High performance liquid chromatography method of macitentan related substances
A technology of high performance liquid chromatography and analysis methods, applied in the direction of analysis materials, material separation, measurement devices, etc., can solve the problem that there are no relevant literature reports on the analysis methods of macitentan raw materials and their preparations, and achieve strong specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Detection equipment and chromatographic conditions:
[0042] High performance liquid chromatography: LC-10AD pump, SPD-M10A detector
[0043] Chromatographic column: AgilentC18 (250×4.6mm, 5μm); mobile phase A: acetonitrile-water-formic acid solution with a volume ratio of 49:51:0.1; mobile phase B: acetonitrile-water-formic acid solution with a volume ratio of 85:15:0.1 Formic acid solution; detection wavelength: 260nm; flow rate: 1.0ml / min; injection volume: 20μl.
[0044] Experimental steps:
[0045] (1) Sample preparation:
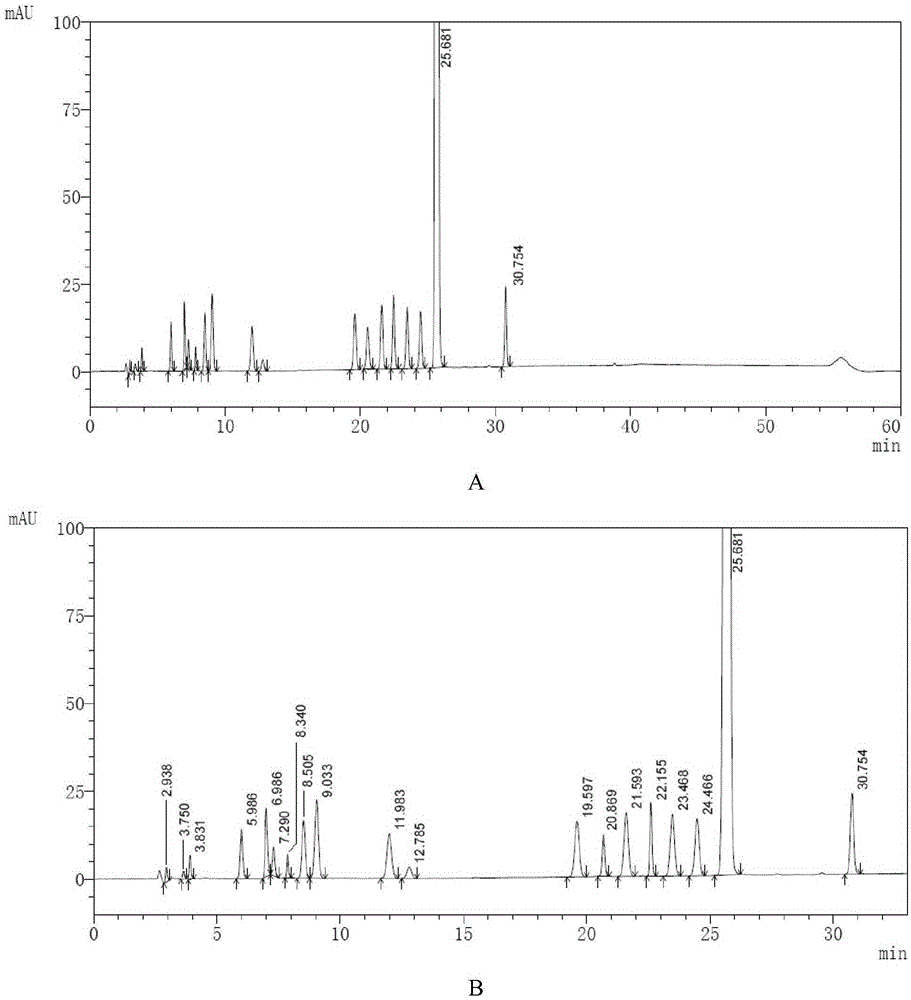
[0046] Take an appropriate amount of macitentan and known impurities A-R, and use ammonium bicarbonate buffer solution with a volume ratio of 1:4 (take 1.58g of ammonium bicarbonate, add 1000ml of water to dissolve, adjust the pH value to 9.0 with ammonia water)-acetonitrile solution for ultrasonication Dissolve and dilute to make a solution containing about 1 mg / ml of macitentan and about 1 μg / ml of impurities A-R, as a sample solution;
[00...
Embodiment 2
[0052] Detection equipment and chromatographic conditions:
[0053] High performance liquid chromatography: LC-10AD pump, SPD-M10A detector
[0054] Chromatographic column: Agilent C18 (250×4.6 mm, 5 μm); mobile phase A: same as Example 1, mobile phase B: same as Example 1; flow rate: 1.0 ml / min; detection wavelength: 260 nm; injection volume: 20 μl.
[0055] (1) Sample preparation:
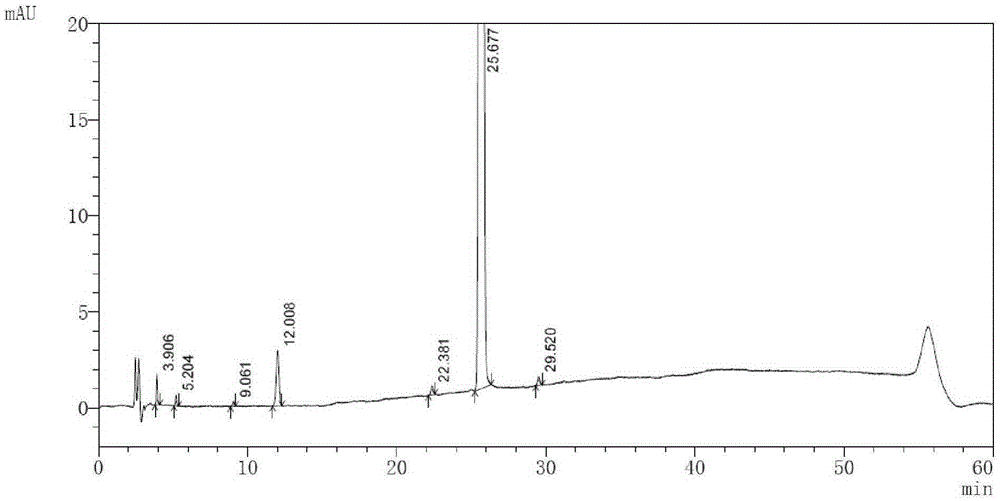
[0056] Acid destruction: Take macitentan tablets, grind them finely, take an appropriate amount of fine powder (approximately equivalent to 50mg containing macitentan), take 50mg of macitentan raw materials, put them in a 50ml measuring bottle, add 5ml of 1mol / L hydrochloric acid solution , placed in a water bath at 80°C for 1 hour, cooled rapidly, added 5ml of 1mol / L sodium hydroxide solution to neutralize, and used ammonium bicarbonate buffer solution with a volume ratio of 1:4 (take 1.58g of ammonium bicarbonate, add 1000ml of water to dissolve, and use ammonia water Adjust the pH value to 9...
Embodiment 3
[0066] Detection equipment and chromatographic conditions:
[0067] High performance liquid chromatography: SSISeries1500 pump, Series1500PDA detector
[0068] Chromatographic column: Agilent C18 (250×4.6 mm, 5 μm); mobile phase A: same as Example 1, mobile phase B: same as Example 1; flow rate: 1.0 ml / min; detection wavelength: 260 nm; injection volume: 20 μl.
[0069] Experimental steps:
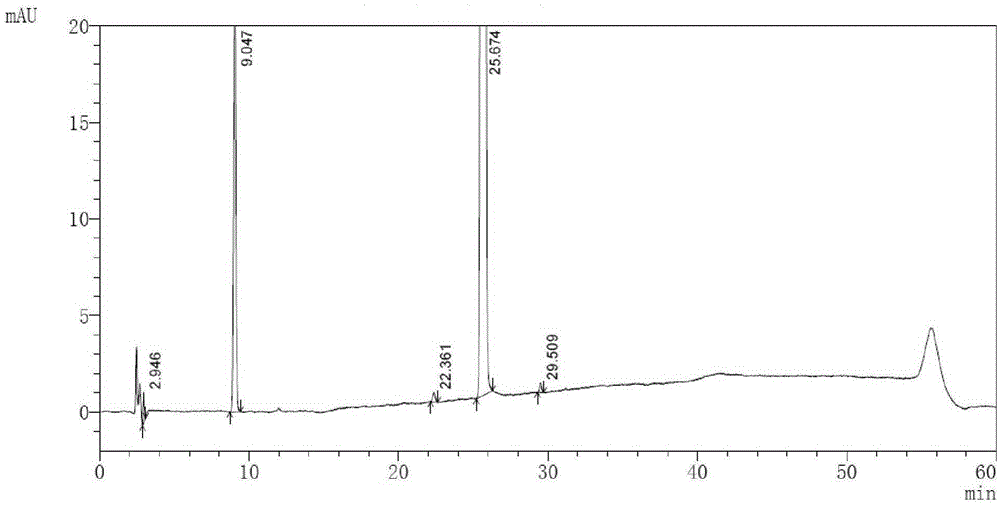
[0070] (1) Sample preparation: take an appropriate amount of macitentan, and use ammonium bicarbonate buffer solution with a volume ratio of 1:4 (take 1.58g of ammonium bicarbonate, add 1000ml of water to dissolve, adjust the pH value to 9.0 with ammonia water)-acetonitrile solution for ultrasound Dissolve and dilute to make a solution containing macitentan 1 mg / ml as the sample solution.
[0071] (2) Gradient elution program setting:
[0072] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
100
0
10
100
0
35
0
100
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com