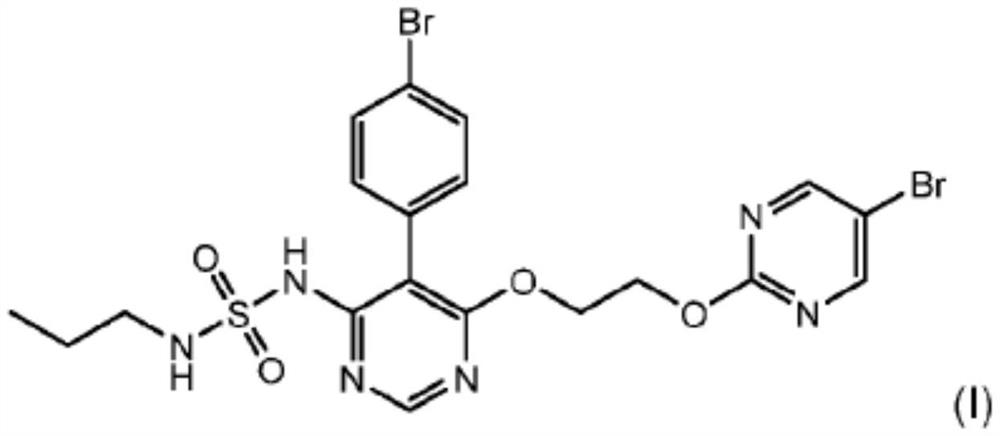
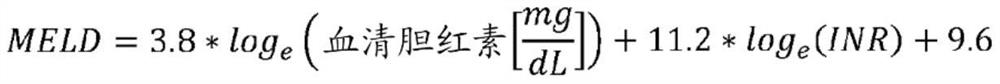Macitentan for use in treating portopulmonary hypertension
A technology of pulmonary arterial hypertension and macitentan, applied in organic active ingredients, cardiovascular system diseases, medical preparations containing active ingredients, etc., can solve problems such as no treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: The PORTICO Study
[0128] The study was conducted in seven countries, including Brazil, Czech Republic, France, Germany, Spain, the United Kingdom and the United States (52 sites initiated and patients enrolled and randomized at 36 sites).
[0129] Research objectives
[0130] main target
[0131] To assess the effect of macitentan versus placebo on PVR in patients with PoPH.
[0132] secondary goal
[0133] To evaluate the effect of macitentan compared with placebo on cardiopulmonary hemodynamics, hepatic portal pressure, disease severity and exercise capacity in patients with PoPH.
[0134] To evaluate the safety and tolerability of macitentan in patients with PoPH.
[0135] To assess the PK of macitentan and its active metabolite ACT-132577 in patients with PoPH (PK subgroup).
[0136] research plan
[0137] A. Overall Study Design and Plan
[0138] A1. Main research
[0139]The study was designed as a randomized, double-blind (DB), ...
Embodiment 2
[0662] Example 2: Analysis of Liver Transplantation
[0663] A. Purpose
[0664] The purpose of the analysis includes :
[0665] - To evaluate the effect of macitentan compared to placebo on N-terminal pro-b-type natriuretic peptide (NT-proBNP) relative to a threshold of 300 ng / L in patients with PoPH.
[0666] - To assess the effect of macitentan compared with placebo on perioperative risk stratification of liver transplantation in patients with PoPH.
[0667] - To evaluate the effect of macitentan compared to placebo on MELD exception eligibility in patients with PoPH.
[0668] - To assess the effect of macitentan compared with placebo on the risk of liver transplant waitlist mortality in patients with PoPH.
[0669] B. method
[0670] (i) Diagnosis and main inclusion criteria :
[0671] The study enrolled adult male or female patients (≥18 years) with confirmed symptomatic PoPH and baseline PVR≥4WU (≥320dyn.s.cm -5 ), and be able to perform a 6-minute walk t...
Embodiment 3
[0724] Example 3 - Post hoc analysis
[0725] This example provides a post hoc analysis of the study conducted in Example 1. These analyzes include:
[0726] • To assess the effect of macitentan on mean pulmonary arterial pressure (mPAP) relative to pretreatment mPAP.
[0727] • To assess the effect of macitentan on pulmonary vascular resistance (PVR) relative to pretreatment PVR.
[0728] For the post hoc analysis the following exploratory endpoints were defined:
[0729] • Changes in mPAP relative to pre-treatment mPAP.
[0730] Changes in PVR relative to pretreatment PVR
[0731] A. Statistical methods
[0732] All analyzes were performed on the full analysis set (FAS, see Randomization, N=85) and no data imputation was performed. FAS included all randomized patients who received at least one dose of study treatment during the double-blind treatment period and had a baseline value for the primary endpoint, PVR.
[0733] Risk categories are recorded in Table 38. Fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com