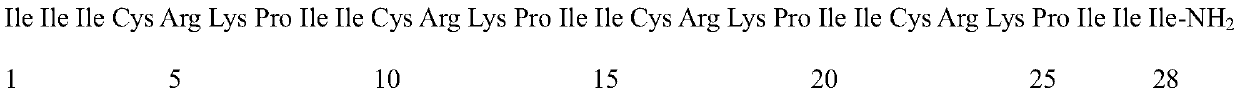Patents
Literature
145 results about "Chymase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
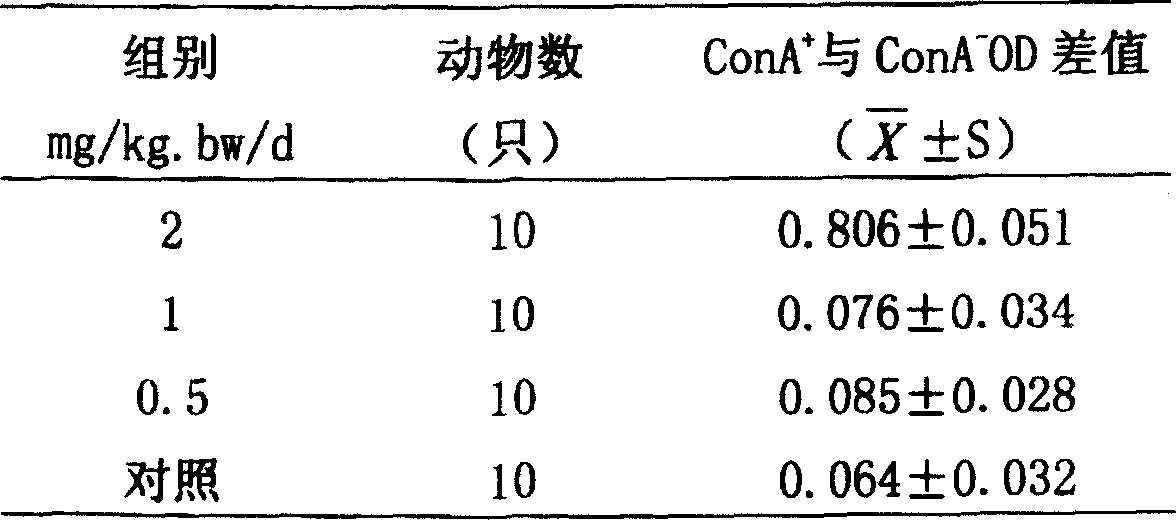
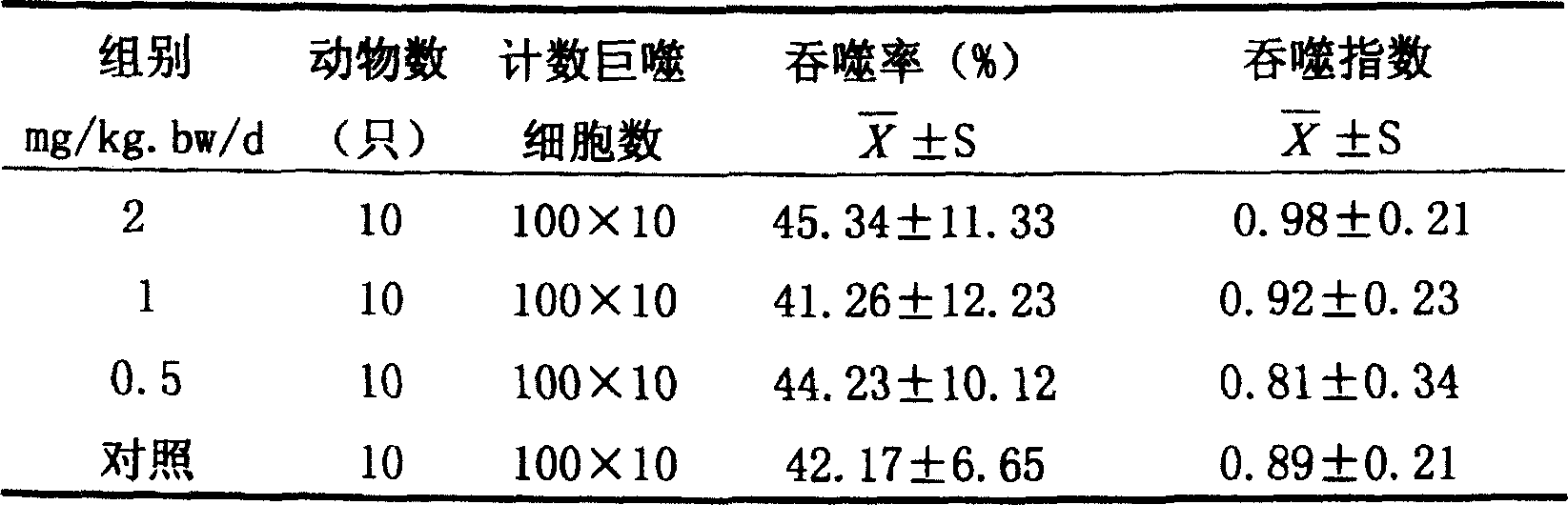
Chymases (EC 3.4.21.39, mast cell protease 1, skeletal muscle protease, skin chymotryptic proteinase, mast cell serine proteinase, skeletal muscle protease) are a family of serine proteases found primarily in mast cells, though also present in basophil granulocytes (e.g. alpha chymase mcpt8). They show broad peptidolytic activity and are involved in a variety of functions. For example, chymases are released by mucosal mast cells upon challenge with parasites and parasite antigens promoting an inflammatory response, and chymase mcp1 and mcp2 are used for marker for mast cell degranulation in parasite infection such as Nematode, Trichuris muris Chymases are also known to convert angiotensin I to angiotensin II and thus play a role in hypertension and atherosclerosis.
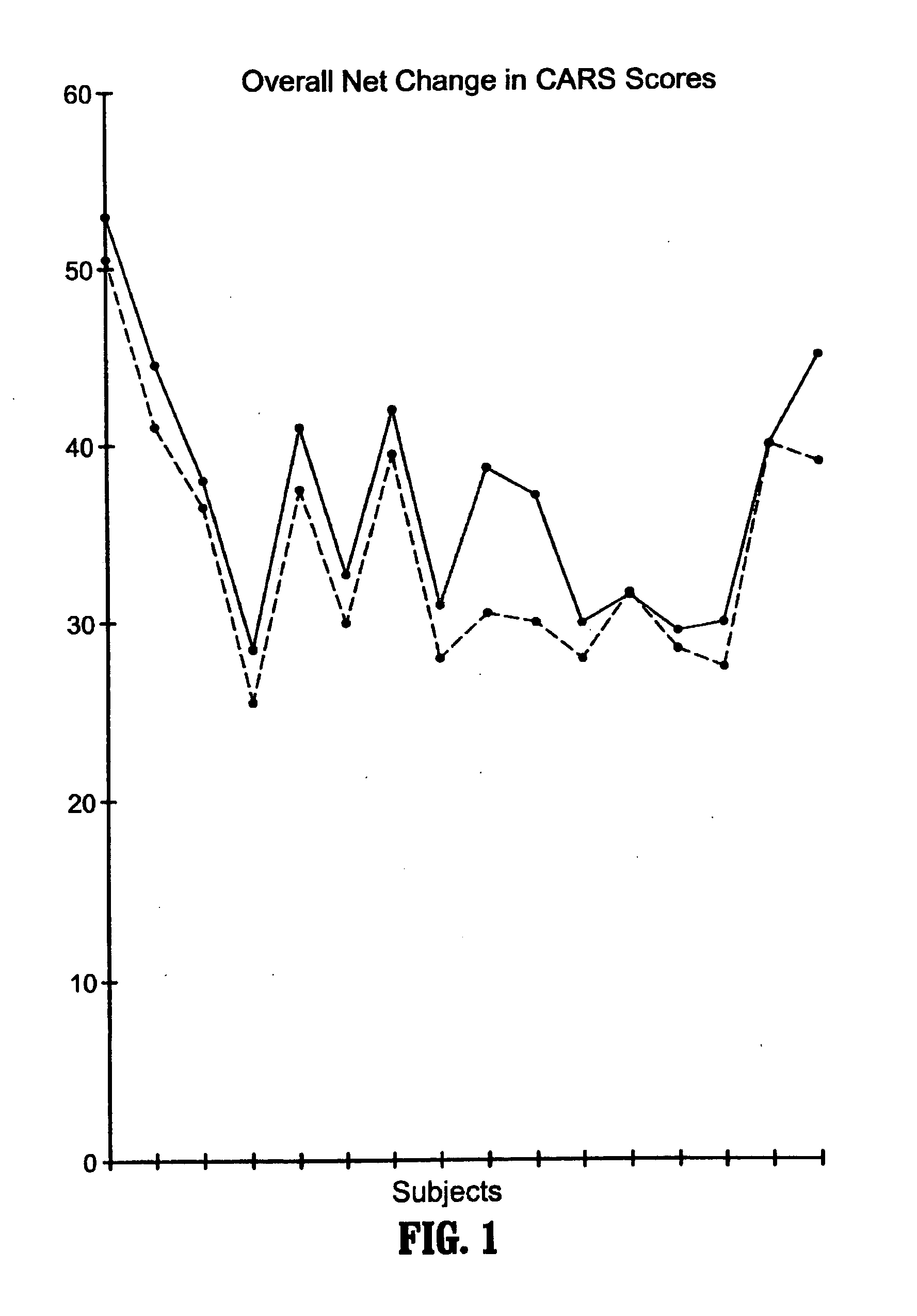
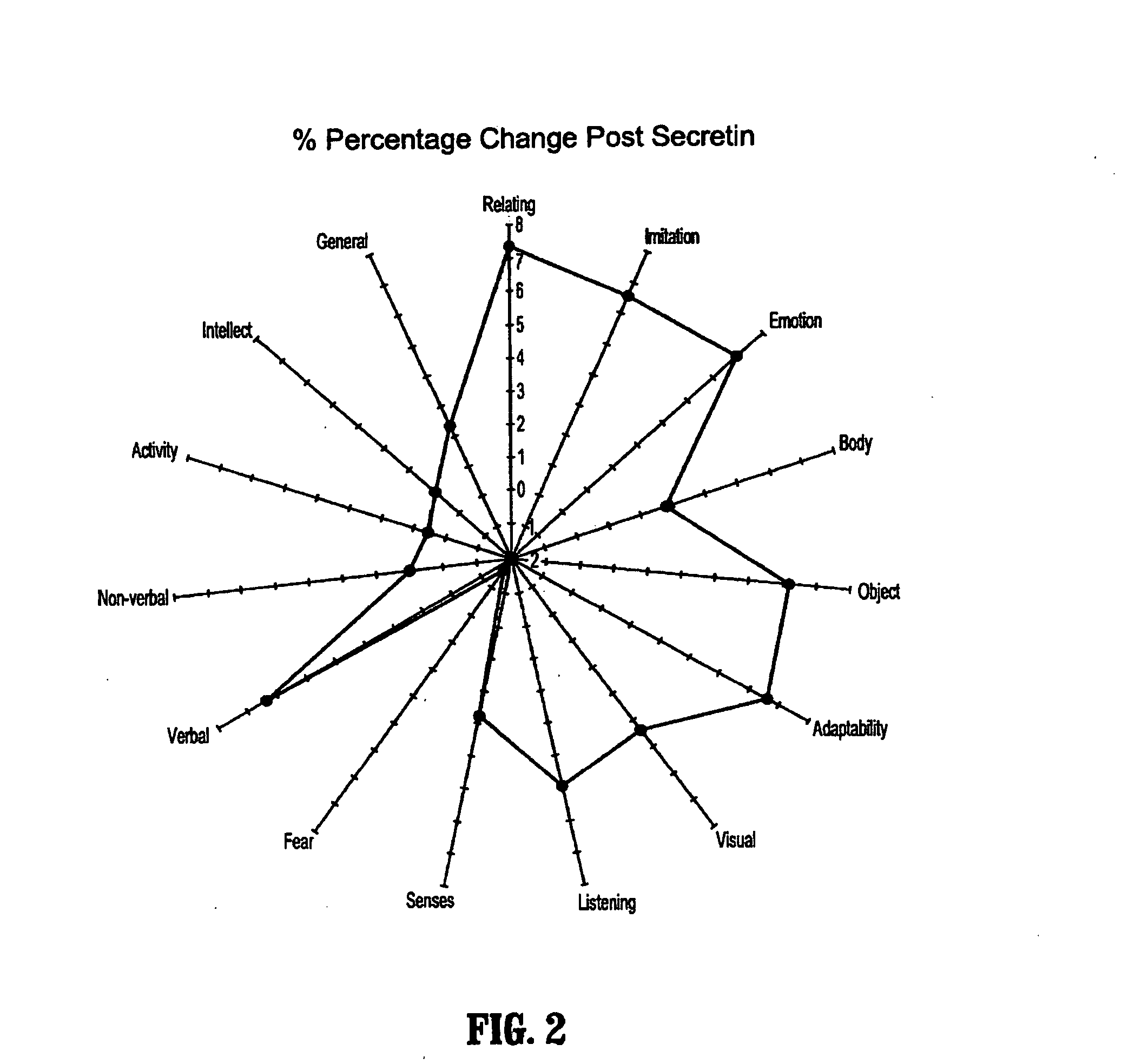
Methods for treating pervasive development disorders
InactiveUS20060183180A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsDiseasePervasive developmental disorder
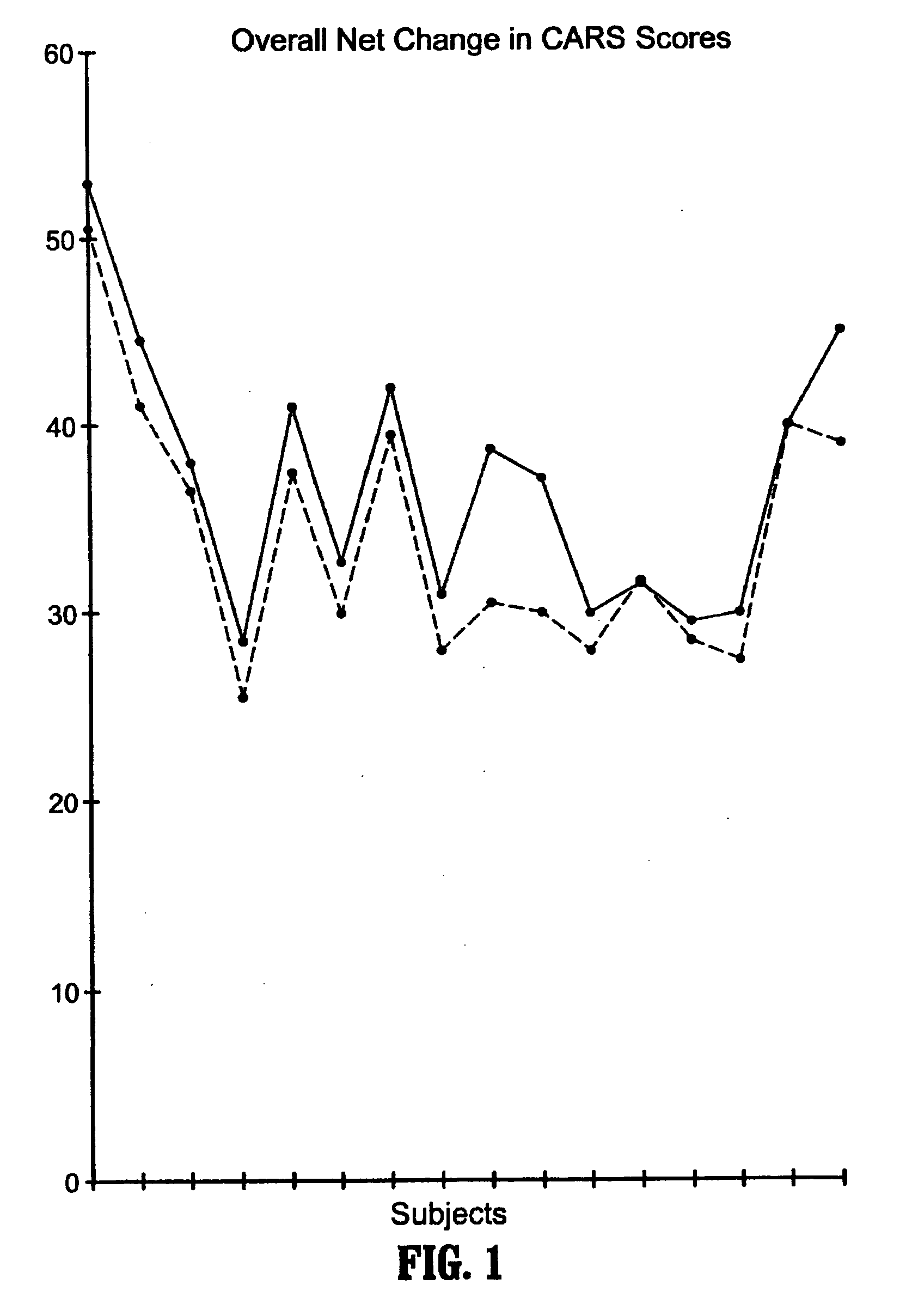
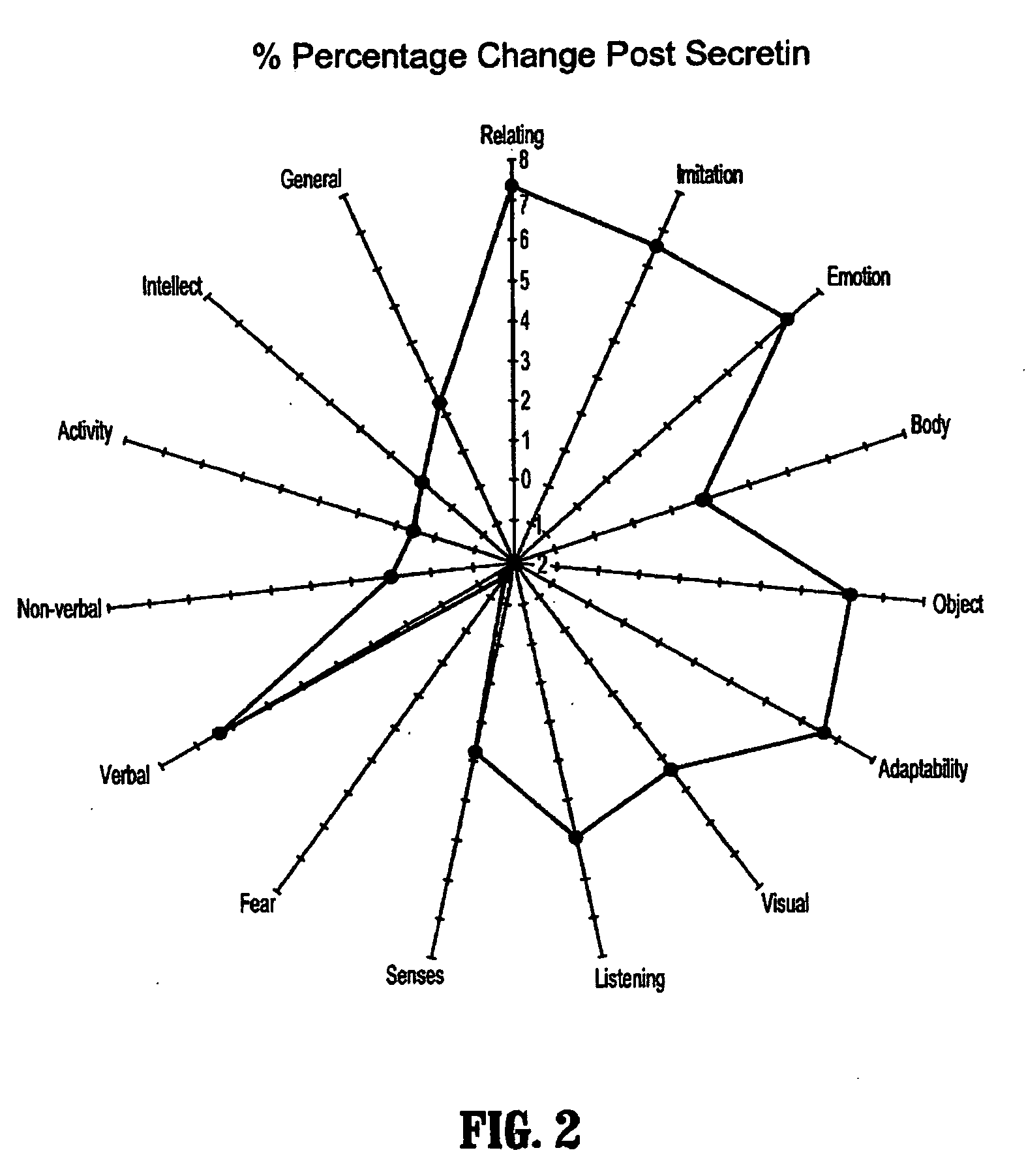
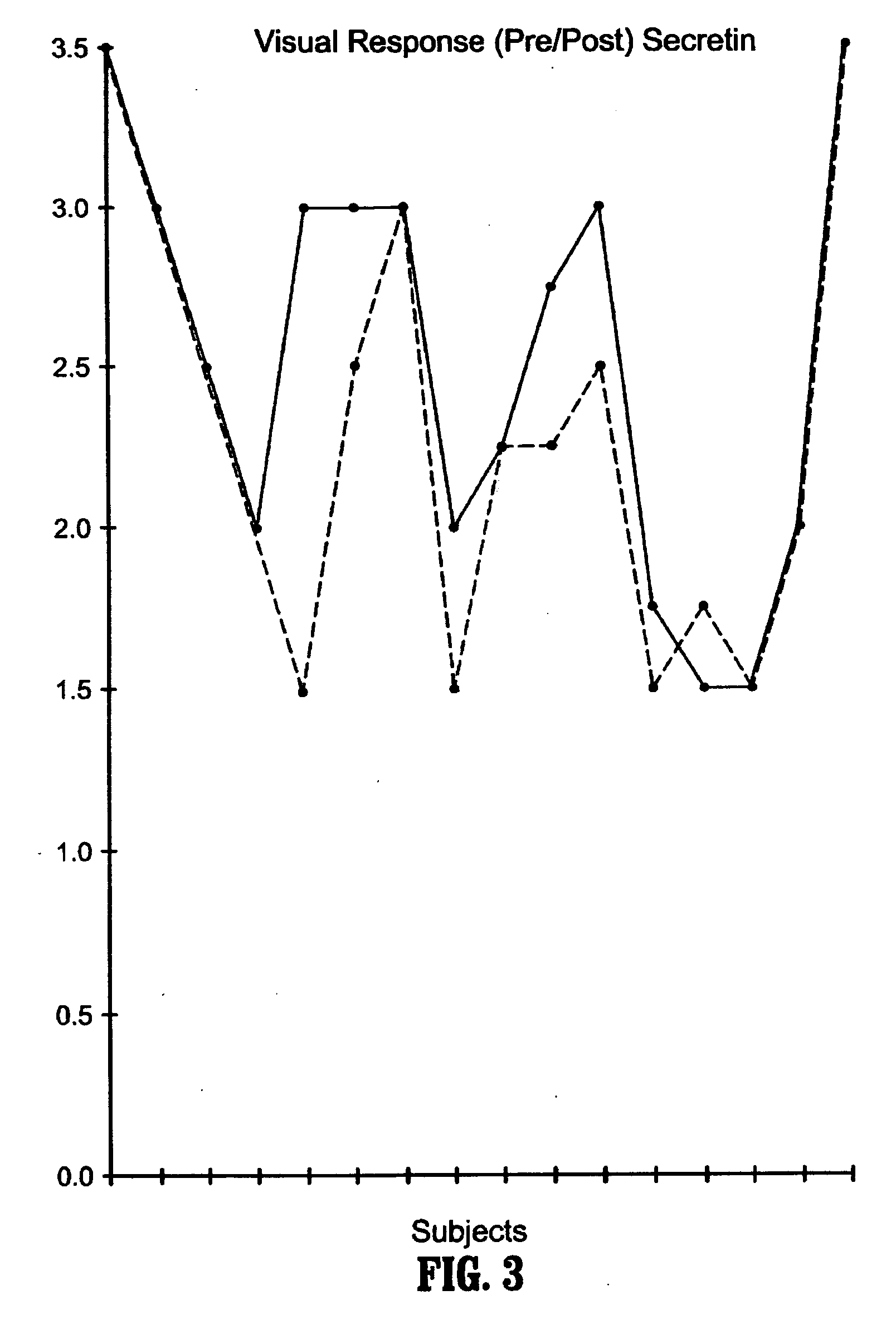
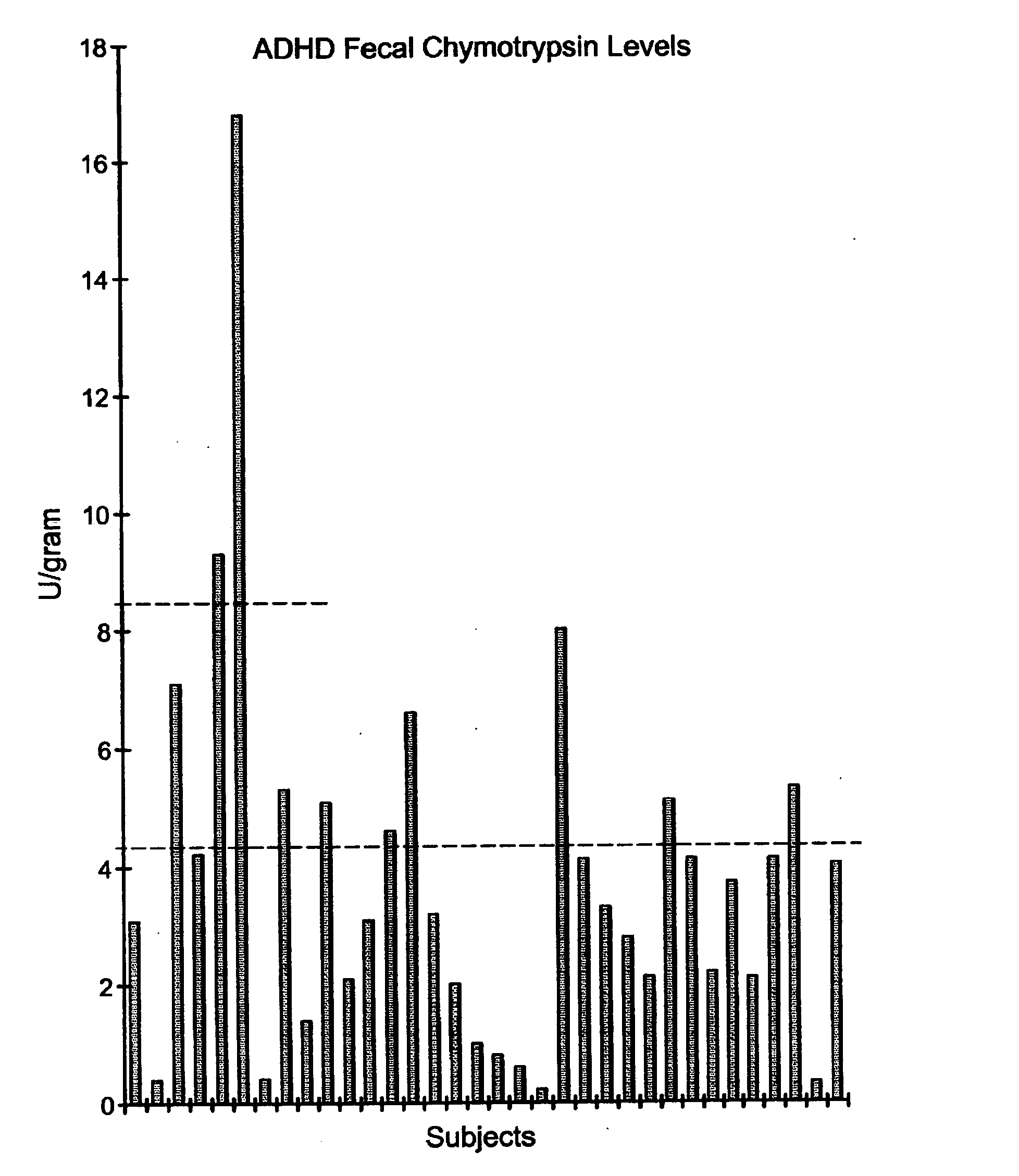
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with i PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Methods for treating pervasive development disorders
InactiveUS20060182728A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsDiseasePervasive developmental disorder
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with i PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
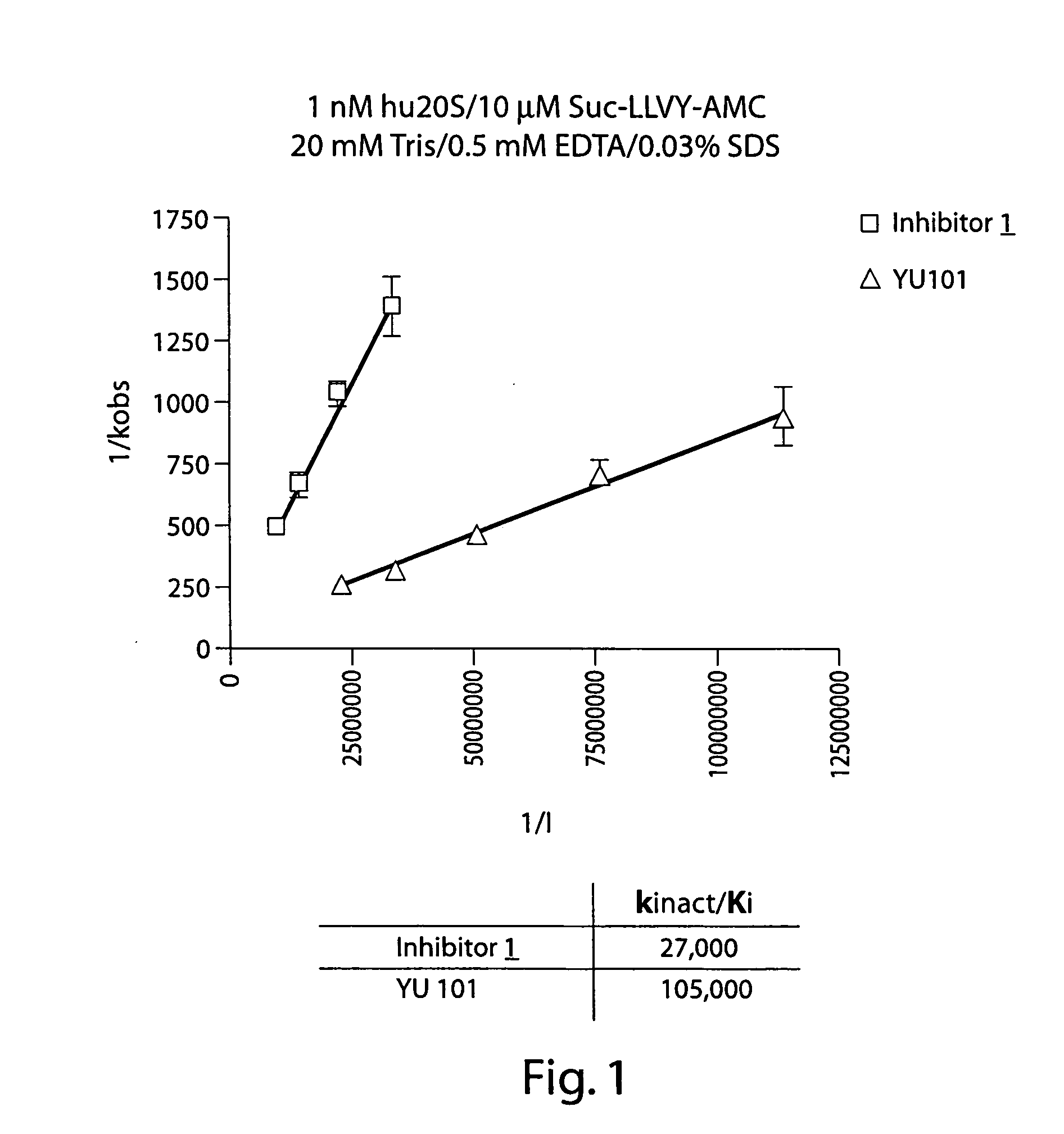
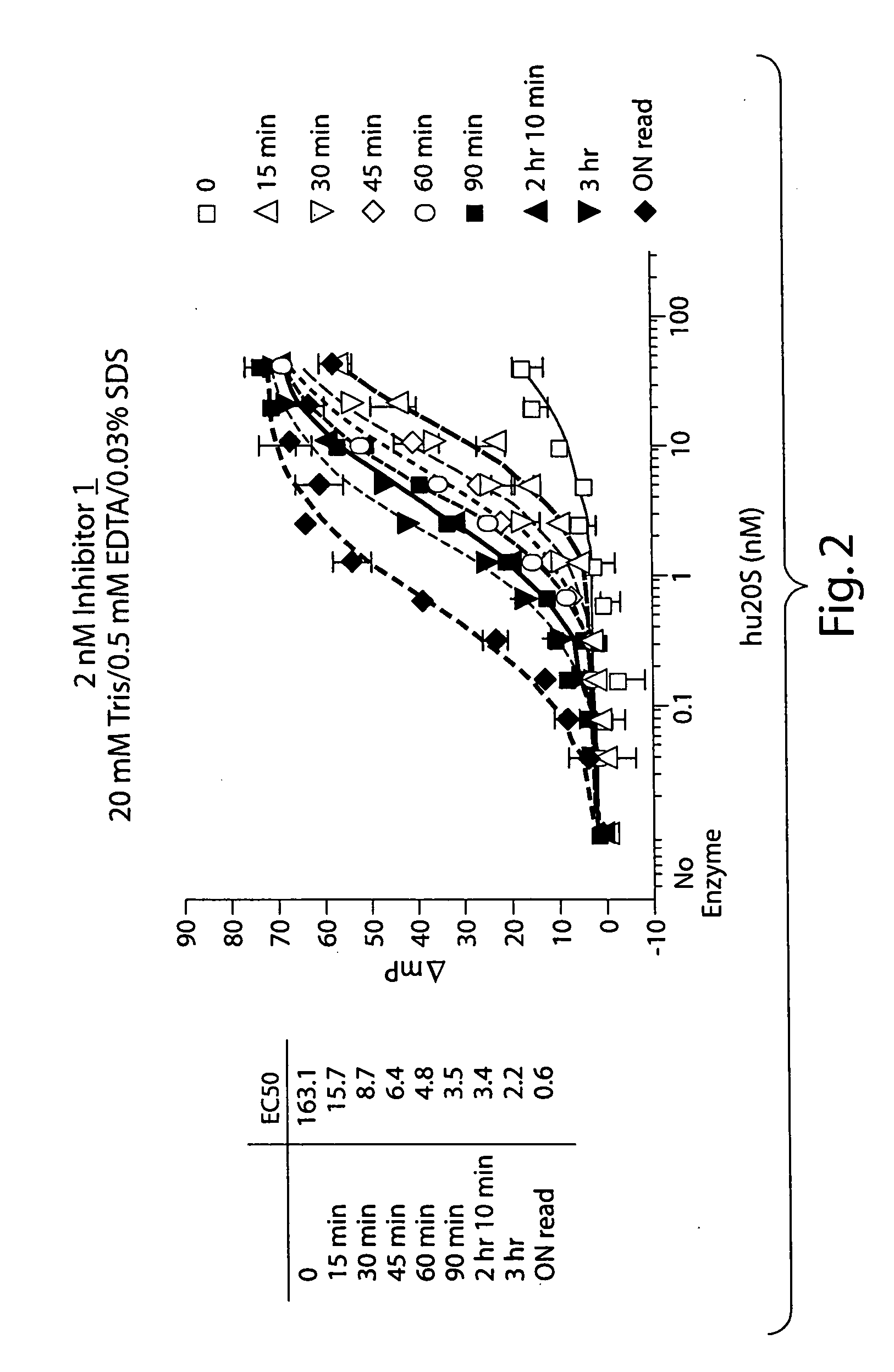
Compounds for enzyme inhibition
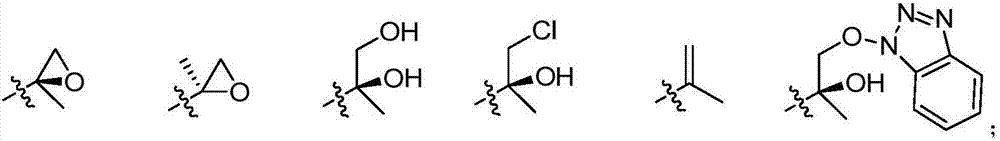
Peptide-based compounds including heteroatom-containing, three-membered rings efficiently and selectively inhibit specific activities of N-terminal nucleophile (Ntn) hydrolases. The activities of those Ntn having multiple activities can be differentially inhibited by the compounds described. For example, the chymotrypsin-like and PGPH activities of the 20S proteasome can be selectively inhibited with the inventive compounds. The peptide-based compounds include at least three peptide units, an epoxide or aziridine, and functionalization at the N-terminus, such as a detectable label. Along with therapeutic utilities, these peptide based compounds can be used in assays useful for screening, monitoring, diagnostic and / or dosing purposes.
Owner:PROTEOLIX INC
Fish serine proteinases and their pharmaceutical and cosmetic use
InactiveUS20020141987A1Avoid injuryInfection-preventing effective amount of the enzymeBiocideCosmetic preparationsAtlantic codSerine proteinases
Fish derived serine proteinases including trypsins and chymotrypsin derived from cod such as Atlantic cod is used for treating and / or preventing a variety of diseases and disorders such as inflammatory diseases, infectious diseases caused by viruses, bacteria and fungal species and diseases where a receptor binding mechanism is involved in the pathogenesis. Pharmaceutical and cosmetic compositions comprising the proteinases are described.
Owner:BJARNASON JON BRAGI
Method for preparing high purity chymotrypsin
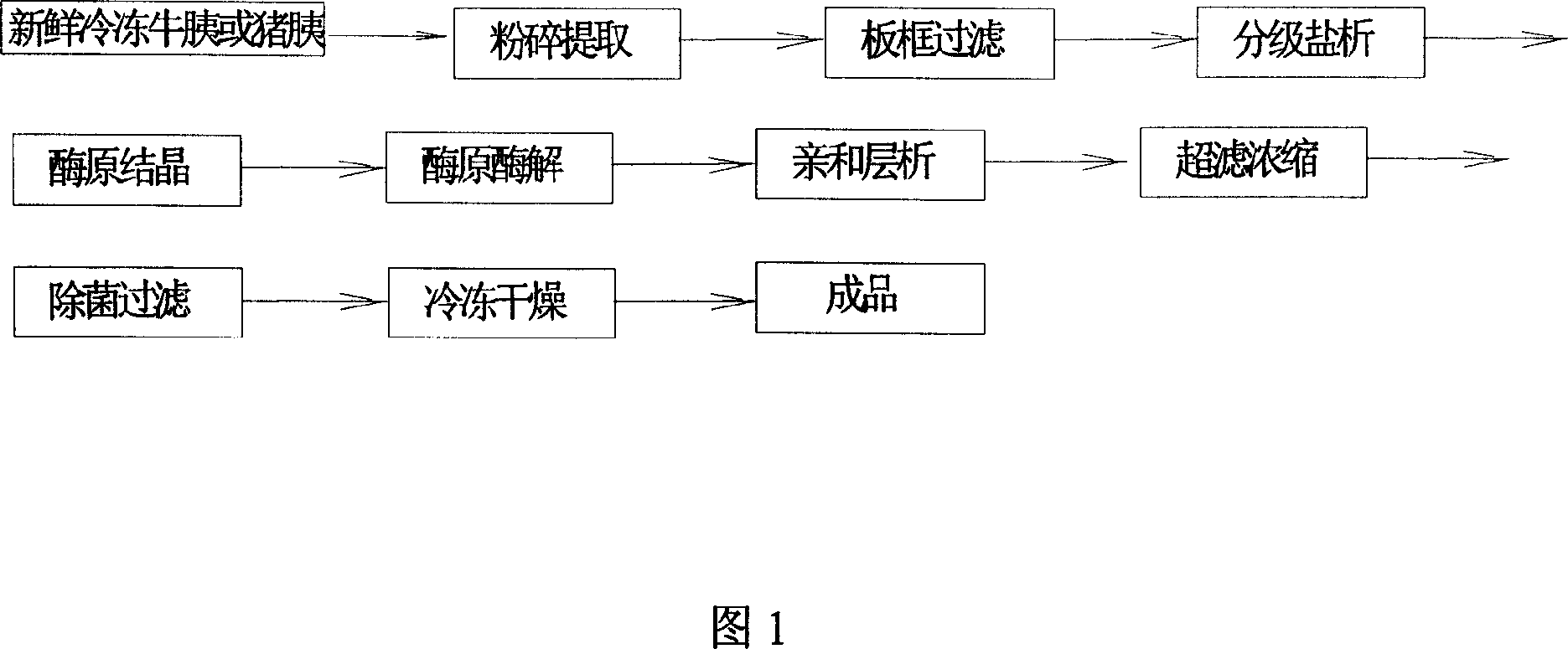
The affinity chromatographic process for producing high purity chymotrypsin includes the following technological steps: freezing fresh ox or pig pancreas as the material, crushing and extracting protein, stepped salting out and crystallizing zymogen, enzymolyzing to obtain coarse product, affinity chromatographic separating and purifying, ultrafiltering and concentrating sterilizing, and vacuum freeze drying to obtain product. Compared with available technology, the present invention has the advantages of suitability for large scale produce, high efficiency, specificity, low cost and high product quality.
Owner:宁波林叶生物科技有限公司
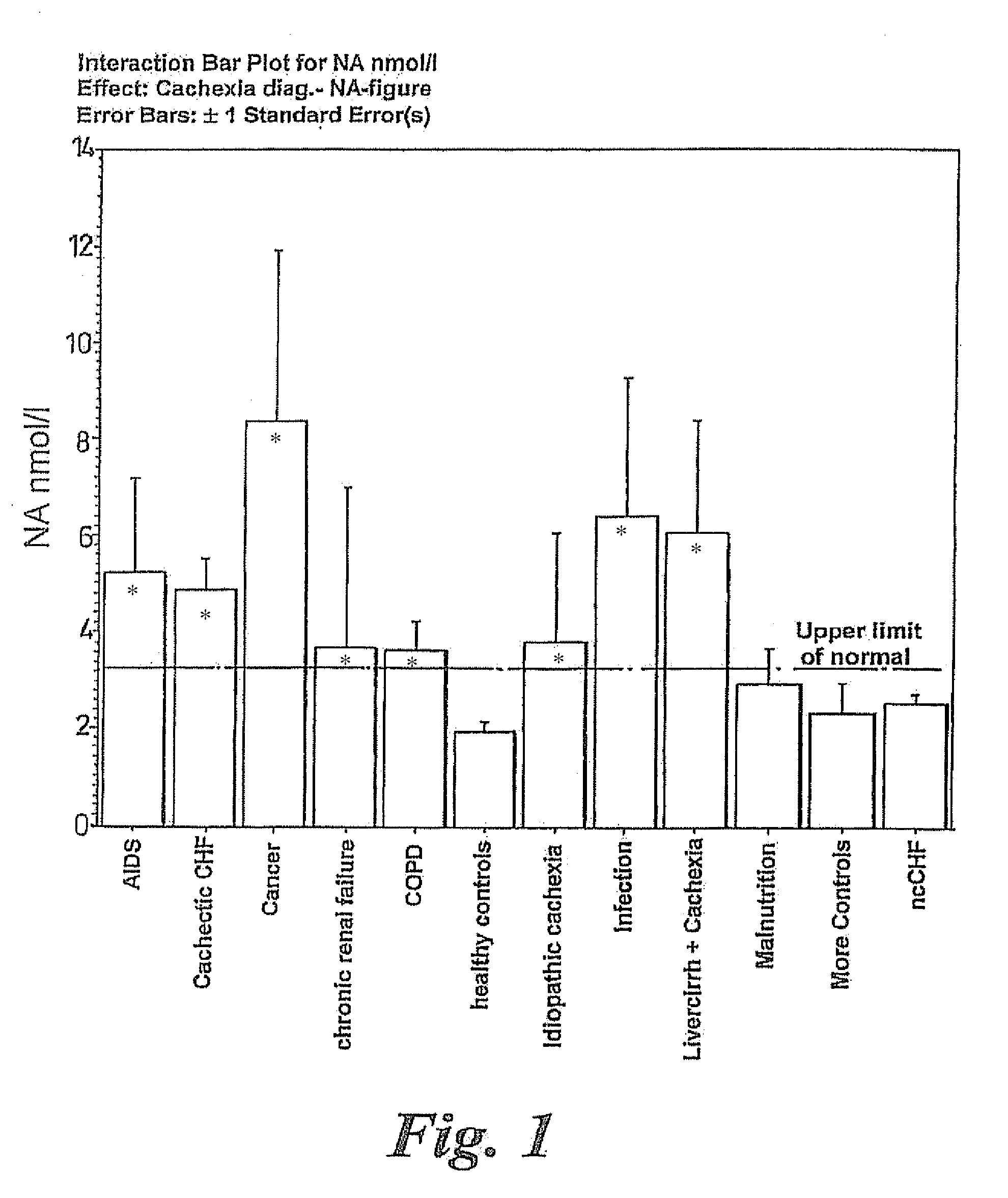
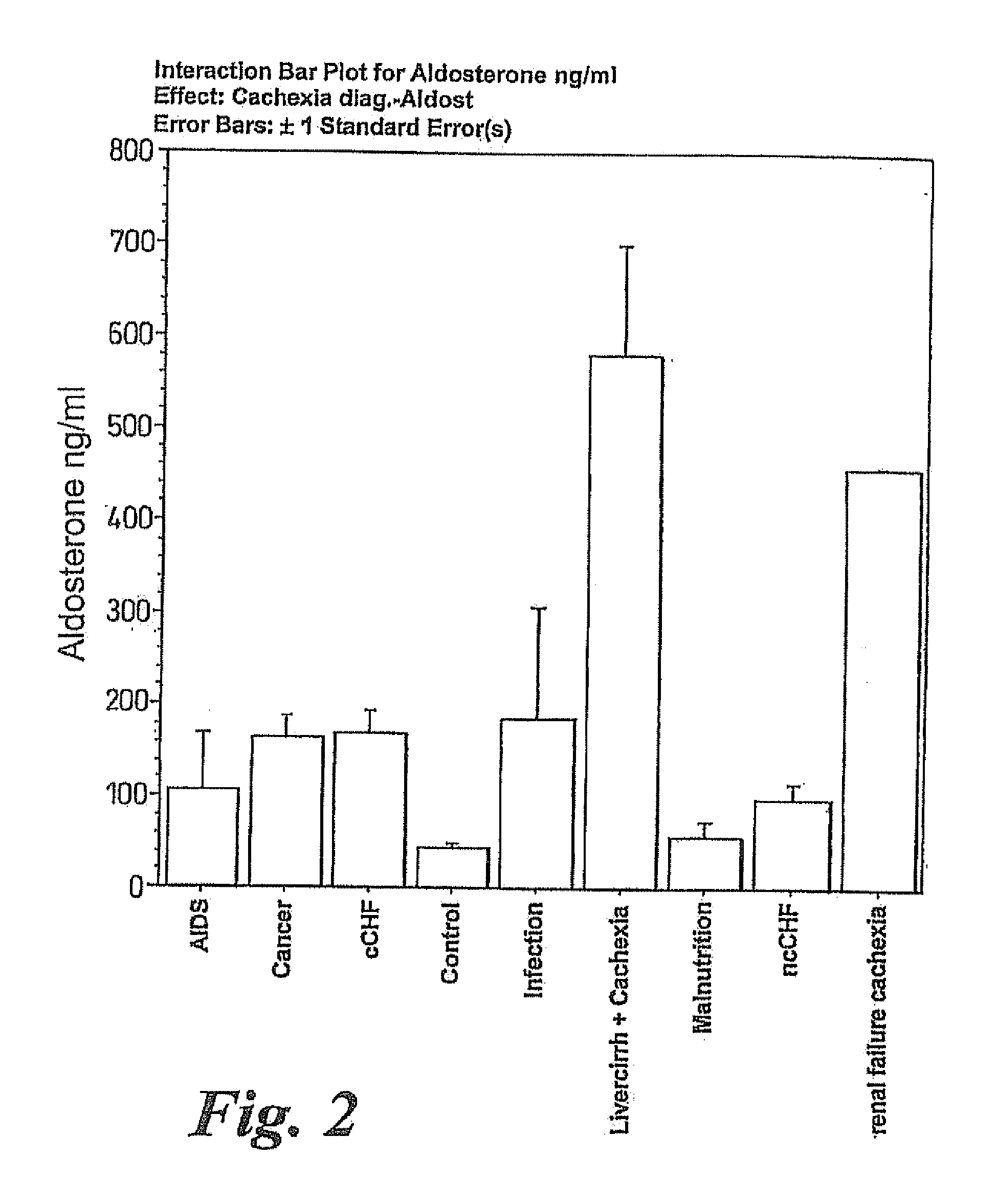
Methods of treating cachexia
InactiveUS7417038B1Reduced metabolic rateIncrease blood flowPeptide/protein ingredientsMetabolism disorderDiseaseImidazoline receptor
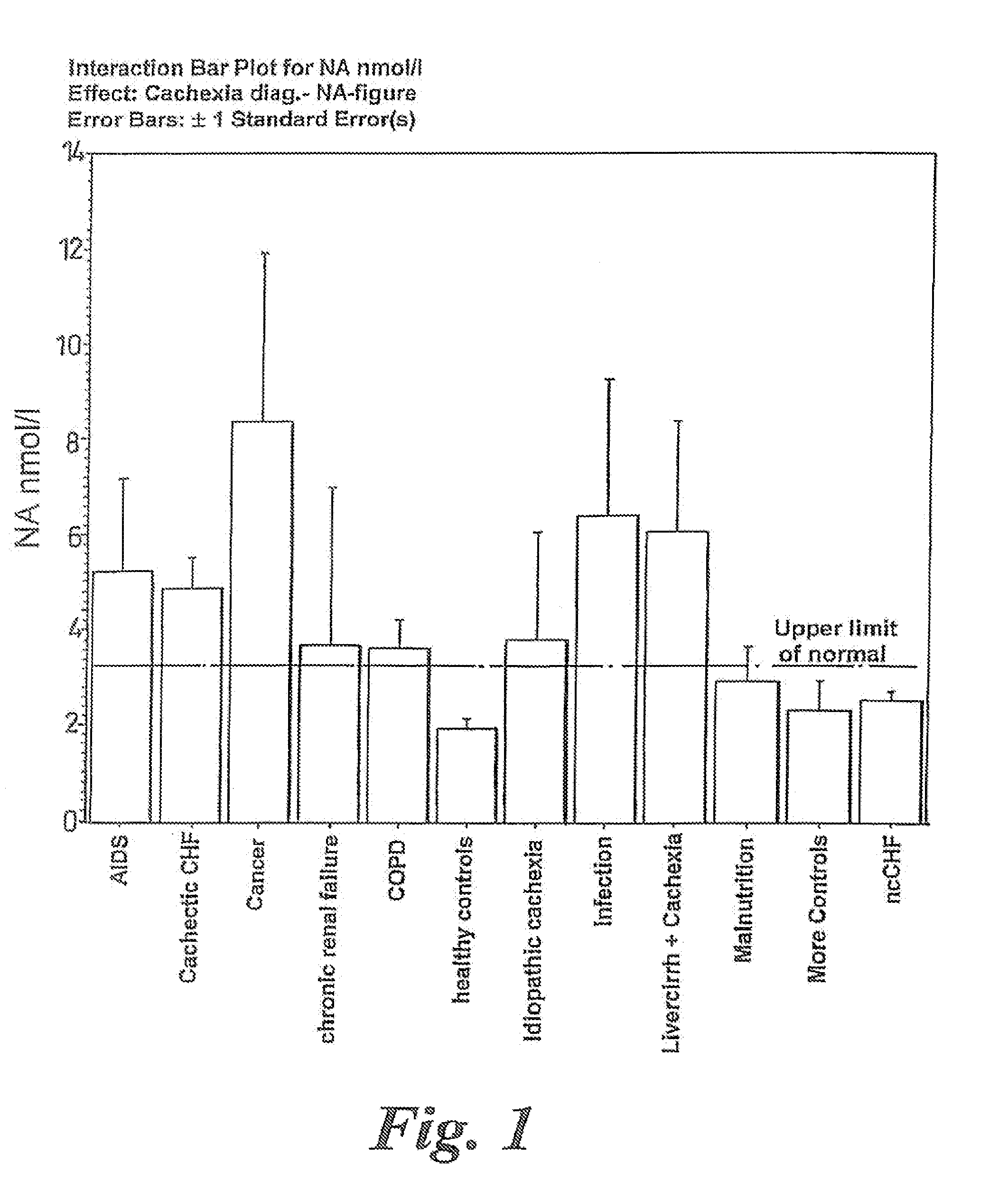
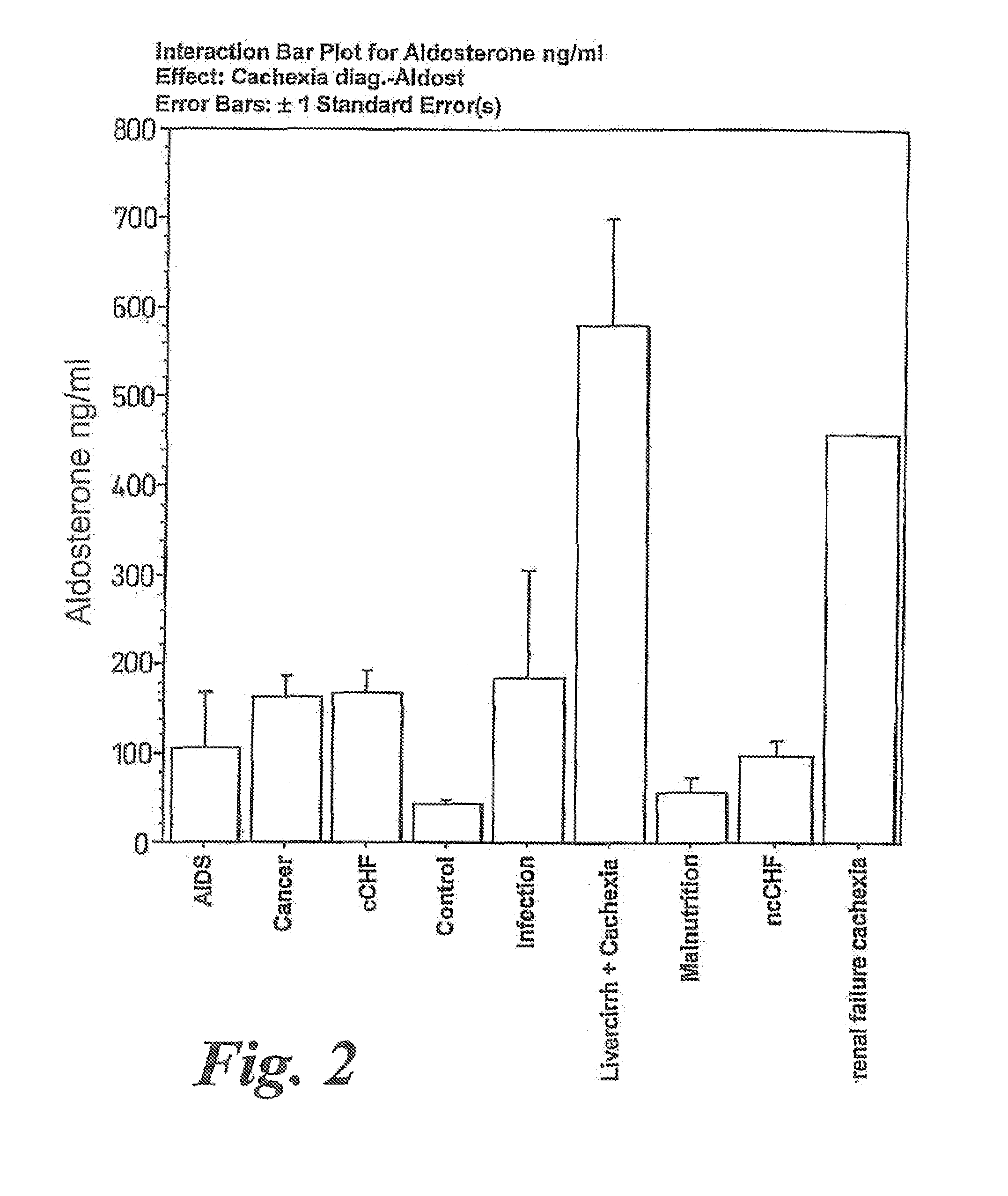
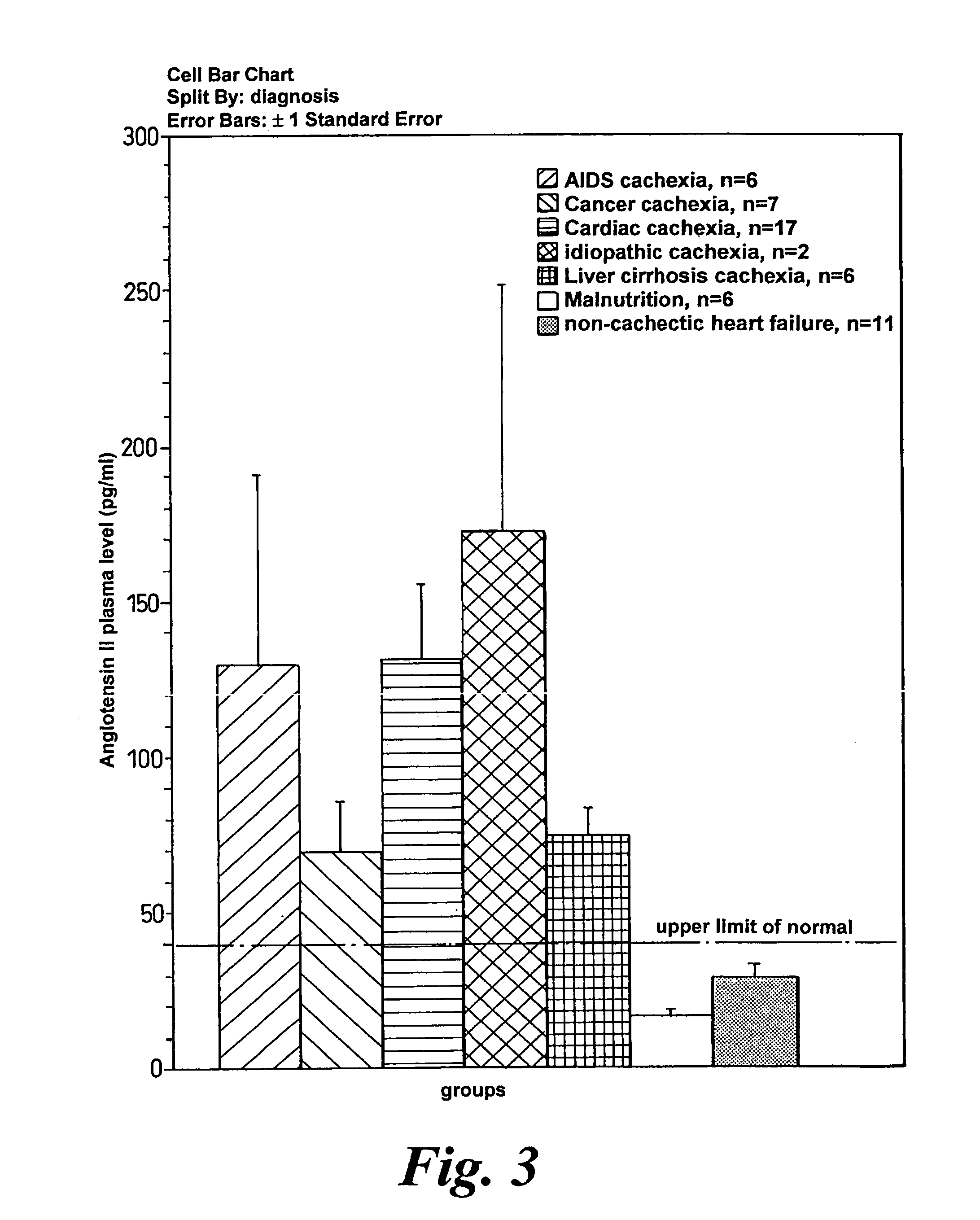
A method of treating weight loss due to underlying disease in a patient the method comprising administering to the patient an effective amount of an agent which reduces sympathetic nervous system activity. A method of treating weight loss due to underlying disease in a patient the 10 method comprising administering to the patient an effective amount of any one or more of the following: a compound which inhibits the effect of aldosterone such as an aldosterone antagonist; a chymase inhibitor; a cathepsin B inhibitor; a 13 receptor blocker; an imidazoline receptor antagonist; a centrally acting tx receptor antagonist; a peripherally acting ct receptor antagonist; a ganglion blocking agent; a drug that has an effect on cardiovascular reflexes and thereby reduce SNS activity such as an opiate; scopolamine; an endothelin receptor antagonist; and a xanthine oxidase inhibitor. The methods are particularly useful in treating cardiac cachexia.
Owner:IMPERIAL INNOVATIONS LTD
Extraction method of trypsin-chymotrypsin
InactiveCN101643723AThe extraction and separation method is simple and feasibleSuitable for industrial productionHydrolasesFreeze-dryingUltrafiltration
The invention relates to the field of biological engineering, in particular to an extraction method of trypsin-chymotrypsin. In the invention, CaCl2 is adopted to activate chymotrypsinogen into the trypsin-chymotrypsin, and simultaneously (NH4)2SO4 is added to generate CaSO4 precipitation and adsorb the trypsin-chymotrypsin; the white freeze-dried powder can be obtained directly through the stepsof elution, salting out, ultrafiltration and freeze-drying, with no need of preparing by the following steps: firstly salting out multiple crystallization joint dialysis and an organic solvent methodof alcohol and acetone and the like is adopted to prepare the trypsin-chymotrypsin crude product, and then the complex steps of CM-cellulose column chromatography and affinity chromatography and the like are carried out for purifying preparation. The extraction method in the invention is convenient and feasible, and is applicable to industrial production, thus saving cost and shortening time whichis shortened to 3 to 4 days from the original required 7 to 8 days. 4.0-4.5 grams of freeze-dried trypsin-chymotrypsin powder can be obtained from per kilogram of pancreas, wherein, the specific activity of chymotrypsin is 355 U. / mg protein (nitrogen) and the specific activity of trypsin is 1361 U. / mg protein (nitrogen).
Owner:马忠仁 +1
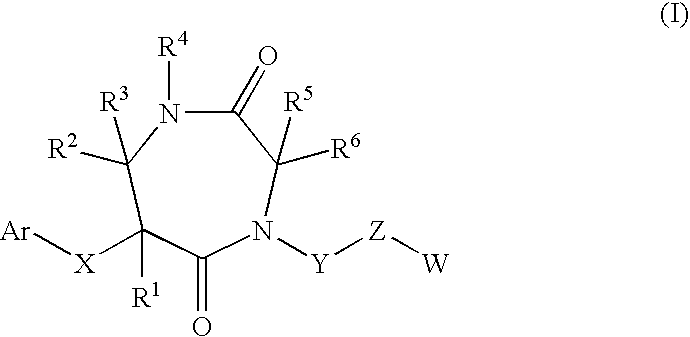
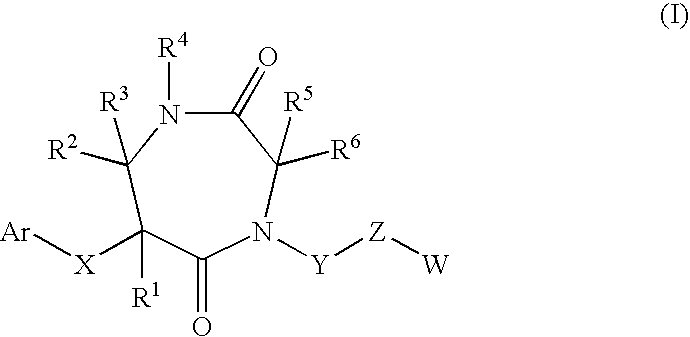
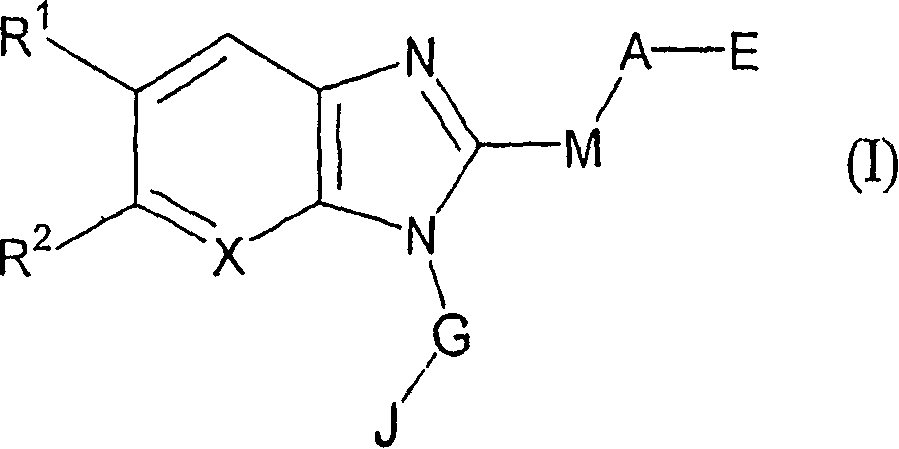
7-membered ring compound and method of production and pharmaceutical application thereof
InactiveUS20090111796A1Prevention and/or treatment of bronchialEfficient productionBiocideSenses disorderDiseaseChymase
A 7-membered heterocyclic compound having the formula (I), or its salt, or a solvate thereof with a chymase inhibitory action and useful for the prevention or treatment of various diseases, in which chymase is involved:a method for producing the same, and a pharmaceutical composition useful for the prevention or treatment of diseases, in which chymase is involved, including the compound of having the formula (I), or its pharmaceutically acceptable salt, or a solvate thereof are provided.
Owner:DAIICHI SANKYO CO LTD
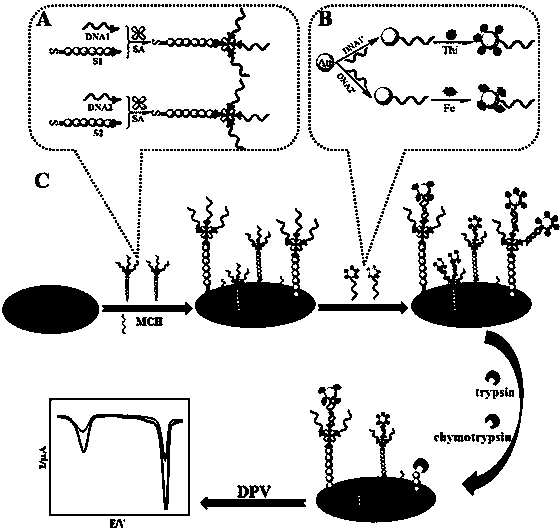
Trypsin-chymotrypsin electrochemical synchronous detection method based on enzyme digestion
InactiveCN104049007APeak current dropMaterial electrochemical variablesEnzyme digestionCarboxyl radical
The invention discloses a trypsin-chymotrypsin electrochemical synchronous detection method based on enzyme digestion and belongs to the technical field of electrochemical sensing. Through gold-mercapto bond action, a DNA-polypeptide compound is fixed to the surface of a gold electrode, and through DNA hybridization reaction, a DNA-gold nanoparticle-electronic mediator nanometer signal probe is captured. When target protease exists, an arginine carboxyl site of one of polypeptides is cut by trypsin and a tyrosine carboxyl site of the other one of the polypeptides is cut by chymotrypsin so that the corresponding nanometer signal probes connected to the polypeptides are separated from the surface of the electrode and thus peak current of the corresponding electronic mediator is reduced. Therefore, the trypsin-chymotrypsin electrochemical synchronous detection method can realize synchronous detection of sensitivity and selectivity of trypsin and chymotrypsin.
Owner:NANCHANG UNIV
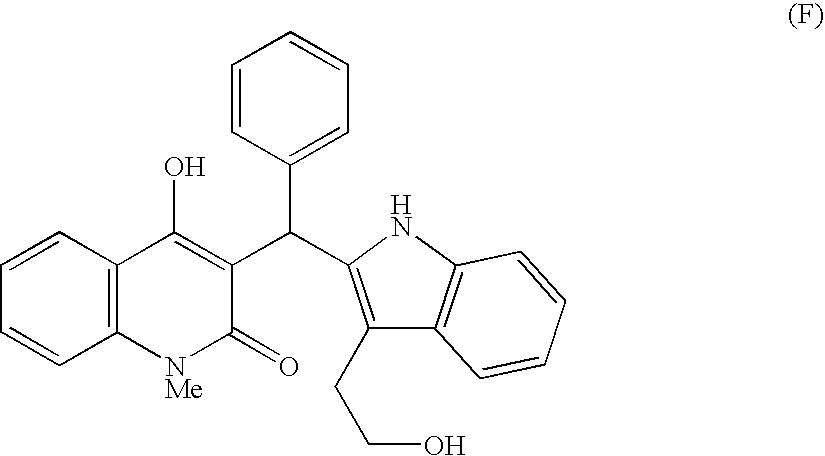
Indole derivatives exhibiting chymase-inhibitory activities and process for preparation thereof
Owner:MEIJI SEIKA KAISHA LTD
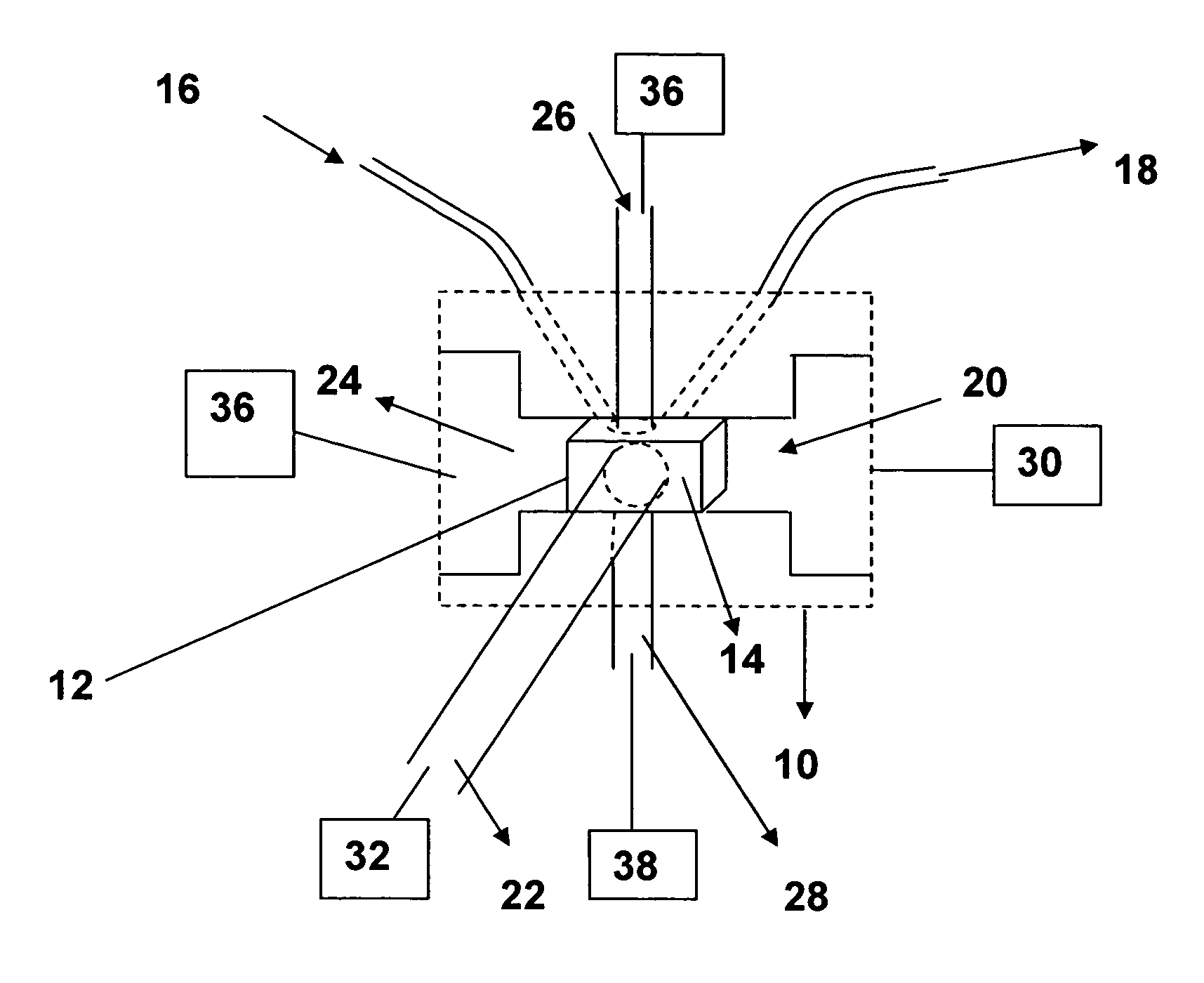
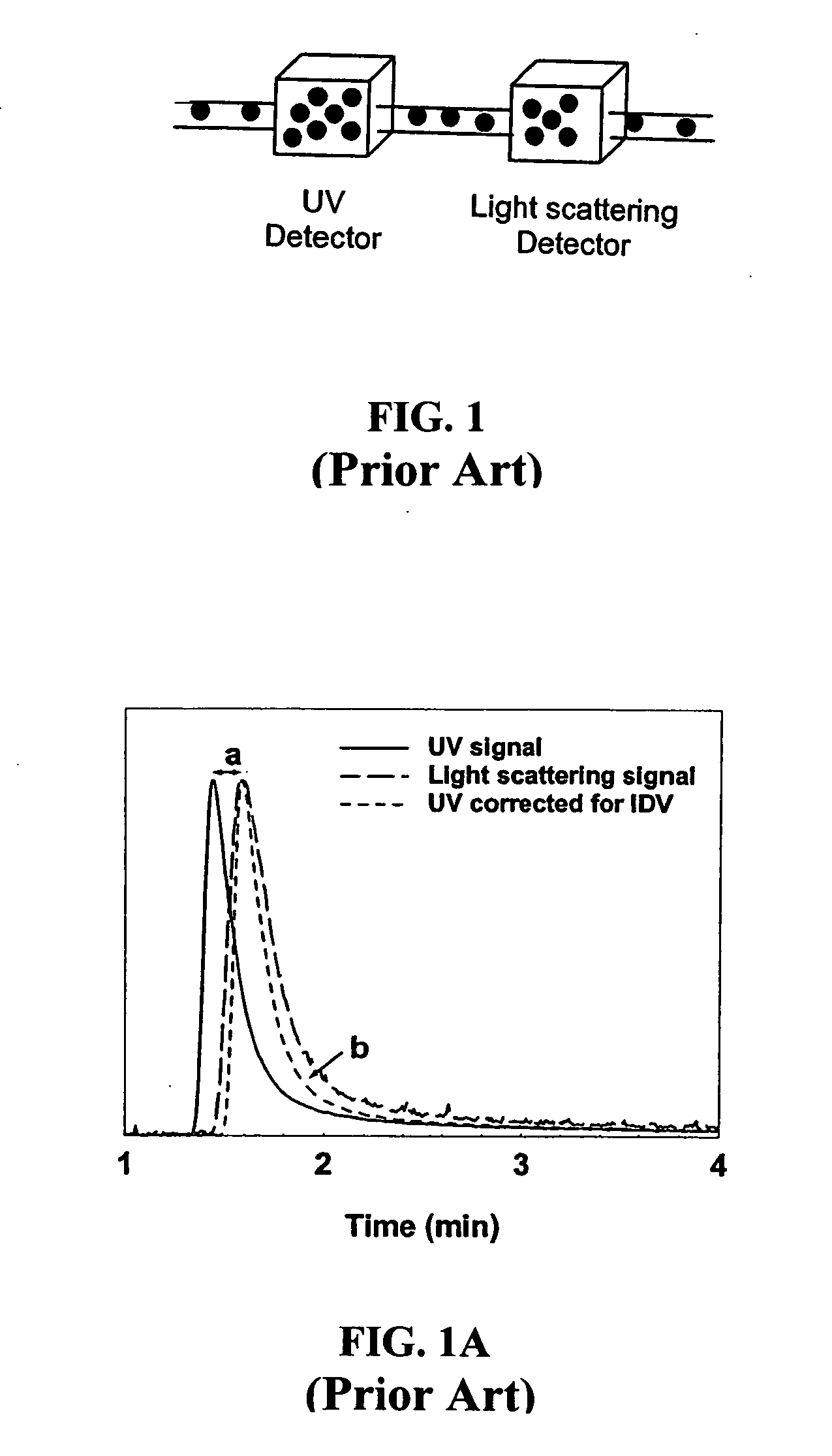
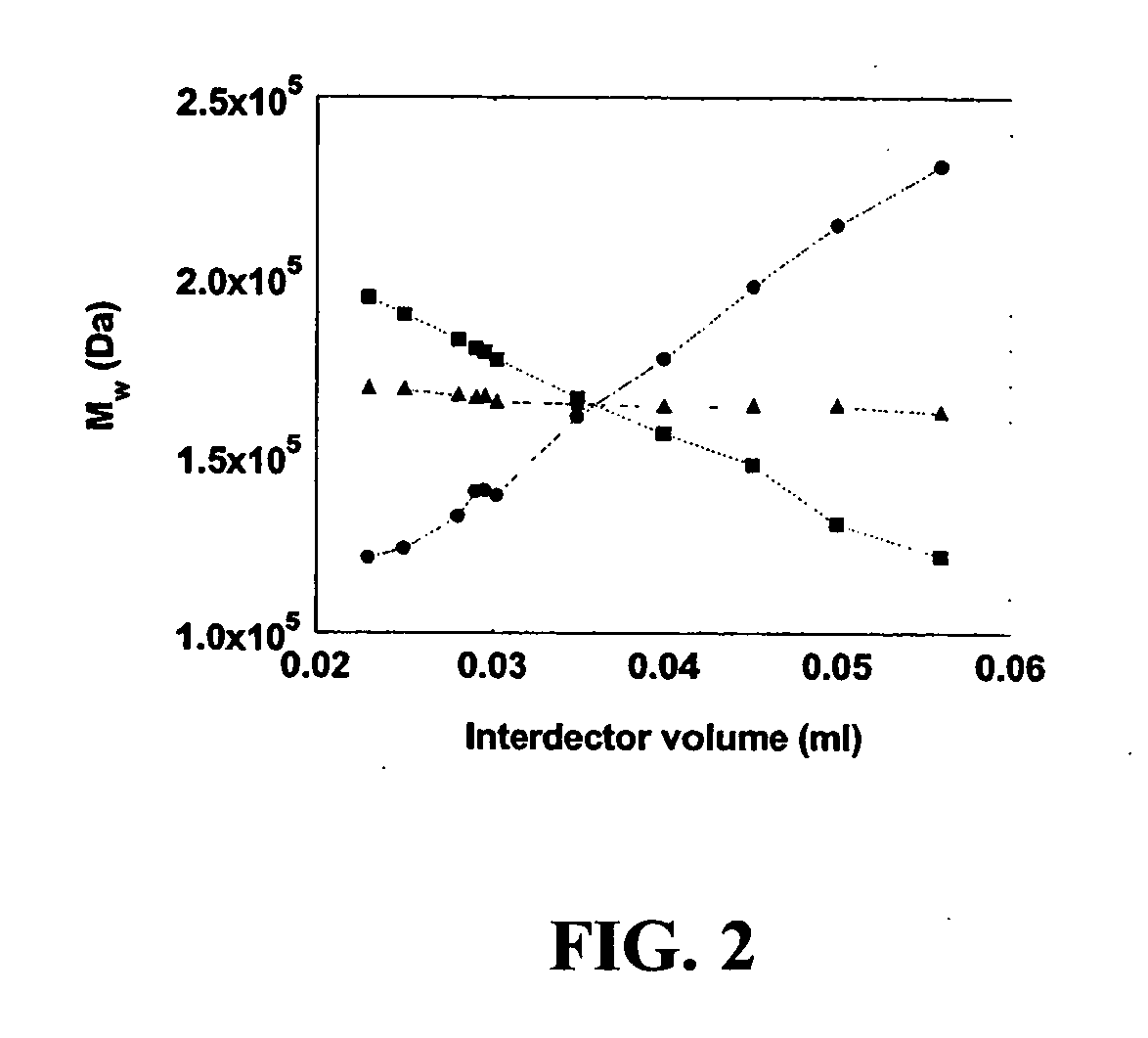
Dual-detector systems and methods having utility in biomolecular measurements
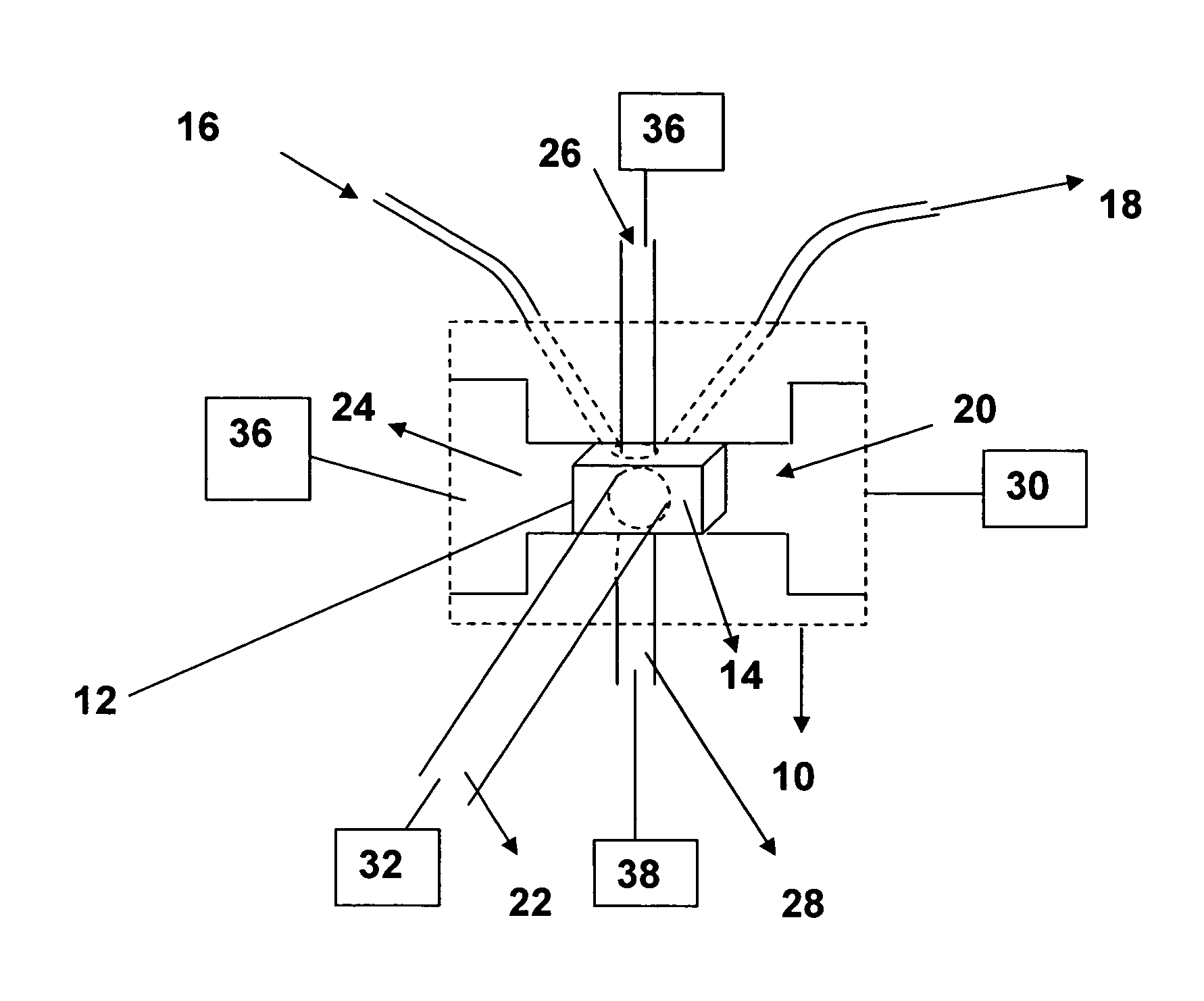
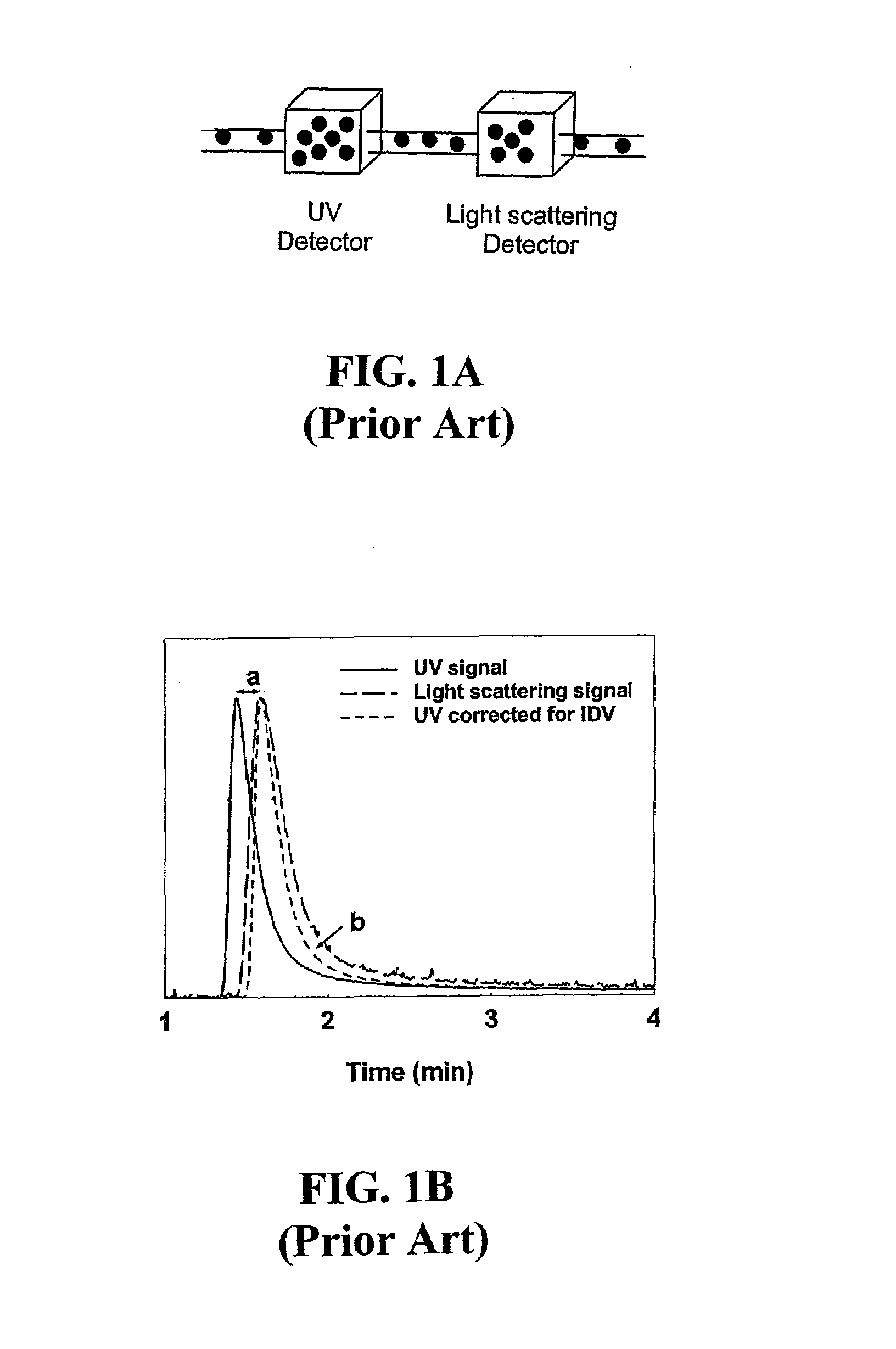
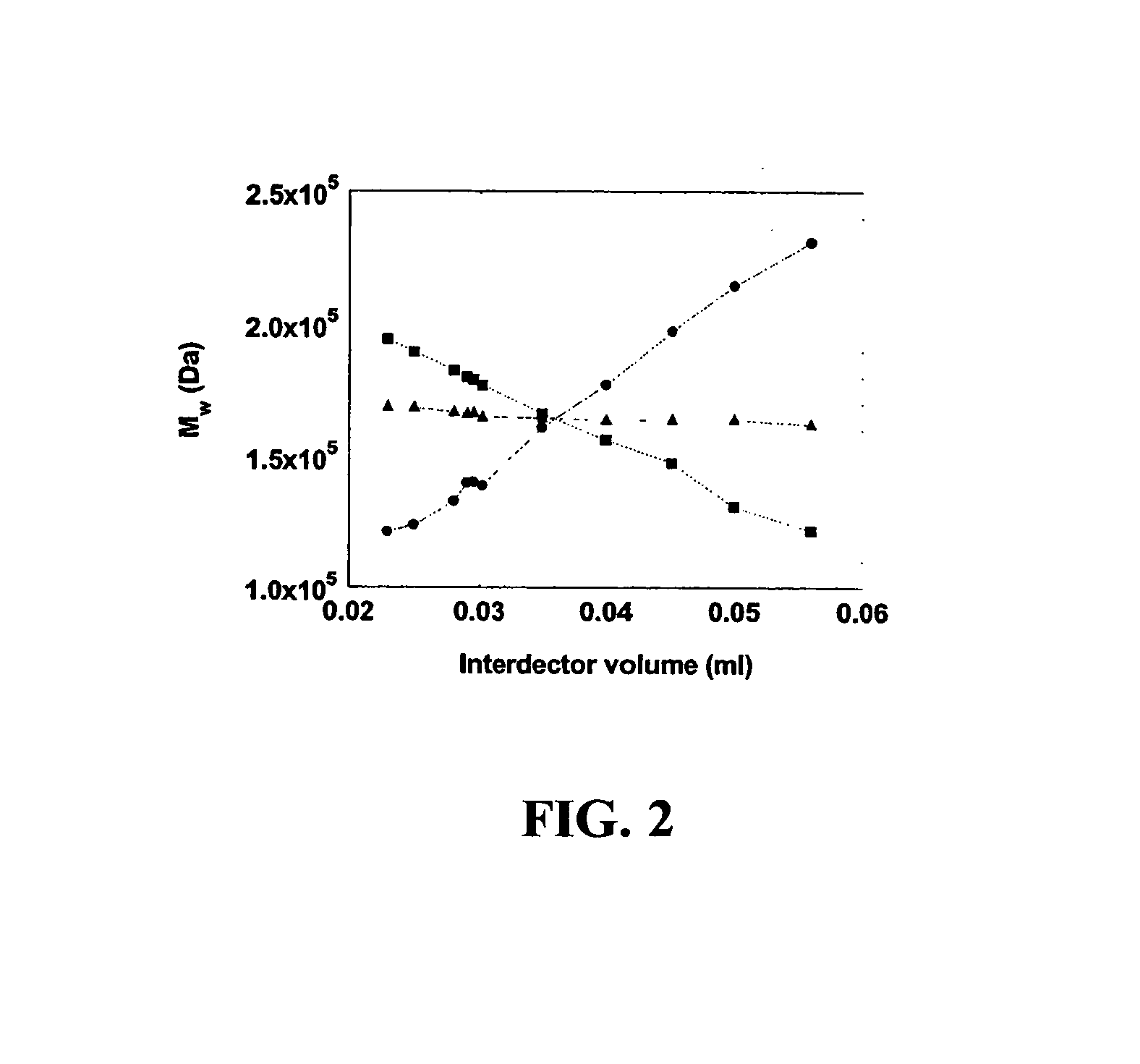
InactiveUS20070178013A1Facilitate measurementFacilitates calculationComponent separationScattering properties measurementsChemistryTear lysozyme
Apparatus, systems and methods for measurement of B22 values of proteins in aqueous solutions in flow-mode utilize a dual-detector cell are provided. Simultaneous measurement of protein concentration and scattered light intensity is facilitated as the protein elutes from a size-exclusion column. Each data point on the resulting chromatograms is converted to Rayleigh's ratio, Rθ, and to concentration c, respectively. The B22 value is calculated from the slope of the Debye plot (Kc / Rθ versus c) generated from a range of the concentrations obtained from these chromatograms for a single protein injection. Measurements may be analyzed using modeling data derived from a predetermined modeling equation to quantify self-association of molecules. The apparatus and method provide a reliable means for determining B22 values for such proteins as lysozyme, chymotrypsinogen, and chymotrypsin in various solution conditions.
Owner:UNIV OF CONNECTICUT
Authentication and uses of adversity specificly induced two-directional expression activity rice promotor CPIP
InactiveCN1807629AImprove stress resistanceFermentationVector-based foreign material introductionAridAbscisic acid
The invention discloses a promotor CPIP from Oryza Sativa L in the plant genetic engineering domain, which is characterized by the following: the promotor CPIP possesses SEQ ID NO: 1 with nucleic acid sequence, which controls two alfapsin inhibition sub-genes simultaneously; the alfapsin inhibition sub-gene is controlled by promotor CPIP in backward-forward direction, which makes answering reaction with arid, salt-forced and abscisic acid(ABA); the promotor region contains a plurality of hostile environment answering cis-form appliance elements; the promotor CPIP controls GUS report gene in backward-forward direction to converse rice; the GUS gene is induced and expressed strongly in the condition of arid and salt-forced.
Owner:HUAZHONG AGRI UNIV
Preparation method for chymotrypsin
The invention provides a preparation method for chymotrypsin. The preparation method comprises the following process procedures: (1), grinding raw material and extracting protein; (2) salting out in a grading manner to obtain a crude product of chymotrypsin; (3), feeding the crude product and crystallizing in a multiple manner; (4), activating; (5), salting out; (6), dialyzing; and (7), freeze-drying. According to the invention, a set of large-scale manufacturing technology is established and has the advantages of simplicity, low cost and the like; the titer of a fine product of prepared chymotrypsin is more than 1,200 unit / milligram; and the prepared chymotrypsin meets the requirements of Chinese pharmacopeia (2010) and has very great market competitiveness.
Owner:QINGDAO JIULONG BIO PHARMA
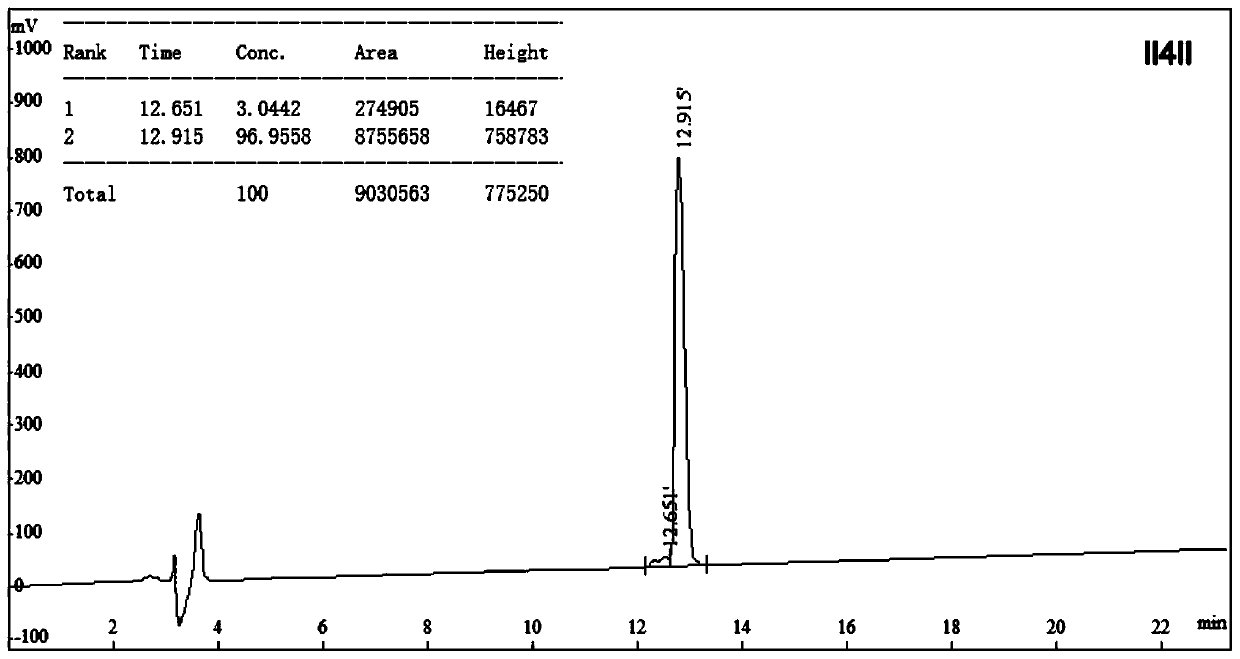
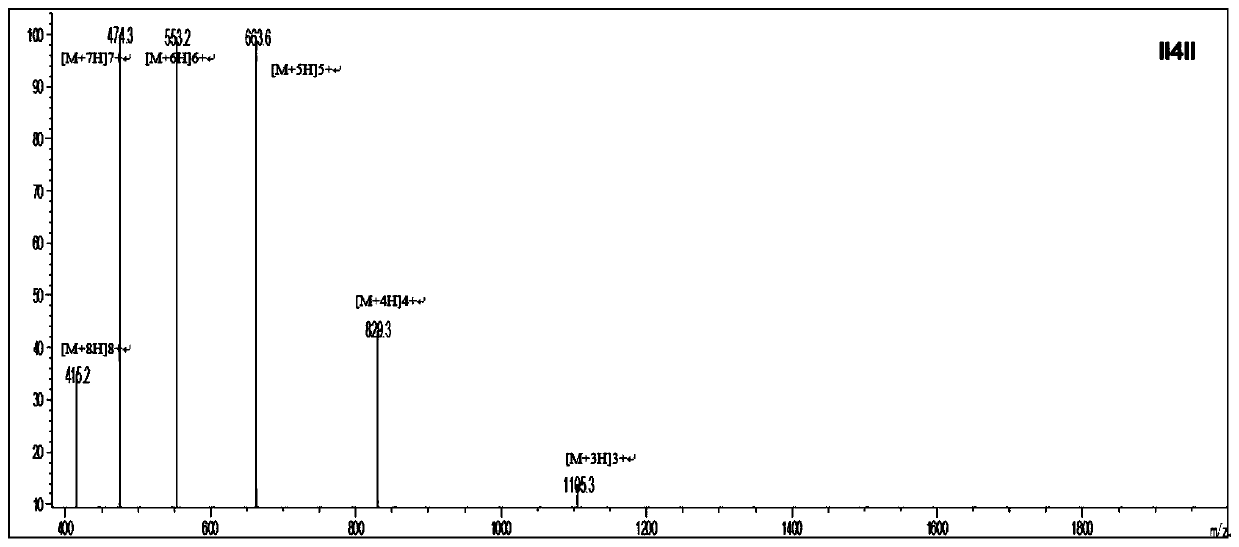
Anti-enzymolysis antibacterial peptide II4II and preparation method and application thereof
ActiveCN111454334AThe experimental technical route is simpleAvoid restriction sitesAntibacterial agentsPeptide/protein ingredientsDiseaseChymase
The invention belongs to the technical field of biology and provides an anti-enzymolysis antibacterial peptide II4II and a preparation method and application thereof. The amino acid sequence of the antibacterial peptide II4II is shown in SEQ ID No. 1. The preparation method comprises the following steps of reasonably arranging amino acids in the sequence based on specific restriction enzyme cutting sites of trypsin, chymotrypsin and pepsin and basic characteristics of the antibacterial peptide, and selecting Ile as a hydrophobic amino acid to obtain the antibacterial peptide II4II. The invention further provides application of the antibacterial peptide II4II in preparation of medicines for treating gastrointestinal tract infection diseases caused by gram-negative bacteria or / and gram-positive bacteria. The antibacterial peptide II4II has a relatively good inhibition effect on multiple bacteria, is relatively low in hemolytic activity, has a treatment index of 80.63, has relatively goodanti-enzymolysis property, is kept unchanged in antibacterial activity after being treated by trypsin, chymotrypsin or pepsin, and has application potential as an antibiotic substitute.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Dual-detector systems and methods having utility in biomolecular measurements
InactiveUS7630076B2Easy to measureEasy to calculateComponent separationScattering properties measurementsChymotrypsinogenBio molecules
Apparatus, systems and methods for measurement of B22 values of proteins in aqueous solutions in flow-mode utilize a dual-detector cell are provided. Simultaneous measurement of protein concentration and scattered light intensity is facilitated as the protein elutes from a size-exclusion column. Each data point on the resulting chromatograms is converted to Rayleigh's ratio, Rθ, and to concentration c, respectively. The B22 value is calculated from the slope of the Debye plot (Kc / Rθ versus c) generated from a range of the concentrations obtained from these chromatograms for a single protein injection. Measurements may be analyzed using modeling data derived from a predetermined modeling equation to quantify self-association of molecules. The apparatus and method provide a reliable means for determining B22 values for such proteins as lysozyme, chymotrypsinogen, and chymotrypsin in various solution conditions.
Owner:UNIV OF CONNECTICUT
Bacteroides signal molecule microecological preparation and its preparation method
InactiveCN101125151ANumber of Spontaneous Activities NoneBlood pressure noBacteria material medical ingredientsPill deliveryAcute toxicity testingCulture fluid
The present invention relates to a b.fragilis signal molecule microbial preparation, the b.fragilis bacterium are inoculated in a culture medium for anaerobic fermentation at 35 to 38 DEG C, after that, the b.fragilis bacteria concentrate is obtained by centrifugal concentration; the bacteria cell walls are broken by high pressure; further, the bacteria concentrate is treated with enzyme hydrolysis by trypsin, chymotrypsin and pepsin, and then the preparation raw powder is obtained by solvent extraction and decompression drying. The preparation raw powder is taken as the main ingredient, and other excipients are added to prepare the oral capsules, tablets, granules, oral liquor and other preparations. The b.fragilis signal molecule product of the present invention is proven to have no acute toxicity, no influence on the times of spontaneous activity, blood pressure and respiration of the animals and can not cause the allergic reactions of the animals by animal trials. The animal trials further prove that: the product has the effects of regulating blood-lipid metabolism, reducing fat accumulation and slimming; at the same time, the present invention can improve the immunity and the anti-tumor effect by adjusting the gene expression and regulating the immune functions.
Owner:SENBAIAO SCI & TECH UNIV DALIAN
Novel imidazolidinedione derivatives and use thereof as drugs
Owner:SENJU PHARMA CO LTD
Purifying process for chymotrypsin
The invention relates to a purifying process for chymotrypsin, which belongs to the field of chymotrypsin in a bio-pharmaceutical technology. The purifying process comprises the following steps of: pulping; salting out, and filtering; adding ammonium sulfate till the saturation degree is 0.4, and standing in a cold chamber over night; filtering and performing ultrafiltration by using an ultrafiltration film of 50kDa; performing ultrafiltration by using an ultrafiltration film of 50kDa; adding ammonium sulfate into a filtrate till the saturation degree is 0.7, and standing at a low temperature over night; filtering and crystallizing; activating; precipitating; performing ultrafiltration by using an ultrafiltration film of 10kDa; purifying with an SP ion exchange column; and performing ultrafiltration by using an ultrafiltration film of 10-30kDa, and sterilizing to obtain the chymotrypsin. In the process, the chymotrypsin is purified by combining the ultrafiltration film with the ion exchange column, so that a good purifying effect is achieved, and an obtained product has high purity; and the chymotrypsin obtained through the process has high activity which is more than 1500 U / mg, the dosage can be reduced, the medical expense of a patient is reduced, the administration safety of the chymotrypsin is improved.
Owner:SHANDONG TOPSCI BIO TECH
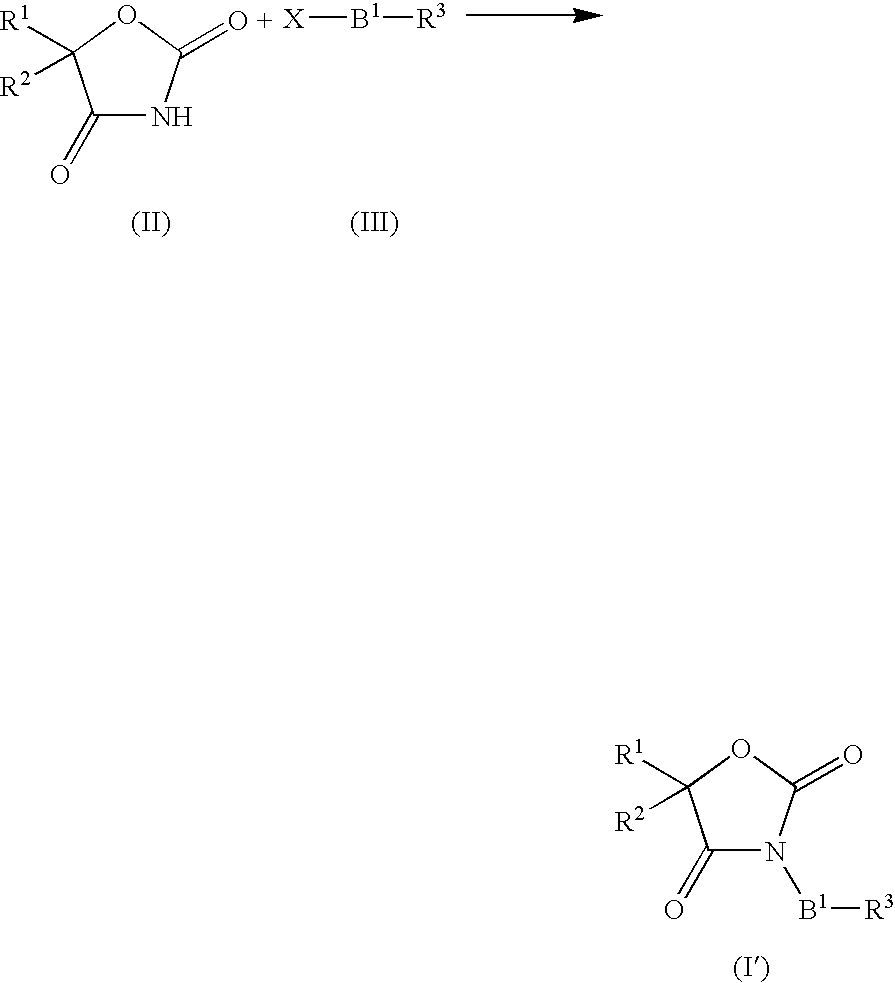
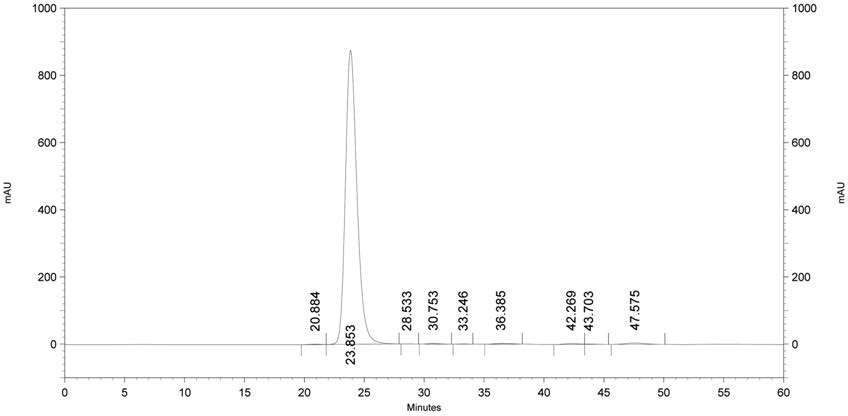
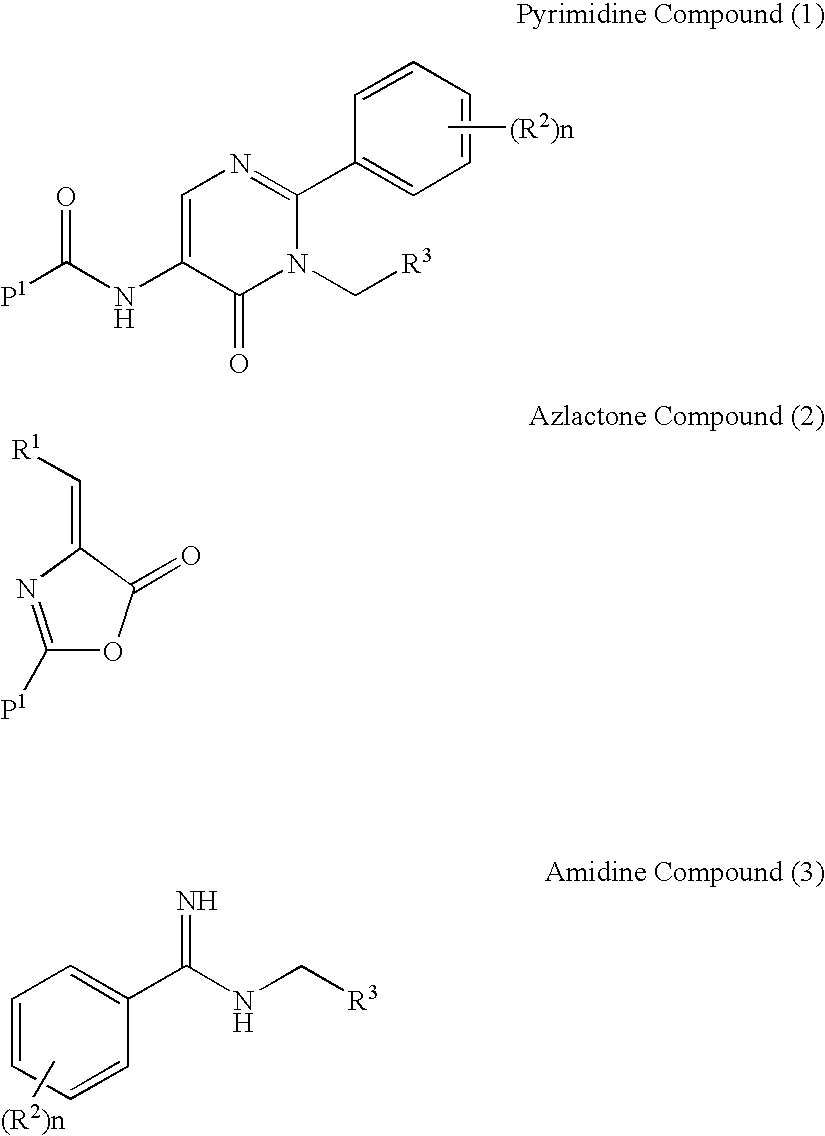
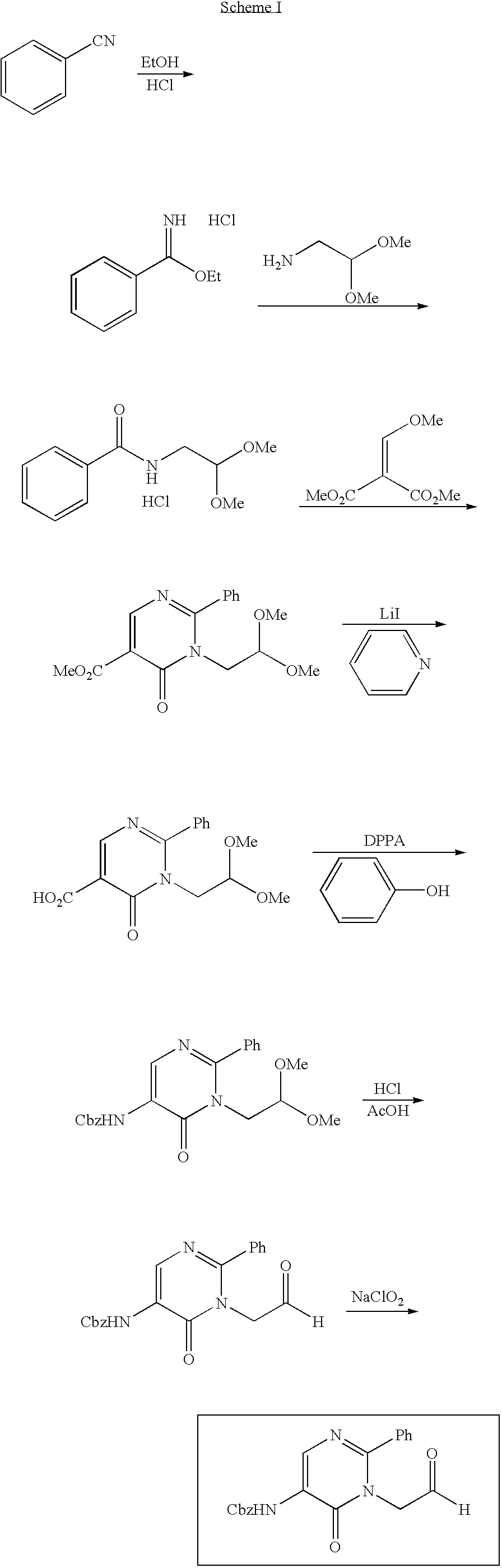
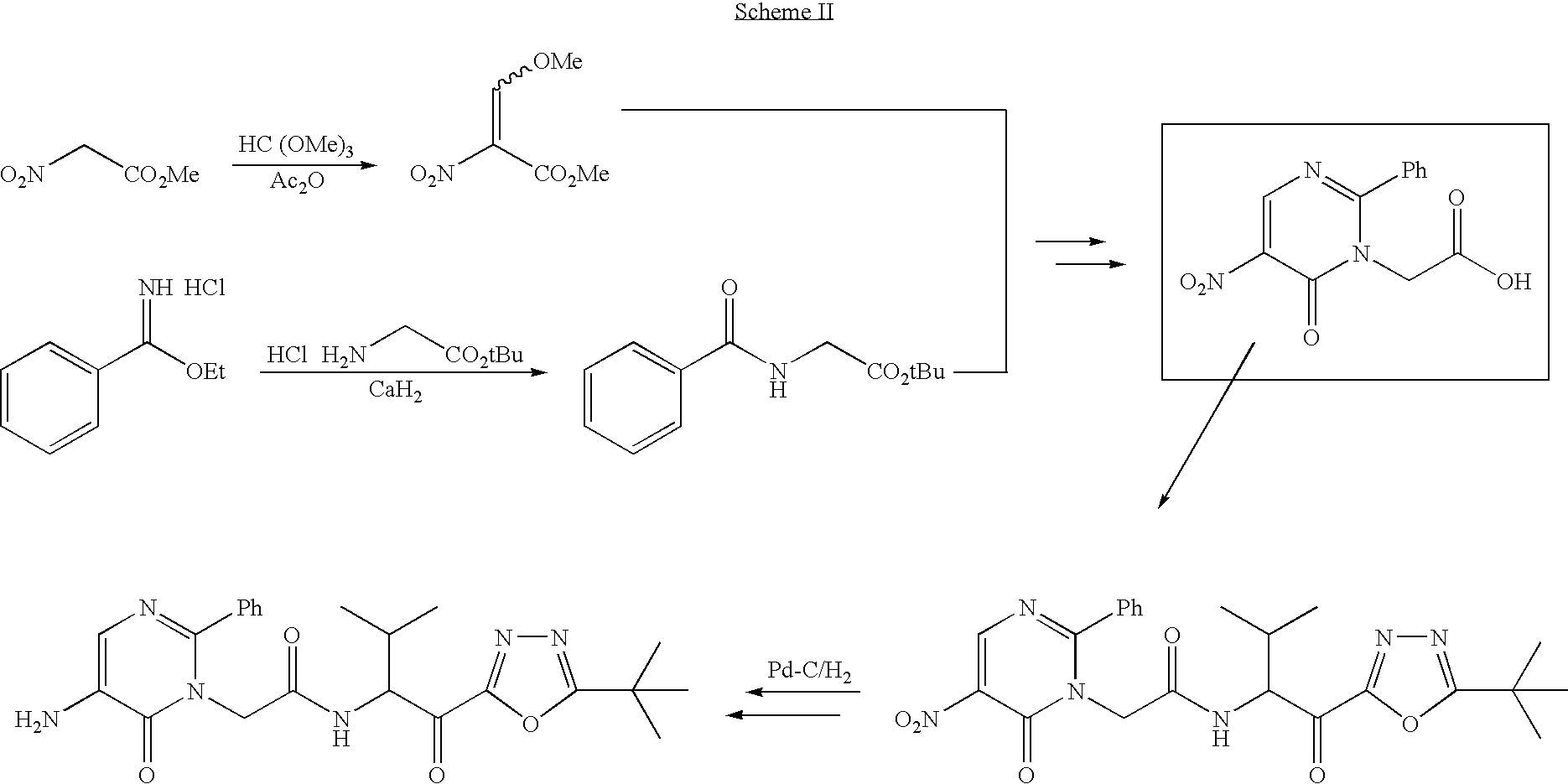
Process for preparing pyrimidine compound
Azlactone Compound (2) is reacted with Amidine Compound (3) to give Pyrimidine Compound (1) which is useful as an intermediate for the production of enzyme inhibitors (e.g., elastase inhibitor, chymase inhibitor etc.): wherein each symbol is as defined in the specification.
Owner:AJINOMOTO CO INC
Microcin C7 heptapeptide and prepared strain thereof
ActiveCN111018950AImprove stabilityStrong heat resistanceBacteriaMicroorganism based processesBiotechnologyMicrocin C7
The invention relates to an microcin C7 heptapeptide with an amino acid sequence of MATINAN. The heptapeptide has stronger biological characteristics of heat resistance, pepsin resistance, trypsin resistance and chymotrypsin resistance, high stability and strong bacteriostatic activity. The invention further provides a high-throughput screening method for obtaining a heat-resistant and protease-resistant strain. A lactobacillus johnsonii strain capable of producing the heptapeptide is screened out; the lactobacillus johnsonii strain is deposited in China General Microbiological Culture Collection Center on October 16th, 2019, and has the accession number CGMCC No.18695. The lactobacillus johnsonii strain has advantages of good stress resistance and high secretion amount and is low in cost.An important foundation is laid for industrial large-scale preparation of microcin C7 heptapeptide in the fields of animal husbandry feed, cosmetics, health care products and the like.
Owner:CHONGQING ACAD OF ANIMAL SCI
Methods of Treatment
A method of treating weight loss due to underlying disease in a patient, the method comprising administering to the patient an effective amount of an agent which reduces sympathetic nervous system activity. A method of treating weight loss due to underlying disease in a patient the method comprising administering to the patient an effective amount of any one or more of the following: a compound which inhibits the effect of aldosterone such as an aldosterone antagonist; a chymase inhibitor; a cathepsin B inhibitor; a β receptor blocker; an imidazoline receptor antagonist; a centrally acting α receptor antagonist; a peripherally acting α receptor antagonist; a ganglion blocking agent; a drug that has an effect on cardiovascular reflexes and thereby reduces SNS activity such as an opiate; scopolamine; an endothelin receptor antagonist; and a xanthine oxidase inhibitor. The methods are particularly useful in treating cardiac cachexia.
Owner:IMPERIAL INNOVATIONS LTD
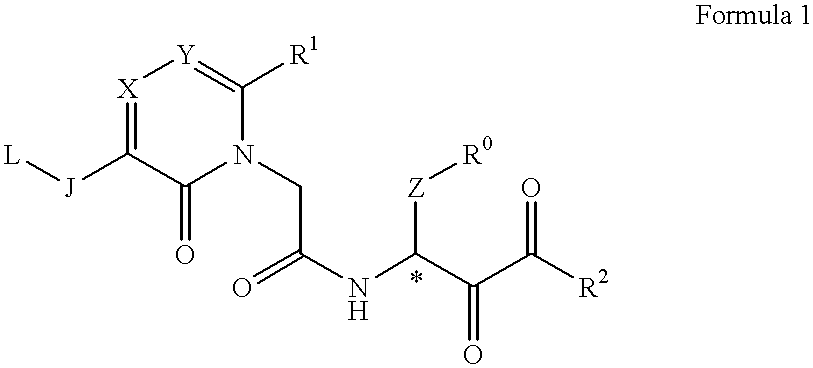
Acetamide derivative and use thereof
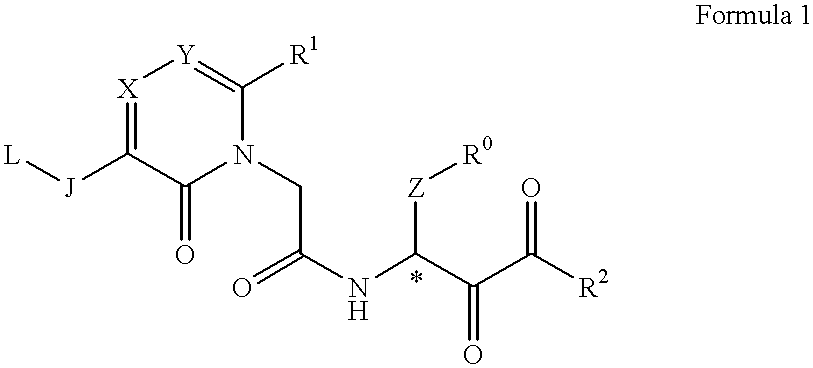
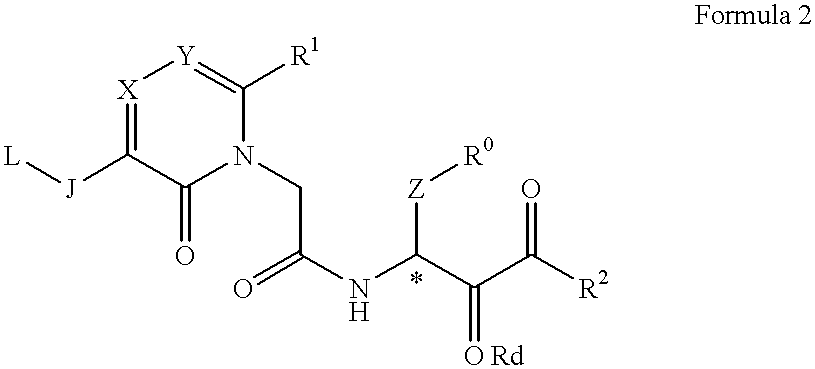
The present Invention relates to a novel acetamide derivative represented by the following Formula 1, which has an inhibition activity for chymotrypsin type proteases and is useful as an inhibitor for the above enzyme, especially as an inhibitor for chymase, and the use thereof as a medicine, for example, an antiasthma drug or a drug for curing vascular injuries complicated with angiogenesis and atheroma.wherein R0 represents a substituted or unsubstituted phenyl group, R1 represents an aryl group, a heteroaryl or an aliphatic lower alkyl group with or without substituents, R2 represents a substituted or unsubstituted alkyl, an aryl alkyl, a heteroaryl alkyl, and a heteroaryloxy alkyl or the like, J represents a carbonyl group, or a methylene group or the like, L represents a methoxy, hydroxyl or acetyloxy group or the like, X and Y independently represents a nitrogen atom or a carbon atom, Z represents a methylene group or a polyethylene group optionally having a substituent.
Owner:NIPPON KAYAKU CO LTD
Novel indole derivatives exhibiting chymase-inhibitory activities and process for preparation thereof
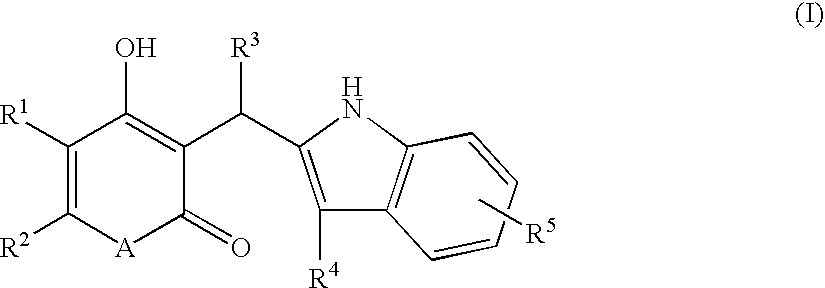
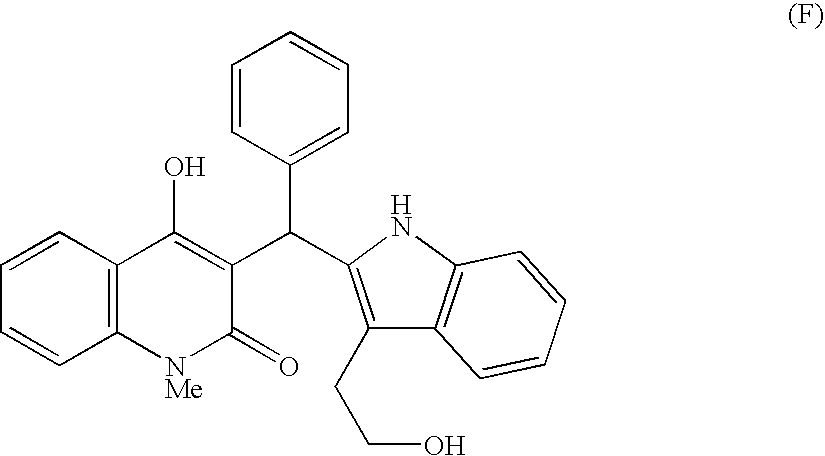
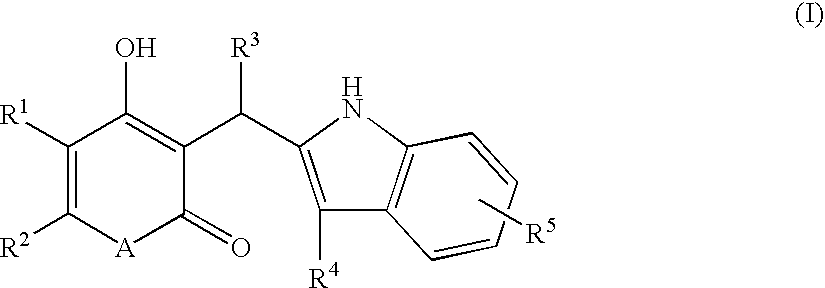
According to this invention, there is provided an indole derivative having the general formula (I) wherein A is an oxygen atom or a nitrogen atom which nitrogen atom is optionally substituted with an alkyl group, and (i) R<1 >and R<2 >each stand for a hydrogen atom or an alkyl group, independently, or (ii) R<1 >and R<2 >as taken together form a cycloalkyl group or an aromatic ring, or (iii) R<1 >and R<2 >as taken together form a heterocyclic ring, and R<3 >is a hydrogen atom, a (C1-C10)alkyl group or others, R<4 >is a substituted alkyl group and R<5 >is a hydrogen atom, a halogen atom, an alkyl group or an alkoxy group and so on, as novel compounds by a novel chemical synthetic process. The indole derivative of formula (I) exhibits a useful chymase inhibitory activity.
Owner:MEIJI SEIKA KAISHA LTD
Traditional chinese medicine composition and the use thereof
ActiveUS20160375083A1Moistening intestineBiocideDispersion deliveryIntestinal structureAdditive ingredient
The present invention relates to the technical field of Traditional Chinese medicine, in particular to a traditional Chinese medicine composition and the use thereof. The traditional Chinese medicine composition comprises the following ingredients: honeysuckle, tangerine peel, stir-baked malt, honey, and hawthorn juice. The traditional Chinese medicine composition of the present invention has significant promotions on the gastric emptying rate, intestinal motility, gastric digestive enzyme (pepsin) activity and pancreatic enzyme (trypsin, chymotrypsin, amylase and lipase) activity of rats, exhibiting that the traditional Chinese medicine composition of the present invention has significant effects of clearing heat-fire and moistening the intestine.
Owner:INFINITUS (CHINA) CO LTD
Methods and compositions for inhibiting angiotensin converting and chymase enzymes
InactiveUS20080206376A1Inhibitory activityMaximize inhibitionBiocideAnimal repellantsAngiotensin-converting enzymePharmacy medicine
The present invention relates to medicinal products, as well as to health and wellness food products, and particularly to a medicinal product or a health and wellness food product for inhibiting Angiotensin Converting Enzyme and Chymase enzyme pathways.
Owner:PALU AFA KEHAATI +4
Methods and compositions for inhibiting angiotensin converting and chymase enzymes
InactiveUS20090022828A1Inhibitory activityMaximize inhibitionBiocideAnimal repellantsAngiotensin-converting enzymePharmacy medicine
The present invention relates to medicinal products, as well as to health and wellness food products, and particularly to a medicinal product or a health and wellness food product for inhibiting Angiotensin Converting Enzyme and Chymase enzyme pathways.
Owner:TAHITIAN NONI INT INC
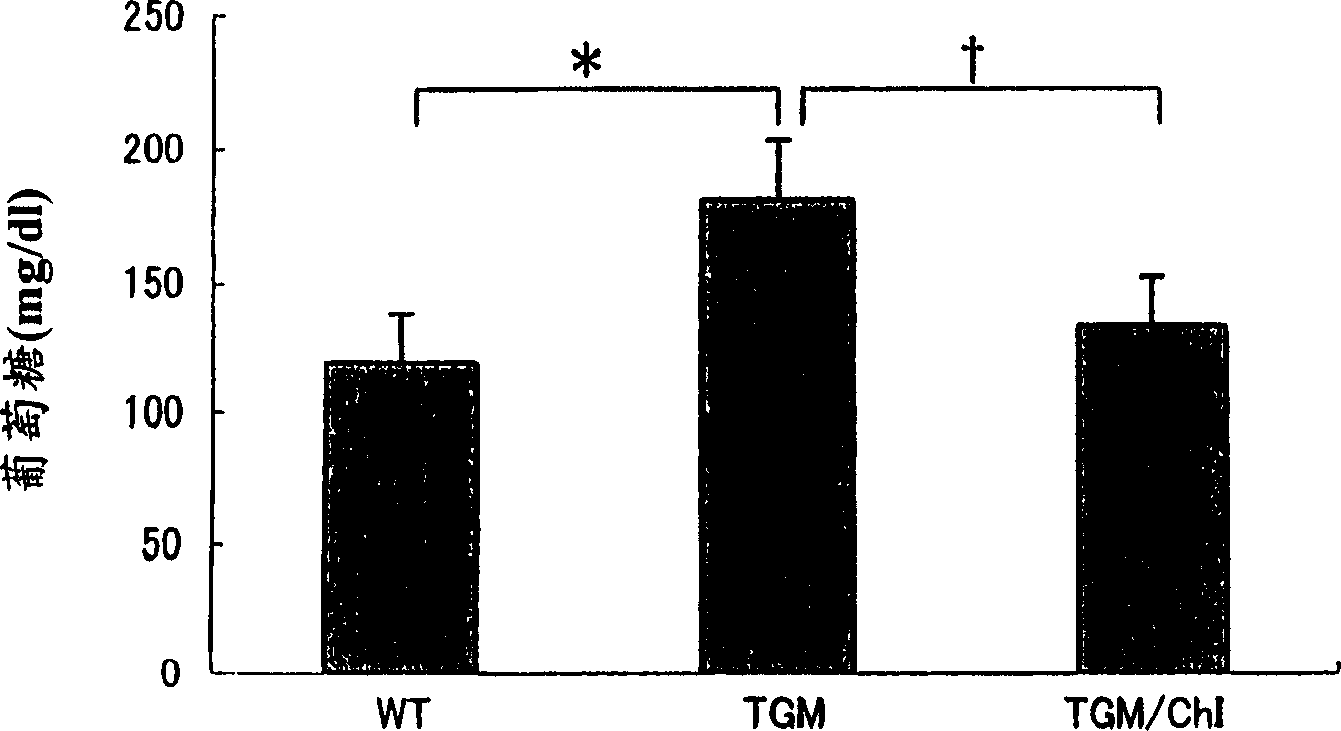
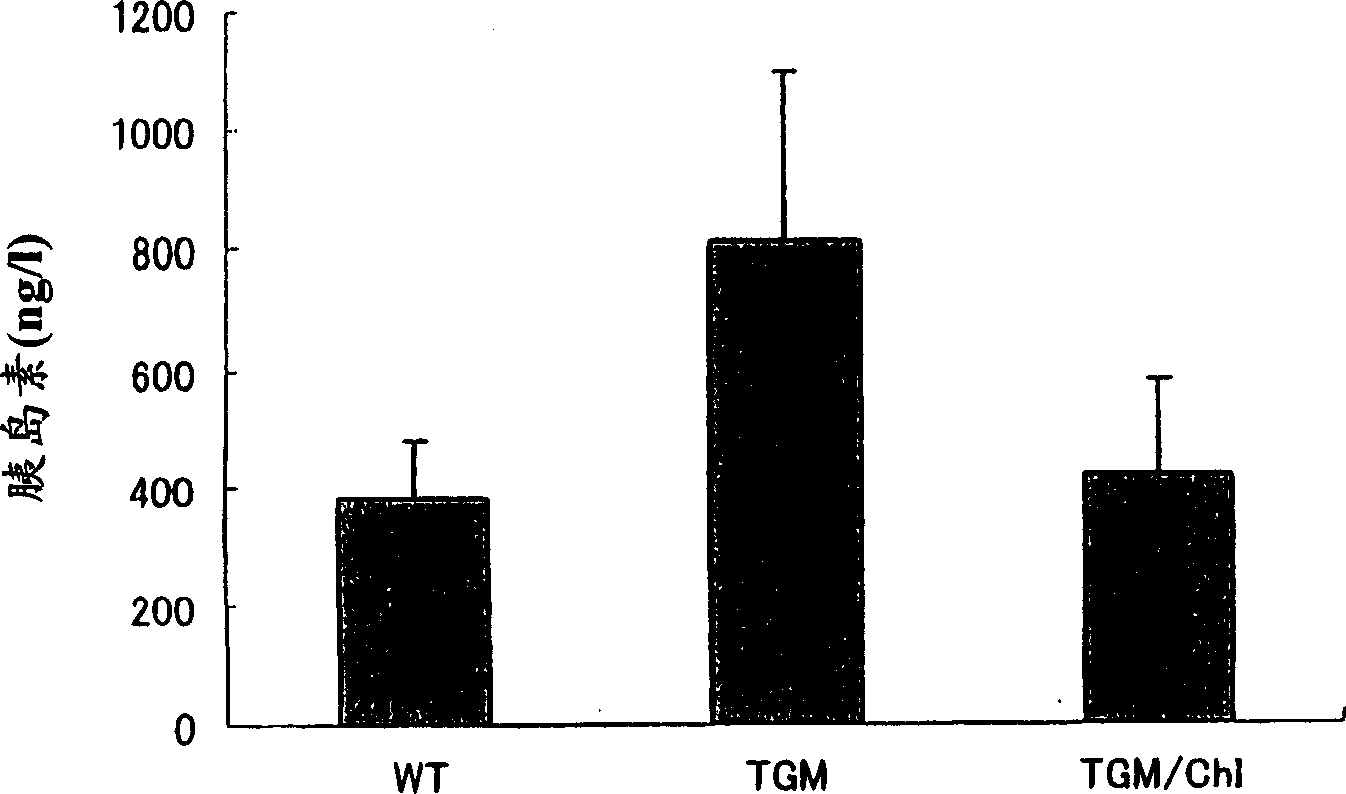
Drug containing chymase inhibitor as the active ingredient
An agent for improving abnormal glucose tolerance or a preventive and / or a remedy for diseases caused by abnormal glucose tolerance containing a chymase inhibitor as the active ingredient. Examples of the diseases caused by abnormal glucose tolerance include diabetes and / or complications of diabetes. Examples of the complications of diabetes include diabetic nephropathy, diabetic retinopathy, diabetic peripheral neuropathy, hyperinsulinemia, insulin resistance syndrome, arteriosclerosis, acute coronary syndrome, arteriosclerosis obliterans, vasculitis, brain infarction, hypertension, renal insufficiency, neuropathy, nephritis, renal aneurysm, renal infarction, obesity and so on.
Owner:TEIJIN PHARMA CO LTD
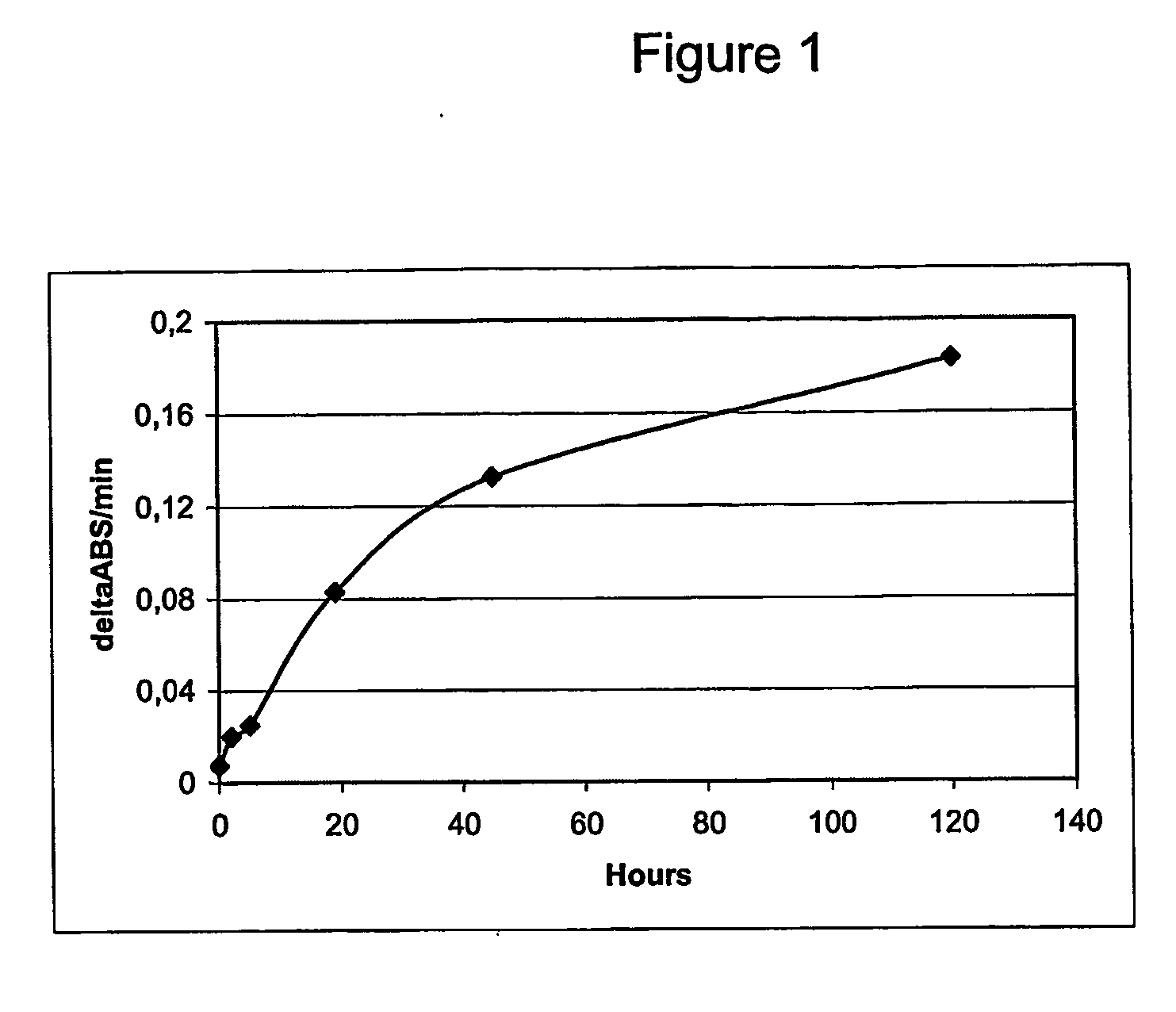
Proteasome inhibitors having chymotrypsin-like activity
Disclosed herein is the use of HLM-008182, as well as its analogues formed via in-house synthesis, as a potent proteasome inhibitors. A new method was developed for HLM-008182 through a four-step protocol and the method was further optimized to a two step protocol. The synthesis in both protocols was regioselective with TiCl4. The reaction was highly efficient with microwave assisted heating and THF as solvent. The modification around the molecule HLM-008182 established primary SAR, indicating that the proteasome inhibition activity was a function of the 2-side chain.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC +1
Cleavage of fusion proteins using granzyme b protease
InactiveUS20060199251A1Efficient crackingImprove versatilitySugar derivativesHydrolasesChymotrypsinogenProteinase activity
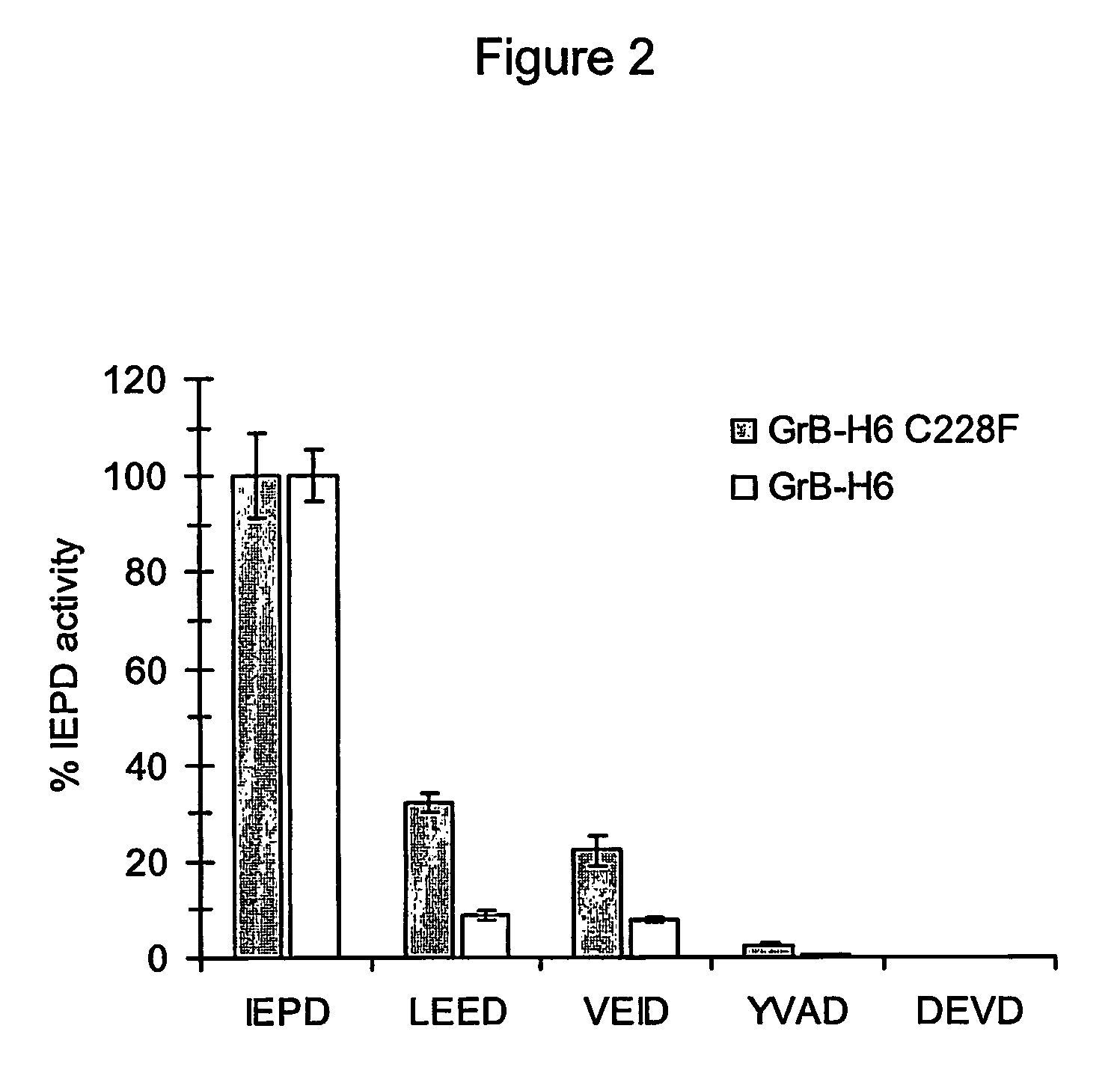
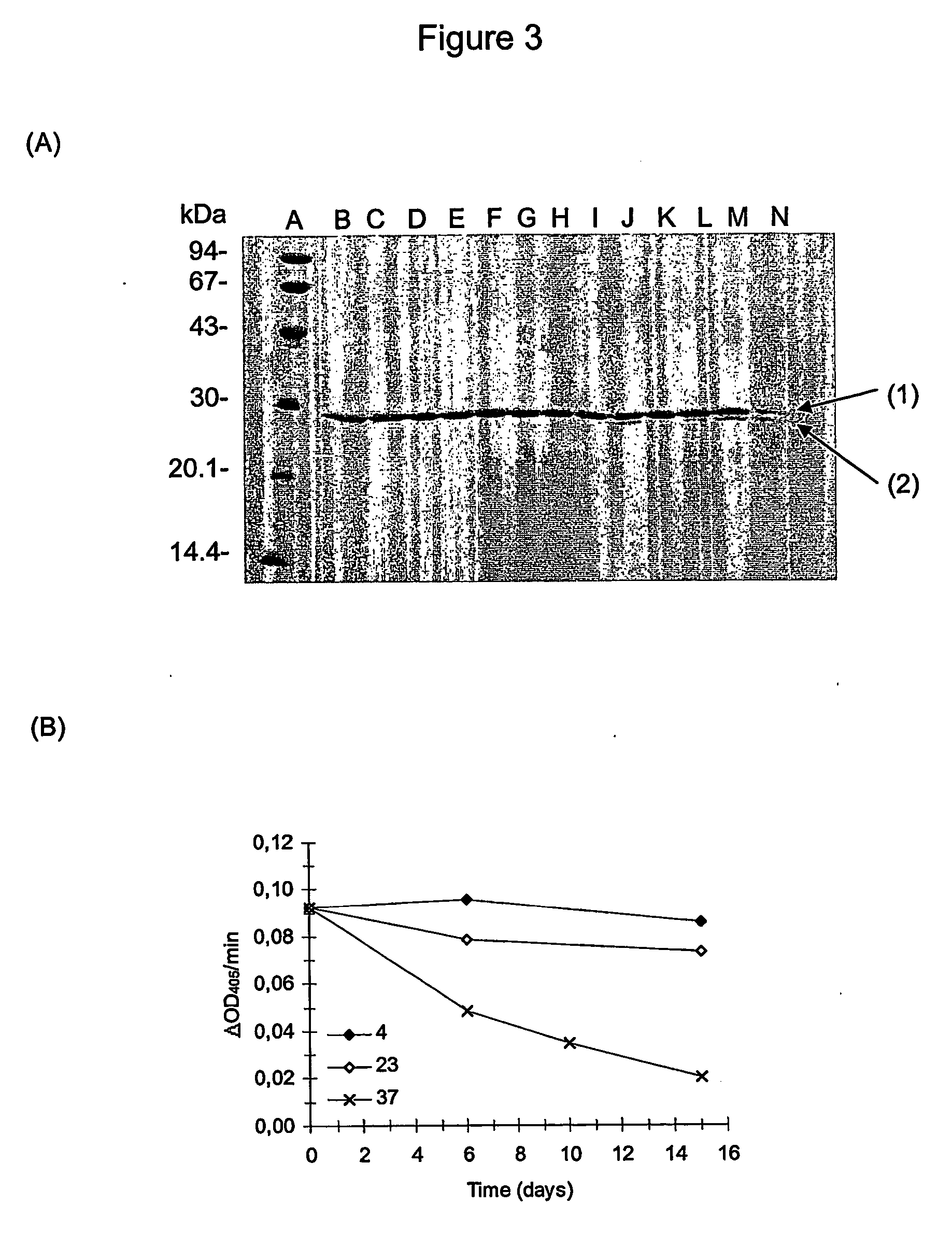
A method for the preparation of a polypeptide of interest in authentic form by enzymatic cleavage of fusion proteins using Granzyme B protease (EC 3.4.21.79). There is also provided fusion proteins comprising a polypeptide of interest and a fusion partner, wherein the junction region between the polypeptide of interest and the fusion partner comprises a Granzyme B protease cleavage site adjacent to the polypeptide of interest, and a human Granzyme B protease variant wherein the Cystein residue no. 228 (chymotrypsinogen numbering) is mutated to Phenylalanine.
Owner:F HOFFMANN LA ROCHE & CO AG
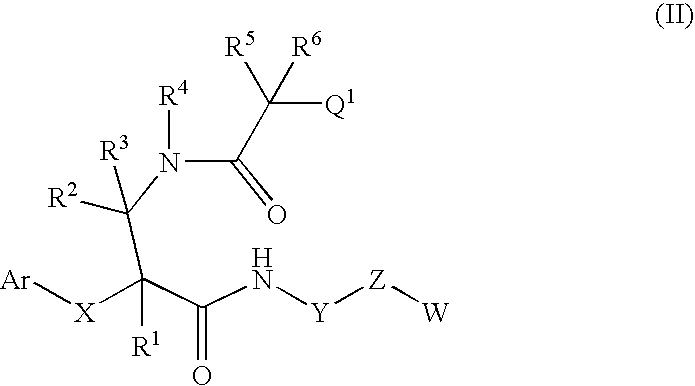
Tetrapeptide epoxy propane derivative, preparation method and application thereof
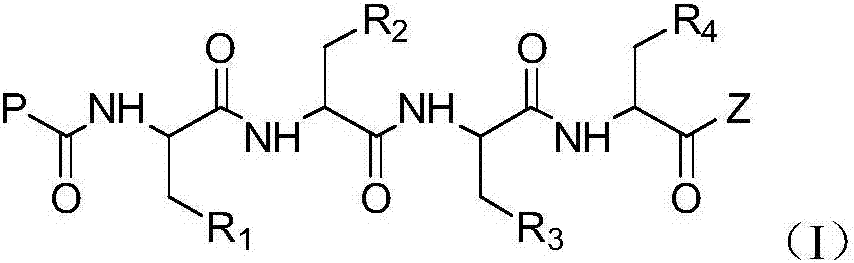
The invention discloses a tetrapeptide epoxy propane derivative or a pharmaceutically acceptable salt thereof, and a preparation method and an application thereof, wherein the tetrapeptide epoxy propane derivative or the pharmaceutically acceptable salt thereof is represented as the formula (I). The compound can selectively inhibit chymotrypsin like activity of 20S proteasome, and also may have anti-inflammation feature and cell proliferation inhibition effect.
Owner:NANJING LINGRUI PHARM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com