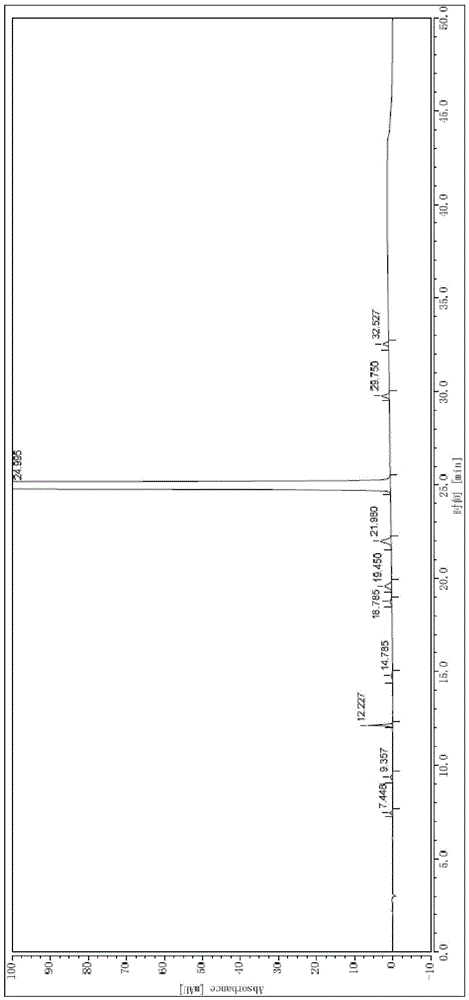
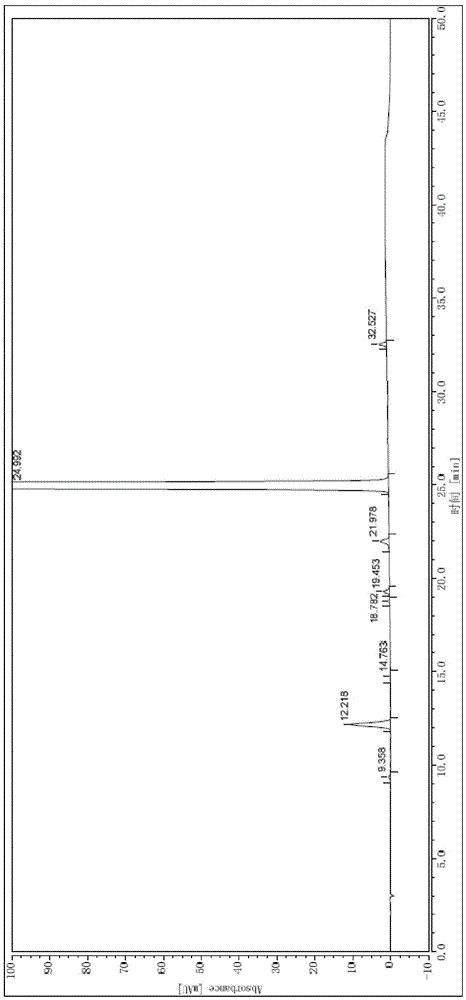
Method for refining high-purity macitentan
A refining method and technology of macitentan, applied in the field of refining endothelin receptor antagonist macitentan, can solve the problems of difficult crystal removal, poor solubility, increase of impurity L and impurity M, etc., and achieve product purity and High yield, low production cost, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Heat 100ml of ethyl acetate at 76-80°C to reflux and boil for 30min. Under the condition of avoiding light, add 20g of macitentan crude product, stir and dissolve at 76-80°C, add 0.2g of activated carbon, stir for decolorization for 10min, press filter while hot, The filtrate was protected from light and cooled to 45-50°C, stirred and crystallized for 1.5h, then slowly cooled to 25-30°C, stirred and crystallized for 1h, filtered, and dried under reduced pressure at 35-45°C for 8h to obtain 18.4g of pure macitentan. Yield 92.0%, purity 99.94%.
Embodiment 2
[0055] Mix 75ml of methanol and 375ml of ethyl acetate, heat and reflux at 68-75°C for 1h, put in 20g of crude macitentan under the condition of avoiding light, stir and dissolve at 60-70°C, add 0.2g of activated carbon, stir for 15min to decolorize, Hot press filtration, the filtrate was protected from light and cooled to 50-55°C, stirred and crystallized for 2 hours, then slowly cooled to 20-25°C, stirred and crystallized for 1.5 hours, filtered, dried under reduced pressure at 35-45°C for 6 hours, and pure macitentan was obtained. Product 17.8g, yield 89.0%, purity 99.97%.
Embodiment 3
[0057] Mix 200ml of ethyl acetate and 300ml of n-hexane, heat and reflux at 70-80°C for 1 hour, put in 20g of the crude product of macitentan under the condition of avoiding light, stir and dissolve at 70-80°C, add 0.2g of activated carbon, stir for 5min to decolorize, Press filter while it is hot, cool the filtrate to 45-50°C in the dark, stir and crystallize for 1.5h, then slowly cool down to 20-25°C, stir and crystallize for 1h, filter, and dry under reduced pressure at 35-45°C for 4h to obtain macitentan Pure product 17.3g, yield 86.5%, purity 99.98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com