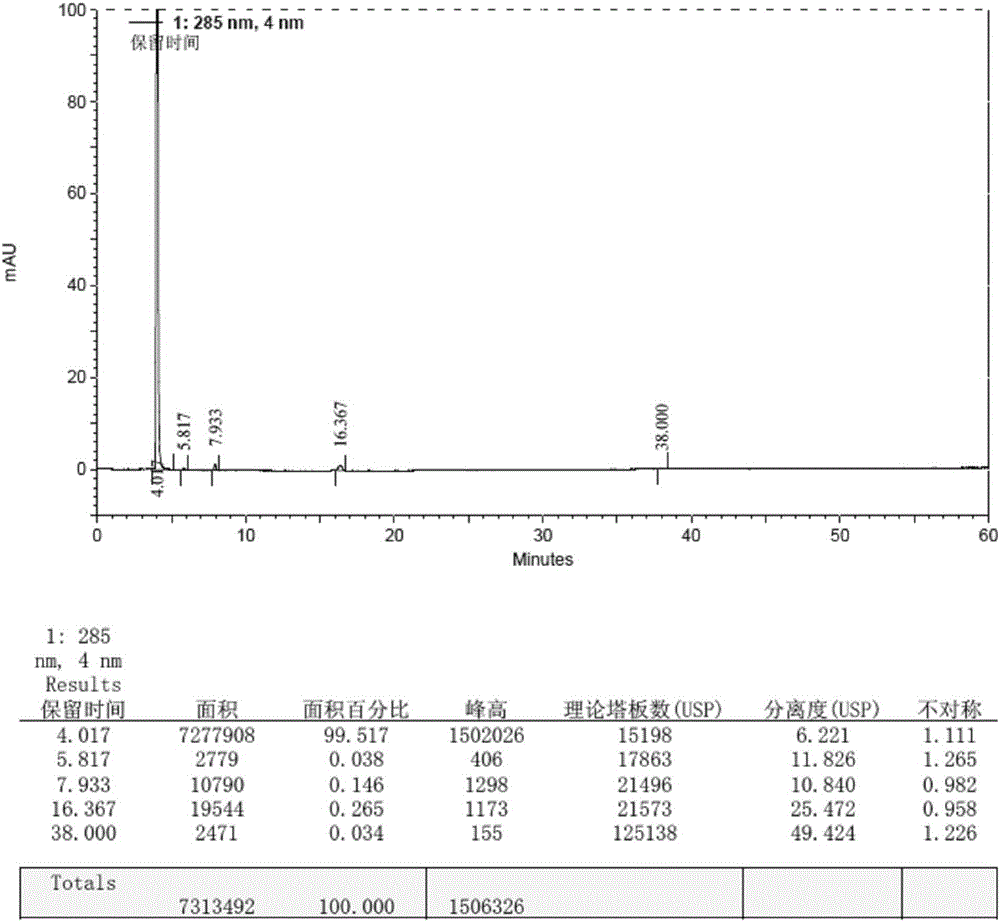
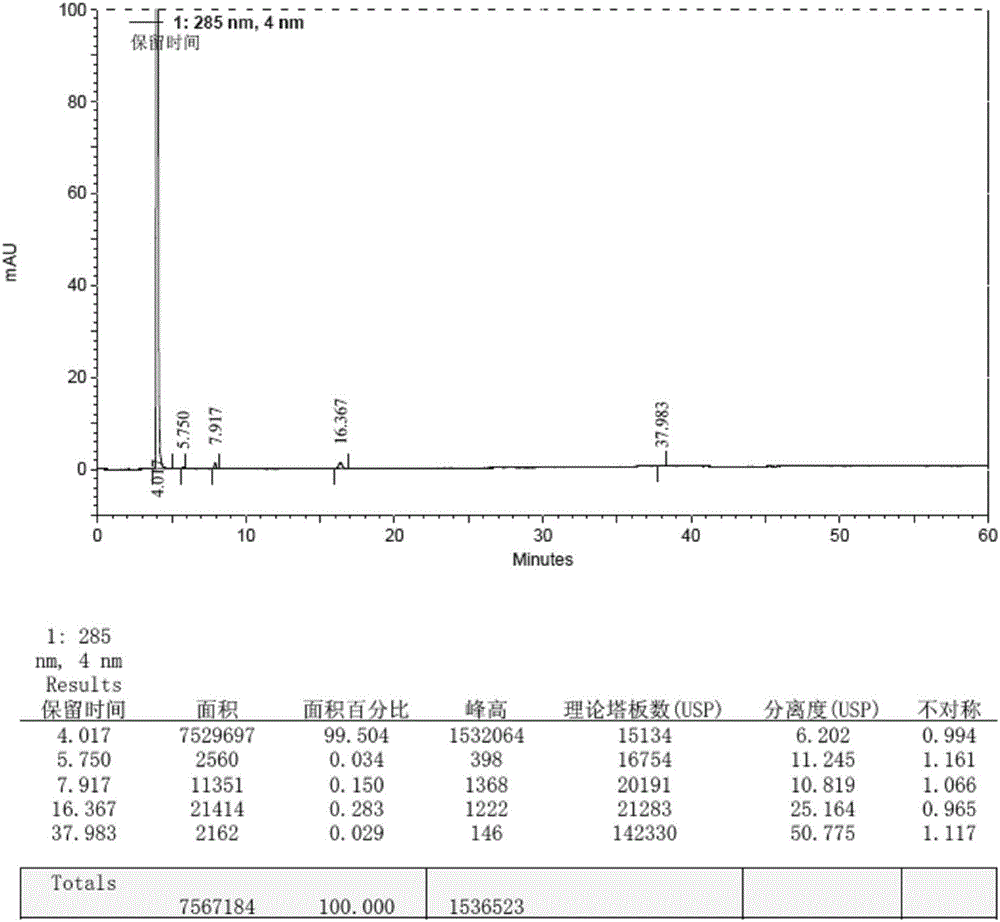
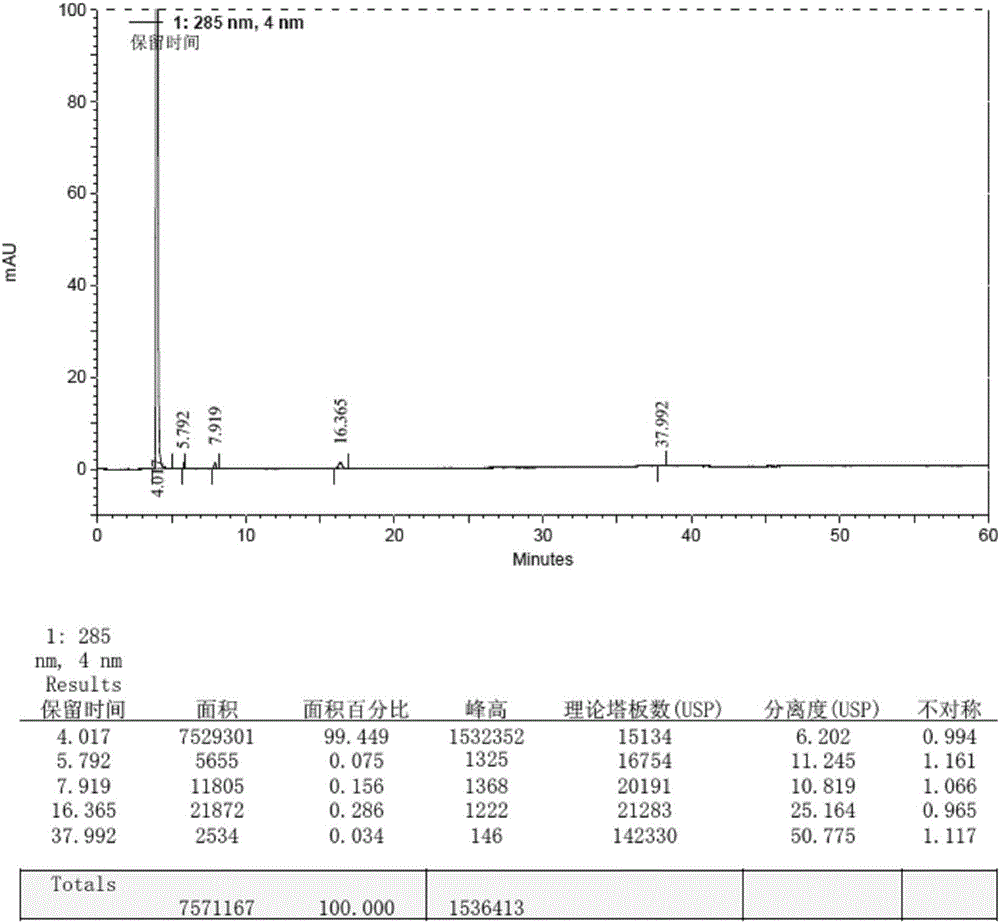
Method for preparing macitentan impurity standard substance
A technology of macitentan and standard products, applied in the field of medicine, can solve the problems of unreported synthesis and content calibration methods, high risk of macitentan, high risk of missed detection, etc., and achieve low synthesis cost and simple preparation process , The effect of short synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]1. Put 50ml of N,N-dimethylformamide, 10g (72.4mmol) of N-propylsulfonamide, and 2.6g (108.3mmol) of sodium hydrogen into a 250ml three-necked reaction flask in sequence, and stir at 10-15°C for 30min. Put in 22g (72.4mmol) of 5-(4-bromophenyl)-4,6-dichloropyrimidine, first stir the reaction at 20-25°C for 8h, then stir the reaction at 35-40°C for 1h, and cool down to 10-15°C Put in 2.2g (35.4mmol) of ethylene glycol and 13.5g (70.8mmol) of p-toluenesulfonyl chloride, raise the temperature to 85-95°C, continue stirring for 8h, cool down to 0-10°C, add 10wt% citric acid aqueous solution to crystallize , filtered, and the filter cake was washed with purified water until the filtrate was neutral, and the solid was collected and dried at 40-50° C. under reduced pressure (vacuum degree ≥ 0.08 MPa) for 6 hours to obtain 21.3 g of impurity crude product, with a yield of 75.2%.
[0049] 2. Add 70ml of ethyl acetate to 21.3g of crude impurity product, heat and stir at 75-85°C to ...
Embodiment 2
[0059] 1. Add 80ml of N,N-dimethylformamide, 10g (72.4mmol) of N-propylsulfonamide, and 5g (208.3mmol) of sodium hydrogen into a 250ml three-necked reaction flask in turn, stir at 10-15°C for 30min, and put in 22g (72.4mmol) of 5-(4-bromophenyl)-4,6-dichloropyrimidine, stirred and reacted at 20-25°C for 8h, then stirred at 35-40°C for 2h, cooled to 10-15°C, Add 2.2g (35.4mmol) of ethylene glycol and 16.8g (88.1mmol) of p-toluenesulfonyl chloride, raise the temperature to 85-95°C, continue stirring for 10h, cool down to 0-10°C, add 10wt% citric acid aqueous solution to crystallize, After filtering, the filter cake was washed with purified water until the filtrate became neutral, and the solid was collected and dried under reduced pressure (vacuum degree ≥ 0.08 MPa) at 40-50°C for 8 hours to obtain 22.2 g of impurity crude product, with a yield of 78.3%.
[0060] 2. Add 100ml of ethyl acetate to 21.3g of impurity crude product, heat and stir at 75-85°C to dissolve, filter while ...
Embodiment 3
[0064] 1. Put 500ml of N,N-dimethylformamide, 100g (724mmol) of N-propylsulfonamide, 26g (1083mmol) of sodium hydrogen into a 1L three-necked reaction flask in turn, stir at 10-15°C for 30min, and put in 5- (4-Bromophenyl)-4,6-dichloropyrimidine 220g (724mmol), first stirred and reacted at 20-25°C for 8h, then stirred and reacted at 35-40°C for 1h, cooled to 10-15°C, and put into ethylene di 22.3g (35.9mmol) of alcohol and 137g (71.9mmol) of p-toluenesulfonyl chloride, heat up to 85-95°C, continue to stir for 9h, cool down to 0-10°C, add 10wt% citric acid aqueous solution to crystallize, filter, filter cake Wash with purified water until the filtrate is neutral, collect the solid, and dry it under reduced pressure (vacuum degree ≥ 0.08 MPa) at 40-50°C for 8 hours to obtain 212.5 g of crude impurity product, with a yield of 73.9%.
[0065] 2. Add 700ml of ethyl acetate to 212.5g of impurity crude product, heat and stir at 75-85°C to dissolve, filter while hot, add 3000ml of met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com