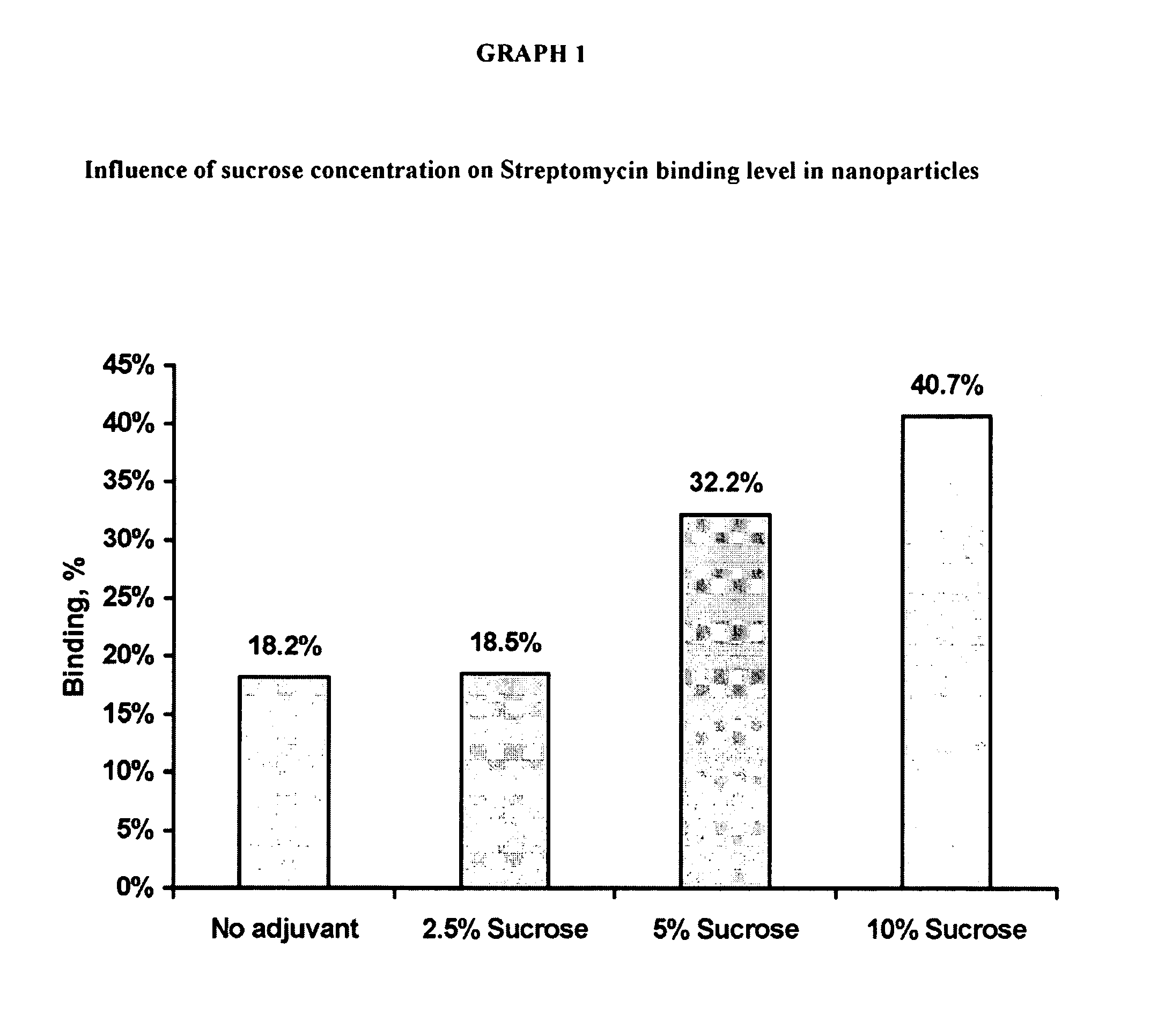
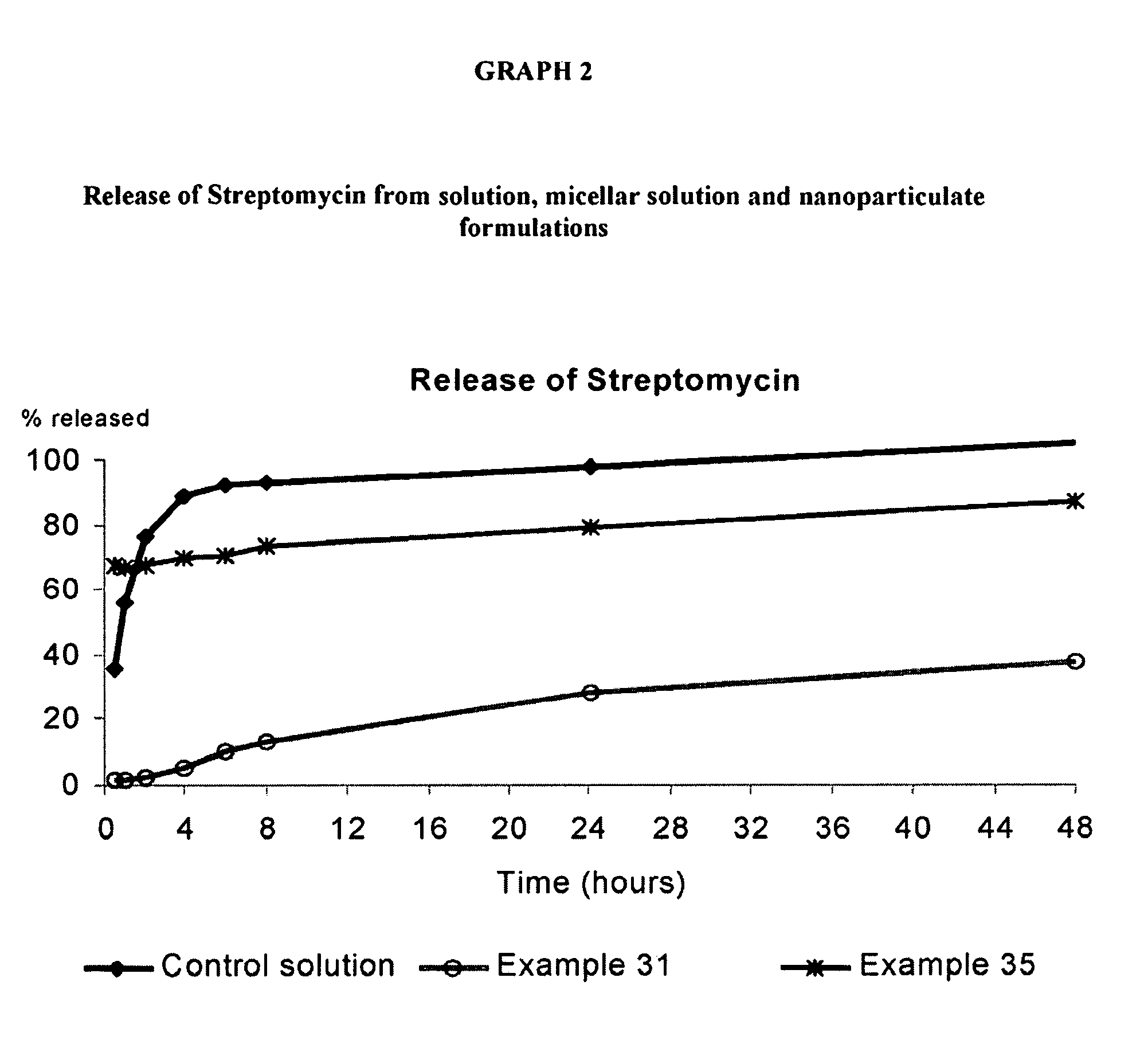
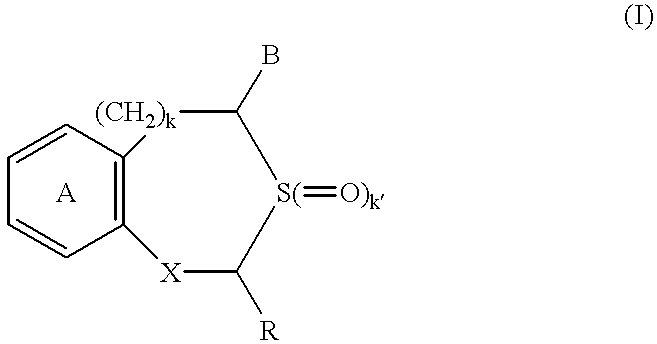
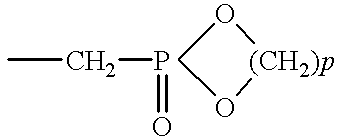
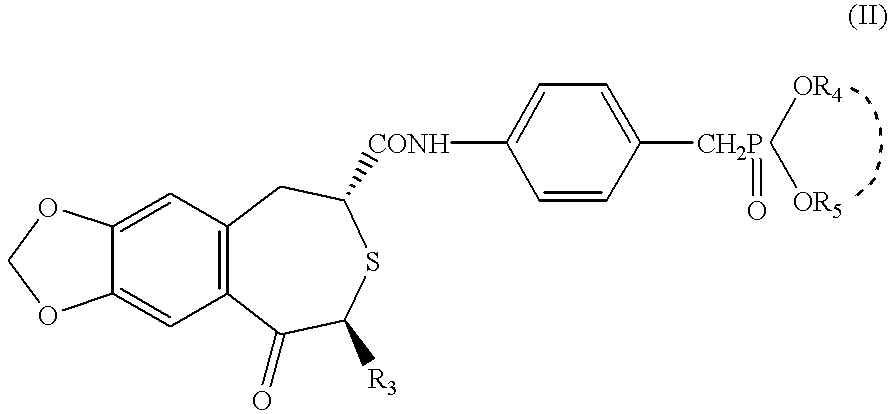
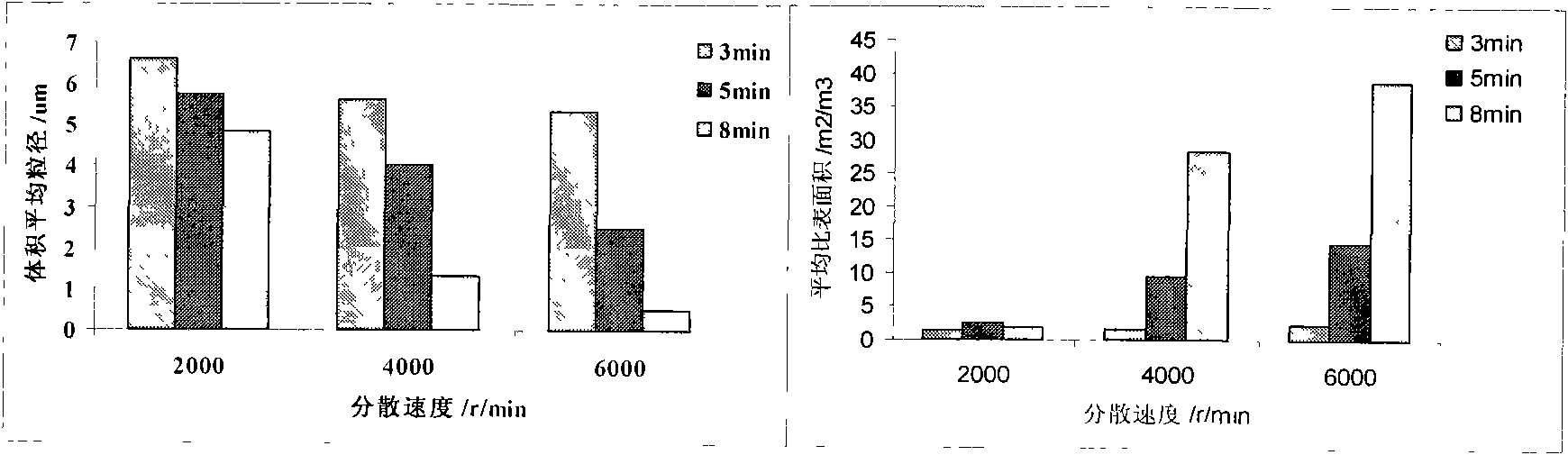
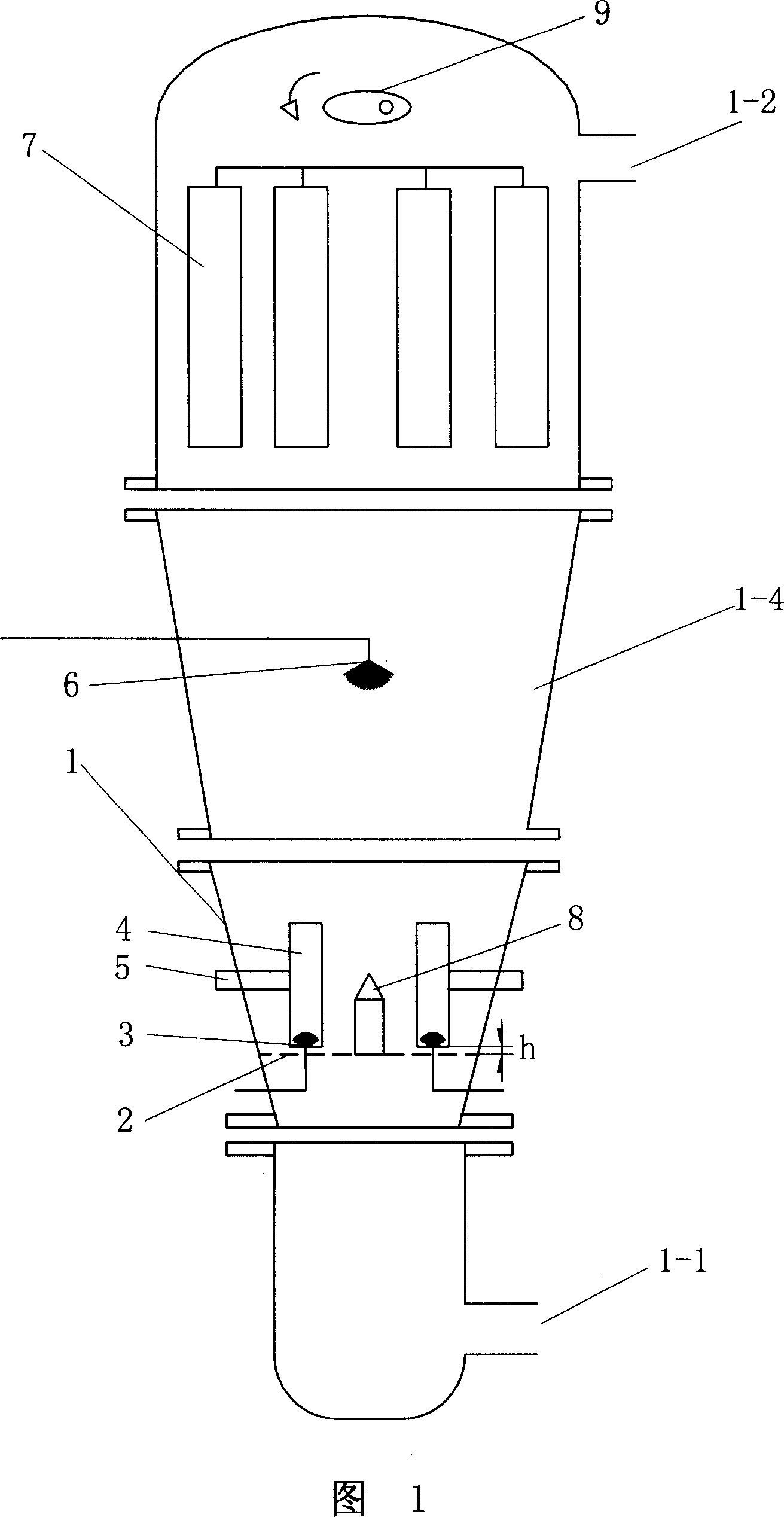
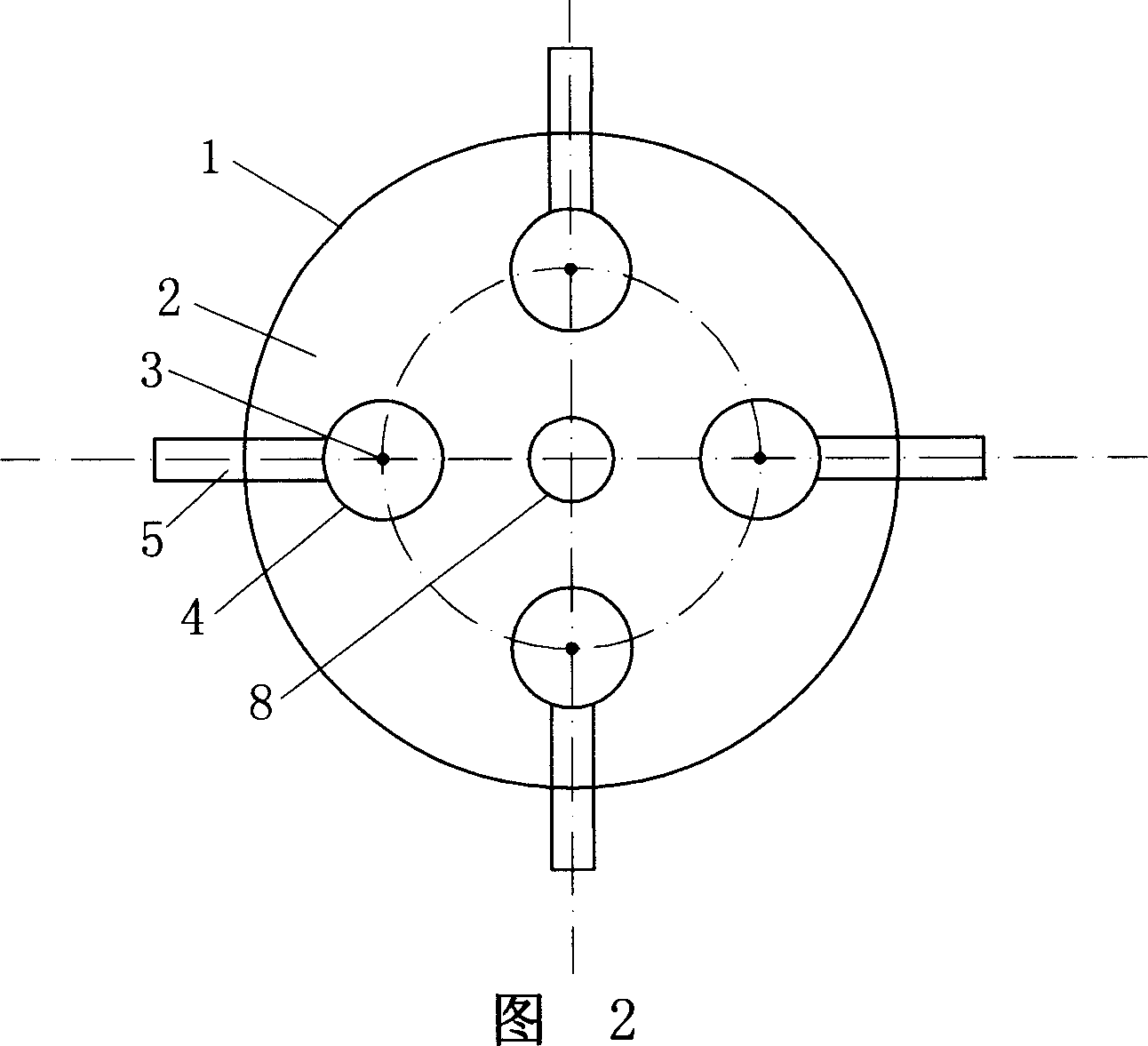
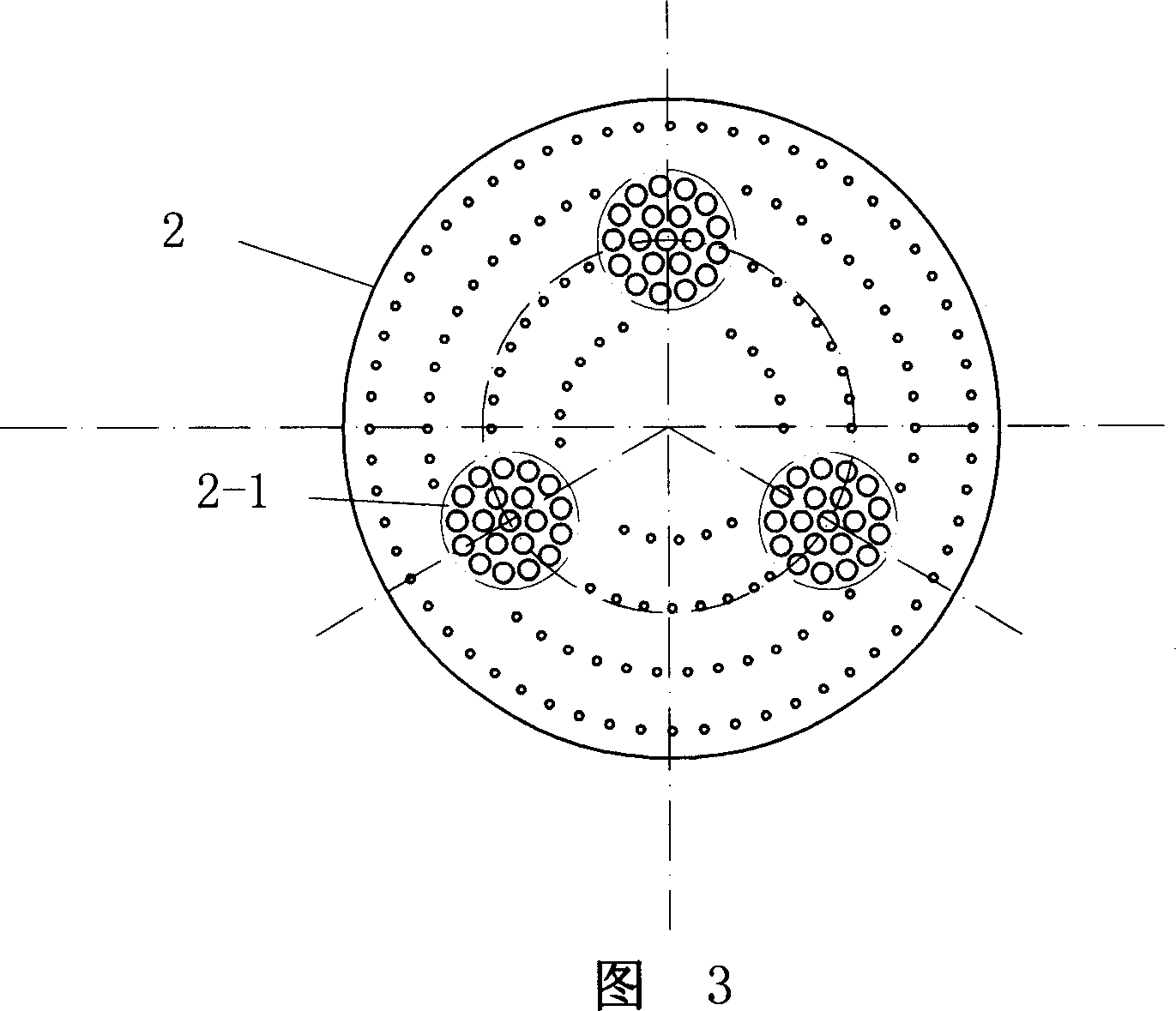
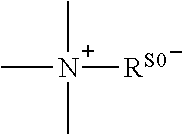Patents
Literature
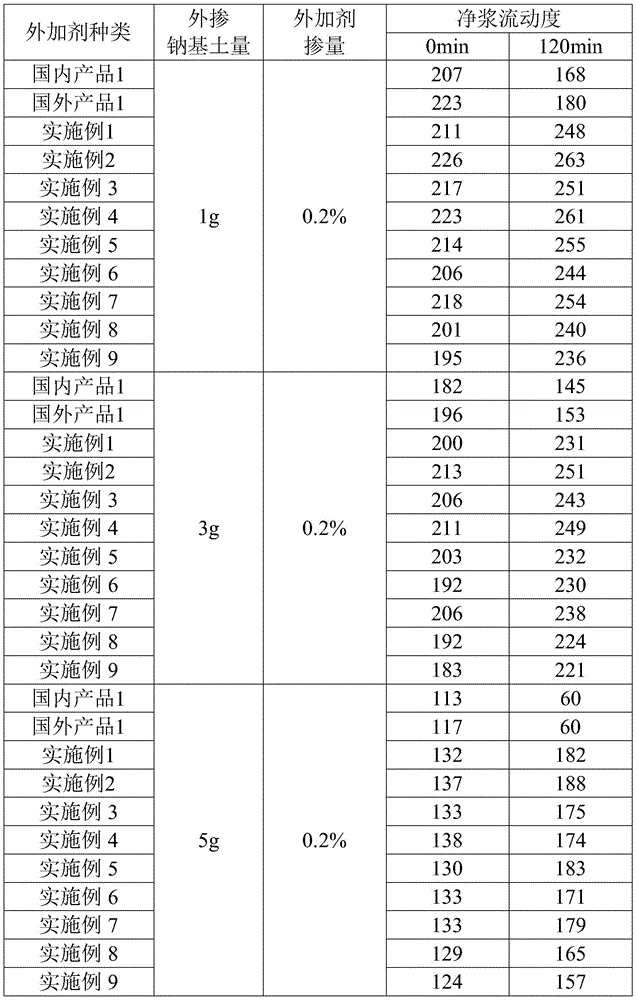
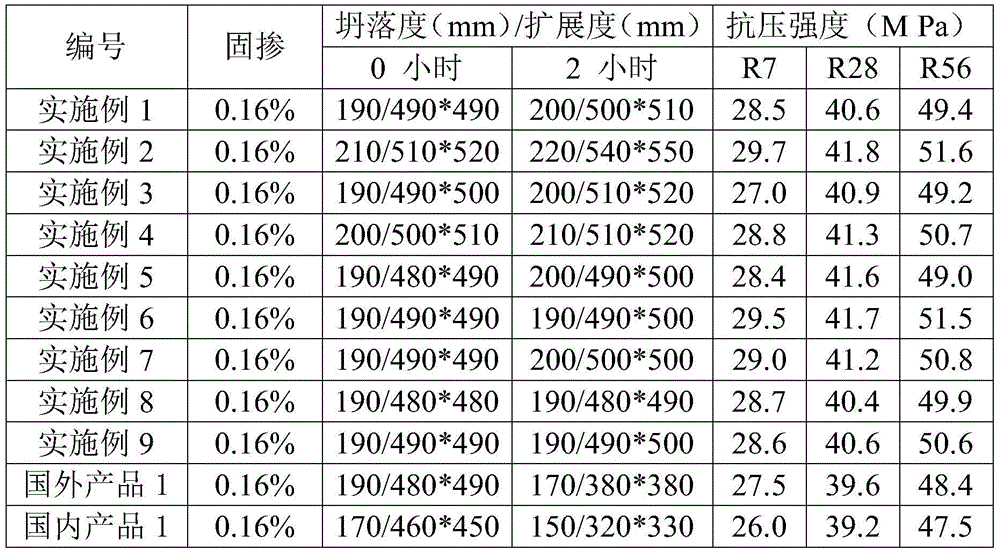
250results about How to "Good sustained release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intravaginal drug delivery devices for the delivery of macromolecules and water-soluble drugs
ActiveUS20090004246A1Good sustained releaseViral antigen ingredientsVirus peptidesIntravaginal administrationPharmacy
An intravaginal drug delivery device comprises a device body comprising a hydrophobic carrier material having at least one channel defining at least one opening to the exterior of said device body, said at least one channel being adapted to receive at least one drug-containing insert; at least one drug-containing insert positioned in said at least one channel, said drug-containing insert capable of releasing a pharmaceutically effective amount of at least one drug suitable for intravaginal administration and containing about 1% to about 70% of at least one water-soluble release enhancer, both the drug and the water-soluble release enhancer dispersed in an insert carrier material; wherein said hydrophobic carrier material and said insert carrier material may be the same or different; and wherein said at least one drug-containing insert is exposed on said exterior of said device body when said intravaginal drug delivery device is in use.
Owner:APTALIS PHARMA
Composition and Method of Treatment of Bacterial Infections
InactiveUS20090061009A1Effective treatmentImprove antibacterial propertiesAntibacterial agentsBiocideNanoparticleAntibacterial drug
The invention is intended for a treatment of severe infections using an injectable drug-delivery system comprising nanoparticles of a biodegradable polymer with incorporated antibacterial drug.
Owner:ALPHARX
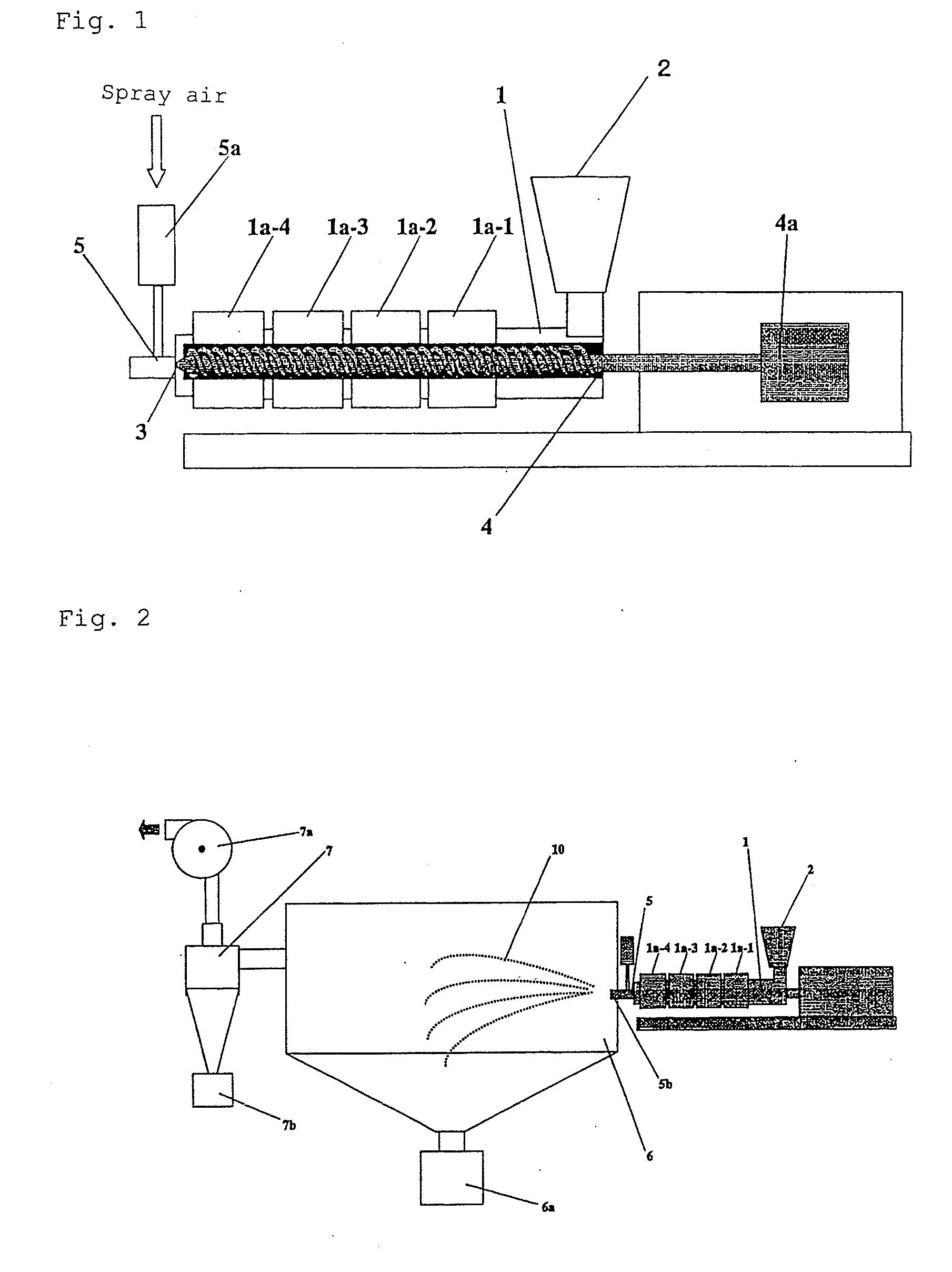
Method of producing drug-containing wax matrix particles, extruder to be used in the method and sustained-release preparation containing cilostazol
InactiveUS20090047357A1Easy to produceSolve low usagePowder deliveryBiocideSpray nozzleSustained-Release Preparations
The present invention aims to provide a method for producing, by a simple method, drug-containing wax matrix granules, particularly drug-containing wax matrix granules having an average particle diameter of 1 mm or lower, while avoiding liquid blockage due to the recrystallization of a molten drug during the period from a melting step to a spray step.Drug-containing wax matrix granules having at least one wax and at least one drug are produced by the following steps (i) and (ii): (i) supplying the at least one drug and the at least one wax to an extruder in which the temperature of a barrel and the temperature of a die are adjusted to be higher than the melting point of the at least one wax; and (ii) while melting and kneading the at least one drug and the at least one wax in the extruder to give a molten kneaded drug and wax, spraying the molten kneaded drug and wax into an atmosphere having a temperature lower than the melting point of the wax from a spray nozzle directly mounted onto a die provided at a top end of the barrel of the extruder, thereby forming the mixture into granules.
Owner:OTSUKA PHARM CO LTD
Apatite-coated solid composition
InactiveUS6344209B1Efficient use ofImprove efficiencyOrganic active ingredientsPowder deliveryApatiteBiodegradable polymer
An apatite-coated solid composition which contains a biodegradable polymer and an apatite-coated solid composition which contains a biodegradable polymer and a medicinal substance have properties of sustained release and of osteoconductive activity.
Owner:TAKEDA PHARMA CO LTD
Controlled and directed local delivery of anti-inflammatory compositions
InactiveUS20060046960A1Good sustained releaseInhibitionPeptide/protein ingredientsAntipyreticControl releaseSkeletal injury
The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by controlled and directed delivery of one or more biological response modifiers to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by implantable or infusion pumps, implantable controlled release devices, or by sustained release compositions comprising biological response modifiers.
Owner:SDGI HLDG
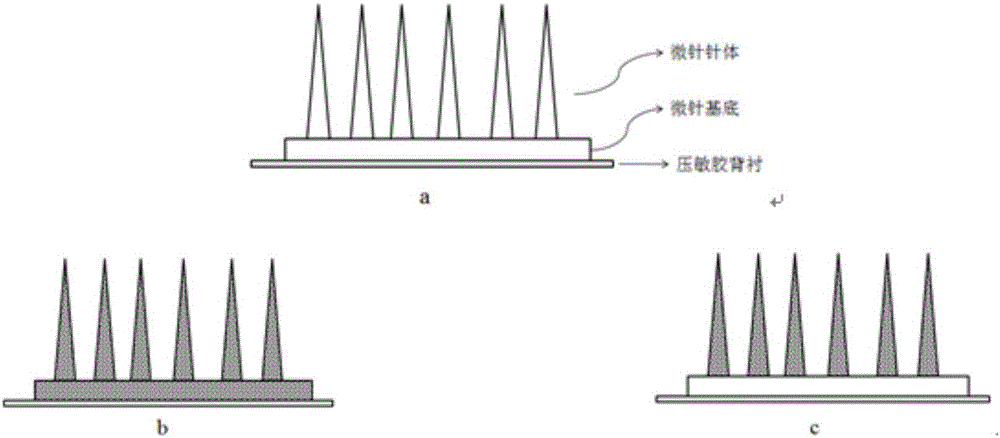
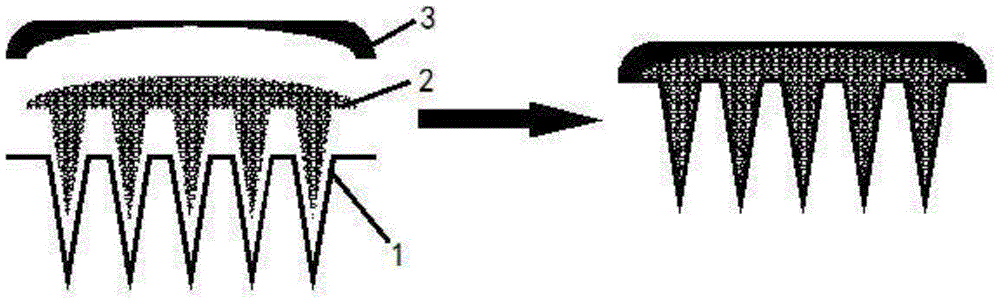
Polymer micro-needle array chip, and preparation method and application thereof
InactiveCN103301092AHigh mechanical strengthEasy piercingSsRNA viruses negative-sensePeptide/protein ingredientsCuticleBiological macromolecule
The invention discloses a polymer micro-needle array chip. The chip comprises a micro-needle array and a substrate used for the standing of the micro-needle array; and the matrix material of the micro-needle array is a polyacrylamide polymer. The invention also discloses a preparation method of the polymer micro-needle array chip, a micro-needle transdermal drug delivery patch prepared through utilizing the polymer micro-needle array chip, and a preparation method of the patch. The micro-needle of the polymer micro-needle array chip has a high mechanical strength and a sharp needle point, so the skin cuticle can be easily pierced; the preparation method avoids a high-temperature processing treatment step, and is in favor of maintaining the activities of biomacromolecules comprising polypeptides, proteins and the like; the polyacrylamide polymer can easily dissolve or swell after meeting with the water-containing environment, and is in favor the sustained release of drugs in skins; and the preparation method of the polymer micro-needle array chip based transdermal drug delivery patch is simple, so the batch produced can be realized through utilizing present manure processing technologies.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Crosslinked polysaccharide gel compositions for medical and cosmetic applications
InactiveUS20130136780A1Enhanced release propertiesGood sustained releasePowder deliveryCosmetic preparationsDiseasePolysaccharide
Methods of producing a biocompatible polysaccharide gel composition having sustained release properties are disclosed. Also disclosed is a biocompatible polysaccharide gel composition having sustained release properties, a method of treating a disease or condition using the present biocompatible polysaccharide gel composition, and a method of controlling rate of release of at least one target solute from the biocompatible polysaccharide gel composition. Pharmaceutical compositions which include the present biocompatible polysaccharide gel composition also are disclosed.
Owner:ALLERGAN INC
Flexible slow-release micro-needle patch and preparation method thereof
InactiveCN106422045AGood dispersionGood sustained releaseOrganic active ingredientsMicroneedlesSolubilityMedicine
The invention discloses a flexible slow-release micro-needle patch and a preparation method thereof. The micro needle patch comprises a micro-needle, a substrate and a back lining, wherein the micro-needle and the substrate form a micro-needle array; a base material of the micro-needle or a base material of the micro-needle array contains a drug in a crystal form; the solubility of the drug in the crystal form in water is less than 100 microgram / ml. The micro-needle patch has the characteristics of simple process, high preparation safety, low process cost and high drug loading capacity.
Owner:BEIJING CAS MICRONEEDLE TECH LTD
Slow-release cationic anti-mud polycarboxylic-type high performance water reducer and preparation method thereof
The present invention discloses slow-release cationic anti-mud polycarboxylic-type high performance water reducer and a preparation method thereof. The slow-release cationic anti-mud polycarboxylic-type high performance water reducer is successfully synthesized from an unsaturated carboxylic acid, a polyether macromonomer, a cationic unsaturated monomer, an unsaturated esters, a crosslinking agent, a chain transfer agent, an oxidizing agent and a reducing agent as raw materials at room temperature without heat source. By introduction of the long-chain-ester crosslinking agent and the cationic unsaturated monomer, the slow-release cationic anti-mud polycarboxylic-type high performance water reducer has good slow-release and anti-mud effects, is low in admixing volume, high in water-reducing rate, excellent in slump retention ability, and not sensitive to mud contained in sand and stones, and the process does not require an additional heat source, an be carried out at room temperature, and is low in investment, low in cost, and suitable for large scale promotion and application.
Owner:GUANGDONG FUTE NEW MATERIALS TECH CO LTD
Separation of platelets from whole blood for use as a healant
InactiveUS20020179537A1Prevent substantial premature releaseImmediate growth factor treatmentSurgical adhesivesDead animal preservationFiltrationBlood plasma
A method is described for separating, retrieving and concentrating platelets from whole blood relying on aggregation of the platelets followed by filtration. This method eliminates the need of a centrifuge for separating said cells from blood. To obtain cellular concentrates of platelets, blood is mixed with compatible agents that will aggregate cells while retaining contained growth factors. The resulting aggregates can then be separated from blood by filtration. If desired, the filter-captured aggregates are subject to a brief washing cycle where they are washed clean of residual aggregating agent, plasma, and red cells. Aggregates can then be partly or wholly deaggregated and the cells retrieved. The result is a suspension of cells and small aggregates with therapeutic levels of concentrated blood cells with included growth factors that are available for delivery to a wound site. A device that accomplishes the aforementioned process is also described.
Owner:MOHAMMAD S FAZAL +1
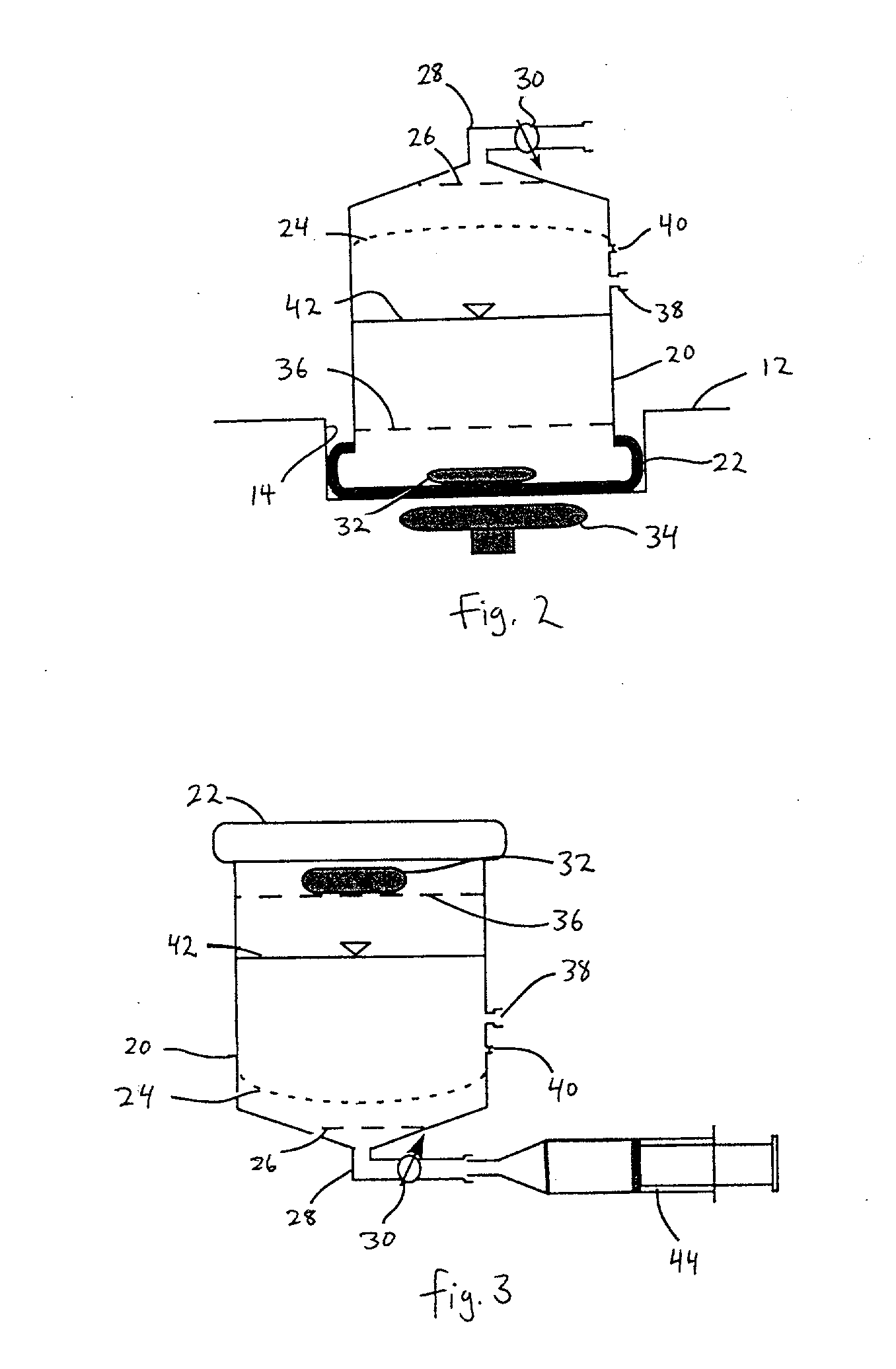
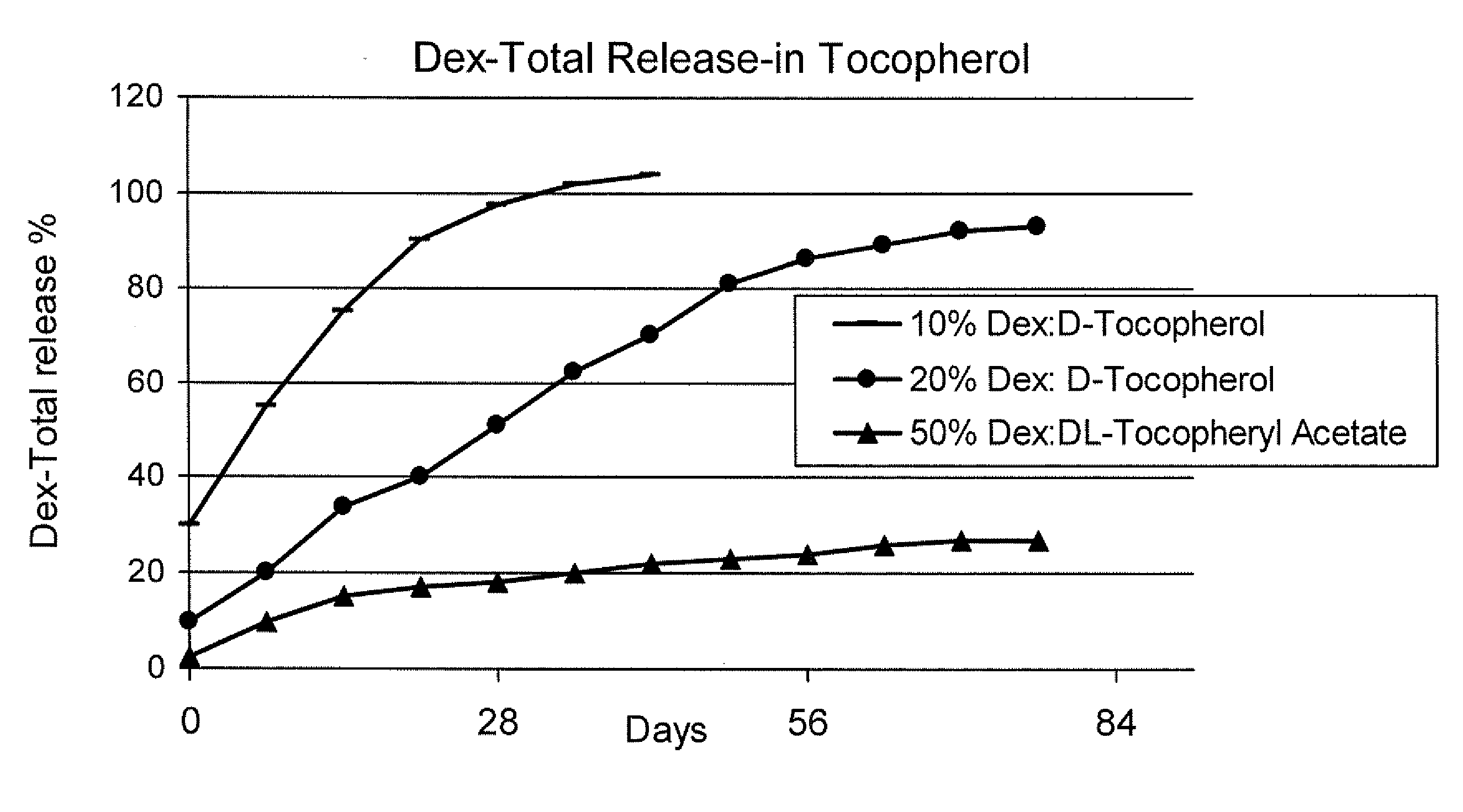
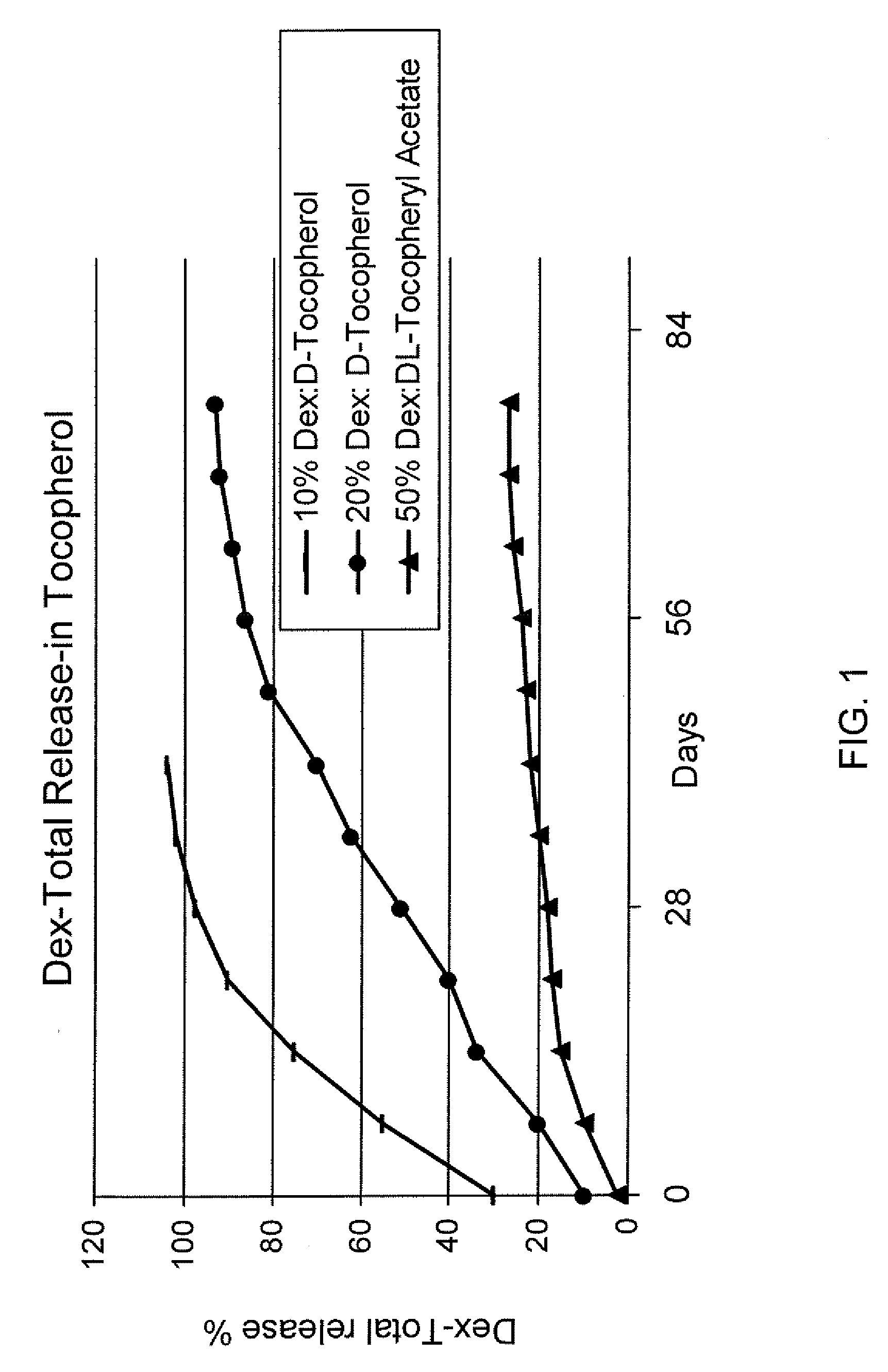
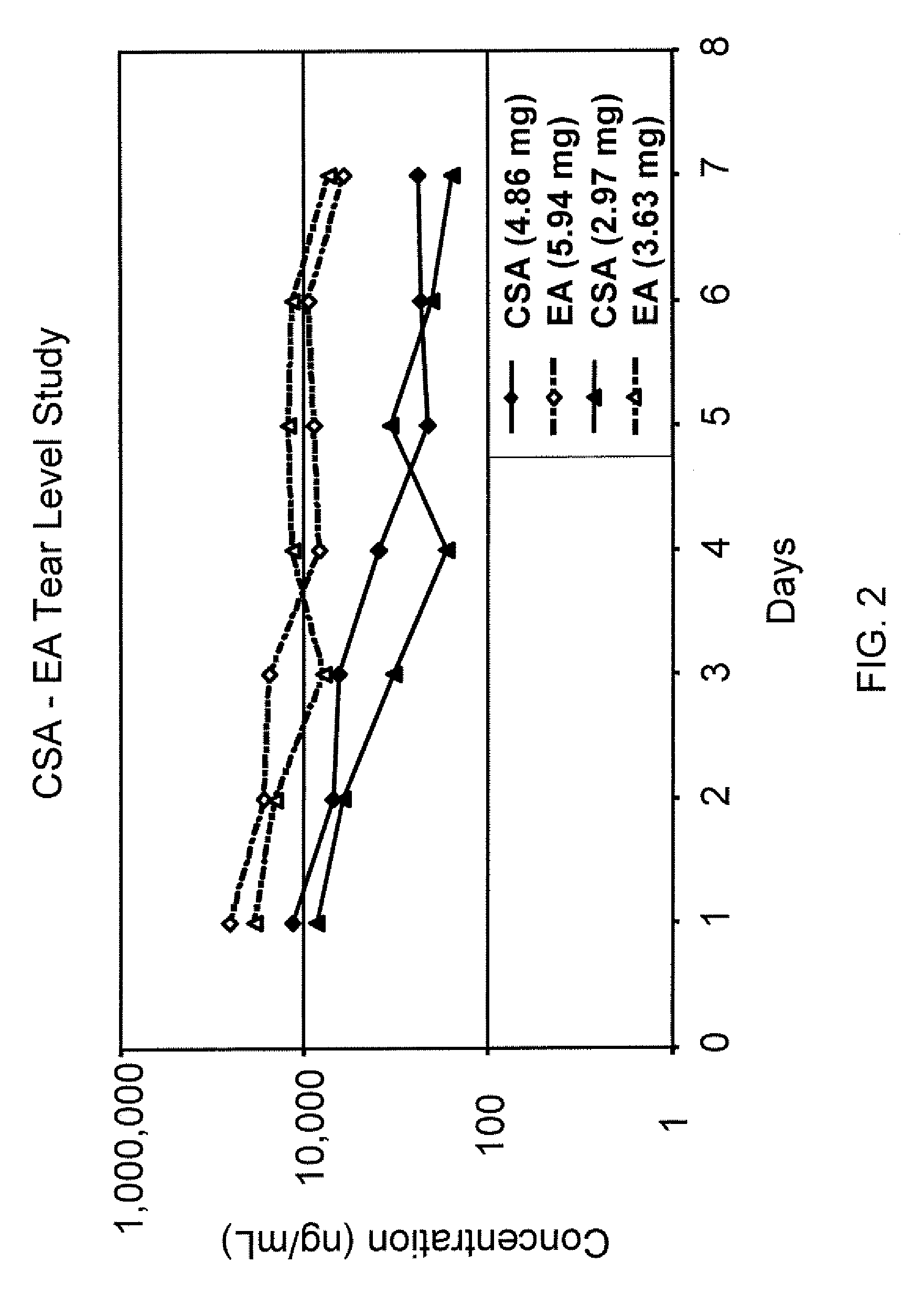
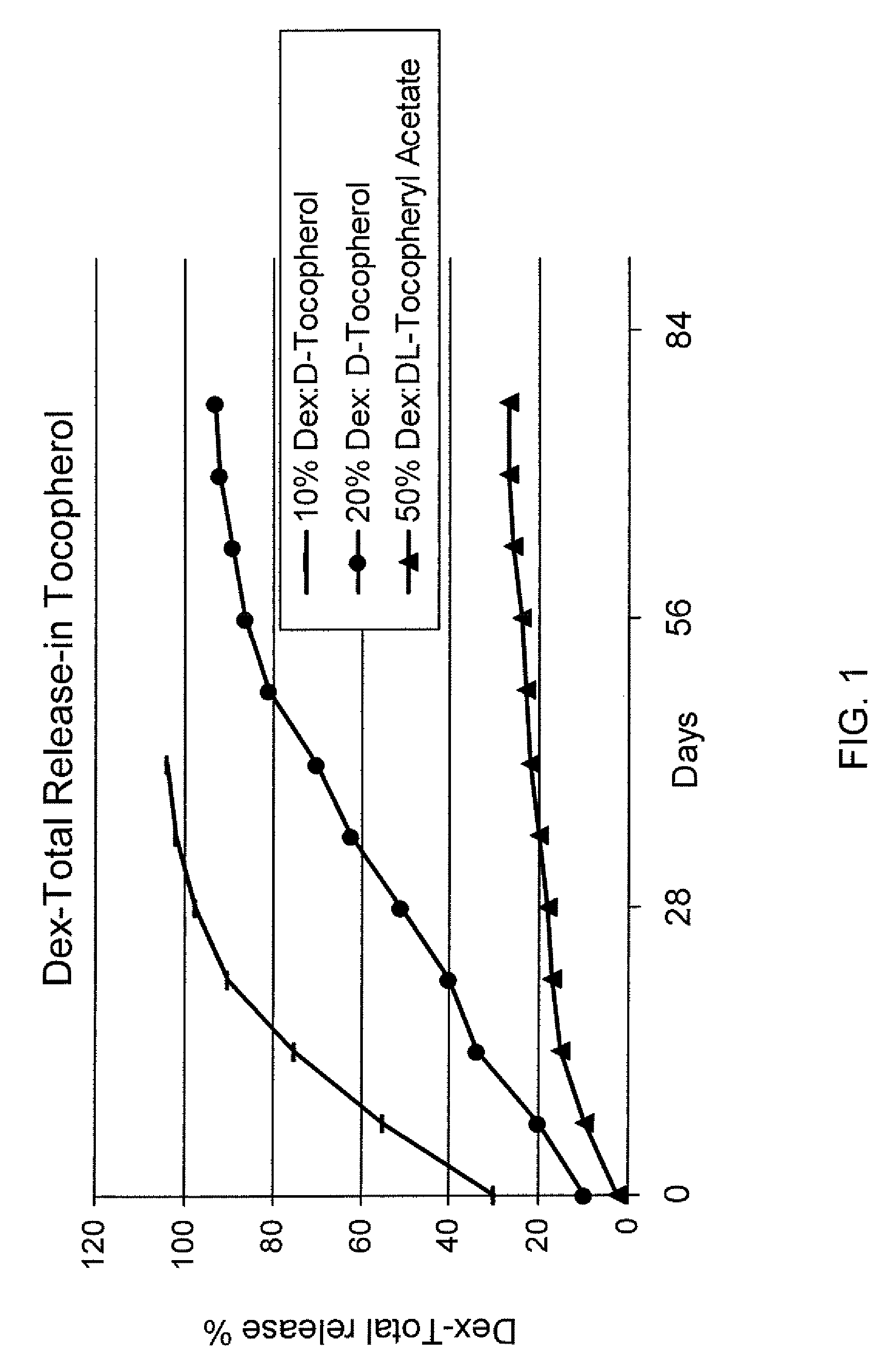
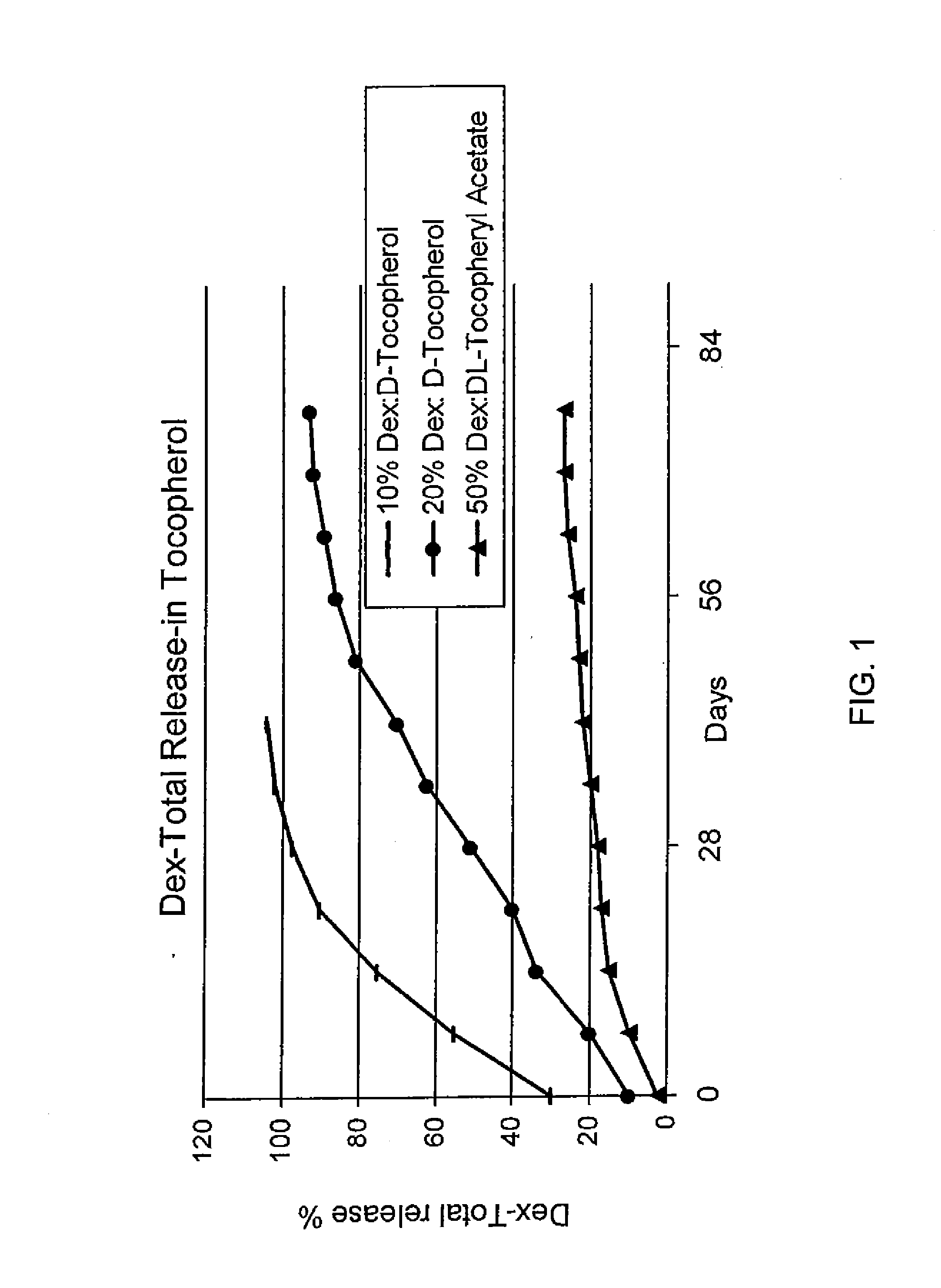
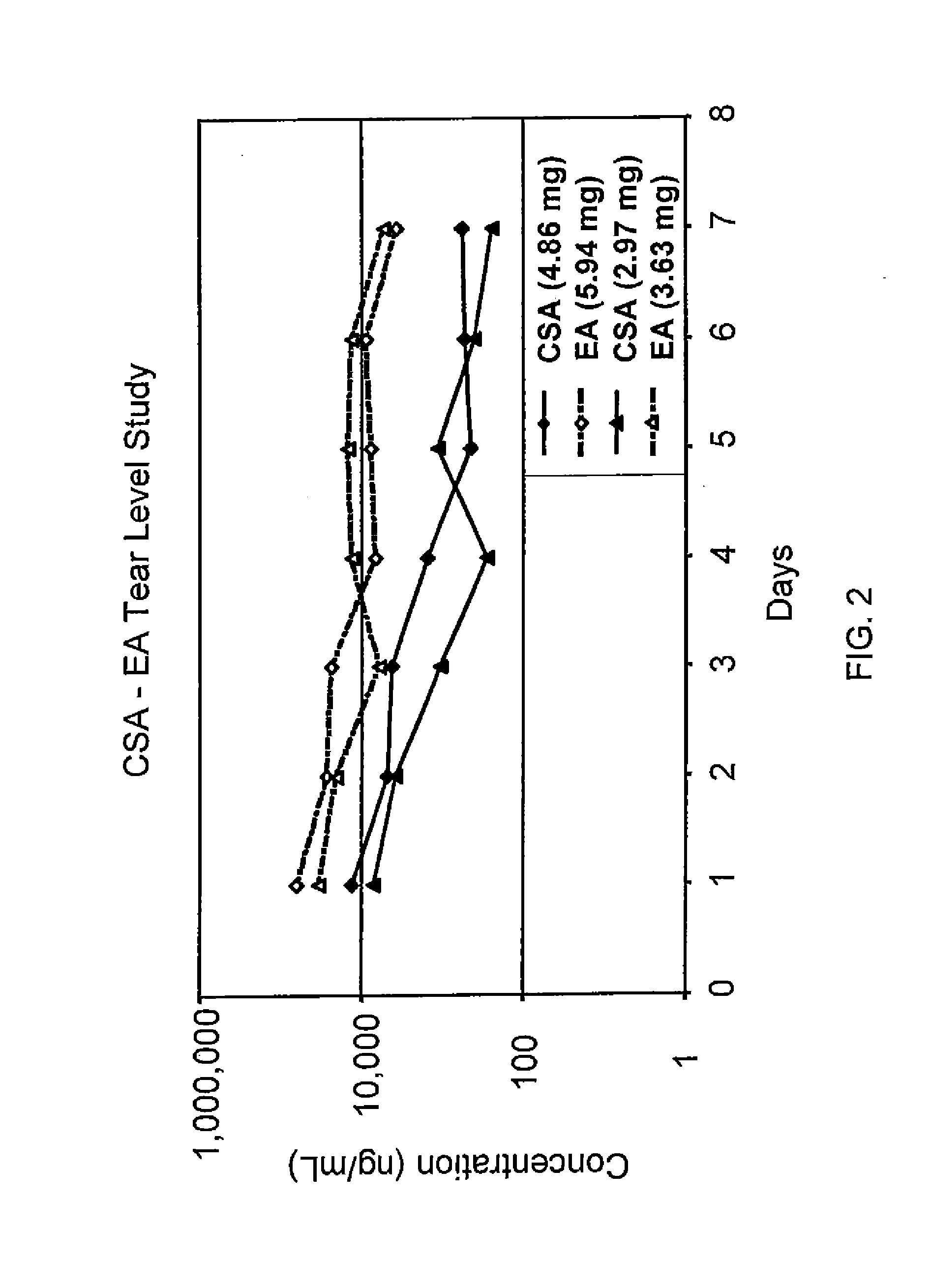
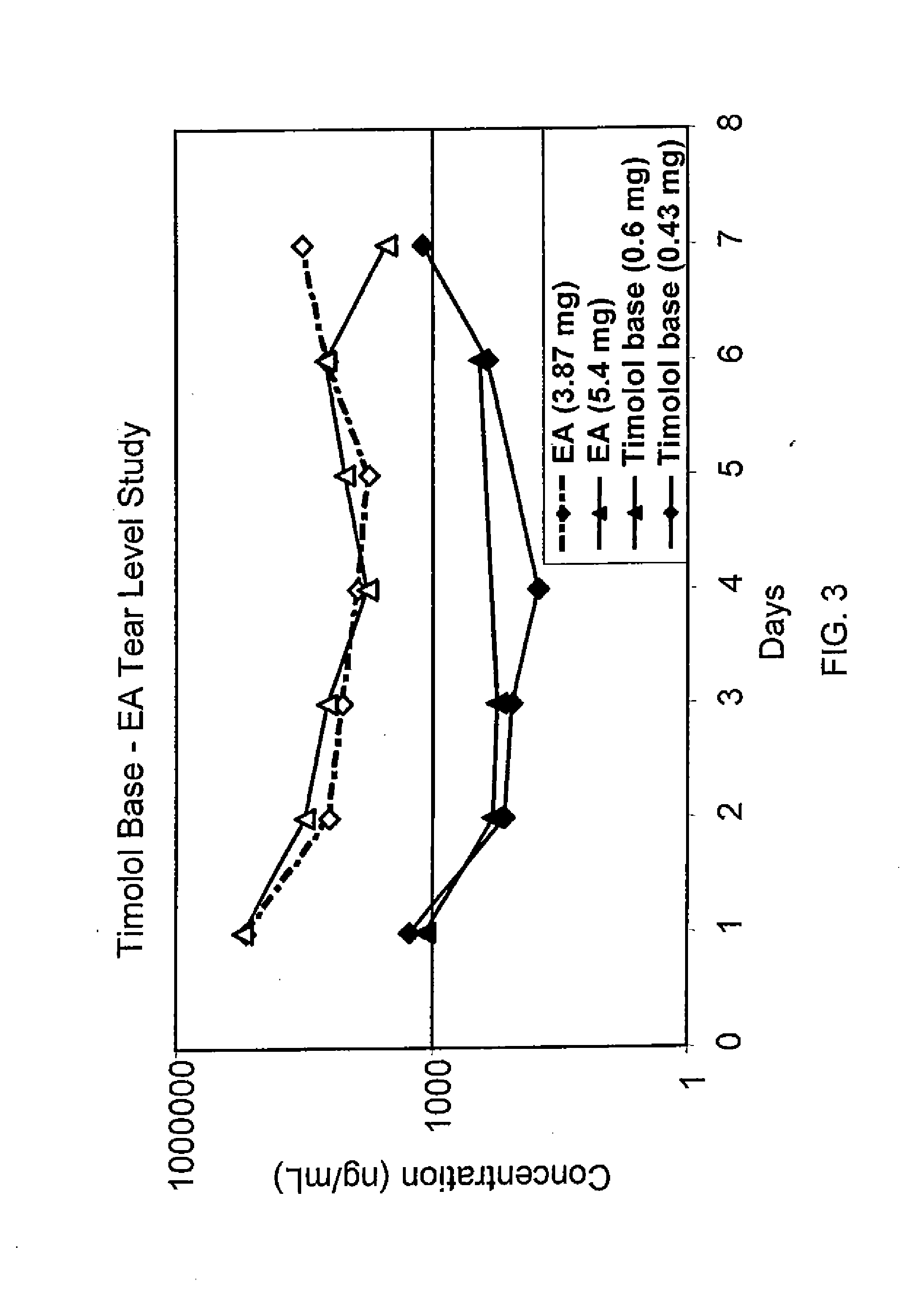
Sustained release eye drop formulations
ActiveUS20090136445A1Economical and practical and efficientEasy to produceAntibacterial agentsBiocideSolubilityIrritation
This invention provides for biocompatible, biodegradable eye drop pharmaceutical formulations useful for the treatment of ocular indications. In particular, tocopherols and their esters of low water solubility, notably α-tocopheryl acetate, are exceptional vehicles for biocompatible, nonirritating topical eye drop formulations that provide sustained release of active agents.
Owner:RAMSCOR
Pharmaceutical compositions and use thereof
InactiveUS20090169635A1Efficient curingIncrease loopAntiinfectivesGranular deliveryAntibacterial activityAntibiotic Y
Colloidal compositions, loaded with non-covalently bonded antibiotics, can be efficiently used for the treatment of severe bacterial pneumonia and other serious lung infections such as tuberculosis. Such formulations, comprised of biodegradable nanoparticles or nanocapsules with incorporated antibiotics, show a significant increase in antibacterial activity, extended and sustained drug release and a decrease in frequency of the drug administration. Antibiotics of various types, such as aminoglycosides, glycopeptides and others can be successfully incorporated into a nanoparticulate colloidal delivery system.
Owner:ALPHARX
Double-layer coated fertilizer and preparation method thereof
InactiveCN104609964AGood sustained releaseImprove water retentionFertilizer mixturesControl releaseMicrocrystalline wax
The invention discloses a double-layer coated fertilizer and a preparation method thereof. The double-layer coated fertilizer is composed of a core fertilizer, and an inner-layer coating material and an outer-layer coating material both covering the core fertilizer; the inner-layer coating material is microcrystalline wax; the outer-layer coating material comprises a water-retaining agent and a coating agent; the mass ratio of the core fertilizer, the inner-layer coating material and the outer-layer coating material is 100:(0.5-2):(3-20); the mass ratio of the water-retaining agent to the coating agent is 100:(5-30). The preparation method comprises the following steps: adding the core fertilizer and the inner-layer coating material to a coating machine, blasting air and heating until the inner-layer coating material is melted to cover the core fertilizer, thereby obtaining the fertilizer covered with the inner-layer coating material; 2) covering the fertilizer covered with the inner-layer coating material with the outer-layer coating material for at least once, thereby obtaining the double-layer coated fertilizer. The double-layer coated fertilizer has better controlled release and water retaining effects; the preparation method is simple; the coating agent is good in dissolving effect, and advantageous for coating; the production method is suitable for batch production.
Owner:RUBBER RES INST CHINESE ACADEMY OF TROPICAL AGRI SCI
Compositions and Methods for Stem Cell Expansion and Differentiation
InactiveUS20070280989A1Free from damagePositively chargedBiocideGenetic material ingredientsCross-linkProgenitor
The present invention relates to compositions comprising stem cells and partially committed progenitor cells and to methods of controlling cell proliferation and differentiation, which can be used for expansion of stem cells and their subsequent differentiation. The present invention provides expanded population of essentially undifferentiated stem cells, which are useful in clinical procedures involving stem cell therapy, and a population derived thereof of which at least part of the cells are differentiated. The cells can be used per se, as a part of a cell-bearing composition comprising cross-linked hyaluronic acid-laminin gels or as a part of a composite implant for tissue regeneration.
Owner:NVR LABS
Method and composition for inhibiting reperfusion injury in the brain
ActiveUS7332159B2Avoid injuryGood sustained releaseNanotechNervous disorderReperfusion injuryNanoparticle
The present invention relates to a method for inhibiting reperfusion injury in the brain. The method involve injecting via the carotid artery or jugular vein an antioxidant-loaded nanoparticle. A nanoparticle formulation containing an inert plasticizer is also provided for sustained release of an active agent.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Crosslinked polysaccharide microparticles and method for their preparation
InactiveUS20070134334A1High recovery rateImprove packaging efficiencyPowder deliverySugar derivativesMicroparticlePolysaccharide
The present invention provides long-acting sustained-release formulations of drugs such as proteins or peptides, which are injectable, completely biodegradable and safe, as well as ensuring efficient encapsulation of the drugs such as proteins or peptides without inhibiting their biological activity. It is possible to achieve injectable sustained-release formulations that ensure efficient encapsulation and long-term sustained release of drugs such as proteins or peptides while retaining their biological activity, when a solution containing a drug and a polysaccharide derivative such as hyaluronic acid having a crosslinkable functional group(s) or a salt thereof is dehydrated in microparticulate form starting from a dilute state, where crosslinking proceeds slowly, to reach a concentration which facilitates crosslinking, to thereby cause crosslinking reaction during concentration, so that the drug is encapsulated into the crosslinked polysaccharide to give drug-carrying microparticles.
Owner:CHUGAI PHARMA CO LTD
Slow released ClO2 air disinfectant
InactiveCN1887356AImprove the activation effectProlong the evaporation timeDeodrantsDiseaseChlorine dioxide
The present invention is slow released ClO2 air disinfectant, and belongs to the field of hygienic disease controlling and sterilizing technology. The slow released ClO2 air disinfectant consists of ClO2 solution in 1-3 weight portions, silicon gel as the carrier of ClO2 solution in 3 and activator in 0.005-0.1 weight portions. The slow released ClO2 air disinfectant has homogeneous and lasting release to maintain proper ClO2 concentration in the air and reach excellent air disinfecting effect. The used silicon gel may be recovered and reused after treatment.
Owner:陈惠 +1
Swelling-type hollow silk fibroin micro-needle drug delivery system and preparation method thereof
ActiveCN104888284AHigh drug loading rateAllergenicity reductionMicroneedlesSurgeryDermal sensitizationProcessing cost
The invention discloses a swelling-type hollow silk fibroin micro-needle drug delivery system and a preparation method thereof. According to the technical scheme of the preparation method, a swelling modified silk fibroin solution is casted in a PDMS mold to be vacuumized, dried and molded as a hollow needle-type housing. After that, a silk fibroin micro-needle array provided with cavities in the hollow needle-type housing is filled with powder-like or solution-like drugs, vacuumizing and drying are carried out to form a drug part. Finally, a layer of modified silk fibroin solution is cast, dried and molded as a cladded coating. The cladded coating is removed to obtain the swelling-type hollow silk fibroin micro-needle drug delivery system. The drugs are wrapped inside the cavities of the silk fibroin micro-needle arrays, so that the system is high in drug loading ratio and low in skin allergenicity and stimulation. The system facilitates the sustained release and the controlled release of micro-needle drugs. Meanwhile, according to the preparation method, since the swelling modified silk fibroin solution is cast, dried and molded in a mild processing condition, the system is low in processing cost and suitable for large-scale production. Drugs are directly wrapped in the cavities of the swelling hollow micro-needles, so that controlled-release drugs are swelled in the micro-needle base material. In this way, the biological activities of the controlled-release drugs are maintained, while the structure of the hollow silk fibroin micro-needle drug delivery system is greatly simplified. The system is better in practicality.
Owner:PHARSUN MEDICAL BIOTECHNICS (SHANGHAI) CO LTD
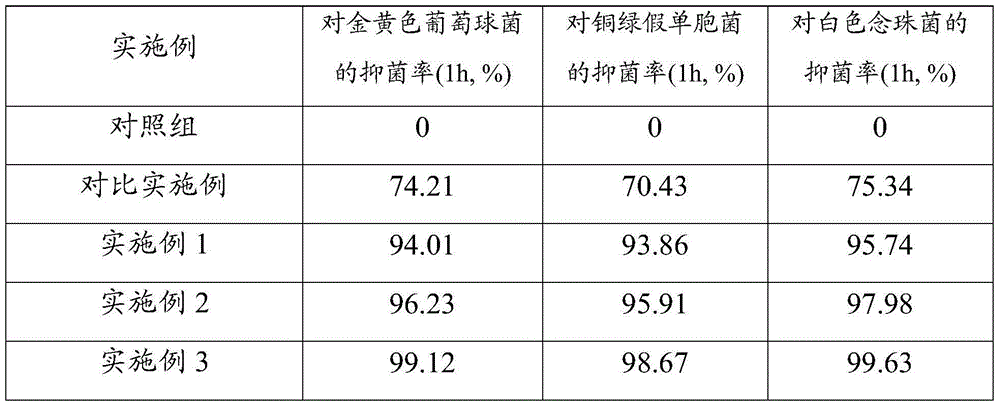
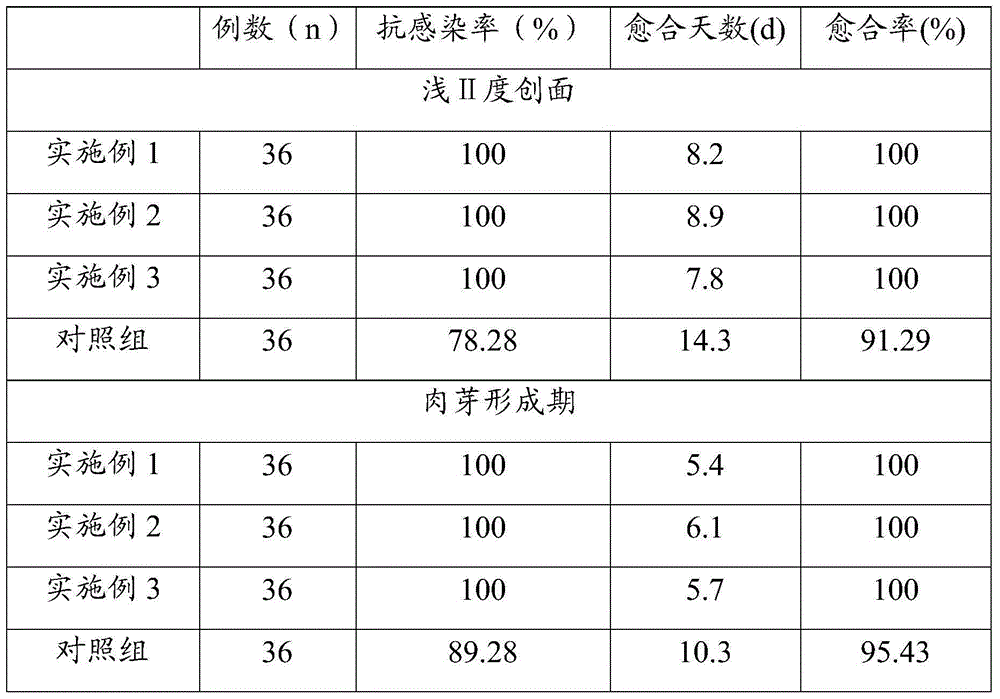
Foam dressing containing composite silver-zinc antibacterial material and preparation method of foam dressing
The invention provides a foam dressing containing a composite silver-zinc antibacterial material and a preparation method of the foam dressing. The foam dressing containing the composite silver-zinc antibacterial material comprises polyurethane foaming plastic and the composite silver-zinc antibacterial material, wherein the composite silver-zinc antibacterial material is adhered to the inside of the polyurethane foaming plastic, comprises the following raw materials in content: nano silver, nano zinc and a nano inorganic carrier; and the composite silver-zinc antibacterial material contains the following raw materials in percentage by mass: 10%-20% of silver and 20%-40% of zinc. The composite silver-zinc antibacterial material is dispersed into the foam dressing in a manner of foam molding or ultrasonic dipping together with foam. The foam dressing containing the composite silver-zinc antibacterial material is capable of effectively absorbing diffusate of the wound surface, and effectively inhibiting growth and breeding of staphylococcus aureus, pseudomonas aeruginosa and candida albicans.
Owner:SHENZHEN TSINGHUA YUANXING NANO MATERIAL CO LTD
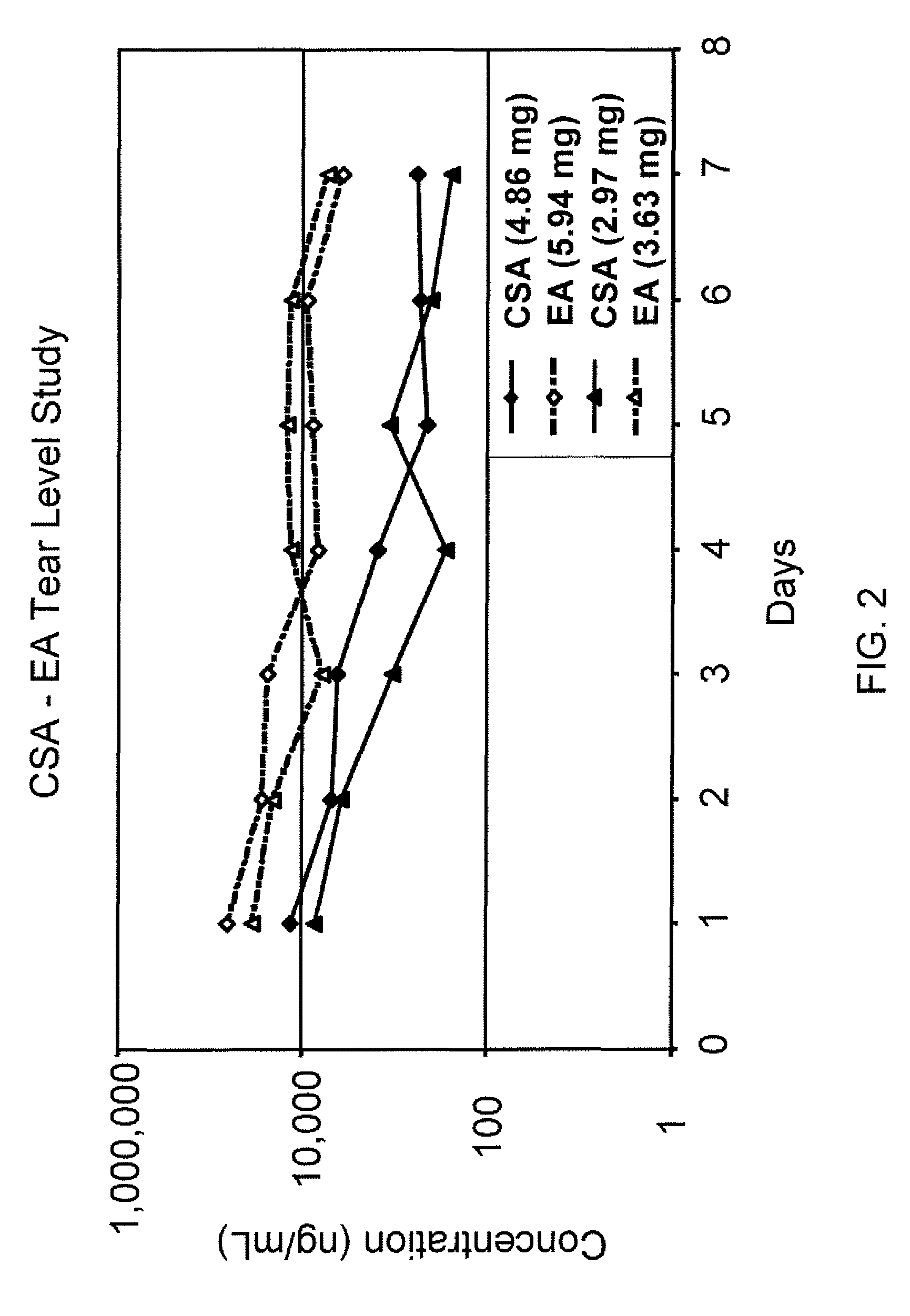
Sustained release eye drop formulations
ActiveUS8541413B2Easy to useEfficient deliveryAntibacterial agentsBiocideActive agentAqueous solubility
This invention provides for biocompatible, biodegradable eye drop pharmaceutical formulations useful for the treatment of ocular indications. In particular, tocopherols and their esters of low water solubility, notably α-tocopheryl acetate, are exceptional vehicles for biocompatible, nonirritating topical eye drop formulations that provide sustained release of active agents.
Owner:RAMSCOR
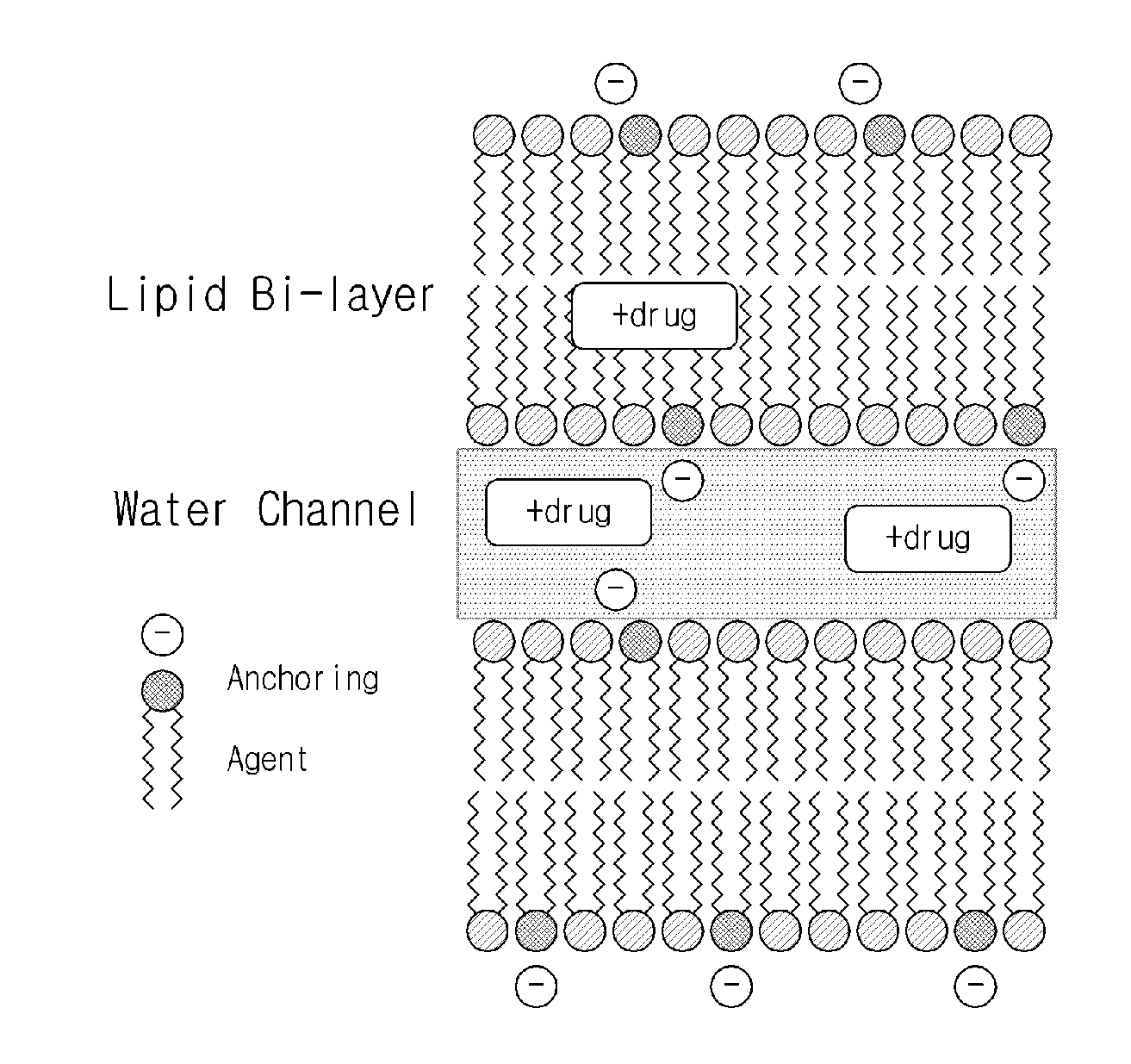
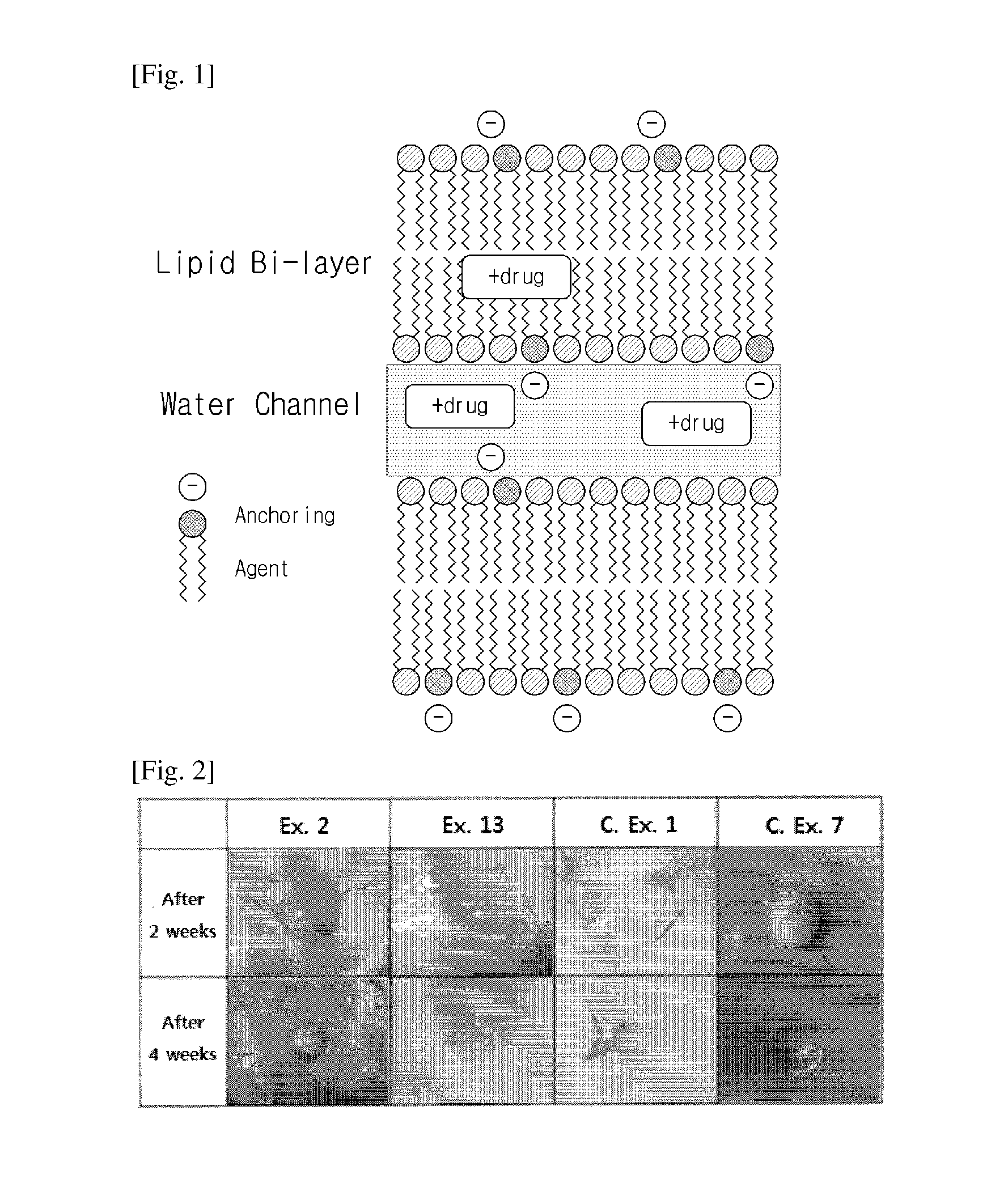
Sustained-release lipid pre-concentrate of cationic pharmacologically active substance and pharmaceutical composition comprising the same
InactiveUS20150265535A1Good sustained releaseImprove sustained releaseBiocideOintment deliveryDrugLiquid crystal
Disclosed is a sustained-release lipid pre-concentrate, comprising: a) at least one liquid crystal former; b) at least one neutral phospholipid; c) at least one liquid crystal hardener; and d) at least one anionic anchoring agent, wherein the sustained-release pre-concentrate exists as a lipid liquid phase in the absence of aqueous fluid and forms into a liquid crystal upon exposure to aqueous fluid. The sustained-release lipid pre-concentrate is configured to enhance the sustained release of cationic pharmacologically active substance through ionic interaction between the anionic anchoring agent and the cationic pharmacologically active substance.
Owner:CHONG KUN DANG PHARMA CORP
Porous composite micro balls for cosmetics and preparation method for porous composite micro balls
InactiveCN102659973AGood parenthoodImprove wettabilityCosmetic preparationsMake-upWater basedFiltration
The invention discloses porous composite micro balls for cosmetics and a preparation method for the porous composite micro balls. According to the method, the porous micro balls are modified by a chemical method, and the porous micro balls have high amphipathicity and can be applied to water-based and oily cosmetic systems well, and the application range of the porous micro balls in the field of the cosmetics is widened. The method comprises the following steps of: adding the prepared porous polymer micro balls, deionized water and ethanol in a mass ratio into a reactor, and dissolving ester compounds or a metal saline solution in the ethanol, dripping into the reactor, stirring for 2 to 8 hours for centrifugal separation, washing, performing suction filtration at room temperature, and performing vacuum drying to obtain the porous composite micro balls, wherein the ester compounds are tetraethoxysilane or butyl titanate; and the metal saline solution is zinc nitrate, zinc acetate, aluminum nitrate, aluminum isopropoxide, aluminum ethylate or aluminum sec-butoxide. Porous structures of the prepared composite micro balls are in an empty cage shape, and the porous structures can be regulated and controlled by changing the process, so that the requirements of different release effects of the cosmetics are met.
Owner:SOUTH CHINA UNIV OF TECH
Polyethylene glycol methyl ether maleate, and preparation method and application thereof
InactiveCN103588969AThe synthesis process is smallLittle impact on product performanceAdditive synthesisPolyethylene glycol
The invention belongs to the concrete additive synthesis field, and concretely discloses a polyethylene glycol methyl ether maleate used for synthesizing a hyper-dispersant, and a preparation method and an application thereof. The preparation method concretely comprises the following steps: adding maleic anhydride and polyethylene glycol monomethyl ether into a reactor, heating to 60DEG C, and carrying out melt mixing; adding a polymerization inhibitor and a catalyst, uniformly stirring, letting in nitrogen, reacting at 90DEG C for 2h to generate an intermediate polyethylene glycol monomethyl ether maleate; and heating to 140-180DEG C, and carrying out a constant temperature stirring reaction for 4-10h to obtain the polyethylene glycol methyl ether maleate. The polyethylene glycol methyl ether maleate prepared through the method can be used for synthesizing hyper-dispersants. The preparation method has the advantages of easy operation, no need of a water carrying agent, simple reaction technology, high esterification rate, few byproudcts, no need of post-tretmetn, realization of the implementation of the reactions under normal pressure, mild reaction conditions, less apparatus investment and low energy consumption.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
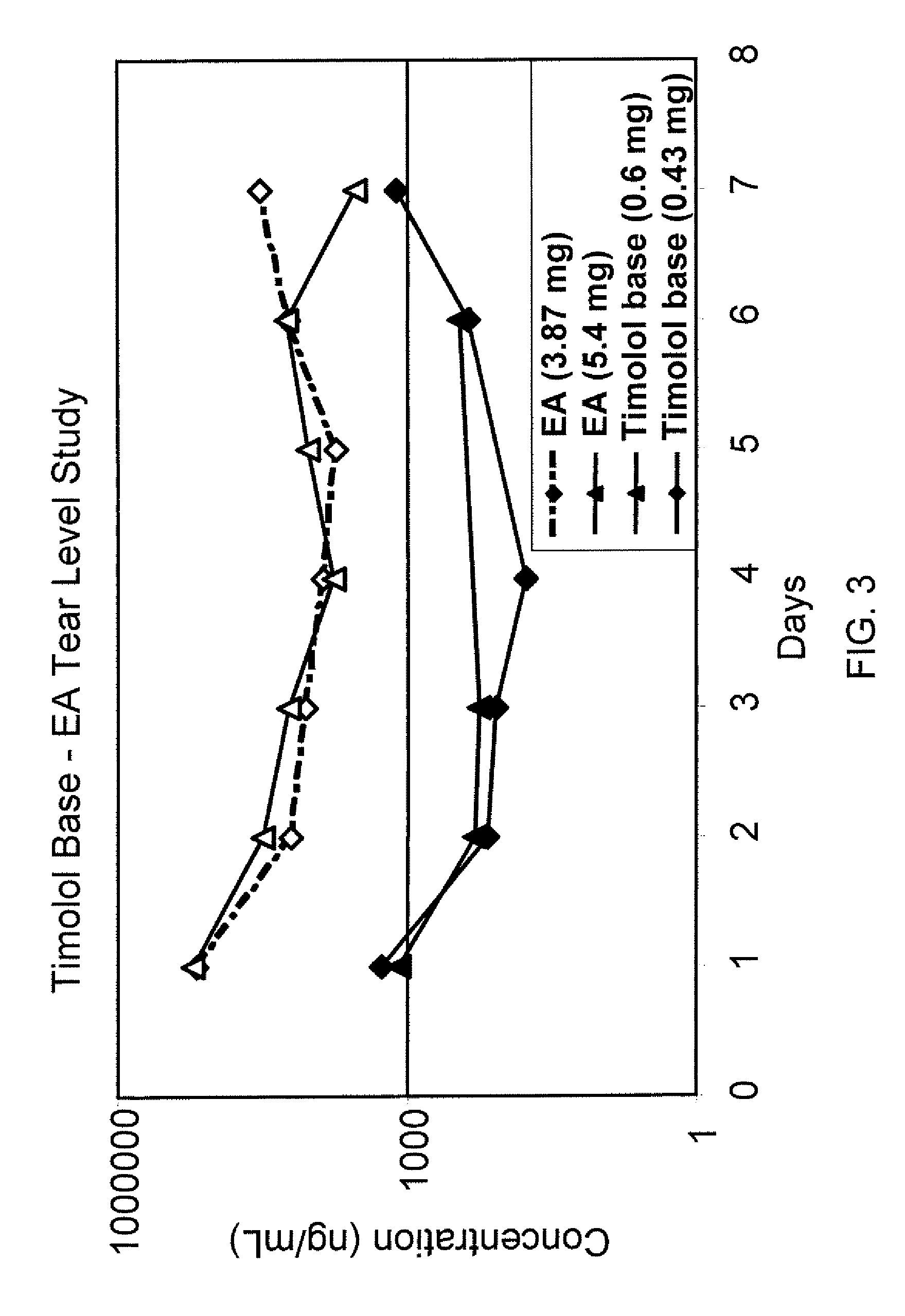
Sustained release eye drop formulations
ActiveUS20130324481A1Easy to useEfficient deliveryBiocideTetracycline active ingredientsSolubilityEye/ear drops
This invention provides for biocompatible, biodegradable eye drop pharmaceutical formulations useful for the treatment of ocular indications. In particular, tocopherols and their esters of low water solubility, notably α-tocopheryl acetate, are exceptional vehicles for biocompatible, nonirritating topical eye drop formulations that provide sustained release of active agents.
Owner:RAMSCOR
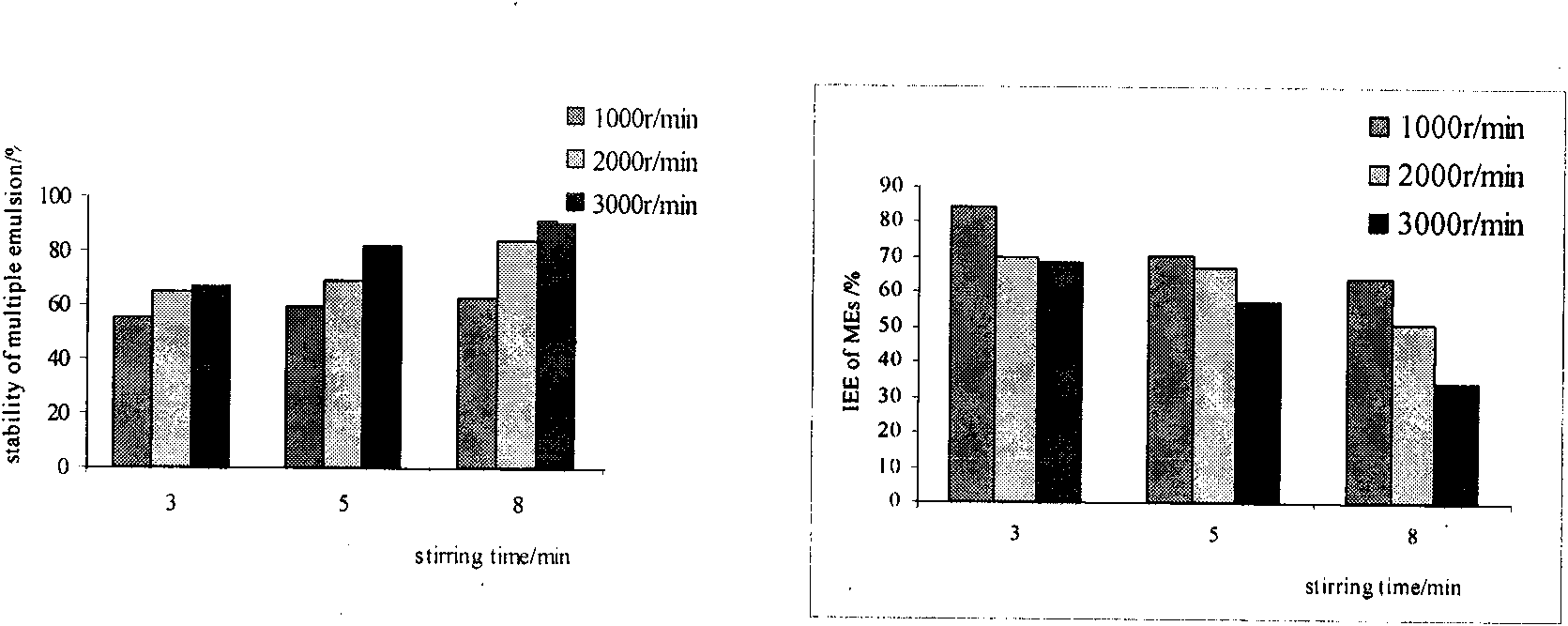
Method for preparing W/O/W type compound emulsion embedding chlorogenic acid, product and application of W/O/W type compound emulsion
ActiveCN101618016APlay a protective effectPlay a storage roleAntibacterial agentsOrganic active ingredientsControlled releaseChlorogenic acid
The invention relates to a method for preparing W / O / W type compound emulsion embedding an chlorogenic acid, belonging to the field of compound emulsion preparation and comprising the following steps: mixing 1 part of aqueous chlorogenic acid solution by weight, 2 parts of oil phases by weight and 0.2-0.4 part of lipophilic emulsifier by weight to be stirred for 4-10 minutes under the speed of 6,000-15,000 turns / minute so as to obtain W / O type colostrums; and then mixing 2-3 parts of the W / O type colostrums and 2-3 parts of external water phrases to be stirred for 2-10 minutes under the speed of 1000-6000 turns / minute so as to obtain the W / O / W type compound emulsion. The method for preparing the W / O / W type compound emulsion can not only play roles of protection and storage on the chlorogenic acid, but also realize the uniform and effective release of the chlorogenic acid in human bodies, and also promotes the slow release, the controlled release and the target effect of absorption and sufficiently plays a role of the chlorogenic acid by embedding the hlorogenic acid with the W / O / W type compound emulsion.
Owner:HUABAO FLAVOURS & FRAGRANCES CO LTD
Fluidized-bed coating machine
InactiveCN101061988AIncrease productionGood sustained releasePharmaceutical product form changeMaterial granulationFluidized bedSpray nozzle
The invention discloses a fluid bed tablet-coating machine, which is characterized by the following: possessing dry air inlet and tank of ventilating port; arranging air flow distribution plate and filter in the tank; arranging at least two big hole area on the same angular of even dispersing on the air flow dispersing plate; setting the perforated ratio of the big hole area at 65%; arranging guide barrel on the upper of the air flow dispersing plate; heading on each big hole area; possessing gap between the floor of the guide barrel and the air flow dispersing plate; arranging a dressing injector; corresponding to each guide barrel; projecting the dressing injector from the central hole of the big hole area of the air flow dispersing plate. This invention possesses high output, which can be fit for big scale industrial production.
Owner:邹龙贵
Sustained release of agents for localized pain management
InactiveUS20080220062A1Improved sustained release deliveryImprove the level ofPowder deliveryNervous disorderLocalized painWhole body
Owner:PSIVIDA US INC
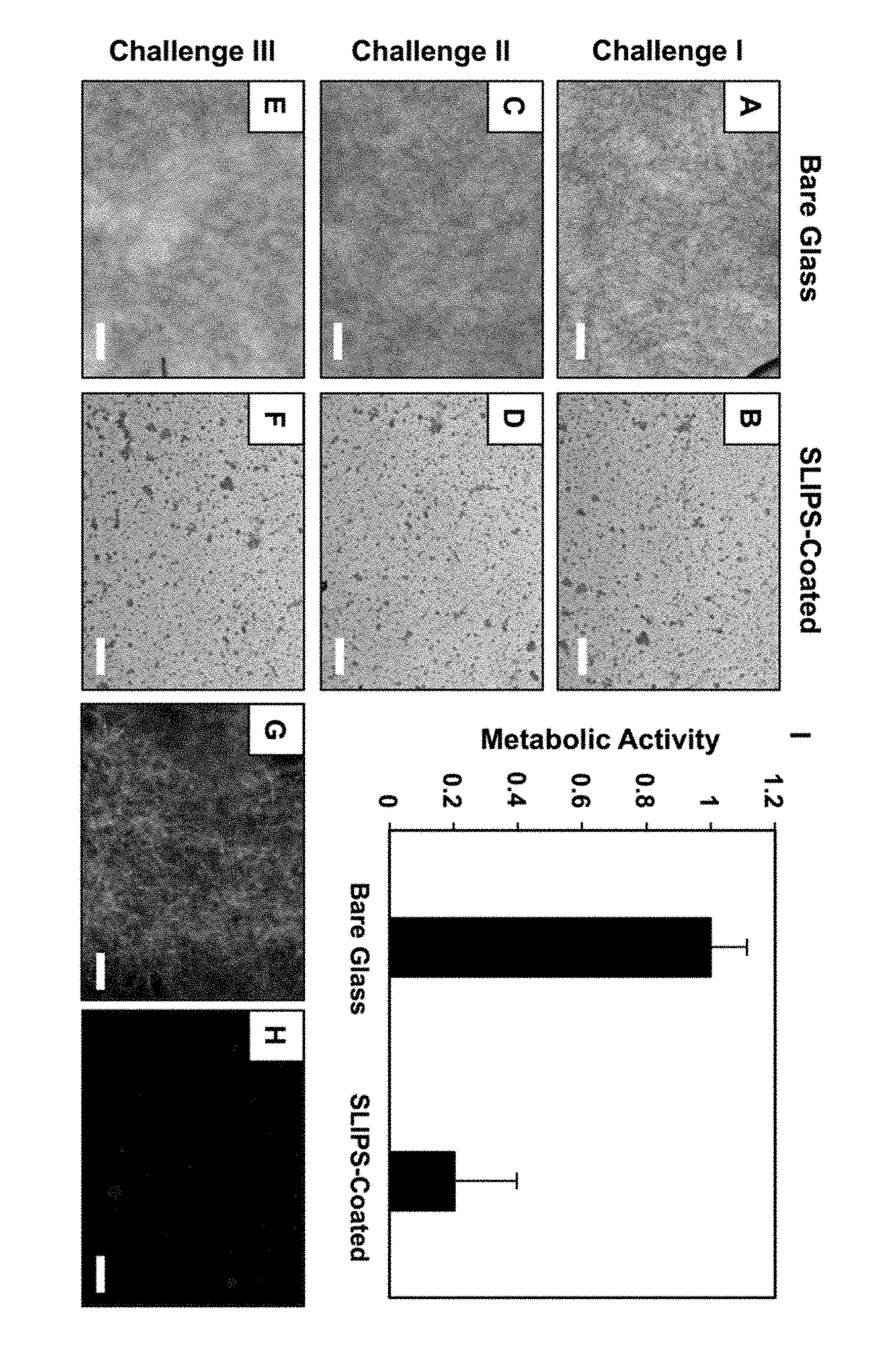
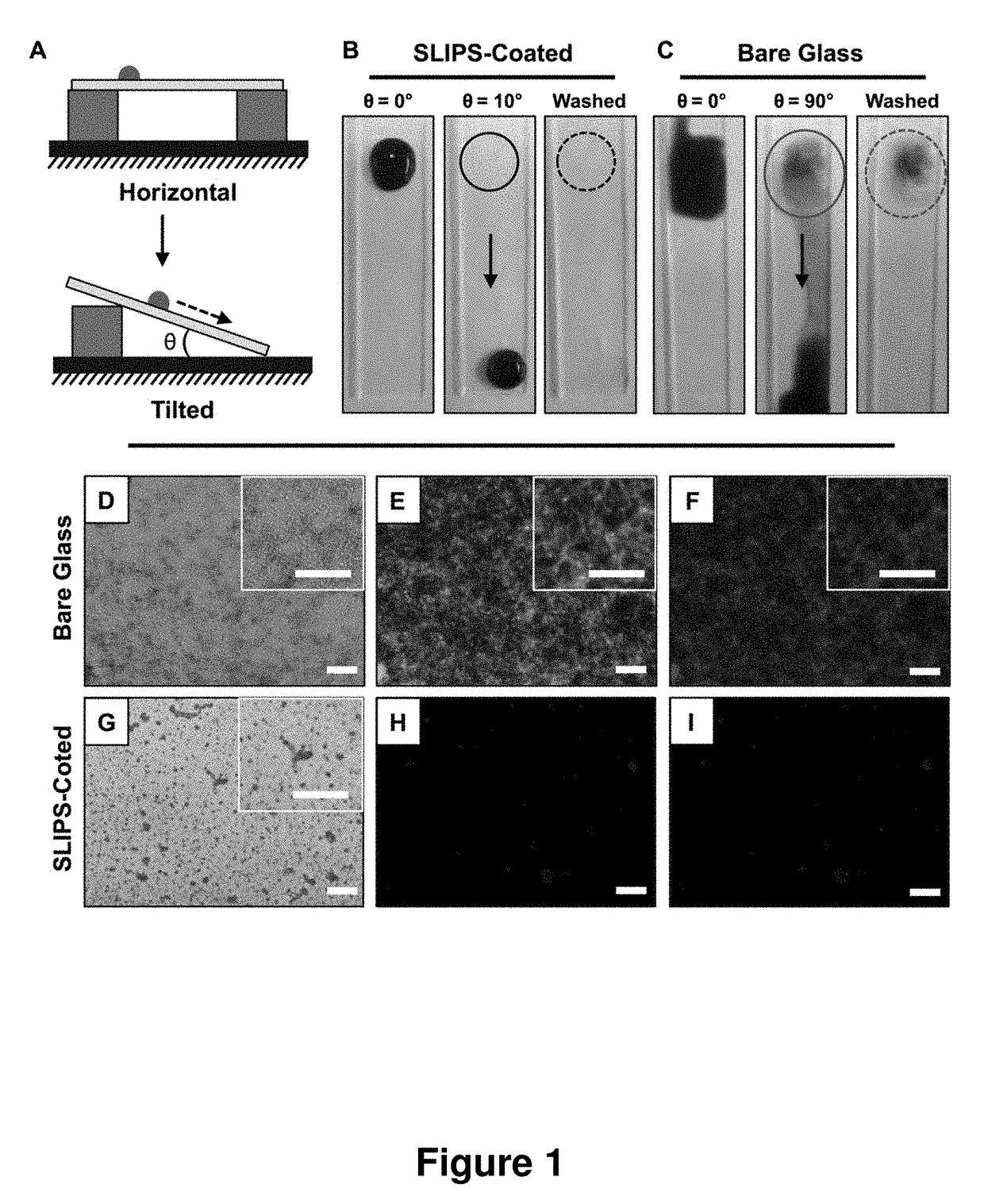
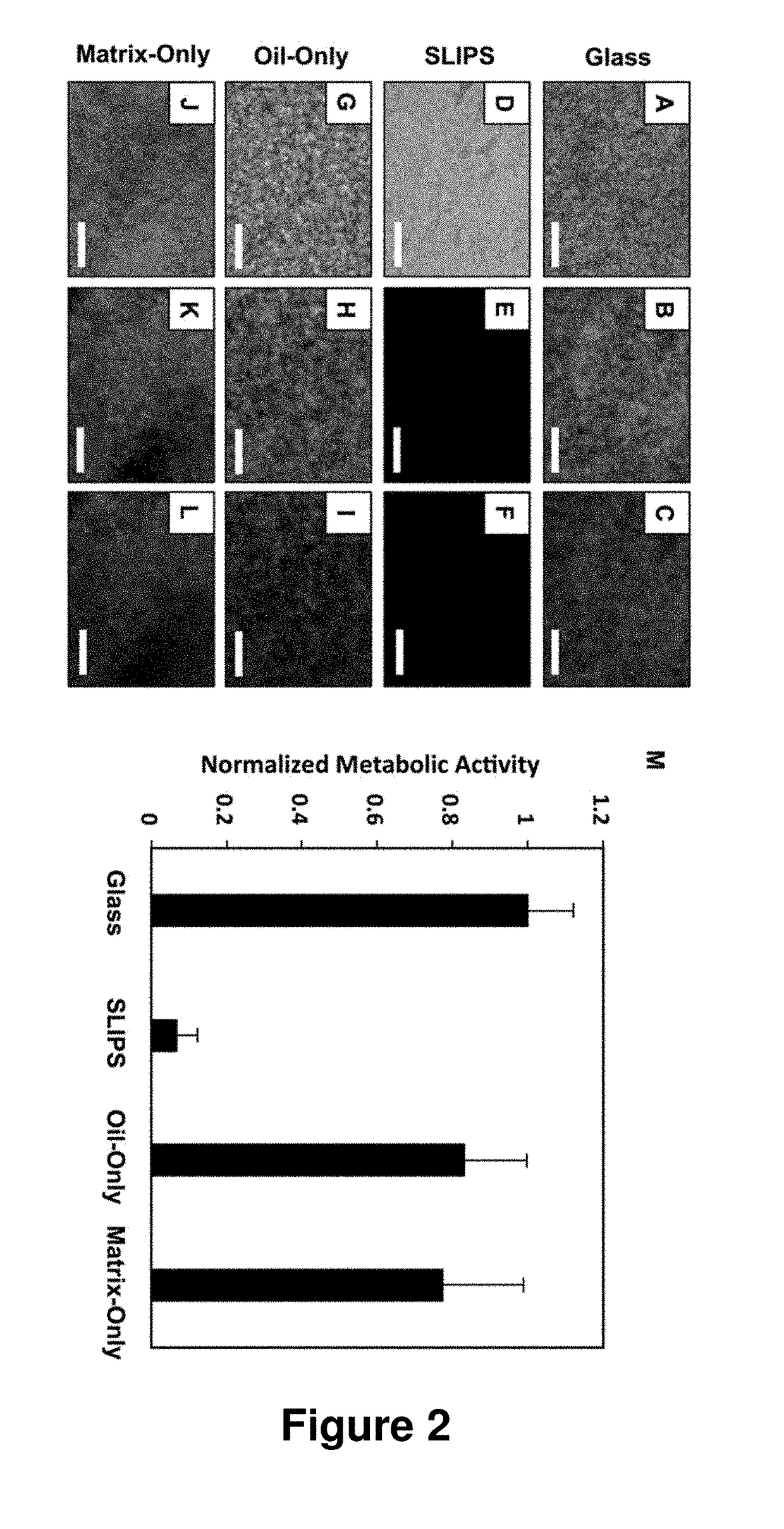
Slippery Liquid-Infused Porous Surfaces that Prevent Microbial Surface Fouling
ActiveUS20180230318A1Good sustained releasePoor surfaceOrganic chemistryAntifouling/underwater paintsMicrobial agentOil phase
The present invention provides polymer-based slippery liquid-infused porous surfaces (SLIPS) that can prevent adhesion and colonization by fungal and bacterial pathogens and also kill and / or attenuate the colonization and virulence of non-adherent pathogens in surrounding media. The present approach exploits the polymer and liquid oil phases in these slippery materials to sustain the release of small molecules such as a broad-spectrum antimicrobial agent, an antifungal agent, an antibacterial agent, an agent that modulates bacterial or fungal quorum sensing, an agent that attenuates virulence, or a combination thereof. This controlled release approach improves the inherent anti-fouling properties of SLIPS, has the potential to be general in scope, and expands the potential utility of slippery, non-fouling surfaces in both fundamental and applied contexts.
Owner:WISCONSIN ALUMNI RES FOUND
Conjugate of polyethylene glycol and anesthetic, and preparation method thereof
ActiveUS10525143B2High drug loadingGood sustained releasePowder deliveryAntipyreticAnalgesia postoperativePolyethylene glycol
A conjugate represented by general formula (I), wherein R0 is a C1-6 alkyl, B is an anesthetic, and A is a linking group, and a quaternary ammonium salt is formed at the linking position between B and R0. The conjugate has a prolonged analgesic effect, and can be used in postoperative analgesia or treatment for chronic pain.
Owner:JENKEM TECH CO LTD TIANJIN
Method for preparing dual-factor carrying type hybrid bionic bone scaffold and application of dual-factor carrying type hybrid bionic bone scaffold
ActiveCN104288839AEasy to set upPromote osteogenic differentiationProsthesisFiberBone-Inducing Protein
The invention relates to a method for preparing a dual-factor carrying type hybrid bionic bone scaffold and application of the dual-factor carrying type hybrid bionic bone scaffold. The preparation method comprises the following steps: immersing a calcium- silicon hybrid bionic bone scaffold in fibers in a chemotactic factor-osteogenesis inducing protein mixed solution with proper concentration; treating under a proper condition to obtain the dual-factor carrying type hybrid bionic bone scaffold. The related application is application of the scaffold material in a bone defect repairing material. The hybrid ossein contains silicon dioxide and hydroxyapatite two minerals, wherein due to the effective combination of massive negative charge-loaded silanol groups existing on the surface of the silicon dioxide and active hydroxyl radicals on basic protein such as SDF-1 or TGF-beta and the specifically strong adsorptivity of nano-hydroxyapatite on osteogenesis induced protein, such as BMP-2 or BMP-7, an extra sustained-release system need not to be added, and hybrid osteocyte recruitment / osteogenesis induction double functionalized modification can be realized.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com