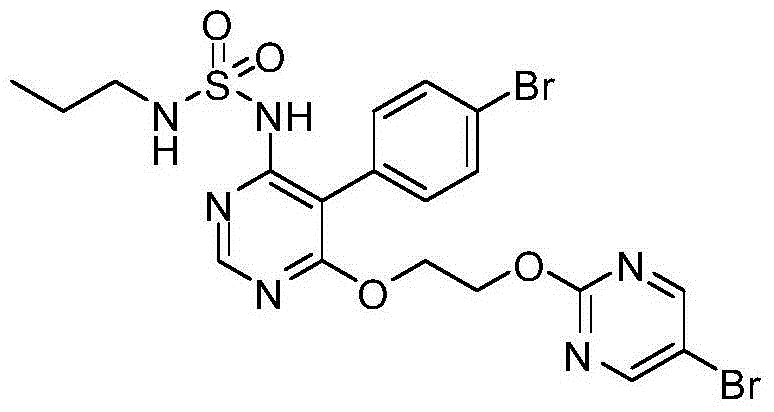
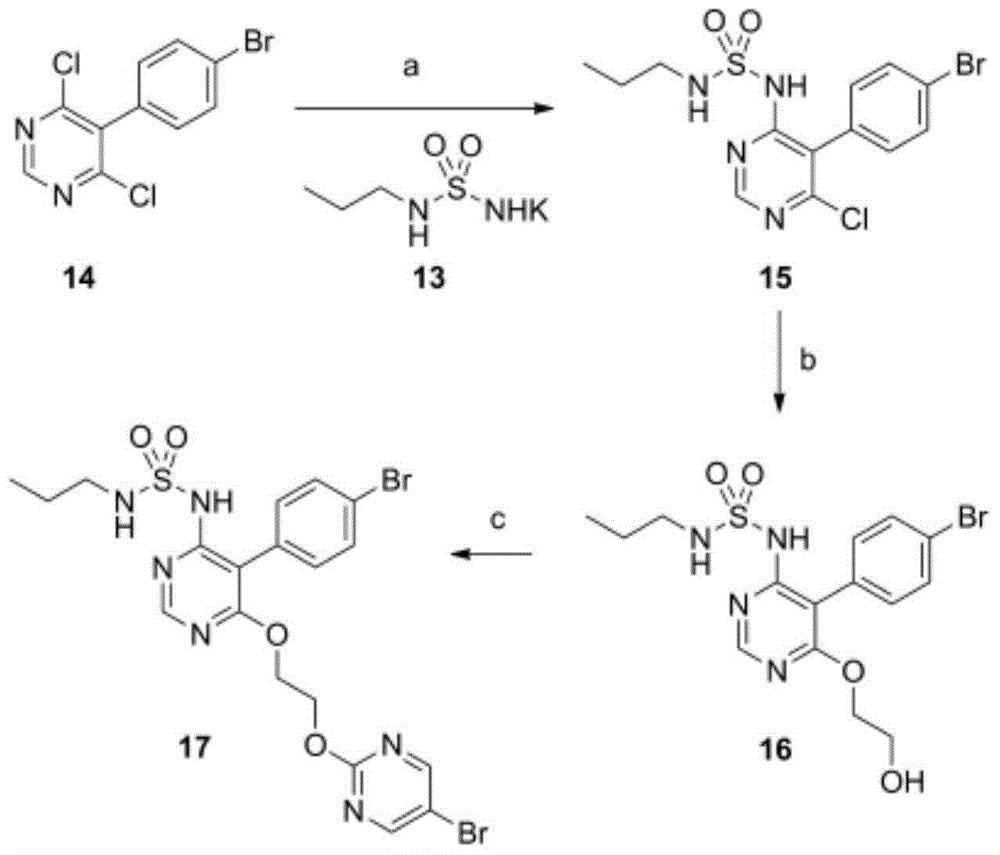
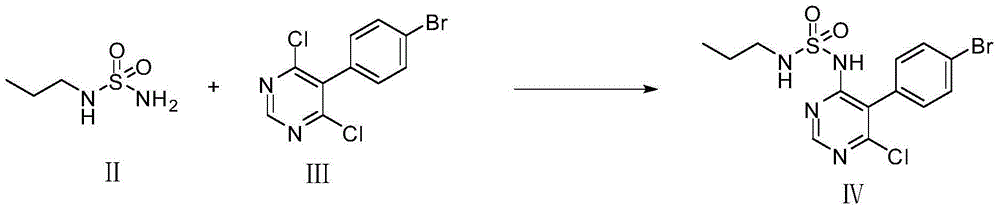
Method for preparing macitentan
A technology of macitentan and compounds, applied in the field of chemical drug synthesis, can solve problems such as long reaction time and unsuitable purification methods for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of macitentan
[0031] (1) N-5-(4-bromophenyl)-6-chloro-4-pyrimidinyl-N , -Synthesis of Propyl Sulfonamide (Formula (Ⅳ))
[0032] 16.8g of N-propylsulfonamide and 150mL of anhydrous toluene were added to a 1L three-necked flask, and then 13g of sodium hydride was added in batches, stirred for 1h, and then slowly added 30g of 5-(4-bromophenyl)-4,6-dichloro Pyrimidine, stirred at room temperature and reacted for 6-8 hours. After the reaction was monitored by TLC, the above reaction solution was poured into 1.2L of ice water, stirred while adding, and the insoluble solid was filtered out by suction. The filtrate was 120ml, 6mol / L dilute hydrochloric acid Adjust the pH to 3~4, a large amount of solids precipitate out, and filter with suction to obtain N-5-(4-bromophenyl)-6-chloro-4-pyrimidinyl-N , -Propylsulfonamide. 26.9g, yield: 67.3%.
[0033] (2) N-5-(4-bromophenyl)-6-(2-2-hydroxyethoxy-)4-pyrimidinyl-N , -Propyl Sulfonamide (Formula (VI) Synthesis
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com