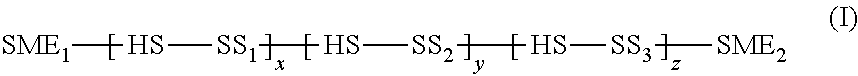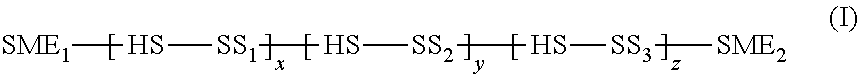Patents
Literature
59 results about "Intravaginal administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intravaginal administration is a route of administration where the substance is applied inside the vagina. Pharmacologically, it has the potential advantage to result in effects primarily in the vagina or nearby structures (such as the vaginal portion of cervix) with limited systemic adverse effects compared to other routes of administration.
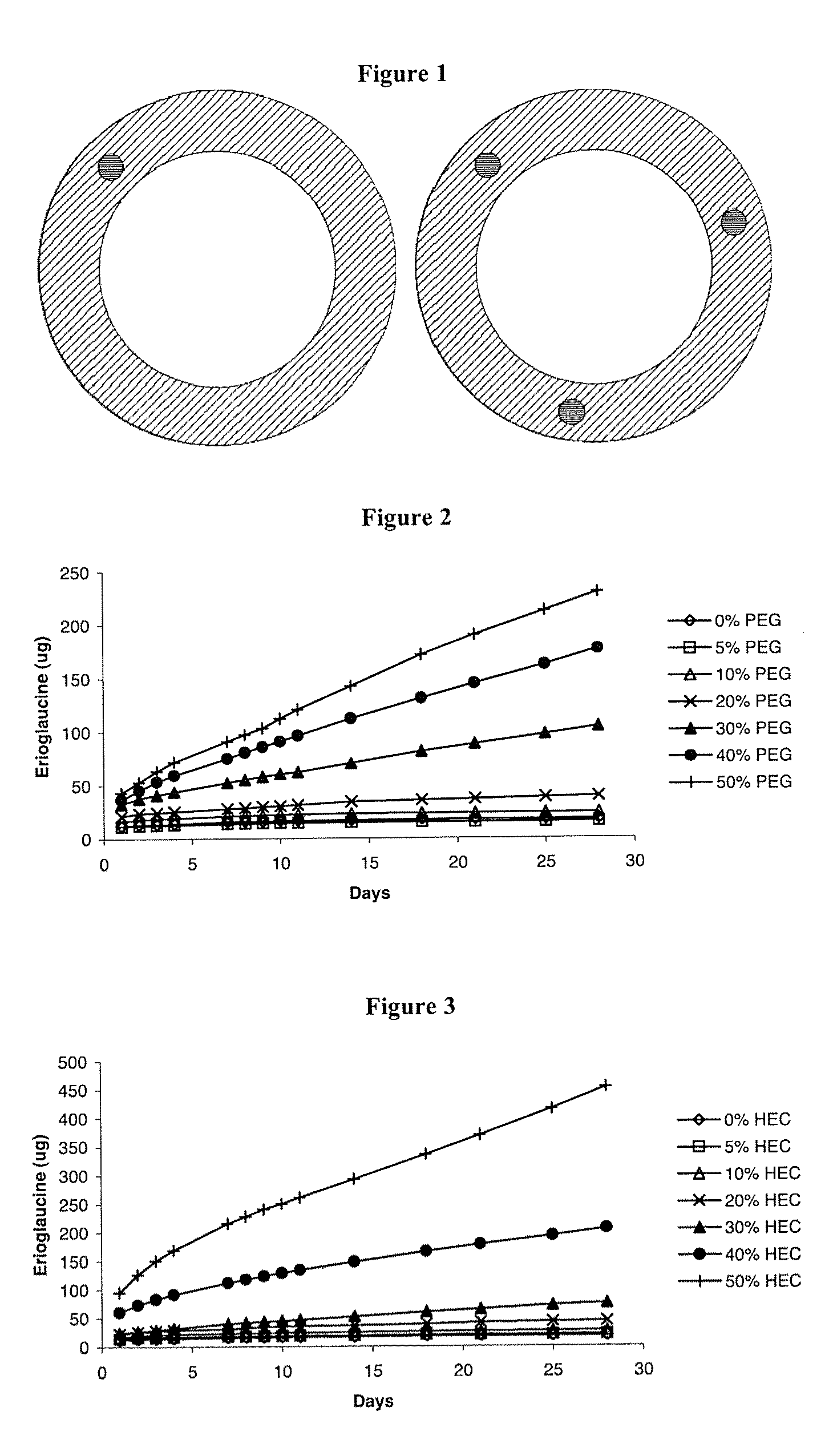
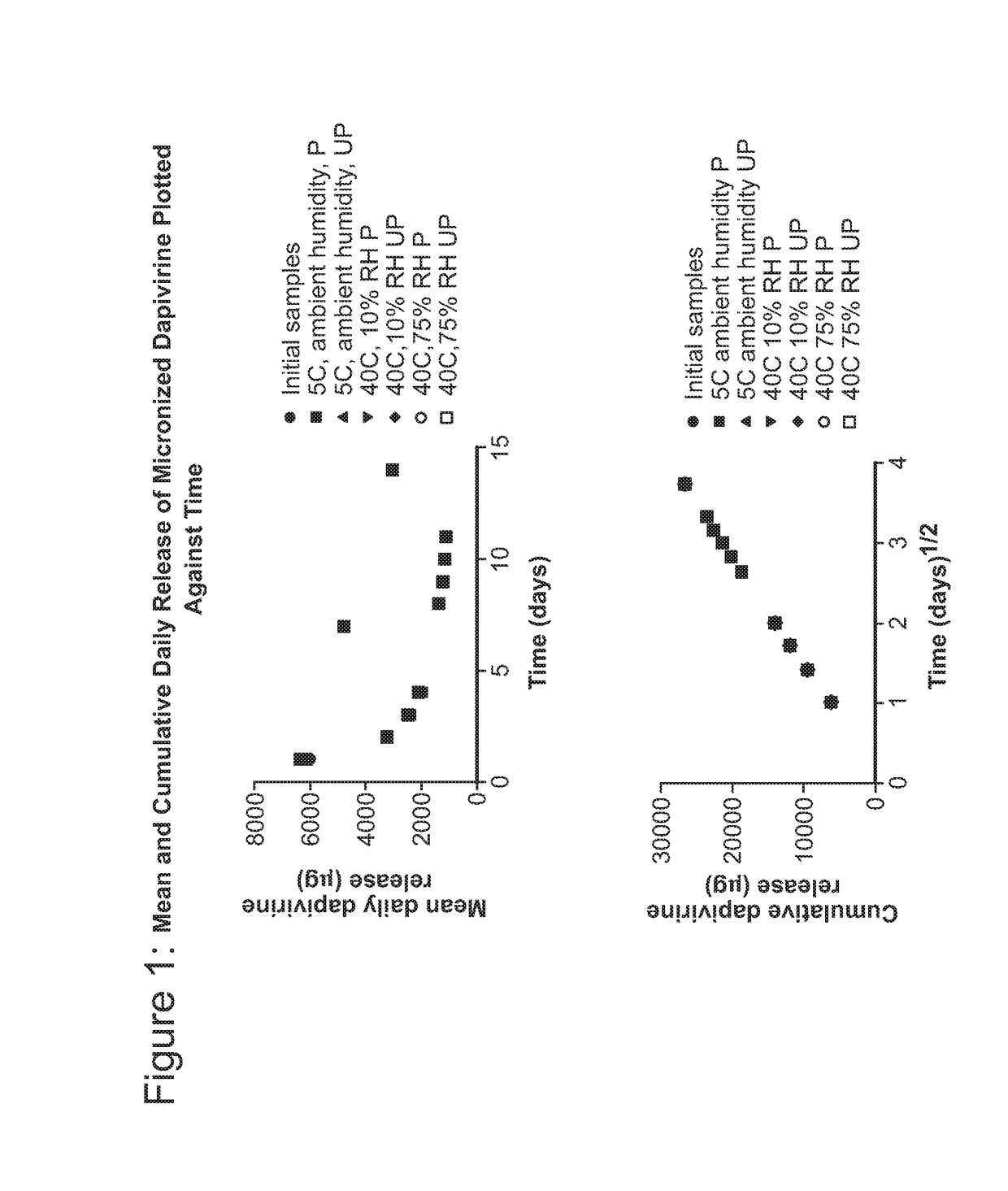
Intravaginal drug delivery devices for the delivery of macromolecules and water-soluble drugs
ActiveUS20090004246A1Good sustained releaseViral antigen ingredientsVirus peptidesIntravaginal administrationPharmacy
An intravaginal drug delivery device comprises a device body comprising a hydrophobic carrier material having at least one channel defining at least one opening to the exterior of said device body, said at least one channel being adapted to receive at least one drug-containing insert; at least one drug-containing insert positioned in said at least one channel, said drug-containing insert capable of releasing a pharmaceutically effective amount of at least one drug suitable for intravaginal administration and containing about 1% to about 70% of at least one water-soluble release enhancer, both the drug and the water-soluble release enhancer dispersed in an insert carrier material; wherein said hydrophobic carrier material and said insert carrier material may be the same or different; and wherein said at least one drug-containing insert is exposed on said exterior of said device body when said intravaginal drug delivery device is in use.
Owner:APTALIS PHARMA
Intravaginal drug delivery device
InactiveUS6103256AExcessive quantityEfficient removalPharmaceutical delivery mechanismMedical devicesIntravaginal administrationActive agent
An intravaginal drug delivery device comprising at least one active agent dispersed in a polymer matrix, wherein the concentration of active agent at the outer surface of the device at the time of use is not substantially higher than the concentration of the active agent in the remainder of the device, a method of treatment therewith and a process for its preparation.
Owner:AVENTIS PHARMA SA (US)
pH-responsive film for intravaginal delivery of a beneficial agent
InactiveUS20060018951A1Reduce transmissionFacilitates the incorporation of biological agentsPowder deliveryTamponsIntravaginal administrationAntioxidant
The present invention provides a delivery system for the intravaginal administration of prophylactic and therapeutic agents. In one embodiment, the invention provides a pH-responsive, biocompatible film for intravaginal administration of a beneficial agent, comprising a biocompatible, hydrophilic polymer that is positively charged at a first pH and in electronically neutral form at a higher pH; an effective amount of a beneficial agent; and, optionally, at least one film-forming binder. The pH responsive film may also include other additives such as plasticizers, sustained release polymers, antioxidants, and antimicrobial agents. In another embodiment, the pH-responsive film of the present invention comprises a laminated composite of (a) a bioadhesive layer that serves to affix the film to a mucosal surface within the vagina and, laminated thereto, (b) at least one reservoir layer comprising at least one beneficial agent and a biocompatible hydrophilic polymer. The pH responsive films of the present invention can be used for contraception, treatment and / or prevention of viral infections, treatment of vaginal infections, relief of vaginal itch, vaginal cleansing, and enhancement of vaginal lubrication.
Owner:SRI INTERNATIONAL
Intravaginal treatment of vaginal infections with metronidazole compositions
InactiveUS20060093675A1Easy to manufactureLess-expensive to manufactureAntibacterial agentsBiocideIntravaginal administrationMicroorganism
The present invention provides a buffered non-flowing composition suitable for the treatment of bacterial vaginosis. The composition includes metronidazole in a concentration of about 0.50% (w / w) to about 1.50% (w / w). The metronidazole is present together with a buffer system in a physiologically tolerable medium. The buffer system provides an acidic buffered pH value for the composition in the range of about 5.0 to about 6.0. The present invention also provides for a method for inhibiting a microorganism. The method includes contacting a microorganism with an effective amount of the composition of the present invention, for a period of time effective to inhibit the microorganism. The present invention also provides for a method for treating bacterial vaginosis in a human patient. The method includes intravaginal administration to a patient in need of such treatment an effective amount of the composition the present invention. The composition is introduced into the vagina at least once a day for a time period of at least one day.
Owner:TOLMAR INC
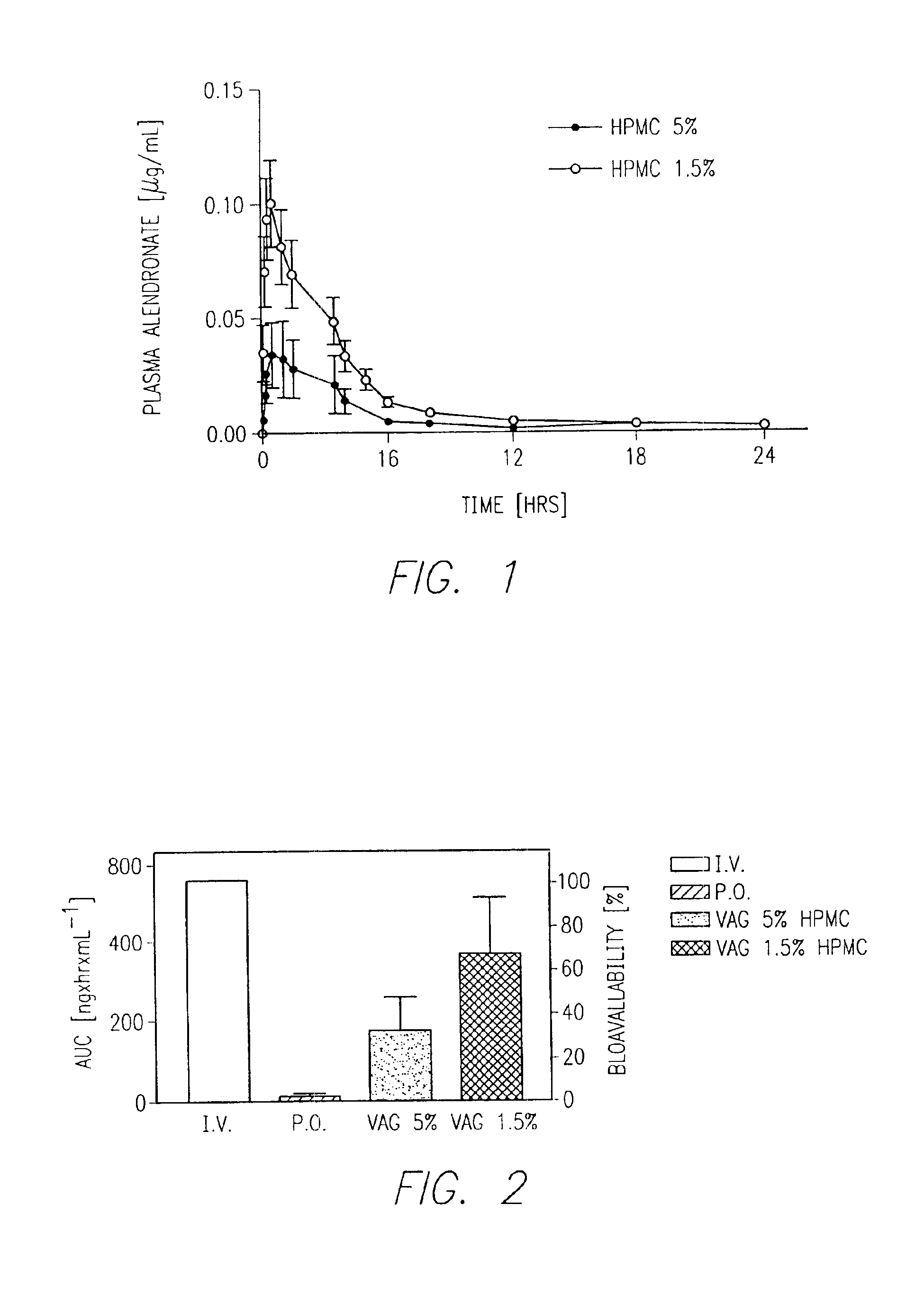
Formulations for transmucosal vaginal delivery of bisphosphonates
InactiveUS6905701B2Effective transmucosal absorptionAvoid developmentBiocideSuppositories deliveryDiseaseIntravaginal administration
Devices, methods, and improved formulation for transmucosal vaginal delivery of bisphosphonates. A targeted site delivery of bisphosphonates to the systemic circulation using a vaginal device comprising an improved bisphosphonate formulation for transmucosal delivery. A method for treatment of osteoporosis and related bone and skeleton diseases, for prevention of bone breakdown and loss of bone mass and strength by intravaginal administration of bisphosphonates to the vagina and transmucosal delivery of bisphosphonates to the general circulation.
Owner:FEMINA PHARMA
Estrogen compositions and therapeutic methods of use thereof
InactiveUS20070036848A1Provide benefitsOrganic active ingredientsUrinary disorderIntravaginal administrationGynecology
A pharmaceutical composition comprises at least one estrogenic compound, the composition being adapted for application in a unit dose amount to a vulvovaginal surface and having at least one nonlipoidal internal phase and at least one lipoidal external phase that is bioadhesive to the vulvovaginal surface, wherein the at least one estrogenic compound is present in an amount of about 5 to about 1000 μg estradiol equivalent per unit dose of the composition, and upon application of the composition to the vulvovaginal surface the at least one estrogenic compound is released over a period of about 3 hours to about 30 days. The composition is useful for vulvovaginal administration to treat atrophic vaginitis or a disorder associated therewith, for example in a menopausal or postmenopausal woman. A method for treating a hypoestrogenism-related condition of the urogenital system of a female patient comprises intravaginal administration of at least one estrogenic compound according to a treatment regimen wherein a series of compositions releasing a progressively increasing daily amount of the at least one estrogenic compound is administered over a period of at least about 1 month.
Owner:DRAGTEK CORP
Intravaginal treatment of vaginal infections with metronidazole compositions
InactiveUS20070231358A1Easy to manufactureLess-expensive to manufactureAntibacterial agentsBiocideIntravaginal administrationMicroorganism
The present invention provides a buffered non-flowing composition suitable for the treatment of bacterial vaginosis. The composition includes metronidazole in a concentration of about 0.50% (w / w) to about 1.50% (w / w). The metronidazole is present together with a buffer system in a physiologically tolerable medium. The buffer system provides an acidic buffered pH value for the composition in the range of about 5.0 to about 6.0. The present invention also provides for a method for inhibiting a microorganism. The method includes contacting a microorganism with an effective amount of the composition of the present invention, for a period of time effective to inhibit the microorganism. The present invention also provides for a method for treating bacterial vaginosis in a human patient. The method includes intravaginal administration to a patient in need of such treatment an effective amount of the composition the present invention. The composition is introduced into the vagina at least once a day for a time period of at least one day.
Owner:TOLMAR INC
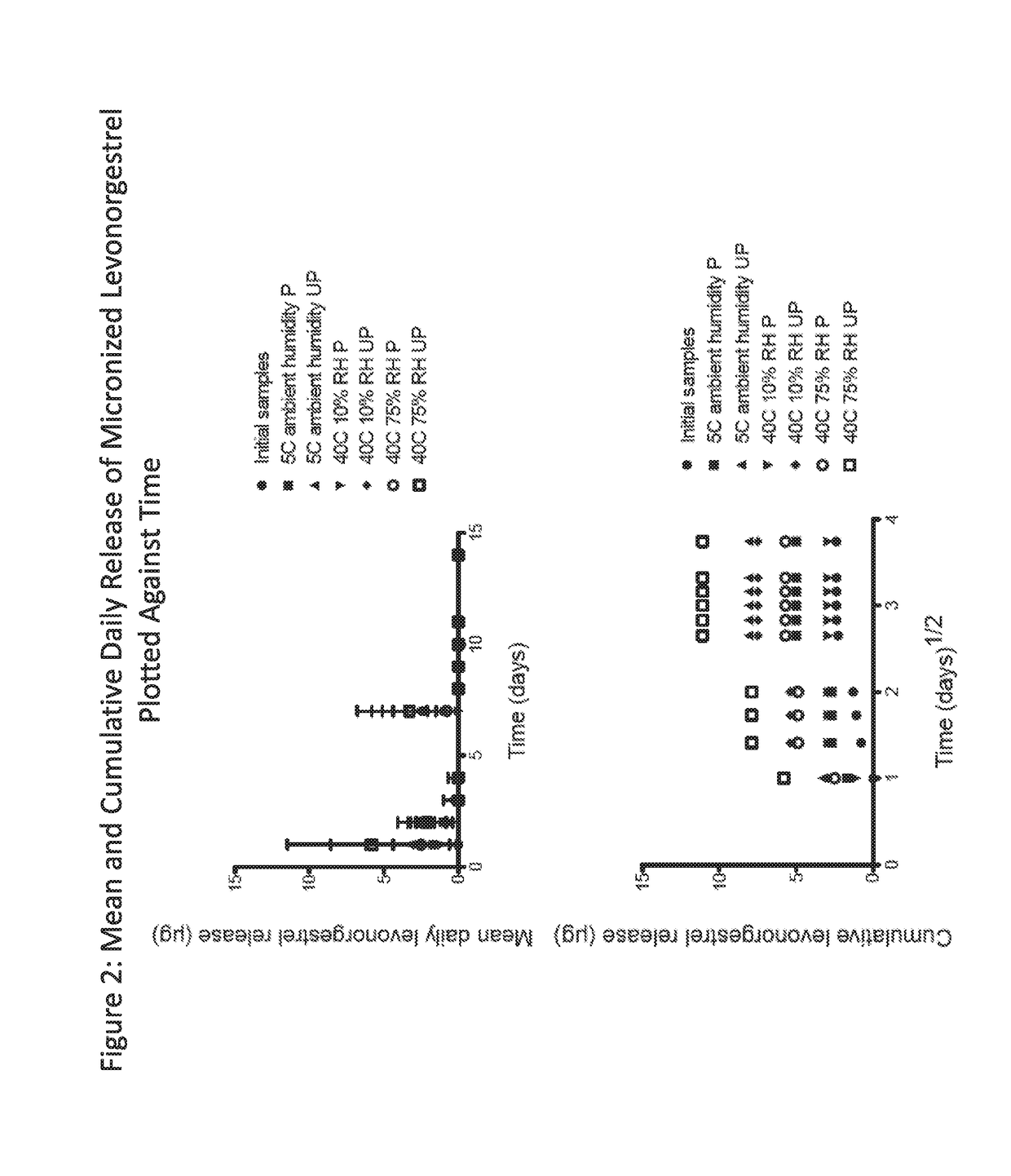
Intravaginal drug delivery device
InactiveUS20110236462A1Antibacterial agentsOrganic active ingredientsIntravaginal administrationActive ingredient
Described herein is an intravaginal drug delivery system. In an embodiment the intravaginal drug delivery system includes a progestin and estrogen compound, and releases the active ingredients in a fixed physiological ratio over a prolonged period of time to produce a contraceptive state in a female.
Owner:EVESTRA
Intravaginal delivery system and process for manufacturing it
The present invention concerns an intravaginal delivery system, said system comprising at least one compartment comprising a core and a membrane encasing the core, wherein the core and the membrane essentially consist of a same or different polymer composition. Additionally the intravaginal delivery system comprises a coupling means to form a closed continuous delivery system. The present invention also concerns a method for manufacturing said intravaginal delivery system. The core and / or the membrane are preferably prepared by injection moulding or by extrusion.
Owner:BAYER OY
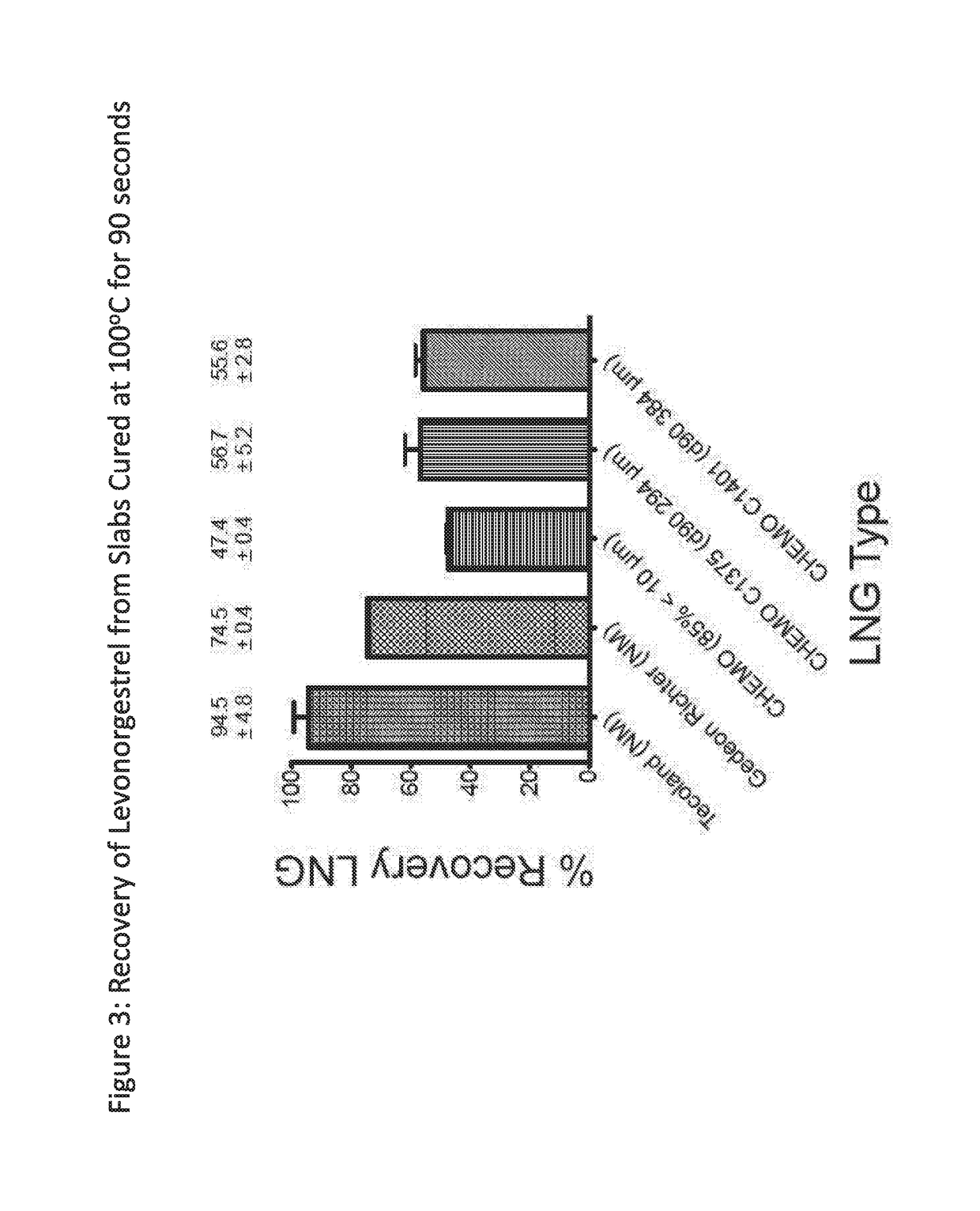
Platinum-catalyzed silicone drug delivery devices and methods of use thereof
InactiveUS20170224823A1Reduce the binding forcePromote recoveryOrganic active ingredientsInorganic non-active ingredientsIntravaginal administrationAdditive ingredient
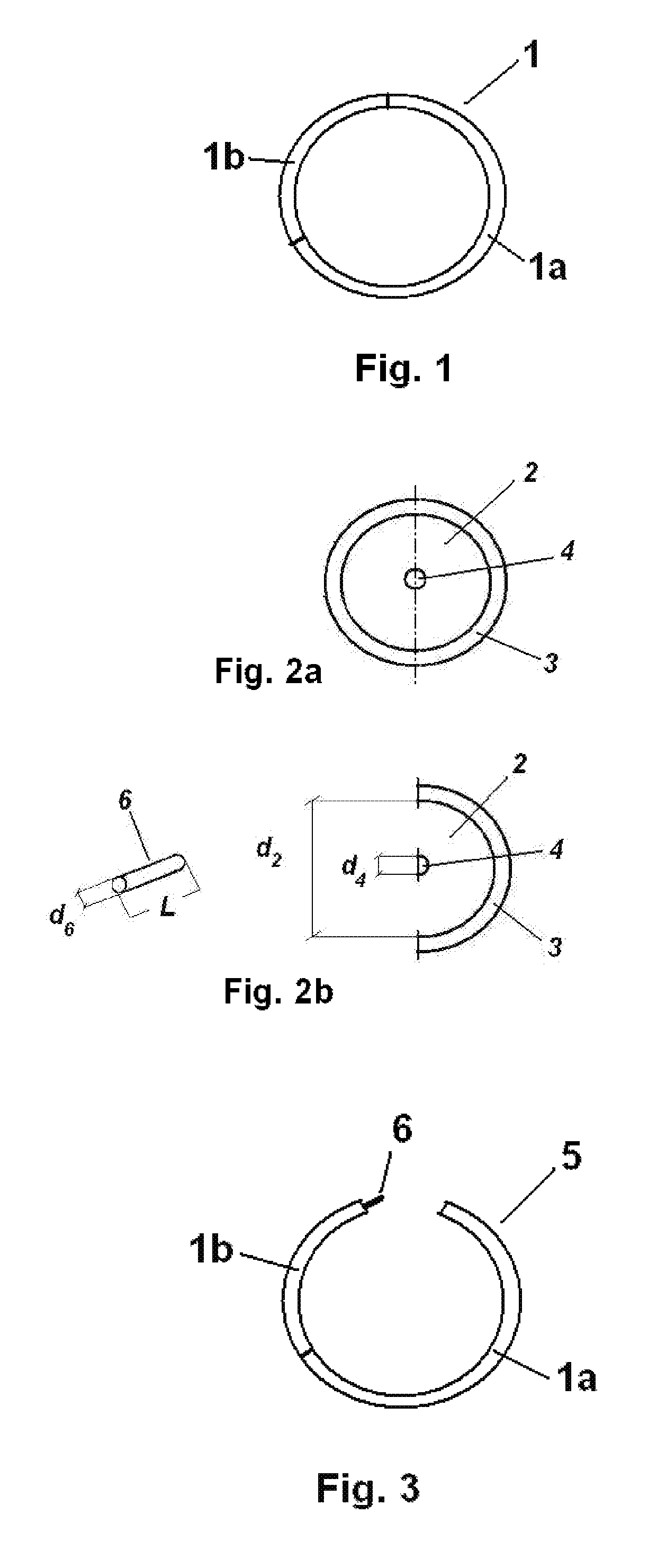
The present invention provides intravaginal drug delivery devices, such as intravaginal rings, comprising active pharmaceutical ingredients (APIs) having terminal alkene, alkyne or carbonyl functionalities. The devices of the invention exhibit increased recovery of the active pharmaceutical ingredient from platinum-catalyzed silicone polymers due to the optimization of drug particle size and cure conditions. The present invention also provides methods of preventing unintended pregnancy in a female human, methods of preventing unintended pregnancy in a female human and HIV infection in a female human, and methods of preparing intravaginal drug delivery devices.
Owner:INT PARTNERSHIP FOR MICROBICIDES
Intravaginal drug delivery devices for the delivery of macromolecules and water-soluble drugs
InactiveCN101827581APeptide/protein ingredientsGenetic material ingredientsIntravaginal administrationPharmacy
An intravaginal drug delivery device comprises a device body comprising a hydrophobic carrier material having at least one channel defining at least one opening to the exterior of said device body, said at least one channel being adapted to receive at least one drug-containing insert; at least one drug-containing insert positioned in said at least one channel, said drug-containing insert capable of releasing a pharmaceutically effective amount of at least one drug suitable for intravaginal administration and containing about 1% to about 70% of at least one water-soluble release enhancer, both the drug and the water-soluble release enhancer dispersed in an insert carrier material; wherein said hydrophobic carrier material and said insert carrier material may be the same or different; and wherein said at least one drug-containing insert is exposed on said exterior of said device body when said intravaginal drug delivery device is in use.
Owner:WARNER CHILCOTT CO LLC
Treatment method
InactiveUS20050049231A1Minimal side-effectsLong-term treatmentBiocideAnimal repellantsIntravaginal administrationSide effect
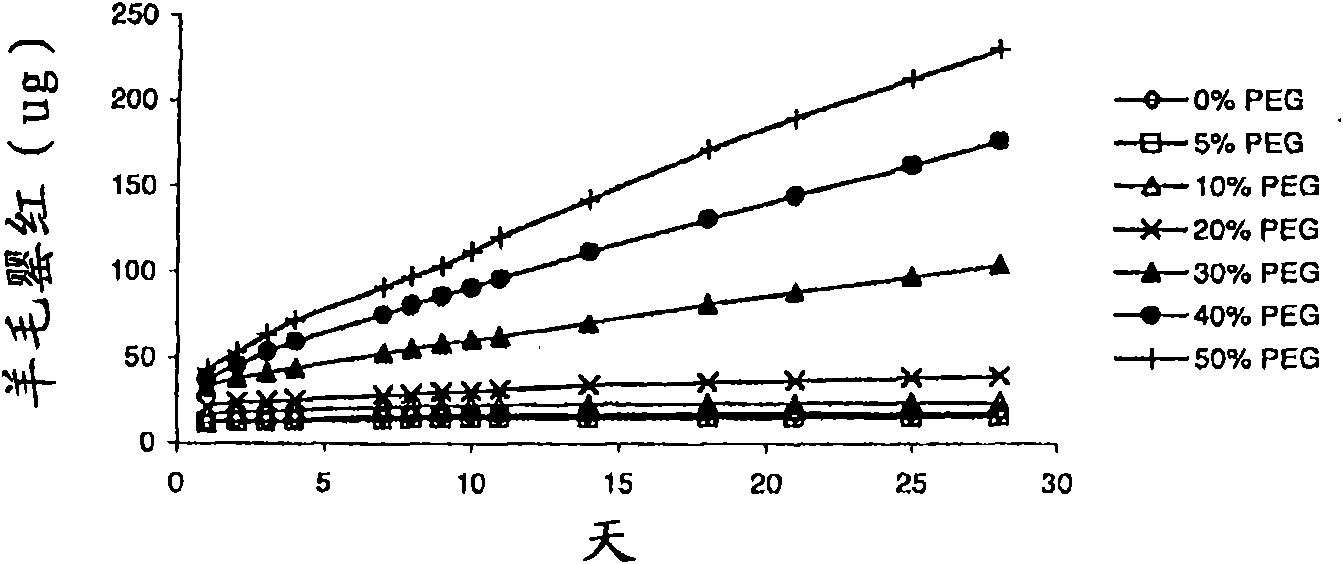
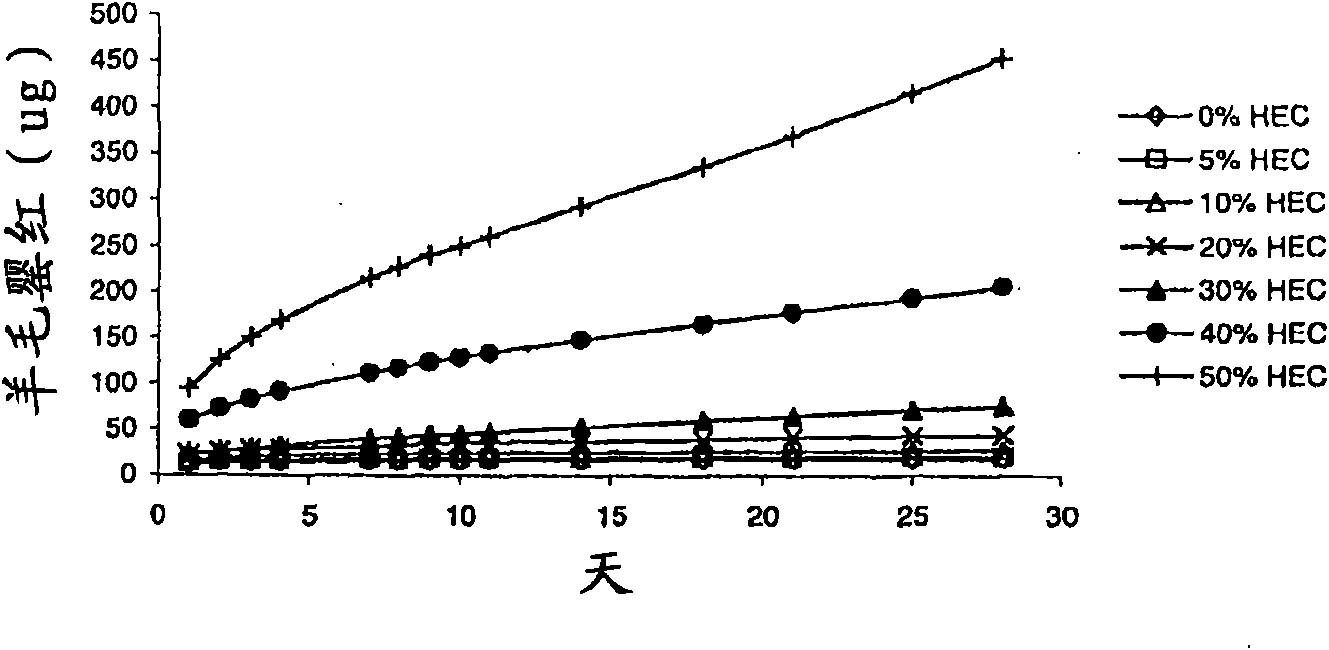
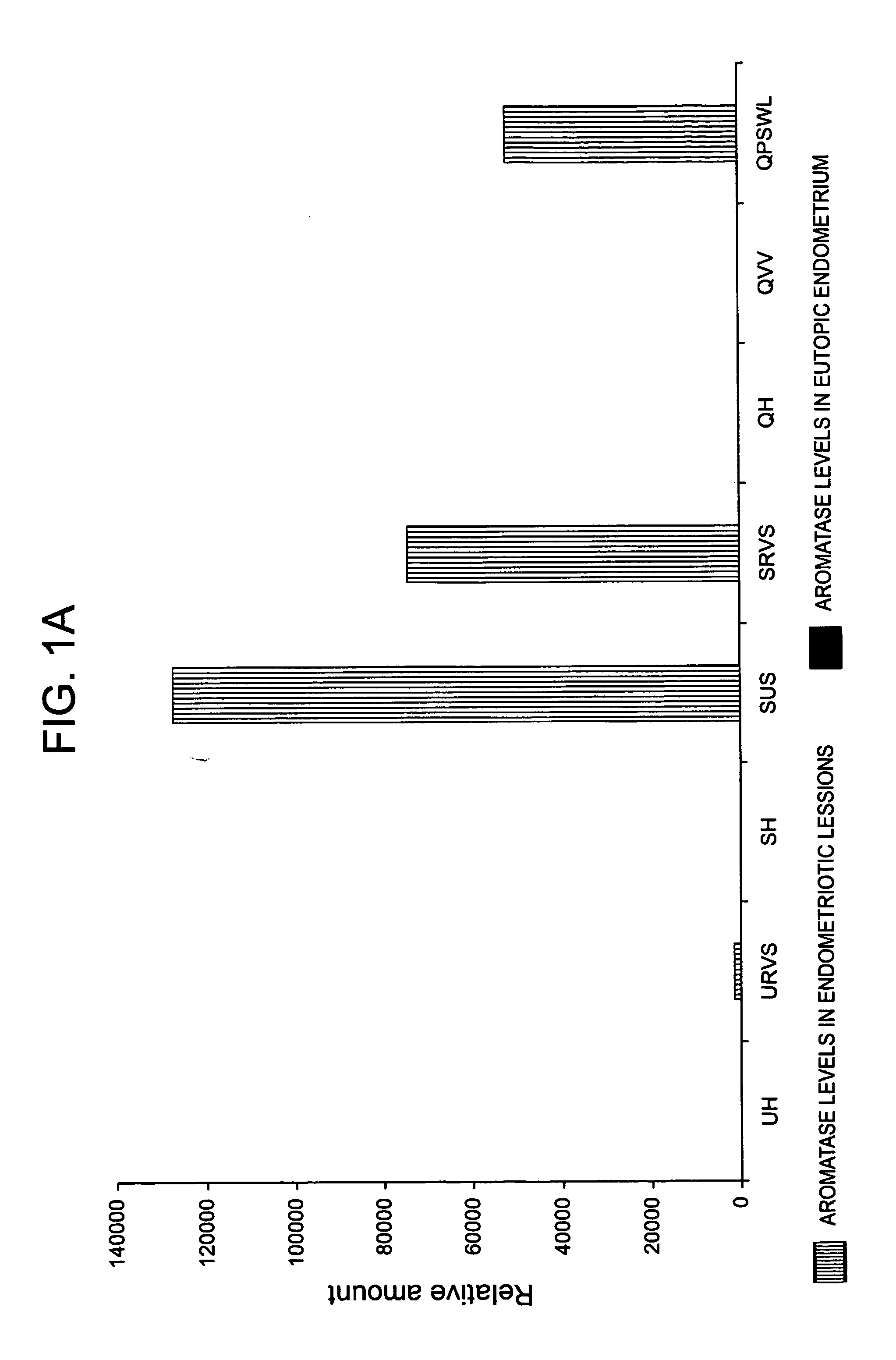
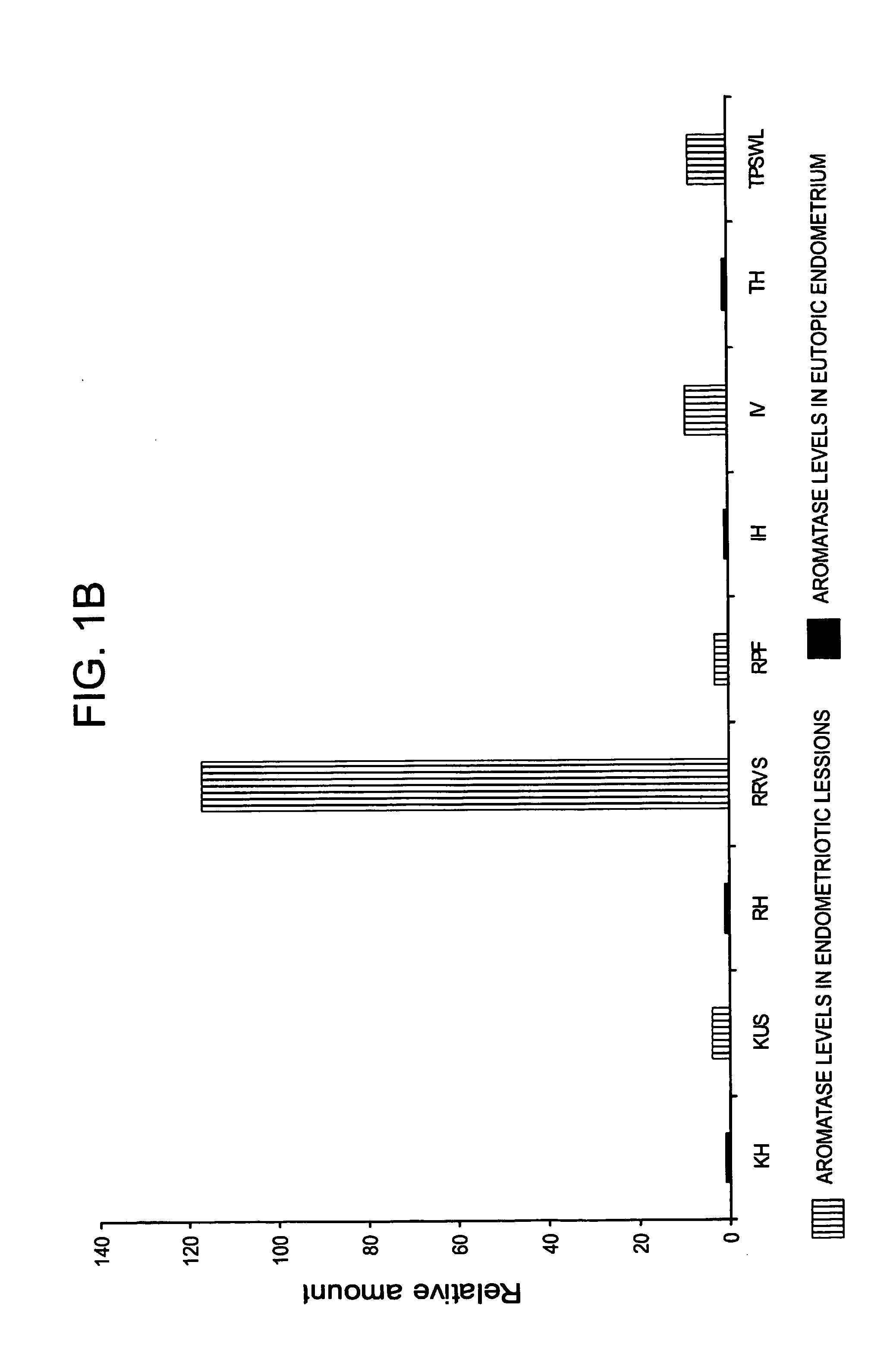
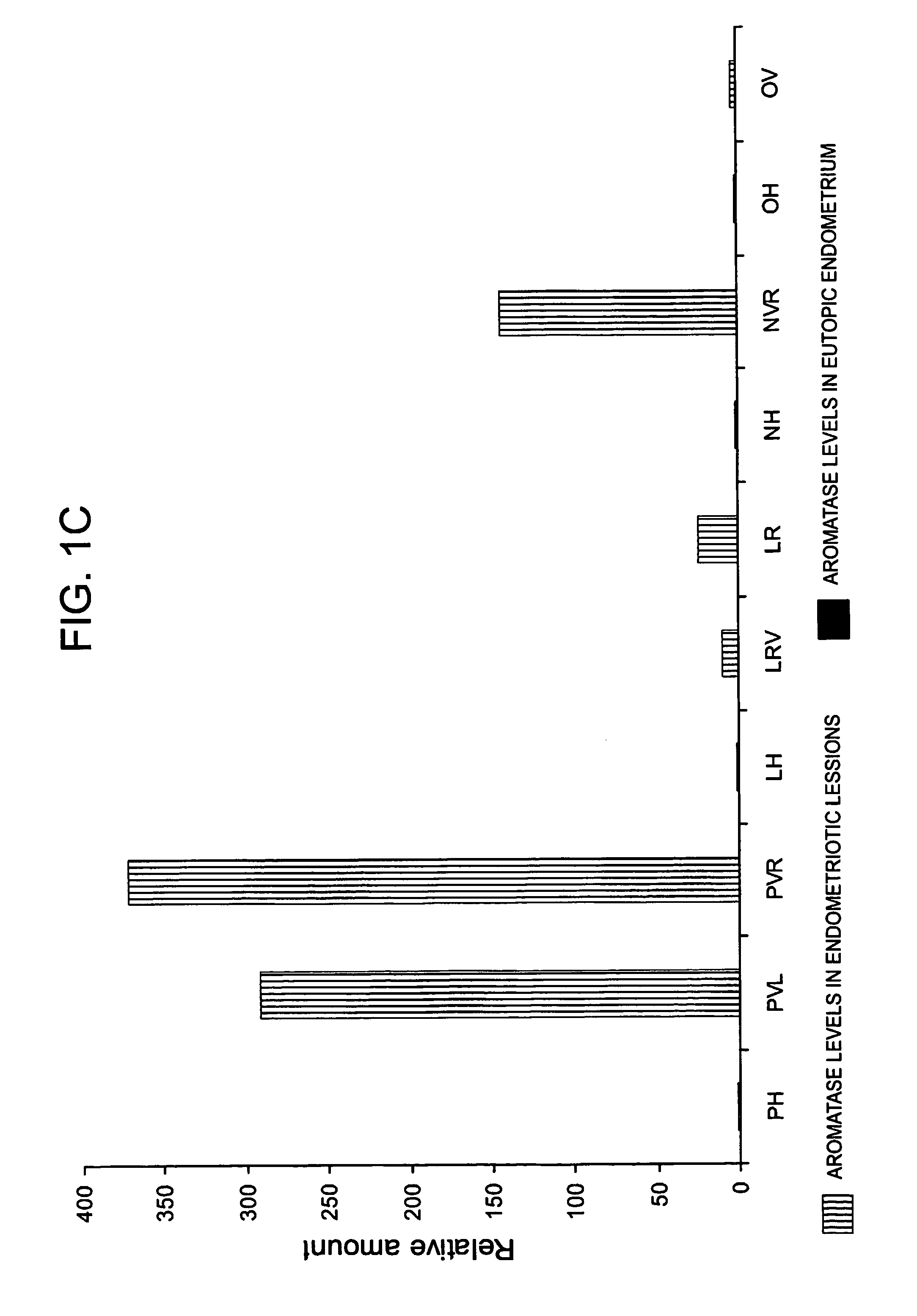
The invention relates to a method for the treatment of an oestrogen-dependent proliferative disorder of the uterus such as endometriosis and uterine fibroids in a patient, by administering an aromatase inhibitor to the patient intravaginally. This achieves high local levels of aromatase inhibitor Locally, and therefore avoids some of the adverse reactions that are observed when aromatase inhibitors are delivered orally. Further, intravaginal delivery allows inhibition of the local lesional production without significantly affecting the circulating levels which have been produced by the ovaries. This results in minimal side-effects and will allow for longer term treatment than current therapies.
Owner:ARES TRADING SA
Medicinal composition for treating vagina disease
InactiveCN1850100ALittle side effectsOrganic active ingredientsAerosol deliveryIntravaginal administrationDisease
The present invention relates to a medicine composition for curing vaginopathy. Said medicine composition contains praestrene, nifurtair and mycostatin which can be used as active component and pharmaceutically-acceptable carrier. Said medicine composition can be made into the dosage forms, such as suppository, capsule, tablet, ointment and lotion suitable for intravaginal administration. Besides, said invention also provides its preparation method.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Medicine composition for treating valval and/or vaginal infection
InactiveCN1857274AImprove the bactericidal effectSignificant local nutritionAntibacterial agentsOrganic active ingredientsIntravaginal administrationDisease
The present invention relates to medicine composition, and especially a kind of medicine composition for treating vulval and / or vaginal infection. The daily dose of the medicine composition includes promestriene 5-10 mg, preferably 8 mg; chlorhexidine acetate 3-8 mg, preferably 6 mg; and metronidazole 100-200 mg, preferably 160 mg. The medicine composition is prepared preferably into vagina administrating preparation forms, such as suppository, capsule, tablet, etc.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Intravaginal drug delivery device
InactiveUS20170319833A1Organic active ingredientsMedical devicesIntravaginal administrationObstetrics
Described herein is an intravaginal drug delivery system. In an embodiment the intravaginal drug delivery system includes a progestin and estrogen compound, and releases the active ingredients in a fixed physiological ratio over a prolonged period of time to produce a contraceptive state in a female.
Owner:EVESTRA
Intravaginal drug delivery devices for the delivery of macromolecules and water-soluble drugs
An intravaginal drug delivery device comprises a device body comprising a hydrophobic carrier material having at least one channel defining at least one opening to the exterior of said device body, said at least one channel being adapted to receive at least one drug-containing insert; at least one drug-containing insert positioned in said at least one channel, said drug-containing insert capable of releasing a pharmaceutically effective amount of at least one drug suitable for intravaginal administration and containing about 1% to about 70% of at least one water-soluble release enhancer, both the drug and the water-soluble release enhancer dispersed in an insert carrier material; wherein said hydrophobic carrier material and said insert carrier material may be the same or different; and wherein said at least one drug-containing insert is exposed on said exterior of said device body when said intravaginal drug delivery device is in use.
Owner:APTALIS PHARMA
Treatment with cholinergic agonists
InactiveUS20110195987A1Effectively preventedEffectively treatedBiocideAntimycoticsIntravaginal administrationUpper urinary tract infection
Methods of treating disorders with cholinergic agonists for example, muscarinic receptor agonists such as pilocarpine and cevimeline are provided. In particular, methods of treating and / or preventing interstitial cystitis, yeast infections, urinary tract infections, atrophic vaginitis, vaginal dryness, and sexual dysfunction associated with vaginal dryness by administering a cholinergic agonist to the subject suffering from the disorders are provided. In addition, intra-vaginal administration of cholinergic agonists such as muscarinic receptor agonists to patients suffering from interstitial cystitis, vaginal dryness, and sexual dysfunction associated with vaginal dryness is also provided.
Owner:NAJARIAN THOMAS M D +1
Vaginal Hydrogel
ActiveUS20190365902A1Antibacterial agentsOrganic active ingredientsIntravaginal administrationReactive site
The vaginal hydrogel may be a composition which includes a glycosaminoglycan, a reactive molecule, and, in some embodiments, a therapeutic agent. The glycosaminoglycan and the reactive molecule may include either thiol groups or thiol reactive sites and have a pH that is within a range of a normal vaginal environment. Some of the thiol groups may interact with the vaginal mucosa and allow the hydrogel to remain in the vagina for a longer period than existing compounds intended for intravaginal administration. Therapeutic agents may be included in the composition. In these embodiments, the hydrogel acts as a vehicle which delivers the therapeutic agent.
Owner:MANN BRENDA K
Drug composition for treating senile vaginitis and preparation method thereof
InactiveCN102652752ABroad spectrum antibacterialHigh antibacterial activityAntibacterial agentsOrganic active ingredientsIntravaginal administrationEstriol
The invention provides a drug composition for treating senile vaginitis and a preparation method thereof. The drug composition takes estriol and moxifloxacin hydrochloride as the main active ingredients, is mainly used for treating such symptoms of senile vaginitis as vaginal wall atrophy, mucosa thinning, local resistance decrease and inflammations caused by bacteria invasion and reproduction and has the functions of recovering the activity of the vaginal epithelial cell and suppressing growth and reproduction of bacteria in the vagina. The drug composition is used for preferably preparing such dosage forms for intravaginal administration as suppositories, gels and ointments.
Owner:ZHUHAI COLLEGE OF JILIN UNIV
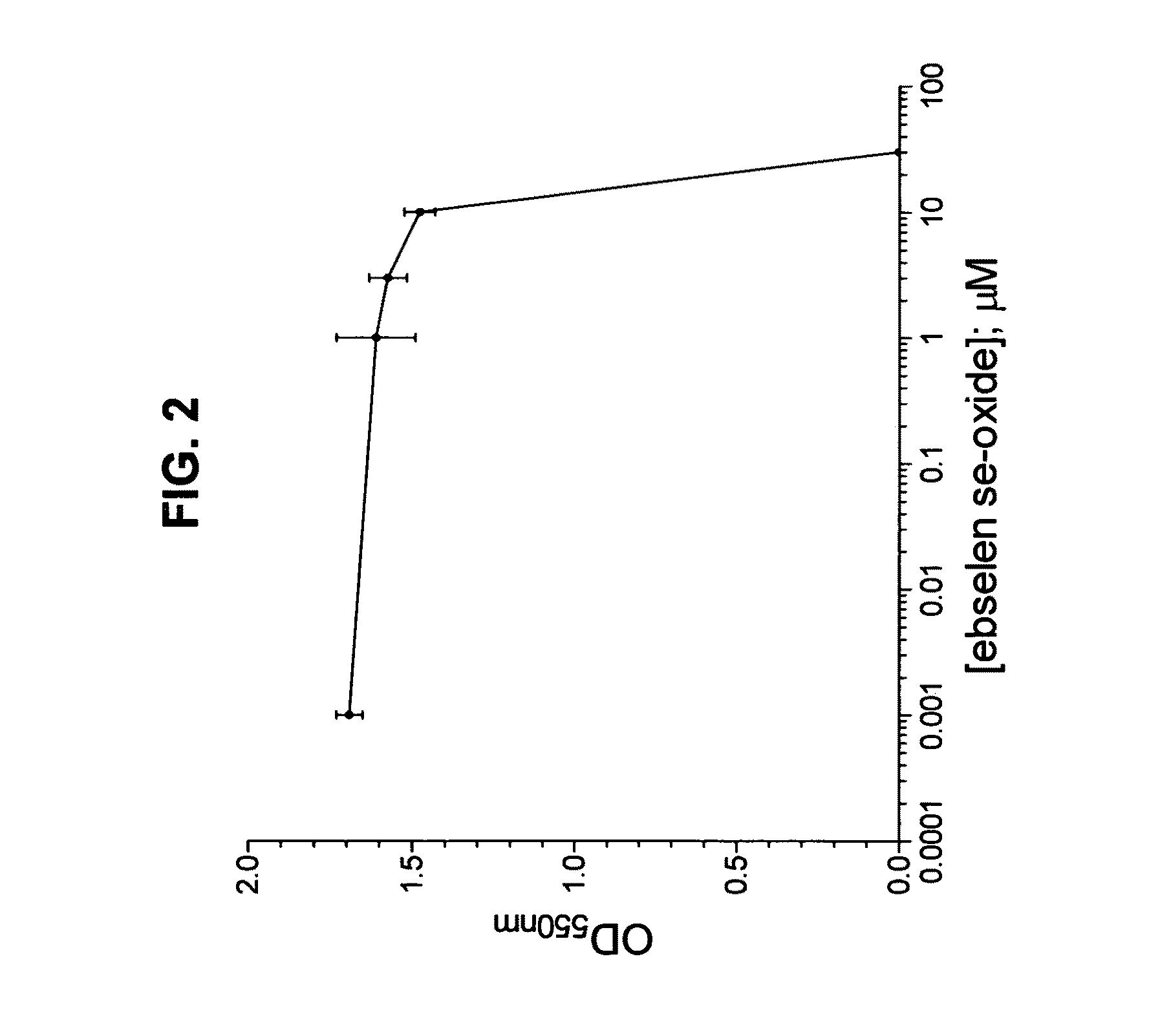
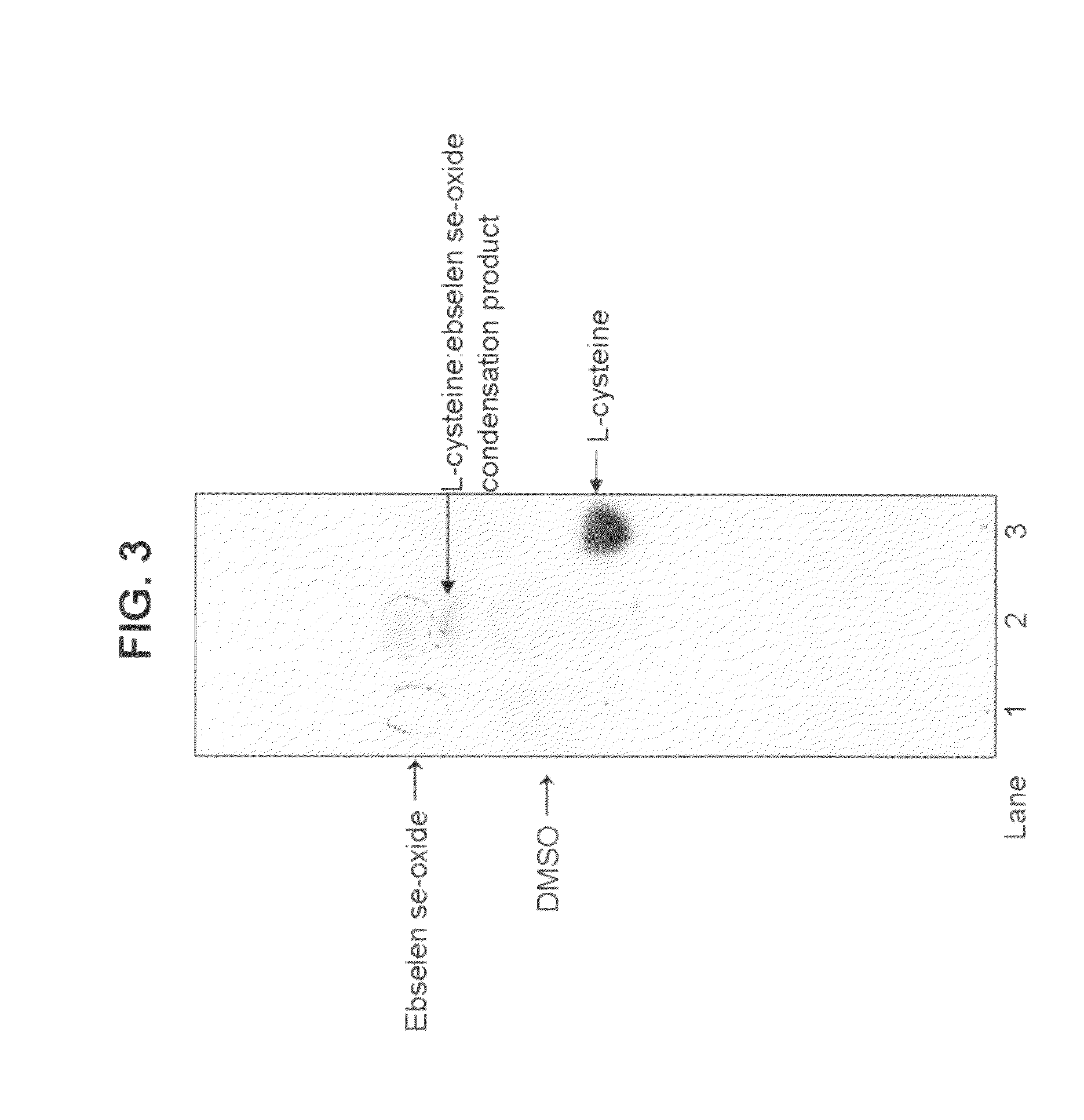
Process for the treatment of bacterial infections using 2-phenyl-1,2-benzisoselenazol-3(2H)-one 1-oxide
Owner:BILLACK BLASE CHRISTOPHER +1
Suppository for teating mycotic vaginitis
InactiveCN1739538APromote hyperplasiaImprove permeabilityOrganic active ingredientsAntimycoticsIntravaginal administrationChlorhexidine Acetate
The suppository for treating mycotic vaginitis contains tinidazole, fungicidin and chlorhexidine in certain proportion, and is prepared through mixing tinidazole, fungicidin and chlorhexidine in certain proportion in some container and filling into administration unit with each suppository weighing 0.5 g. The suppository is administrated via vagina and absorbed through mucous membrane to kill fungi in vagina. It may also promote the proliferation of epidermis cell in vagina, regulate pH value in vagina, raise antifungus capacity, improve the permeability of cell membrane and reduce exudation and has no negative effect.
Owner:马海龙
Novel secnidazole soft gelatin capsule formulations and uses thereof
InactiveUS20190008784A1Antibacterial agentsOrganic active ingredientsIntravaginal administrationBacterial vaginosis
Embodiments described herein are directed to novel soft gelatin capsule formulations for intravaginal administration comprising secnidazole compounds and methods and uses of these pharmaceutical compositions in the treatment of bacterial vaginosis.
Owner:LUPIN INC
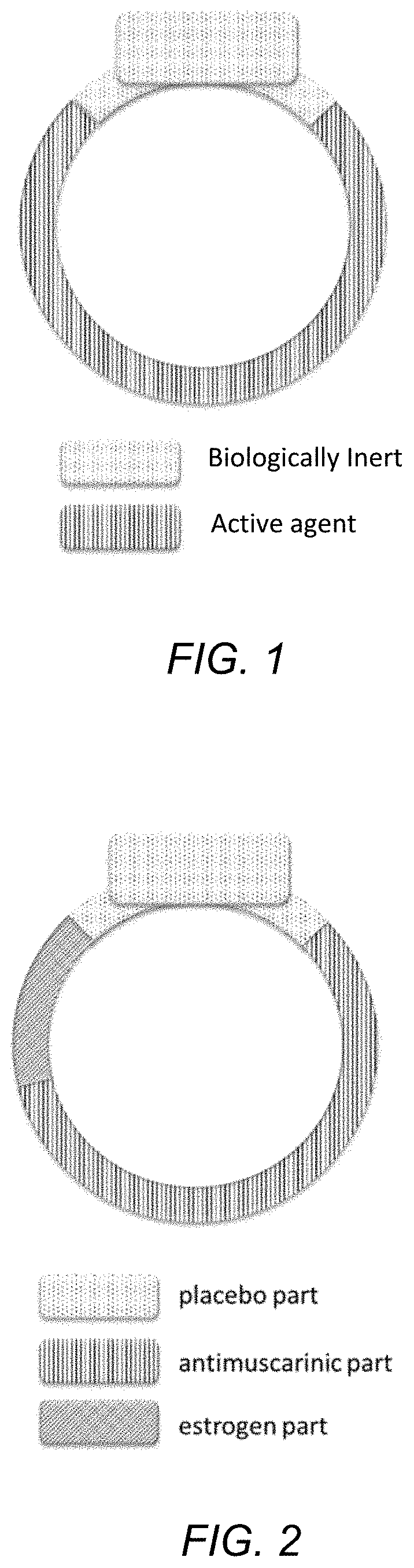
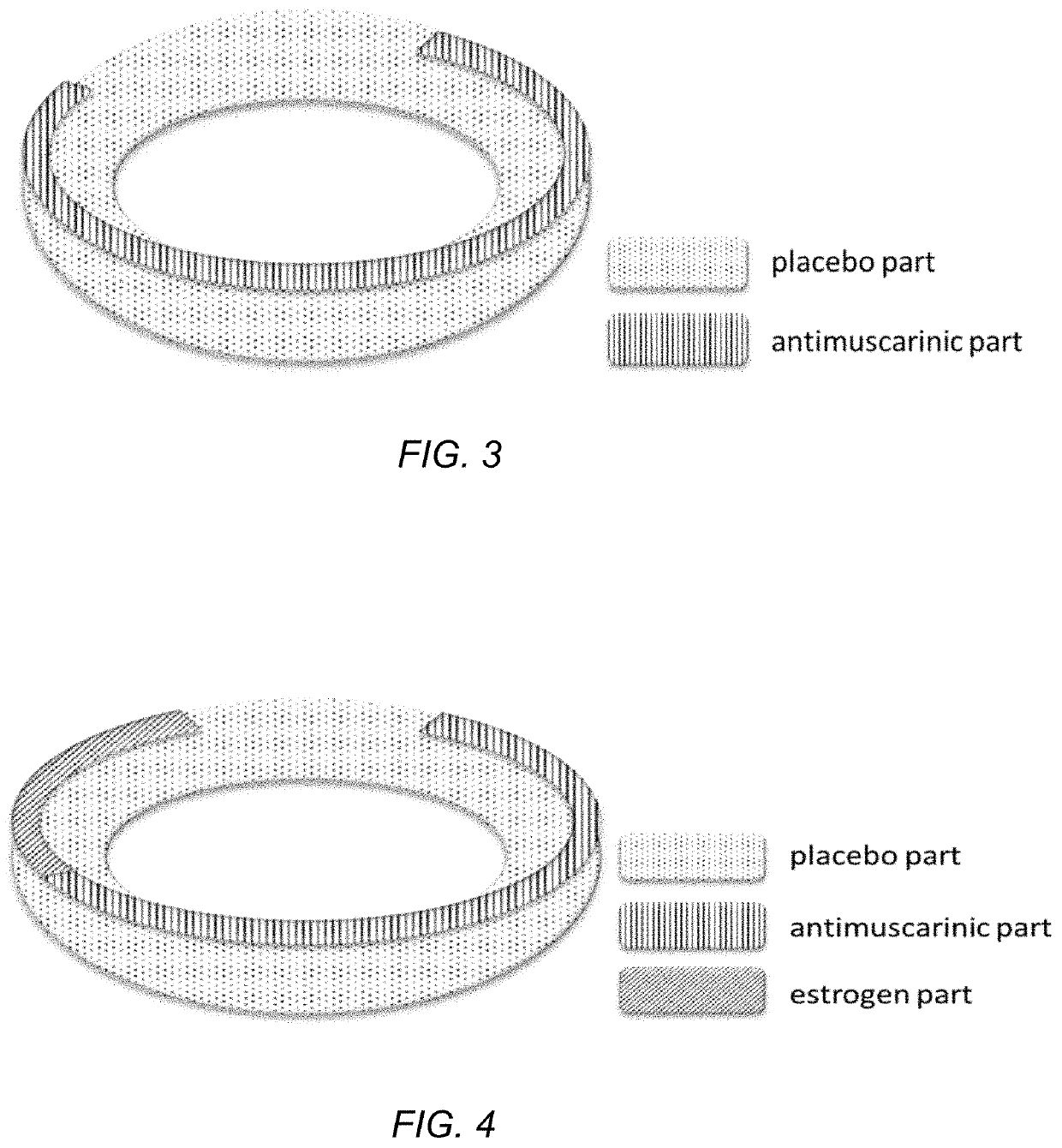
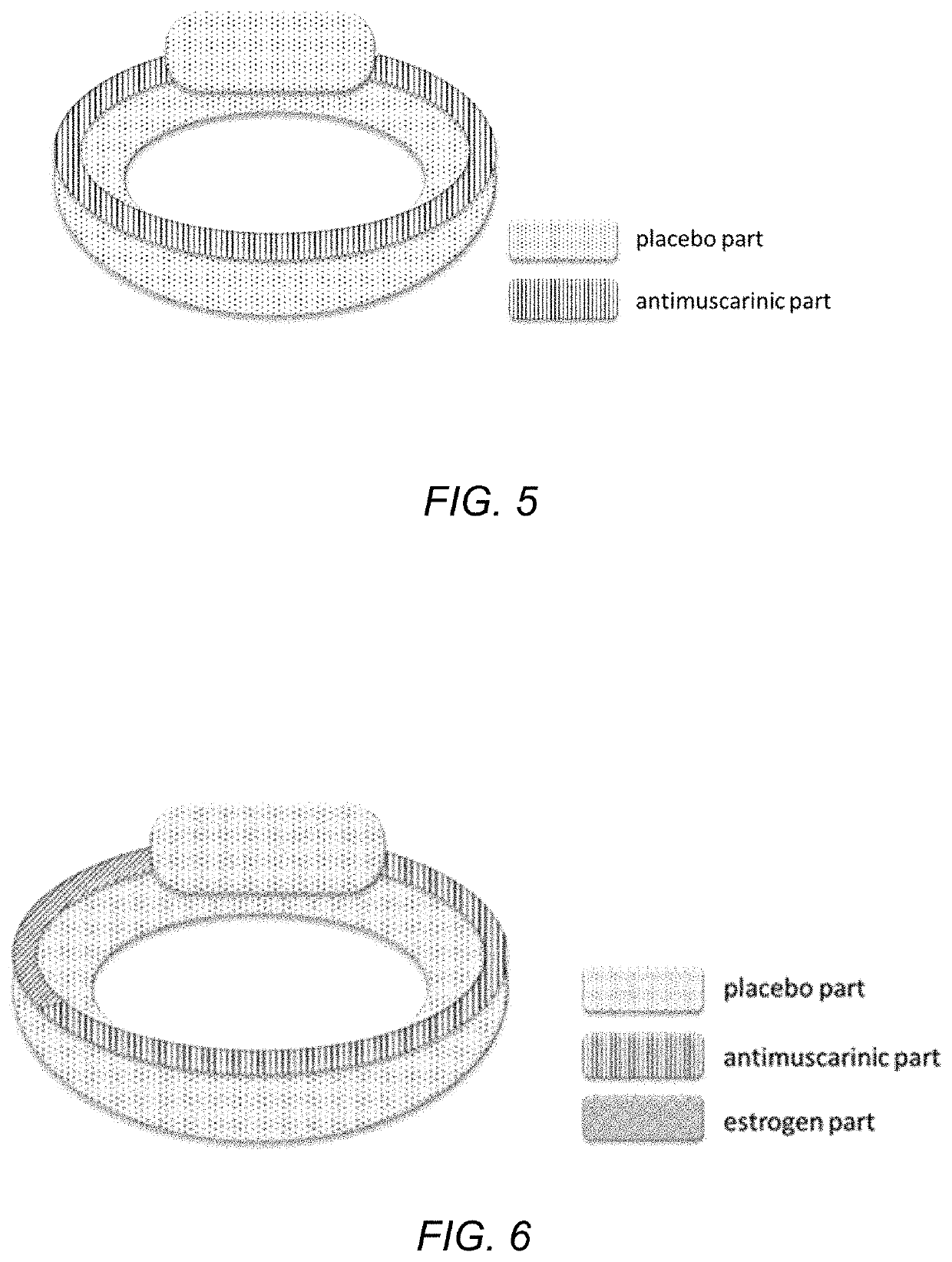
Drug device combination for the effective treatment of incontinence in women
InactiveUS20200237656A1Alters release rateOrganic active ingredientsSuppositories deliveryIntravaginal administrationEffective treatment
In embodiments described herein, an intravaginal device for treating incontinence in women includes one or more treatment modalities. The treatment modalities include: 1) incorporating an antimuscarinic agent into an intravaginal drug delivery device; 2) incorporating an estrogenic agent into an intravaginal drug delivery device, and 3) using a pessary. One or more of these modalities may be used, alone or in combination, to treat incontinence in women.
Owner:EVESTRA
Chinese-western medicine compound preparation used for treating cervical leukoplakia and preparation method thereof
InactiveCN105288628AComprehensive treatment is fast and effectiveOvercome deficienciesSuppositories deliveryPharmaceutical non-active ingredientsIntravaginal administrationSide effect
The invention discloses a Chinese-western medicine compound preparation used for treating cervical leukoplakia and a preparation method thereof. The Chinese-western medicine compound preparation used for treating cervical leukoplakia is mainly prepared from the following main materials and accessory materials: the main materials comprise all-grass of purple cyathocline, root of Juncus setchuensis Buch., Flos Edgeworthlae, Clerodendron petasites, medicinal citron seed, root of clammy hopseedbush, Maesa perlarius and a non-steroid anti-inflammatory agent; and the accessory materials comprise stringy stonecrop herb, dandelion, glossy privet fruit, smallfruit fig aerial root, Pilea japonica, Cynanchum wilfordii, chachi citrus leaf, Cleome viscosa L., propylene glycol alginate and a chitosan-glucan compound. Effective concomponents in traditional Chinese medicines are extracted for preparation of a suppository. According to the invention, traditional Chinese medicinal materials are reasonably selected and combined according to the theory of traditional Chinese medicine and are cooperatively used with western medicinal components; the prepared compound preparation is delivered in the vagina, can locally exert antibacterial, anti-inflammatory, necrotic tissue-removing and granulation-promoting effects and is capable of entering blood circulation under the action of absorption by the vaginal canal to exert liver-coursing, spleen-invigorating, dampness-eliminating, heat-clearing, blood-nourishing and yin-nourishing effects; and the compound preparation comprehensively performs drug effect to realize comprehensive treatment of cervical leukoplakia, and is fast, effective, safe and free of side-effect.
Owner:张雪梅
Gynecological nursing dosing device enabling medicine to be evenly diffused in vagina
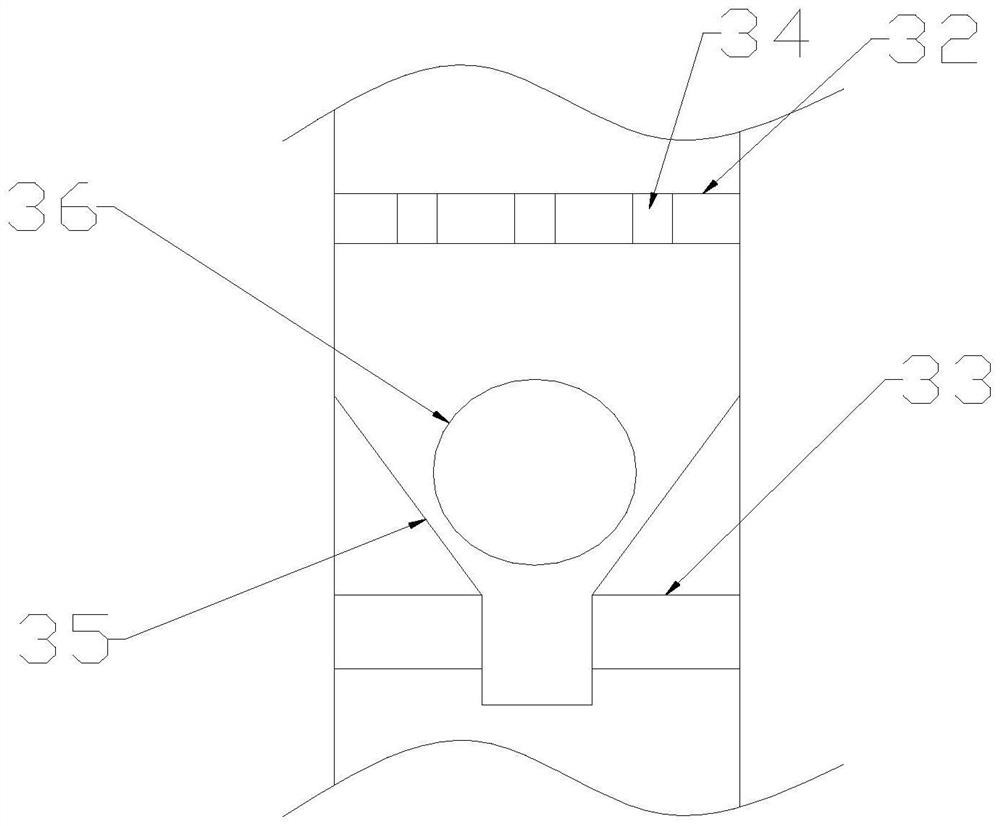
InactiveCN114681777AFlow rate regulationSpread evenlyMedical devicesIntravaginal administrationPharmacy medicine
The invention discloses a gynecological nursing dosing device capable of enabling medicine to be uniformly diffused in the vagina, belongs to the technical field of gynecological nursing, and solves the technical problems that liquid medicine cannot be uniformly diffused in the vagina during dosing in the vagina, and part of the liquid medicine flows back during vagina contraction. The device comprises an outer cylinder, a piston is arranged in the outer cylinder in a sliding connection mode, the right end of the piston is fixedly connected with a hand push rod, a liquid inlet is formed in the side wall of the outer cylinder, and a liquid inlet cover is arranged on the liquid inlet. A connecting cylinder is fixedly arranged at the left end of the outer cylinder, a liquid outlet pipe is arranged at the left end of the outer cylinder and located in the connecting cylinder in a communicating mode, the liquid outlet pipe comprises a large-diameter pipe and a small-diameter pipe, and the right end of the large-diameter pipe is communicated with the left end of the small-diameter pipe in a smooth transition mode. A spherical adjusting body is arranged in the liquid outlet pipe and located at the joint of the large-diameter pipe and the small-diameter pipe, and a hole channel horizontally penetrating through the spherical adjusting body is formed in the spherical adjusting body.
Owner:王瑞芳
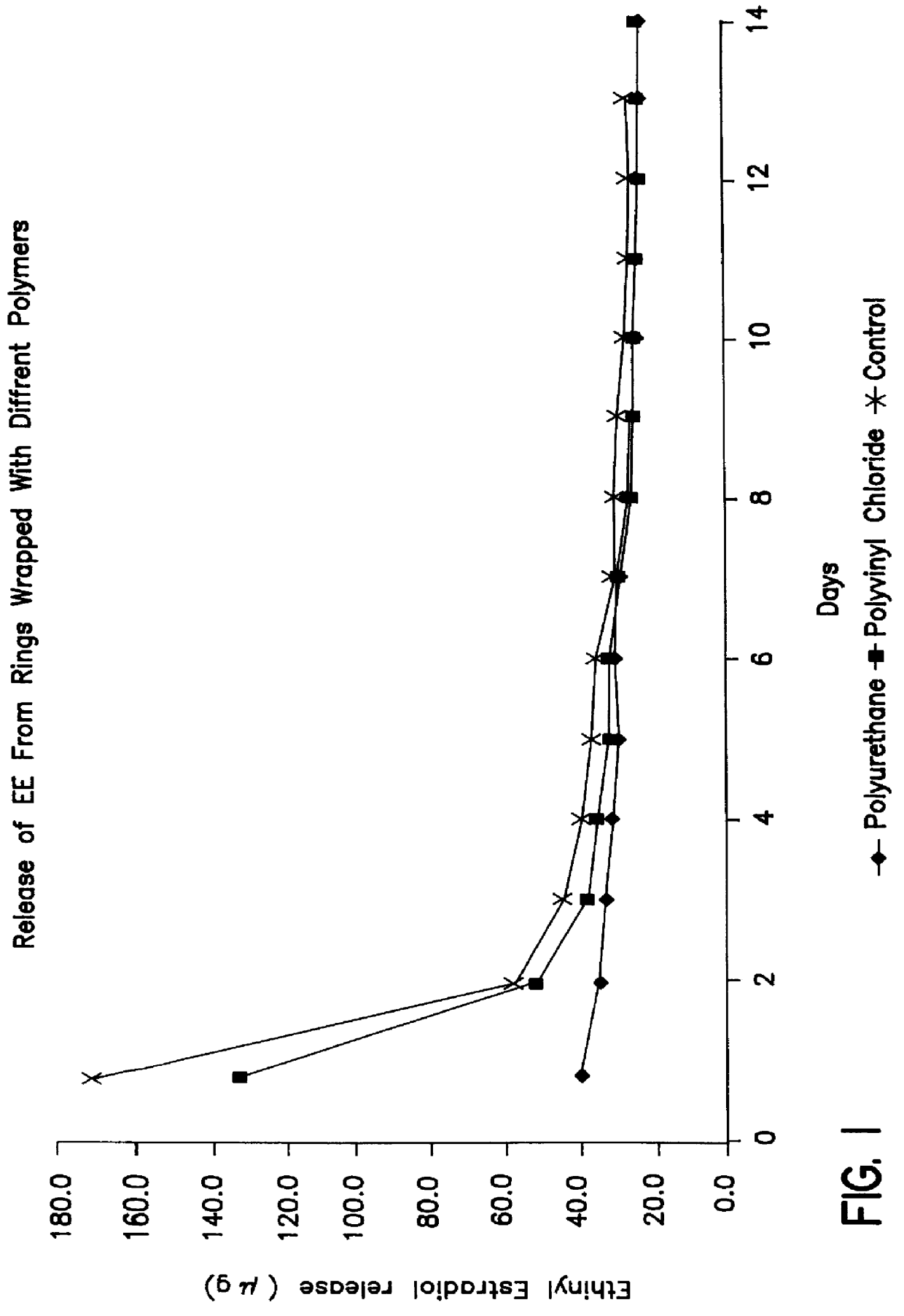
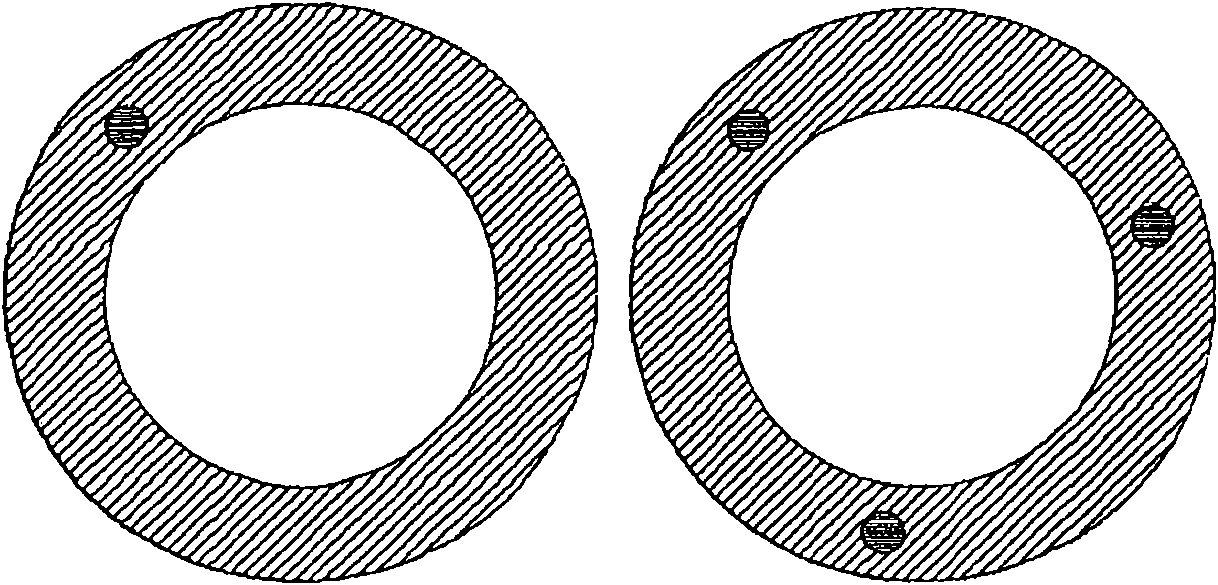
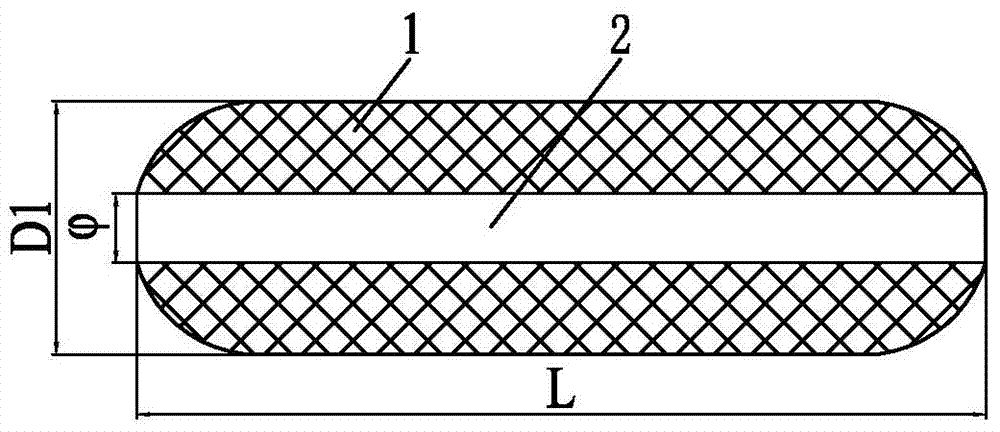
Intravaginal ring comprising polyurethane composition for drug delivery
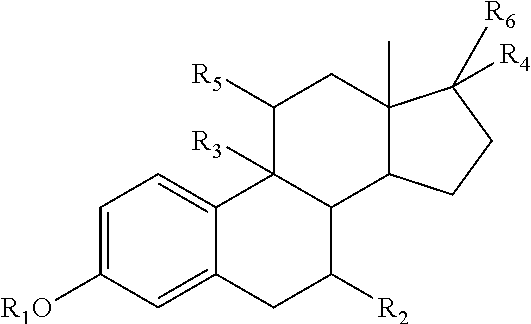
The invention is directed to an intravaginal drug delivery device comprising at least one pharmaceutically active substance, and a polyurethane copolymer, wherein the copolymer has the structure according to formula (I):The invention is further directed to administering one or more pharmaceutically active substances to a patient in need thereof.
Owner:DSM IP ASSETS BV
Endometriosis treatment
InactiveUS20130184245A1Organic active ingredientsOintment deliveryIntravaginal administrationDanazol
A pharmaceutical composition comprises at least one nonlipoidal internal phase and at least one lipoidal external phase that is bioadhesive to a vaginal mucosal surface, and comprises danazol in an amount of about 3% to about 30% by weight of the composition, wherein upon application of the composition to the vaginal mucosal surface the danazol is released over a period of about 1 to about 10 days. The composition is useful for intravaginal administration to treat a condition such as endometriosis for which danazol is indicated.
Owner:LUMARA HEALTH IP
Truncated secretory aspartyl proteinase 2
InactiveUS20100285109A1Improving immunogenicityOrganic active ingredientsAntimycoticsIntravaginal administrationCandida famata
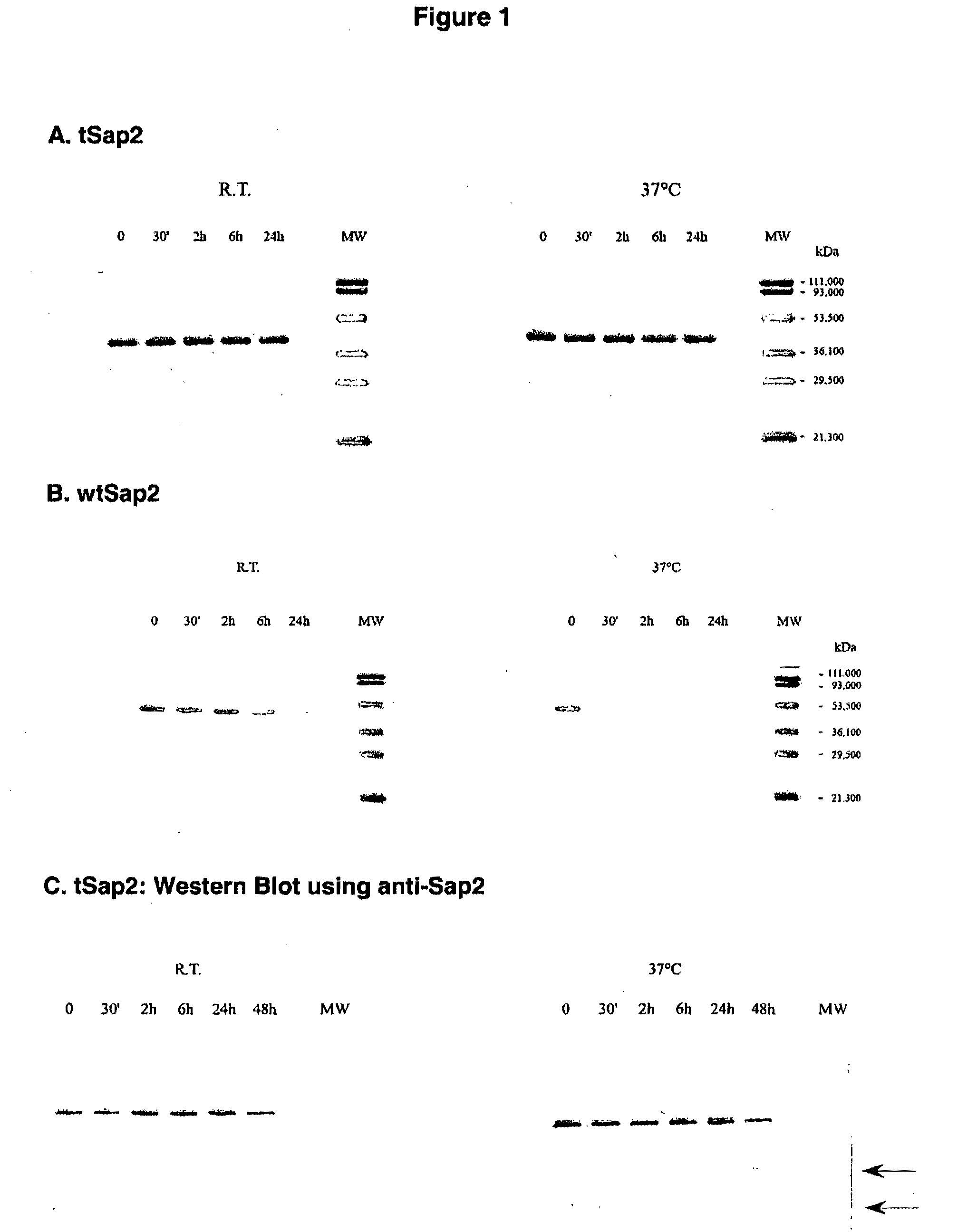
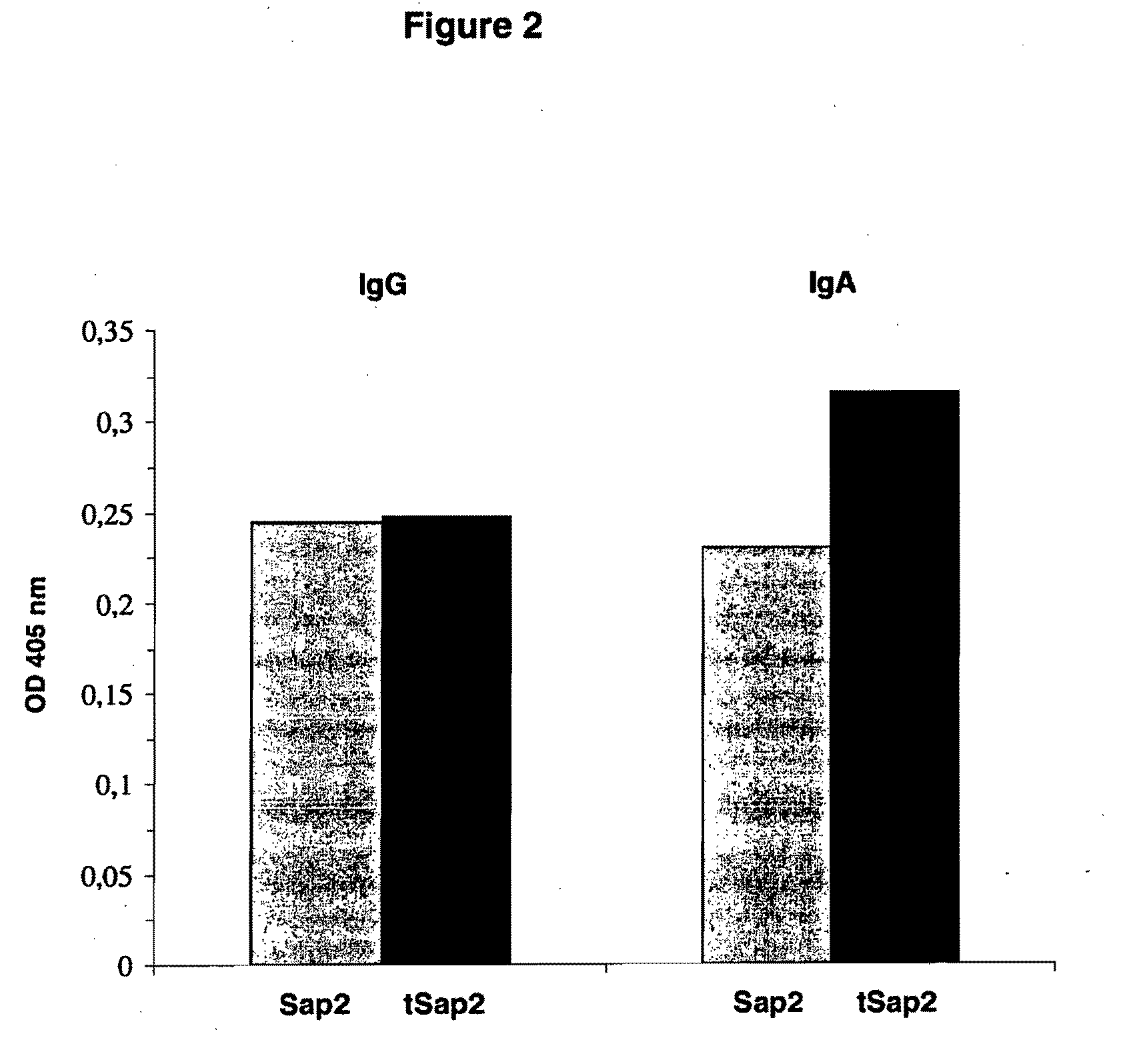
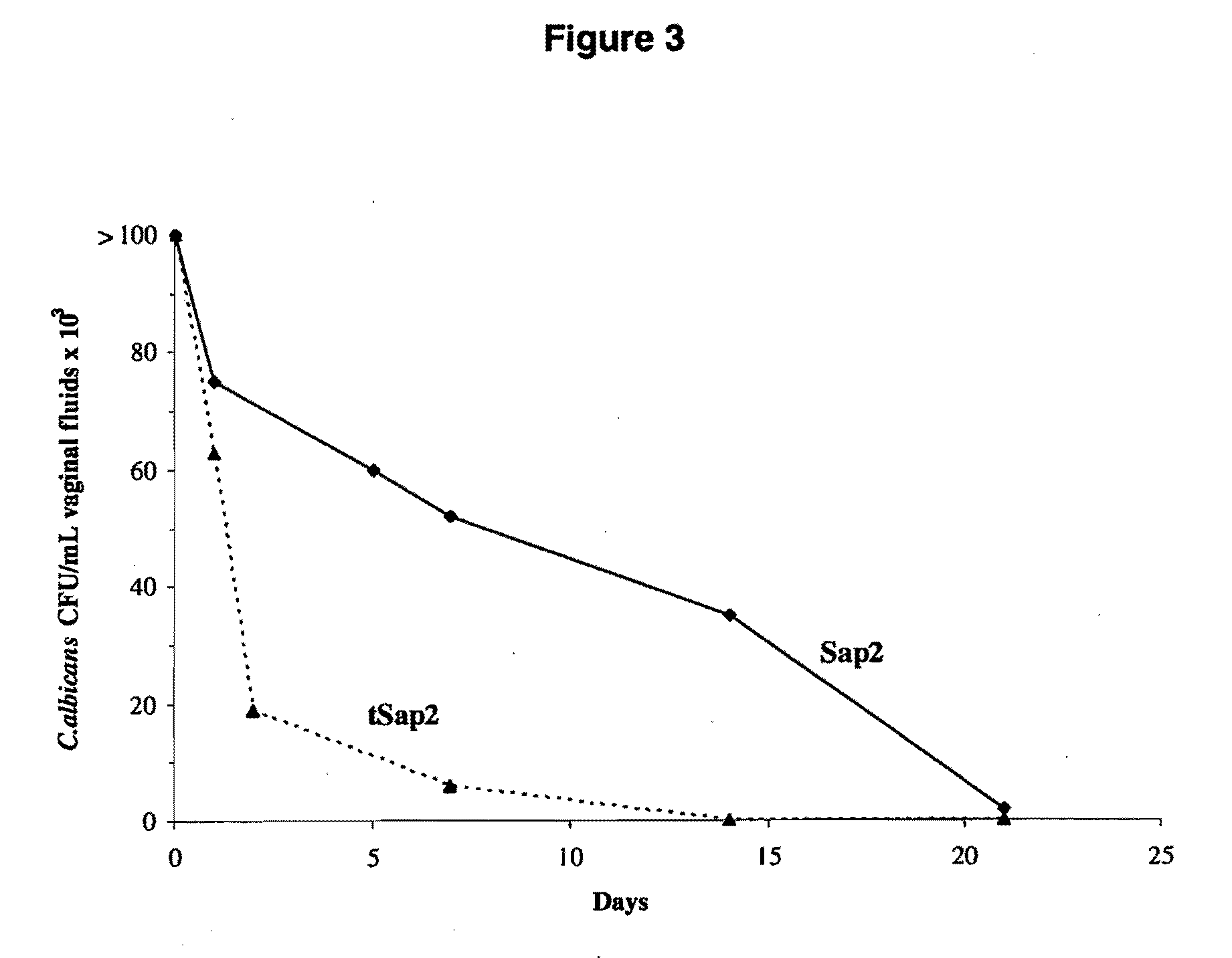
The present invention relates to a truncated form of the secretory aspartyl proteinase 2 (Sap2), as well as to nucleic acid molecules encoding same. This truncated Sap2 polypeptide (tSap2) is surprisingly stable and devoid of enzymatic activity but retains full immunogenicity upon intravaginal administration and confers full protection against intravaginal challenge by the Candida fungus. The present invention further relates to compositions comprising tSap2 and to the use of tSap2 in the preparation of such compositions.
Owner:INST SUPERIORE DI SANITA +1
Endovaginal implantable treatment device
InactiveCN104117135AStrong water absorption and retention capacityControl release speedMedical devicesIntravaginal administrationSecondary hyperlipidemia
The invention discloses an endovaginal implantable treatment device, and belongs to the field of medical apparatuses. The endovaginal implantable treatment device aims at solving the technical problem that an existing vagina drug application device can not carry out endovaginal drug application in the menstrual periods and is short in treatment time in the non-menstrual periods and poor in clinic adaptability. The endovaginal implantable treatment device comprises a cylinder, treatment drugs are contained in the cylinder, and at least one axial through hole is formed in the cylinder in the axial direction of the cylinder, and / or at least one groove penetrating through the cylinder is formed in the outer wall of the cylinder in the axial direction of the cylinder. The total volume of the endovaginal implantable treatment device is larger than or equal to 10 cm<3>; materials of the cylinder have the hydrophilia or the hydroscopicity and can not be slaked or can not be dissolved into water, the cylinder is flexible, smooth in surface and free of viscidity in the water saturation state, and meanwhile the water lubricating performance is achieved. Vagina drug application is adopted for the endovaginal implantable treatment device, and the endovaginal implantable treatment device is used for treating the colpitis, the diabetes mellitus, the hyperlipidemia and the like.
Owner:崔仁海
Medicine composition for treating valval and/or vaginal diseases
The present invention relates to medicine composition for treating vulval and / or vaginal diseases. The medicine composition for treating vulval and / or vaginal diseases contains promestriene and miconazole in the weight ratio of 1 to 5-1 to 8 as the active components. The daily dose of the medicine composition includes promestriene 6-10 mg, preferably 7 mg; and miconazole 30-80 mg, preferably 50 mg. The medicine composition is prepared into vagina administrating preparation forms, such as suppository, capsule, tablet, etc.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com