Drug containing chymase inhibitor as the active ingredient
A chymotrypsin-like and chymotrypsin-like technology, which is applied in the field of medicines containing chymotrypsin-like inhibitors as active ingredients
- Summary
- Abstract
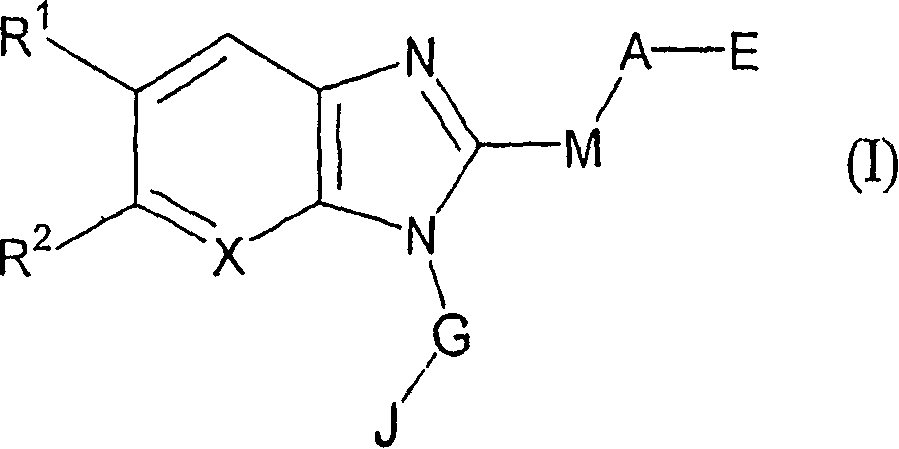
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0235] Improving Effects of Impaired Glucose Tolerance
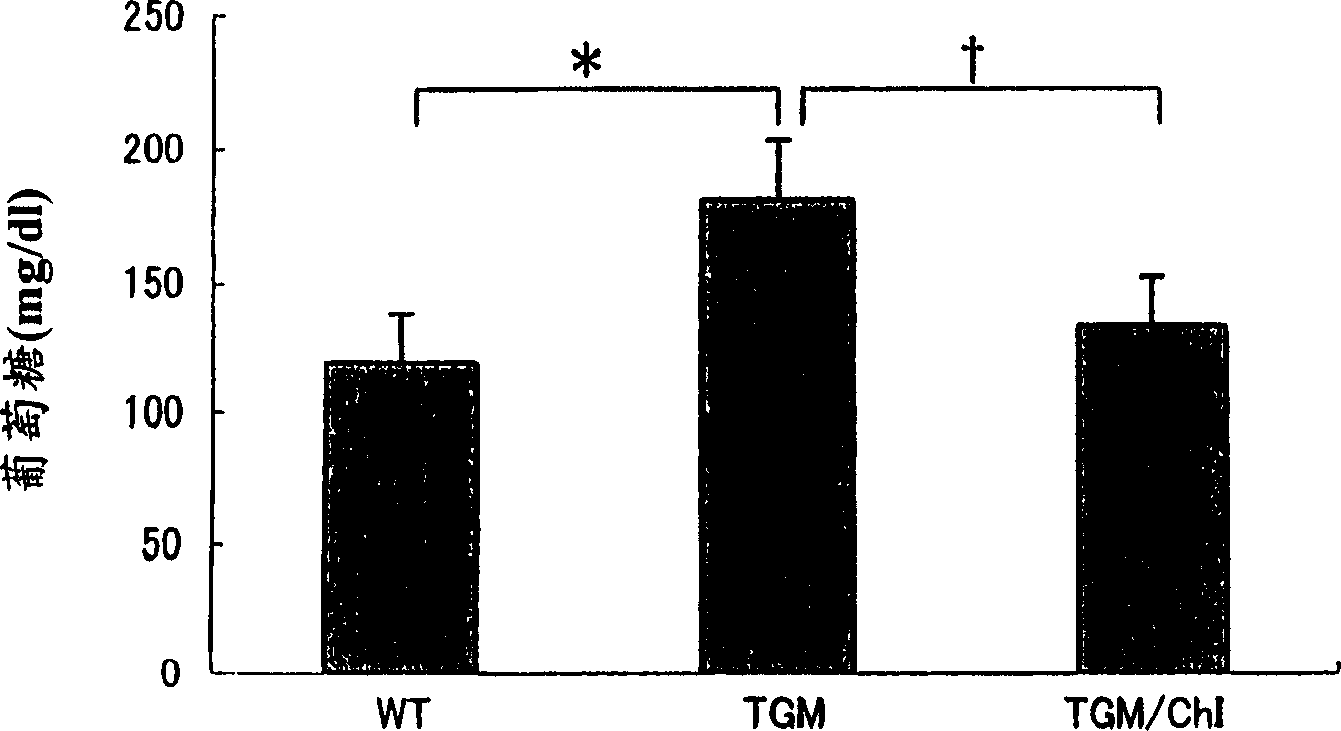
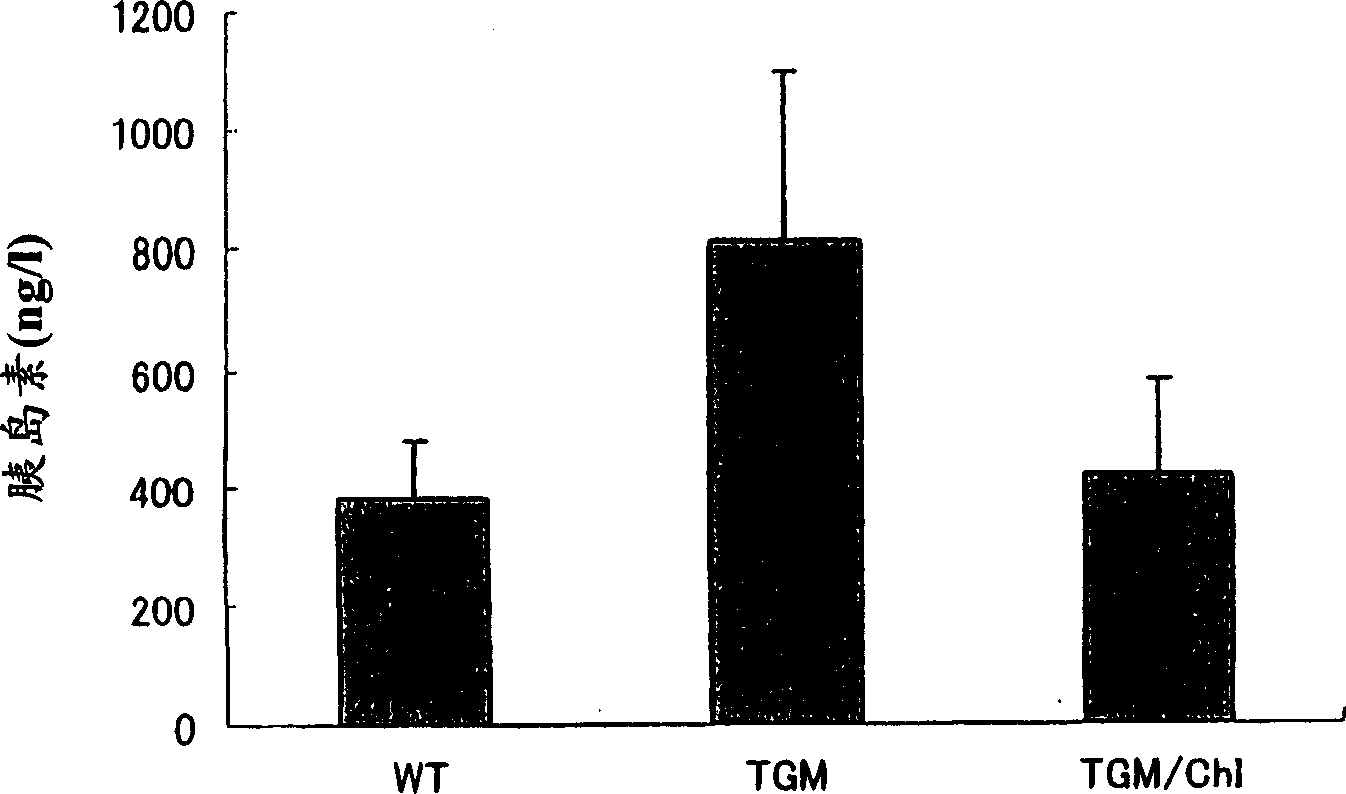
[0236] 22 weeks of wild-type mice (C57Black) (Wild), TGM (mice expressing human chymotrypsin gene in it) and by mixing 0.1% chymotrypsin inhibitor (Chymase Inhibitor: ChI, compound number 58 Sulfate, the IC of compound 58 50 The value is 1nM-10nM) from 10 weeks to 12 consecutive weeks of TGM (TGM / ChI) 3 groups, orally administered 1.5g / kg of glucose after overnight fasting, blood glucose concentration and insulin concentration after 60 minutes were measured .
[0237] result:
[0238] At 60 minutes after the sugar load, the blood glucose concentrations were 119±20 mg / dl for Wild, 181±22 mg / dl for TGM, and 134±18 mg / dl for TGM / ChI* (mean±standard deviation, for Wild*pfigure 1 ).
[0239] On the other hand, the blood insulin concentration at the same time was 386±97ng / l for Wild, 809±228ng / l for TGM, and 425±158ng / l for TGM / ChI (mean±standard deviation). Glucose concentration also increased significantly, but showed a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com