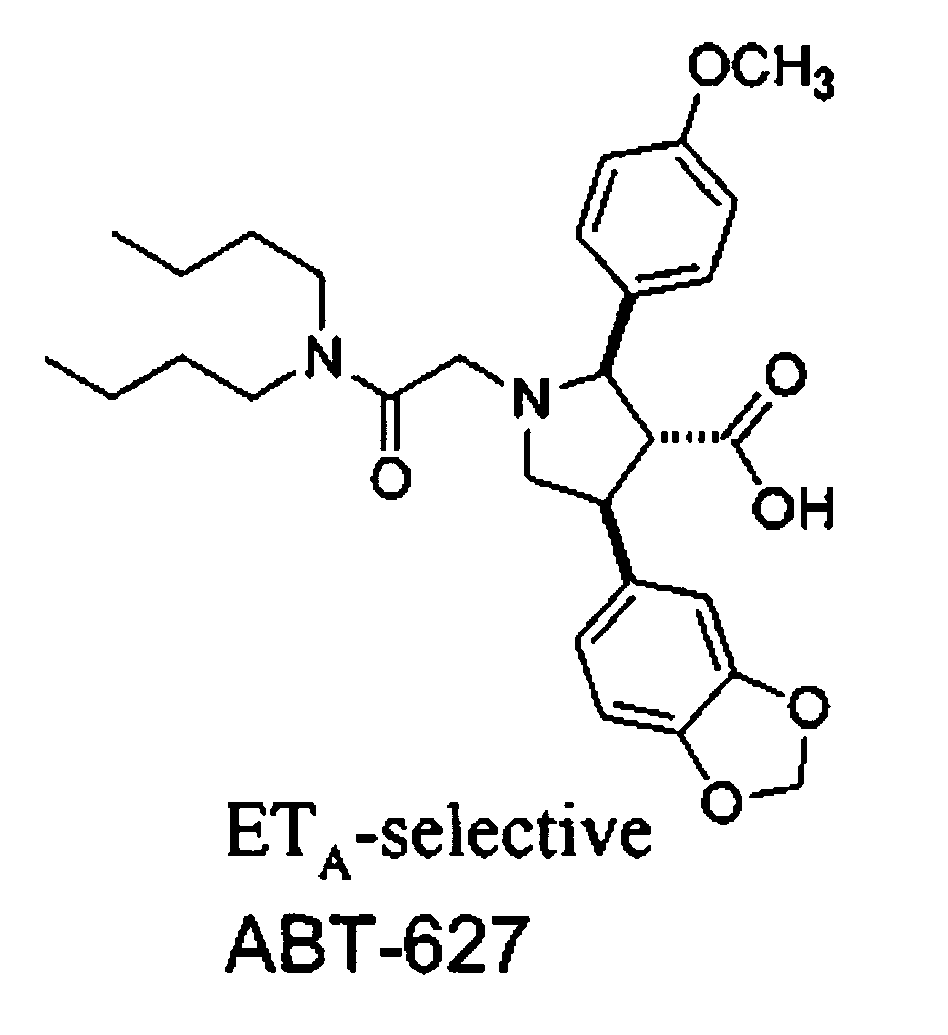
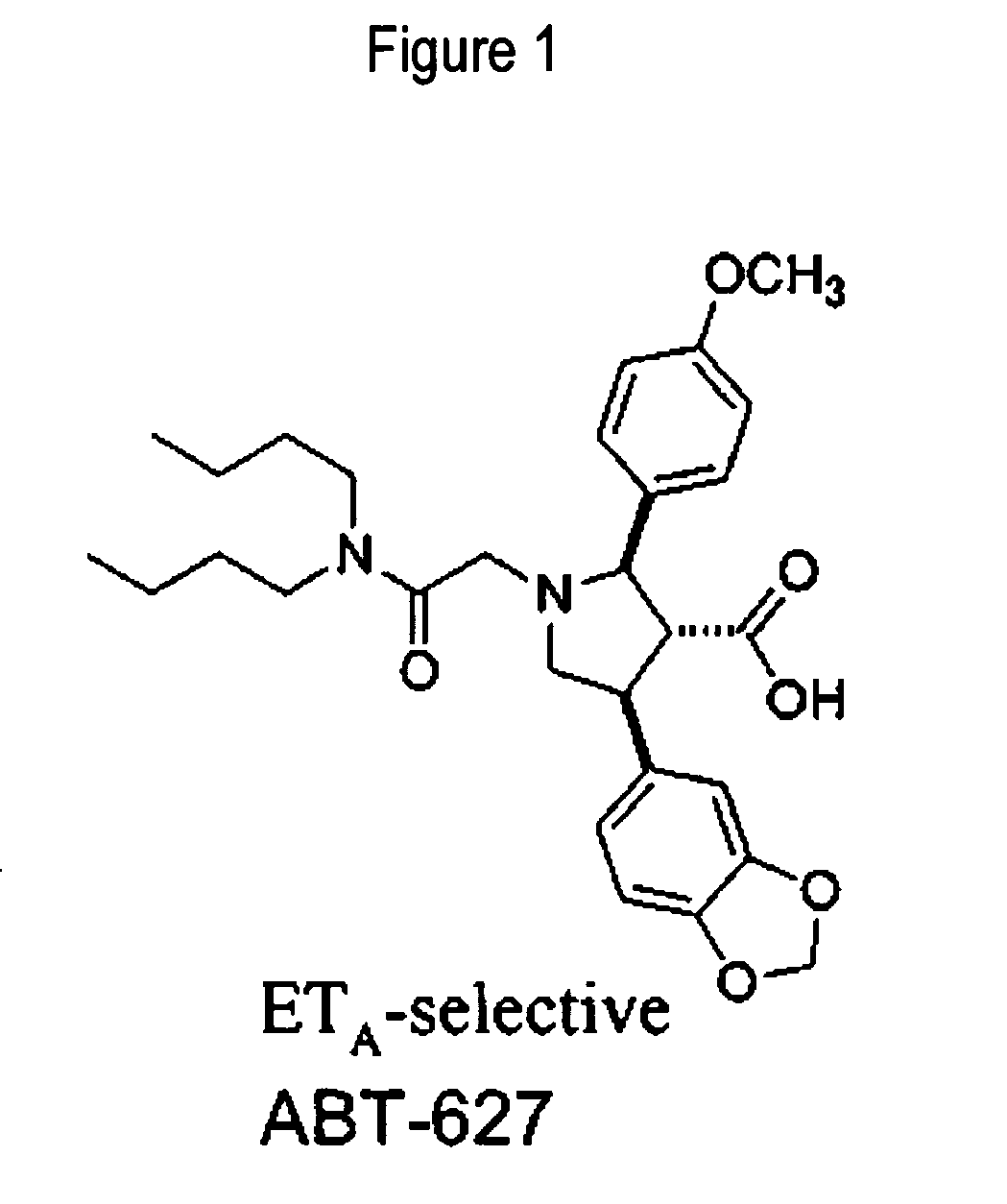
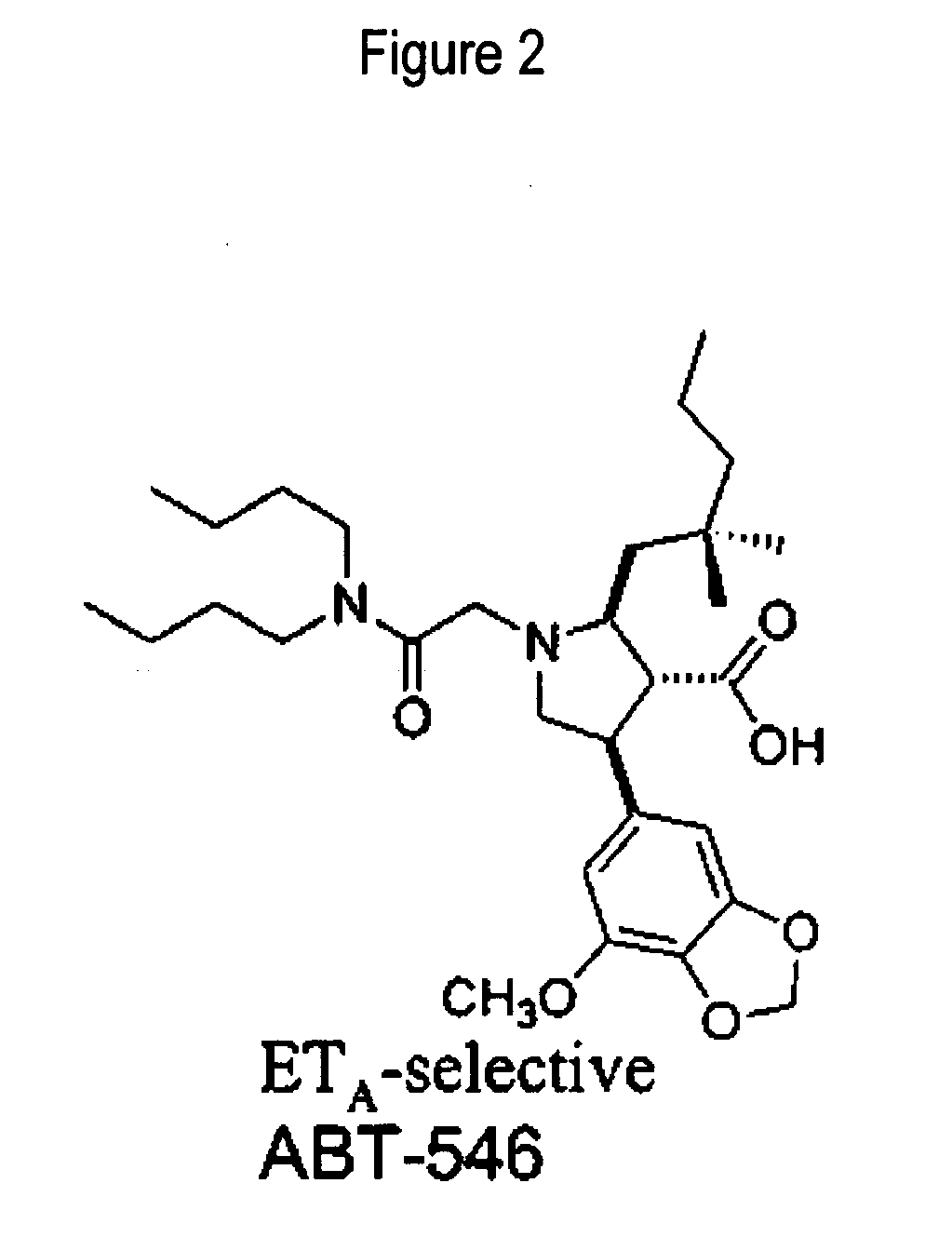
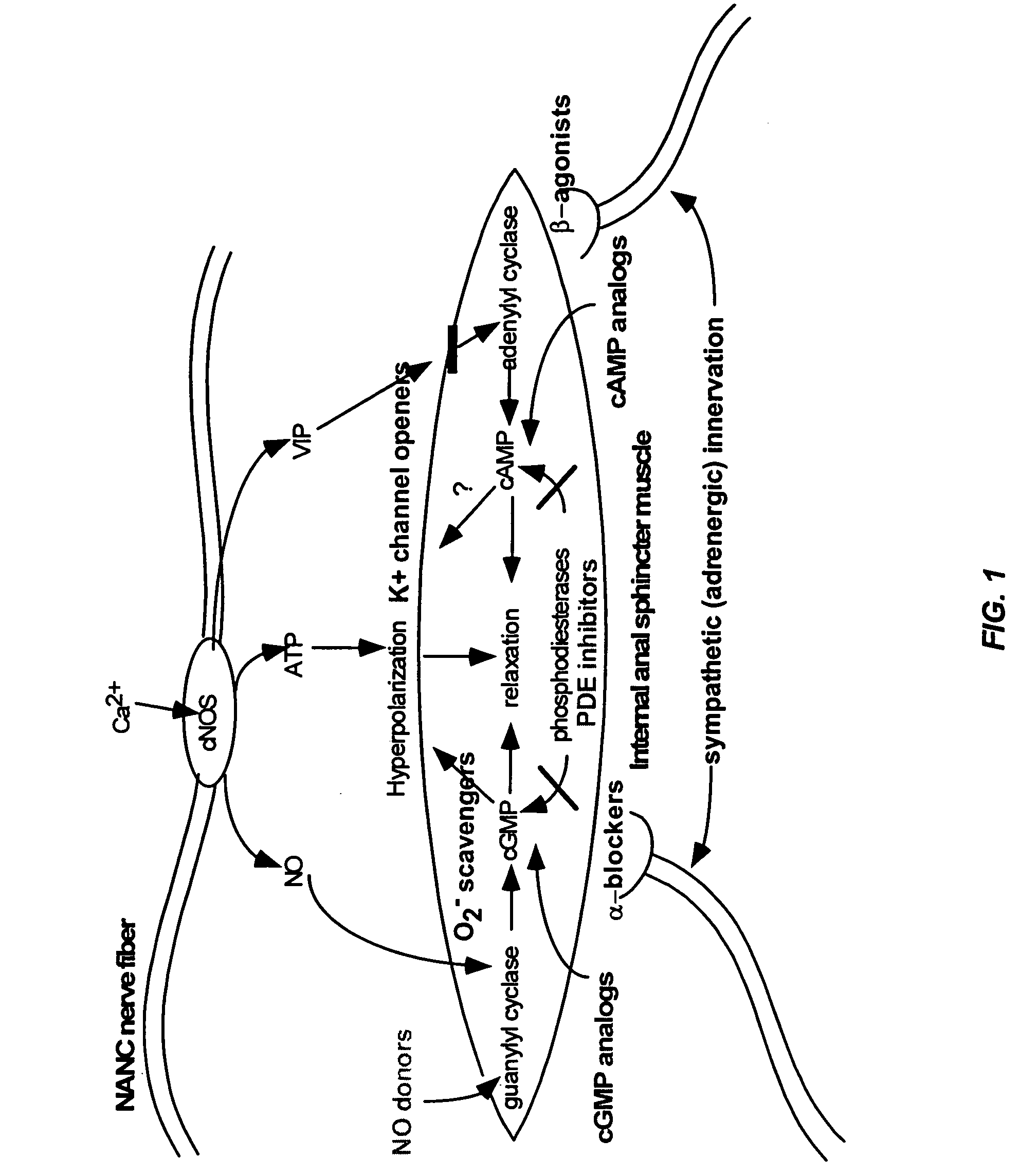
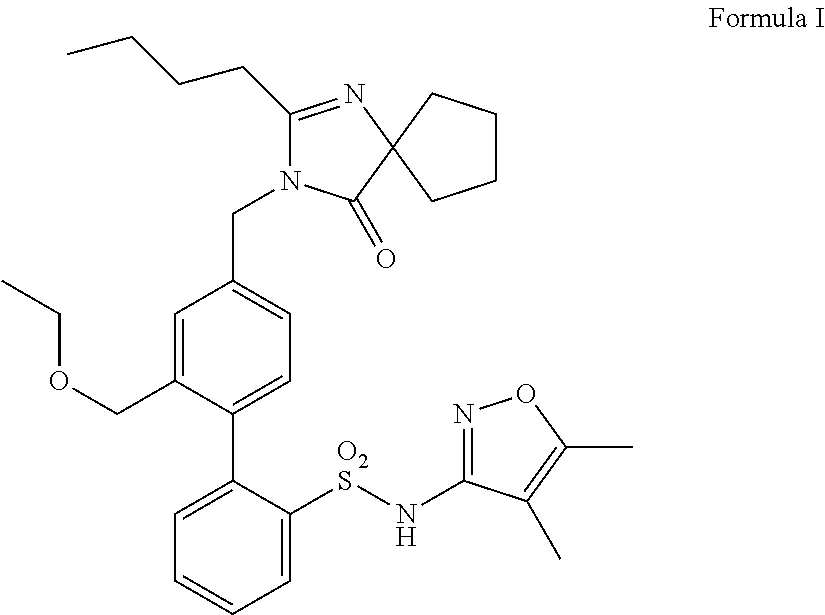
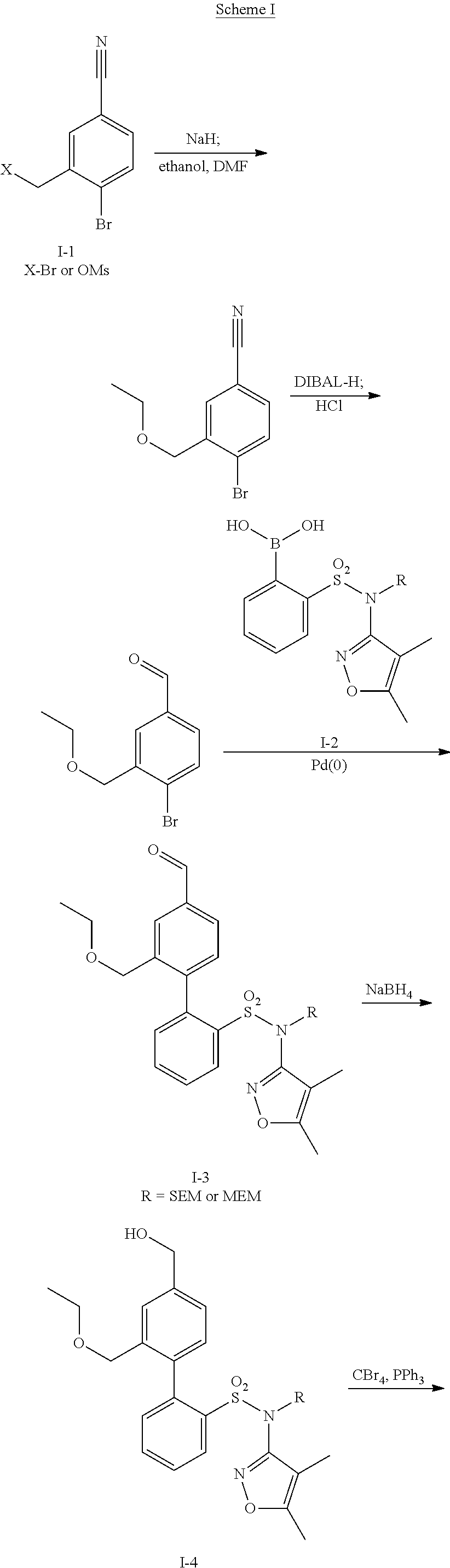
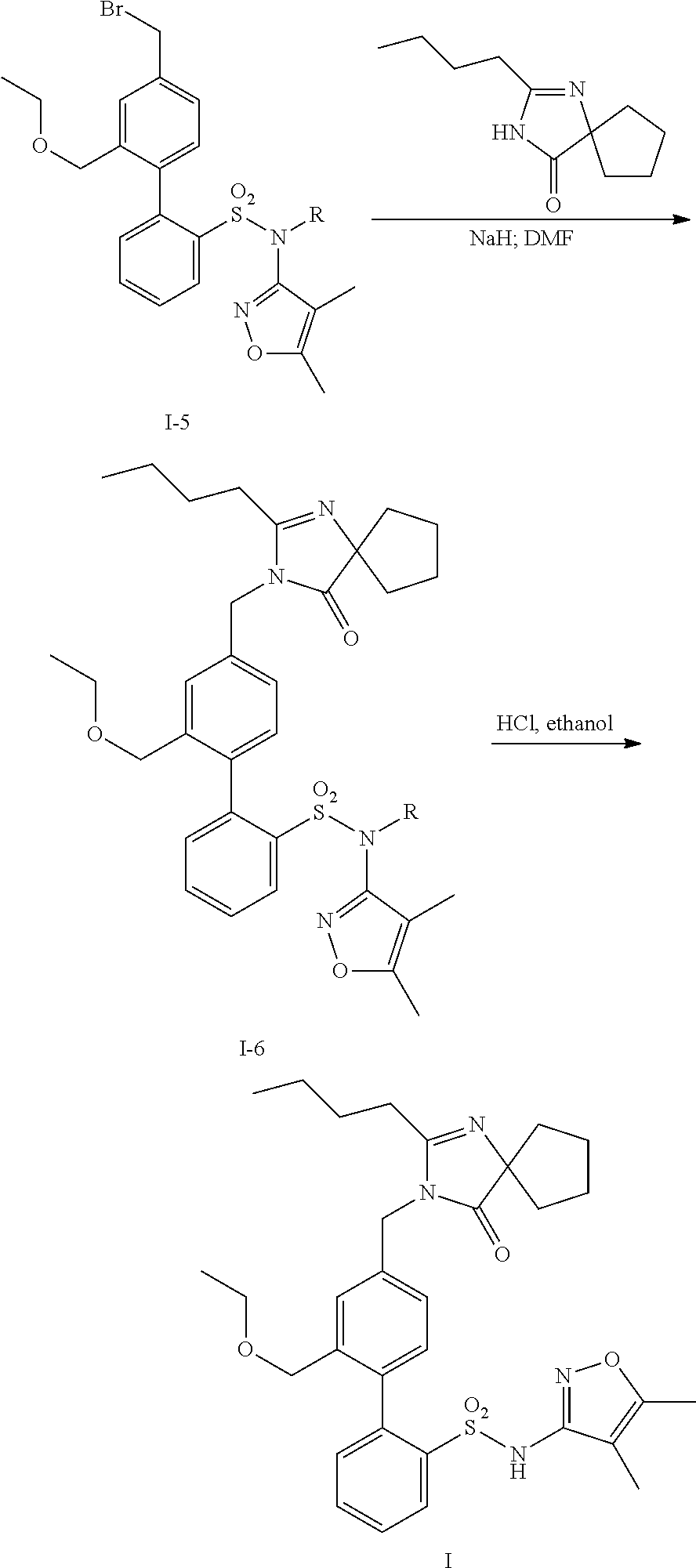
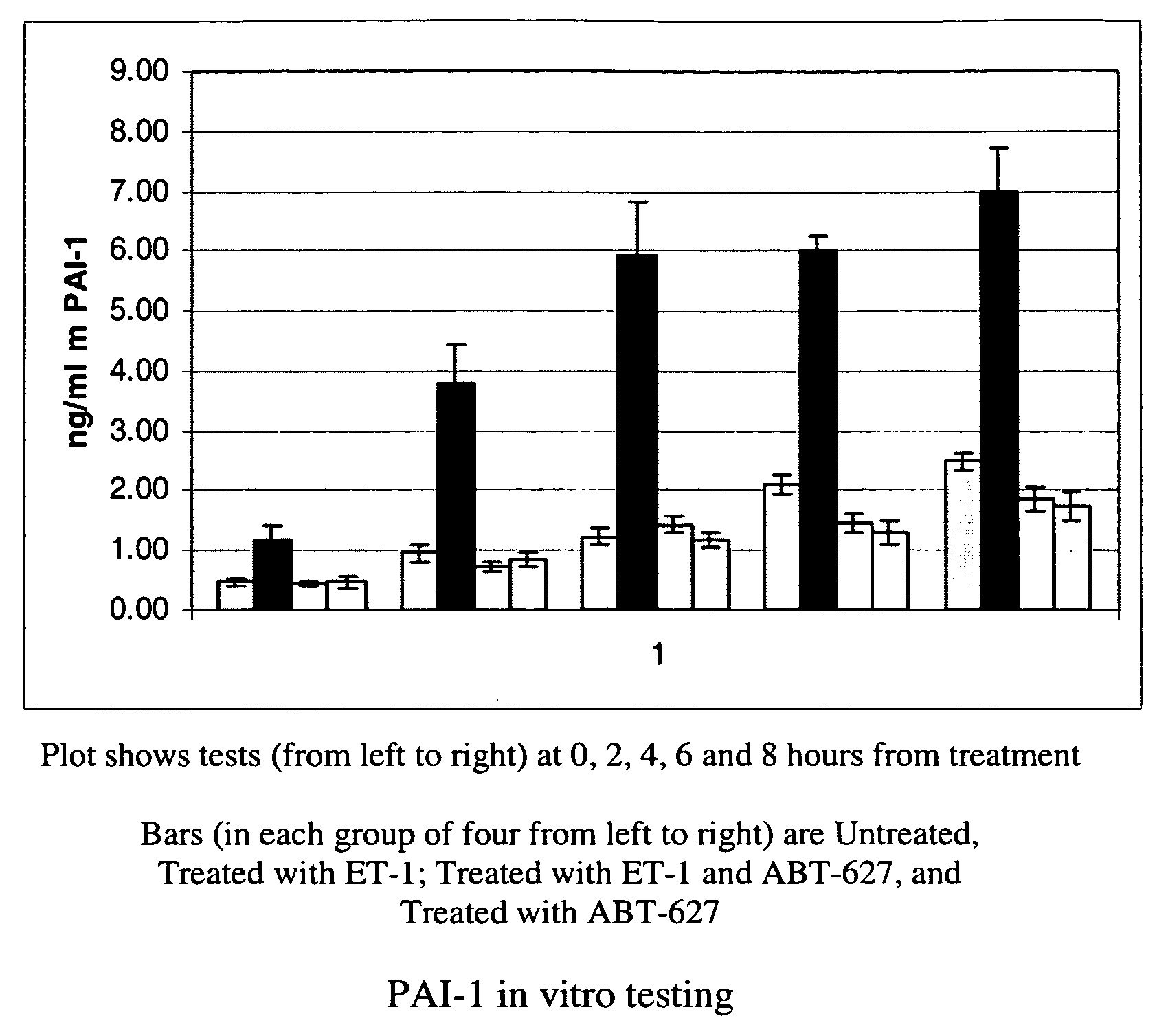
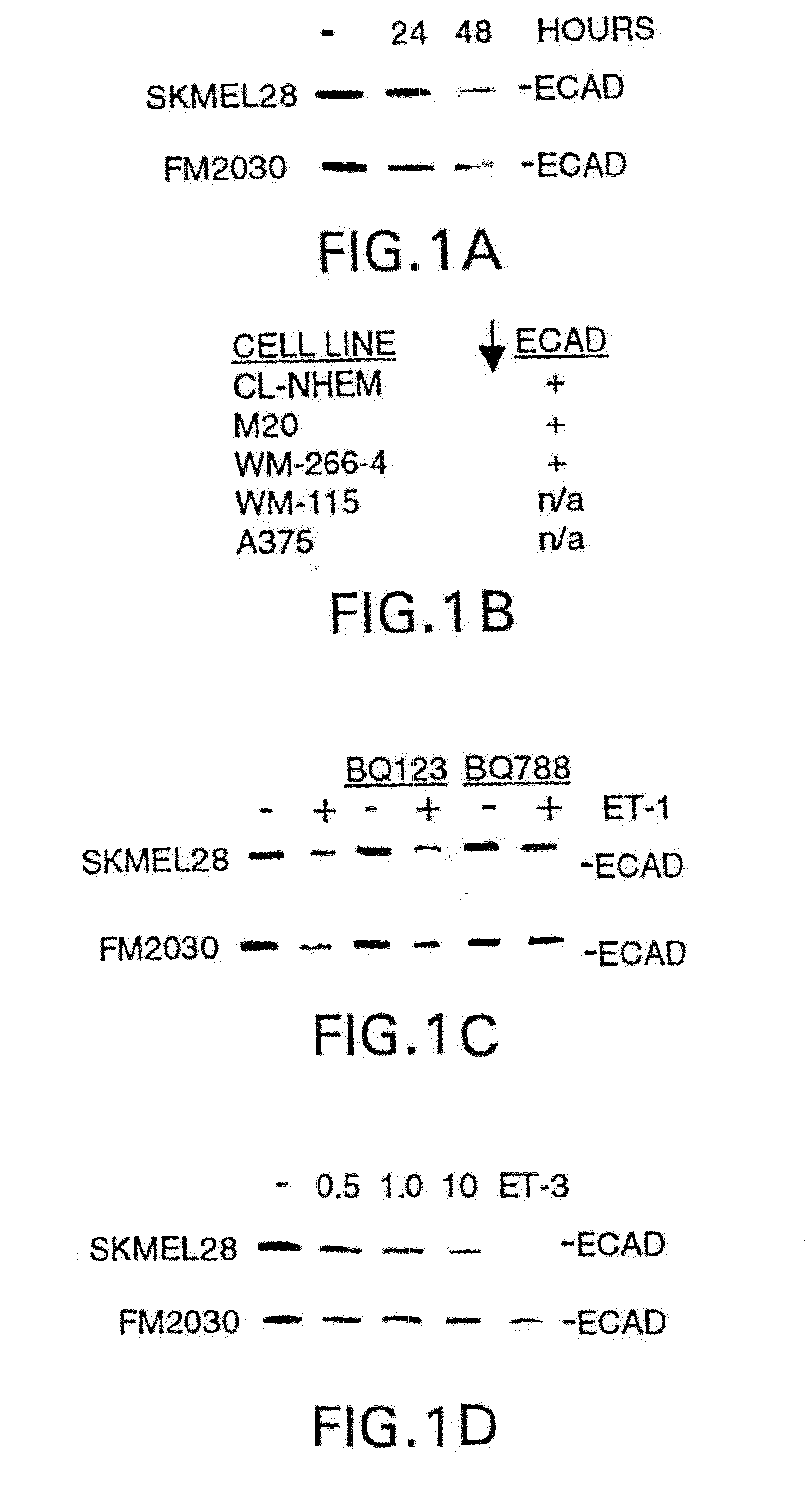
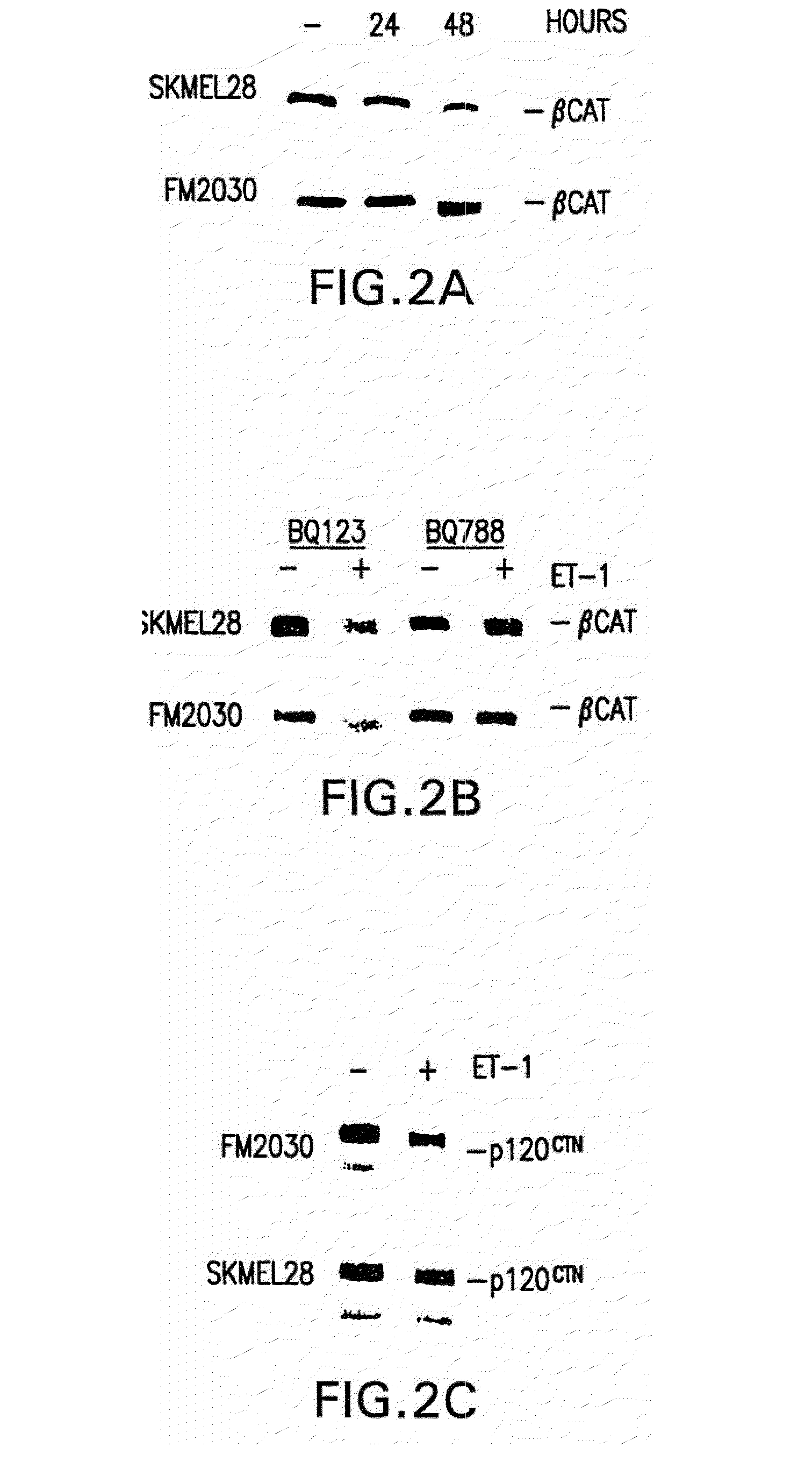
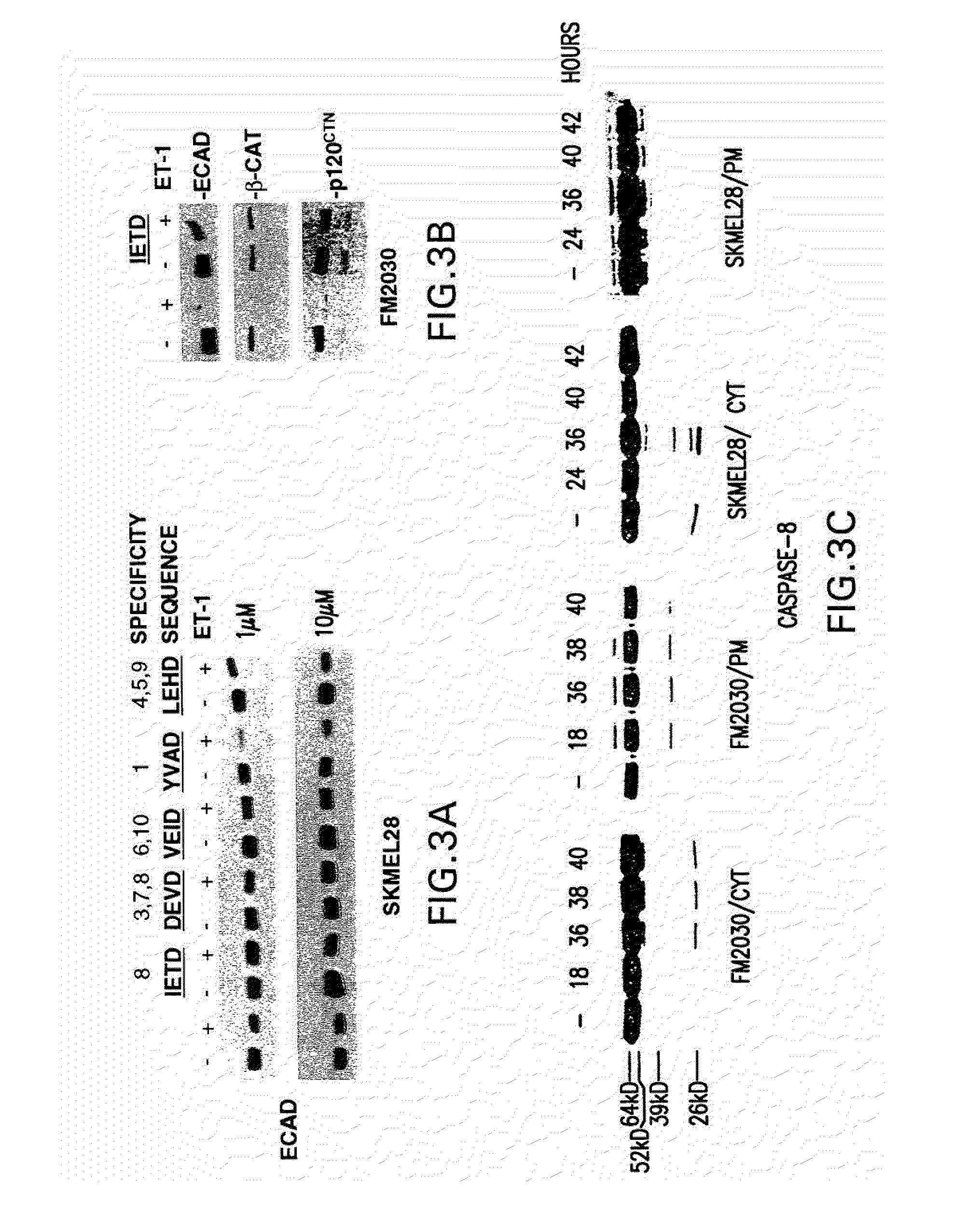
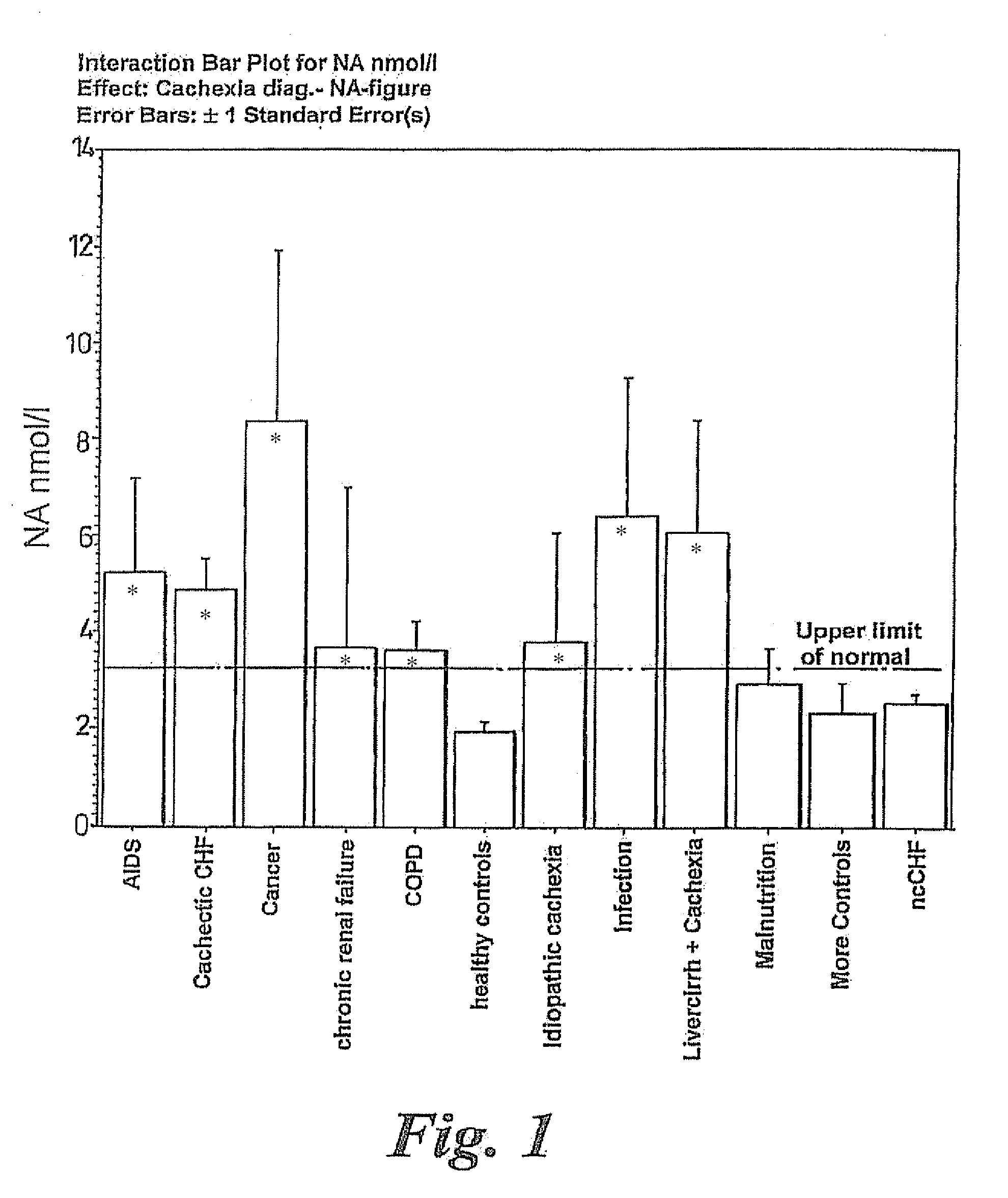
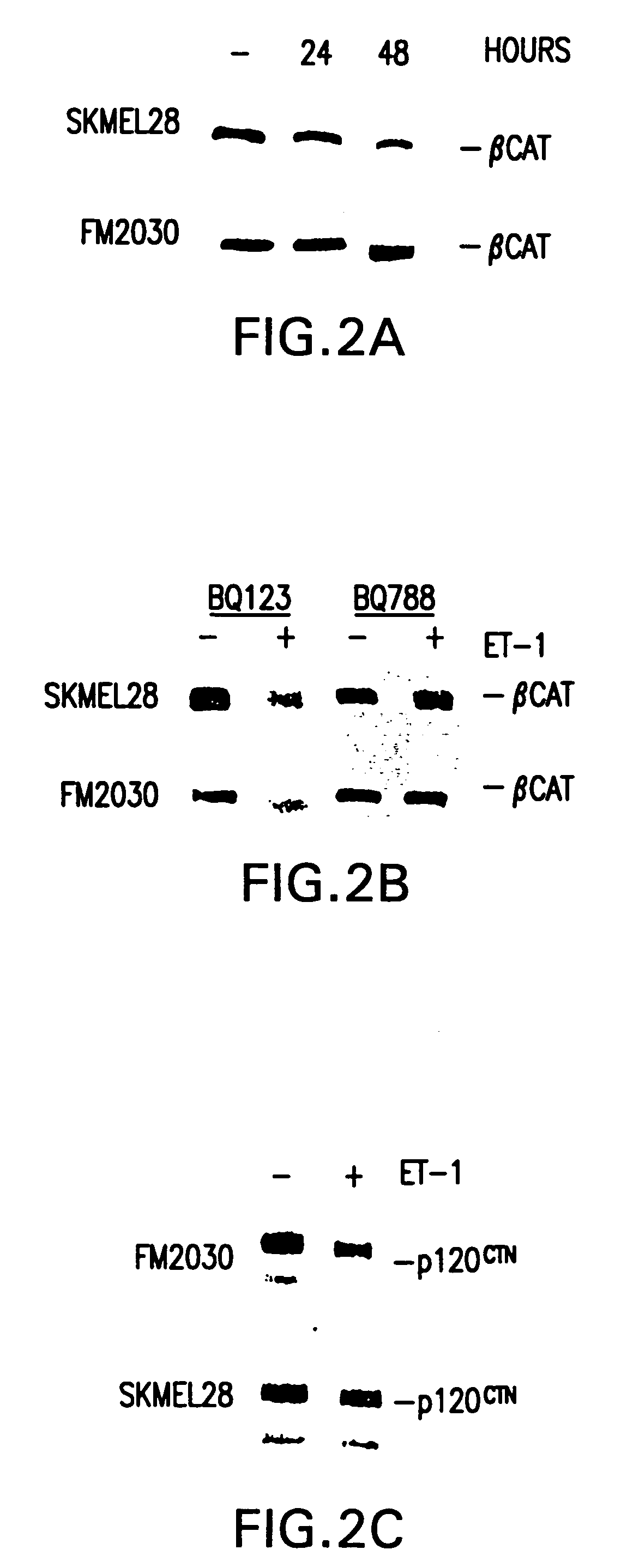
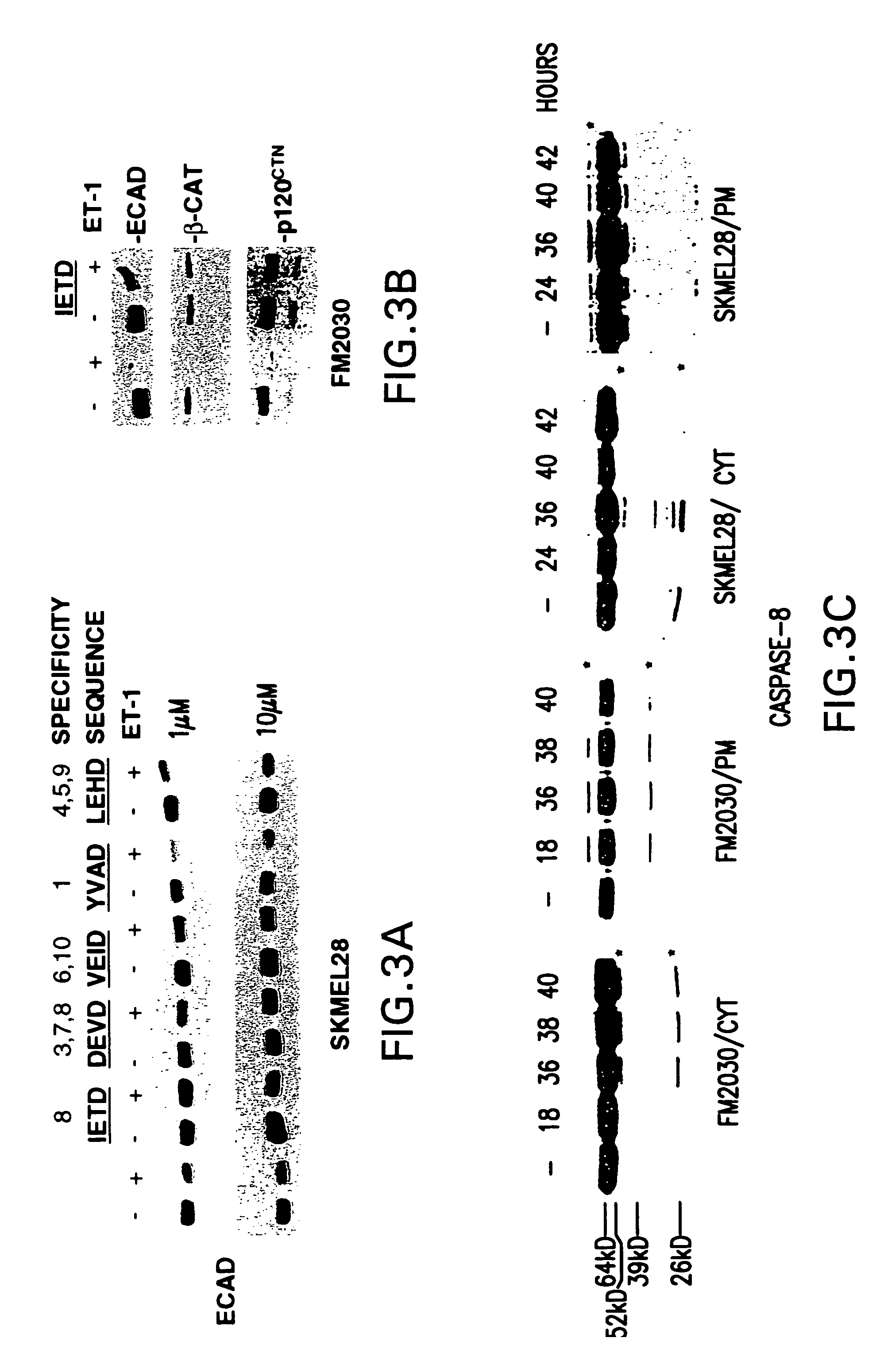
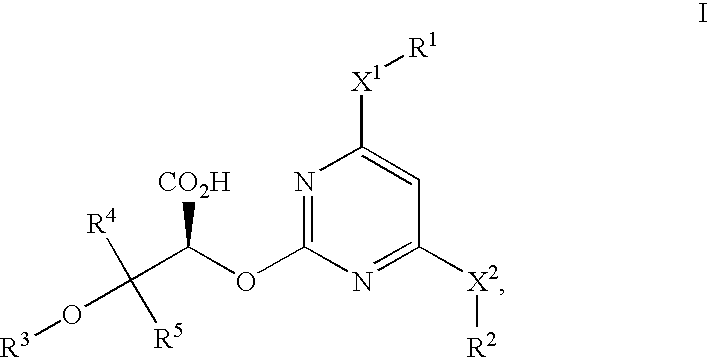
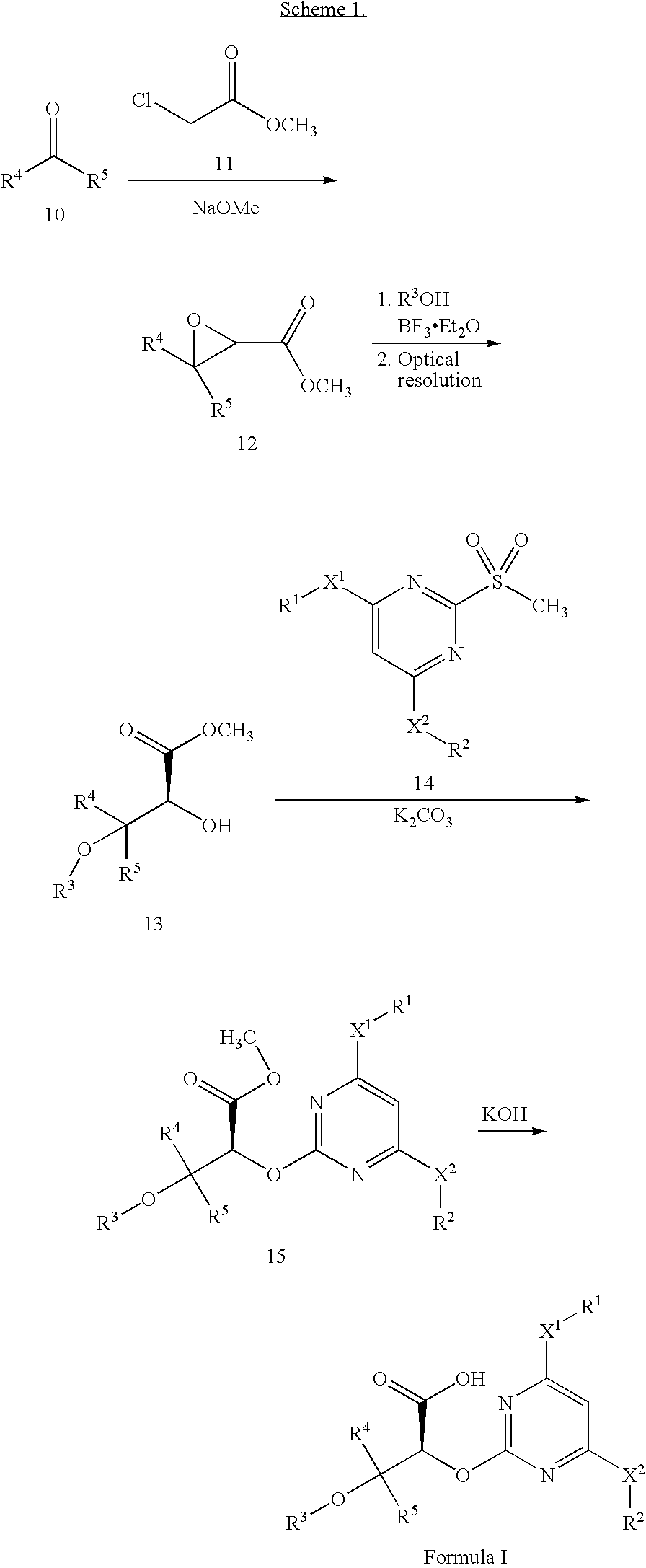
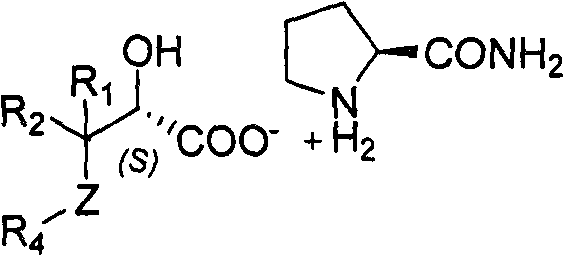Patents
Literature
69 results about "Endothelin receptor antagonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
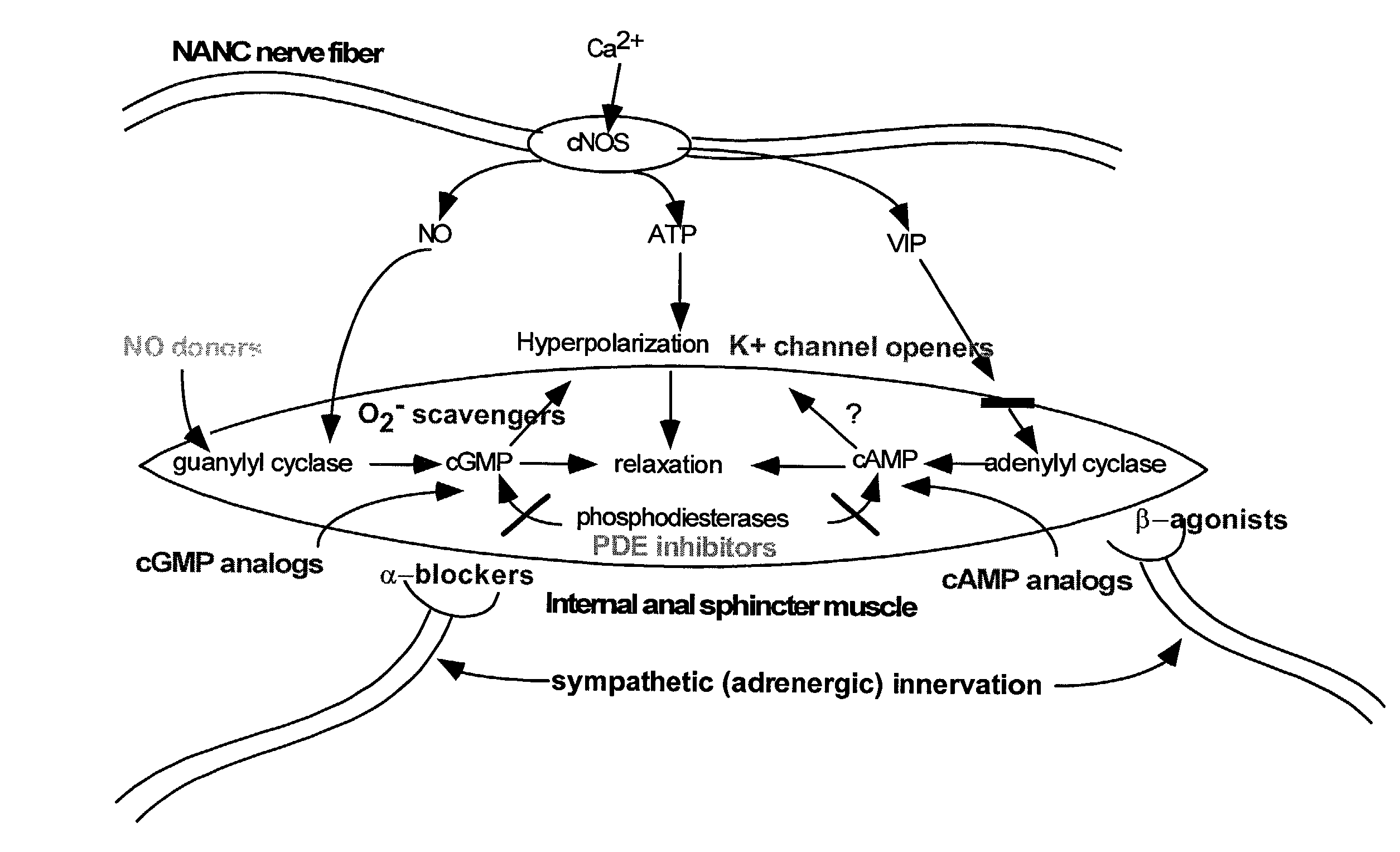
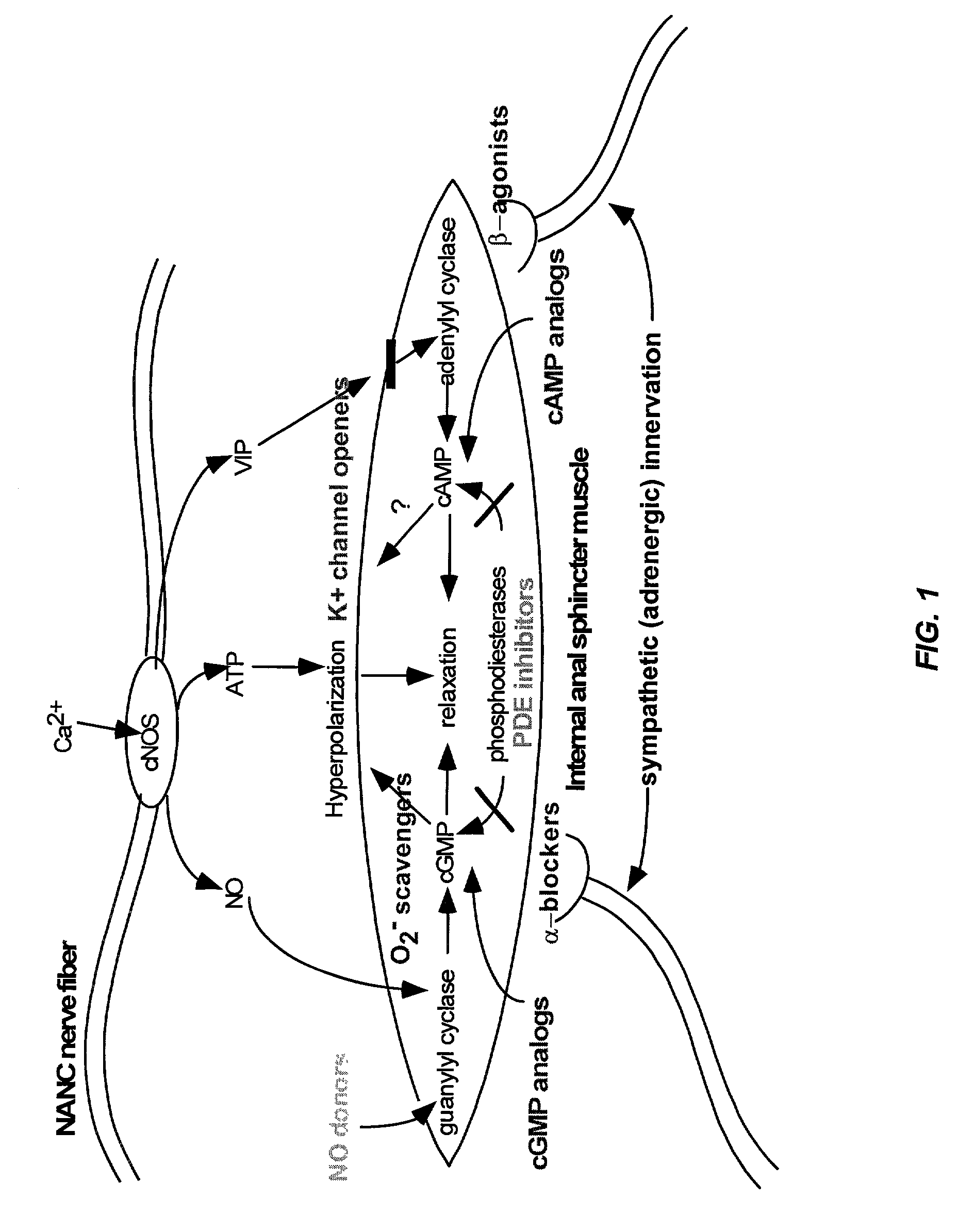
An endothelin receptor antagonist (ERA) is a drug that blocks endothelin receptors.
Compounds and methods for the treatment of urogenital disorders
InactiveUS6987129B2Reduce painLess discomfortBiocidePeptide/protein ingredientsDiseaseFemale Sexual Arousal Disorder
The present invention provides methods for treating a variety of urogenital disorders, such as, for example, vaginismus, dyspareunia, vulvodynia (including vulvar vestibulitis), interstitial cystitis, nonspecific urethriris (i.e., nonspecific pain and / or burning of the urinary tract) and sexual dysfunctions, such as, for example, female sexual arousal disorders and female sexual orgasmic disorders, using a variety of compounds, including, but not limited to, NO donors, calcium channel blockers, cholinergic modulators, α-adrenergic receptor antagonists, β-adrenergic receptor agonists, phosphodiesterase inhibitors, cAMP-dependent protein kinase activators (e.g., cAMP mimetics), superoxide scavengers, potassium channel activators, estrogen-like compounds, testosterone-like compounds, benzodiazepines, adrenergic nerve inhibitors, antidiarrheal agents, HMG-CoA reductase inhibitors, smooth muscle relaxants, adenosine receptor modulators, adenylyl cyclase activators, endothelin receptor antagonists, bisphosphonates and cGMP-dependent protein kinase activators (e.g., cGMP mimetics).
Owner:STREHKEHN INT LTD
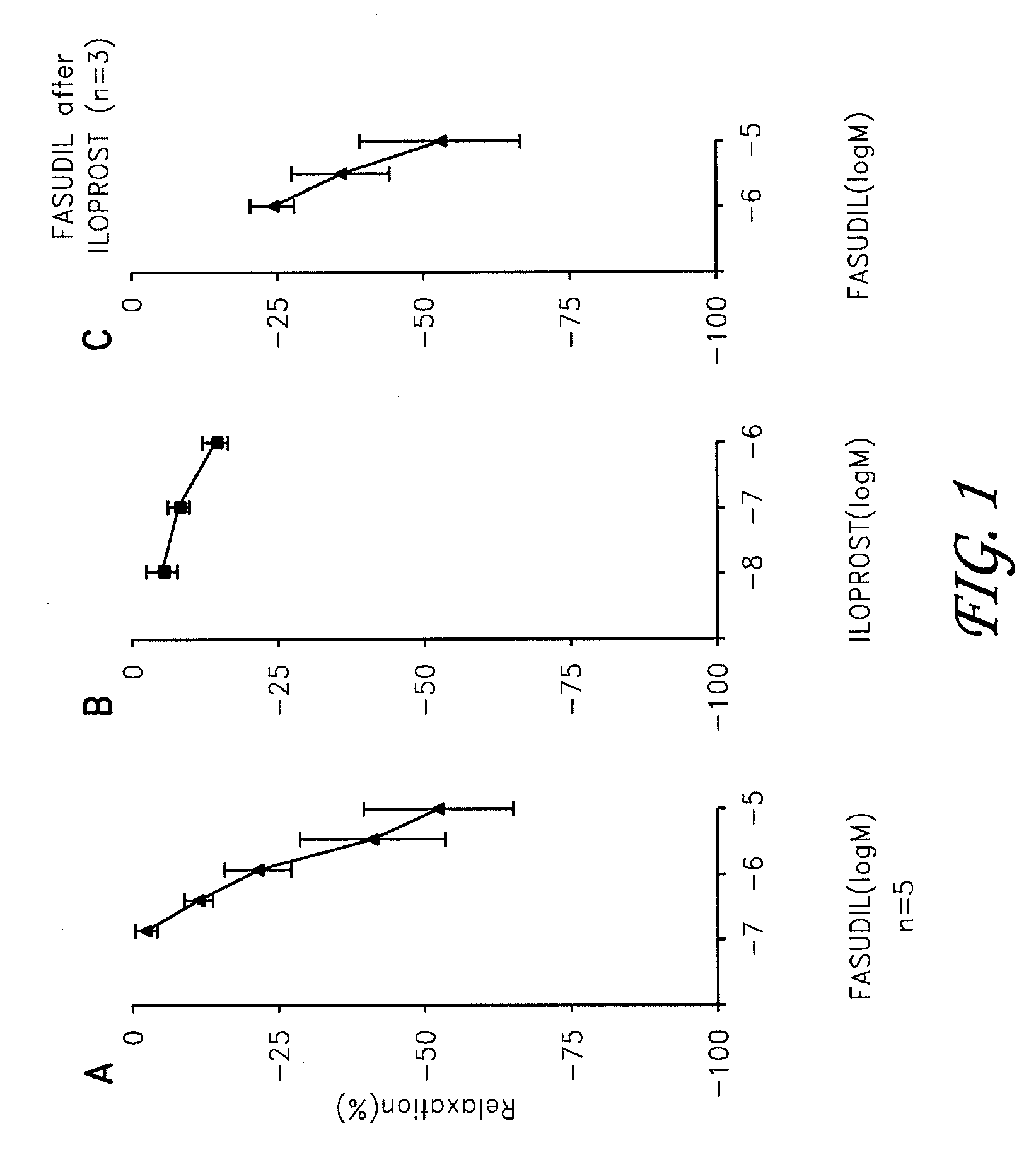
Iloprost in combination therapies for the treatment of pulmonary arterial hypertension
InactiveUS20050101608A1BiocideElcosanoid active ingredientsEndothelin receptor antagonistPDE Inhibitor
Preferred embodiments of the present invention are related to novel therapeutic drug combinations and methods for treating pulmonary arterial hypertension. More particularly, aspects of the present invention are related to using a combination of iloprost and at least one additional agent, selected from the group consisting of an endothelin receptor antagonist and a PDE inhibitor.
Owner:COTHERIX INC
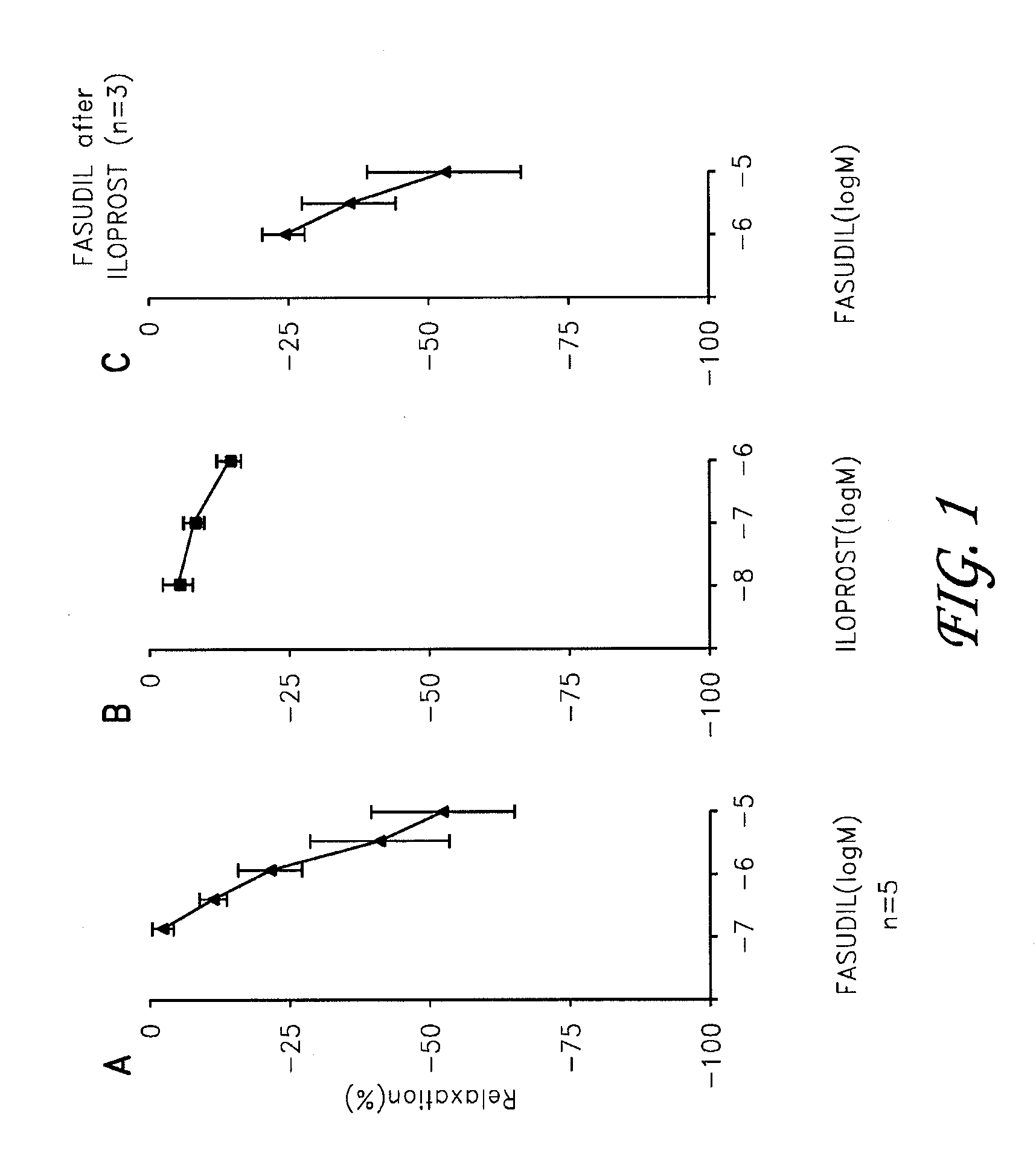
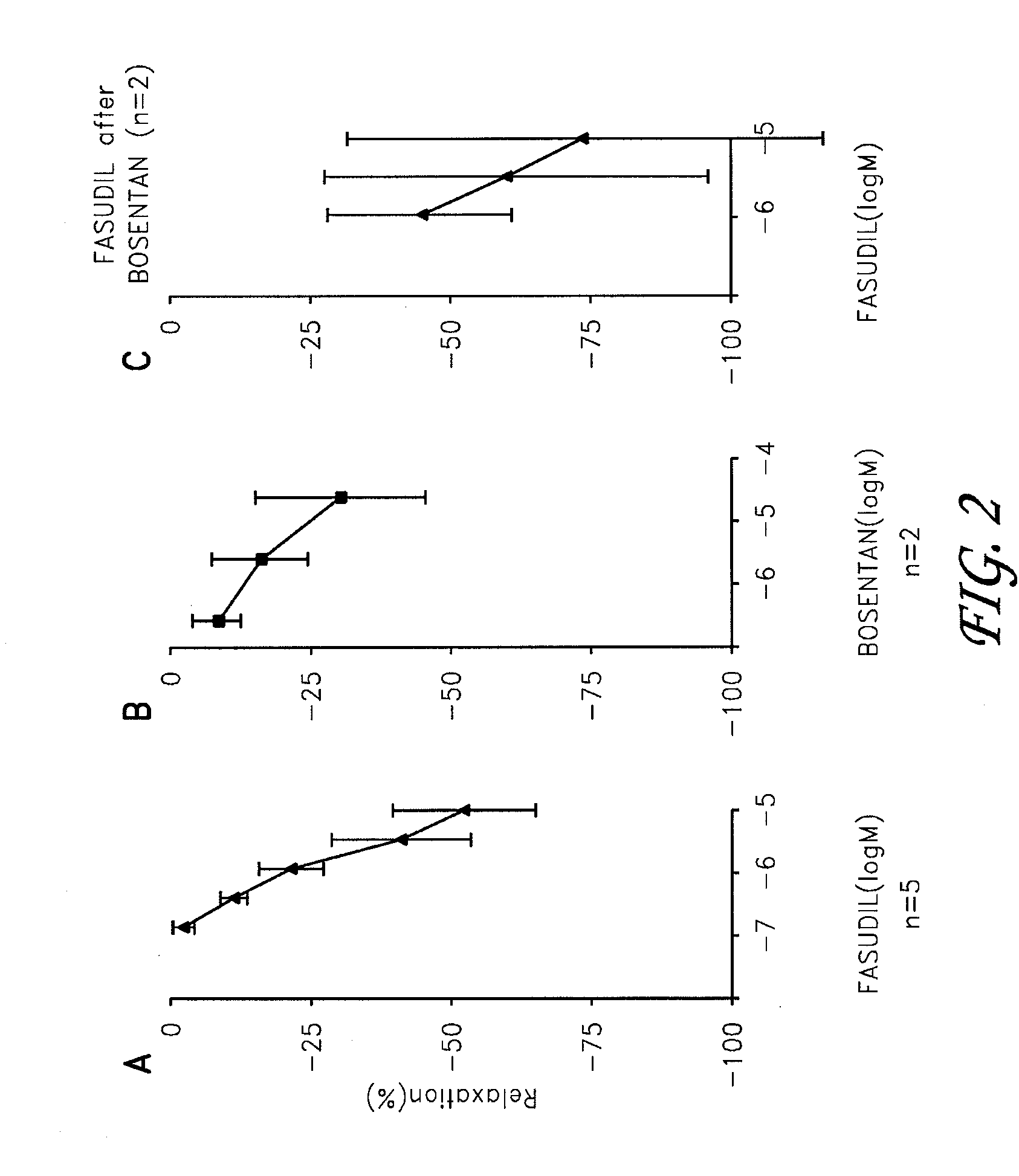
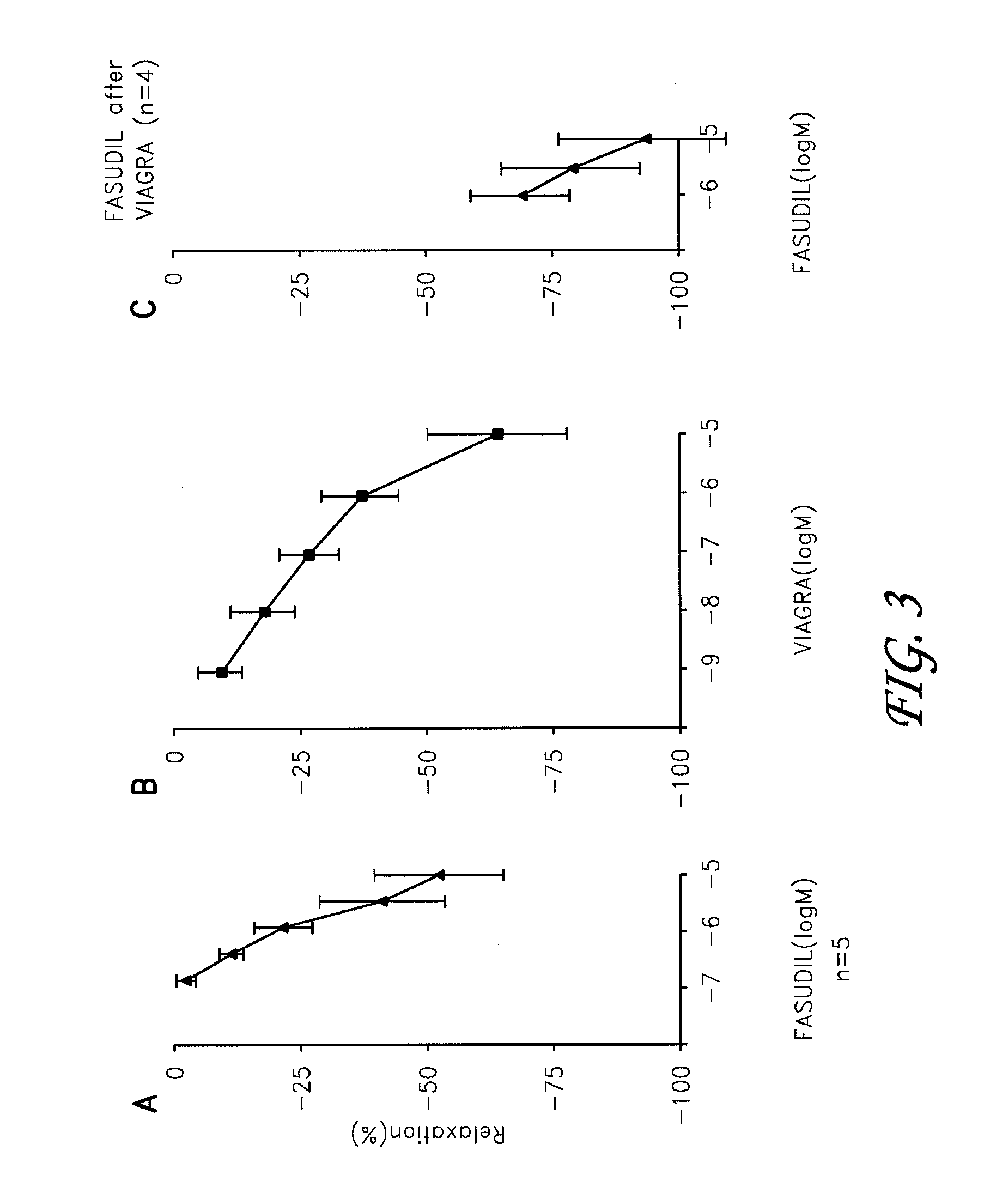
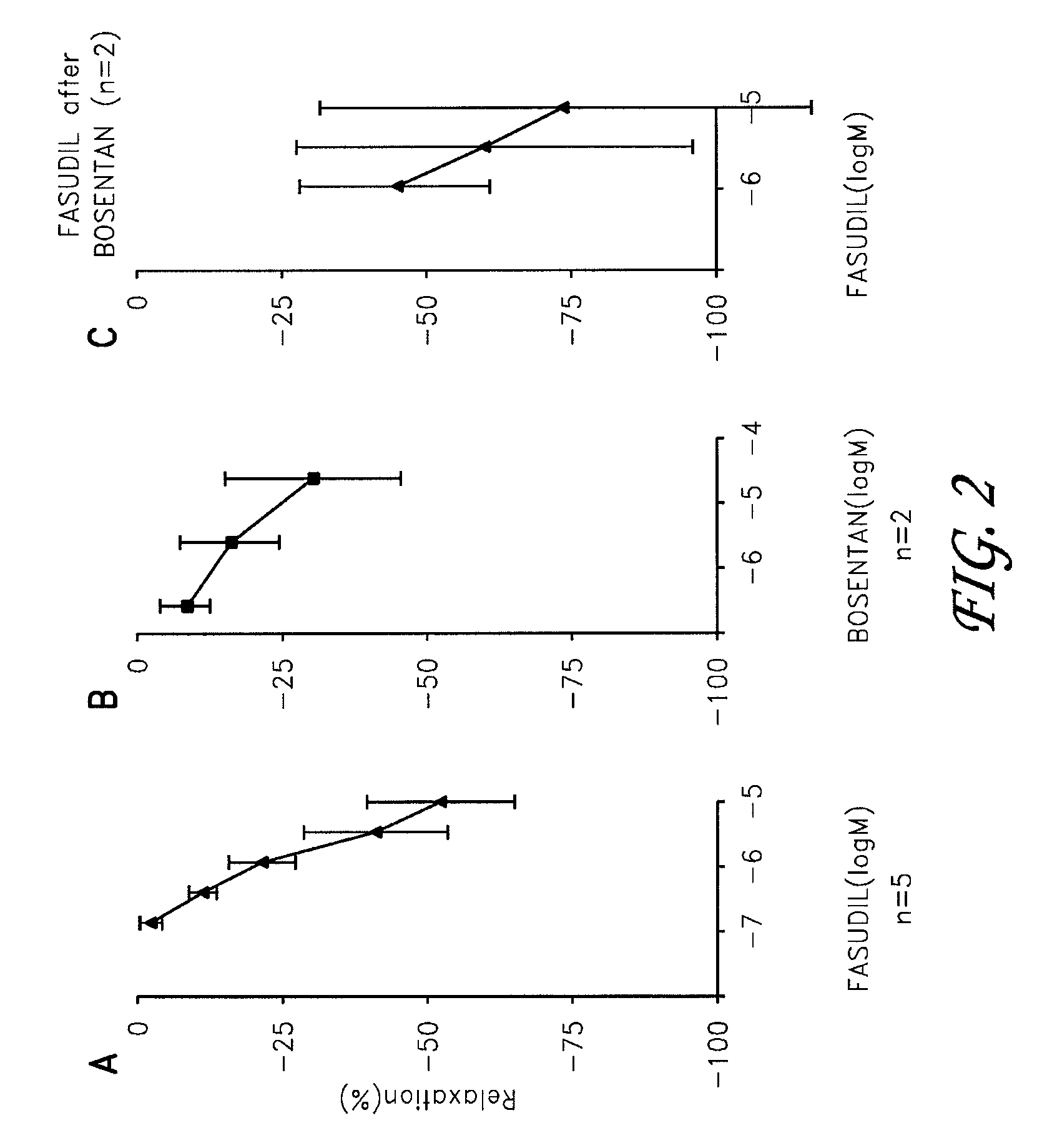
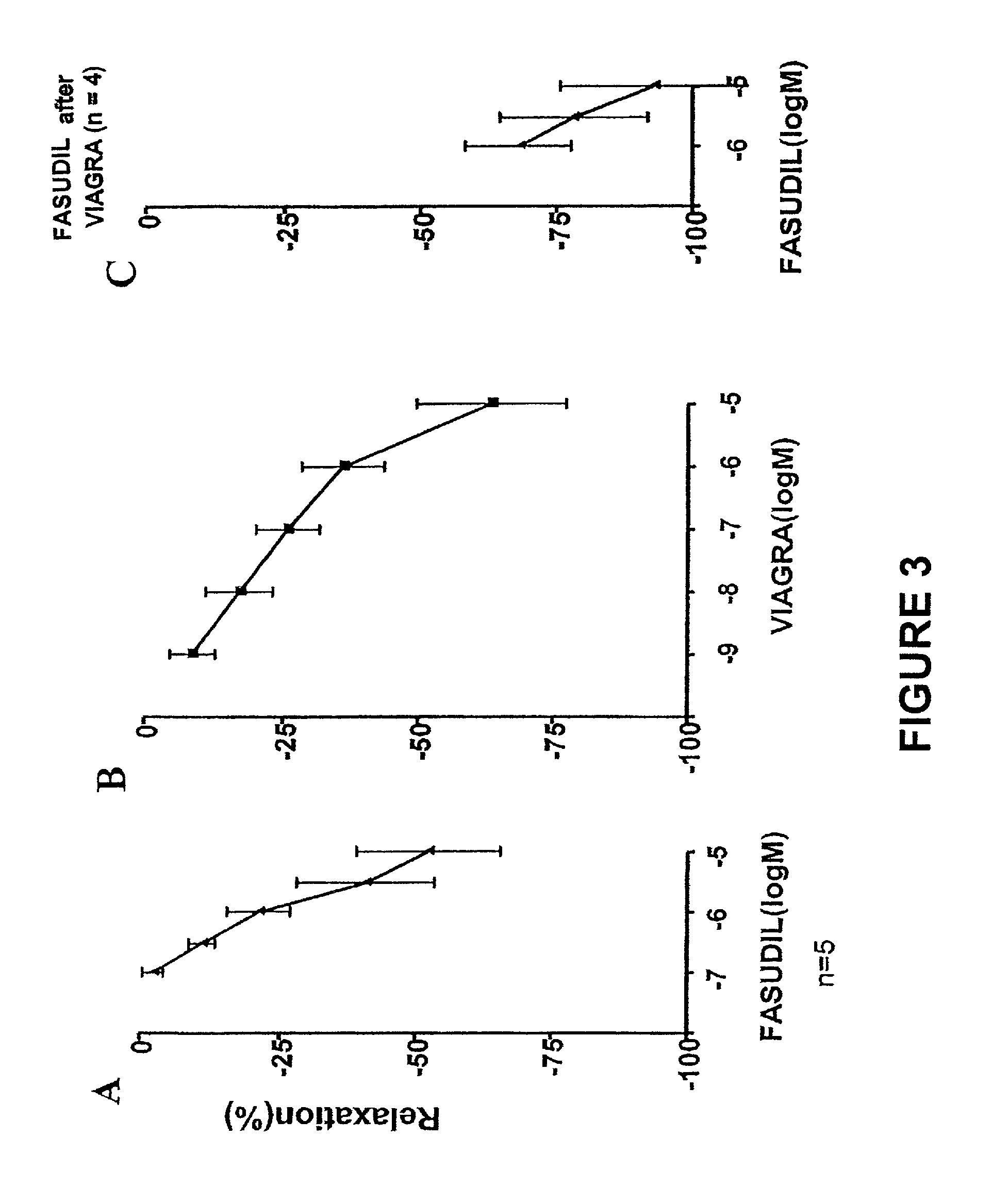
Fasudil in combination therapies for the treatment of pulmonary arterial hypertension
Preferred embodiments of the present invention are related to novel therapeutic drug combinations and methods for treating and / or preventing pulmonary arterial hypertension and / or stable angina. More particularly, aspects of the present invention are related to therapeutic combinations comprising a Rho-kinase inhibitor, such as fasudil, and one or more additional compounds selected from the group consisting of prostacyclins, such as iloprost, endothelin receptor antagonists, PDE inhibitors, calcium channel blockers, 5-HT2A antagonists, such as sarpogrelate, selective serotonin reuptake inhibitors, such as fluoxetine, statins, and vascular remodeling modulators, such as Gleevec.
Owner:ASAHI KASEI PHARMA
Galactose-pronged polysaccharides in a formulation for antifibrotic therapies
Methods and compositions for reducing fibrosis and cirrhosis are provided in which an effective dose of an admixture of a polysaccharide compound and, for example, a compound selected from the group consisting of antibodies specific to intracellular or cell-surface: (i) beta-PDGF receptors; (ii) synaptophysin; (iii) zvegf3; (iv) CCR1 receptors; (v) connective tissue growth factor; (vi) alpha 1-smooth muscle actin; (vii) matrix metalloproteinases MMP 2 and MMP9; (viii) matrix metalloproteinase inhibitors TIMP1 and TMP2; (ix) integrins; (x) TFG-β1; (xi) endothelin receptor antagonists; and (xii) collagen synthesis and degradation modulating compounds; (xiii) actin synthesis and degradation modulating compounds; and (xiv) tyrosine kinases is administered to an animal in order to treat fibrosis.
Owner:GALECTIN THERAPEUTICS
Endothelin a receptor antagonists in combination with phosphodiesterase 5 inhibitors and uses thereof
InactiveUS20060205733A1Eliminate side effectsLow toxicityBiocideNervous disorderDiseasePhosphodiesterase 5 inhibitor
The invention relates generally to combination therapies comprising an endothelin A receptor (ETA) antagonist and a phosphodiesterase 5 (PDE5) inhibitor, pharmaceutical compositions comprising ETA antagonist and PDE5 inhibitor and methods of treating various disorders comprising administering an ETA antagonist and a PDE5 inhibitor. In particular, the combination therapies and pharmaceutical compositions are useful for the treatment and / or prevention of cardiac disorders such as pulmonary arterial hypertension (PAH).
Owner:ENCYSIVE PHARMA INC
Compounds and methods for the treatment of urogenital disorders
The present invention provides methods for treating a variety of urogenital disorders, such as, for example, vaginismus, dyspareunia, vulvodynia (including vulvar vestibulitis), interstitial cystitis, nonspecific urethriris (i.e., nonspecific pain and / or burning of the urinary tract) and sexual dysfunctions, such as, for example, female sexual arousal disorders and female sexual orgasmic disorders, using a variety of compounds, including, but not limited to, NO donors, calcium channel blockers, cholinergic modulators, α-adrenergic receptor antagonists, β-adrenergic receptor agonists, phosphodiesterase inhibitors, cAMP-dependent protein kinase activators (e.g., cAMP mimetics), superoxide scavengers, potassium channel activators, estrogen-like compounds, testosterone-like compounds, benzodiazepines, adrenergic nerve inhibitors, antidiarrheal agents, HMG-CoA reductase inhibitors, smooth muscle relaxants, adenosine receptor modulators, adenylyl cyclase activators, endothelin receptor antagonists, bisphosphonates and cGMP-dependent protein kinase activators (e.g., cGMP mimetics).
Owner:STREHKEHN INT LTD
Use of endothelin antagonists to prevent restenosis
InactiveUS20050175667A1Improve efficiencyImprove efficacyBiocideOrganic active ingredientsPercent Diameter StenosisThrombus
Provided are devices and methods for treating or preventing smooth muscle cell proliferation caused by endothelin-mediated conditions. In particular, a medical device comprising a structure which is implantable within a body lumen and means on or within the structure for releasing an endothelin (A) receptor antagonist at a rate effective to inhibit smooth muscle cell proliferation. The device can be, for example, an expansible stent or a graft, and the means can include a matrix coating, wherein the endothelin (A) receptor antagonist can be dispersed within the coating or disposed directly on the structure and under the matrix. The methods and devices of this invention can be used to decrease the incidence of restenosis as well as other thromboembolic complications resulting from implantation of medical devices.
Owner:EDWARDS LIFESCIENCES LLC
Oral formulations of diphenylsulfonamide endothelin and angiotensin ii receptor agonists to treat elevated blood pressure and diabetic nephropathy
InactiveUS20140142149A1Improves friabilityEasy to compressBiocideSenses disorderEndothelin receptor antagonistAgonist
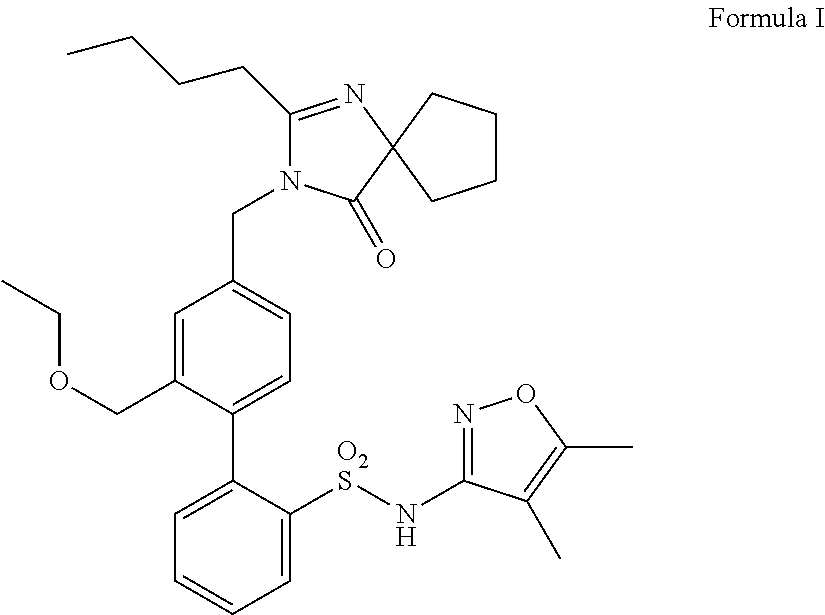
Methods of administering and pharmaceutical compositions of a biphenyl sulfonamide compound which is a dual angiotensin and endothelin receptor antagonist are disclosed for treating diseases.
Owner:LIGAND PHARMA INC
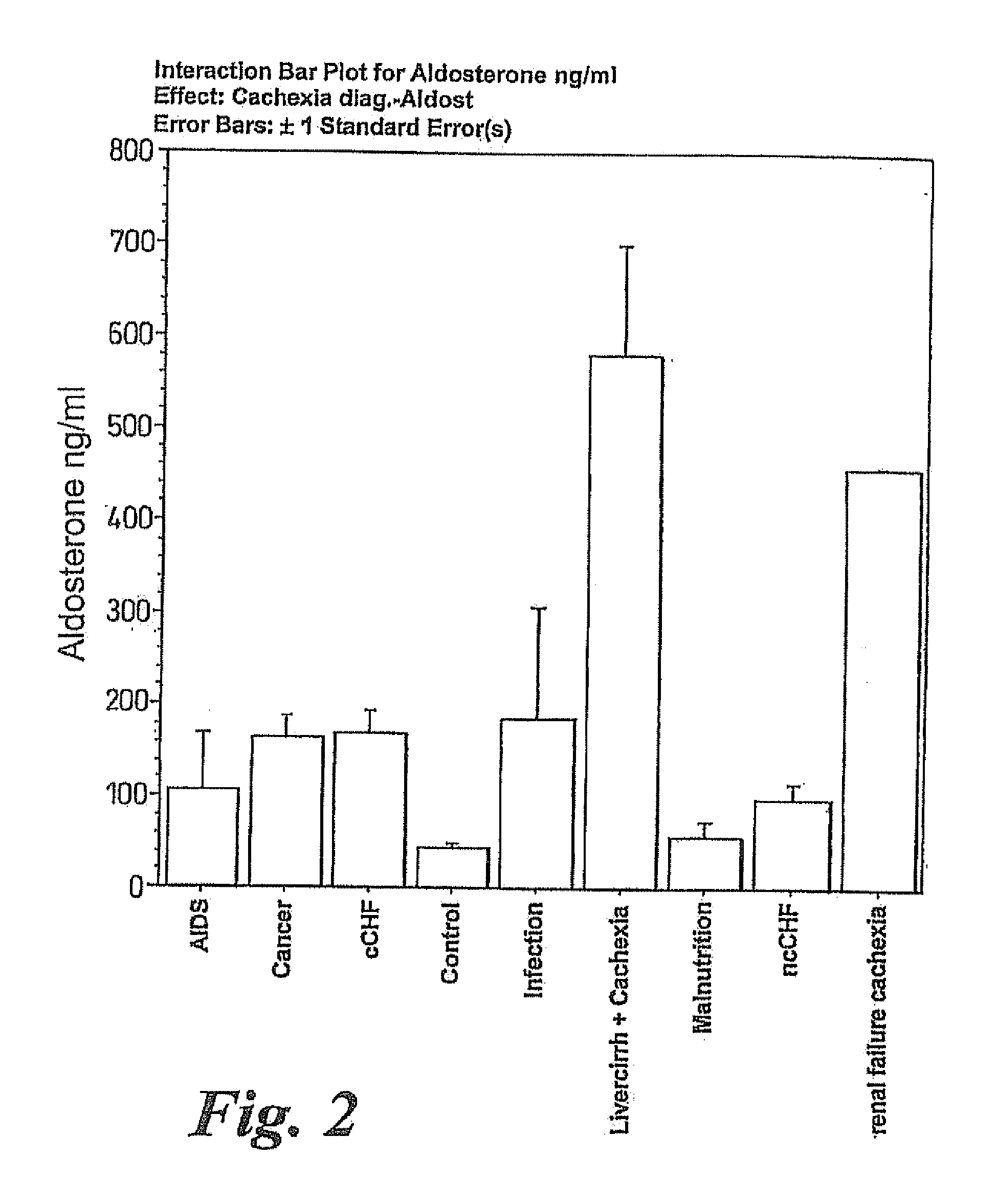
Methods of treating cachexia
InactiveUS7417038B1Reduced metabolic rateIncrease blood flowPeptide/protein ingredientsMetabolism disorderDiseaseImidazoline receptor
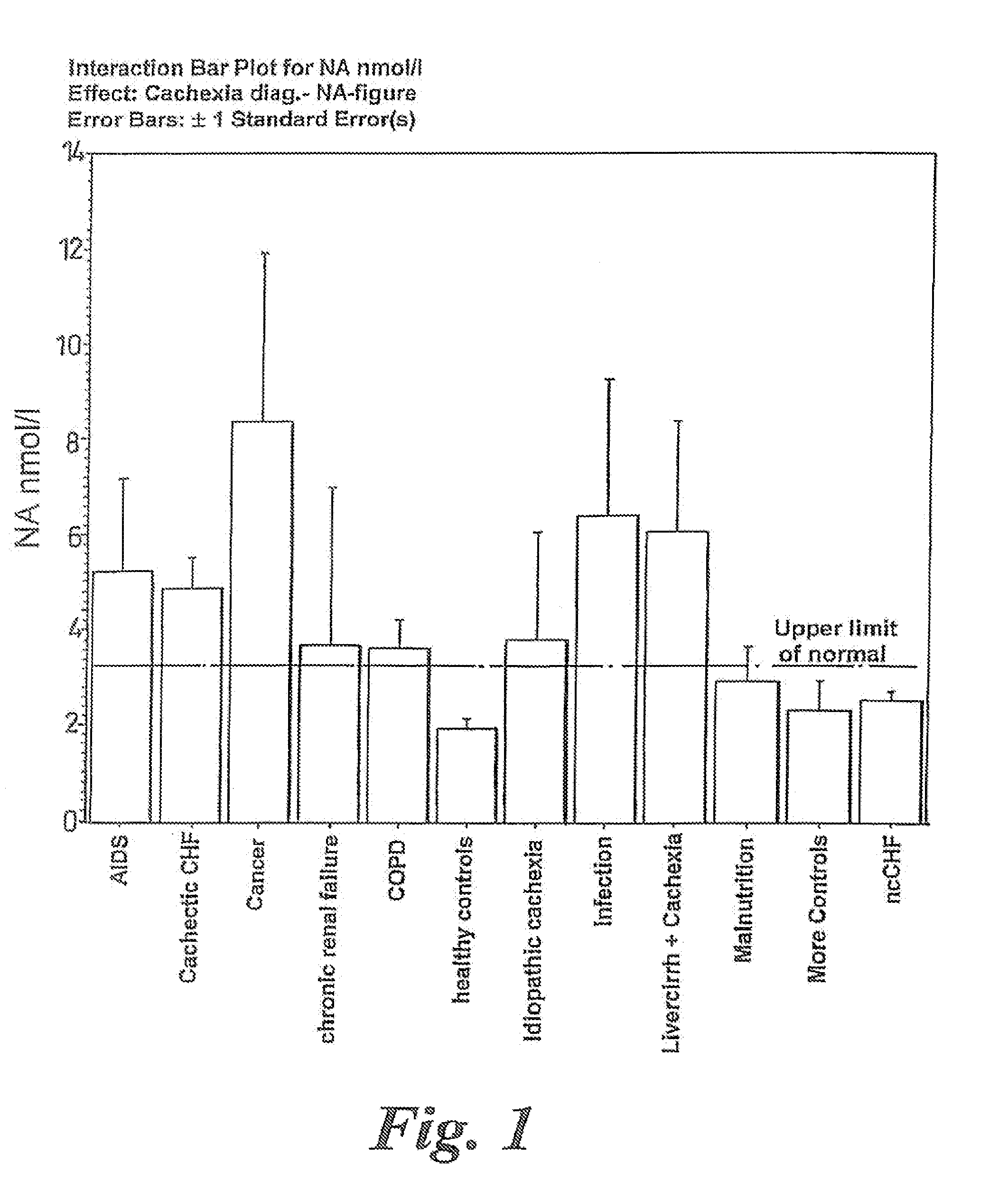
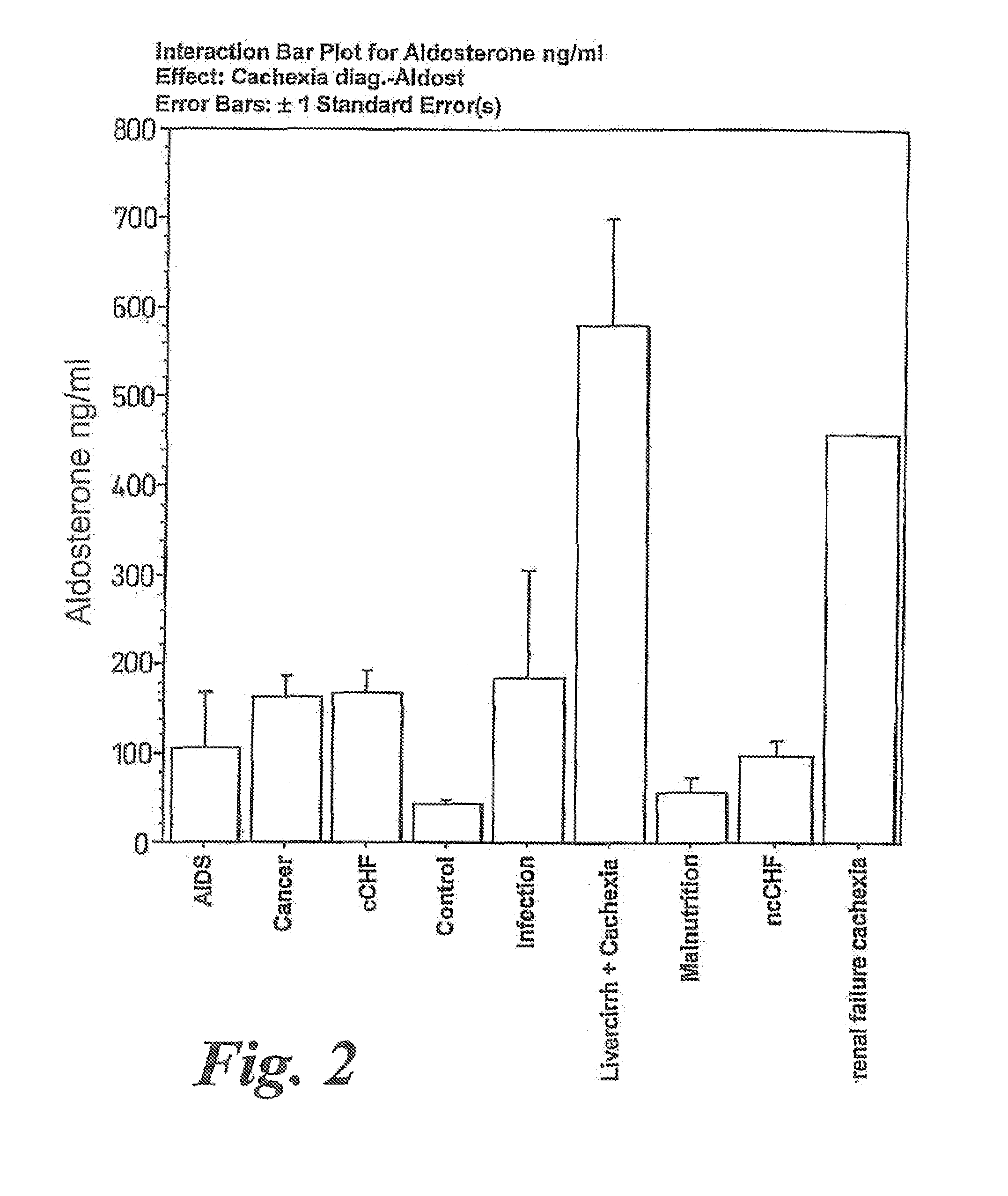
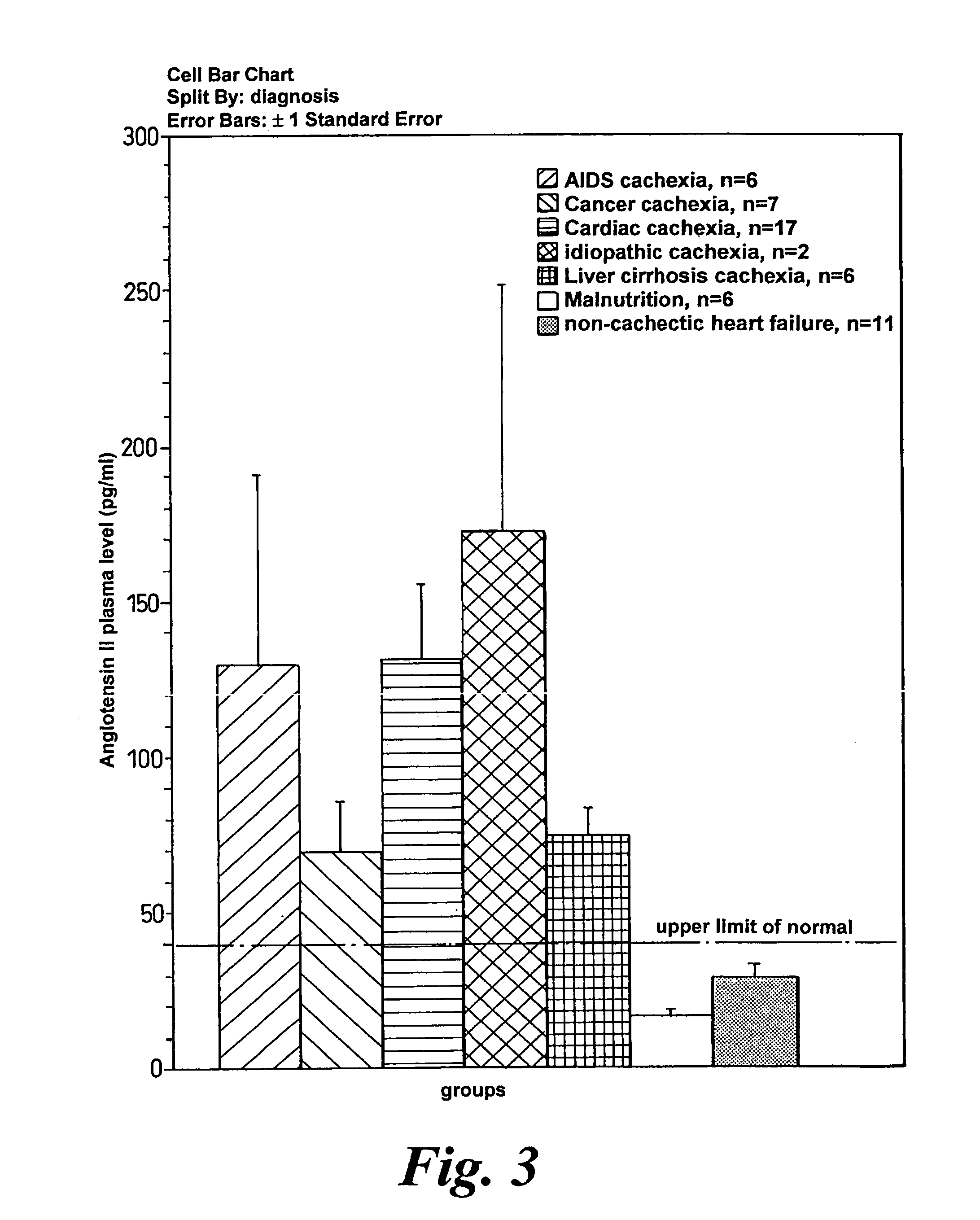
A method of treating weight loss due to underlying disease in a patient the method comprising administering to the patient an effective amount of an agent which reduces sympathetic nervous system activity. A method of treating weight loss due to underlying disease in a patient the 10 method comprising administering to the patient an effective amount of any one or more of the following: a compound which inhibits the effect of aldosterone such as an aldosterone antagonist; a chymase inhibitor; a cathepsin B inhibitor; a 13 receptor blocker; an imidazoline receptor antagonist; a centrally acting tx receptor antagonist; a peripherally acting ct receptor antagonist; a ganglion blocking agent; a drug that has an effect on cardiovascular reflexes and thereby reduce SNS activity such as an opiate; scopolamine; an endothelin receptor antagonist; and a xanthine oxidase inhibitor. The methods are particularly useful in treating cardiac cachexia.
Owner:IMPERIAL INNOVATIONS LTD
Oral formulations of diphenylsulfonamide endothelin and angiotensin ii receptor agonists to treat elevated blood pressure and diabetic nephropathy
ActiveUS20150164865A1Lower systolic blood pressureLower diastolic blood pressureBiocideSenses disorderDiseaseAngiotensin II receptor type 1
Methods of administering and pharmaceutical compositions of a biphenyl sulfonamide compound which is a dual angiotensin and endothelin receptor antagonist are disclosed for treating diseases.
Owner:LIGAND PHARMA INC
Method for screening for endothelin-receptor antagonist activity and for treating conditions caused by endothelin
InactiveUS20050008710A1Potent effectGood blood pressureBiocideCompound screeningFluorescenceEvaporation
Aliquots of extracts from ethnopharmacological plants that have activity against the effects of sarafotoxins present in snake venom are isolated and identified as antagonists of endothelin using a fluorescence-based assay. A process is provided for the identification of an antagonist of an endothelin selected from the group consisting of endothelin-1, endothelin-2, endothelin-3 and mixtures thereof. The process comprises extraction of ethnopharmacological plants with a solvent followed by evaporation of the solvent to form an aliquot containing at least one component of the extract, optionally purifying and isolating one or more component by chromatography, and subjecting the aliquot or purified component to a competitive fluorescent binding assay using biotinylated endothelin-1, wherein the plants having activity against the effects of one or more sarafotoxins present in snake venom.
Owner:PHYTOMYCO RES CORP
Methods and compositions for emergency contraception using endothelin receptor antagonists
ActiveUS20090203591A1Inhibit ovulationEfficient implementationBiocideOrganic chemistryEndothelin receptor antagonistEmergency medicine
Disclosed are methods and compositions containing endothelin receptor antagonists for emergency contraception.
Owner:THE POPULATION COUNCIL INC
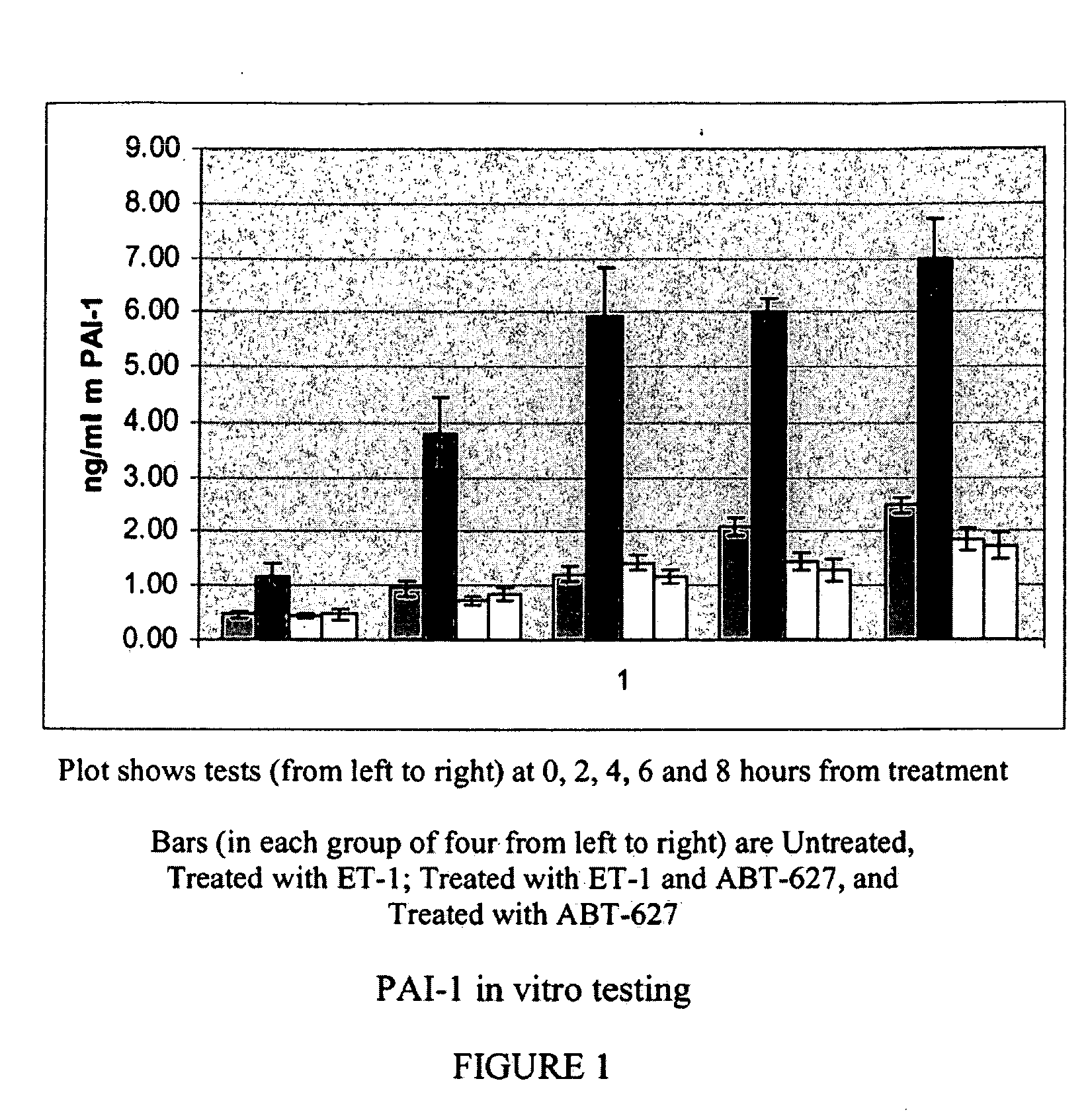
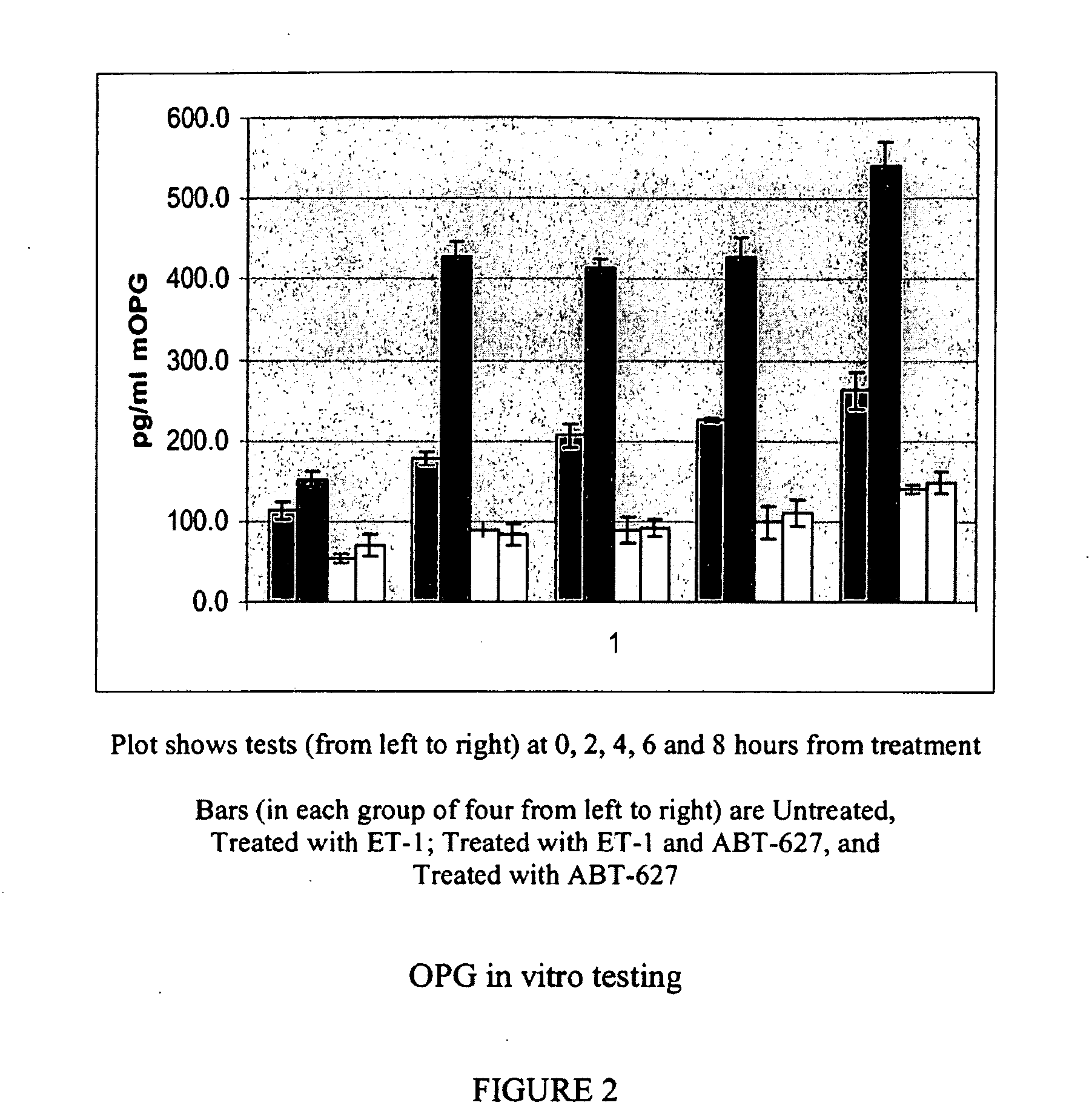
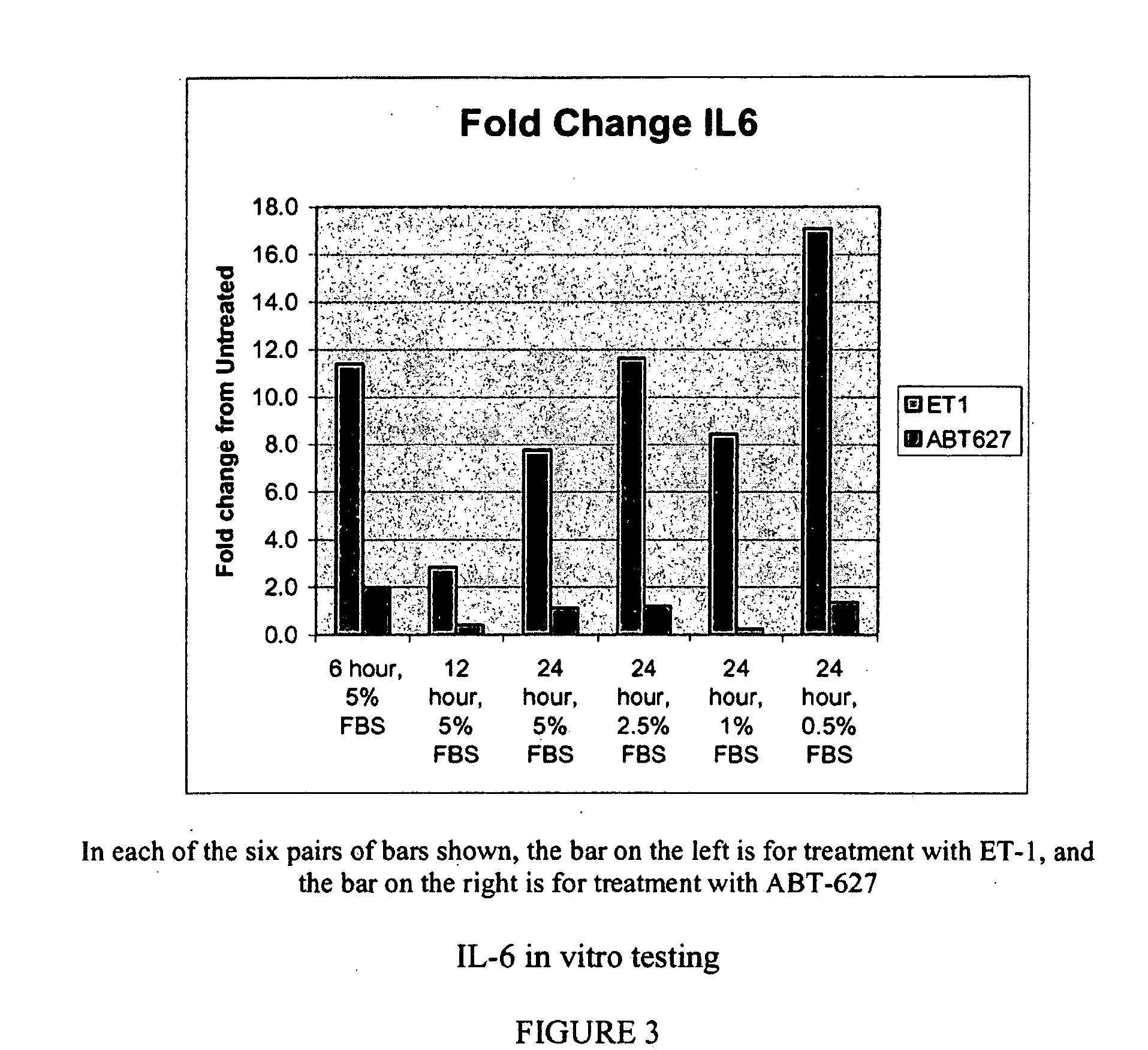
Companion diagnostic assays for endothelin receptor antagonists
InactiveUS20080102451A1Increase choiceMicrobiological testing/measurementBiological testingMedicineTissue sample
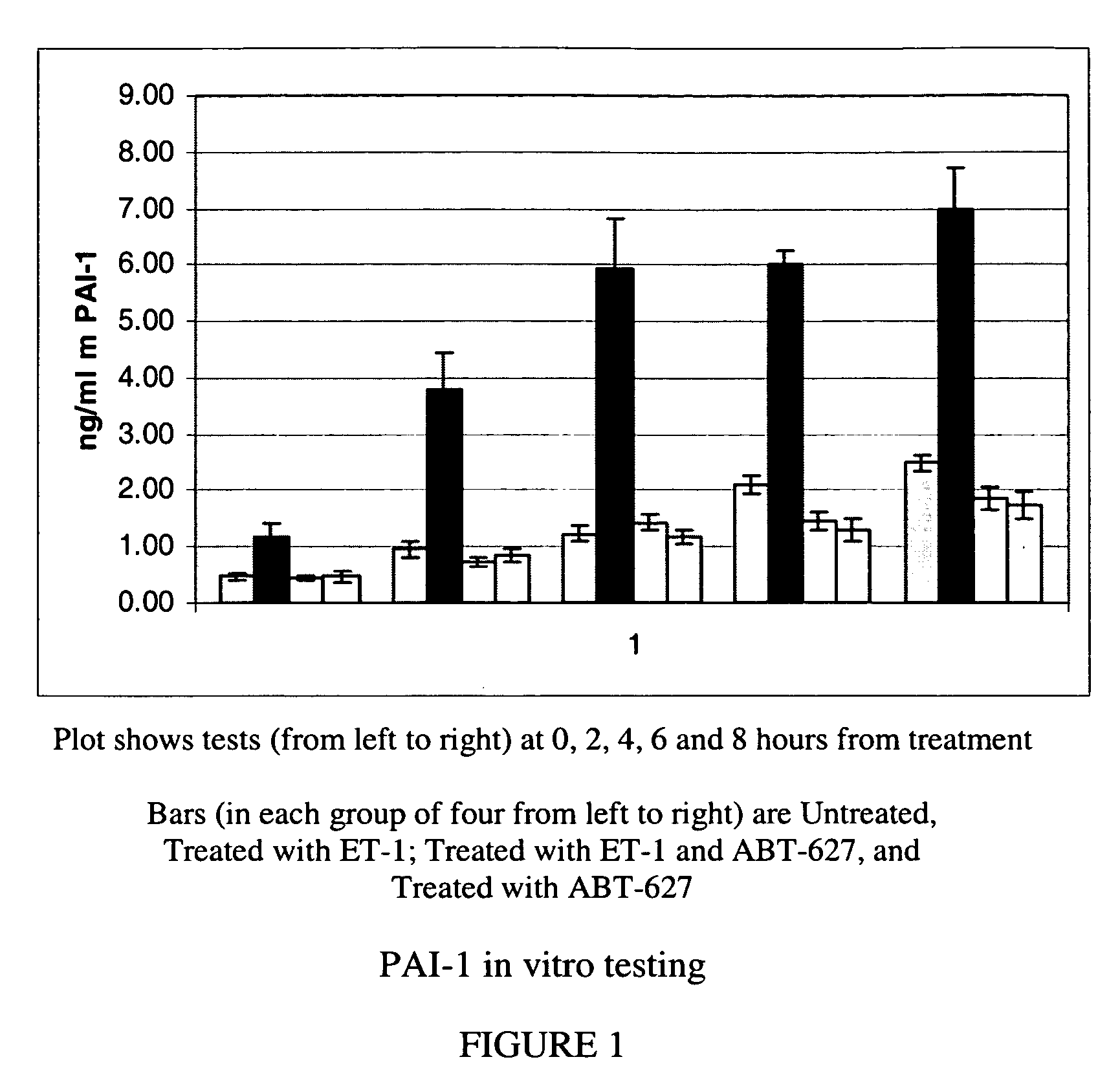
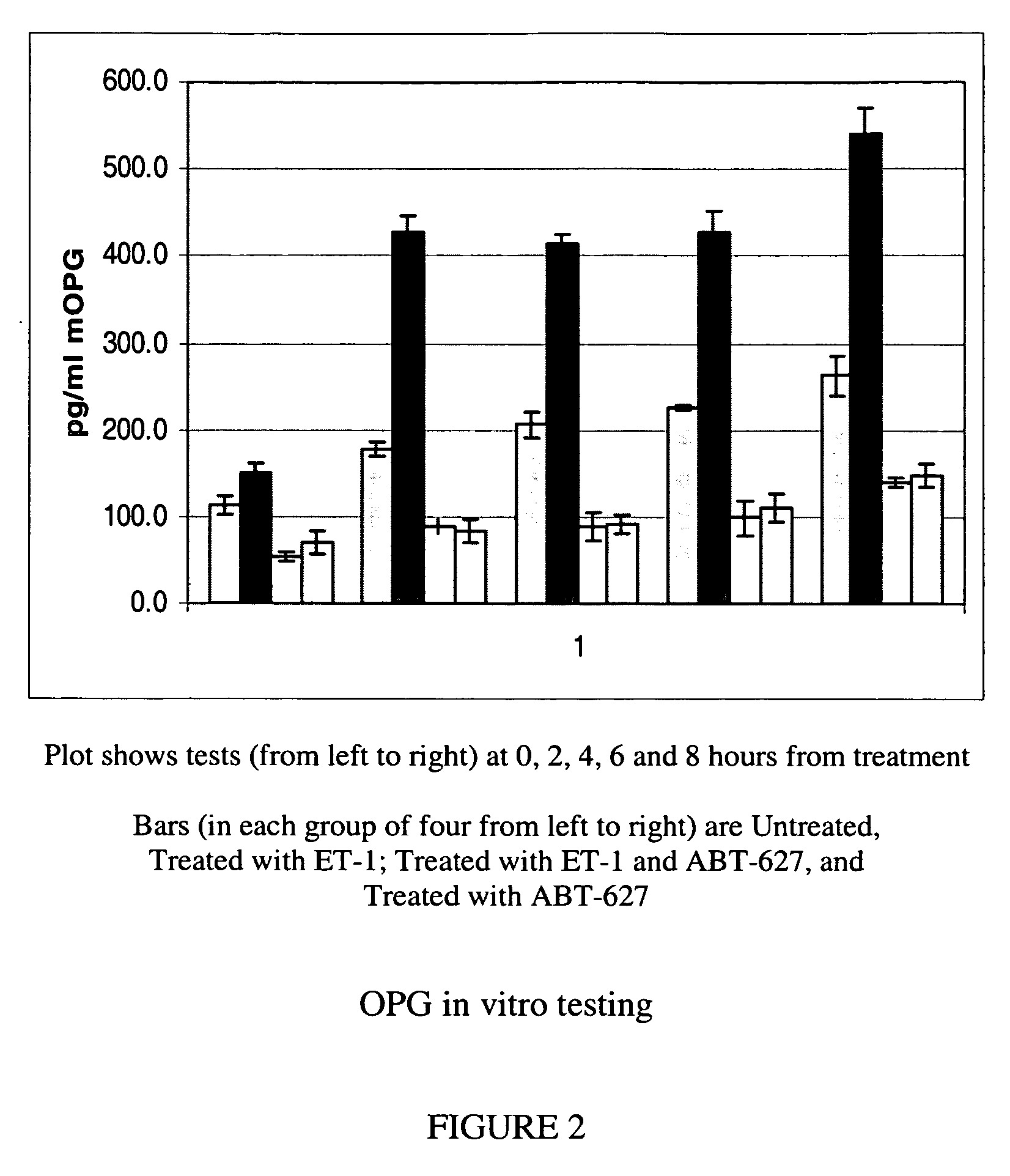
Methods for identifying cancer patients eligible to receive endothelin receptor antagonist therapy and for monitoring patient response to endothelin receptor antagonist therapy comprise assessment of the expression levels of at least one of PAI-1, uPA, TGFbeta2, IL-6, IL-8 and OPG in a patient tissue sample. The methods of the invention allow more effective identification of patients to receive endothelin receptor antagonist therapy and of determination of patient response to the therapy.
Owner:ABBOTT LAB INC
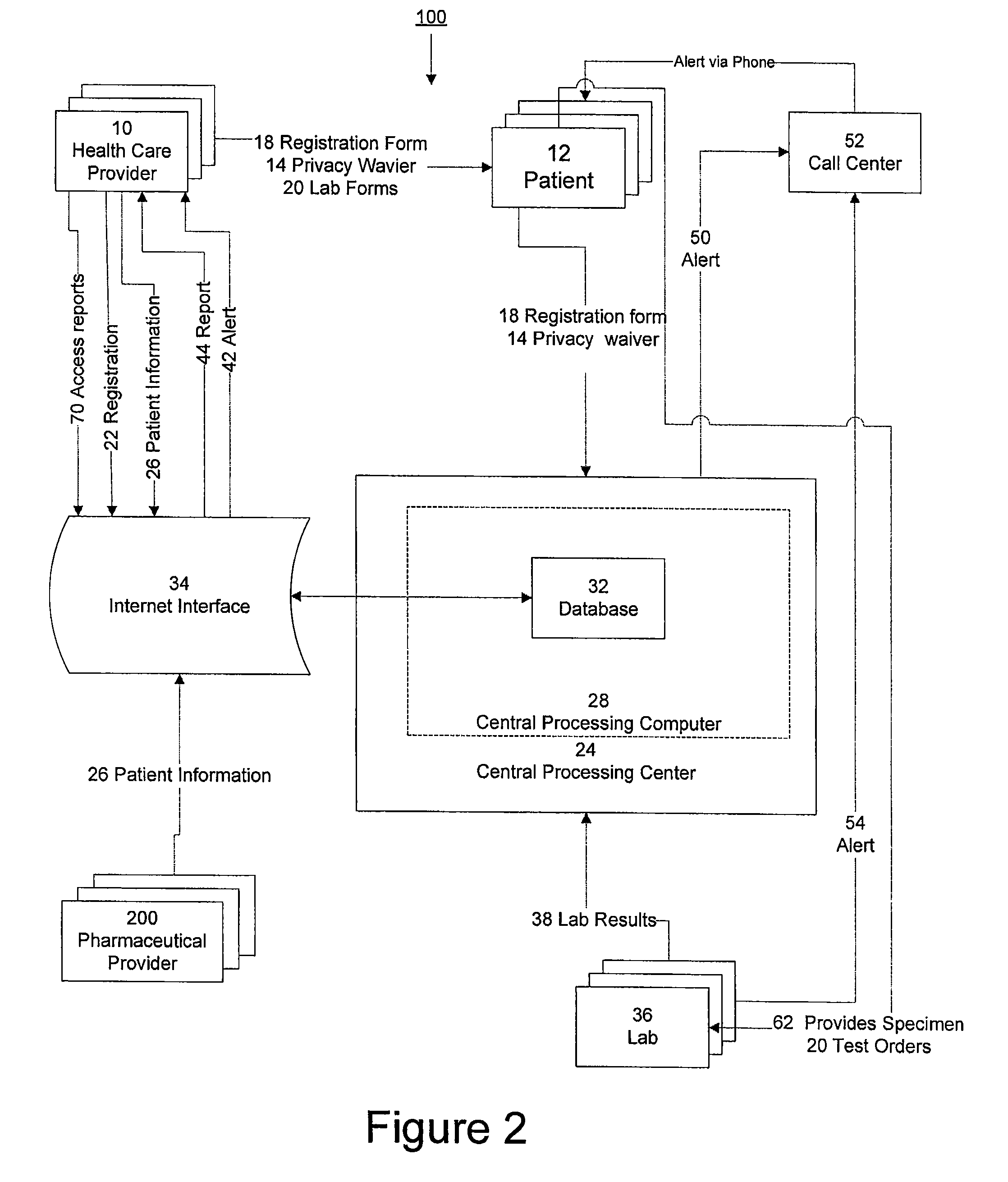
System for managing laboratory test results for patients taking an endothelin receptor antagonist
InactiveUS20090171694A1Computer-assisted medical data acquisitionResourcesTime scheduleLaboratory Test Result
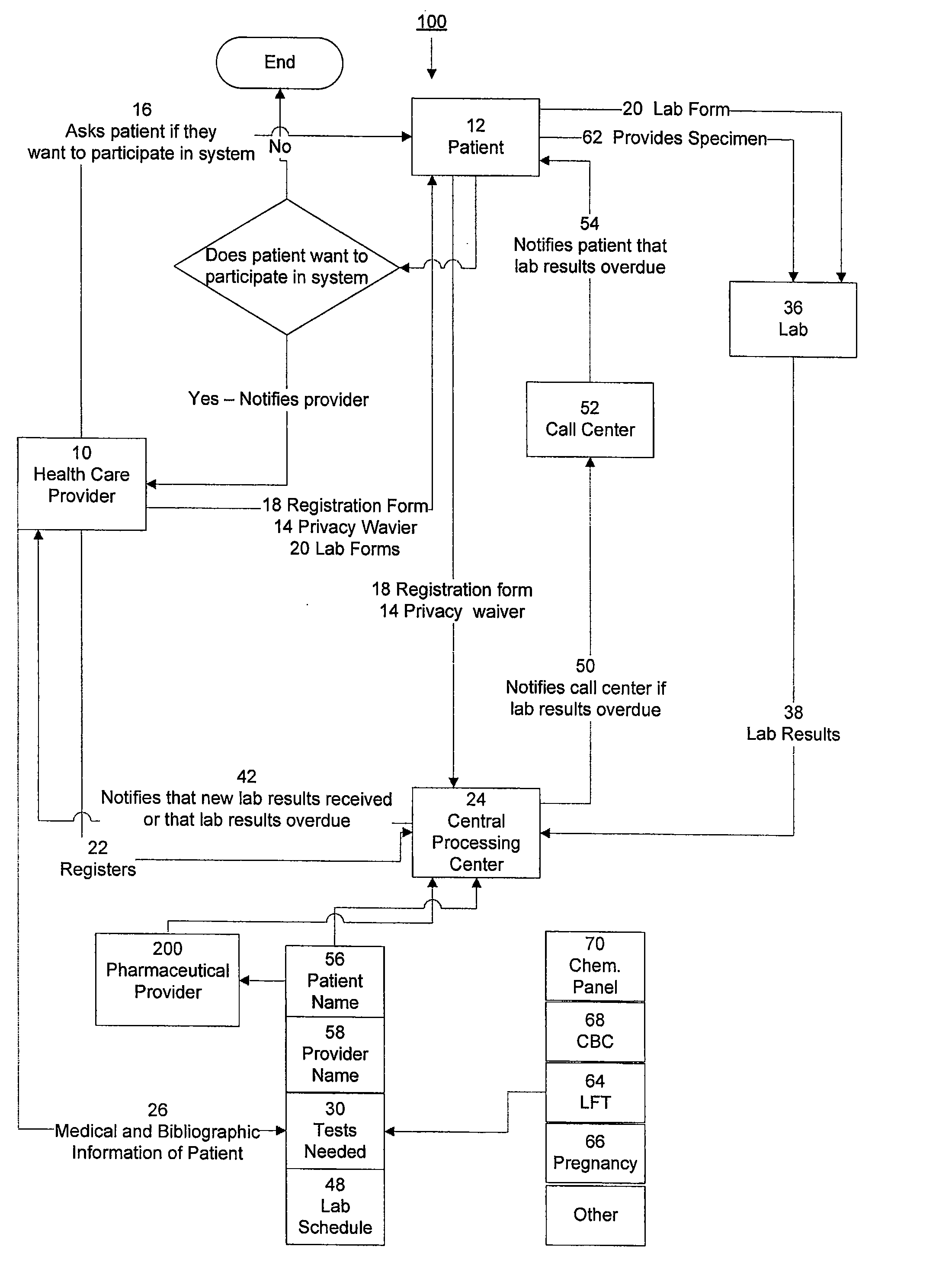
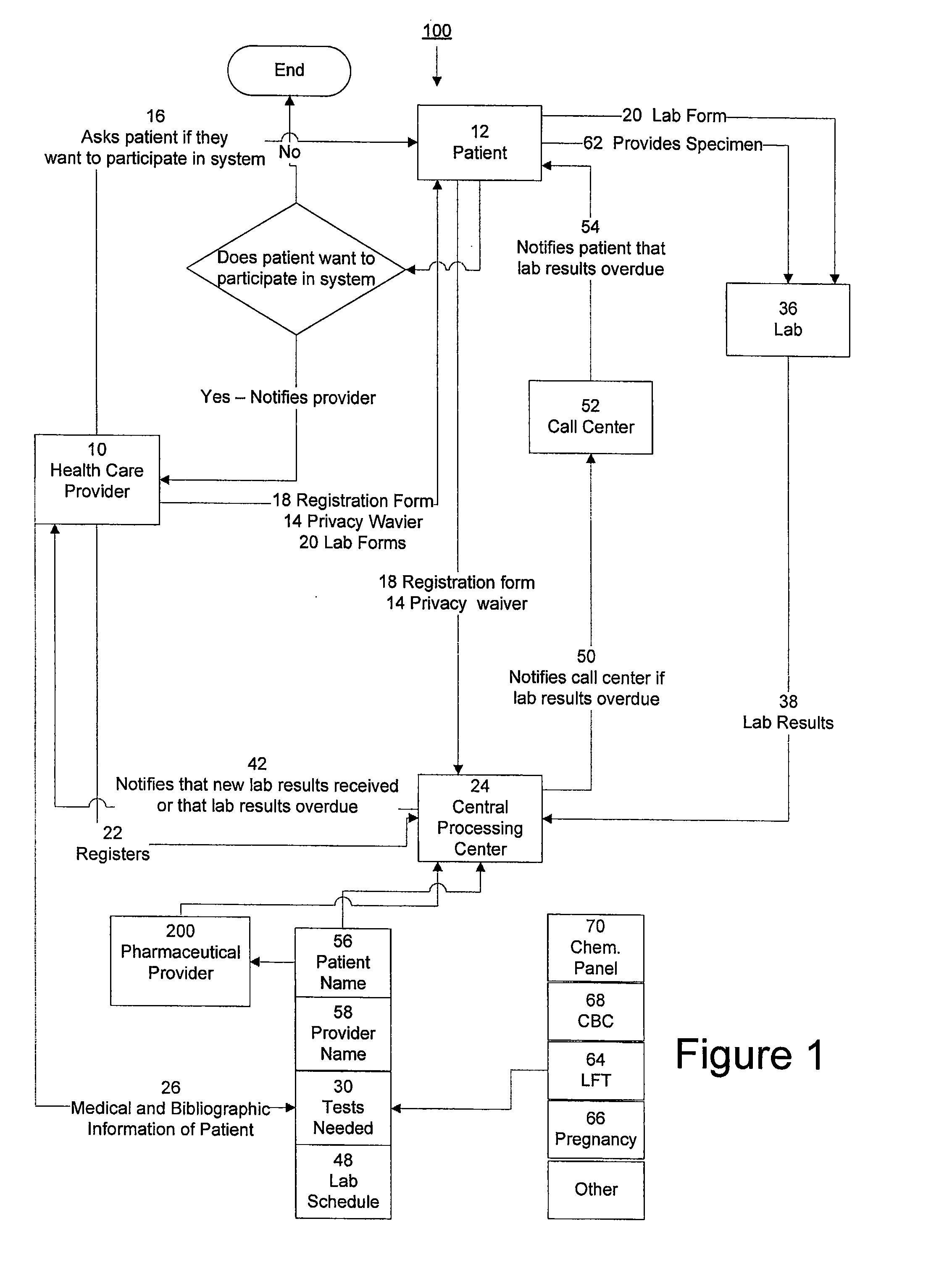
The invention provides a system and method of collecting, storing and managing laboratory test results of patients. In one embodiment the invention is directed towards collecting, storing and managing laboratory test results of patients taking an endothelin receptor antagonist (ERA). The system provides a way of securely collecting and tracking information from a plurality of patients, a plurality of laboratories, and a plurality of health care providers by use of a database. The method comprises receiving patient information from health care providers, receiving a laboratory test schedule for each patient, and laboratory tests needed for each patient. This data is entered into a central processing computer. The patients then go to any laboratory to have the monthly testing done due to the ERA prescription. The laboratory test data collected is sent to the central processing computer. The central processing computer can generate an automated report on the laboratory results and supply the results to the health care provider. Additionally, if no laboratory test data for the patient is entered into the central processing computer in accordance with the laboratory test schedule for the patient, the central processing computer will notify a call center which will call the patient, laboratory or health care provider informing them of the overdue laboratory test data.
Owner:ACTELION PHARMA US
Companion Diagnostic Assays For Endothelin Receptor Antagonists
InactiveUS20100184026A1Increase choiceMicrobiological testing/measurementDisease diagnosisMedicineEndothelin receptor antagonist
Methods for identifying cancer patients eligible to receive endothelin receptor antagonist therapy and for monitoring patient response to endothelin receptor antagonist therapy comprise assessment of the expression levels of at least one of PAI-1, uPA, TGFbeta2, IL-6, IL-8 and OPG in a patient tissue sample. The methods of the invention allow more effective identification of patients to receive endothelin receptor antagonist therapy and of determination of patient response to the therapy.
Owner:ABBOTT MOLECULAR INC
Cancer treatment with endothelin receptor antagonists
The present invention relates to therapeutic protocols and pharmaceutical compositions designed to treat and prevent cancer. More specifically, the present invention relates to a novel method of treating cancer using antagonists to the endothelin B receptor (ETB) or inactive mimic forms of endothelin-1. The pharmaceutical compositions of the invention are capable of selectively inhibiting the early events associated with the development of cancer. The present invention further relates to screening assays to identify compounds which inhibit ETB activation.
Owner:NEW YORK UNIV
Methods of Treatment
A method of treating weight loss due to underlying disease in a patient, the method comprising administering to the patient an effective amount of an agent which reduces sympathetic nervous system activity. A method of treating weight loss due to underlying disease in a patient the method comprising administering to the patient an effective amount of any one or more of the following: a compound which inhibits the effect of aldosterone such as an aldosterone antagonist; a chymase inhibitor; a cathepsin B inhibitor; a β receptor blocker; an imidazoline receptor antagonist; a centrally acting α receptor antagonist; a peripherally acting α receptor antagonist; a ganglion blocking agent; a drug that has an effect on cardiovascular reflexes and thereby reduces SNS activity such as an opiate; scopolamine; an endothelin receptor antagonist; and a xanthine oxidase inhibitor. The methods are particularly useful in treating cardiac cachexia.
Owner:IMPERIAL INNOVATIONS LTD
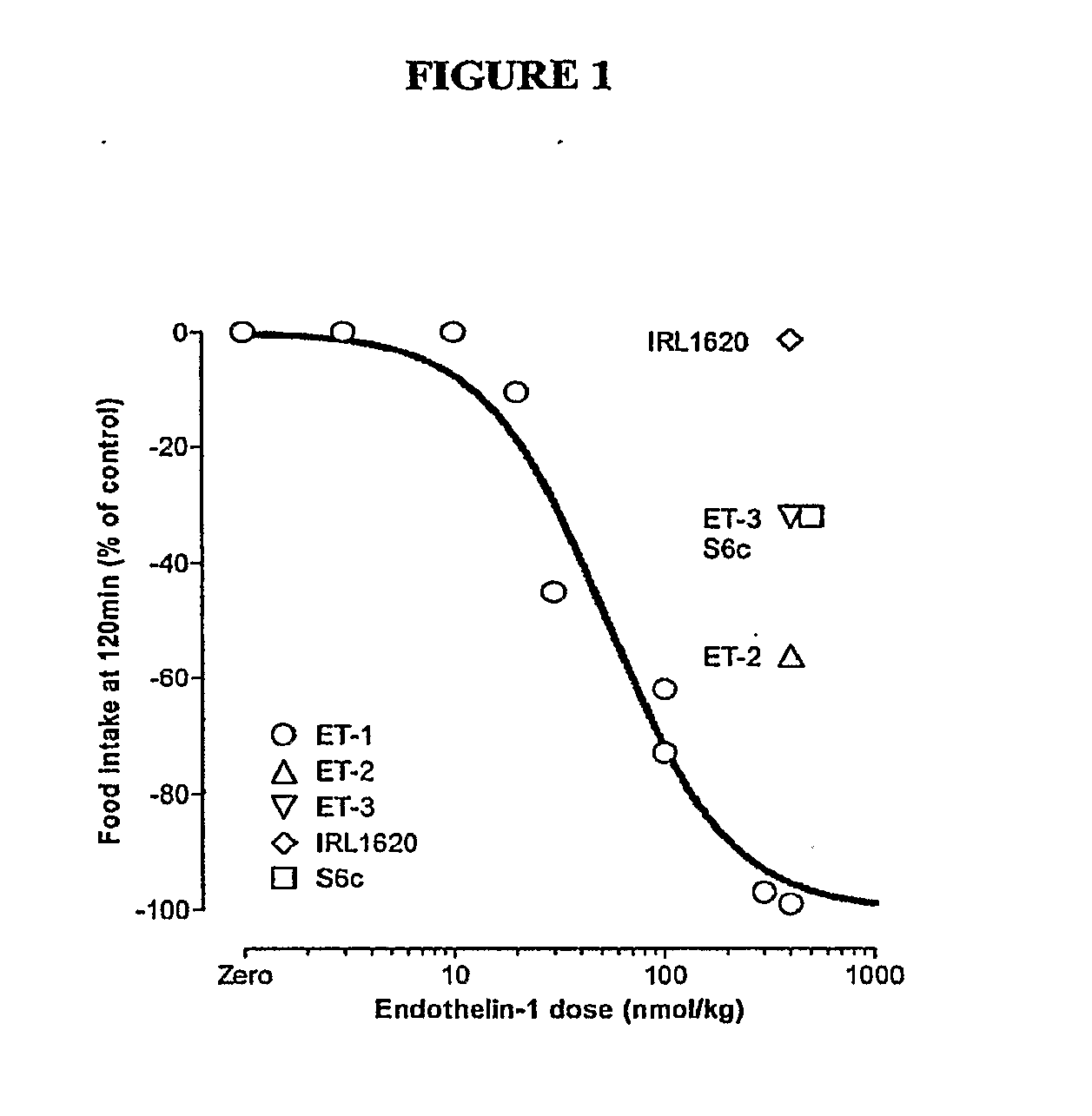
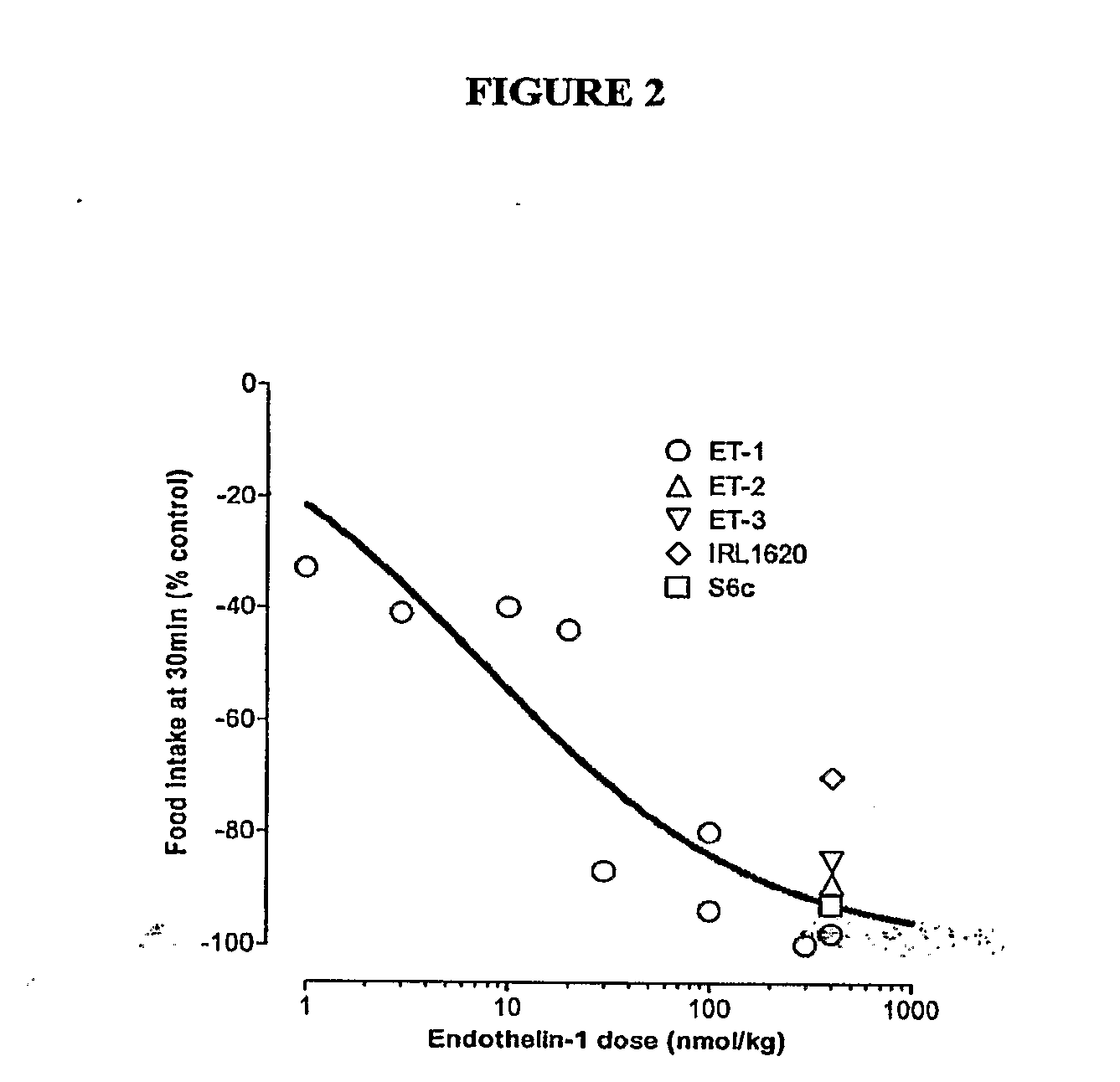
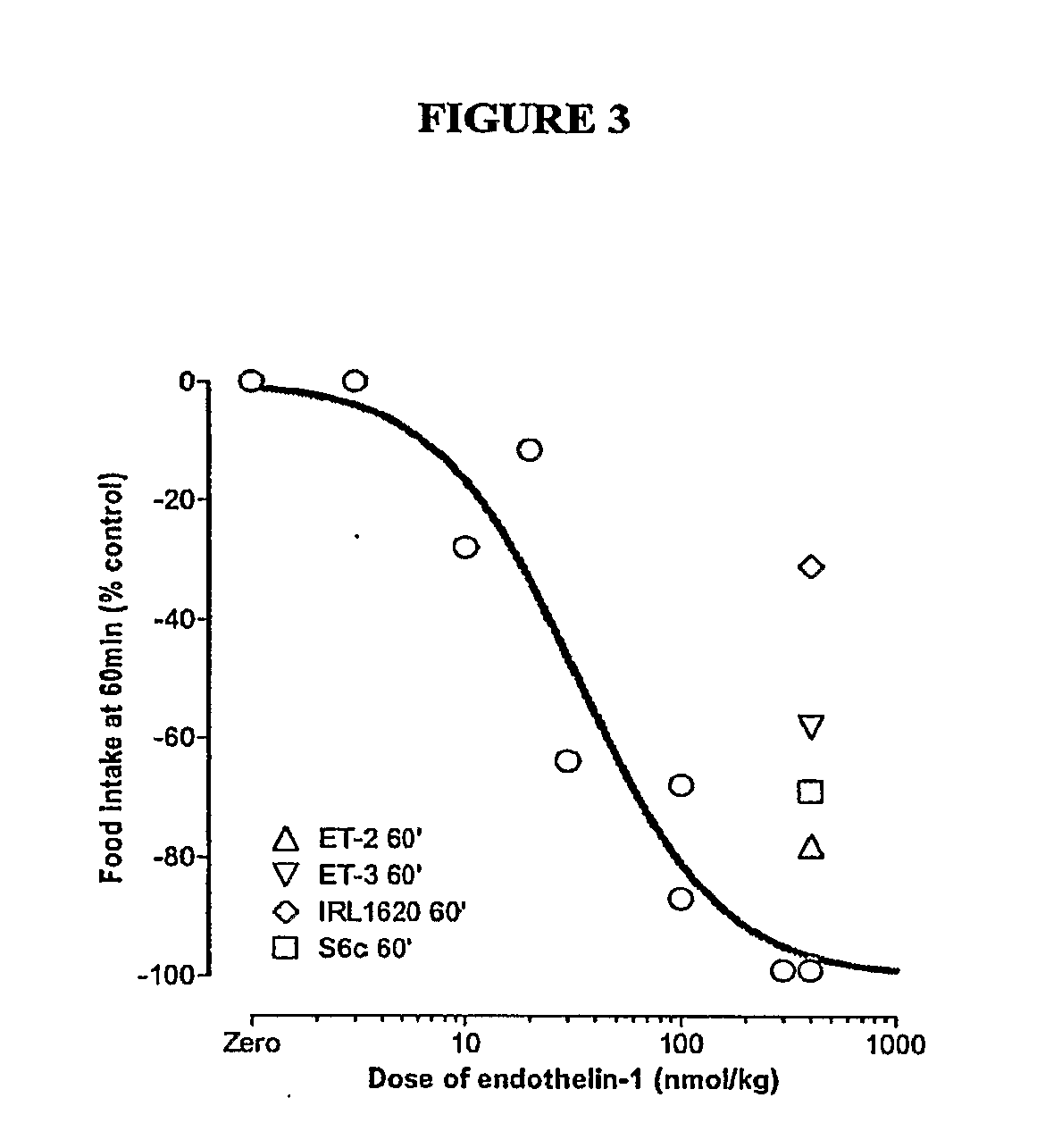
Endothelin and Endothelin Receptor Agonists in the Treatment of Metabolic Diseases
InactiveUS20100004166A1Reduce food intakeInduce satietyPeptide/protein ingredientsMetabolism disorderFeeding disorderInsulin resistance
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an endothelin or an endothelin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:AMYLIN PHARMA INC
Compositions and methods for reducing visual loss
InactiveUS20160346224A1Reduce visual lossOrganic active ingredientsSenses disorderParticulatesSide effect
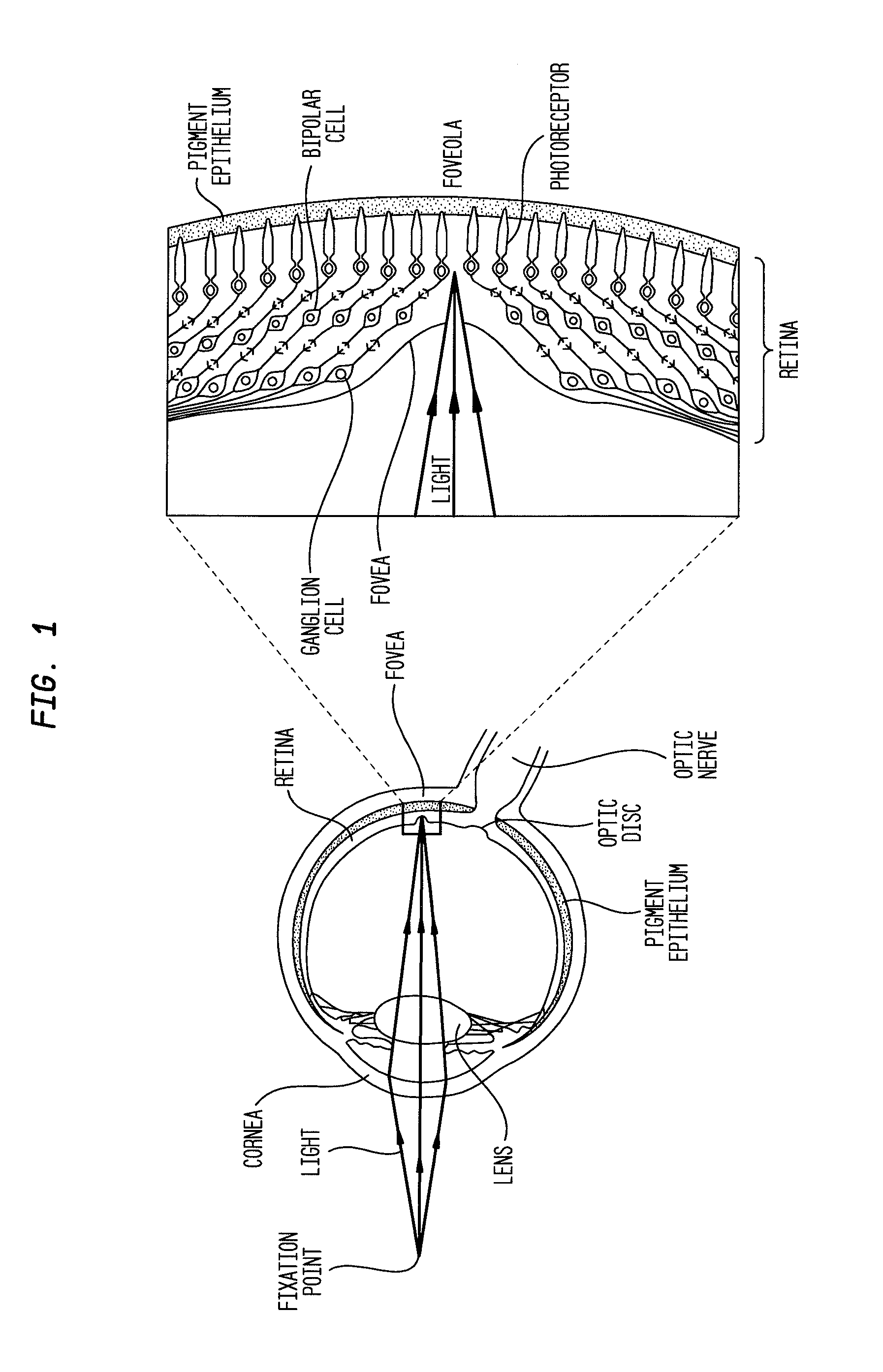
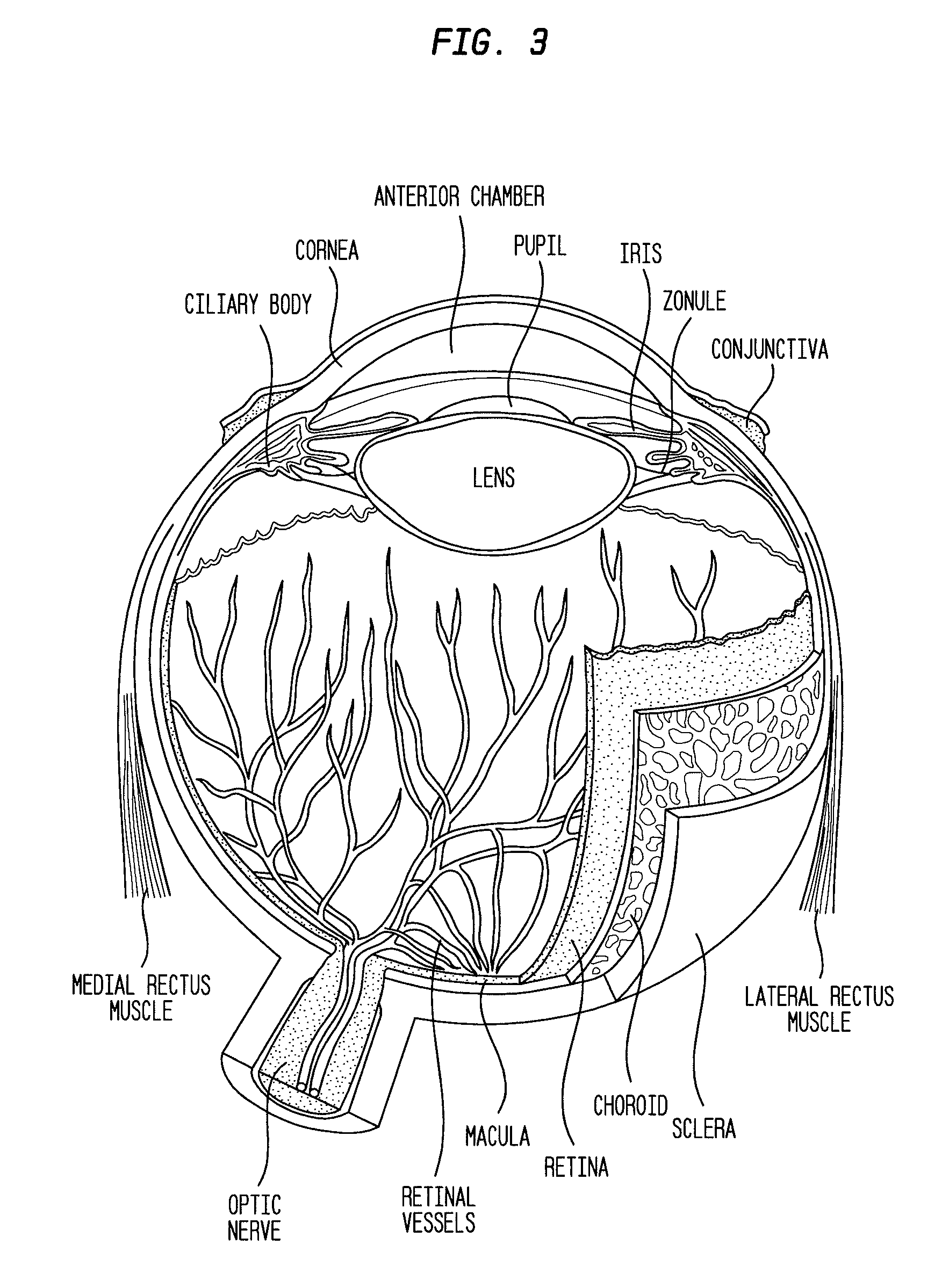
The described invention provides a method for reducing visual loss and for treating one or more of adverse consequence of an eye disease, including abnormal intraocular pressure, retinal vascular disease, retinal ganglion cell death, or a combination thereof in order to reduce visual loss. The method entails providing a flowable particulate composition that contains a particulate formulation comprising a plurality of particles of uniform size distribution, a therapeutic amount of a therapeutic agent selected from a voltage-gated calcium channel antagonist, an endothelin receptor antagonist, or a combination thereof, and optionally an additional therapeutic agent, wherein the particles are of uniform size distribution, and wherein each particle comprises a matrix; and a pharmaceutically acceptable carrier. The pharmaceutical composition is characterized by: dispersal of the therapeutic agent throughout each particle, adsorption of the therapeutic agent onto the particles, or placement of the therapeutic agent in a core surrounded by a coating, sustained release of the therapeutic agent and optionally the additional therapeutic agent from the composition, and a local therapeutic effect that is effective to reduce signs or symptoms of the adverse consequence without entering systemic circulation in an amount to cause unwanted side effects. The method further entails administering a therapeutic amount of the pharmaceutical composition by a means for administration at a site of administration. The administering includes topically, parenterally, or by implantation. Sites of administration include intraocularly, intraorbitally, or into subconjunctival space.
Owner:EDGE THERAPEUTICS
Cancer treatment with endothelin receptor antagonists
InactiveUS7566452B1Compound screeningOrganic active ingredientsCancer preventionEndothelin B receptor
The present invention relates to therapeutic protocols and pharmaceutical compositions designed to treat and prevent cancer. More specifically, the present invention relates to a novel method of treating cancer using antagonists to the endothelin B receptor (ETB) or inactive mimic forms of endothelin-1. The pharmaceutical compositions of the invention are capable of selectively inhibiting the early events associated with the development of cancer. The present invention further relates to screening assays to identify compounds which inhibit ETB activation.
Owner:NEW YORK UNIV
Selective endothelin type-a antagonists
InactiveUS20080262006A1Avoid interactionBiocideOrganic active ingredientsDiseaseEndothelin receptor antagonist
This invention relates to novel endothelin receptor antagonists that selectively inhibit the interaction between Endothelin-1 (ET-1) and endothelin type-A receptors, their derivatives, acceptable acid addition salts. This invention also provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions that are beneficially treated by endothelin receptor antagonists, particularly those diseases and conditions that are beneficially treated by selective inhibitors of endothelin type-A receptors.
Owner:CONCERT PHARMA INC
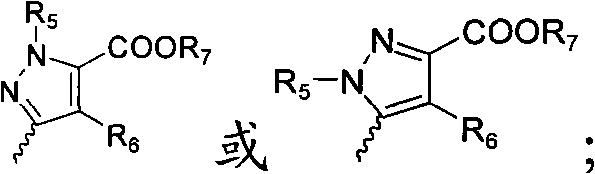
Antagon of endostadin receptor pyrazole carboxylic acids
A pyrazole-carboxylic acid kind of endothelin receptor antagon for treating cardiovascular and cerebrovascular diseases, tumor, diabetes, nephretis and asthma is disclosed.
Owner:CHINA PHARM UNIV
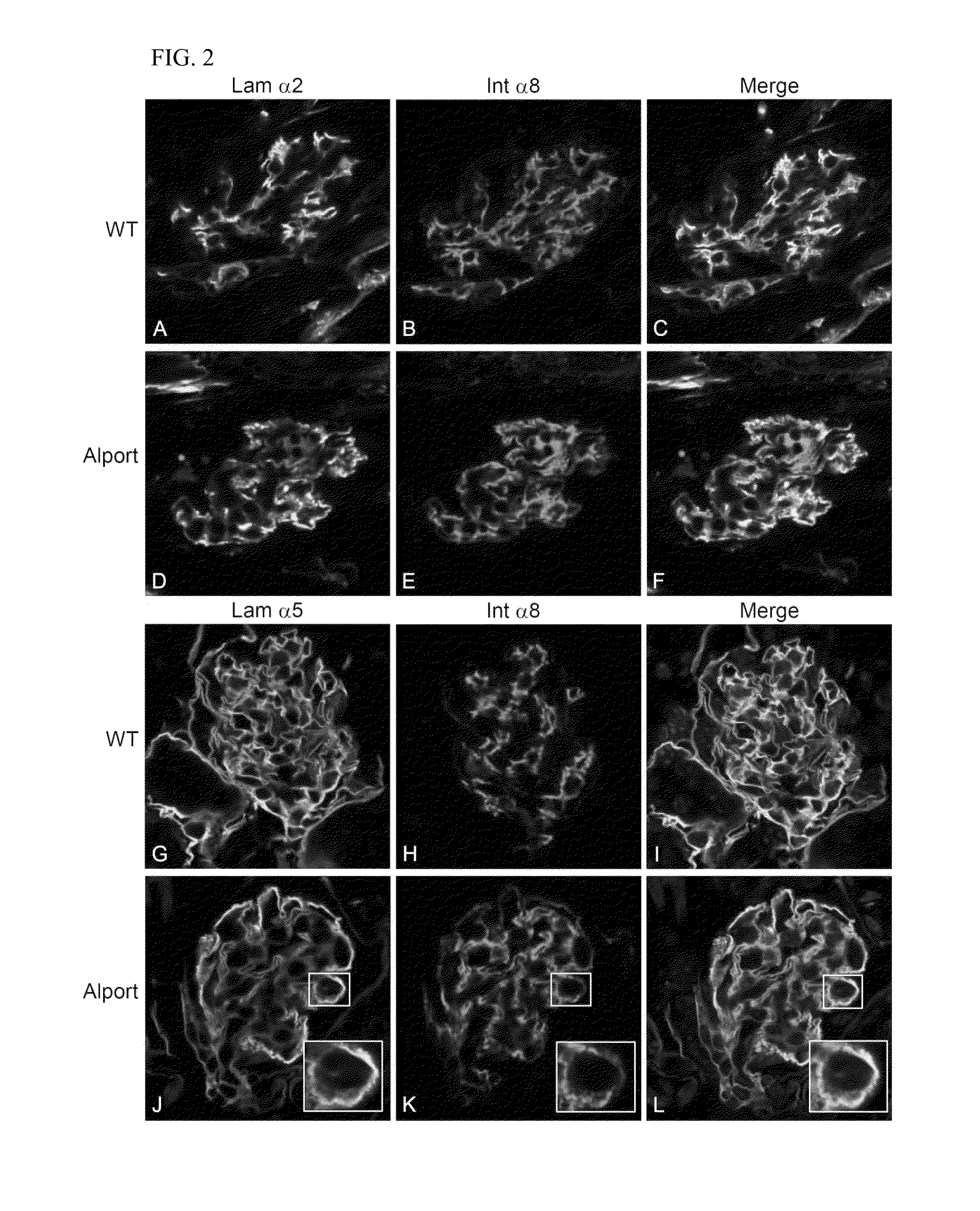
Rac1 inhibitors for the treatment of alport glomerular disease
The present invention provides methods of treating Alport syndrome in a subject by the administration of an agent that can blocks the activation of RAC1 / CDC42 members of the rho family of small GTPases. Such agents include, but are not limited to, the endothelin receptor antagonists such as bosentan and letairis and neutralizing antibodies to endothelin-1. Such administration prevents invasion of the glomerular capillary tufts by mesangial lamellipodial / filopodial processes, blocks mesangial process invasion abrogates the deposition of laminin 211 in the GBM, and prevents the activation of maladaptive expression of proteins known to contribute to glomerular disease progression.
Owner:FATHER FLANAGANS BOYS HOME DOING BUSINESS AS BOYS TOWN NAT RES HOSPITAL
Fasudil in combination therapies for the treatment of pulmonary arterial hypertension
Owner:ASAHI KASEI PHARMA
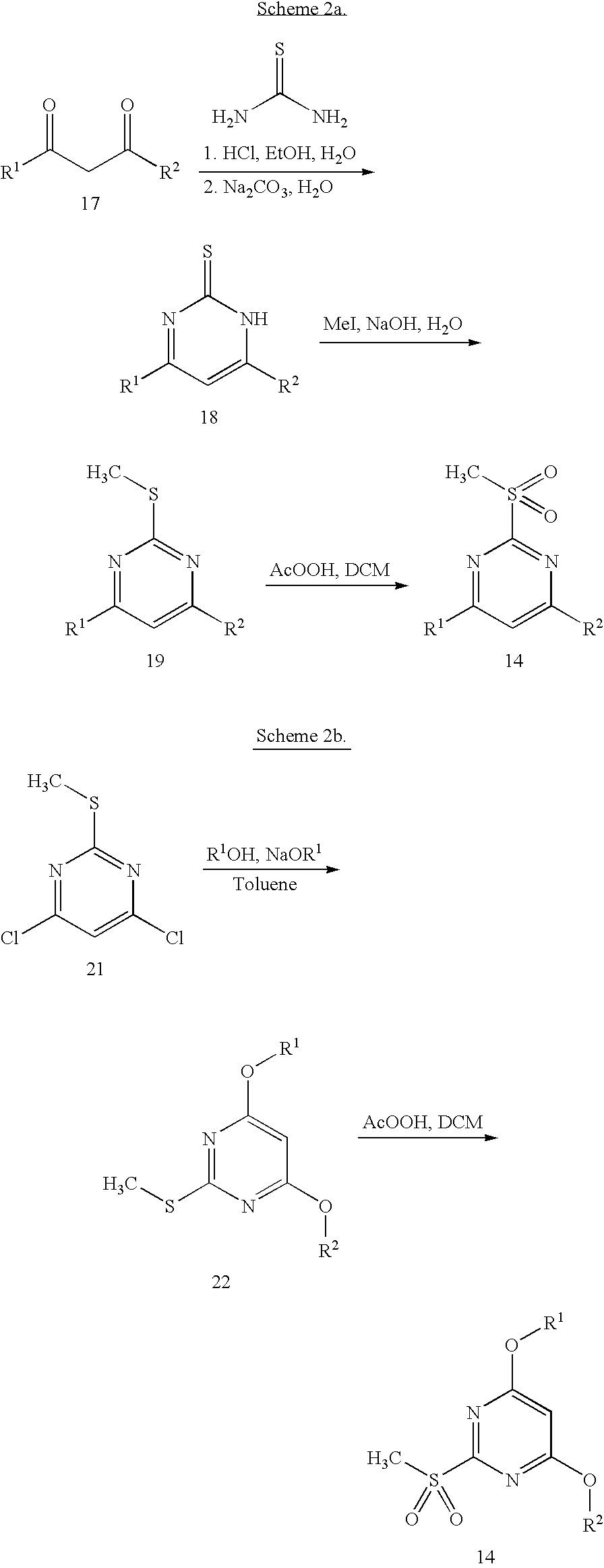
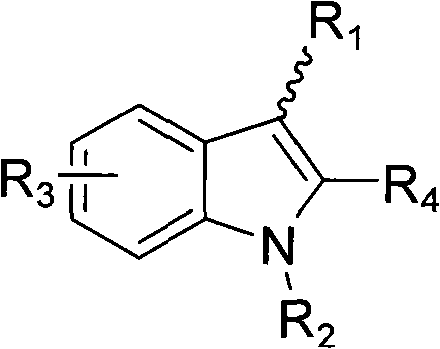
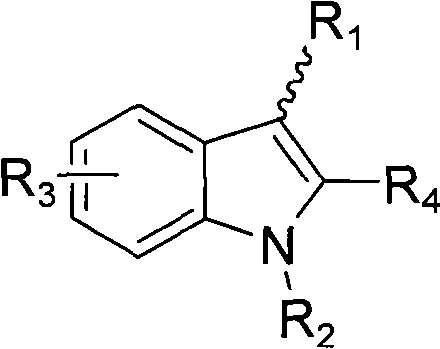
Indole ring substituted pyrazole carboxylic acid endothelin receptor antagonist as well as preparation method and application thereof
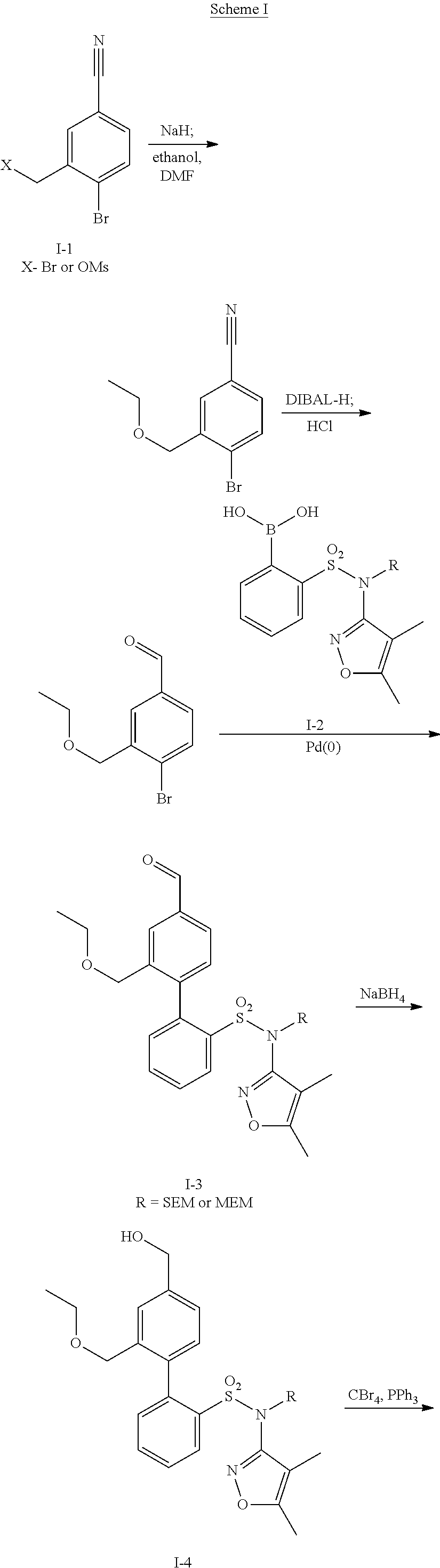
The invention provides a compound shown in the general formula I or pharmaceutically acceptable salts thereof. In the general formula I, R1 is shown in the specification; R2 and R5 are respectively H, alkyl, cycloalkyl, carboxyl, aryl, substituted aryl, benzyl or substituted benzyl independently; R3, R4 and R6 are respectively H, halogen, nitryl, hydroxy, alkyl, cycloalkyl, alkoxy, aryl, substituted aryl or OR' independently; R' is alkyl, cycloalkyl, aryl, substituted aryl, benzyl or substituted benzyl; and R7 is H or alkyl. The invention also provides a method for preparing the compound and an application of the compound. The compound shown in the general formula I or salts thereof have the function of resisting endothelin, can serve as active ingredients for preparing the drugs for treating hypertension or pulmonary arterial hypertension and are effective in a quite wide dosage range.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
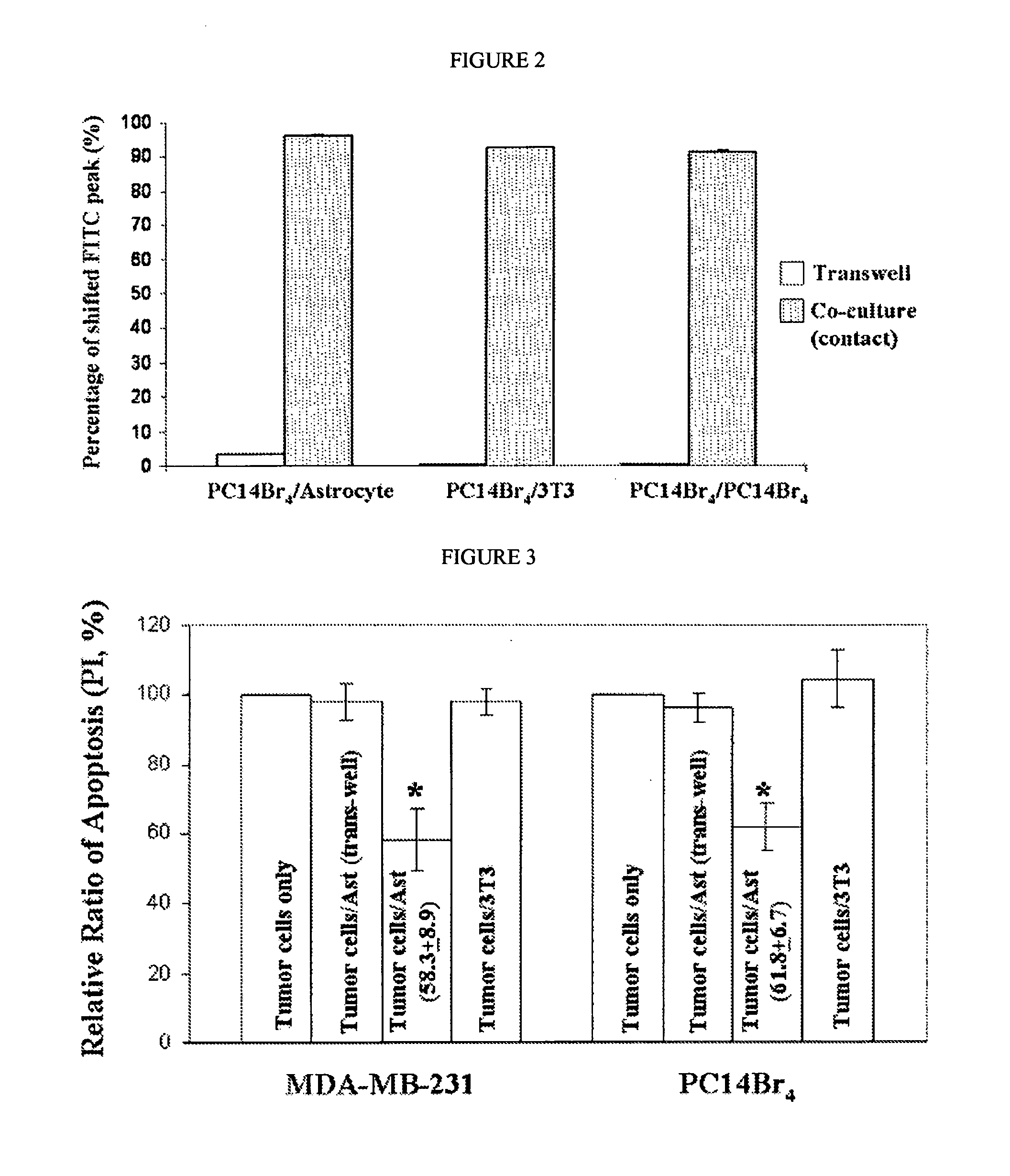
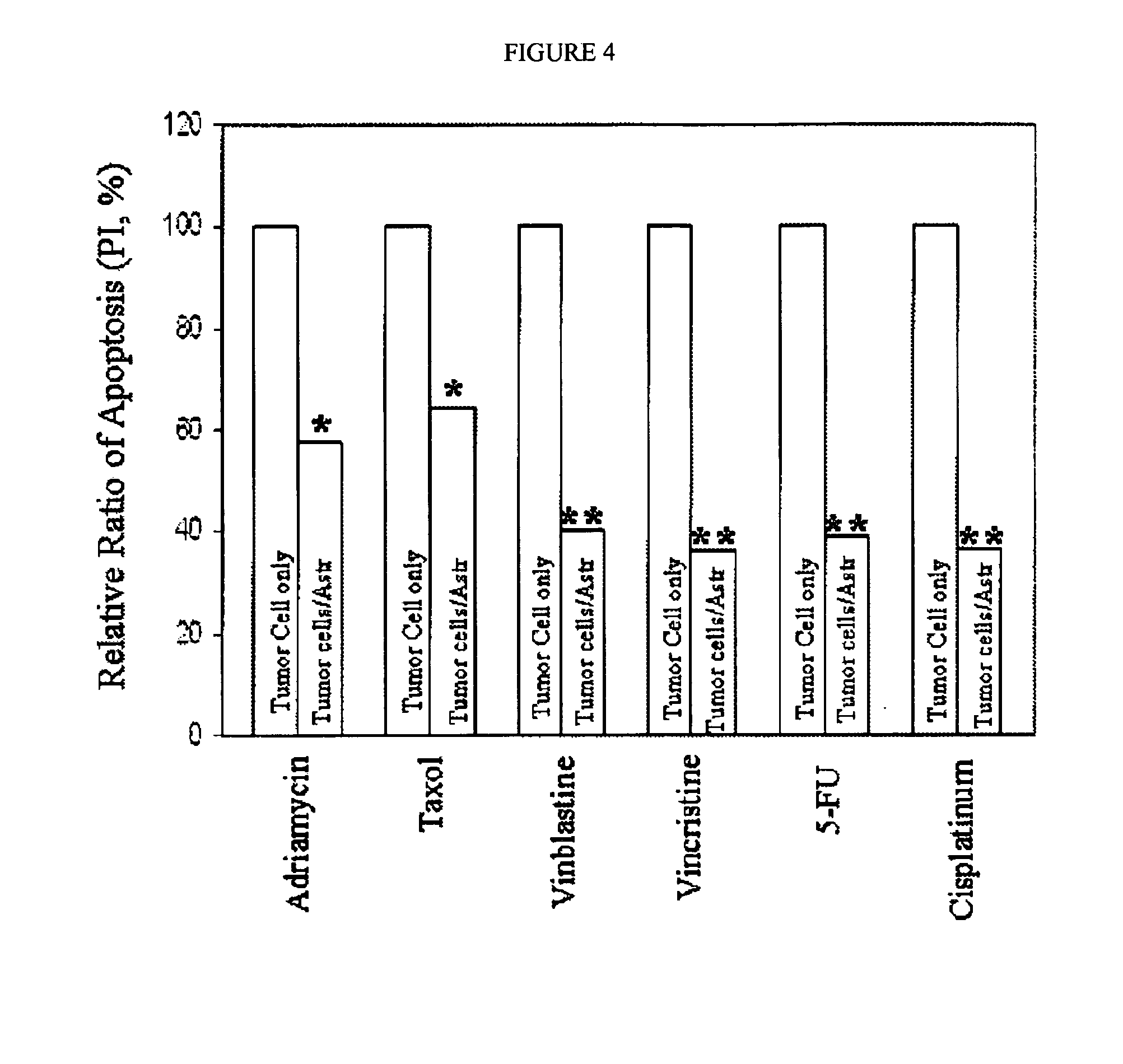
Treatment of astrocytes-tumor cells inhibitors of endothelin receptors
InactiveUS20150352113A1Maintain steady stateProtect neuronsOrganic active ingredientsBiocideCytotoxicityEndothelin receptor antagonist
The disclosure relates to an endothelin receptor antagonist for use in the prevention or treatment of brain metastases in combination with a cytotoxic chemotherapy agent, radiotherapy or both. The endothelin receptor antagonist may for example be bosentan, macitentan or a mixture of bosentan and macitentan.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Endothelin receptor antagonists
This invention relates to novel endothelin receptor antagonists, derivatives, acceptable acid addition salts thereof. The invention also provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions that are beneficially treated by compounds that block the endothelin signaling pathway that leads to vasoconstriction and in particular those diseases or conditions beneficially treated by endothelin receptor antagonists.
Owner:SUN PHARMA IND INC
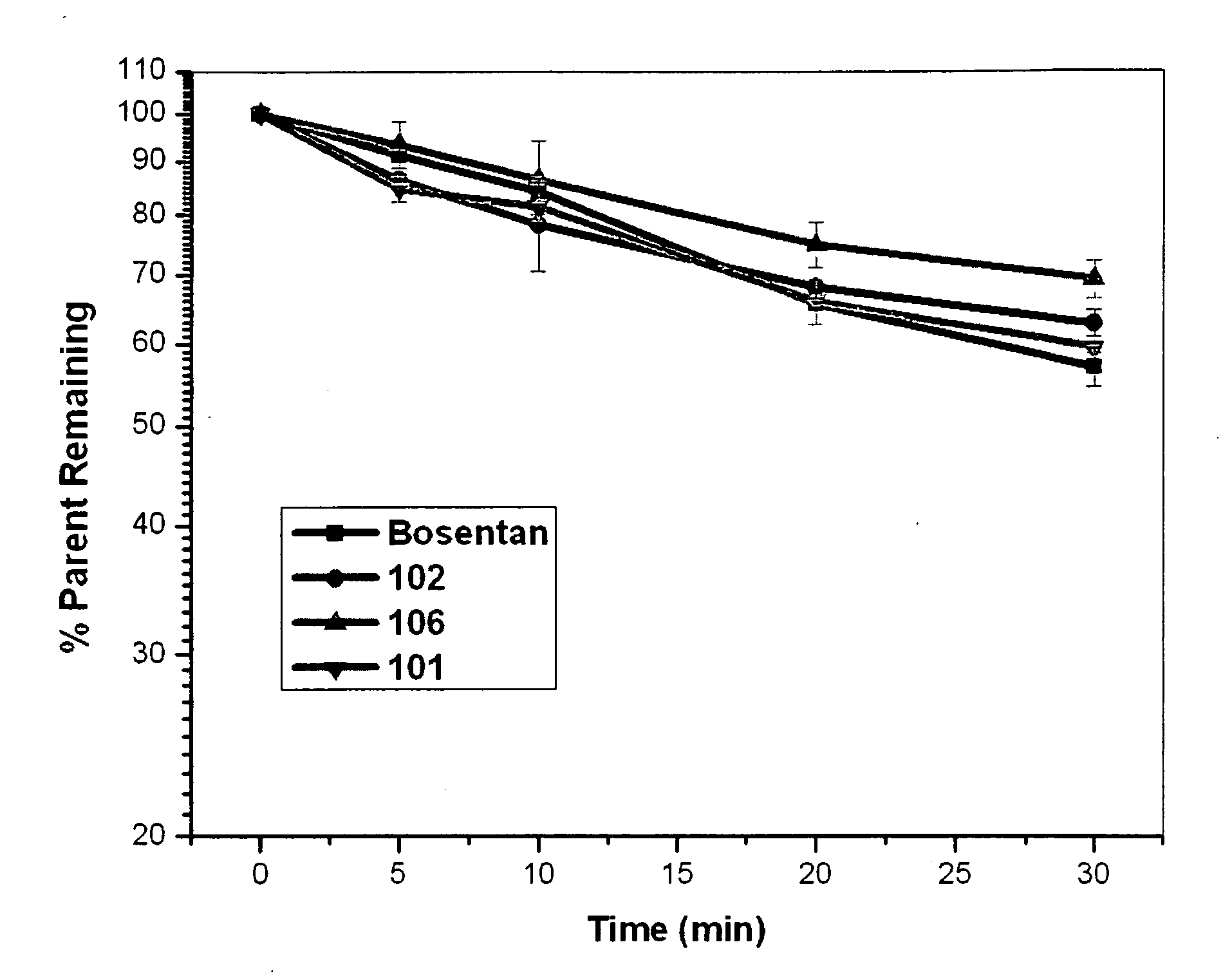
Improved Process For The Preparation Of Endothelin Receptor Antagonists
InactiveUS20110263854A1Commercially viableProcess environmental protectionOrganic compound preparationCarboxylic acid esters preparationEndothelin receptor antagonistChemistry
The present invention relates to improved processes for the preparation of Endothelin receptor antagonists, their salts and intermediates.
Owner:MSN LAB PTE LTD
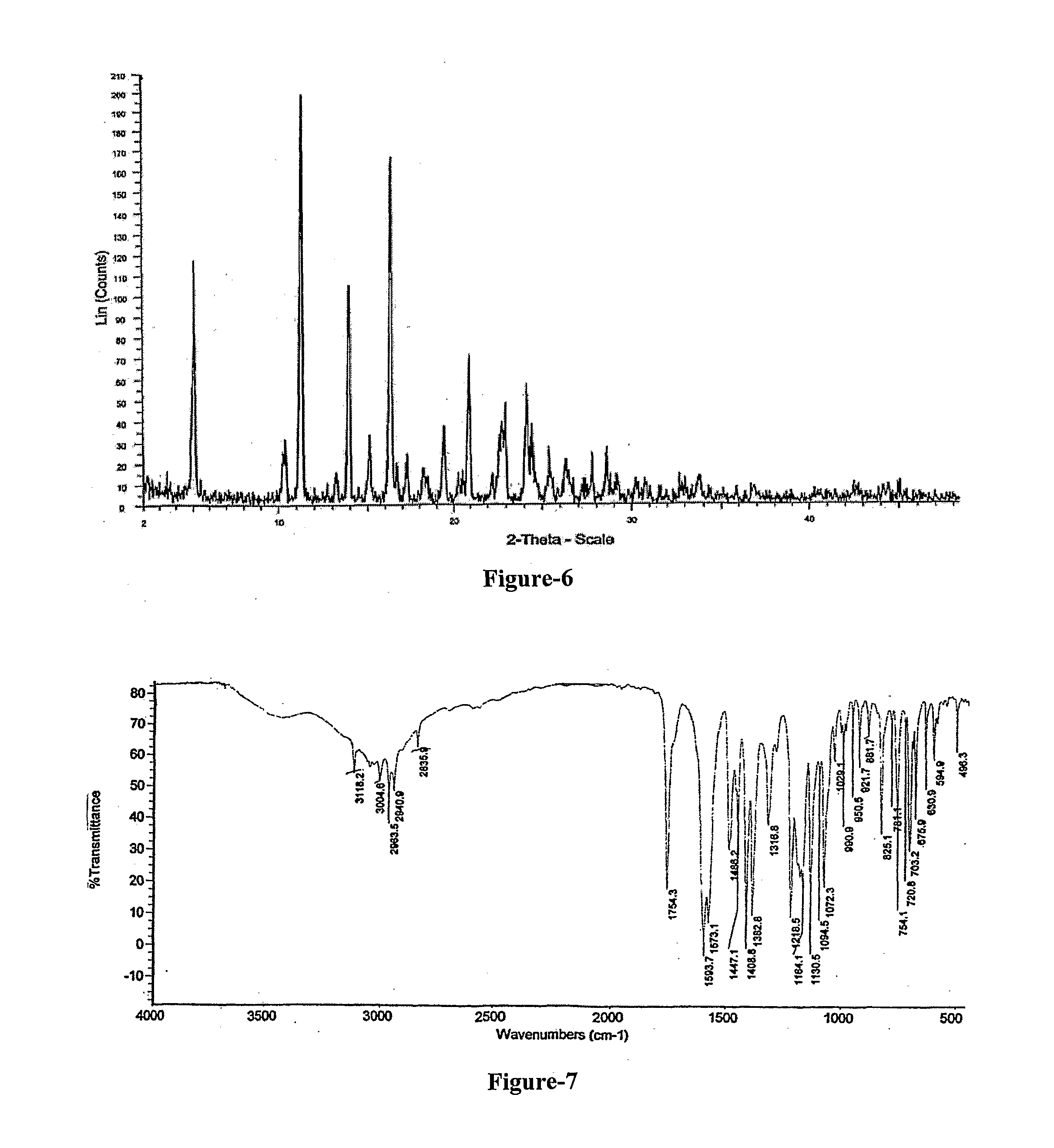
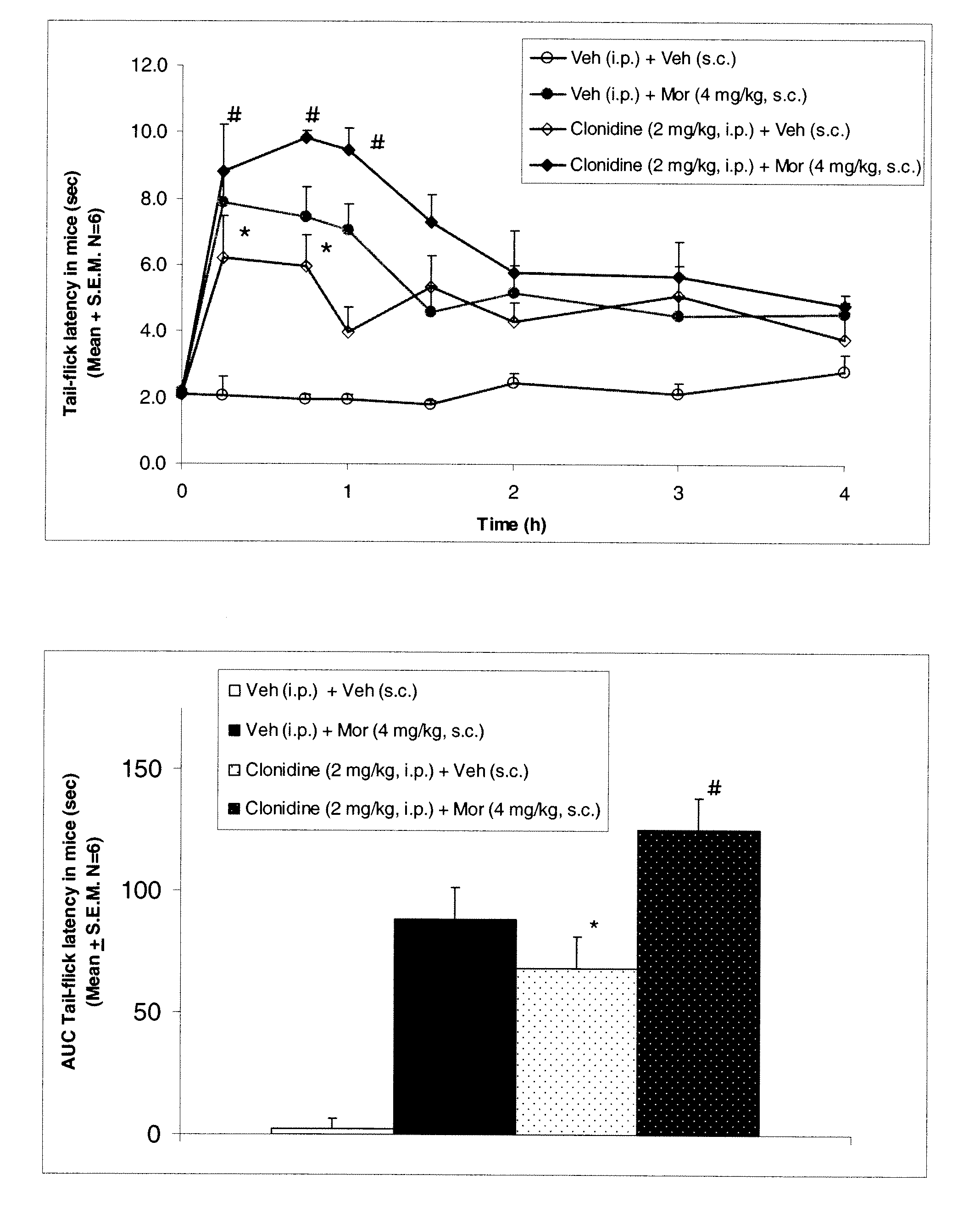
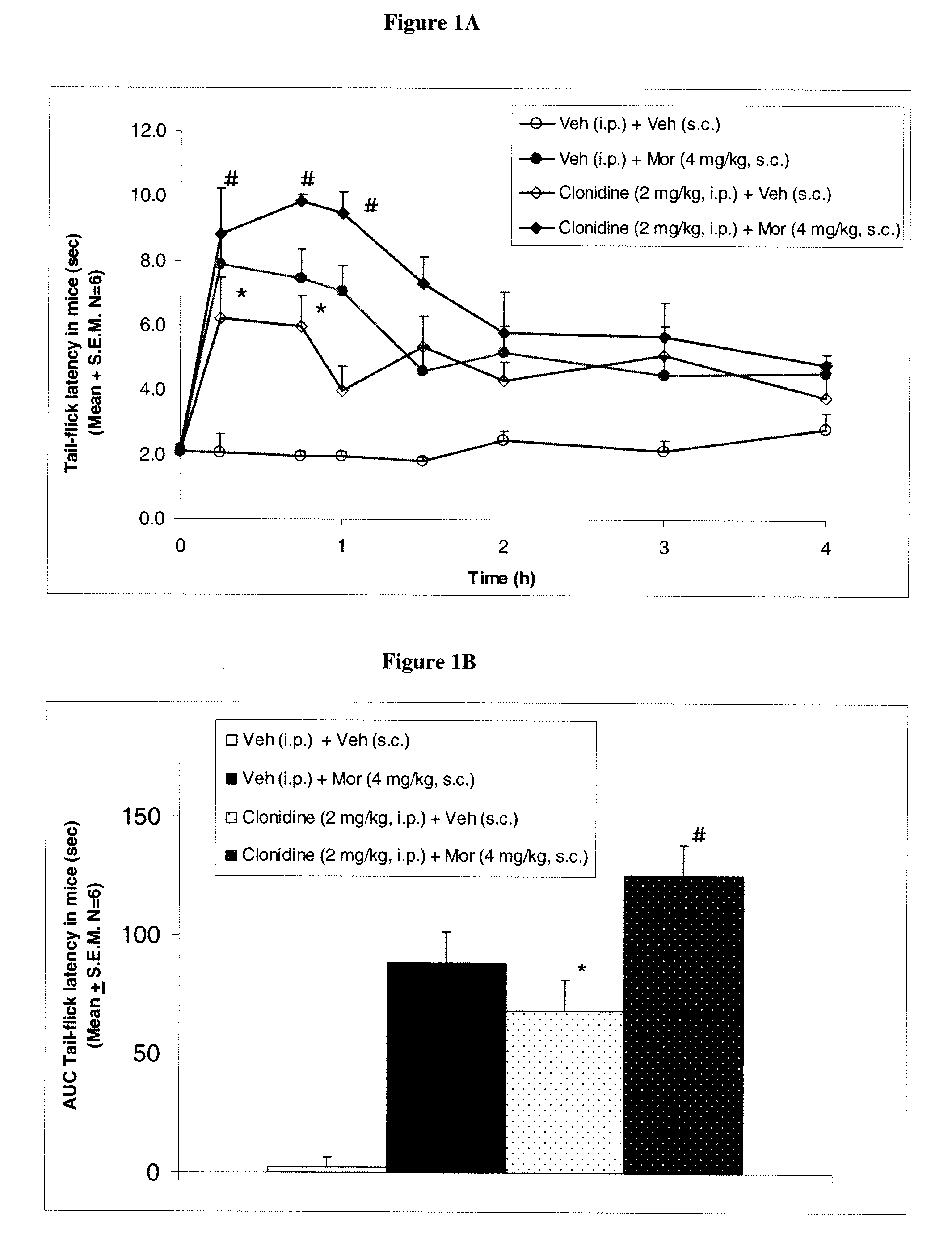
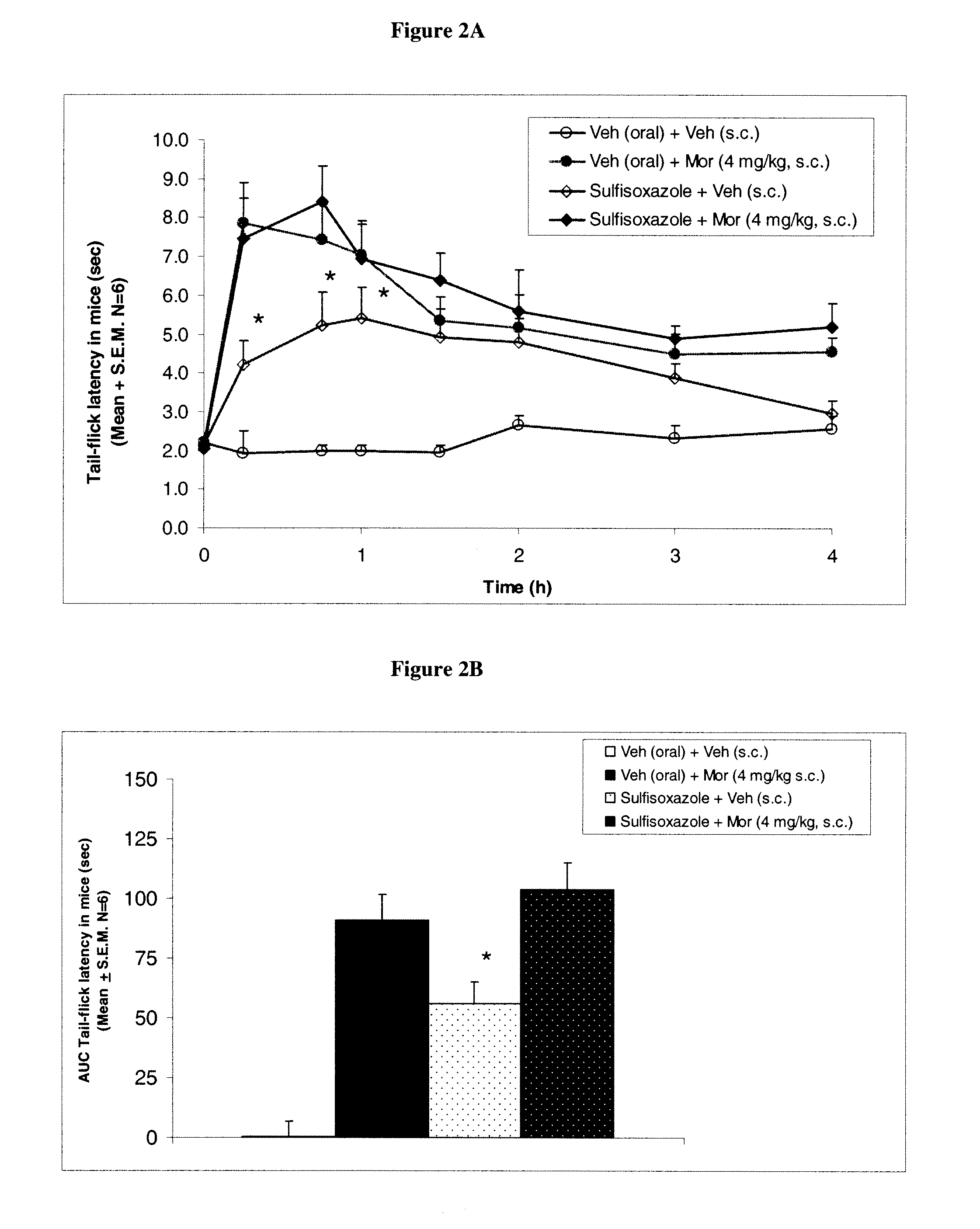
Methods to treat pain using an alpha-2 adrenergic agonist and an endothelin antagonist
InactiveUS20110263542A1Good analgesic effectProduce analgesiaBiocideNervous disorderEndothelin receptor antagonistEndothelin Antagonists
The present invention relates, in general to treatment of pain comprising administering an alpha-2 adrenergic agonist and an endothelin antagonist, wherein administration of the agents acts as an analgesic and ameliorates pain in a subject.
Owner:ENDOGENX
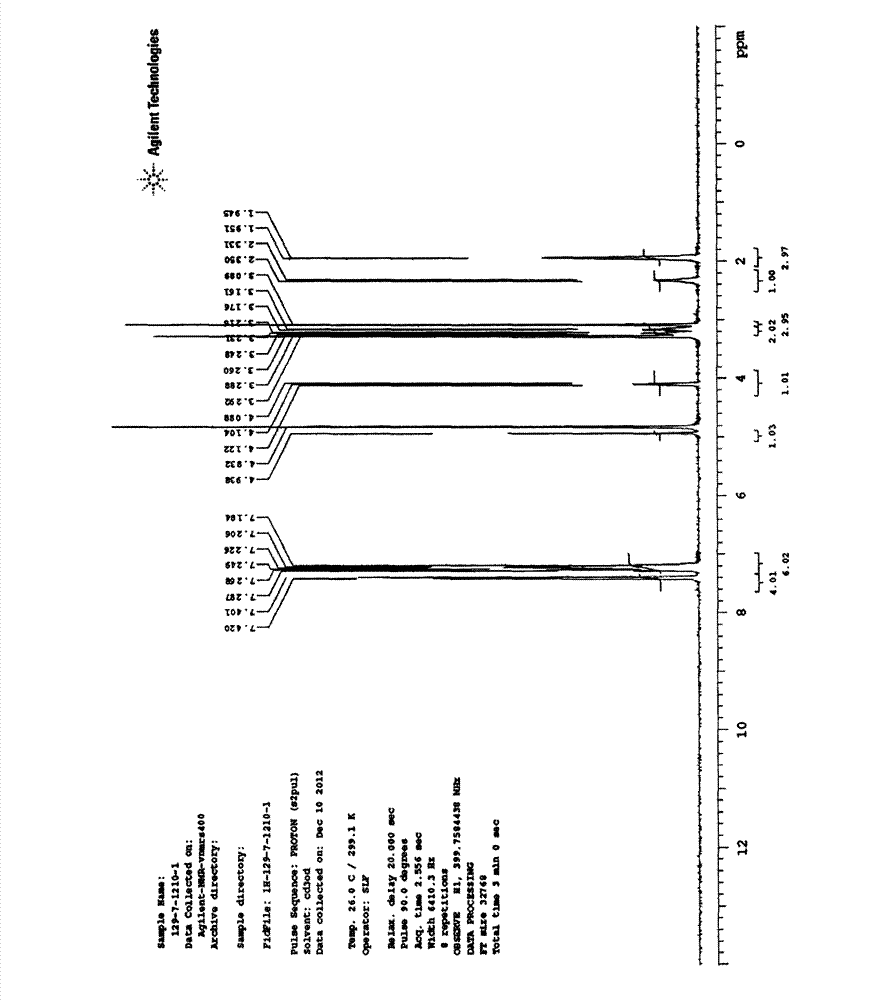
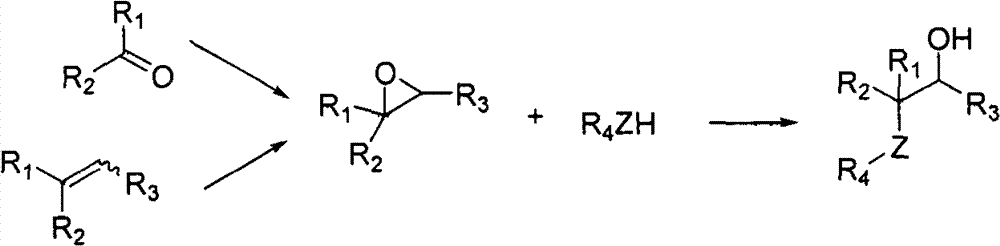
Splitting method of 2-hydracrylic acid racemic mixture
ActiveCN103086877ALow priceLow costOrganic compound preparationCarboxylic compound preparationEconomic benefitsEndothelin receptor antagonist
The invention relates to a splitting method of 2-hydracrylic acid racemic mixture. The method comprises the following steps of: carrying out optical active alkali reaction on 2-hydracrylic acid racemic mixture and L-prolinamide, separating a single isomer L-prolinamide salt of 2-hydroxyl acrylic acid compound; and taking the single isomer of the 2-hydroxyl acrylic acid compound as important intermediate for synthetizing endothelin receptor antagonist for treating cardiovascular disease. Therefore, the single isomer with high optical purity is separated by the splitting method which is simple to operate and is provided by the invention; and the method is cheap in required material and reagent, and suitable for industrial production, and has good economic benefit.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com