Macitentan preparation method
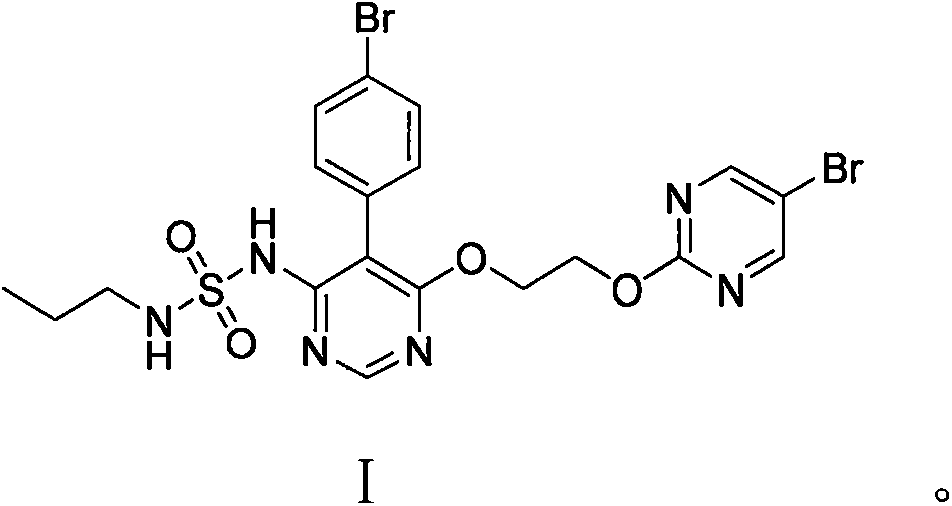
一种马西替坦、化合物的技术,应用在药物化学合成领域,能够解决增加溶剂回收和三废处理费用、不能满足原料药质量要求、产物马西替坦纯度低等问题,达到反应时间缩短、简化溶剂回收、后处理简单的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
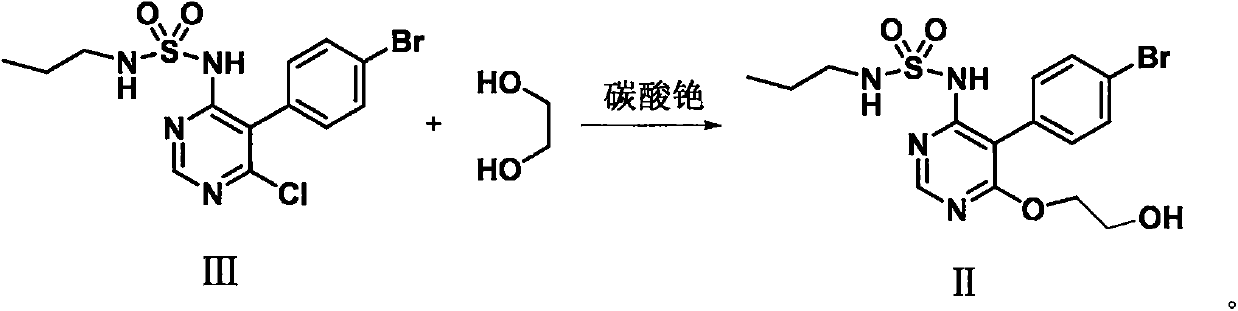
[0074] Example 1 The preparation of formula III compound
[0075] In a 10L flask, add 608g (2mol) of compound of formula IV, 880g (5mol) of potassium salt of compound of formula V and 5L of DMF, under nitrogen protection, heat up to 70°C, keep warm for 4 hours, recover 3.6L of DMF by distillation under reduced pressure, and the residue Add 3L of water, adjust the pH to 6-7 with hydrochloric acid, precipitate a solid, suction filter, dry and dehydrate the solid to obtain a crude product, recrystallize with 2L of acetonitrile to obtain 700g of the compound of formula III, a white solid, the molar yield is 86%, and the HPLC purity is 99.7% .
[0076] 1 H NMR (400MHz, CDCl 3 ): δ8.65(s, 1H), 7.68-7.74(m, 2H), 7.16-7.21(m, 2H), 6.90(s br, 1H), 5.52(s br, 1H), 2.94-3.03(m , 2H), 1.50-1.66 (m, 2H), 0.96 (t, J=7.6Hz, 3H).
Embodiment 2
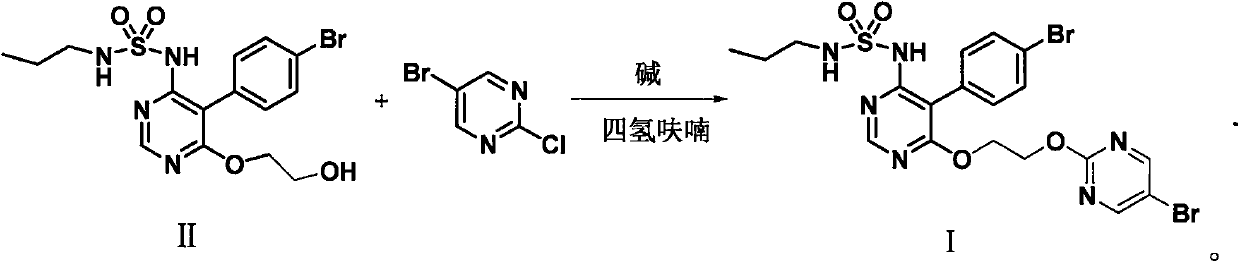
[0077] Example 2 The preparation of formula II compound
[0078] In a 2000mL flask, add 40.6g (0.1mol) of the compound of formula III prepared in Example 1 and 250mL of ethylene glycol, add 65g of cesium carbonate under stirring, raise the temperature to 130°C under nitrogen protection, and keep the reaction for 5 hours. Cool the reaction solution below 50°C, add 300mL water and 300mL ethyl acetate, adjust the pH to 2-3 with hydrochloric acid, separate the layers, wash the organic phase with 100mL water, separate the layers, concentrate the organic phase to dry the solvent, and wash the residue with 300mL acetic acid Isopropyl ester was recrystallized to obtain 38.8g of compound of formula II, HPLC purity: 99.4%, impurity A content: 0.15%, impurity B content: 0.20%, molar yield: 90%.
[0079] 1 H NMR (400MHz, CDCl 3 ): δ8.48(s, 1H), 7.61-7.67(m, 2H), 7.13-7.22(m, 2H), 5.70(s br, 1H), 4.47-4.51(m, 2H), 3.82-3.86( m, 2H), 2.98 (t, J = 7.0 Hz, 2H), 1.50-1.66 (m, 2H), 0.95 (...
Embodiment 3
[0080] Example 3 The preparation of formula II compound
[0081] In a 2000mL flask, add 40.6g (0.1mol) of the compound of formula III and 500mL of ethylene glycol, add 98g of cesium carbonate under stirring, raise the temperature to 110°C under nitrogen protection, and keep the reaction for 4 hours. Cool the reaction solution below 50°C, add 400mL water and 300mL ethyl acetate, adjust the pH to 2-3 with hydrochloric acid, separate the layers, wash the organic phase with 100mL water, separate the layers, concentrate the organic phase to dry the solvent, and wash the residue with 300mL methanol Recrystallized to obtain 39.6g of compound of formula II, HPLC purity: 99.5%, impurity A content: 0.12%, impurity B content: 0.20%, molar yield: 92%.
[0082] 1 H NMR (400MHz, CDCl 3 ): δ8.48(s, 1H), 7.61-7.67(m, 2H), 7.13-7.22(m, 2H), 5.70(s br, 1H), 4.47-4.51(m, 2H), 3.82-3.86( m, 2H), 2.98 (t, J = 7.0 Hz, 2H), 1.50-1.66 (m, 2H), 0.95 (t, J = 7.0 Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com