Patents
Literature
51 results about "Laboratory Test Result" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The outcome of a laboratory test.
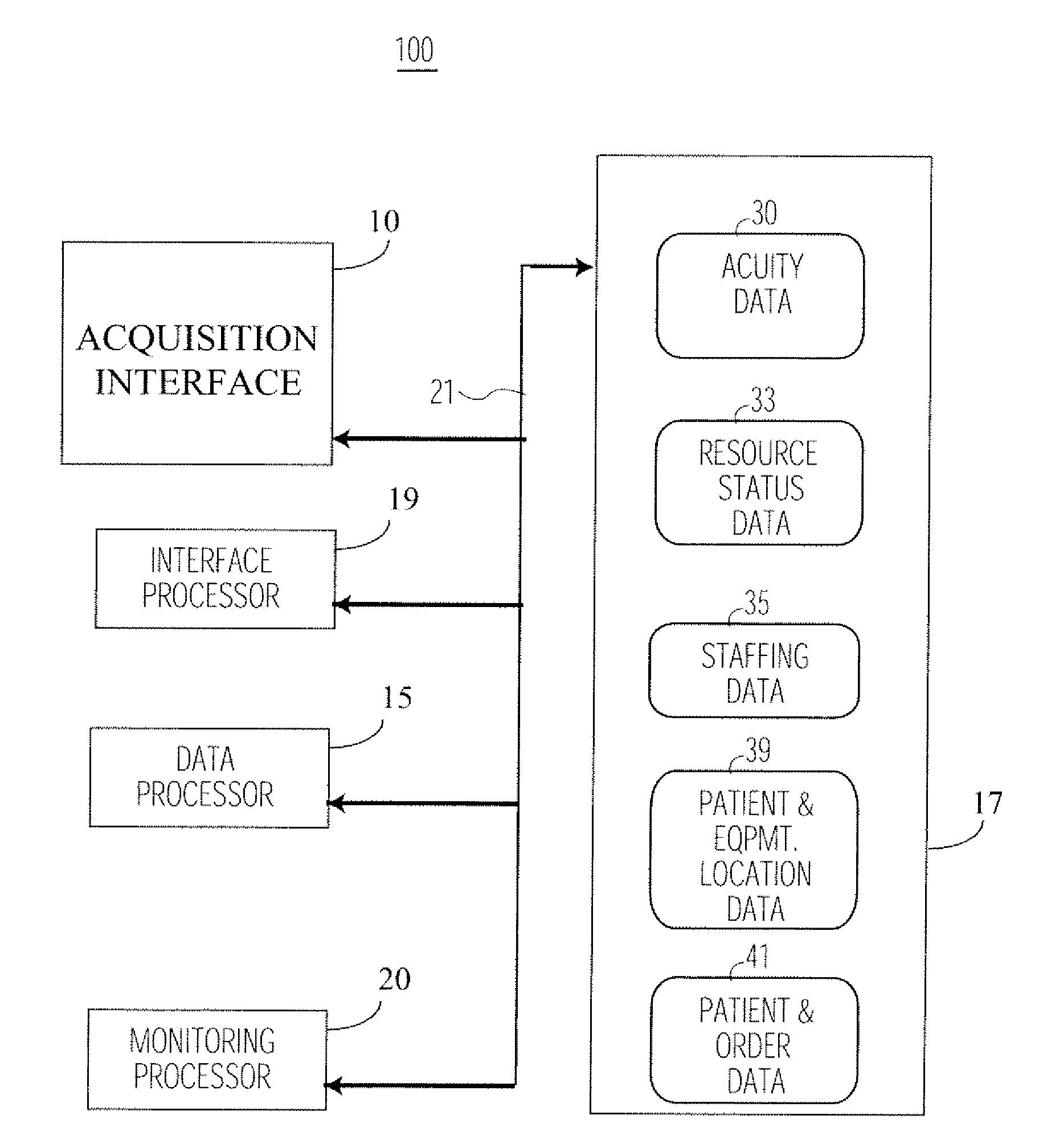
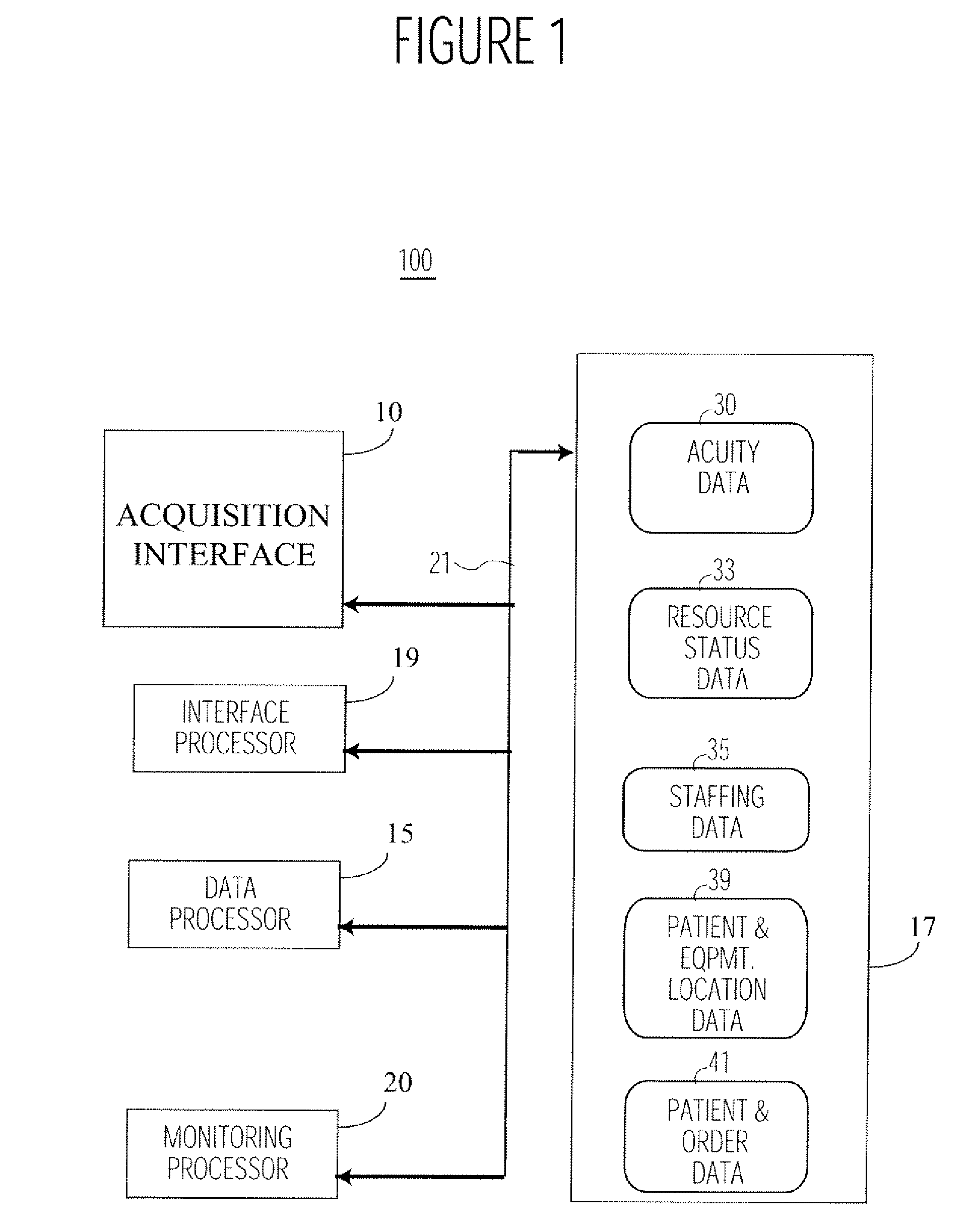
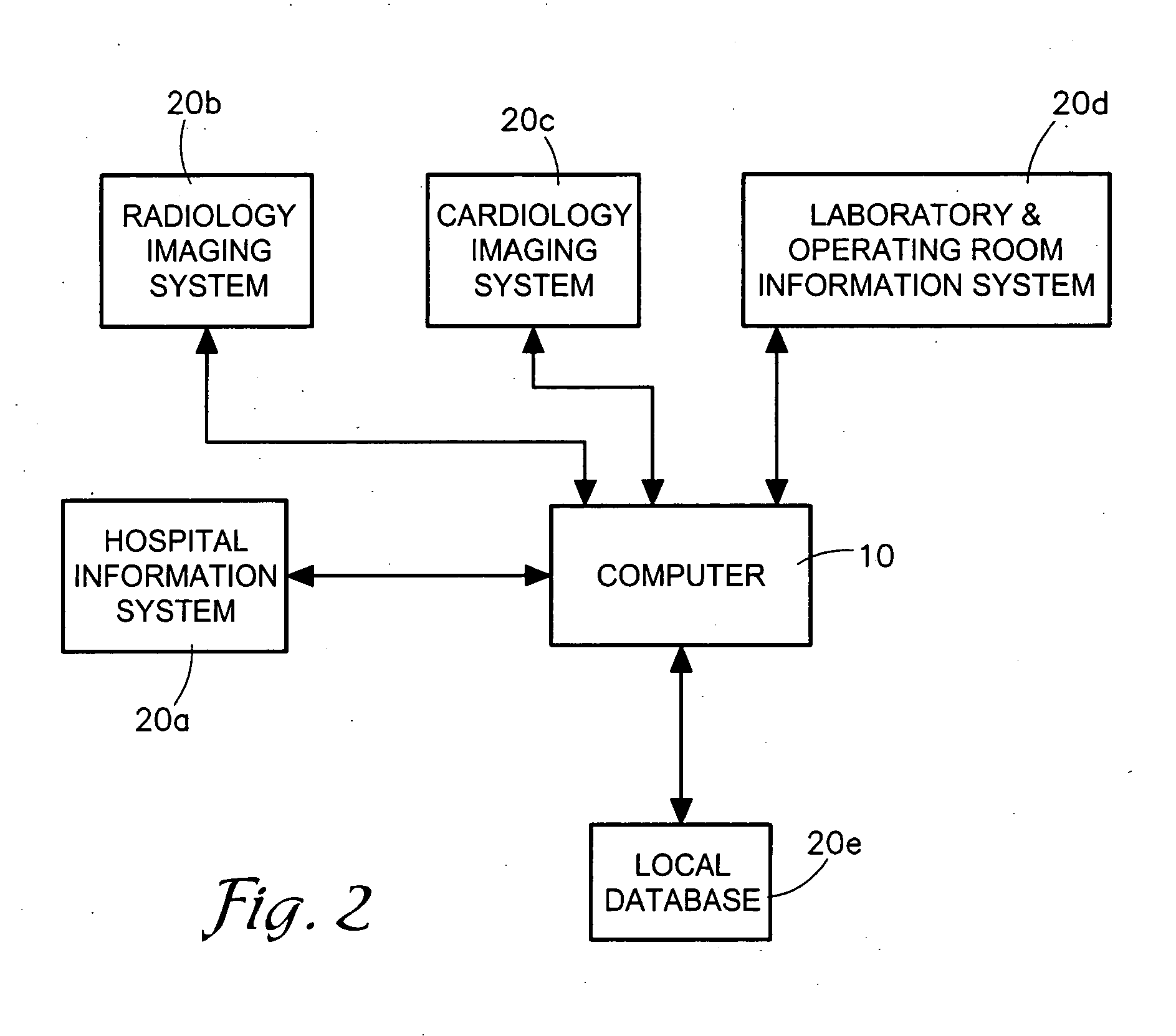
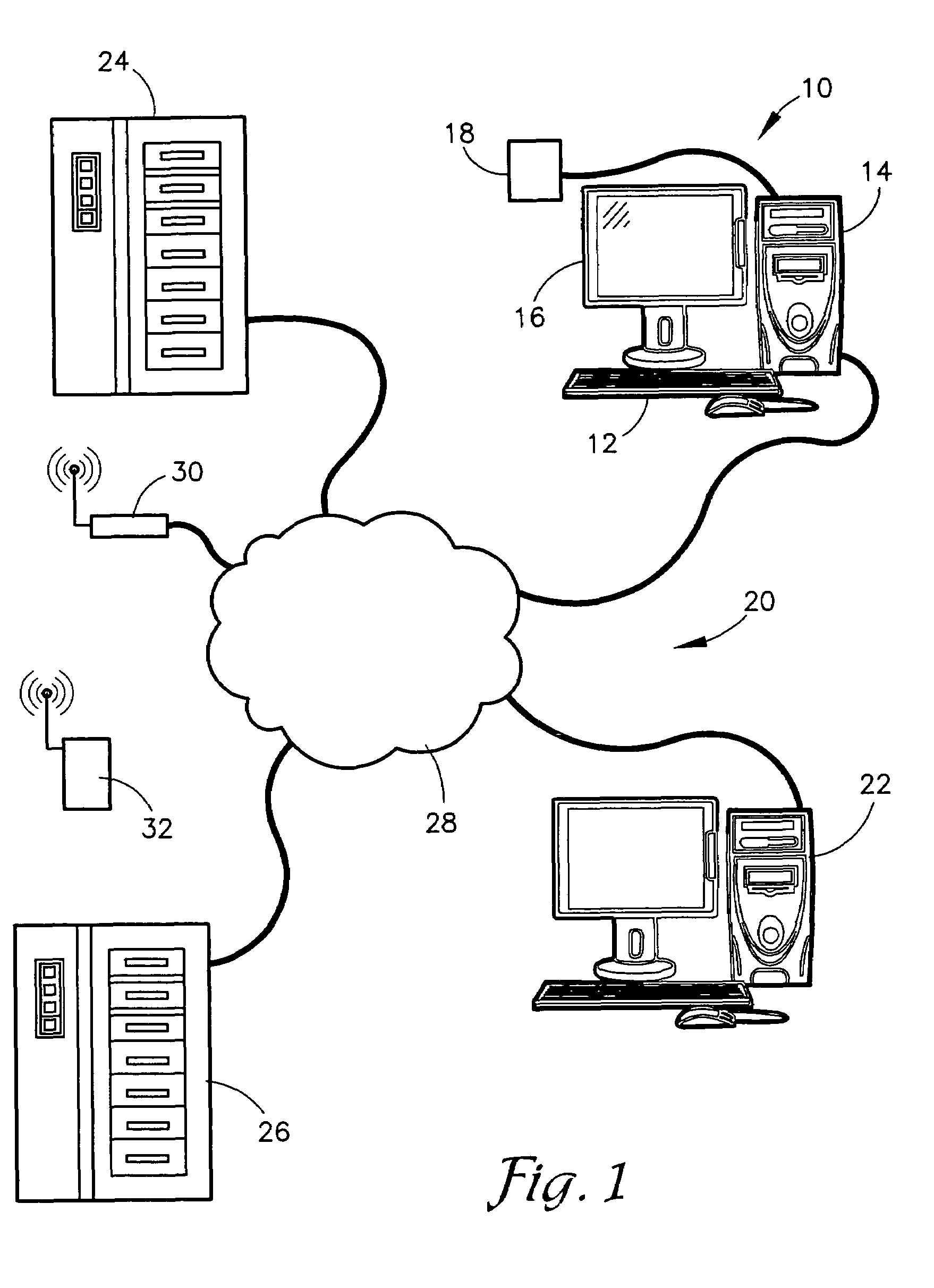
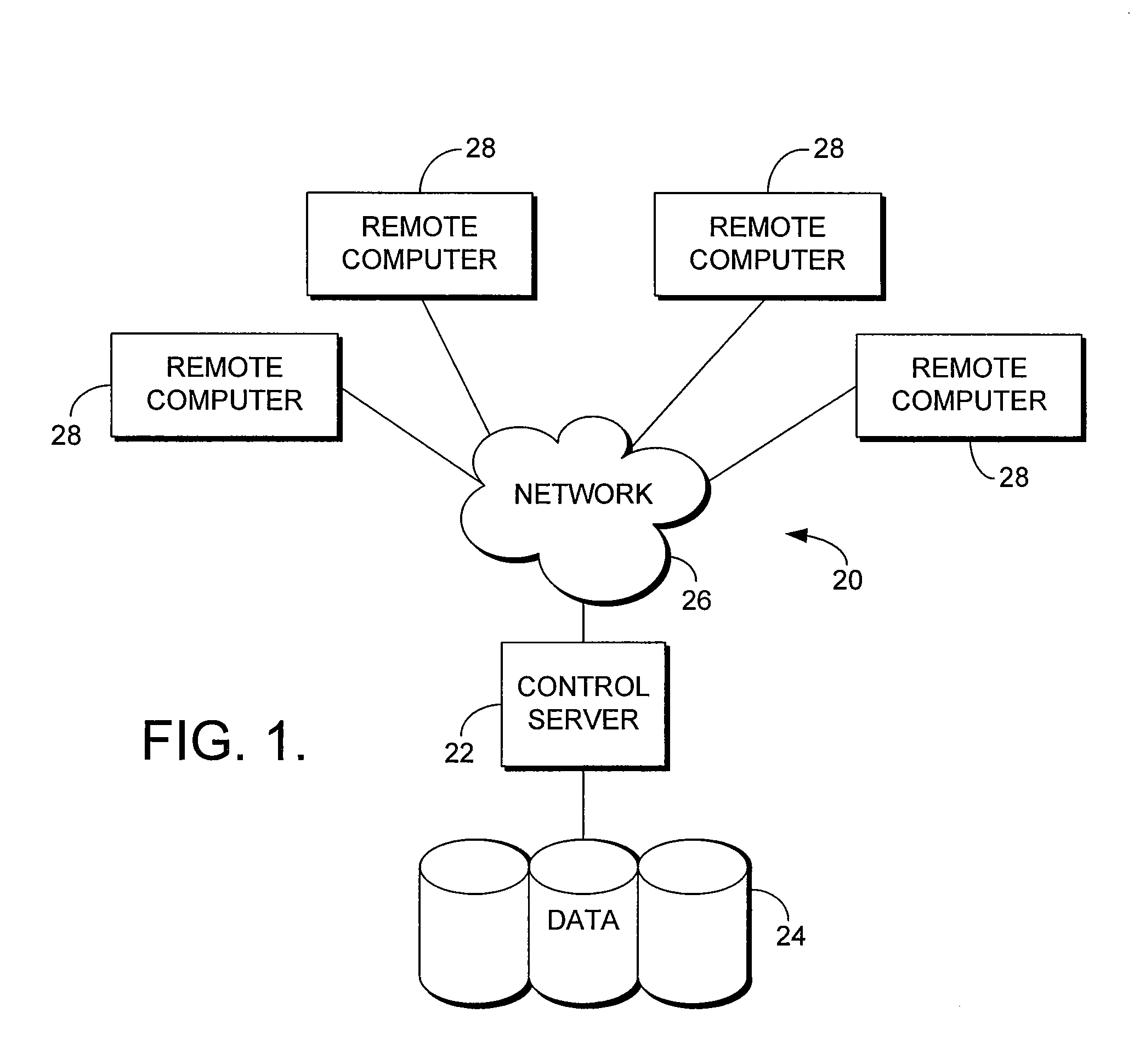
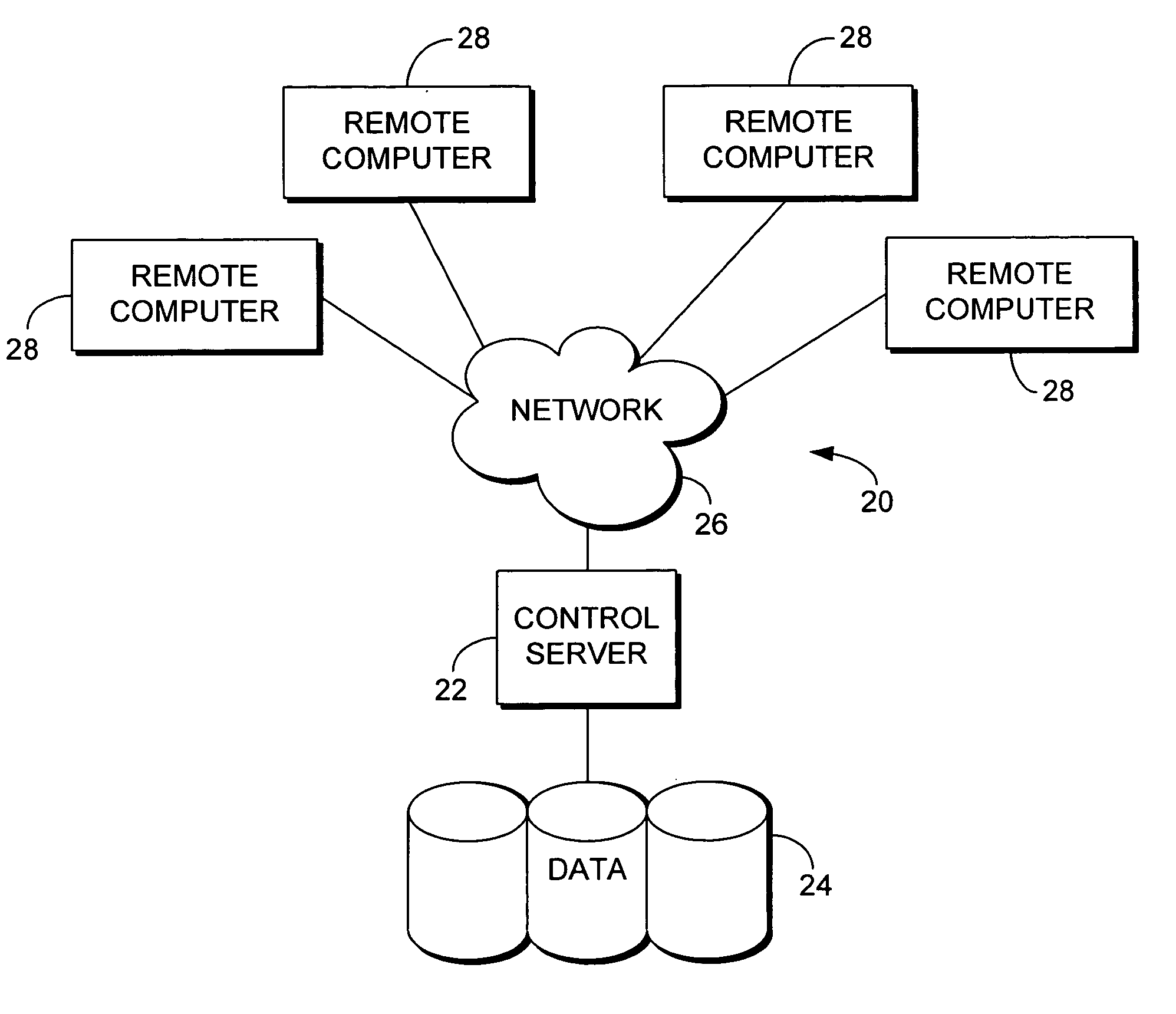
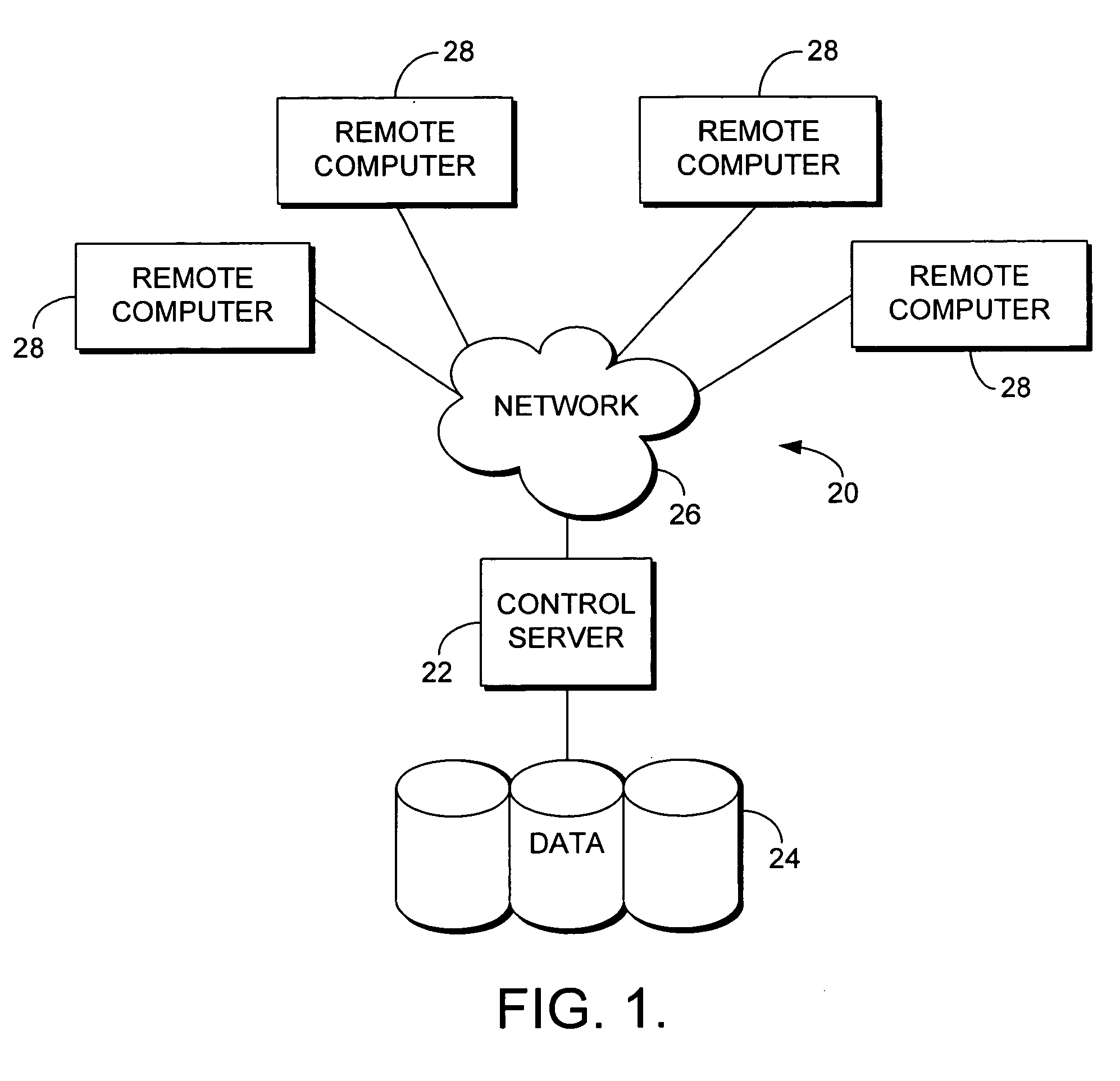
System for Monitoring Healthcare Related Activity In A Healthcare Enterprise
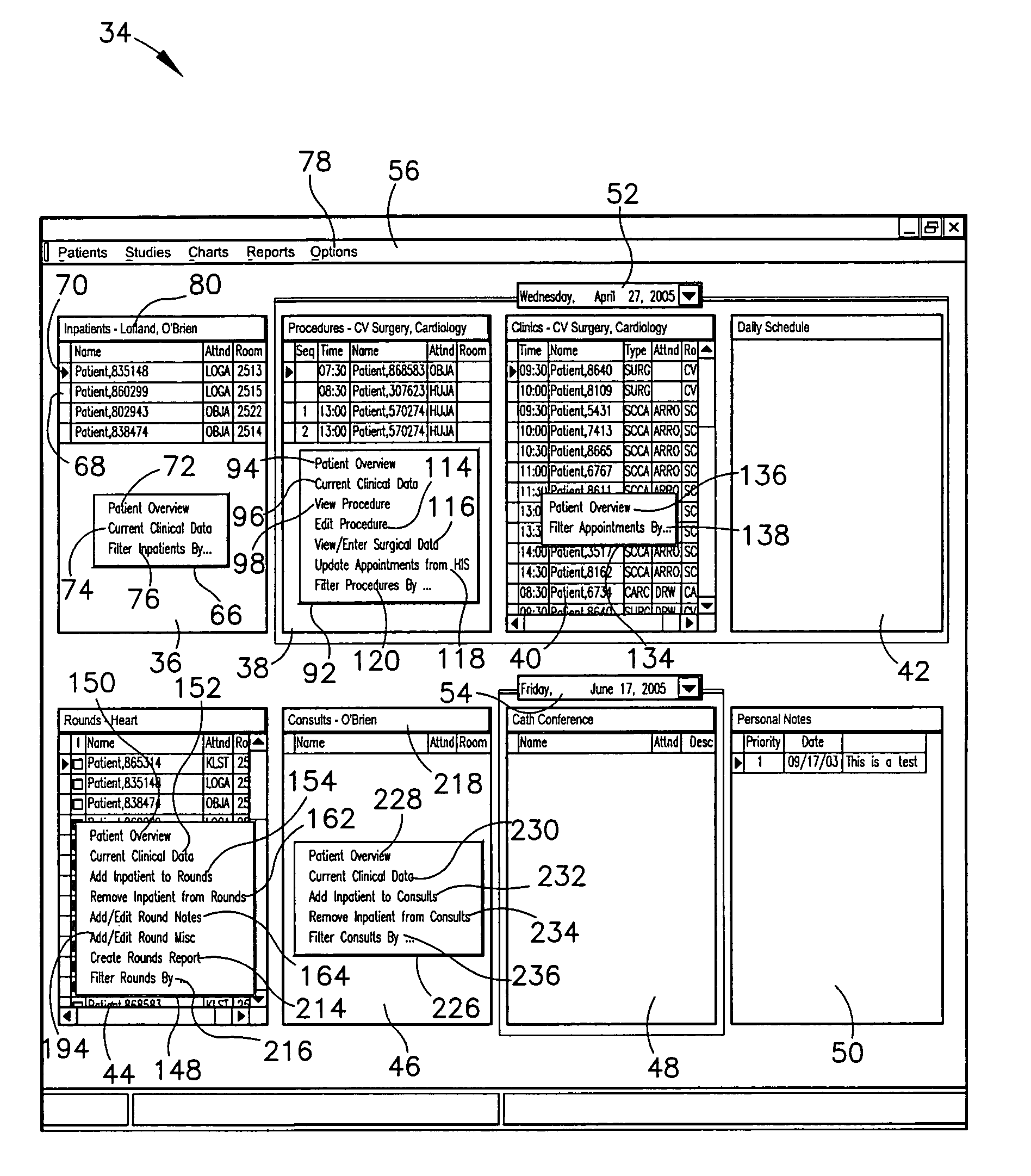
A system provides comprehensive patient and resource status information (manpower and equipment required to maintain optimal patient care) in an organization (e.g., a hospital) for use in adjusting resources to meet existing and future conditions. A system for monitoring activity in a healthcare enterprise includes an acquisition interface for acquiring acuity data representative of severity of medical condition of an individual patient, for multiple different patients. A monitoring processor monitors data identifying orders initiated for treatment to be provided to an individual patient and data identifying laboratory test results received for an individual patient, for multiple different patients. A data processor generates data representing status of healthcare activity for multiple patients in response to the data identifying orders and laboratory test results and the acuity data, for multiple different patients. An interface processor provides the data representing status of healthcare activity to a healthcare worker.
Owner:SIEMENS MEDICAL SOLUTIONS HEALTH SERVICES CORPORAT
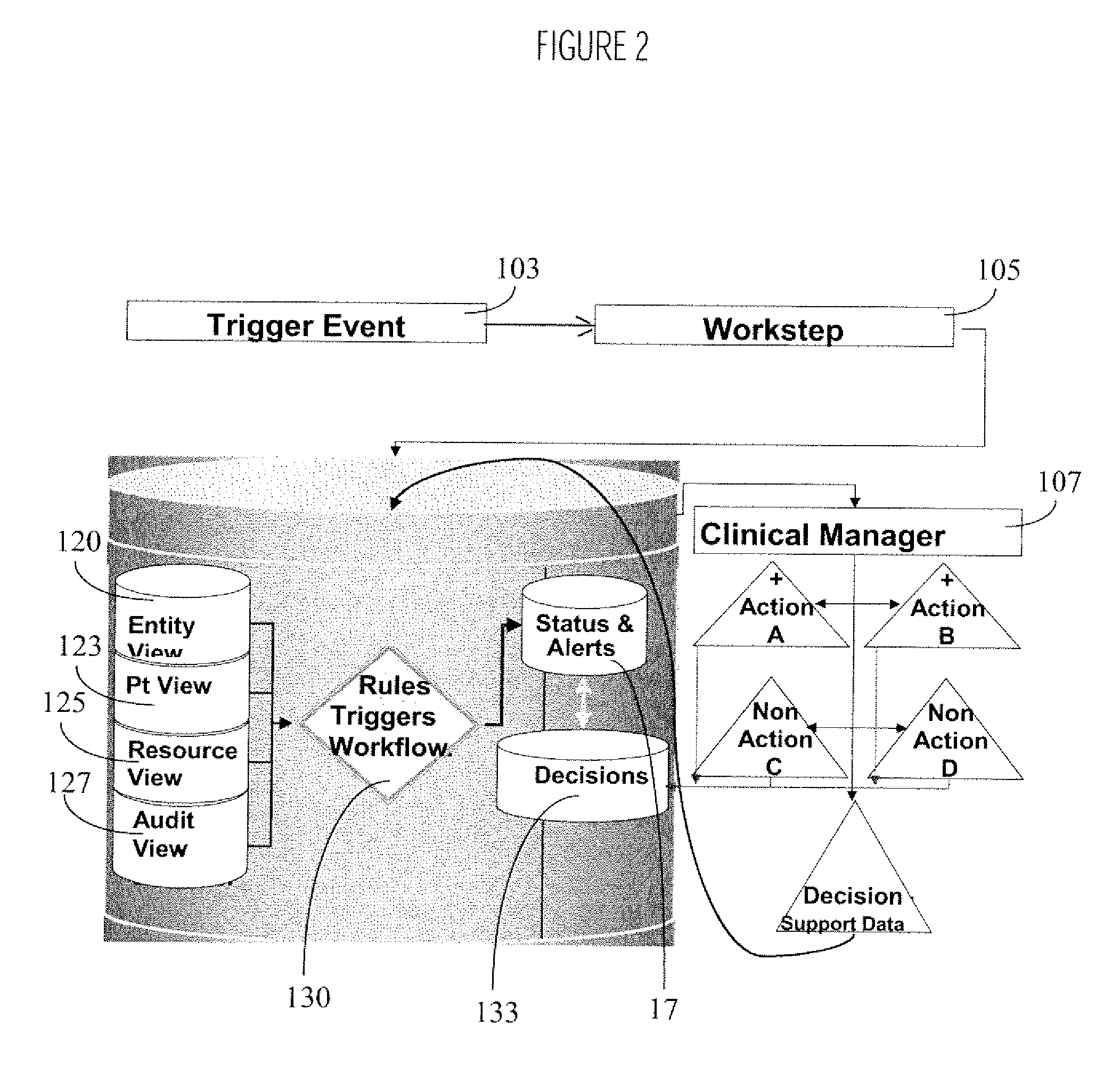
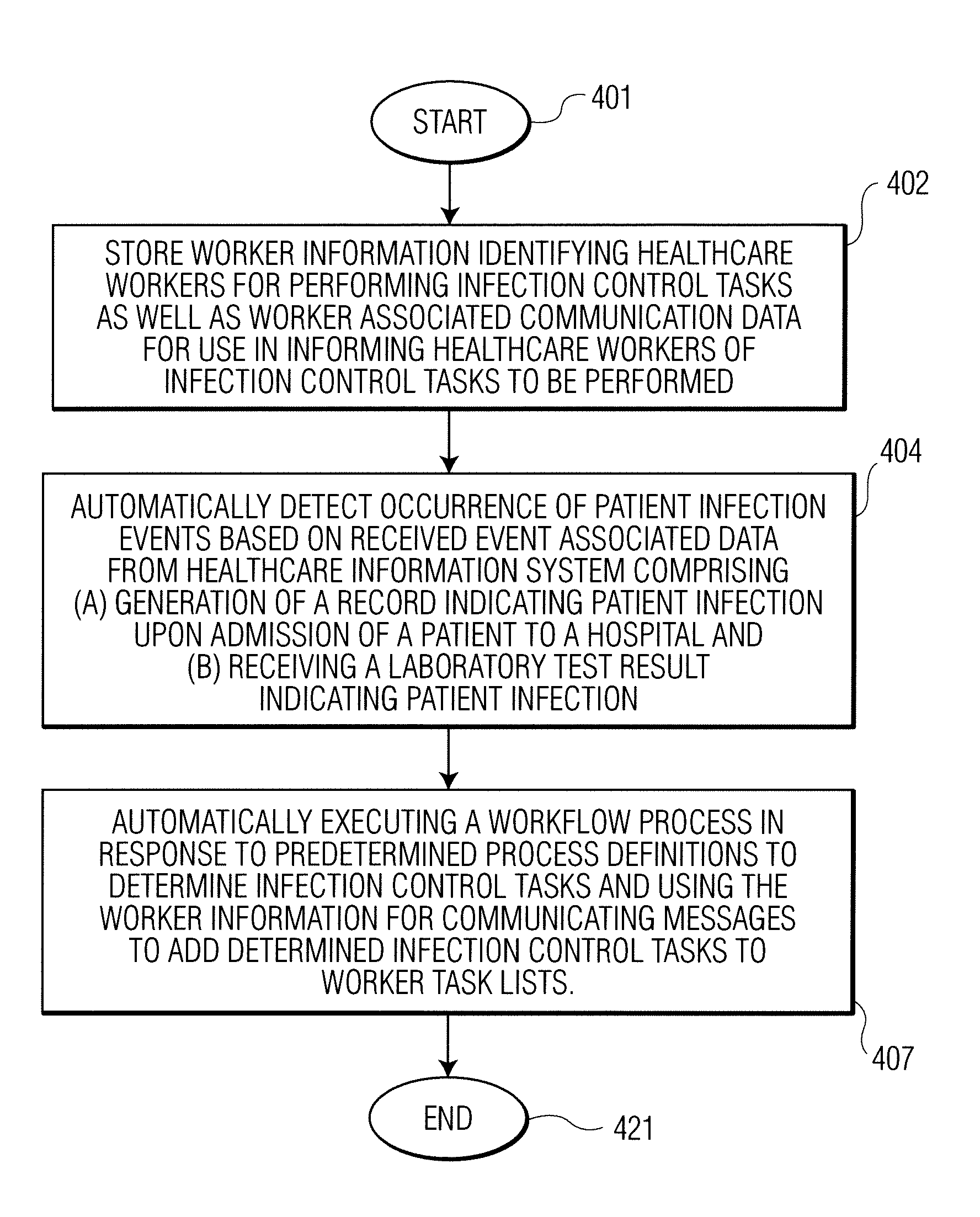
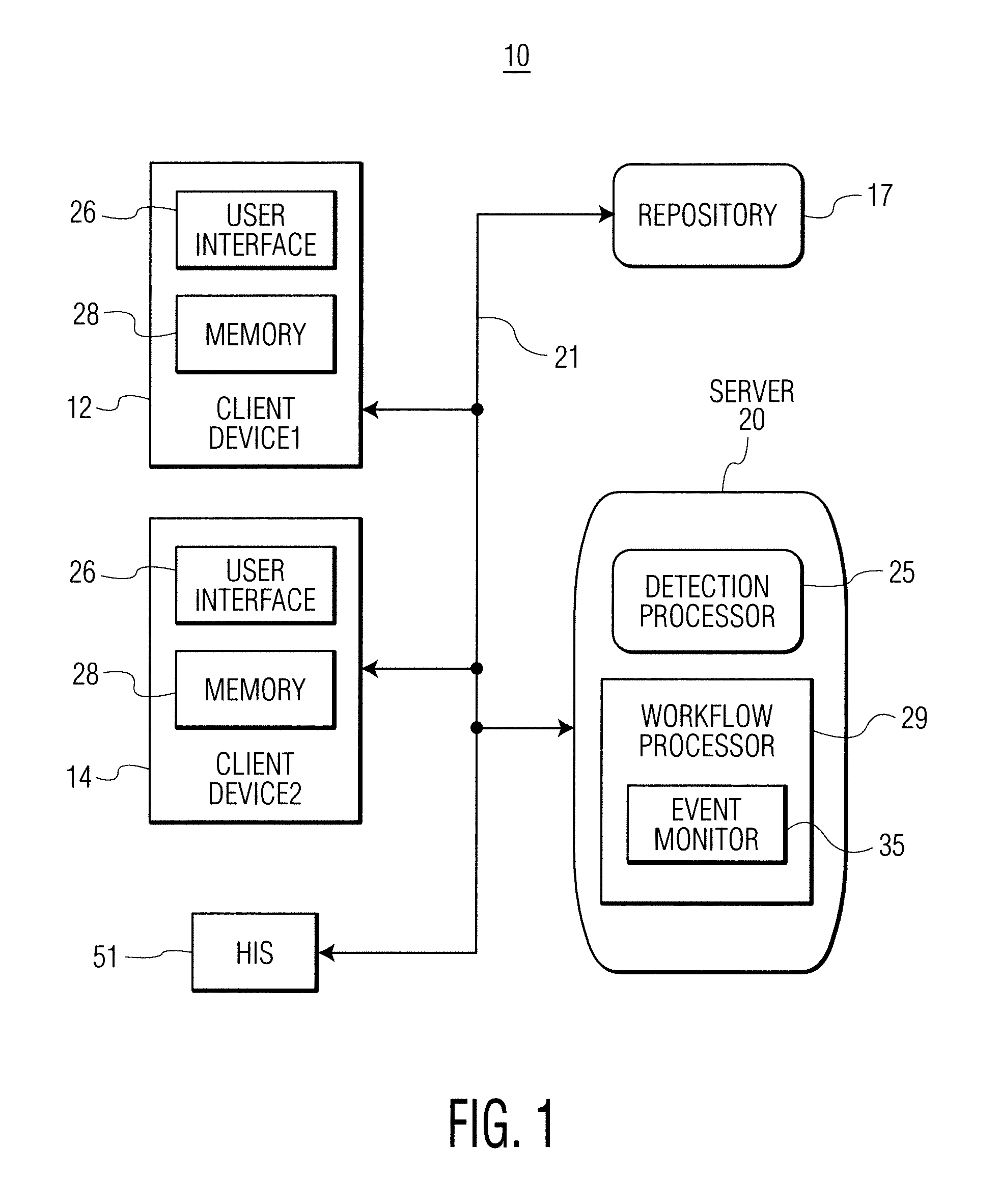
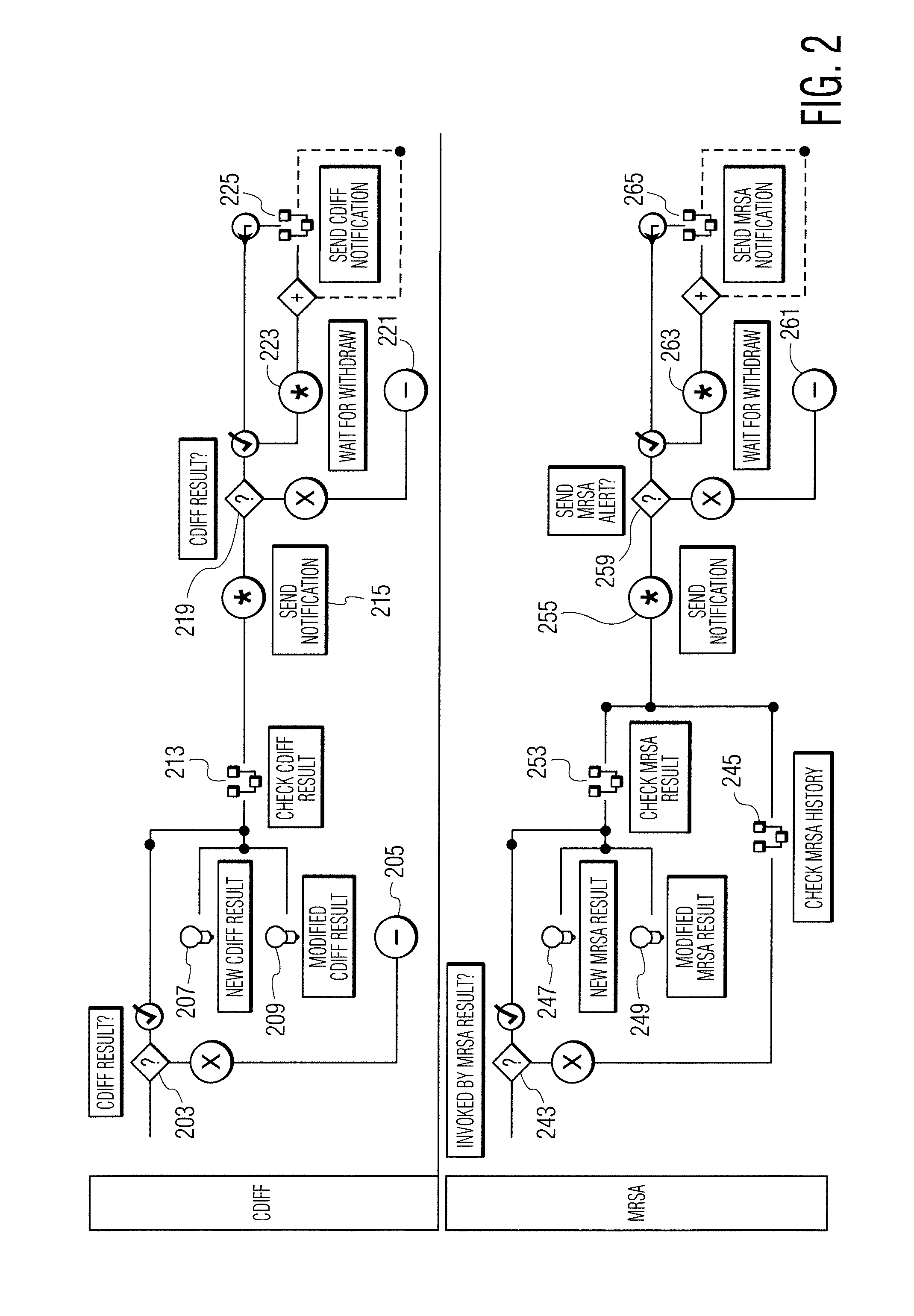
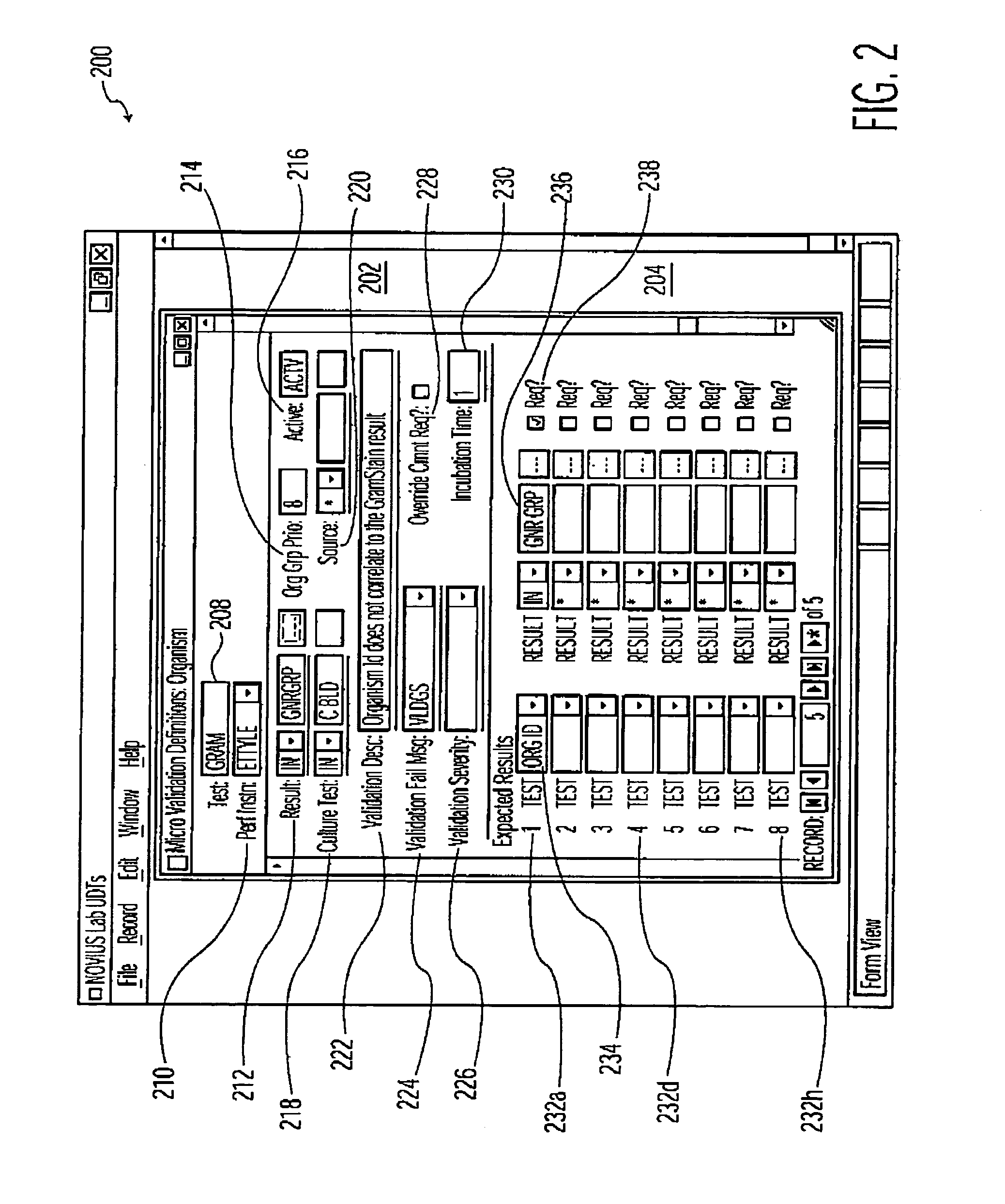
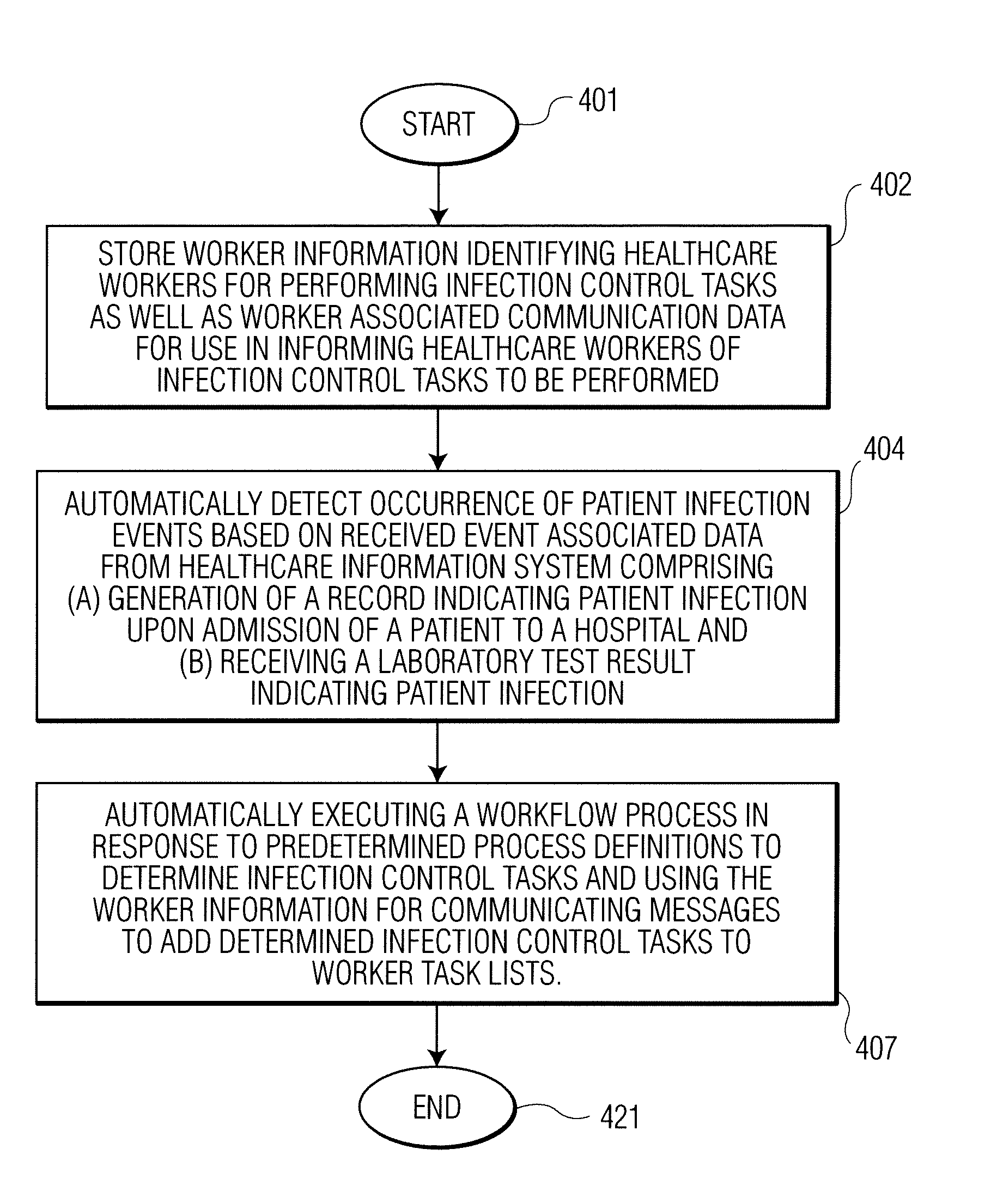
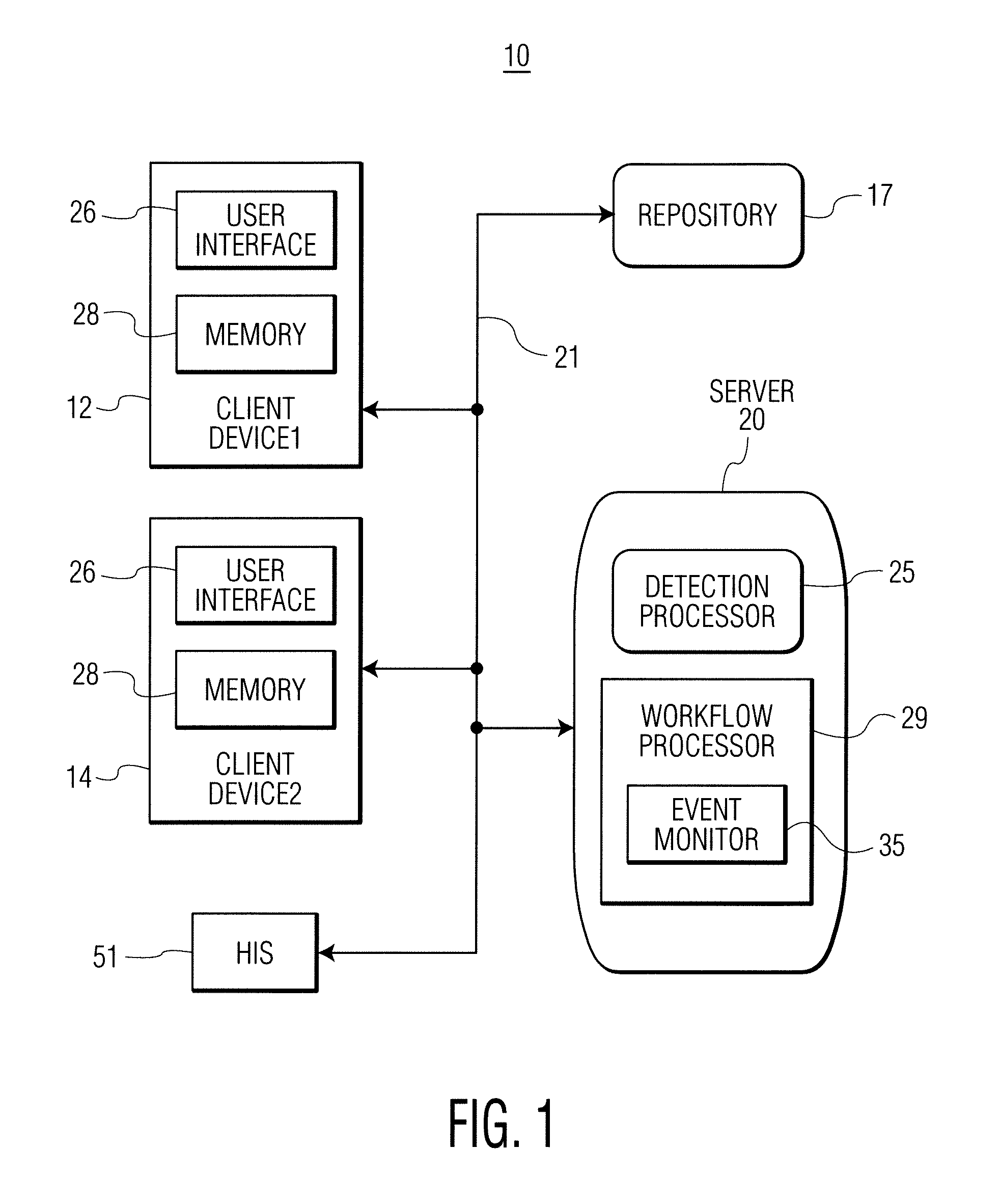
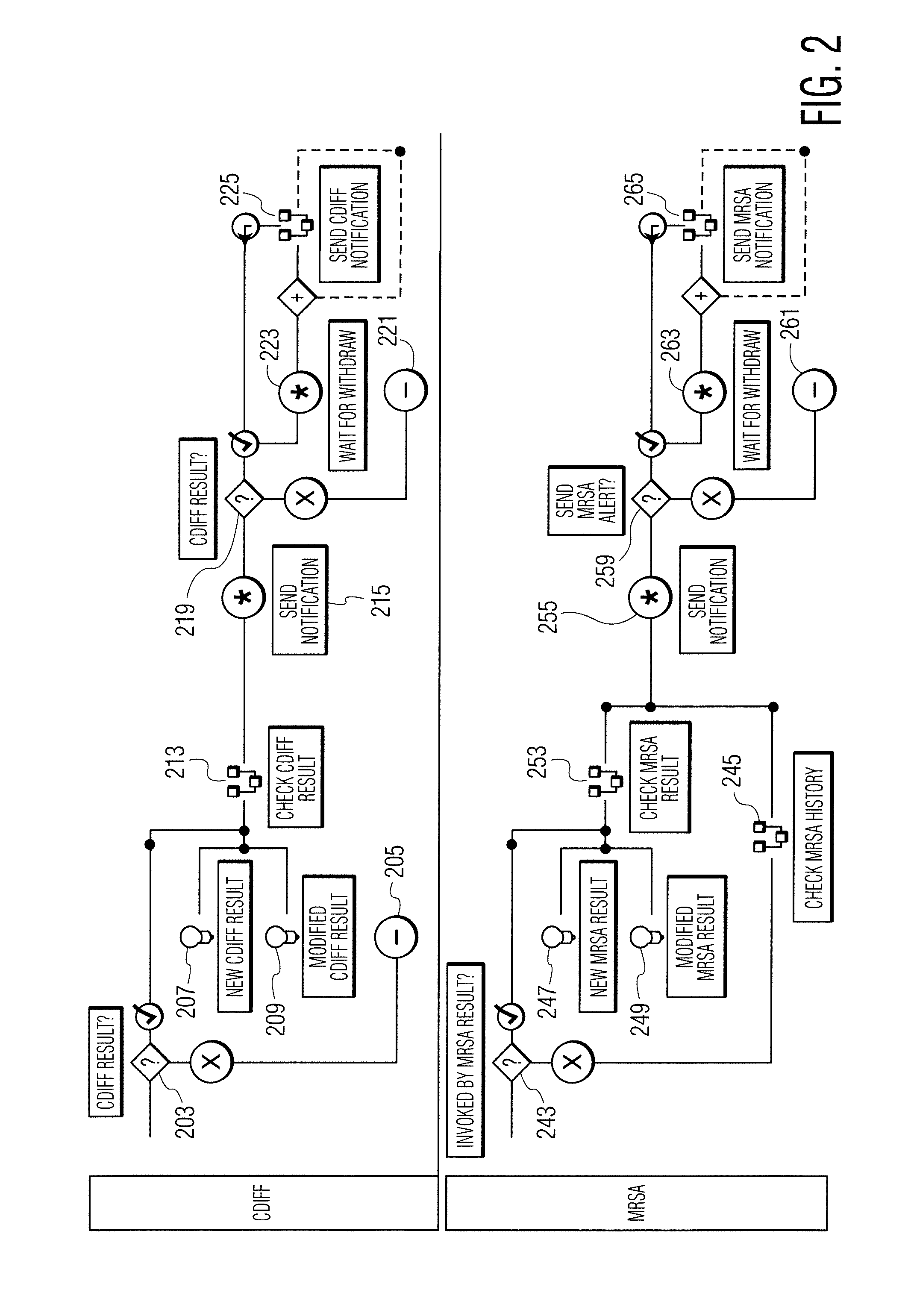
Infection control management and workflow system
A system identifies multiple medical conditions, observations, and laboratory test results using active sensors and predetermined rules to identify infected patients. An infection control and workflow management system includes a repository of worker information identifying healthcare workers for performing infection control tasks as well as worker associated communication data for use in informing healthcare workers of infection control tasks to be performed. A detection processor automatically detects infection in patients from multiple different sources including from at least one of, (a) a medical record evaluated upon admission of a patient to a hospital and (b) a laboratory test result. A workflow processor uses the worker information for automatically communicating a message to inform a healthcare worker of a task to be performed to initiate infection control tasks using communication data in response to detection of an infected patient.
Owner:CERNER INNOVATION
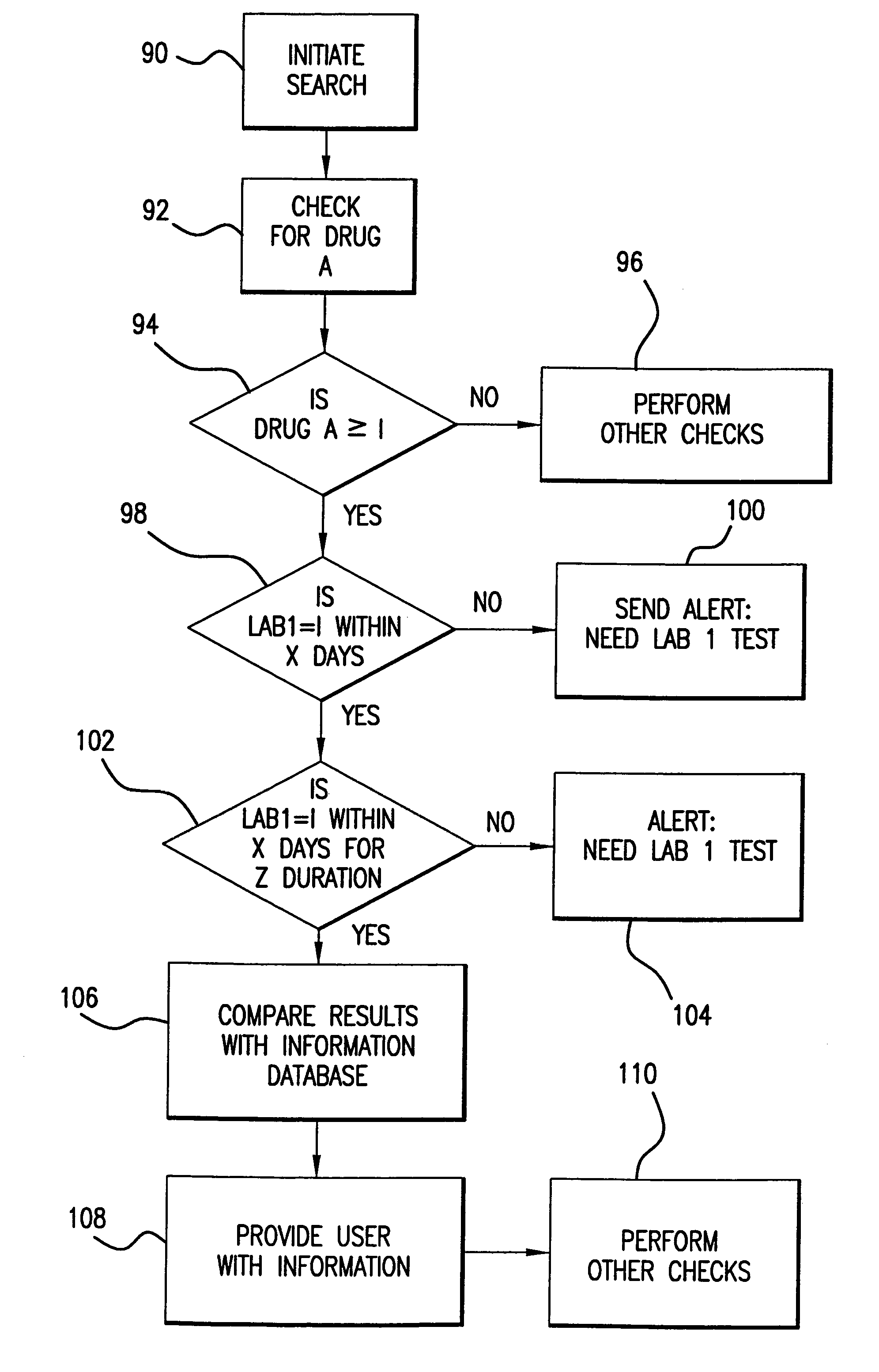
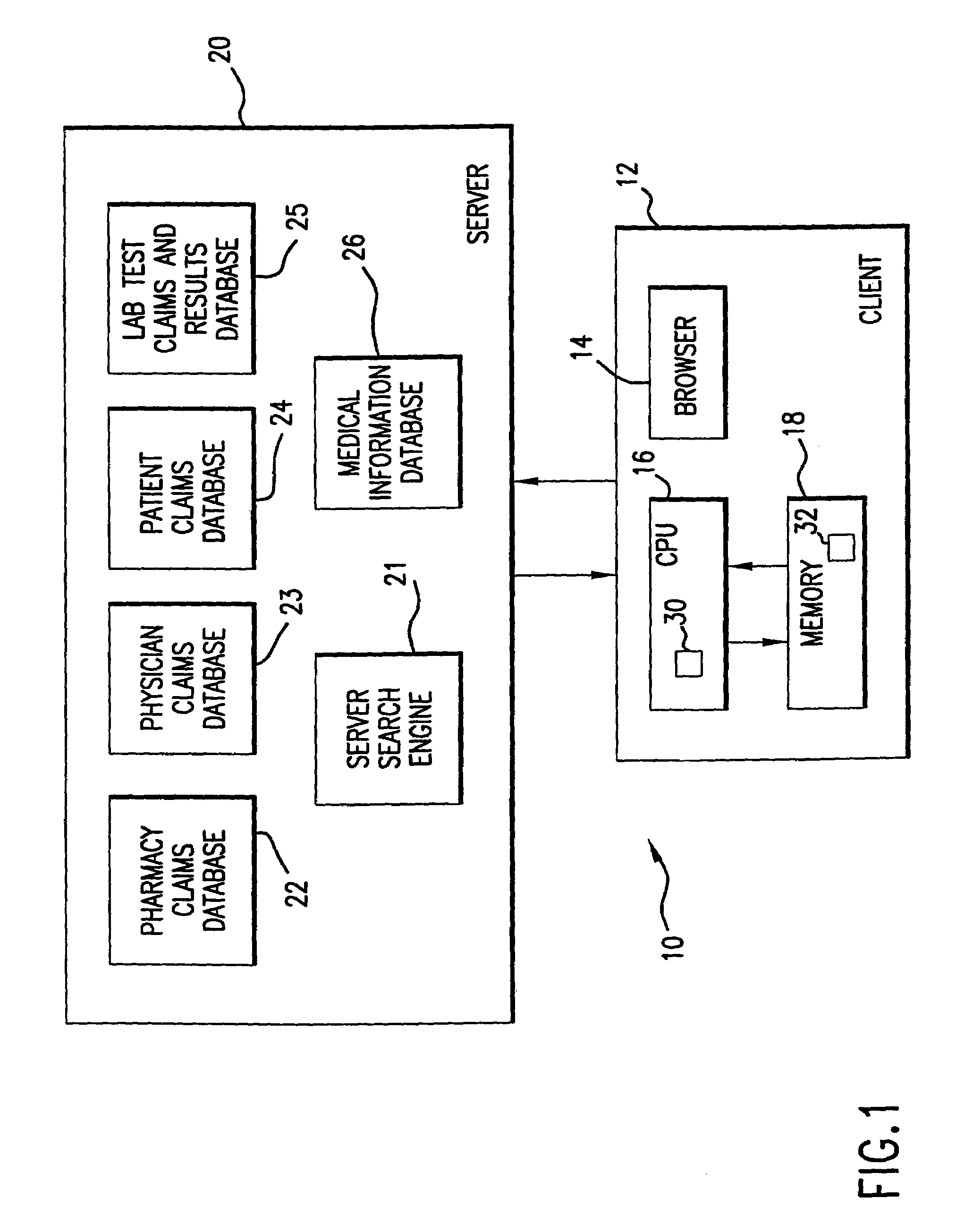
System for monitoring regulation of pharmaceuticals from data structure of medical and labortory records
InactiveUS7124031B1Strong specificityHigh strengthPhysical therapies and activitiesDigital data processing detailsDiseaseLaboratory Test Result
A system is provided that integrates of records of clinical laboratory services into the assessment and optimization of patient health care and, in particular, regulation of the use of pharmaceuticals. Laboratory test result records are used in conjunction with other health care benefits records to monitor regulation of use of pharmaceuticals by patients. The incorporation of laboratory tests and results into such a utilization system allows improvement in the management of a patient's therapy based on a more precise picture of the patient's level of illness as revealed by the laboratory test results. The system of the present invention also allows optimization of the selection of laboratory tests to be performed, and also provides an outcome assessment of the risk of hospitalization due to pharmaceutical treatments resulting in physician intervention, leading to a change in physician prescribing behavior and, accordingly, a decrease in drug induced hospitalizations and improved quality of patient care and savings of health care costs.
Owner:PROVANTAGE INC +1
System and method for collecting, organizing, and presenting visit-oriented medical information
InactiveUS20070016441A1Quick selectionMechanical/radiation/invasive therapiesDrug and medicationsLaboratory Test ResultRelevant information
A method and computer program collects patients' medical information and presents the information to a user via an interactive user interface (400) that includes a plurality of information tabs. Each tab presents information about a particular patient from a certain phase of the patient's visit to a healthcare providing facility. The information tabes include diagnoses (402), heart lab (404), radiology (406), pre-operative (408), operative (410), post-operative (412), reports (414), discharge (416), and follow up (418). The post-operative tab presents laboratory test results in the form of a table (514) and a chart (516), wherein the chart includes laboratory test results graphed over time and corresponding event and normal value indicators.
Owner:CHILDRENS MERCY HOSPITAL
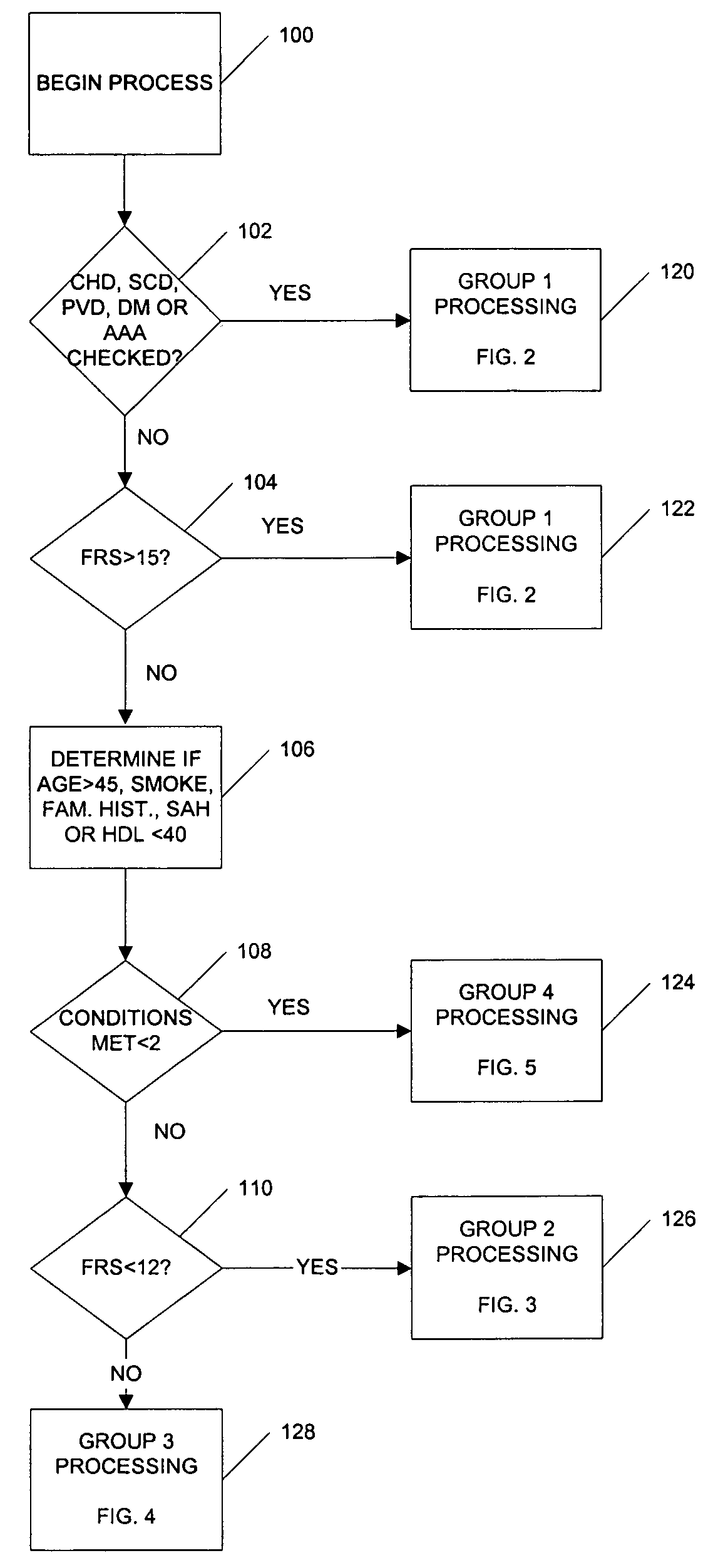
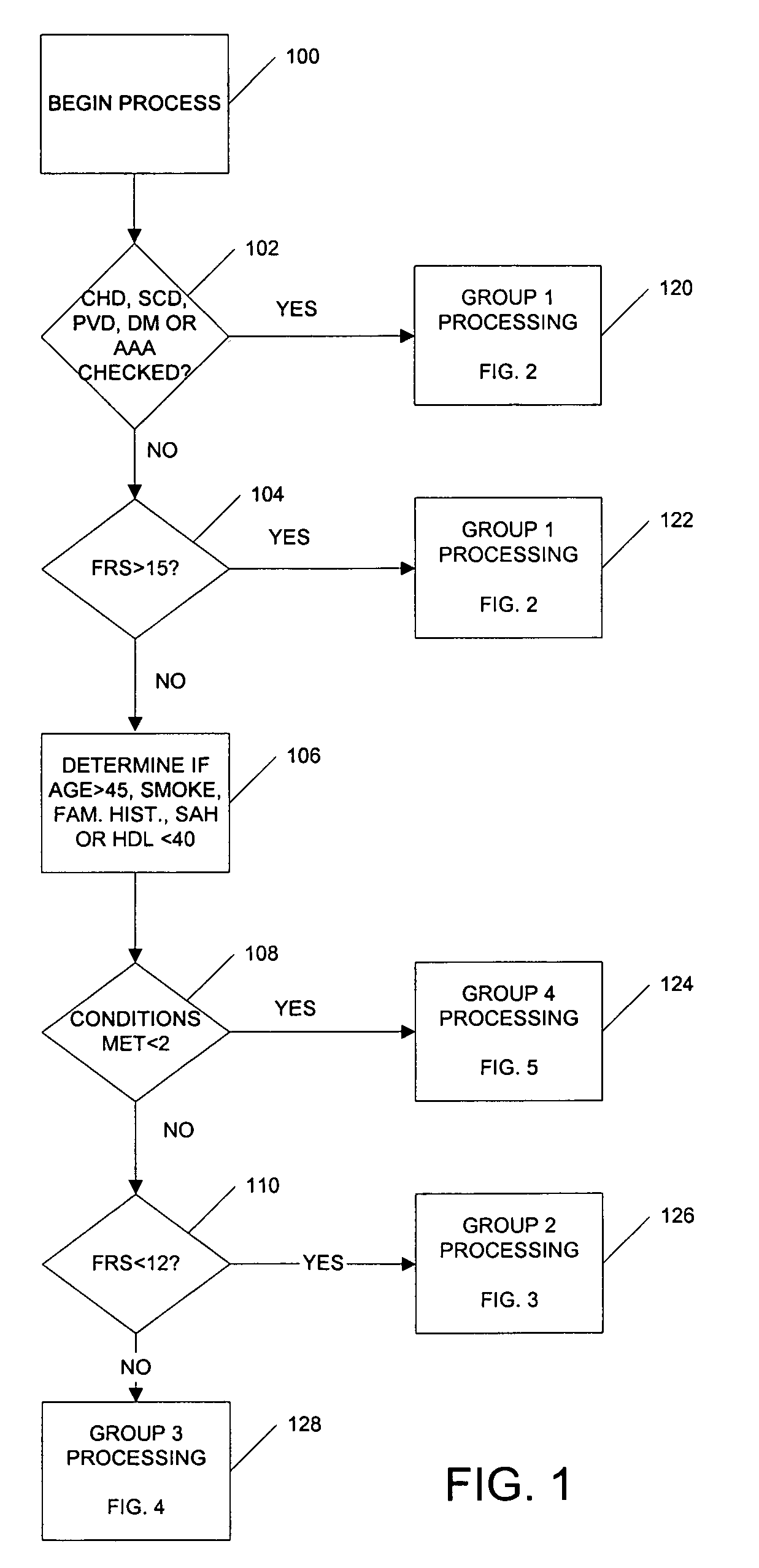
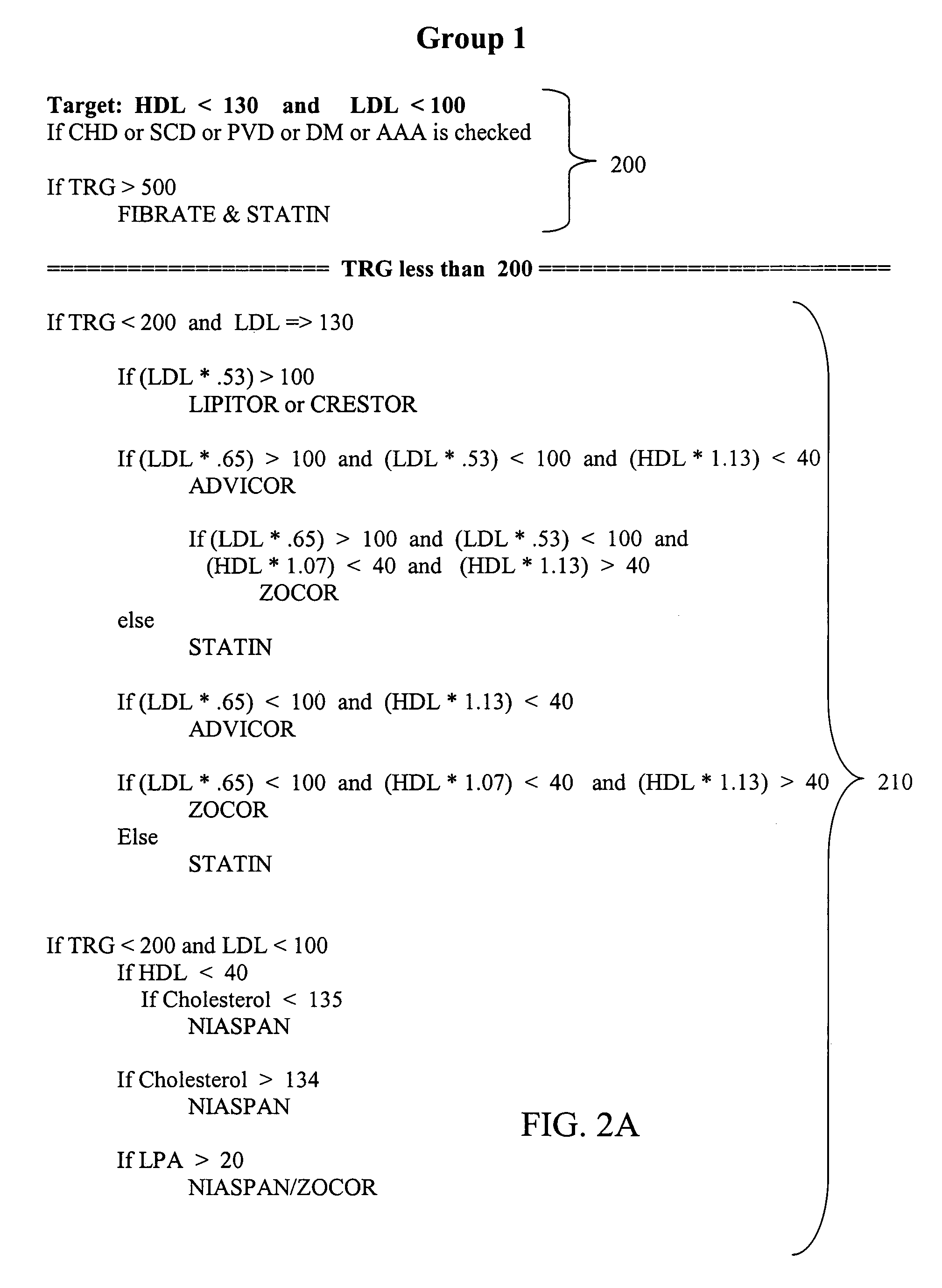
Medical risk assessment method and program product
ActiveUS7306562B1Physical therapies and activitiesData processing applicationsGuidelineRisk classification
A medical risk assessment method and computer program product resident on a computer or a hand-held device that allows a physician to determine the best strategy for primary and secondary cardiovascular disease prevention utilizing current guidelines and published medical literature. The computer program product evaluates a number of risk factors to determine specific recommendations for an individual patient, including Framingham risk scoring (FRS), pertinent medical history, individual lipid panel and advanced lipoprotein profiling, patient laboratory test results, and published literature on the effects of anti-lipid medicines on plasma concentration and / or composition of lipoprotein molecules and clinical outcomes. The risk assessment method establishes a cardiovascular treatment therapy strategy for a patient by determining a cardiac risk classification group, determining a cardiovascular treatment therapy based on the patient's lipoprotein profile and the patient's cardiac group risk classification, and presenting the cardiovascular treatment therapy for the patient to a medical practitioner on a patient evaluation display.
Owner:MEDICAL SOFTWARE
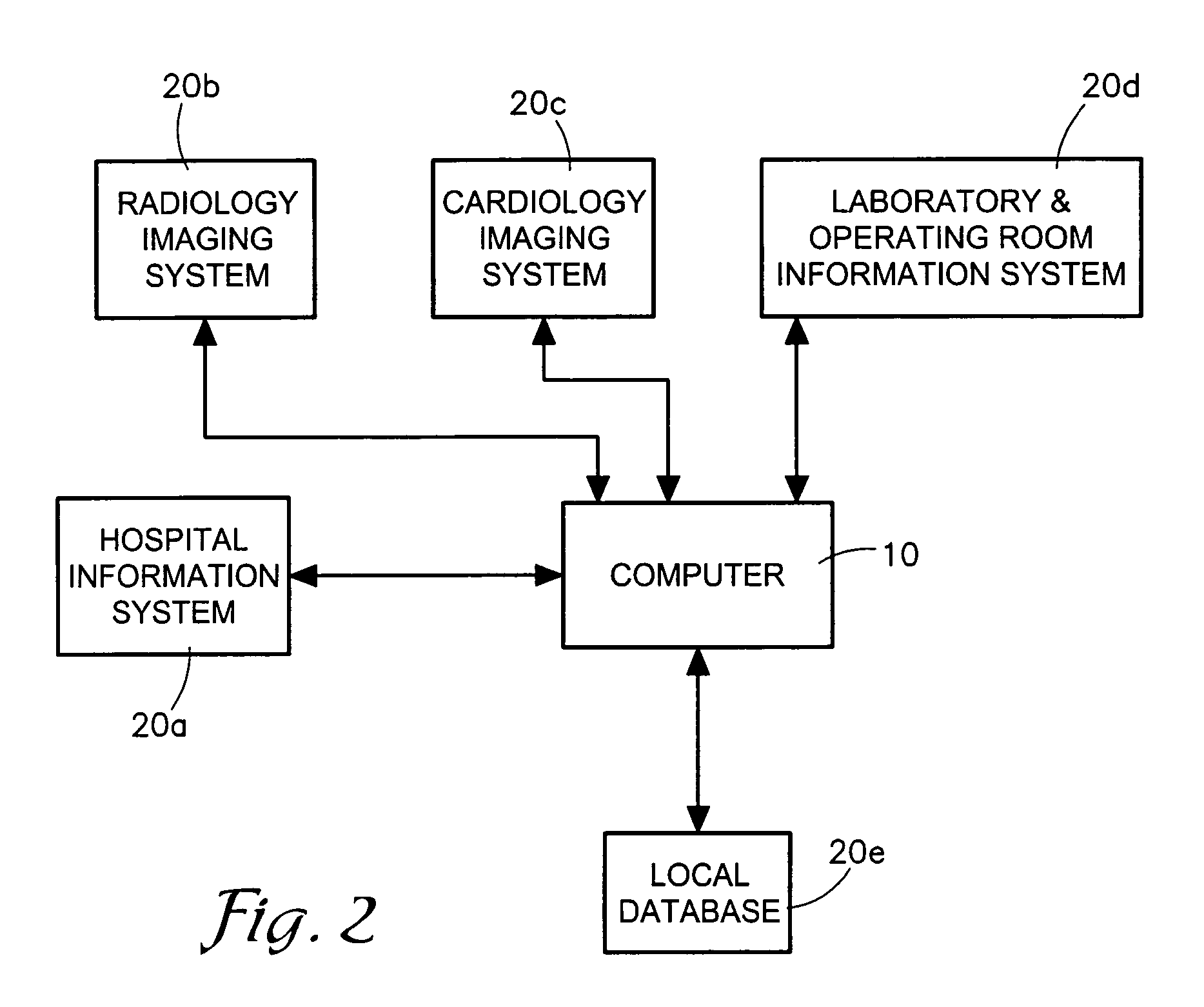
System and method for collecting, organizing and presenting research-oriented medical information
InactiveUS20070016440A1Local control/monitoringComputer-assisted medical data acquisitionLaboratory Test ResultLibrary science
A method and computer program manages medical research study information and laboratory test result information. The program generates an interactive user interface with elements for setting up a study (702), managing study member information (704), managing patient information (706), receiving and displaying comments (708), and configuring data to be stored in a database associated with the research study (710). The program also receives medical event information from a user and laboratory test result information from a laboratory information system (20d), and generates a chart (934) that correlates the test results and the medical events. The program also receives a normal range pertaining to a test result from the laboratory information system (20d) and provides a normal range indicator (952) for the test result.
Owner:CHILDRENS MERCY HOSPITAL
System for monitoring regulation of pharmaceuticals from data structure of medical and laboratory records
InactiveUS20070033075A1Leveling precisionEasy to managePhysical therapies and activitiesDrug and medicationsDiseaseLaboratory Test Result
A system is provided that integrates of records of clinical laboratory services into the assessment and optimization of patient health care and, in particular, regulation of the use of pharmaceuticals. Laboratory test result records are used in conjunction with other health care benefits records to monitor regulation of use of pharmaceuticals by patients. The incorporation of laboratory tests and results into such a utilization system allows improvement in the management of a patient's therapy based on a more precise picture of the patient's level of illness as revealed by the laboratory test results. The system of the present invention also allows optimization of the selection of laboratory tests to be performed, and also provides an outcome assessment of the risk of hospitalization due to pharmaceutical treatments resulting in physician intervention, leading to a change in physician prescribing behavior and, accordingly, a decrease in drug induced hospitalizations and improved quality of patient care and savings of health care costs.
Owner:EXPRESS SCRIPTS STRATEGIC DEV INC +1
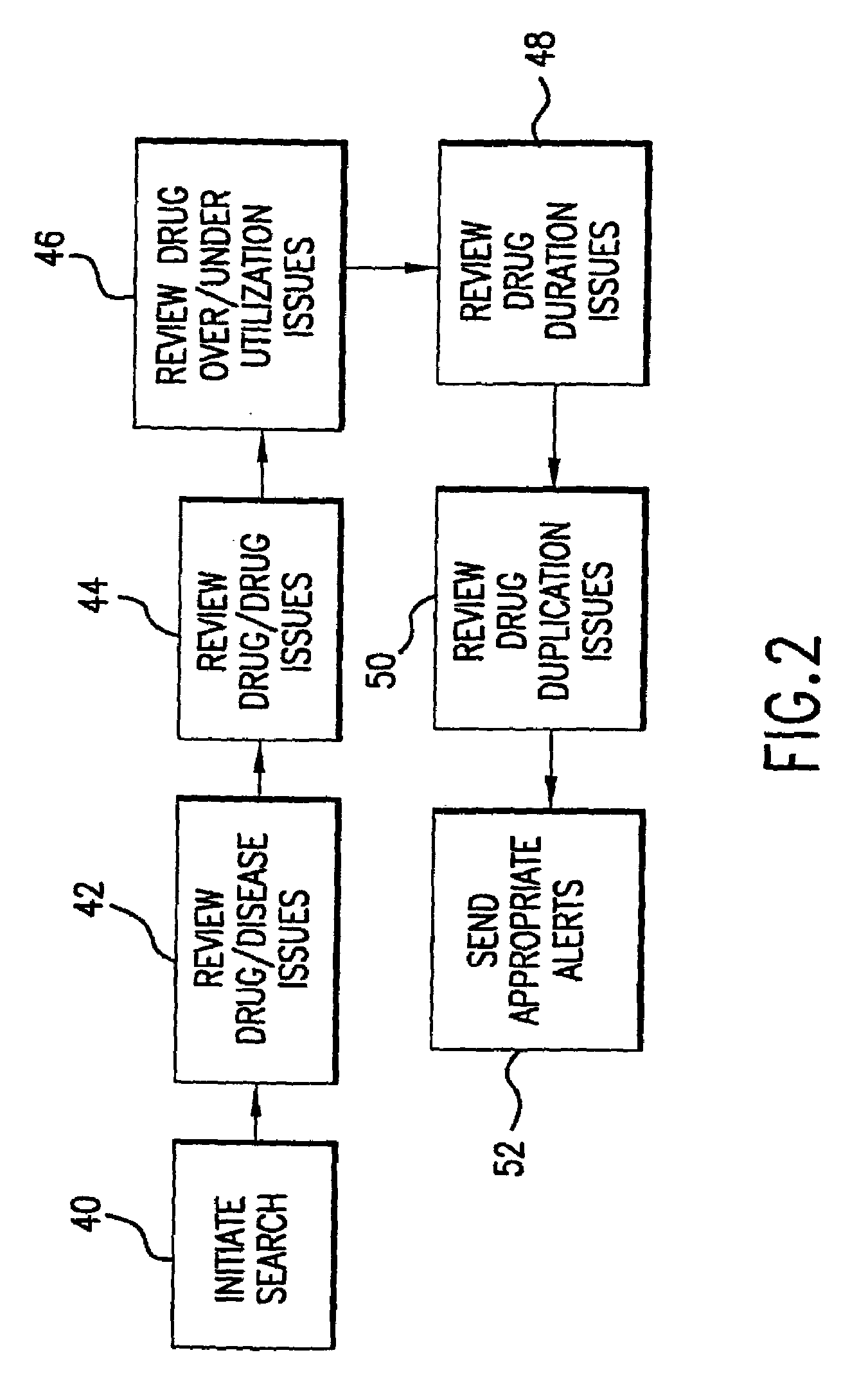
Multi-phase anchor-based diagnostic decision-support method and system
InactiveUS20110257988A1Efficient conductionData processing applicationsHealth-index calculationLaboratory Test ResultHealth professionals
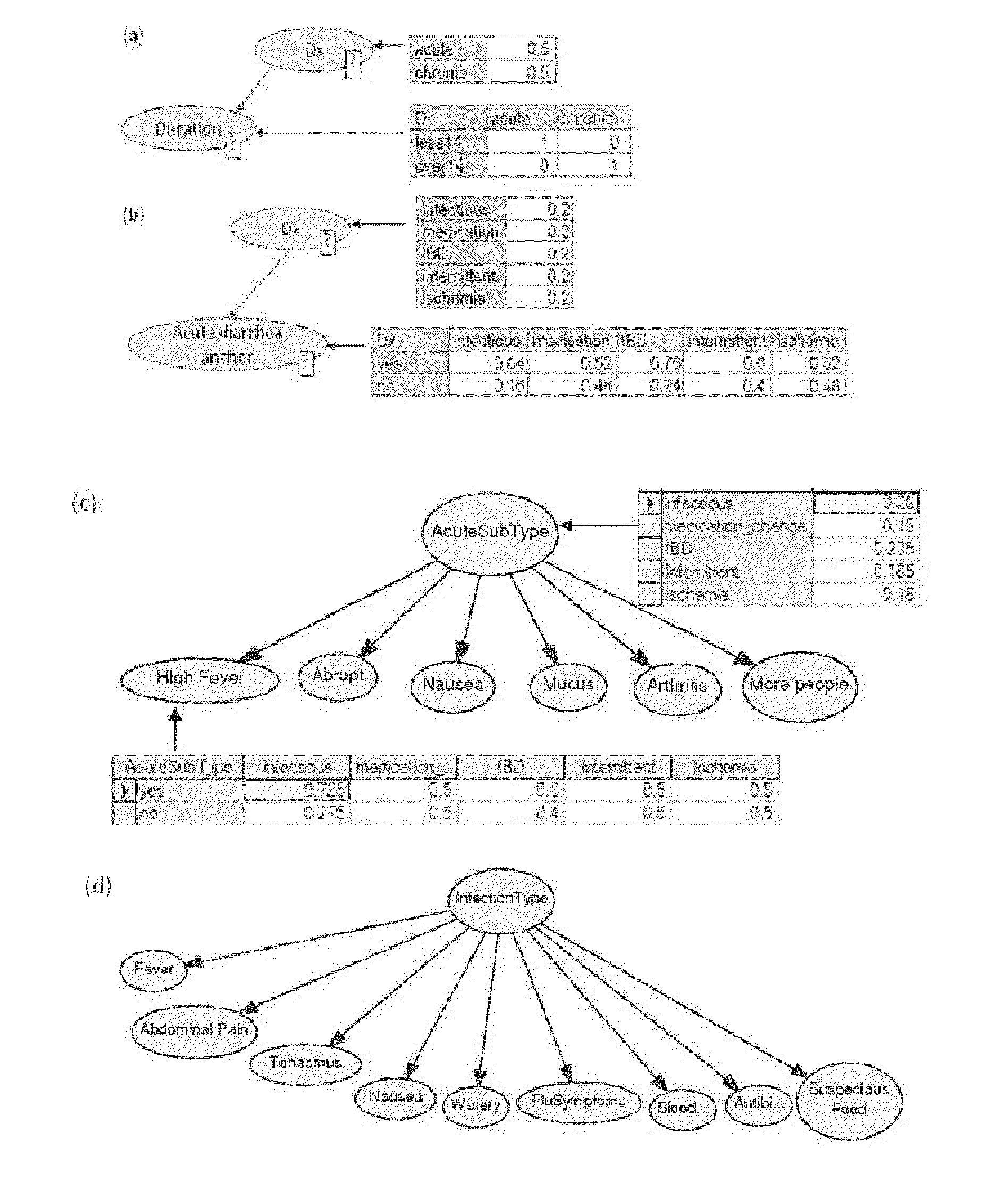
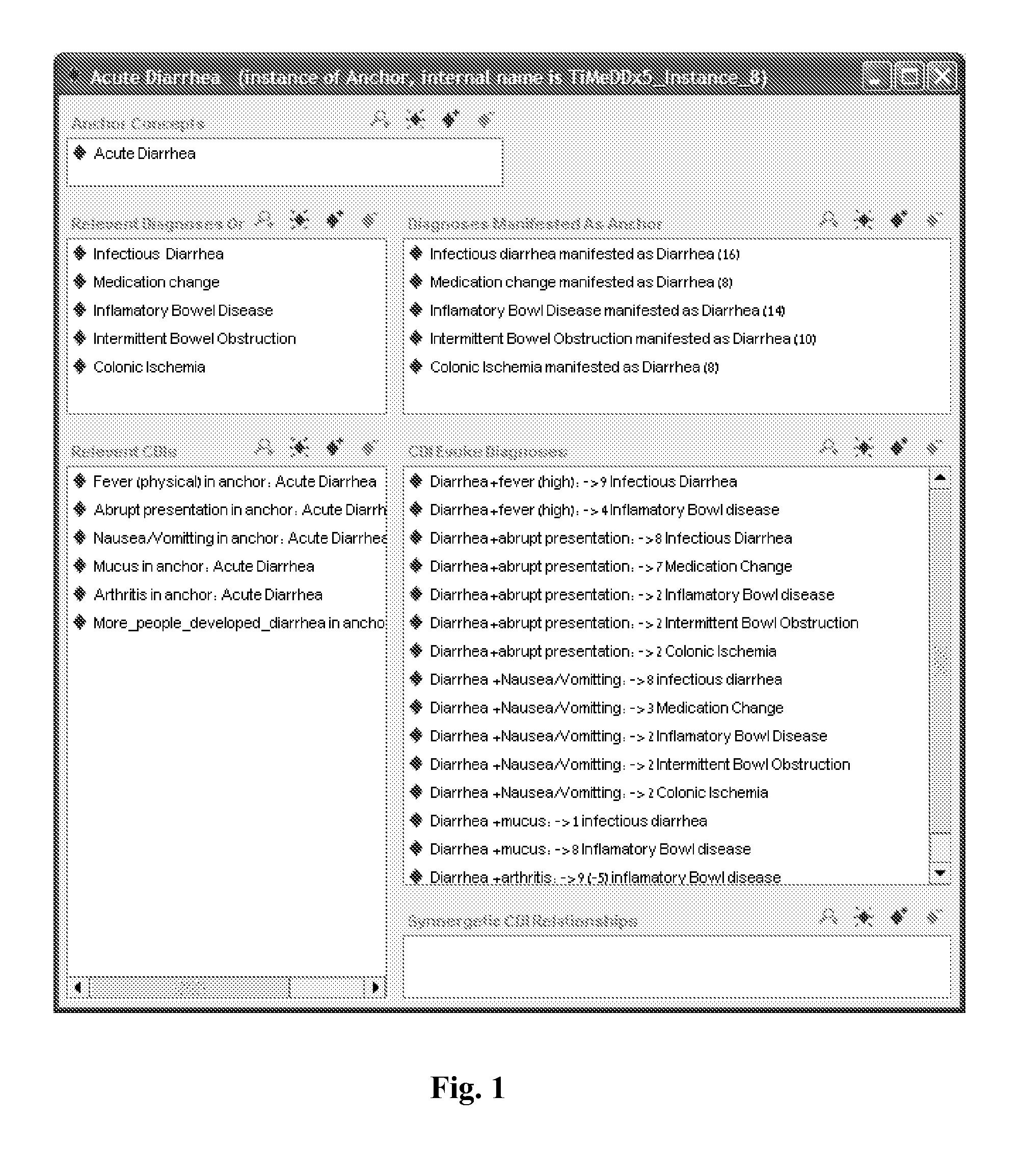
A medical diagnosis decision support system for assisting a health professional to diagnose a medical condition. The system is first provided with an anchor condition that can be a symptom, a sign, a laboratory test result, or an imaging test results or any combination thereof The system then guides users in a series of predetermined phases regarding abstract or concrete diagnosis groups that should be considered and appropriate data that should be collected during the clinical investigation process. The system suggests history and physical examination clinical data items, laboratory, and imaging tests that should be collected in order to differentiate among alternative diagnoses. In each phase, possible diagnoses are listed and ranked.
Owner:MOR RES APPL LTD +1
Enhanced recovery response prediction
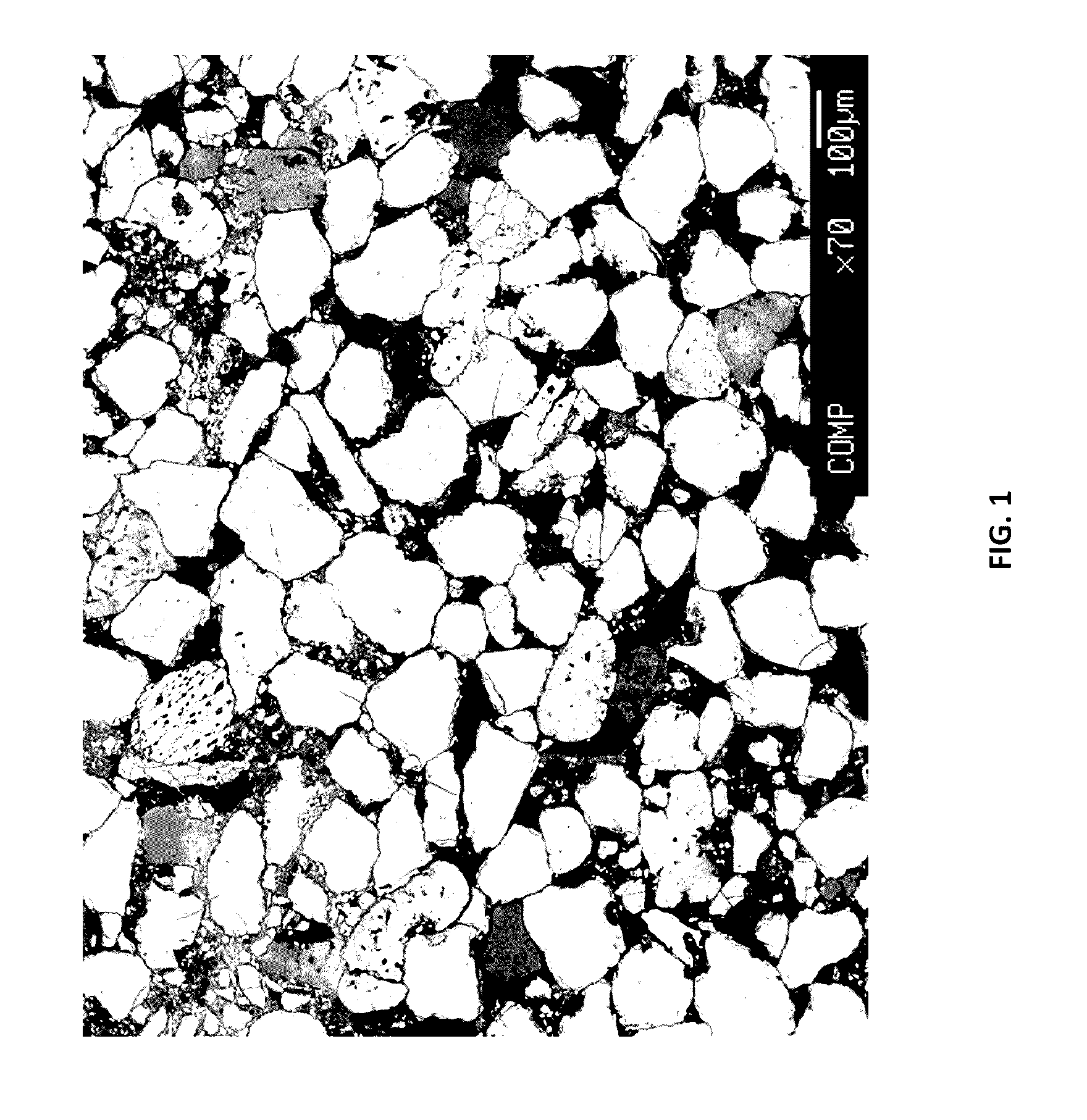
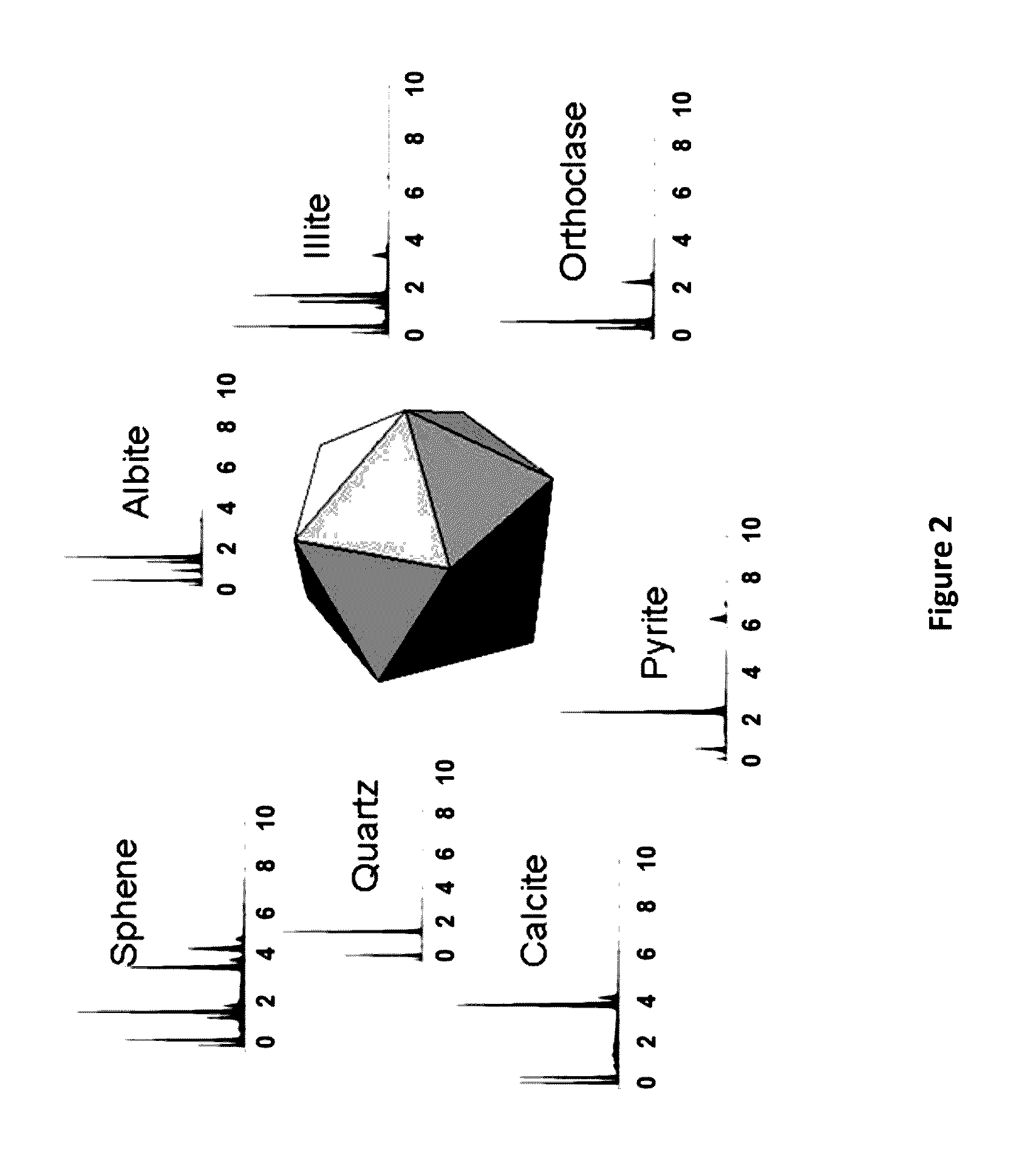
ActiveUS20170017011A1Easy to explainImprove aspectEarth material testingPermeability/surface area analysisLaboratory Test ResultRock sample
Owner:CONOCOPHILLIPS CO
System and method for collecting, organizing and presenting research-oriented medical information
InactiveUS7512541B2Digital data processing detailsComputer-assisted medical data acquisitionLaboratory Test ResultNormal range
A method and computer program manages medical research study information and laboratory test result information. The program generates an interactive user interface with elements for setting up a study (702), managing study member information (704), managing patient information (706), receiving and displaying comments (708), and configuring data to be stored in a database associated with the research study (710). The program also receives medical event information from a user and laboratory test result information from a laboratory information system (20d), and generates a chart (934) that correlates the test results and the medical events. The program also receives a normal range pertaining to a test result from the laboratory information system (20d) and provides a normal range indicator (952) for the test result.
Owner:CHILDRENS MERCY HOSPITAL
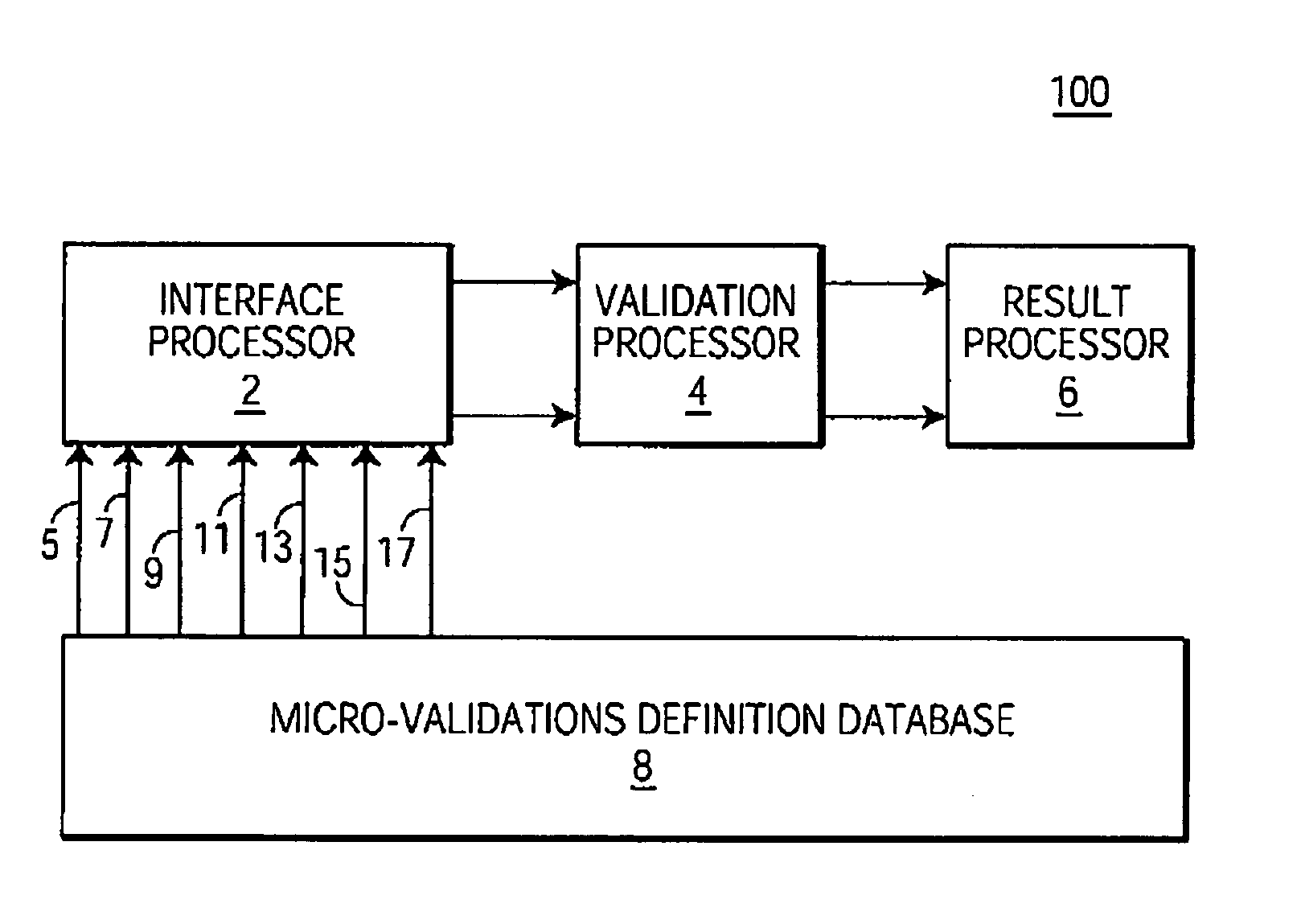
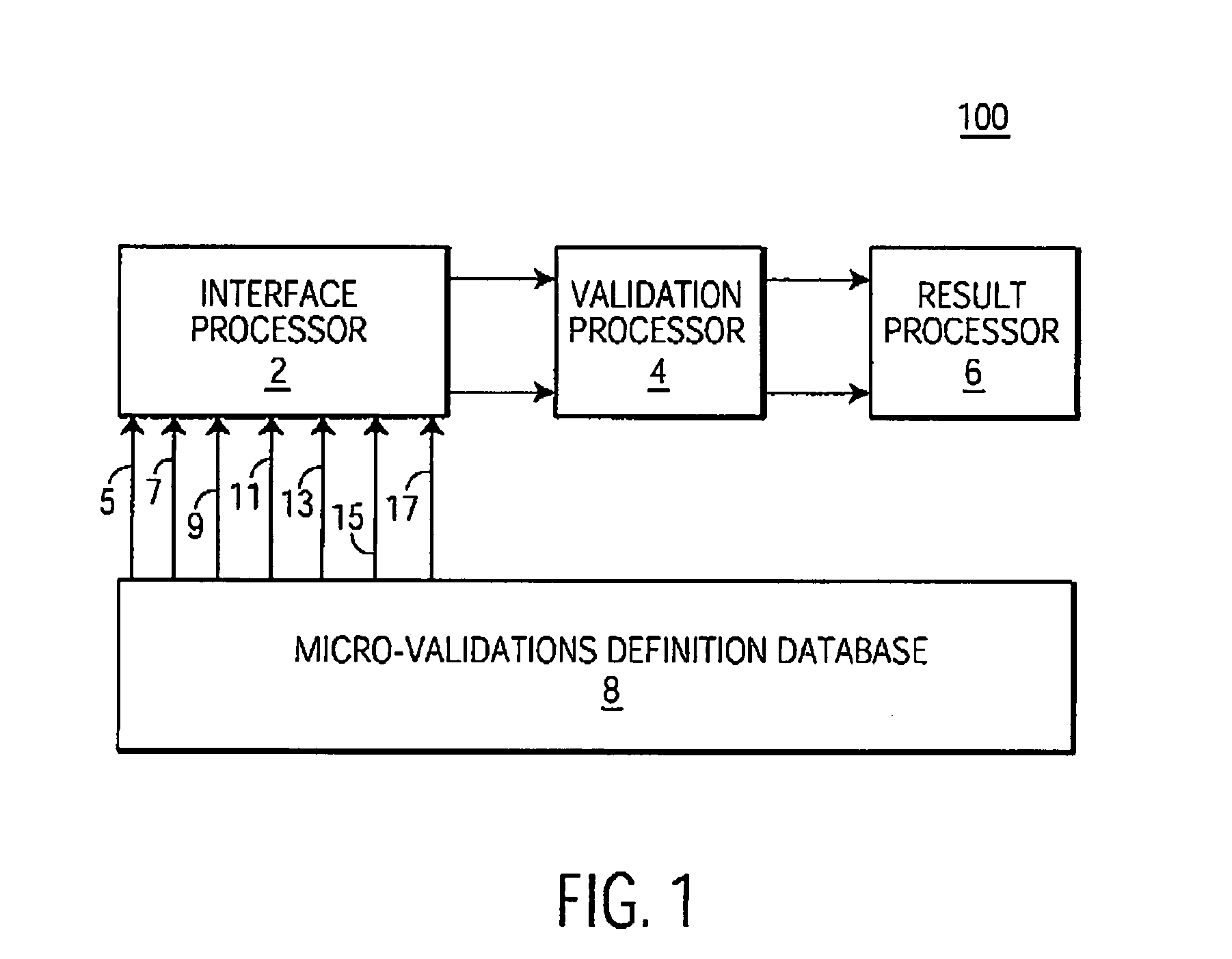
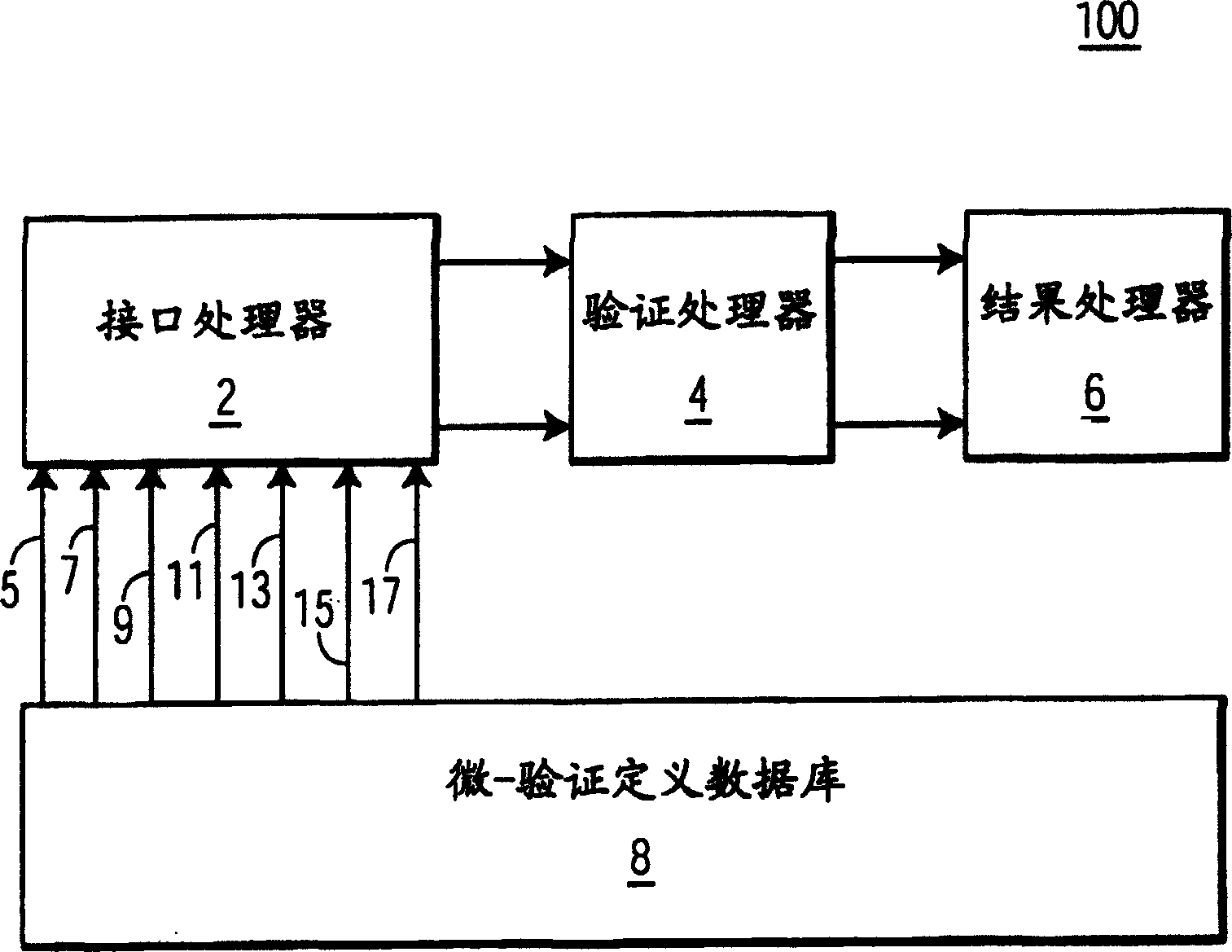
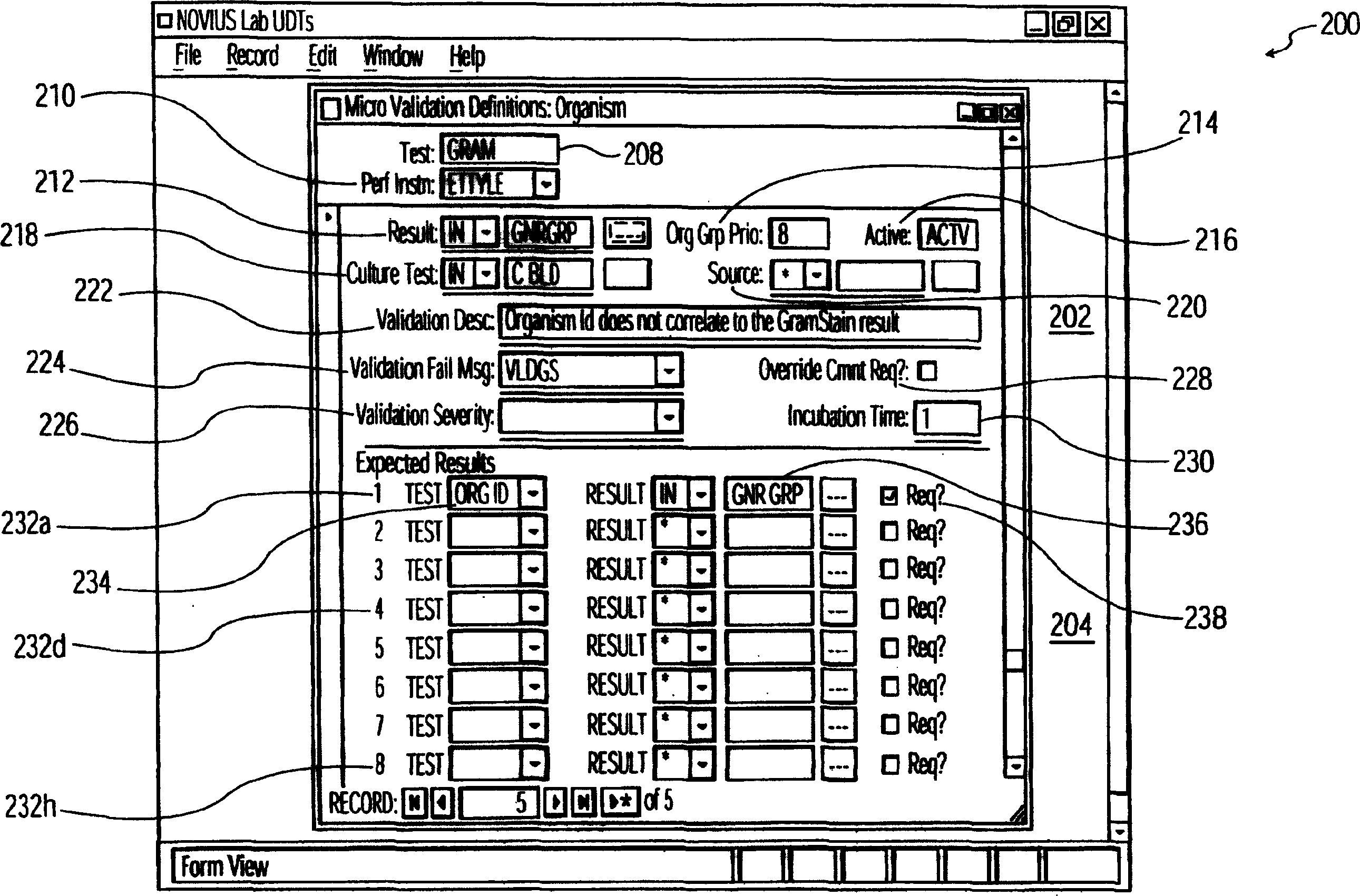
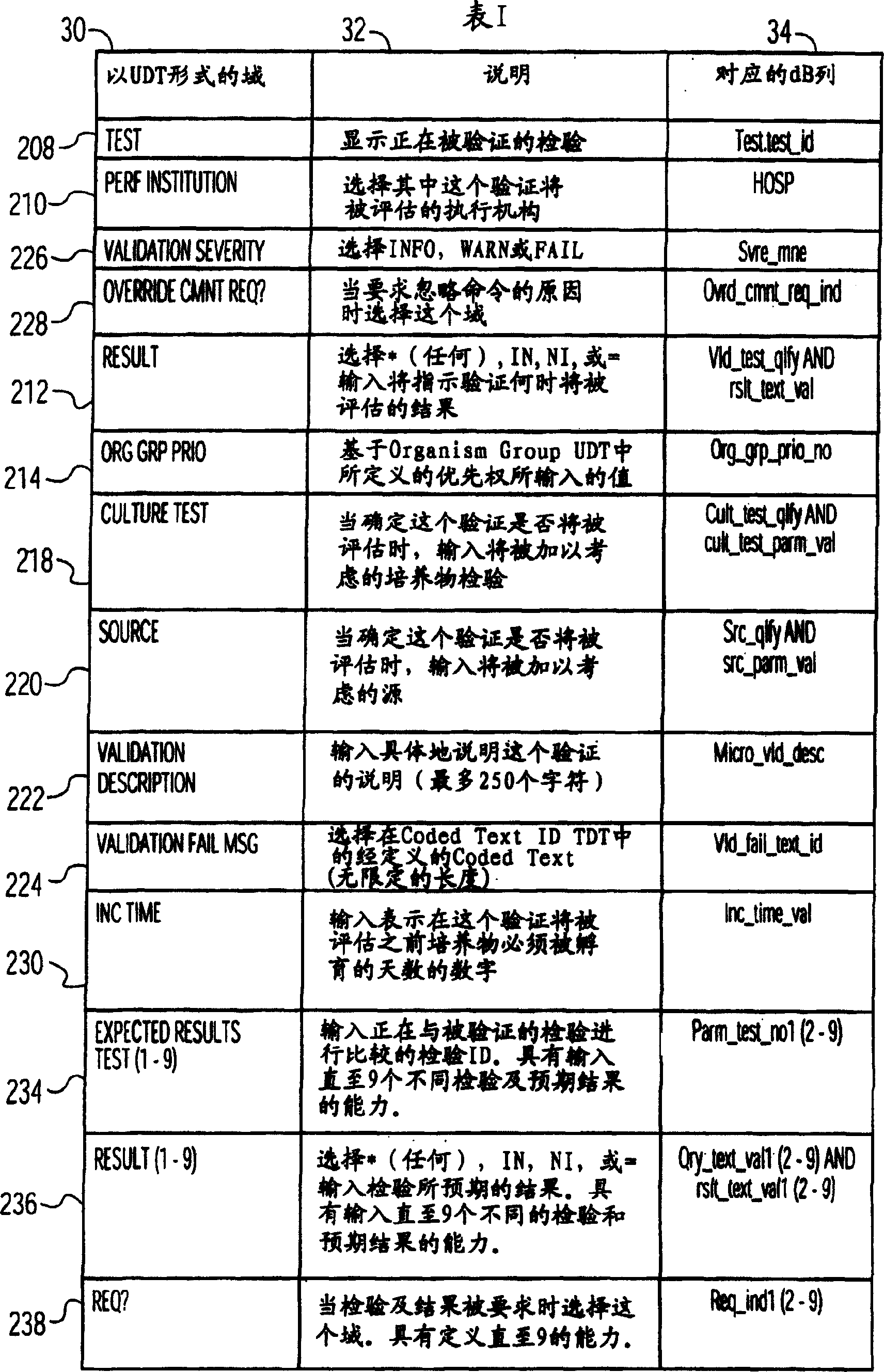
System and method for processing information related to laboratory tests and results
ActiveUS7158890B2Enhanced authenticationSpeed up the processMicrobiological testing/measurementDigital computer detailsLaboratory Test ResultComputer science
A system and method are provided that addresses the need for an improved microbiology validation system that monitors and validates clinical culture laboratory test results as they occur throughout the testing lifecycle of a clinical culture. Techniques are provided that consider the results of earlier performed tests when entering results for later performed tests so that inconsistent or wrong test results of a patient are not inadvertently released to a physician for review.
Owner:CERNER INNOVATION
Infection Control Management and Workflow System
A system identifies multiple medical conditions, observations, and laboratory test results using active sensors and predetermined rules to identify infected patients. An infection control and workflow management system includes a repository of worker information identifying healthcare workers for performing infection control tasks as well as worker associated communication data for use in informing healthcare workers of infection control tasks to be performed. A detection processor automatically detects infection in patients from multiple different sources including from at least one of, (a) a medical record evaluated upon admission of a patient to a hospital and (b) a laboratory test result. A workflow processor uses the worker information for automatically communicating a message to inform a healthcare worker of a task to be performed to initiate infection control tasks using communication data in response to detection of an infected patient.
Owner:CERNER INNOVATION
Medical information validation system
ActiveUS8364499B2Data processing applicationsHospital data managementLaboratory Test ResultWork flow
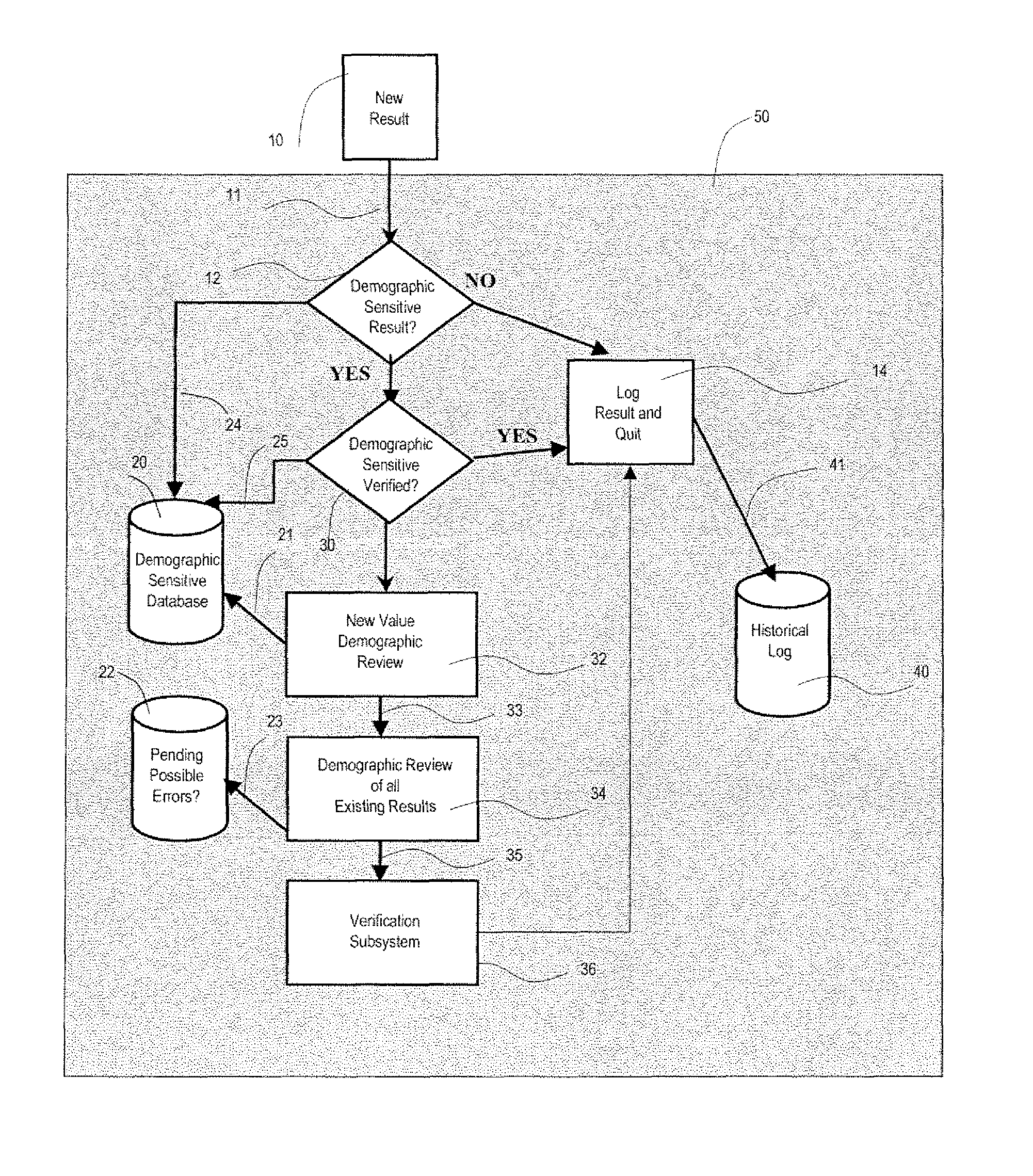
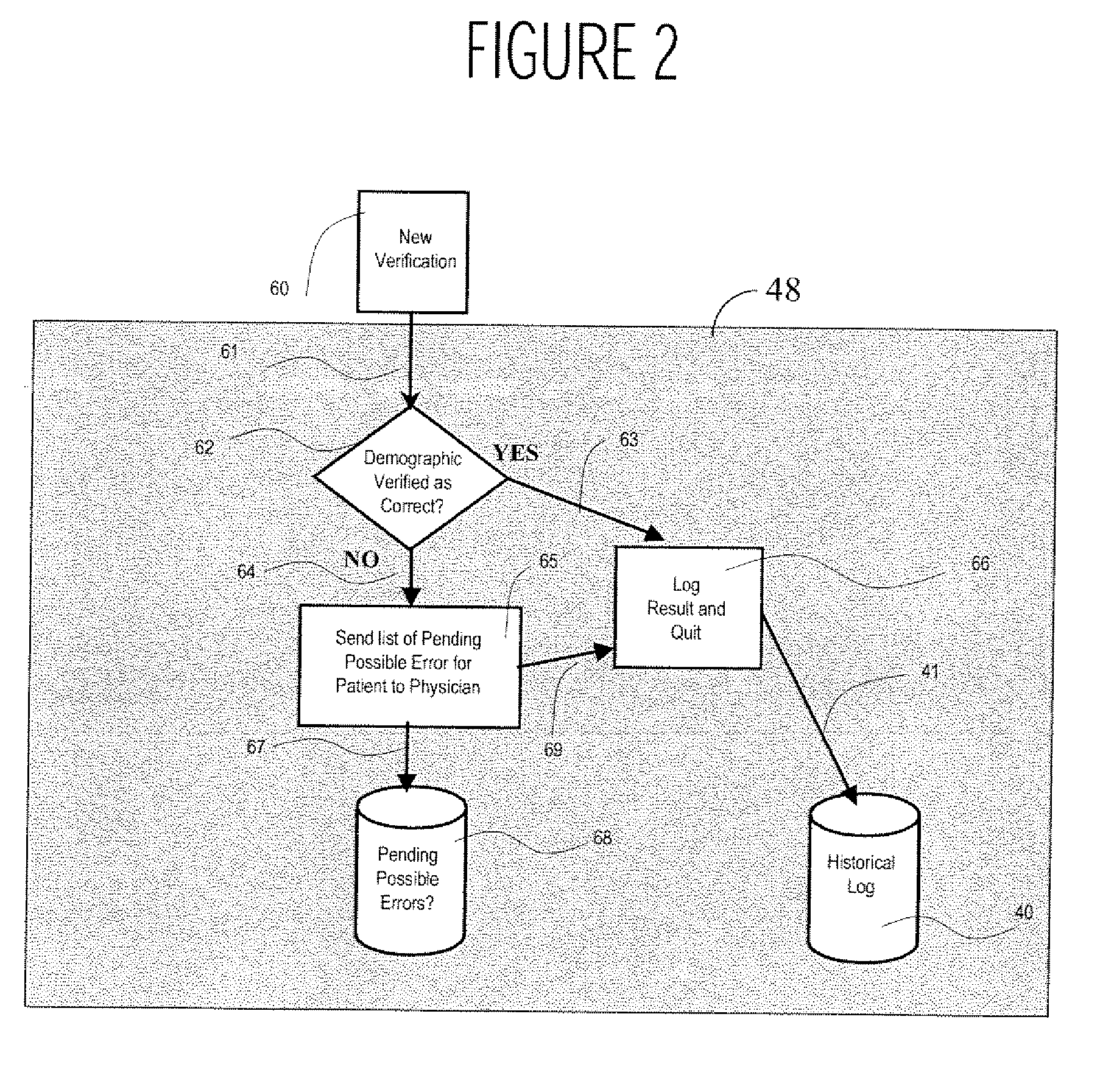
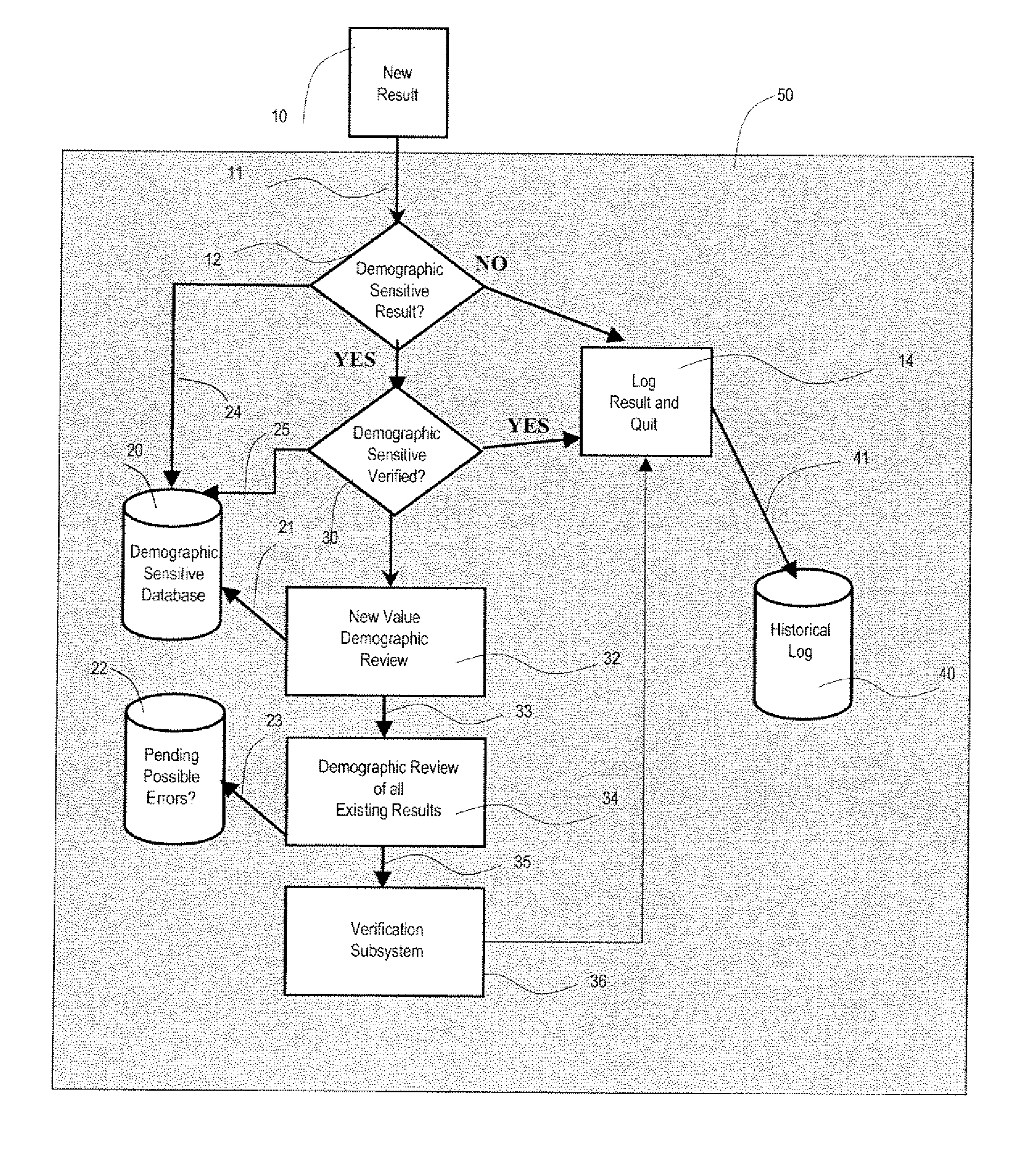
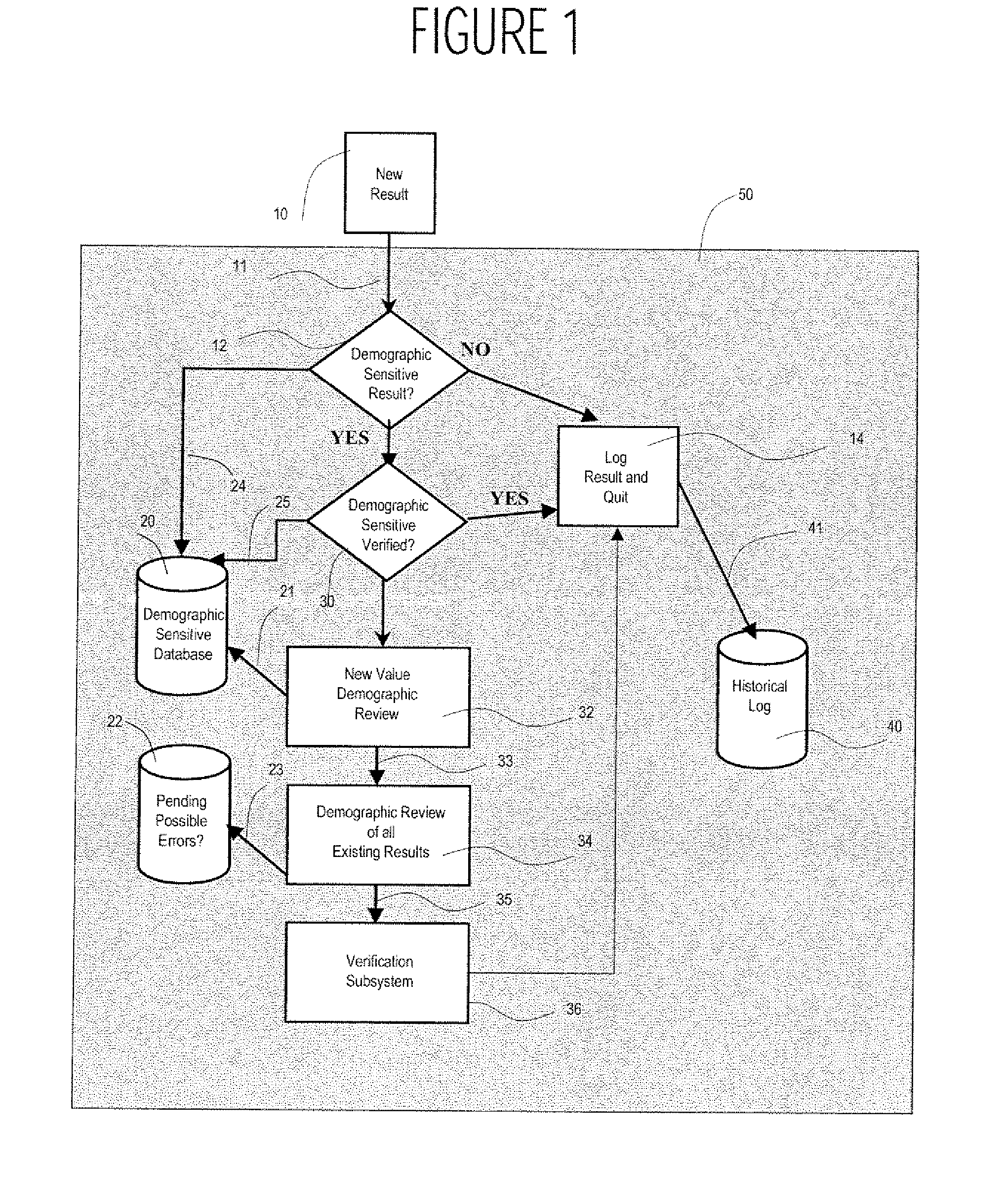
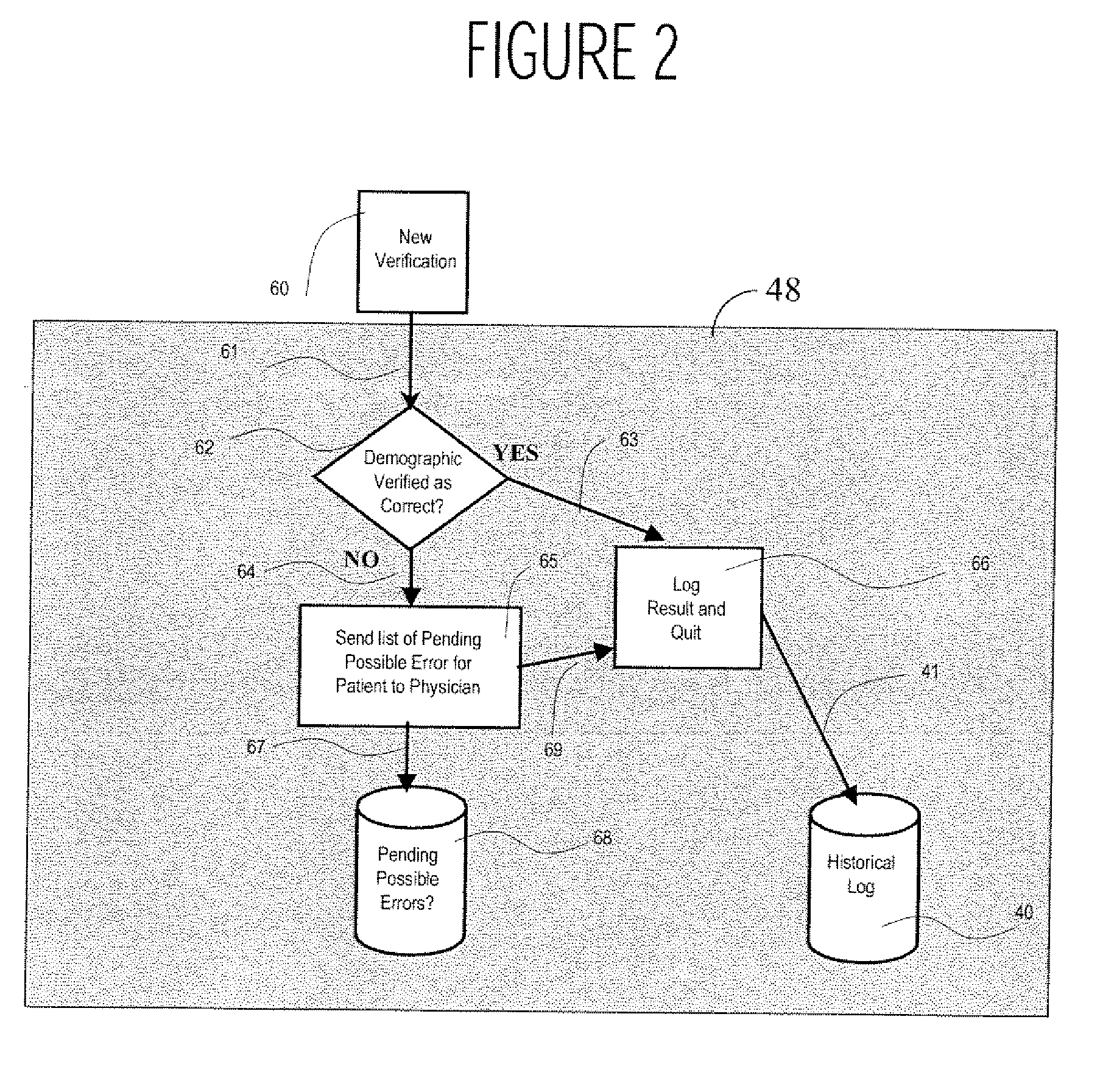
A system uses clinical data to help identify incorrect demographic information (such as gender or age) of a patient that may cause an erroneous critical or abnormal indication in medical tests and uses results of a clinical test to indicate possible demographic errors and various methods to modify a workflow to incorporate alternative tasks or to request verification of specific demographic facts. A system for validating patient medical information includes an input processor for receiving a laboratory test result value of a particular patient. A data processor automatically compares the received laboratory test result value with a predetermined normal value range for a patient having demographic characteristics of the particular patient, and compares the received laboratory test result value with a predetermined normal value range for a patient having different demographic characteristics than the particular patient. A communication processor initiates generation of an indication to a user indicating stored demographic information of the particular patient may be inaccurate.
Owner:CERNER INNOVATION
Medical Information Validation System
ActiveUS20070112858A1Generate inaccurateHospital data managementHealthcare resources and facilitiesLaboratory Test ResultWork flow
A system uses clinical data to help identify incorrect demographic information (such as gender or age) of a patient that may cause an erroneous critical or abnormal indication in medical tests and uses results of a clinical test to indicate possible demographic errors and various methods to modify a workflow to incorporate alternative tasks or to request verification of specific demographic facts. A system for validating patient medical information includes an input processor for receiving a laboratory test result value of a particular patient. A data processor automatically compares the received laboratory test result value with a predetermined normal value range for a patient having demographic characteristics of the particular patient, and compares the received laboratory test result value with a predetermined normal value range for a patient having different demographic characteristics than the particular patient. A communication processor initiates generation of an indication to a user indicating stored demographic information of the particular patient may be inaccurate.
Owner:CERNER INNOVATION
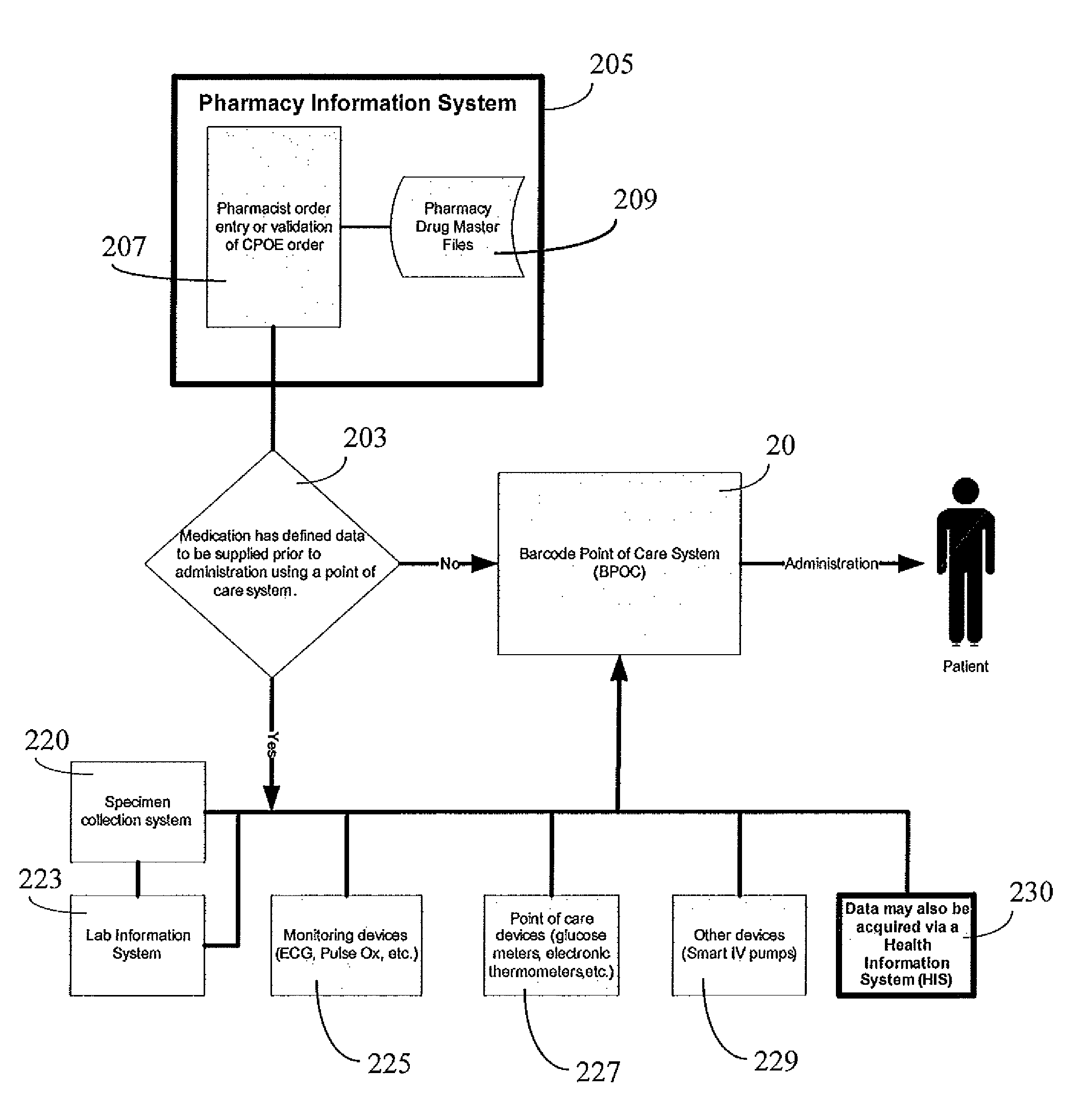
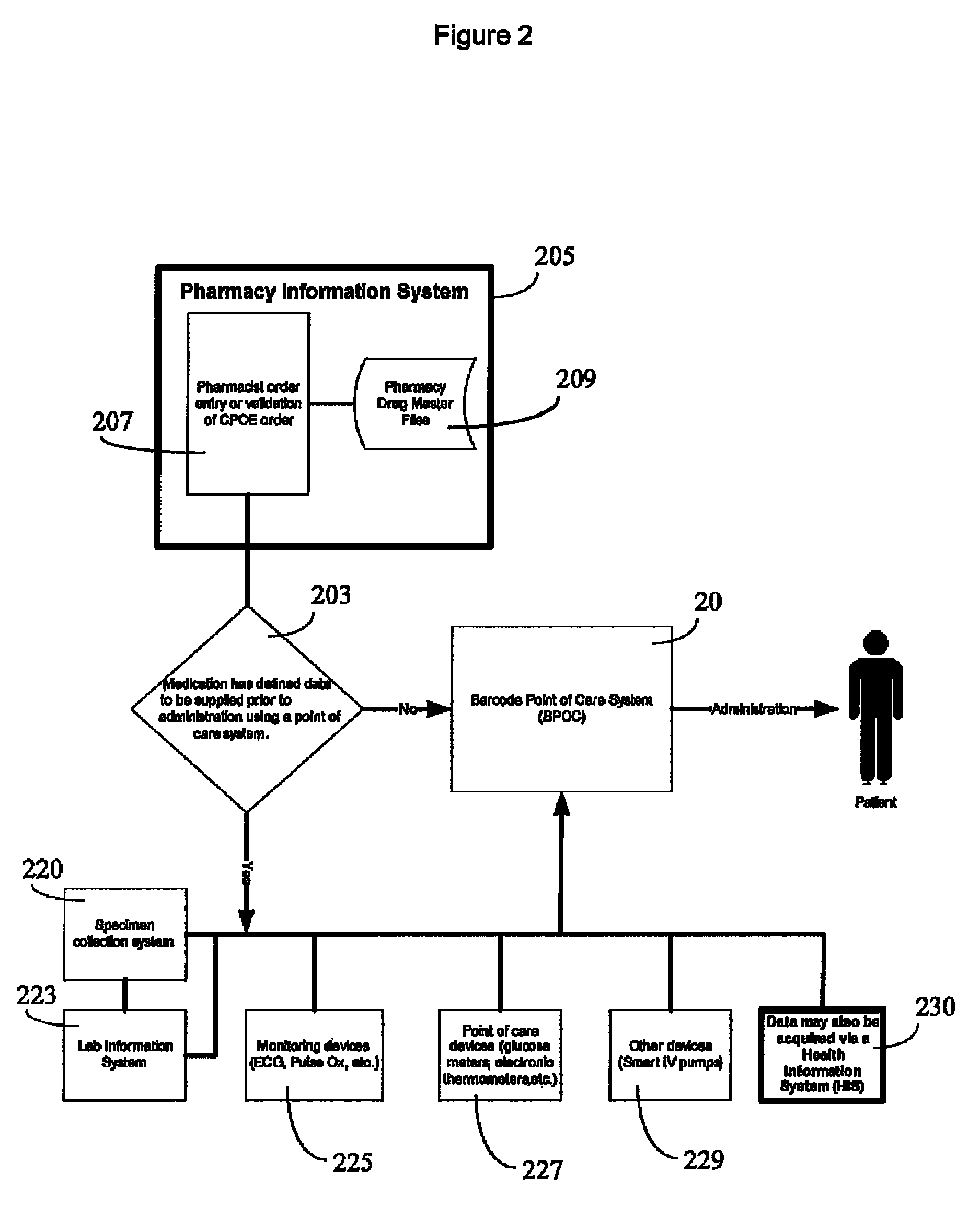
Integrated point of care medication administration information system
ActiveUS8150709B2Data processing applicationsDrug and medicationsLaboratory Test ResultPoint of care
An integrated point of care medication administration system comprises a point of care medication administration system including an interface for communicating with the at least one repository and information sources and including a processor. The processor uses the information and the interface in automatically acquiring for a particular patient, data representing particular patient parameters and laboratory test results associated with a particular individual medication in response to user initiation of an order for the particular individual medication to be administered to the particular patient. A display device presents at least one display image indicating acquired particular patient parameters and laboratory test results of the particular patient and identifying a particular patient parameter or laboratory test result needing to be acquired prior to administration of the particular individual medication to the particular patient.
Owner:CERNER INNOVATION
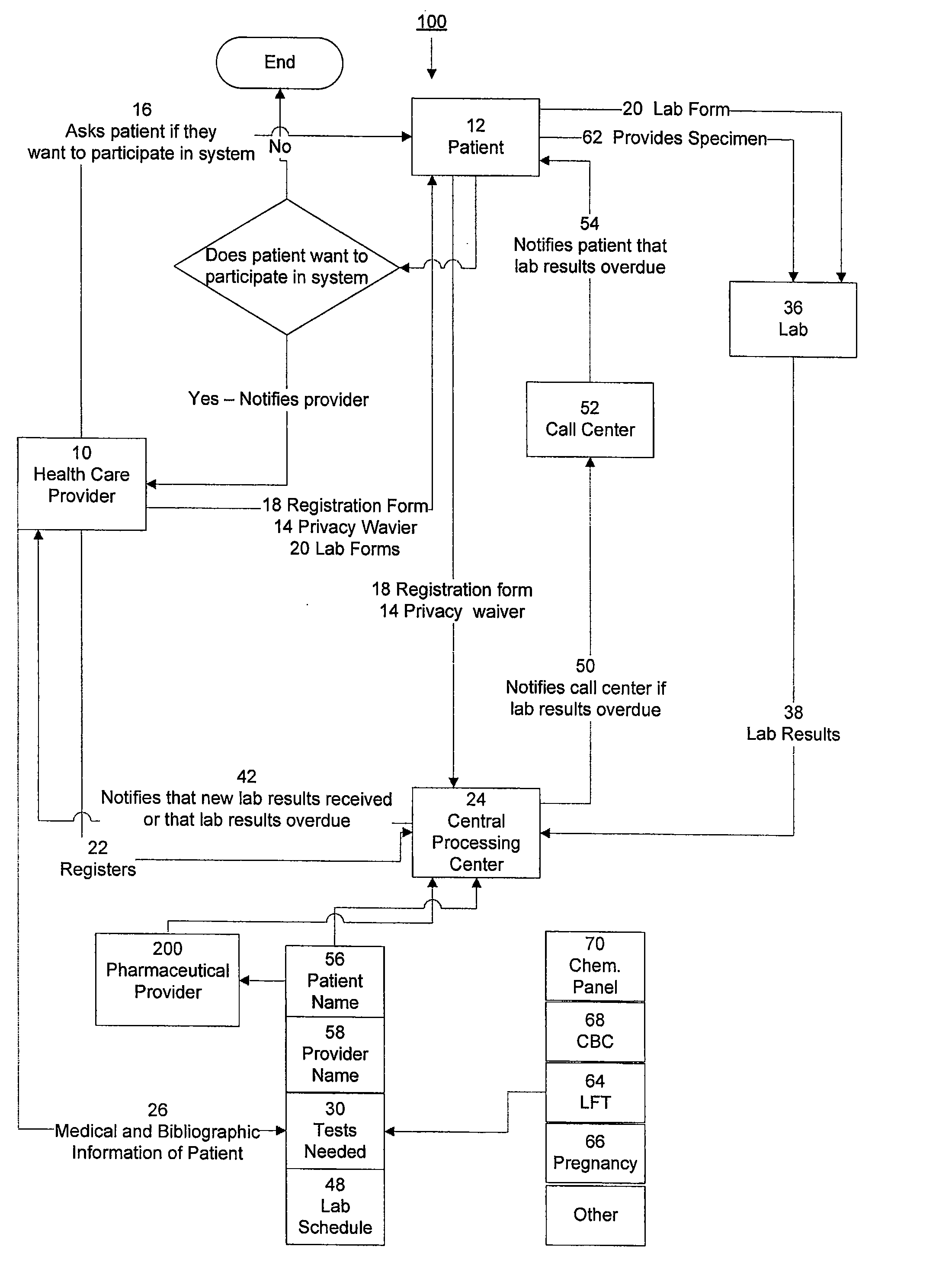
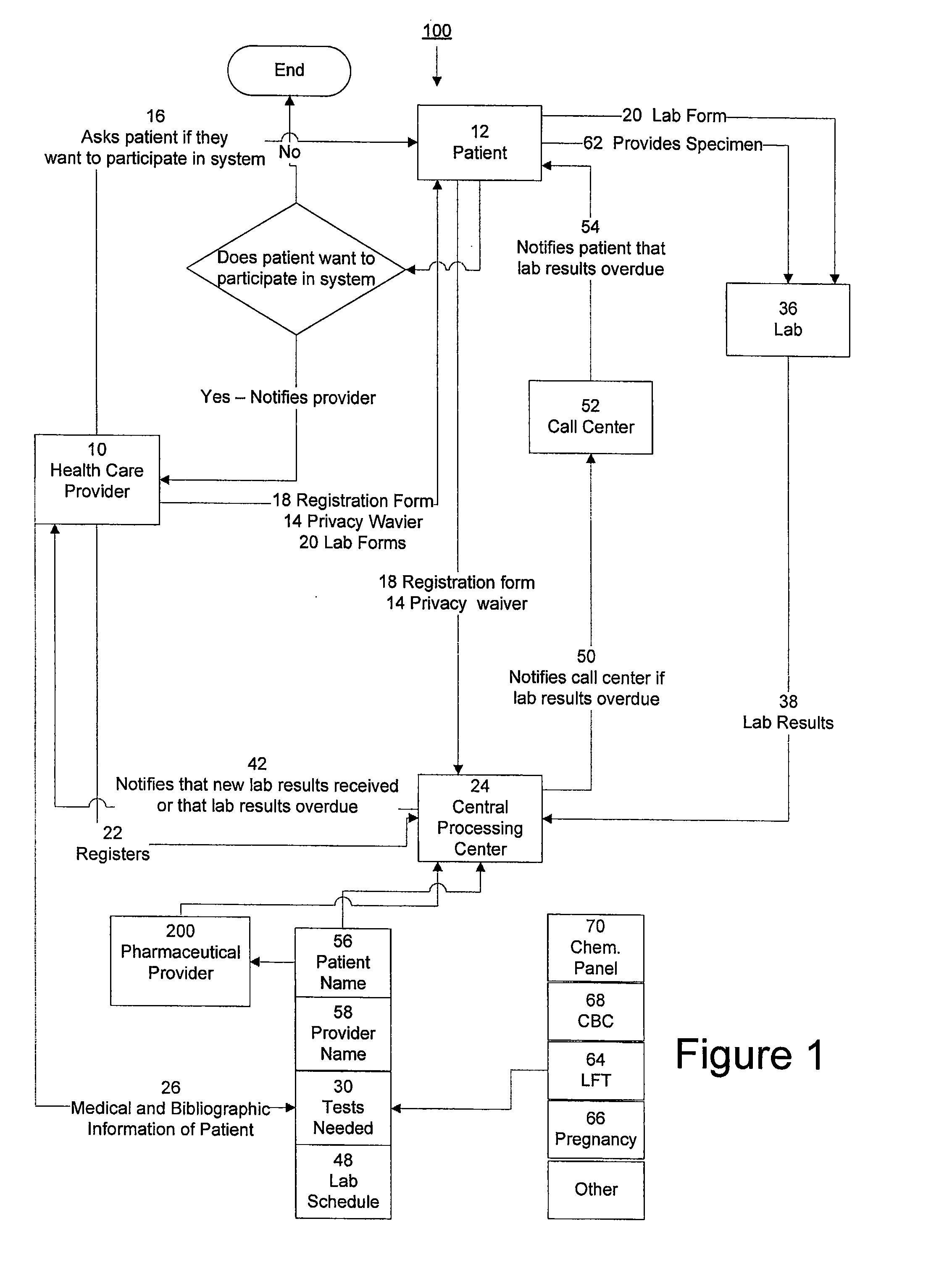
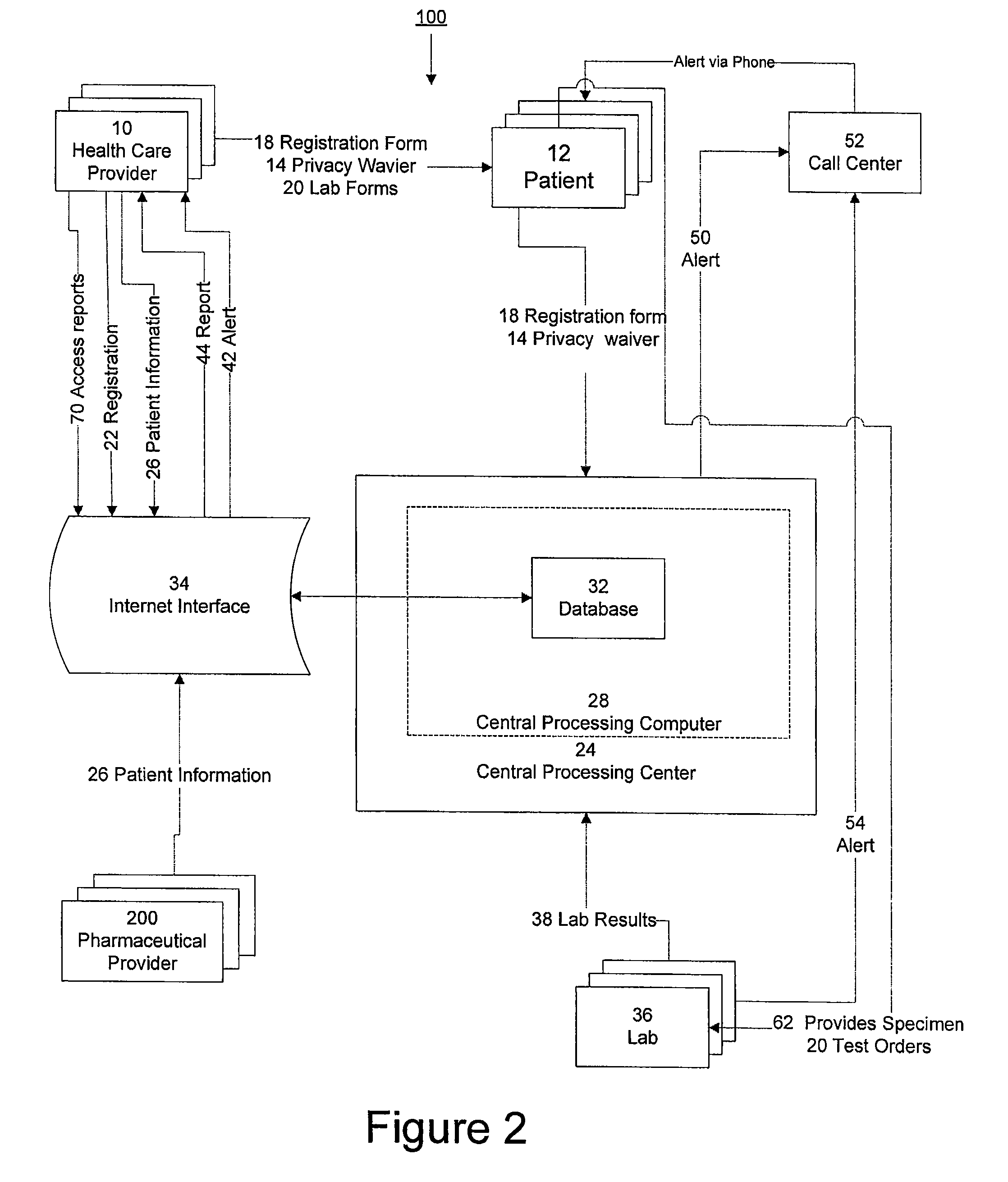
System for managing laboratory test results for patients taking an endothelin receptor antagonist
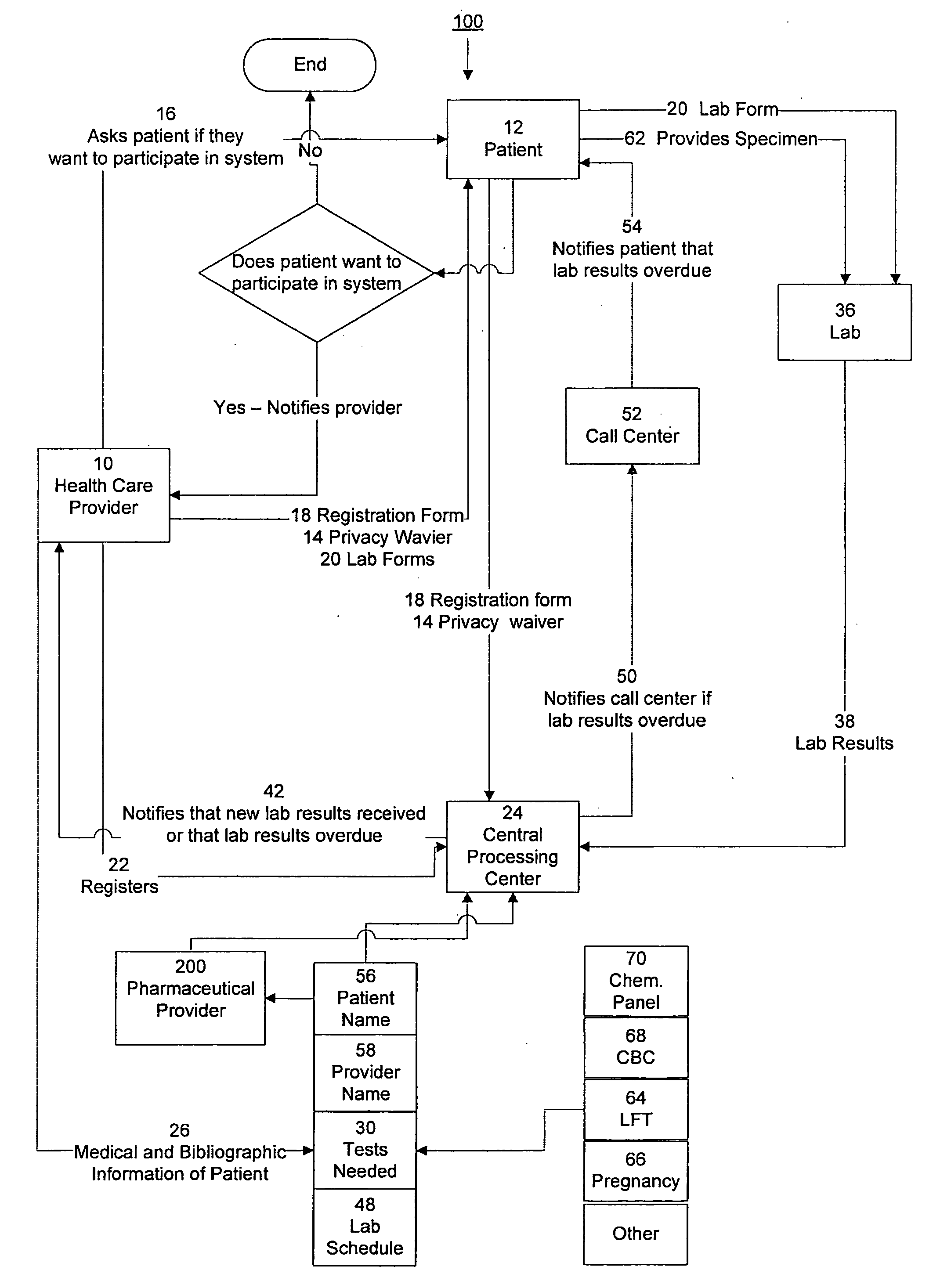
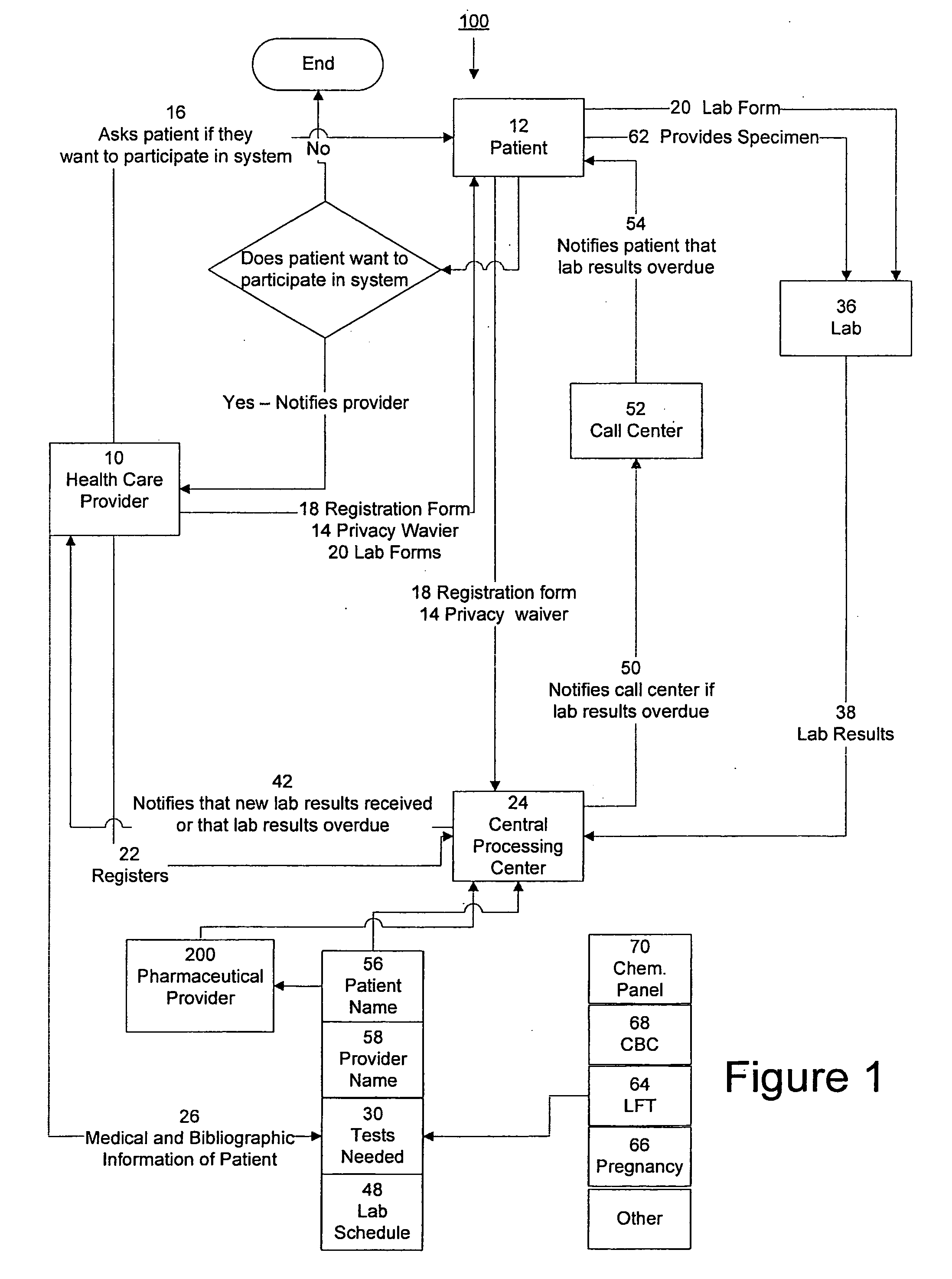
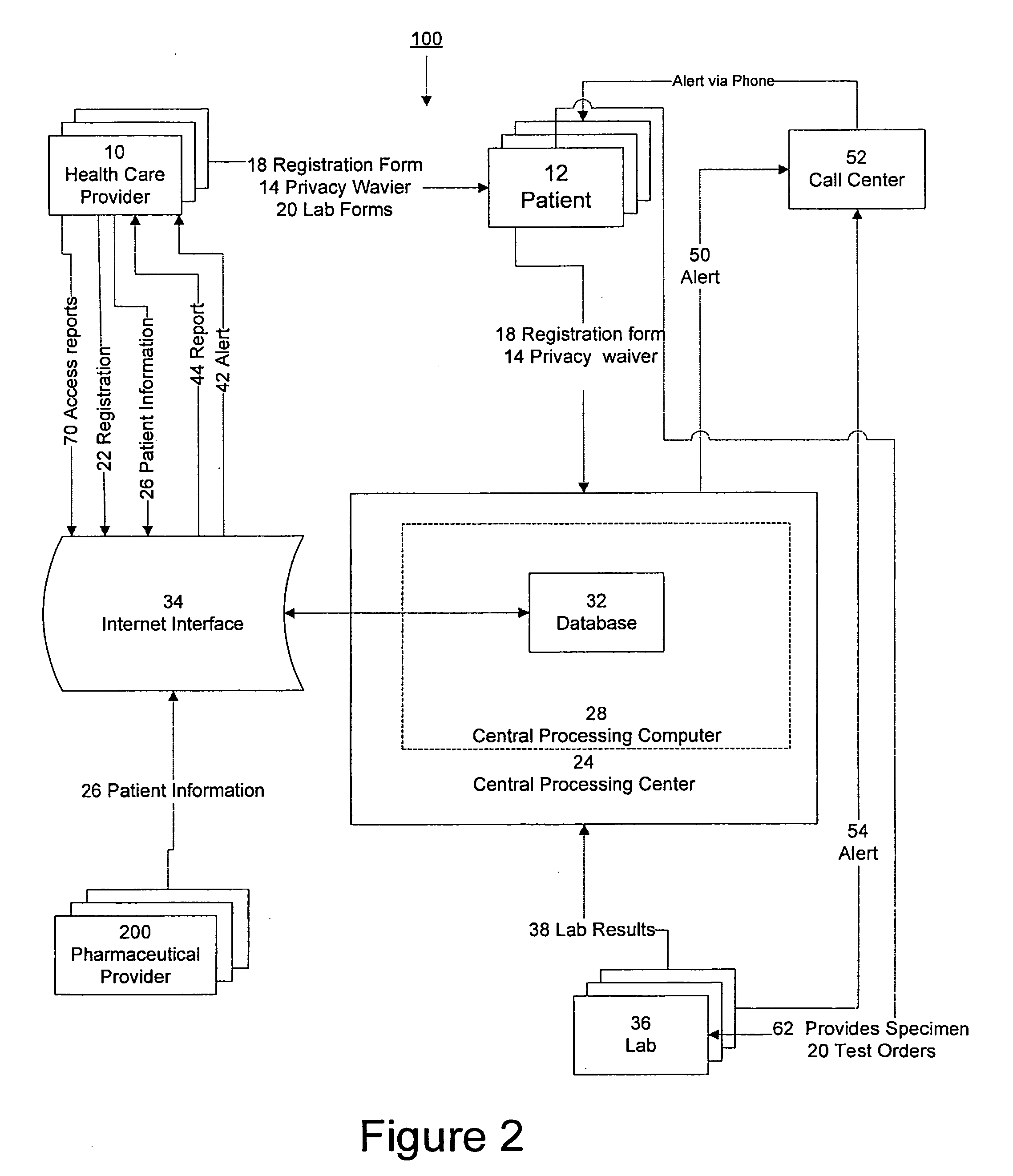
InactiveUS20090171694A1Computer-assisted medical data acquisitionResourcesTime scheduleLaboratory Test Result
The invention provides a system and method of collecting, storing and managing laboratory test results of patients. In one embodiment the invention is directed towards collecting, storing and managing laboratory test results of patients taking an endothelin receptor antagonist (ERA). The system provides a way of securely collecting and tracking information from a plurality of patients, a plurality of laboratories, and a plurality of health care providers by use of a database. The method comprises receiving patient information from health care providers, receiving a laboratory test schedule for each patient, and laboratory tests needed for each patient. This data is entered into a central processing computer. The patients then go to any laboratory to have the monthly testing done due to the ERA prescription. The laboratory test data collected is sent to the central processing computer. The central processing computer can generate an automated report on the laboratory results and supply the results to the health care provider. Additionally, if no laboratory test data for the patient is entered into the central processing computer in accordance with the laboratory test schedule for the patient, the central processing computer will notify a call center which will call the patient, laboratory or health care provider informing them of the overdue laboratory test data.
Owner:ACTELION PHARMA US
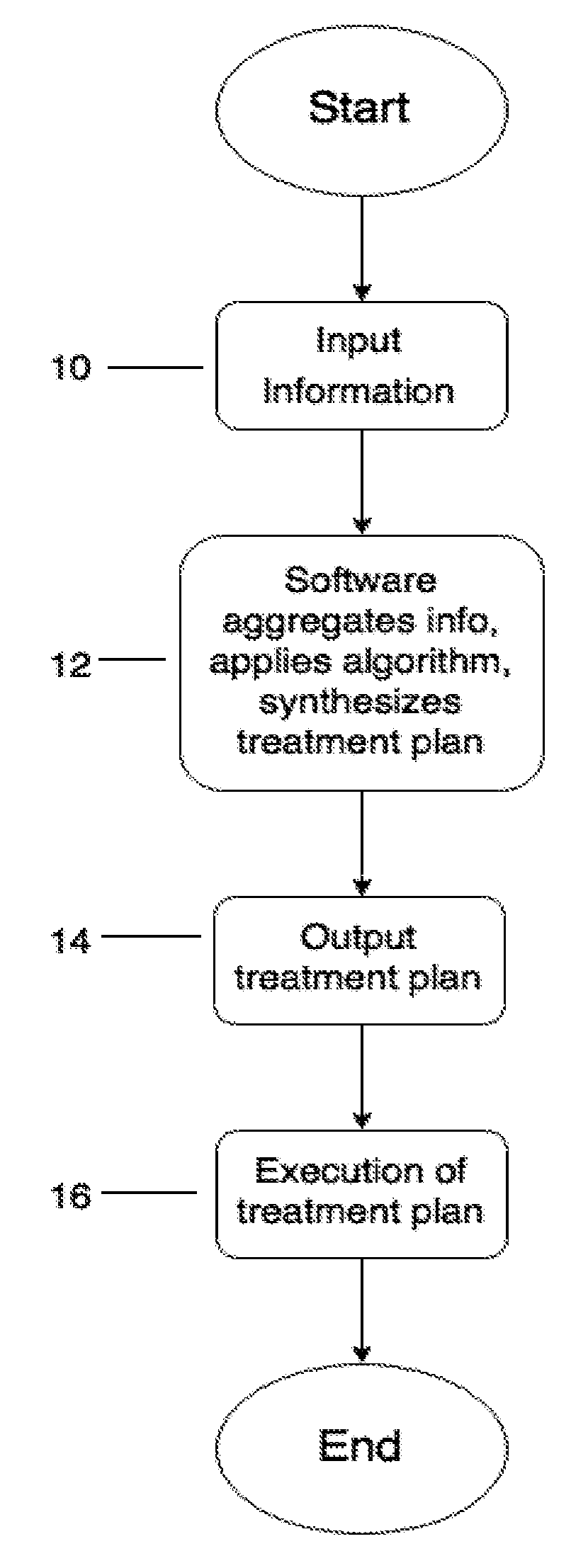
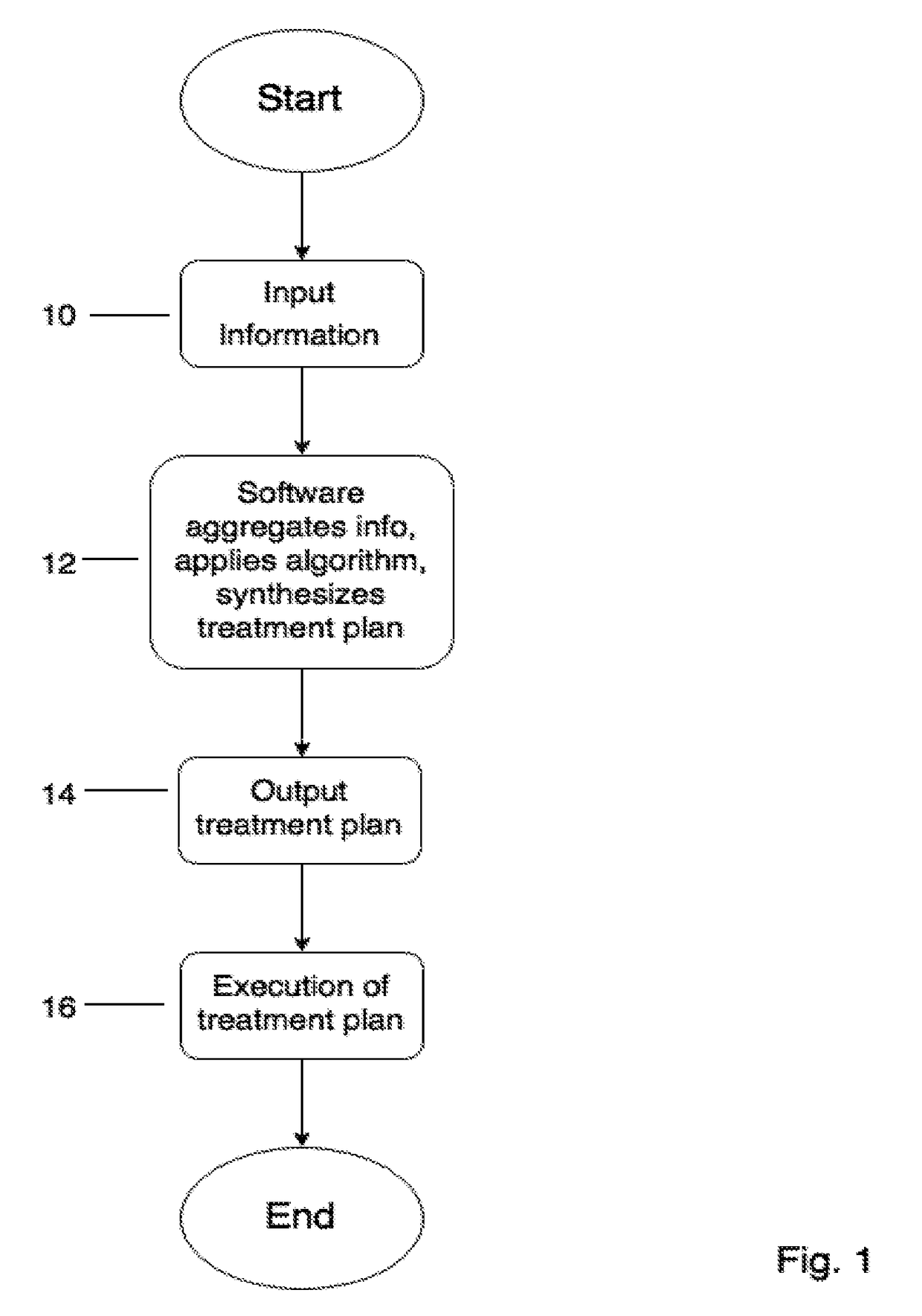
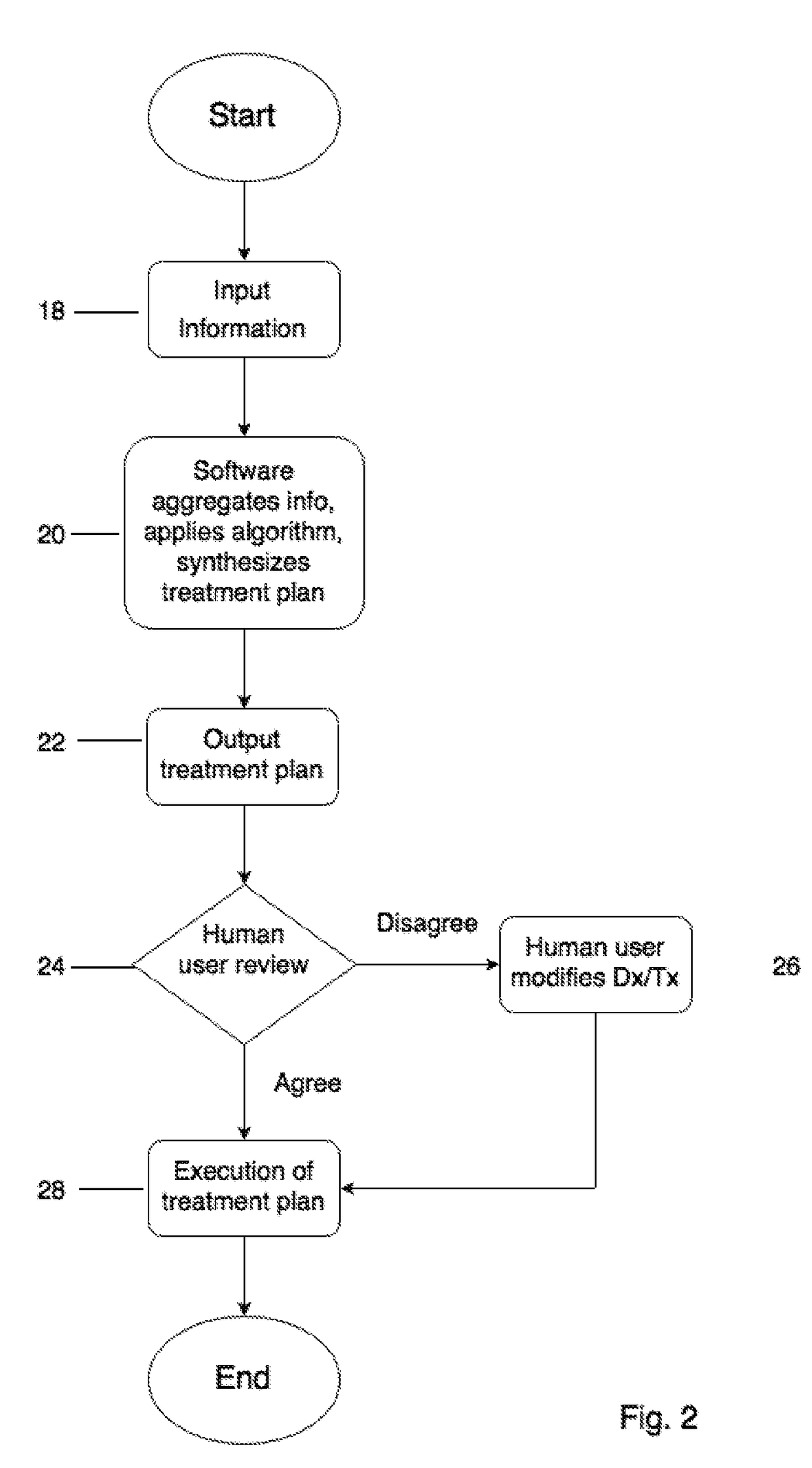
Computerized system, device, method and program product for medical treatment automation
InactiveUS20170109486A1Drug and medicationsComputer-assisted medical data acquisitionAnalysis dataPain management
A computerized system and method that automates diagnosing and prescribing treatment plans for patients with medical conditions, such as: dermatological (e.g. acne, dark spots, anti-aging), high blood pressure, diabetes, pre and post-surgical care, pain management, simple infections, and other medical conditions with standardized treatment protocols. The user inputs data comprising symptoms on their mobile device or a kiosk, e.g. a digital photograph of the user's body and / or face made with the computer's camera. The data is analyzed locally or on a remoter server, and an email is returned comprising the diagnosis and treatment plan. If a doctor's approval is required, the server will electronically communicate with a doctor's office computer, and receive the approval, to include authorization for a medical-drug prescription. User medical data input further comprises medical diagnostic equipment and / or laboratory test results that are transmitted to the user's mobile device, kiosk, or directly to the remote server.
Owner:TRAN HIEN THANH
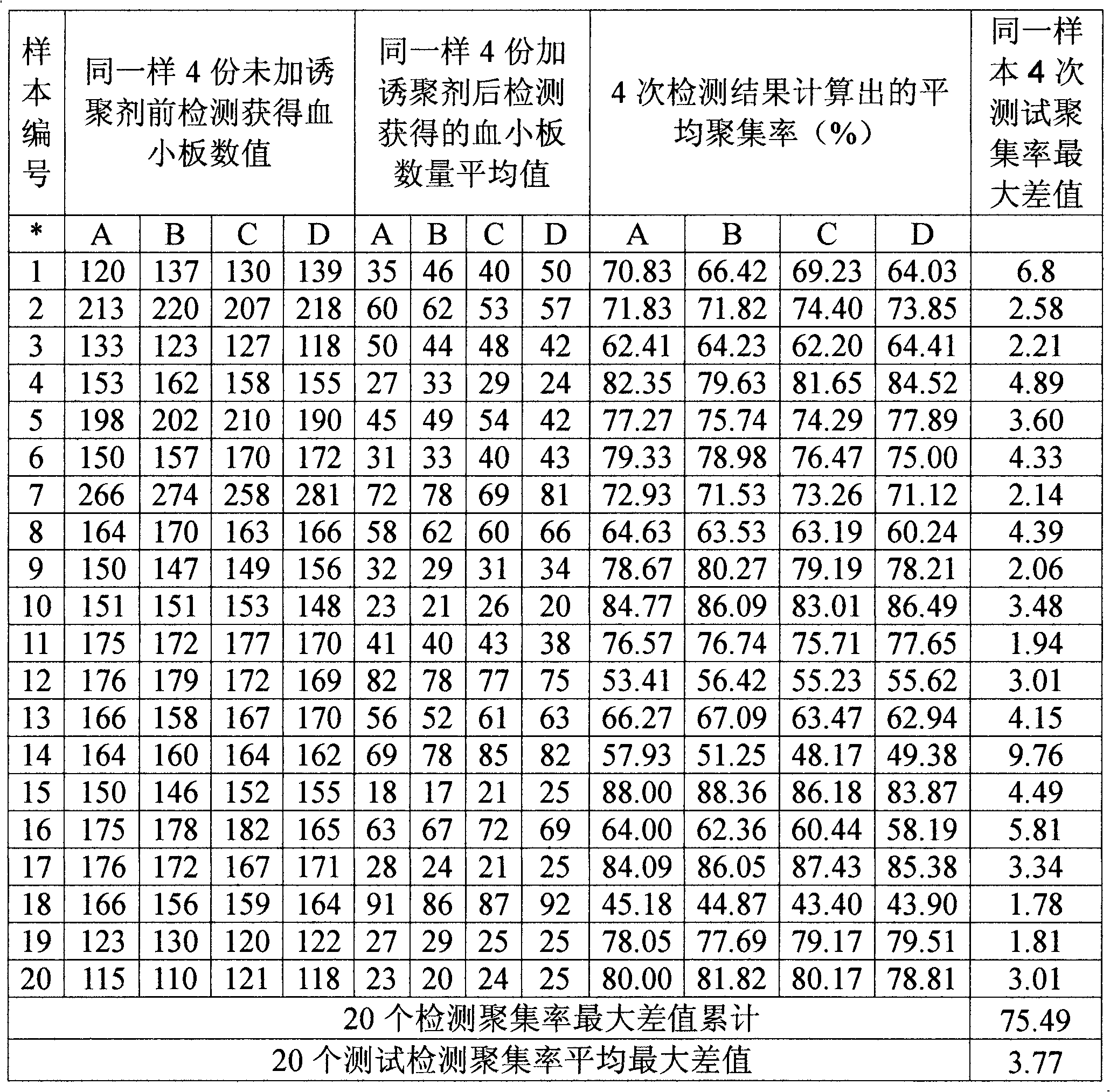
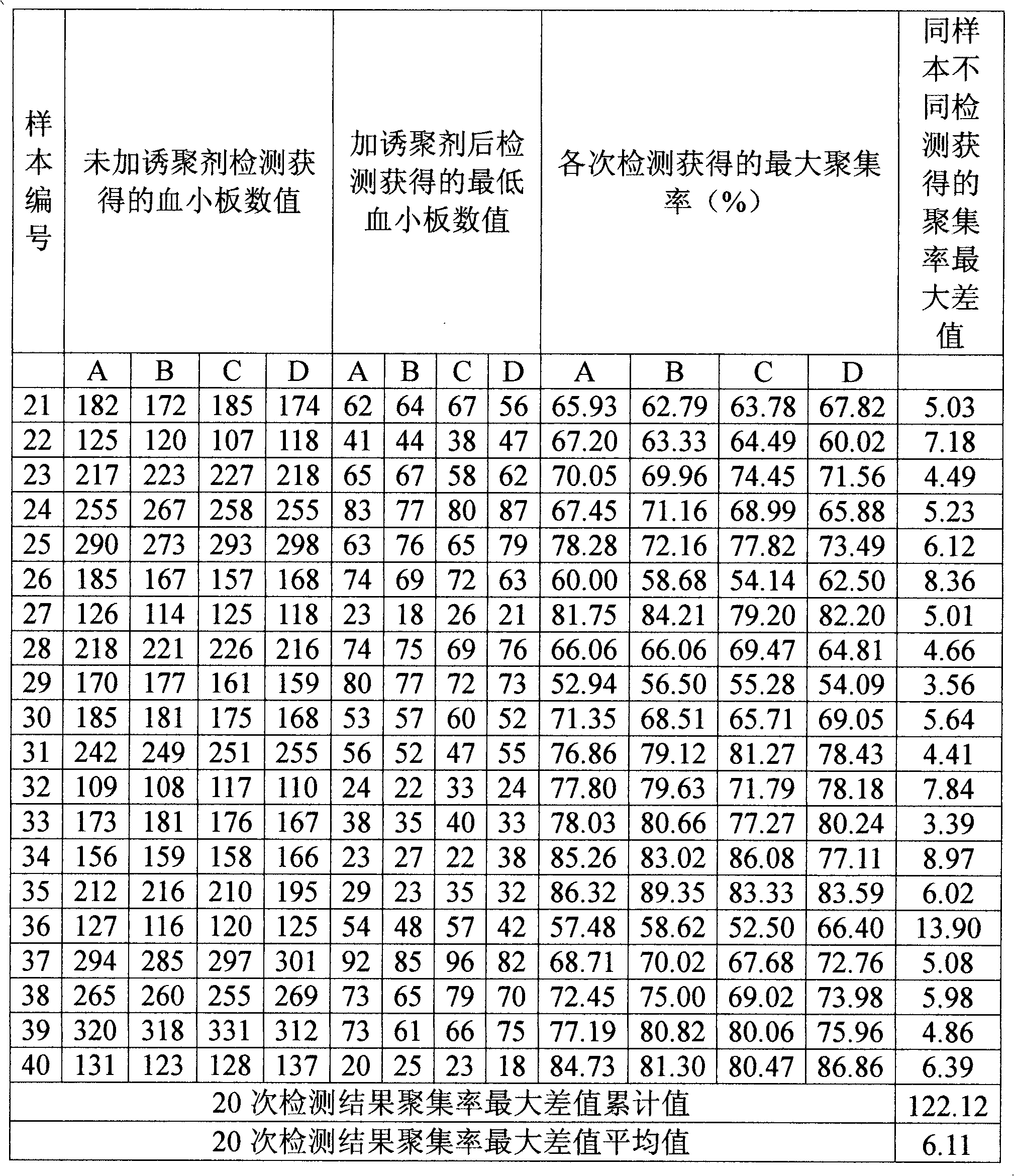
Optimized automation-adaptable platelet aggregation function inspection and analysis method
The invention provides an optimized automation-adaptable platelet aggregation function inspection and analysis method, and belongs to the field of clinical laboratory medicine. The method is an improvement and optimization of a platelet aggregation function inspection and analysis method using an automation counting method. The method is suitable for platelet aggregation function automatic analysis instruments using the counting method. According to the method, the number of platelets is inspected and recorded and an aggregation rate is calculated. A laboratory test result shows that the method is better in repeatability of the obtained result of platelet aggregation rate compared with a previous largest aggregation rate method.
Owner:SINNOWA MEDICAL SCI & TECH
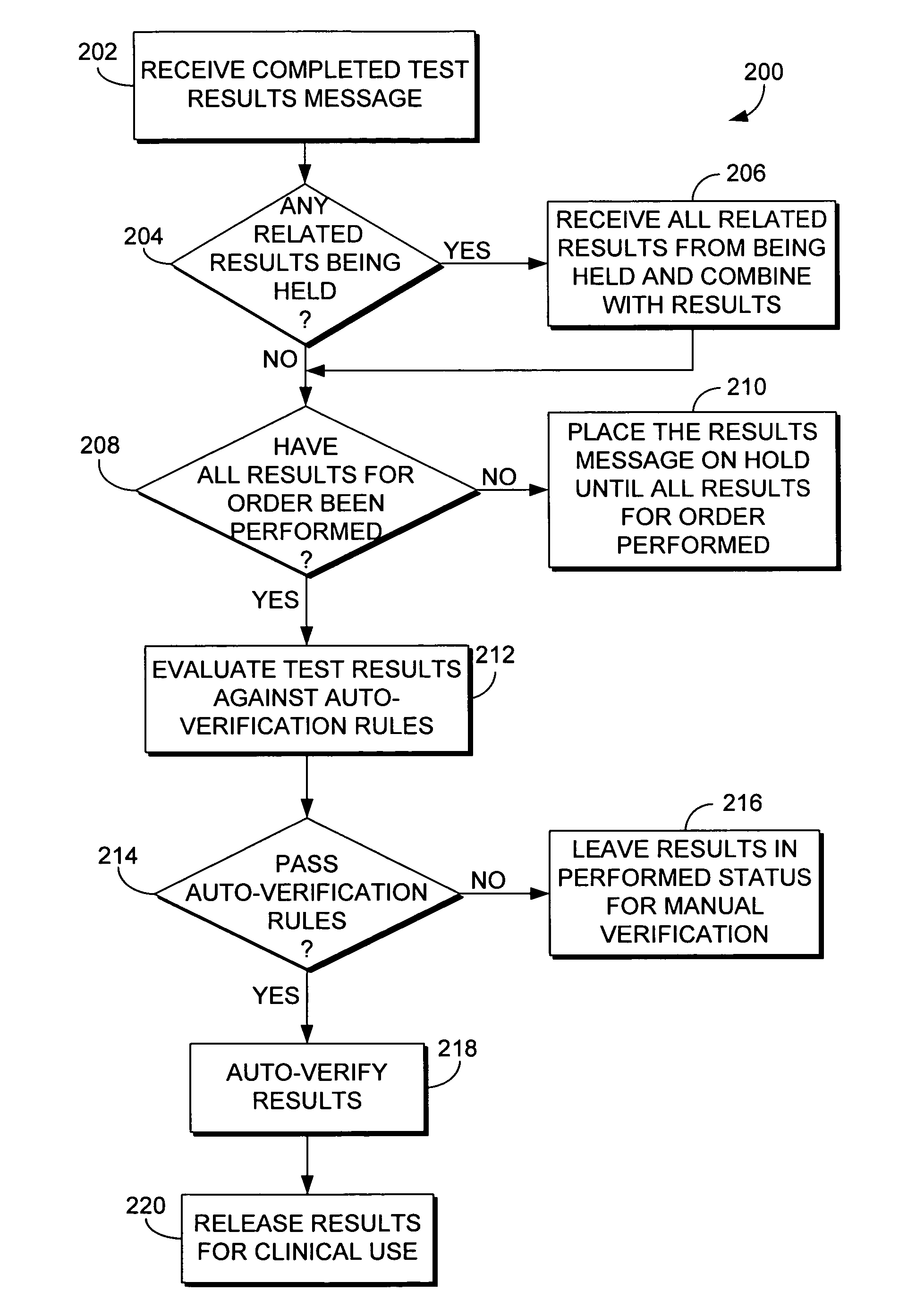
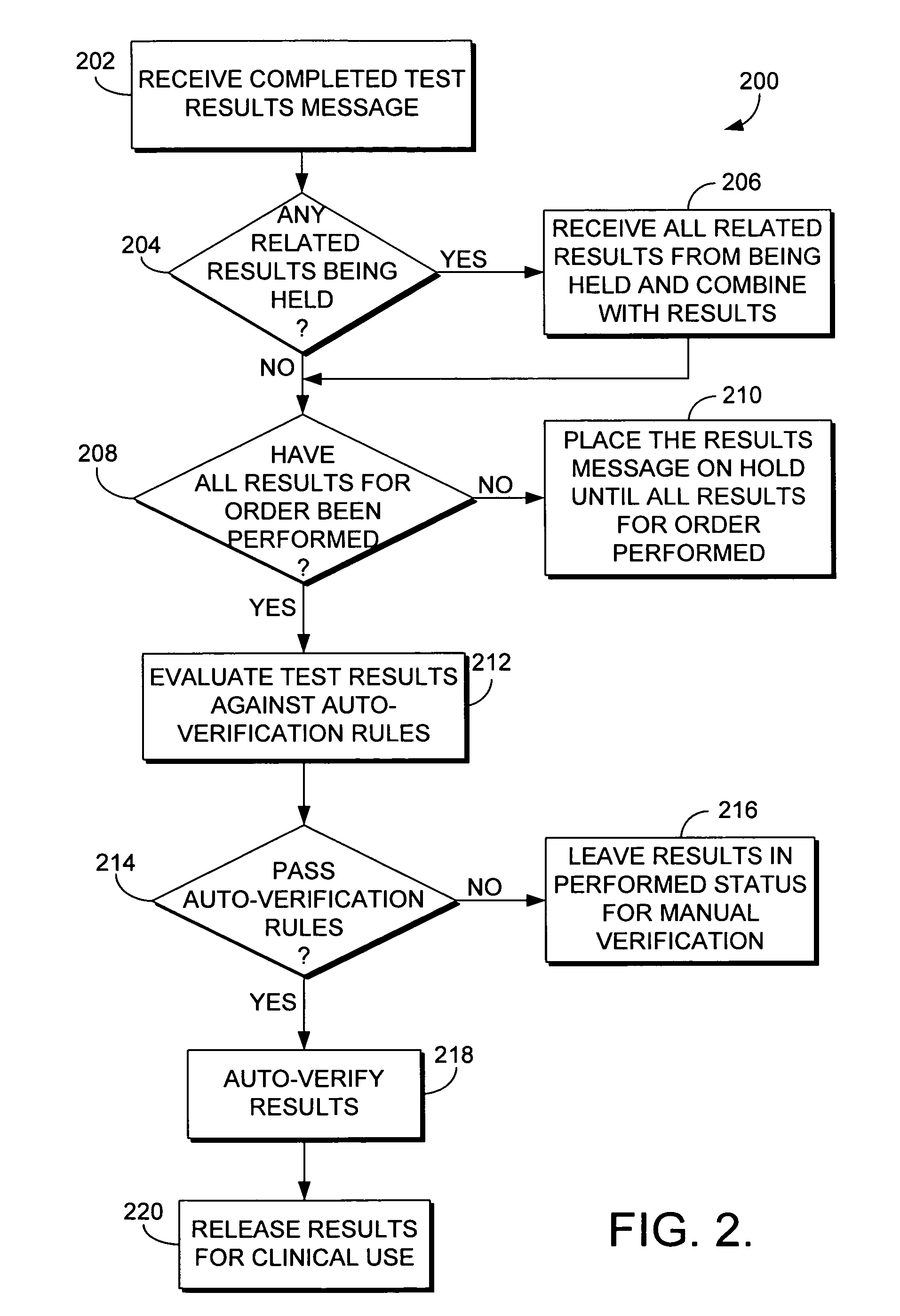
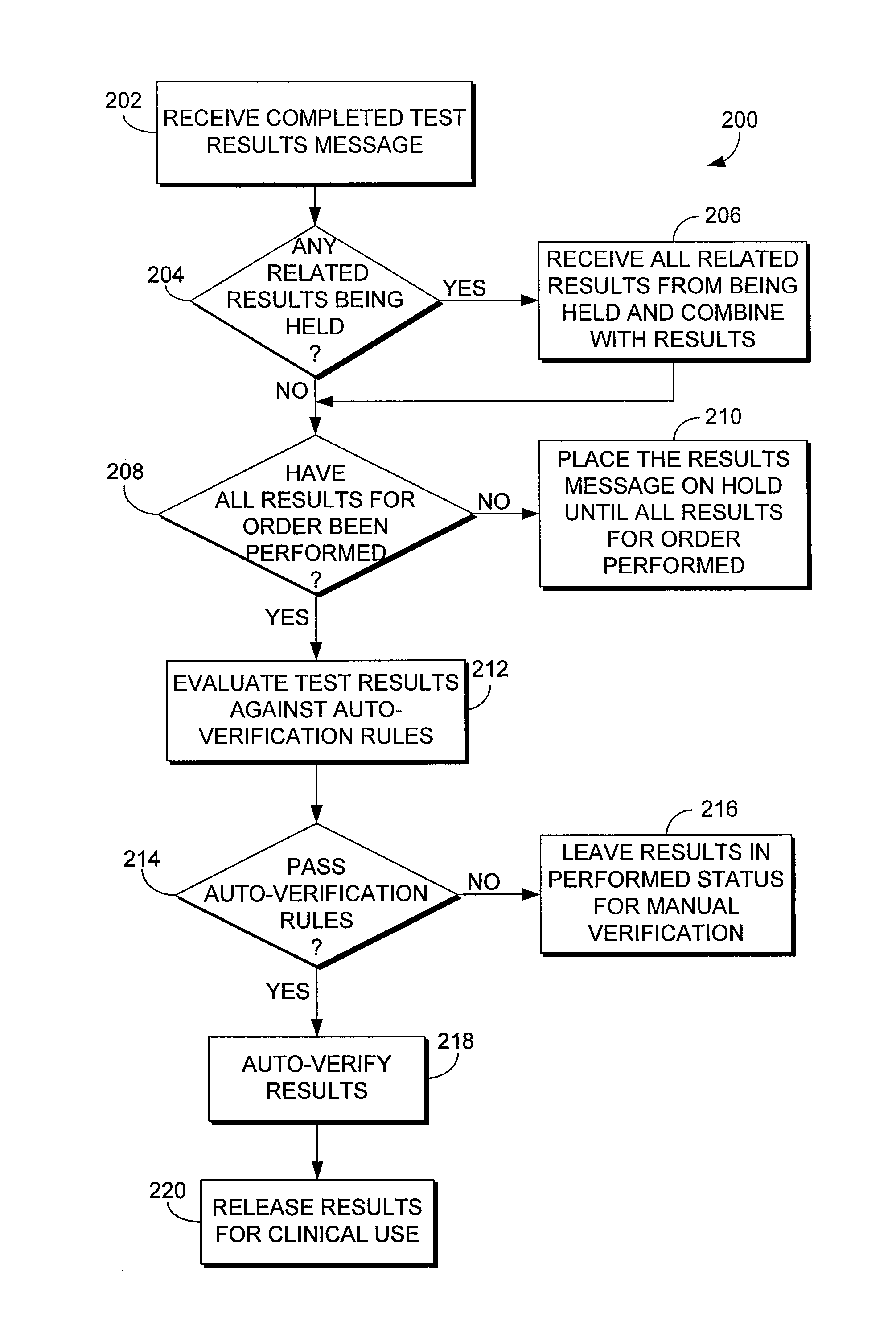
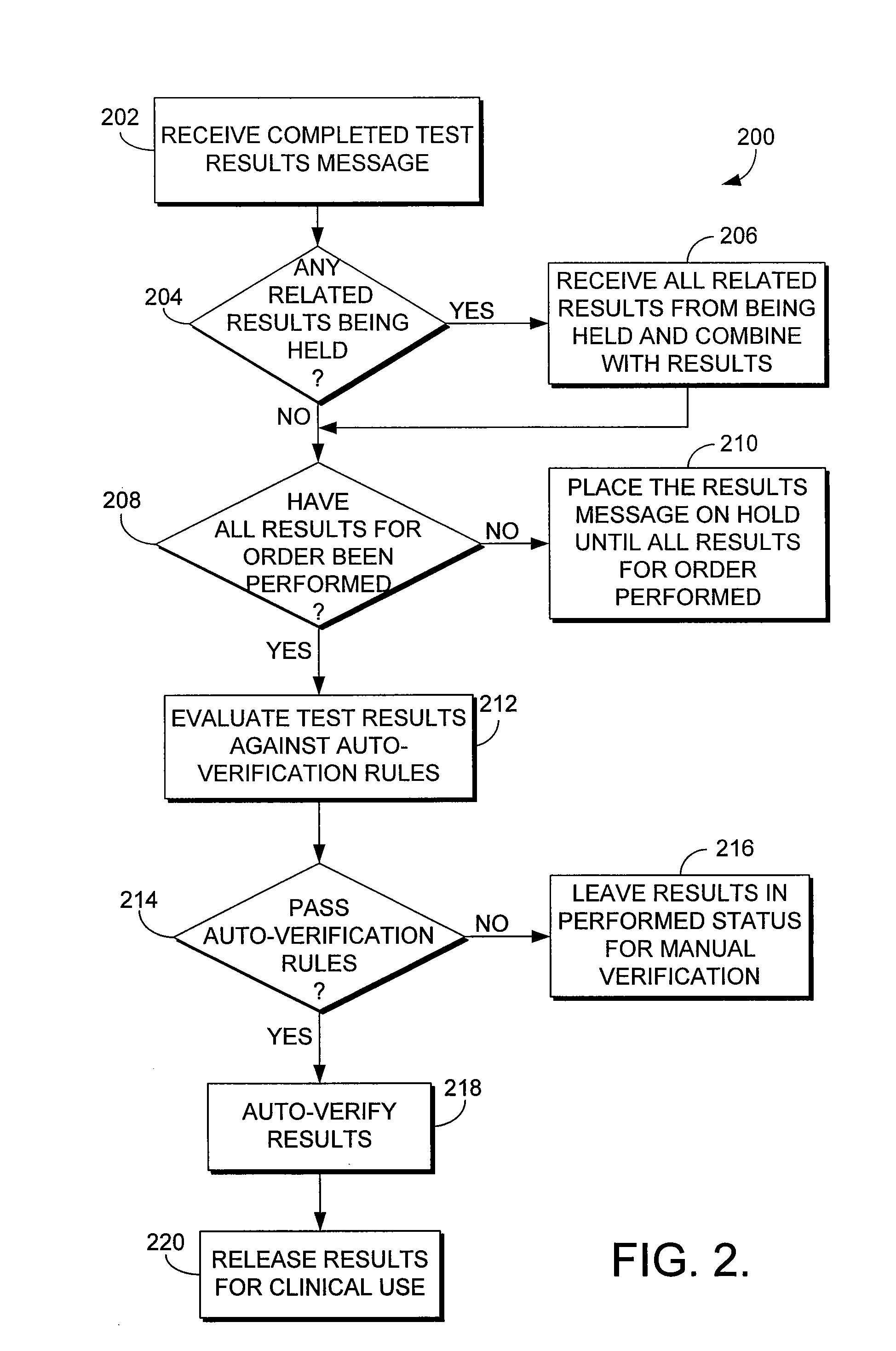
System and method for automatically verifying multiple laboratory test results in a computerized environment
The present invention relates to a method of holding laboratory test results in a computerized environment. The method receives a laboratory test result for a healthcare order for a patient. The healthcare order comprises multiple laboratory tests. The method determines whether all of the test results for the healthcare order have been received. If all of the test results for the healthcare order have not been received, the laboratory test result is held until all of the results for the healthcare order have been received. If all of the results for the healthcare order have been received, the method compares each test result of the healthcare order to predefined criteria to determine whether each test result for the healthcare order is within a predetermined acceptable range. If each of the test results for the healthcare order is within the acceptable range, the entire order is automatically verified.
Owner:CERNER INNOVATION
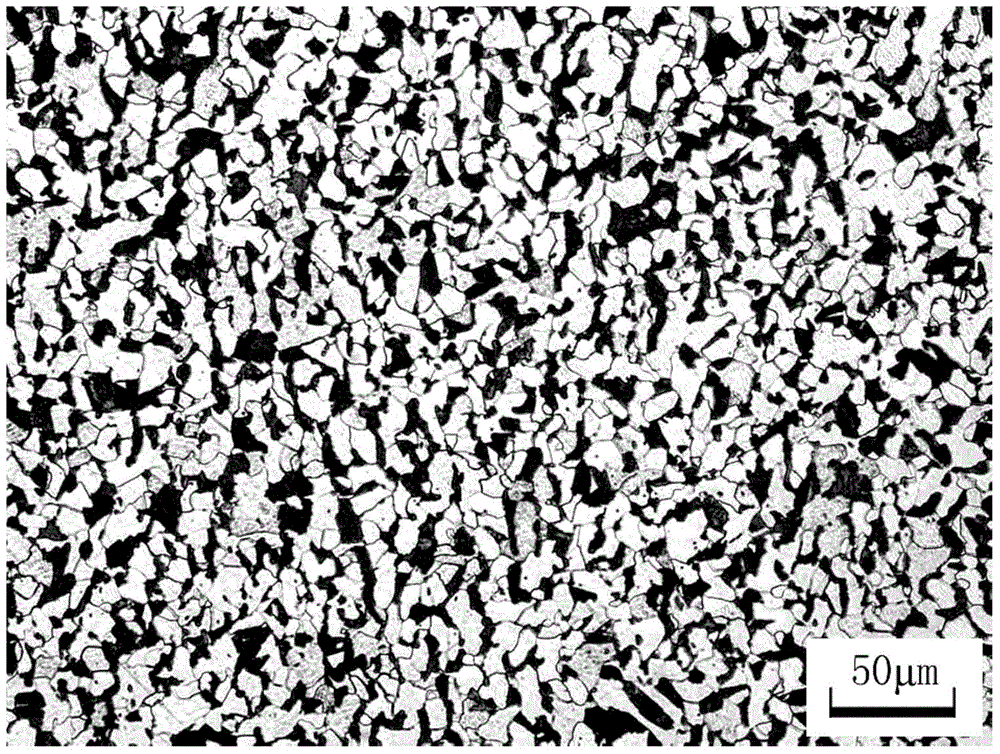
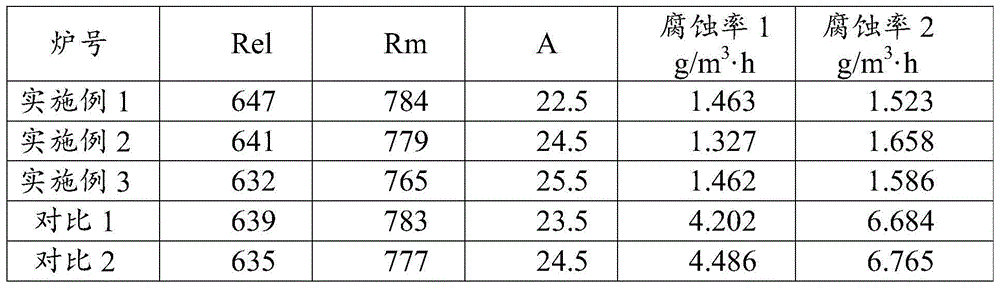
Alkali corrosion resistance high strength steel and production method thereof
The present invention discloses an alkali corrosion resistance high strength steel and a production method thereof, wherein the steel comprises, by mass, 0.15-0.20% of C, 0.45-0.75% of Si, 0.75-1.35% of Mn, less than or equal to 0.015% of P, less than or equal to 0.015% of S, 0.2-0.3% of Cu, 0.1-0.25% of Ni, 0.01-0.03% of Ti, 0.8-1.3% of Cr, 0.001-0.003% of B, 0.08-0.12% of V, 0.008-0.0120% of N, and the balance of Fe and unavoidable impurities. Compared with the existing ordinary building steel, the steel of the present invention has the following characteristic that the corrosion resistance in the alkaline environment is significantly improved, wherein laboratory test results show that the corrosion resistance is more than 2 times the corrosion resistance of the ordinary reinforcing steel bar. Compared with the epoxy resin coating reinforcing steel bar, the steel of the present invention has the excellent corrosion resistance, wherein the peeling phenomenon is easily generated on the epoxy resin coating reinforcing steel bar surface and the corrosion rate is high after the peeling. Compared with the stainless steel, the steel of the present invention has the substantially reduced production cost.
Owner:武汉钢铁有限公司
System and method for automatically verifying multiple laboratory test results in a computerized environment
ActiveUS20100256989A1Diagnostic recording/measuringSensorsLaboratory Test ResultProgramming language
The present invention relates to a method of holding laboratory test results in a computerized environment. A first laboratory test result is received, and is related to one or more other laboratory tests that comprise a healthcare order. It is determined whether the test results of the order have been received. If they have all been received, the test results are compared to predefined criteria and are determined to be considered normal. Once determined to be considered normal, the order is automatically verified. If the tests results of the healthcare order have not all been received, the first laboratory test result is held until all of the test results have been received.
Owner:CERNER INNOVATION
System and method for processing information and user interface system
InactiveCN1603834ASpecial data processing applicationsMaterial analysisLaboratory Test ResultVerification system
A system and method are provided that addresses the need for an improved microbiology validation system that monitors and validates clinical culture laboratory test results as they occur throughout the testing lifecycle of a clinical culture. Techniques are provided that consider the results of earlier performed tests when entering results for later performed tests so that inconsistent or wrong test results of a patient are not inadvertently released to a physician for review.
Owner:SIEMENS MED SOLUTIONS HEALTH
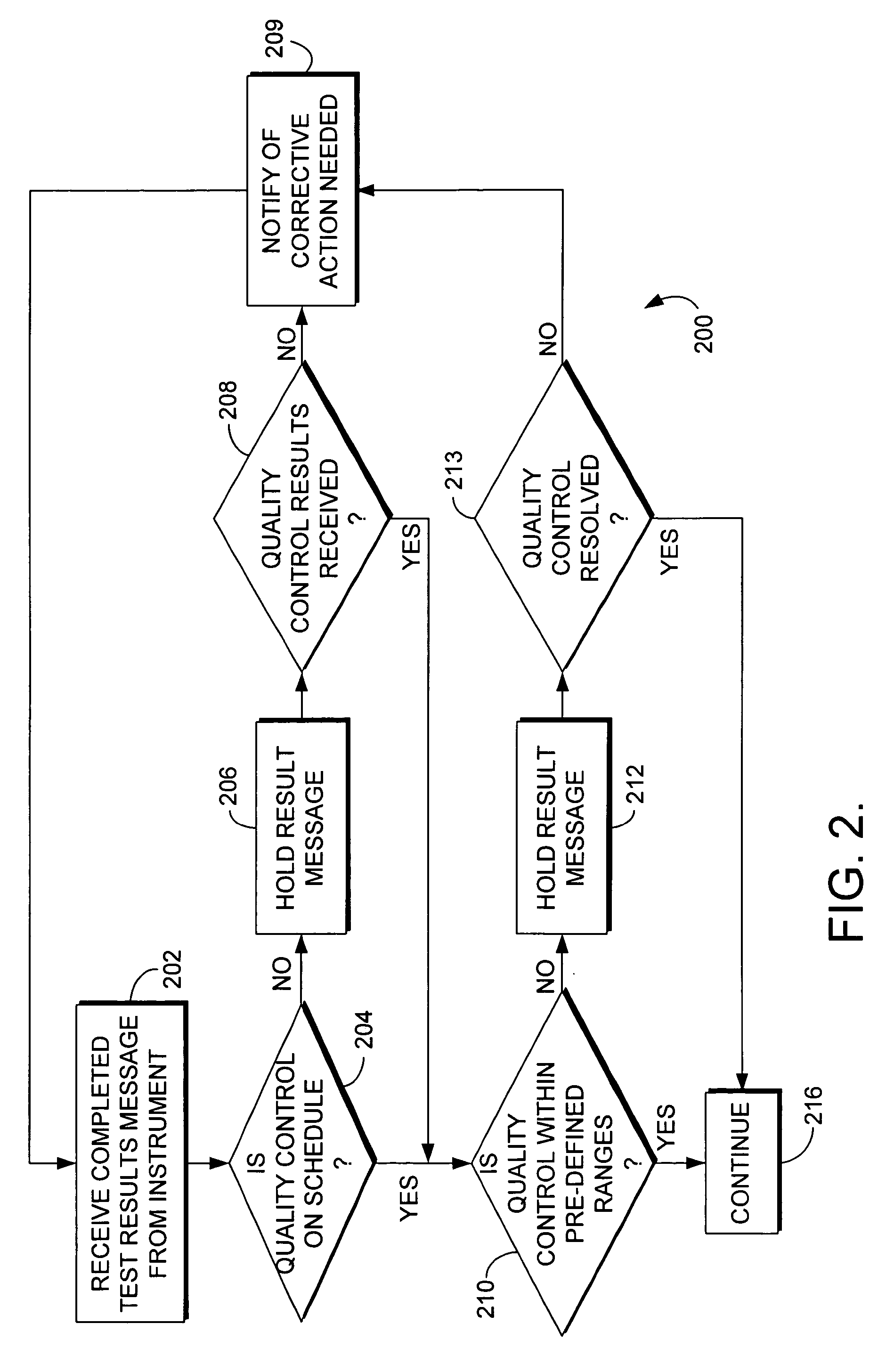
System and method for reviewing quality control of instruments performing laboratory tests in a computerized environment
InactiveUS20060149487A1Computer-assisted medical data acquisitionSpecial data processing applicationsLaboratory Test ResultQuality control
The present invention relates to a method and system for suspending the processing of laboratory test results in a computerized environment. One or more test results from an instrument in a healthcare laboratory are received. It is determined whether the instrument has had a quality control procedure performed within a predefined period. If not, the processing of the one or more test results is suspended until the quality control procedure has been performed on the instrument. If the quality control procedure has been performed within the predefined period, it is determined whether the quality control procedure performed indicates that the instrument is performing properly. If not, the processing of the one or more test results is suspended until the quality control issue is resolved.
Owner:CERNER INNOVATION
System for managing laboratory test results for patients taking an endothelin receptor antagonist
The invention provides a system and method of collecting, storing and managing laboratory test results of patients. In one embodiment the invention is directed towards collecting, storing and managing laboratory test results of patients taking an endothelin receptor antagonist (ERA). The system provides a way of securely collecting and tracking information from a plurality of patients, a plurality of laboratories, and a plurality of health care providers by use of a database. The method comprises receiving patient information from health care providers, receiving a laboratory test schedule for each patient, and laboratory tests needed for each patient. This data is entered into a central processing computer. The patients then go to any laboratory to have the monthly testing done due to the ERA prescription. The laboratory test data collected is sent to the central processing computer. The central processing computer can generate an automated report on the laboratory results and supply the results to the health care provider. Additionally, if no laboratory test data for the patient is entered into the central processing computer in accordance with the laboratory test schedule for the patient, the central processing computer will notify a call center which will call the patient, laboratory or health care provider informing them of the overdue laboratory test data.
Owner:ROSS III ERNEST OSGOOD +3
Full-size photovoltaic module IAM testing device and testing method thereof
PendingCN114189211AEnsure power generation efficiencyIncrease incomePhotovoltaic monitoringPhotovoltaic energy generationLaboratory Test ResultEngineering
The invention relates to the technical field of testing devices, in particular to a full-size photovoltaic module IAM testing device and a testing method thereof.The full-size photovoltaic module IAM testing device is provided with a transverse rod, rotating shafts are symmetrically and vertically arranged at the positions, opposite to the ground, of the lower end of the transverse rod, and a bearing penetrates through the joint of one end of each rotating shaft and the transverse rod; the other end of the rotating shaft is fixed on a support, and a photovoltaic assembly is fixed on the support. According to the full-size photovoltaic module IAM test device and the test method thereof, the power generation performance of the full-size photovoltaic module at different angles can be measured, so that a laboratory test result is closer to the actual power generation efficiency of a photovoltaic power station; the power generation efficiency of the photovoltaic module is guaranteed, and the income of the whole photovoltaic power station is improved.
Owner:常州华阳检验检测技术有限公司
Method for discriminating hydrophobic migration characteristics of silicone rubber based on different salt components
ActiveCN108760579ATest results closelyClose resultsSurface/boundary effectLaboratory Test ResultSilicone rubber
The invention provides a method for discriminating hydrophobic migration characteristics of silicone rubber based on different salt components. The method comprises the following steps: respectively preparing soiling solutions with the salt components of NaCl, CaCl2 and (NH4)2SO4 and an ash component of diatomite; testing the hydrophobicity of samples treated by the soiling solutions with different salt components at different migration times, wherein the testing time is 24h, 48h, 96h and 144h after migration respectively; determining a saturation value of a hydrophobic migration angle, calculating indexes of the hydrophobic migration characteristics according to hydrophobic migration testing results, and discriminating the hydrophobic migration characteristics. By the method, the seven indexes of the hydrophobic migration characteristics are creativity used for comprehensively evaluating the characteristics of the silicone rubber; by the method, through a simulation test based on a variety of soiling solutions, the hydrophobic migration characteristics of the silicone rubber are comprehensively evaluated, so that laboratory test results are more closely related to field application.
Owner:ELECTRIC POWER RESEARCH INSTITUTE OF STATE GRID SHANDONG ELECTRIC POWER COMPANY +1
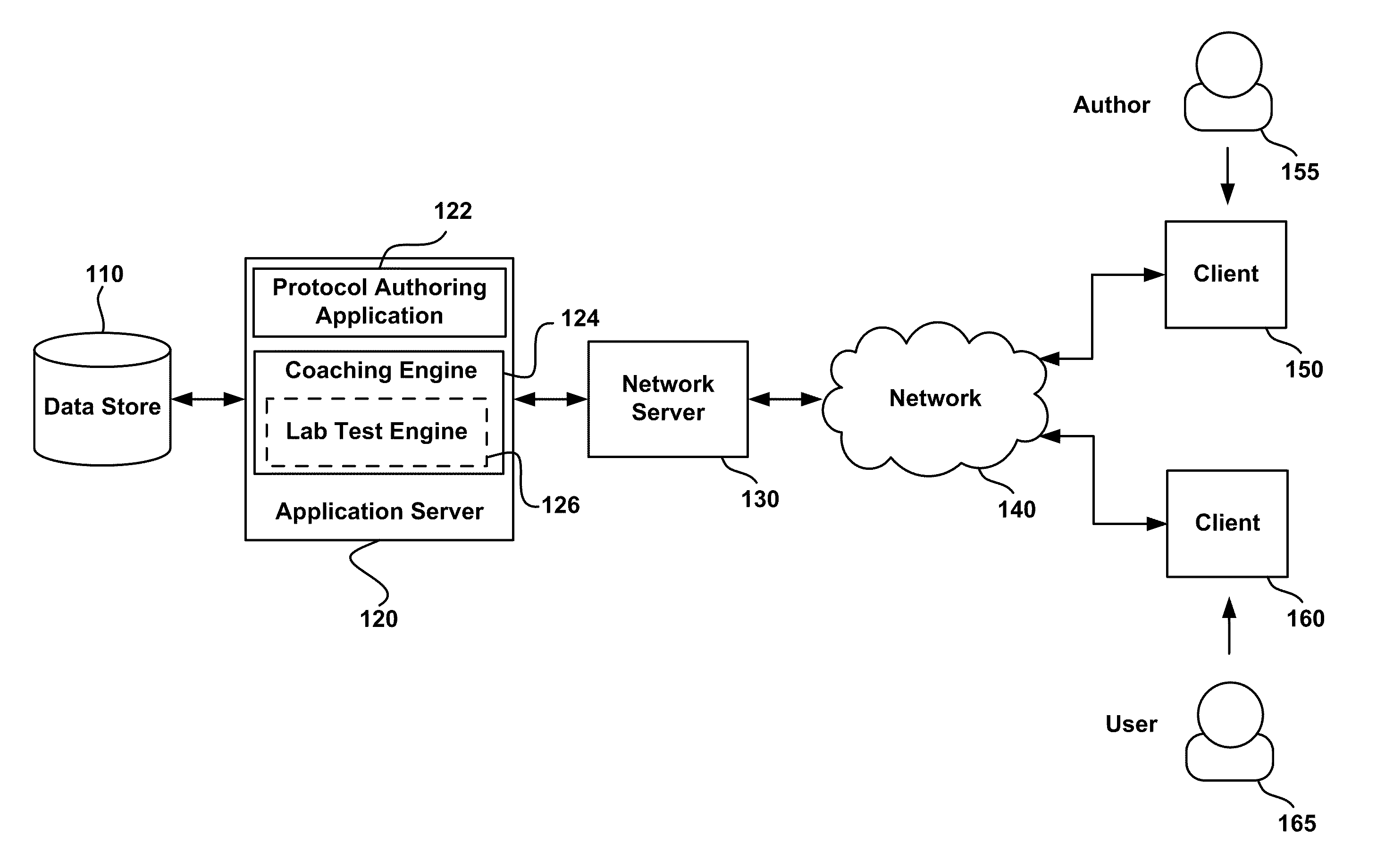
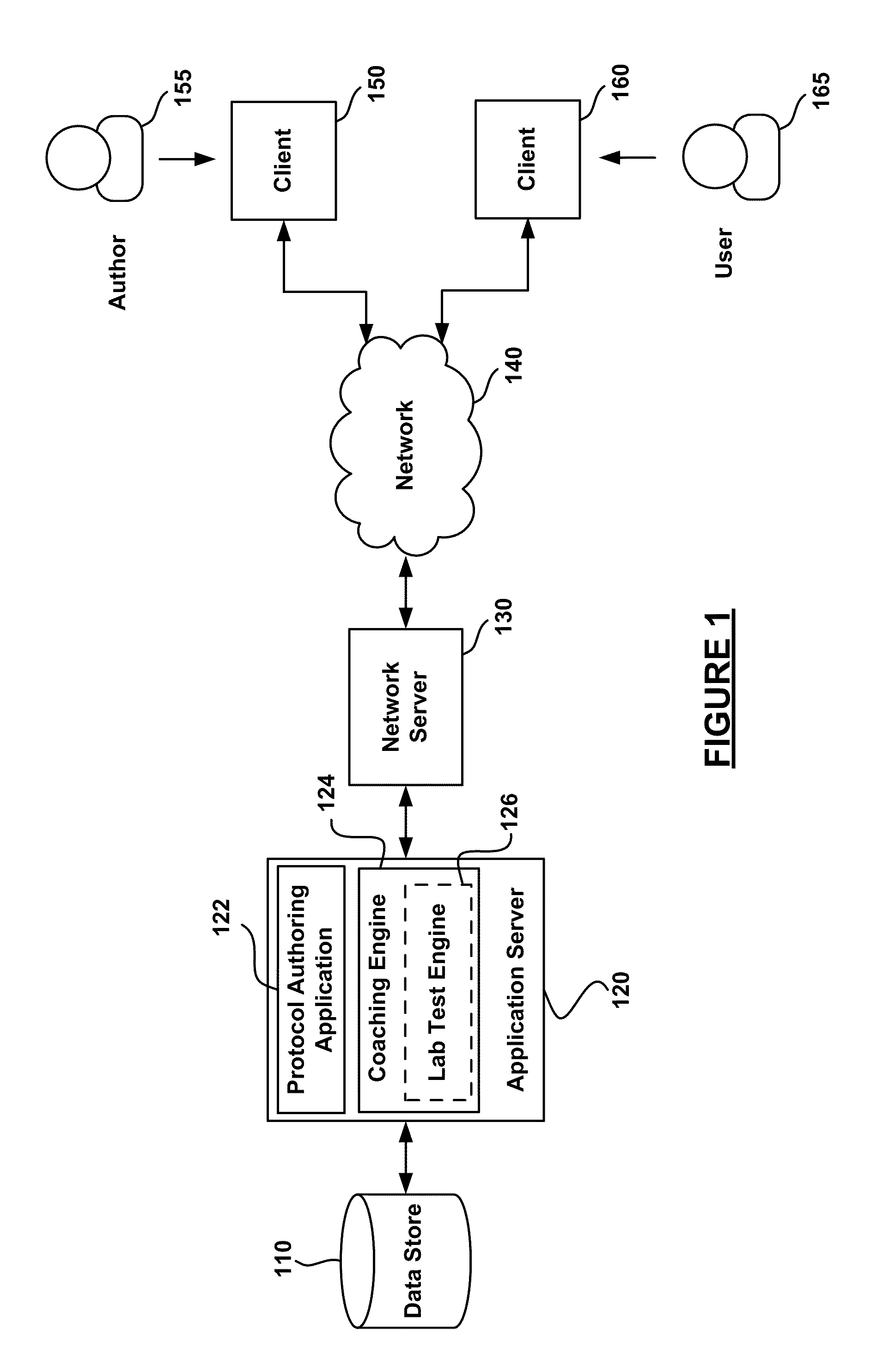
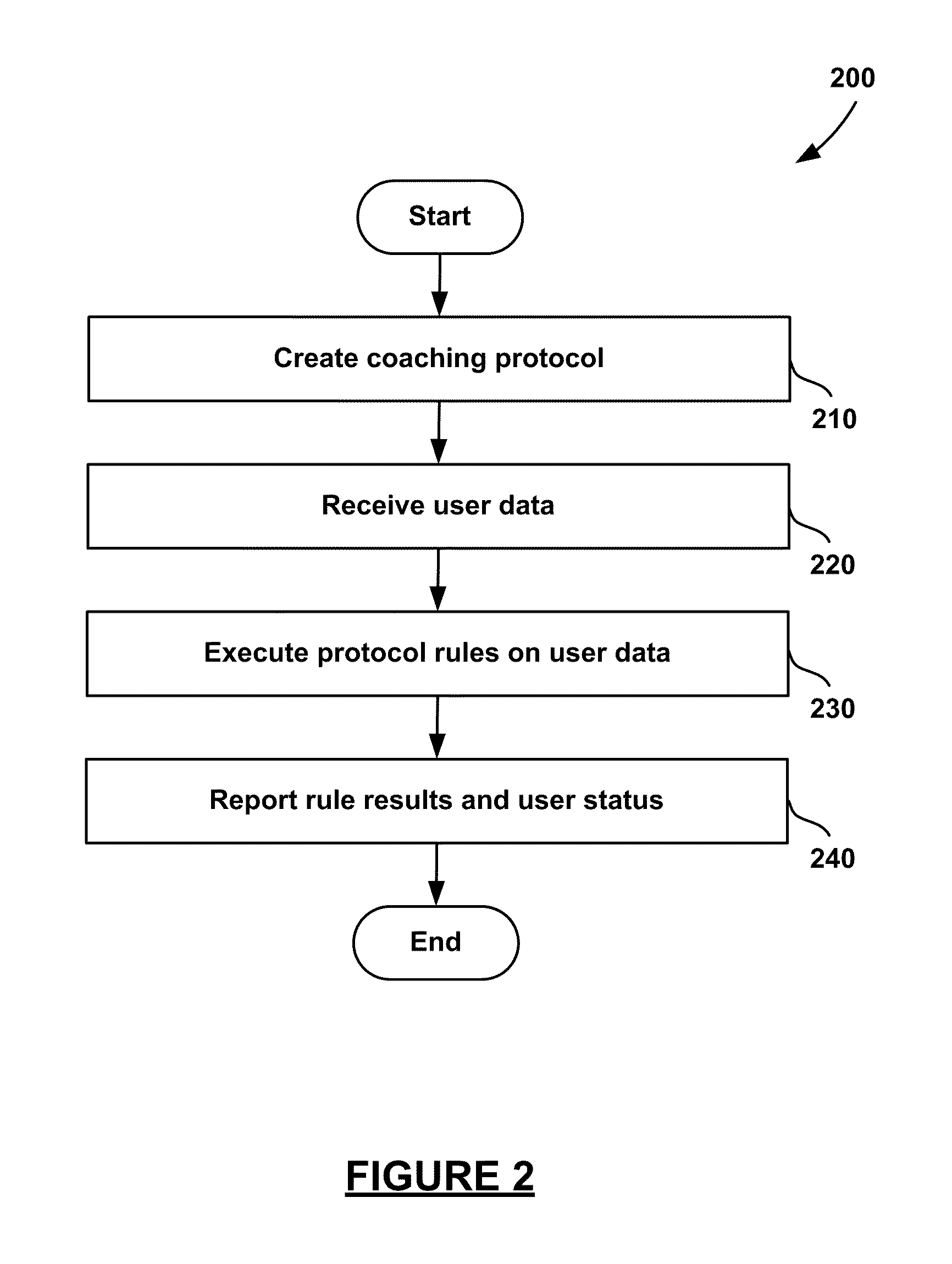
Analysis of User Laboratory Test Results
InactiveUS20110153347A1Data processing applicationsComputer-assisted medical data acquisitionLaboratory Test ResultComputer science
Lab tests may be processed for a user through execution of a lab test coaching engine stored in memory and executable by a processor. The lab test coaching engine accesses user lab test results and processes the user lab test results based on a coaching protocol and user health data. The results of the lab test results are reported to the user and a health care professional and the lab test coaching engine may perform an action based processed lab test results.
Owner:KEAS
System and method to test nutritional supplements
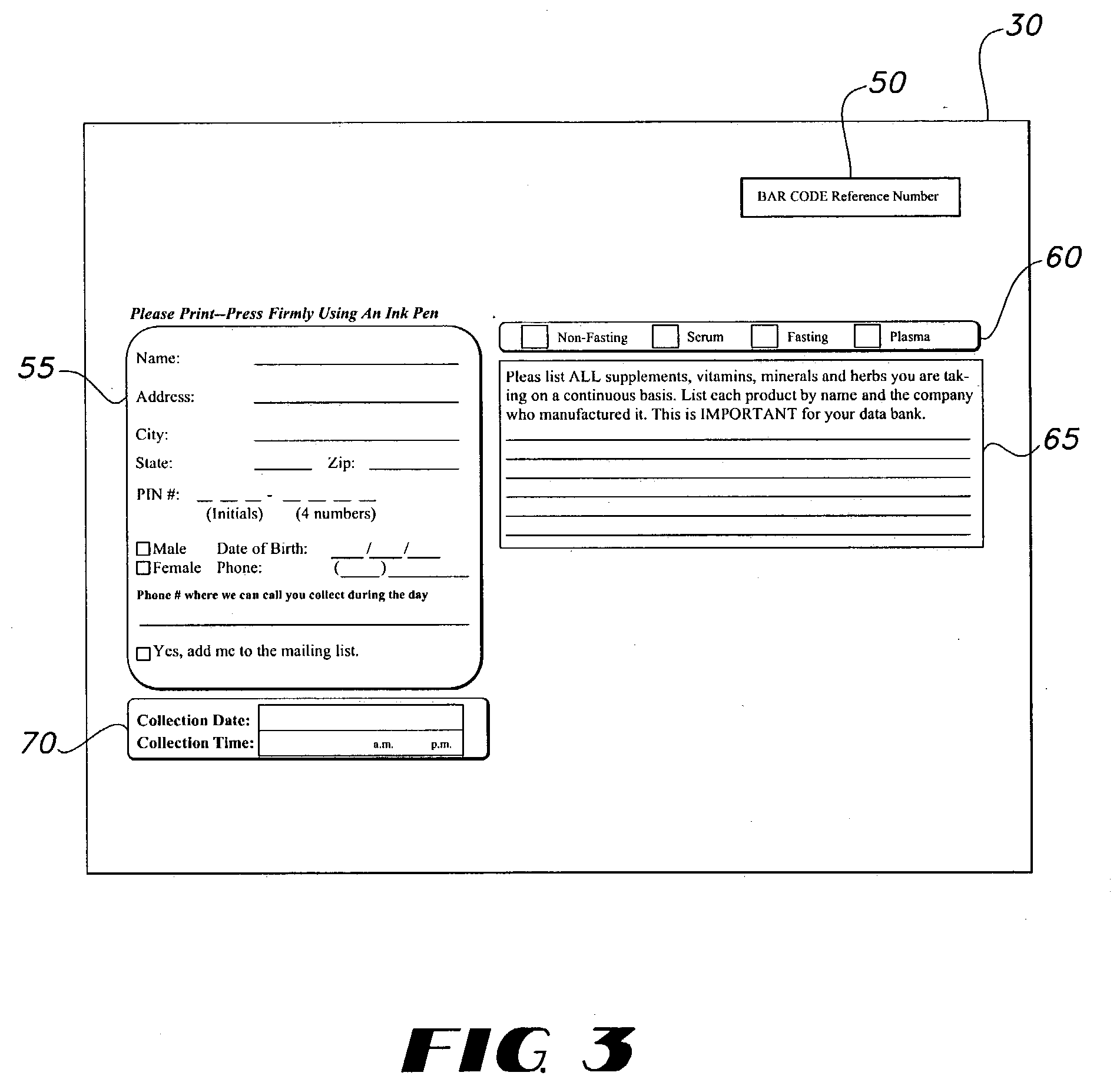
This invention provides a fluid sample retrieval kit, including a sample transfer tube, to facilitate a method of confidentially providing laboratory test results to a consumer of the fluid sample retrieval kit. Additionally, this invention provides a method to compare clinical chemistry values from a plurality of consumers for a designated nutritional supplement, or a combination of nutritional supplements. Additionally, this method provides a fluid sample retrieval system.
Owner:DUNN JACKY F
Method for inducing DC-CIK by utilizing muramyl dipeptide
ActiveCN103710308AEnhance phagocytosisEnhance antigen presentationMammal material medical ingredientsBlood/immune system cellsLaboratory Test ResultMHC restriction
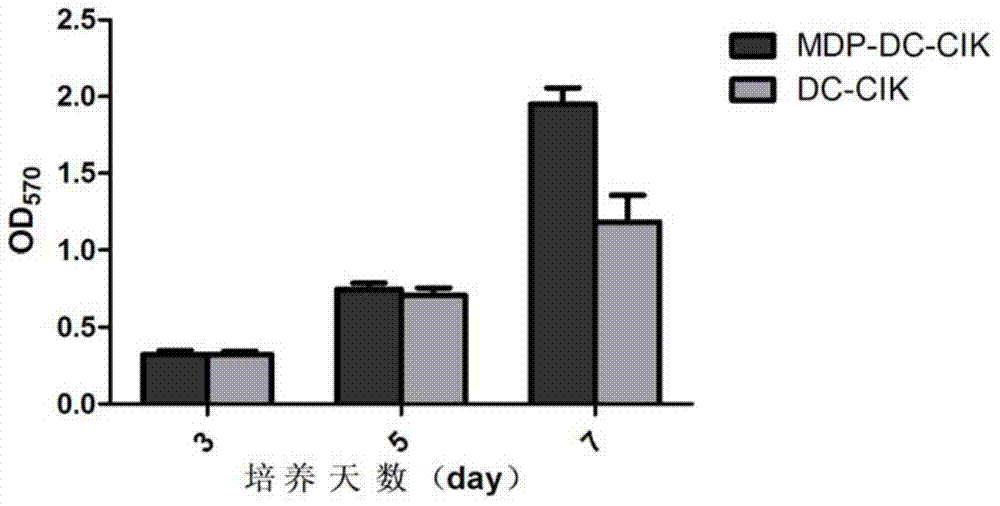
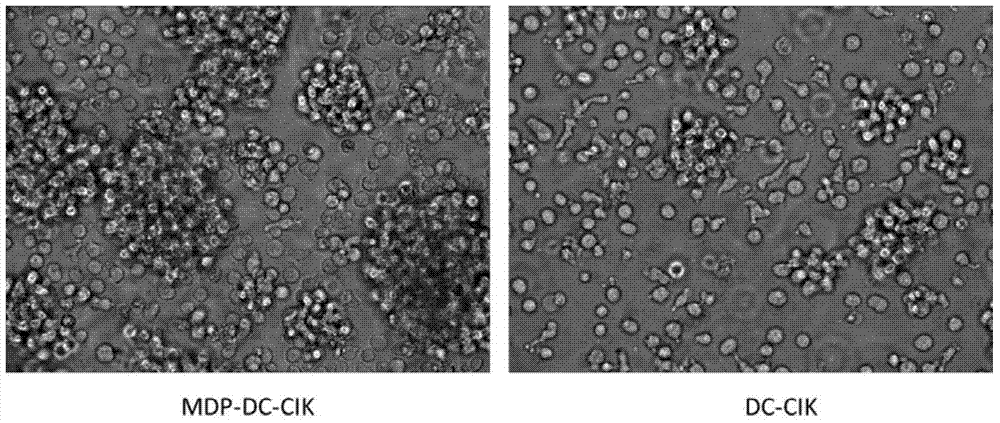
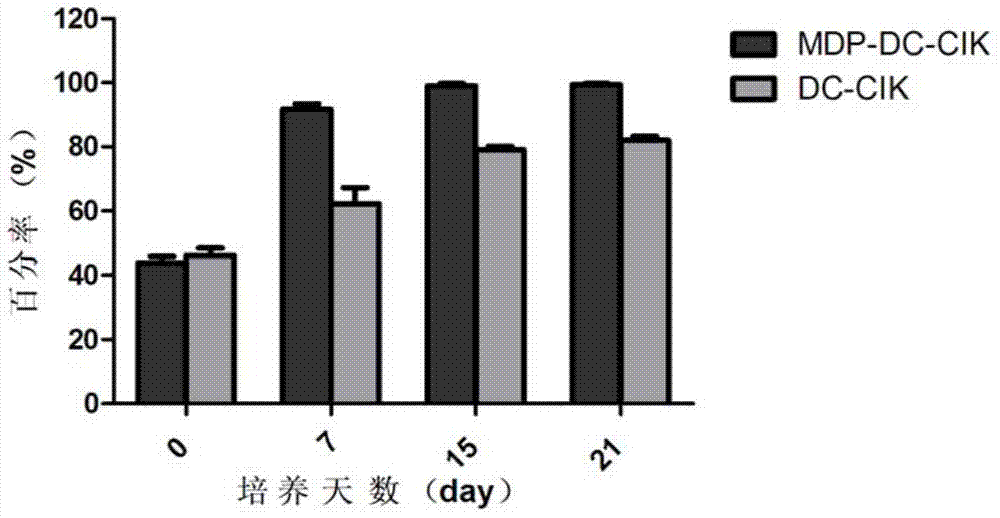
The invention provides a method for inducing DC-CIK by utilizing muramyl dipeptide, that is to say, the muramyl dipeptide is added into a CIK or DC-CIK culture solution to induce proliferation and differentiation of CIK or DC-CIK cells, so as to improve the tumor-killing activity of the CIK or DC-CIK cells. The method comprises the following steps: peripheral blood collection, tumor antigen acquisition, mononuclear cell separation, mononuclear cell collection, mononuclear cell washing, MDP-DC-CIK cell induction, and culture. MDP-DC-CIK cells introduced and cultured by the method are detected by a flow cytometry, the ratio of CD3+CD56+ non-MHC restricted NKT cells is found to be up to 80% or more; and at the same time, the activity of tumor killer cells is much higher than that of DC-CIK cells by conventional induction culture, and a laboratory test result of the tumor-killing percentage reaches 99% or more.
Owner:中海峡福建细胞生物科技有限公司
Filter collector for pituitary tumor surgical specimens
InactiveCN103040490AEasy to collectHigh precisionSurgical needlesWound drainsLaboratory Test ResultPituitary tumors
The invention relates to a filter collector for pituitary tumor surgical specimens. The filter collector for pituitary tumor surgical specimens comprises a suction tube (2) and a negative pressure suction tube (3). One end of the suction tube (2) is provided with a suction hose (4) used to suck specimen tissues. The other end of the suction tube (2) is provided with a connector. The connector is connected with a filter tube (1). A filter (5) is arranged inside the filter tube (1). The other end of the filer tube (1) matches with a connection of the negative pressure suction tube (3) in shape and is disposed on the connection of the negative pressure suction tube (3). The filter collector enables collection of tumor specimens for brain tumor surgery to be convenient collection to be faster, and acquisition of large specimen tissues to be easy, so that accuracy in laboratory test results is increased and related fundamental research is facilitated.
Owner:SHANGHAI CHANGZHENG HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com