Patents
Literature
112results about How to "Minimize migration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
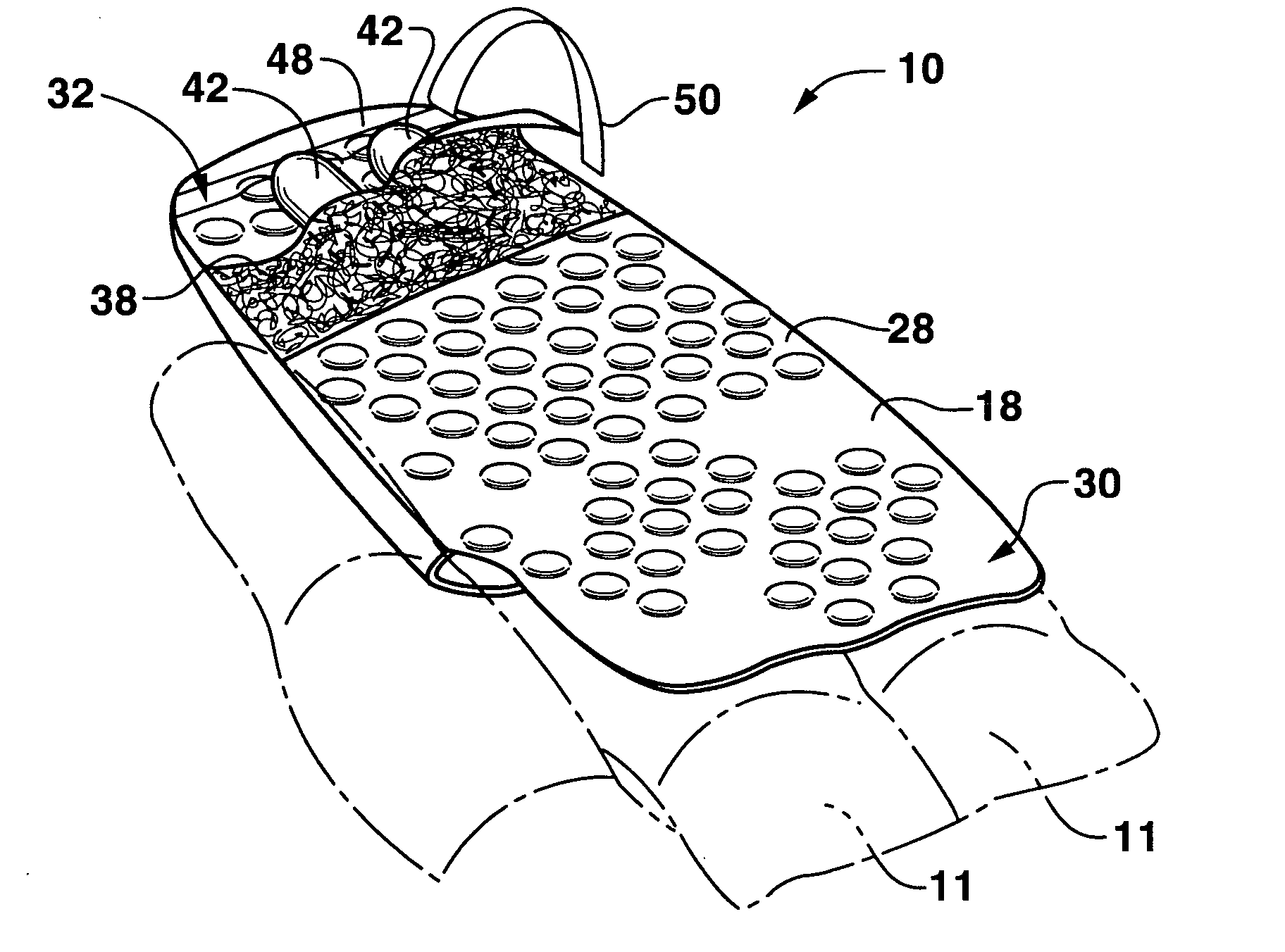
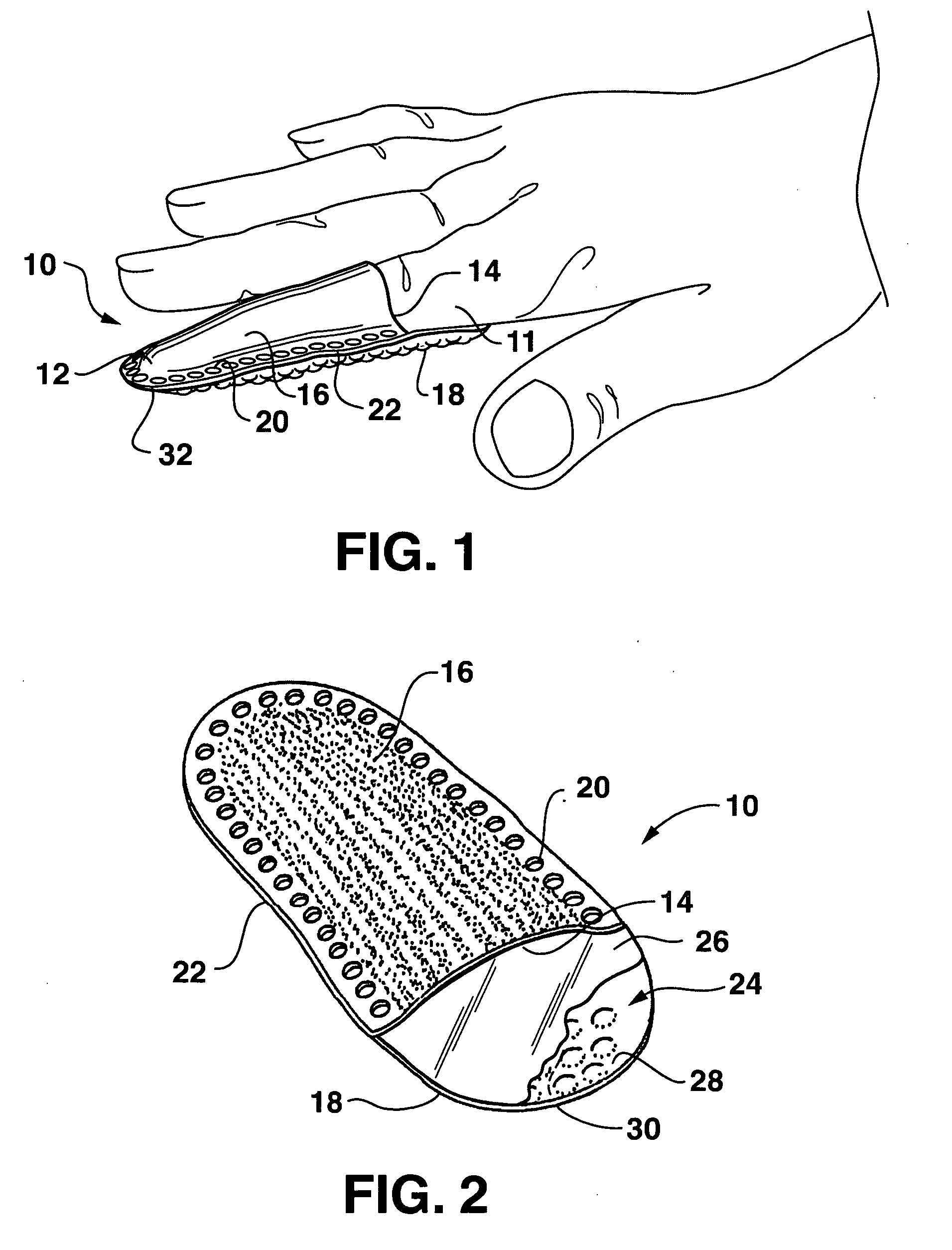
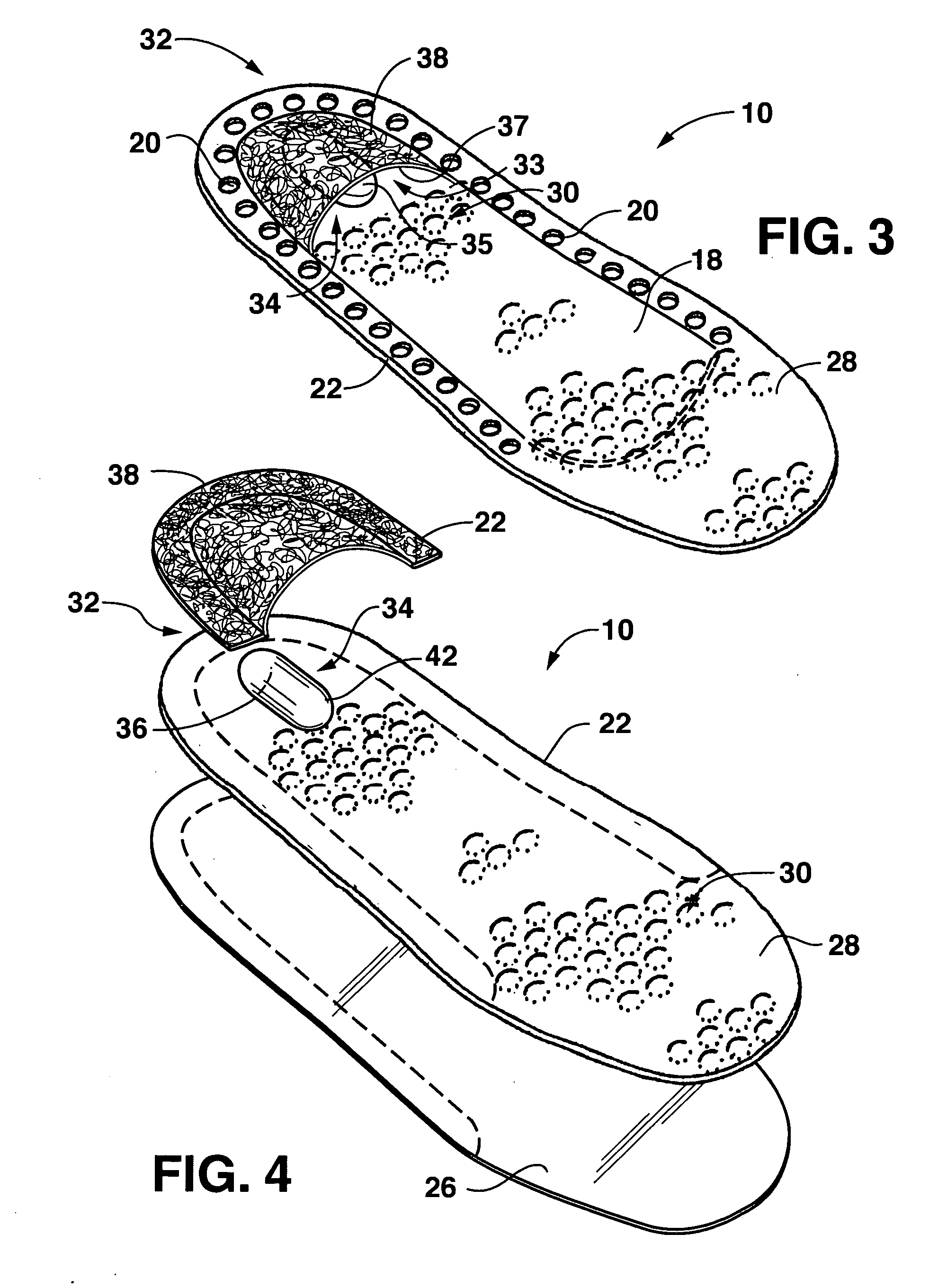
Drug depot implant designs
ActiveUS7727954B2Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injuryChronic pain
Owner:WARSAW ORTHOPEDIC INC
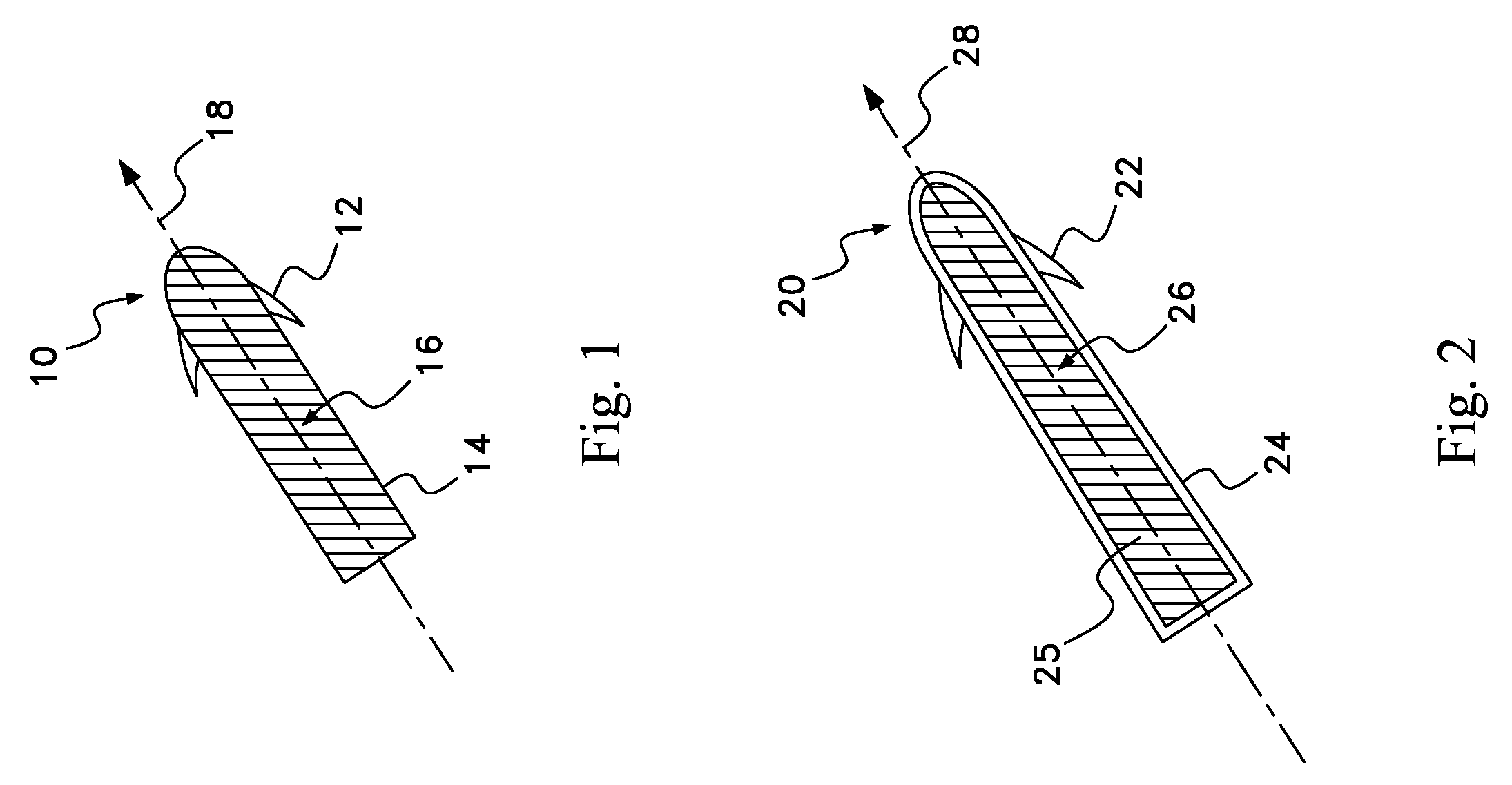
Drug depot implant designs and methods of implantation
ActiveUS20070243225A1Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injurySacroiliac joint
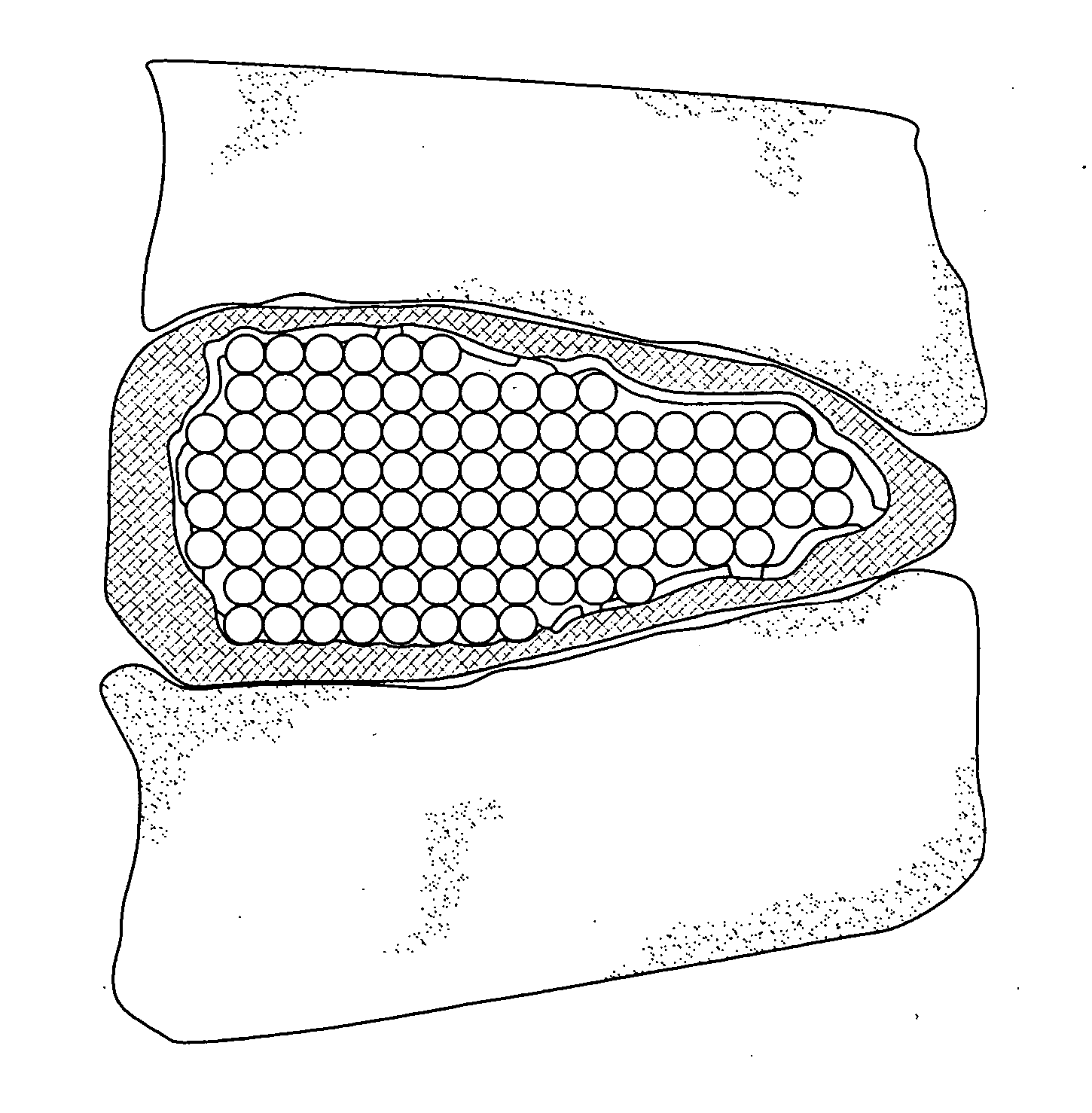
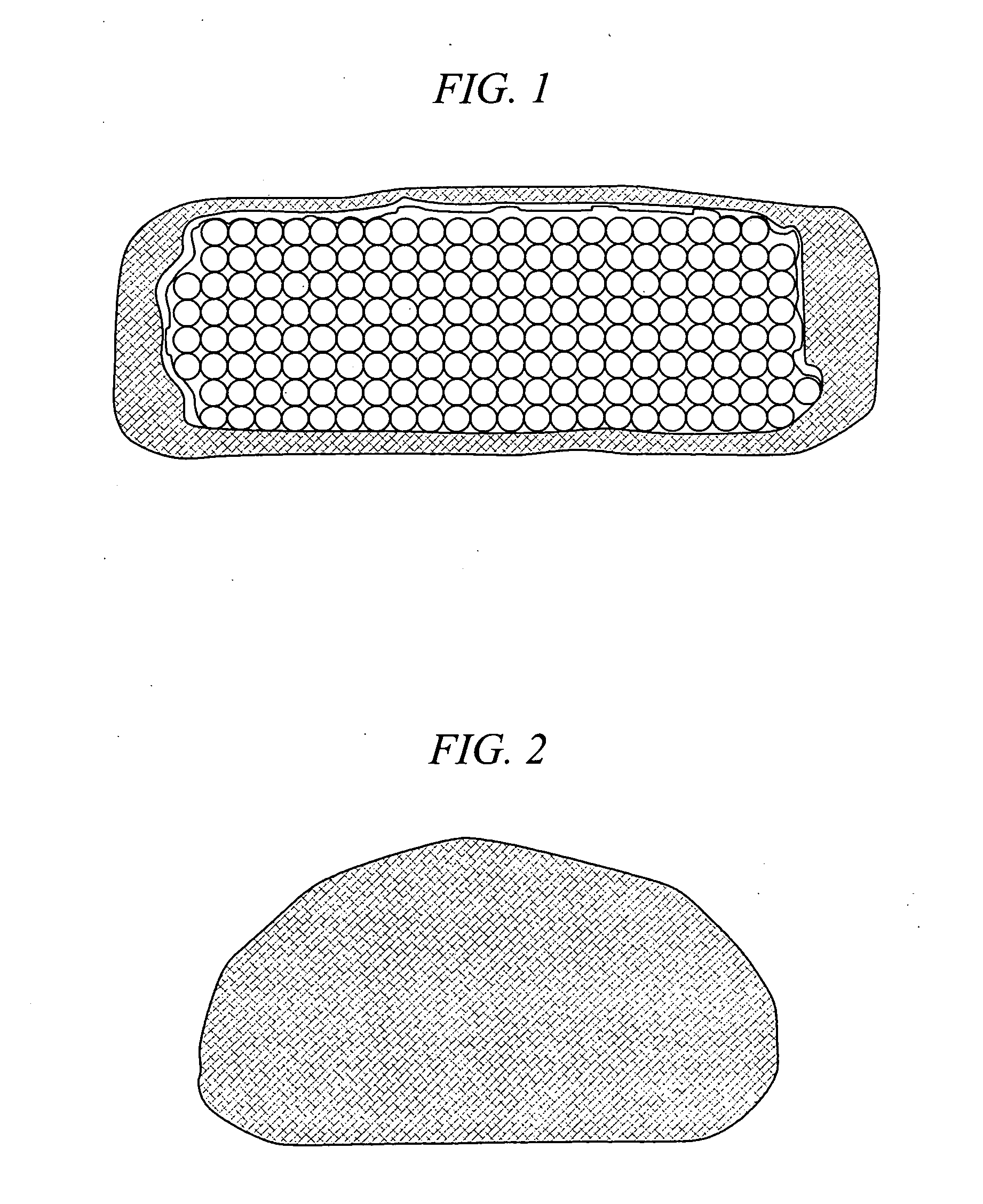
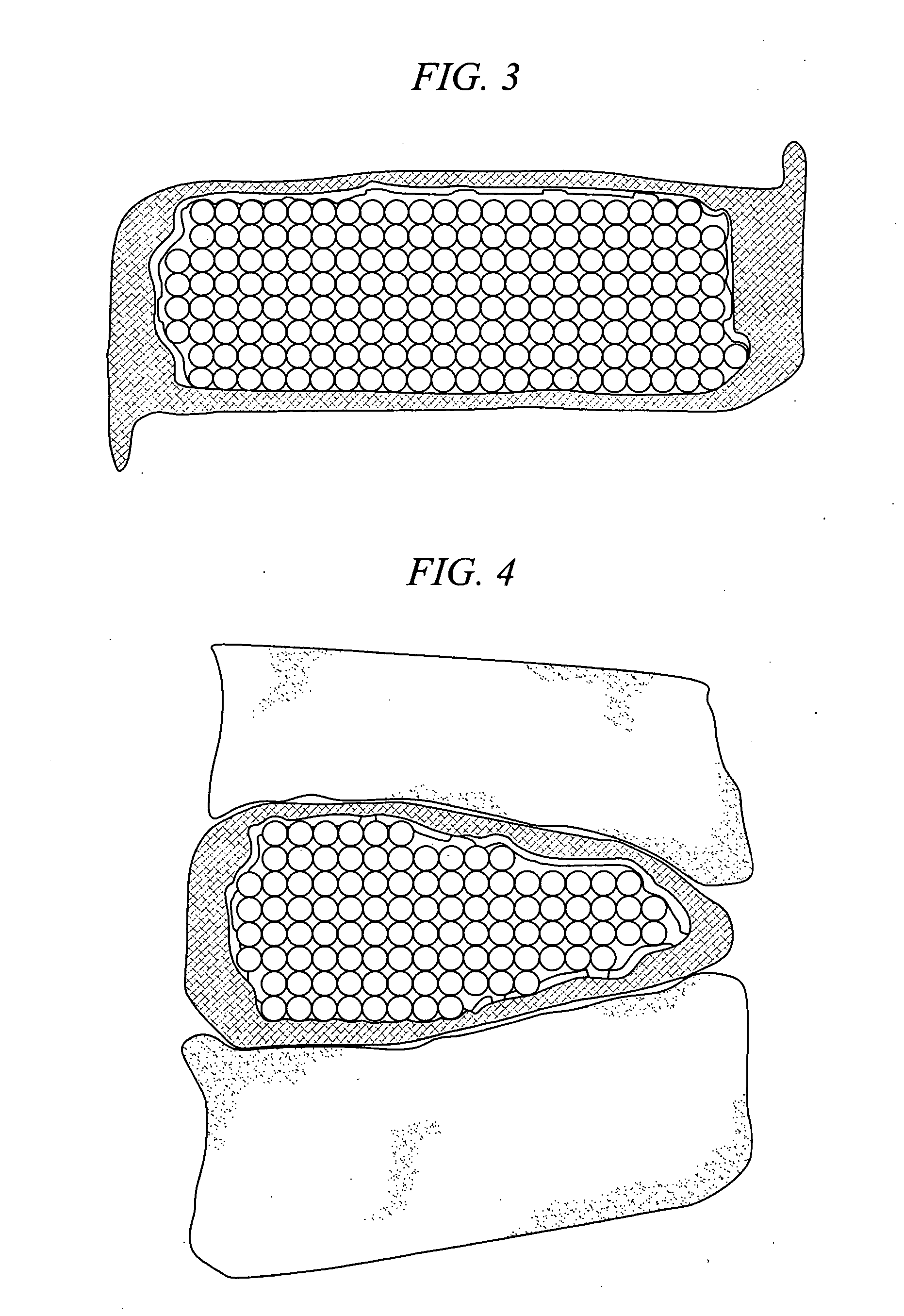
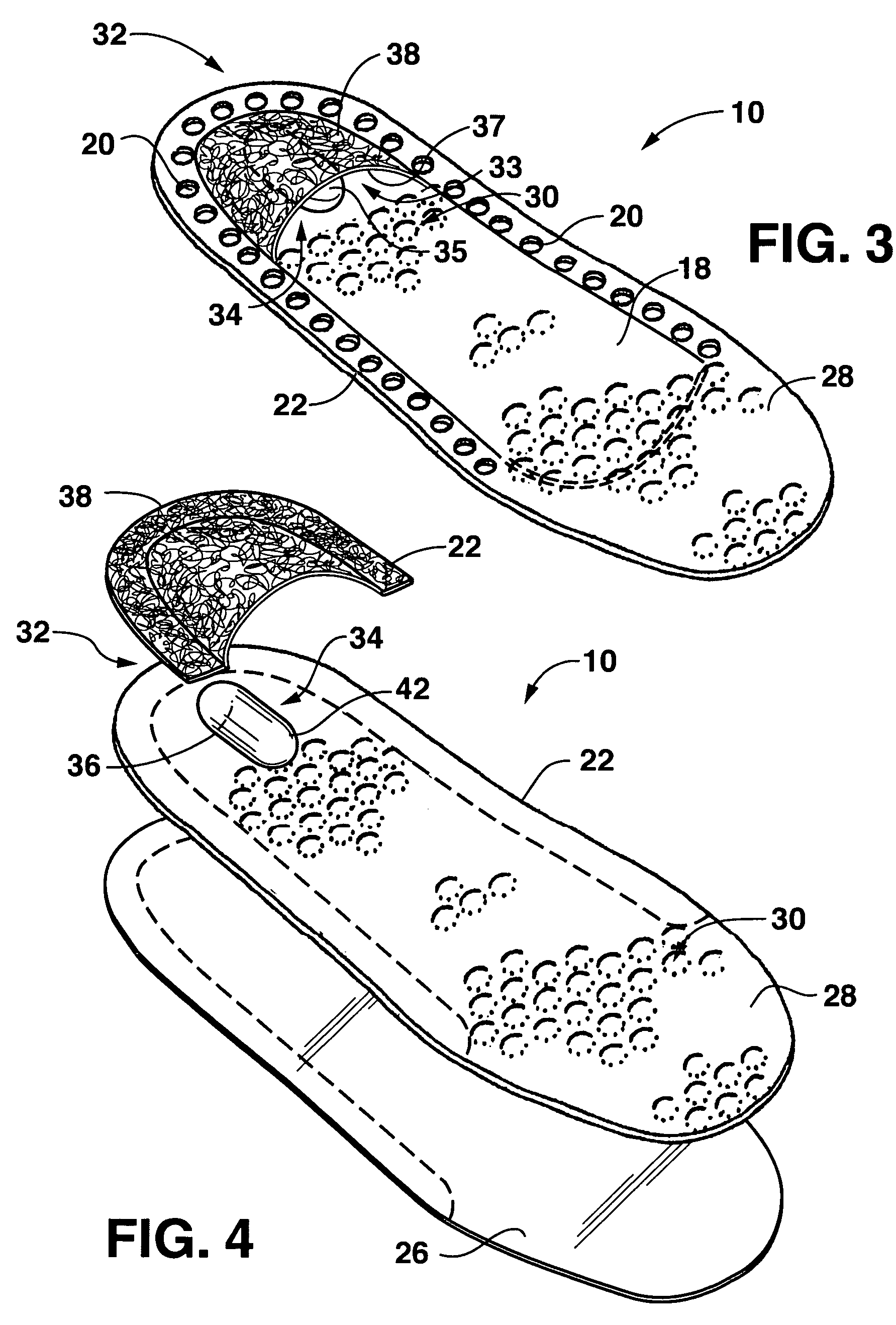
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
Drug depot implant designs and methods of implantation
ActiveUS20070243228A1Uniform drug distributionMinimal disruptionBiocidePeptide/protein ingredientsSkeletal injurySacroiliac joint
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
Methods for embolizing blood vessels
InactiveUS6335384B1Reduce molecular weightEasy to adjustHeavy metal active ingredientsOrganic active ingredientsParticulatesMedicine
Disclosed are methods useful for treating vascular lesions wherein a non-particulate agent such as a metal coil is introduced into a vascular site (e.g., an aneurysm cavity) in conjunction with an embolizing composition comprising a biocompatible polymer and a biocompatible solvent.The biocompatible solvent is miscible or soluble in blood and also solubilizes the polymer during delivery. The biocompatible polymer is selected to be soluble in the biocompatible solvent but insoluble in blood. Upon contact with the blood, the biocompatible solvent dissipates from the embolic composition whereupon the biocompatible polymer precipitates. Precipitation of the polymer in the presence of the non-particular agent permits the agent to act as a structural lattice for the growing polymer precipitate.In another embodiment, the biocompatible polymer composition can be replaced with a biocompatible prepolymer composition containing a biocompatible prepolymer.
Owner:MICRO THEREPEUTICS INC
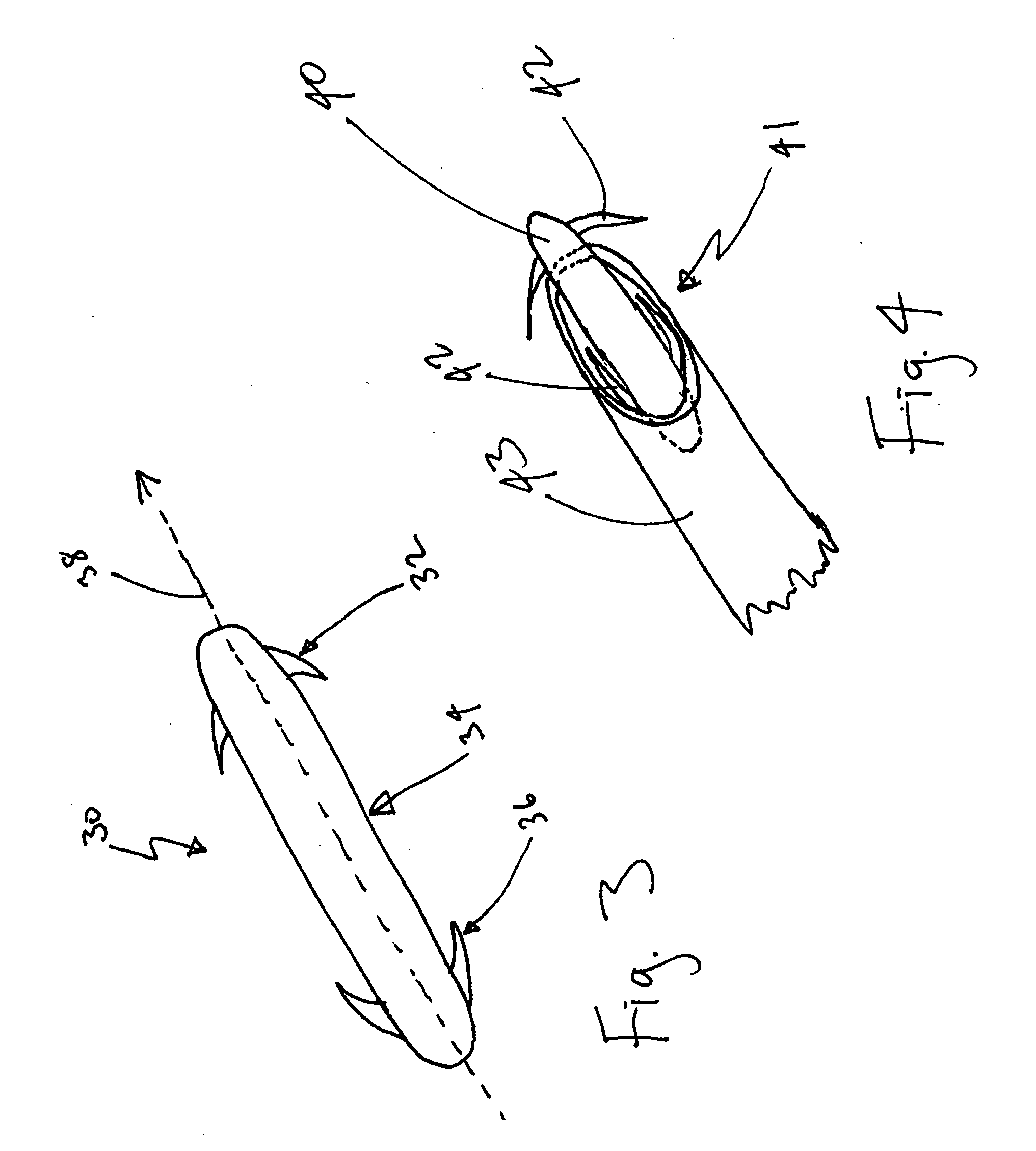
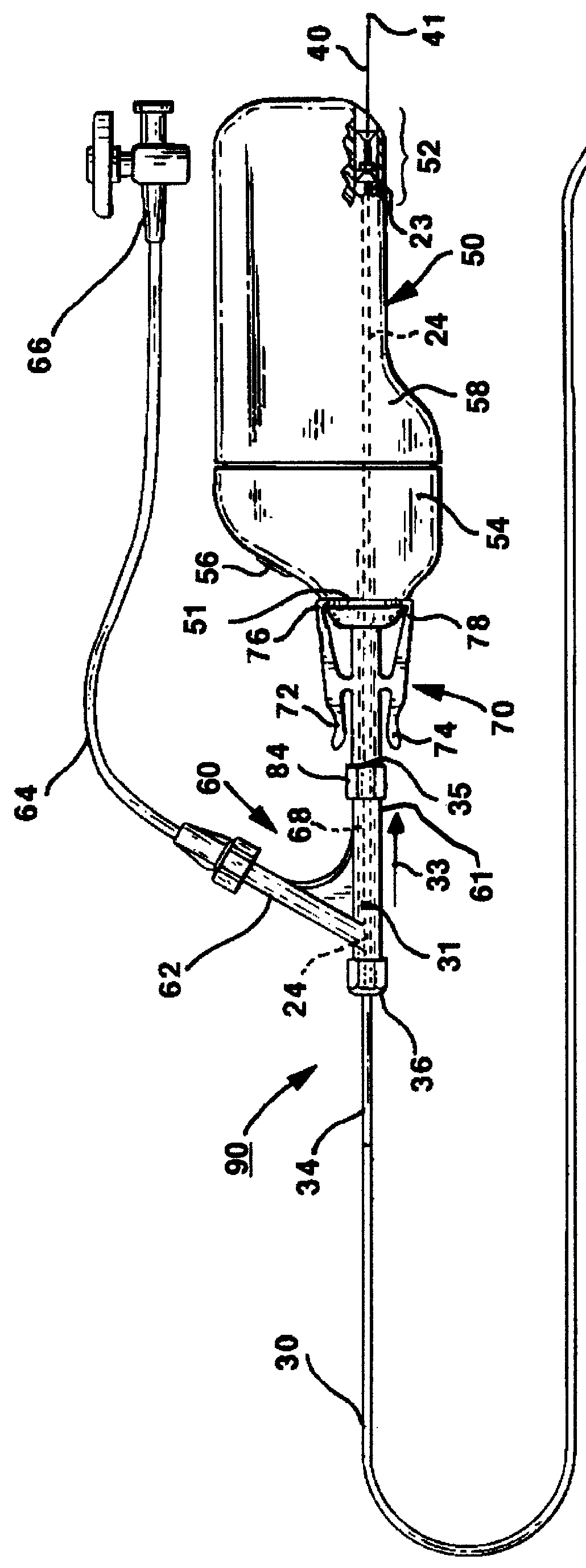
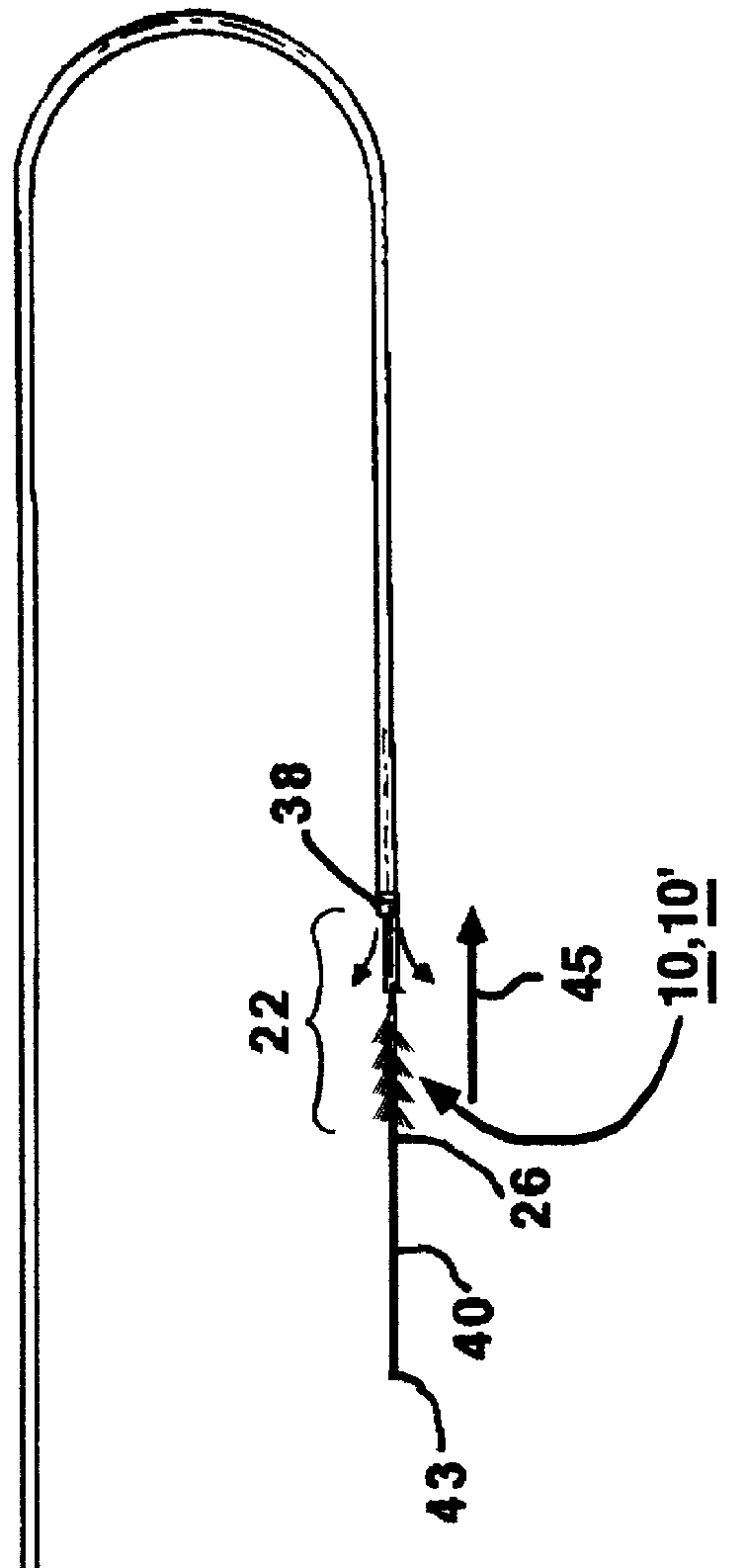
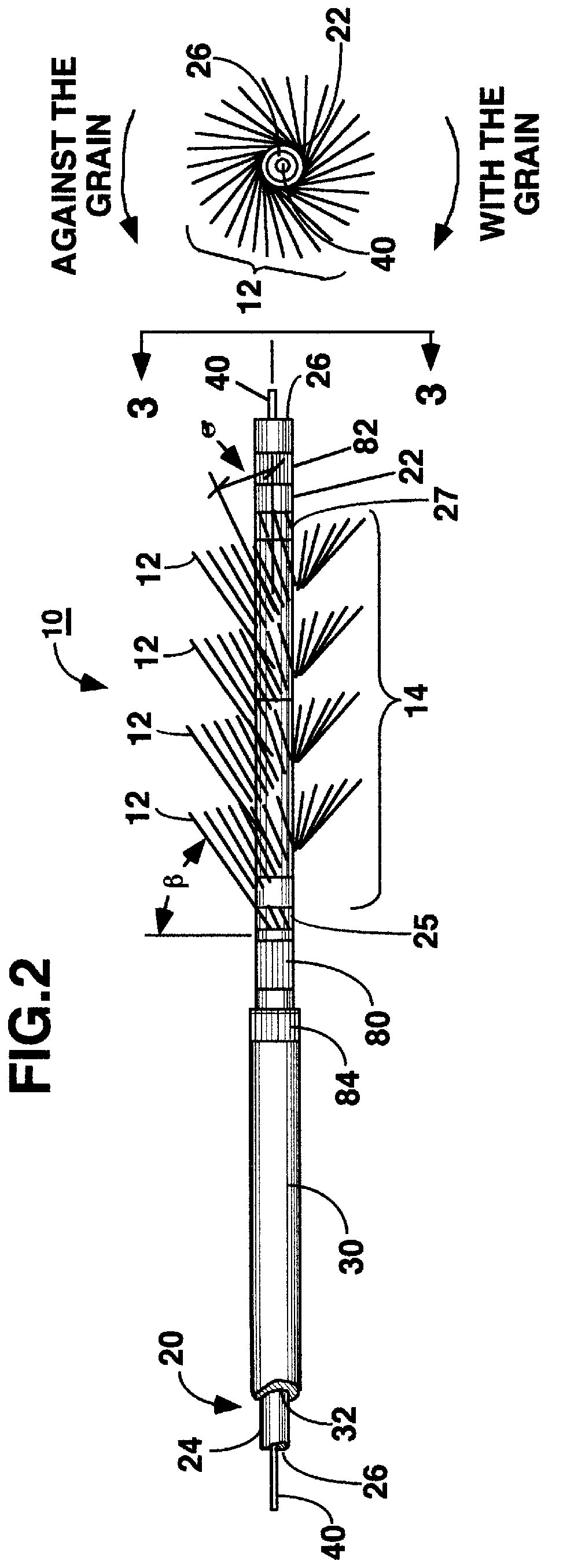
Miniaturized medical brush
A miniaturized brush particularly adapted for medical use formed at the distal end of an elongated brush drive shaft having a hollow lumen formed therein for introduction over a guidewire. The brush drive shaft is enclosed in the lumen of a brush delivery catheter and other components of a brush sub-assembly adapted to deliver infusate through the catheter lumen and to be coupled to a drive motor unit for rotating the brush drive shaft and brush. The brush bristles of the distal brush are adapted to be garaged in a distal end section of the brush delivery catheter lumen during introduction through a body lumen. The brush bristles are formed of a thin sheet of rigid plastic material that is shaped to have a plurality of fringe elements extending in parallel from a mounting web. The mounting web is wound about and attached to a distal end section of the drive shaft outer circumference so that the fringe elements extend outward from the drive shaft surface and obliquely of the drive shaft axis. Preferably, proximal and distal spiral brush sections are formed in this manner so that fluids are impelled distally and proximally, respectively, between the proximal and distal brush sections as infusate is delivered.
Owner:TYCO HEALTHCARE GRP LP
Methods for embolizing blood vessels
InactiveUS6017977AReduce molecular weightEasy to adjustHeavy metal active ingredientsOrganic active ingredientsPrepolymerSolvent
Disclosed are methods useful for treating vascular lesions wherein a non-particulate agent such as a metal coil is introduced into a vascular site (e.g., an aneurysm cavity) in conjunction with an embolizing composition comprising a biocompatible polymer and a biocompatible solvent. The biocompatible solvent is miscible or soluble in blood and also solubilizes the polymer during delivery. The biocompatible polymer is selected to be soluble in the biocompatible solvent but insoluble in blood. Upon contact with the blood, the biocompatible solvent dissipates from the embolic composition whereupon the biocompatible polymer precipitates. Precipitation of the polymer in the presence of the non-particulate agent permits the agent to act as a structural lattice for the growing polymer precipitate. In another embodiment, the biocompatible polymer composition can be replaced with a biocompatible prepolymer composition containing a biocompatible prepolymer.
Owner:MICRO THEREPEUTICS INC
Antimicrobial brush
InactiveUS6108847ACost-effectiveNon-toxic, durableBiocideAntifouling/underwater paintsBristleChemical compound
A brush having antimicrobial characteristics that inhibit bacterial growth. The antimicrobial agents, compounds or chemicals are embedded in either the body or bristles or both of the brush. Further, the present invention is a method of manufacturing a brush having antimicrobial characteristics that inhibit bacterial growth. An antimicrobial additive is incorporated in resin concentrate form into the amorphous zones of the molecular structure of the polymer from which brush handles are injection molded, thereby incorporating the antimicrobial agent into the brush handle. The antimicrobial additive in the body of the brush, incorporated in the manner above, results in substantive controlled migration from the body to the bristles, until a point of equilibrium is reached. The invention is suitable for any brush in which bristles are embedded in plastic, including toothbrushes, hair brushes, scrub brushes, toilet bowl brushes, cosmetic brushes, lip-color brushes, etc.
Owner:MICROBAN PROD CO INC
Highly efficient gas permeable devices and methods for culturing cells
ActiveUS20080227176A1Minimize potentialEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsCulture cellPolystyrene
Owner:WILSON WOLF MFG
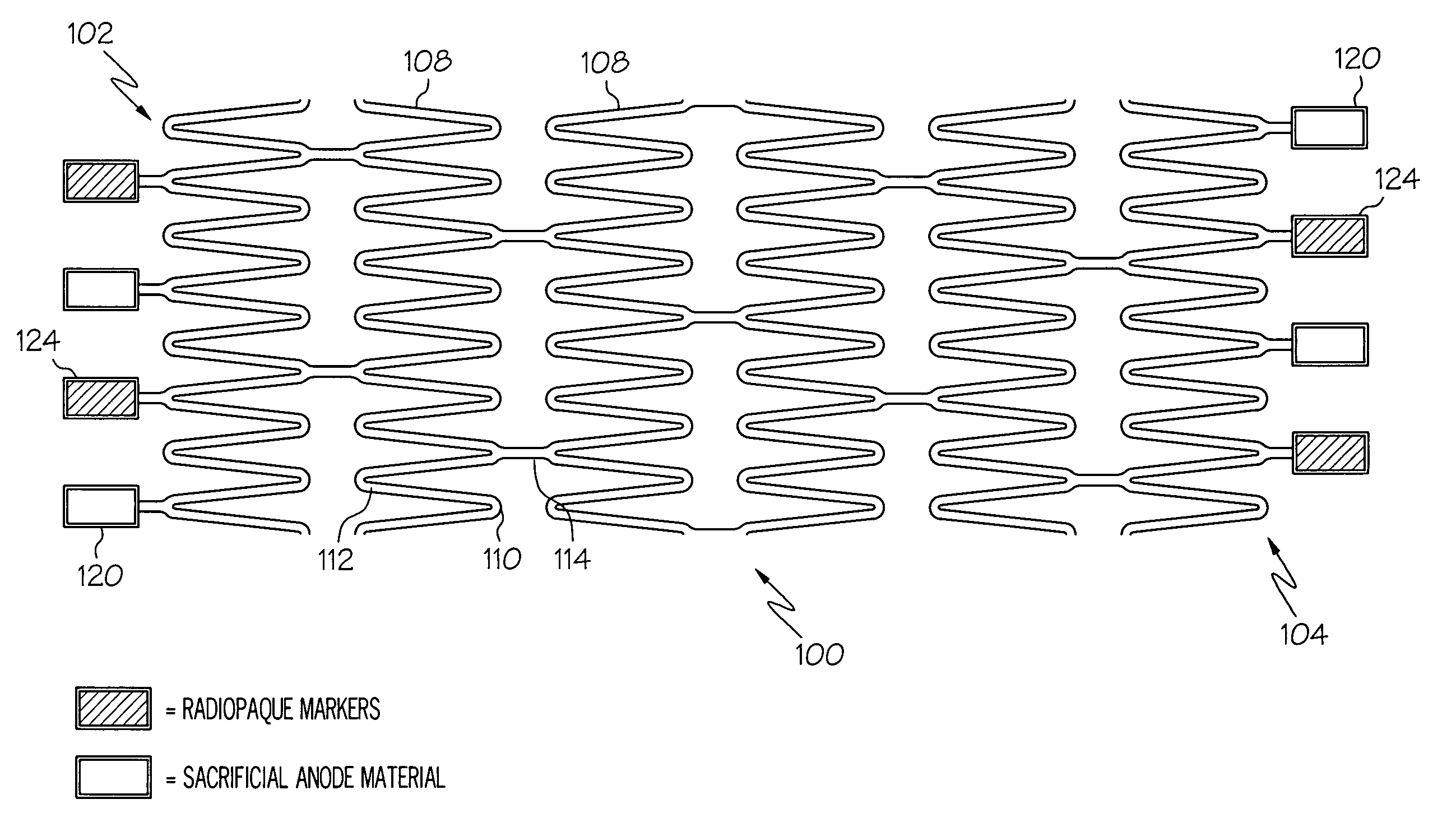
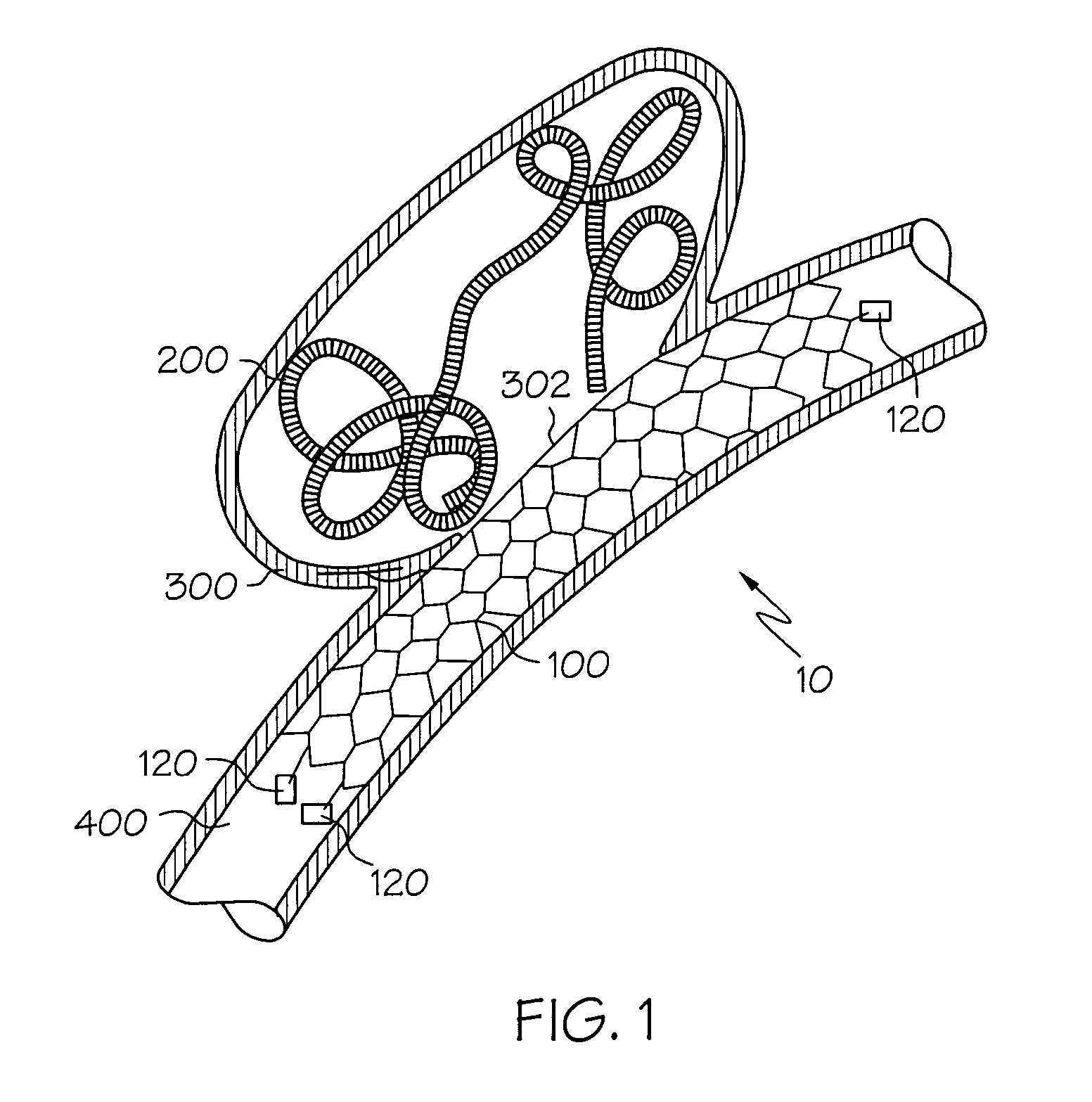
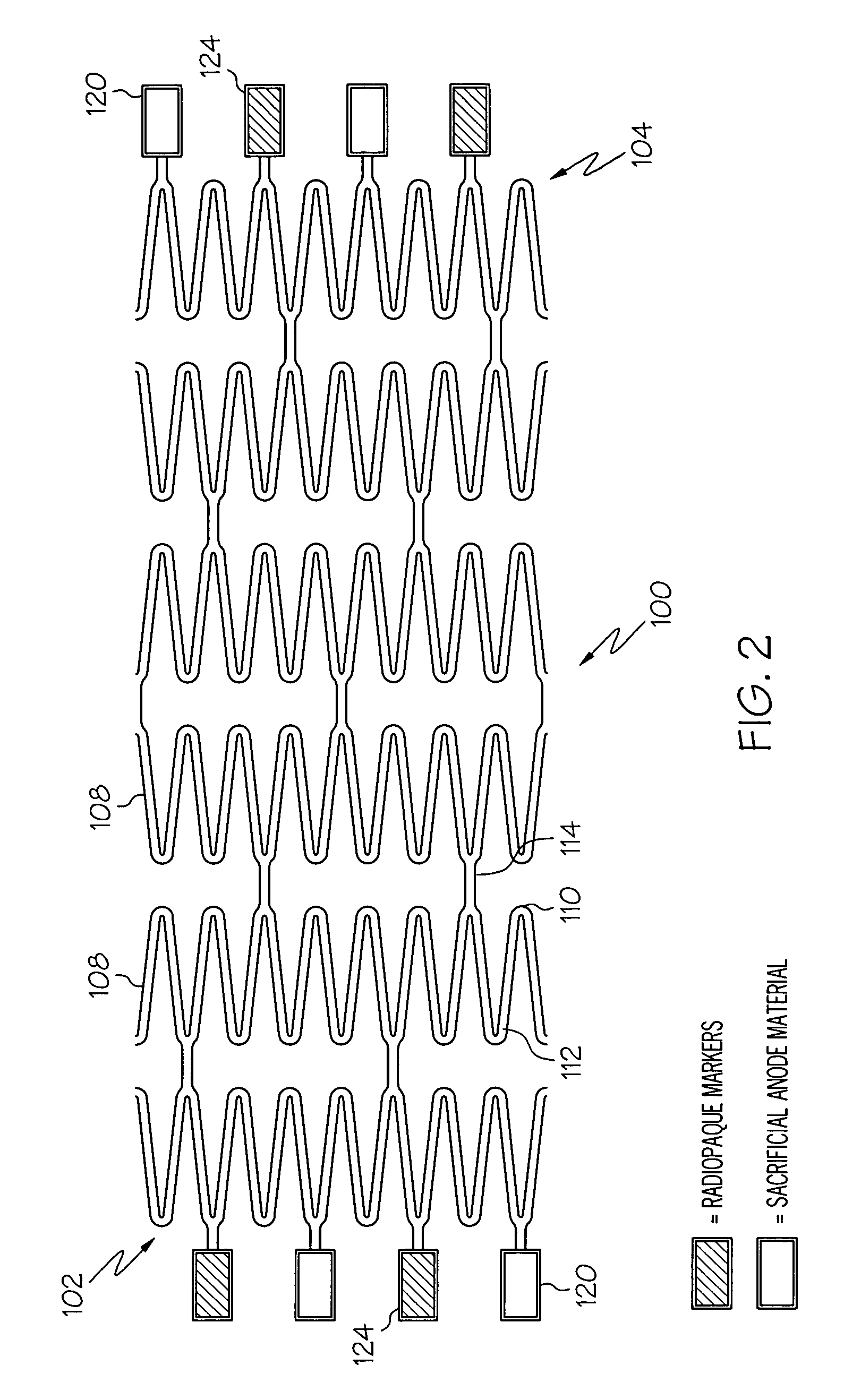
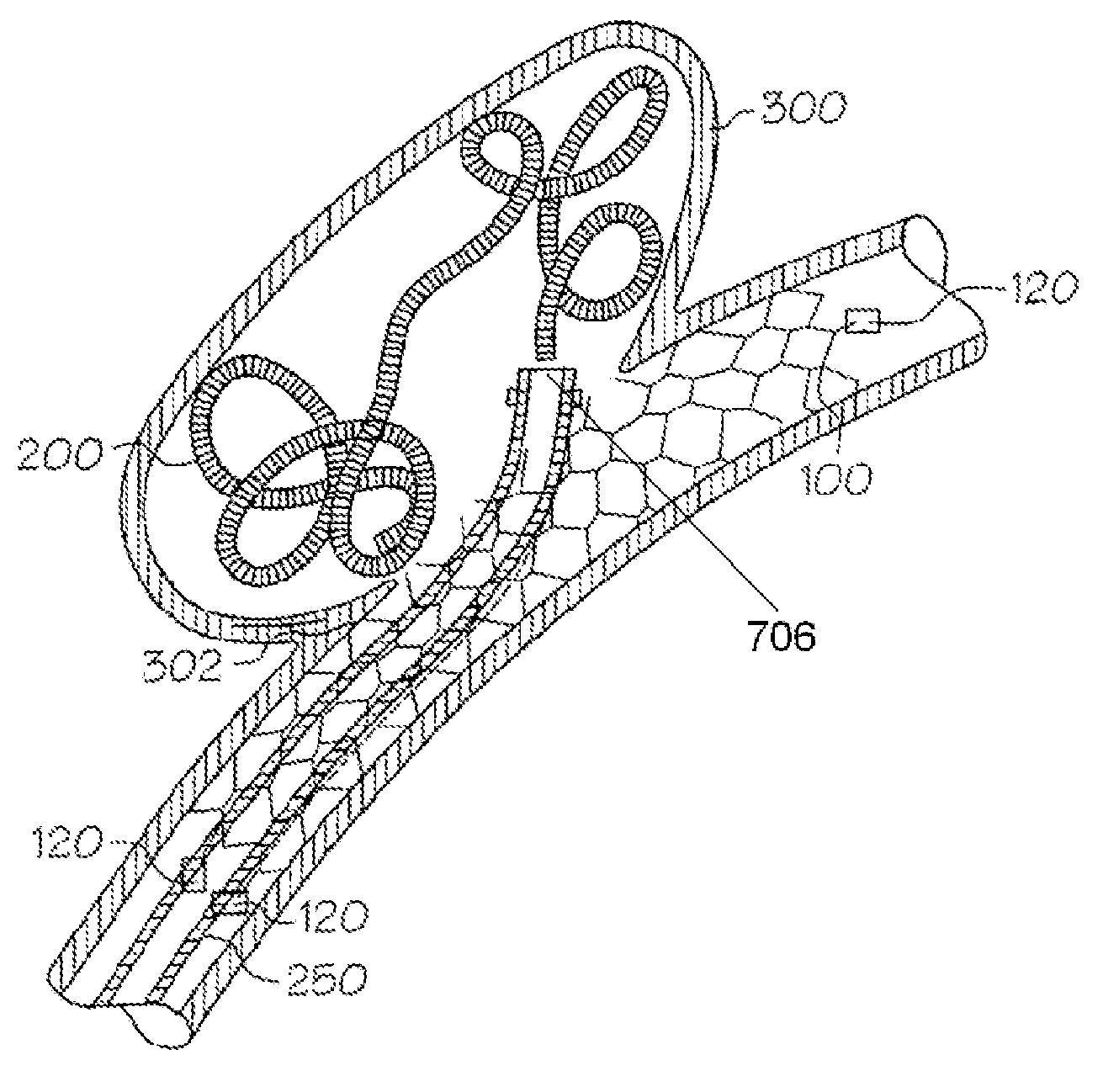
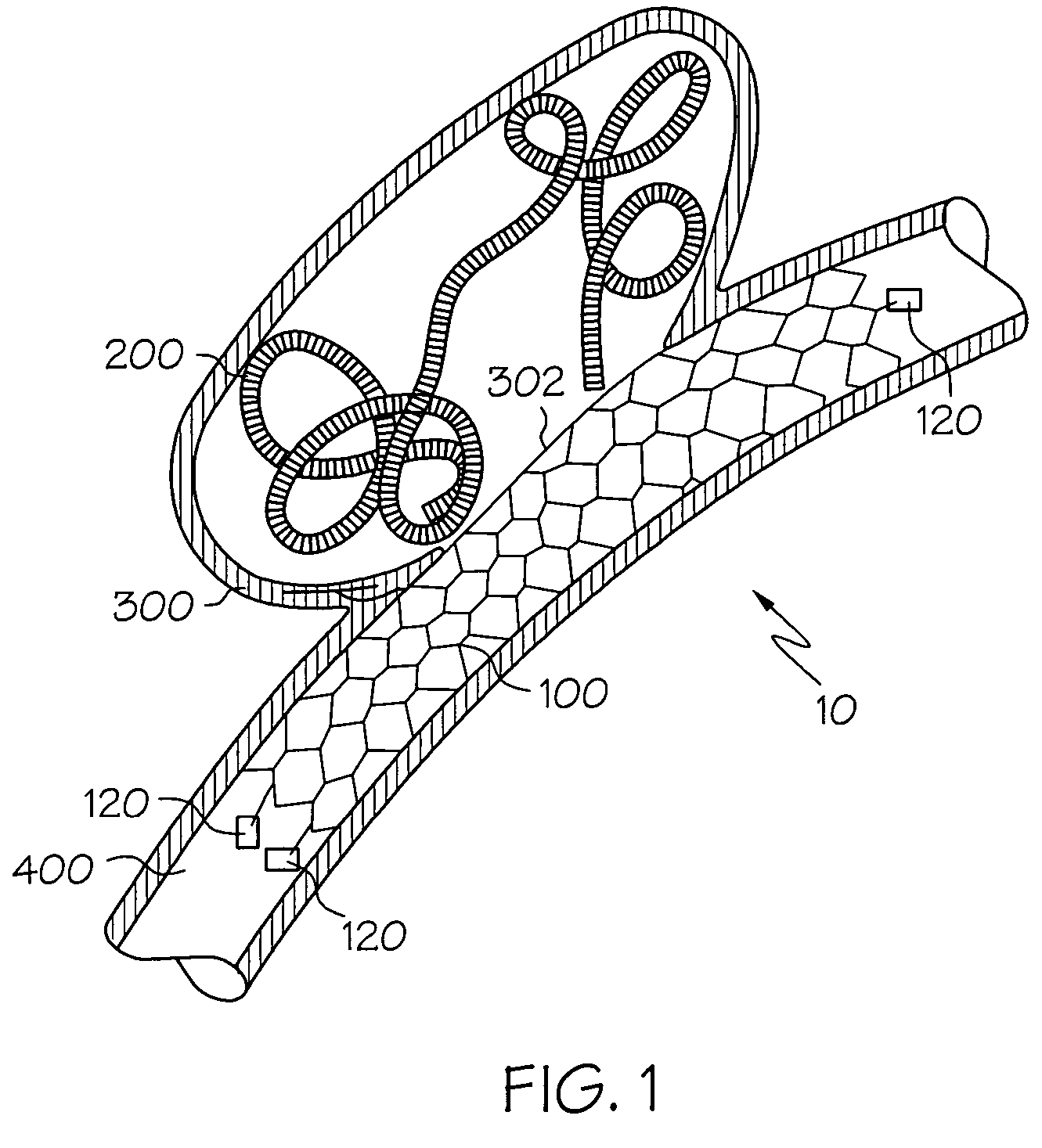
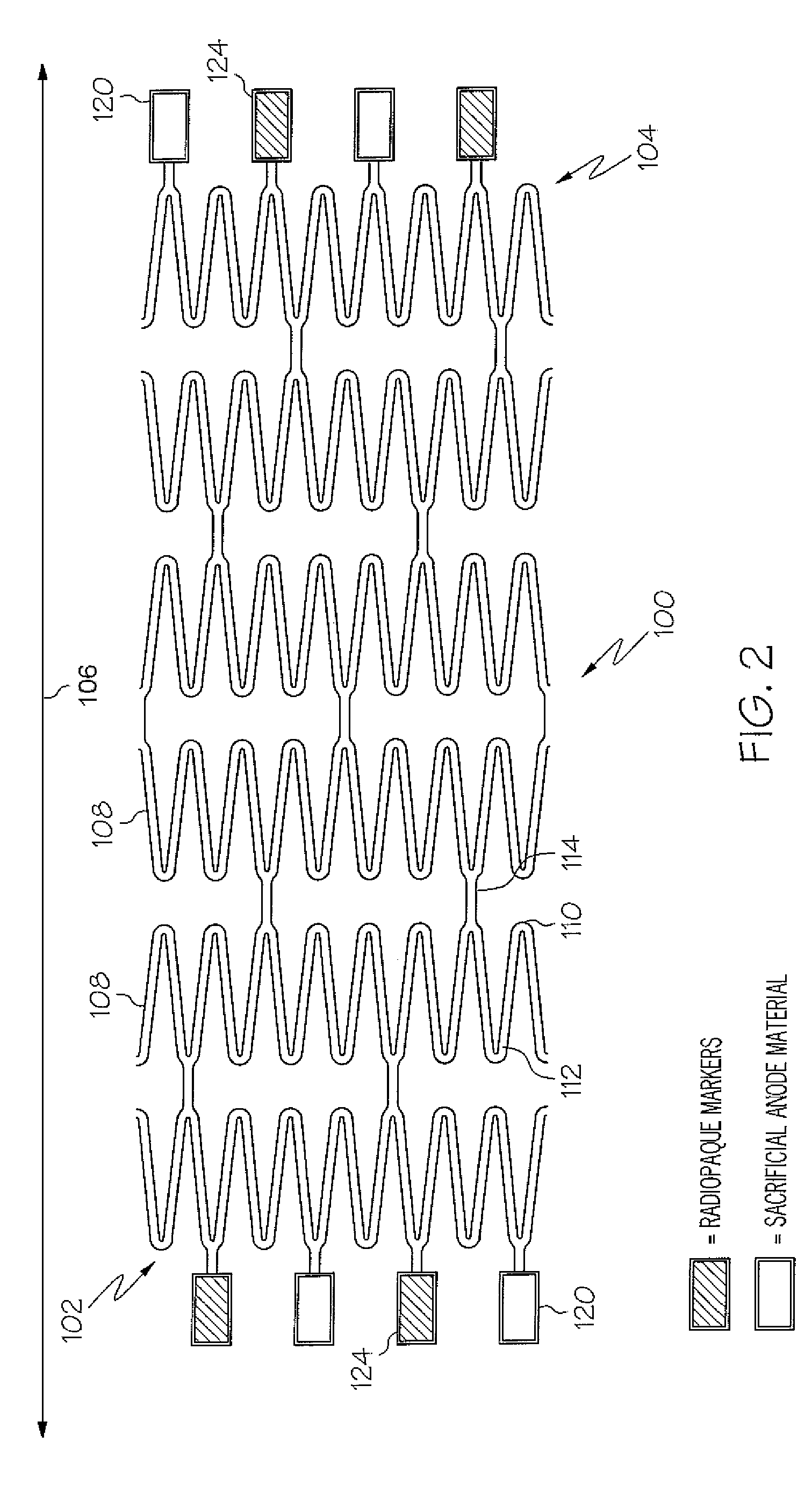
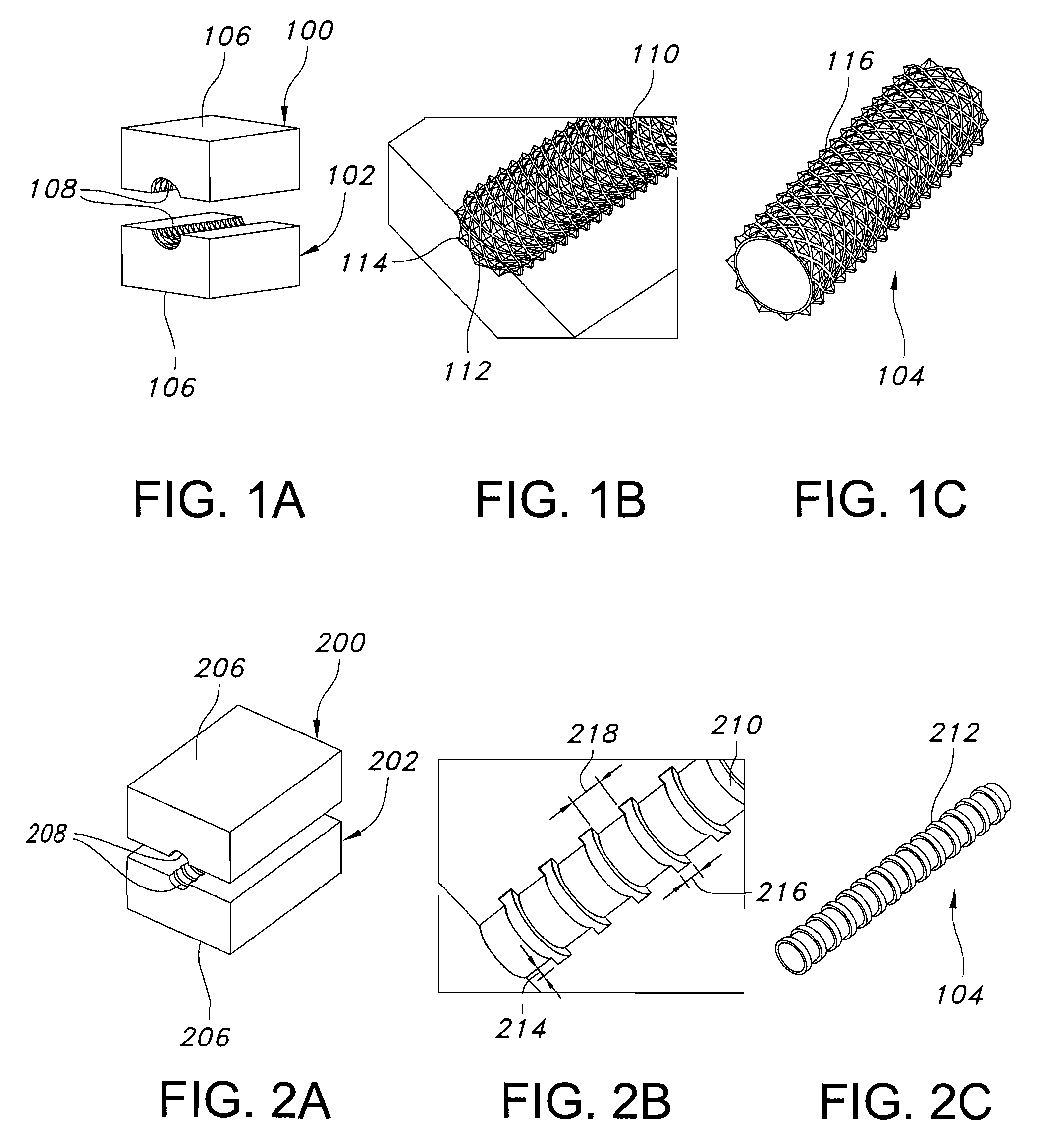
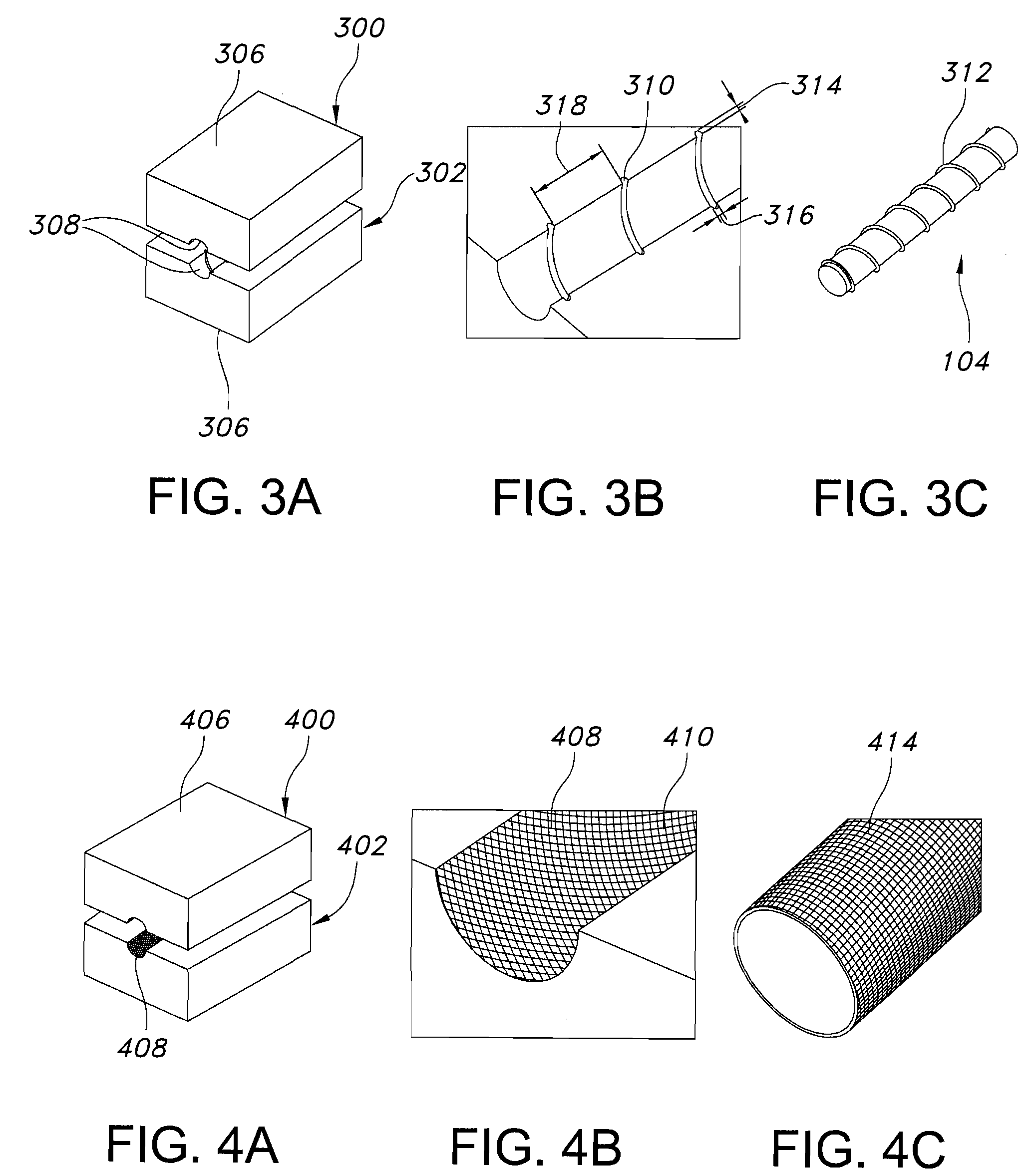
Galvanic Corrosion Methods and Devices for Fixation of Stent Grafts
Methods and devices are provided to contribute to improved stent graft fixation within vessels at treatment sites. Improved stent graft fixation within vessels at treatment sites is provided by providing stent grafts and methods of making and using stent grafts having structural scaffoldings which undergo controlled galvanic corrosion in situ. Other embodiments include stent grafts having galvanic cells attached to the vessel luminal wall-contacting sides. Still other embodiments include stent grafts that undergo controlled galvanic corrosion and include at least one additional cell growth promoting factor.
Owner:MEDTRONIC VASCULAR INC
Low leakage liquid atomization device
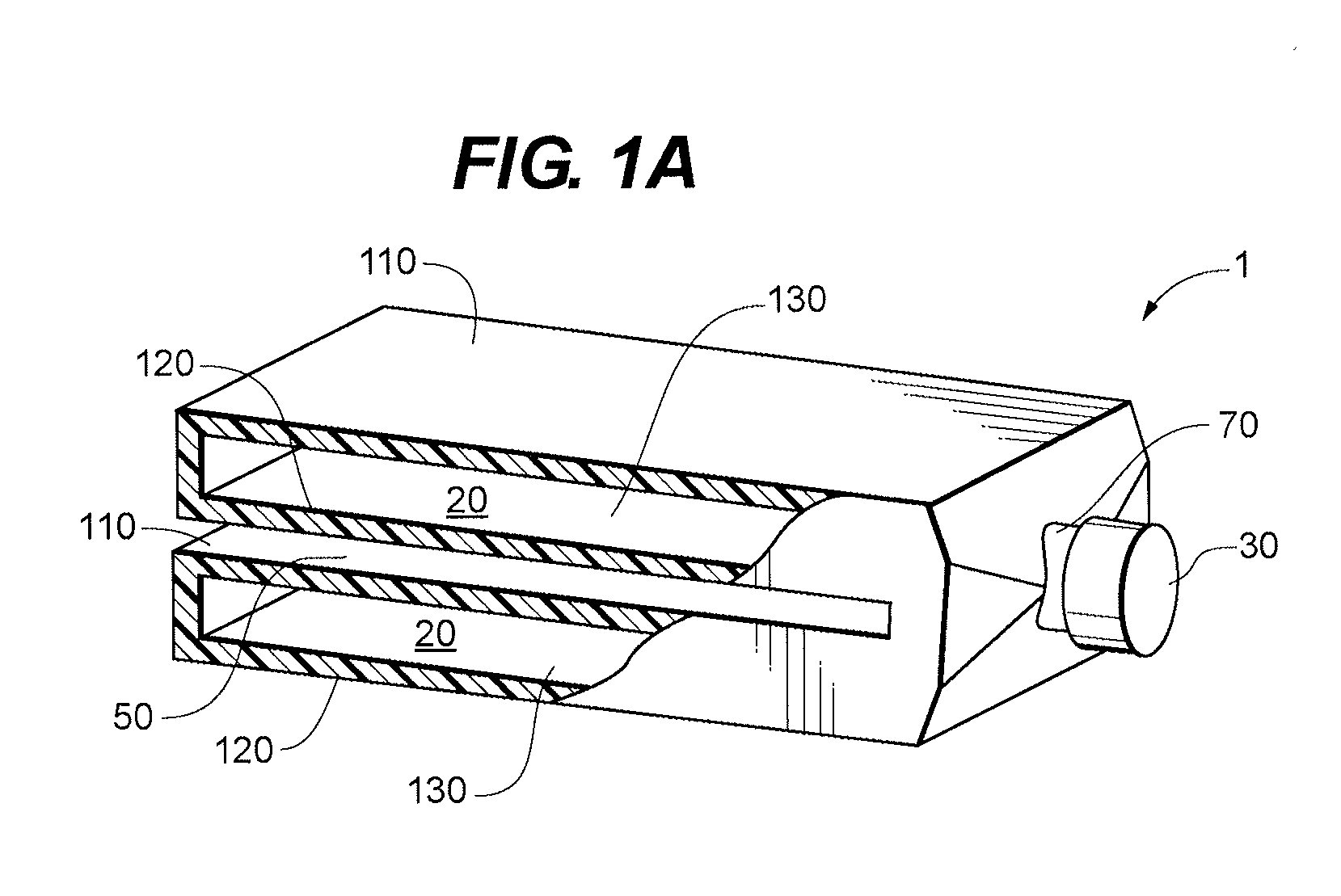
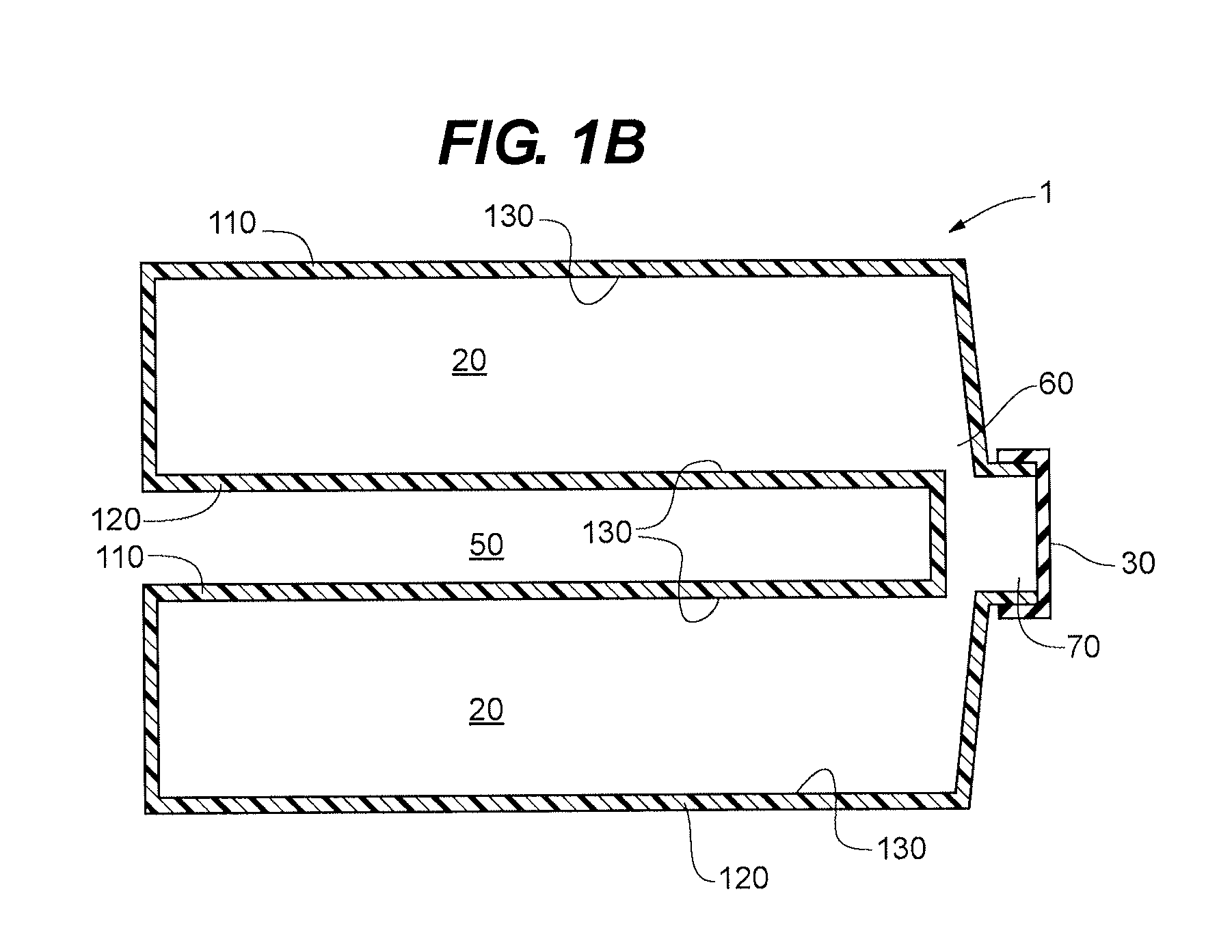
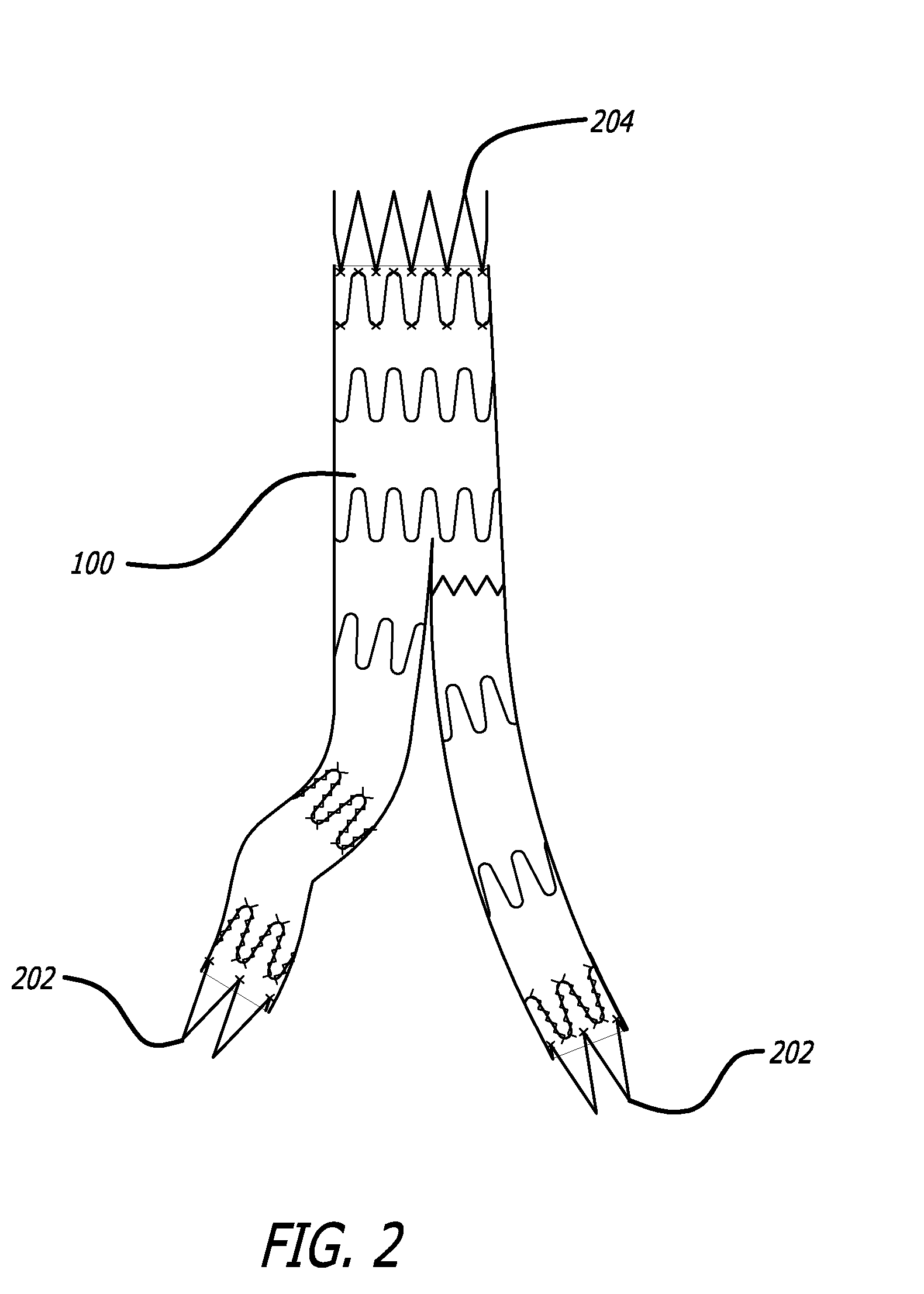
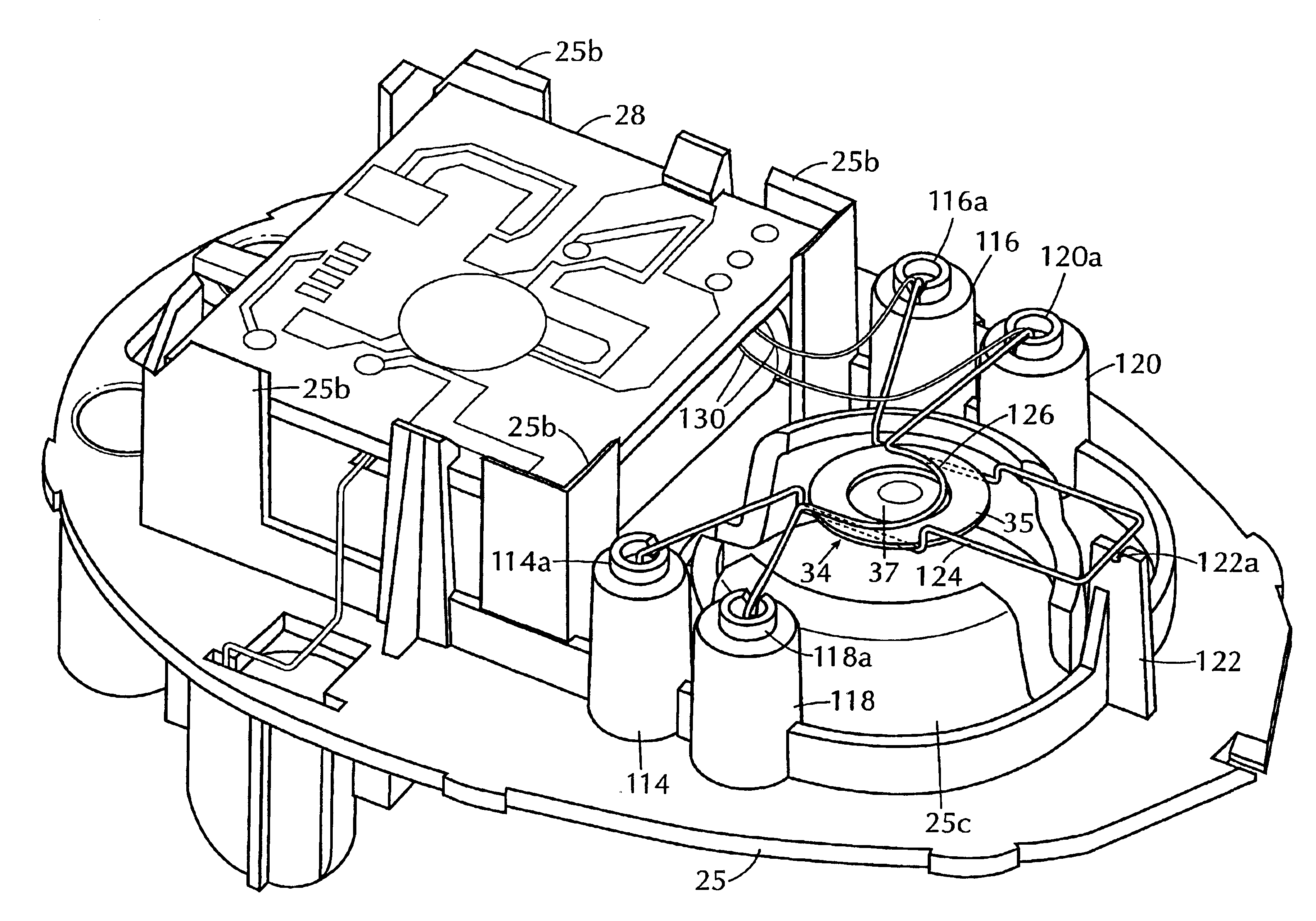
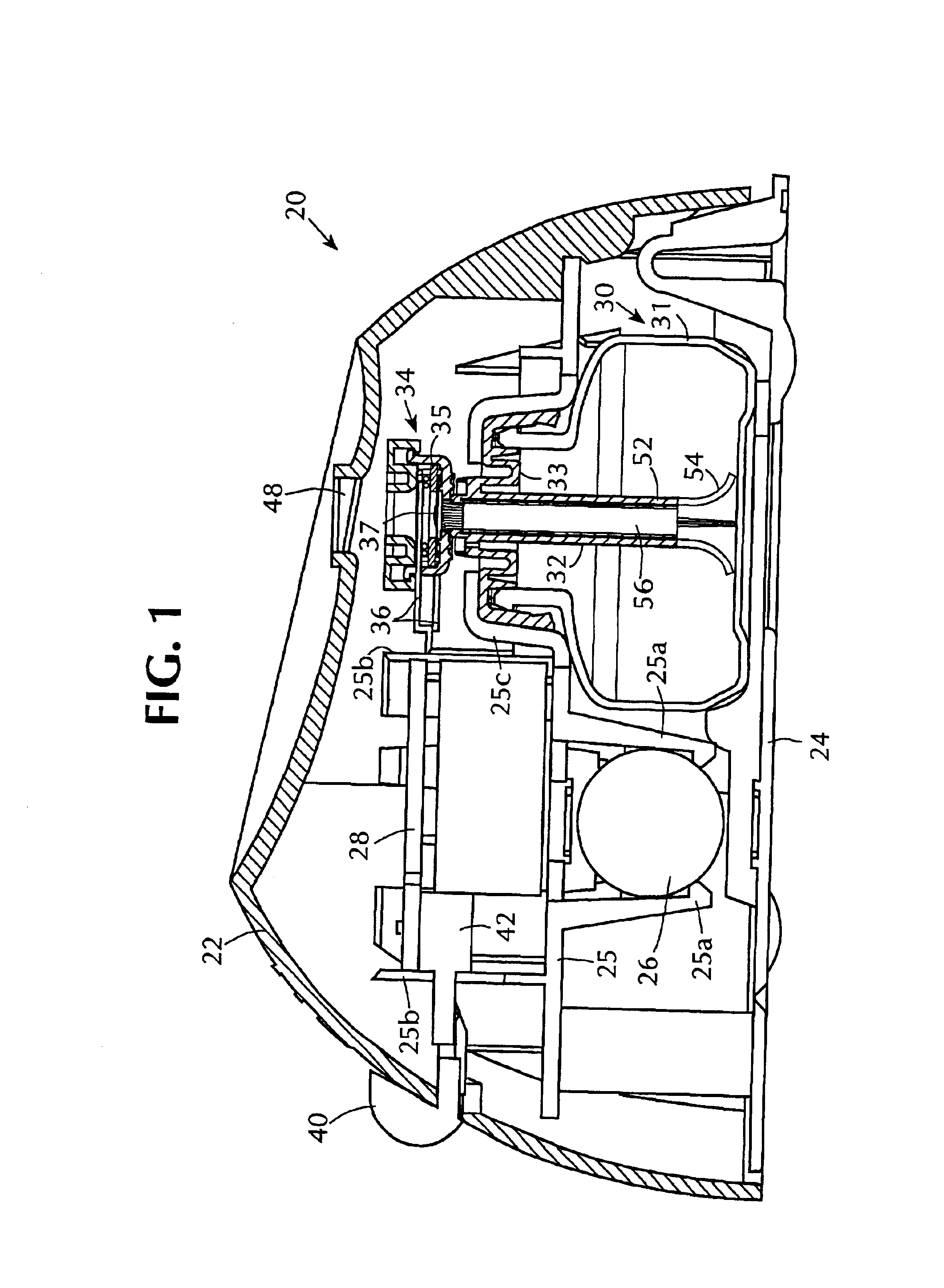
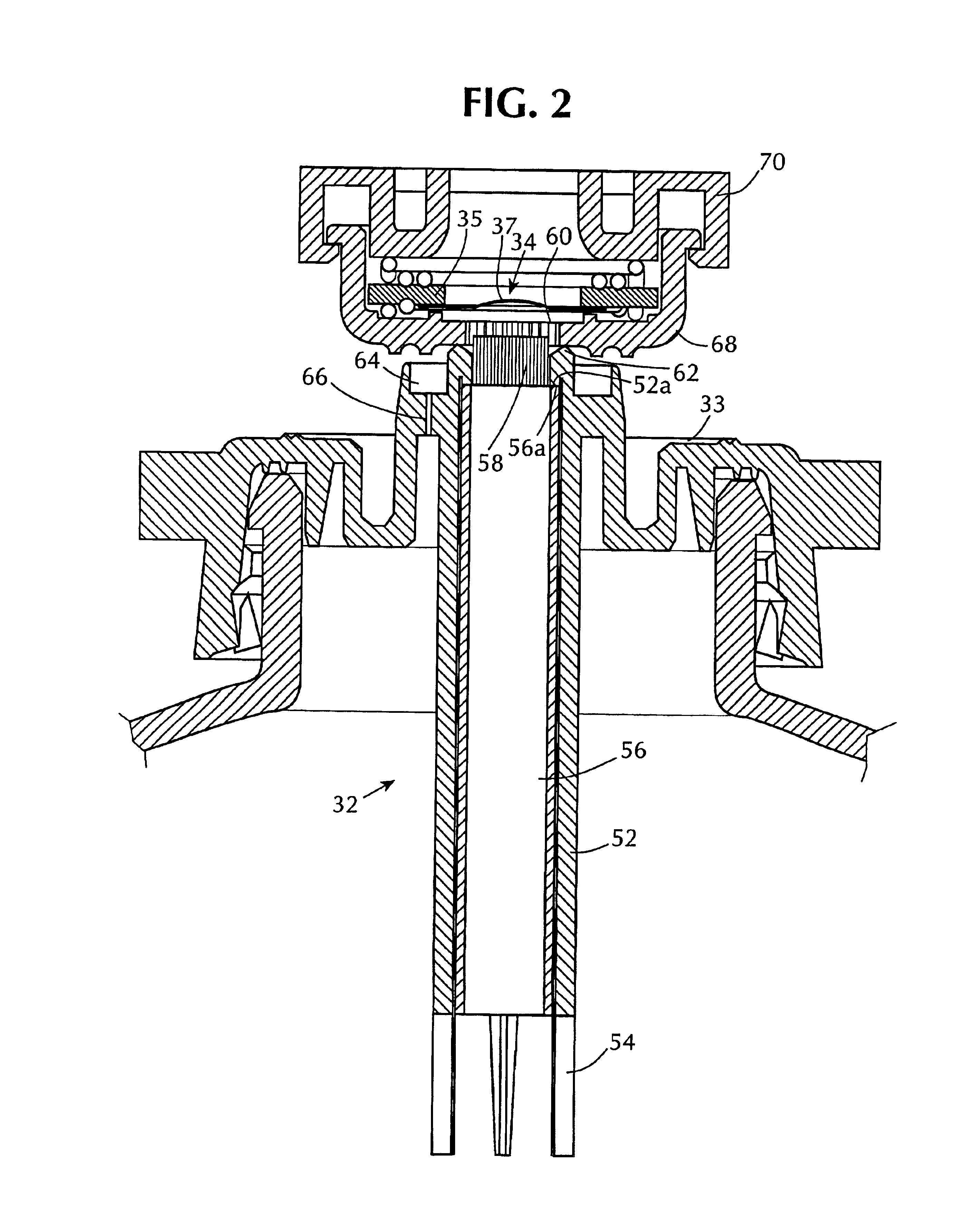
InactiveUS6843430B2Minimize migrationImprove device performanceMovable spraying apparatusSpray nozzlesElectricityNebulizer
A battery operated atomizer device comprising, in a housing (22), a liquid reservoir (30) from which a capillary type liquid delivery system (38) extends to contact a piezoelectric actuator an atomization plate assembly (34), the assembly (34) being supported by means of wire-like elements (36) in cantilever fashion over the liquid delivery system, the liquid delivery system comprising an outer tubular member (52) and a solid rod (56) which have facing surfaces configured to define between them, longitudinal capillary liquid passages.
Owner:SC JOHNSON & SON INC
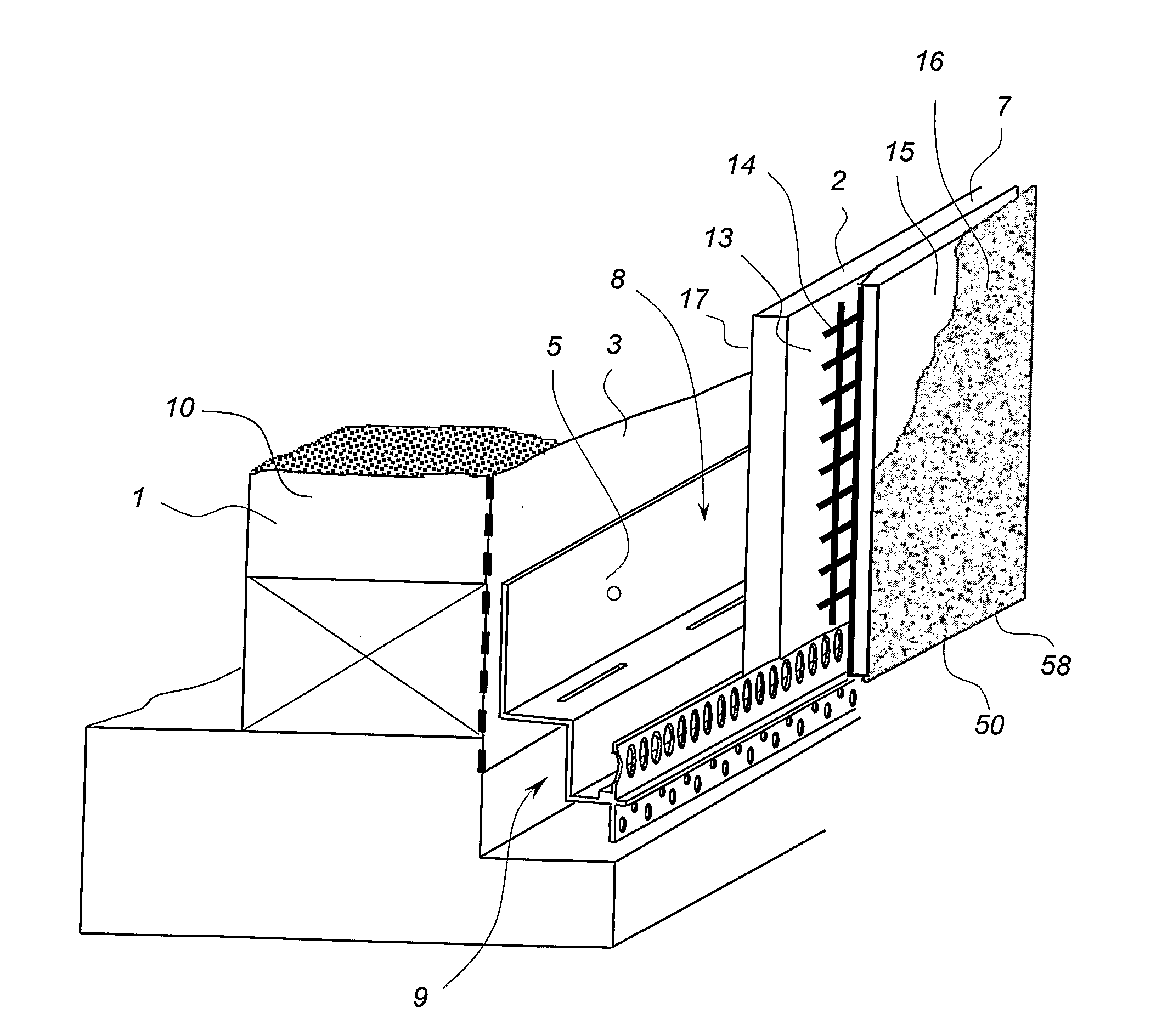
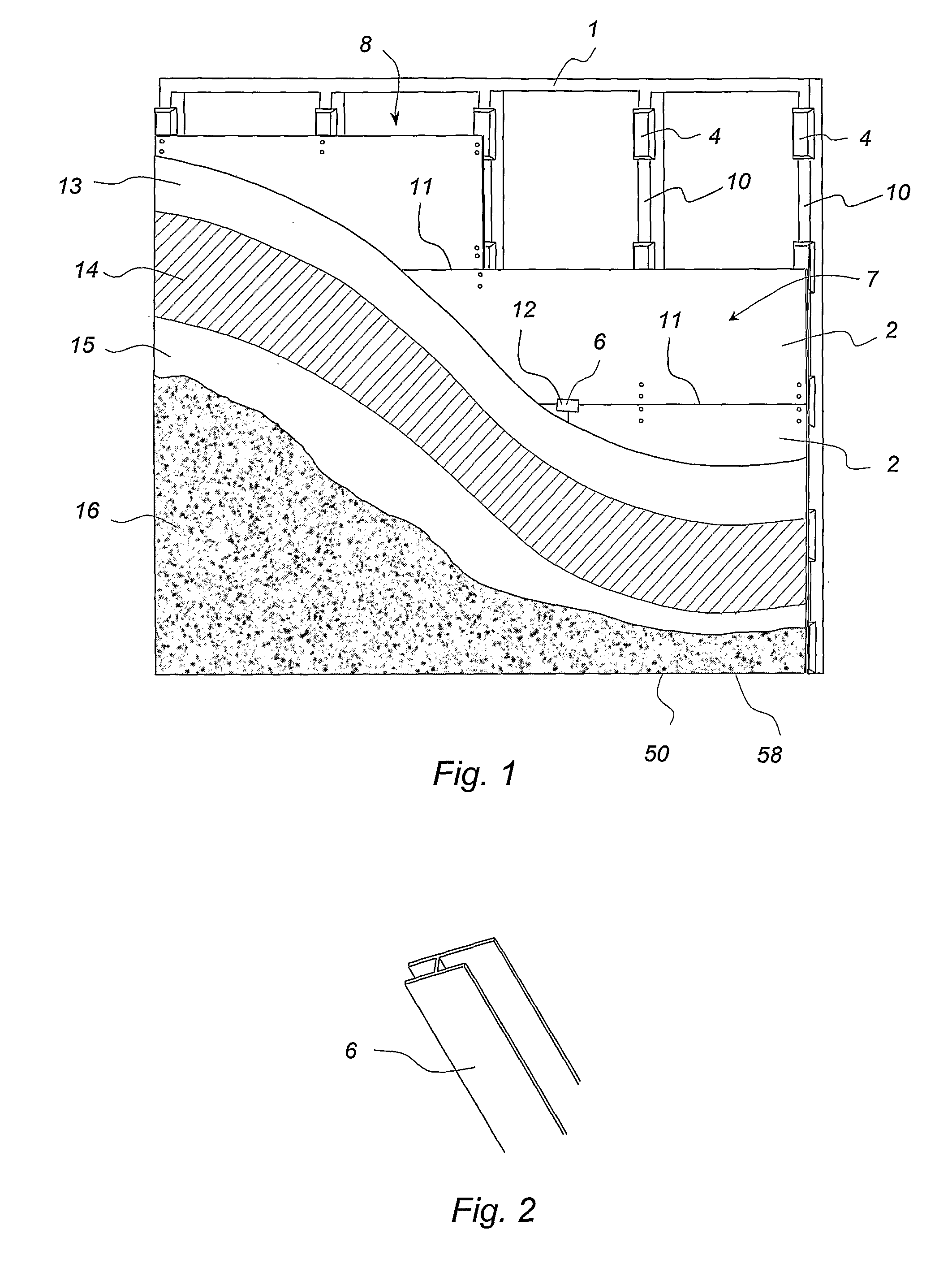
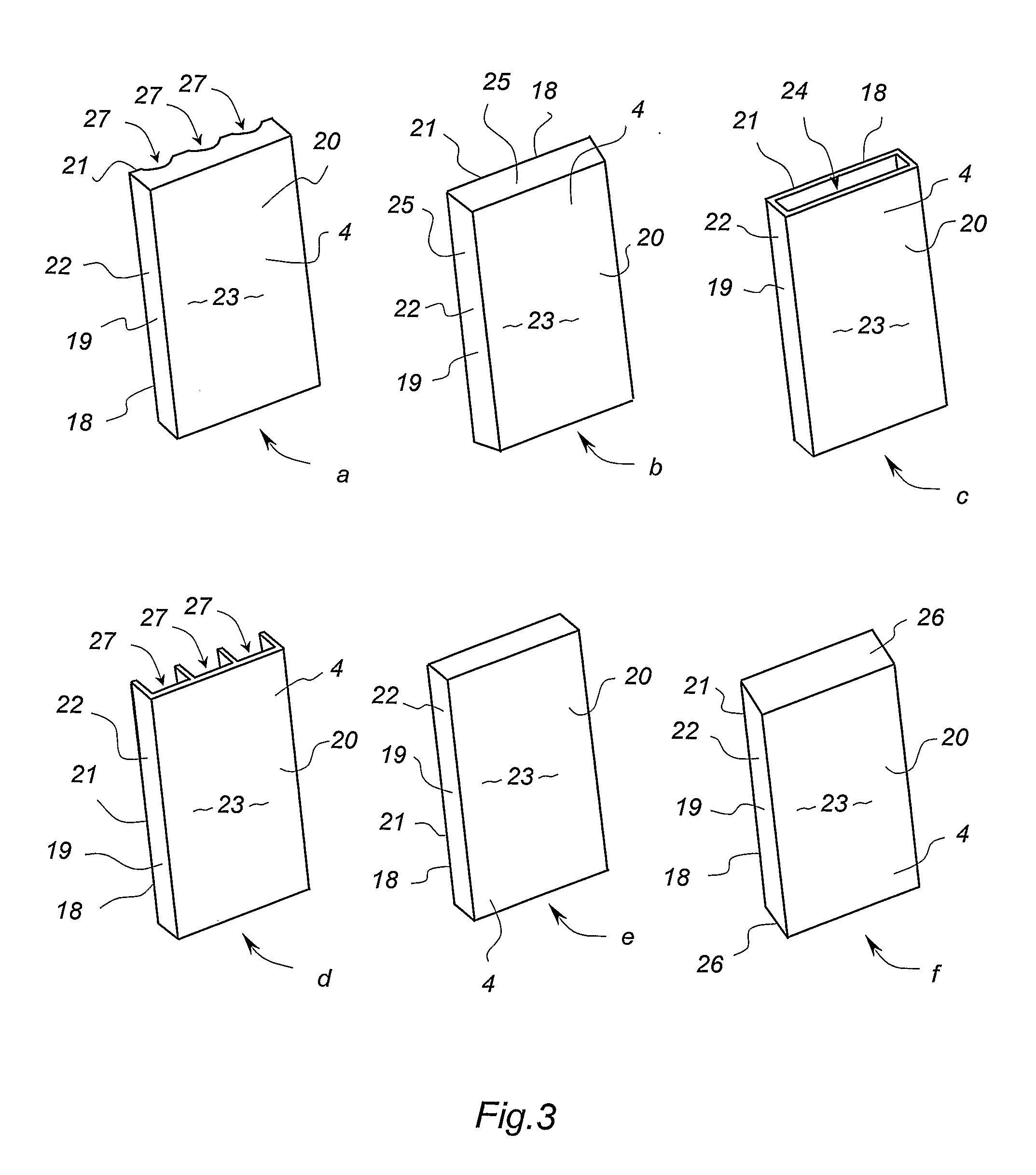
Cavity Wall System
InactiveUS20080104918A1Keep dryImprove thermal insulation propertiesCovering/liningsWallsStructural engineeringCavity wall
A cavity wall system and a method of forming a cavity wall including: a wall structure (1); a plurality of outer wall cladding panels (2); and a plurality of discrete mounting elements (4, 5, 6) for mounting the cladding panels a predetermined distance away from the wall structure (1) so as to form a substantially flat exterior wall surface (7) and a substantially uninterrupted internal wall cavity (8) between the cladding panels (2) and the wall structure (1); the mounting elements (4, 5, 6) being sized and arranged so as to allow substantially uninterrupted fluid flow throughout the cavity (8); and the system further including moisture control means defining a moisture control plane to minimise migration of liquid moisture from the cavity (8) into the wall structure (1). The invention also provides a mounting member (4) and a termination member (5) for use as mounting elements in the cavity wall system and method.
Owner:JAMES HARDIE TECH LTD
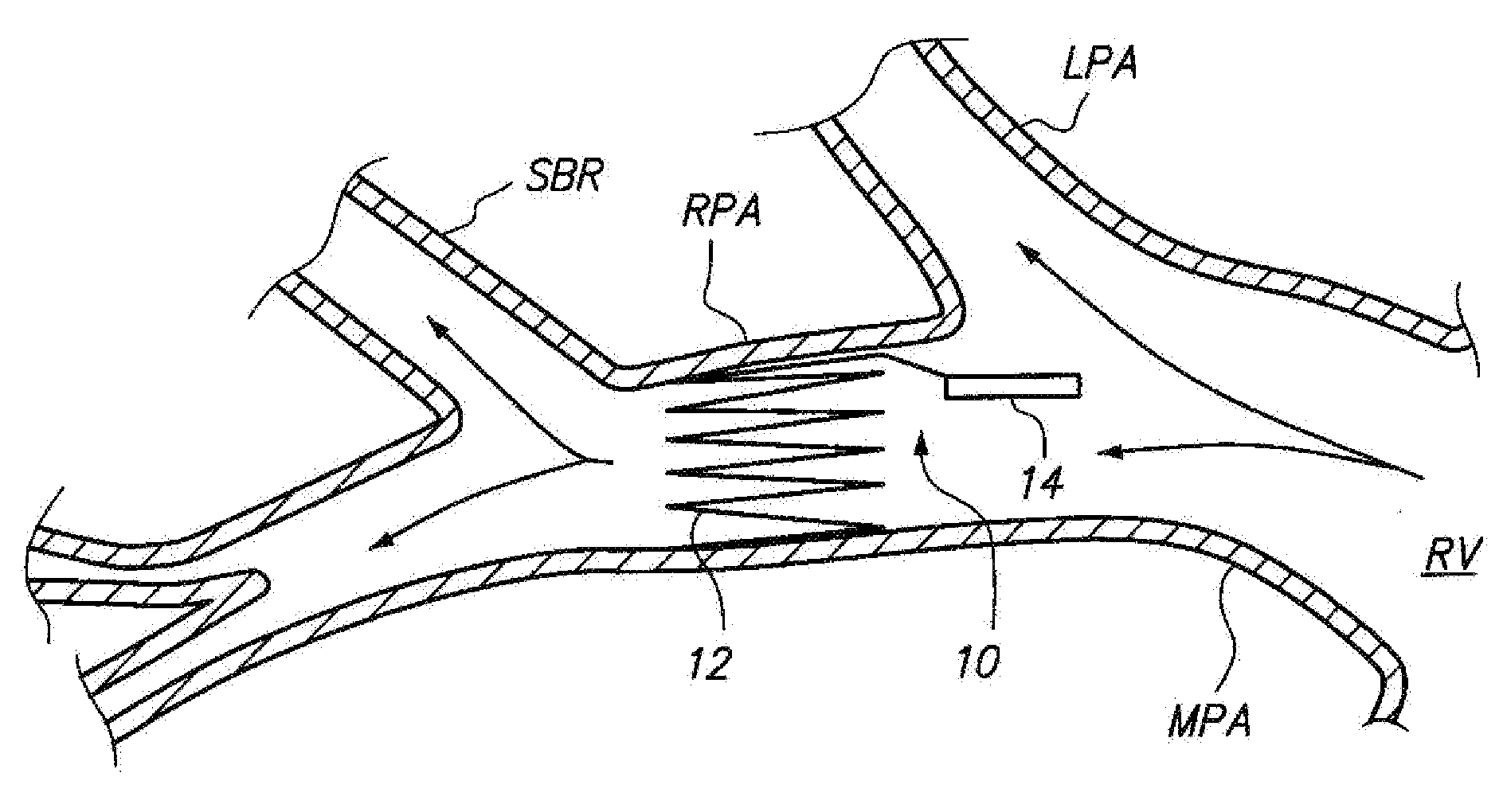
Devices For Fixing A Sensor In A Lumen
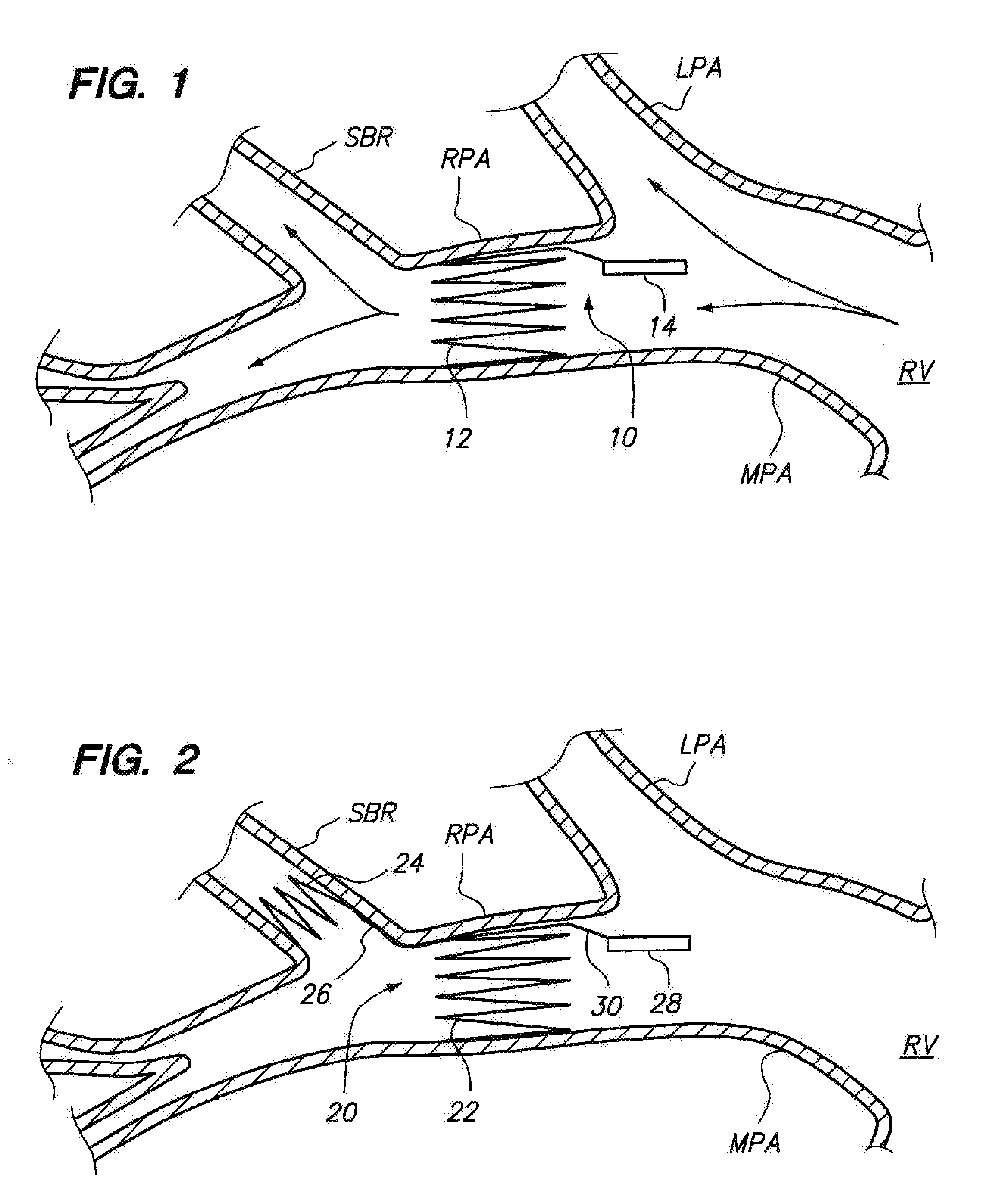
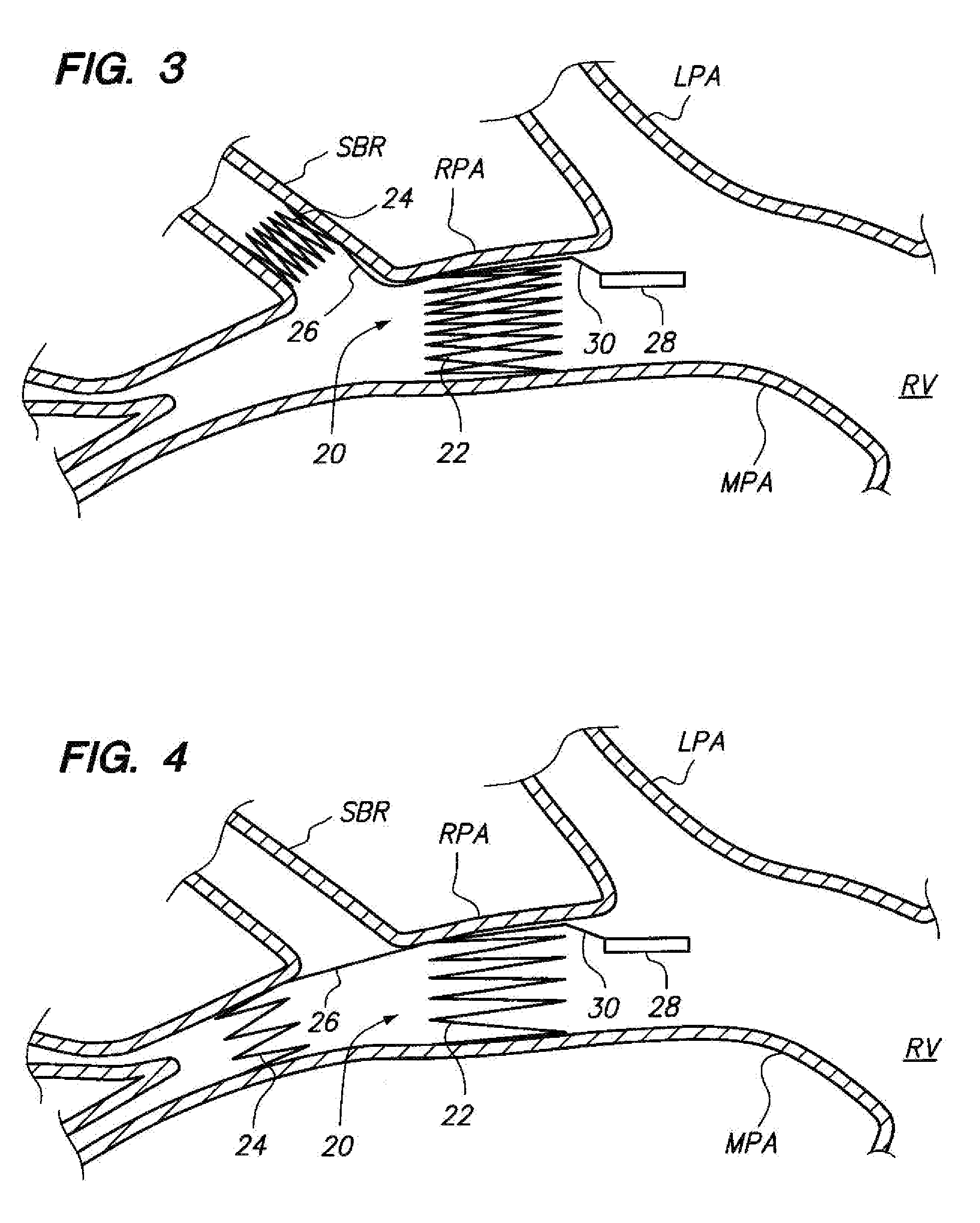
Sensing devices and methods of implanting sensing devices within an anatomical vessel network of a patient are provided. In one method, a fixation element (e.g., a stent or coil) of the sensing device is expanded into firm contact with a wall of a main anatomical vessel (e.g., a right pulmonary artery), and a stabilization element of the sensing device is placed into contact with a wall of an anatomical vessel branch of the main anatomical vessel. In another method, a first fixation element of the sensing device is expanded into firm contact with the wall of the main anatomical vessel (e.g., a right pulmonary artery) at a longitudinal location proximal to the anatomical vessel branch, and a second fixation element of the sensing device is expanded into firm contact with the wall of the main anatomical vessel at a longitudinal location distal to the anatomical vessel branch.
Owner:REMON MEDICAL TECH
Systems and methods of blood-based therapies having a microfluidic membraneless exchange device
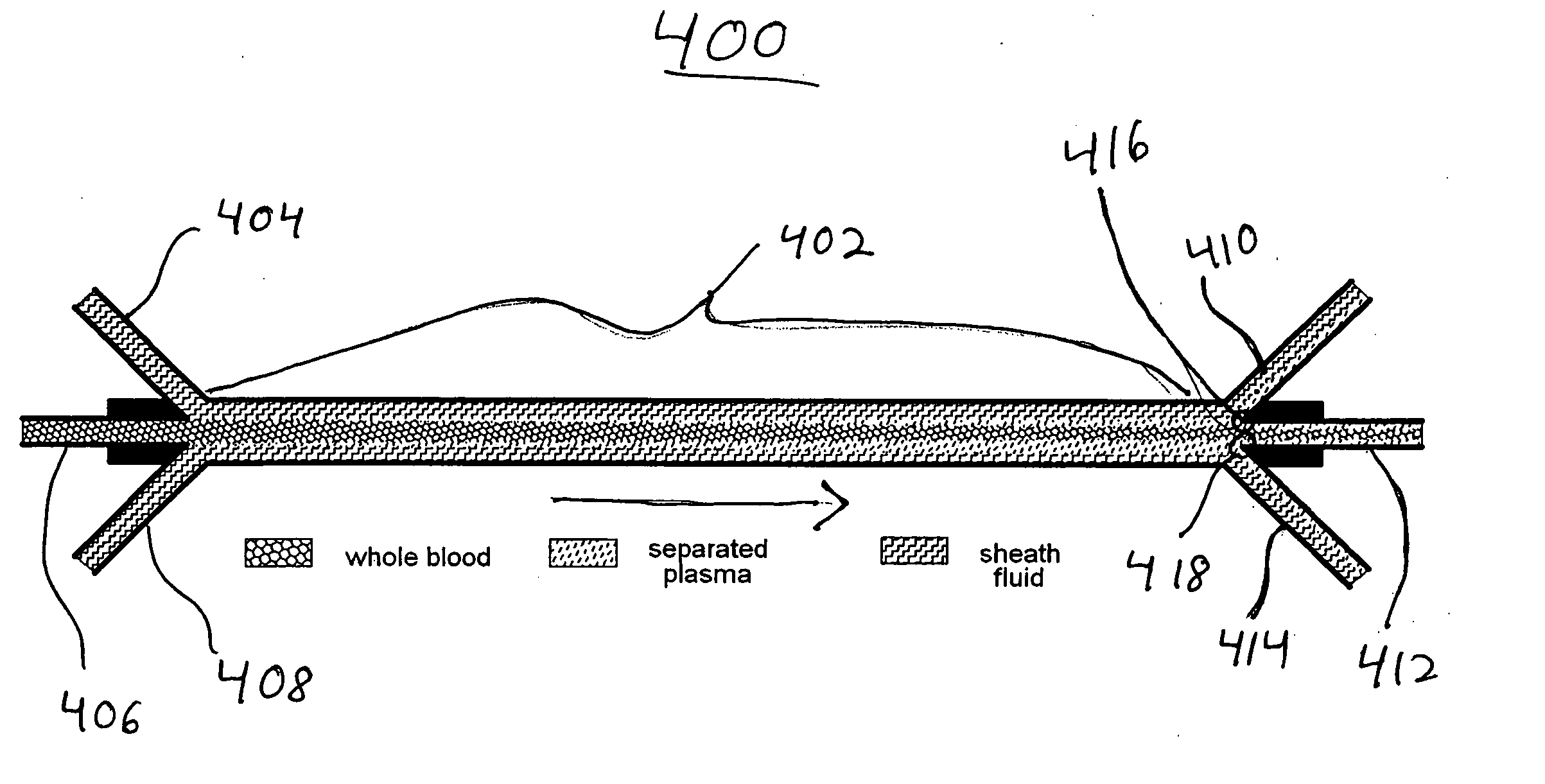
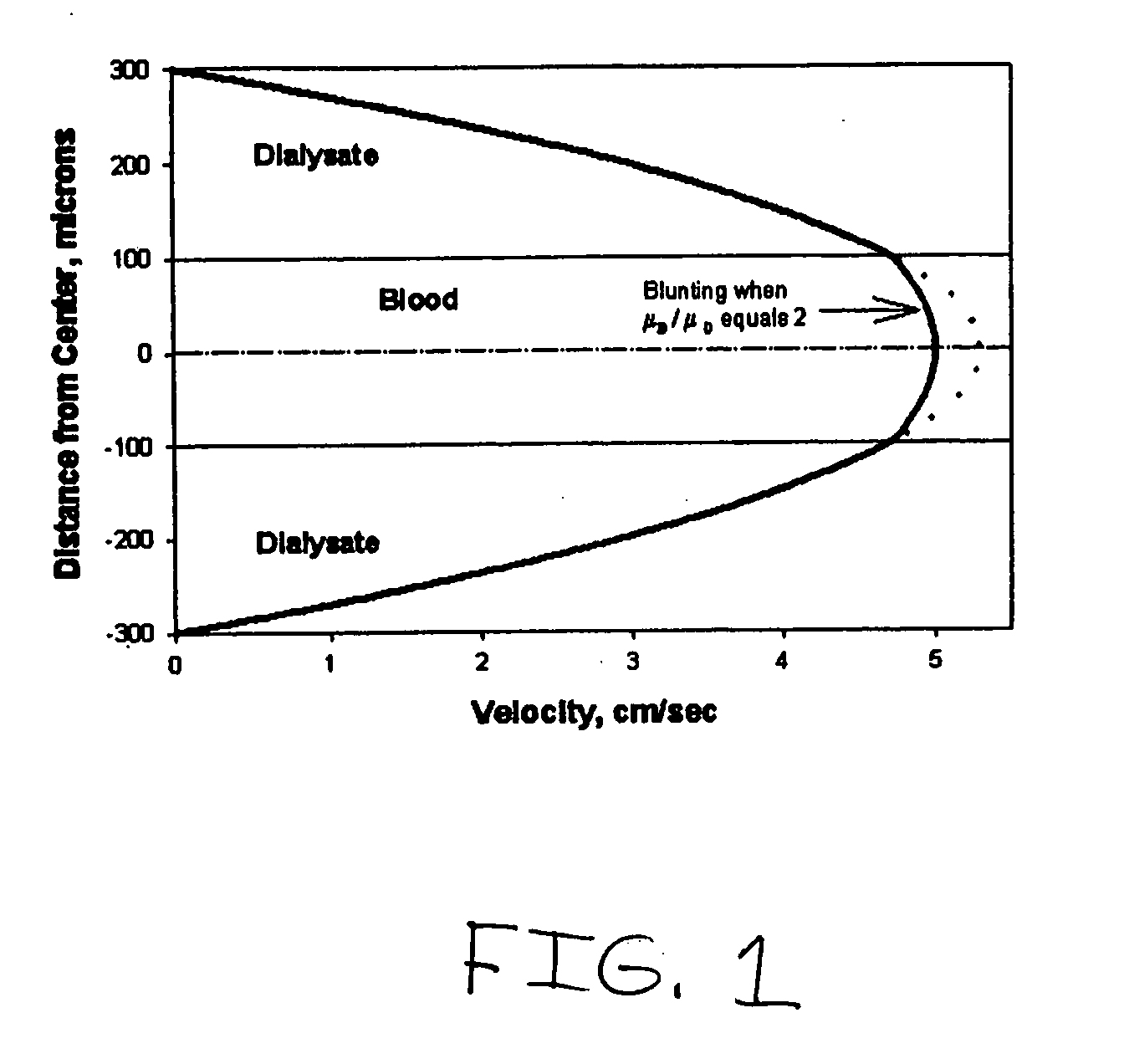
InactiveUS20060076295A1Reduces undesirable activationMinimizing bioincompatibilitiesComponent separationMedical devicesDiffusionThin layer
The present invention is directed to devices, systems and methods for removing undesirable materials from a sample fluid by contact with a second fluid. The sample fluid flows as a thin layer adjacent to, or between, concurrently flowing layers of the second fluid, without an intervening membrane. In various embodiments, a secondary separator is used to restrict the removal of desirable substances and effect the removal of undesirable substances from blood. The invention is useful in a variety of situations where a sample fluid is to be purified via a diffusion mechanism against an extractor fluid. Moreover, the invention may be used for the removal of components from a sample fluid that vary in size. When blood is the sample fluid, for example, this may include the removal of ‘small’ molecules, ‘middle’ molecules, macromolecules, macromolecular aggregates, and cells, from the blood sample to the extractor fluid.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
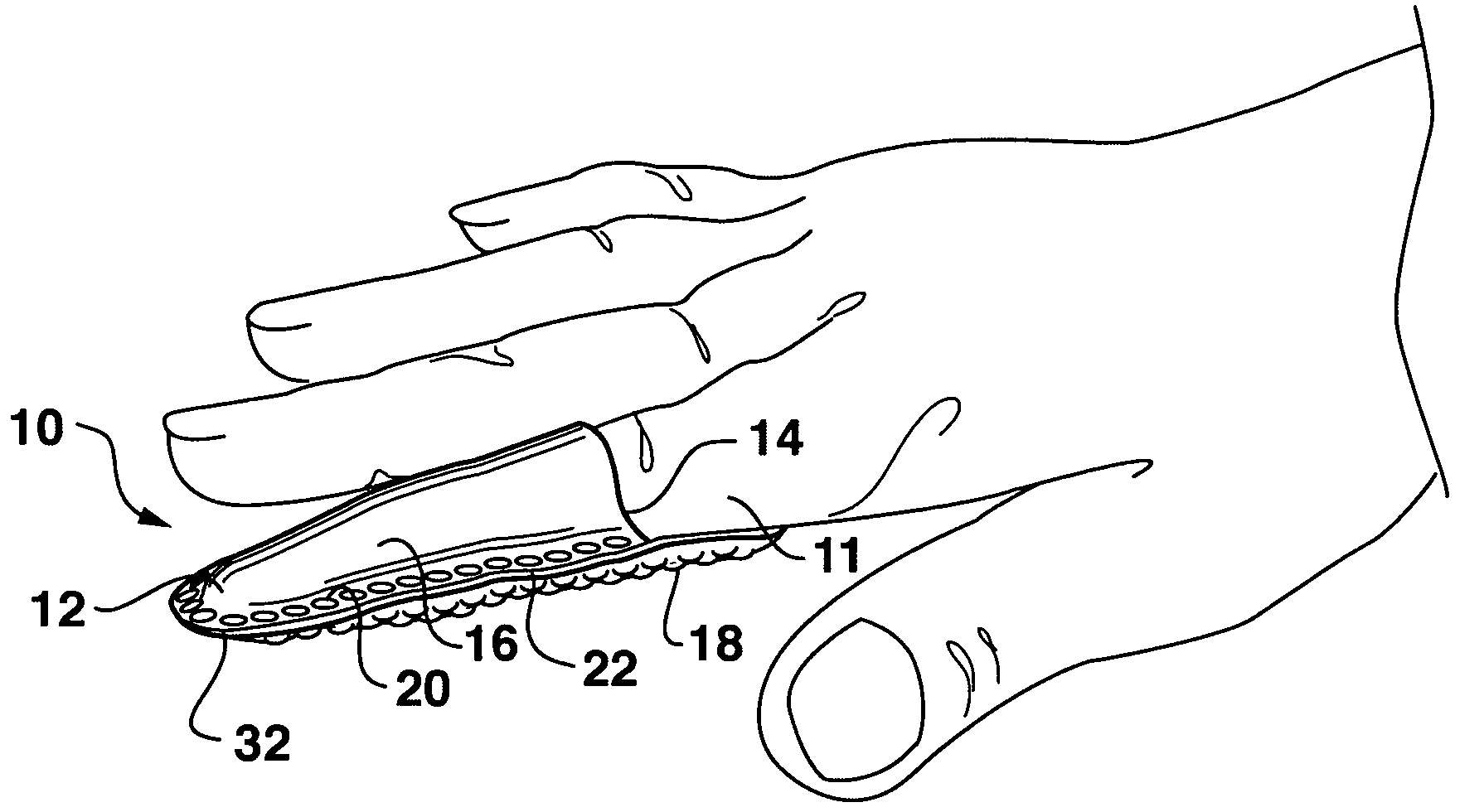
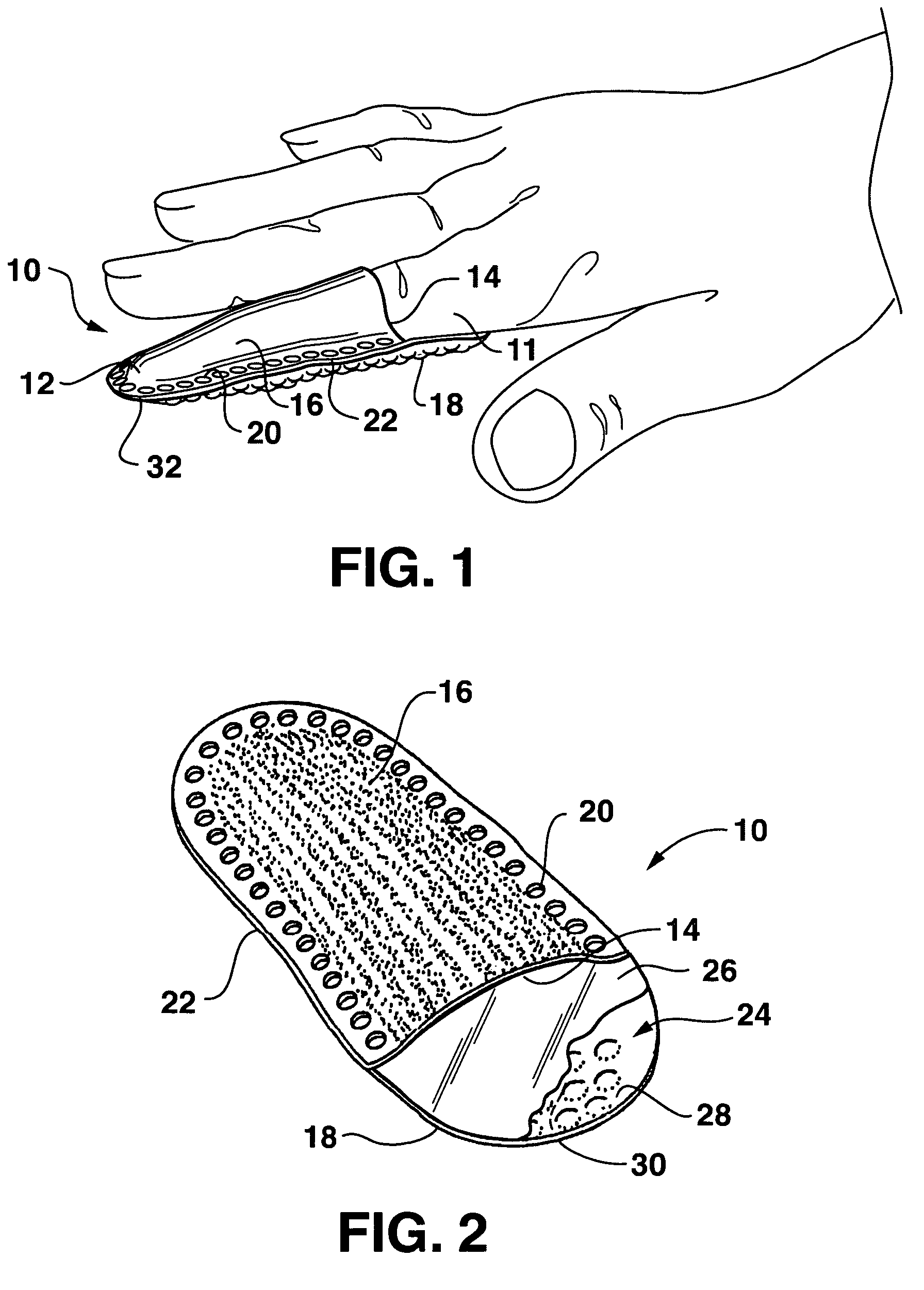
Gastrointestinal anchor compliance
ActiveUS7976488B2Improve abilitiesEasy to keepOesophagiIntravenous devicesDiseaseIntestinal structure
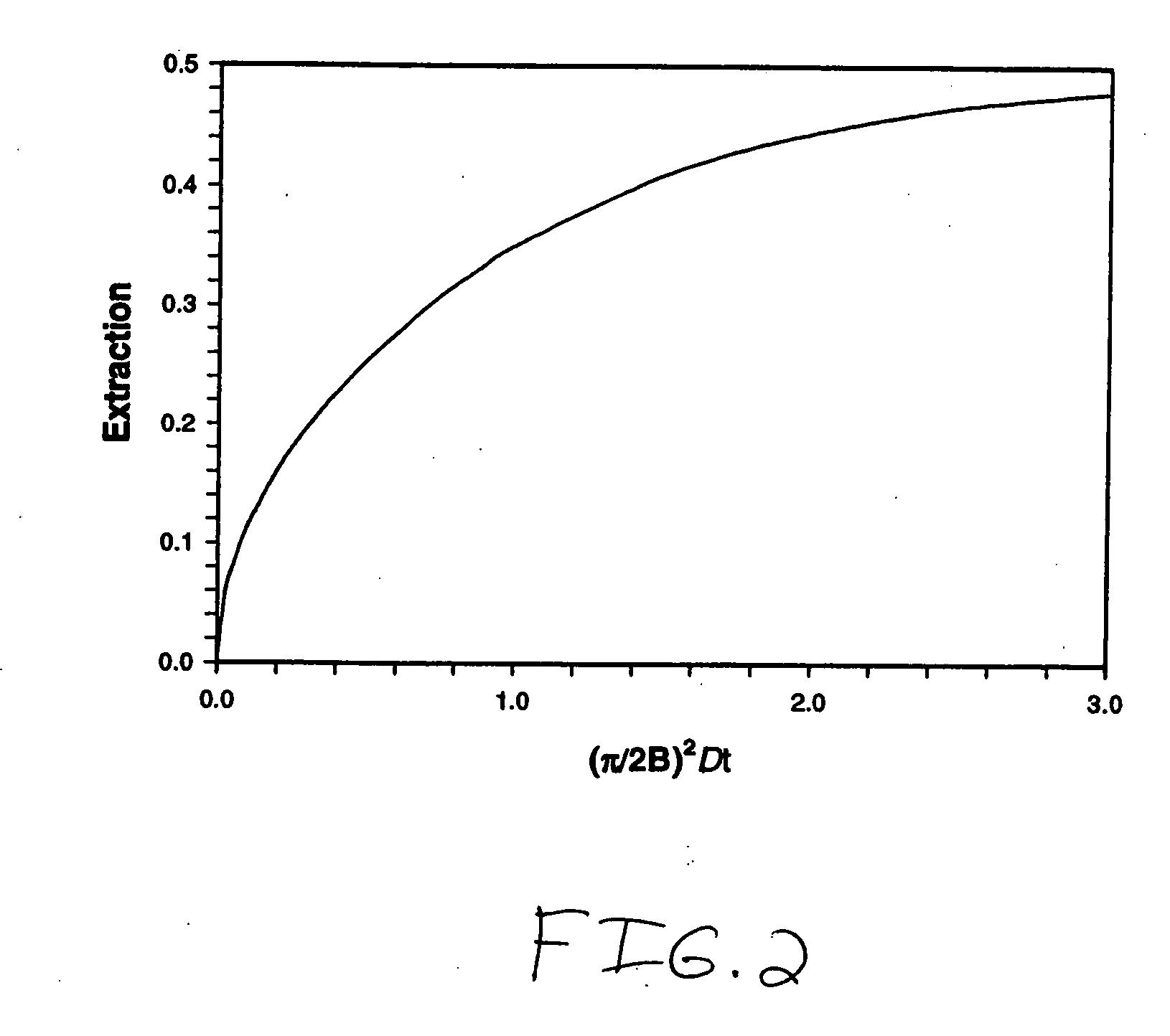
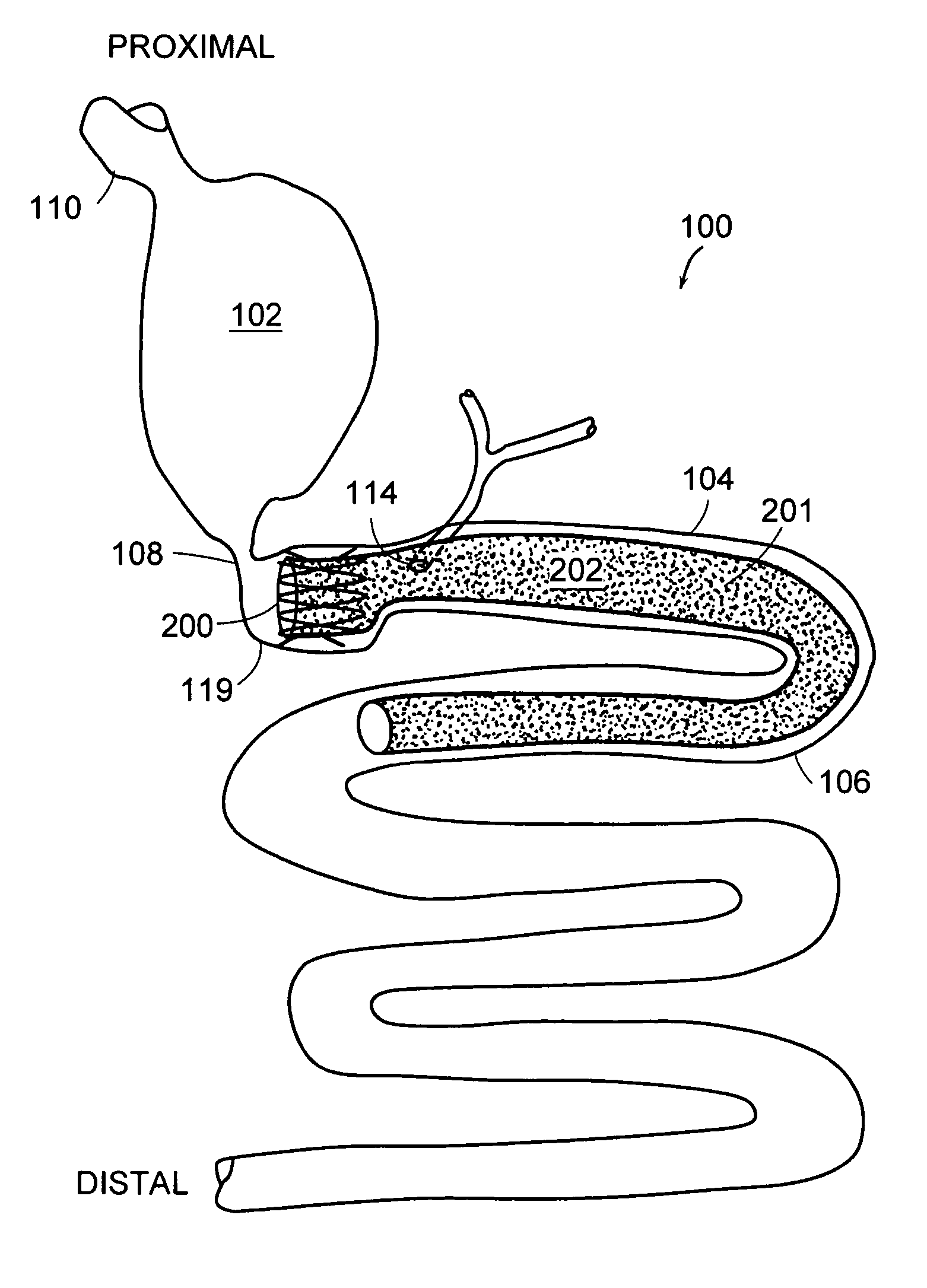
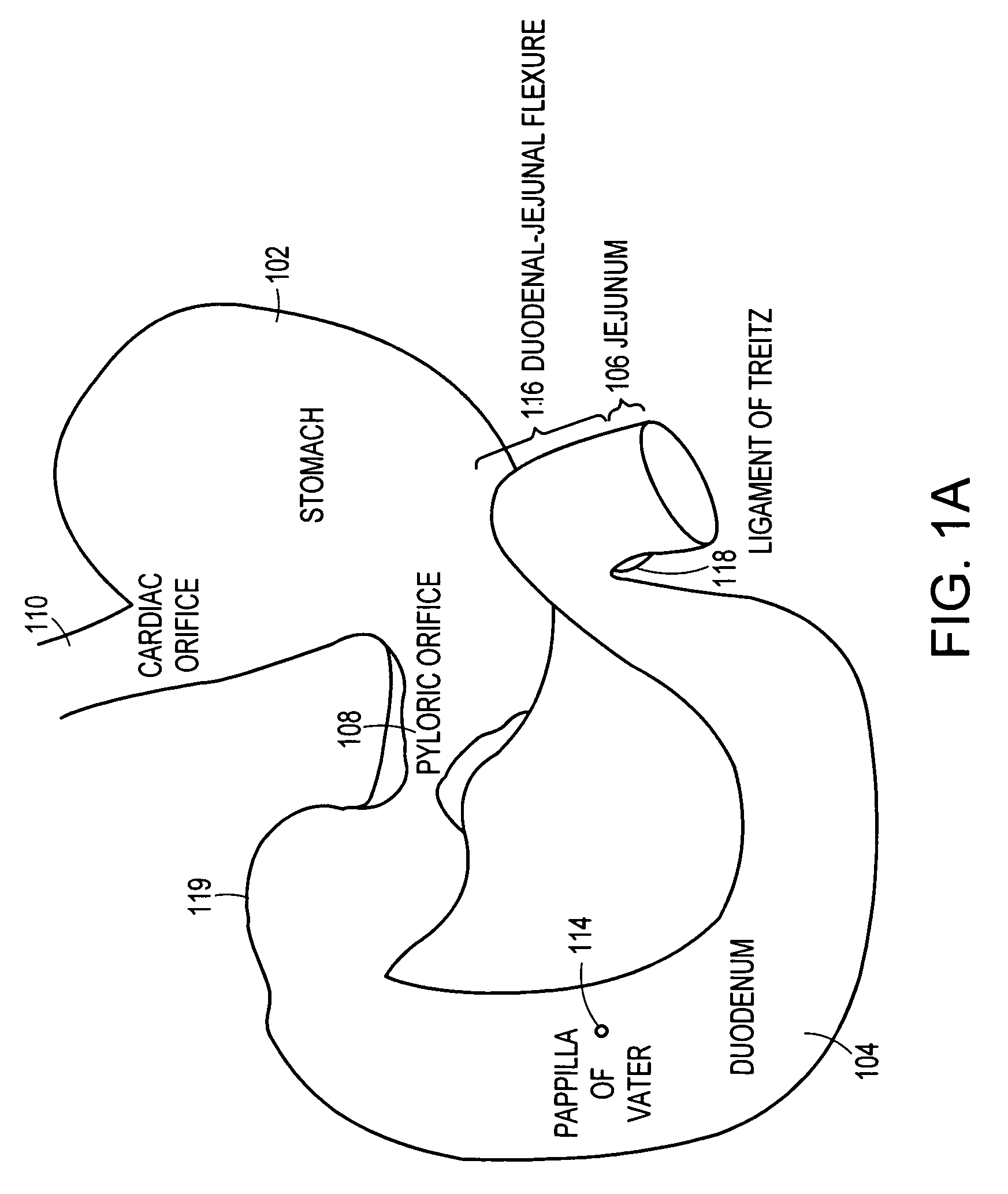
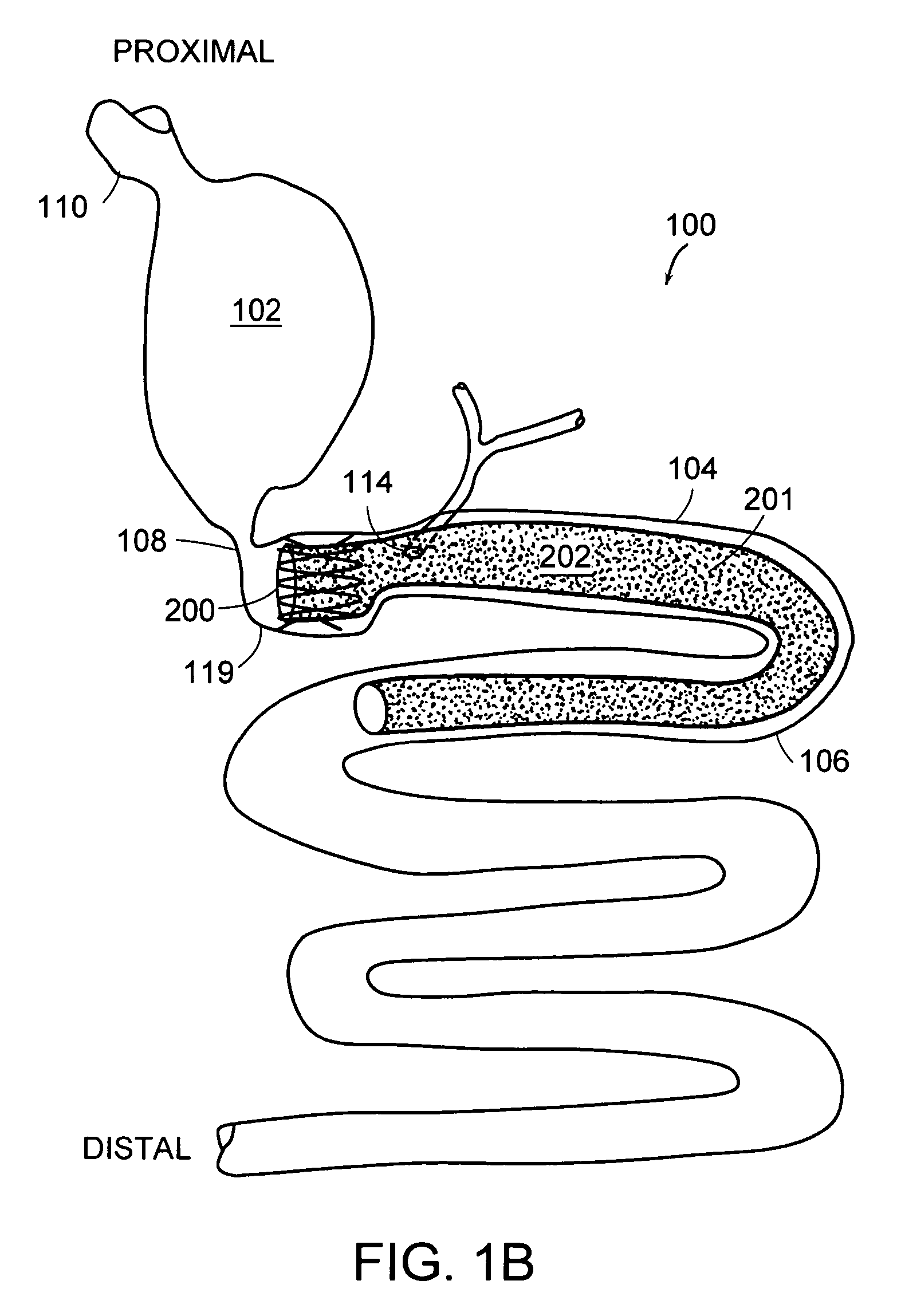
A collapsible gastrointestinal anchor can be characterized in various embodiments by a radial force of about 0.1 Newtons (N) or greater at a compressed diameter of 25 millimeters (mm); by an average spring rate of about 13 Newtons / meter (N / m) or greater in a range of motion between a relaxed diameter and a compressive elastic deformation diameter; or by a radial force over the range of motion of about 0.1 N or greater. Typically, the anchor can be adapted to be retained within a subject's intestine, more typically in the duodenum, or particularly in the duodenal bulb just distal to the pylorus.A gastrointestinal implant device includes the collapsible gastrointestinal anchor and a floppy sleeve. The sleeve is open at both ends and adapted to extend into a subject's intestine, the anchor being coupled to a proximal portion of the sleeve.Also include are methods of implanting the gastrointestinal implant device in a subject, and methods of treating a subject for disease.The disclosed gastrointestinal invention leads to an improved ability to secure anchors and devices in the gastrointestinal tract while tending to minimize migration.
Owner:GI DYNAMICS
Prostheses for spine discs having fusion capability
InactiveUS20060052874A1Reduced flexibilityMiniaturization exerciseSpinal implantsProsthesisEngineering
The present invention provides both a device and a method. The device is a human made replacement for the soft discs in the spine. A fabric pouch encloses a central hydraulic element made up of small soft beads. Two pouches with beads are implanted into a prepared disc space to function as an intervertebral disc. The method is conversion of the device into a fusion element.
Owner:UNIV OF SOUTH FLORIDA
Highly efficient devices and methods for culturing cells
InactiveUS20080176318A1Minimize migrationMinimize potentialBioreactor/fermenter combinationsBiological substance pretreatmentsChemistryCulture cell
This invention relates to methods and devices that improve cell culture efficiency. They include the use of gas permeable culture compartments that reduce the use of space while maintaining uniform culture conditions, and are more suitable for automated liquid handling. They include the integration of gas permeable materials into the traditional multiple shelf format to resolve the problem of non-uniform culture conditions. They include culture devices that use surfaces comprised of gas permeable, plasma charged silicone and can integrate traditional attachment surfaces, such as those comprised of traditional tissue culture treated polystyrene. They include culture devices that integrate gas permeable, liquid permeable membranes. A variety of benefits accrue, including more optimal culture conditions during scale up and more efficient use of inventory space, incubator space, and disposal space. Furthermore, labor and contamination risk are reduced.
Owner:WILSON WOLF MFG
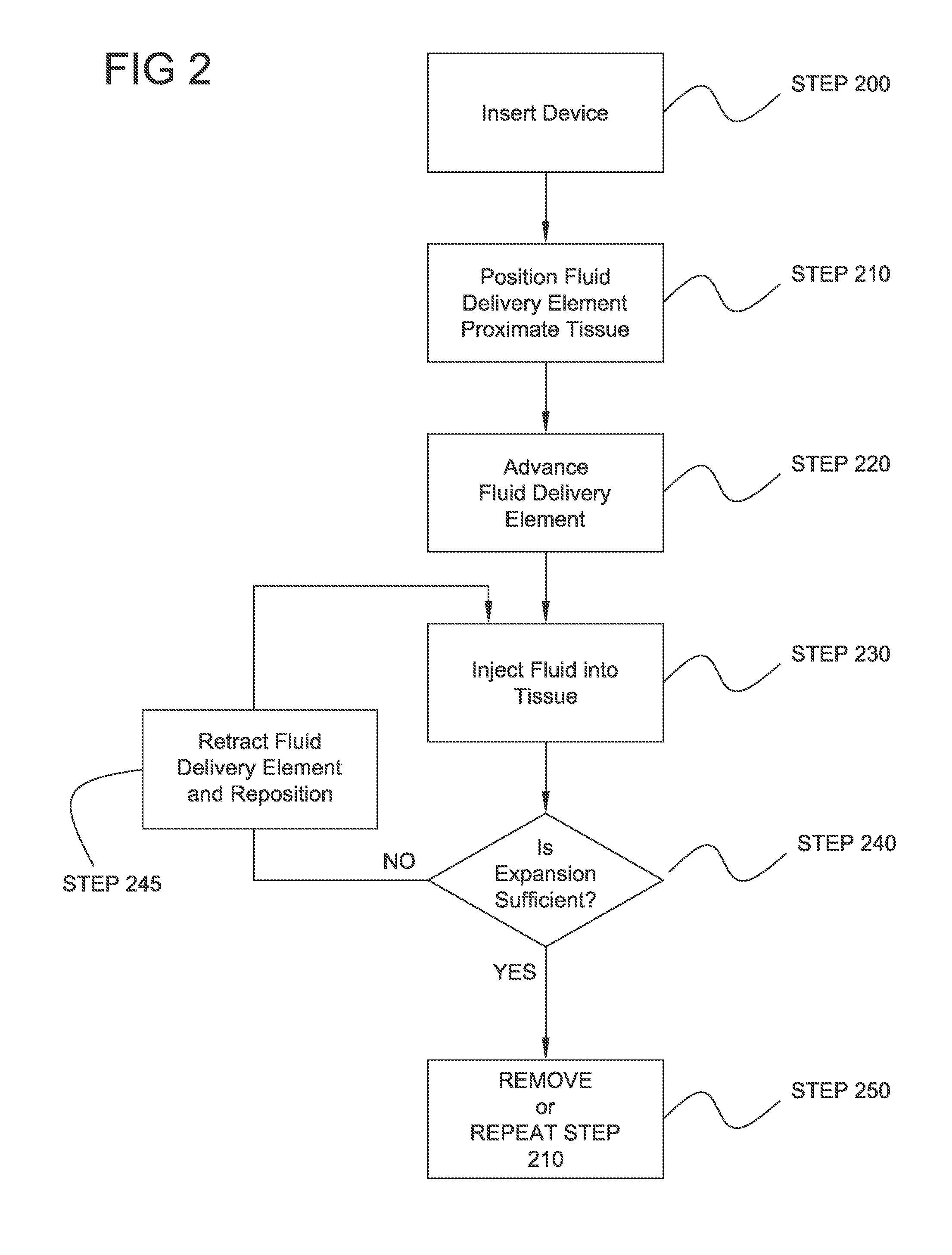
Tissue expansion devices, systems and methods
A device for expanding tissue comprises at least one fluid delivery tube and at least one fluid delivery element in fluid communication with the at least one fluid delivery tube. The at least one fluid delivery tube comprises a proximal end, a distal end, and a lumen therebetween. The device is constructed and arranged to perform a near full circumferential expansion of luminal wall tissue. Systems and methods are also provided, including a system for expanding tissue layers and treating tissue proximate to the expanded tissue layers.
Owner:FRACTYL HEALTH INC
Control of shoot/foliar feeding pests with pesticide seed treatments
InactiveUS20080092256A1Effective protectionAvoid damageBiocideDead animal preservationShootAdemetionine
A method of preventing damage to the shoots and foliage of a plant by a pest includes treating a seed having an exogenous gene that encodes for the production of a protein having activity against European corn borer or corn root worm with a composition comprising at least one pyrethrin or synthetic pyrethroid that is selected on the basis of having an activity against an insect other than European corn borer or corn root worm. Treated seeds are also provided.
Owner:MONSANTO TECH LLC
Crosslinked polysaccharide gel compositions for medical and cosmetic applications
InactiveUS20130136780A1Enhanced release propertiesGood sustained releasePowder deliveryCosmetic preparationsDiseasePolysaccharide
Methods of producing a biocompatible polysaccharide gel composition having sustained release properties are disclosed. Also disclosed is a biocompatible polysaccharide gel composition having sustained release properties, a method of treating a disease or condition using the present biocompatible polysaccharide gel composition, and a method of controlling rate of release of at least one target solute from the biocompatible polysaccharide gel composition. Pharmaceutical compositions which include the present biocompatible polysaccharide gel composition also are disclosed.
Owner:ALLERGAN INC
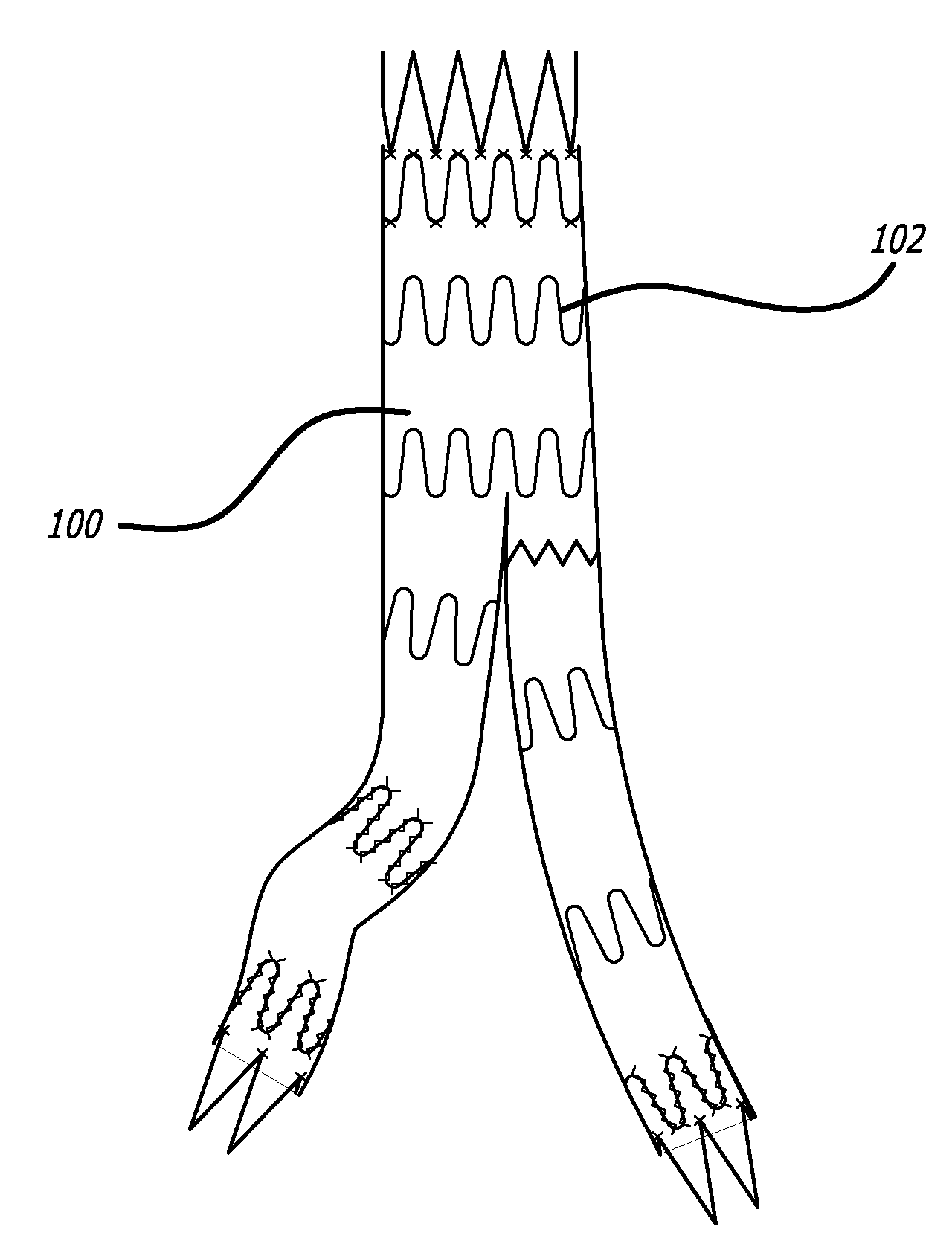
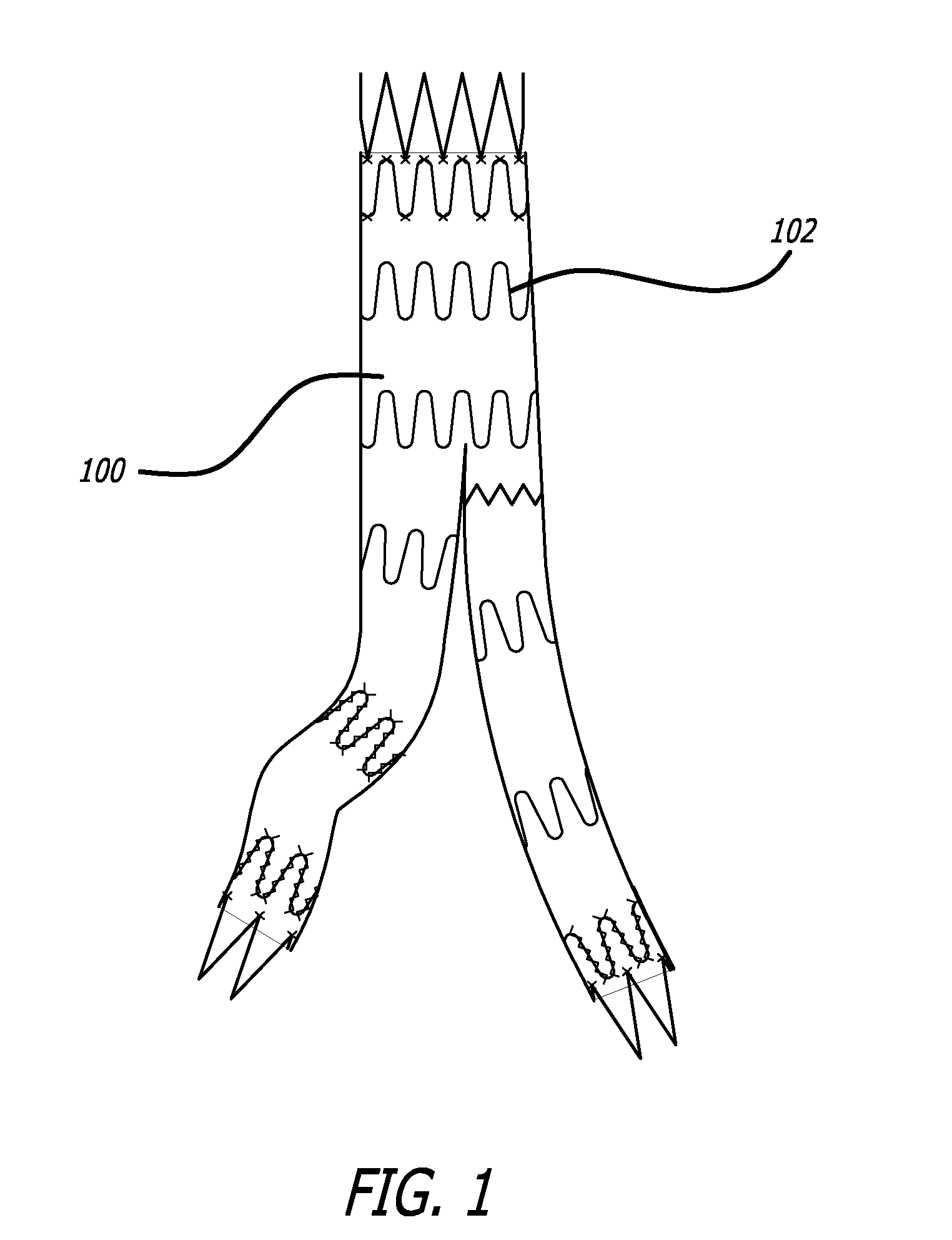
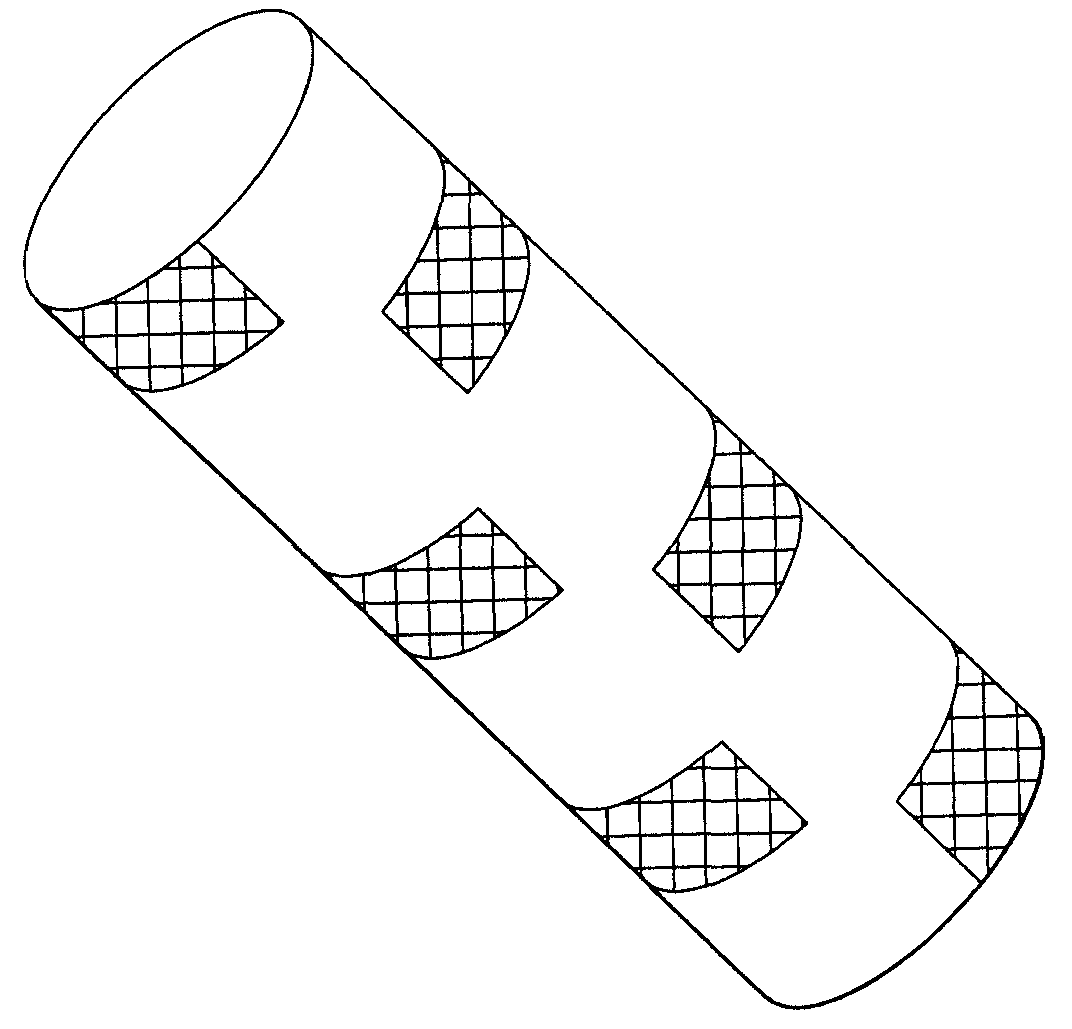
Anti-migration features and geometry for a shape memory polymer stent
The present invention relates to a radially-expandable stent for implantation in a bodily passageway, being expandable from an initial unexpanded state to an expanded state, having an outer surface with a geometric pattern covering said outer surface to minimize migration after implantation.
Owner:BOSTON SCI SCIMED INC
Disposable wipe with liquid storage and application system
InactiveUS7674058B2Inhibit migrationMinimize migrationCosmetic preparationsMake-upLiquid storageUrology
Owner:KIMBERLY-CLARK WORLDWIDE INC
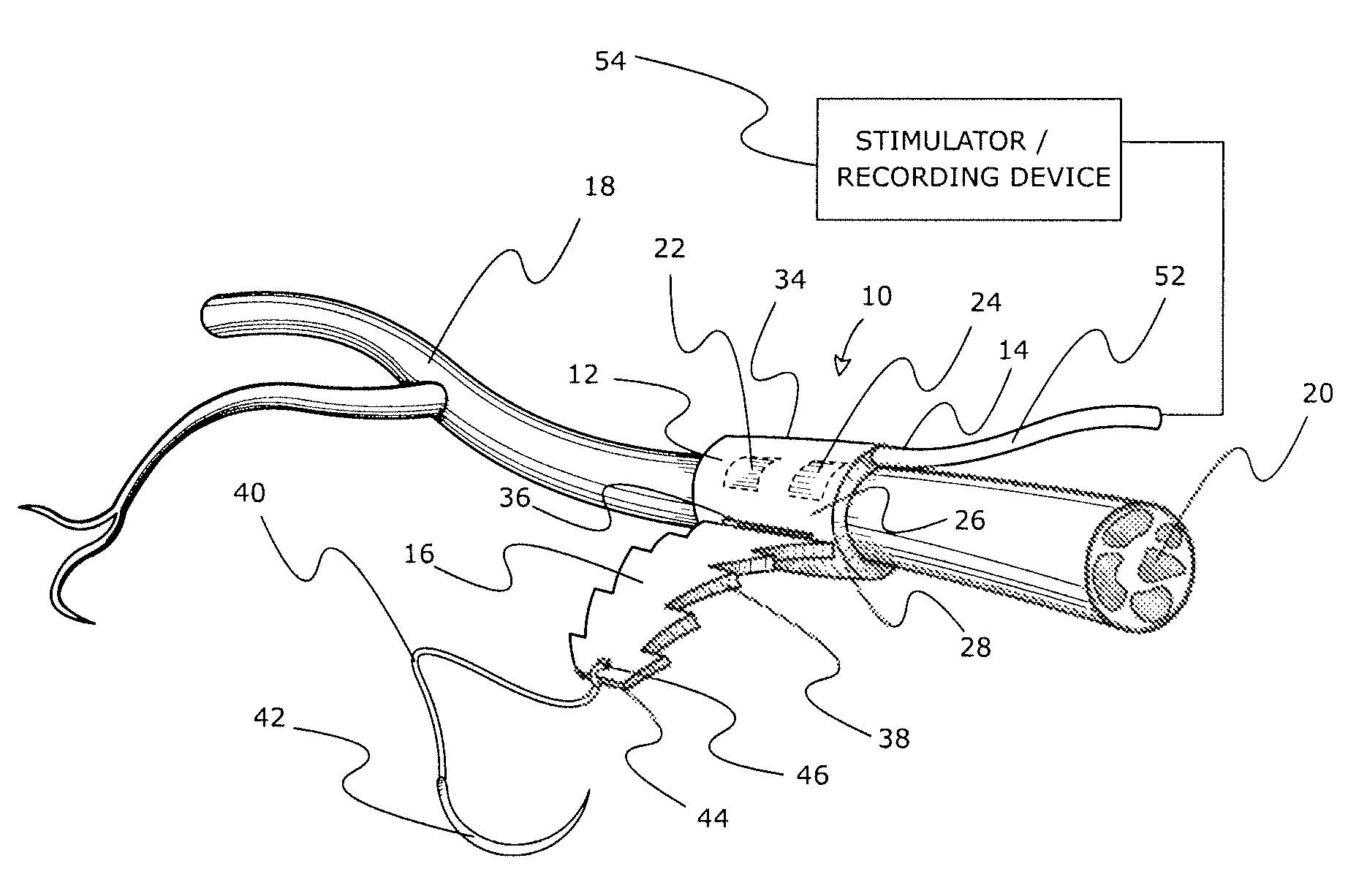
System and method for lead fixation
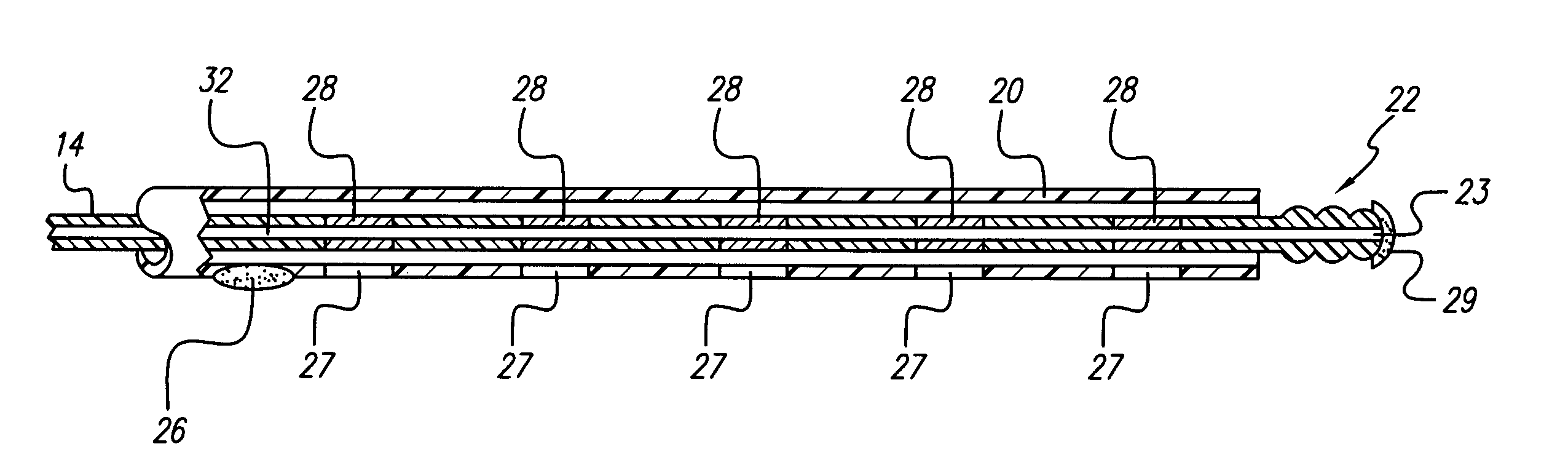
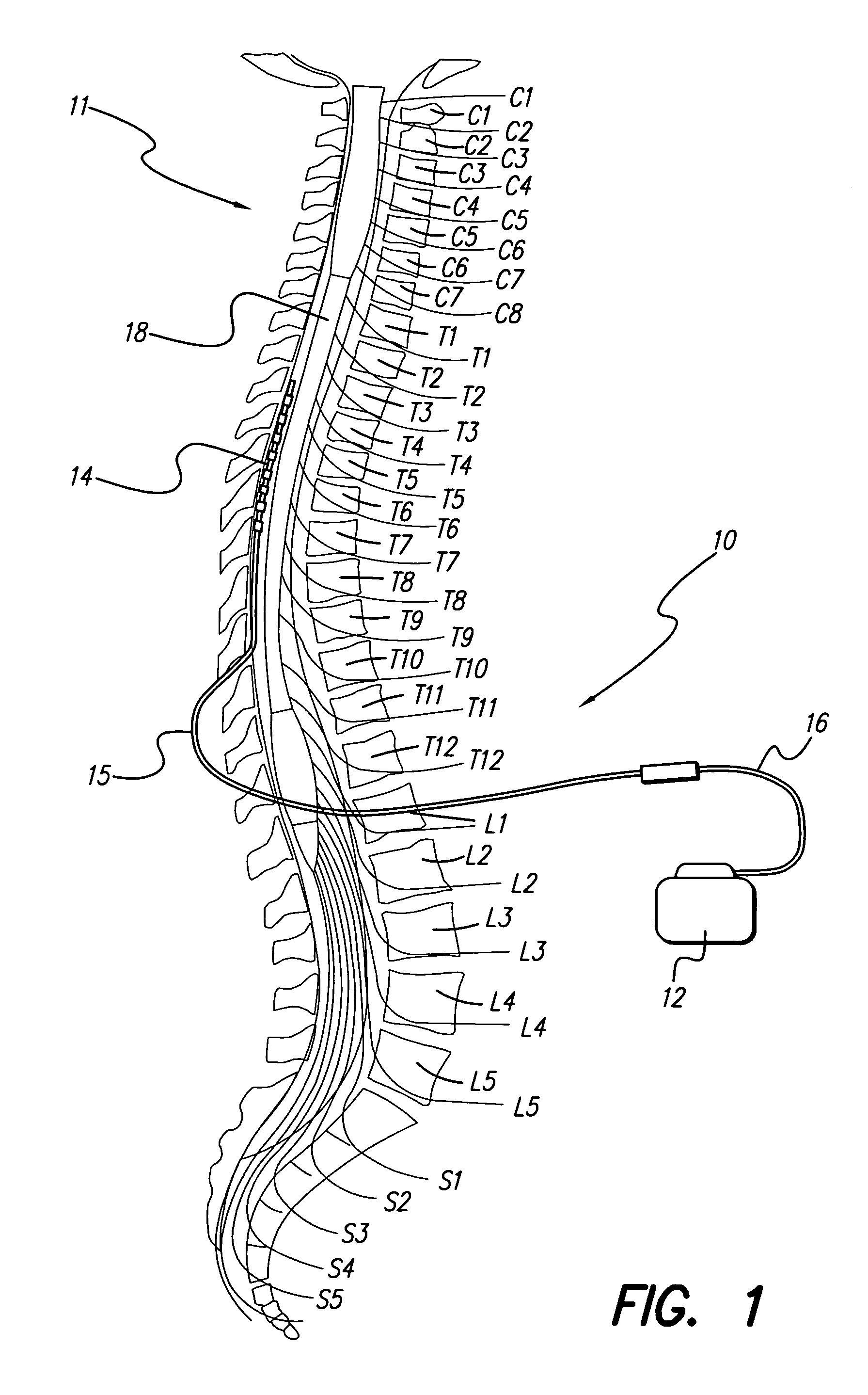
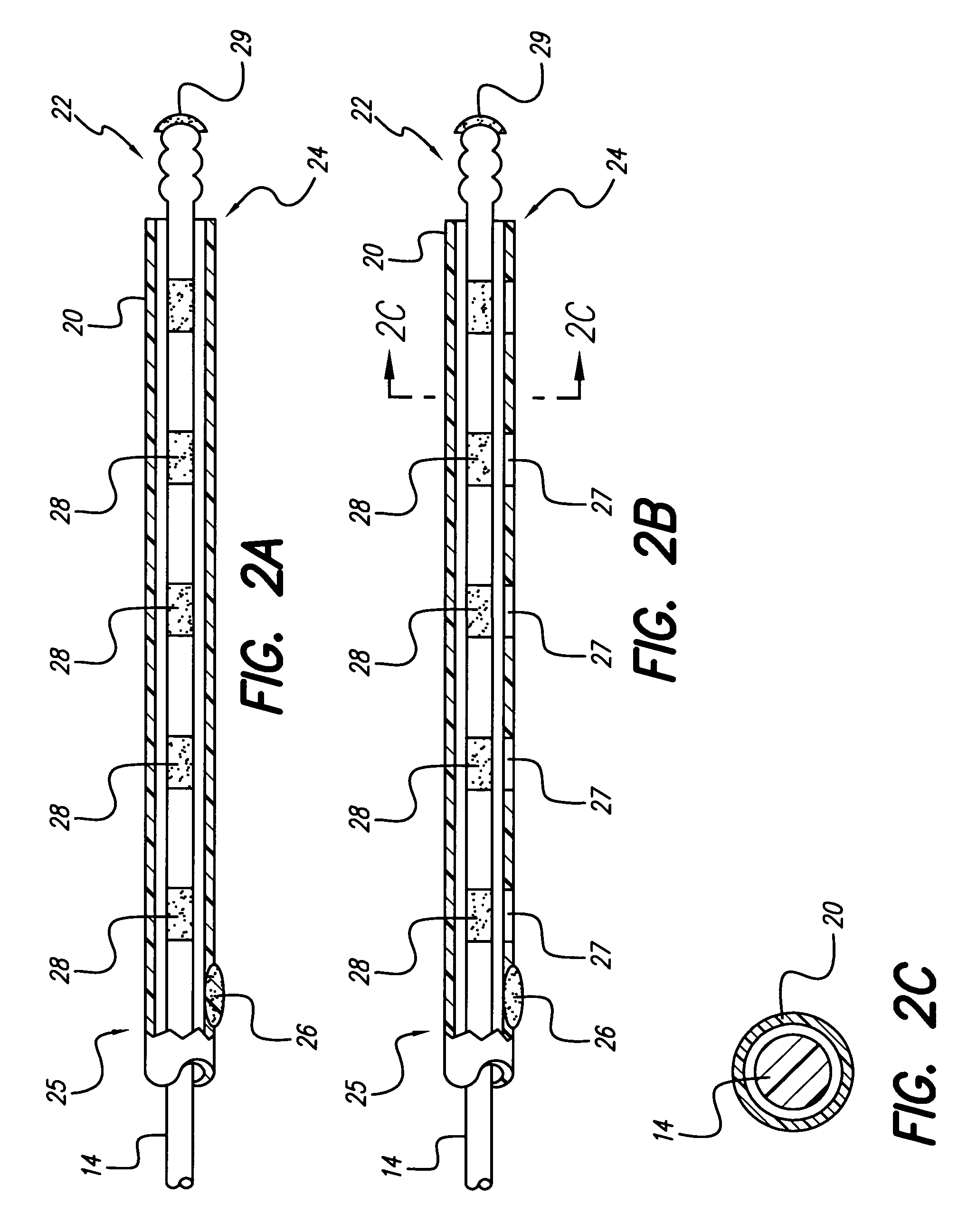
A medical lead includes a pitted, grooved or threaded electrode array tip and a flexible tube or sheath encompassing the electrode array located near the lead tip. In some embodiments, the electrode array adheres to tissue, the tube or sheath adheres to the electrode array at the distal end of the electrode array or the tube or sheath adheres to tissue at the proximal end of the tube or sheath. Embodiments of the tube or sheath may be made from biodegradable material and can include electrode windows spaced along the tube or sheath corresponding to placement of electrode contacts of the electrode array.
Owner:BOSTON SCI NEUROMODULATION CORP
Ultraviolet light screening compositions
InactiveUS6869596B1Minimize migrationReduce productionMaterial nanotechnologyCosmetic preparationsUltraviolet lightsUltraviolet A Radiation
Owner:OXFORD UNIV INNOVATION LTD
Adjustable tissue or nerve cuff and method of use
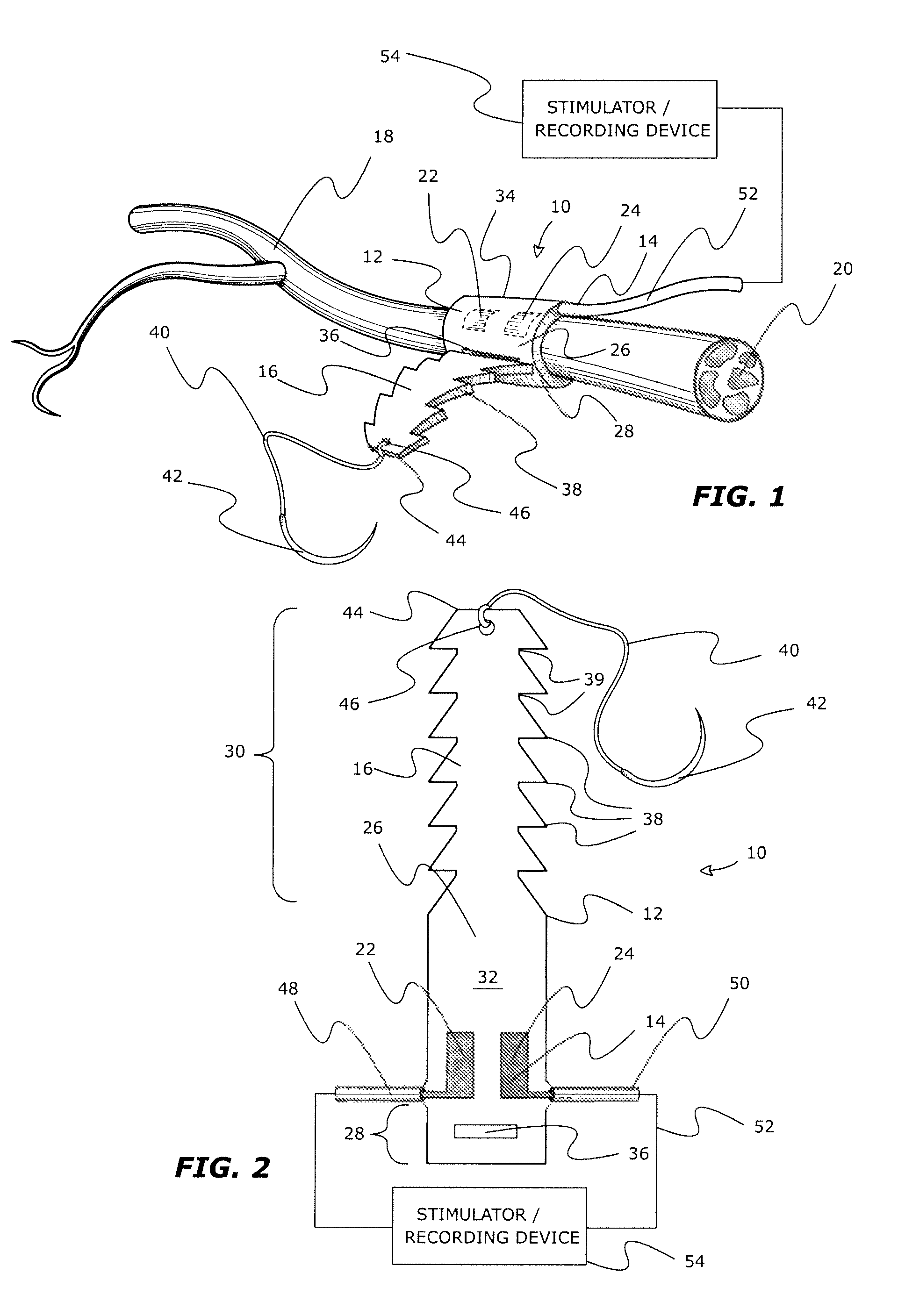
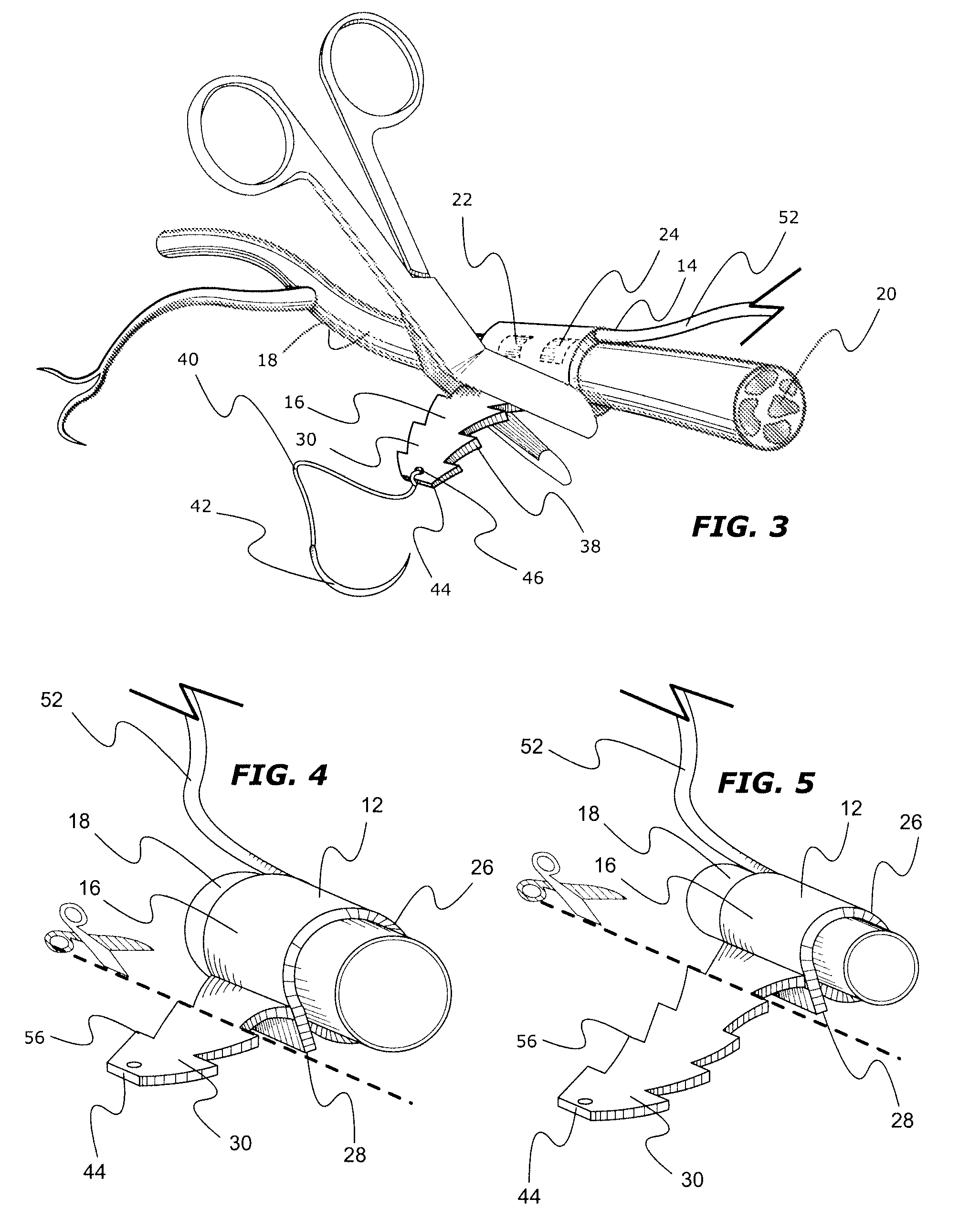
InactiveUS8116882B2Minimize of failureMinimize migrationSpinal electrodesSensorsNerve cuffConductive materials
An implantable adjustable body tissue cuff, apparatus and method. The cuff is an elastomeric strap of biocompatible non-conductive material. The strap's tail and head provide adjustable length fastening by a) the tail being formed with longitudinally spaced, laterally paired locking projections and the head being formed with one or more locking apertures; or b) the tail being formed with longitudinally spaced locking apertures and the head being formed with one or more laterally paired locking projections. The locking projection shape allows for passage through the locking aperture, while restricting movement in a reverse direction. The cuff accommodates devices such as tissue stimulators or recorders, with conductive elements attached, imbedded, or printed on the strap body. The cuff is intra-operatively adjusted to optimize placement and contact between conductive elements and body tissue, without tissue damage. This cuff accommodates varying tissue diameters and simplifies manufacture and surgical placement.
Owner:ANGELTEAR SOLUTIONS
Container seal with radio frequency identification tag, and method of making same
ActiveUS20120217244A1Minimize migrationCapsContainer decorationsElectromagnetic interferenceEngineering
A container defines an interior volume, an opening into the interior volume, and a sealing surface bordering the opening. A closure assembly may be attachable over the opening and includes at least one of an induction seal liner, an inner seal, and a sealable film. A radio frequency identification tag includes a microprocessor and an antenna for receiving, storing, and transmitting selected digitized information. The induction seal liner, inner seal, and sealable film minimizes migration of fluids between the interior volume and the container exterior when the closure assembly may be engaged with the sealing surface. The radio frequency identification tag may be included in the closure assembly, or within the interior volume, or on the container exterior, or may be incorporated into the induction seal liner, inner seal, or sealable film, without the closure assembly electromagnetically interfering with the receiving, storing, or transmitting of digitized information.
Owner:EAGILE
Process for preparing a flexographic printing plate from a photopolymerizable element
InactiveUS20060160025A1High color contrastSufficient color contrastPhotosensitive materialsSemiconductor/solid-state device manufacturingLeuco dyeEngineering
This invention relates to a photosensitive element for use as a flexographic printing plate and a process for preparing the plate from the element. The photosensitive element has at least one photopolymerizable elastomeric layer that comprises a binder, a monomer, a photoinitiator, an onium salt, and a leuco dye. Upon exposure to actinic radiation, the onium salt and leuco dye react resulting in a change of color in polymerized portions of the photopolymerizable layer. The color change provides enhanced image color contrast in the photosensitive element.
Owner:DUPONT ELECTRONICS INC
Substantially dry, silica-containing soot, fused silica and optical fiber soot preforms, apparatus, methods and burners for manufacturing same
InactiveUS20050120752A1Minimizes incorporationEfficient preparationGlass shaping apparatusGlass deposition burnersWater productionSilicon dioxide
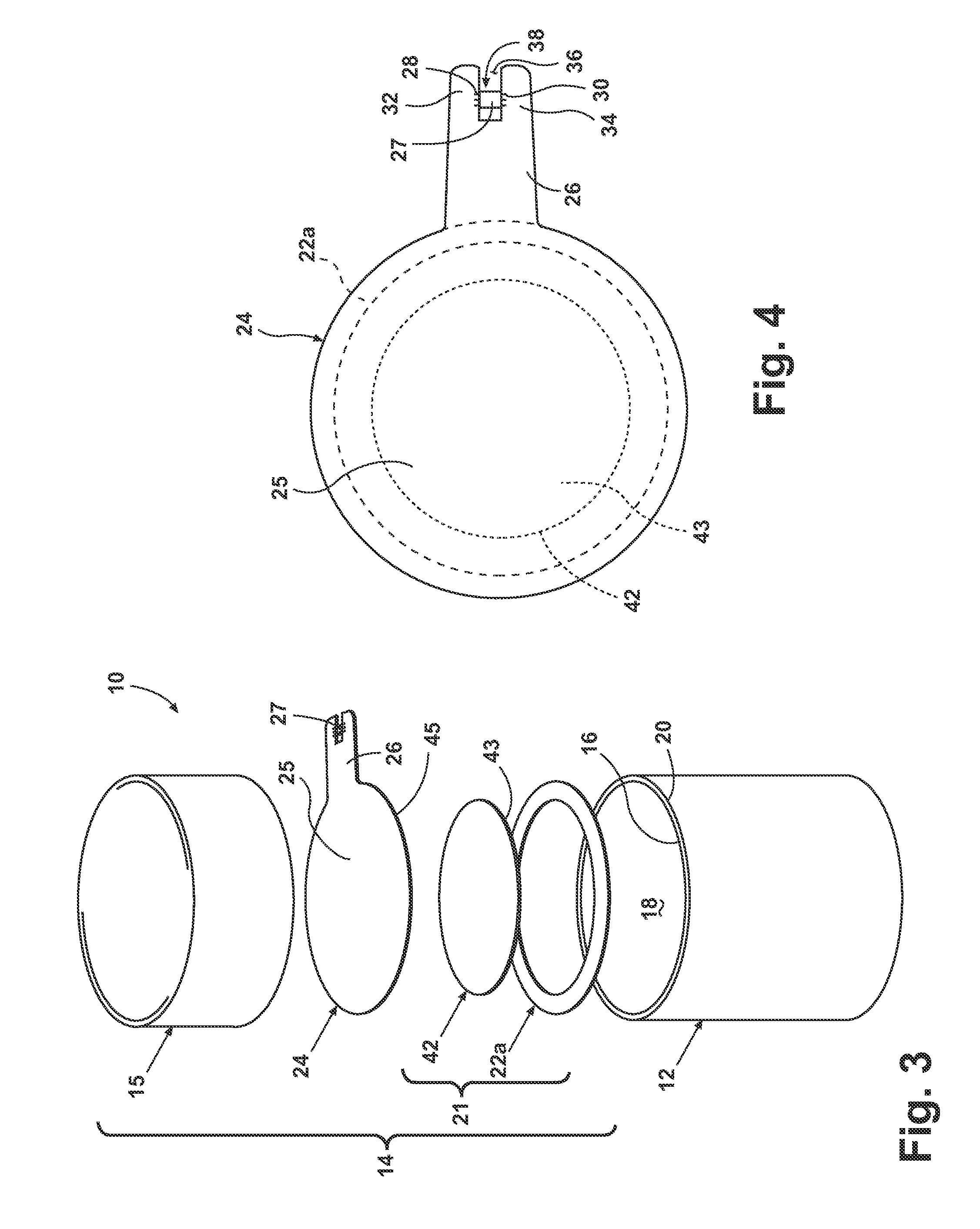
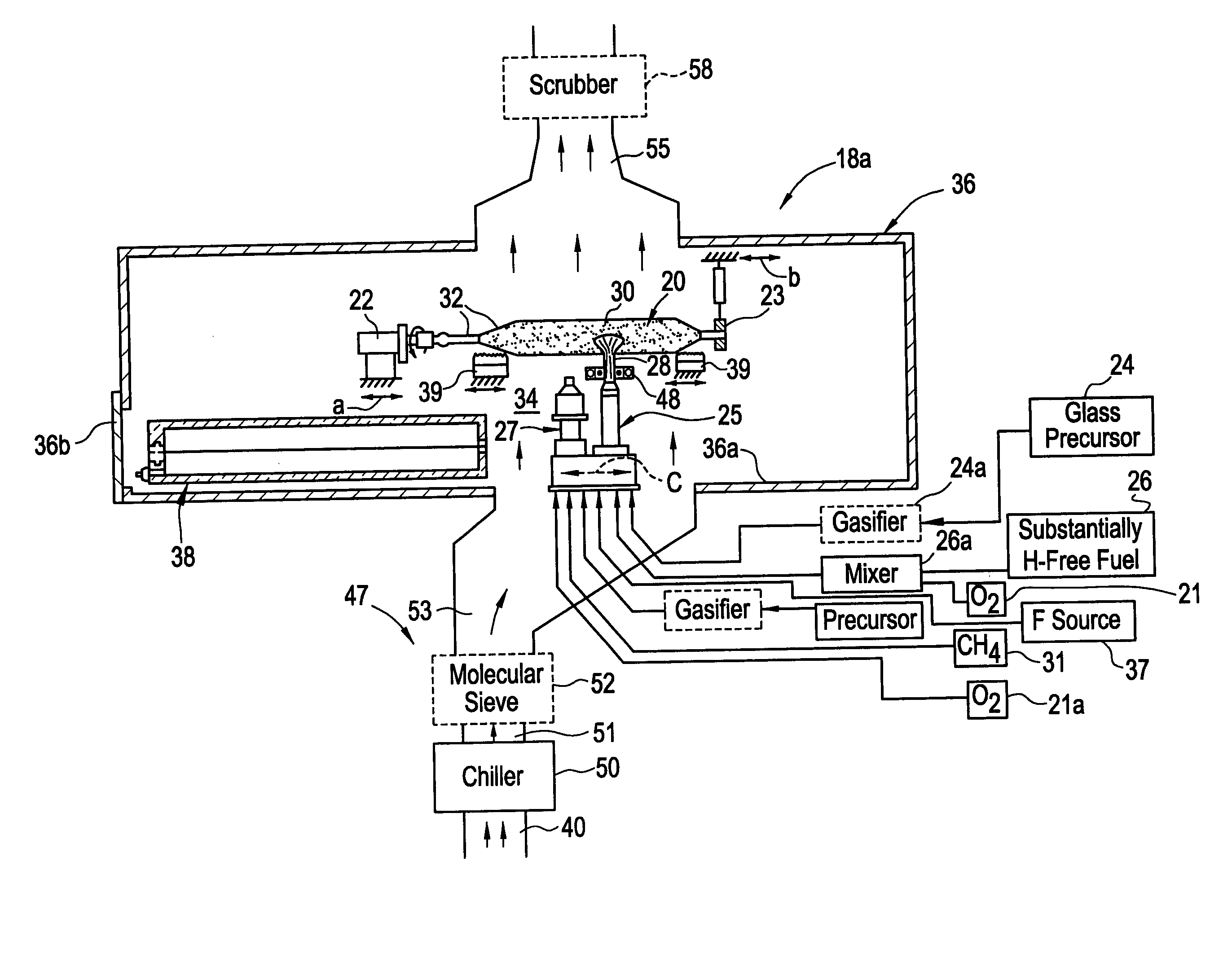
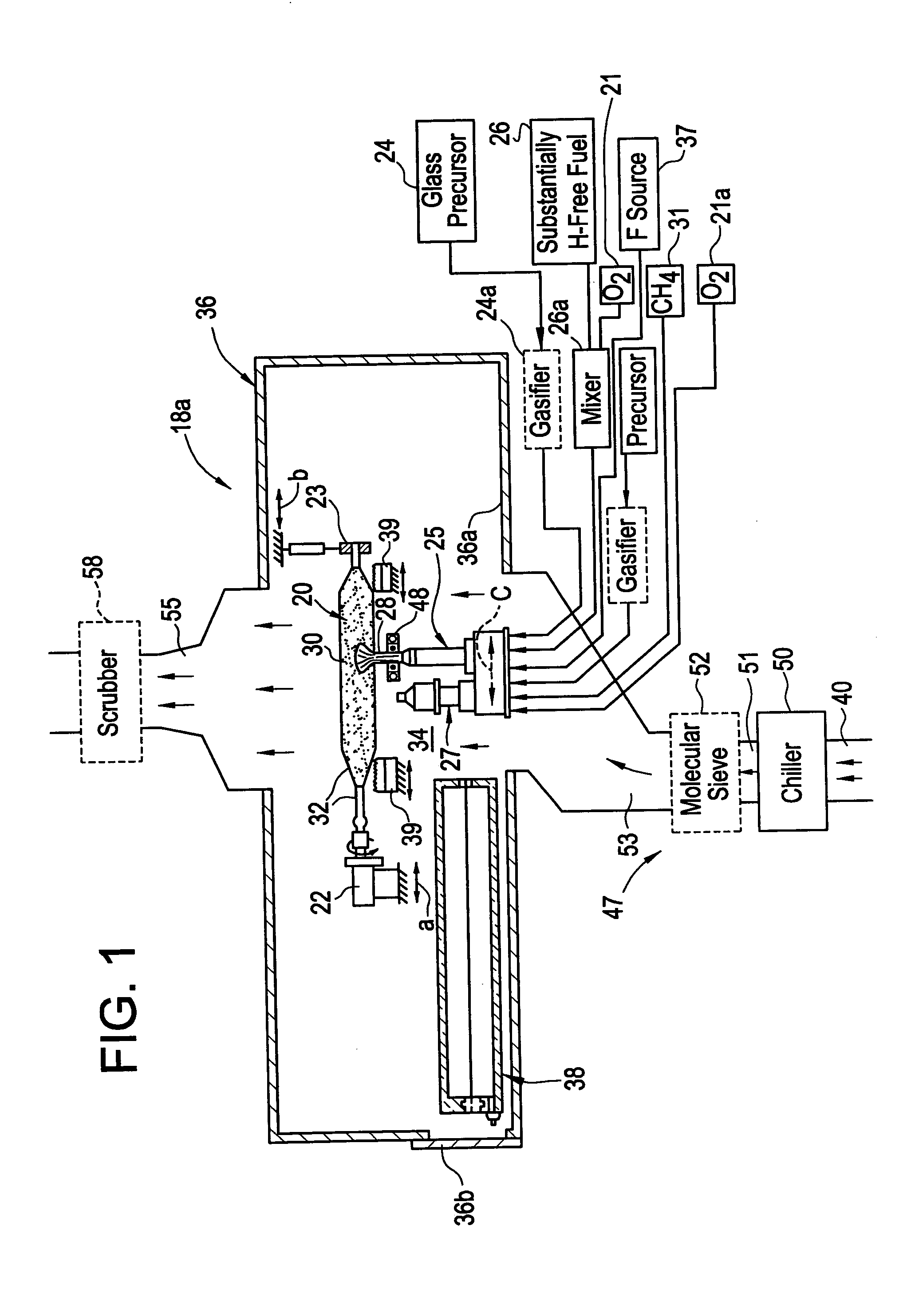
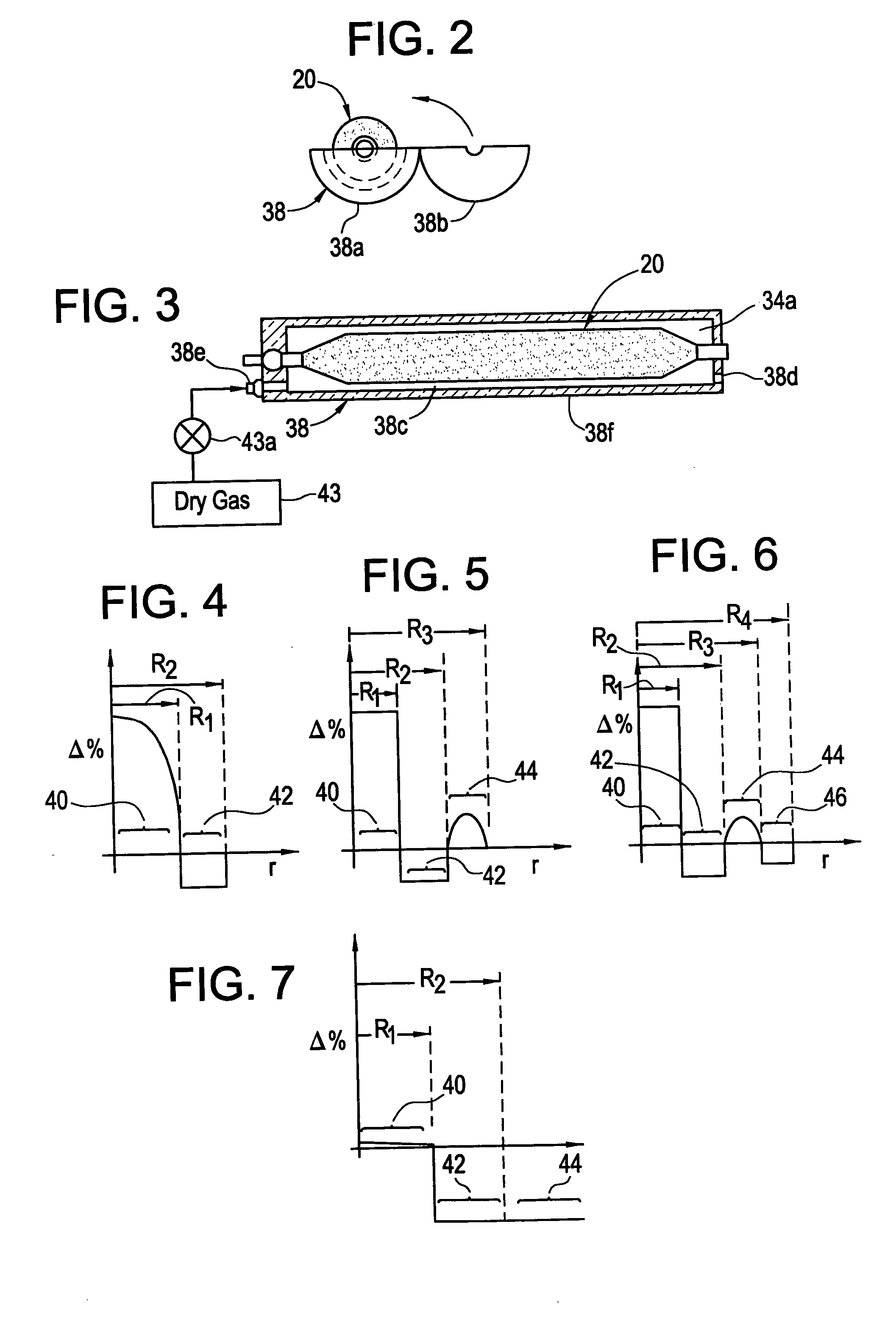
Methods, apparatus and precursors for producing substantially water-free silica soot, preforms and glass. The methods and apparatus make substantially water-free fused silica preforms or glass by removing water as a reaction product, removing water from the atmosphere, removing water from the transport process, or combinations thereof. In a first embodiment, substantially water-free soot, preforms or glass are achieved by using a hydrogen-free fuel, such as carbon monoxide, in the deposition process. In another embodiment, a soot producing burner has parameters that enable operation on a substantially hydrogen-free fuel. End burners, which minimize water production, are also described. Such water-free methods are useful in depositing fluorine-doped soot because of the low water present and the efficiency in which fluorine is incorporated. In another embodiment, glassy barrier layer methods and apparatus are described for minimizing dopant migration, especially fluorine. Laser and induction methods and apparatus for forming the barrier layer are depicted. A chlorine, fluorine and silica precursor, such as chlorofluorosilane, may be utilized to form fluorinated soot. Other methods and apparatus are directed to combinations of conventional and substantially water-free processes. One embodiment is directed to combustion enhancing additives for addition to the substantially hydrogen-free fuels. The methods and apparatus in accordance with the invention are particularly useful for producing photomask substrates and optical fiber preforms.
Owner:BROWN JOHN T +5
Disposable wipe with liquid storage and application system
InactiveUS20070045135A1Inhibit migrationMinimize migrationCosmetic preparationsMake-upUrologyLiquid storage
Owner:KIMBERLY-CLARK WORLDWIDE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com