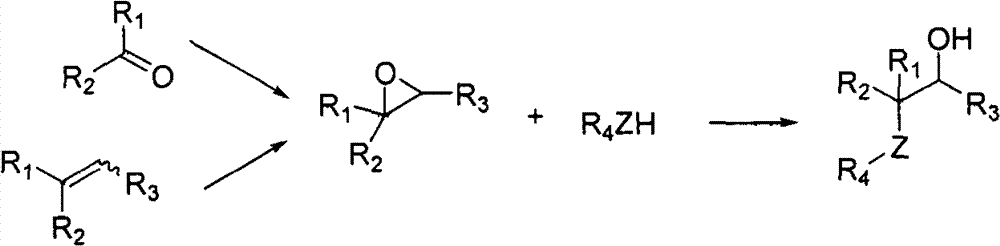
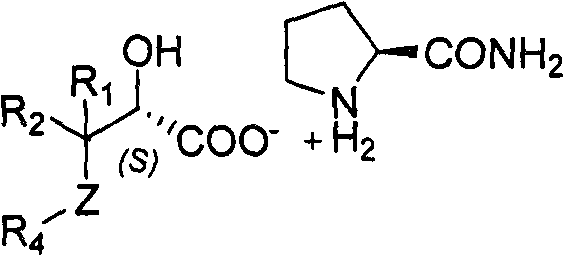Splitting method of 2-hydracrylic acid racemic mixture
A technology for hydroxypropionic acids and racemates, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve the problems of large yield loss, inconvenient operation, high market price, etc., and achieve reduction Production energy consumption, improved operability, and low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
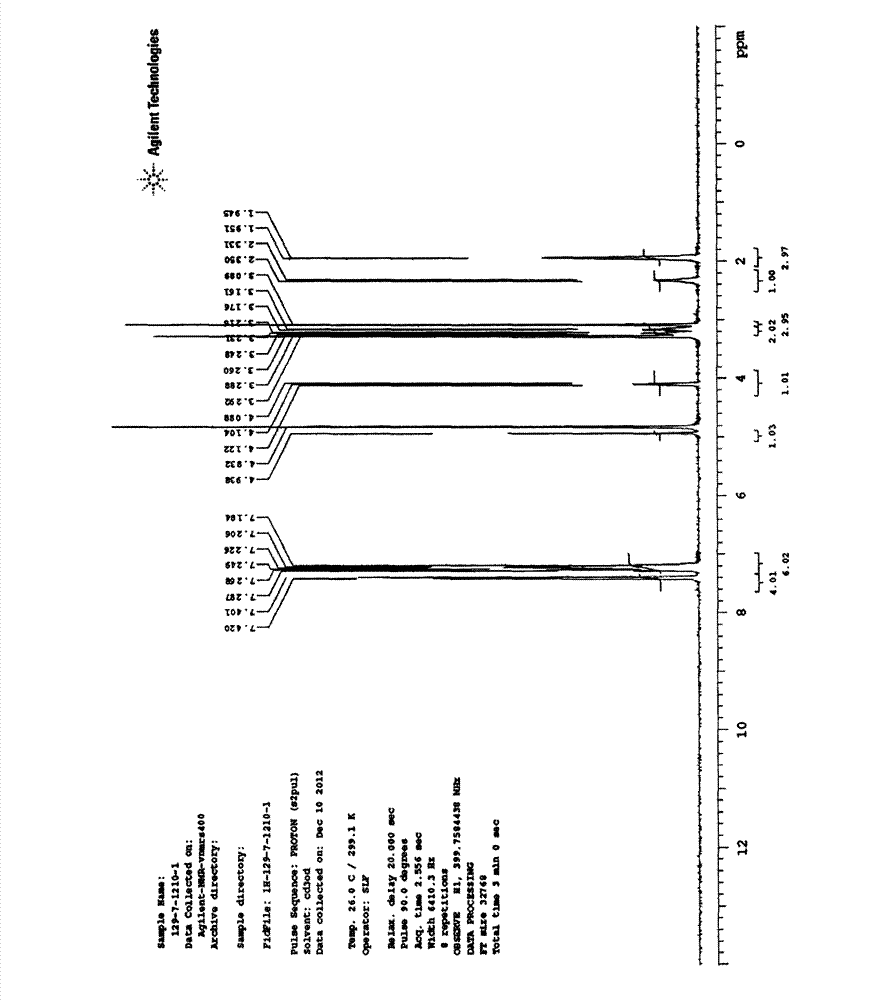
[0026] Preparation of (S)-2,3-dihydroxy-3,3-diphenylpropionic acid by resolution of racemate
[0027] In reaction bottle, drop into 150g ethanol, 75g ethyl acetate, 20g (77.2mmol) 2,3-dihydroxyl-3,3-diphenylpropionic acid and 4.8g (42.5mmol) L-proline amide, under stirring The temperature of the reaction solution was raised to 56°C, and after 30 minutes of heat preservation and stirring, the temperature of the reaction solution was slowly cooled to 0-5°C and filtered to obtain (S)-2,3-dihydroxy-3,3-diphenylpropionic acid L-proline Aminoamide salt. .
[0028] Add 100 g of ethyl acetate and 100 g of water to the filter cake, stir, and adjust the pH of the solution to 2 to 3. After adjustment, separate the layers, and extract the water layer once with 50 g of ethyl acetate. The organic layers were combined, washed once with 50 g of water, and separated. The organic layer was concentrated under reduced pressure to obtain 140 g of ethyl acetate. After the concentration was compl...
Embodiment 2
[0032] Preparation of (S)-2-hydroxy-3-methyl-3,3-diphenylpropionic acid by resolution of racemate
[0033] Drop into 250g methanol and 120g methyl tert-butyl ether, 20g (75.7mmol) 2-hydroxyl-3-methyl-3,3-diphenyl propionic acid and 4.5g (39.4mmol) L-proline in the reaction flask Amide, the temperature of the reaction solution was raised to 80.5°C under stirring, and the temperature of the reaction solution was slowly cooled to 0-5°C after stirring for 30 minutes, and filtered to obtain (S)-2-hydroxy-3-methyl-3,3-di Phenylpropionate L-Prolinamide Salt.
[0034]Add 100 g of ethyl acetate and 50 g of water to the filter cake, stir, and adjust the pH of the solution to 2 to 3. After adjustment, separate the layers, and extract the water layer once with 50 g of ethyl acetate. The organic layers were combined, washed once with 50 g of water, and separated. The organic layer was concentrated under reduced pressure to obtain 140 g of ethyl acetate. After the concentration was comple...
Embodiment 3
[0038] Preparation of (S)-2-hydroxy-3-methoxy-3,3-diphenylpropionic acid by resolution of racemate
[0039] 200g of isopropanol is pumped into the reactor, and then 10kg (36.5mmol) of 2-hydroxyl-3methoxy-3,3-diphenylpropionic acid and 2.3g (20mmol) of L-prolinamide are dropped into the kettle , the temperature of the reaction solution was raised to 75°C under stirring, and the reaction solution was slowly cooled to 25°C after 30 minutes of insulated stirring, and filtered to obtain (S)-2-hydroxy-3-methoxy-3,3-diphenyl Propionic acid L-prolinamide salt.
[0040] Add 50 g of ethyl acetate and 20 g of water to the filter cake, stir to adjust the pH of the solution to 2 to 3, after adjustment, separate the layers, and extract the water layer once with 25 g of ethyl acetate. The organic layers were combined, washed once with 10 g of water, and separated. The organic layer was concentrated under reduced pressure to obtain 70 g of ethyl acetate. After the concentration was complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com