analyzing the signal, to apply an electrical waveform to the at least one electrode. In various configurations, the device may be used alternatively or additionally to treat fecal incontinence, interstitial cystitis, chronic pelvic pain, or urine retention.
Owner:ASTORA WOMENS HEALTH
Remotely enabled pacemaker and implantable subcutaneous cardioverter/defibrillator system
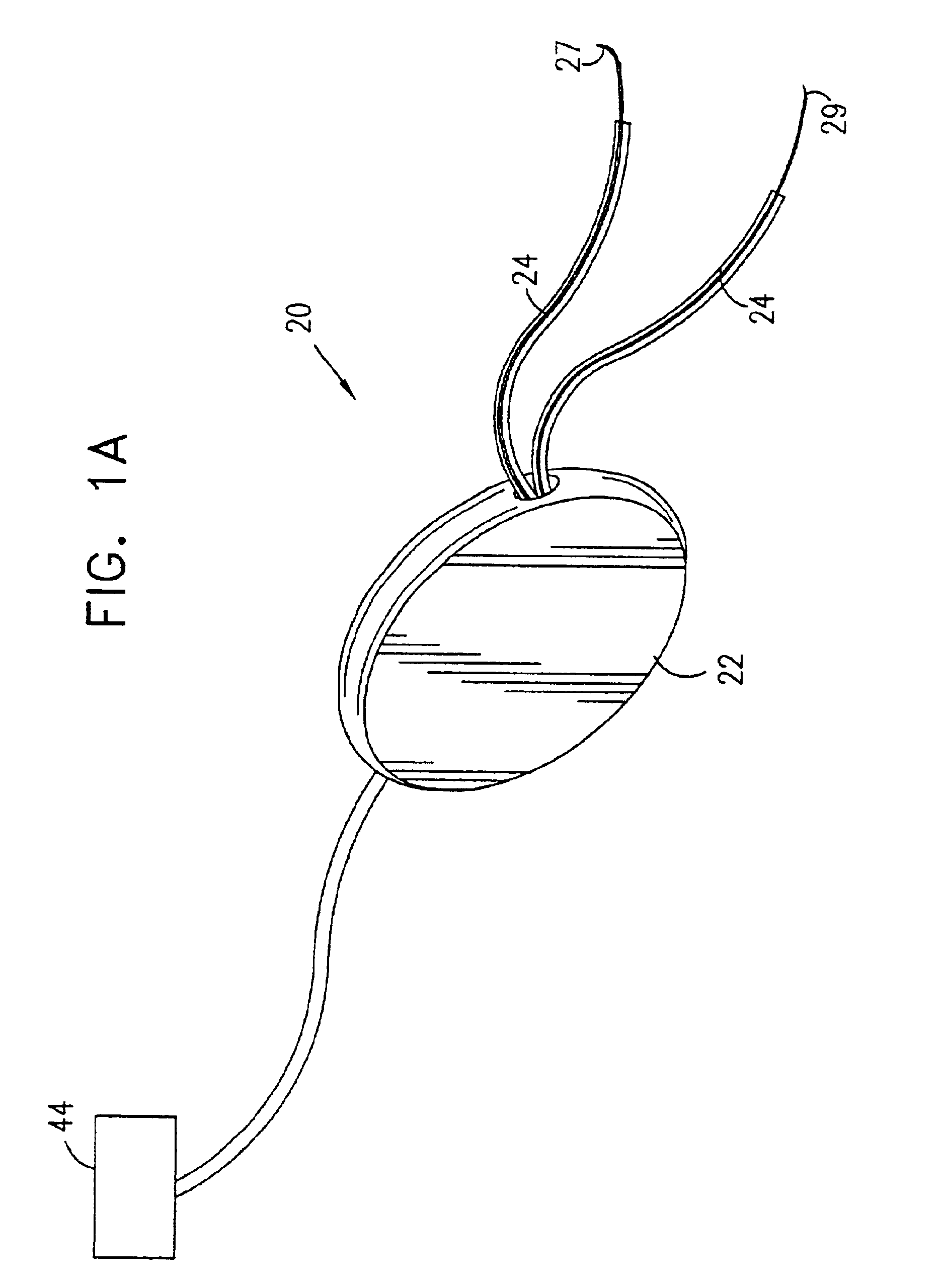
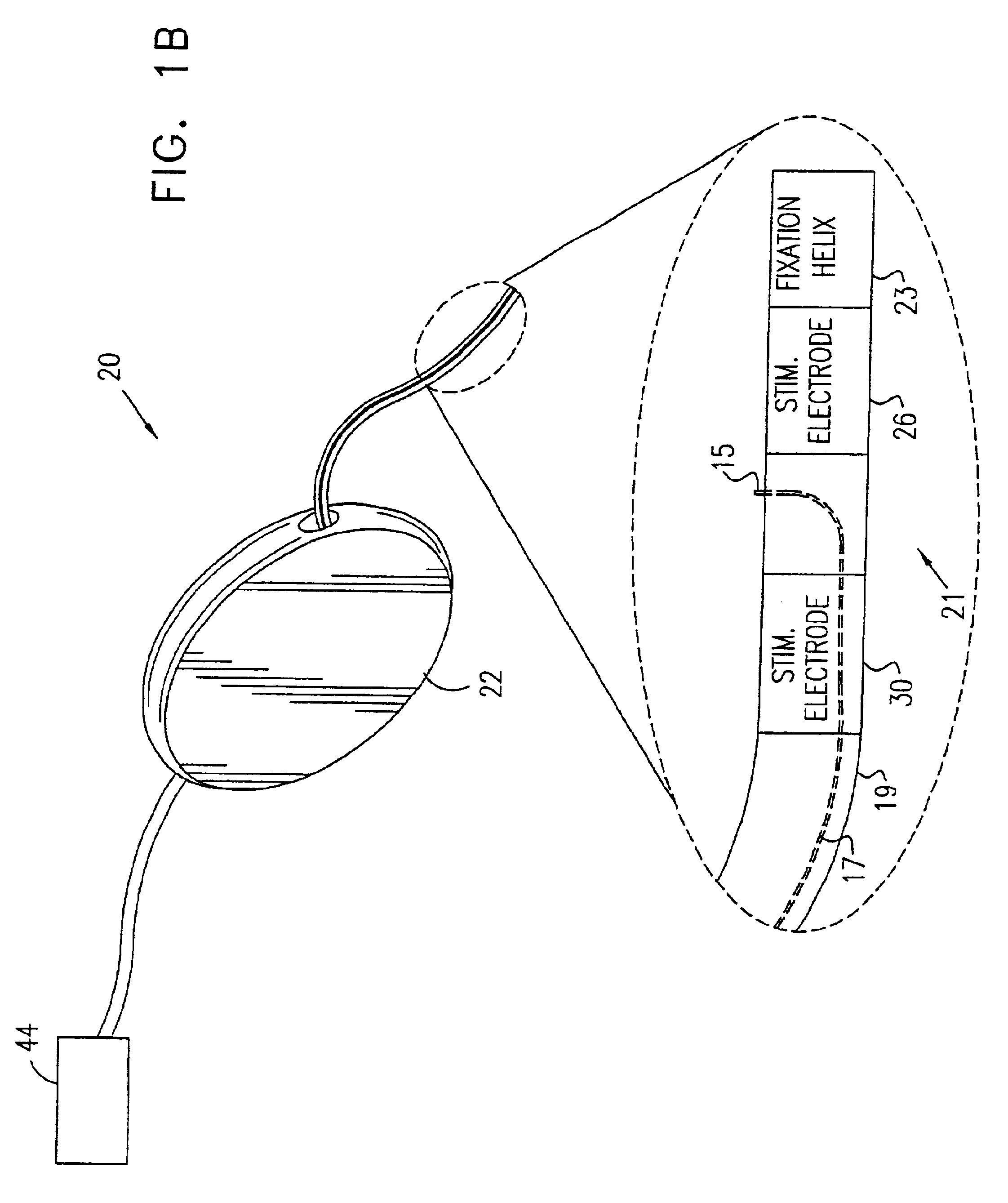
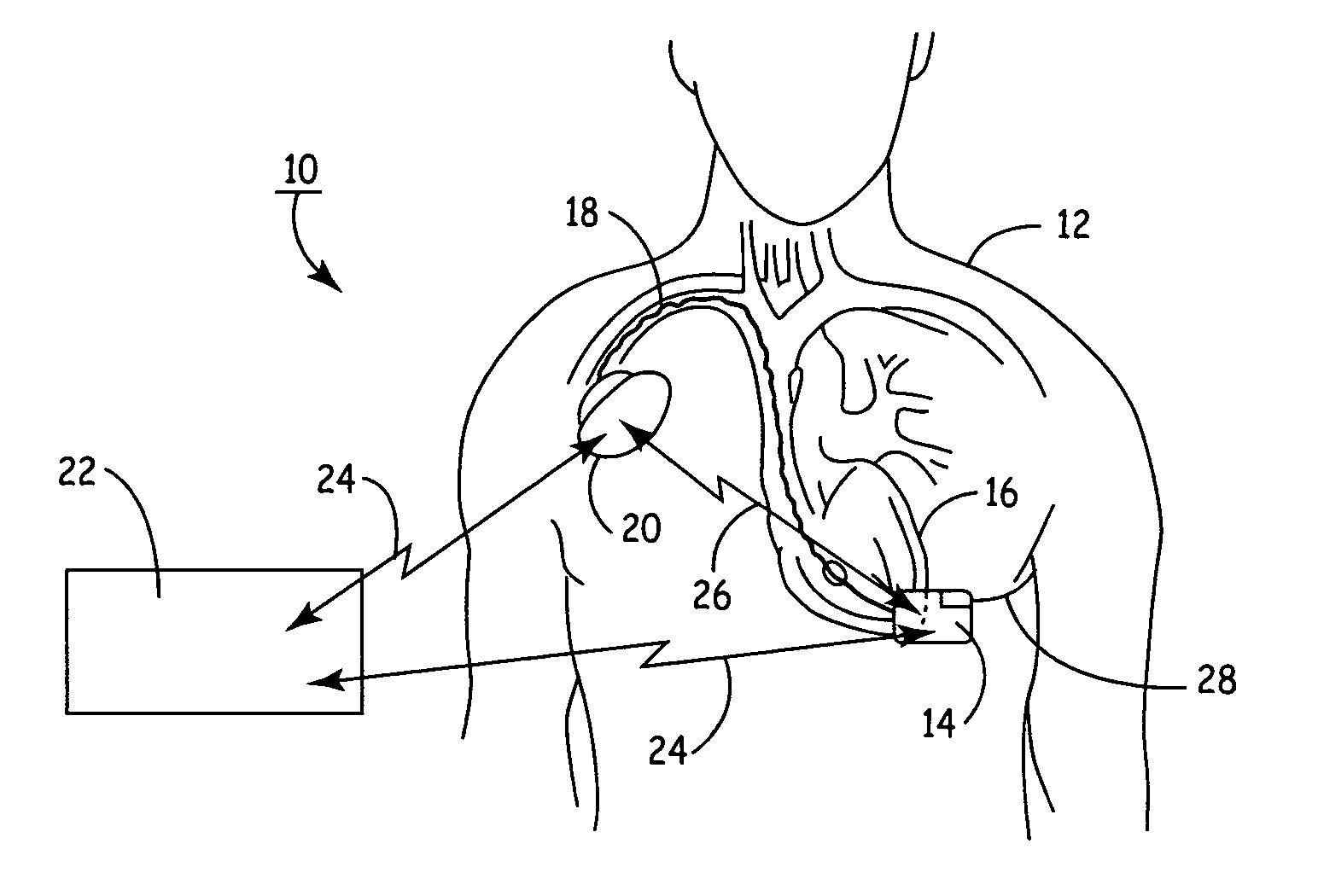
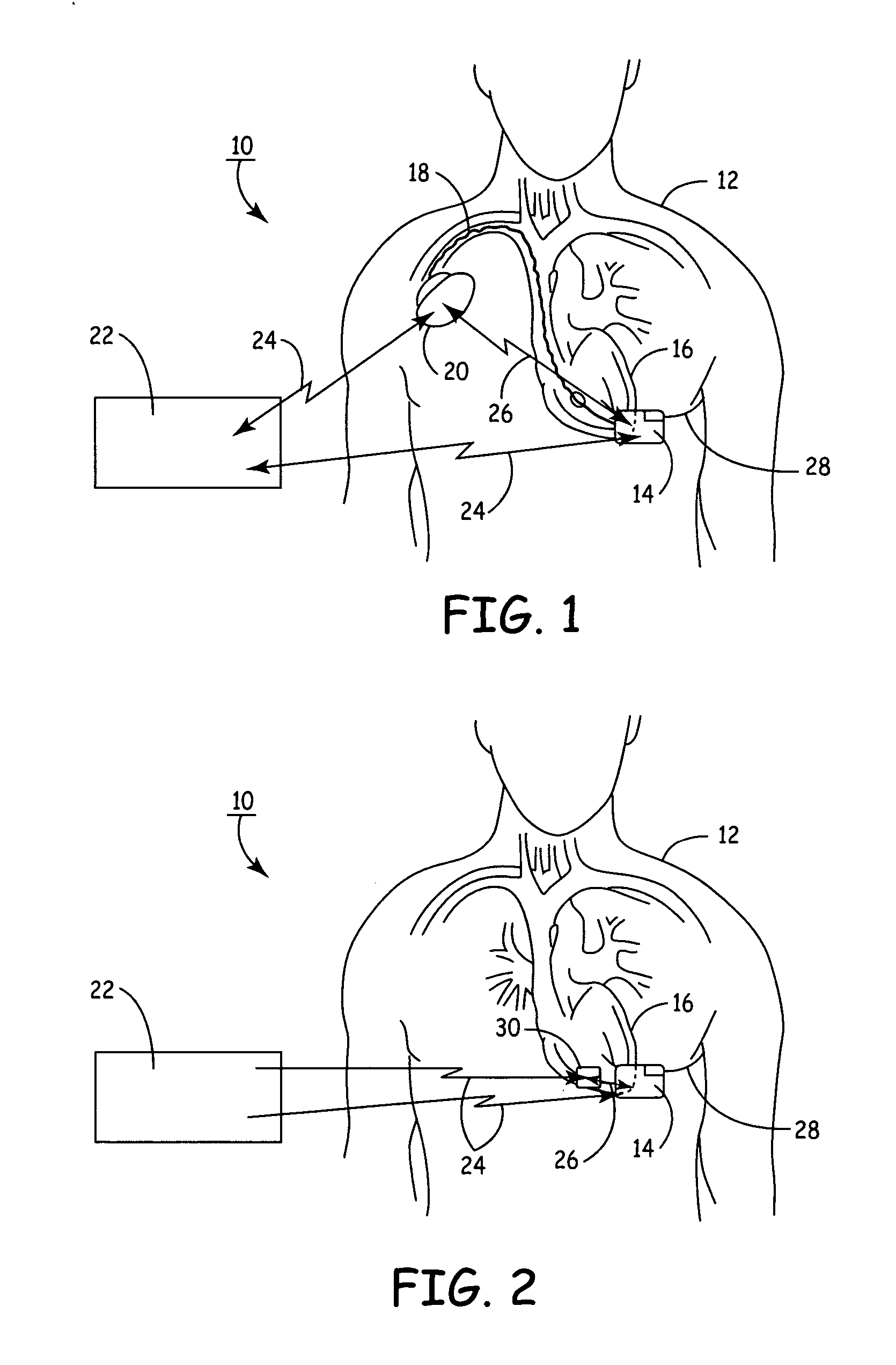
ActiveUS20060241701A1Relieve painSafe and effective operationHeart defibrillatorsSubcutaneous implantationCardiac pacemaker electrode
Subcutaneous Implantable cardioverter-defibrillators (SubQ ICDS) are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. The SubQ ICD is implemented with other implantable and external medical devices and communicates to provide drugs and therapy in a coordinated and synergistic manner.
Owner:MEDTRONIC INC
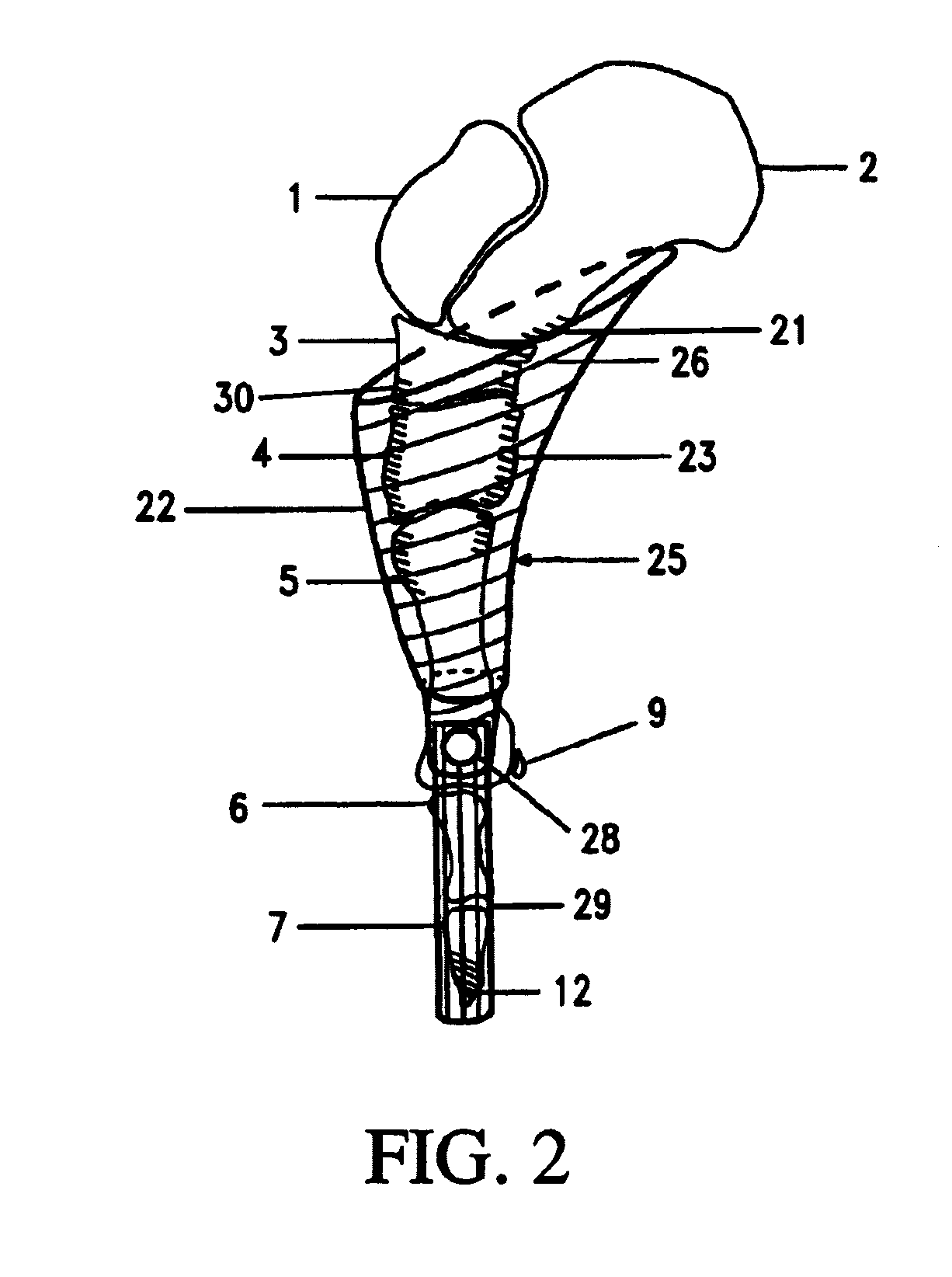
Prosthetic acetabular cup and prosthetic femoral joint incorporating such a cup
ActiveUS20050060040A1Natural angular movementReduce the possibility of misalignmentInternal osteosythesisJoint implantsSpherical bearingProsthesis
An acetabular prosthesis having an outer member for engaging the acetabulum. The outer member has a part-spherical bearing surface terminating in a distal rim. The rim has a contour such that the portion thereof to be located between the ischium and the pubis extends distally further from an equator of the bearing surface than the contour to be implanted between the pubis and the illium and between the ischium and the illium.
Owner:STRYKER EURO OPERATIONS HLDG LLC
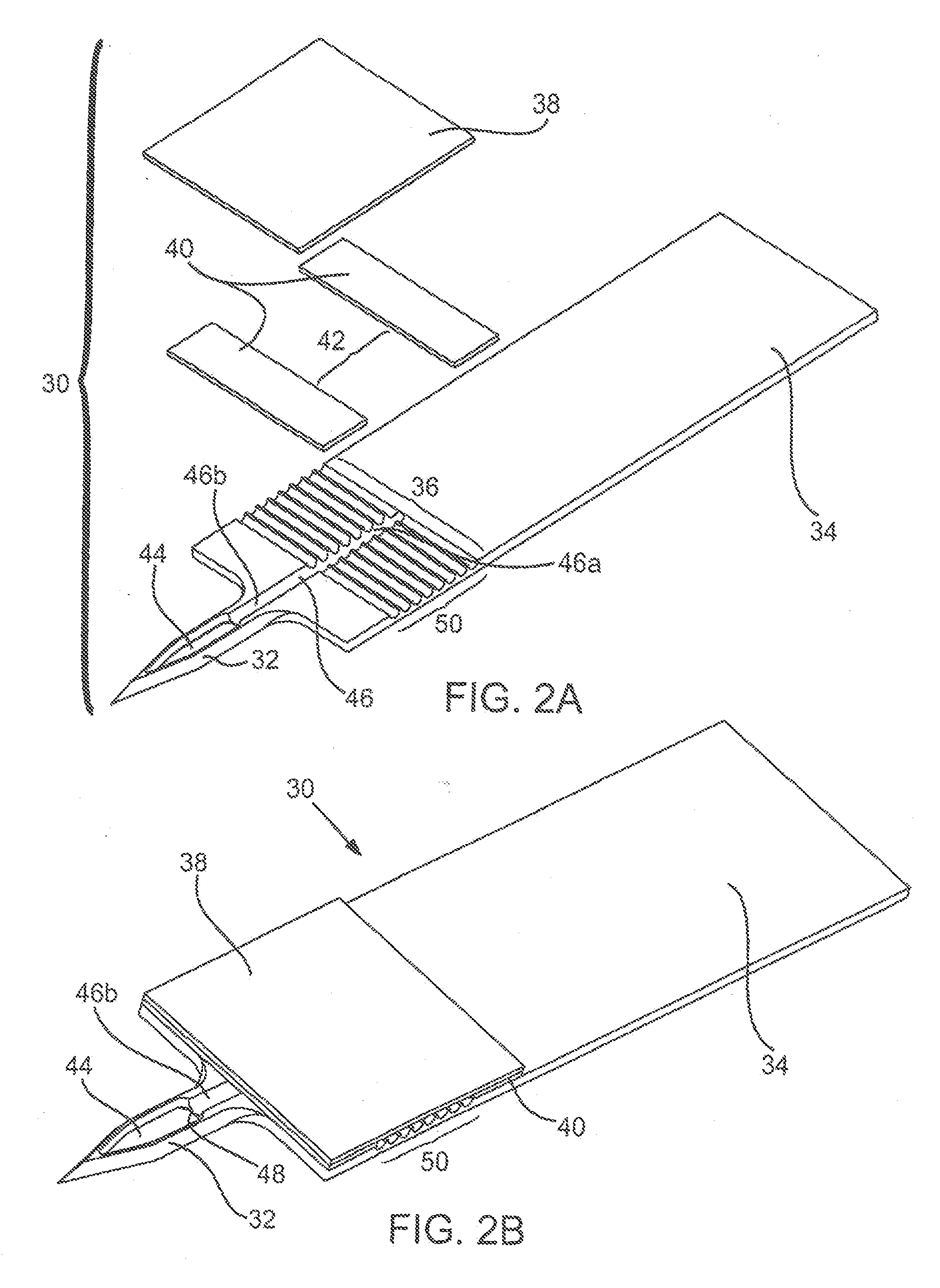
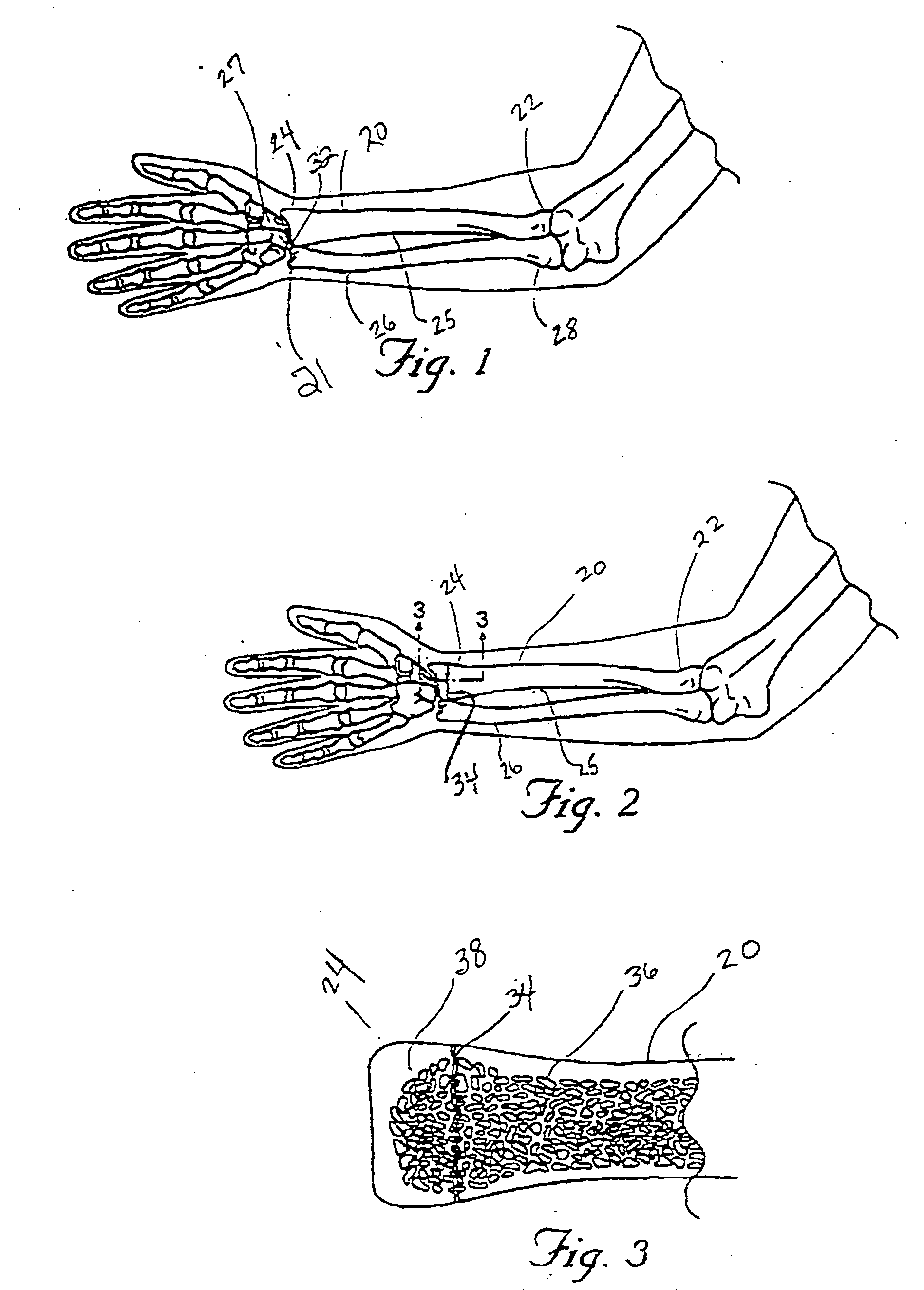
Drug depot implant designs and methods of implantation
ActiveUS20070243225A1Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injurySacroiliac joint
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
Devices and methods for accessing and analyzing physiological fluid
InactiveUS7343188B2Convenient angleReduce painSensorsBiological testingLiquid glucosePhysiological fluid
Systems, devices and methods for determining the concentration of physiological fluid analytes are provided. The subject systems have a plurality of biosensor devices present on a disposable cartridge. Each biosensor device includes a biosensor and a skin penetration means. In practicing the subject methods, a movement means of the device is used to move each biosensor device in a first direction that provides for penetration of the skin-piercing means into a skin layer followed by movement of the biosensor in a second direction that provides for removal of the skin-piercing means from the skin layer, where this movement profile provides for physiological fluid access and analyte concentration determination by the analyte sensor means. The subject systems, devices and methods for using the same find use in determining the concentration of a variety of different physiological fluid analytes, and are particularly suited for use in detection of physiological fluid glucose concentration.
Owner:LIFESCAN IP HLDG LLC
Drug depot implant designs and methods of implantation
ActiveUS20070243228A1Uniform drug distributionMinimal disruptionBiocidePeptide/protein ingredientsSkeletal injurySacroiliac joint
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
Lancet for blood collection and puncture needle unit
InactiveUS20050131441A1Safely discardedEasy to useSensorsBlood sampling devicesBlood collectionEngineering
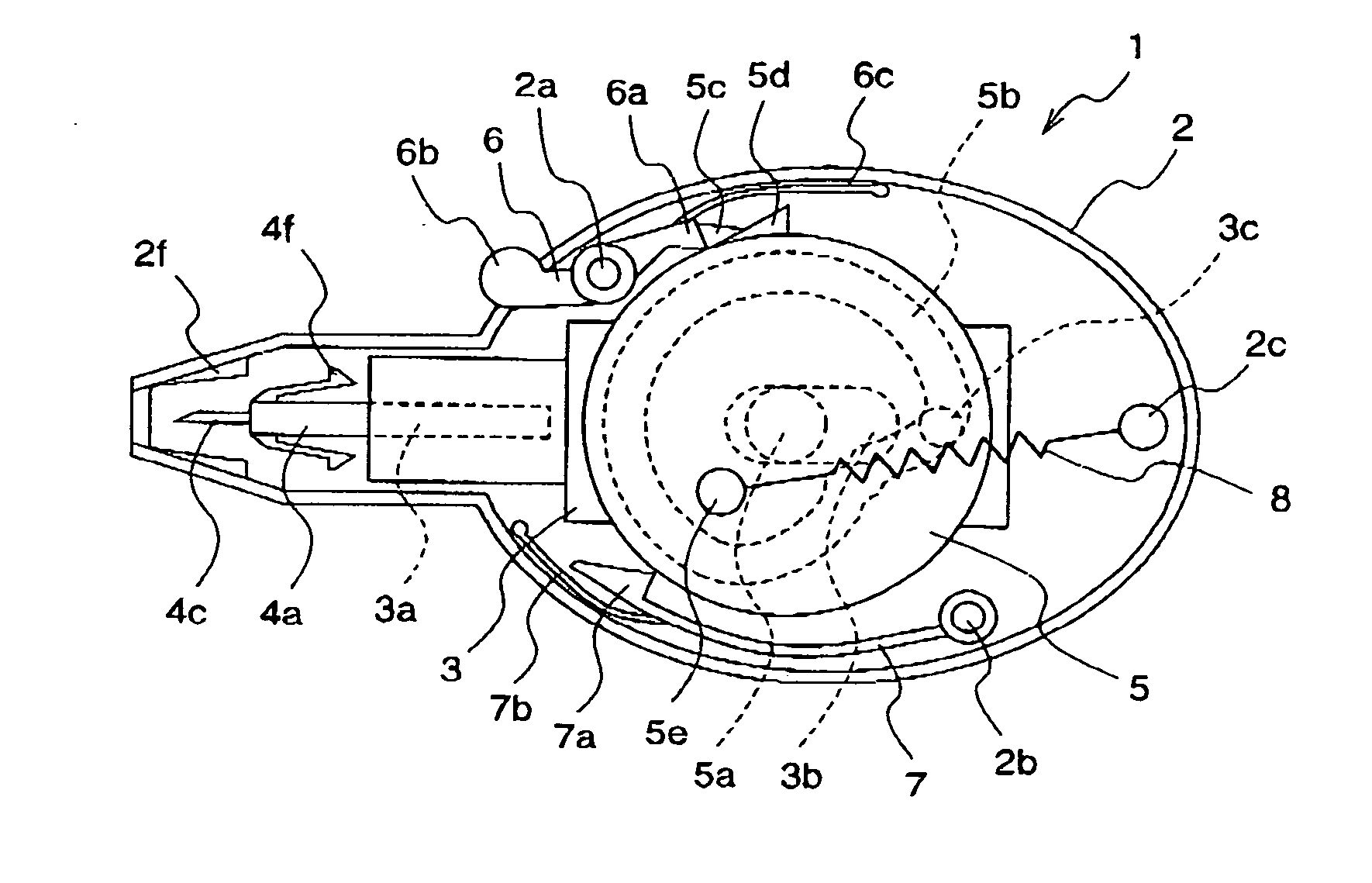
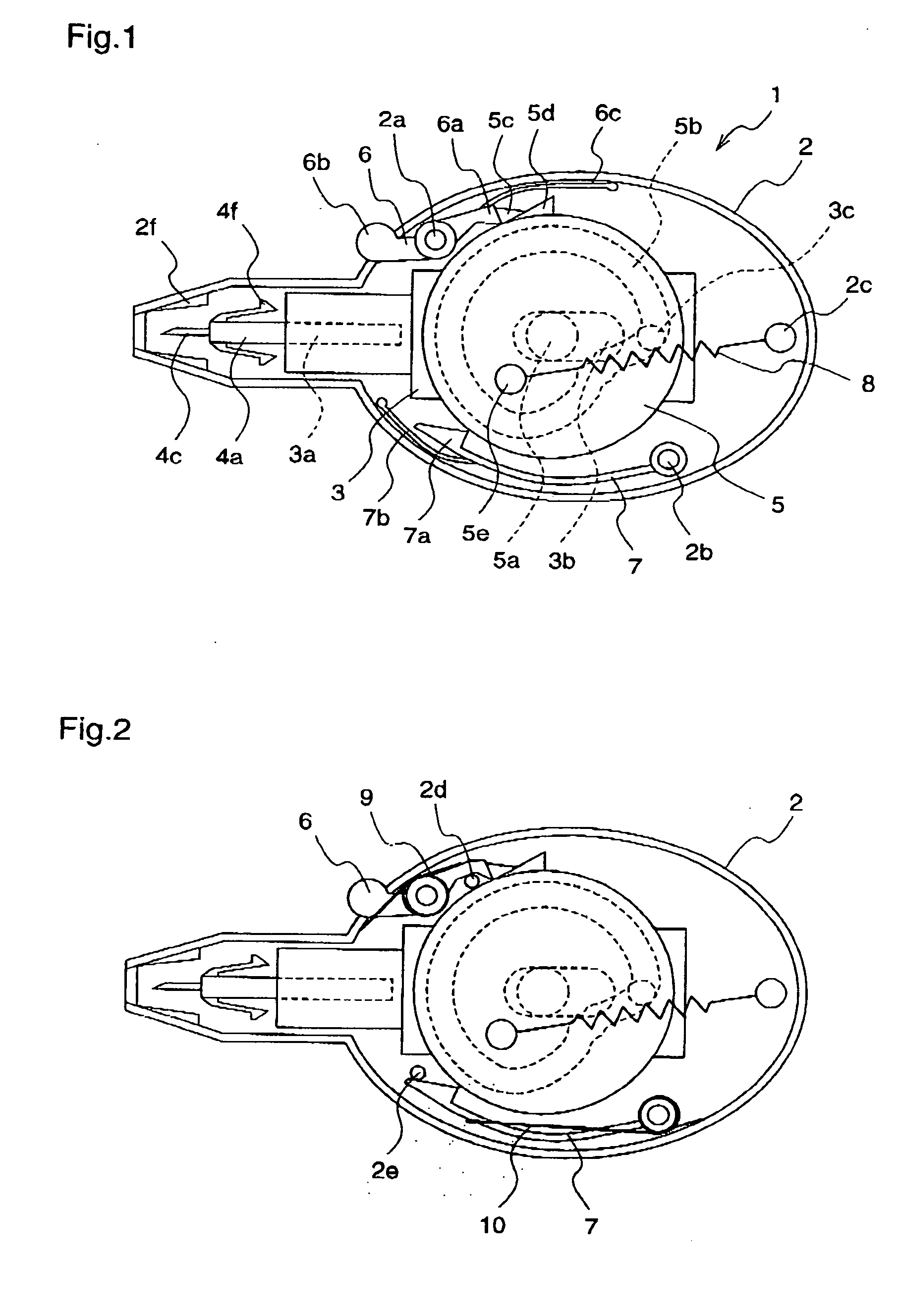
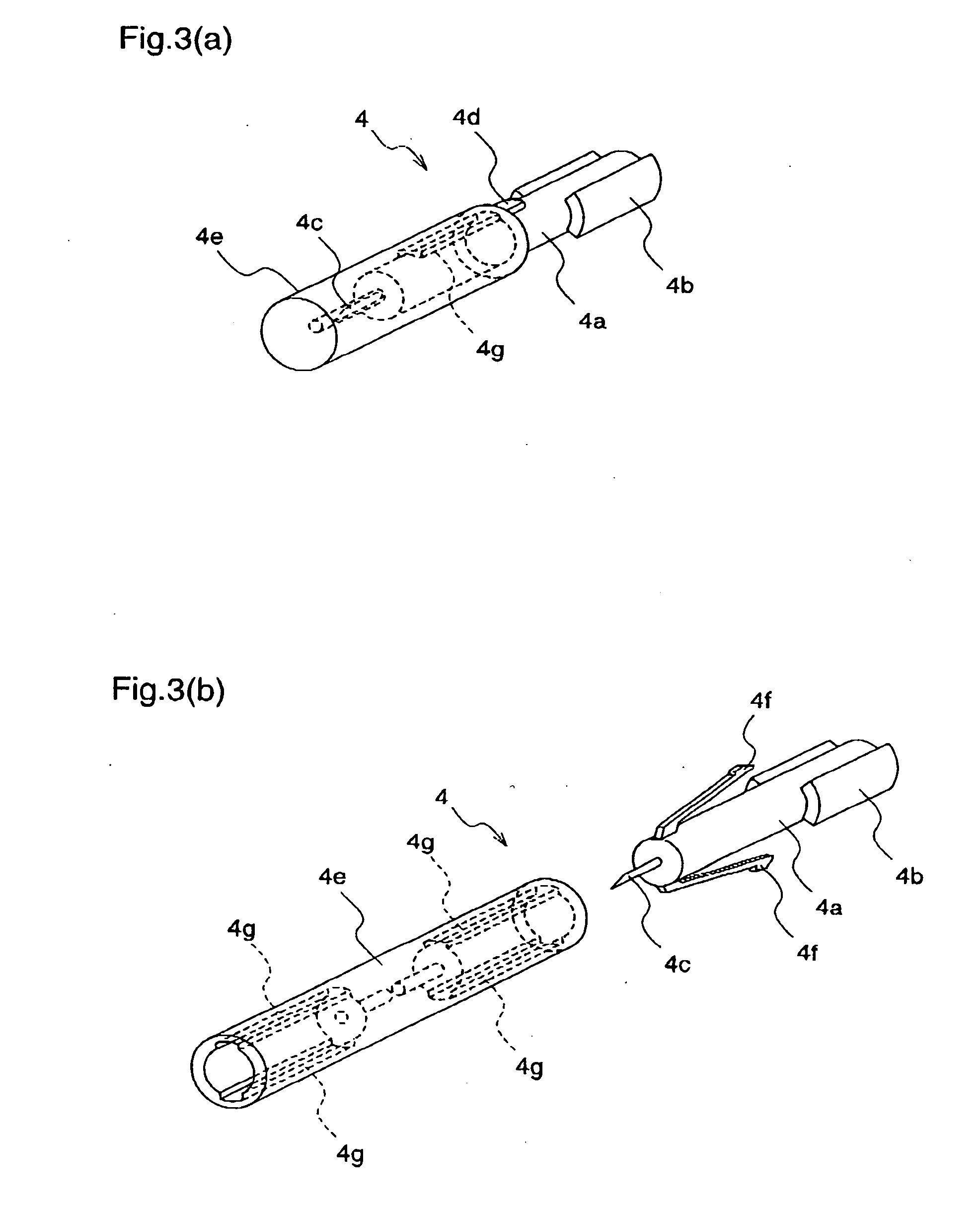
A lancet comprises a slider having a puncture needle holding mechanism at its one end; a cam ring which has a continuous cam groove, is rotatable about a support shaft, and has a cam ring claw and an anti-return claw for restricting the rotation; a ring spring for applying a force to rotate the cam ring; a rotatable stopper arm for holding and releasing the rotation of the cam ring; and a rotatable ratchet for restricting the direction of rotation of the cam ring. A puncture needle unit comprises a puncture needle body which is integrally molded with a protrusion to be fitted to the lancet, a rotation stop rib, and a puncture needle; and a puncture needle cap which is lightly pressed into the puncture needle body.
Owner:PHC HLDG CORP
Apparatus and method for wound, cavity, and bone treatment
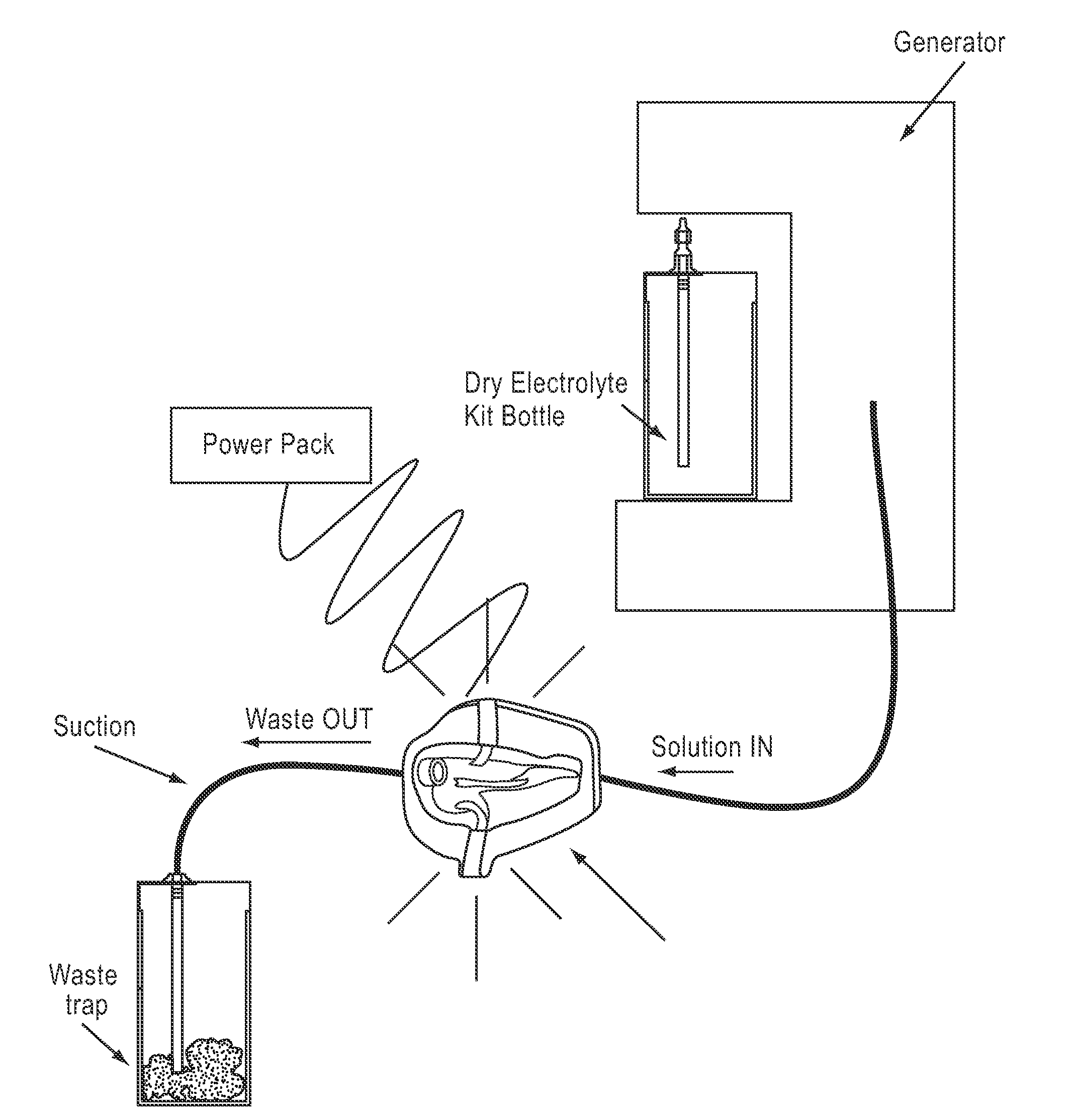
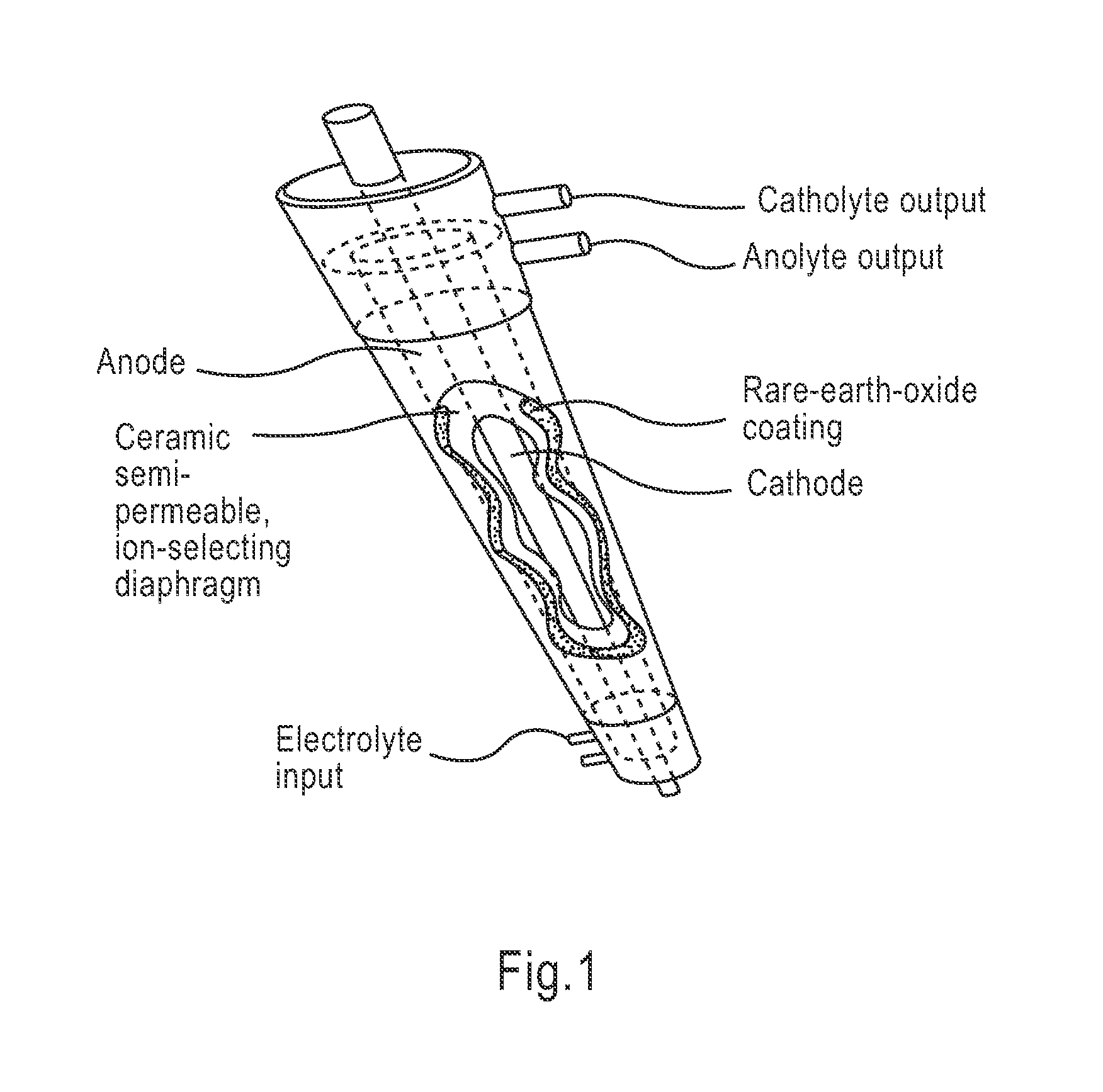
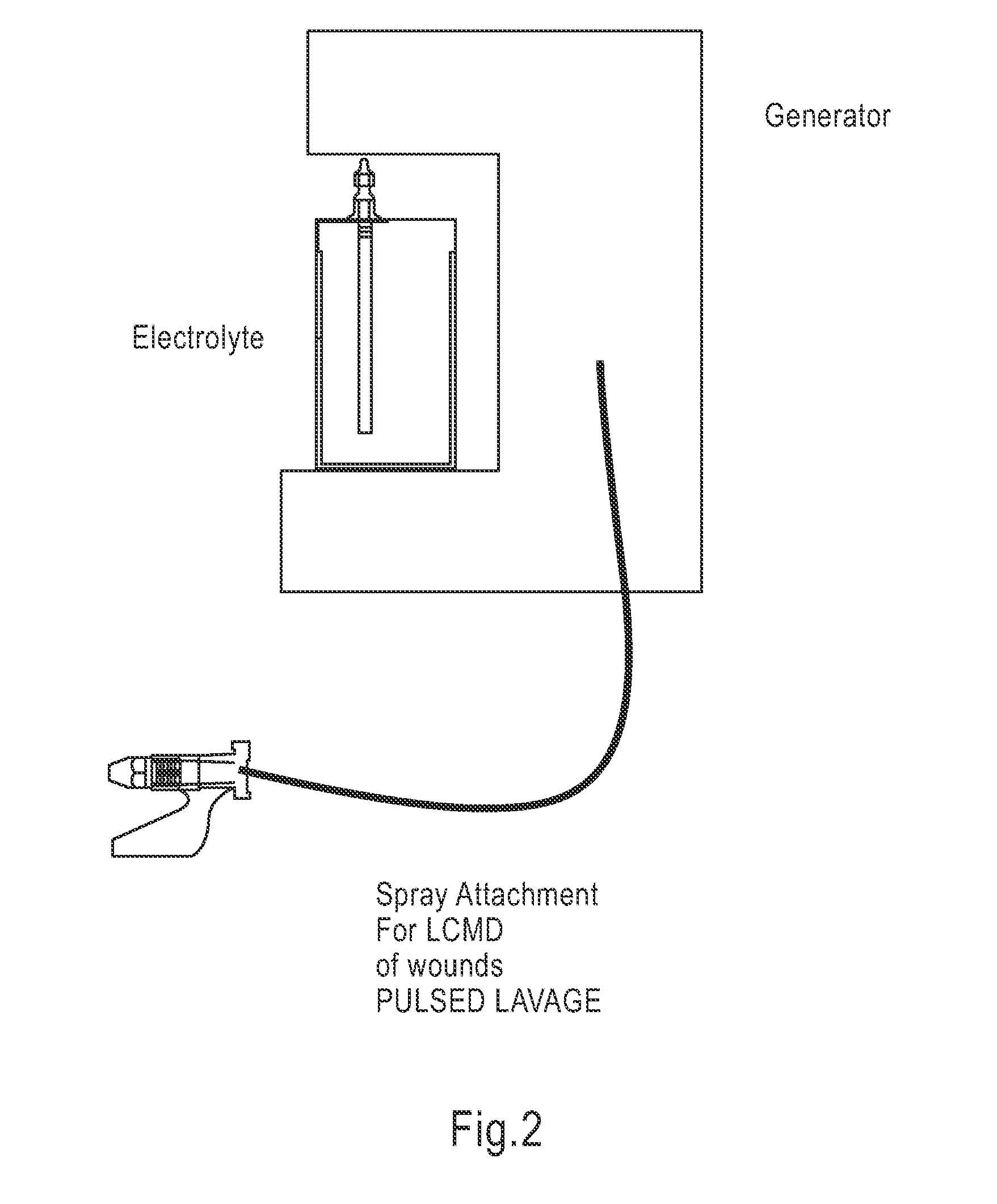
InactiveUS20100030132A1Quicker wound closureSafer wound care environmentElectrolysis componentsCannulasNeed treatmentBiomedical engineering
The present invention provides a treatment apparatus. The apparatus contains a reservoir or generator for a treatment solution, a mechanism for delivering the treatment solution to a wound site, and a mechanism for applying the solution to a wound, tissue, bone or surgical cavity for treatment. The apparatus may apply the solution (e.g., a solution containing hypohalous acid) with, for example, an occlusive wound dressing, pulsative lavage device, hydrotherapy, hydrosurgical device, and / or ultrasound. A waste container may be operably connected to the apparatus for collecting waste from the wound by run-off, or by applying negative pressure (e.g. a vacuum). Because the apparatus of the invention can optionally be portable or mobile, the invention is suitable for use in hospitals and nursing homes, as well as for home wound care. The invention also provides a method for treating a wound (or other area needing treatment), and / or for reducing wound bioburden, by supplying a hypochlorous acid solution to the site, such as a wound colonized or infected with drug resistant bacteria, before, during, or after negative pressure wound therapy.
Owner:PURICORE
Lancet device and method
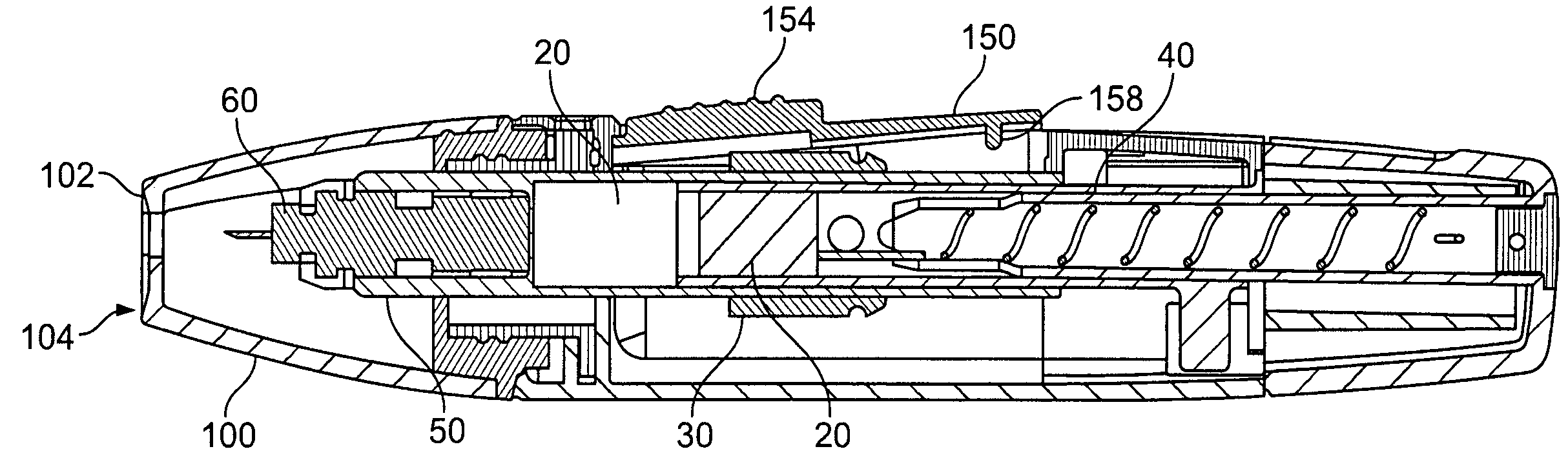
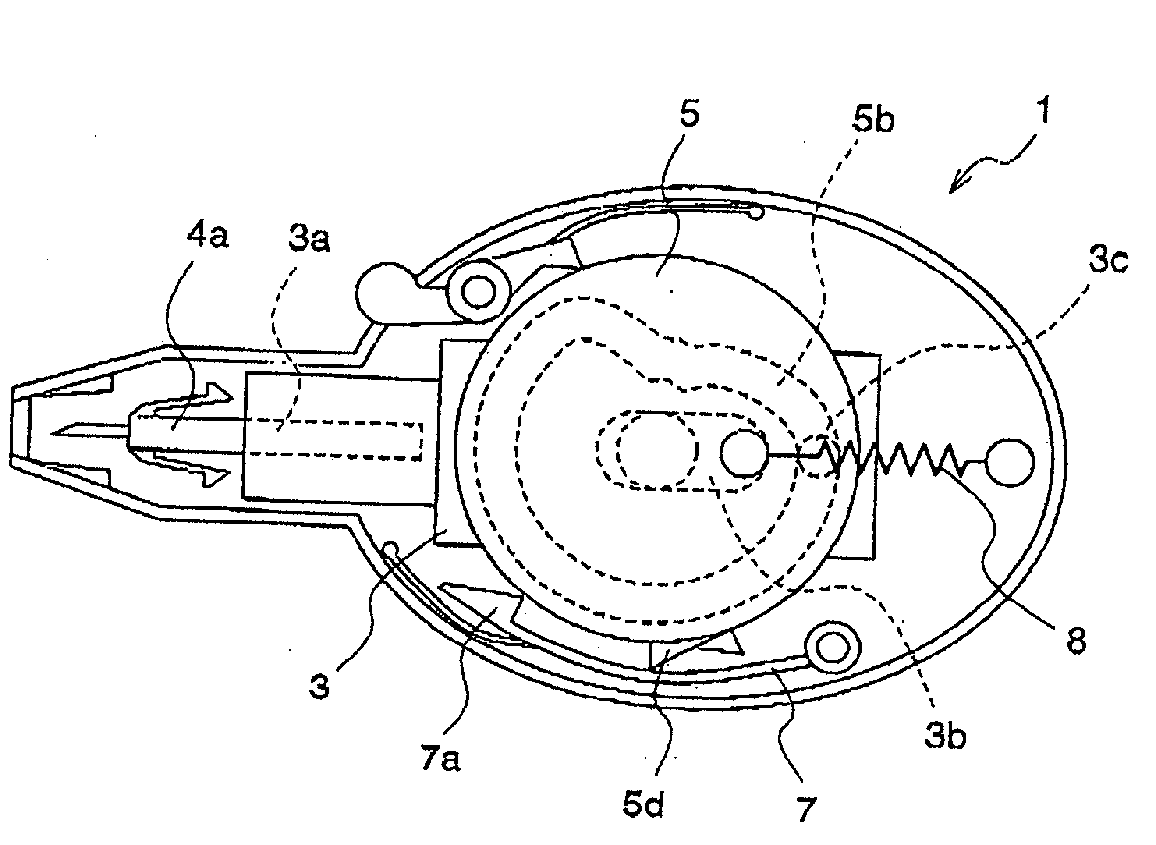
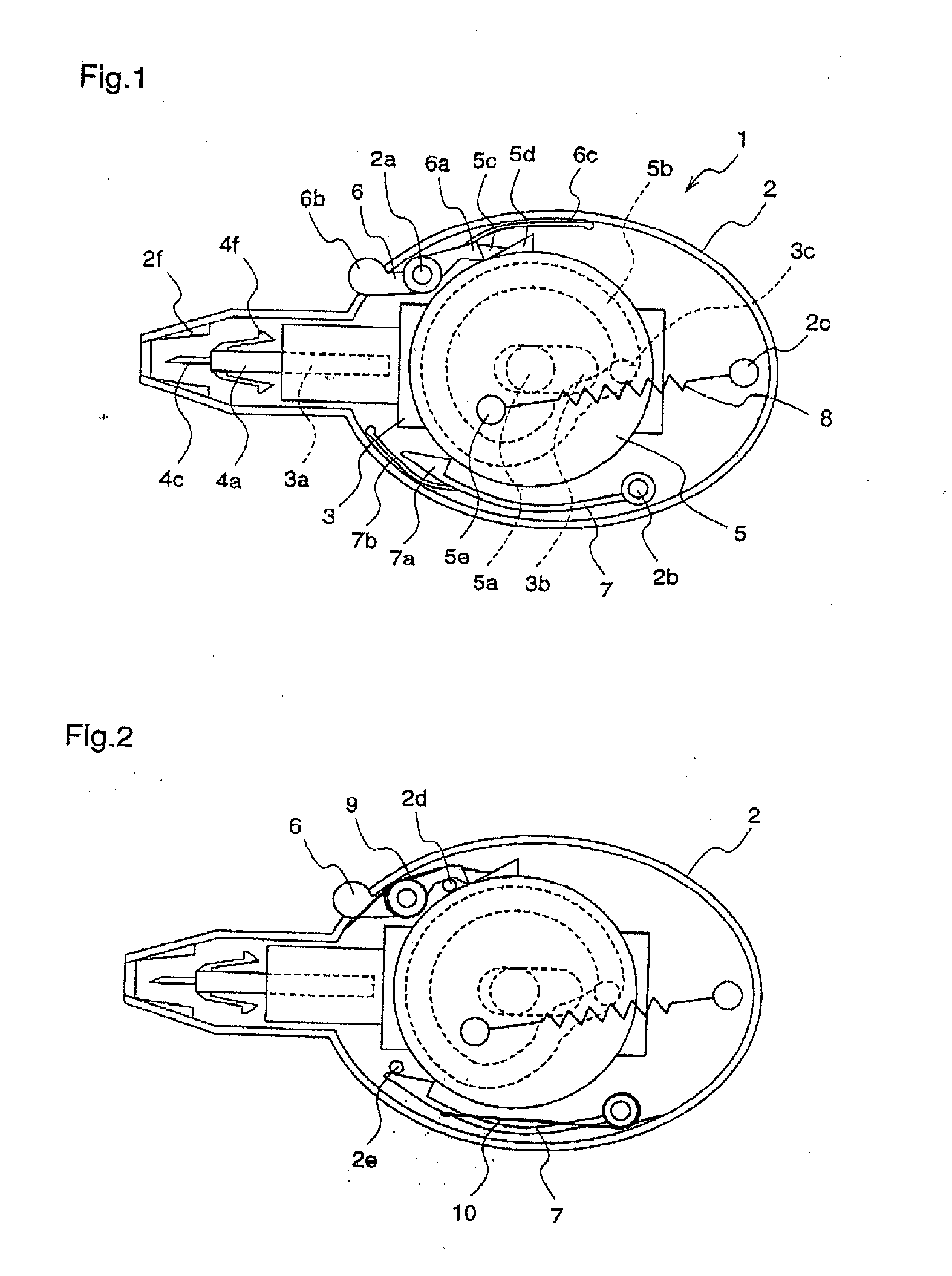
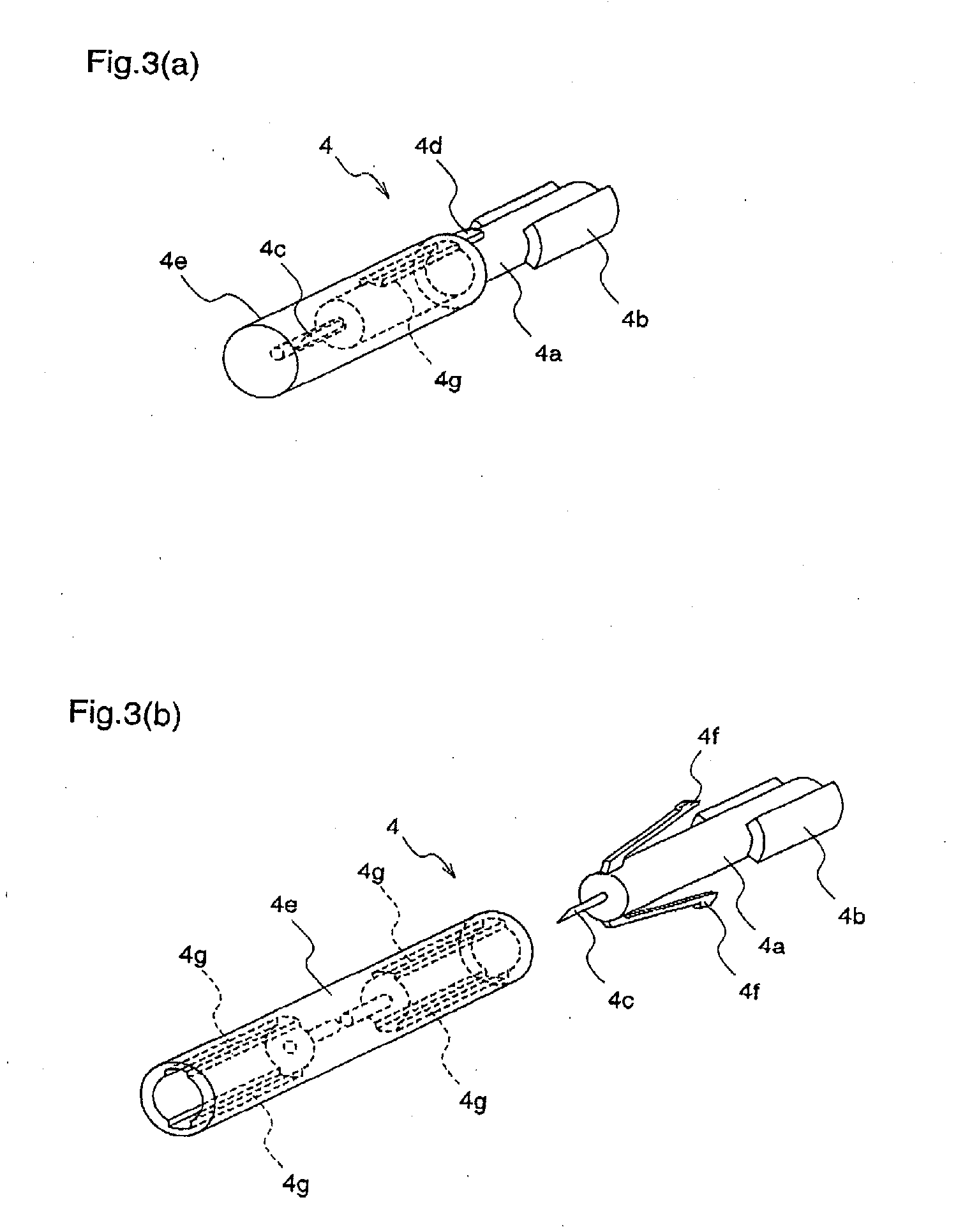
InactiveUS20050125019A1Reduce painEasily adjust and controlCatheterExcision instrumentsPuncturingMagnet
A lancet device (10) having an internal magnet (20) and the forces emanating therefrom driving and retracting a collar (30) and a lancet (60). The device has a steady state condition (SS) wherein the device is at rest and in equilibrium. The device can be armed and activated to puncture. Once puncturing has occurred the device returns to a steady state condition. A lancet method involves positioning both a magnet (20) and a member (30) capable of being affected thereby with a lancet (60) in communications with one of either the magnet or the member, positioning either the member (30) or the magnet (20) to an armed position wherein the magnetic forces from the magnetic affect the member, and releasing the one of either the member or the magnet from the armed position permitting movement between the member and magnet by at least, in part, the magnetic forces, resulting in the movement of the lancet from a withdrawn position to the piercing position.
Owner:VIROTEK LLC +1
Devices and Methods for Accessing and Analyzing Physiological Fluid
InactiveUS20080009768A1Irritation and pain be avoidRapidly and accurately detect occurrenceSensorsBiological testingSkin penetrationSubject specific
Systems, devices and methods for determining the concentration of physiological fluid analytes are provided. The subject systems have a plurality of biosensor devices present on a disposable cartridge. Each biosensor device includes a biosensor and a skin penetration means. In practicing the subject methods, a movement means of the device is used to move each biosensor device in a first direction that provides for penetration of the skin-piercing means into a skin layer followed by movement of the biosensor in a second direction that provides for removal of the skin-piercing means from the skin layer, where this movement profile provides for physiological fluid access and analyte concentration determination by the analyte sensor means. The subject systems, devices and methods for using the same find use in determining the concentration of a variety of different physiological fluid analytes, and are particularly suited for use in detection of physiological fluid glucose concentration.
Owner:LIFESCAN INC
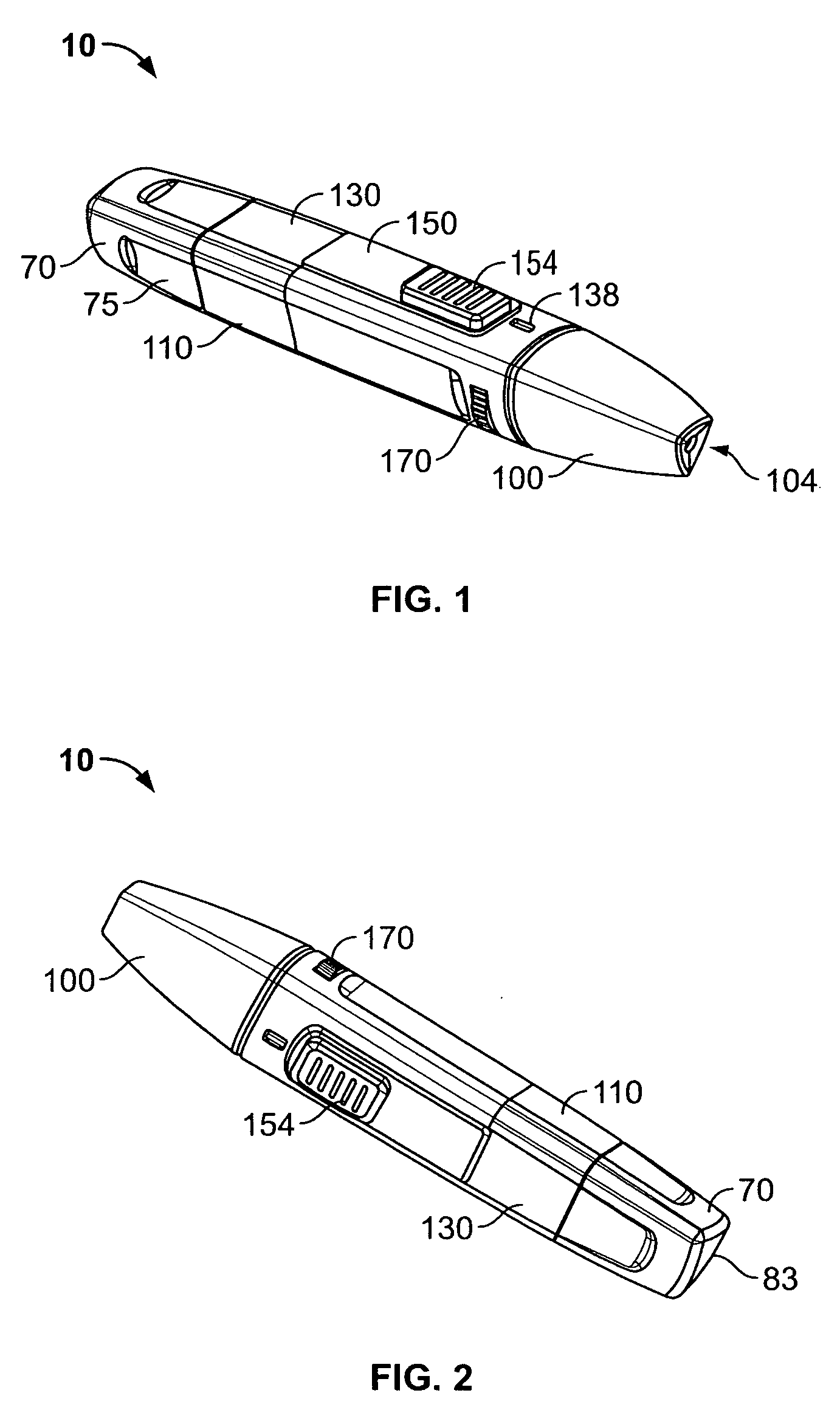
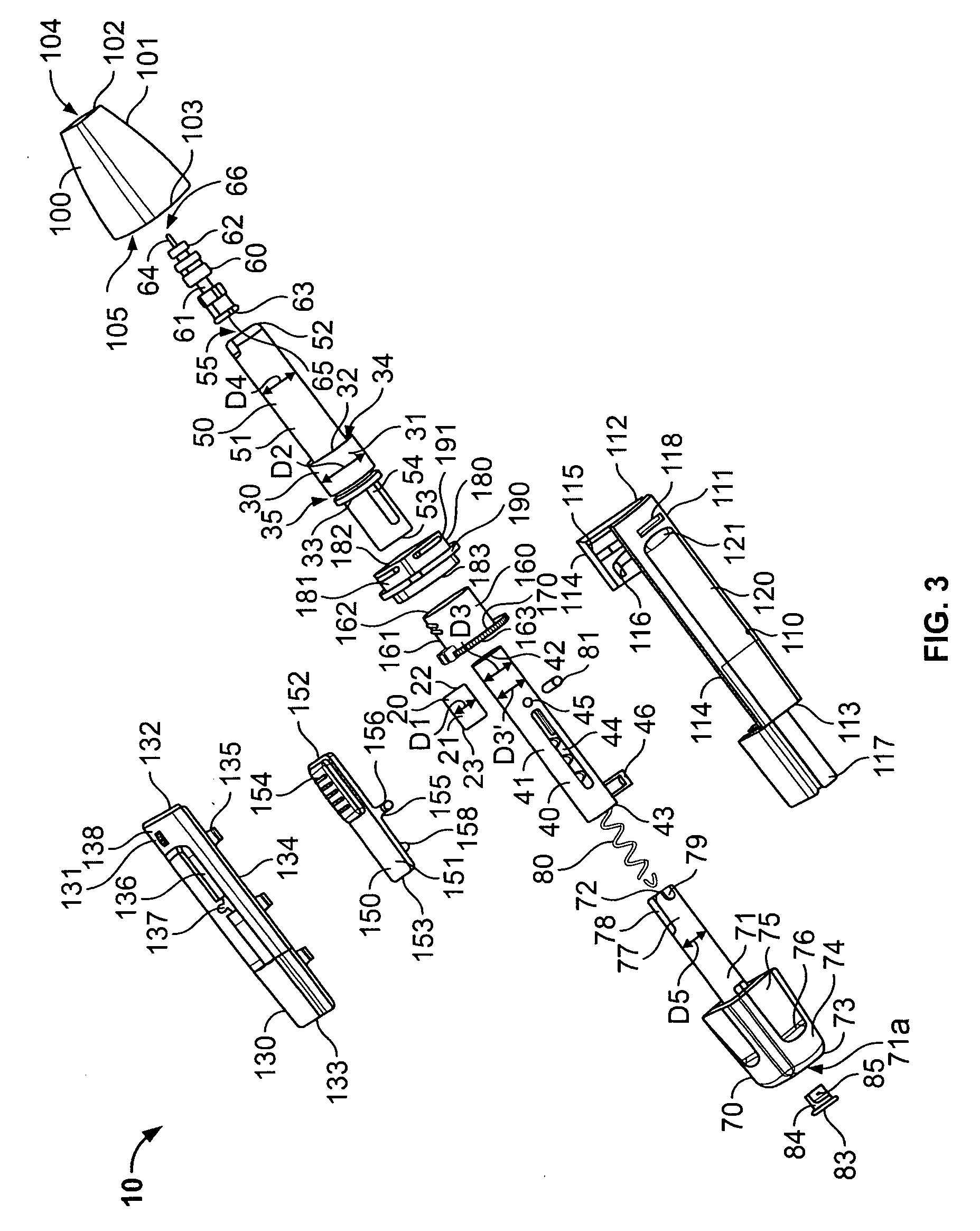
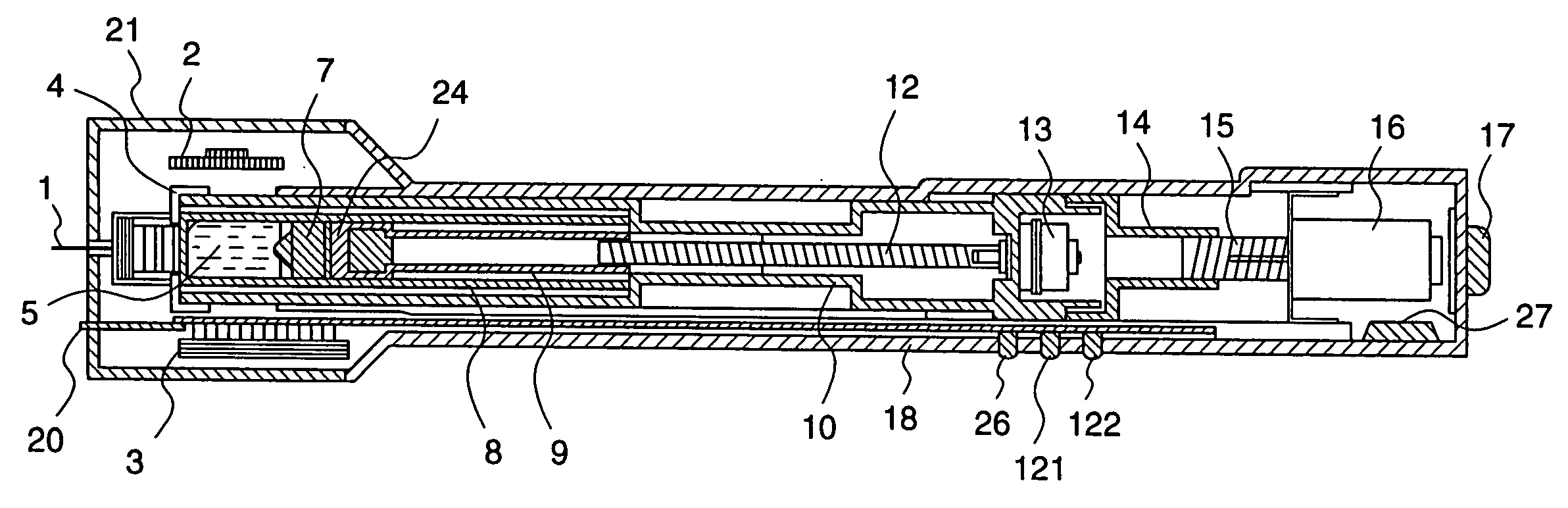
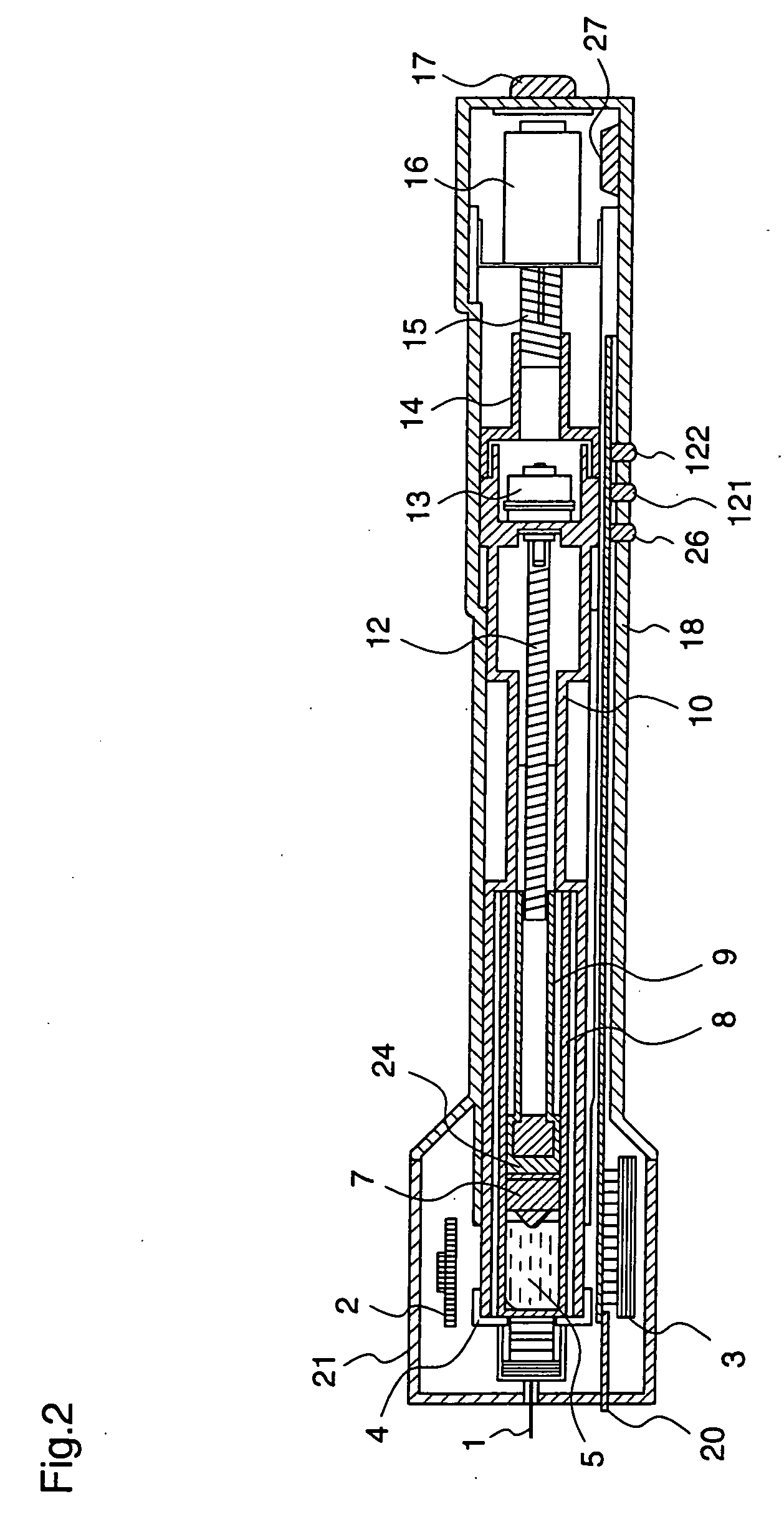
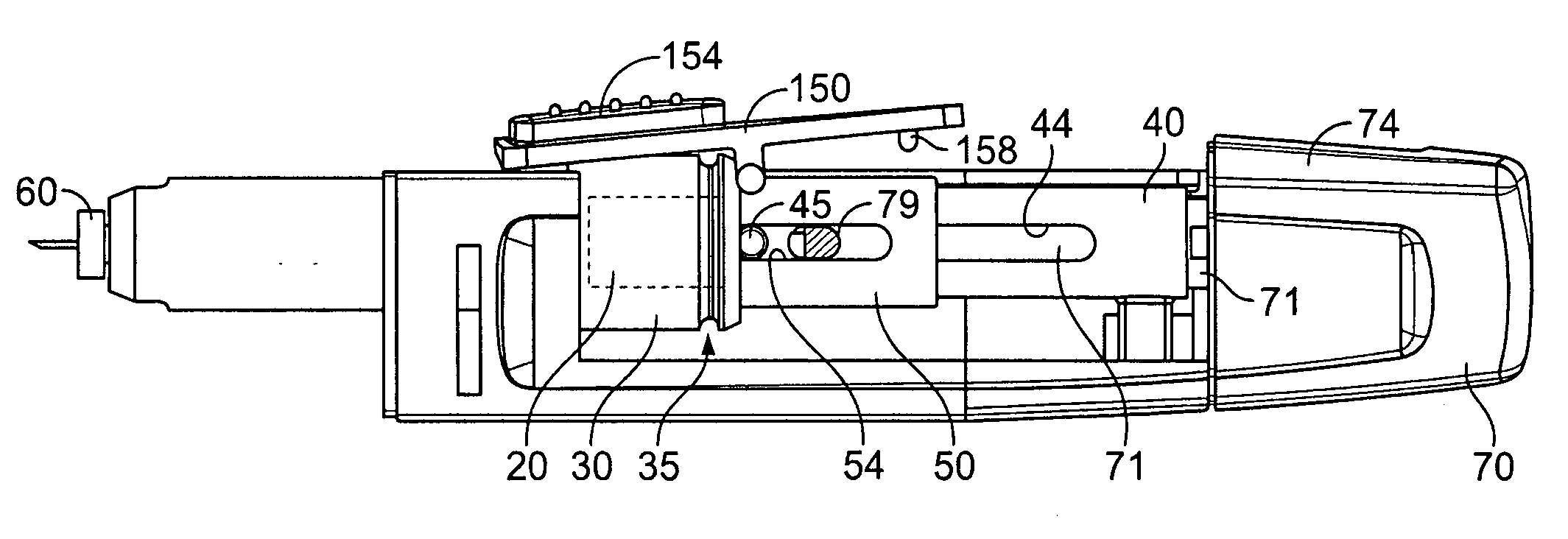
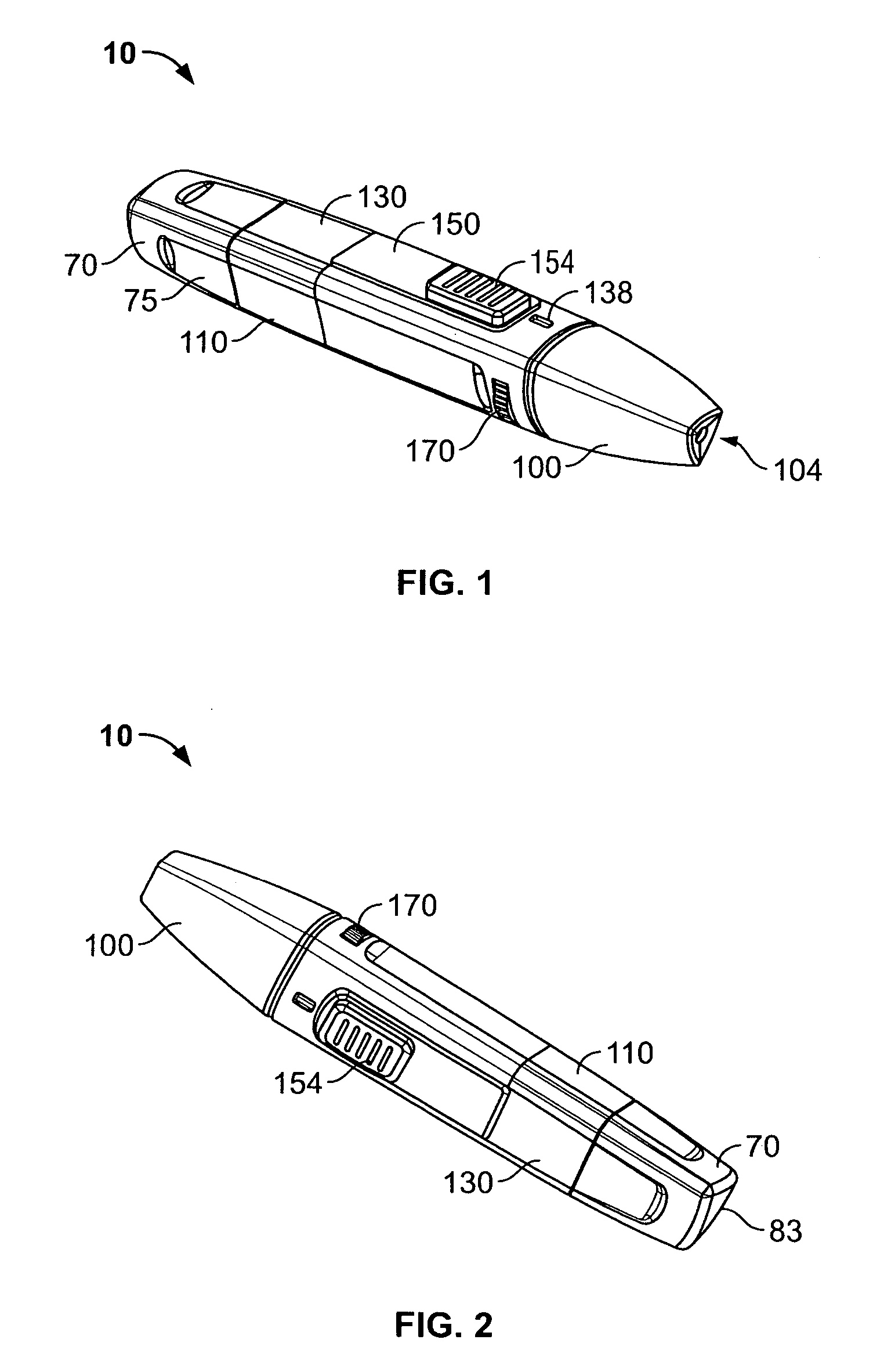
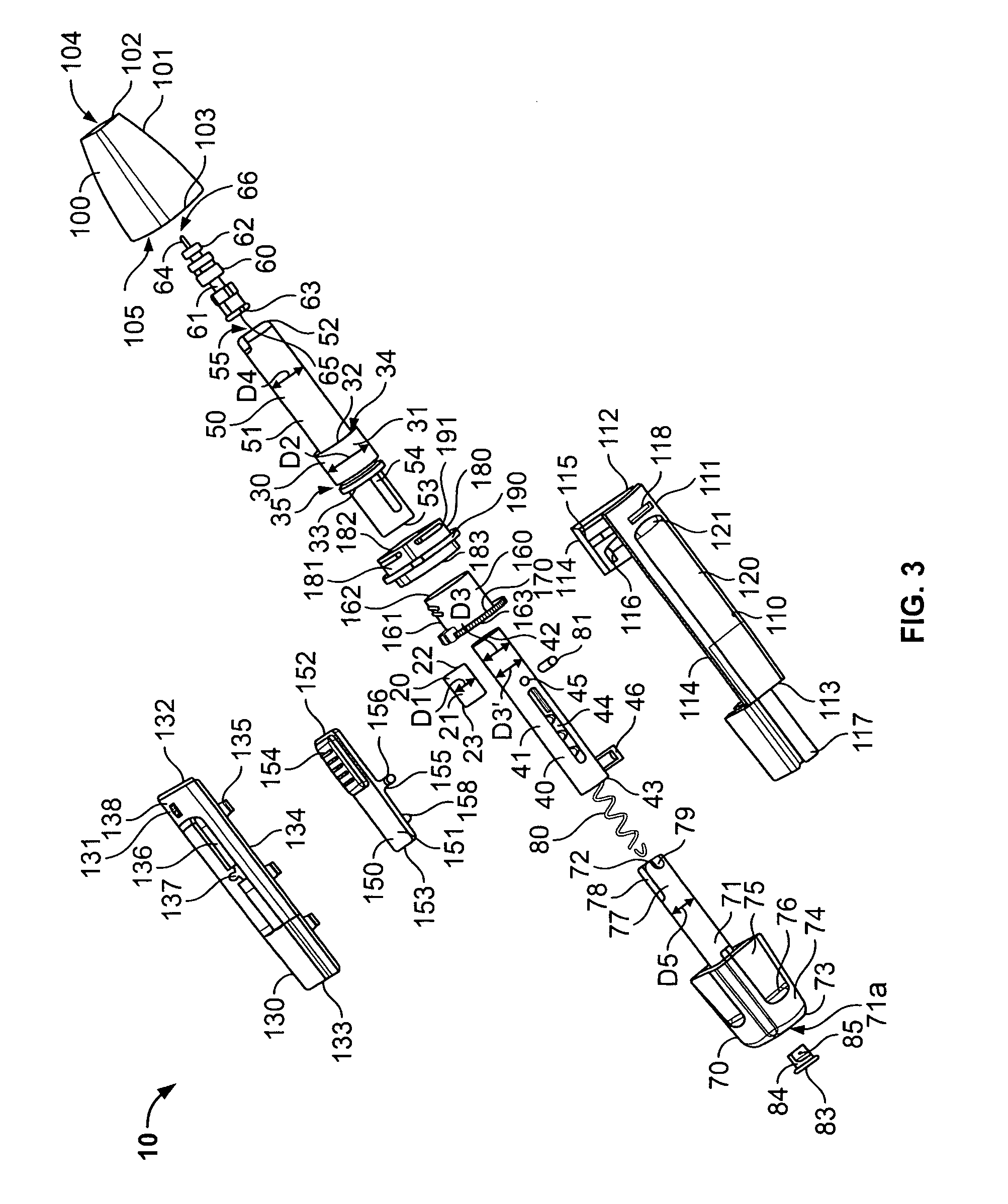
Medical automatic medicator
ActiveUS20050209569A1Control depthReduce painAmpoule syringesAutomatic syringesDrugs solutionNeedle insertion
After pressing a part of the exterior of a body of an administration instrument against a body region of a patient to which a drug solution is to be administered, an injection needle that is housed in the instrument body is automatically protruded from the body to insert the needle into the body region, and further, the injection needle being inserted into the body region is automatically housed in the instrument body to remove the needle from the body region. Thereby, the pain of the patient during needle insertion and needle removal is reduced, and administration at a constant speed is possible during injection of the drug solution. Furthermore, even when two kinds of drug solutions or a dissolving and mixing type drug solution are / is used, mixing can be easily and reliably carried out.
Owner:PHC HLDG CORP
Drugs as well as their production and use in the treatment of pain-associated neuropathies
InactiveUS20090162421A1Reduction of evoked neuropathic painReduce painBiocideAntipyreticPeripheral neuropathic painTreatment pain
The present invention relates to the use of tarenflurbil and / or a pharmaceutically tolerable salt or derivative thereof in enantiomerically-pure and / or essentially enantiomerically-pure form or a form that is enriched with respect to flurbiprofen racemate and / or a racemate of said salt or derivative, for the production of a drug for the treatment of pain-associated neuropathy, pain-associated neuropathy that is simultaneously accompanied by states of nociceptive pain, peripheral and / or predominantly peripheral neuropathic pain or central and / or predominantly central neuropathic pain.
Owner:PAZ ARZNEIMITTEL ENTWICKLUNGSGMBH
Methods for Reducing Discomfort During Electrostimulation, and Compositions and Apparatus Therefor
ActiveUS20110319975A1Reduce inflammationReduce injuriesPowder deliveryExternal electrodesSkin surfaceElectrode gel
An electrode assembly for neuro-cranial stimulation includes an electrode, a conductive gel, and an adapter including an interior compartment for positioning the electrode relative to the adapter and for receiving and retaining the conductive gel. The conductive gel contacts the electrode along an electrode-gel interface. An orifice at one end of the interior compartment and adjacent to a positioning surface of the adapter for positioning the electrode assembly against a skin surface of a user enables the conductive gel is able to contact the skin surface of the user to define a gel-skin interface, such that a minimum distance between the electrode-gel interface and the gel-skin interface is maintained between 0.25 cm and 1.3 cm. An electrode assembly mounting apparatus is provided for adjustably positioning a plurality of electrode assemblies against target positions on the cranium.
Owner:RES FOUND THE CITY UNIV OF NEW YORK
Rigid articulated Pointe shoe
InactiveUS7036244B1Reduce downward forceRelieves high load of forceUpperBootlegsTransverse axisEngineering
The invention is a Pointe shoe for ballet. It has a rigid mid-foot section and a rigid toe loop connected by a transverse axis joint located at the metatarsal-phalange joint (M-P). With the foot in Pointe position, the weight of the dancer is supported by the rigid mid-foot section. The downward force is passed through the M-P joint to the front of the toe loop. None of the weight of the dancer needs to be supported by the toes. In contrast, prior art Pointe shoes have a rigid shank and toe cup to assist the toes in supporting the weight of the dancer. The toes have small bones, muscles, and ligaments. This often results in pain and injury to the toes. The shoe of the invention has a mid-foot section that is shaped with support surfaces for the sole of the heel bone and the dorsal side of the cuneiform and metatarsal bones. These bones are larger and stronger than the bones of the toes. This shoe provides a larger area of bone and tissue to support the weight of the dancer on Pointe. It is more comfortable to use and results in fewer injuries.
Owner:FINCH DENNIS
Devices and methods for facilitating fluid transport
ActiveUS20070078358A1Easy to transportShorten the timeDiagnostic recording/measuringSensorsFluid transportEngineering
Arrangements are provided including a base having a bore disposed therein extending from a first surface of the base through a second surface of the base, a fluid transport tube having a first end, a second end opposite the first end, and a lumen having an inner diameter, at least the second end of the tube being received within the bore of the base, and at least one fluid transport enhancing groove having at least a first section disposed in the second surface of the base and in fluid communication with the bore.
Owner:INTUITY MEDICAL INC
Methods and devices for deployment into a lumen
InactiveUS20070227544A1Facilitate cellularFacilitate tissue ingrowthMale contraceptivesFallopian occludersBiomedical engineeringNon traumatic
Owner:BAYER HEALTHCARE LLC
Actuation system for a bodily fluid extraction device and associated methods
ActiveUS7169116B2Reduce painLess discomfortOperating means/releasing devices for valvesVacuum evaporation coatingEngineeringBody fluid
An actuation system for a bodily fluid extraction device includes a detector cap, at least one vibration sensor connected to the detector cap, and at least one signal processing unit in communication with the vibration sensor. In addition, the signal processing unit is configured to receive an output signal from the vibration sensor, analyze the received output signal, and to send an actuation signal to the bodily fluid extraction device based on the analysis of the received output signal.
Owner:CILAG GMBH INT +1
Lancet device and method
A lancet device (10) having an internal magnet (20) and the forces emanating therefrom driving and retracting a collar (30) and a lancet (60). The device has a steady state condition (SS) wherein the device is at rest and in equilibrium. The device can be armed and activated to puncture. Once puncturing has occurred the device returns to a steady state condition. A lancet method involves positioning both a magnet (20) and a member (30) capable of being affected thereby with a lancet (60) in communications with one of either the magnet or the member, positioning either the member (30) or the magnet (20) to an armed position wherein the magnetic forces from the magnetic affect the member, and releasing the one of either the member or the magnet from the armed position permitting movement between the member and magnet by at least, in part, the magnetic forces, resulting in the movement of the lancet from a withdrawn position to the piercing position.
Owner:VIROTEK LLC +1
Method for treating degenerative disc disease using noninvasive capacitively coupled electrical stimulation device
A method for treatment of degenerative disc disease using capacitively coupled electrical stimulation. In one embodiment, a subject diagnosed as having degenerative disc disease is treated by placing first and second electrodes on the subject's body at the site of an identified disc in a state of degenerative disc disease, and applying an electric field to the identified disc via the first and second electrodes with the intent to treat the degenerative disc disease. The electric field is created with an electrical signal having a frequency within a range of 20 to 100 kHz and having a symmetrical waveform with an amplitude within a range of 0.1 to 20 volts peak-to-peak, preferably a frequency of approximately 60 kHz and an amplitude of approximately 5 volts peak-to-peak.
Owner:EUROPEAN BIOINFORMATICS INSTITUTE
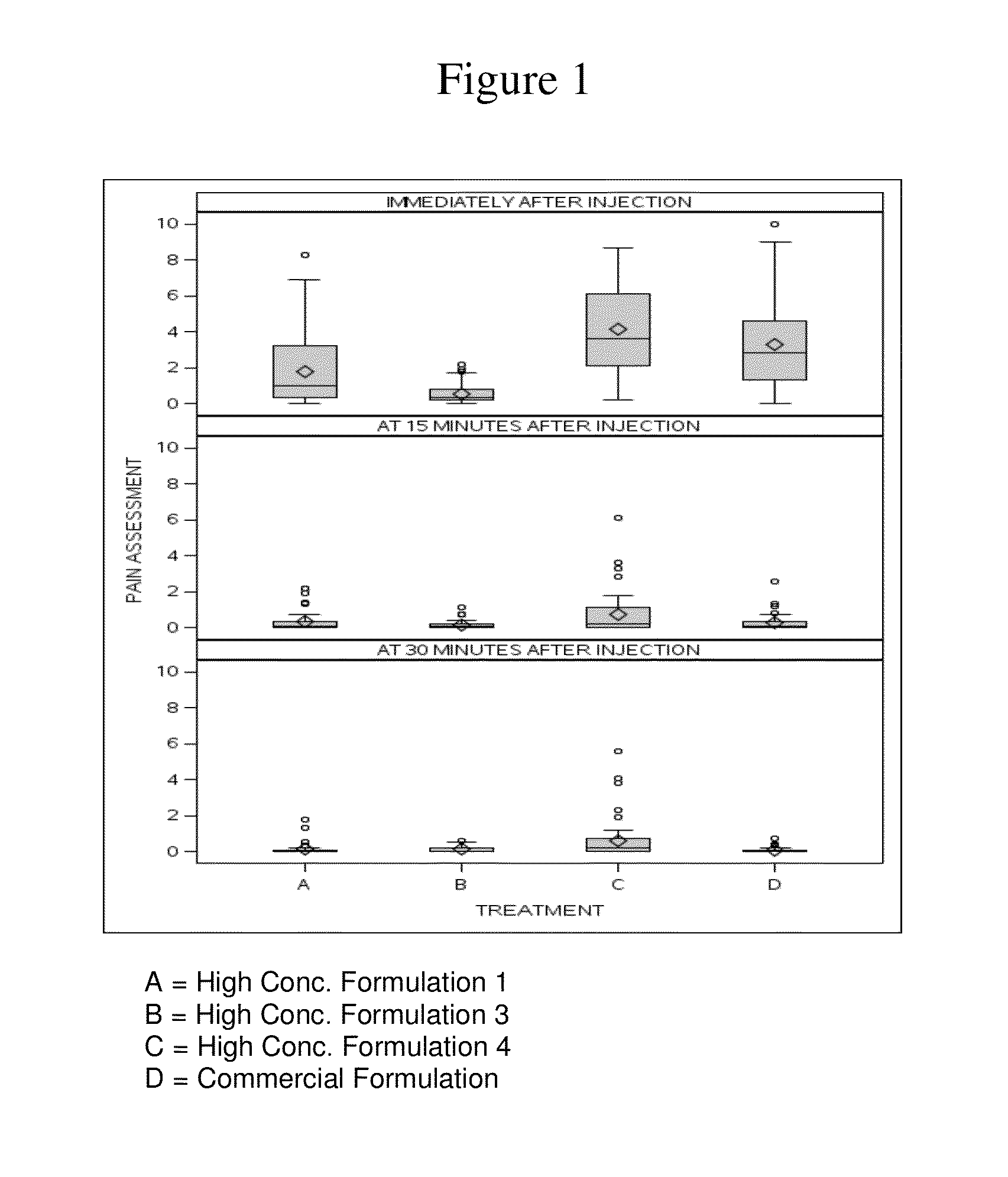
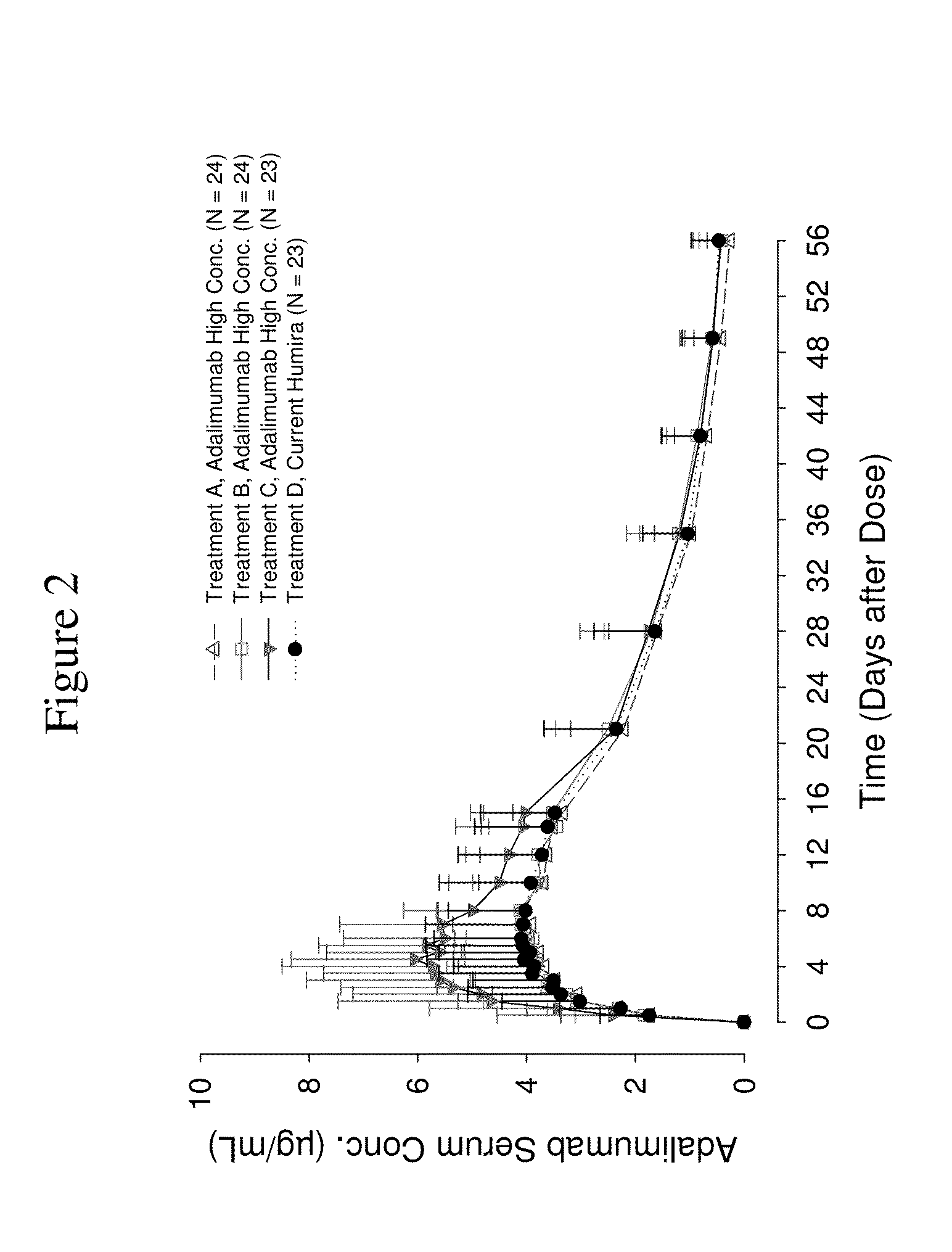
High concentration anti-TNFα antibody liquid formulations
ActiveUS8821865B2Improve bioavailabilityRelieve painSenses disorderNervous disorderAntiendomysial antibodiesTherapeutic protein
The invention provides a liquid aqueous pharmaceutical formulation comprising a human anti-TNFa antibody, or antigen-binding portion thereof, which reduces pain associated with injection in a subject by at least about 50% when compared to injecting an otherwise identical formulation comprising at least one salt and / or at least one buffer. The invention also provides a liquid aqueous pharmaceutical formulation comprising a human anti-TNFa antibody, or antigen-binding portion thereof, having increased bioavailability upon subcutaneous administration into a subject. The formulation may comprise a therapeutic protein, such as a human anti-TNF-alpha antibody, or an antigen-binding portion thereof, or a biosimilar thereof.
Owner:ABBVIE BIOTECHNOLOGY LTD
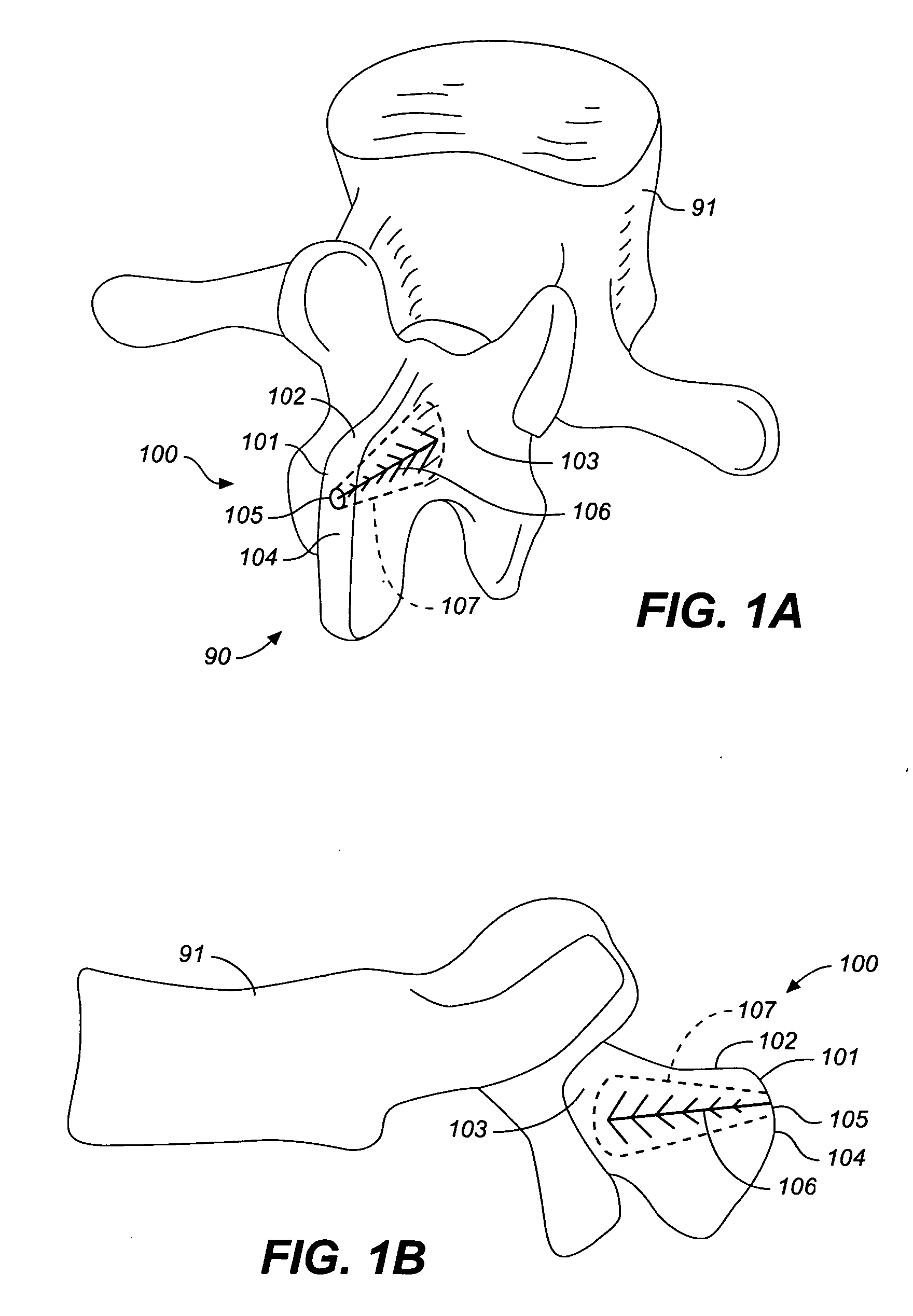
Device and method for correcting a spinal deformity
ActiveUS20100100130A1Less discomfortAlleviating or deformityAdditive manufacturing apparatusInternal osteosythesisMedicineDirect device
A device and method for correcting a spinal deformity are provided. A spinal implant for correcting a spinal deformity includes a multipoint connector that connects to at least one vertebra of a spine at a plurality of locations and a force directing device that applies a force to the vertebra through the multipoint connector. The force directing device may include a rod which extends generally along an axis of the spine and a force directing member which is adjustably coupled to both the rod and the multipoint connector and which applies a corrective force to the at least one vertebra.
Owner:K2M +1
Lancet for blood collection and puncture needle unit
A lancet having a slider with a puncture needle holding mechanism at one end thereof, and having a cam ring with a continuous cam groove, rotatable about a support shaft, and with a cam ring claw and an anti-return claw restricting the rotation. The lancet also having a ring spring applying a force to rotate the cam ring, having a rotatable stopper arm holding and releasing the rotation of the cam ring, and having a rotatable ratchet restricting the direction of rotation of the cam ring. A puncture needle unit including a puncture needle body integrally molded with a protrusion fitted to the lancet, a rotation stop rib, a puncture needle, and a puncture needle cap lightly pressed into the puncture needle body.
Owner:PHC HLDG CORP
Systems and methods for reducing fractured bone using a fracture reduction cannula
InactiveUS20070118143A1Raise the possibilitySooner resumptionSurgical furnitureSurgical needlesFracture reductionMedicine
Systems and methods provide for the fixation of osteoporotic and non-osteoporotic long bones, especially Colles' fractures. A cannula having a circumferential opening is inserted into cancellous bone and directed such that the circumferential opening faces the fracture. The cannula is further adapted to receive an expandable structure, the expandable structure being inserted through the cannula until it is in registration with the circumferential opening. The expandable structure is expanded through the circumferential opening into cancellous bone and toward the fracture. The expansion of the expandable structure through the circumferential opening toward the fracture causes compression of cancellous bone and moves fractured cortical bone, thus creating a cavity proximal to the fracture. The cavity is then filled with a flowable bone filling material and the material allowed to harden.
Owner:ORTHOPHOENIX
Linearly expanding spine cage for enhanced spinal fusion
ActiveUS20080188941A1Reduce painImprove functioningBone implantSpinal implantsVertebraSpinal arthrodesis
A linearly expanding spine cage has a minimized diameter in its unexpanded state that is equal to the diameter of an insertion groove cut into adjacent vertebral bodies. The cage conformably engages between the endplates of adjacent vertebrae to effectively distract the disc space, widen neuroforamina, stabilize the motion segments and eliminate pathologic spine motion. Angular deformities can be corrected, and natural curvatures maintained. The cage enhances spinal arthrodesis by creating a rigid spine segment. Expanding linearly (vertically, along the vertical axis of the adjacent spine) rather than uniformly, the cage height increases and holds the vertebrae with fixation forces greater than adjacent bone and soft tissue failure forces. Stability is thus achieved immediately, enabling patient function by eliminating painful motion. The cage width remains stable, so as to decrease impingement upon a second cage, or upon soft tissue structures in the immediate vicinity, including neural or vascular elements.
Owner:HOWMEDICA OSTEONICS CORP
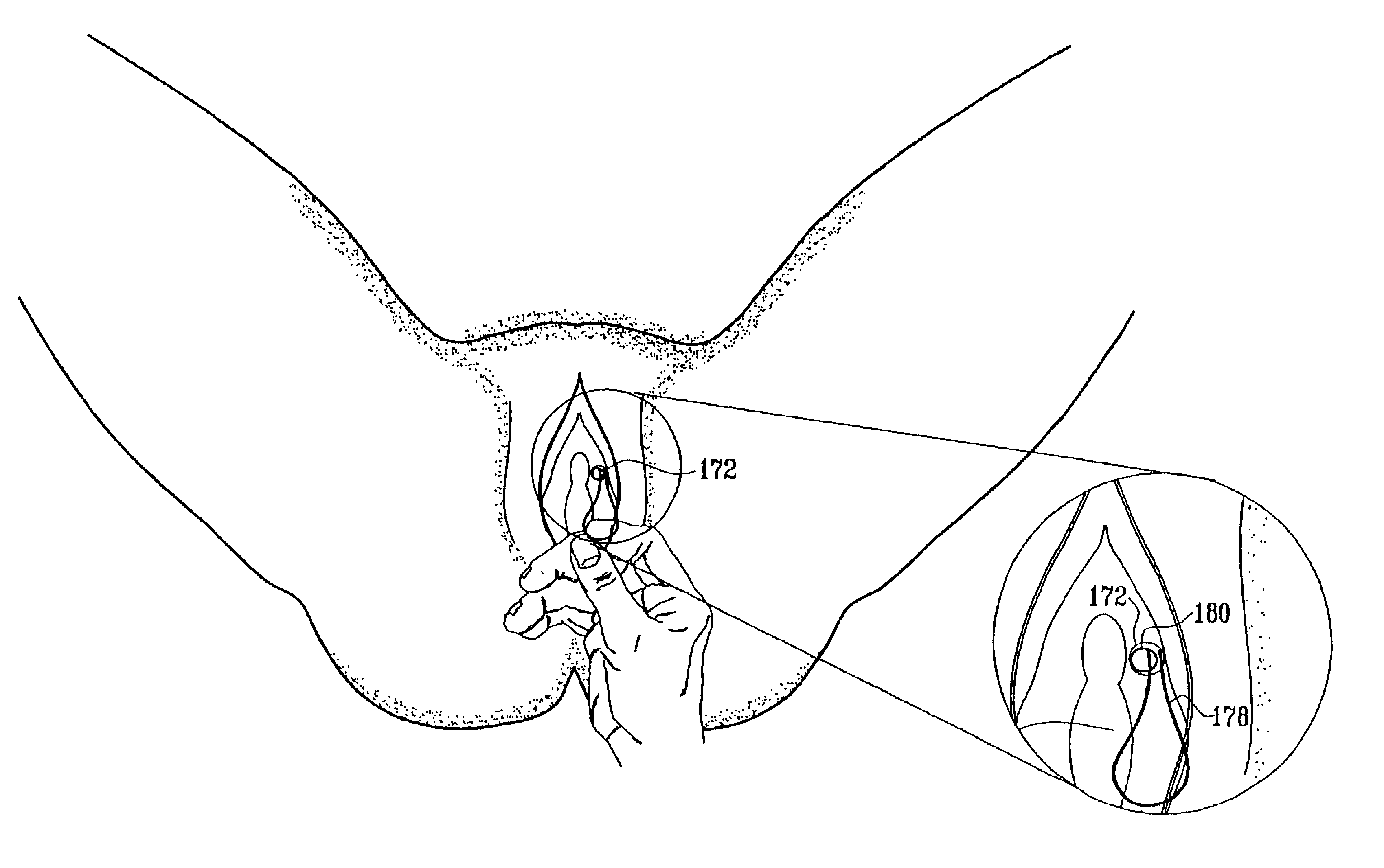
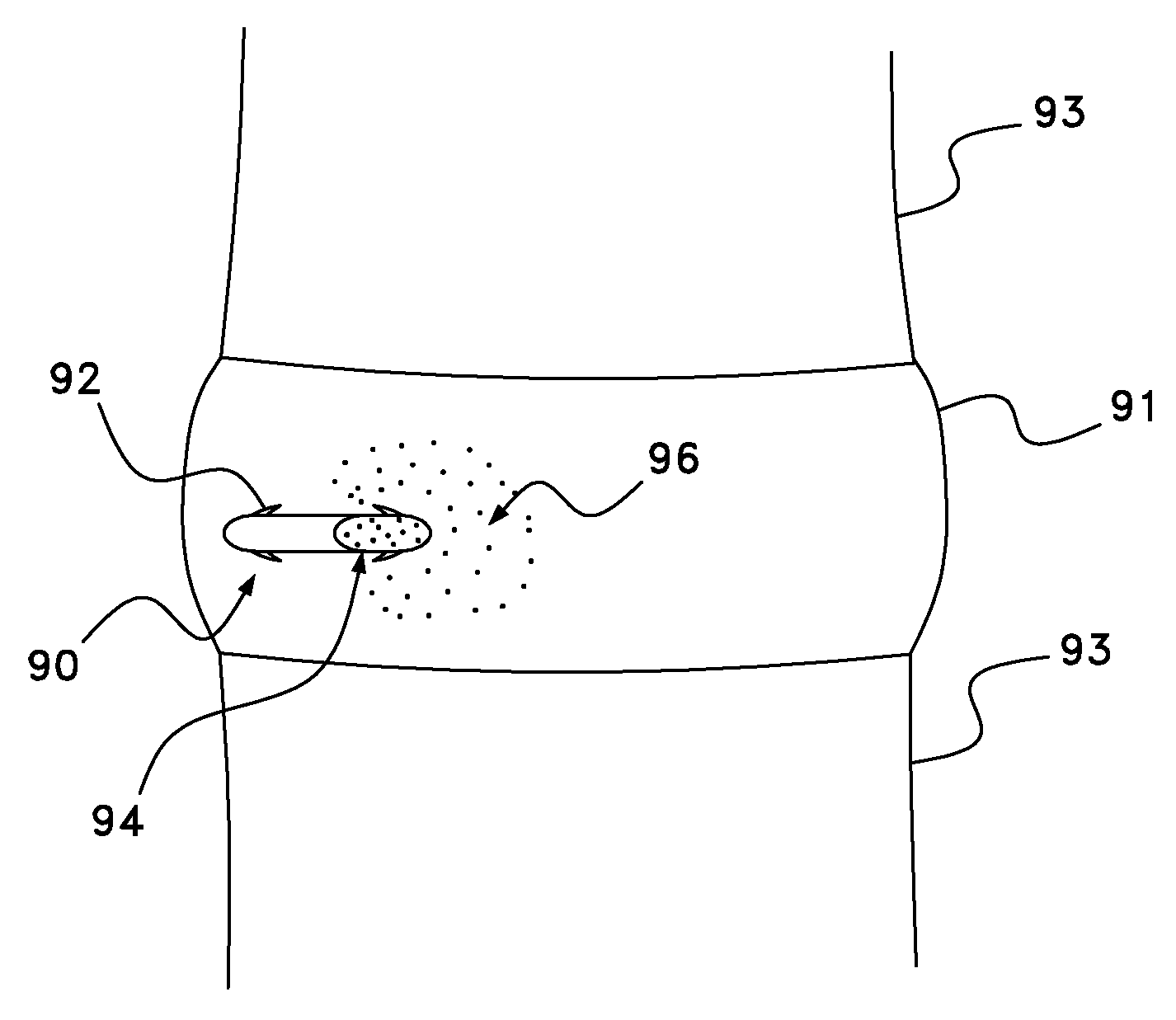
Methods and apparatus for utilization of barbed sutures in human tissue including a method for eliminating or improving blood flow in veins
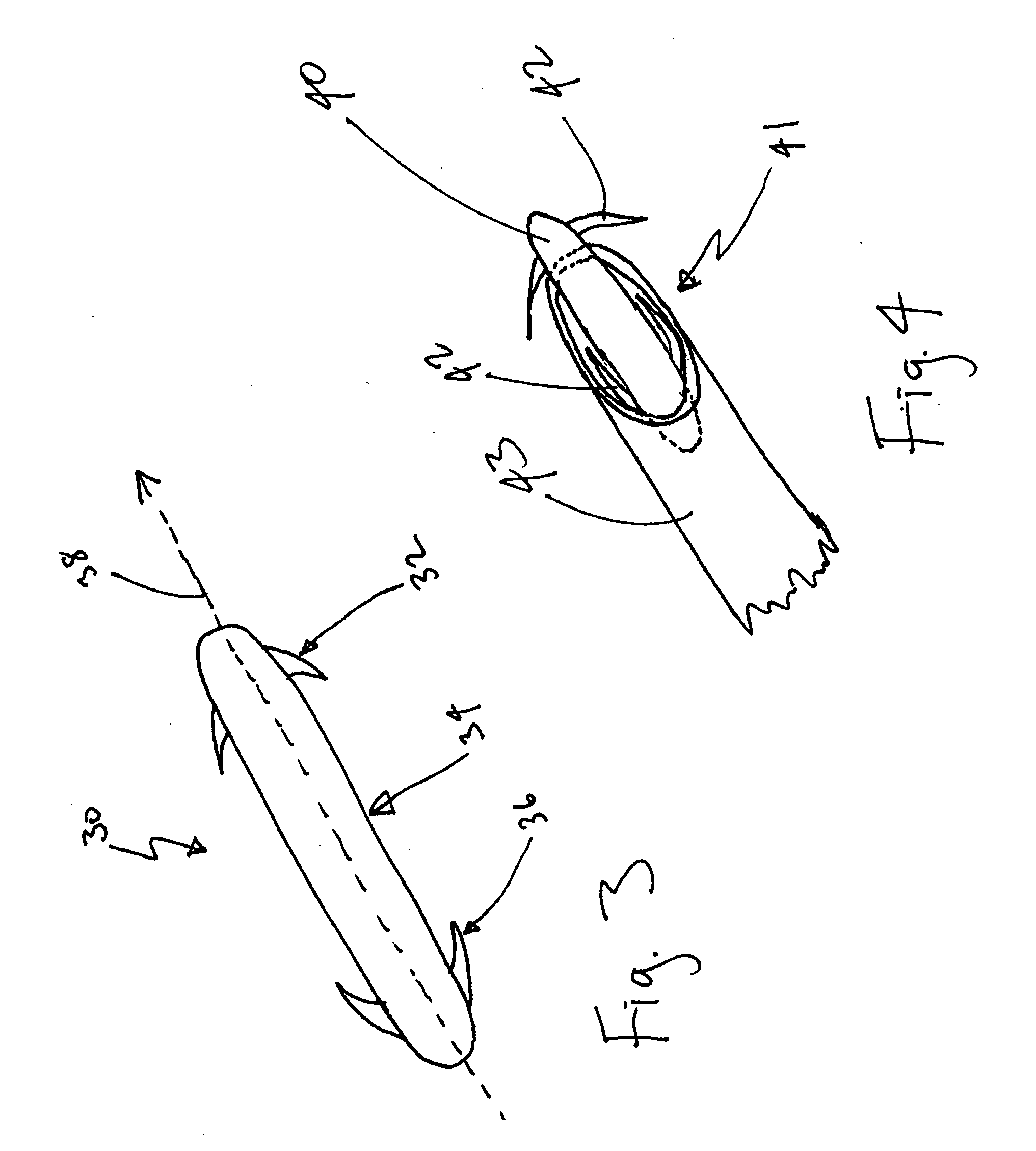
InactiveUS20080215072A1Reduce painPrecise positioningSuture equipmentsDiagnosticsBreaking pointBarbed suture
A method and apparatus for eliminating or improving blood flow within incompetent veins to correct venous insufficiency using a barbed bidirectional suture with predetermined breaking point. Said suture may also be utilized for other tissue applications. A two-way barb suture is placed in an insertion device comprised of a tubular body with or without a pointed distal tip. The inserting device and one-way or two-way suture are placed in a position to effectively close off the vein or leave a device within the vein. The insertion device is then withdrawn leaving the suture in place. This barbed suture with predetermined breaking point is used by the method of the present invention to prevent reflux, and as a method to join body tissue, attach dissimilar body tissues, attach devices to body tissues, and alter the position of body tissues by remote percutaneous access with the assistance of ultrasound or fluoroscopy and with the assistance of endoscopic devices.
Owner:KELLY GRAHAM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com