Endovaginal implantable treatment device
A treatment device and implanted technology, applied in the field of medical devices, can solve the problems of poor clinical adaptability, inability to administer intravaginally during menstruation, and short treatment time during non-menstrual period, and achieve adjustable drug release speed and drug release. The effect of continuous and stable process and good sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
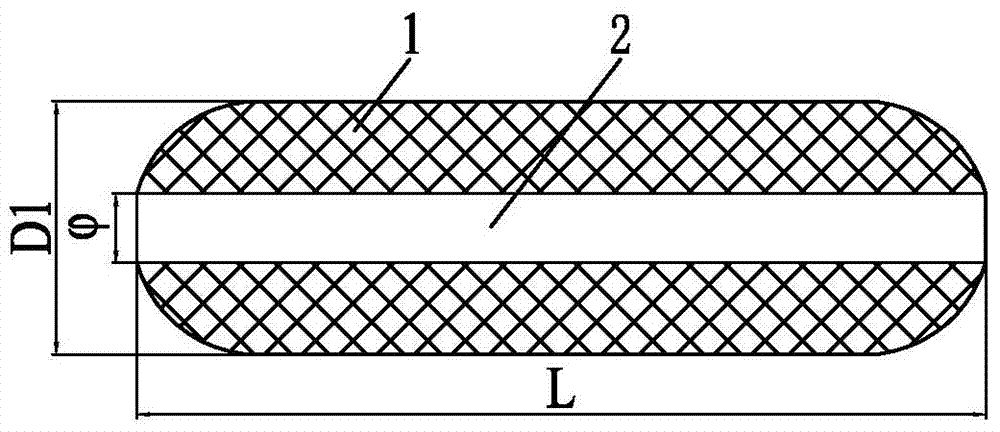
[0084] Specific implementation mode one: combine figure 1 and 2 To illustrate, in the present embodiment, the intravaginal implantable treatment device includes a cylinder 1. The cylinder 1 is a cylinder with a diameter D1 of 2.5 cm. The front end and the tail of the cylinder 1 are semi-arc surfaces. An axial through hole 2 is arranged at the center along the axial direction of the cylinder 1, and the diameter of the axial through hole 2 is The total length L of the column body 1 is 5.5 cm; the material of the column body 1 is starch-grafted acrylate copolymer gel, and the therapeutic medicine is dispersed in the column body 1 .
[0085] In this embodiment, the intravaginal implantable treatment device maintains its structure basically unchanged during the treatment process, and the axial through hole provides an excretion channel for menstruation and vaginal secretions, which can be used during menstruation, and the normal excretion function can also reduce the patient's D...
specific Embodiment approach 2
[0086] Specific implementation mode two: combination image 3 and 4 To illustrate, in this embodiment, the intravaginal implantable treatment device includes a column body 1, which is an ellipsoid with a maximum major axis length D3 of 3 cm and a maximum short axis length D2 of 2 cm, and therapeutic drugs are dispersed in the column body 1 , the center of the cylinder 1 is provided with an axial through hole 2 along the axial direction of the cylinder 1. The through hole 2 is an elliptical cylindrical hole with a major axis length 4 of 6 mm and a minor axis length of 4 mm. The total length of the cylinder 1 L is 6 cm; the material of column 1 is cross-linked polyacrylamide gel.
specific Embodiment approach 3
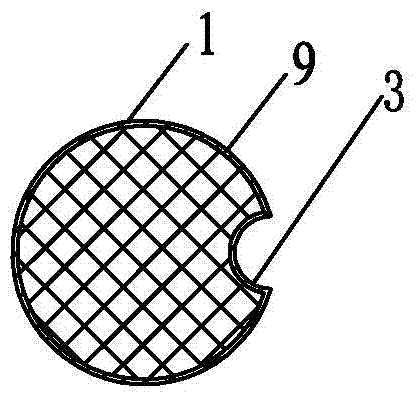
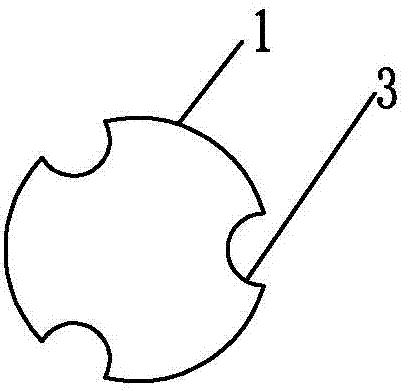
[0087] Specific implementation mode three: combination Figure 5 and 6 To illustrate, in this embodiment, the intravaginal implantable treatment device includes a cylinder 1. The cylinder 1 has a side length D4 of 2.5 cm, a cube with rounded corners, and the front end and tail are smooth arc surfaces. At least one axial through hole 2 is arranged at the center of the cylinder 1 along the axial direction of the cylinder 1, and the diameter of the through hole 2 is The total length L of the column body 1 is 7 cm, and the material of the column body 1 is cellulose grafted acrylamide copolymer gel.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com