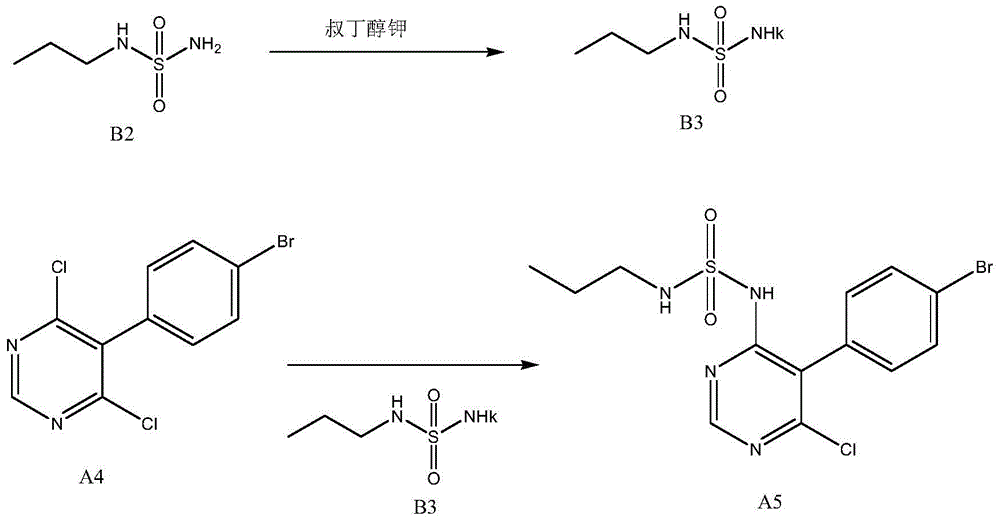
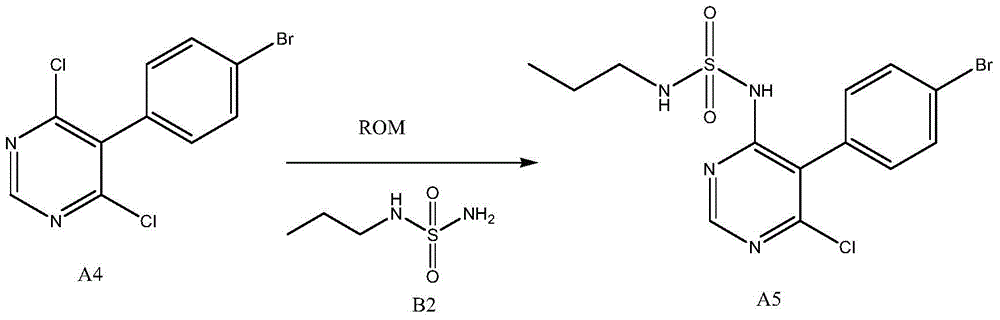
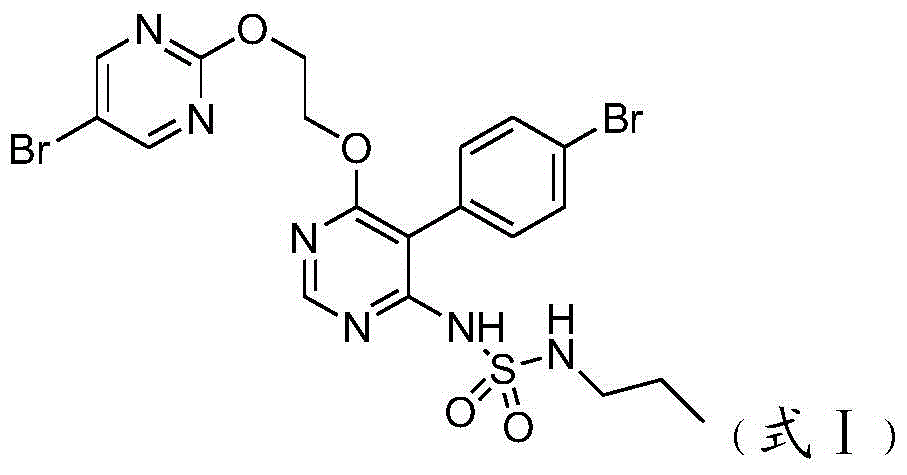
A kind of new preparation method of macitentan intermediate
A technology of volume ratio and reaction time, applied in organic chemistry and other fields, can solve the problems of N-propylsulfamate potassium salt transfer, storage difficulties, industrialization difficulties, and large amount of three wastes, so as to shorten the preparation cycle, The effect of large solvent capacity and large amount of waste treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of N-(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propylaminosulfonamide
[0031] The preparation method of N-(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propyl aminosulfonamide comprises the following steps:
[0032] 1) Add 272g (2.00mol) N-propylsulfamate (0.2% moisture) to 500g (1.65mol) of 5-(4-bromophenyl)-4,6-dichloropyrimidine and 5000ml of dimethyl In the base sulfoxide, then add 220g (2.00mol) of potassium tert-butoxide, at room temperature, stir, and react for 4 hours;
[0033] 2) Add 25000ml of saturated brine and 25000ml of ethyl acetate to the reaction solution for extraction, take the organic layer and concentrate it, and recrystallize the obtained concentrate with 2000ml of methanol to obtain N-(5-(4-bromophenyl)-6-chloro - 473 g of 4-pyrimidinyl)-N'-propylsulfamate (calculated as N-propylsulfamate, the molar yield is 58.4%).
[0034] According to the detection method of the present invention, the HPLC purity of N-(5-(4-bromo...
Embodiment 2
[0035] Example 2 Preparation of N-(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propylaminosulfonamide
[0036] The preparation method of N-(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propyl aminosulfonamide comprises the following steps:
[0037] 1) Add 272g (2.00mol) N-propylsulfamate (0.2% moisture) to 500g (1.65mol) of 5-(4-bromophenyl)-4,6-dichloropyrimidine and 5000ml of dimethyl In the base sulfoxide, add the potassium ethylate of 168g (2.00mol) again, room temperature, stir, react 4 hours;
[0038]2) Add 25000ml of saturated brine and 25000ml of ethyl acetate to the reaction solution for extraction, take the organic layer and concentrate, and recrystallize the obtained concentrate with 2000ml of methanol to obtain N-(5-(4-bromophenyl)-6-chloro- 446 g of 4-pyrimidinyl)-N'-propylsulfamate (calculated as N-propylsulfamate, the molar yield is 55.0%).
[0039] According to the detection method of the present invention, the HPLC purity of N-(5-(4-bromophenyl)-6-chloro...
Embodiment 3
[0040] Example 3 Preparation of N-(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propylaminosulfonamide
[0041] The preparation method of N-(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)-N'-propyl aminosulfonamide comprises the following steps:
[0042] 1) Add 272g (2.00mol) N-propylsulfamate (moisture content 0.2%) to 500g (1.65mol) of 5-(4-bromophenyl)-4,6-dichloropyrimidine and 5000ml of tetrahydrofuran , then add 220g (2.00mol) of potassium tert-butoxide, stir at room temperature, and react for 6 hours;
[0043] 2) Add 25000ml of saturated brine and 25000ml of ethyl acetate to the reaction solution for extraction, take the organic layer and concentrate, and recrystallize the obtained concentrate with 2000ml of methanol to obtain N-(5-(4-bromophenyl)-6-chloro- 387 g of 4-pyrimidinyl)-N'-propylsulfamate (calculated as N-propylsulfamate, the molar yield is 47.7%).
[0044] According to the detection method of the present invention, the HPLC purity of N-(5-(4-bromophenyl)-6-chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com