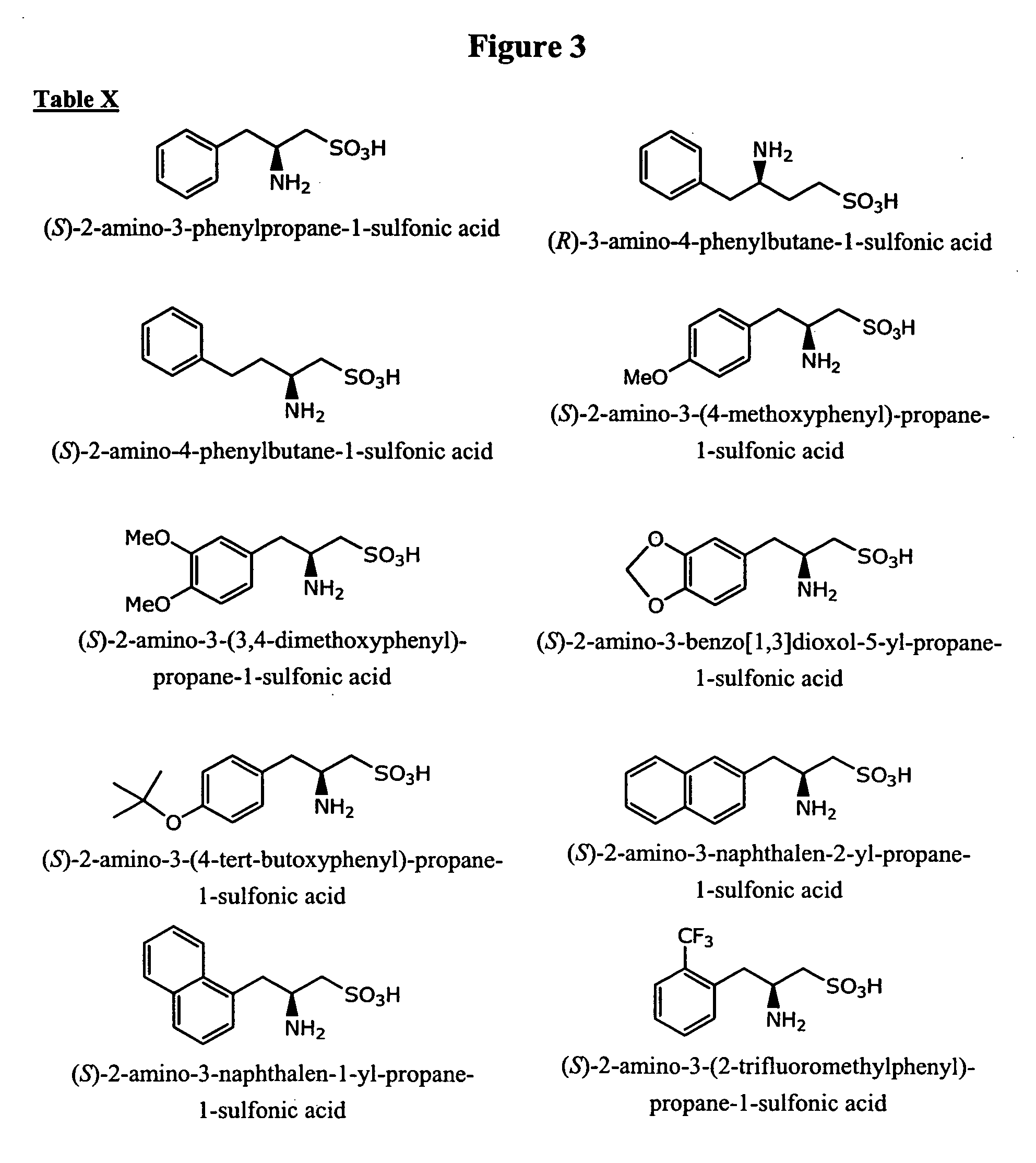
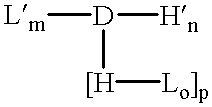Patents
Literature
2174 results about "Therapy medication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
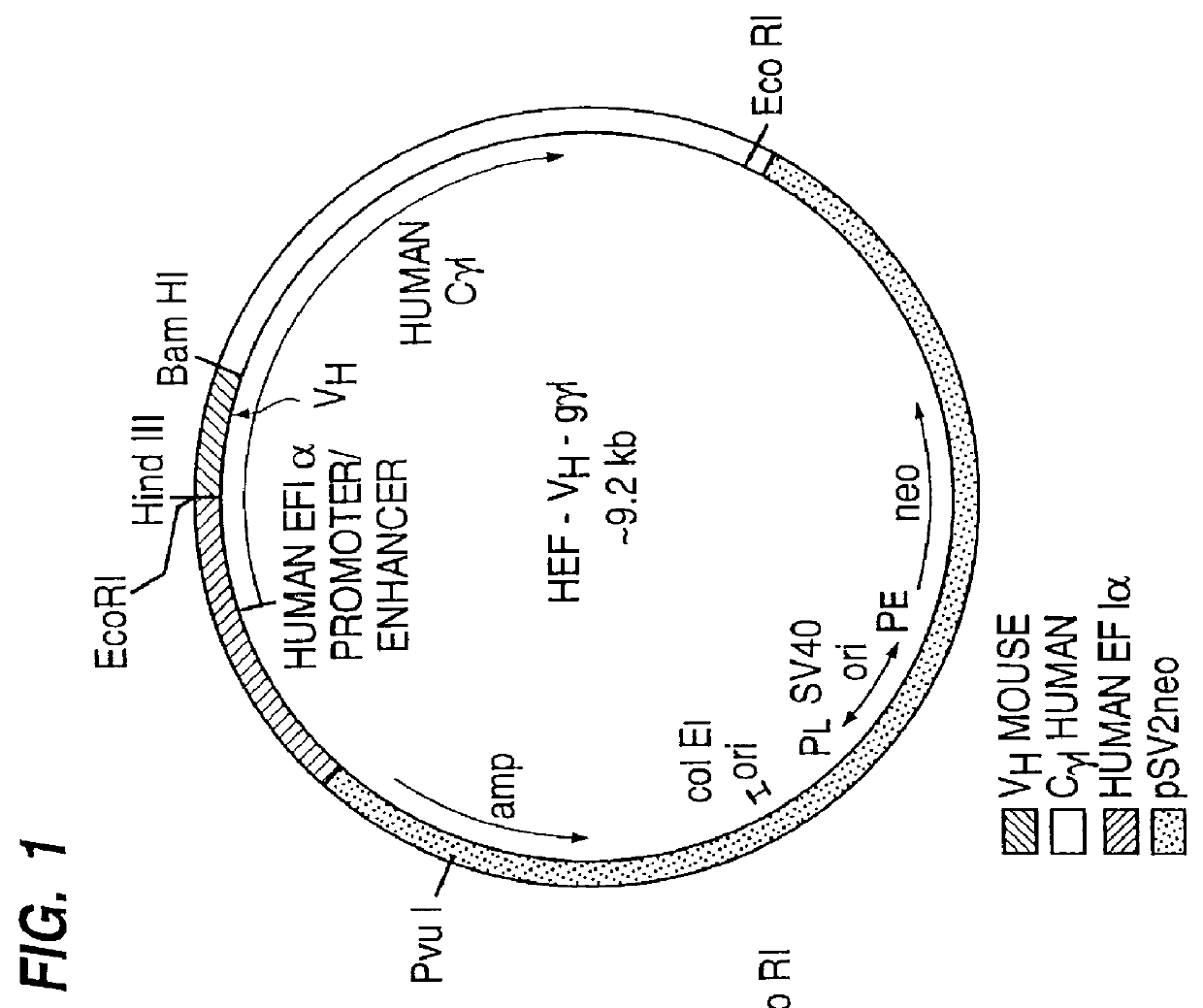
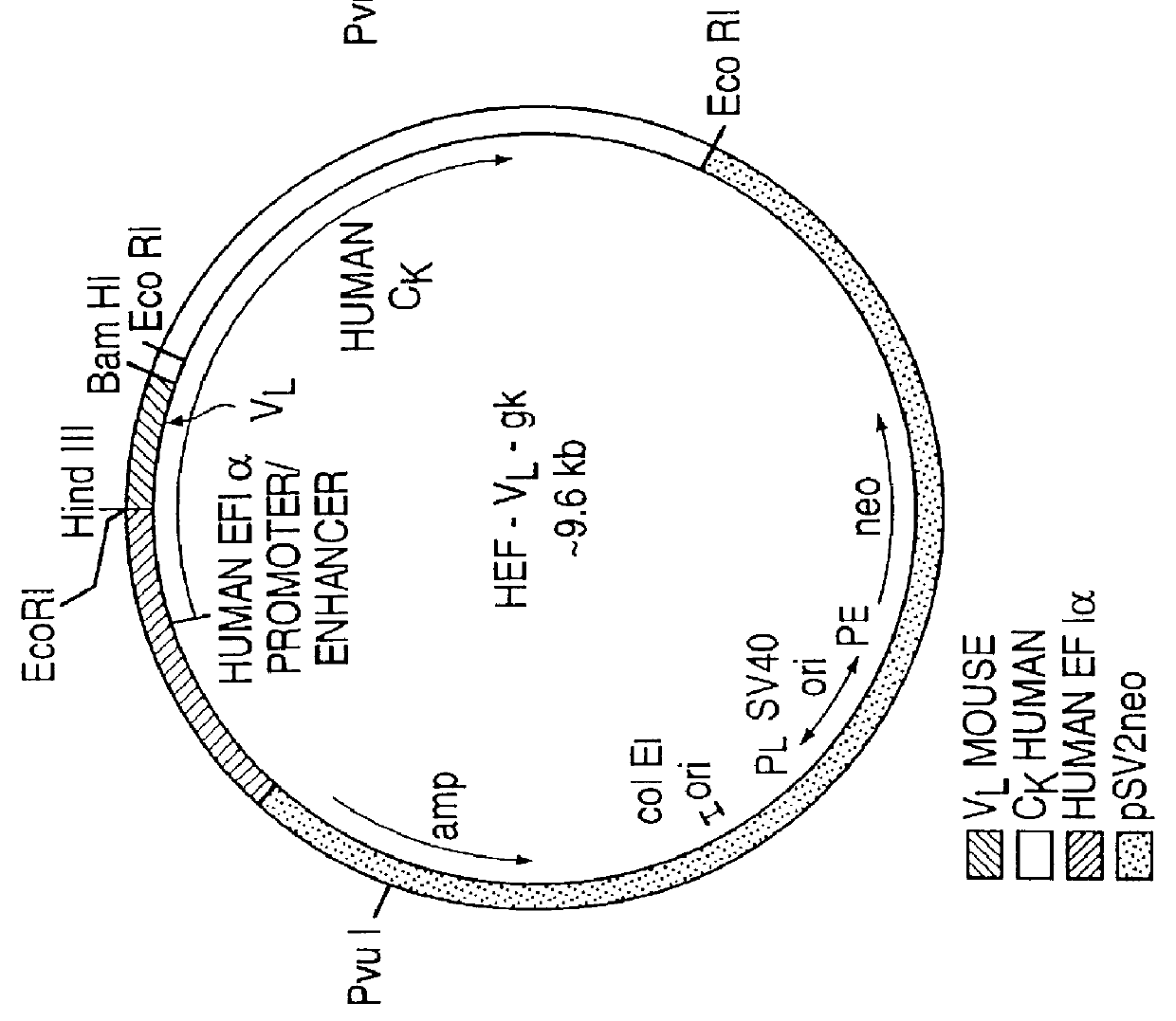
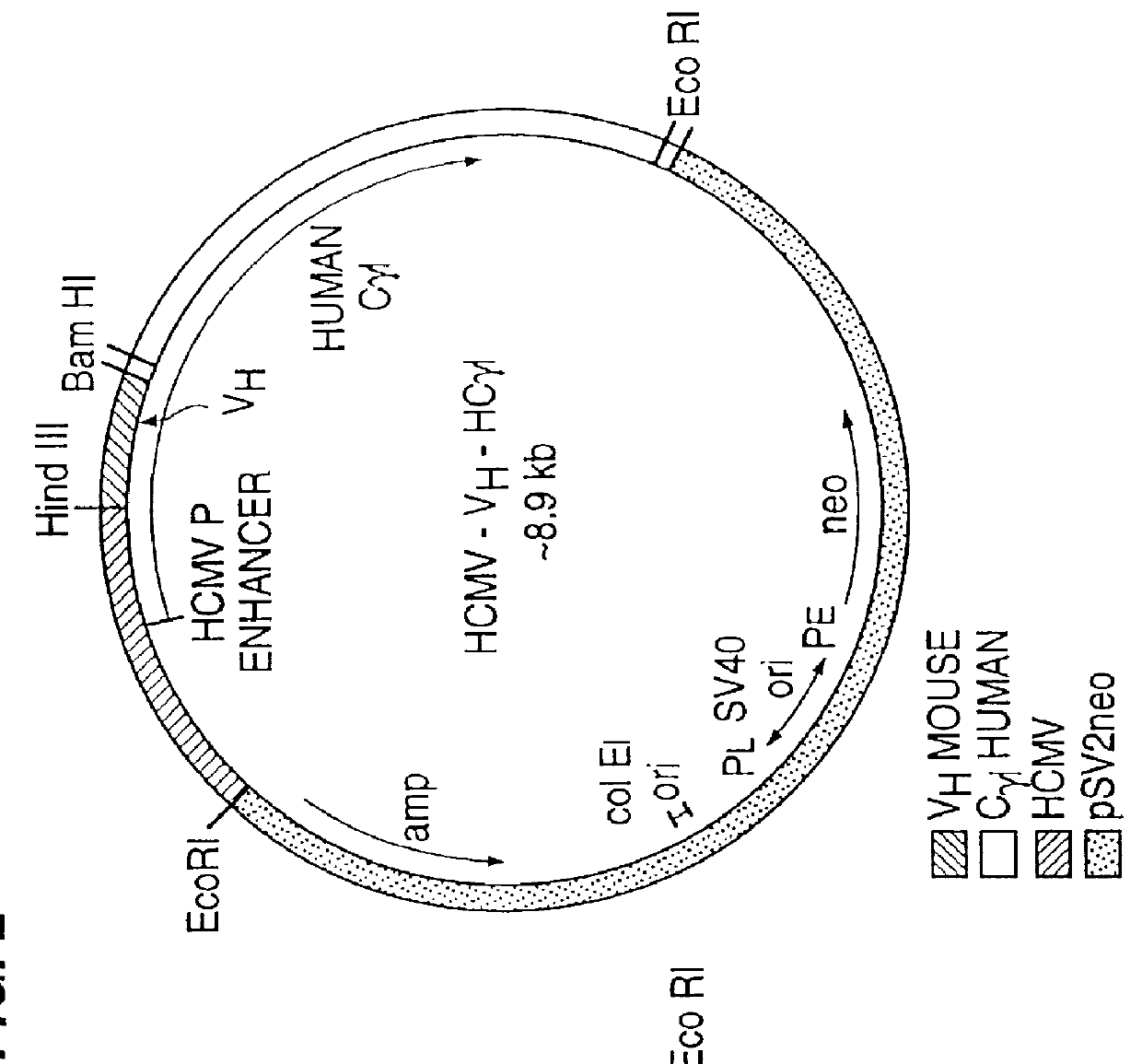
Drug releasing anastomosis devices and methods for treating anastomotic sites
ActiveUS7108701B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH
Coated endovascular AAA device
InactiveUS6852122B2Reduce drug toxicityGood curative effectSuture equipmentsStentsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Therapeutic formulations for the treatment of beta-amyloid related diseases
InactiveUS20050031651A1Impairs daily activityAvoid impairmentPharmaceutical delivery mechanismPharmaceutical active ingredientsCytotoxicityAmyloid
This invention relates to methods and pharmaceutical compositions for treating amyloid-β related diseases, including Alzheimer's disease. The invention, for example, includes a method of concomitant therapeutic treatment of a subject, comprising administering an effective amount of a first agent and a second agent, wherein said first agent treats an amyloid-β disease, neurodegeneration, or cellular toxicity; and said second agent is a therapeutic drug or nutritive supplement.
Owner:BELLUS HEALTH (INT) LTD (CH)
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
Fluid component analysis system and method for glucose monitoring and control
Disclosed are methods and apparatuses for determining analyte concentration in a sample such as bodily fluid. Systems and methods disclosed herein can also include a treatment dosing system to infuse or inject a treatment dose (e.g. insulin, dextrose, etc.) and provide glycemic control. The dose of the treatment drug may be based on the patient's calculated sensitivity to treatment dosing, for example. The dose of the treatment drug may be based on the concentration of the analyte or the average value for the concentration of the analyte and / or the rate of change of the value of the concentration of the analyte. Delivery of the treatment drug can be cut off if the determined analyte concentration indicates that continued delivery would be harmful to the patient.
Owner:OPTISCAN BIOMEDICAL +1
Bioabsorbable brachytherapy device
InactiveUS6575888B2Controlled release rateMinimally shieldsRadioactive preparation formsX-ray/gamma-ray/particle-irradiation therapyBrachytherapy deviceRadiopaque medium
A bioabsorbable brachytherapy device includes a tubular housing with sealed ends and an enclosed radioactive material. The radioactive material includes a radioisotope, such as palladium-103 or iodine-125. The tubular housing is made from a biocompatible and bioabsorbable polymeric material, and is sealed by means such as heat welding or solvent fixing. The device may further include a radiopaque medium and one or more therapeutic drugs.
Owner:FERRING BV
System and method for therapeutic drug monitoring
InactiveUS20050054942A1Accurate assessmentCost-effective and frequentNervous disorderElectrotherapyNoseEnvironmental health
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA
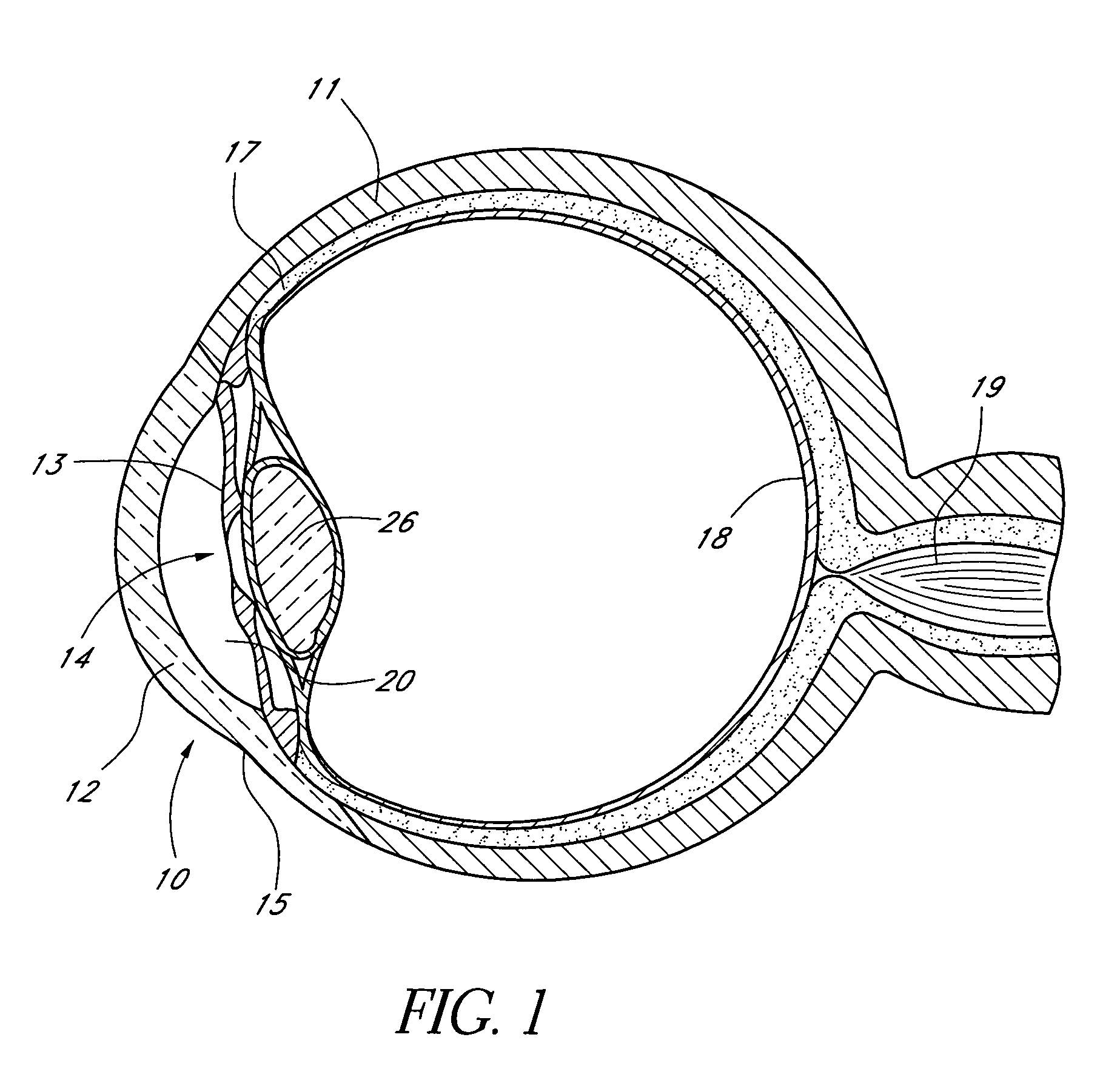
Ocular implant with therapeutic agents and methods thereof
InactiveUS7708711B2Simple treatmentPromote recoveryEye surgeryAnaesthesiaAqueous humorOcular implant
Devices and methods are provided for the treatment of ocular disorders. An ocular implant has a body comprising material that includes a therapeutic drug. The body has an inlet portion and an outlet portion. The inlet portion is configured to reside in an anterior chamber of an eye when the outlet portion is disposed in a physiological outflow pathway of the eye. The outlet portion has an outflow opening such that the body drains fluid from the anterior chamber to the physiological outflow pathway. One method of treating an ocular disorder involves introducing an implant comprising a therapeutic drug into the eye such that the implant drains aqueous humor into a physiological outflow pathway and the therapeutic drug reaches eye tissue.
Owner:DOSE MEDICAL CORP
Drug-eluting stent cover and method of use
An intravascular stent includes an eluting sheath fabricated from a mesh for controlled release of therapeutic drugs and for delivery of the therapeutic drugs in localized drug therapy in a blood vessel. The eluting sheath is attached to at least a portion of an outside surface area of the stent structure and is fabricated from a mesh designed to neck down in response to a radially outward directed force resulting in the uniform expansion of the stent. The eluting sheath can be loaded with at least one therapeutic drug for the release thereof at a treatment site to facilitate repair of a damaged vessel. The stent has a high degree of flexibility in the longitudinal direction, yet has adequate vessel wall coverage and radial strength sufficient to hold open an artery or other body lumen.
Owner:ABBOTT CARDIOVASCULAR
Intravascular Tissue Disruption
InactiveUS20120116438A1Reduce high blood pressureSmall volumeGuide needlesStentsNovel agentsTarget tissue
Owner:SHIFAMED HLDG
Coating for controlled release of a therapeutic agent
InactiveUS20050033417A1Minimize potential risk of damageReduce frictionSuture equipmentsOrganic active ingredientsBlood vesselPolymer
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
Fluid component analysis system and method for glucose monitoring and control
Disclosed are methods and apparatus for determining analyte concentration in a sample such as bodily fluid. Systems and methods disclosed herein can also include a treatment dosing system to infuse or inject a treatment drug (e.g. insulin or glucose) and provide glycemic control. The dose of the treatment drug may be based on the concentration of the analyte or the average value for the concentration of the analyte and / or the rate of change of the value of the concentration of the analyte.
Owner:INSULET CORP +1
Coated medical devices for the treatment of vulnerable plaque
InactiveUS7195640B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesPercent Diameter StenosisCoating
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. In addition to reducing or substantially eliminating a biological organism's reaction to the introduction of the medical device to the organism, the medical device in combination with one or more therapeutic drugs, agents and / or compounds may be utilized to treat various vascular diseases, for example, restenosis and vulnerable plaque. In the case of vulnerable plaque, one or more drugs, agents or compounds may be utilized to treat the various aspects of vulnerable plaque and these drugs, agents and / or compounds may be released with a given release profile for the most effective treatment. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH +1
Reshaped human antibody to human interleukin-6
InactiveUS6121423AUseful purposeAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsDiseaseV region
A reshaped antibody comprising: (A) L chains comprising: (1) a human C region, and (2) an L chain V region comprising human L chain FRs and L chain CDRs of a mouse monoclonal antibody; and (B) H chains comprising: (1) a human H chain C region, and (2) an H chain V region comprising human H chain FRs, and H chain CDRs of a mouse monoclonal antibody to human IL-6. Since the major portions of the reshaped human antibody are derived from human, and the mouse CDRs are less immunogenic, then the present reshaped human antibody is less immunogenic, and therefore inhibits information transfer by IL-6, and is promising as a therapeutic agent for diseases caused by IL-6.
Owner:CHUGAI PHARMA CO LTD
Local vascular delivery of trichostatin a alone or in combination with sirolimus to prevent restenosis following vascular injury
ActiveUS20050136090A1Minimize potential risk of damageReduce frictionBiocideSurgeryPercent Diameter StenosisBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
Device for the delivery of a cardioprotective agent to ischemic reperfused myocardium
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. Implantable medical devices may be coated or otherwise have affixed thereto agents for healing ischemic tissue.
Owner:WYETH LLC
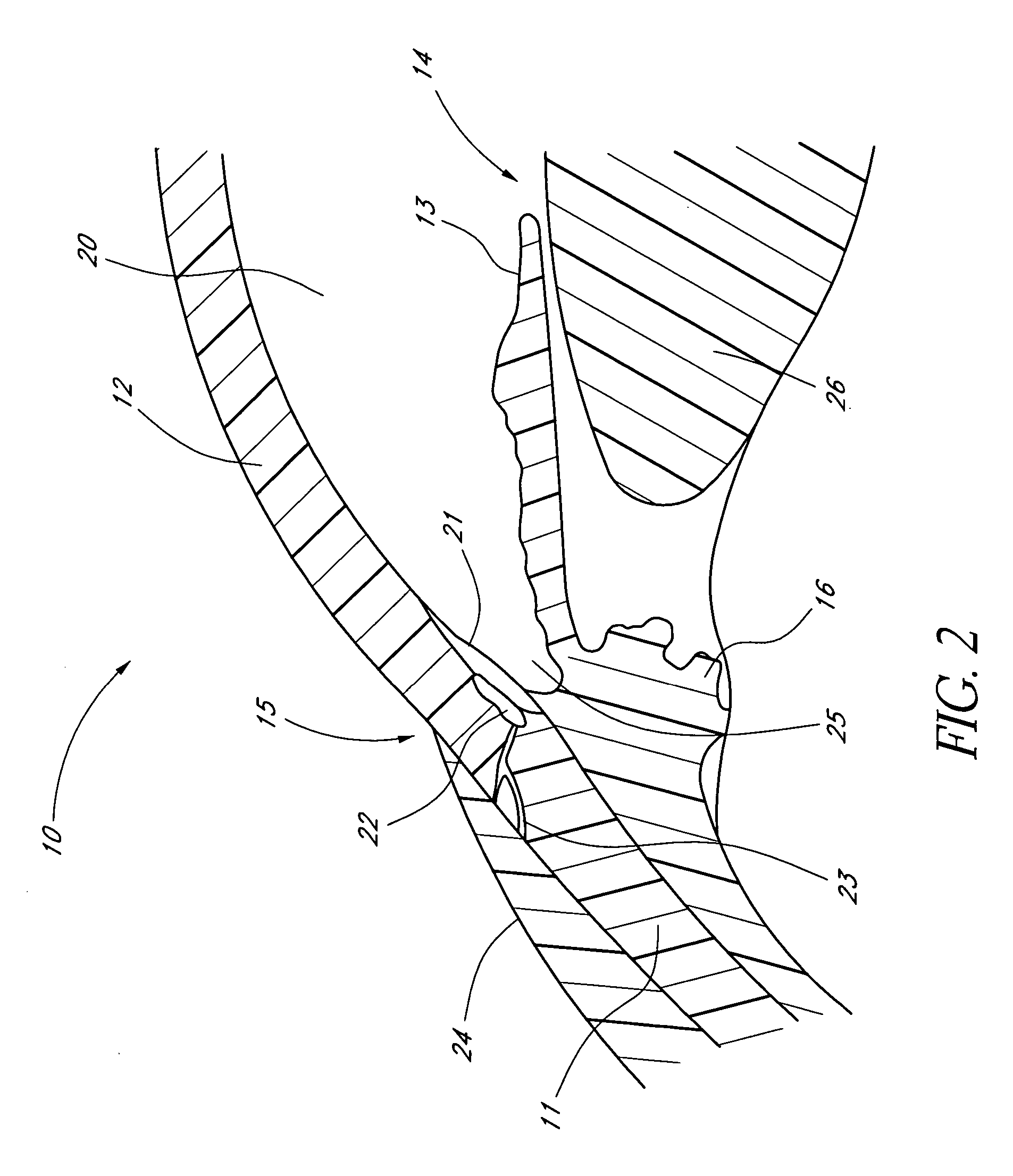
Combined treatment for cataract and glaucoma treatment
InactiveUS20070118147A1Maintain pressureFast and safe and less-expensiveEye surgerySurgeryGlaucoma proceduresSchlemm's canal
A method is provided for treatment of cataract in combination with a glaucoma procedure while maintaining the intraocular pressure by permitting aqueous to flow out of an anterior chamber of the eye through a surgically stented pathway. A trabecular stent is adapted for implantation within the trabecular meshwork of an eye such that intraocular liquid flows controllably from the anterior chamber of the eye to Schlemm's canal, bypassing the trabecular meshwork. Depending upon the specific treatment contemplated, pharmaceuticals may be utilized in conjunction with the trabecular stent enabling post-cataract healing processes.
Owner:GLAUKOS CORP
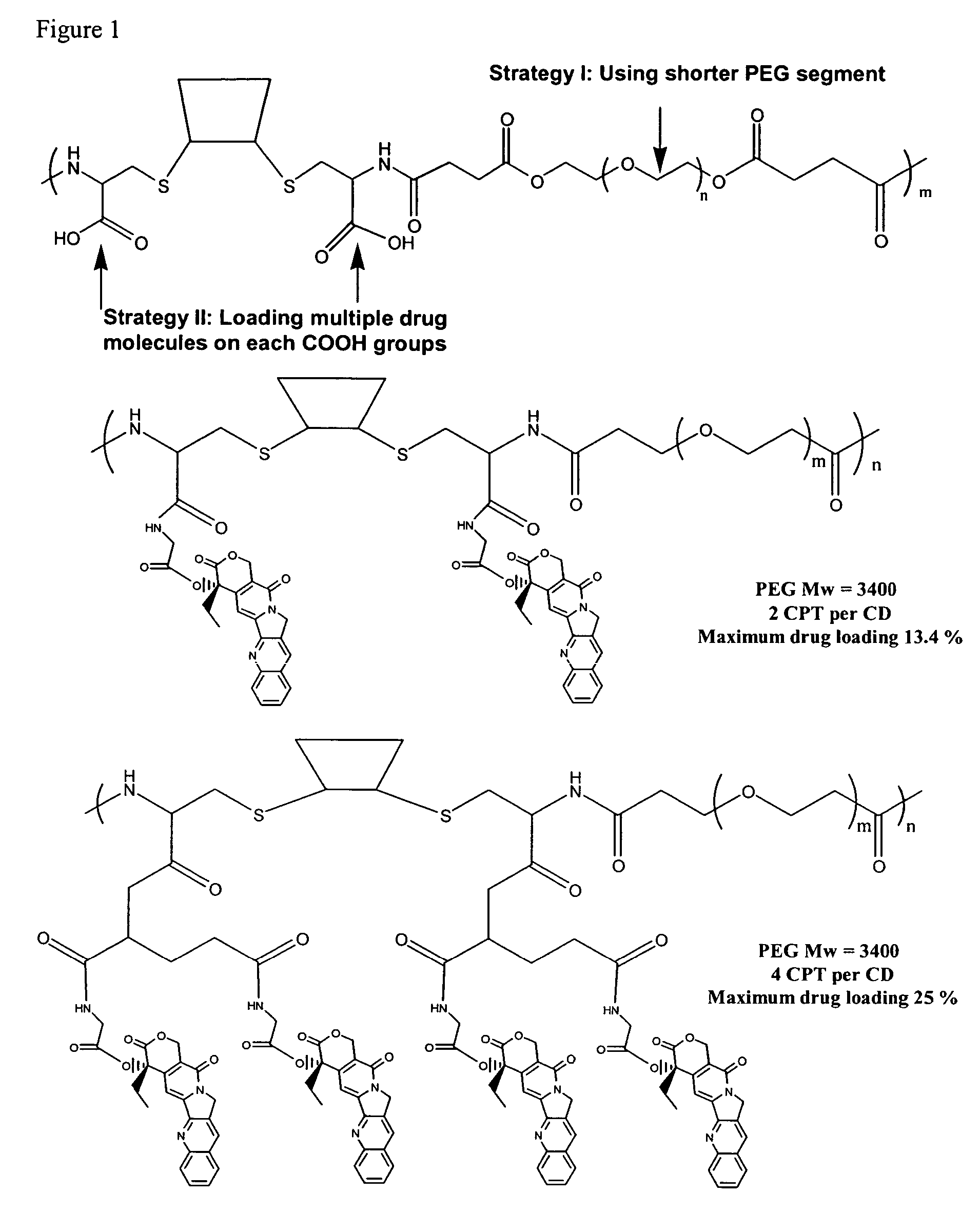
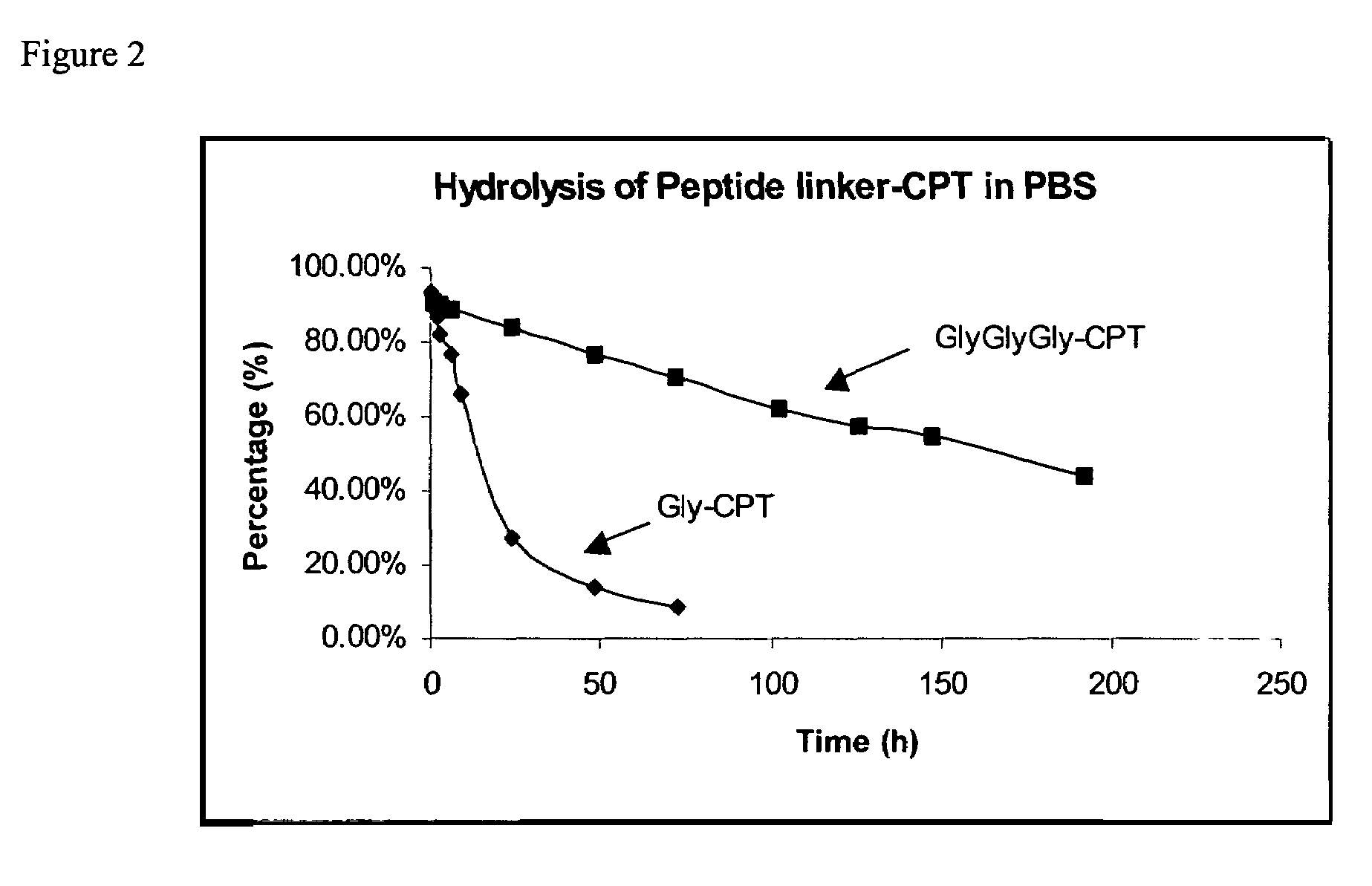
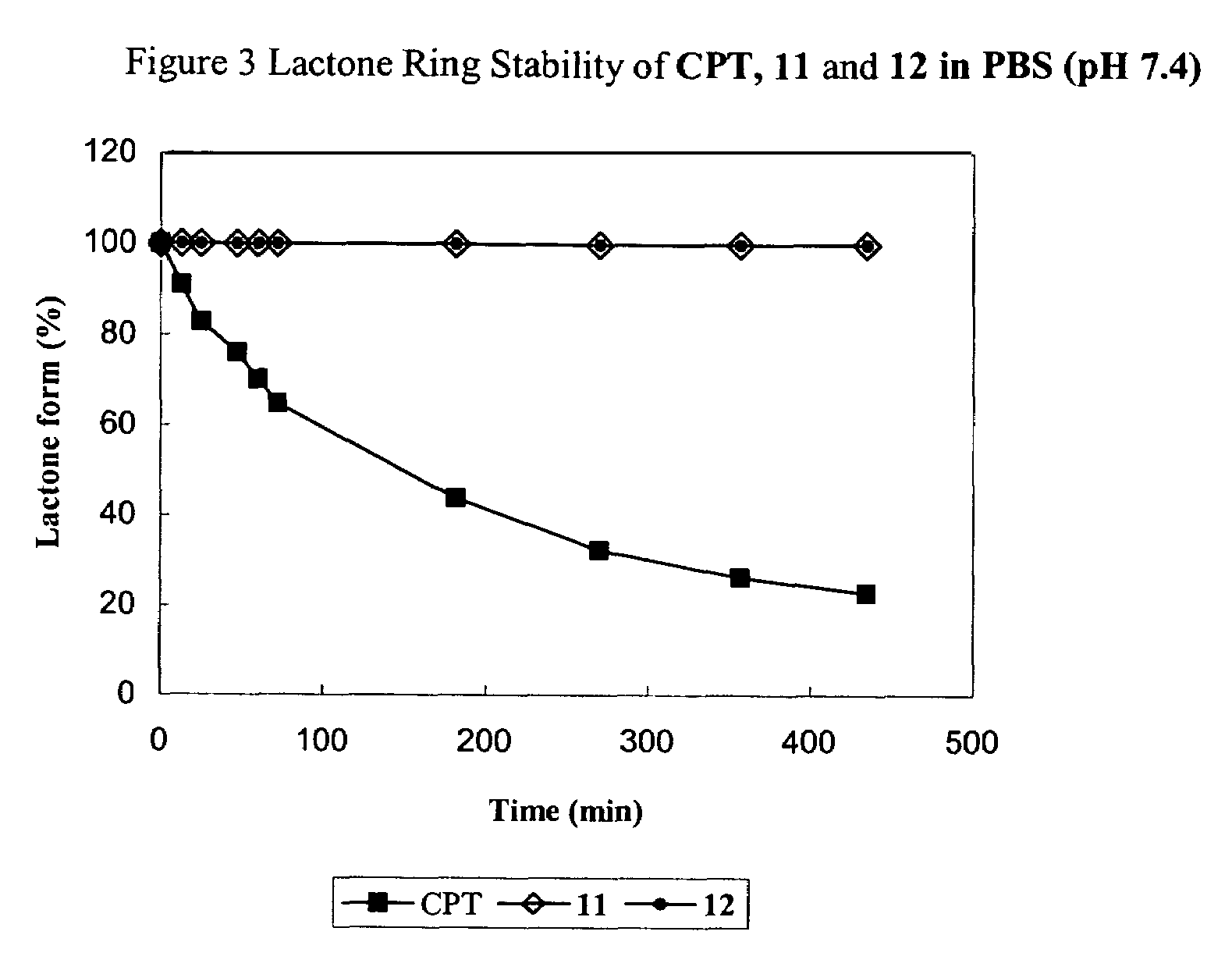
Cyclodextrin-based polymers for therapeutics delivery
ActiveUS7270808B2Improve drug stabilityImprove solubilityAntibacterial agentsBiocideSolubilityPresent method
The present invention relates to novel compositions of therapeutic cyclodextrin containing polymeric compounds designed as a carrier for small molecule therapeutics delivery and pharmaceutical compositions thereof. These cyclodextrin-containing polymers improve drug stability and solubility, and reduce toxicity of the small molecule therapeutic when used in vivo. Furthermore, by selecting from a variety of linker groups and targeting ligands the polymers present methods for controlled delivery of the therapeutic agents. The invention also relates to methods of treating subjects with the therapeutic compositions described herein. The invention further relates to methods for conducting pharmaceutical business comprising manufacturing, licensing, or distributing kits containing or relating to the polymeric compounds described herein.
Owner:CERULEAN PHARMA
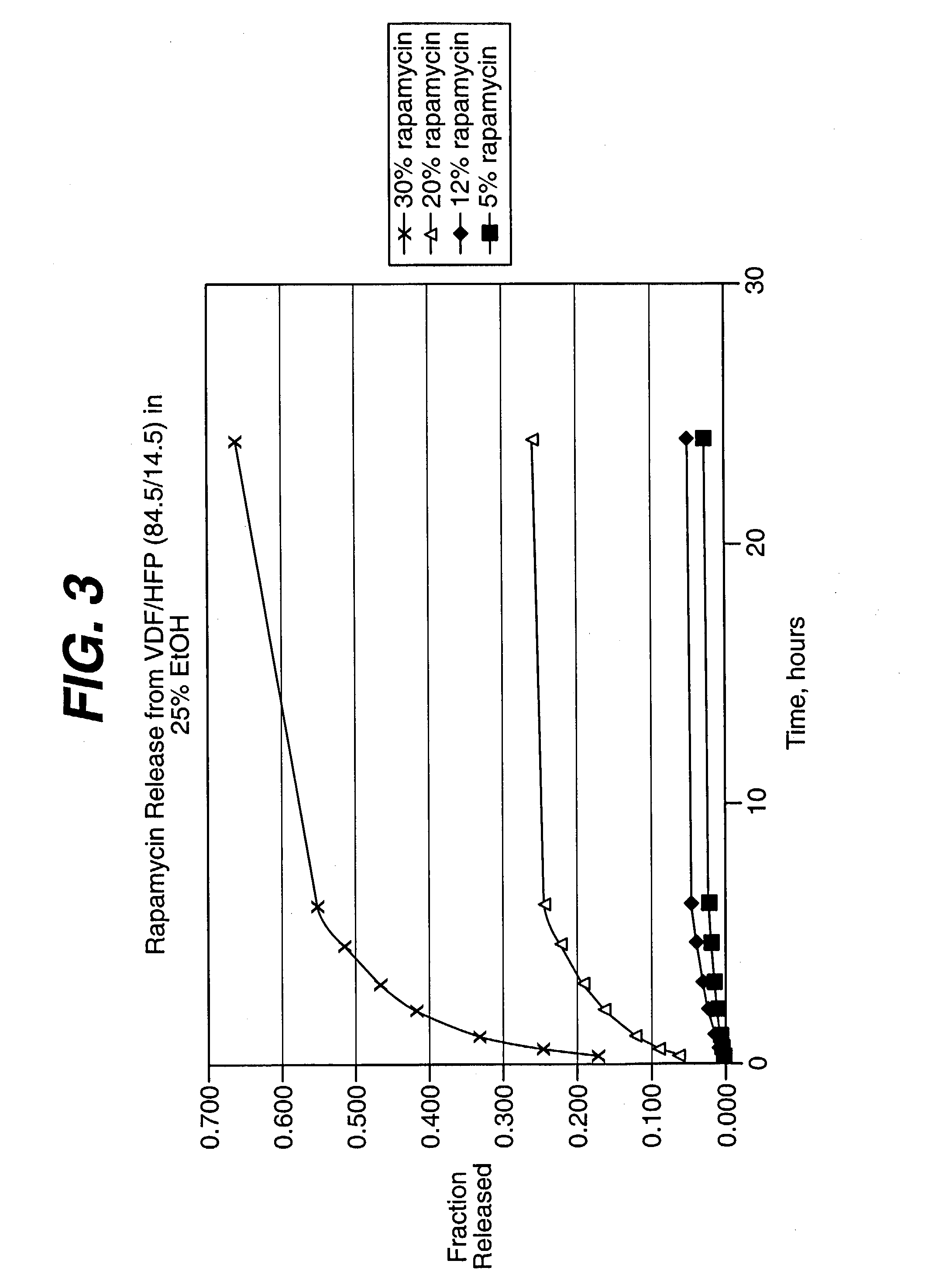
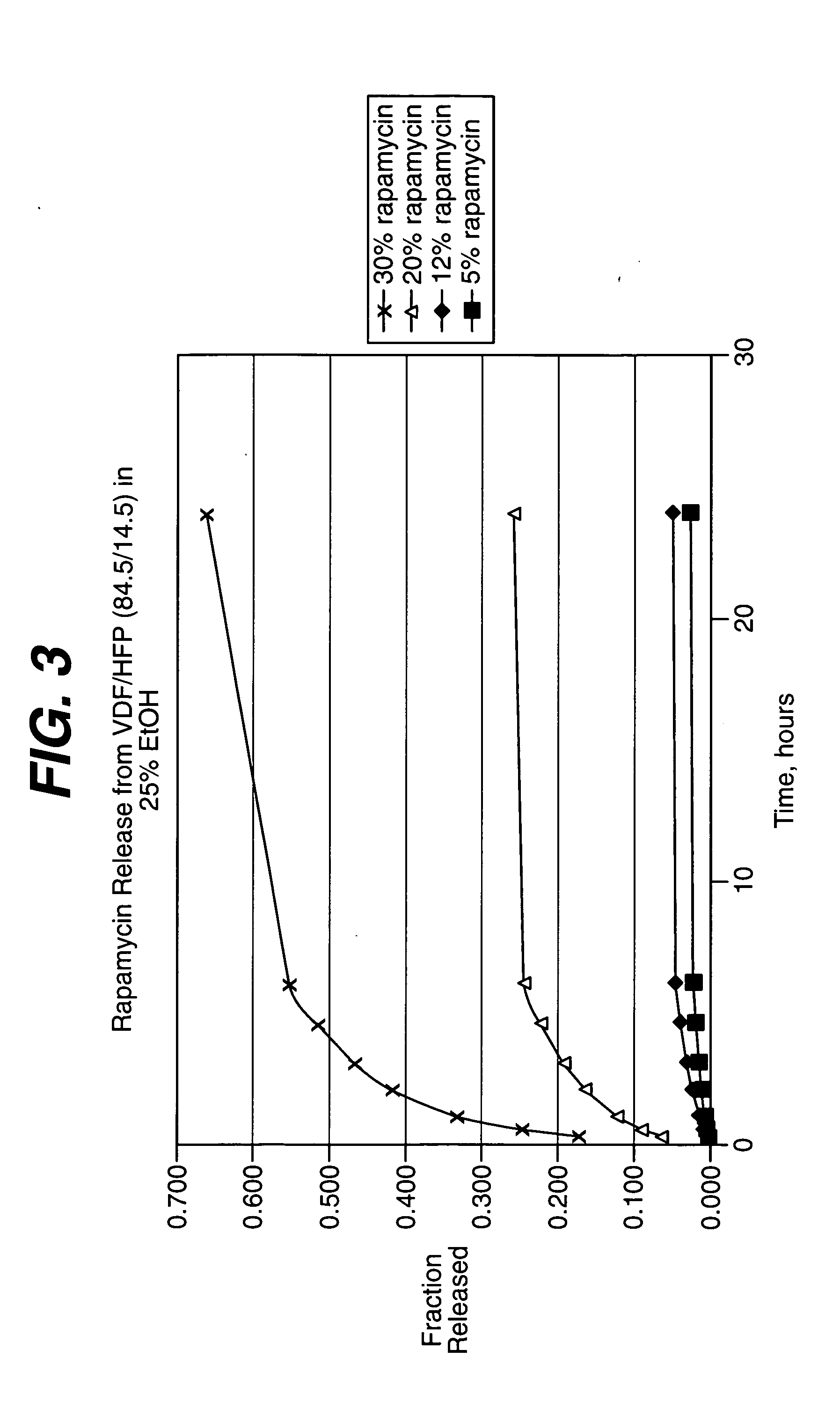
Solution formulations of sirolimus and its analogs for CAD treatment
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Injectable formulations of taxanes for cad treatment
InactiveUS20050272806A1Minimize potential risk of damageReduce frictionBiocideOrganic active ingredientsAntioxidantBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. Liquid formulations, including solutions and suspensions of the various drugs, agents and / or compounds, may be locally or regionally delivered. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Intraluminal medical devices in combination with therapeutic agents
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
Application of bacteroides fragilis in preparation of composition for treating inflammatory bowel diseases
InactiveCN103156888AEnhance pharmacological effectsGood treatment effectMilk preparationBacteria material medical ingredientsPharmacologic actionBowels diseases
The invention relates to the technical field of application of bacteroides fragilis, and in particular relates to application of bacteroides fragilis in preparation of a composition for treating inflammatory bowel diseases. The experiments show that bacteroides fragilis is safe and nontoxic and strong in pharmacologic action, and has good treating effect of treating inflammatory bowel diseases, thereby indicating that the bacteroides fragilis has good edible and medicinal prospects. According to the invention, a novel use of bacteroides fragilis is explored and a novel application field is developed. Bacteroides fragilis as a probiotic can be used for preparing foods or medical compositions for treating inflammatory bowel diseases so as to provide health-cared foods or treating medicines suitable for human body to take.
Owner:广东知光生物科技有限公司
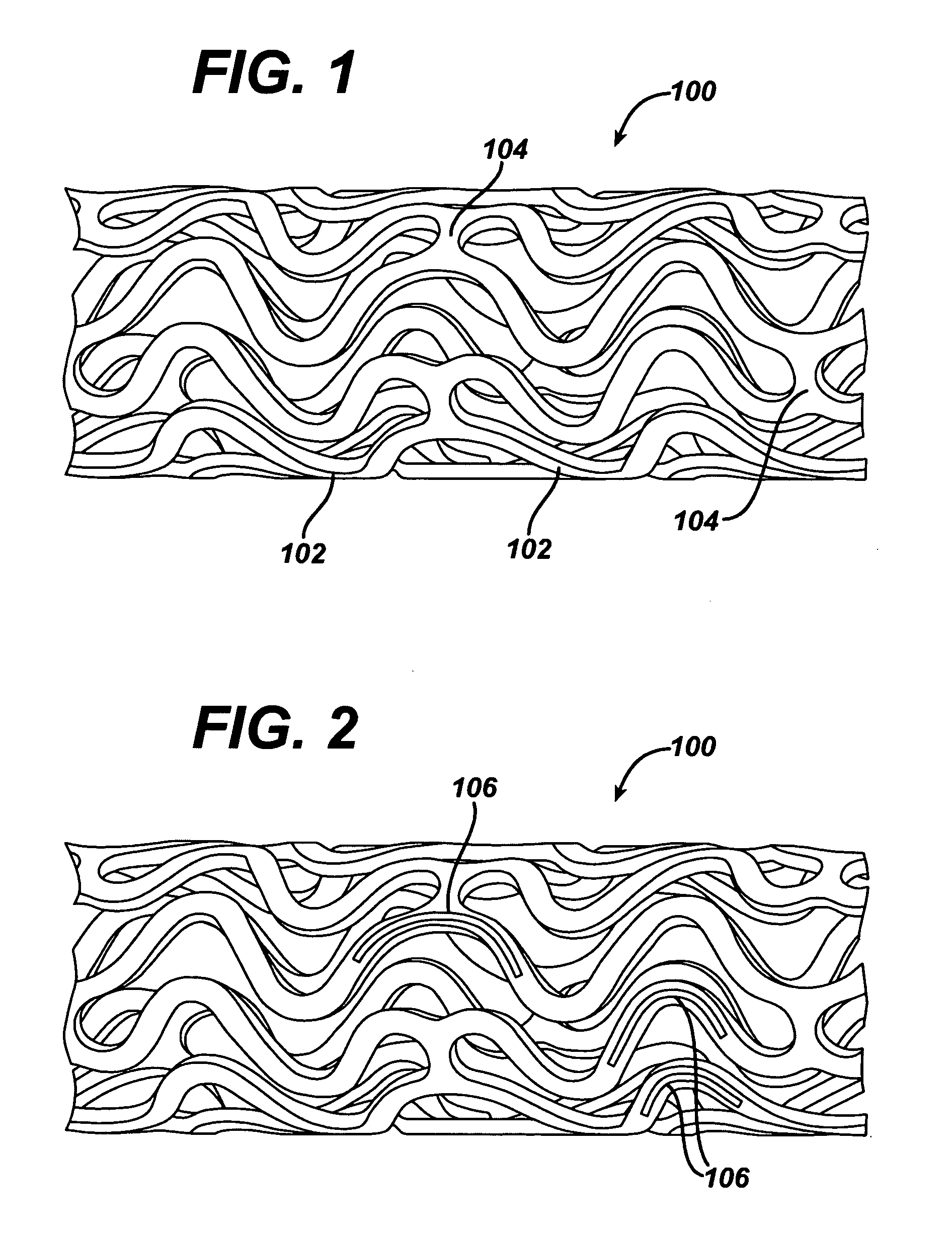
Thin-film nitinol based drug eluting stent
InactiveUS20070173787A1Reduce drug toxicityGood curative effectStentsPharmaceutical delivery mechanismDiseaseBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. These devices may also comprise thin films that perform a number of functions. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:CORDIS CORP
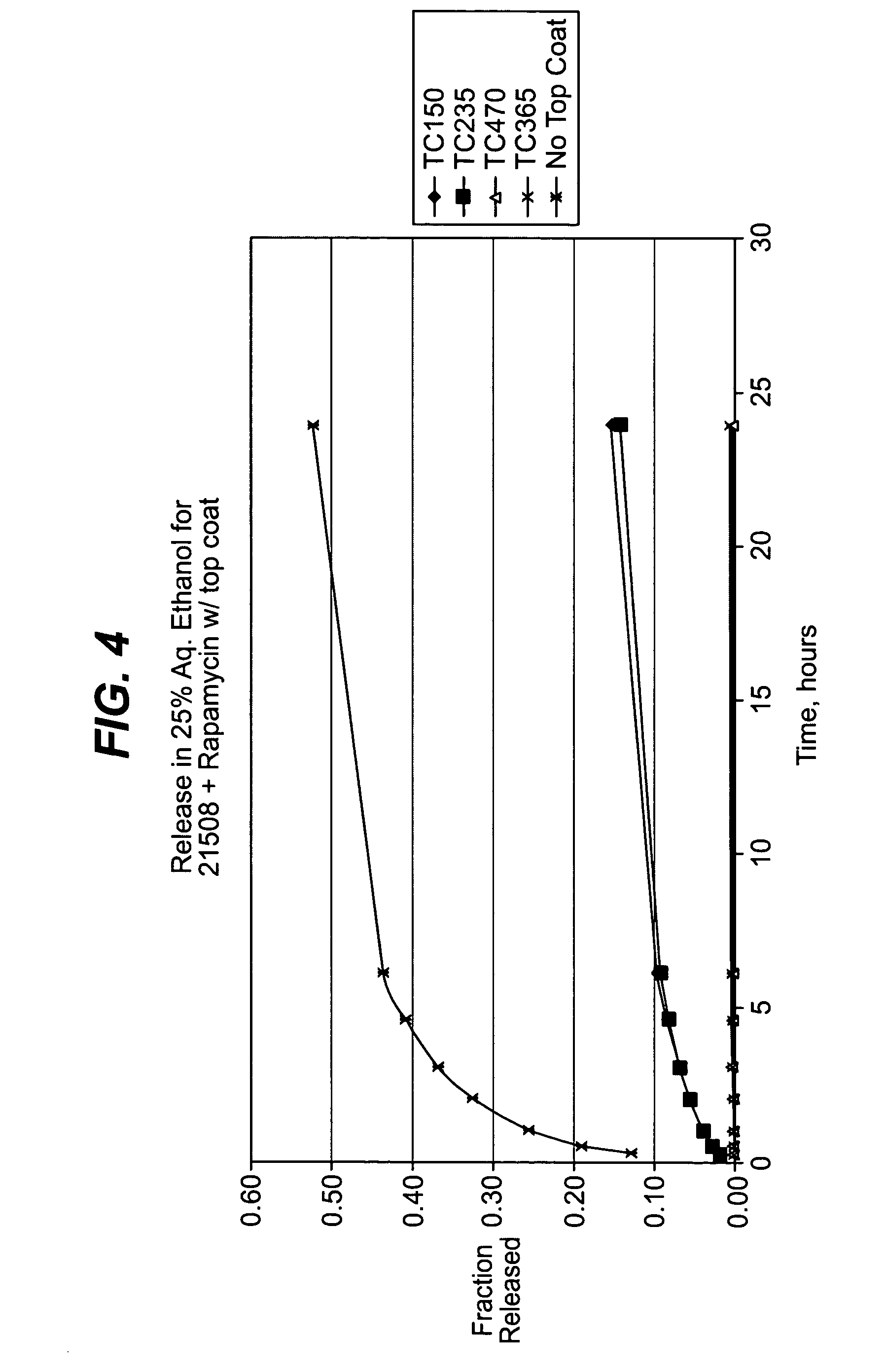
Medical devices, drug coatings and methods for maintaining the drug coatings thereon
InactiveUS7056550B2Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyMedical device
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC +1
Ultrasound imaging and treatment
InactiveUS7078015B2Low costSuitable for useUltrasonic/sonic/infrasonic diagnosticsCosmetic preparationsSound energyUltrasonic imaging
Methods of and apparatus for preparing temperature activated gaseous precursor-filled liposomes are described. Gaseous precursor-filled liposomes prepared by these methods are particularly usefull for example, in ultrasonic imaging applications and in therapeutic drug delivery systems.Gas, gaseous precursors and perfluorocarbons are presented as novel potentiators for ultrasonic hyper-thermia. The gas, gaseous precursors and perfluorocarbons which may be administered into the vasculature, interstitially or into any body cavity are designed to accumulate in cancerous and diseased tissues. When therapeutic ultrasonic energy is applied to the diseased region heating is increased because of the greater effectiveness of sound energy absorption caused by these agents.
Owner:IMARX THERAPEUTICS
Coated aneurysmal repair device
InactiveUS20050249776A1Reduce drug toxicityGood curative effectBiocideStentsBlood vesselDisease cause
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
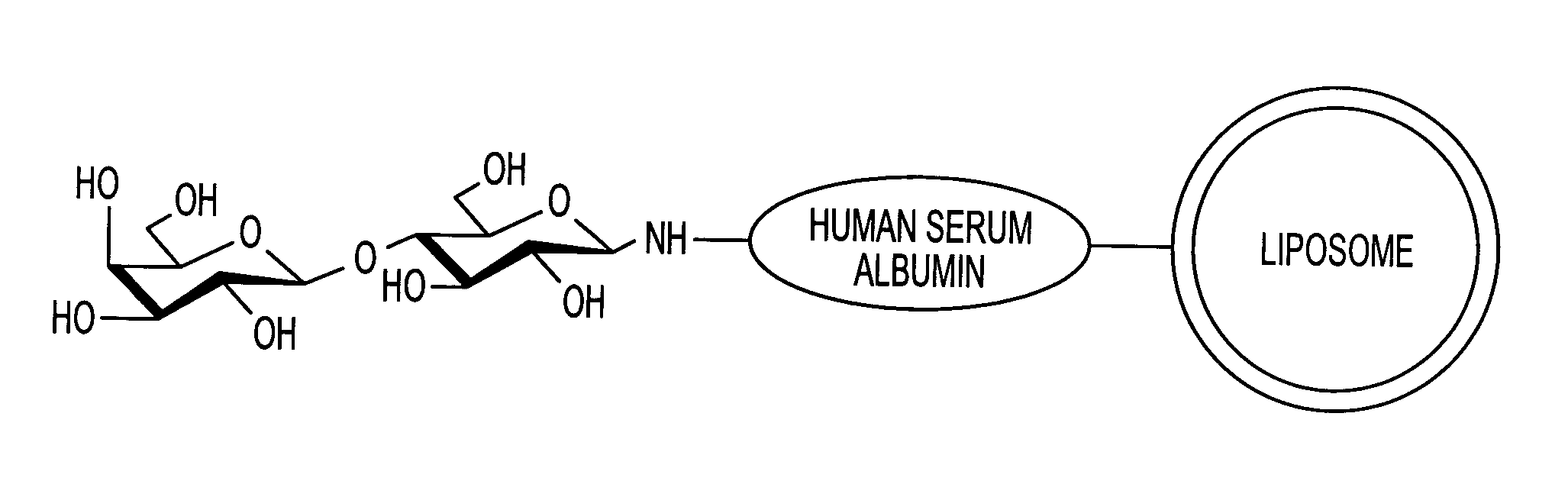
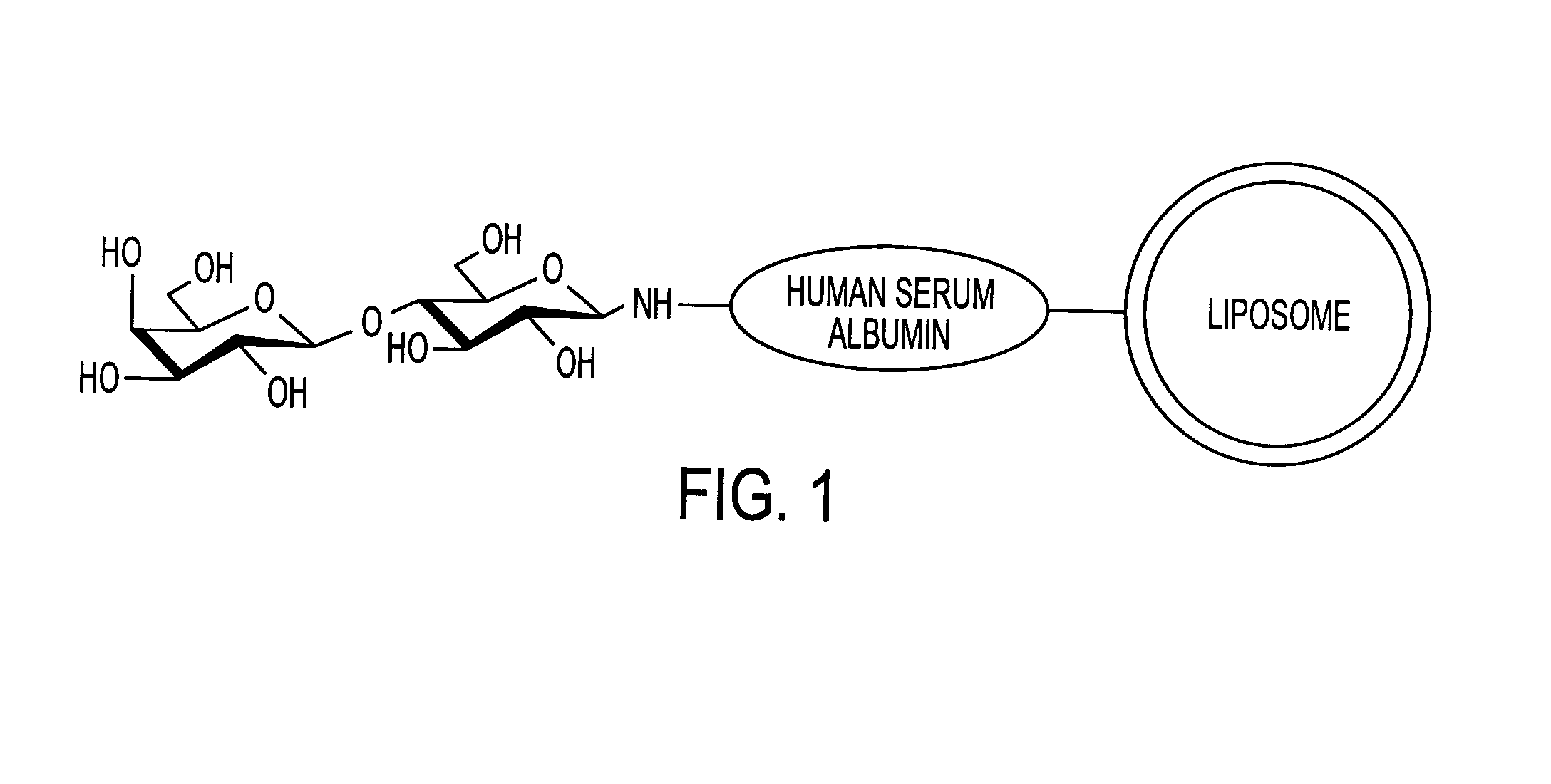
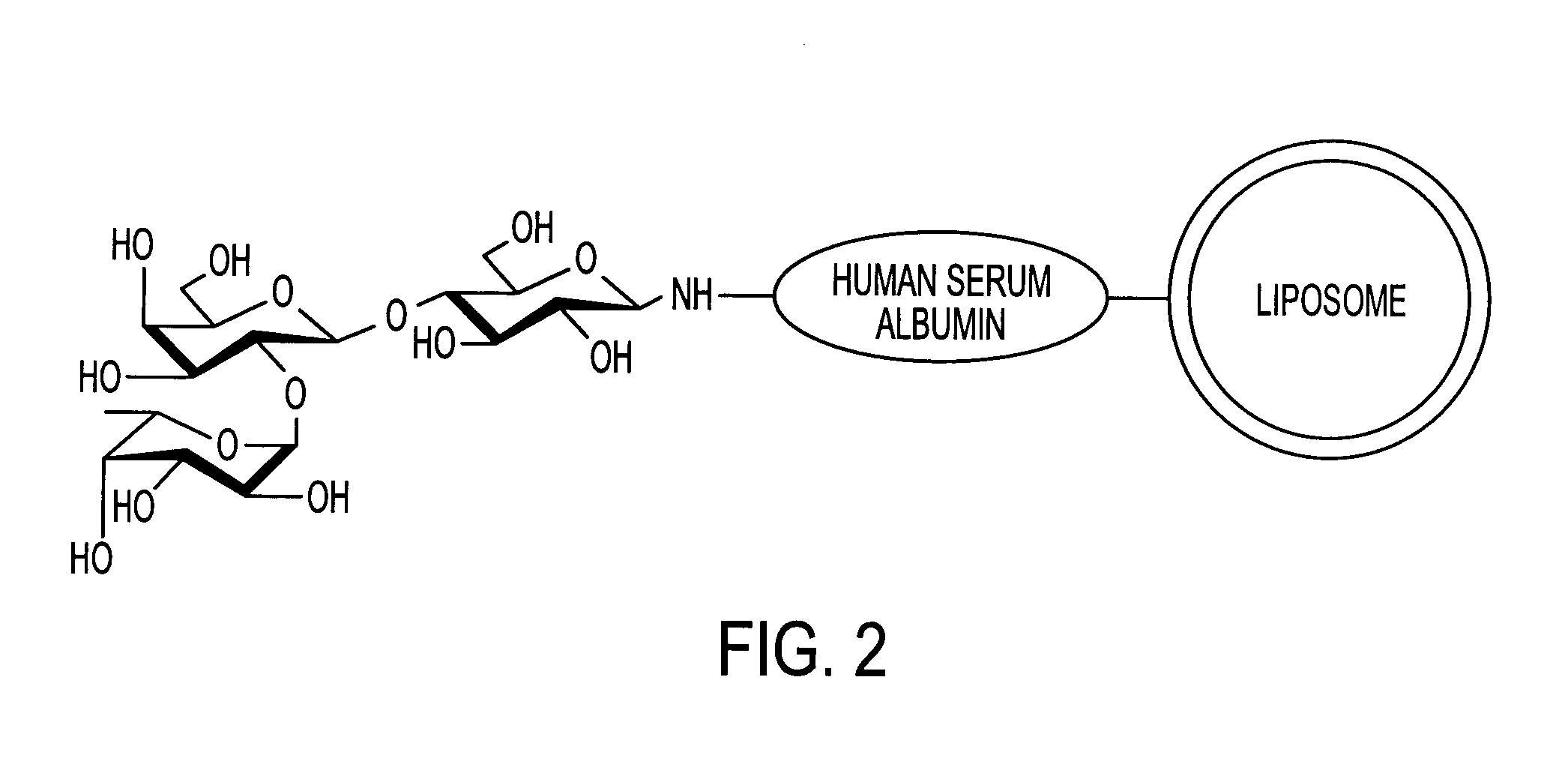
Sugar-modified liposome and products comprising the liposome
InactiveUS7070801B2Improve absorption qualityQuality improvementUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsIntestinal structureCancer cell
The present invention provides a sugar-modified liposome having a sugar chain bonded to its membrane surface, preferably through a linker protein, and having excellent absorption qualities, particularly in the intestine. The molecular structure and quantity of the sugar chain is selectively varied to allow the liposome to be delivered in a targeted manner to selected cells and tissues. The liposome is applicable to medicinal drugs, cosmetics and other various products in the medical / pharmaceutical fields, and it is especially useful in a therapeutic drug delivery system that recognizes target cells and tissues, such as cancer cells, and in the delivery of drugs or genes locally to a selected region, or in a diagnostic cell / tissue sensing probe.
Owner:YAMAZAKI DDS
Device for local and/or regional delivery employing liquid formulations of therapeutic agents
InactiveUS20080181927A1Elimination reactionReduce riskBiocideSurgeryDiseasePercent Diameter Stenosis
Medical devices may be utilized for local and regional therapeutic agent delivery. These therapeutic agents or compounds may reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including restenosis, vulnerable plaque, and atherosclerosis in type 2 diabetic patients. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Iloprost in combination therapies for the treatment of pulmonary arterial hypertension
InactiveUS20050101608A1BiocideElcosanoid active ingredientsEndothelin receptor antagonistPDE Inhibitor
Preferred embodiments of the present invention are related to novel therapeutic drug combinations and methods for treating pulmonary arterial hypertension. More particularly, aspects of the present invention are related to using a combination of iloprost and at least one additional agent, selected from the group consisting of an endothelin receptor antagonist and a PDE inhibitor.
Owner:COTHERIX INC
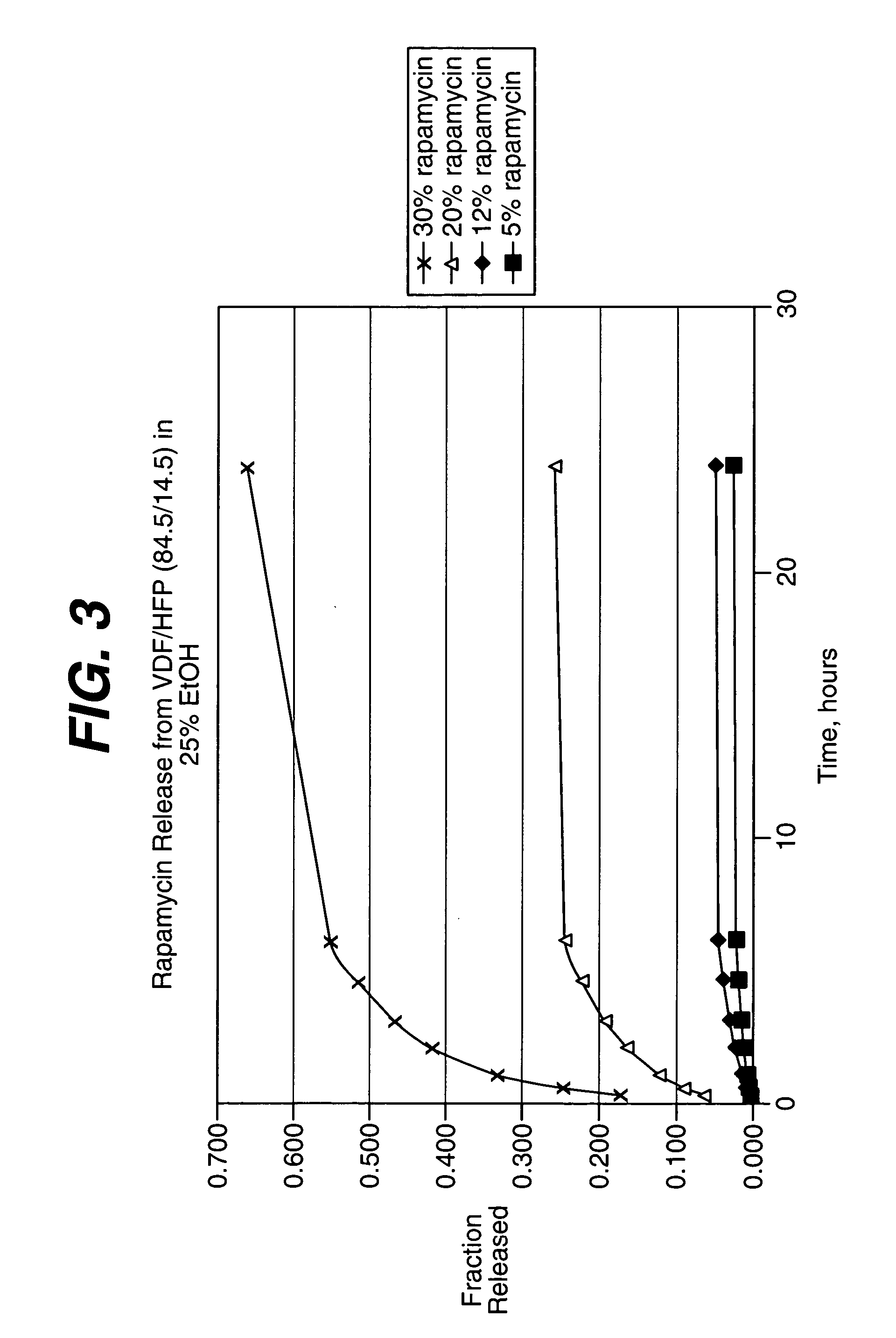
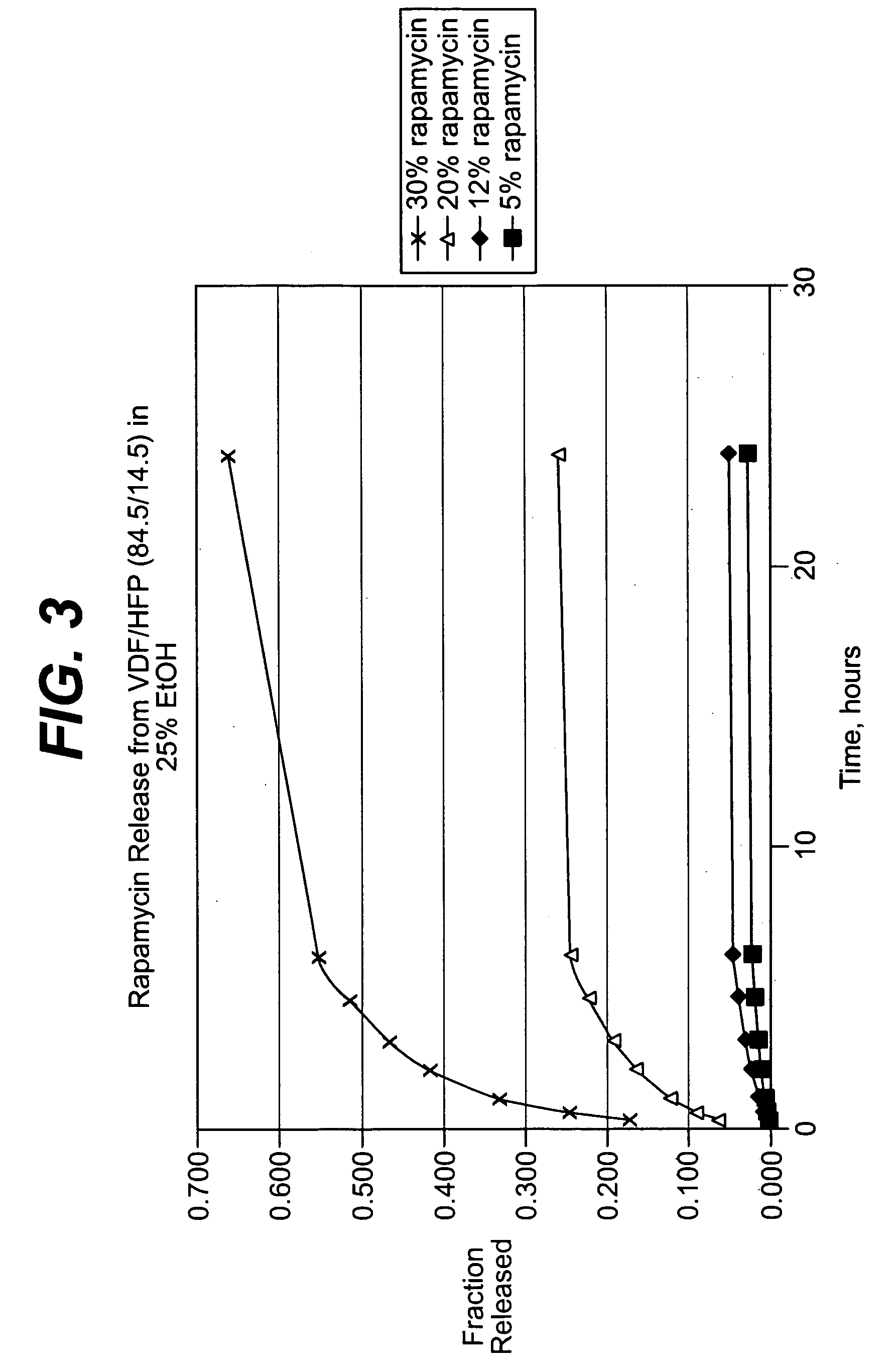
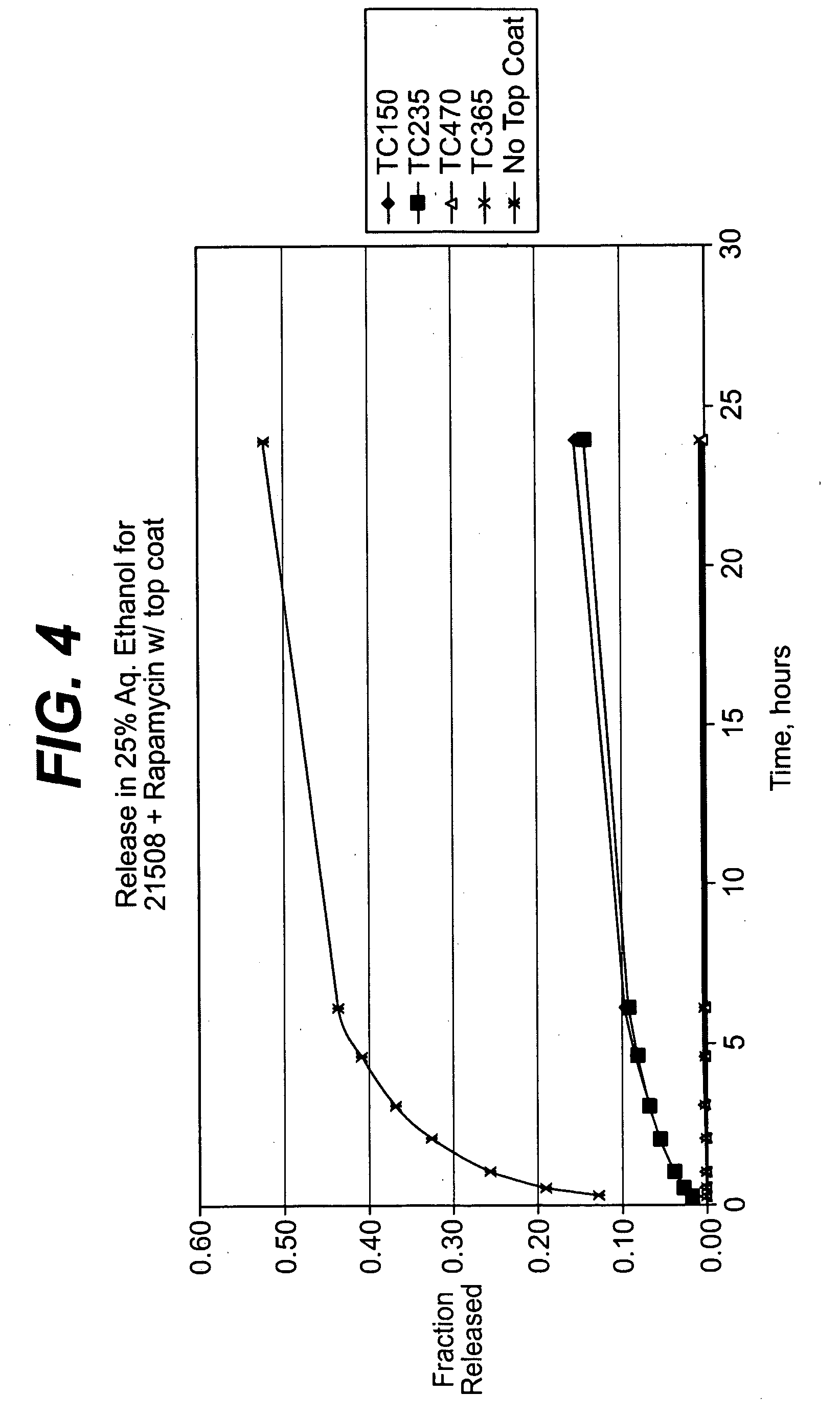
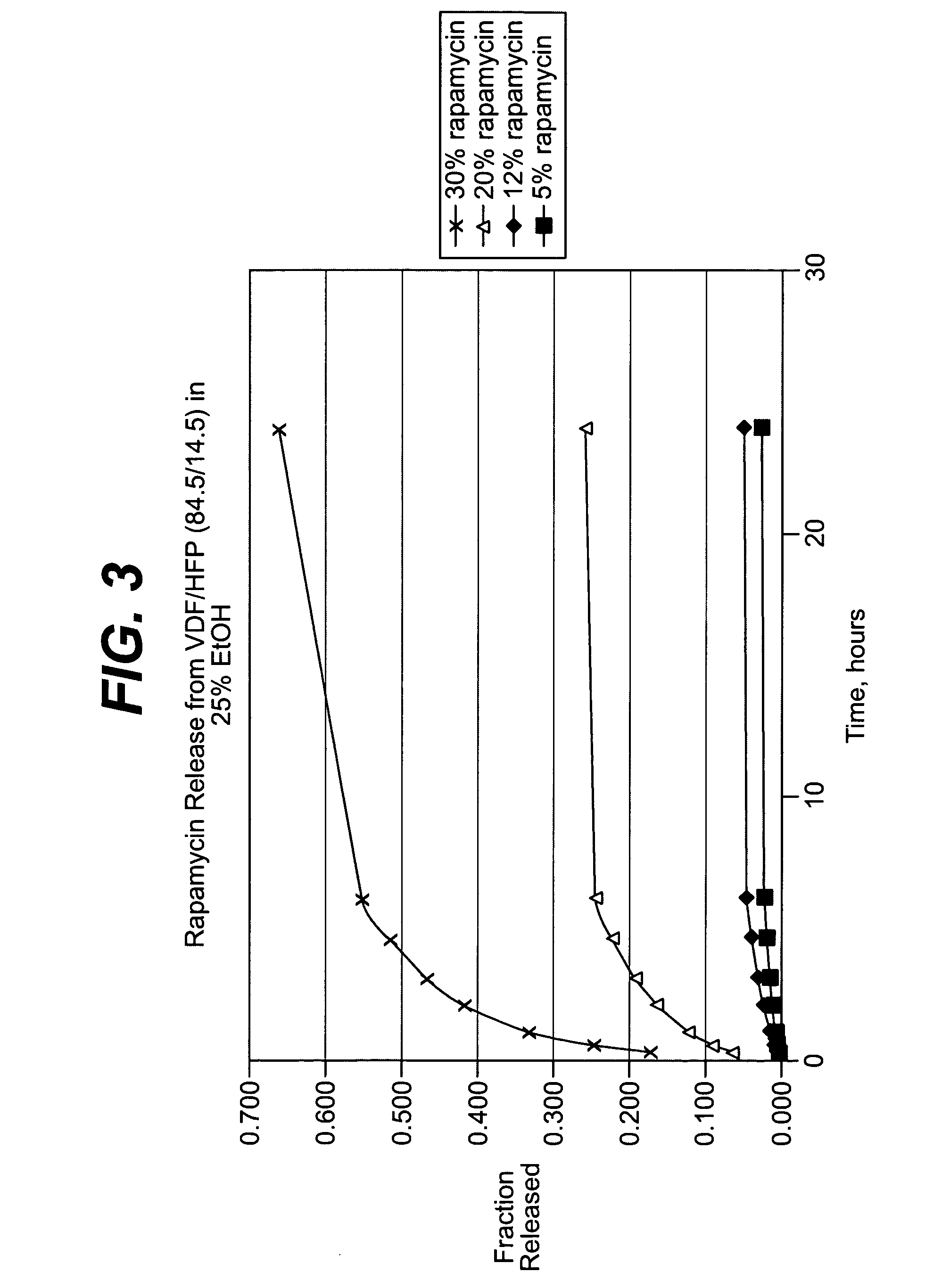
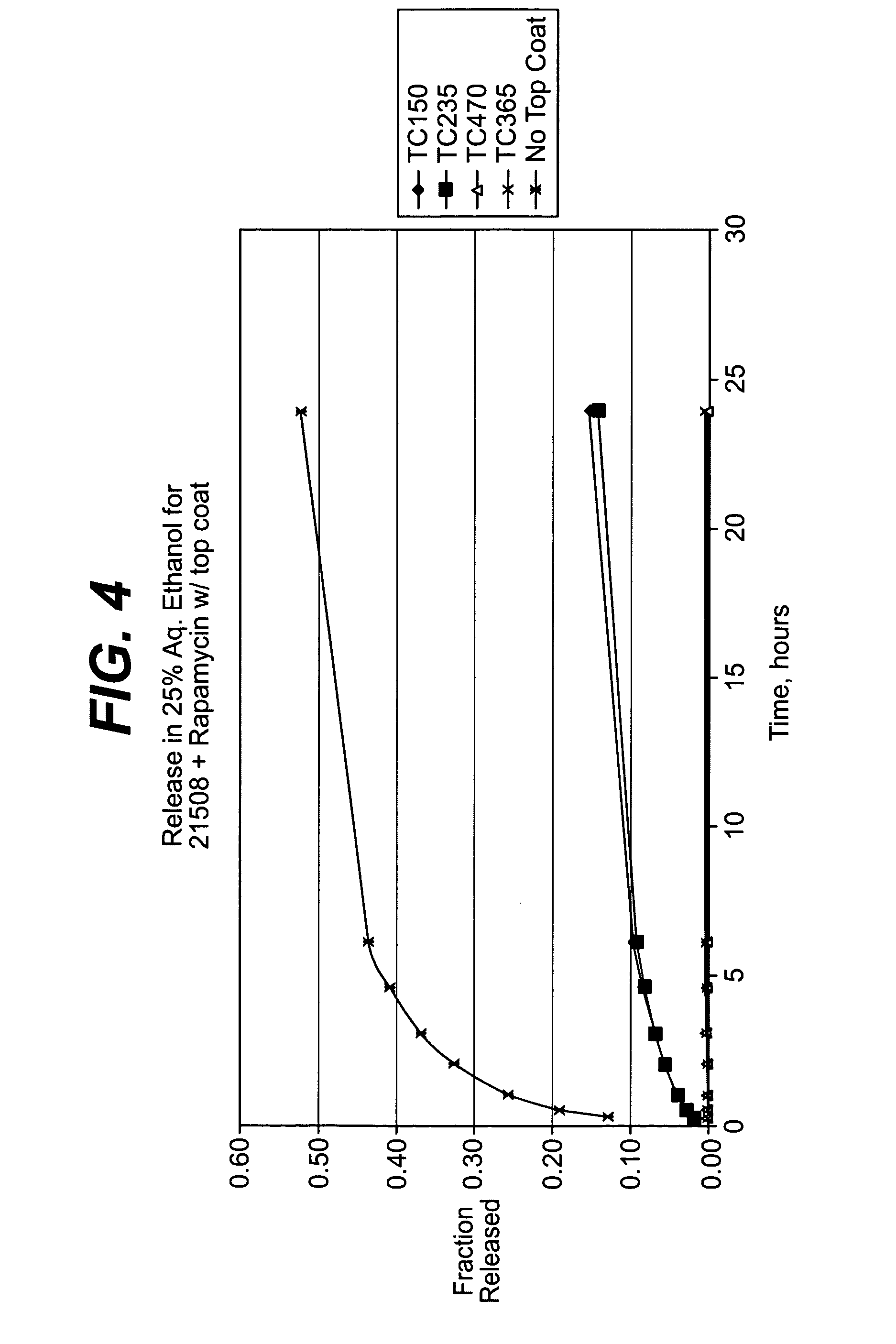
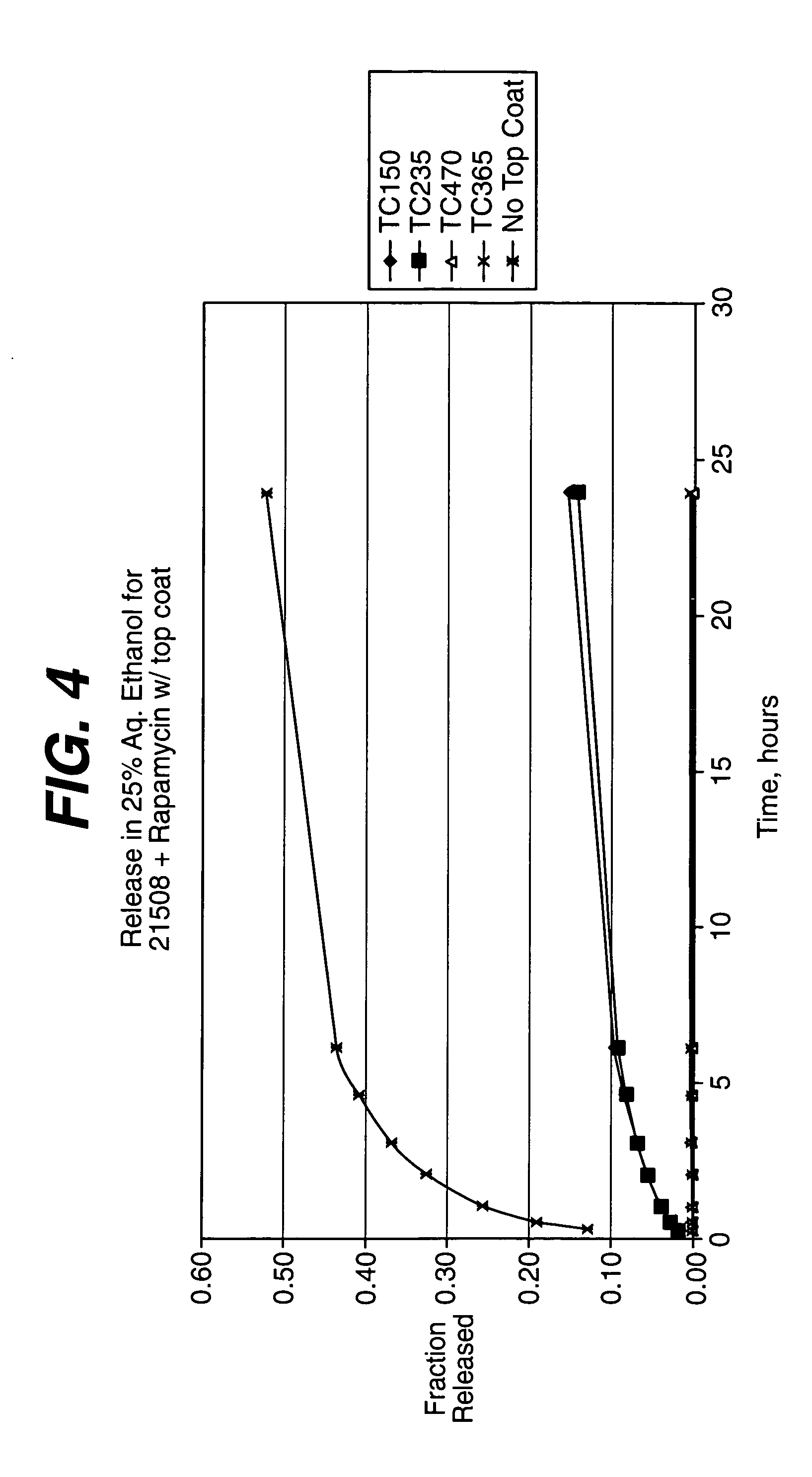
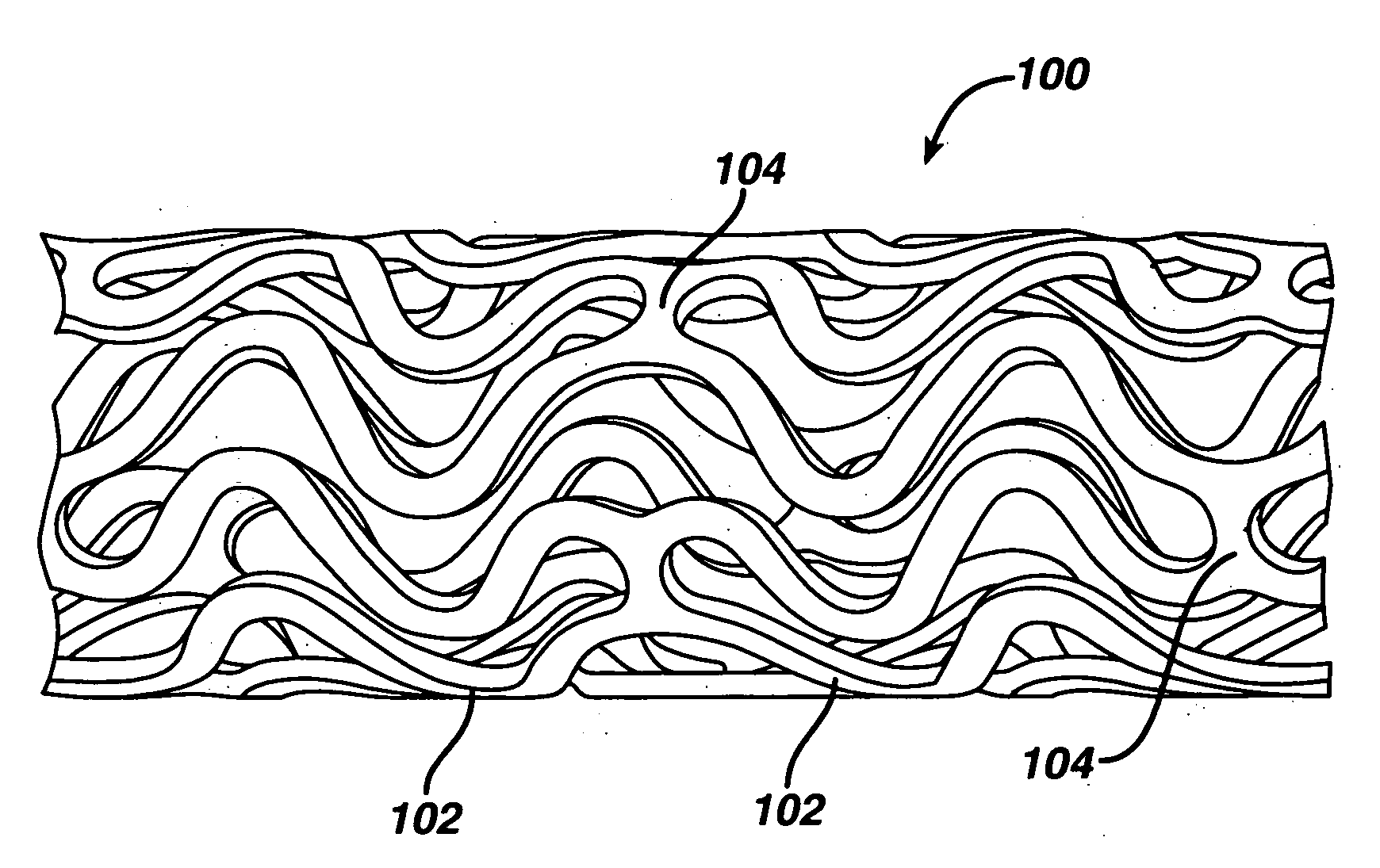
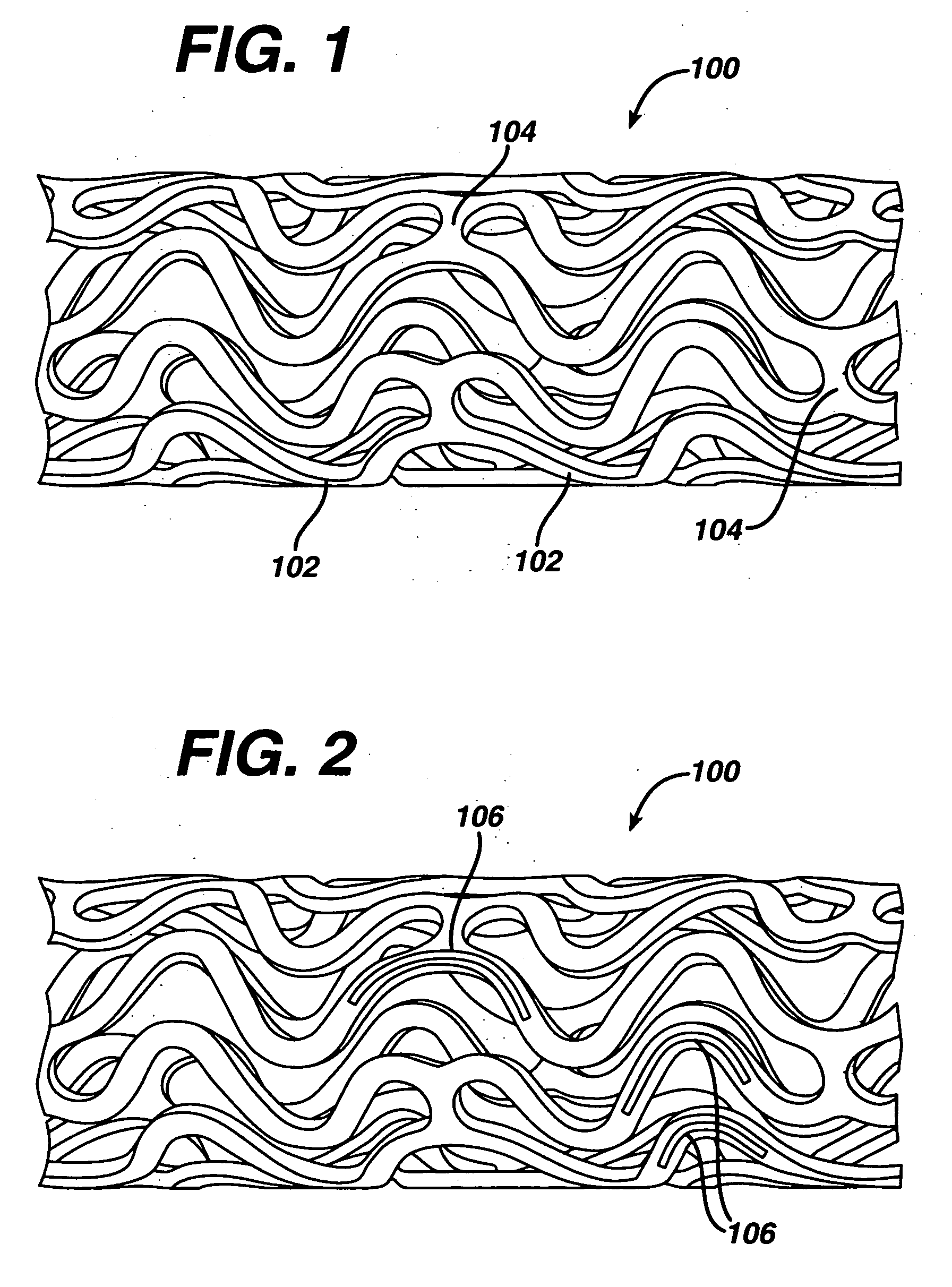
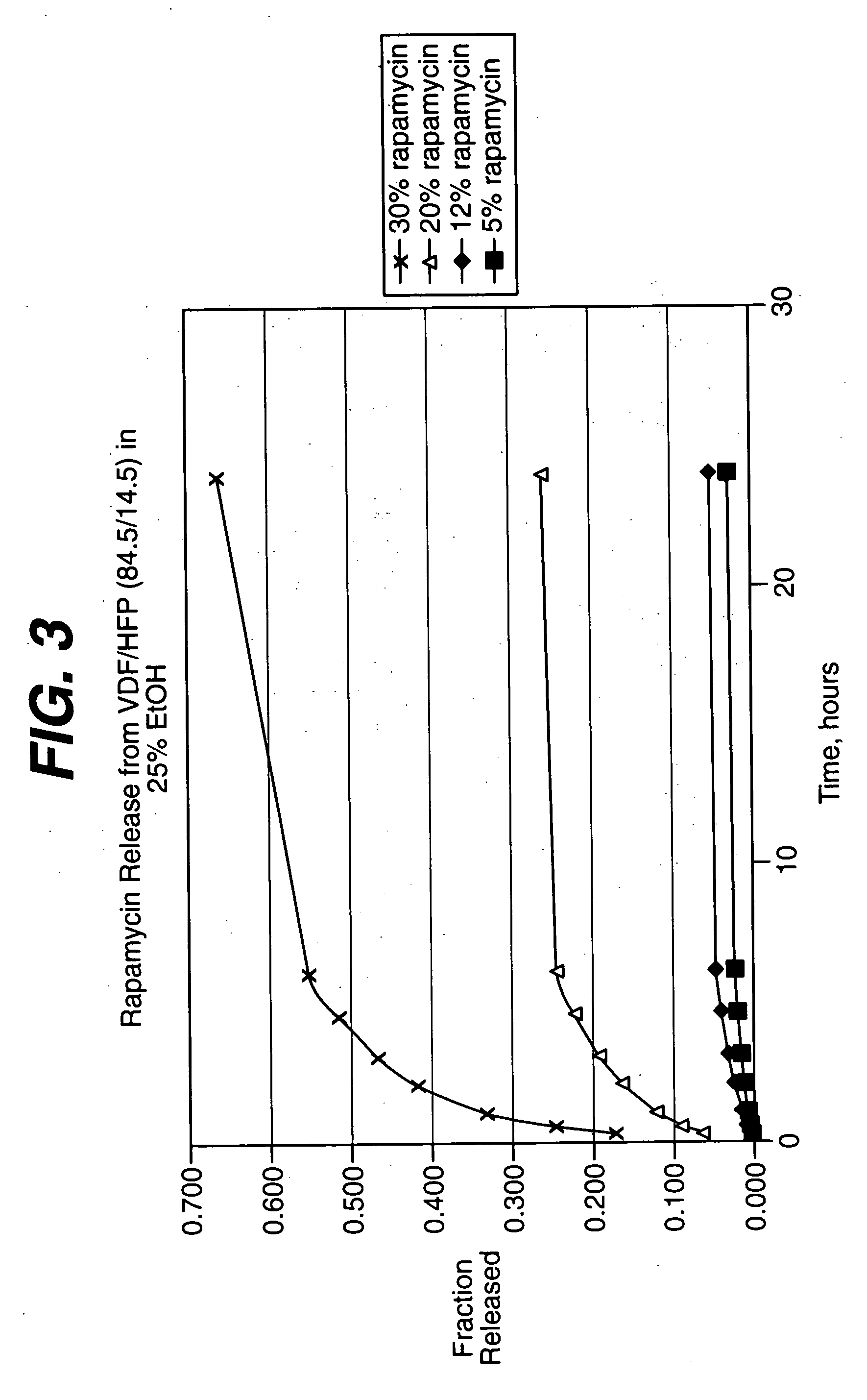
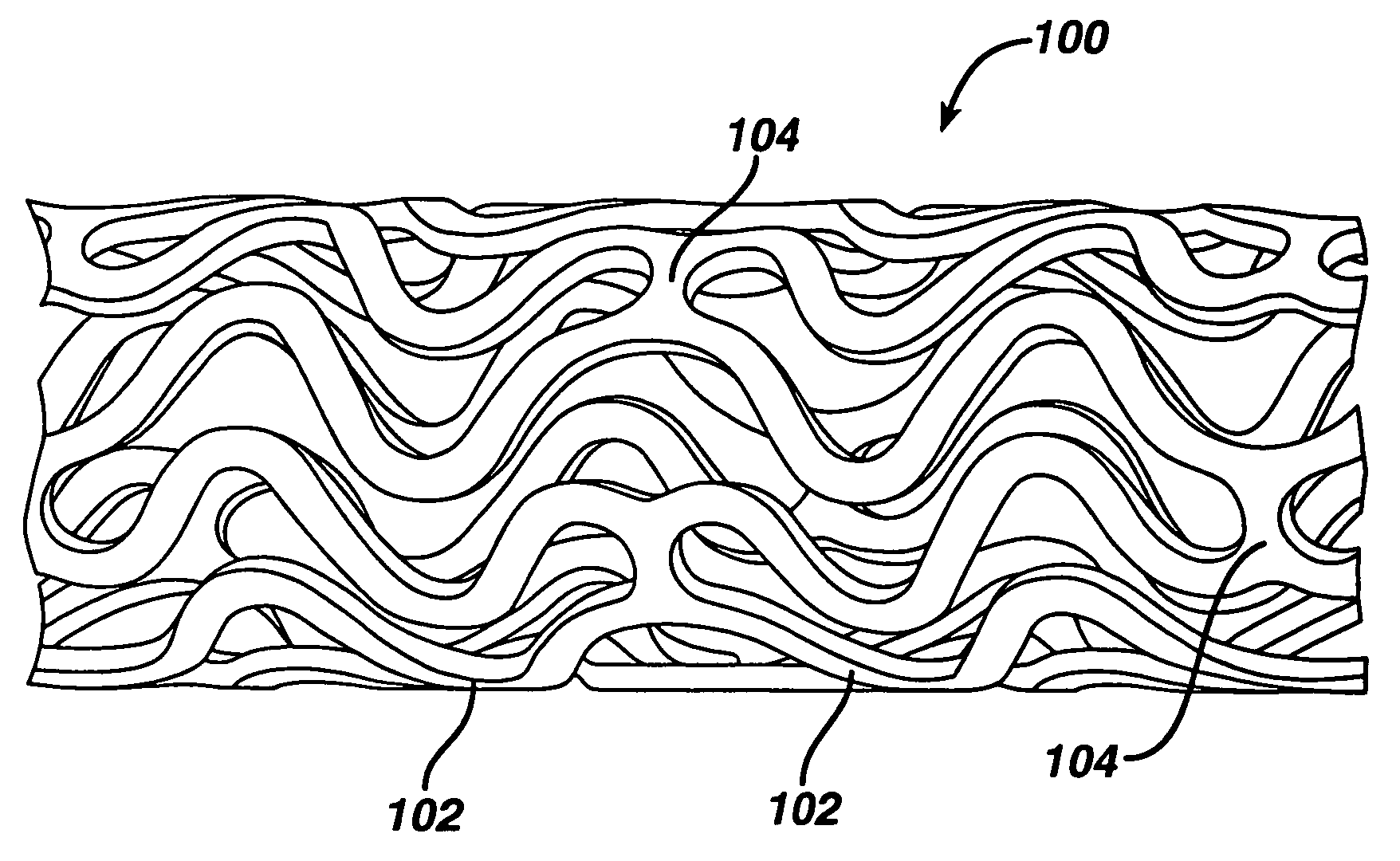
Rapamycin coated expandable devices
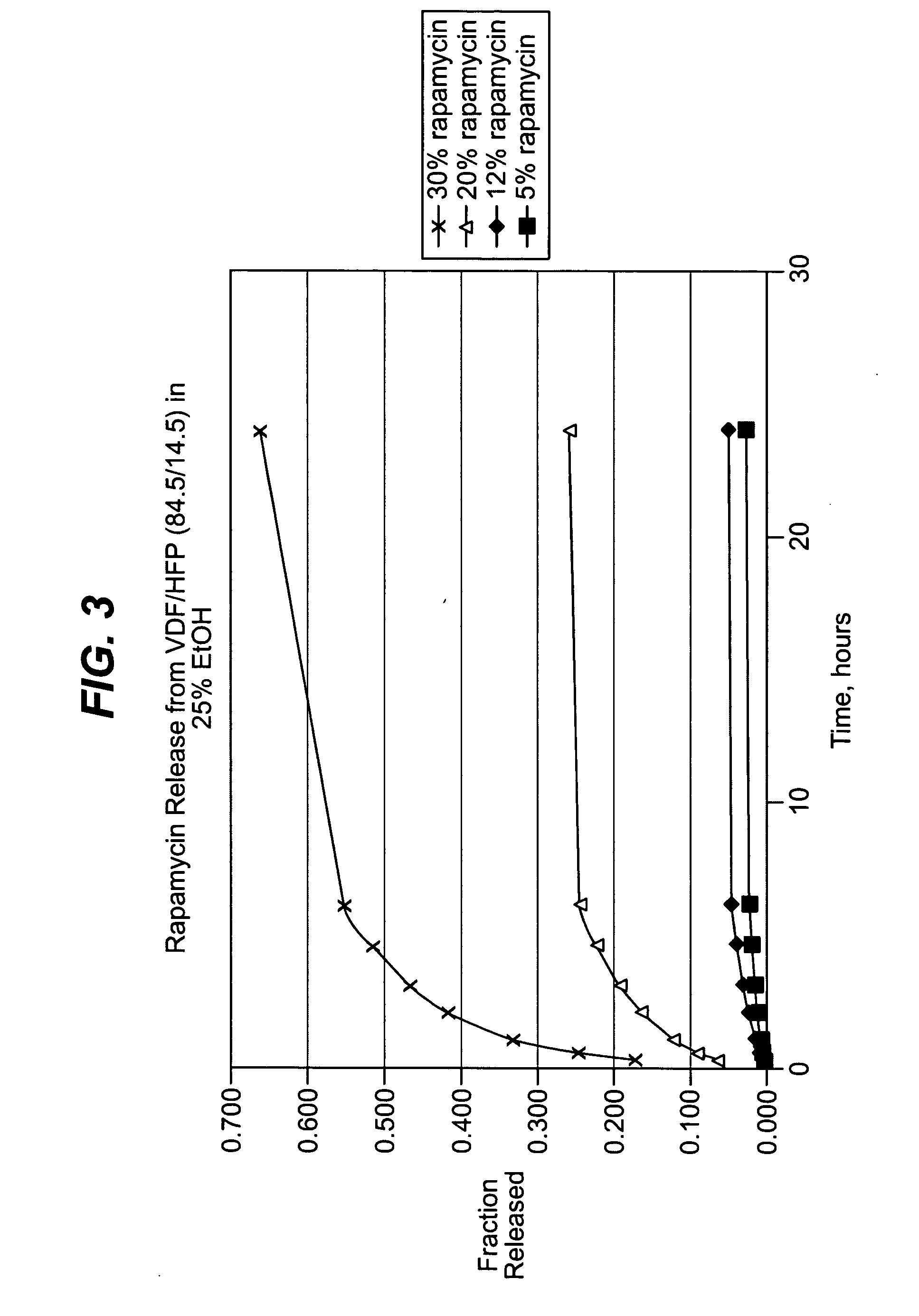
Medical devices may be utilized for local and regional therapeutic agent delivery. These therapeutic agents or compounds may reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including restenosis, vulnerable plaque, and atherosclerosis in type 2 diabetic patients.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com