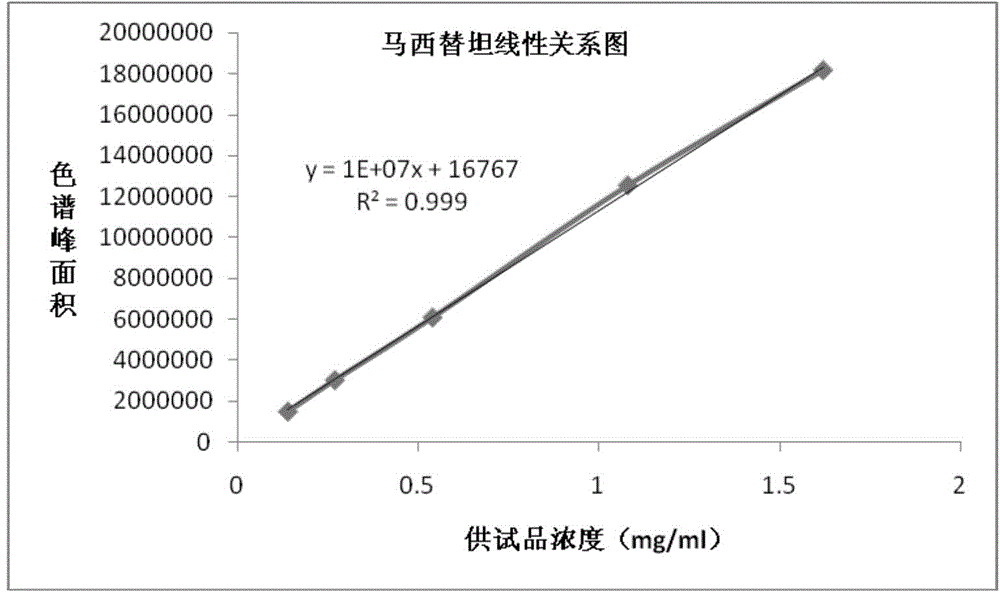
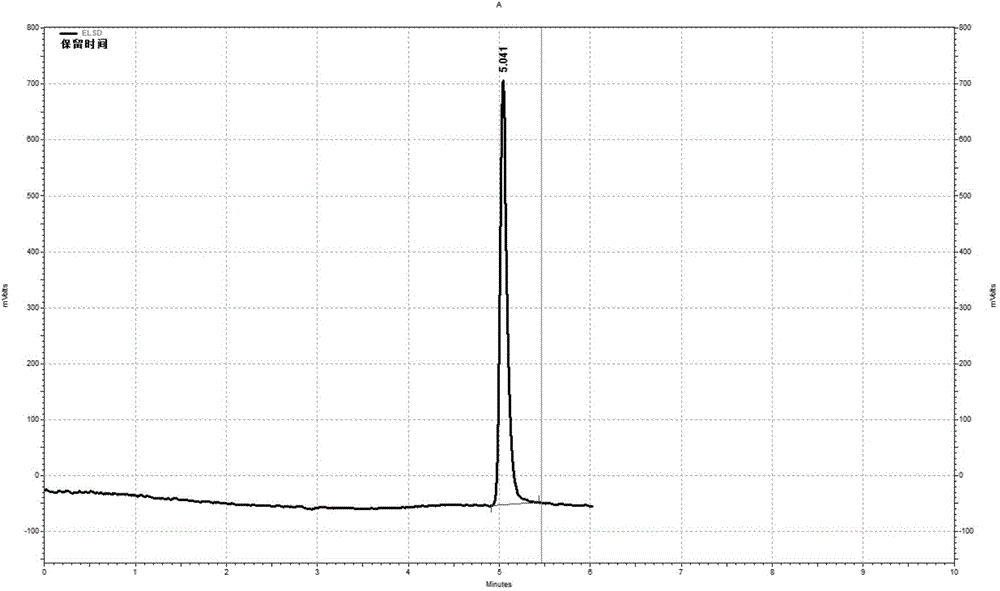
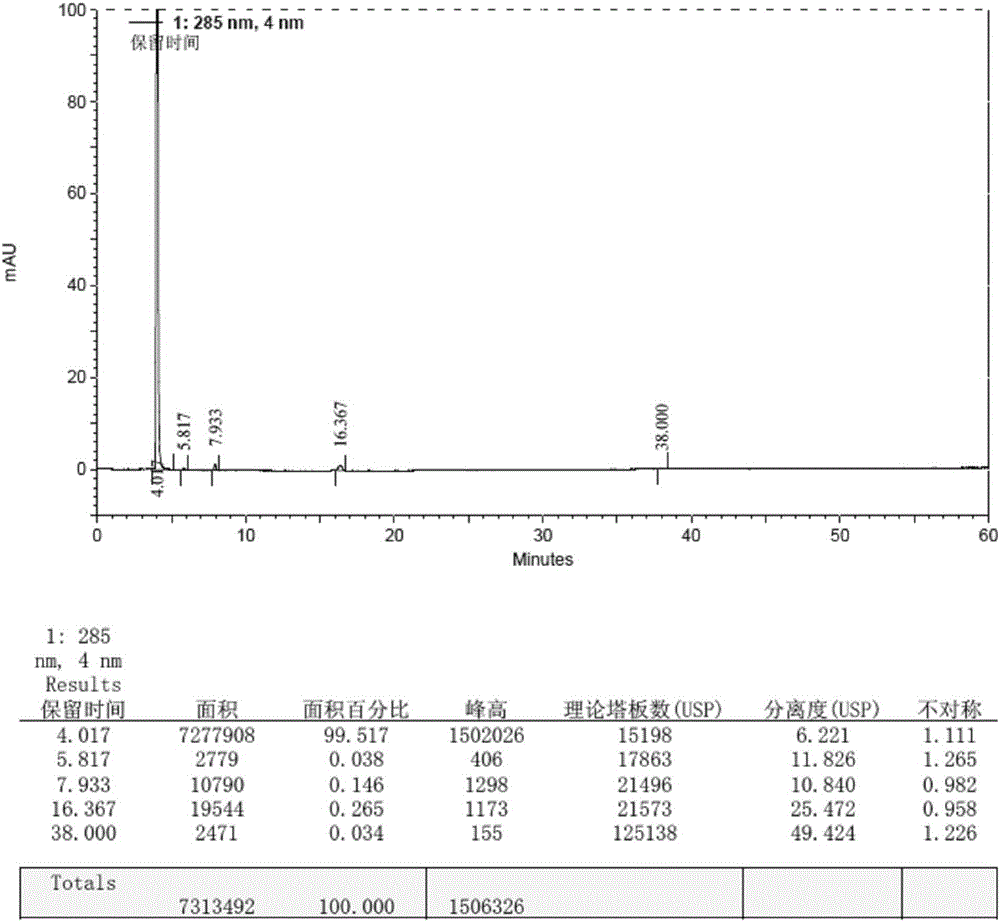
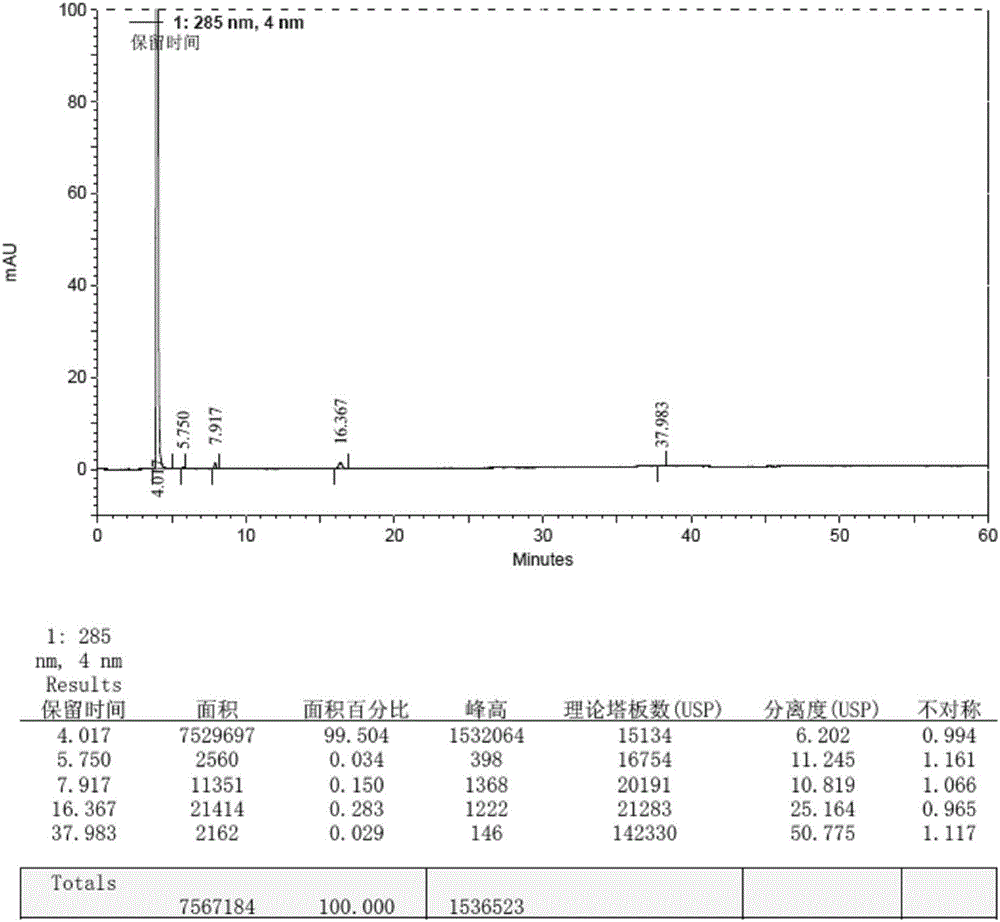
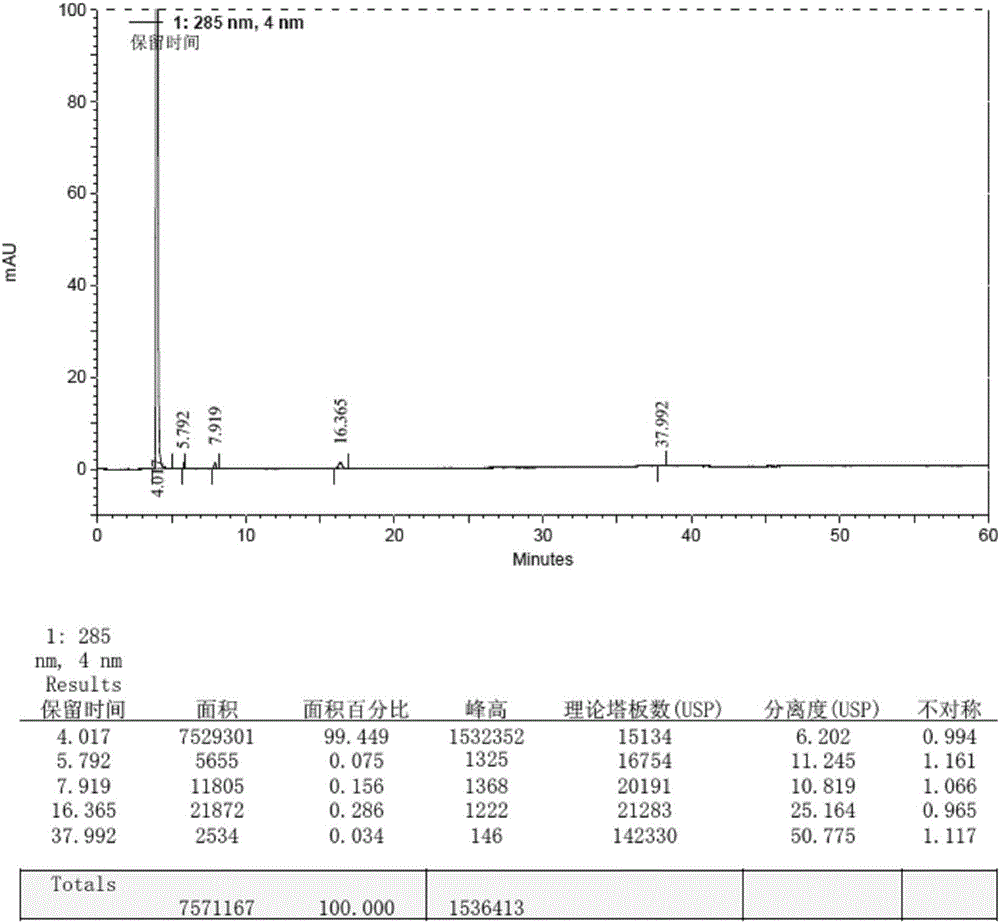
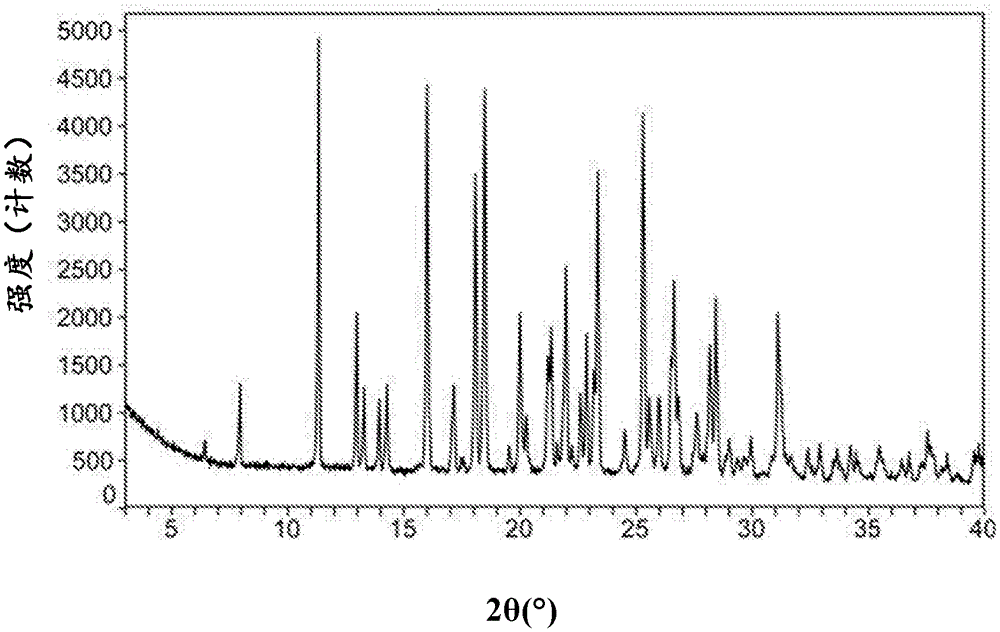
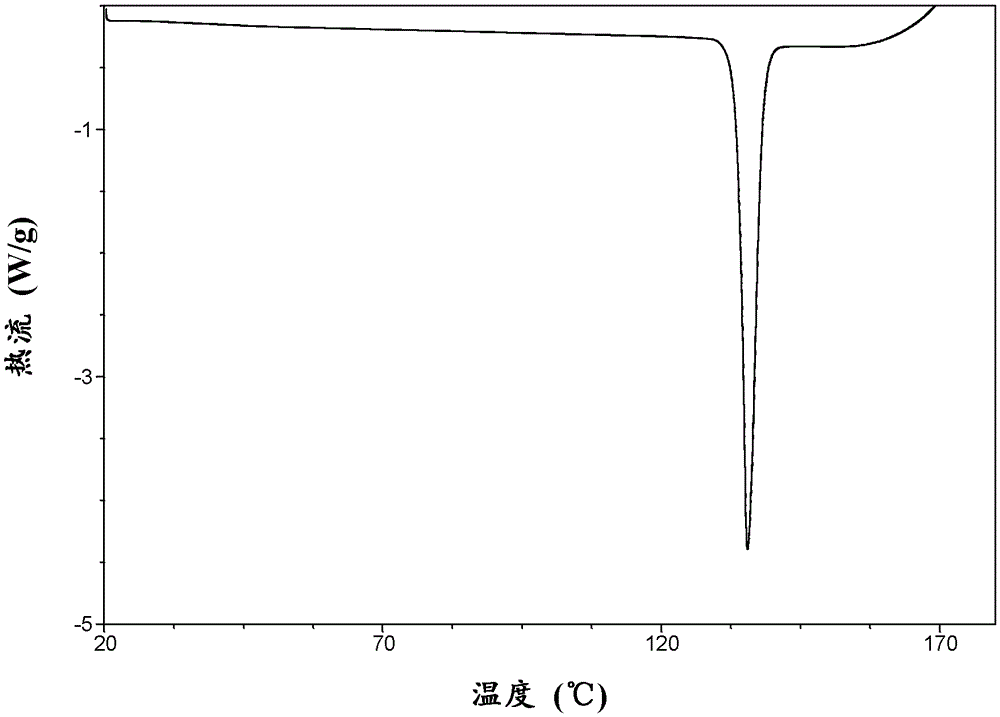
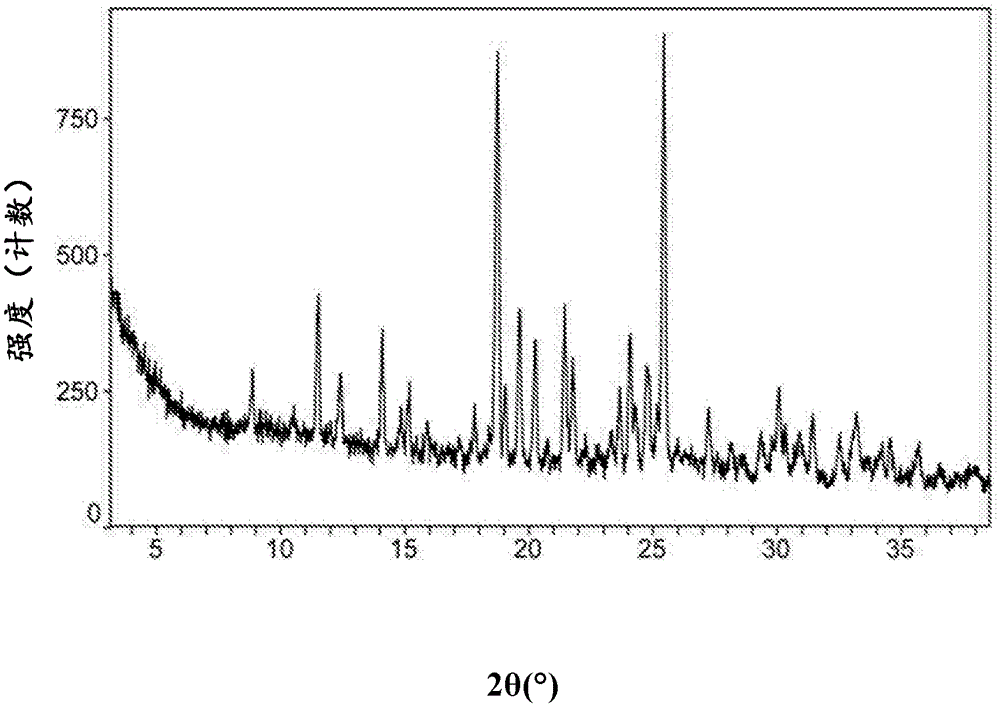
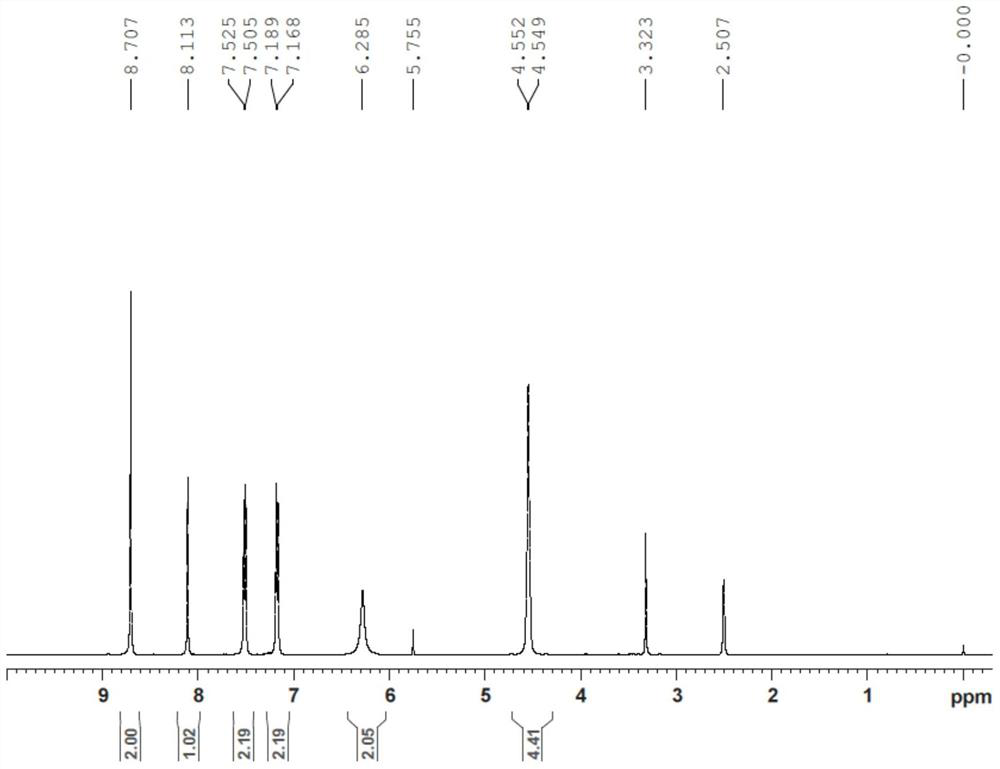
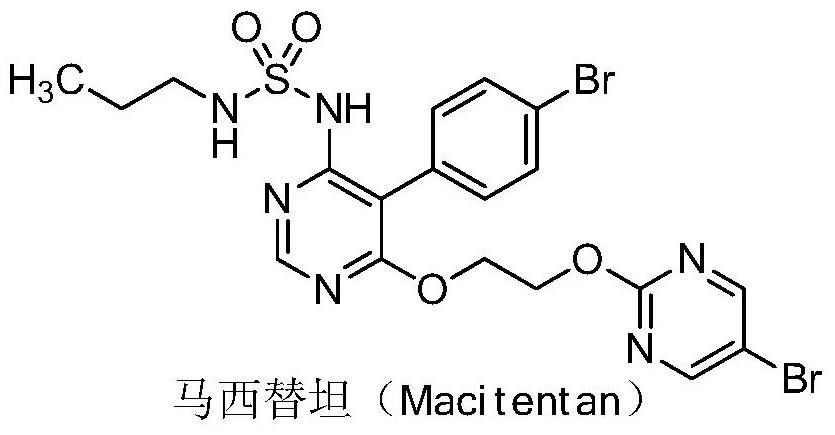
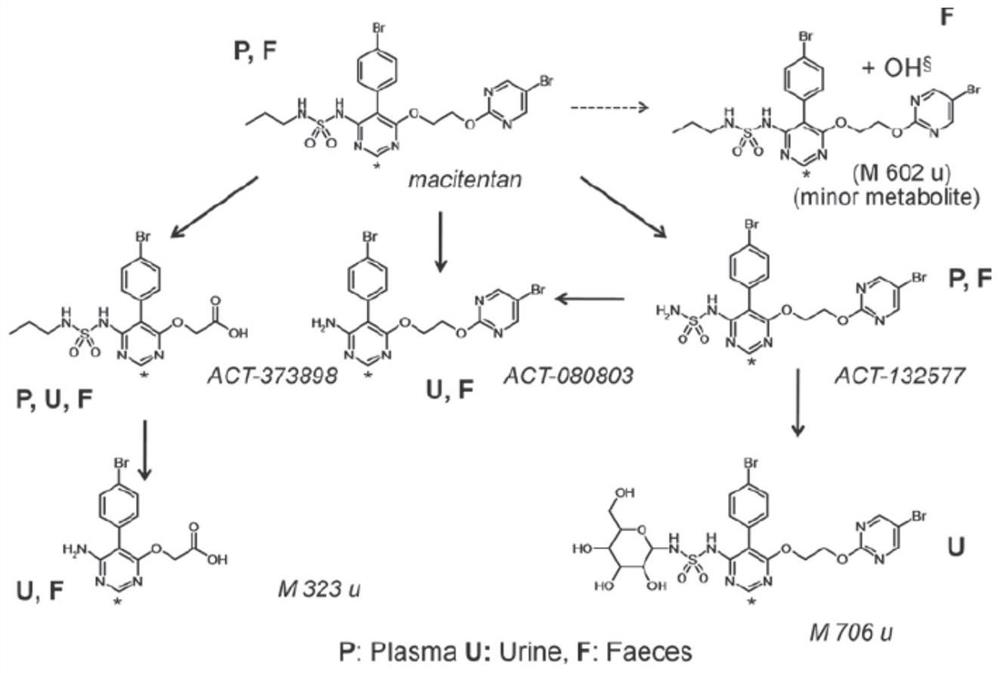
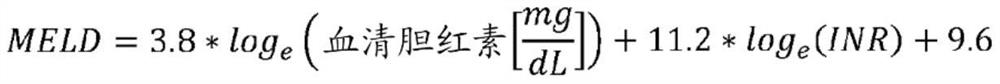Patents
Literature
35 results about "Macitentan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
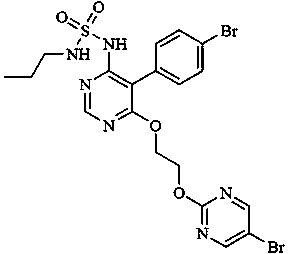
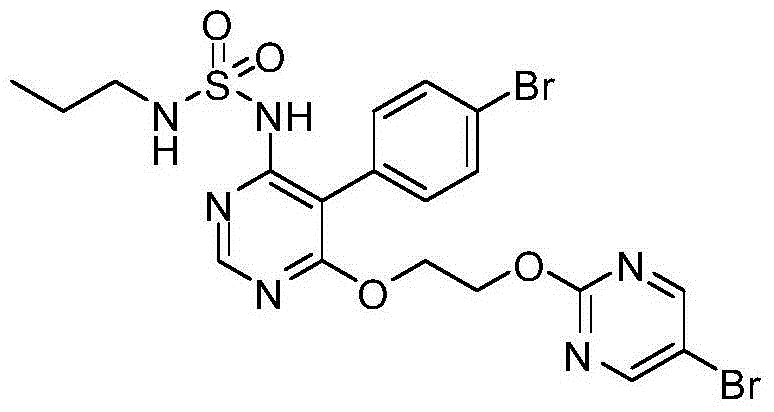
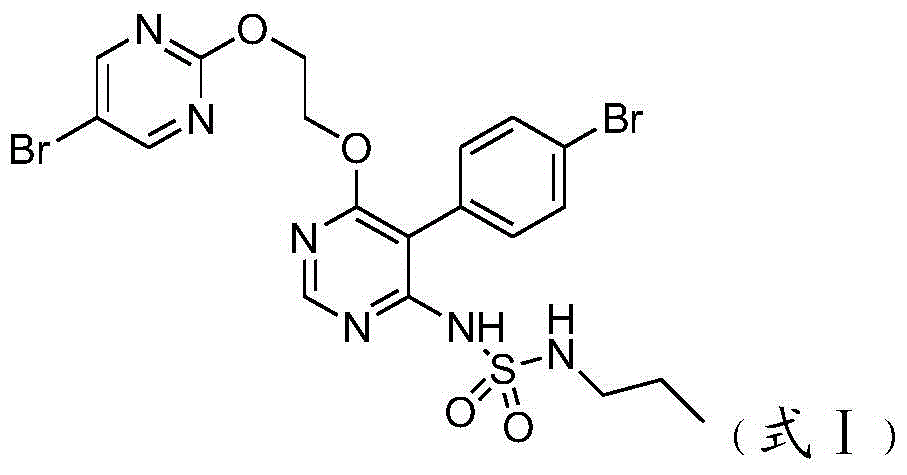
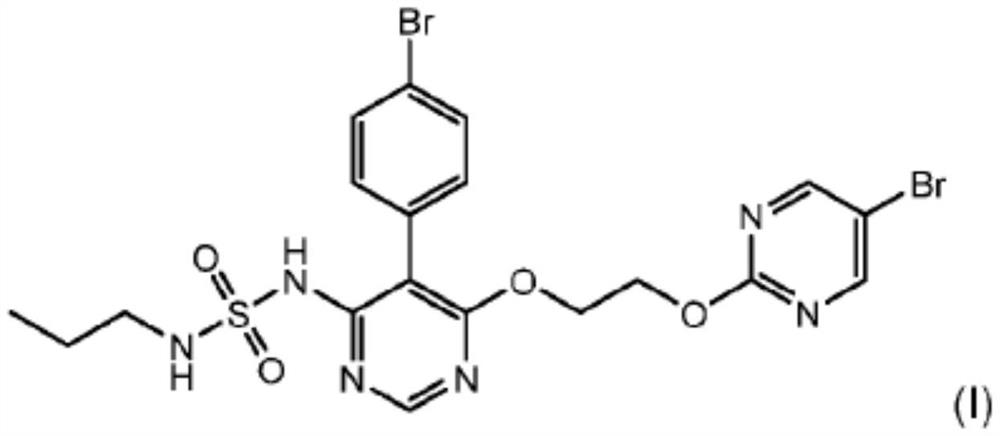
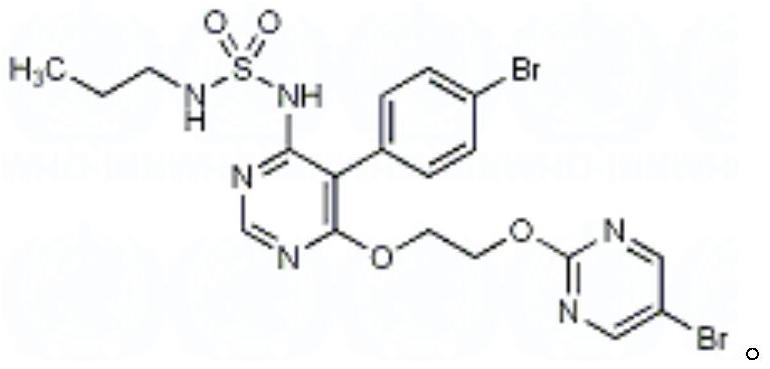
Macitentan (trade name Opsumit) is an endothelin receptor antagonist (ERA) approved for the treatment of pulmonary arterial hypertension (PAH). The other two ERAs marketed as of 2014 are bosentan and ambrisentan. Macitentan is a dual ERA, meaning that it acts as an antagonist of two endothelin (ET) receptor subtypes, ETA and ETB. However, macitentan has a 50-fold increased selectivity for the ETA subtype compared to the ETB subtype. The drug received approval from the U.S. Food and Drug Administration (FDA) on October 13, 2013.
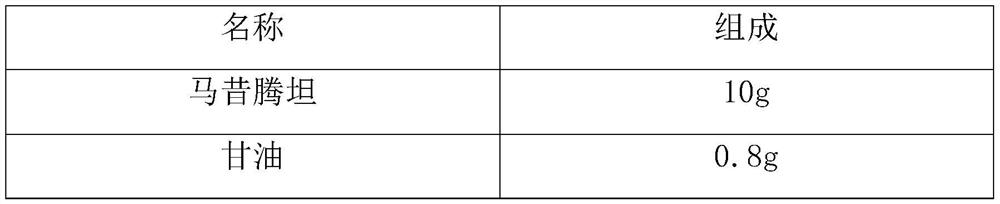
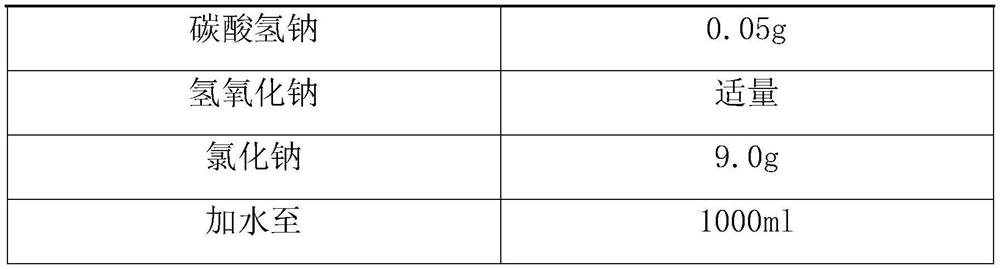
Macitentan oral disintegrating tablet for treating PAH (pulmonary arterial hypertension) and preparation method of macitentan oral disintegrating tablet
InactiveCN107913256ADisintegrates quicklyGreat tasteOrganic active ingredientsRespiratory disorderDiseaseSolubility
The invention discloses a macitentan orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet is a drug mixture comprising macitentan, flavoring agent, filler, disintegrating agent, lubricant, wetting agent and binder, wherein the physiologically active ingredient macitentan is The weight percentage of disintegrating tablets is 5-10%. The solid pharmaceutical preparation provided by the invention adopts solid dispersion technology. By mixing and grinding the main drug and the filler, the solubility of the main drug is improved, the dissolution effect is improved, and the dissolution rate of the preparation is ensured. High content uniformity and high bioavailability. In addition, the macitentan orally disintegrating tablet with convenient administration, rapid onset of effect and high bioavailability of the present invention can improve the drug compliance of patients and is beneficial to the treatment of diseases.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
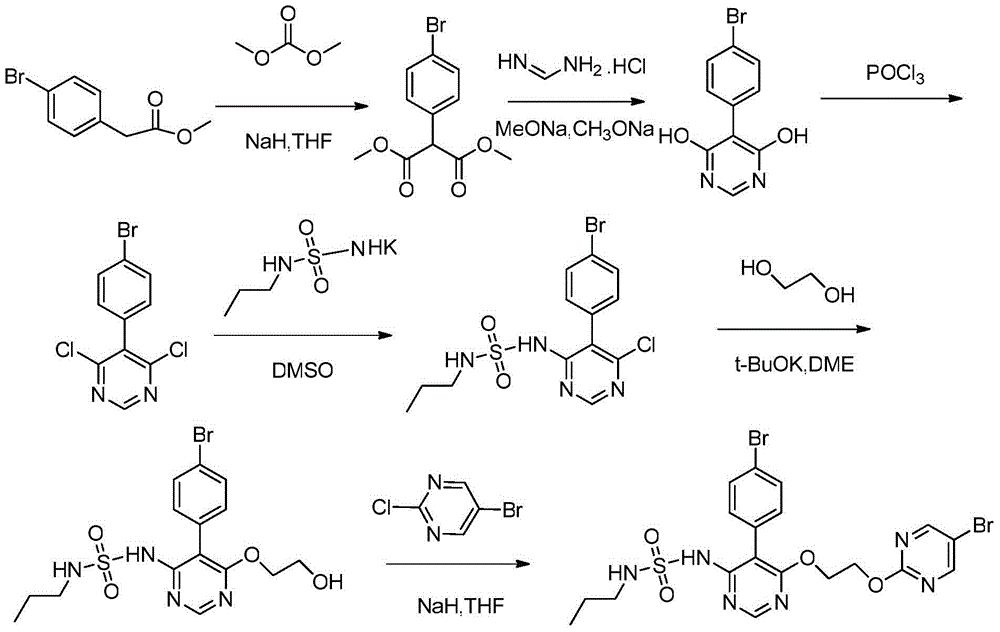
Method for preparing macitentan
InactiveCN104447572AHigh yieldAvoid the disadvantage of long reaction timeOrganic chemistryAfter treatmentProcess conditions
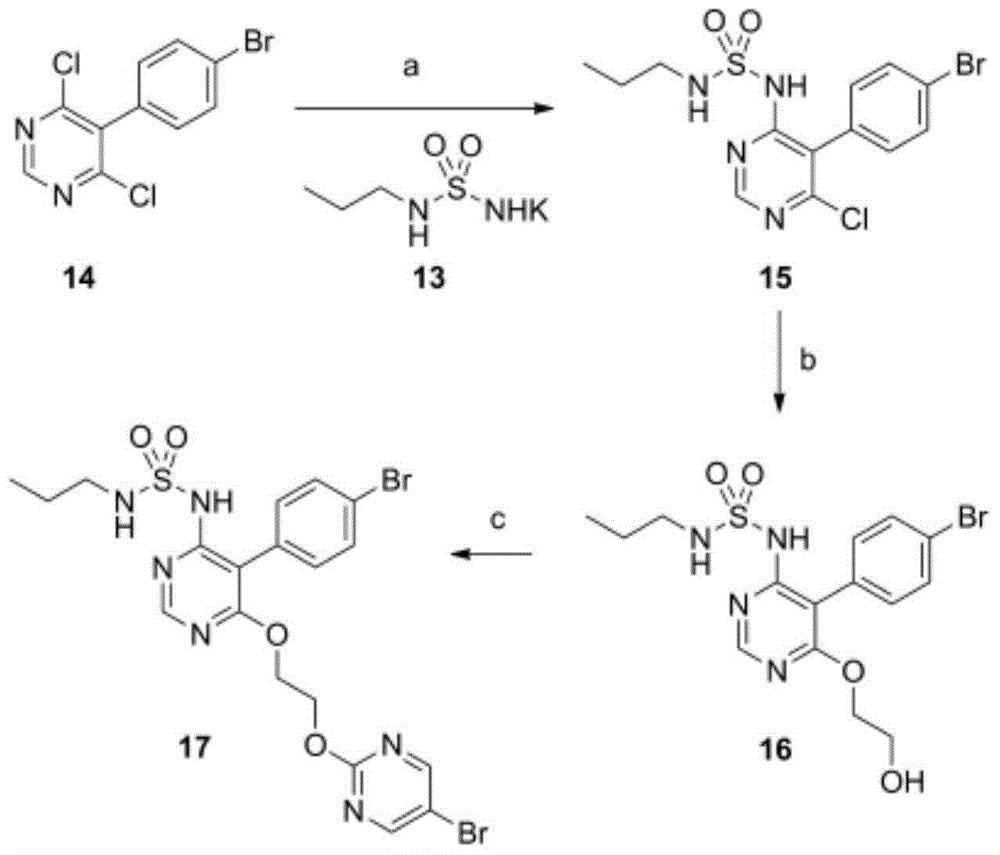
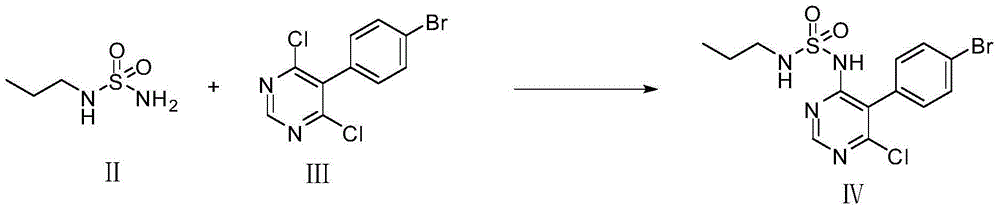
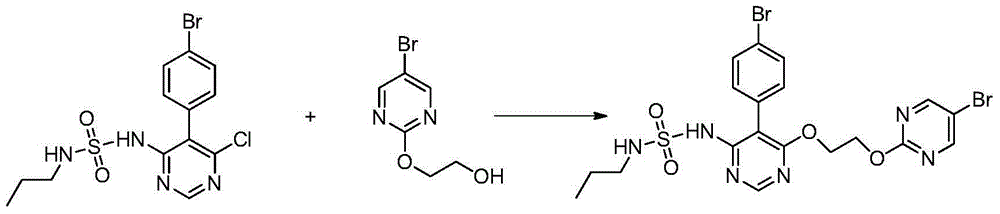
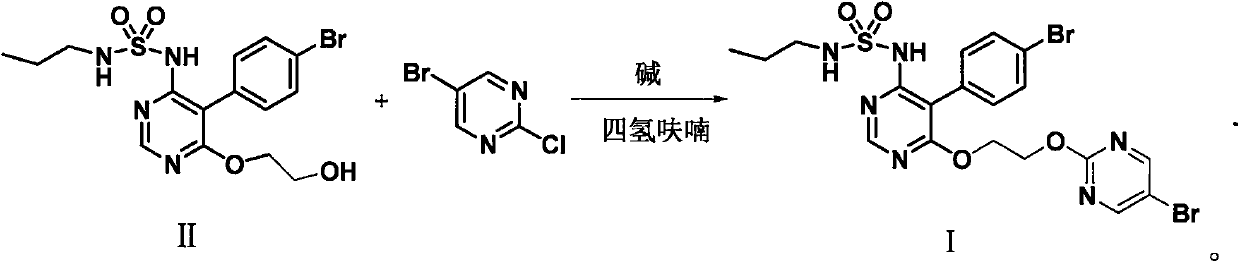
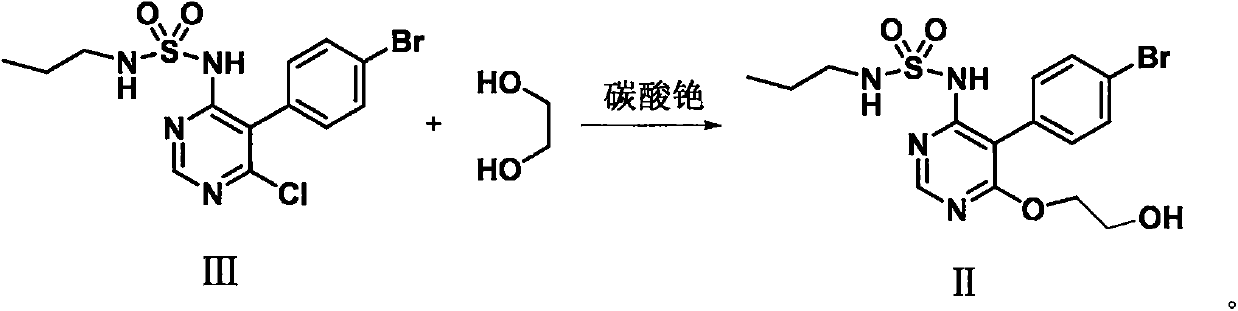
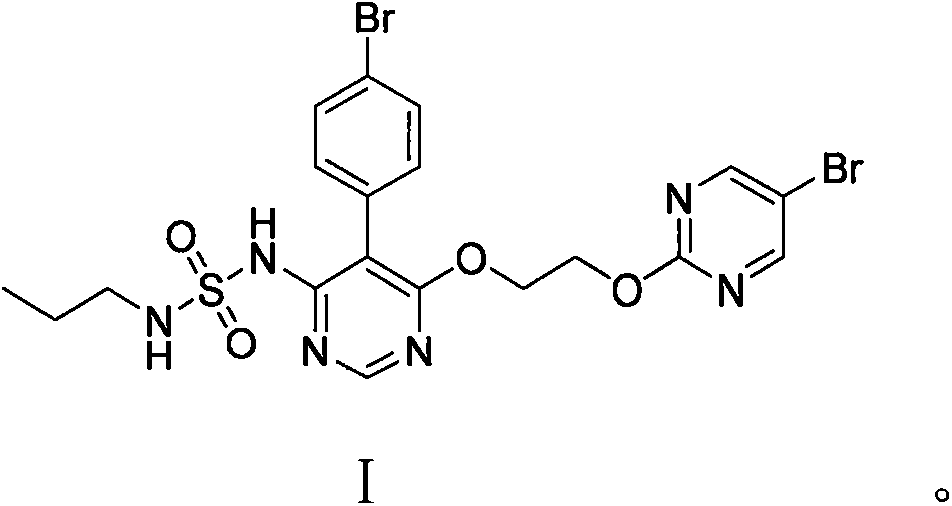
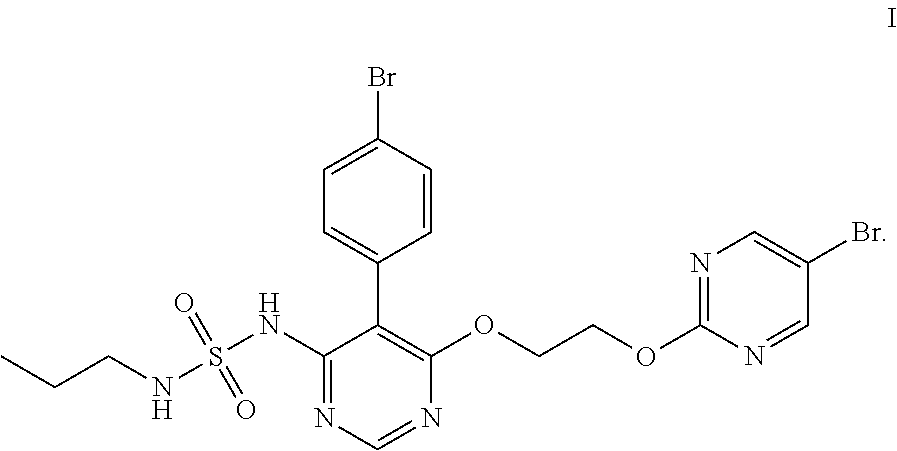
The invention discloses a method for preparing macitentan. The method comprises the following steps: (1) carrying out a substitution reaction between a compound N-propanesulfonamide of a formula (II) and a compound 5-(4-bromophenyl)-4,6-dichloropyrimidine of a formula (III) so as to convert into a compound of a formula (IV); (2) carrying out a substitution reaction between the compound of the formula (IV) and a compound of a formula (V) in the presence of a strong base, thereby obtaining a compound of a formula (VI); and (3) carrying out a substitution reaction between the compound of the formula (VI) and a compound of a formula (VII) in the presence of a strong base, thereby obtaining the compound (macitentan) of the formula (I). The process conditions of the method for preparing macitentan are mild, the reaction time is greatly shortened, the after-treatment is simple, the purity is high, the reaction cost is low, and the industrial production is easily realized.
Owner:NANJING CORE TECH CO LTD
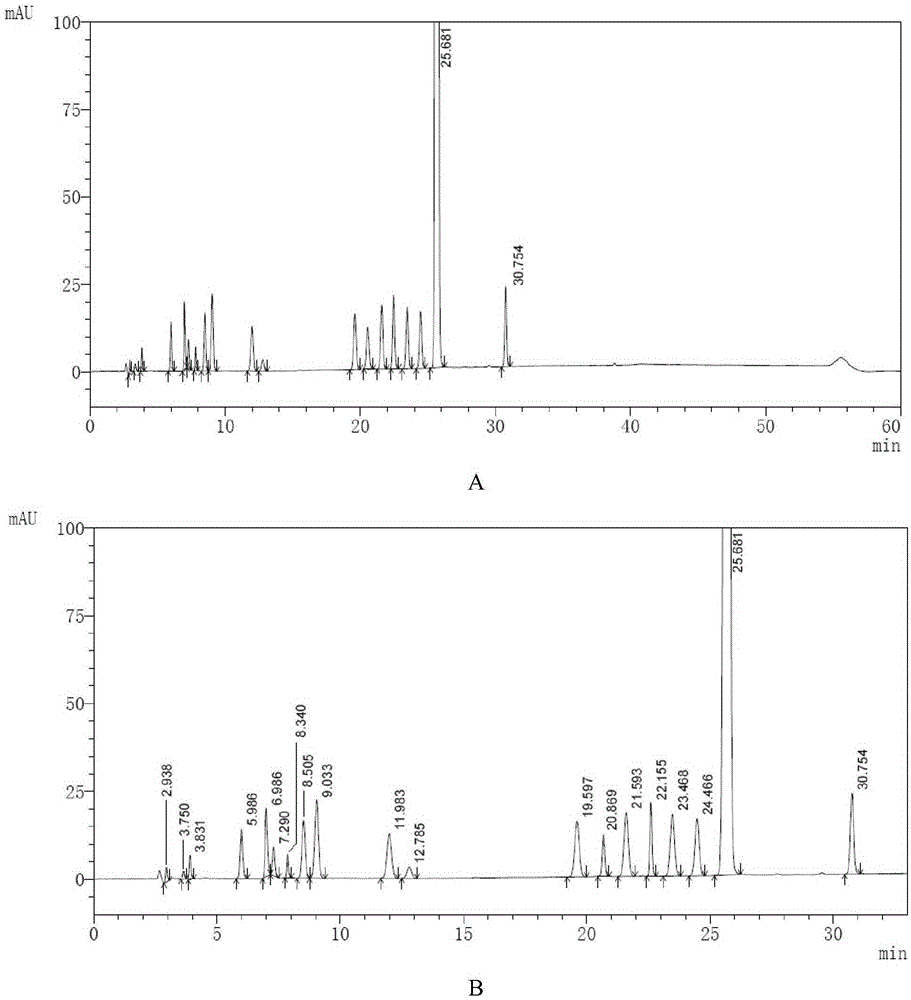
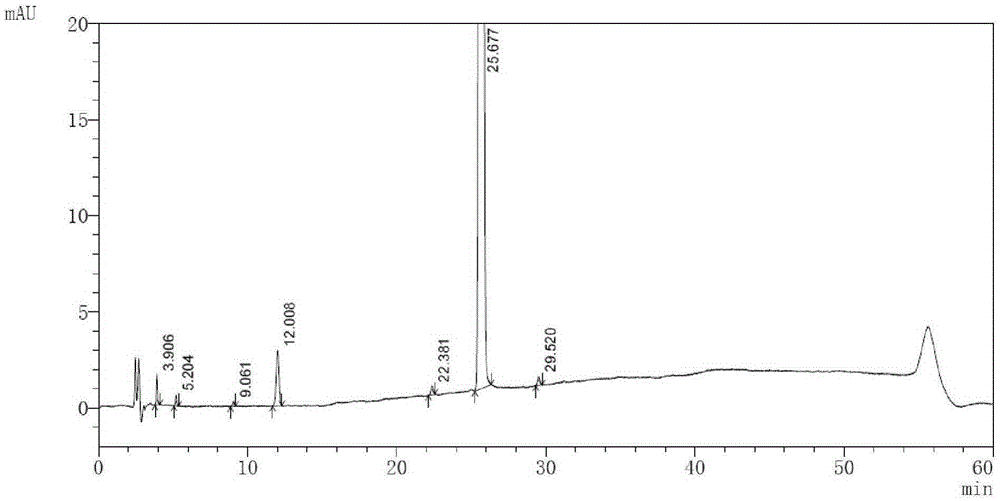
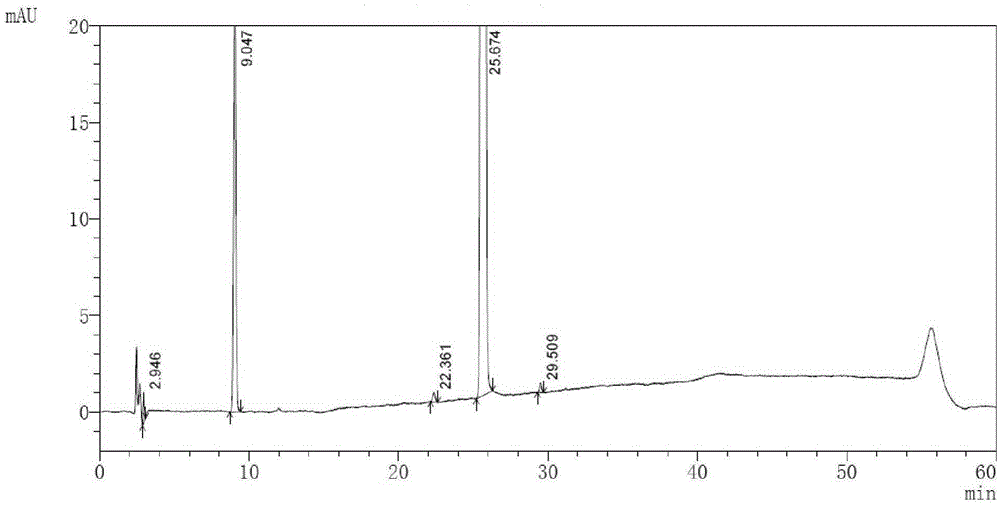
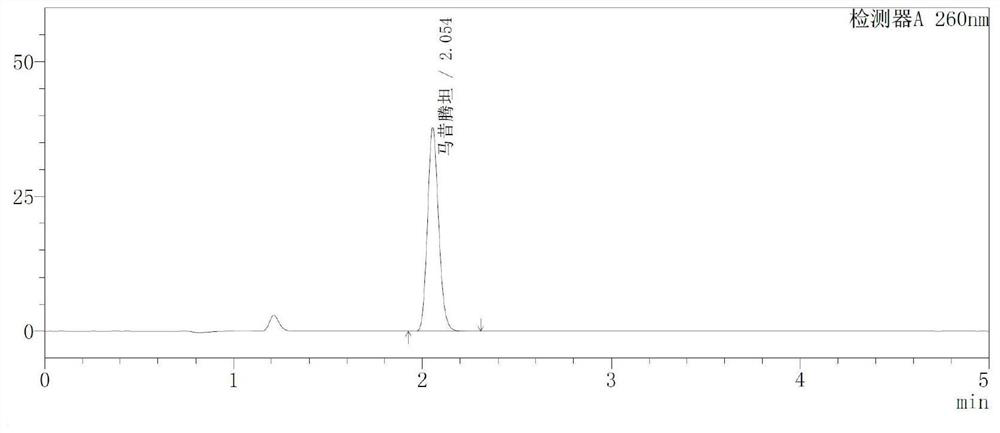
High performance liquid chromatography method of macitentan related substances
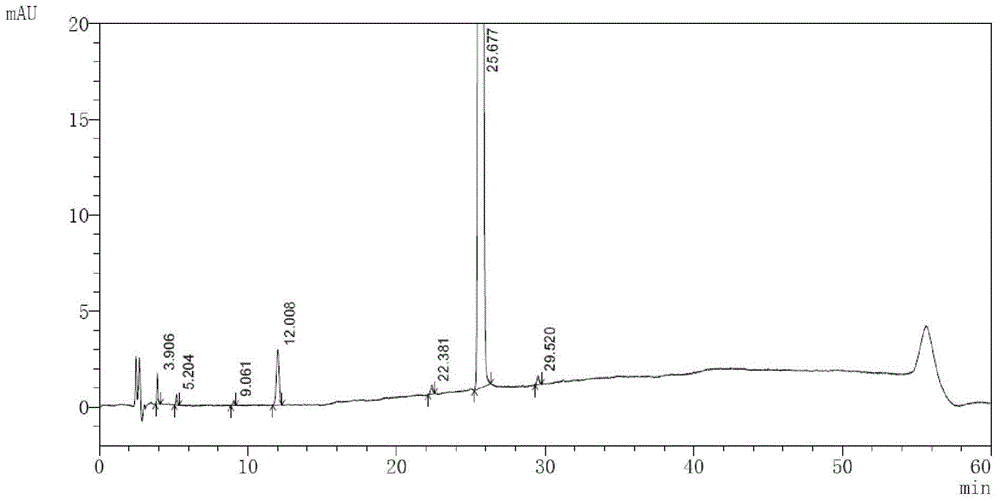
The invention discloses a high performance liquid chromatography method of macitentan related substances. The method adopts a reversed phase chromatographic column and an ultraviolet detector, and adopts acetonitrile-water-formic acid as a mobile phase to carry out gradient elution. The method can be used to simultaneously analyze all known impurities in a macitentan raw material and preparations thereof, and also allows the content of the known impurities to be effectively controlled through a correction factor-containing main component self-contrasted technology, the resolution among all impurity peaks and the resolution between a main peak and an adjacent impurity peak are respectively greater than 1.5, and the peak purities of the main peak and all the impurity peaks are 1.0. The method is a simple and reliable analysis method for quality control of the macitentan raw material and the preparations thereof.
Owner:合肥启旸生物科技有限公司
Method for preparing macitentan
InactiveCN105272923ARaw materials are cheap and easy to getReduce manufacturing costOrganic chemistryChemistryMacitentan
The invention discloses a method for preparing macitentan. The method comprises a following synthesis route. As a result of experiments, the method provided by the invention has the advantages of simple operation, mild reaction conditions, low requirement on equipment, inexpensive and easy-to-obtain raw materials, and low production cost. The method can be easily applied in large-scale productions. The method meets the requirements of macitentan industrialized production, and has industrial application value.
Owner:重庆瑞泊莱医药科技有限公司
Method for refining high-purity macitentan
The invention discloses a method for refining high-purity macitentan. The method comprises the steps of heating and dissolving crude macitentan by using a newly-boiled solvent in the absence of light, adding active carbon to perform decoloration for 5-15 min, performing filter pressing while the solution is hot, cooling filtrate to 45-55 DEG C in the absence of light and performing crystallization for 1-2 h with stirring, performing cooling to 20-30 DEG C to perform crystallization for 1-2 with stirring, and performing filtration and drying to obtain pure macitentan. The method has the advantages that the method is simple and convenient to operate and high in yield and the product is pure, the yield is 85% or above, and the purity of pure macitentan is 99.9% or above (according to high performance liquid chromatography (HPLC) detection).
Owner:HEFEI JIUNUO MEDICAL TECH
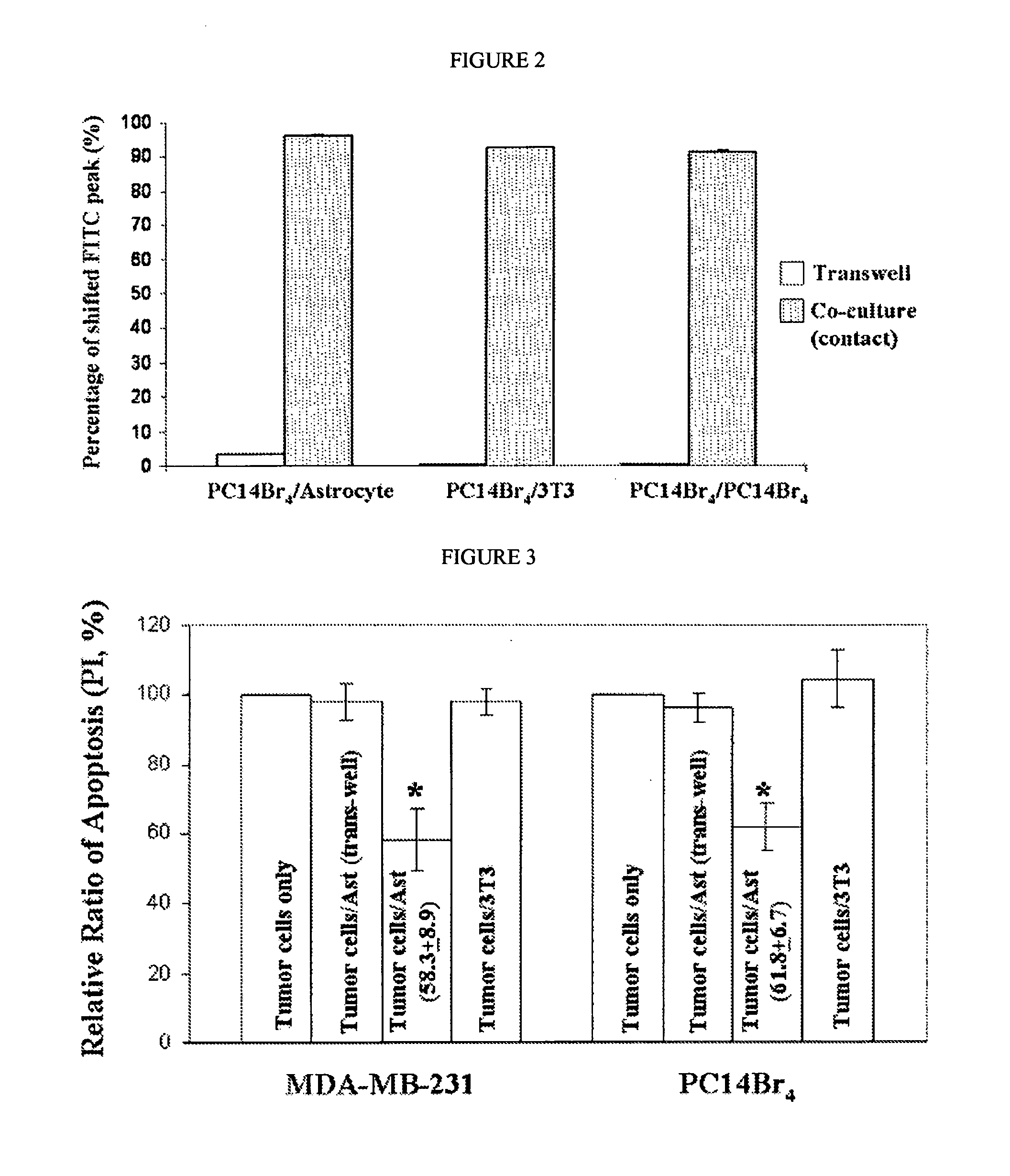
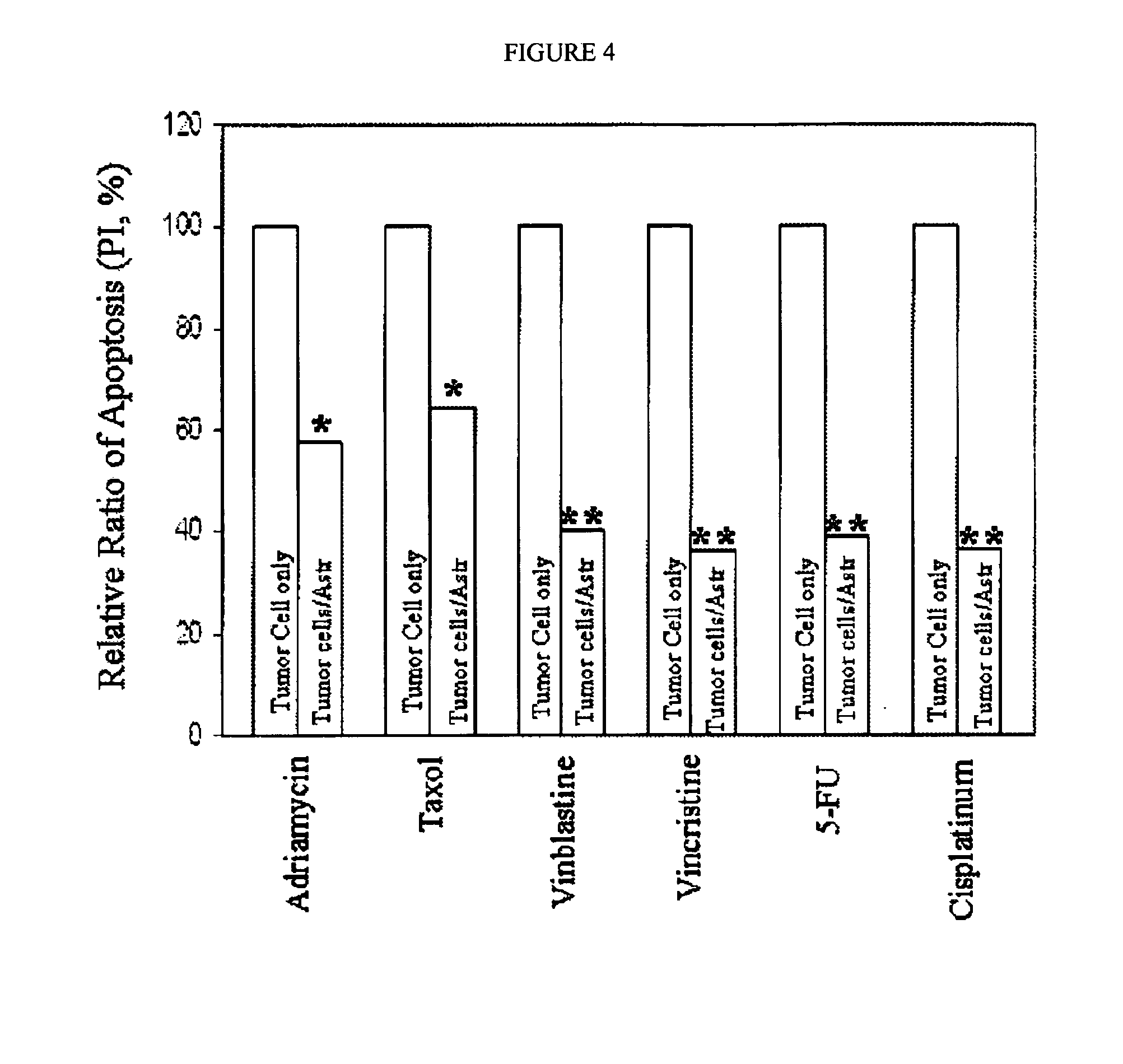
Treatment of astrocytes-tumor cells inhibitors of endothelin receptors
InactiveUS20150352113A1Maintain steady stateProtect neuronsOrganic active ingredientsBiocideCytotoxicityEndothelin receptor antagonist
The disclosure relates to an endothelin receptor antagonist for use in the prevention or treatment of brain metastases in combination with a cytotoxic chemotherapy agent, radiotherapy or both. The endothelin receptor antagonist may for example be bosentan, macitentan or a mixture of bosentan and macitentan.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Detection method for macitentan intermediate and application thereof
ActiveCN104897833AEasy to operateFast dissolutionComponent separationQuantitative determinationAnalysis method
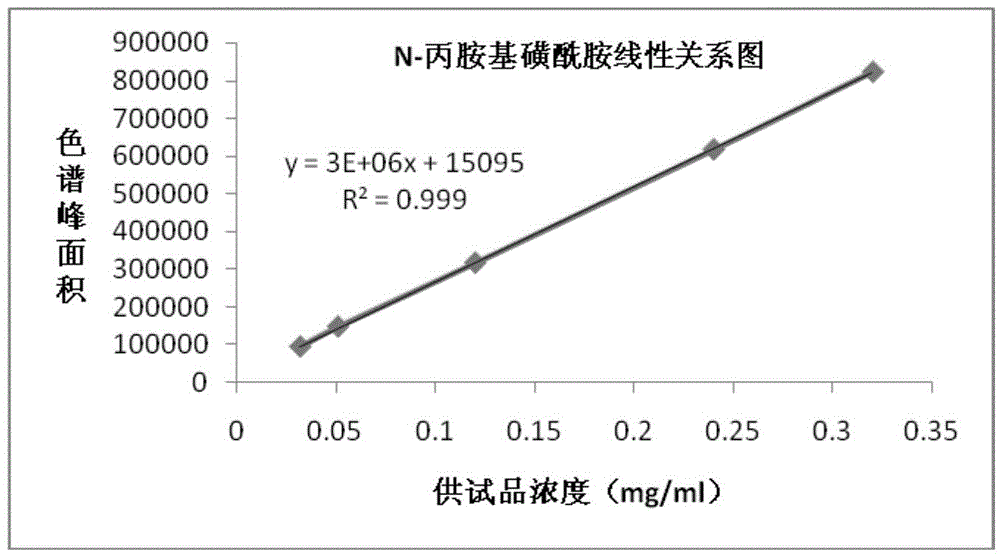
The invention provides a detection method for a macitentan intermediate and application thereof. The intermediate is N-propylamino sulfonamide or its salt. The method is characterized in that it employs high performance liquid chromatography to conduct detection, the detector is an evaporative light scattering detector (ELSD), and the mobile phase contains an alkaline additive of certain proportion. The invention also provides application of the detection method. The detection method provided by the invention can fully separate the main component from other materials very well, and can accurately determine the content of impurities. The method has the advantages that: the sample dissolving speed is fast, the same can be completely dissolved within 1min, and the analysis time is generally within 30min; preparation of the mobile phase is simple in operation; the N-propylamino sulfonamide detection limit of the method is 15.75ng, and the sensitivity is high; with the method, the recovery rate of N-propylamino sulfonamide is more than 90%, and the average recovery rate can reach 98.16%, thus meeting the quantitative determination requirements of N-propylamino sulfonamide. In addition, the prepared solution is stable when it is determined within 36h. In summary, the analysis method is stable and reliable, and has high sensitivity.
Owner:CHENGDU CLIMB PHARMA TECH
Method for preparing macitentan impurity standard substance
InactiveCN106478522ASimple preparation processShort synthesis cycleOrganic chemistryEtherConventional analysis
The invention discloses a method for preparing a macitentan impurity standard substance. The method includes the steps of using 5-(4-bromophenyl)-4,6-dichloropyrimidine as a raw material, conducting condensation of 5-(4-bromophenyl)-4,6-dichloropyrimidine, N-propanesulfonamide and ethylene glycol to prepare a 1,2-di[[N-[5-(4-bromophenyl)]-N'-propanesulfonamide-6-oxo]pyrimidyl] ethyl diether crude product, refining the crude product to obtain a pure product, and calibrating the content of the pure product by a conventional analysis method. The method has a simple process and a short preparation period, and the calibrated product content is more than 99.0%. The provided macitentan impurity can serve as the impurity standard substance and be applied to qualitative and quantitative research and detection of impurities in macitentan raw materials and preparations thereof.
Owner:HEFEI JIUNUO MEDICAL TECH
Macitentan crystal and its preparation method, its pharmaceutical composition and use
ActiveCN104411691BSuitable for useOrganic active ingredientsSenses disorderPharmaceutical drugCombinatorial chemistry
The present invention relates to a new crystalline form of macitentan, which has advantages in terms of solubility. The present invention also relates to the preparation method of the new crystal form, its pharmaceutical composition and its use in the preparation of medicines for treating hypertension and pulmonary arterial hypertension.
Owner:倪云
Macitentan preparation method
ActiveCN107868055ANot easy to accumulateQuality improvementOrganic chemistryBiochemical engineeringProcess engineering
The invention relates to a Macitentan preparation method. According to the preparation method, the process is simple, convenient and easy to control, reaction selectivity is good, product quality is high, and the method is suitable for industrial production.
Owner:SEASONS BIOTECHNOLOGY (TAIZHOU) CO LTD
Macitentan crystal, preparation method thereof, pharmaceutical composition and application thereof
ActiveCN105693624AOrganic active ingredientsSenses disorderPharmaceutical drugCombinatorial chemistry
The invention relates to a novel crystal form of macitentan. The novel crystal has advantages on the aspect of solubility. The invention also relates to a preparation method of the novel crystal form, a pharmaceutical composition thereof and an application of the pharmaceutical composition in preparation of drugs for treating hypertension and pulmonary hypertension.
Owner:倪云
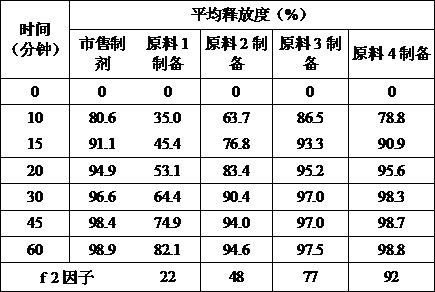
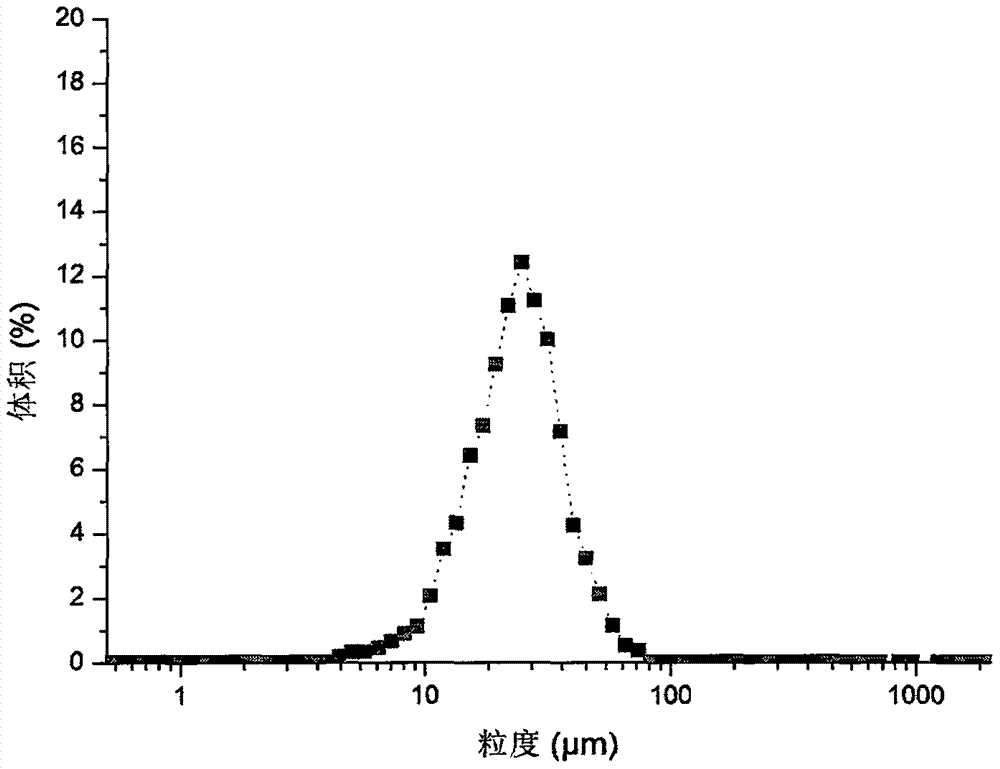
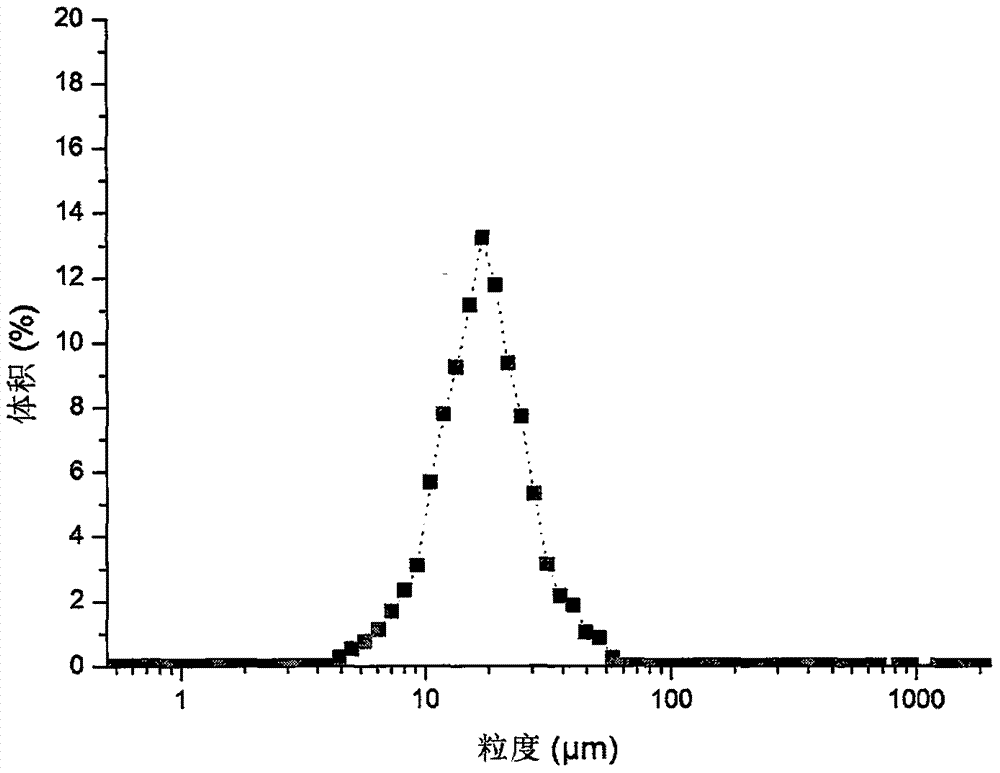
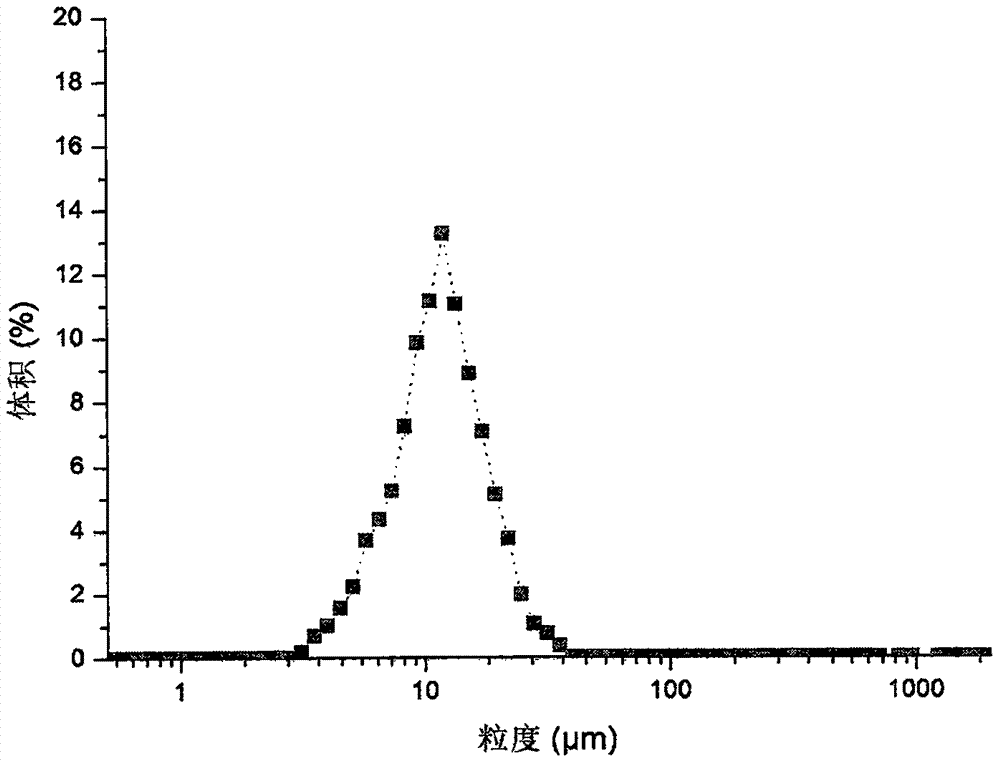
Method for rapidly controlling and evaluating release of macitentan tablet
InactiveCN112336693AImprove product qualityIncreased production requirementsOrganic active ingredientsComponent separationFluid phaseProcess engineering
The invention discloses an important method for rapidly controlling and evaluating the release of a macitentan tablet. The method comprises the following steps that the macitentan tablet is simply prepared, and the release speed is mainly influenced by controlling the particle size of a bulk drug and a dissolution medium; in the particle size range, the particle size of raw materials is preferablyabout 10-50 um; the dissolution medium is preferably selected from 0.02%-0.1% of hexadecyl trimethyl ammonium bromide; and high performance liquid chromatography is adopted for determination, and determination is completed within 3 minutes. The quality of the macitentan tablet meets the standard, the macitentan tablet has release similarity with similar preparations sold in the market, quality research and control are carried out before production, rework can be effectively reduced, the production cost is saved, energy consumption is reduced, and the production efficiency is improved.
Owner:南京斯泰尔医药科技有限公司
Macitentan microsphere and preparation method thereof
InactiveCN106924190AOrganic active ingredientsPharmaceutical non-active ingredientsMicrosphereOil phase
The invention provides a macitentan microsphere preparation and a preparation method thereof. The macitentan microsphere provided by the invention consists of macitentan, a polymer carrier material, an emulsifier and a stabilizer. The preparation method of the macitentan microsphere preparation provided by the invention comprises the following steps: (1) dissolving the polymer carrier material and the macitentan in an oil-phase solvent so as to obtain an oil phase, and taking a water solution of the emulsifier as an aqueous phase; (2) adding the oil phase to the aqueous phase and conducting homogenizing so as to obtain an O / W emulsion; (3) adding the emulsion to a water solution of the stabilizer, so that the solvent of the oil phase completely volatilizes; and (4) separating, washing and drying the microsphere so as to obtain the microsphere preparation provided by the invention.
Owner:HYBIO PHARMA
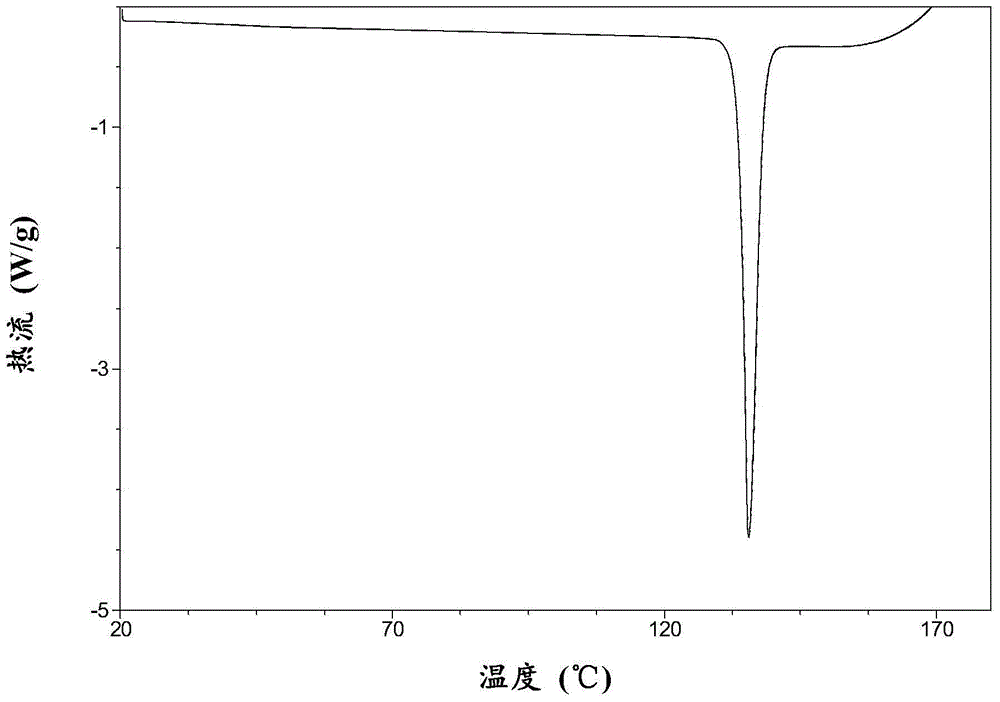
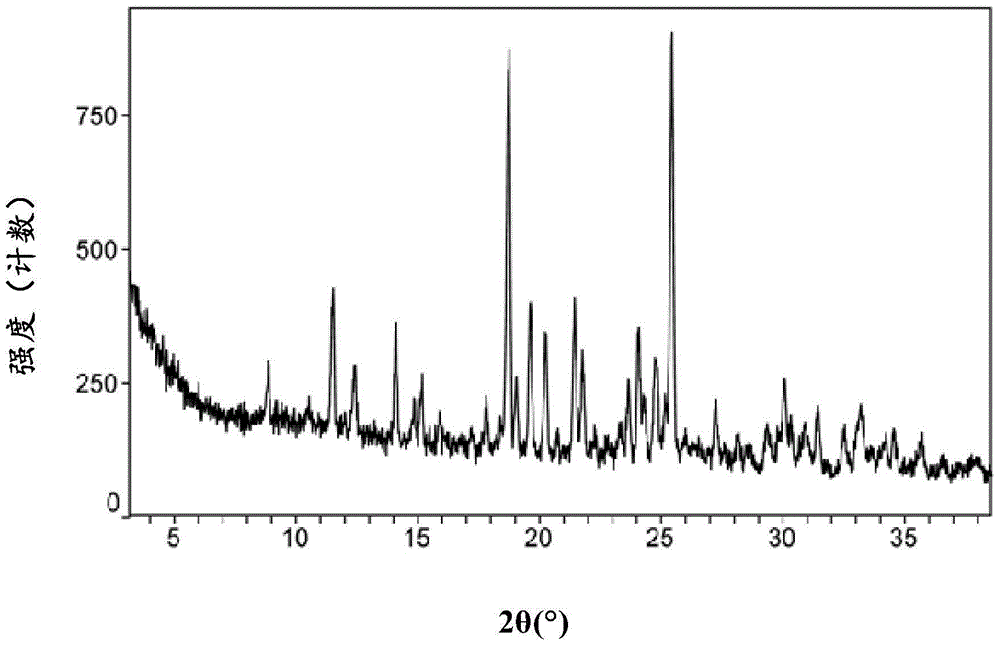
Macitentan crystal form and preparation method thereof
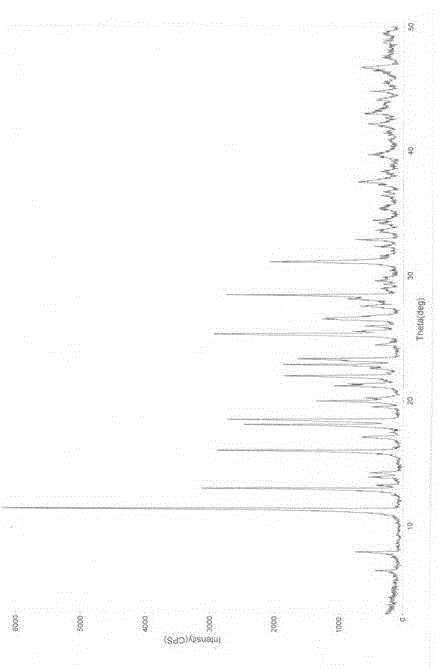
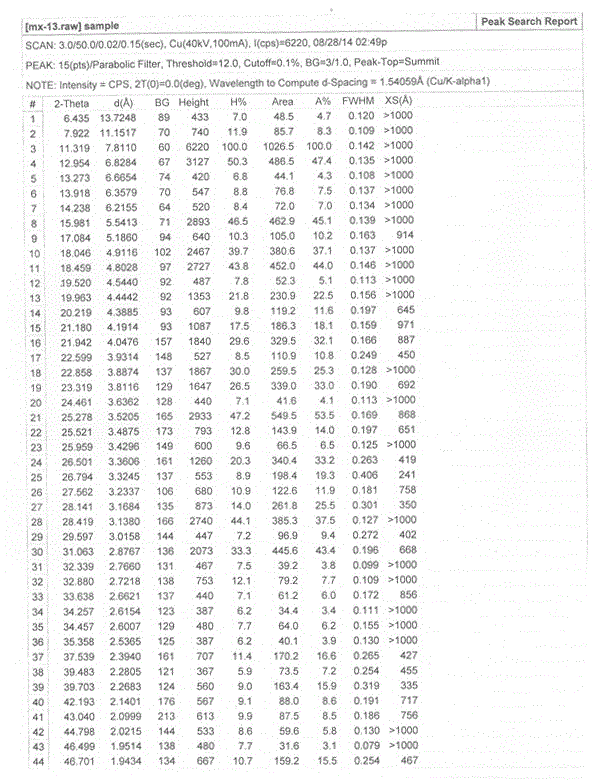
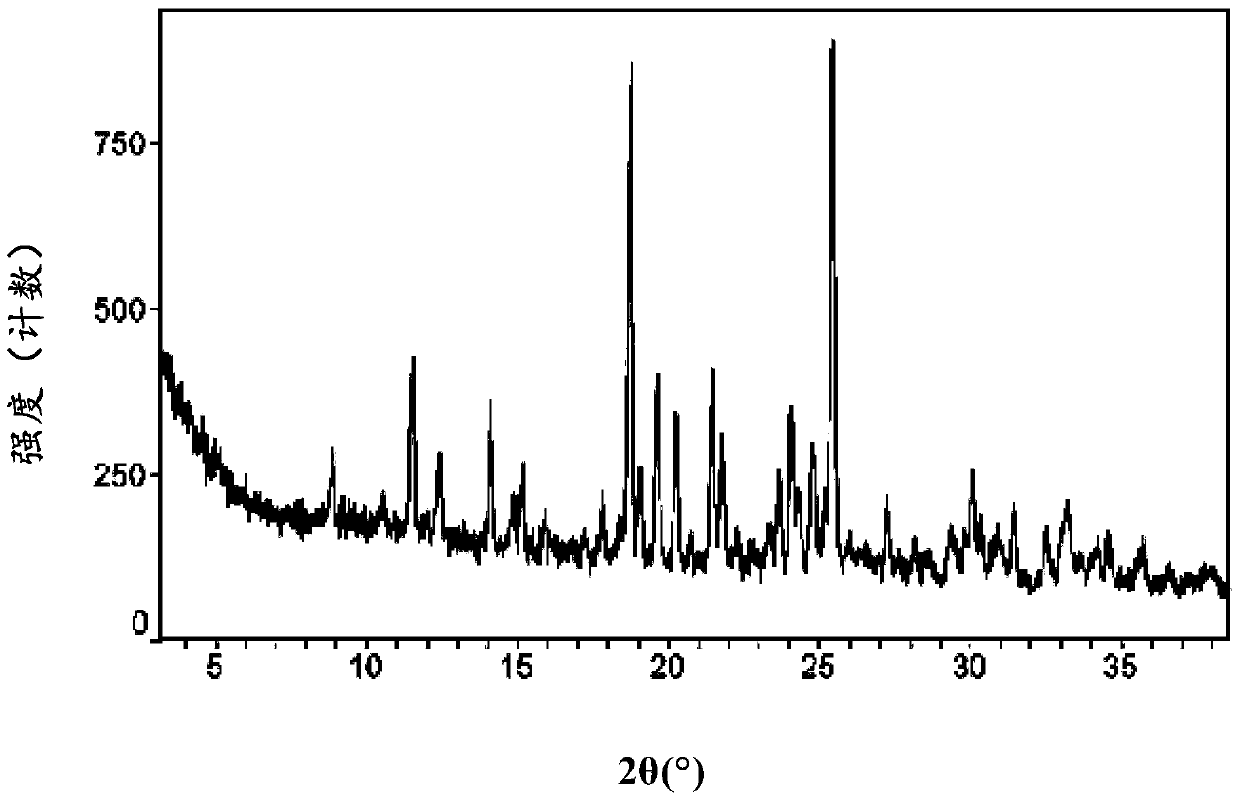
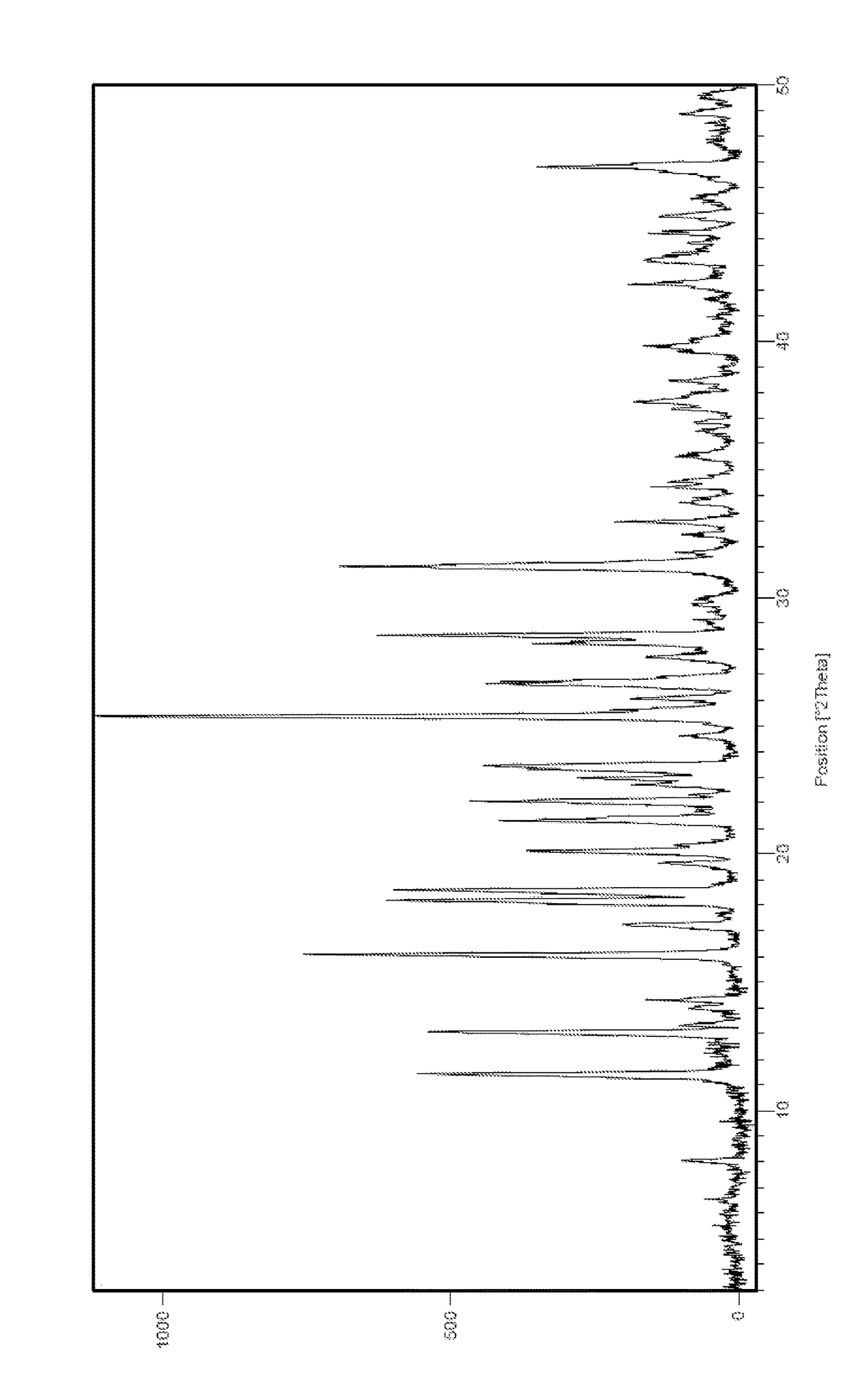
InactiveCN105461637AGood crystal stabilityEasy to operateOrganic active ingredientsOrganic chemistryCondensed matter physicsPowder diffraction
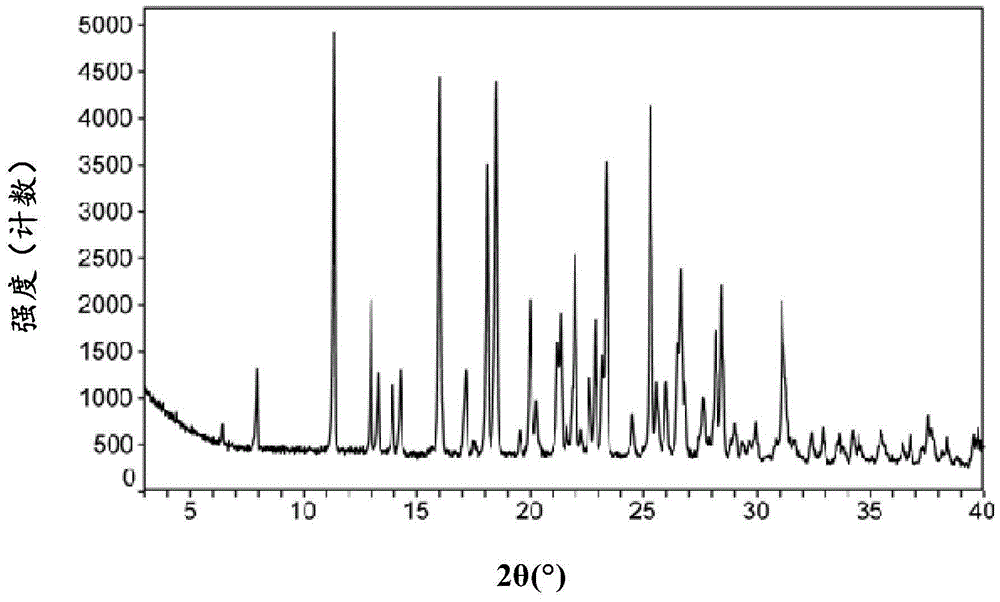
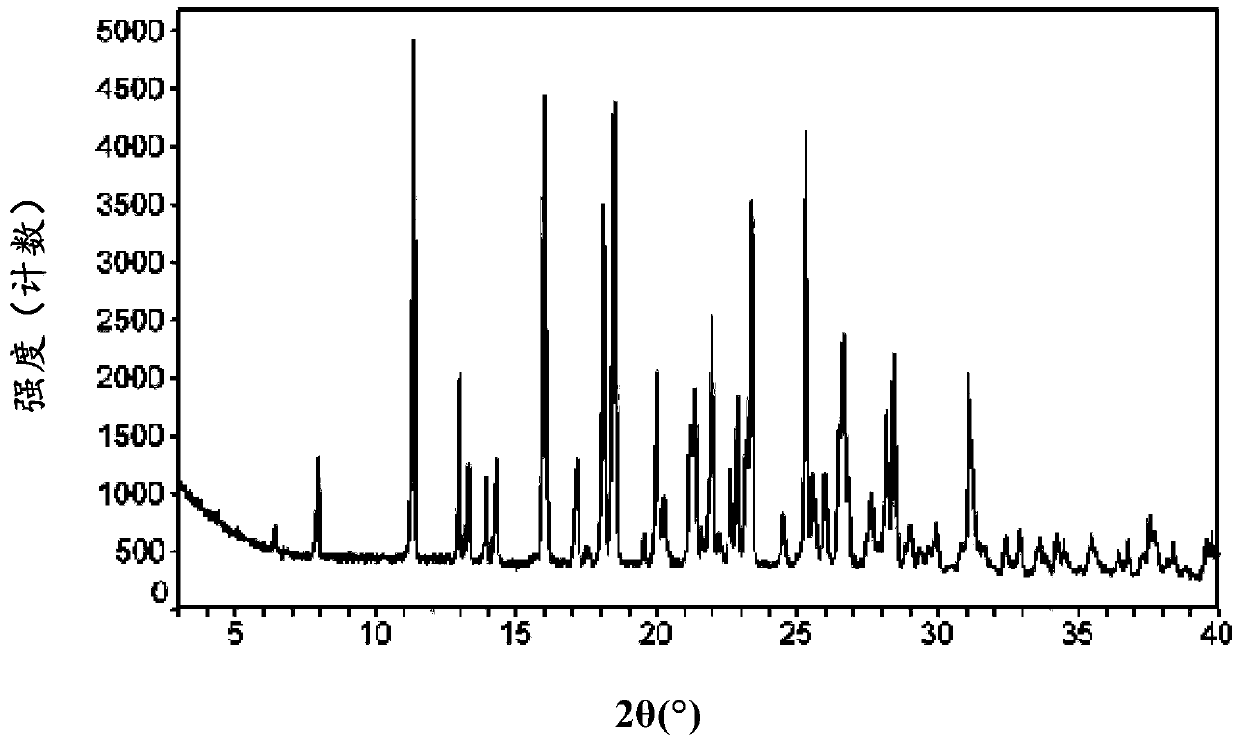
The present invention relates to a macitentan crystal form and a preparation method thereof, wherein the characteristic absorption peaks exist when the diffraction angle (2[theta]) is 11.32+ / -0.2, 12.94+ / -0.2, 15.98+ / -0.2, 18.05+ / -0.2, 18.46+ / -0.2, 21.94+ / -0.2, 23.32+ / -0.2, 25.28+ / -0.2, 26.50+ / -0.2, 28.42+ / -0.2, and 31.06+ / -0.2 in the X-ray powder diffraction pattern of the crystal form. The macitentan crystal form prepared by using the method of the present invention has characteristics of good stability, simple operation, easy production, and easy storage.
Owner:天津市医药集团技术发展有限公司
Topical ophthalmic formulations of endothelin receptor antagonists
ActiveUS20180110728A1Organic active ingredientsSenses disorderDiabetes mellitusEndothelin receptor antagonist
The present invention relates to a topical ophthalmic formulation comprising at least one antagonist of the endothelin receptor, preferably selected from sitaxentan, ambrisentan, atrasentran, bosentan, macitentan and tezosentan, or a mixture thereof, more preferably bosentan. It also relates to the use of a topical ophthalmic formulation comprising at least one antagonist of the endothelin receptor, preferably selected from sitaxentan, ambrisentan, atrasentran, bosentan, macitentan and tezosentan, or a mixture thereof, more preferably bosentan, as active ingredient for preventing and / or treating the retinal neurodegeneration induced by diabetes and / or aging.
Owner:RETINSET SL
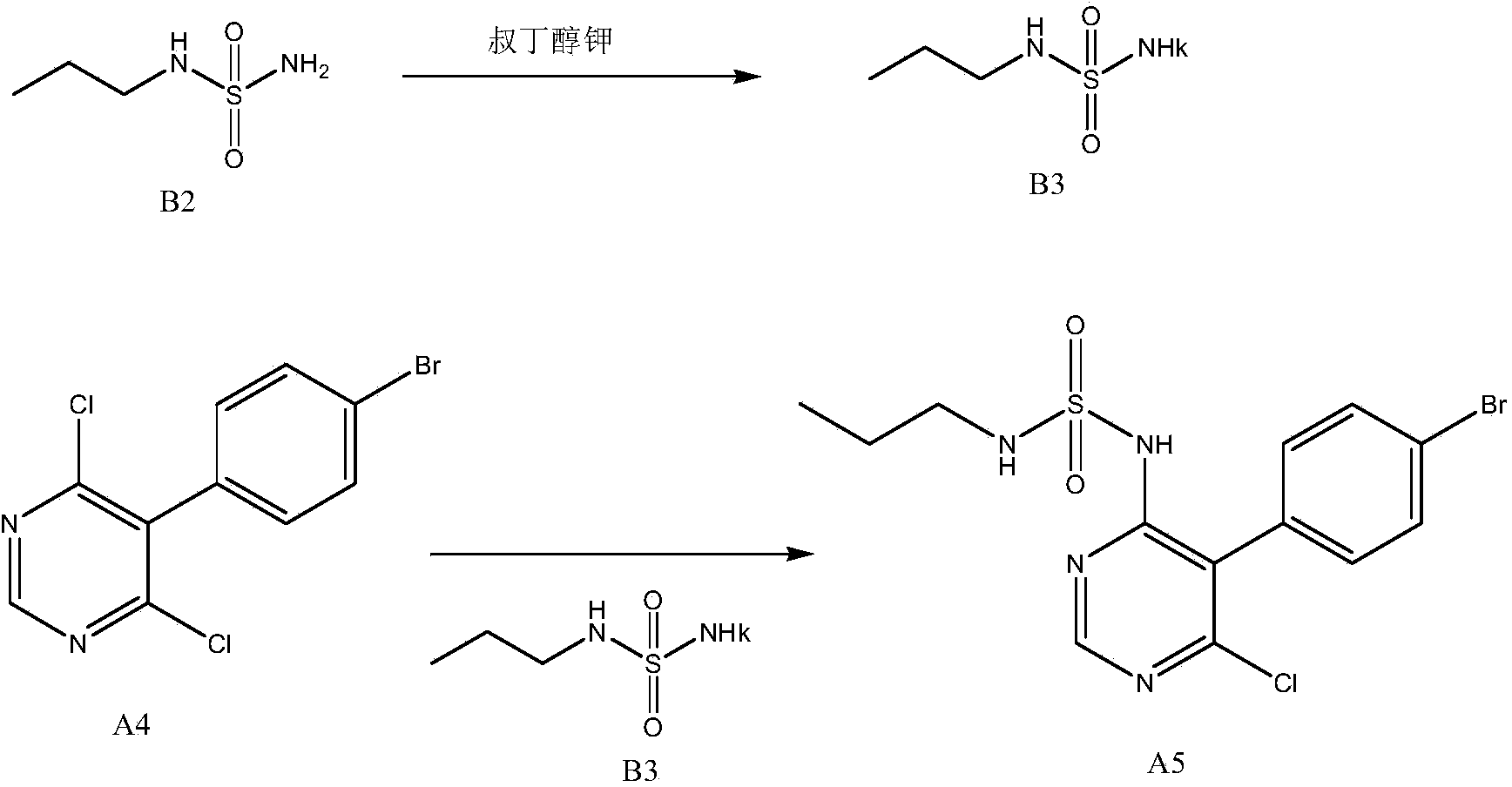
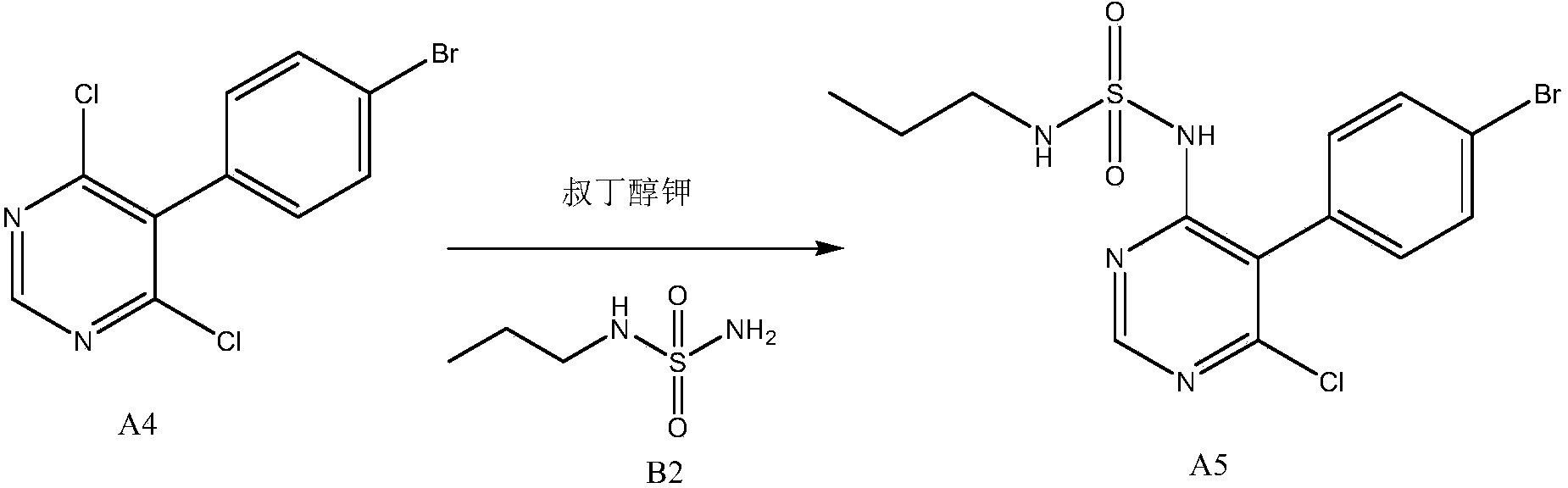
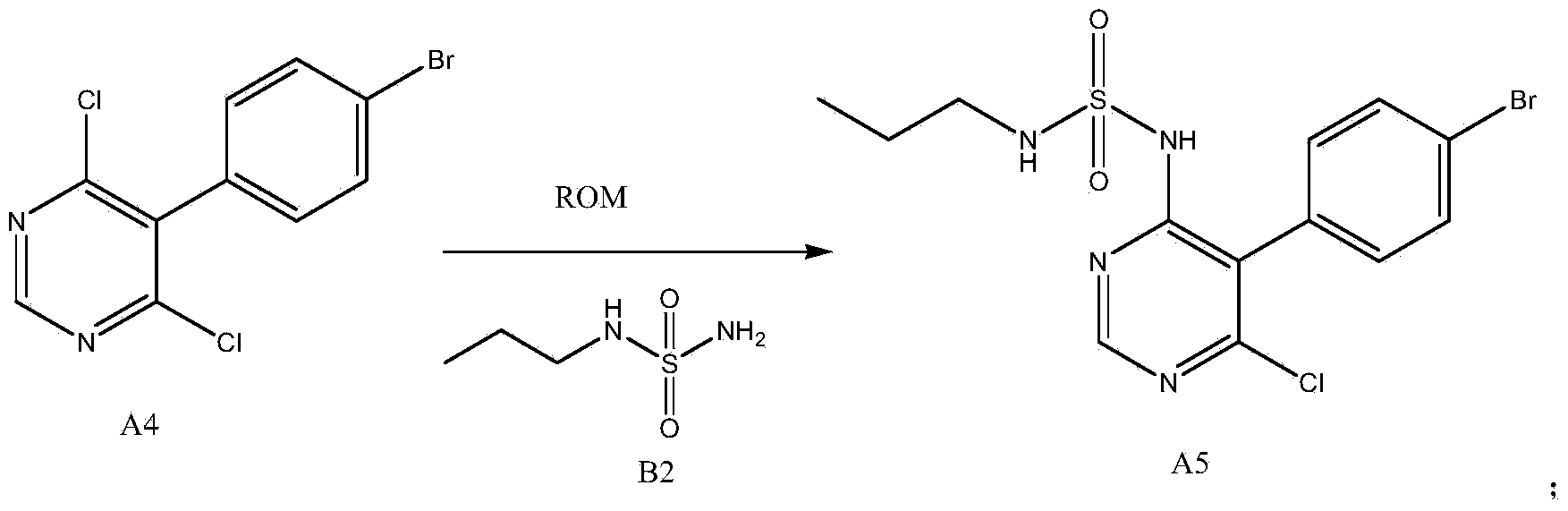
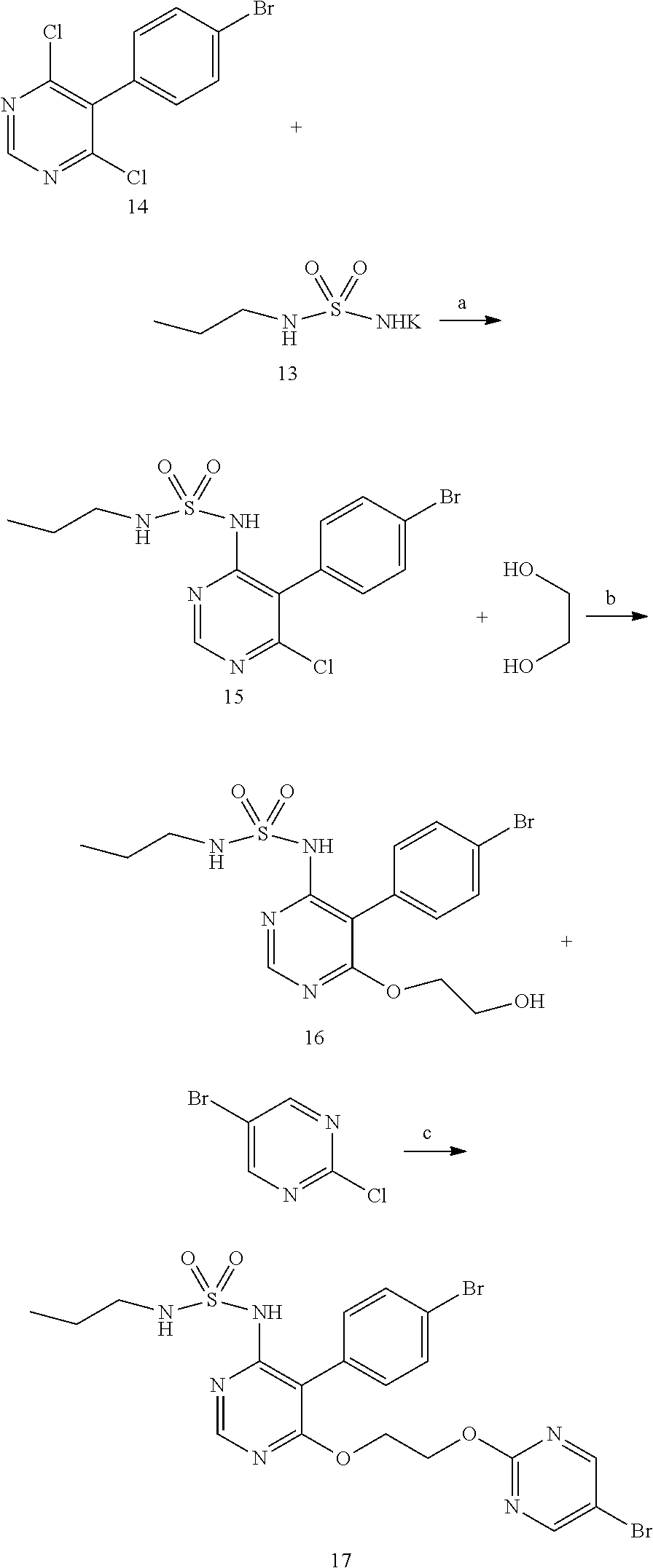
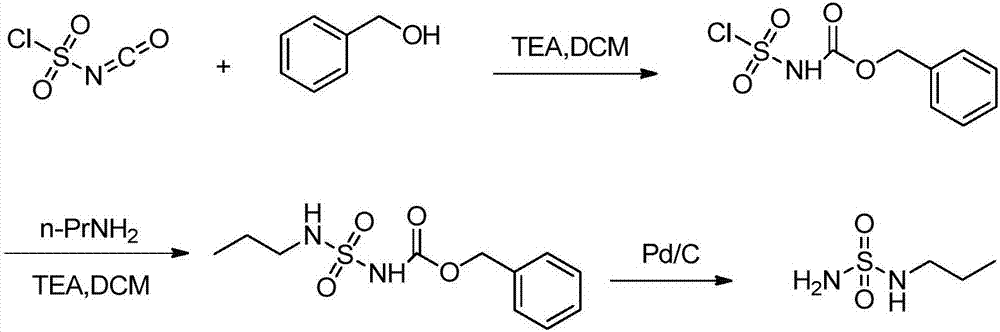
New preparation method of macitentan intermediate
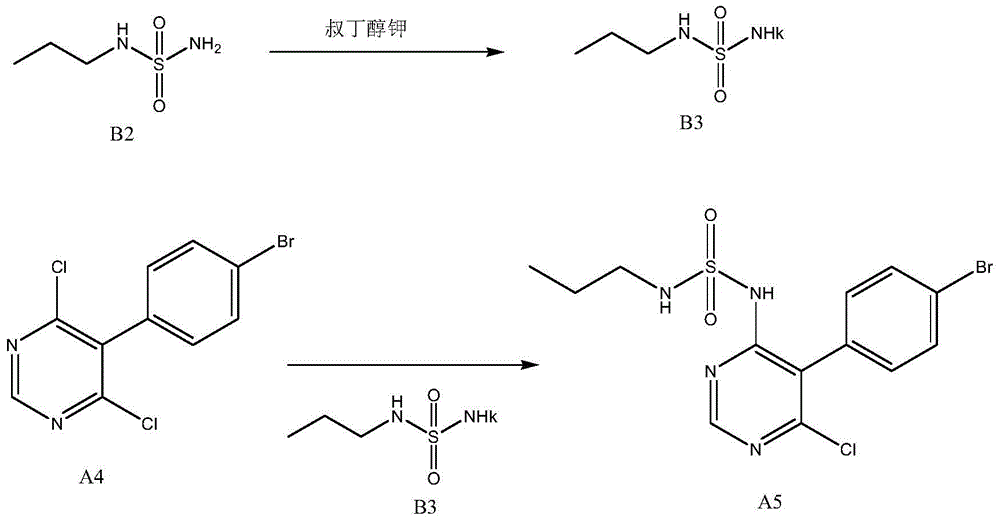
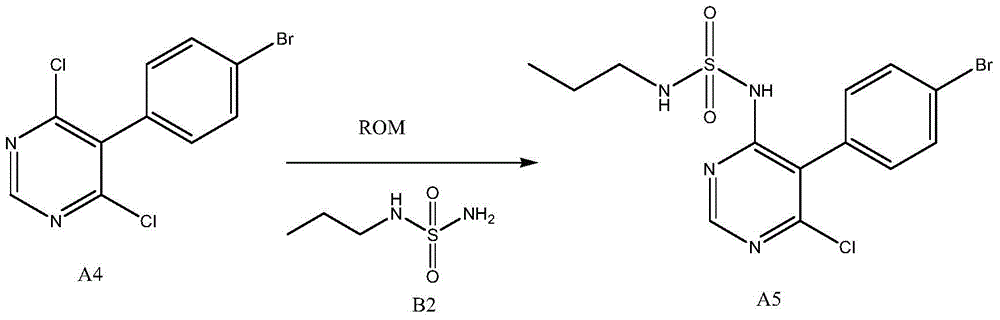
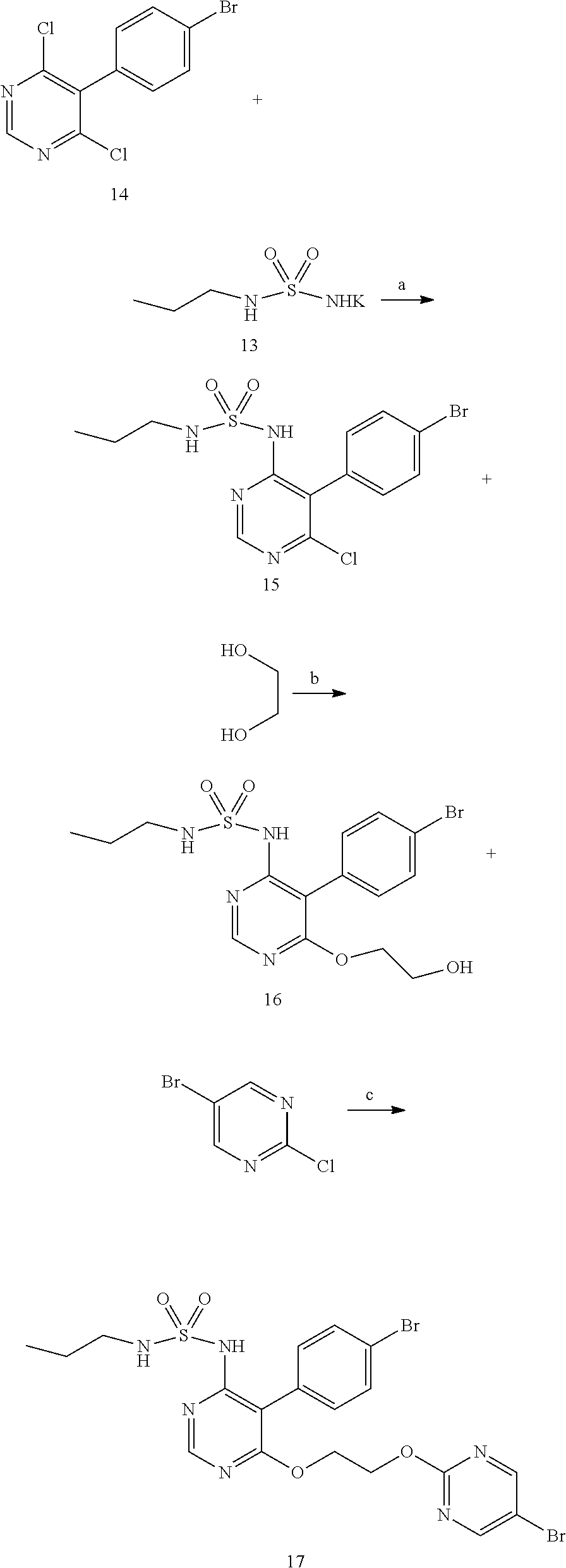
The invention discloses a new preparation method of a macitentan intermediate, wherein the intermediate is N-(5-(4-bromophenyl)-6-chloro-4-pyrimidyl)-N'-propylsulfamide; the new preparation method comprises the steps of adding N-propylsulfamide and 5-(4-bromophenyl)-4,6-dichloropyrimidine to dimethyl sulfoxide, and then adding a metal alkoxide ROM, and stirring for reacting to obtain the N-(5-(4-bromophenyl)-6-chloro-4-pyrimidyl)-N'-propylsulfamide. The synthetic process of the new preparation method is simplified, and also the production efficiency is also improved virtually and the dosage of a solvent is reduced, and therefore, the new preparation method is more applicable to popularization and application at home and abroad. The yield is 51% higher than a documentary value, and the purity of the product is higher; the step of salification is omitted, so that the production efficiency is improved and the dosage of the solvent is reduced, and as a result, the preparation method is more environmentally friendly.
Owner:CHENGDU CLIMB PHARMA TECH
An improved process for the preparation of macitentan
The present invention relates to an improved process for the preparation of macitentan and pharmaceutical acceptable salts thereof. Further present invention also relates to methylene chloride solvate of macitentan and their use in the preparation of pure macitentan.
Owner:LAURUS LABS
A kind of new preparation method of macitentan intermediate
The invention discloses a new preparation method of a macitentan intermediate, wherein the intermediate is N-(5-(4-bromophenyl)-6-chloro-4-pyrimidyl)-N'-propylsulfamide; the new preparation method comprises the steps of adding N-propylsulfamide and 5-(4-bromophenyl)-4,6-dichloropyrimidine to dimethyl sulfoxide, and then adding a metal alkoxide ROM, and stirring for reacting to obtain the N-(5-(4-bromophenyl)-6-chloro-4-pyrimidyl)-N'-propylsulfamide. The synthetic process of the new preparation method is simplified, and also the production efficiency is also improved virtually and the dosage of a solvent is reduced, and therefore, the new preparation method is more applicable to popularization and application at home and abroad. The yield is 51% higher than a documentary value, and the purity of the product is higher; the step of salification is omitted, so that the production efficiency is improved and the dosage of the solvent is reduced, and as a result, the preparation method is more environmentally friendly.
Owner:CHENGDU CLIMB PHARMA TECH
Macitentan for use in treating portopulmonary hypertension
PendingCN113795257AOrganic active ingredientsDigestive systemLiver transplantsPortopulmonary hypertension
The present invention provides methods for treating portopulmonary hypertension, comprising administering to a patient in need thereof, a therapeutically effective amount of macitentan. Preferably, the methods are clinically proven safe and / or effective. Also provided are methods of improving liver transplant perioperative mortality risk category, improving MELD exception eligibility, and reducing the risk of removal from a liver transplant waitlist in a patient with portopulmonary hypertension and liver disease, comprising administering to a patient in need thereof a therapeutically effective amount of macitentan.
Owner:ACTELION PHARM LTD
Macitentan solution for inhalation and preparation method of macitentan solution
PendingCN112569210AAccurate measurementGood curative effectOrganic active ingredientsDispersion deliveryPreservative freeInhalation
The invention relates to a macitentan solution for inhalation and a preparation method of the macitentan solution. The macitentan solution comprises an active ingredient macitentan, a stabilizer, an isoosmotic adjusting agent, a pH value adjusting agent and a solvent. The single dose specification of the macitentan solution for inhalation is 2ml, and the macitentan solution is accurate in meteringand good in curative effect; and the macitentan solution has the advantages of no preservatives, stable quality, few side reactions and the like.
Owner:INCREASEPHARM TIANJIN INST CO LTD
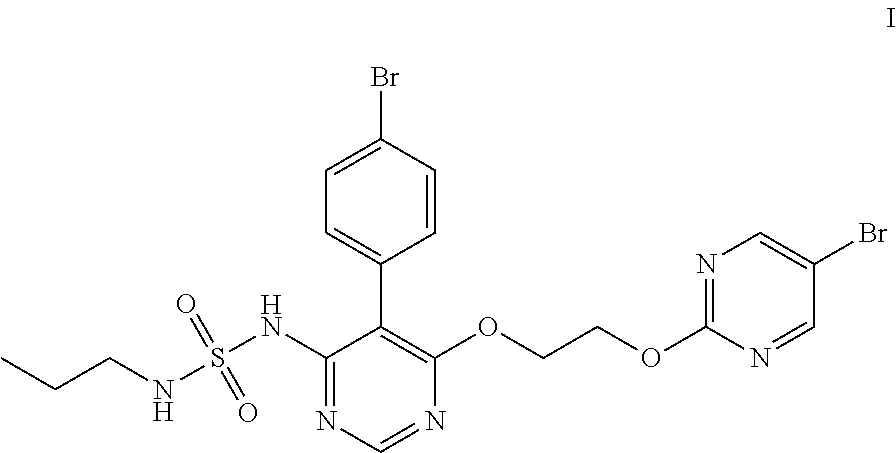
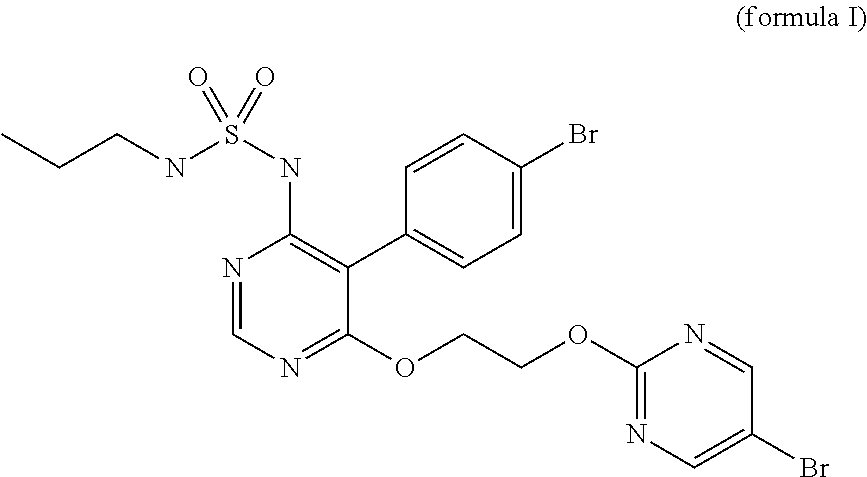
Macitentan related substances, and preparing methods and uses thereof
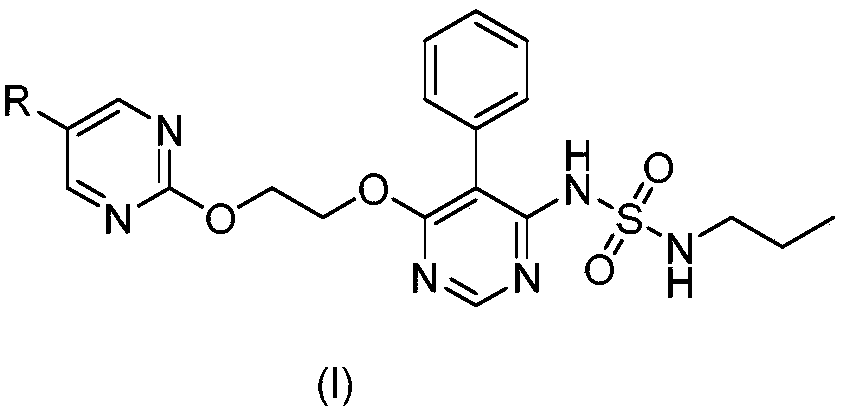
Macitentan related substances shown as a formula (I), uses thereof as macitentan impurity reference substances and a high performance liquid chromatography method for separation and measurement of macitentan and the compounds of the formula (I). Preparing methods provided are mild in reaction condition and simple in after-treatment, and can prepare the compounds of the formula (I) with purity meeting requirements in a large scale, with the compounds being adopted as impurity reference substances in macitentan quality analysis.
Owner:NANJING CHIA TAI TIANQING PHARMA
A kind of high performance liquid chromatography analysis method of macitentan related substances
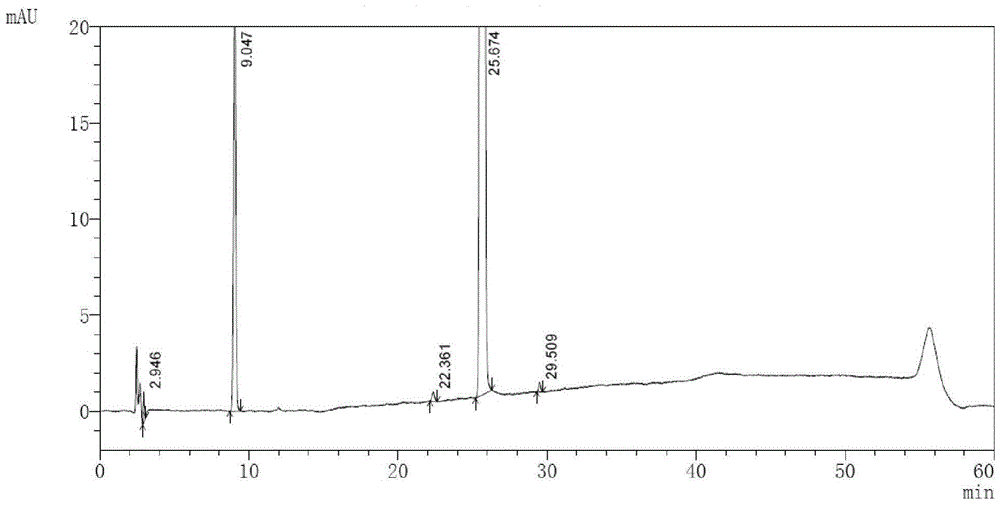
The invention discloses a high performance liquid chromatography method of macitentan related substances. The method adopts a reversed phase chromatographic column and an ultraviolet detector, and adopts acetonitrile-water-formic acid as a mobile phase to carry out gradient elution. The method can be used to simultaneously analyze all known impurities in a macitentan raw material and preparations thereof, and also allows the content of the known impurities to be effectively controlled through a correction factor-containing main component self-contrasted technology, the resolution among all impurity peaks and the resolution between a main peak and an adjacent impurity peak are respectively greater than 1.5, and the peak purities of the main peak and all the impurity peaks are 1.0. The method is a simple and reliable analysis method for quality control of the macitentan raw material and the preparations thereof.
Owner:合肥启旸生物科技有限公司
Macitentan crystal and its preparation method, its pharmaceutical composition and use
The present invention relates to a new crystalline form of macitentan, which has advantages in terms of solubility. The present invention also relates to the preparation method of the new crystal form, its pharmaceutical composition and its use in the preparation of medicines for treating hypertension and pulmonary arterial hypertension.
Owner:倪云
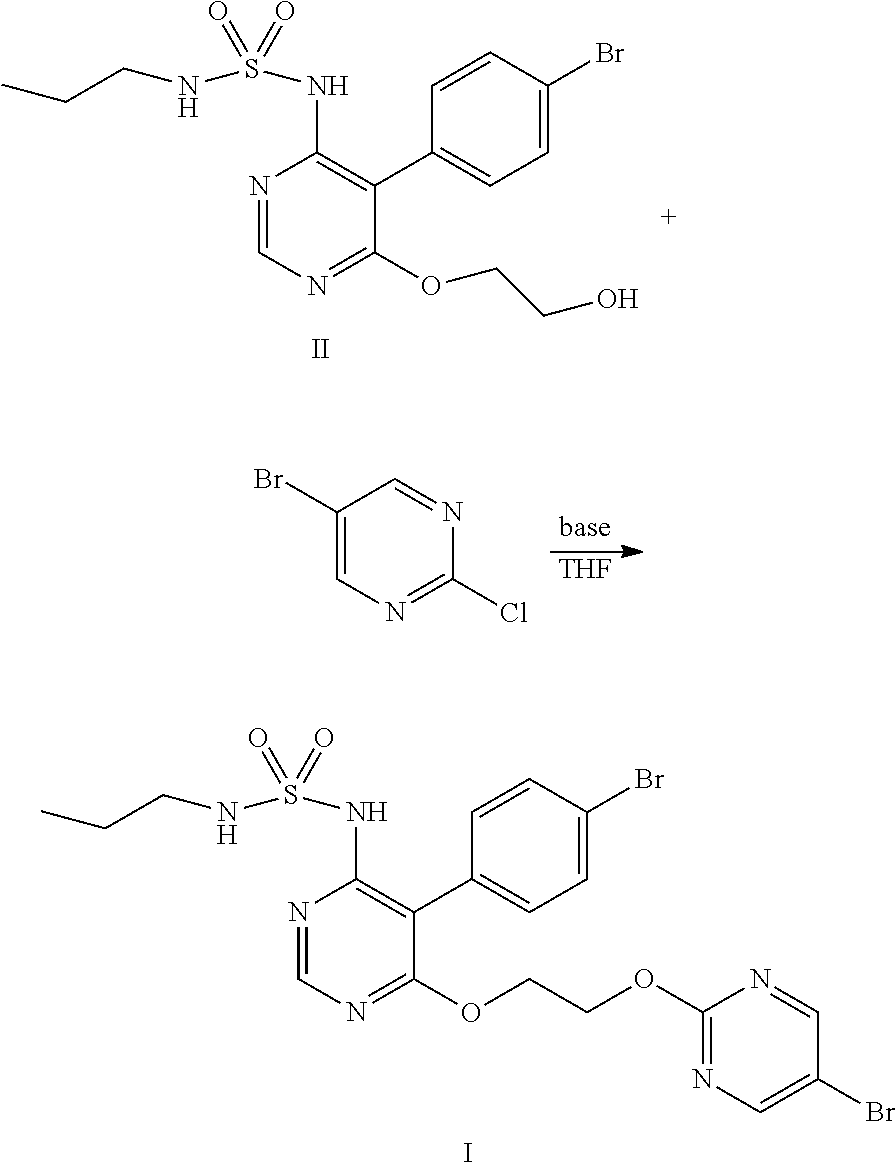
Method for preparing macitentan and intermediate compound thereof
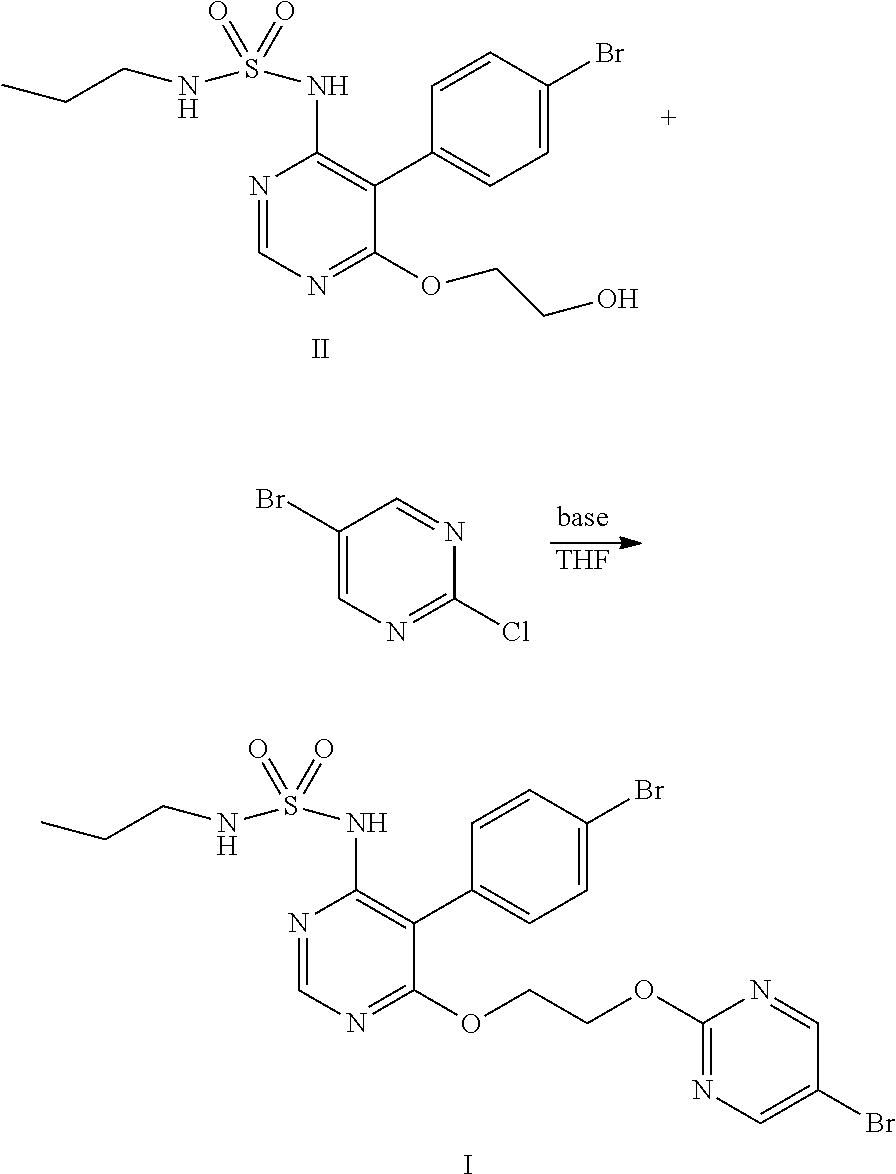
The present invention relates to technical field of chemical synthesis of drugs, and provides a preparation method of Macitentan and intermediate compound thereof. Adding THF solution containing compound II and 5-bromo-2-chloropyrimidine slowly into THF solution containing base to react, or adding THF solution containing compound II and THF solution containing 5-bromo-2-chloropyrimidine slowly at the same time into THF solution containing base to react and obtain Macitentan (shown as compound I), wherein the base is selected from sodium hydride, potassium hydride, lithium hydride or lithium bis(trimethylsilyl)amide. The selectivity of the preparation method is very good, which is suitable for industrial production. The obtained product Macitentan has good quality and high yield. And the product compound II also has good quality and high yield, its HPLC purity is up to 99.0%, the content of impurity A is less than 0.20%, the content of impurity B is less than 0.25%.
Owner:SEASONS BIOTECHNOLOGY (TAIZHOU) CO LTD
Method for preparing macitentan and intermediate compound thereof
ActiveUS20210300880A1Easy to controlGood reaction selectivityOrganic chemistryChemical synthesisTrimethylsilyl
The present invention relates to technical field of chemical synthesis of drugs, and provides a preparation method of Macitentan and intermediate compound thereof. Adding. THF solution containing compound II and 5-bromo-2-chloropyrimidine slowly into THF solution containing base to react, or adding THF solution containing compound II and THF solution containing 5-bromo-2-chloropyrimidine slowly at the same time into THF solution containing base to react and obtain Macitentan (shown as compound I), wherein the base is selected from sodium hydride, potassium hydride, lithium hydride or lithium bis(trimethylsilyl)amide. The selectivity of the preparation method is very good, which is suitable for industrial production. The obtained product Macitentan has good quality and high yield. And the product compound II also has good quality and high yield, its HPLC purity is up to 99.0%, the content of impurity A is less than 0.20%, the content of impurity B is less than 0.25%.
Owner:SEASONS BIOTECHNOLOGY (TAIZHOU) CO LTD
Preparation method of macitentan intermediate
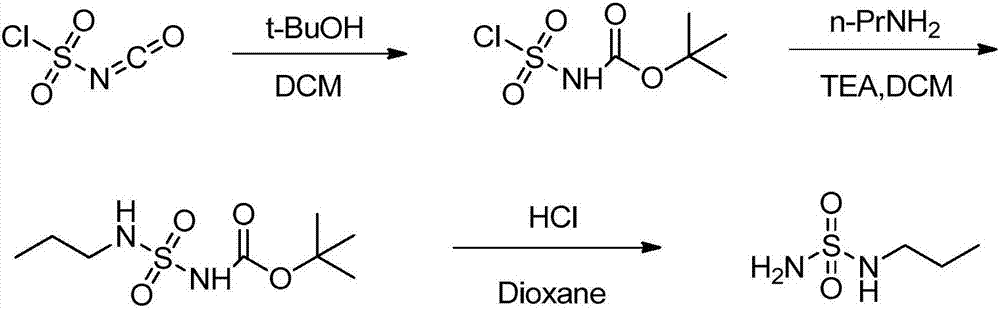
InactiveCN107141238AEasy to operateReactive shortSulfuric acid amide preparationMacitentanSulfonyl chloride
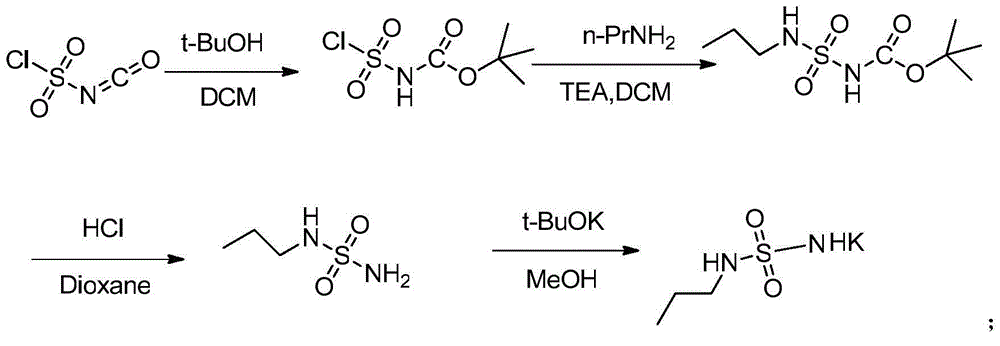
The invention discloses a preparation method of n-propylamine sulfonamide serving as a macitentan intermediate. The preparation method comprises the following steps: by taking n-propylamine and sulfonyl chloride as start raw materials and taking triethylamine as an acid-binding agent, preparing an n-propylamine sulfonamide coarse product through two-step reaction, and performing post-treatment distillation, so as to obtain the n-propylamine sulfonamide. According to the preparation method disclosed by the invention, raw materials which are low in cost and readily available are used; reactions and operations in all the steps are simple; the reaction steps and the reaction time are short; the n-propylamine sulfonamide is easy to popularize, high in yield, low in cost and suitable for industrial large-scale production.
Owner:吴宁怡
Pharmaceutical composition containing crystalline macitentan
ActiveUS9730932B2Good chemical stabilityAvoiding undesired conversionOrganic active ingredientsFlexible coversFree baseMacitentan
Owner:SANDOZ AG
Preparation method of small-particle-size macitentan
The invention relates to a preparation method of small-particle-size macitentan, and belongs to the technical field of raw material medicine preparation. According to the preparation method of the small-particle-size macitentan, firstly, an macitentan crude product is dissolved in an isopropanol-n-heptane mixed solvent, and filtering is conducted; then obtained filter liquid is cooled to 5 to 15 DEG C, the temperature is maintained, under stirring, acetone with volume 0.8-1.2 times of that of the solvent in the first step is added in a flowing mode, stirring speed is 200-230 rpm, the speed ofadding the acetone in a flowing mode is 8-12 ml / min, and after flowing adding is completed, stirring is conducted continuously for 3-5 hours; filtering is conducted, and drying is conducted at 50-60 DEG C. The invention provides the preparation method of the small-particle-size macitentan. The prepared macitentan D90 can be controlled to be between 3.5 micrometers and 38.06 micrometers, and a rawmaterial medicine which can be used directly is provided for an macitentan preparation.
Owner:WEIHAI YUNRUI INFORMATION TECH CO LTD
Preparation method of macitentan related substances
ActiveCN106279043BEfficient preparationHigh quality preparationOrganic chemistryPharmaceutical SubstancesBiology
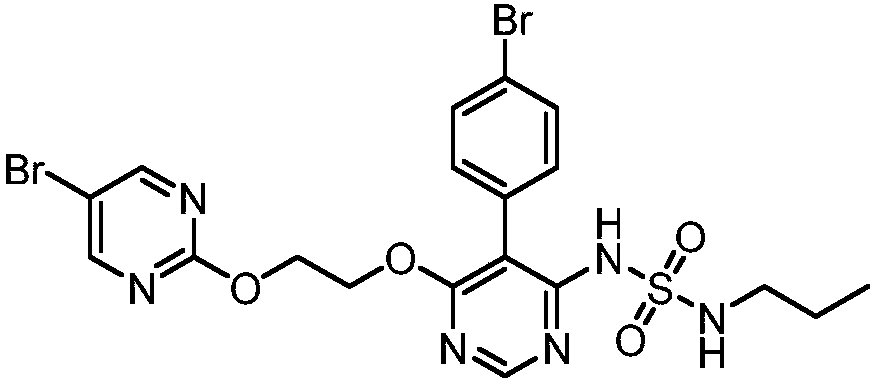
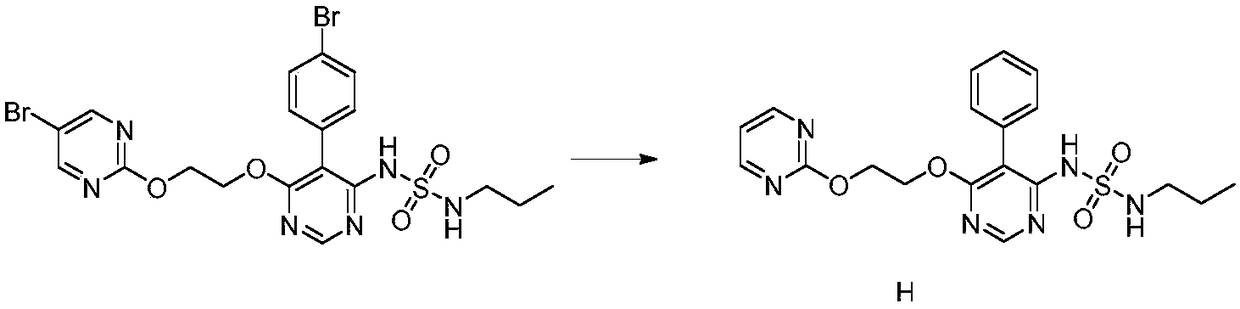
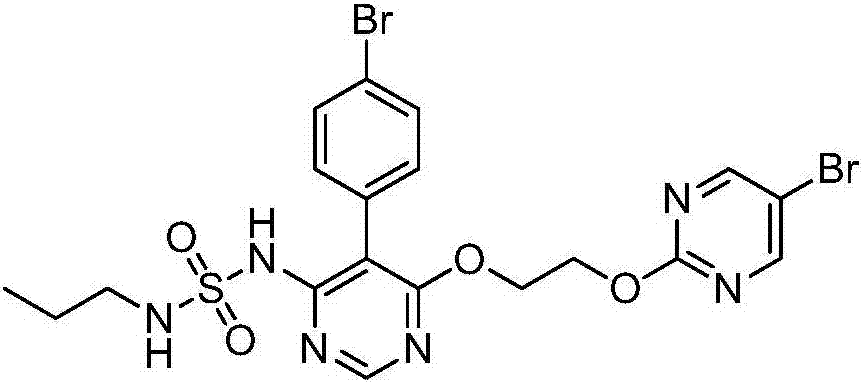
The invention discloses a method for preparing a macitentan related substance. Specifically, the invention relates to a method for preparing a metabolic product (a compound of a formula I) of a pulmonary hypertension-resistant drug macitentan (N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidyl)oxo] ethyoxyl]-4-pyrimidyl]-N'-propanesulfonamide). The method comprises the following steps: carrying out a heating reaction on macitentan, and obtaining a target compound. According to the method, the target product can be efficiently prepared at high purity. The structural formula is as shown in the specification.
Owner:JIANGSU HANSOH PHARMA CO LTD
Topical ophthalmic formulations of endothelin receptor antagonists
The present invention relates to a topical ophthalmic formulation comprising at least one antagonist of the endothelin receptor, preferably selected from sitaxentan, ambrisentan, atrasentran, bosentan, macitentan and tezosentan, or a mixture thereof, more preferably bosentan. It also relates to the use of a topical ophthalmic formulation comprising at least one antagonist of the endothelin receptor, preferably selected from sitaxentan, ambrisentan, atrasentran, bosentan, macitentan and tezosentan, or a mixture thereof, more preferably bosentan, as active ingredient for preventing and / or treating the retinal neurodegeneration induced by diabetes and / or aging.
Owner:RETINSET SL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com